Abstract
Cerebral small vessel diseases (SVDs) encompass a group of genetic and sporadic pathological processes leading to brain lesions, cognitive decline, and stroke. There is no specific treatment for SVDs, which progress silently for years before becoming clinically symptomatic. Here, we examine parallels in the functional defects of parenchymal arterioles in CADASIL, a monogenic form of SVD, and in response to subarachnoid hemorrhage, a common type of hemorrhagic stroke that also targets the brain microvasculature. Both animal models exhibit dysregulation of the voltage-gated potassium channel, KV1, in arteriolar myocytes, an impairment that compromises responses to vasoactive stimuli and impacts cerebral blood flow autoregulation and local dilatory responses to neuronal activity (neurovascular coupling). However, the extent to which this channelopathy-like defect ultimately contributes to these pathologies is unknown. Combining experimental data with computational modeling, we describe the role of KV1 channels in the regulation of myocyte membrane potential at rest and during the modest increase in extracellular potassium associated with neurovascular coupling. We conclude that parenchymal arteriole resting membrane potential and myogenic tone depend strongly on KV1.2/1.5 channel density, and that reciprocal changes in KV channel density in CADASIL and subarachnoid hemorrhage produce opposite effects on extracellular potassium-mediated dilation during neurovascular coupling.
Keywords: voltage-gated potassium channel, cerebral blood flow, cerebral small vessel disease, CADASIL, subarachnoid hemorrhage
INTRODUCTION
Dementia and stroke rank among the most pressing health issues worldwide (1,2). Cerebral small vessel diseases (SVDs), termed for various pathologies associated with small vessel dysfunction in the brain, have emerged as a central link between these two major co-morbidities. SVDs account for at least 40 % of dementia cases and more than 30 % of strokes (1,3). They encompass multiple distinct diseases that can be separated based on their underlying genetic defects, risk factors, and clinical presentations. Despite the severe nature of these diseases, there are no treatments with proven efficacy against cerebral SVDs.
Major progress has been made in identifying overlapping mechanisms involved in the functional defects of small (parenchymal) arterioles within the brain in response to subarachnoid hemorrhage (SAH) (4) and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a monogenic form of SVD (5). Although, strictly speaking SAH is not a cerebral SVD, an increasing body of evidence suggests that SAH-induced perfusion deficits originate from the parenchymal microvasculature (6–13). These deficits appear to be driven by changes in the balance of matrix metalloproteinase (MMP) activity which supports the novel concept that perturbations of the extracellular matrix can be a point of convergence between these cerebral small vessel pathologies (14). These changes, in turn, alter the number of voltage-gated K+ (KV) channels at the arteriolar smooth muscle cells (SMCs) plasma membrane (15–18). The KV channel superfamily, comprising 12 subfamilies (KV1–KV12), is one of the most diverse families of K+ channels identified to date (19). Molecular cloning of K+ channels from different cell types has revealed that KV subfamilies share a common primary structure, reflecting hetero- or homo-multimeric assembly of four pore-forming α subunits and auxiliary β subunits, with the identity of the α subunit being the primary determinant of biophysical and pharmacological properties of individual channels (20–22). In vascular SMCs, KV channels are active under physiological conditions, and the K+ efflux that they mediate serves as a key negative feedback control mechanism that opposes moment-to-moment pressure-induced constriction (23). Thus, any increase or decrease in functional KV channel density on the plasma membrane of arteriolar SMCs is predicted to impact arteriolar diameter.
The potential dire consequences of disrupting the number of functional SMC KV channels are evident in the two divergent cerebral small vessel pathologies presented here—SAH and CADASIL. In the hemorrhagic stroke (i.e. subarachnoid hemorrhage) model, MMP activation is understood to cause KV channel endocytosis through activation of the epidermal growth factor receptor (EGFR), resulting in increased vasoconstriction and decreased cerebral blood flow (CBF) (15,16). On the other hand, in a mouse model of CADASIL, an increase in tissue inhibitor of metalloproteinase-3 (TIMP-3) inhibits MMP (ADAM17) activity, leading to an increased number of functional KV channels in the plasma membrane of arteriolar SMCs and attenuation of vasoconstriction (17,18). These findings support the concept that the number of KV channels are tuned to provide the appropriate membrane potential control in response to changes in intravascular pressure, and that any change in channel number will adversely affect cerebral arteriolar function.
Here, we weave experimental data and computational modeling to describe small vessel dysfunction after SAH and in CADASIL from a KV channelopathy perspective, revealing how the “Yin and Yang” of SMC KV channels—the balance between upregulation and downregulation—is crucial for maintaining parenchymal arteriole (PA) function, CBF and, ultimately, brain health.
1. KV CHANNELS IN MYOCYTES PROFOUNDLY REGULATE THE MYOGENIC TONE OF BRAIN PARENCHYMAL ARTERIOLES
In vivo, small diameter arteries and arterioles exist in a partially constricted state, largely owing to the vasoconstrictor influence of intravascular pressure. These blood vessels can constrict further as pressure increases and, conversely, dilate when the pressure lessens (24). This response, referred to as myogenic tone, supports CBF autoregulation and protects capillaries from the disruptive effects of high blood pressure (25). Within the brain, myogenic tone also provides the vasodilatory reserve necessary for PAs to locally dilate and increase blood delivery in response to neuronal activity. This use-dependent increase in blood flow, termed functional hyperemia, is supported by a variety of mechanisms collectively referred to as neurovascular coupling (NVC) (1,26–29). Thus, the myogenic response within the cerebral vasculature, specifically in small diameter arterioles, is crucial for CBF control.
This myogenic response can be reproduced ex vivo using an arteriography system by increasing intravascular pressure through a cannula inserted into the arteriolar lumen, enabling the study of the molecular players that contribute to this phenomenon. Intravascular pressure causes a graded membrane potential (Vm) depolarization of SMCs that leads to an increase in the open-state probability of voltage-dependent Ca2+ channels (VDCCs), thereby enhancing Ca2+ influx and ultimately causing myogenic constriction (23,30). In mouse PAs, elevation of luminal pressure from 10 mmHg to 40 mmHg typically causes an 18-mV depolarization of the SMC membrane from −53 mV to −35 mV; the associated doubling in the level of myogenic tone manifests as a constriction that represents approximately a 35–40% decrease in arterial diameter (17).
Like most biological processes, myogenic tone is modulated by negative feedback elements (31). Among them, K+ channels in SMCs and endothelial cells (ECs) of arterioles can serve as a brake on pressure-induced depolarization and constriction (23). At a physiological extracellular K+ concentration ([K+]o) of 3 mM and an intracellular K+ concentration of 140 mM, the equilibrium potential for K+ (EK) is −102.7 mV. At 40 mm Hg, the estimated pressure experienced in vivo by cerebral arterioles of this size (32), the SMC membrane potential is about −35 mV, creating a 68-mV driving force for K+ efflux. Consequently, opening of K+ channels exerts a rapid and strong hyperpolarizing effect that acts to oppose pressure-induced constriction and, more broadly, provides a vasodilatory influence.
