Abstract
Background:
In the United States, one in six children are affected by neurodevelopmental disorders, and polybrominated diphenyl ethers (PBDEs) in flame-retardant chemicals are measured ubiquitously in children.
Objective:
We conducted a systematic a systematic review regarding developmental exposure to PBDEs and intelligence or Attention Deficit/Hyperactivity Disorder (ADHD) and attention-related behavioral conditions in humans.
Methods:
We searched articles published up to 26 September 2016, and included original studies that quantified exposures to PBDEs incurred any time in proximity to conception or during in utero, perinatal, or childhood time periods. We evaluated the risk of bias of individual studies and the overall quality and strength of the evidence according to the Navigation Guide systematic review methodology. We established criteria in advance to identify studies that could be combined using random effects meta-analyses (DerSimonian-Laird method).
Results:
Fifteen studies met the inclusion criteria; 10 studies met the criteria for intelligence and nine for attention-related problems. We rated studies generally with “low” to “probably low” risk of bias and rated the overall body of evidence as “moderate” quality with “sufficient” evidence for an association between Intelligence Quotient (IQ) and PBDEs. Our meta-analysis of four studies estimated a 10-fold increase (in other words, times 10) in PBDE exposure associated with a decrement of 3.70 IQ points (95% confidence interval: 0.83, 6.56). We concluded the body of evidence was of “moderate” quality for ADHD with “limited” evidence for an association with PBDEs, based on the heterogeneity of association estimates reported by a small number of studies and the fact that chance, bias, and confounding could not be ruled out with reasonable confidence.
Conclusion:
We concluded there was sufficient evidence supporting an association between developmental PBDE exposure and reduced IQ. Preventing developmental exposure to PBDEs could help prevent loss of human intelligence. https://doi.org/10.1289/EHP1632
Introduction
The prevalence of neurodevelopmental disorders such as autism and Attention-Deficit/Hyperactivity Disorder (ADHD) has increased over the past four decades (Grandjean and Landrigan 2006; Newschaffer et al. 2005; Prior 2003; Rutter 2005; Visser et al. 2010), currently estimated to affect about 15% of children in the U.S. (Boyle et al. 2011; U.S. EPA 2013). This increase cannot be completely explained by genetics, improved diagnostics, or known environmental risk factors (Hertz-Picciotto and Delwiche 2009; Landrigan et al. 2012; NRC 2000; Newschaffer et al. 2005), although increased diagnosis and awareness of the disorders could play a role. Emerging science has identified the potential role of toxic environmental chemicals as being an underevaluated modifiable risk factor that may interfere with brain development in fetuses and children (Bennett et al. 2016). Environmental chemical exposures are widespread in the population, and modest associations characteristic of environmental risks can translate into adverse population-level effects (Bellinger 2012; Institute of Medicine 1981).
Polybrominated diphenyl ethers (PBDEs) are a group of synthetic chemicals used as chemical flame retardants to inhibit or resist the spread of fire (ATSDR 2004). PBDEs comprise 209 possible congeners, with the major congeners detected in human and environmental samples being BDE-47, BDE-99, BDE-100, and BDE-153 (Darnerud et al. 2001; Frederiksen et al. 2009; Hites 2004; Sjodin et al. 2008). PBDEs have been used in polyurethane foam and hard plastics and can be found in a variety of everyday products, such as upholstered furniture, cars, mattresses, building materials, textiles, and computers and other electronic equipment (ATSDR 2004; Birnbaum and Staskal 2004). Because they can be present in significant quantities in products (5–30% by weight) (Darnerud et al. 2001; World Health Organization 1994) and because they are additives rather than covalently bound to consumer products, there is higher potential for leaching, volatilization, or degradation, leading to consumer and environmental exposures (Darnerud et al. 2001; Gill et al. 2004; Watanabe and Sakai 2003). Human exposures are ubiquitous beginning in utero (Morello-Frosch et al. 2016; Woodruff et al. 2011b), which is a highly vulnerable period of human brain development (Grandjean et al. 2008), and PBDEs have been found pervasively in U.S. household dust samples (Darnerud et al. 2001; Frederiksen et al. 2009; Mitro et al. 2016). Levels of PBDEs measured in Americans are the highest in the world, due to greater historic use of these chemicals in the U.S. than elsewhere because of differences in regulatory standards across countries (Besis and Samara 2012; Frederiksen et al. 2009). Despite the recent phase-out of production and use of PBDEs, exposures are expected to continue for decades because they are widely prevalent in existing consumer goods, such as furniture, and they are highly persistent in the environment and bioaccumulate up the food chain (Herbstman et al. 2010; Hites 2004; Norstrom et al. 2002; Sjodin et al. 2008).
