ABSTRACT
Chlamydia has been detected in the gastrointestinal tracts of humans and animals. We now report that gastrointestinal Chlamydia muridarum is able to induce robust transmucosal protection in mice. C. muridarum colonization in the gastrointestinal tract correlated with both a shortened course of C. muridarum genital tract infection and stronger protection against subsequent genital tract challenge infection. Mice preinoculated intragastrically with C. muridarum became highly resistant to subsequent C. muridarum infection in the genital tract, resulting in prevention of pathology in the upper genital tract. The transmucosal protection in the genital tract was rapidly induced, durable, and dependent on major histocompatibility complex (MHC) class II antigen presentation but not MHC class I antigen presentation. Although a deficiency in CD4+ T cells only partially reduced the transmucosal protection, depletion of CD4+ T cells from B cell-deficient mice completely abolished the protection, suggesting a synergistic role of both CD4+ T and B cells in the gastrointestinal C. muridarum-induced transmucosal immunity. However, the same protective immunity did not significantly affect C. muridarum colonization in the gastrointestinal tract. The long-lasting colonization with C. muridarum was restricted to the gastrointestinal tract and was nonpathogenic to either gastrointestinal or extragastrointestinal tissues. Furthermore, gastrointestinal C. muridarum did not alter the gut microbiota or the development of gut mucosal resident memory T cell responses to a nonchlamydial infection. Thus, Chlamydia may be developed into a safe and orally deliverable replicating vaccine for inducing transmucosal protection.
KEYWORDS: Chlamydia muridarum, oral inoculation, transmucosal immunity, nonpathogenic, Chlamydia, gastrointestinal infection, mucosal immunity, oral vaccines
INTRODUCTION
Chlamydial organisms have been detected in the gastrointestinal (GI) tracts of both animals (1) and humans (2–5). However, the medical significance of GI tract Chlamydia remains unclear. Although the GI tract Chlamydia organisms may serve as a reservoir for the potential autoinoculation of the genital tract (6, 7), this hypothesis has not been tested, and there is no direct evidence from either studies with animal models or investigations with humans supporting this hypothesis. On the contrary, it was recently reported that Chlamydia muridarum failed to spread from the GI tract into the genital tract of the same mice after colonization of the GI tract for 70 days (8). Although the finding made in mice cannot be used to exclude the possibility that Chlamydia trachomatis bacteria from the GI tract autoinoculate the genital tract in humans, it clearly suggests that more studies are required to address the significance of the GI tract Chlamydia before any conclusions may be drawn. C. trachomatis is a sexually transmitted bacterial pathogen that causes pathologies in the genital tract (9, 10). Although C. trachomatis lymphogranuloma venereum (LGV) serovars are known to cause proctitis in men who have sex with men (11, 12), the association of C. trachomatis serovars D to K with human GI tract pathologies remains unclear (13–18). A better differentiation of antibodies induced by C. trachomatis serovars D to K from those induced by other chlamydial species is required for study of this association (18). Since C. trachomatis is a known pathogen in the human genital tract, the medically relevant question at this moment is whether GI tract C. trachomatis can affect the susceptibility of the genital tract to C. trachomatis infection and pathogenicity.
Intravaginal inoculation of C. muridarum causes hydrosalpinx and infertility in mice (19–21), closely mimicking the tubal adhesion/infertility observed in women (22–24), which is why the murine model has been extensively used for studying the mechanisms of C. trachomatis pathogenesis and immunity (25–30). C. muridarum also colonizes the mouse GI tract (6, 8, 31–33). Genital tract C. muridarum can spread to the GI tract (34) via a hematogenous route (35) to establish long-lasting colonization in the GI tract. However, it remains unknown how the long-lasting C. muridarum colonization in the GI tract may impact the susceptibility of the mouse genital tract to subsequent C. muridarum infection. Answers to this question may provide the information needed to address how GI tract C. trachomatis may affect human susceptibility to C. trachomatis infection in the genital tract.
It has been shown that plasmid-free C. muridarum, which is unable to induce hydrosalpinx, produces a relatively normal genital tract infection (36, 37). In susceptible CBA/1J mice, plasmid-free C. muridarum caused a robust infection with prolonged genital tract shedding of C. muridarum (38). This prolonged shedding correlated with a delayed/reduced spreading of plasmid-free C. muridarum to the GI tract, suggesting that GI tract C. muridarum may be able to induce immunity for limiting C. muridarum replication in the genital tract. Prior intravaginal infection with wild-type C. muridarum is known to induce robust immunity against reinfection in the genital tract (39–42). However, intravaginal infection with plasmid-free C. muridarum was less effective in preventing challenge infection with wild-type C. muridarum in the genital tract (36), again correlating the reduced spreading of plasmid-free C. muridarum into the GI tract with an insufficient induction of protective immunity in the genital tract.
In the current study, we found that the prolonged shedding of plasmid-free C. muridarum from the genital tract was shortened by coinoculation of wild-type C. muridarum into the mouse GI tract, indicating that GI tract C. muridarum can induce immunity limiting the replication of C. muridarum in the genital tract. Indeed, mice intragastrically inoculated with C. muridarum became highly resistant to subsequent infection with C. muridarum in the genital tract, resulting in the transmucosal prevention of genital tract C. muridarum from inducing hydrosalpinx. The transmucosal protection was dependent on major histocompatibility complex (MHC) class II (MHC-II) antigen presentation but not MHC class I (MHC-I) antigen presentation. CD4+ T cells and B cells may synergistically mediate the transmucosal protection. Despite the robust protective immunity induced by GI tract C. muridarum, the long-lasting C. muridarum colonization in the GI tract was nonpathogenic. It did not significantly alter the gut microbiota or mucosal immune responses to nonchlamydial antigens. The observations presented above together suggest that C. muridarum may be developed into a safe and orally deliverable replicating vaccine for inducing transmucosal protection.
RESULTS
GI tract C. muridarum induces transmucosal protection against genital tract infection.
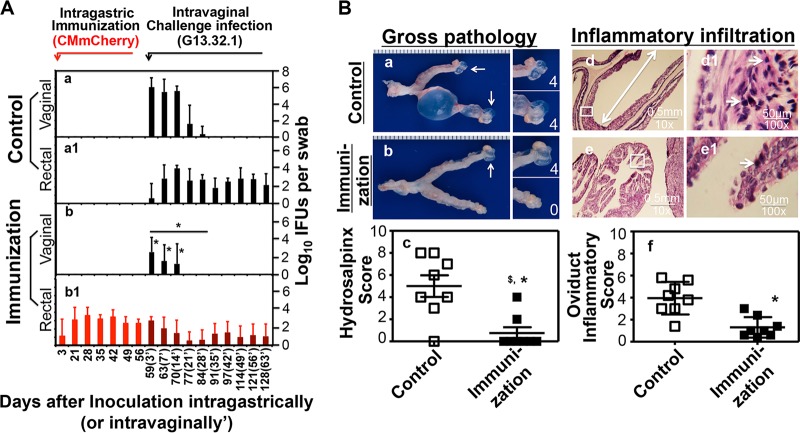
C. muridarum colonization in the gastrointestinal (GI) tract correlated with reduced C. muridarum infection in the genital tract of the same mice. First, the extent to which C. muridarum organisms spread from the genital tract into the GI tract inversely correlated with their course of shedding in the genital tract (38). Second, the coinoculation of C. muridarum organisms into the GI tracts of mice infected vaginally with plasmid-deficient C. muridarum significantly shortened the course of vaginal infection (see Fig. S1 in the supplemental material). Finally, the reduced spreading of plasmid-free C. muridarum into the GI tract also minimized immunity against reinfection in the genital tract (Fig. S2) (36). To directly test whether GI tract C. muridarum can induce transmucosal protection in the genital tract, mice were inoculated intragastrically with C. muridarum bacteria that express mCherry (CM-mCherry; to differentiate the bacteria used for intragastric immunization from the subsequent intravaginal challenge organisms) and subsequently challenged vaginally with wild-type C. muridarum clone G13.32.1 (which does not express mCherry) (Fig. 1). Mice inoculated with CM-mCherry in the GI tract and colonized for 56 days were highly resistant to intravaginal challenge infection with G13.32.1. This transmucosal protection prevented the mice from developing hydrosalpinx, as validated both macroscopically and microscopically. Thus, C. muridarum colonization in the GI tract can function as immunization to induce transmucosal protection against C. muridarum challenge infection in the genital tract.
FIG 1.
Effect of intragastric inoculation as an oral vaccination on genital tract susceptibility to C. muridarum challenge infection. C57BL/6J mice intragastrically inoculated with buffer only (control group, n = 8) (a and a1) or 2 × 105 IFU of wild-type C. muridarum (clone CM-mCherry, immunization group, n = 8) (b and b1) were challenged intravaginally on day 56 with 2 × 105 IFU of wild-type C. muridarum clone G13.32.1. (A) Mice were monitored for live organism shedding by the collection of both vaginal (a and b) and rectal (a1 and b1) swab specimens over the time course displayed along the x axis. The results are expressed as the log10 number of IFU per swab specimen along the y axis. Black bars, titers of G13.32.1; red bars, titers of CM-mCherry; dark red bars, titers of both G13.32.1 and CM-mCherry. Note that on days 3, 7, and 14 after intravaginal challenge (designated in parentheses as 3′, 7′, and 14′, respectively) after intragastric immunization, immunized mice displayed a >1,000-fold decrease in the number of IFU by evaluation of vaginal swab specimens at each time point (*, P < 0.05, Wilcoxon rank-sum test). The overall shedding course was also significantly reduced (*, P < 0.05, Wilcoxon rank-sum test, AUC, for panel b versus panel a). (B) All mice were sacrificed on day 128 after intragastric immunization (or day 63′ after challenge) for evaluation of the upper genital tract pathology both macroscopically (a and b) and microscopically (d and e). (a and b) Representative macroscopic images of one entire genital tract from the control (a) and immunization (b) groups are shown. White arrows, oviducts positive for hydrosalpinges. Magnified images of oviduct/ovary regions are shown on the right of the overall genital tract images, with the white numbers indicating the hydrosalpinx scores. (c) Both the incidence and the severity of hydrosalpinx were quantitated. The group immunized in the GI tract developed a significantly lower incidence ($, P < 0.05, Fisher's exact test) and a reduced score (*, P < 0.05, Wilcoxon rank-sum test) compared with those for the control mice. (d and e) Microscopically, severely dilated oviducts (marked with a white line with arrows at both ends) were easily identified from control mice, as shown in the representative image (d), while the immunized mice mostly displayed normal oviduct cross sections (e). (d1 and e1) The inflammatory cells were identified using a 100× objective lens, as shown in the representative images from the control (d1) and immunized (e1) mice. The areas observed with a 100× objective lens are marked with white squares in the 10× images. (f) The severity of the inflammatory infiltration was semiquantitated using the criteria described in the Materials and Methods section. Note that the immunized mice developed scores significantly decreased (*, P < 0.05, Wilcoxon rank-sum test) compared with those for the control mice.