Arteriolar SMCs predominantly express four types of K+ channels: ATP-sensitive (KATP), large conductance Ca2+-activated (BK), inward rectifier (KIR), and voltage-gated (KV) (23,25,33,34). Interestingly, it appears that only KIR and KV channels are active under physiological conditions in PA SMCs examined in vitro. KATP channels are expressed in large cerebral pial arteries on the surface of the brain (35), but the absence of PA dilation in response to the KATP agonist cromakalim suggests a minimal role for this channel type within the brain cortex (36). The apparent lack of functional KATP channels in PAs is consistent with previous reports that small cerebral arterioles fail to dilate in response to cromakalim (37–40). In many vascular beds, including cortical surface arteries, BK channels are activated by local Ca2+-release events (Ca2+ sparks) through ryanodine receptors (RyRs), and exert tonic negative feedback on myogenic tone (41,42). Consistent with this tonic negative feedback element opposing myogenic tone, treatment with BK channel blockers such as paxilline causes significant and sustained constriction of pial cerebral arteries (43). In contrast, BK channel blockade does not alter PA diameter, suggesting that BK channel-mediated K+ efflux does not constitute a significant feedback mechanism against myogenic tone in PAs under physiological conditions (17,44–46), possibly because Ca2+ spark activity is very low in these arterioles (at least in rat and mouse) (47–50). However, considering that BK currents are detectable in isolated PA SMCs (9,46), and can be activated in certain conditions such as acidosis (36,50), a role for BK (and KATP) channels under all physiological and pathological conditions or in all species is possible.
KIR channels, predominantly KIR2.1 channels, are present in both PA SMCs (51–53) and ECs (54). KIR2.1 channels conduct strong inward current at Vm negative to EK and a small outward current that peaks and then decreases as Vm becomes increasingly positive to EK (23,54). The role of KIR channels in regulating smooth muscle membrane potential has been described in previous reviews (23,34,40). Inhibition of this channel with 30 µM Ba2+ or genetic ablation in ECs does not affect PA resting diameter (55). This is likely because channel activity at −35 mV, the resting Vm of PAs at 40 mmHg, is very low, with a conductance, gKIR, about a 10,000-time less than the maximal conductance of this channel (54,56,57). In addition to its aforementioned dependence on Vm, KIR is also activated by external K+. This enables SMCs and ECs to sense elevations in extracellular K+ in response to neuronal activity and mediate NVC (7,45,51,55). Therefore, the physiological role of KIR in the brain microcirculation during NVC has been likened to that of a “vascular K+ electrode”, as defined by Longden and Nelson (54). The voltage and K+ dependence of KIR synergize in response to small increases in external K+, leading to dramatic hyperpolarization that is capable of bringing Vm close to EK and causing near maximal dilation (45,51,55).
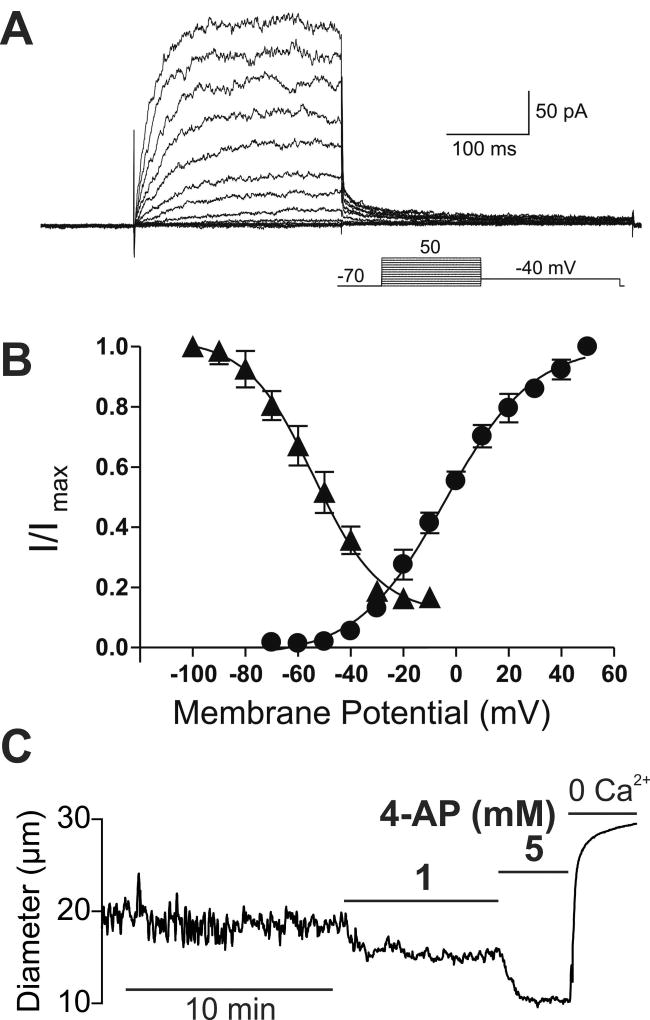
We have established that KV channels play a tonic and profound role in opposing vasoconstrictor influences (e.g., myogenic tone) in PAs from rodent brains (17,58,59). SMC membrane depolarization in response to pressure or vasoconstrictors increases KV channel open probability, creating a hyperpolarizing K+ efflux that counterbalances depolarizing current through VDCCs and other Na+/Ca2+ permeable channels to act as a break on vasoconstriction. KV channels in vascular SMCs show fast activation kinetics in response to membrane potential depolarization, with activation time constants (τact) on the order of tens of milliseconds (Figure 1A). In addition, the steady-state activation and inactivation properties of KV allow significant K+ currents between −40 and +10 mV, implicating KV channels in the regulation of PA SMC membrane potential at physiological intravascular pressures (Figure 1B). The contribution of KV channels to the regulation of resting PA diameter is illustrated by the significant constriction caused by inhibition of KV channels with 1 mM 4-aminopyridine (4-AP) at physiological pressure (40 mmHg) (Figure 1C) (17,23,58,60,61).
Figure 1. KV channels exert a tonic dilatory influence on the diameter of intracerebral arterioles.
(A) Families of KV currents from an isolated arteriolar smooth muscle cell elicited by voltage pulses from −70 mV to +50 mV in the presence of 100 nM iberiotoxin to inhibit large conductance (BK) currents. (B) steady-state activation (circles) and inactivation (triangles) properties of KV currents measured from isolated arteriolar smooth muscle cells. Solid lines, Boltzmann fits to the data. (C) Typical recording of the internal diameter of a pressurized parenchymal arteriole (40 mm Hg) showing the constriction caused by the perfusion of the KV blocker 4-AP, 1 and 5 mM. A and B are from (58) and C is from (17).
The voltage dependence of KV channels has been described using a Boltzmann-type steady-state activation term (Figure 2). The characteristics of this voltage dependence—voltage at half-maximal activation (V0.5) and slope factor (kKv)—appear to vary significantly among channel subtypes (17,22,23). The involvement of KV1 channel family members, predominantly KV1.2 and KV1.5 subtypes, in the regulation of arteriolar tone has been documented in the cerebral vasculature of multiple species (17,58,60,62,63). Studies have also reported that KV2 channels, primarily those of the KV2.1 subfamily, contribute to counteracting myogenic constriction in rat pial cerebral arteries (64–67). Other groups have also provided evidence supporting the contribution of KV7 channels (KV7.1, KV7.4, and KV7.5 subtypes) to the regulation of pial cerebral arterial diameter (61,68). Regarding intracerebral PAs, a number of lines of experimental evidence support the view that KV1.2 and KV1.5 subtypes are predominantly expressed and primarily regulate PA SMC Vm and arteriolar diameter. First, V0.5 values measured in PA SMCs were −3.2 mV in rat (58) and +6 mV in mouse PA SMCs (17), similar to values reported for heteromultimers of KV1.2/1.5 channels (69). In contrast, the V0.5 value of KV7 channels are ~ −30 mV (70). Second, RT-PCR revealed mRNA expression of KV1.2 and KV1.5, but not KV1.1, KV1.3, KV1.4, KV1.6 or KV2.1 in rat PAs (58). Third, stromatoxin, an inhibitor of KV2 channels (66,71), does not significantly constrict mouse parenchymal arterioles (17). Furthermore, it has been shown that the 4-AP sensitivity of K+ currents, Vm, and tone are dependent on the expression of KV1.2 and KV1.5 subtypes (60,69,72–74).