Several animal and human studies have explored associations between developmental exposures to PBDEs and decrements in motor development, cognitive development, and attention-related behaviors (Chao et al. 2007; Chen et al. 2014; Costa and Giordano 2007; Gascon et al. 2011, 2012; Herbstman et al. 2010; Hoffman et al. 2012; Roze et al. 2009). Studies in children have mostly focused on Intelligence Quotient (IQ) and ADHD-related outcomes. IQ is the most commonly studied neurological endpoint in children, representing a combined score of a child’s function across several cognitive domains. IQ measured at school age is an important indicator of child brain health and predictive of academic and occupational success (Neisser et al. 1996). Reduced IQ is predictive of diminished lifetime earnings (Salkever 2014), increased risk for mortality, depression, diagnosis for certain medical conditions, and poorer health generally (Batty et al. 2007a, 2007b, 2009; Der et al. 2009). ADHD and attention-related behavioral conditions may have implications for children’s academic and social abilities, as well as their respective families’ functioning (Bagwell et al. 2001; Faraone et al. 2001; Harpin 2005; Johnston and Mash 2001). Furthermore, symptoms may persist into adulthood, creating concern for long-term effects of the disorder (Barkley 2002; Gudjonsson et al. 2012; Nijmeijer et al. 2008; Spencer et al. 2014; Wehmeier et al. 2010; Weiss and Hechtman 1993).
To assess the evidence of PBDEs’ contribution to neurodevelopmental disorders, we conducted a systematic review of human studies examining developmental exposure to PBDEs and 1) quantitative measures of intelligence and 2) ADHD and attention-related behavioral problems, such as hyperactivity, inattention, impulsivity, or response inhibition.
Methods
Systematic Review Methodology
Although systematic review methods have been used for decades in the clinical sciences (Guyatt et al. 2008; Higgins and Green 2011), detailed methods for conducting a systematic review directly applicable to the decision context and evidence streams in environmental health have only recently been developed and utilized in the field of environmental health sciences (Johnson et al. 2014, 2016; Koustas et al. 2014; Lam et al. 2014; Rooney et al. 2014; Vesterinen et al. 2014; Woodruff et al. 2011a; Woodruff and Sutton 2014). We conducted our review using the Navigation Guide, a systematic review methodology for evaluating environmental evidence based on methods used in the clinical sciences (Johnson et al. 2014, 2016; Koustas et al. 2014; Lam et al. 2014, 2016; Vesterinen et al. 2014; Woodruff et al. 2011a), i.e., the Cochrane Collaboration and Grading of Recommendations Assessment Development and Evaluation (GRADE) (Guyatt et al. 2008; Higgins and Green 2011; Woodruff et al. 2011a). As is standard practice for systematic reviews and the Navigation Guide, we developed a protocol prior to initiating the review and registered it in PROSPERO (Lam et al. 2015a).
Study Question
Our objective was to answer the questions: “Does developmental exposure to PBDEs in humans affect a) quantitative measures of intelligence, or b) ADHD and attention-related behavioral conditions?” The “Participants,” “Exposure,” “Comparator,” and “Outcomes” (PECO) statement is briefly outlined below with additional specifics available in our protocol.
Participants. The study? population was humans.
Exposure. The review examined studies of any developmental exposure to PBDEs that occurred prior to the assessment of intelligence or ADHD and attention-related behavioral problems. We decided in advance to include only studies that measured PBDE exposure using biomarkers (i.e., measured in human biological samples) because these represent an integrated measure of exposure from multiple sources (household dust, food, electronics, textiles, etc.) and because of their demonstrated reliability (Makey et al. 2014; Sjodin et al. 2004).
Comparator. Humans exposed to lower levels of PBDEs than humans exposed to higher levels.
Outcomes. Any clinical diagnosis or other continuous or dichotomous scale assessment of a) quantitative measures of intelligence, or b) ADHD and attention-related behavioral problems.
Data Sources
We searched the databases PubMed, ISI Web of Science, Biosis Previews, Embase, Google Scholar, and Toxline on March 5, 2015, using the search terms shown in Table S1. We did not limit our search by language or initial publication date. We used the Medical Subject Headings (MeSH) database to compile synonyms for PBDE, IQ, and ADHD and attention-related behavioral condition outcomes (Lam et al. 2015a). We updated the search on September 27, 2016, to identify any new studies. We also supplemented these results by searching toxicological and grey literature databases (See Table S2); consulting with subject matter experts; and hand-searching references of included studies, review papers on the topic, and references cited by and citing included studies.