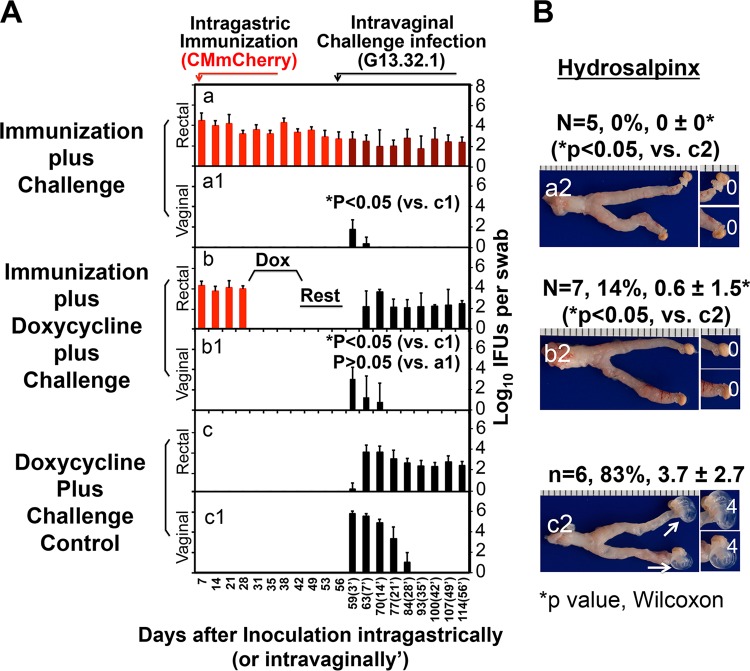
Transmucosal protection is rapidly induced, durable, and independent of sustained C. muridarum colonization in the gastrointestinal tract.
Both the time required for GI tract C. muridarum induction of transmucosal protection and the duration of protection were determined (Fig. 2). One week after intragastric inoculation with CM-mCherry, mice gained significant resistance to intravaginal challenge infection with G13.32.1, with G13.32.1 shedding being reduced by >100-fold on day 3 and the course of infection being shortened by ∼1 week, leading to a significant reduction in both the overall infection course and the upper genital tract pathology. The protection was enhanced over time (Fig. 1) and lasted ≥20 weeks. Whether the transmucosal protection was dependent on ongoing CM-mCherry colonization in the GI tract was further determined (Fig. 3). Mice with or without CM-mCherry in the GI tract for 28 days were either left untreated or treated with doxycycline daily for 2 weeks. After resting for another 2 weeks, the mice were vaginally challenged with G13.32.1. Mice colonized with CM-mCherry in the GI tract for 56 days became highly resistant to intravaginal challenge infection and hydrosalpinx induction, as described above. Importantly, after the immunized mice received daily doxycycline treatment between days 28 and 42, which completely cured the GI tract CM-mCherry infection, the mice still maintained a robust resistance to intravaginal challenge infection and hydrosalpinx development. Thus, within 4 weeks, intragastrically inoculated C. muridarum induced a robust memory response that was protective. Mock-immunized mice similarly treated with doxycycline developed severe hydrosalpinx after the same intravaginal challenge, suggesting that the doxycycline treatment protocol did not affect chlamydial pathogenicity in the upper genital tract. It is worth noting that although the immunized mice were resistant to challenge infection with C. muridarum in the genital tract, the GI tract remained susceptible to colonization by the C. muridarum organisms.
FIG 2.
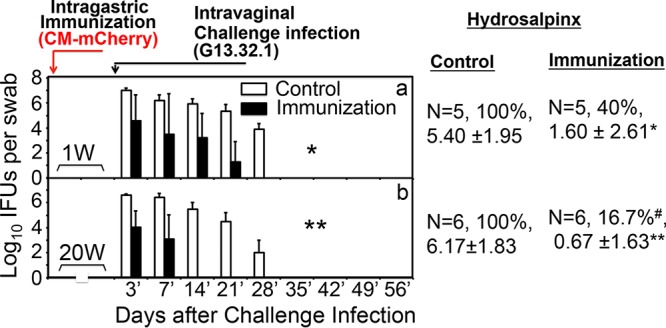
Intragastric immunization elicits rapid and durable protective immunity to genital tract challenge. C57BL/6J mice with (n = 5) or without (n = 5) prior intragastric immunization with 2 ×105 IFU of CM-mCherry for 1 week (1W) (a) or 20 weeks (20W) (b) were challenged vaginally with clone G13.32.1. The mice were monitored for C. muridarum shedding by evaluation of both vaginal and rectal (not shown) swab specimens on days 3 and 7 postinfection (3′ and 7′, respectively) and weekly thereafter. The results are expressed as the log10 number of IFU per swab specimen. Mice were significantly resistant to a genital tract challenge only 1 week after immunization in the GI tract (*, P < 0.05, Wilcoxon rank-sum test, AUC), and the resistance increased and lasted for up to 20 weeks (**, P < 0.01, Wilcoxon rank-sum test, AUC). All mice were sacrificed on day 56 after the challenge infection, and the upper genital tract was evaluated for the incidence (in percent) of hydrosalpinx and the severity score (mean ± standard deviation). Immunization via the GI tract resulted in significant protection against hydrosalpinx induced by the vaginal infection (#, P < 0.05, Fisher's exact test; *, P < 0.05, Wilcoxon rank-sum test; **, P < 0.01, Wilcoxon rank-sum test).
FIG 3.
The durable transmucosal protection induced by intragastric immunization is not dependent on long-term gastrointestinal infection. Groups of C57BL/6J mice immunized intragastrically with 2 × 105 IFU of CM-mCherry (n = 5 for panel a and n = 7 for panel b) or not immunized (n = 6) (c) were treated on day 28 with doxycycline (20 μg/kg of body weight intragastrically once daily) for 2 weeks (days 28 to 42) (b and c) or were not treated with doxycycline (a). The doxycycline-treated mice were then rested for 2 weeks (days 43 to 56). On day 56 after immunization in the GI tract, all mice were intravaginally challenged with 2 × 105 IFU of clone G13.32.1. (A) Mice were monitored for the shedding of chlamydiae by evaluation of both vaginal (a to c) and rectal (a1 to c1) swab specimens over the course of infection (the days after challenge infection are designated 3′ to 56′ in parentheses). Results are expressed as the log10 number of IFU per swab specimen. Mice in the immunization plus doxycycline treatment group displayed no IFU in the rectal swab specimens prior to the intravaginal challenge (days 31 to 56) (b) but maintained transmucosal protection against chlamydial infection in the genital tract (*, P < 0.05, Wilcoxon rank-sum test, for panel b1 versus panel c1), equivalent to the findings for immunized mice not treated with doxycycline (*, P < 0.05, Wilcoxon rank-sum test, for panel a1 versus panel c1). These two groups maintained similar levels of protection (*, P > 0.05, Wilcoxon rank-sum test, for panel b1 versus panel a1). (b and c) The genital tract G13.32.1 organisms spread to the GI tracts. (a and a1) Black bars, G13.32.1 alone; dark red bars; both CM-mCherry and G13.32.1. (B) On day 114 after intragastric immunization, all mice were sacrificed to evaluate the upper genital tract pathology macroscopically. Representative images of the entire genital tracts from the groups receiving immunization without doxycycline treatment (a2), immunization plus doxycycline treatment (b2), or doxycycline treatment without immunization (c2) are shown. White arrows, oviducts positive for hydrosalpinges. Magnified images of oviduct/ovary regions are shown on the right of the overall genital tract images, with the white numbers indicating the hydrosalpinx scores. Both the incidence of hydrosalpinx and the hydrosalpinx severity score (mean ± standard deviation) are listed above the corresponding images. Regardless of doxycycline treatment, immunized mice were significantly protected from the development of hydrosalpinx (*, P < 0.05, Wilcoxon rank-sum test, for the immunization alone group in panel a2 versus panel c2 and for the immunization plus doxycycline treatment group in panel b2 versus panel c2).
The gastrointestinal C. muridarum-induced transmucosal immunity is mediated by MHC class II-restricted but not MHC class I-restricted immune responses, and both CD4+ T cells and B cells contribute to protective immunity.
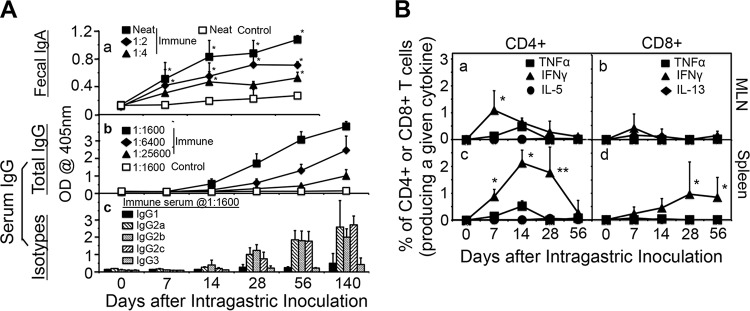
To define the mechanism(s) of transmucosal immunity induced by intragastric immunization with C. muridarum, we first monitored both the humoral and cellular immune responses (Fig. 4 and Fig. S3). GI tract C. muridarum induced robust gut (fecal) IgA and systemic IgG antibodies that recognized epitopes exposed on the surface of the elementary bodies (EBs) coated on enzyme-linked immunosorbent assay (ELISA) plates. A significant level of IgA was detected as early as day 7 after immunization, but the level quickly plateaued by day 14. In contrast, the serum IgG level continued to rise up to 20 weeks after intragastric immunization, with IgG2 being the dominant isotype. When the T cell responses were monitored in the same mice, gamma interferon (IFN-γ)-producing T cell responses were dominant. The EB-specific T cell responses peaked on day 7 in the mesenteric draining lymph nodes (MLN) and on day 14 or 28 in the spleen. The number of IFN-γ-producing CD4+ T cells was significantly higher than that of IFN-γ-producing CD8+ T cells in both the MLN and spleen, which may be relevant to antichlamydial immunity since IFN-γ-producing CD4+ T cells but not IFN-γ-producing CD8+ T cells are known to play important roles in controlling C. muridarum infection in the genital tract (27). The levels of IFN-γ-producing CD4+ and CD8+ T cells peaked earlier in the MLN than in the spleen, which is consistent with antigen-specific T cells being induced in the GI tract.