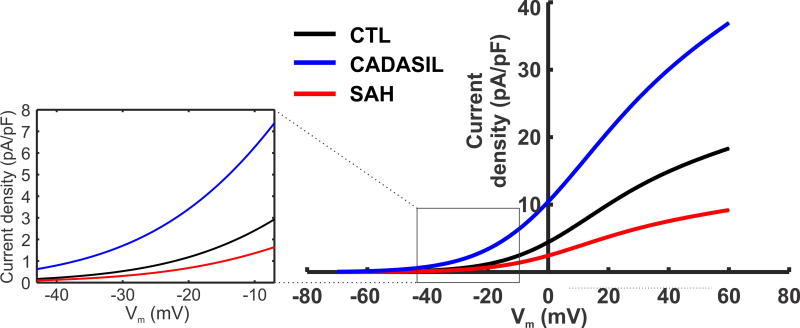
Figure 2. Steady state KV current density in PA SMCs from normal and diseased animal models.
Steady state KV current densities for CTL, CADASIL (TgNotch3R169C), and SAH models are fitted using a linear equation with a Boltzmann-type activation term , from experimental data (17) and Koide & Wellman unpublished data. Cm is the membrane capacitance; Vm is the membrane potential; GKv is the whole-cell conductance of KV channels; EK is the reversal potential for K+. At physiological membrane potentials pA differences in KV currents are predicted (Figure inset). Model parameters: Gkv = 1.6 [nS]; VKv,0.5 = 6 [mV]; kkv = 14 [mV] for control; Gkv = 0.8 [nS]; VKv,0.5 = 6 [mV]; kkv = 14 [mV] for SAH; and Gkv = 3.2 [nS]; VKv,0.5 = 2.6 [mV]; kkv = 15.8 [mV] for CADASIL model; Cm = 12.8 [pF]; [K+]i = 150 [mM]; [K+]o = 3 [mM].
2. IMPACT OF PATHOLOGICAL INCREASES (CADASIL) OR DECREASES (SAH) IN PA KV CHANNEL NUMBERS
The structural organization of the cerebral circulation may explain the formation of regional pathological lesions, which are associated with small vessel dysfunction in SVDs. The two-dimensional network of surface (pial) arteries branches out and dives down into the brain parenchyma as PAs, which ultimately transition into capillary networks of interconnected vessels with a considerable capacity for redirecting blood flow (75). However, PAs upstream of the interconnected capillary networks control the blood supply of a specific volume of brain territory; as such, they represent bottlenecks to blood flow and thus are the element most vulnerable to cerebral SVDs (76,77). PAs also exhibit unique features, including the lack of extrinsic innervation and the presence of astrocytic processes that enwrap almost their entire basolateral surface (78–80). PAs are the last smooth muscle-containing vessels upstream of the capillary bed, and their active luminal diameter in mouse brain is typically < 20 µm—about one-third the width of a human hair. These anatomical features make ex vivo experimental approaches for studying their function extremely challenging, which has limited the investigation of this pathophysiologically important vascular bed.
In both SAH and CADASIL, dysregulation of brain PA reactivity precedes the onset of neurological deficits. Using well-established rabbit (15,43,81), rat (6,7) and mouse (12,13) models of SAH, and a transgenic mouse model of CADASIL (TgNotch3R169C) in which a human NOTCH3 receptor mutation—the molecular cause of CADASIL—is overexpressed (17,82), we have discovered that KV channel activity in PA SMCs is abnormal in both pathological conditions (17,18,59). Although abnormal KV activity could conceivably reflect changes in channel gating properties or recruitment of new KV channel family members, our experimental data demonstrated unchanged τact, V0.5, or k in both disease models. Instead, the observed changes in KV channel activity better correlates with a change in the number of functional channels at the SMC plasma membrane (Figure 2). Specifically, SAH causes a decrease in functional KV channels and enhanced vasoconstriction. Conversely, CADASIL model mice exhibit an increase in SMC membrane KV channel numbers and a decrease in cerebral arteriolar tone. One possible mechanism underlying both of these vascular pathologies is altered trafficking of KV channels. In this context, altered shedding of the epidermal growth factor receptor ligand, HB-EGF, caused by aberrant MMP and/or ADAM (a disintegrin and metalloproteinase) activity, leads to enhanced (SAH) or decreased (CADASIL) EGFR-mediated endocytosis of KV channels (14–18).
Based on current density data obtained experimentally using the perforated-patch configuration of the patch-clamp technique (17) (Koide & Wellman unpublished data), we calculated the number of KV channels in control (CTL), CADASIL and SAH conditions at a given voltage (−40 mV) using the Goldman–Hodgkin–Katz constant field equation ((83); see (17) for calculation details). We found that the average number of functional KV channels per myocyte in CTL mice was 3,060 ± 479 (6 cells from 6 animals), a number that increased to 4,970 ± 655 (6 cells from 6 animals) in CADASIL (TgNotch3R169C) mice. A comparison of CTL and SAH rats revealed the opposite change, with 3,465 ± 346 channels per myocyte observed in CTL rats (6 cells from 5 animals) compared with 1809 ± 423 in SAH rats (6 cells from 6 animals) (Koide & Wellman unpublished data). In each case, the cell capacitances of myocytes were similar, indicating a similar membrane surface area in all three conditions. Therefore, the CADASIL-causing mutation results in a ~57 % increase in KV channel number, whereas SAH reduces KV channel number by ~48 %. The differences in KV current densities between disease and control animals, at different membrane potentials, are depicted in Figure 2. Steady-state KV current data for CTL and CADASIL mice are fitted to a Boltzmann-type Vm-dependent activation, as previously done (17). The voltage dependency of KV1 in SAH is predicted based on a 48% reduction in KV1 conductance relative to CTL. SMC outward KV1 current increases dramatically at depolarized potentials, reflecting KV channel opening with depolarization, and current density correlated with the KV channel number in all conditions.