Study Selection
We included original studies that quantified PBDEs (in the form of any individual congener or sum of multiple congeners) measured in human biological samples and reported associations with either ADHD and attention-related behavioral problems or a quantitative measure of intelligence. We screened references in duplicate for inclusion using DistillerSR (Evidence Partners). Two of four possible reviewers (N.D., L.D., J.M., P.S.) independently reviewed titles and abstracts of each reference to determine eligibility. References not excluded were then independently screened through full-text review by two of the same four reviewers above. An additional reviewer (JL) screened 5% of the titles/abstracts and full texts for quality assurance.
We excluded studies if: a) the report did not contain original data; b) the article did not involve human subjects; c) there was no quantitative measure of developmental PBDE exposure in human biological samples; d) a study did not assess ADHD and attention-related behavioral problems or a quantitative measure of intelligence; or e) there was no comparator–control group or exposure-range comparison (see Supplemental Material, “List of Excluded Studies”). We used the term “attention-related behavioral problems” or “conditions” or “outcomes” to represent a spectrum of behavioral deficits that may be examined in epidemiological studies of neurodevelopment and that have been identified in previous reviews as relevant to ADHD or attention (Eubig et al. 2010).
Data Extraction
We extracted data from studies in duplicate using a database from DRAGON, an online data review and integration tool (ICF International; available at: http://www.icfi.com/insights/products-and-tools/dragon-online-tool-systematic-review). Two of three authors (N.D., L.D., J.M.) and a University of California, San Francisco, research assistant (H. Tesoro) independently extracted data related to study characteristics and outcome measures (Table S3) from each included article. A third author (J.L.) reviewed all the studies to resolve any discrepancies between the two independent extractors and further ensure the accuracy of extracted data. We extracted all relevant estimates of association reported in the article relating PBDE exposure (for any individual congener or sum of multiple congeners) with intelligence or ADHD and attention-related behavior problems. For the meta-analysis for intelligence outcomes, we extracted adjusted regression estimates (for articles reporting multiple models adjusting for different sets of covariates, we selected estimates from the fully adjusted model, including the most confounders) and standard errors or 95% confidence interval (CI) limits and standardized to a continuous increment in exposure (i.e., per 1-unit increase in log-transformed PBDE exposure) when possible. We contacted 11 of 15 corresponding study authors to request additional data for both intelligence and ADHD-related outcomes missing from their published articles and received usable data from seven authors.
Rate the Quality and Strength of the Evidence
Assessing the risk of bias for each included study.
We evaluated risk of bias for each of the included studies using a modified instrument based on the Cochrane Collaboration’s “Risk of Bias” tool and the Agency for Healthcare Research and Quality’s (AHRQ) domains (i.e., selection bias, confounding, performance bias, attrition bias, detection bias, and reporting bias) (Higgins and Green 2011; Viswanathan et al. 2012). Possible ratings for each domain were “low,” “probably low,” “probably high,” or “high” risk of bias, with customized instructions for each domain based on the type of evidence anticipated beforehand (see Supplemental Material, “Instructions for Making Risk of Bias Determinations”). For example, we determined that for a study to be rated “low” risk of bias for the confounding domain, the analysis must either adjust for all of the following confounders or report that these confounders were evaluated and omitted because inclusion did not substantially affect the results: HOME Inventory, maternal age, maternal education, marital status, maternal use of alcohol during pregnancy, maternal depression, household income/poverty, gestational exposure to environmental tobacco smoke, child sex, exposure to other neurotoxic agents (i.e., lead), birth weight or gestational age, number of children in the home, father’s presence in the home, preschool and out-of-home child care facility attendance, psychometrician, location and language of the assessment (see Supplemental Material, “Instructions for Making Risk of Bias Determinations”). These confounders were collectively identified in our protocol for inclusion prior to screening studies by review authors with subject matter expertise on intelligence, ADHD, or PBDEs (DAA, BPL, JM) and with knowledge gathered from the literature (Watkins et al. 2013).
Two of six possible review authors with subject-matter expertise (D.A.A., B.P.L., P.S., D.B., J.M., J.L.) and one additional consultant with subject-matter and risk-of-bias rating expertise (P.I.J.) independently recorded risk-of-bias determinations for each included study, separately by outcome. We also ultimately reviewed risk-of-bias ratings for each study and across the body of evidence as a group to develop consensus on the rationale for all ratings and to ensure consistency in our ratings.