FIG 4.
Induction of humoral and cellular immune responses by intragastric immunization with C. muridarum. (A) C57BL/6J mice with intragastric immunization with CM-mCherry (solid symbols, n = 5) and naive mice (open symbols, n = 5) were monitored for fecal IgA (a) and serum IgG (b) as well as serum IgG isotypes (c) on different days after immunization (days 0 to 56 for IgA and days 0 to 140 for serum IgG). Samples collected from mice prior to immunization were defined as day 0 samples. CM-mCherry EB were used as antigens and were used to coat the ELISA plates. For the measurement of IgA, undiluted fecal suspensions (neat) from the immunized mice or fecal suspensions from the immunized mice diluted 1:2 or 1:4 along with neat samples from the control mice were tested. Serum samples from the same mice were 4-fold serially diluted, and to assess serum anti-C. muridarum IgG responses, 1:1,600, 1:6,400, and 1:25,600 dilutions were included for immunized mice and 1:1,600 dilutions were included for control mice. Immunization via the GI tract induced significantly higher levels of anti-C. muridarum IgA in the gut and IgG antibodies in the serum (*, P < 0.05, Wilcoxon rank-sum test) compared with the levels of the other antibodies. (c) For the isotyping of serum IgG, the binding of serum samples from immunized mice (at a 1:1,600 dilution) to the plate-immobilized EBs was probed by the use of HRP-conjugated goat anti-mouse IgG1, IgG2a, IgG2b, IgG2c, and IgG3. The ELISA results were expressed as raw OD values, as displayed on the y axis. Immunization via the GI tract induced significantly higher levels of anti-C. muridarum IgG2 antibodies than IgG1 and IgG3 antibodies. (B) C57BL/6J mice intragastrically immunized with 2 × 105 IFU of CM-mCherry or control mice were sacrificed on days 0 (n = 2), 7 (n = 5 for immunized mice, n = 2 for control mice), 14 (n = 4 for immunized mice, n = 2 for control mice), 28 (n = 8 for immunized mice, n = 2 for control mice), or 56 (n = 10 for immunized mice, n = 2 for control mice) to detect the intracellular cytokines TNF-α, IFN-γ, IL-5, and IL-13 in CD4+ (left) and CD8+ (right) T cells from mesenteric draining lymph nodes (MLN) (top) and spleen (bottom). Results are expressed as the percentage of CD4+ or CD8+ T cells that express a given cytokine. Immunized mice developed dominant IFN-γ-producing CD4+ and CD8+ T cell responses in both the MLN and spleen. P values are for the number of IFN-γ-producing T cells versus the number of T cells that produced TNF-α, IL-5, or IL-13. *, P < 0.05, Wilcoxon rank-sum test; **, P < 0.01, Wilcoxon rank-sum test.
To further determine the immune cells required for the transmucosal immunity, we compared the transmucosal protection between mice with and mice without deficiencies in different immune components (Fig. 5). The intragastric immunization induced significant transmucosal protection against C. muridarum challenge infection in the genital tracts of wild-type, MHC-I knockout (KO), CD4 KO, or μ chain KO mice, suggesting that MHC class I antigen presentation, CD4+ T cells, or B cells are not essential for GI tract C. muridarum-induced transmucosal immunity. Careful comparison revealed that the protection in the immunized CD4 KO mice was not as robust as that in the immunized wild-type, MHC-I KO, or B cell KO mice, suggesting that CD4+ T cells are relatively more important in the C. muridarum induction of transmucosal immunity. Importantly, MHC-II KO mice remained highly susceptible to C. muridarum infection in the genital tract, regardless of the GI tract immunization, demonstrating that MHC-II antigen presentation is essential for GI tract C. muridarum induction of transmucosal immunity. This conclusion is further supported by the observation that depletion of CD4+ T cells in B cell KO mice completely blunted the transmucosal protection, suggesting that both CD4+ T cells and B cells are synergistically required for full protection.
FIG 5.
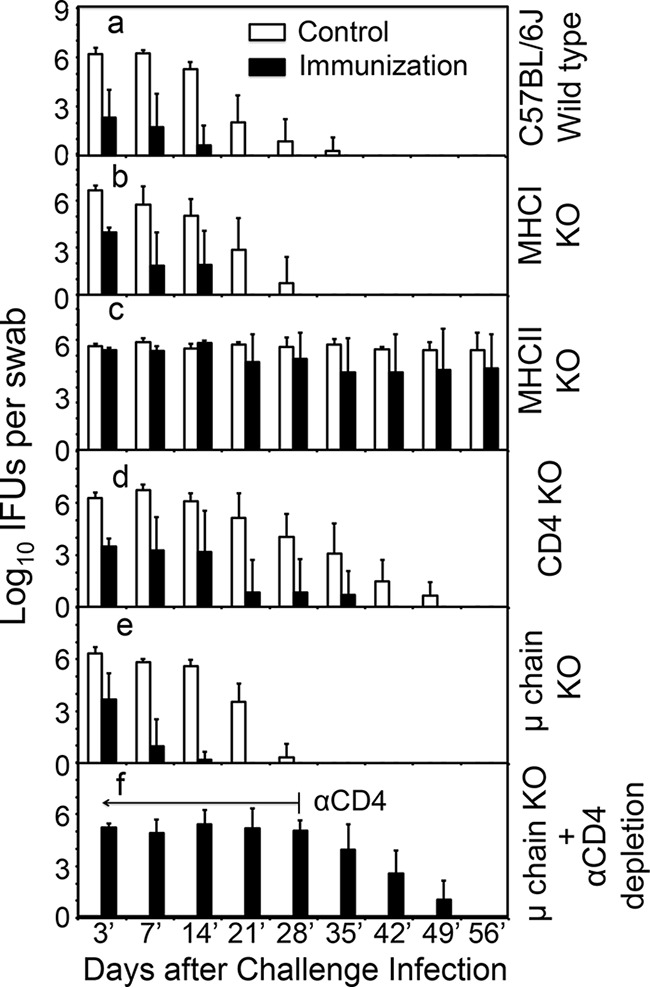
Effect of gene deficiency on intragastric immunization-induced transmucosal protection against genital tract infection. C57BL/6J mice without a deficiency (n = 20) (a) or with a deficiency in MHC class I (MHC-I KO; n = 10) (b), MHC class II (MHC-II KO; n = 10) (c), CD4+ T cells (CD4 KO; n = 10) (d), or B cells (μ chain KO; n = 10 for panel e and n = 5 for panel f) were intragastrically immunized with 2 × 105 IFU of CM-mCherry (n = 5 or 10 per group) or buffer alone (control; n = 5 or 10 per group). On day 35 after the immunization, the mice were intravaginally challenged with 2 × 105 IFU of clone G13.32.1 and live organism shedding was monitored by evaluation of vaginal swab specimens at various time points after the intravaginal infection (from days 3′ to 56′). Results are expressed on the y axis as the log10 number of IFU per swab specimen. Intragastric immunization with C. muridarum protected against challenge infection in the genital tracts of wild-type (a), MHC-I KO (b), CD4 KO (d), or μ chain KO (e) mice (P < 0.05, Wilcoxon rank-sum test). However, MHC-II KO mice (c) remained highly susceptible to C. muridarum colonization regardless of their immunization status (P > 0.05 for immunized versus control mice). Although CD4 KO mice were also induced to develop significant protection, the protection was not as robust as that in the wild-type, MHC-I KO, or B cell KO mice (P < 0.05 for immunized CD4 KO mice versus immunized wild-type, MHC-I KO, or μ chain KO mice). (f) Anti-CD4 antibody was applied to a group of immunized μ chain KO mice 5 days prior to the intravaginal challenge infection and then twice every week for a total of 5 weeks, which completely blunted the B cell-independent transmucosal protection.
Gastrointestinal tract Chlamydia muridarum is nonpathogenic.
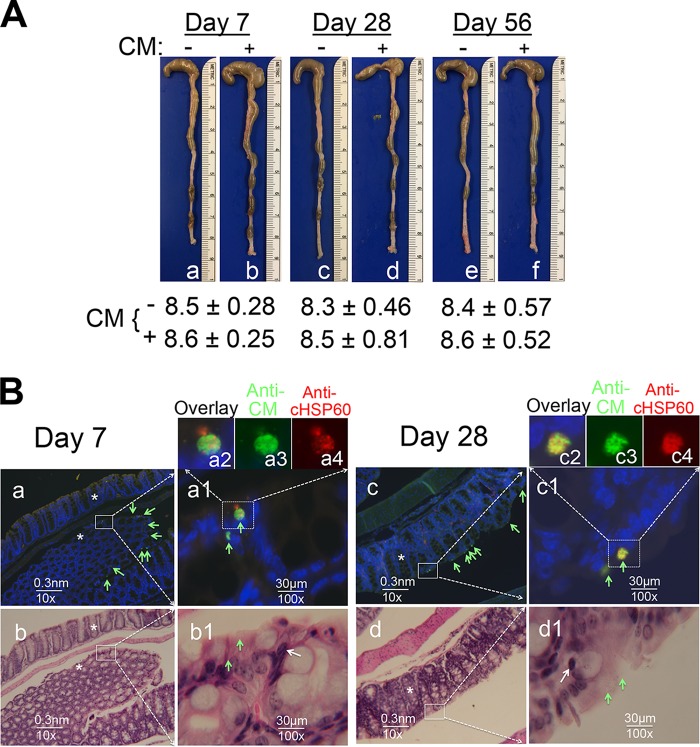
Having demonstrated the strong transmucosal protective immunity induced by GI tract C. muridarum, we next evaluated whether C. muridarum colonization in the GI tract is pathogenic. Since long-lasting C. muridarum colonization is restricted to the cecum, colon, and rectum (8, 35), we carefully examined the mouse colons. As shown in Fig. 6 and S4, there was no significant difference in the gross appearance or length of the cecum, colon, and rectum between mice with C. muridarum colonization and mice without C. muridarum colonization for 7, 28, or 56 days, suggesting that C. muridarum did not cause colitis. C. muridarum inclusions were microscopically localized in the colon mucosal epithelial cells. Despite the presence of clusters of C. muridarum-infected epithelial cells, the epithelial tissue architecture remained intact when the adjacent sections were examined following hematoxylin-eosin (H&E) staining. Furthermore, there was a general lack of significant inflammatory infiltration, although scattered inflammatory cells were always detectable. Compared to control colonic tissue, no significant difference was found between infected and noninfected mice (data not shown).