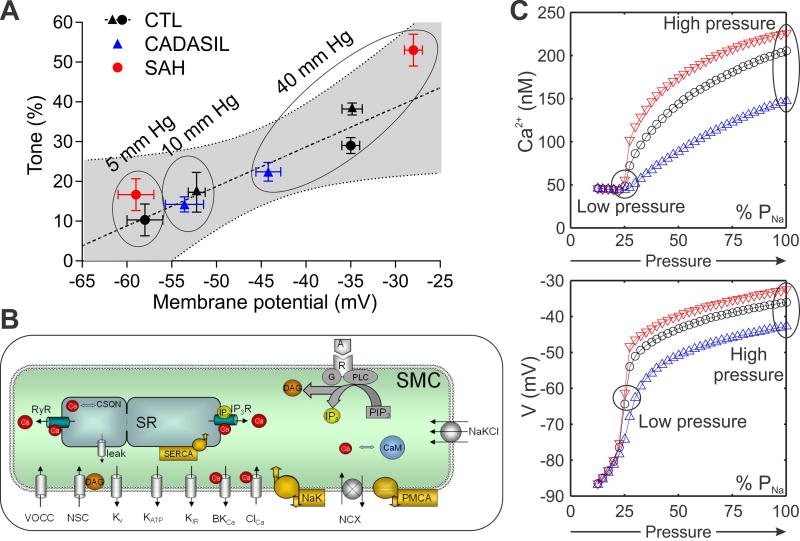
Given the role of K+ efflux through KV channels in opposing pressure-induced membrane depolarization and vasoconstriction, the prediction is that increased (CADASIL) (17) or decreased (SAH) (6,84) KV channel numbers would alter the degree of negative-feedback control, resulting in hyperpolarization (CADASIL) or depolarization (SAH) of PA SMCs. Consistent with this prediction, experimentally measured Vm was hyperpolarized (−45 mV) (17) and depolarized (−28 mV) (6) in pressurized (40 mmHg) PAs obtained from CADASIL mice and SAH model animals, respectively, compared to CTL groups (−35 mV). The relationship between myogenic tone and membrane potential in CTL, CADASIL and SAH animals is summarized in Figure 3A. At lower intravascular pressure (5 and 10 mmHg), tone and membrane potential did not differ among the three groups. However, as pressure is increased to a more physiological level (40 mmHg), Vm in PAs from CADASIL mice remained almost 10 mV more hyperpolarized than that in CTL PAs (−45 mV vs. −35 mV), whereas Vm was pulled to −28 mV in SAH (6,17).
Figure 3. Relationship between myogenic tone and membrane potential.
(A) Values of membrane potential and myogenic tone at different intravascular pressure (mm Hg) from CADASIL (TgNotch3R169C, blue triangles) and SAH (red circles) animals are consistent with the linear regression obtained from CTL animals (black triangles represent CTL mice from (17) and black circles represent CTL rats from (6)) showing a similar relationship between tone and membrane potential. (B) A detailed model of SMC membrane potential and Ca2+ dynamics was adapted from (102) and modified by incorporating the KV1 current of PA SMCs from CTL animals (Figure 2), while adjusting other transmembrane currents (KIR, NSC, VDCC, NaK, PMCA) to produce resting Vm and Ca2+ concentration in agreement with experimental data (17,103). The effect of altered KV1 channel density in CADASIL (blue triangles) and SAH (red triangles) was examined assuming all other model parameters remain the same as in CTL (black circles). (C) The effect of increasing pressure was simulated by depolarizing SMC membrane through increasing Na+ permeability (PNa). Model simulations, in agreement with the corresponding experiments in (A), show differences between CADASIL and SAH animals in Vm (bottom) and Ca2+ (top) as pressure increases and highlight the inhibitory role of KV channels and the effect KV channel density on myogenic tone. Parameters as in reference (102) except: PVDCC=6.3×10−5 cm/s; PNaNSC=1.23×10−6 cm/s; IPMCA=8.58 pA; INaK=7.76 pA/pF; GKIR = 0.5 nS/(mM)0.5; GNa,leak=0.12 nS; Cm = 12.8 pF.
We also used a mathematical model of SMC membrane permeability to examine whether the reported changes in Vm could be explained by measured differences in KV density between CADASIL and SAH animals and CTL groups (6,17) (Figure 3B). This model accounts for the activity of important SMC components, including KV1, KIR, BK, KATP, VDCC, and non-selective cation (NSC) channels, as well as Na+/K+ ATPases (NaK) and plasma membrane Ca2+ pumps (PMCA). Figure 3C demonstrates the effect of increased (CADASIL) and decreased (SAH) KV channel density under the assumption that the presence of all these other components remains unchanged. Simulations show the effect of increasing transmembrane Na+ permeability (PNa) accounts for the pressure-induced smooth muscle depolarization in myogenic response, presumably through the opening of stretch activated NSC channels. In agreement with the experimental data (Figure 3A; 5–10 mmHg), the difference in membrane potential between control and disease states is negligible at low PNa current (i.e., low intraluminal pressure) as the contribution of KV currents to the total transmembrane current diminishes (Figure 3B and 3C). The model predicts that high PNa current (i.e., high intraluminal pressure) leads to depolarization and opening of KV channels, highlighting the inhibitory role of KV channels on the myogenic response. A larger number of KV channels (CADASIL) provide greater negative feedback, resulting in hyperpolarized Vm, less VDCC activity, lower intracellular Ca2+, and less myogenic tone. Conversely, a decrease in the number of KV channels (SAH) provides less negative-feedback, resulting in more depolarized Vm, increased VDCC activity, higher intracellular Ca2+, and increased myogenic tone. Thus, the model demonstrates that changes in SMC KV channel density can account for changes in Vm, intracellular Ca2+ concentration and myogenic tone, consistent with observations from CADASIL and SAH model animals. In both pathological conditions, a change in the gain of the KV-mediated negative feedback loop is expected to have profound effects on the responses of arterioles to pressure fluctuations (CBF autoregulation), but also to impact the effect of external K+ variations during NVC.
3. IMPACT OF SMALL VESSEL PATHOLOGIES ON THE INTERPLAY BETWEEN KV AND KIR CHANNELS DURING NVC
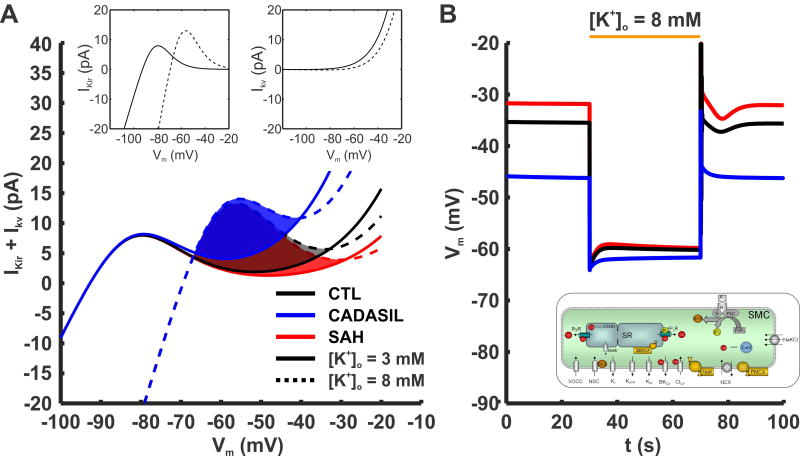
NVC is the process that links localized neuronal activity in the brain to vasodilation of proximate PAs so as to ensure adequate delivering of oxygen and nutrients to metabolically active regions of the central nervous system. A key component of NVC is the KIR-dependent SMC hyperpolarization and vasodilation that results from modest increases in [K+]o within the perivascular space (51,52,85). In isolated PAs, raising [K+]o from 3 mM to 8 mM hyperpolarizes the SM Vm to near the new EK of −76 mV, causing near maximum vasodilation by decreasing Ca2+ influx (51). This dramatic Vm hyperpolarization is achieved by KIR activation. Raising [K+]o from 3 mM to 8 mM activates KIR channels and results in a 50-fold increase in membrane K+ permeability (86). In addition to a modest rise in [K+]o (e.g., from 3 mM to 8 mM), membrane hyperpolarization itself increases KIR channel activity, causing a rightward shift of the KIR current-voltage relationship and increased outward current amplitude (Figure 4A, left inset) as a result of the positive shift in EK and an increase in KIR conductance (87). In contrast, a modest rise in [K+]o monotonically decreases outward KV current due to a reduction in the K+ chemical gradient (Figure 4A, right inset). Additionally, modest increases in [K+]o and the associated KIR channel activation and Vm hyperpolarization would be expected to decrease KV (and possibly BK) channel open probability. Thus, the interaction between KIR and KV/BK channels during modest increases in [K+]o creates a “tug-of-war” dynamic that would be expected to play an important role in determining SMC K+ permeability and PA diameter during NVC. Sufficient KIR activation is required to overcome the concurrent decrease in KV/BK channel activity to produce Vm hyperpolarization and vasodilation. Under physiological conditions, this is the case, with modest increases in [K+]o associated with neuronal activation leading to SMC Vm hyperpolarization, vasodilation, and increased local CBF during NVC.