Statistical analyses.
Prior to study selection, we developed a list of study characteristics to identify studies suitable for meta-analysis (i.e., study features, characterization of the study populations, exposure assessment method, and outcome assessment method). An initial decision applicable for both outcomes concerned a minimum age of children in a study at time of neurological assessment. We decided that measurements of intelligence or ADHD and attention-related behavioral problems that have been measured at an early age (i.e., old) would not be combined in meta-analyses with other studies measuring at later ages, because some evidence from longitudinal birth cohort studies exists showing that statistical associations for neurodevelopmental outcomes are more detectable as children mature (Chen et al. 2014; Karagas et al. 2012; Rauh et al. 2006). We decided beforehand that studies of Full Scale IQ (FSIQ) and McCarthy Scales of Children’s Abilities (MSCA) (Levin 2011) were combinable if a) children included in the study were selected from the general population and at least 3 y old at the time of the assessment (for better accuracy of intelligence measurement at older ages); b) exposure was measured in any biological matrix (i.e., maternal serum, cord blood, breastmilk, etc.) as lipid-adjusted BDE-47 and/or a sum of congeners including at least lipid-adjusted BDEs 47, 99, 100, and 153 (the most common congeners in terms of population exposure) because like dioxins and polychlorinated biphenyls (PCBs), PBDEs are lipophilic and measurements in different biologic matrices are combinable when adjusted for lipid content [e.g., when exposure is expressed as nanograms of PBDE per gram of lipid) (Alaee 2016; Hites 2004)]; and c) exposure was measured during pregnancy or near birth. FSIQ and MSCA tests are both standardized with mean scores of 100 and a standard deviation of 15, so no rescaling was necessary to combine scores from studies using MSCA with those from studies using FSIQ. For studies repeating assessments as children aged, we selected the latest assessment time point for inclusion in our meta-analysis. We also identified beforehand that because Bayley Scales of Infant Development (BSID) (Michalec 2011) are generally administered to children too young for IQ testing, these measures would be inappropriate to combine with estimates such as FSIQ or MSCA.
For ADHD, we determined beforehand that it would be appropriate to combine in a meta-analysis the studies that reported ADHD total score (Child Behavior Checklist (CBCL), Conners’ ADHD/Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV Scales (CADS) (Conners 2001), Parental Strength and Difficulties Questionnaire (SDQ) (Goodman 1997) if a) the children included in the study were selected from the general population and were at least 4 y old at the time of the assessment, and b) BDE-47 and/or a sum of congeners including at least BDEs 47, 99, 100, and 153 was measured during pregnancy or near delivery.
Random effects meta-analyses were performed using the DerSimonian-Laird method (DerSimonian and Laird 1986). Statistical heterogeneity across study estimates in the meta-analyses was evaluated using Cochran’s Q statistic (with as our cut-off for statistical significance) and (Higgins and Green 2011; Johnson et al. 2014; Koustas et al. 2014; Lam et al. 2014). For other outcomes that were not amenable to a meta-analysis (i.e., due to insufficient number of studies or existence of heterogeneity across study design), we displayed the estimates of association in tables and considered these findings in the final rating of the overall body of evidence.
To investigate the effect that publication bias may have on our meta-analysis, we quantitatively evaluated the potential effect that a new study might have on changing the interpretation of our overall results. Specifically, the association estimate of a new or unpublished study necessary to alter the results of the meta-analysis was calculated under two scenarios, so that a) the 95% CI of the meta-analysis overlapped zero, b) the meta-analysis central association estimate was greater than zero (moves to the opposite direction—i.e., such that increases in PBDE exposures would be associated with increases in intelligence). In making this calculation, we assumed that the new hypothetical study would have a standard error of 2.3, equal to the smallest in our group of studies (Eskenazi et al. 2013).
Rating the quality of evidence across studies.
We rated the quality of the overall body of evidence as “high,” “moderate,” or “low.” We assigned an initial rating of “moderate” quality to human observational studies based on the previously described rationale (Johnson et al. 2014; Koustas et al. 2014; Vesterinen et al. 2014; Woodruff and Sutton 2014), and then we considered potential adjustments (“downgrades” or “upgrades”) to the quality rating based on eight categories of considerations: risk of bias, indirectness, inconsistency, imprecision, potential for publication bias, large magnitude of effect, dose response, and whether residual confounding would minimize the overall effect estimate (Balshem et al. 2011); the specific factors and instructions to review authors considered are summarized in Table 1 and detailed in our protocol (Lam et al. 2015b). Possible ratings were 0 (no change from initial quality rating), (1 level downgrade) or (2 level downgrade), (1 level upgrade) or (2 level upgrade). Review authors independently evaluated the quality of the evidence, and then compared ratings as a group, and recorded the consensus and rationale for each final decision.