FIG 6.
Comparison of colons from mice with C. muridarum colonization and mice without C. muridarum colonization. (A) For macroscopic comparison, colons collected from female C57BL/6J mice with (+) (b, d and f) or without (−) (a, c, and e) intragastric immunization with 2 ×105 IFU of CM-mCherry on days 7, 28, and 56 following infection were examined for signs of colitis. Images of the cecum, colon, and rectum from one mouse in each group are shown with the cecum on top. The length (in centimeters) of the colon from each group (mean ± standard deviation) is listed below the corresponding images. Data are for 5 mice in each group (each treatment at each time point). The lengths of the colons remained similar regardless of whether the mice were immunized or when the colon samples were collected. (B) Some of the colon tissues described above were selected for microscopic examination. Colonic sections from mice sacrificed on day 7 (n = 5 mice colonized with CM-mCherry, n = 4 mice not colonized with CM-mCherry) or 28 (n = 5 mice colonized with CM-mCherry, n = 4 mice not colonized with CM-mCherry) were subjected to immunofluorescence (a, a1, c, and c1) or H&E (b, b1, d, and d1) staining. Representative images from one immunized mouse sacrificed on day 7 (a and b) and one immunized mouse sacrificed on day 28 (c and d) are presented. C. muridarum inclusions labeled with a rabbit anti-C. muridarum antibody (Anti-CM, green) and a mouse anti-chlamydial HSP60 monoclonal antibody (Anti-cHSP60, red) are indicated with green arrows. (a1 and c1) A selected area from the images visualized with a 10× objective lens (a and c) was magnified with a 100× objective lens. (a2 and c2) An infected cell from the images visualized with a 100× objective lens was further magnified digitally as an overlay, and images of anti-CM labeling (a3 and c3) (green) and anti-cHSP60 labeling (a4 and c4) (red) are shown. The adjacent sections were subjected to H&E staining and visualized under a 10× (b and d) and a 100× (b1 and d1) objective lens. The images visualized under the 100× objective lens were taken from the areas marked with white squares in the images visualized with a 10× objective lens. Putative inflammatory cells in the images visualized with a 100× objective lens are marked with white arrows. Representative longitudinal and cross-section crypts are indicated with asterisks in both the images with immunofluorescence staining (a and c) and the images with H&E staining (b and d). When both the number of C. muridarum inclusions and the extent of colonic inflammatory infiltration were semiquantitatively scored, no significant difference was found between mice with Cm-mCherry colonization and mice without Cm-mCherry colonization.
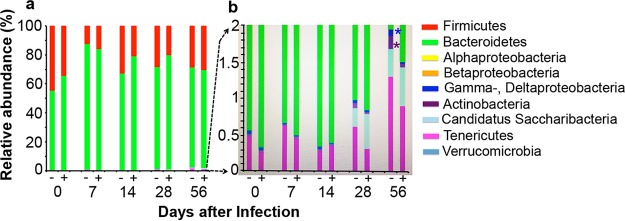
Next we evaluated the effects of C. muridarum colonization in the GI tract on the mouse gut microbiota profiles (Fig. 7). When fecal samples collected from C57BL/6J mice with or without C. muridarum GI tract colonization were analyzed for 7 major bacteria phyla, including the 4 subclasses of the phylum Proteobacteria (Table S1), all mice maintained a stable ratio of Firmicutes versus Bacteroidetes throughout the time course, regardless of C. muridarum colonization. When the less abundant phyla were closely examined, we found that by day 28 mice started to acquire “Candidatus Saccharibacteria,” a recently identified phylum, based on 16S rRNA gene sequence detection. On day 56, the phylum Tenericutes displayed an increase. These time-dependent changes to the gut microbiota were not affected by GI tract C. muridarum. Interestingly, the GI tract C. muridarum colonization seemed to significantly dampen a time-dependent increase in the phyla Actinobacteria, Deltaproteobacteria, and Gammaproteobacteria, suggesting that C. muridarum may be able to stabilize the gut microbiota.
FIG 7.
Effect of C. muridarum gastrointestinal colonization on gut microbiota. Fecal samples were collected from C57BL/6J female mice with (+; n = 5) or without (−; n = 5) C. muridarum colonization in the GI tract for various lengths of time, as described in the Fig. 6 legend. Fecal samples collected prior to intragastric inoculation (day 0) were used for the baseline. Total genomic DNA extracted from the fecal samples was used for quantitation of 16S rRNA genes by qPCR using primers specific for 7 bacteria phyla, including the 4 classes of the Proteobacteria phylum, listed in the key on the right. (a) The relative abundance of each phylum or class is expressed in percent, as shown along the y axis. All mice maintained a stable ratio of Firmicutes to Bacteroidetes throughout the time course, regardless of whether the mice were colonized with C. muridarum. (b) To display the phyla occupying less than 2% abundance, the portion of the overall plot containing the less abundant phyla was magnified with a maximal y axis scale setting of 2%. C. muridarum colonization did not affect the time-dependent acquisition of the phyla “Candidatus Saccharibacteria” and Tenericutes but slowed the increase in the Actinobacteria (*, P < 0.05, Wilcoxon rank-sum test) and the Gammaproteobacteria and Deltaproteobacteria (*, P < 0.05, Wilcoxon rank-sum test).
We further assessed whether GI tract C. muridarum affects the gut immune responses to nonchlamydial infection (Fig. 8). When the development of gut-resident memory T cells in response to lymphocytic choriomeningitis virus (LCMV) infection was compared between mice with GI tract C. muridarum and mice without GI tract C. muridarum, we found that LCMV induced significant levels of antigen-specific CD8+ T cells in the spleens of all mice to which naive P14 CD8+ T cells (CD45.1) were adoptively transferred, regardless of whether they were colonized with C. muridarum in the gastrointestinal tract. The responses of both the donor-derived P14 T cells and the endogenous CD8+ T cells were unaltered. More importantly, when the levels of gut intraepithelial CD8+ T cells positive for both CD103 and CD69 (defined as tissue-resident memory T cells [Trm cells]) were compared in mice with or without prior colonization with C. muridarum, it was found that both groups of mice developed similar levels of gut Trm cells. Since the adoptively transferred P14 T cells with T cell receptors (TCR) engineered to recognize a single LCMV epitope were monitored, this assay was very sensitive. Thus, we can conclude that the gastrointestinal tract C. muridarum does not affect either the systemic or the gastrointestinal mucosal immune responses to nonchlamydial antigen stimulation.
FIG 8.
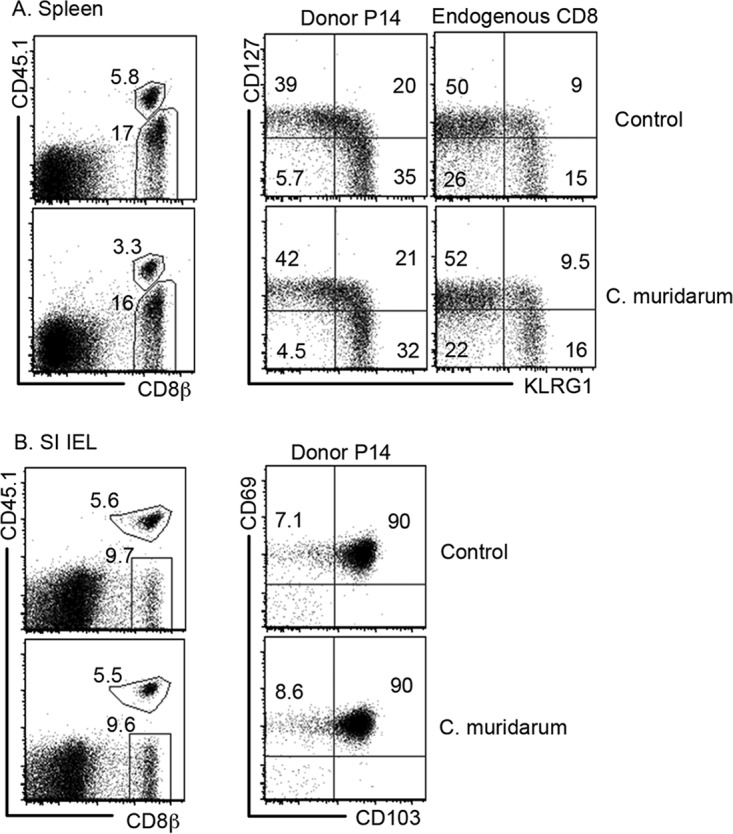
Effect of C. muridarum gastrointestinal colonization on intestinal immune responses to nonchlamydial infection. C57BL/6J female mice without (top; Control) or with (bottom, C. muridarum) CM-mCherry colonization in the GI tracts for 22 days were intravenously injected with 10,000 naive CD8+ T cells purified from P14 mice (Donor P14) and immediately infected intraperitoneally with 2 × 105 PFU of LCMV. Seventeen days after LCMV infection, the mice were sacrificed and splenocytes and intestinal intraepithelial lymphocytes (IEL) were isolated. Splenocytes were used for monitoring the donor P14 mouse and endogenous CD8+ T cell populations (A), while the small intestine (SI) IELs were used for measuring LCMV epitope-specific gut-resident memory cells using flow cytometry (B). Representative fluorescence-activated cell sorting profiles are shown. Three mice from each group were analyzed, and similar results were observed for each mouse. LCMV infection induced similar numbers of P14 IELs positive for both CD103 and CD69 (gut-resident memory CD8+ T cells) in mice with or without C. muridarum colonization.
Finally, we also evaluated whether the gastrointestinal C. muridarum colonization caused significant pathologies in extragastrointestinal organs/tissues, including the lung and genital tract (Fig. 9), as well as the liver, kidney, and spleen (data not shown). Using a 4× objective lens, no significant structural alterations were observed in any of the organ sections of mice that had been intragastrically immunized for 56 days or not. Under a 100× objective lens, there was no significant difference in inflammatory infiltration between the control and immunized mice. Apparently, GI tract C. muridarum colonization did not result in any significant chronic inflammation in these organs. Similar results were observed from other organs, including the kidney and liver.