Figure 4. Effect of PA SMC KV current density and the interplay with KIR current on Vm dynamics at rest and during potassium challenge.
(A) Combined contribution of KIR and Kv currents in healthy and diseased models during rest and [K+]o stimulus. Activation of KIR current by [K+]o and hyperpolarization is accounted: where Gkir,max is the maximal KIR conductance. Solid lines show the sum of the two currents at rest, and dashed lines are during elevation of [K+]o from 3 to 8 mM. The shaded regions show the range of voltages within which KIR current increases more than KV current decreases during the K+ stimulus, i.e. the resting Vm window where the K+ challenge will result in hyperpolarization. As KV current density increases (from SAH, red lines; to CTL black lines; to CADASIL, blue lines) the window shrinks in size and shifts to more hyperpolarized potentials. (B) Representative simulation using the model of PA SMCs from Figure 3. SMCs from CTL (black line), CADASIL (blue line) and SAH (red line) conditions hyperpolarize following an increase in extracellular K+, [K+]o, from 3 mM to 8 mM. The change in membrane potential is less for CADASIL as a result of the more hyperpolarized resting Vm prior to the K+ challenge. GKIR = 0.76 [nS/(mM)0.5]; kKIR = 7 [mV]; VKIR,0.5= EK+12 [mV].
However, evidence indicates impaired NVC in CADASIL (14,18) and SAH (7,13) animal models. Since KV channel number in PA SMCs is significantly altered in these two pathologies, we used computational modeling to investigate the effect of different KV channel densities on SMC membrane potential when [K+]o is elevated from 3 mM to 8 mM. The current-voltage curves of KV currents obtained from Figures 1 and 2 are summed with representative PA SMC KIR currents in Figure 4A. Solid lines represent combined KV and KIR currents at rest ([K+]o = 3 mM), and dashed lines represent currents when [K+]o is elevated to 8 mM. Shaded areas show the increase in net hyperpolarizing current predicted by shifting [K+]o from 3 mM to 8 mM and highlight the Vm window where the KIR influence is dominant (i.e., overcomes the opposing influence of decreased KV channel activity), producing a net increase in membrane K+ permeability. Within this Vm range, or “KIR window”, hyperpolarization can be achieved during increases in [K+]o to 8 mM, whereas outside of this window, the K+ stimulus will result in unstable membrane potential or even depolarization. Therefore, K+-induced hyperpolarization is possible when the resting Vm is within the KIR window (Figure 4A shaded areas). Despite differences in KV current density, our modeling indicates that conditions exist where increasing [K+]o from 3 mM to 8 mM would be predicted to hyperpolarize PA SMCs of CTL, CADASIL, and SAH animals (Figure 4B).
In SAH, the prediction is that decreased KV channel density extends the Vm window where the KIR influence is dominant (Figure 4A red region). This is consistent with our results showing that parenchymal arterioles isolated from the brains of SAH animals and pressurized ex vivo dilate in response to modest increases in extracellular K+ (7). However, SAH is associated with an inversion of NVC in animal models; that is, instead of causing vasodilation, neuronal activation causes vasoconstriction in these animals, both in brain slices and in vivo (7,13,88). We have proposed that this inversion of NVC is the result of a pathological increase in basal [K+]o reflecting enhanced K+ efflux by astrocytic endfeet, rather than impaired KIR function. When summed with neurally evoked K+ efflux, the net elevation in basal [K+]o leads to a more depolarized SMC EK that lies outside of the influence of the KIR window. Thus, the polarity of the vascular response is switched from dilation to constriction due to Vm depolarization and enhanced Ca2+ entry through VDCCs (7,13,88).
In CADASIL, the representative model predicts that an increase in KV channel density reduces the size of the KIR window, but also shifts the window to more hyperpolarized potentials (Figure 4A, blue region). However, increased KV channel density in CADASIL model mice also brings resting Vm to more hyperpolarized values (17). According to the simulation, this limits the impact of the increased KV density on a 8-mM [K+]o challenge, and allow K+-induced dilations to occur. Consistent with this, PAs from CADASIL model mice respond to 8, 15 and 20 mM [K+]o with near maximum dilation ex vivo, comparable to that in CTL animals (17). Nevertheless, an altered hyperpolarization window may compromise K-induced dilation under some conditions, for example when Vm is more depolarized by the presence of an additional vasoconstrictor. Also, a higher KV channel density in CADASIL decreases the vasodilatory reserve, because PAs are in a less constricted state compared with controls. This could lead to deficits in vascular autoregulation and the ability of K+-induced signaling to efficiently redirect blood flow in the vascular network during NVC, as previously reported (14,18).
CONCLUSIONS
Our understanding of cerebral SVDs has advanced greatly over the past decade. Recent work has established the contribution of the yin and yang of the KV channel balance to the pathological progression of cerebral small vessel dysfunction. Here, we combine data from different animal models with detailed computational modeling to further understand elements of this pathology.
KV channels, estimated to number 3,000–3,500 per SMC in physiological conditions, play a profound role in regulating PA SMC resting Vm and PA myogenic tone. These channels are sensitive to inhibition by 4-AP, and the current footprint obtained in native PA myocytes aligns with the properties of KV1.2 and KV1.5 subunits, results supported by the expression of mRNAs for these subunits (17,58,59). Changing the number of channels per SMC results in abnormal SMC Vm and myogenic responses—a yin and yang dynamic that helps to account for cerebral microvascular defects in both CADASIL and SAH. Indeed, in addition to being dramatically altered by CADASIL and SAH, KV channel expression and function in the vascular wall can also be disrupted in the context of other major causes of cerebral SVDs, such as diabetes (58), aging (89), and hypertension (34,90). Thus, targeting KV1 channels in the vascular wall with the aim of restoring normal hemodynamic function may be a future therapeutic option for such disorders (91).
Secondly, an in-depth characterization of the SMC membrane K+ permeability control by the KV−KIR interplay during NVC will enable further advances in our understanding of the impact of SVDs on functional hyperemia. Here, we establish an inroad to this analysis, introducing the new concept that changes in the number of KV channels impact the tug-of-war between activation of SMC KIR channels and deactivation of KV channels, which can impair sensitivity to vasoreactive signaling.
Overall, increased KV channel density in CADASIL narrows the Vm range over which KIR channel can induce dilation, but also brings resting Vm to more hyperpolarized values, partially counteracting the impact of the tug-of-war between KV and KIR channels at the expense of the vasodilatory reserve. In SAH, decreased KV channel density leads to depolarization of resting Vm and increases myogenic tone. Compromised NVC in this disease model may be attributable to high extracellular K+ levels resulting from excessive K+ efflux from astrocytes that depolarizes rather than hyperpolarizes SMCs.