Table 1.
Summary of rating quality and strength of the body of human evidence for developmental exposures to PBDEs.
| Category | Summary of criteria for downgrades | Final rating for downgrades | Rationale |
|---|---|---|---|
| (A) IQ outcome | |||
| Risk of bias | Study limitations – a substantial risk of bias across body of evidence | 0 | Risk of bias for studies of IQ was generally “low” or “probably low” across studies and domains. Studies that received “probably high ratings” evaluated outcomes related to IQ, such as infant/toddler assessments of intelligence (i.e., Bayley Scales), and these studies were not included in the meta-analysis that informed our final decision. As such, we agreed that these limitations within certain studies were not strong enough to warrant downgrading for risk of bias across all studies. |
| Indirectness | Evidence was not directly comparable to the question of interest (i.e., population, exposure, comparator, outcome) | 0 | IQ outcomes were measured in humans and in populations that are directly relevant to the population of the study question, as outlined in the PECO statement. |
| Inconsistency | Widely different estimates of effect in similar populations (heterogeneity or variability in results) | 0 | All estimates of associations reported in studies included in the meta-analysis were consistently “positive,” (i.e., reporting increased decrements in IQ or MSCA with increasing BDE-47 exposure). Confidence intervals overlapped across all four studies and were similar in width except Gascon et al. (2011), which had wider confidence intervals (CIs) and also included the fewest subjects (). Estimates from the meta-analysis indicate that statistical heterogeneity was not present () and the combined association estimate was statistically significant. |
| For the IQ studies not combinable in the meta-analysis, the majority of estimates assessing BSID reported poorer outcomes with increasing BDE-47 exposure, although one study reported an association in the opposite direction (but not statistically significant). Confidence intervals for studies overlapped across studies evaluating the same assessment tool and reporting the same association measure. We determined that the number of studies using the same assessment tool at the same age and reporting similar association measures was small and thus the available evidence, while not fully consistent across BSID studies, did not provide strong enough evidence to warrant downgrading for Inconsistency. | |||
| Imprecision | Studies had few participants and few events (wide CIs as judged by reviewers) | 0 | We judged that the width of the CI around the estimate of association from the meta-analysis was sufficiently narrow given the sample size and thus that the evidence did not warrant downgrading for imprecision. |
| Publication bias | Studies missing from body of evidence, resulting in an over or underestimate of true effects from exposure | 0 | Number of studies included in the meta-analysis were too small (i.e., ) for a statistical evaluation of potential publication bias. We identified findings from the grey literature through our comprehensive search, and many studies that reported findings that were not statistically significant. Our quantitative analysis to determine what measure of association would need to be reported by a hypothetical new study to change our meta-analysis effect to no longer be statistically significant or to move it in the opposite direction minimized concern that an unpublished null study would likely change our conclusion. |
| Summary of Criteria for Upgrading | Upgrades | ||
| Large magnitude of effect | Upgraded if modeling suggested confounding alone unlikely to explain associations with large effect estimate as judged by reviewers | 0 | The overall effect size from the meta-analysis was quite large for an environmental epidemiology study (3.70 decrement in IQ per 10-fold increase (in other words, times 10) in PBDE exposure—approximately half the association that has been reported for lead exposure and IQ outcome), but not all reported effect sizes are consistently large and we judged the magnitude of effect not large enough to warrant upgrading the evidence. |
| Dose–response | Upgraded if consistent relationship between dose and response in one or multiple studies, and/or dose response across studies | There was evidence of a dose-response gradient reported in some studies (Adgent et al. 2014), whereas other studies reported significant differences for higher categories of exposure compared to lower, but no statistically significant trend across all categories (Herbstman et al. 2010; Zhang et al. 2016). The results from our meta-analysis reported a statistically significant decrement in intelligence with increased PBDE exposure assuming a linear relationship in studies with high relevance to the study question. We felt this was convincing to assign a upgrade to the overall body of evidence. | |
| Category | Summary of criteria for upgrades | Final rating for upgrades | Rationale |
|---|---|---|---|
| Confounding minimizes effect | Upgraded if consideration of all plausible residual confounders or biases would underestimate the effect or suggest a spurious effect when results show no effect | 0 | We identified some studies that might have residual confounding because they did not account for all important confounders as listed in the protocol. However, we did not expect that omission of any of these confounders would have led to underestimating our meta-analysis association estimate and therefore did not upgrade for this consideration. |