FIG 9.
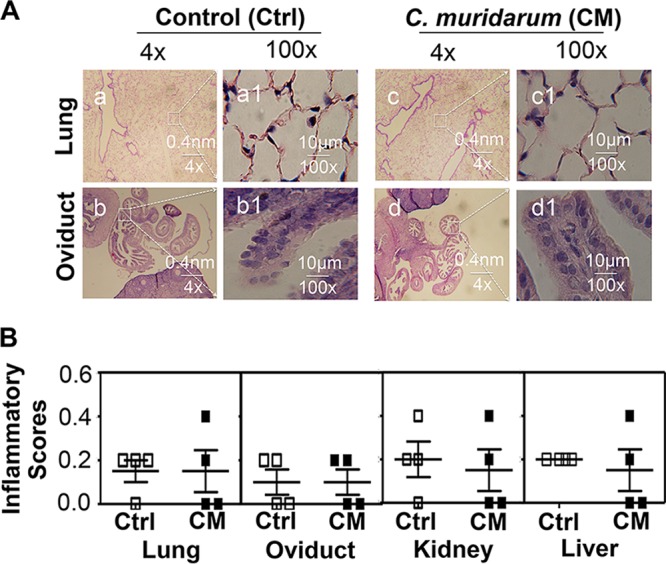
Effect of gastrointestinal C. muridarum on inflammatory pathologies of extragastrointestinal tract organs/tissues. Organs/tissues other than the gastrointestinal tracts were harvested from the mice described in the Fig. 6 legend. (A) Representative images of H&E-stained lung (a, a1, c, and c1) and oviduct (b, b1, d, and d1) sections from one mouse sacrificed on day 56 after intragastric immunization are presented. Under a 4× objective lens, no significant structural alteration was observed regardless of intragastric immunization. When the images were viewed with a 100× objective lens, there was no significant difference in inflammatory infiltration between the control and immunized mice. (B) The inflammatory infiltration was further semiquantitatively scored from sections of lung, genital tract, kidney, and liver under the 100× objective lens. No significant difference was found in any of these organs between the control mice (Ctrl) and the C. muridarum-immunized mice (CM).
DISCUSSION
Chlamydia trachomatis is spread sexually between humans and is also frequently detected in human rectal swab specimens (3, 4, 43, 44). However, the medical significance of GI tract C. trachomatis remains unknown. Women diagnosed with urogenital C. trachomatis infection are susceptible to reinfection at rates ranging from 12% to 39% (45–49), with many women appearing to be resistant to reinfection (47–50). Since the study populations also contained women with C. trachomatis infection in the GI tract (2–5, 43, 51), it is unclear whether women positive for C. trachomatis in the GI tract are more resistant or susceptible to reinfection in the genital tract. Directly addressing this question may require clinical studies comparing the genital tract reinfection rates between women with GI tract C. trachomatis infection and women without GI tract C. trachomatis infection.
To acquire data for supporting the relevant clinical studies, in the current study, we used a mouse model of C. muridarum infection to evaluate the effects of GI tract C. muridarum on the genital tract susceptibility to challenge infection with C. muridarum. We found that intragastric inoculation of C. muridarum induced robust transmucosal protection against subsequent C. muridarum infection in the genital tract. The protection was rapidly induced and lasted for long periods of time. Oral immunization for 1 week was sufficient to induce protection in the genital tract. During the first week after oral inoculation, most C. muridarum organisms were localized in the small intestine (data not shown) (7), suggesting that the protective immunity might be induced by C. muridarum in the small intestine. Efforts are under way to further localize the tissue site where C. muridarum induces transmucosal immunity. Once it is induced, the immunity lasted for long periods of time. By 20 weeks after oral immunization, robust transmucosal immunity was still evident. However, the long-lasting protection was not dependent on active C. muridarum colonization since oral doxycycline treatment for the clearance of C. muridarum GI tract colonization did not negatively affect the transmucosal immunity.
It is worth noting that although the GI tract C. muridarum-induced transmucosal immunity protected mice against subsequent genital tract infection with C. muridarum, it did not affect the colonization of C. muridarum in the GI tract. How did the C. muridarum-induced immunity selectively spare the GI tract C. muridarum bacteria? Long-lasting GI tract colonization with C. muridarum is mainly restricted to the cecum and colon (8). C. muridarum may have established itself as a normal commensal in the epithelial tissues of the cecum and colon. Like other commensal microbial species, despite the robust IgA responses to them in the gut, they remain in the gut for long periods of time. Indeed, high levels of anti-C. muridarum IgA were detected in fecal samples (6, 7) (Fig. 4). Intestinal secretory IgA is considered essential for maintaining the homeostasis of the gut commensal species (52), although the mechanisms by which host immune responses may stabilize commensal species in the GI tract remain unknown.
The GI tract C. muridarum-induced transmucosal immunity was dependent on MHC class II-restricted responses but not on MHC class I-restricted responses, which is consistent with the essential roles of MHC-II-restricted responses in controlling C. muridarum infection in the genital tract (53). However, because MHC class II-deficient mice failed to resolve the primary genital tract infection, it was not possible to evaluate the role of MHC class II-restricted responses in preventing secondary genital tract infection. In the current study, mice deficient in MHC class II restricted C. muridarum to the GI tract without dissemination to the genital tract. Thus, we could still challenge these mice intravaginally. We found that mice deficient in MHC class II developed robust live organism shedding courses regardless of whether they had been immunized with C. muridarum, which provides the first direct evidence demonstrating the essential role of MHC class II antigen presentation in preventing a secondary infection in the mouse genital tract. Since a deficiency in MHC class II-restricted responses causes a dearth of CD4+ lymphocytes in the thymus and spleen (54), we further compared mice with a deficiency in either CD4+ T cells or B cells for the development of transmucosal immunity. Mice with or without a deficiency in CD4+ T cells or B cells still developed robust transmucosal immunity, suggesting that CD4+ T cells and B cells can compensate for each other. Depletion of CD4+ T cells from B cell-deficient mice completely blocked the transmucosal protection, reproducing the phenotype of MHC class II knockout mice. These observations together suggest that CD4+ T cells or B cells alone may be dispensable for the GI tract C. muridarum-induced transmucosal immunity, but both may function synergistically to promote robust transmucosal immunity. Morrison and Morrison have recently demonstrated a central role of antibody in C. muridarum-induced immunity against reinfection in the genital tract (39), which is dependent on the activation of an effector cell population in genital tract tissues by CD4+ T cells (55). These authors further revealed that IFN-γ from the CD4+ T cells is responsible for activating the effector cell population that functions in antibody-mediated antichlamydial immunity (40). The effector cells can be epithelial cells and/or phagocytes. A similar effector mechanism may also play a critical role in the transmucosal immunity induced by GI tract C. muridarum.
Despite the long-lasting colonization of C. muridarum in the GI tract, C. muridarum has not been associated with any significant pathology in the GI tract (7, 8, 31, 32, 34, 35). In the current study, we carefully examined not only the GI tract tissues but also the non-GI tract tissues of mice colonized with C. muridarum in the GI tract. The productive C. muridarum infection in the intestinal epithelial cells did not cause significant inflammatory infiltration. Macroscopically, there were also no signs of colitis. Microscopically, the clusters of C. muridarum-infected epithelial cells did not lead to any significant alteration of the gut microbiota or a response to nonchlamydial infection. When non-GI tract tissues, such as those of the genital tract and the lungs, from the same mice were examined, there was no sign of significant inflammatory infiltration. Thus, the long-lasting colonization of C. muridarum in the GI tract is nonpathogenic to mice. Interestingly, C. trachomatis colonization in the human GI tract also appears to be nonpathogenic (16). Although C. trachomatis lymphogranuloma venereum (LGV) serovars are known to cause proctitis in men who have sex with men (11, 12), the sexually transmitted C. trachomatis serovars D to K have not been associated with any significant pathology in the human GI tract, despite the frequent detection of C. trachomatis in rectal swab specimens (14–18).
C. muridarum colonization of the GI tract is nonpathogenic for either GI tract tissues (Fig. 5 to 8) (7, 31) or extra-GI tract tissues (Fig. 9) and can induce robust transmucosal immunity in the genital tract (Fig. 1 to 4). GI tract C. muridarum may be considered an orally deliverable replicating vaccine. First, the oral uptake of attenuated live bacterial or viral vaccines has been well accepted. Although the reversion of orally administered attenuated vaccines into pathogens has caused some concerns (56), our studies have shown that C. muridarum behaves like a normal commensal species in the mouse gut. However, clinical studies are required to determine whether C. trachomatis also behaves like a normal commensal species in the human gut and is able to induce transmucosal protection in the human genital tract. Second, when C. muridarum is delivered into the mouse GI tract (8) or the blood (35), it is restricted to the GI tract and does not autoinoculate the genital tract. However, it has been proposed that GI tract C. trachomatis may serve as a reservoir for autoinoculation of the human genital tract (6, 7). Thus, clinical studies to directly test this hypothesis are urgently needed. Third, GI tract C. muridarum can be cleared using doxycycline without affecting the transmucosal immunity induced, thus providing a safeguard measure for preventing any potential pathogenicity. However, the optimal conditions for effectively clearing C. trachomatis from the human GI tract still need to be worked out. Finally, mutant C. muridarum strains that are attenuated in inducing a pathology in the mouse upper genital tract have been identified (57, 58), suggesting that these mutants can be safer oral vaccines. However, although attenuated plasmid-free C. trachomatis strains have been identified or created (59–65), neither their safety nor their efficacy after they are orally taken up into the human GI tract is known.
It is worth noting that although in the current study we did not find any significant pathologies or C. muridarum colonization in the extra-GI tract tissues of mice precolonized with C. muridarum in the GI tract, Perry and Hughes (32) have previously reported that C. muridarum spreads into extra-GI tract tissues. This discrepancy might be caused by the different strains of C. muridarum used. The Nigg strain was used in the current study, while the Weiss strain used by Perry and Hughes (32). The Weiss strain is known to be more invasive than the Nigg strain (66). Thus, a more virulent C. muridarum strain may still be able to spread to and cause pathology in extra-GI tract tissues. In addition, the information obtained from the current study is purely based on the mouse model of C. muridarum infection, and this information should not be extrapolated to human Chlamydia infections. Caveats should be considered even when using the knowledge to guide clinical studies.
MATERIALS AND METHODS
Chlamydial organism growth.