FUTURE DIRECTIONS
Recent studies by our group and others have identified a previously unanticipated role for the endothelium, specifically KIR channels in capillary ECs, in sensing neural activity at the capillary level and translating it into a propagating hyperpolarizing electrical signal that dilates upstream arterioles (55,92,93). These findings highlight the importance of conducted hyperpolarization along PAs and hold the promise of resolving controversies regarding SVD-induced neurovascular dysfunction, potentially providing a paradigm-shifting concept. The recent development of cell-type–specific, genetically encoded fluorescent voltage sensors brings the possibility of in vivo and ex vivo optical electrophysiology within reach (94–97). In the context of electrical signaling between capillaries and arterioles, this may allow us to image how changes in KV channels alter the regenerative hyperpolarization of the endothelium during NVC. Importantly, CADASIL is caused by mutations in the NOTCH3 receptor, which is expressed not only in vascular SMCs, but also in pericytes (5). The role of pericytes, and their possible dysfunction in NVC, has recently been brought to light by several groups (93,98–101). Although consensus on this point has remained elusive, the increasing number of studies suggests that pericytes provide an additional layer of CBF regulation and therefore could play a key role in facilitating or dampening capillary-to-arteriole signaling. However, whether KV channels are expressed in pericytes, and whether they are up-regulated in CADASIL and potentially down-regulated in SAH, remains unknown. Resolving these questions may prove critical to a full understanding of the regulation of CBF by KV channels in the intracerebral microcirculation.
PERSPECTIVE
KV channels located in SMCs of brain parenchymal arterioles oppose pressure-induced depolarization. Changes in KV channel density occur in pathological processes that target the brain microcirculation, impairing intracerebral arteriole constriction in response to changes in intravascular pressure. This blunting of a fundamental vascular function is expected to impact cerebral blood flow autoregulation and local dilation in response to neuronal activity (functional hyperemia). Interventions aimed at restoring KV channel function to improve brain perfusion may thus be a future therapeutic direction in the treatment of cerebral small vessel pathologies.
Acknowledgments
The authors gratefully acknowledge Amanda Mirando and Dr. Adam Strand for their insightful discussion and editorial assistance. This study was supported by a Scientist Development Grant (14SDG20150027 to M.K.) and Grant-in-Aid (17GRNT33700280 to G.C.W.) from the American Heart Association, and grants the Totman Medical Research Trust (to M.T.N.), Fondation Leducq (to M.T.N.), European Union’s Horizon 2020 research and innovation programme (grant agreement No 666881, SVDs@target, to M.T.N.), and National Institutes of Health (P01-HL-095488, R01-HL-121706, R37-DK-053832, 7UM-HL-1207704 and R01-HL-131181 to M.T.N.; and R01-HL-136636 to F.D.).
Abbreviations used
- 4-AP
4-aminopyridine
- [K+]o
external K+ concentration
- ADAM
a disintegrin and metalloproteinase
- BK
large conductance Ca2+- activated K+ channel
- CADASIL
cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- CBF
cerebral blood flow
- CTL
control
- EC
endothelial cell
- EGFR
epidermal growth factor receptor
- EK
reversal potential for K+
- HB-EGF
heparin-binding epidermal growth factor-like growth factor
- KATP
ATP-sensitive K+ channel
- KIR
inward rectifier K+ channel
- KV
voltage-gated K+ channel
- MMP
matrix metalloproteinase
- NaK
Na+/K+ ATPase
- NSC
non-selective cation channels
- NVC
neurovascular coupling
- PA
parenchymal arteriole
- PMCA
plasma membrane Ca2+ ATPase
- RyR
ryanodine receptor
- SAH
subarachnoid hemorrhage
- SMC
smooth muscle cell
- SVD
small vessel disease
- TIMP-3
tissue inhibitor of metalloproteinase-3
- VDCC
voltage-dependent Ca2+ channel
- Vm
membrane potential
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Z, Lin P-J, Levey A. Monetary costs of dementia in the United States. N Engl J Med. 2013;369(5):489. doi: 10.1056/NEJMc1305541. [DOI] [PubMed] [Google Scholar]
- 3.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 4.Bederson JB, Connolly ES, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Jr, Harbaugh RE, Patel AB, Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 5.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser M-G. CADASIL. Lancet Neurol. 2009;8(7):643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 6.Nystoriak MA, O'Connor KP, Sonkusare SK, Brayden JE, Nelson MT, Wellman GC. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol. 2011;300(3):H803–H812. doi: 10.1152/ajpheart.00760.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koide M, Bonev AD, Nelson MT, Wellman GC. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc Natl Acad Sci USA. 2012;109(21):E1387–1395. doi: 10.1073/pnas.1121359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Østergaard L, Aamand R, Karabegovic S, Tietze A, Blicher JU, Mikkelsen IK, Iversen NK, Secher N, Engedal TS, Anzabi M, Jimenez EG, Cai C, Koch KU, Naess-Schmidt ET, Obel A, Juul N, Rasmussen M, Sørensen JC. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2013;33(12):1825–1837. doi: 10.1038/jcbfm.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wellman GC, Koide M. Impact of subarachnoid hemorrhage on parenchymal arteriolar function. Acta Neurochir Suppl. 2013;115:173–177. doi: 10.1007/978-3-7091-1192-5_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tso MK, Macdonald RL. Subarachnoid hemorrhage: a review of experimental studies on the microcirculation and the neurovascular unit. Transl Stroke Res. 2014;5(2):174–189. doi: 10.1007/s12975-014-0323-4. [DOI] [PubMed] [Google Scholar]
- 11.Terpolilli NA, Brem C, Bühler D, Plesnila N. Are We Barking Up the Wrong Vessels? Cerebral Microcirculation After Subarachnoid Hemorrhage. Stroke. 2015;46(10):3014–9. doi: 10.1161/STROKEAHA.115.006353. [DOI] [PubMed] [Google Scholar]
- 12.Balbi M, Koide M, Schwarzmaier SM, Wellman GC, Plesnila N. Acute changes in neurovascular reactivity after subarachnoid hemorrhage in vivo. J Cereb Blood Flow Metab. 2017;37(1):178–187. doi: 10.1177/0271678X15621253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balbi M, Koide M, Wellman GC, Plesnila N. Inversion of neurovascular coupling after subarachnoid hemorrhage in vivo. J Cereb Blood Flow Metab. 2017;7(11):3625–3634. doi: 10.1177/0271678X16686595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joutel A, Haddad I, Ratelade J, Nelson MT. Perturbations of the cerebrovascular matrisome: A convergent mechanism in small vessel disease of the brain? J Cereb Blood Flow Metab. 2016;36(1):143–157. doi: 10.1038/jcbfm.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishiguro M, Morielli AD, Zvarova K, Tranmer BI, Penar PL, Wellman GC. Oxyhemoglobin-Induced Suppression of Voltage-Dependent K+ Channels in Cerebral Arteries by Enhanced Tyrosine Kinase Activity. Circ Res. 2006;99(11):1252–1260. doi: 10.1161/01.RES.0000250821.32324.e1. [DOI] [PubMed] [Google Scholar]
- 16.Koide M, Penar PL, Tranmer BI, Wellman GC. Heparin-binding EGF-like growth factor mediates oxyhemoglobin-induced suppression of voltage-dependent potassium channels in rabbit cerebral artery myocytes. Am J Physiol Heart Circ Physiol. 2007;293(3):H1750–H1759. doi: 10.1152/ajpheart.00443.2007. [DOI] [PubMed] [Google Scholar]