| Overall quality of evidence | Moderate | Although we applied a rating for the “Dose–response” consideration, we did not feel that the dose–response evidence was strong enough to warrant upgrading the overall quality rating. | |
| Overall strength of evidencea | Sufficient | A positive relationship is observed between exposure and outcome where chance, bias, and confounding can be ruled out with reasonable confidence. The available evidence includes results from multiple well-designed, well-conducted studies, and the conclusion is unlikely to be strongly affected by the results of future studies. | |
| (B) ADHD outcome | |||
| Summary of criteria for downgrades | Downgrades | ||
| risk of bias | Widely different estimates of effect in similar populations (heterogeneity or variability in results) | 0 | Risk of bias was generally “low” or “probably low” across studies and domains. Generally, the domain of confounding was most frequently judged to be other than “low” risk of bias; however, we did not judge that this warranted downgrading for risk of bias across all studies. |
| Indirectness | Widely different estimates of effect in similar populations (heterogeneity or variability in results) | 0 | ADHD-related outcomes are measured in humans and in populations that are directly relevant to the population of the study question, as outlined in the PECO statement. |
| Inconsistency | Widely different estimates of effect in similar populations (heterogeneity or variability in results) | 0 | The majority of studies reported association estimates showing increased risk of ADHD symptoms with increasing PBDE exposures, although some studies did report associations in the opposite direction. Confidence intervals for studies overlapped across studies evaluating the same assessment tool and reporting the same association measure. We determined that the number of studies evaluating the same assessment tool at the same age and reporting similar association measures was small and did not provide strong enough evidence to warrant downgrading for Inconsistency. |
| Imprecision | Studies had few participants and few events (wide CIs as judged by reviewers) | 0 | We judged that the width of the CI around the estimate of association was sufficiently narrow given the sample size and did not feel there was any reason to downgrade the overall body of evidence for Imprecision. |
| Publication bias | Studies missing from body of evidence, resulting in an over or underestimate of true effects from exposure | 0 | Number of studies included was too small (i.e., ) for a statistical evaluation of potential publication bias. We identified findings from the grey literature through our comprehensive search, and many studies reported findings that were not statistically significant |
| Summary of Criteria for Upgrading | Upgrades | ||
| Large magnitude of effect | Upgraded if modeling suggested confounding alone unlikely to explain associations with large effect estimate as judged by reviewers | 0 | Studies that reported positive associations between exposure and outcome were interpreted as primarily minimal-to-moderate magnitudes; review authors judged that there was insufficient evidence to upgrade for Large Magnitude of Effect. |
| Dose–response | Upgraded if consistent relationship between dose and response in one or multiple studies, and/or dose response across studies | 0 | There was not enough evidence to evaluate existence of a dose–response relationship, primarily due to the small number of studies and the heterogeneity in reporting of effect estimates (i.e., Spearman’s Rho correlation coefficient, adjusted linear regression results, adjusted odds ratios, adjusted incidence rate ratios, and adjusted relative risks). We therefore concluded there was insufficient evidence to warrant upgrading for dose–response. |
| Confounding minimizes effect | Upgraded if consideration of all plausible residual confounders or biases would underestimate the effect or suggest a spurious effect when results show no effect | 0 | We identified some studies that might have residual confounding because they did not account for all important confounders as listed in the protocol. However, we did not expect that omission of any of these confounders would have led to underestimating the association estimate and therefore did not upgrade for this consideration. There were not enough combinable studies to perform a meta-analysis. |
| Category | Summary of criteria for downgrades | Final rating for downgrades | Rationale |
|---|---|---|---|
| Overall quality of evidence | Moderate | No upgrades or downgrades applied to the overall quality of evidence. | |
| Overall strength of evidencea | Limited | An association is generally observed between exposure and adverse outcome, but chance, bias, and confounding could not be ruled out with reasonable confidence. Confidence in the relationship is constrained by such factors as: the number, size, assessment, measure of association or quality of individual studies, or inconsistency of findings across individual studies. As more information becomes available, the observed effect could change, and this change may be large enough to alter the conclusion. |
Detailed instructions to authors on how to apply these criteria are presented in the Protocol, Appendix VII, Instructions for Grading the Quality and Strength of Evidence (Lam et al. 2015b). Language for the definitions of the rating categories were adapted from descriptions of levels of certainty provided by the U.S. Preventive Services Task Force Levels of Certainty Regarding Net Benefit. https://www.uspreventiveservicestaskforce.org/Page/Name/update-on-methods-estimating-certainty-and-magnitude-of-net-benefit.