All Chlamydia muridarum clones used in the current study were derived from strain Nigg3 (GenBank accession number CP009760.1), including a passaged clone designated G13.32.1 (58) and plasmid-free clone CMUT3G5 (GenBank accession number CP006974.1). CMUT3G5 was used for transformation with pmCherry:CM to create CM-mCherry, as described previously (67, 68). Both G13.32.1 and CM-mCherry were propagated in HeLa cells and purified as elementary bodies (EBs) as reported previously (34, 69). Aliquots of the purified EBs were stored at −80°C until use.
Mouse immunization, antibody depletion, and challenge infection.
Mouse experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council (70). The protocol was approved by the Committee on the Ethics of Laboratory Animal Experiments of the University of Texas Health Science Center at San Antonio.
Purified C. muridarum EBs were used to inoculate 6- to 7-week-old female mice (The Jackson Laboratory, Inc., Bar Harbor, ME) intragastrically (as an immunization route), intrarectally, or intravaginally as described previously (8, 21, 34, 71). The following mouse strains were used in the current study. We started with CBA/J mice (stock number 000656; The Jackson Laboratory) since they are highly susceptible to C. muridarum induction of hydrosalpinx (21). After observing the transmucosal protection induced by GI tract C. muridarum, we switched to C57BL/6J mice (stock number 000664; The Jackson Laboratory) in order to investigate the mechanisms. Various gene-deficient mice, including MHC class I (MHC-I) knockout (KO) mice (stock number 002087; The Jackson Laboratory), MHC class II (MHC-II) KO mice (stock number 003584; The Jackson Laboratory), CD4 KO mice (stock number 002663; The Jackson Laboratory), and μ chain (B cell) KO mice (stock number 002288; The Jackson Laboratory), were used. Furthermore, some C57BL/6J mice were used to make bone marrow-derived dendritic cells (DCs), while B6;D2-TCR LCMV (P14) mice carrying congenic marker CD45.1 (stock number 004694; The Jackson Laboratory) were used to provide donor T cells. Inoculation of viable C. muridarum bacteria intragastrically was used to mimic oral immunization. Following each inoculation, both vaginal and rectal swab specimens were periodically taken to monitor viable C. muridarum colonization or organs/tissues were harvested (after the mice were sacrificed) to titrate viable organisms as described previously (8, 34, 35). In some experiments, mice were treated with anti-CD4 antibody (clone number GK1.5, rat IgG2b, purified as described previously [40] or purchased from Bio X Cell, West Lebanon, NH) 5 days prior to challenge infection and then twice a week after infection for a total length of 5 weeks. Each injection was administered intraperitoneally with 500 μg IgG in 500 μl phosphate-buffered saline (PBS) as described previously (39, 40).
Evaluating organ/tissue inflammatory pathology macroscopically and microscopically.
At various time points after intragastric immunization or intravaginal challenge infection, as indicated above and in the figures for the individual experiments, mice were euthanized for the evaluation of organ pathologies. For the genital tract, the focus was on upper genital tract hydrosalpinx. After documentation of the oviduct hydrosalpinx using high-resolution digital photography, the oviduct hydrosalpinx was scored both macroscopically (hydrosalpinx severity score) and microscopically (oviduct dilation and inflammatory infiltration scores) as described previously (58). For the GI tract, the focus was on the colon. After the appearance of the colon (dark or bright) was observed and its length was measured, the small and large intestines were fixed and cut open longitudinally and separately rolled in melting agarose to make wax blocks. Sections were cut as described previously for genital tract tissues (58), and three representative sections were immunostained for the detection of C. muridarum using an immunofluorescence assay as described previously (37, 72) and below. Sections adjacent to those positive for C. muridarum inclusions were stained with H&E for evaluation of the inflammatory infiltration, with a focus on the positions corresponding to the areas positive for C. muridarum. The inflammatory infiltrates were scored on an ordinal scale as described previously for oviduct tissue sections (58). For other organs, the overall appearances were carefully examined and any abnormalities were recorded. The organs were then fixed with formalin, embedded, sectioned, and stained with H&E (for evaluation of inflammatory infiltration) or evaluated by immunofluorescence as described above for the GI tract tissues.
Immunofluorescence assay.
For immunofluorescence labeling of the C. muridarum bacteria in HeLa cells, a rabbit antibody (designated R1604, raised with purified C. muridarum EBs) was used as a primary antibody to label C. muridarum and was visualized with goat anti-rabbit IgG conjugated with Cy2 (green; catalog number 111-225-144; Jackson ImmunoResearch Laboratories, Inc., West Grove PA). The DNA dye Hoechst 3328 (blue; Sigma-Aldrich, St. Louis, MO) was used to visualize the nuclei. The doubly labeled samples were used to count the C. muridarum bacteria under a fluorescence microscope (model AX70; Olympus) equipped with a charge-coupled-device camera (Hamamatsu). For samples containing two different types of C. muridarum bacteria, such as the G13.32.1 and CM-mCherry strains, we used a rat anti-mCherry monoclonal antibody (MAb; M11217; Thermo Fisher Scientific) together with R1604 as the primary antibody to differentiate them. To differentiate G13.32.1 from the plasmid-free CMUT3G5, we used mouse anti-pGP3 or GlgA antibodies (67, 68, 73) in combination with R1604 as the primary antibody. For the labeling of the C. muridarum bacteria in mouse organ/tissue sections, besides rabbit antibody R1604, mouse MAb BC7.1 was used as another primary antibody for the detection of chlamydial HSP60, which was visualized with goat anti-mouse IgG conjugated with Cy3 (red). Together with the DNA staining (blue), the tissue sections were triply stained and evaluated using the AX70 fluorescence microscope. Color images were taken individually and superimposed digitally into tricolor as described previously (69, 74).
ELISA for measuring mouse fecal IgA and serum IgG antibodies.
For measuring IgA antibodies, fecal samples from each mouse were thoroughly resuspended in PBS solution at a final concentration of 1 mg/10 μl. After centrifugation at 13,000 rpm in a microcentrifuge for 5 min, the supernatants were applied neat or after 2-fold serial dilution to 96-well plates precoated with purified C. muridarum EBs (CM-mCherry clone). IgA binding was detected with goat anti-mouse IgA conjugated with horseradish peroxidase (HRP; catalog number 626720; Invitrogen, Waltham, MA) plus a soluble substrate, ABTS [2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt; catalog number 30931-67-0; Sigma-Aldrich, St. Louis, MO]. The absorbance at 405 nm was detected with a Synergy H4 microplate reader (BioTek, Winooski, VT), and the results were expressed as raw optical density (OD) values. For the detection of IgG, serum samples collected from the mouse tail vein were subjected to 4-fold serial dilution starting at 1:1,600. IgG binding to the plate-coated EBs was detected with a goat anti-mouse IgG-HRP conjugate (catalog number 31430; Thermo Fisher Scientific) as described above. For isotyping of IgG, the same ELISA scheme described above was used. The serum samples diluted 1:1,600 were applied to C. muridarum-coated plates. Mouse IgG1, IgG2a, IgG2b, IgG2c, and IgG3 bound to the plate-immobilized EBs were detected using the corresponding secondary antibodies, including goat anti-mouse IgG1 (catalog number A10551), IgG2a (catalog number A10685), IgG2b (catalog number M32407), IgG2c (catalog number pa129288), and IgG3 (catalog number m32707) (all from Thermo Fisher Scientific), respectively. The results were expressed as raw OD values.
Flow cytometry for profiling of T cell intracellular cytokines.
To monitor intracellular cytokine production by T cells in C57BL/6J mice with or without intragastric immunization with C. muridarum for various times, both mesenteric draining lymph node (MLN) and spleen cells were collected and restimulated in vitro for 24 h with dendritic cells (DCs) that had been prepulsed overnight with or without C. muridarum EBs. To prepare the DCs, the femur and tibia bones from the same strain of mice were harvested to make a bone marrow cell suspension 7 days prior to the restimulation assay. The bone marrow cells were cultured in granulocyte-macrophage colony-stimulating factor-containing medium. The loosely attached cells that contained DCs were selectively collected on day 6, and the DCs were further enriched by removing the macrophages through repeated attachment as described previously (75). The enriched DCs were then pulsed overnight with live C. muridarum EBs at a multiplicity of infection of 10. On the next day, the EB-pulsed DCs were used to present C. muridarum antigens to T cells. After overnight in vitro antigen presentation or restimulation (10 to 14 h), GolgiStop protein transport inhibitor (brefeldin A; catalog number B5936; Sigma-Aldrich) was added to the culture at a final concentration of 25 μg/ml 6 h before detection of intracellular cytokines. To identify T cell subsets and to detect their intracellular cytokines, lymphocytes restimulated in vitro were blocked with a rat anti-mouse Fc receptor (CD16/CD32) antibody (clone 2.4G2; BD Biosciences, San Jose, CA). The blocked lymphocytes were labeled with anti-CD4 (peridinin chlorophyll protein-Cy5.5; catalog number RM4-5; BioLegend, San Diego, CA) and anti-CD8β (Efluor 450; catalog number eBioH35-17.2; eBioscience) antibodies. Following fixation and permeabilization, the following antibodies were added: anti-tumor necrosis factor alpha (anti-TNF-α; conjugated with fluorescein isothiocyanate; clone MP6-XT22; eBioscience), anti-IFN-γ (conjugated with phycoerythrin [PE]-Cy7; clone XMG1.2; BioLegend), anti-interleukin-5 (anti-IL-5; conjugated with allophycocyanin; catalog number TRFX5; BioLegend), and anti-IL-13 (conjugated with PE; catalog number eBio13A; eBioscience). After antibody staining, the number of cells stained with a given combination of antibodies in the cell samples was counted using an LSRII flow cytometer (BD Bioscience) as described previously (74, 76). The data were analyzed using FlowJo software (FlowJo, LLC).
qPCR for profiling of fecal microbiota.