- 17.Dabertrand F, Krøigaard C, Bonev AD, Cognat E, Dalsgaard T, Domenga-Denier V, Hill-Eubanks DC, Brayden JE, Joutel A, Nelson MT. Potassium channelopathy-like defect underlies early-stage cerebrovascular dysfunction in a genetic model of small vessel disease. Proc Natl Acad Sci USA. 2015;112(7):E796–E805. doi: 10.1073/pnas.1420765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capone C, Dabertrand F, Baron-Menguy C, Chalaris A, Ghezali L, Domenga-Denier V, Schmidt S, Huneau C, Rose-John S, Nelson MT, Joutel A. Mechanistic insights into a TIMP3-sensitive pathway constitutively engaged in the regulation of cerebral hemodynamics. Elife. 2016;5:e17536. doi: 10.7554/eLife.17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stühmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharm Rev. 2005;57(4):473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 20.Jan LY, Jan YN. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54(1):537–55. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- 21.Jan LY, Jan YN. Cloned potassium channels from eukaryotes and prokaryotes. Annu Rev Neurosci. 1997;20(1):91–123. doi: 10.1146/annurev.neuro.20.1.91. [DOI] [PubMed] [Google Scholar]
- 22.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 23.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268(4 Pt 1):C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 24.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28(3):220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79(2):387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 26.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66(1):8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 27.Cipolla MJ. The Cerebral Circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2009. [PubMed] [Google Scholar]
- 28.Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107(51):22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernard C. Lectures on the phenomena of life common to animals and plants. 1885 [Google Scholar]
- 32.Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41(1):50–55. doi: 10.1161/01.hyp.0000042427.05390.5c. [DOI] [PubMed] [Google Scholar]
- 33.Jackson WF. Potassium Channels in the Peripheral Microcirculation. Microcirculation. 2005;12(1):113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tykocki NR, Boerman EM, Jackson WF. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr Physiol. 2017;7(2):485–581. doi: 10.1002/cphy.c160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jansen-Olesen I, Mortensen CH, El-Bariaki N, Ploug KB. Characterization of KATP-channels in rat basilar and middle cerebral arteries: studies of vasomotor responses and mRNA expression. Eur J Pharmacol. 2005;523(1–3):109–118. doi: 10.1016/j.ejphar.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 36.Dabertrand F, Nelson MT, Brayden JE. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ Res. 2012;110(2):285–294. doi: 10.1161/CIRCRESAHA.111.258145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarron JG, Quayle JM, Halpern W, Nelson MT. Cromakalim and pinacidil dilate small mesenteric arteries but not small cerebral arteries. Am J Physiol. 1991;261(2 Pt 2):H287–H291. doi: 10.1152/ajpheart.1991.261.2.H287. [DOI] [PubMed] [Google Scholar]
- 38.McPherson GA, Stork AP. The resistance of some rat cerebral arteries to the vasorelaxant effect of cromakalim and other K+ channel openers. Br J Pharmacol. 1992;105(1):51–58. doi: 10.1111/j.1476-5381.1992.tb14209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagao T, Ibayashi S, Sadoshima S, Fujii K, Ohya Y, Fujishima M. Distribution and physiological roles of ATP-sensitive K+ channels in the vertebrobasilar system of the rabbit. Circ Res. 1996;78(2):238–243. doi: 10.1161/01.res.78.2.238. [DOI] [PubMed] [Google Scholar]
- 40.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77(4):1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 41.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 42.Nelson MT, Cheng H, Rubart M, Santana LF, bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270(5236):633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 43.Koide M, Nystoriak MA, Krishnamoorthy G, OConnor KP, Bonev AD, Nelson MT, Wellman GC. Reduced Ca2+ spark activity after subarachnoid hemorrhage disables BK channel control of cerebral artery tone. J Cereb Blood Flow Metab. 2011;31(1):3–16. doi: 10.1038/jcbfm.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horiuchi T, Dietrich HH, Hongo K, Dacey RG. Mechanism of Extracellular K+-Induced Local and Conducted Responses in Cerebral Penetrating Arterioles. Stroke. 2002;33(11):2692–2699. doi: 10.1161/01.str.0000034791.52151.6b. [DOI] [PubMed] [Google Scholar]
- 45.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA. 2010;107(8):3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab. 2011;31(5):1175–1186. doi: 10.1038/jcbfm.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol. 2011;300(5):H1616–H1630. doi: 10.1152/ajpheart.00728.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westcott EB, Goodwin EL, Segal SS, Jackson WF. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol. 2012;590(8):1849–1869. doi: 10.1113/jphysiol.2011.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dabertrand F, Mironneau J, Macrez N, Morel J-L. Full length ryanodine receptor subtype 3 encodes spontaneous calcium oscillations in native duodenal smooth muscle cells. Cell Calcium. 2008;44(2):180–189. doi: 10.1016/j.ceca.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Dabertrand F, Nelson MT, Brayden JE. Ryanodine receptors, calcium signaling, and regulation of vascular tone in the cerebral parenchymal microcirculation. Microcirculation. 2013;20(4):307–316. doi: 10.1111/micc.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 52.Longden TA, Dabertrand F, Hill-Eubanks DC, Hammack SE, Nelson MT. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci USA. 2014;111(20):7462–7467. doi: 10.1073/pnas.1401811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Povlsen GK, Longden TA, Bonev AD, Hill-Eubanks DC, Nelson MT. Uncoupling of neurovascular communication after transient global cerebral ischemia is caused by impaired parenchymal smooth muscle Kir channel function. J Cereb Blood Flow Metab. 2016;36(7):1195–1201. doi: 10.1177/0271678X16638350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation. 2015;22(3):183–196. doi: 10.1111/micc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20(5):717–726. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quayle JM, McCarron JG, Brayden JE, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol. 1993;265(5 Pt 1):C1363–1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- 57.Knot HJ, Zimmermann PA, Nelson MT. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol. 1996;492(Pt 2):419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Straub SV, Girouard H, Doetsch PE, Hannah RM, Wilkerson MK, Nelson MT. Regulation of intracerebral arteriolar tone by KV channels: effects of glucose and PKC. Am J Physiol Heart Circ Physiol. 2009;297(3):C788–796. doi: 10.1152/ajpcell.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koide M, Wellman GC. SAH-induced suppression of voltage-gated K+ (KV) channel currents in parenchymal arteriolar myocytes involves activation of the HB-EGF/EGFR pathway. Acta Neurochir Suppl. 2013;115:179–184. doi: 10.1007/978-3-7091-1192-5_34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rush NJ. Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels. J Physiol. 2009;551(3):751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong XZ, Harhun MI, Olesen SP, Ohya S, Moffatt JD, Cole WC, Greenwood IA. Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol. 2010;588(17):3277–3293. doi: 10.1113/jphysiol.2010.192823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel [KValpha1] subunits in terminal arterioles of rabbit. J Physiol. 2001;534(Pt 3):691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheong A, Dedman AM, Xu SZ, Beech DJ. KValpha1 channels in murine arterioles: differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol. 2001;281(3):H1057–H1065. doi: 10.1152/ajpheart.2001.281.3.H1057. [DOI] [PubMed] [Google Scholar]
- 64.Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem. 2004;279(45):47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- 65.Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Heart Cell Physiol. 2006;291(2):C348–C356. doi: 10.1152/ajpcell.00086.2006. [DOI] [PubMed] [Google Scholar]