Rating the strength of the evidence across studies.
We assigned an overall strength of evidence rating based on four considerations: a) Quality of body of evidence (i.e., the rating from the previous step); b) Direction of effect; c) Confidence in effect (likelihood that a new study could change our conclusion); and d) Other compelling attributes of the data that may influence certainty, e.g., specificity of the association when the outcome is rare or unlikely to have multiple causes (NTP 2015). Possible ratings were “sufficient evidence of toxicity,” “limited evidence of toxicity,” “inadequate evidence of toxicity,” or “evidence of lack of toxicity” (Table 2), based on categories used by the International Agency for Research on Cancer (IARC), the U.S. Preventive Services Task Force, and U.S. Environmental Protection Agency (EPA) (IARC 2006; Sawaya et al. 2007; U.S. EPA 1991, 1996). Review authors independently evaluated the quality of the evidence and then compared ratings as a group and recorded the consensus and rationale.
Table 2.
Strength of evidence definitions for human evidence.
| Strength rating | Definition |
|---|---|
| Sufficient evidence of toxicity | A positive relationship is observed between exposure and outcome where chance, bias, and confounding can be ruled out with reasonable confidence. The available evidence includes results from one or more well-designed, well-conducted studies, and the conclusion is unlikely to be strongly affected by the results of future studies.a |
| Limited evidence of toxicity | A positive relationship is observed between exposure and outcome where chance, bias, and confounding cannot be ruled out with reasonable confidence. Confidence in the relationship is constrained by such factors as: the number, size, or quality of individual studies, or inconsistency of findings across individual studies.a As more information becomes available, the estimated association could change, and this change may be large enough to alter the conclusion. |
| Inadequate evidence of toxicity | The available evidence is insufficient to assess effects of the exposure. Evidence is insufficient because of: the limited number or size of studies, low quality of individual studies, or inconsistency of findings across individual studies. More information may allow an assessment of effects. |
| Evidence of lack of toxicity | No relationship is observed between exposure and outcome, and chance, bias and confounding can be ruled out with reasonable confidence. The available evidence includes consistent results from more than one well-designed, well-conducted study at the full range of exposure levels that humans are known to encounter, and the conclusion is unlikely to be strongly affected by the results of future studies.a The conclusion is limited to the age at exposure and/or other conditions and levels of exposure studied. |
Note: The Navigation Guide rates the quality and strength of evidence of human and non-human evidence streams separately as “sufficient,” “limited,” “inadequate,” or “evidence of lack of toxicity” and then these two ratings are combined to produce one of five possible statements about the overall strength of the evidence of a chemical’s reproductive/developmental toxicity. The methodology is adapted from the criteria used by the International Agency for Research on Cancer (IARC) to categorize the carcinogenicity of substances (IARC 2006), except as noted.