Fecal samples were collected from female C57BL/6J mice on different days after intragastric inoculation with or without C. muridarum. Prior to the intragastric inoculation, fecal samples were collected from all mice to establish a baseline for each mouse and were defined as day 0 samples. The fecal samples were rapidly frozen in liquid nitrogen and stored at −80°C until DNA extraction. Total genomic DNA was extracted from each sample (150 mg) using a QIAamp DNA stool minikit (Qiagen Inc., Germantown, MD) according to the manufacturer's instructions. Ratios of the absorbance at 260 nm/absorbance at 280 nm were determined to quantify and assess the purity of the DNA samples. A SYBR green-based real-time quantitative PCR (qPCR) with iTaq universal SYBR green super mix 500 from Bio-Rad was used to quantitate the bacterial 16S rRNA genes in each fecal sample with the primers specific for the bacterial phyla and classes listed in Table S1 in the supplemental material, including the Bacteroidetes, Firmicutes, Actinobacteria, “Candidatus Saccharibacteria,” Verrucomicrobia, Tenericutes, Alphaproteobacteria, Betaproteobacteria, Epsilonproteobacteria, and Gammaproteobacteria, as well as a pair of universal primers. The following PCR conditions were used: 95°C for 15 s and 60°C for 30 s for 45 cycles. The number of PCR cycles required to achieve a given level of amplicon with each pair of primers was used to calculate the relative abundance of the template targeted by a given pair of primers, as described previously (77).
Monitoring the development of gut-resident memory T cells in response to LCMV infection.
C57BL/6J female mice with or without C. muridarum G13.321 colonization in the GI tract for 28 days were intravenously injected with 10,000 naive CD8+ T cells purified from donor P14 mice carrying congenic marker CD45.1 (B6;D2-TCR lymphocytic choriomeningitis virus [LCMV]). Immediately following the adoptive transfer, each recipient mouse was infected intraperitoneally with 2 × 105 PFU of LCMV Armstrong (78). On day 17 after LCMV infection, the mice were sacrificed and the spleens and small intestines were harvested to prepare splenocytes and intestinal intraepithelial lymphocytes (IELs), respectively. The IELs were isolated from the small intestine as described previously (78). The splenocytes were used for monitoring the donor P14 and endogenous CD8+ T cell populations, while the IELs were used for measuring LCMV epitope-specific gut-resident memory cells using flow cytometry, as described elsewhere (78). Briefly, both splenocytes and IELs were gated for double positivity for CD8 and CD45.1 for use as donor CD8+ T cells. The spleen-derived double-positive cells were plotted for expression of KLRG1 and CD127 to distinguish memory precursor effector cells (MPEC) and short-lived effector cells (SLEC) (79), while the gut-derived IELs were plotted for expression of CD103 and CD69 to identify gut-resident memory CD8+ T cells.
Statistical analyses.
All data, including the time course of live organism shedding as the number of inclusion-forming units (IFU), genome copy numbers, and infection rates, were compared using the area under the curve (AUC) between two groups and the Wilcoxon rank-sum test. The individual IFU data points and pathology scores were analyzed by the Wilcoxon rank-sum test, while categorical data, including the percentage of mice positive for live organism shedding or hydrosalpinx, were analyzed using Fisher's exact test.
Supplementary Material
ACKNOWLEDGMENTS
This study is supported in part by a U.S. NIH grant (R01AI121989 to G.Z.), the Central South University 3rd Xiangya Hospital Endowment Fund (to L.W.), and a postdoctoral fellowship from the China Scholarship Council (to C.Z.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00630-17.
REFERENCES
- 1.Campos-Hernandez E, Vazquez-Chagoyan JC, Salem AZ, Saltijeral-Oaxaca JA, Escalante-Ochoa C, Lopez-Heydeck SM, de Oca-Jimenez RM. 2014. Prevalence and molecular identification of Chlamydia abortus in commercial dairy goat farms in a hot region in Mexico. Trop Anim Health Prod 46:919–924. doi: 10.1007/s11250-014-0585-6. [DOI] [PubMed] [Google Scholar]
- 2.Peters RP, Dubbink JH, van der Eem L, Verweij SP, Bos ML, Ouburg S, Lewis DA, Struthers H, McIntyre JA, Morre SA. 2014. Cross-sectional study of genital, rectal, and pharyngeal Chlamydia and gonorrhea in women in rural South Africa. Sex Transm Dis 41:564–569. doi: 10.1097/OLQ.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 3.Gratrix J, Singh AE, Bergman J, Egan C, McGinnis J, Drews SJ, Read R. 2014. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis 41:589–591. doi: 10.1097/OLQ.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 4.Gratrix J, Singh AE, Bergman J, Egan C, Plitt SS, McGinnis J, Bell CA, Drews SJ, Read R. 2015. Evidence for increased Chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis 60:398–404. doi: 10.1093/cid/ciu831. [DOI] [PubMed] [Google Scholar]
- 5.Musil K, Currie M, Sherley M, Martin S. 2016. Rectal chlamydia infection in women at high risk of chlamydia attending Canberra Sexual Health Centre. Int J STD AIDS 27:526–530. doi: 10.1177/0956462415586317. [DOI] [PubMed] [Google Scholar]
- 6.Yeruva L, Spencer N, Bowlin AK, Wang Y, Rank RG. 2013. Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog Dis 68:88–95. doi: 10.1111/2049-632X.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rank RG, Yeruva L. 2014. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun 82:1362–1371. doi: 10.1128/IAI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Zhang Q, Zhang T, Zhang Y, Zhu C, Sun X, Zhang N, Xue M, Zhong G. 2016. The Chlamydia muridarum organisms fail to auto-inoculate the mouse genital tract after colonization in the gastrointestinal tract for 70 days. PLoS One 11:e0155880. doi: 10.1371/journal.pone.0155880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2015. Chlamydial infections. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/std/tg2015/chlamydia.htm. [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2009. 2008 sexually transmitted disease surveillance. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/std/stats08/toc.htm. [Google Scholar]
- 11.Quinn TC, Goodell SE, Mkrtichian E, Schuffler MD, Wang SP, Stamm WE, Holmes KK. 1981. Chlamydia trachomatis proctitis. N Engl J Med 305:195–200. doi: 10.1056/NEJM198107233050404. [DOI] [PubMed] [Google Scholar]
- 12.de Vries HJ, Zingoni A, White JA, Ross JD, Kreuter A. 2014. 2013 European guideline on the management of proctitis, proctocolitis and enteritis caused by sexually transmissible pathogens. Int J STD AIDS 25:465–474. doi: 10.1177/0956462413516100. [DOI] [PubMed] [Google Scholar]
- 13.Elliott PR, Forsey T, Darougar S, Treharne JD, Lennard-Jones JE. 1981. Chlamydiae and inflammatory bowel disease. Gut 22:25–27. doi: 10.1136/gut.22.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KJ, Kim J, Shin DH, Jung JO, Koh S, Kim KY, Lee JM. 2015. Chlamydial proctitis in a young man who has sex with men: misdiagnosed as inflammatory bowel disease. Chonnam Med J 51:139–141. doi: 10.4068/cmj.2015.51.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orda R, Samra Z, Levy Y, Shperber Y, Scapa E. 1990. Chlamydia trachomatis and inflammatory bowel disease—a coincidence? J R Soc Med 83:15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mardh PA, Ursing B, Sandgren E. 1980. Lack of evidence for an association between infection with Chlamydia trachomatis and Crohn's disease, as indicated by micro-immunofluorescence antibody tests. Acta Pathol Microbiol Scand B Microbiol Immunol 88:57–59. [DOI] [PubMed] [Google Scholar]
- 17.McGarity BH, Robertson DA, Clarke IN, Wright R. 1991. Deoxyribonucleic acid amplification and hybridisation in Crohn's disease using a chlamydial plasmid probe. Gut 32:1011–1015. doi: 10.1136/gut.32.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss TR, Darougar S, Woodland RM, Nathan M, Dines RJ, Cathrine V. 1993. Antibodies to Chlamydia species in patients attending a genitourinary clinic and the impact of antibodies to C. pneumoniae and C. psittaci on the sensitivity and the specificity of C. trachomatis serology tests. Sex Transm Dis 20:61–65. doi: 10.1097/00007435-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 20.de la Maza LM, Pal S, Khamesipour A, Peterson EM. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 62:2094–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. doi: 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. doi: 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodgers AK, Wang J, Zhang Y, Holden A, Berryhill B, Budrys NM, Schenken RS, Zhong G. 2010. Association of tubal factor infertility with elevated antibodies to Chlamydia trachomatis caseinolytic protease P. Am J Obstet Gynecol 203:494.e7–494.e14. doi: 10.1016/j.ajog.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlcek KR, Li W, Manam S, Zanotti B, Nicholson BJ, Ramsey KH, Murthy AK. 2016. The contribution of Chlamydia-specific CD8(+) T cells to upper genital tract pathology. Immunol Cell Biol 94:208–212. doi: 10.1038/icb.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Maza LM, Peterson EM. 2002. Vaccines for Chlamydia trachomatis infections. Curr Opin Investig Drugs 3:980–986. [PubMed] [Google Scholar]
- 27.Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu C, Peng B, Li Z, Lei L, Li Z, Chen L, He Q, Zhong G, Wu Y. 2013. Induction of protective immunity against Chlamydia muridarum intravaginal infection with the chlamydial immunodominant antigen macrophage infectivity potentiator. Microbes Infect 15:329–338. doi: 10.1016/j.micinf.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson RM, Kerr MS, Slaven JE. 2014. An atypical CD8 T-cell response to Chlamydia muridarum genital tract infections includes T cells that produce interleukin-13. Immunology 142:248–257. doi: 10.1111/imm.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockey DD, Wang J, Lei L, Zhong G. 2009. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines 8:1365–1377. doi: 10.1586/erv.09.98. [DOI] [PubMed] [Google Scholar]