- 66.Zhong XZ, Abd-Elrahman KS, Liao CH, El-Yazbi AF, Walsh EJ, Walsh MP, Cole WC. Stromatoxin-sensitive, heteromultimeric Kv2.1/Kv9.3 channels contribute to myogenic control of cerebral arterial diameter. J Physiol. 2010;588(22):4519–4537. doi: 10.1113/jphysiol.2010.196618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nieves-Cintron M, Nystoriak MA, Prada MP, Johnson K, Fayer W, Dell'Acqua ML, Scott JD, Navedo MF. Selective down-regulation of KV2.1 function contributes to enhanced arterial tone during diabetes. J Biol Chem. 2015;290(12):7918–7929. doi: 10.1074/jbc.M114.622811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jepps TA, Olesen SP, Greenwood IA. One man's side effect is another man's therapeutic opportunity: targeting Kv7 channels in smooth muscle disorders. Br J Pharmacol. 2012;168(1):19–27. doi: 10.1111/j.1476-5381.2012.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kerr PM, Clement-Chomienne O, Thorneloe KS, Chen TT, Ishii K, Sontag DP, Walsh MP, Cole WC. Heteromultimeric Kv1.2–Kv1.5 channels underlie 4-aminopyridine-sensitive delayed rectifier K+ current of rabbit vascular myocytes. Circ Res. 2001;89(11):1038–44. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- 70.Blom SM, Schmitt N, Jensen HS. The Acrylamide (S)-2 As a positive and negative modulator of Kv7 channels expressed in xenopus laevis oocytes. PLoS One. 2009;4(12):e8251. doi: 10.1371/journal.pone.0008251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Escoubas P, Diochot S, Célérier M-L, Nakajima T, Lazdunski M. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol Pharmacol. 2002;62(1):48–57. doi: 10.1124/mol.62.1.48. [DOI] [PubMed] [Google Scholar]
- 72.Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K+ channels of vascular smooth muscle. Circ Res. 2001;89(11):1030–1037. doi: 10.1161/hh2301.100817. [DOI] [PubMed] [Google Scholar]
- 73.Plane F, Johnson R, Kerr P, Wiehler W, Thorneloe K, Ishii K, Chen T, Cole W. Heteromultimeric Kv1 channels contribute to myogenic control of arterial diameter. Circ Res. 2005;96(2):216–224. doi: 10.1161/01.RES.0000154070.06421.25. [DOI] [PubMed] [Google Scholar]
- 74.Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. Key role of Kv1 channels in vasoregulation. Circ Res. 2006;99(1):53–60. doi: 10.1161/01.RES.0000229654.45090.57. [DOI] [PubMed] [Google Scholar]
- 75.Shih AY, Rühlmann C, Blinder P, Devor A, Drew PJ, Friedman B, Knutsen PM, Lyden PD, Mateo C, Mellander L, Nishimura N, Schaffer CB, Tsai PS, Kleinfeld D. Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation. 2015;22(3):204–218. doi: 10.1111/micc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci USA. 2007;104(1):365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci. 2013;16(1):55–63. doi: 10.1038/nn.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23(27):9254–62. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2005;100(3):1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 80.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 81.Link TE, Murakami K, Beem-Miller M, Tranmer BI, Wellman GC. Oxyhemoglobin-induced expression of R-type Ca2+ channels in cerebral arteries. Stroke. 2008;39(7):2122–8. doi: 10.1161/STROKEAHA.107.508754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joutel A, Monet-Leprêtre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, Lemaire-Carrette B, Domenga V, Schedl A, Lacombe P, Hubner N. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120(2):433–445. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alvarez O, Latorre R. The enduring legacy of the “constant-field equation” in membrane ion transport. J Gen Physiol. 2017;149(10):911–920. doi: 10.1085/jgp.201711839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koide M, Wellman GC. SAH-induced MMP activation and KV current suppression is mediated via both ROS-dependent and ROS-independent mechanisms. Acta Neurochir Suppl. 2015;120:89–94. doi: 10.1007/978-3-319-04981-6_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang C, Tabatabaei M, Bélanger S, Girouard H, Moeini M, Lu X, Lesage F. Astrocytic endfoot Ca2+ correlates with parenchymal vessel responses during 4-AP induced epilepsy: An in vivo two-photon lifetime microscopy study. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17725417. 271678X17725417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Longden TA, Hill-Eubanks DC, Nelson MT. Ion channel networks in the control of cerebral blood flow. J Cereb Blood Flow Metab. 2016;36(3):492–512. doi: 10.1177/0271678X15616138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shah AK, Cohen IS, Datyner NB. Background K+ current in isolated canine cardiac Purkinje myocytes. Biophys J. 1987;52(4):519–525. doi: 10.1016/S0006-3495(87)83241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pappas AC, Koide M, Wellman GC. Purinergic signaling triggers endfoot high-amplitude Ca2+ signals and causes inversion of neurovascular coupling after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2016;36(11):1901–1912. doi: 10.1177/0271678X16650911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang LS, Kim S, Dominguez JM, Sindler AL, Dick GM, Muller-Delp JM. Aging and muscle fiber type alter K+ channel contributions to the myogenic response in skeletal muscle arterioles. J Applied Physiol. 2009;107(2):389–398. doi: 10.1152/japplphysiol.91245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cox RH. Changes in the expression and function of arterial potassium channels during hypertension. Vasc Pharmacol. 2002;38(1):13–23. doi: 10.1016/s1537-1891(02)00122-2. [DOI] [PubMed] [Google Scholar]
- 91.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009 Dec;8(12):982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EMC. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3(3):e000787–7. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei HS, Kang H, Rasheed I-YD, Zhou S, Lou N, Gershteyn A, McConnell ED, Wang Y, Richardson KE, Palmer AF, Xu C, Wan J, Nedergaard M. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron. 2016;91(4):851–862. doi: 10.1016/j.neuron.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gong Y, Huang C, Li JZ, Grewe BF, Zhang Y, Eismann S, Schnitzer MJ. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science. 2015;350(6266):1361–6. doi: 10.1126/science.aab0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marshall JD, Li JZ, Zhang Y, Gong Y, St-Pierre F, Lin MZ, Schnitzer MJ. Cell-type-specific optical recording of membrane voltage dynamics in freely moving mice. Cell. 2016;167(6):1650–1662. e15. doi: 10.1016/j.cell.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.St-Pierre F, Marshall JD, Yang Y, Gong Y, Schnitzer MJ, Lin MZ. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat Neurosci. 2014;17(6):884–889. doi: 10.1038/nn.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lou S, Adam Y, Weinstein EN, Williams E, Williams K, Parot V, et al. Genetically targeted all-optical electrophysiology with a transgenic cre-dependent optopatch mouse. J Neurosci. 2016;36(43):11059–11073. doi: 10.1523/JNEUROSCI.1582-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87(1):95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, Boas DA, Sakadžić S, Zlokovic BV. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci. 2017;20(3):406–416. doi: 10.1038/nn.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Underly RG, Levy M, Hartmann DA, Grant RI, Watson AN, Shih AY. Pericytes as inducers of rapid, matrix metalloproteinase-9-dependent capillary damage during ischemia. J Neurosci. 2017;37(1):129–140. doi: 10.1523/JNEUROSCI.2891-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of Ca2+ dynamics in rat mesenteric smooth muscle cell: agonist and NO stimulation. J Theor Biol. 2008;253(2):238–260. doi: 10.1016/j.jtbi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 103.Cipolla MJ, Sweet J, Chan S-L, Tavares MJ, Gokina N, Brayden JE. Increased pressure-induced tone in rat parenchymal arterioles vs. middle cerebral arteries: role of ion channels and calcium sensitivity. J Applied Physiol. 2014;117(1):53–59. doi: 10.1152/japplphysiol.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]