- 31.Igietseme JU, Portis JL, Perry LL. 2001. Inflammation and clearance of Chlamydia trachomatis in enteric and nonenteric mucosae. Infect Immun 69:1832–1840. doi: 10.1128/IAI.69.3.1832-1840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry LL, Hughes S. 1999. Chlamydial colonization of multiple mucosae following infection by any mucosal route. Infect Immun 67:3686–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeruva L, Melnyk S, Spencer N, Bowlin A, Rank RG. 2013. Differential susceptibilities to azithromycin treatment of chlamydial infection in the gastrointestinal tract and cervix. Antimicrob Agents Chemother 57:6290–6294. doi: 10.1128/AAC.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q, Huang Y, Gong S, Yang Z, Sun X, Schenken R, Zhong G. 2015. In vivo and ex vivo imaging reveals a long-lasting chlamydial infection in the mouse gastrointestinal tract following genital tract inoculation. Infect Immun 83:3568–3577. doi: 10.1128/IAI.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai J, Zhang T, Wang L, Shao L, Zhu C, Zhang Y, Failor C, Schenken R, Baseman J, He C, Zhong G. 2016. Intravenous inoculation with Chlamydia muridarum leads to a long-lasting infection restricted to the gastrointestinal tract. Infect Immun 84:2382–2388. doi: 10.1128/IAI.00432-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Connell CM, Ingalls RR, Andrews CW Jr, Scurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 37.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. 2014. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun 82:983–992. doi: 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao L, Melero J, Zhang N, Arulanandam B, Baseman J, Liu Q, Zhong G. 2017. The cryptic plasmid is more important for Chlamydia muridarum to colonize the mouse gastrointestinal tract than to infect the genital tract. PLoS One 12:e0177691. doi: 10.1371/journal.pone.0177691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison SG, Morrison RP. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun 69:2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naglak EK, Morrison SG, Morrison RP. 6 September 2016. IFNγ is required for optimal antibody-mediated immunity against genital Chlamydia infection. Infect Immun. doi: 10.1128/IAI.00749-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Lei L, Chang X, Li Z, Lu C, Zhang X, Wu Y, Yeh IT, Zhong G. 2010. Mice deficient in MyD88 develop a Th2-dominant response and severe pathology in the upper genital tract following Chlamydia muridarum infection. J Immunol 184:2602–2610. doi: 10.4049/jimmunol.0901593. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Lei L, Zhou Z, He J, Xu S, Lu C, Chen J, Yang Z, Wu G, Yeh IT, Zhong G, Wu Y. 2013. Contribution of interleukin-12 p35 (IL-12p35) and IL-12p40 to protective immunity and pathology in mice infected with Chlamydia muridarum. Infect Immun 81:2962–2971. doi: 10.1128/IAI.00161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dukers-Muijrers NH, Speksnijder AG, Morre SA, Wolffs PF, van der Sande MA, Brink AA, van den Broek IV, Werner MI, Hoebe CJ. 2013. Detection of anorectal and cervicovaginal Chlamydia trachomatis infections following azithromycin treatment: prospective cohort study with multiple time-sequential measures of rRNA, DNA, quantitative load and symptoms. PLoS One 8:e81236. doi: 10.1371/journal.pone.0081236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Javanbakht M, Gorbach P, Stirland A, Chien M, Kerndt P, Guerry S. 2012. Prevalence and correlates of rectal Chlamydia and gonorrhea among female clients at sexually transmitted disease clinics. Sex Transm Dis 39:917–922. doi: 10.1097/OLQ.0b013e31826ae9a2. [DOI] [PubMed] [Google Scholar]
- 45.Gaydos CA, Wright C, Wood BJ, Waterfield G, Hobson S, Quinn TC. 2008. Chlamydia trachomatis reinfection rates among female adolescents seeking rescreening in school-based health centers. Sex Transm Dis 35:233–237. doi: 10.1097/OLQ.0b013e31815c11fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kampman C, Koedijk F, Driessen-Hulshof H, Hautvast J, van den Broek I. 2016. Retesting young STI clinic visitors with urogenital Chlamydia trachomatis infection in the Netherlands; response to a text message reminder and reinfection rates: a prospective study with historical controls. Sex Transm Infect 92:124–129. doi: 10.1136/sextrans-2015-052115. [DOI] [PubMed] [Google Scholar]
- 47.Geisler WM, Lensing SY, Press CG, Hook EW III. 2013. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis 207:1850–1856. doi: 10.1093/infdis/jit094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu F, Stoner BP, Taylor SN, Mena L, Tian LH, Papp J, Hutchins K, Martin DH, Markowitz LE. 2011. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol 118:231–239. doi: 10.1097/AOG.0b013e3182246a83. [DOI] [PubMed] [Google Scholar]
- 49.Hosenfeld CB, Workowski KA, Berman S, Zaidi A, Dyson J, Mosure D, Bolan G, Bauer HM. 2009. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis 36:478–489. doi: 10.1097/OLQ.0b013e3181a2a933. [DOI] [PubMed] [Google Scholar]
- 50.Geisler WM, Wang C, Morrison SG, Black CM, Bandea CI, Hook EW III. 2008. The natural history of untreated Chlamydia trachomatis infection in the interval between screening and returning for treatment. Sex Transm Dis 35:119–123. doi: 10.1097/OLQ.0b013e318151497d. [DOI] [PubMed] [Google Scholar]
- 51.Dukers-Muijrers NH, Schachter J, van Liere GA, Wolffs PF, Hoebe CJ. 2015. What is needed to guide testing for anorectal and pharyngeal Chlamydia trachomatis and Neisseria gonorrhoeae in women and men? Evidence and opinion. BMC Infect Dis 15:533. doi: 10.1186/s12879-015-1280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathias A, Pais B, Favre L, Benyacoub J, Corthesy B. 2014. Role of secretory IgA in the mucosal sensing of commensal bacteria. Gut Microbes 5:688–695. doi: 10.4161/19490976.2014.983763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison RP, Feilzer K, Tumas DB. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun 63:4661–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. 1999. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A 96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farris CM, Morrison SG, Morrison RP. 2010. CD4+ T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infect Immun 78:4374–4383. doi: 10.1128/IAI.00622-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stern A, Yeh MT, Zinger T, Smith M, Wright C, Ling G, Nielsen R, Macadam A, Andino R. 2017. The evolutionary pathway to virulence of an RNA virus. Cell 169:35–46.e19. doi: 10.1016/j.cell.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen C, Zhou Z, Conrad T, Yang Z, Dai J, Li Z, Wu Y, Zhong G. 2015. In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract. Infect Immun 83:1881–1892. doi: 10.1128/IAI.03158-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conrad TA, Gong S, Yang Z, Matulich P, Keck J, Beltrami N, Chen C, Zhou Z, Dai J, Zhong G. 2015. The chromosome-encoded hypothetical protein TC0668 is an upper genital tract pathogenicity factor of Chlamydia muridarum. Infect Immun 84:467–479. doi: 10.1128/IAI.01171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kari L, Whitmire WM, Olivares-Zavaleta N, Goheen MM, Taylor LD, Carlson JH, Sturdevant GL, Lu C, Bakios LE, Randall LB, Parnell MJ, Zhong G, Caldwell HD. 2011. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J Exp Med 208:2217–2223. doi: 10.1084/jem.20111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding H, Gong S, Tian Y, Yang Z, Brunham R, Zhong G. 2013. Transformation of sexually transmitted infection-causing serovars of Chlamydia trachomatis using blasticidin for selection. PLoS One 8:e80534. doi: 10.1371/journal.pone.0080534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Connell CM, AbdelRahman YM, Green E, Darville HK, Saira K, Smith B, Darville T, Scurlock AM, Meyer CR, Belland RJ. 2011. Toll-like receptor 2 activation by Chlamydia trachomatis is plasmid dependent, and plasmid-responsive chromosomal loci are coordinately regulated in response to glucose limitation by C. trachomatis but not by C. muridarum. Infect Immun 79:1044–1056. doi: 10.1128/IAI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sigar IM, Schripsema JH, Wang Y, Clarke IN, Cutcliffe LT, Seth-Smith HM, Thomson NR, Bjartling C, Unemo M, Persson K, Ramsey KH. 2014. Plasmid deficiency in urogenital isolates of Chlamydia trachomatis reduces infectivity and virulence in a mouse model. Pathog Dis 70:61–69. doi: 10.1111/2049-632X.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.An Q, Radcliffe G, Vassallo R, Buxton D, O'Brien WJ, Pelletier DA, Weisburg WG, Klinger JD, Olive DM. 1992. Infection with a plasmid-free variant Chlamydia related to Chlamydia trachomatis identified by using multiple assays for nucleic acid detection. J Clin Microbiol 30:2814–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyashita N, Matsumoto A, Matsushima T. 2000. In vitro susceptibility of 7.5-kb common plasmid-free Chlamydia trachomatis strains. Microbiol Immunol 44:267–269. doi: 10.1111/j.1348-0421.2000.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 65.Yeow TC, Wong WF, Sabet NS, Sulaiman S, Shahhosseini F, Tan GM, Movahed E, Looi CY, Shankar EM, Gupta R, Arulanandam BP, Hassan J, Abu Bakar S. 2016. Prevalence of plasmid-bearing and plasmid-free Chlamydia trachomatis infection among women who visited obstetrics and gynecology clinics in Malaysia. BMC Microbiol 16:45. doi: 10.1186/s12866-016-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramsey KH, Sigar IM, Schripsema JH, Denman CJ, Bowlin AK, Myers GA, Rank RG. 2009. Strain and virulence diversity in the mouse pathogen Chlamydia muridarum. Infect Immun 77:3284–3293. doi: 10.1128/IAI.00147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Chen C, Gong S, Hou S, Qi M, Liu Q, Baseman J, Zhong G. 2014. Transformation of Chlamydia muridarum reveals a role for Pgp5 in suppression of plasmid-dependent gene expression. J Bacteriol 196:989–998. doi: 10.1128/JB.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. doi: 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 71.Shao L, Zhang T, Liu Q, Wang J, Zhong G. 2017. Chlamydia muridarum with mutations in chromosomal genes tc0237 and/or tc0668 is deficient in colonizing the mouse gastrointestinal tract. Infect Immun 85:e00321-. doi: 10.1128/IAI.00321-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, Zhong G. 2013. Oviduct infection and hydrosalpinx in DBA1/j mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum. PLoS One 8:e71649. doi: 10.1371/journal.pone.0071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gong S, Yang Z, Lei L, Shen L, Zhong G. 2013. Characterization of Chlamydia trachomatis plasmid-encoded open reading frames. J Bacteriol 195:3819–3826. doi: 10.1128/JB.00511-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhong G, Reis e Sousa C, Germain RN. 1997. Production, specificity, and functionality of monoclonal antibodies to specific peptide-major histocompatibility complex class II complexes formed by processing of exogenous protein. Proc Natl Acad Sci U S A 94:13856–13861. doi: 10.1073/pnas.94.25.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu H, Zhong G. 1999. Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live Chlamydia trachomatis infection. Infect Immun 67:1763–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhong G, Reis e Sousa C, Germain RN. 1997. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility complex class II complexes after soluble protein exposure in vivo or in vitro. J Exp Med 186:673–682. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang YW, Chen MK, Yang BY, Huang XJ, Zhang XR, He LQ, Zhang J, Hua ZC. 2015. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in mouse feces. Appl Environ Microbiol 81:6749–6756. doi: 10.1128/AEM.01906-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang N, Bevan MJ. 2013. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.