Language for the definitions of the rating categories were adapted from descriptions of levels of certainty provided by the U.S. Preventive Services Task Force Levels of Certainty Regarding Net Benefit.
Results
All Qualifying Studies
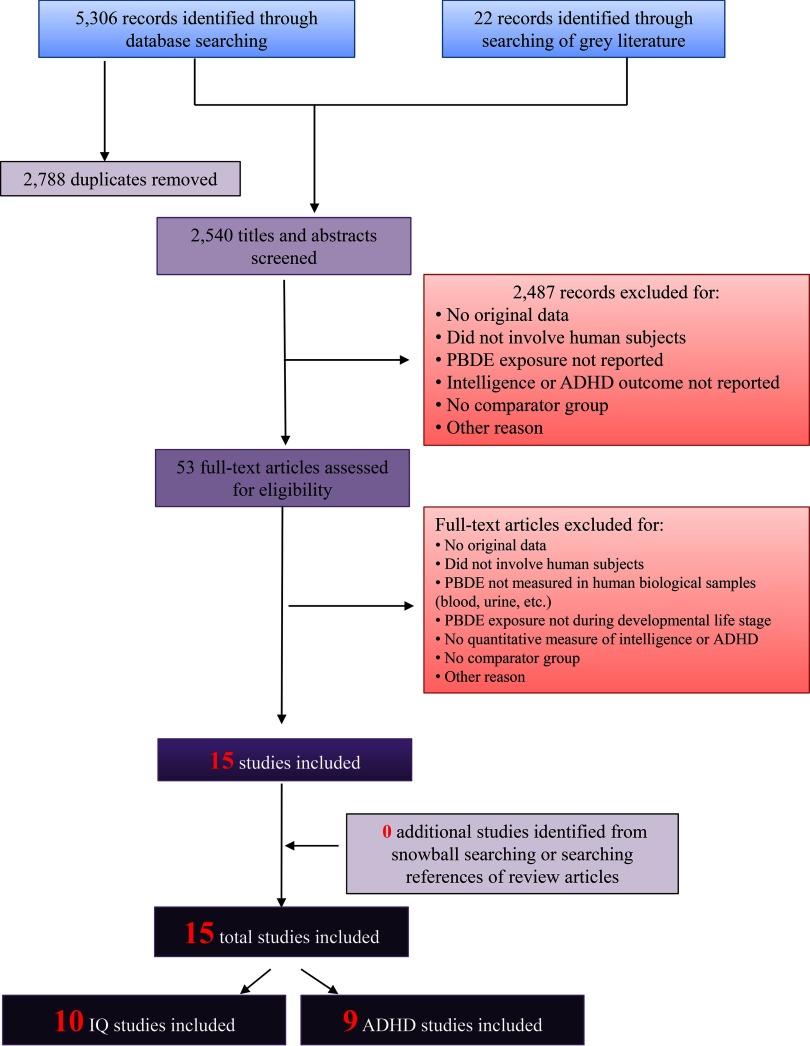
Our search retrieved 2,540 unique records as follows: the March 2015 search retrieved a total of 1,824 unique records, of which 12 met the inclusion criteria; the September 2016 search update added 716 unique records, of which an additional three studies met the inclusion criteria (Cowell et al. 2015; Sagiv et al. 2015; Zhang et al. 2016) (Figure 1). Of the 15 total included studies, 10 were relevant to the outcome of intelligence (Adgent et al. 2014; Chao et al. 2011; Chen et al. 2014; Eskenazi et al. 2013; Gascon et al. 2011, 2012; Herbstman et al. 2010; Lin et al. 2010; Shy et al. 2011; Zhang et al. 2016), and nine to the outcome of ADHD and attention-related behavioral conditions (Adgent et al. 2014; Chen et al. 2014; Cowell et al. 2015; Eskenazi et al. 2013; Gascon et al. 2011; Gump et al. 2014; Hoffman et al. 2012; Roze et al. 2009; Sagiv et al. 2015). Included studies were published from 2009 to 2016 and involved 35–622 study participants, for a total of almost 3,000 mother–child pairs from eight populations around the world (Table 3). All studies measured PBDE exposure in maternal/child serum, cord blood, child whole blood, or breastmilk and adjusted for lipid content (i.e., the units of exposure were nanograms of PBDE per gram of lipids). The majority of included studies adjusted for maternal age, sex of child, mother’s parity, and some measure of socioeconomic status (Table 3).
Figure 1.
Flowchart showing the literature search and screening process for studies relevant to PBDE exposure and IQ/ADHD outcomes. The primary goal of our search was to obtain comprehensive results; therefore, our search was not limited by language or publication date. The search terms used for each database are provided in Table S1.
Table 3.
Human studies included in our systematic review of developmental exposure to PBDEs and IQ and/or ADHD in children.
| A. Studies measuring intelligence outcomes | |||||||
|---|---|---|---|---|---|---|---|
| Study reference (Cohort, if applicable) | Study Population | Location | Sample size | Congeners evaluated | PBDE range | Exposure matrix | Intelligence-related outcomes |
| Prospective birth cohort | |||||||
| Herbstman et al. 2010 | Women pregnant on September 11, 2001 | New York City, NY, USA | 152 mother–child pairs | 47, 99, 100, 153 | BDE-47 range: lipid | Cord blood | BSID-II MDI and PDI assessed at 12, 24, and 36 months. WPPSI-R FSIQ assessed at 48, 72 months |
| Note: LOD when no analytical background was detected in blank samples was defined as a signal-to-noise . When analytical background was detected in the blanks, the LOD was defined as three times the SD of the blanks. was 83.6% for BDE-47 and between 55.9-69.1% for BDEs 99, 100 and 153. For concentrations below the LOD, authors used the . Confounders: maternal age, maternal education, maternal IQ, material hardship during pregnancy, breast-feeding status, language and location of interview and assessment, consumption of fish/seafood when pregnant, cord blood mercury and lead concentrations, child’s age at testing, sex of child, ethnicity, environmental tobacco smoke exposure in home, gestational age at birth. | |||||||
| Lin et al. 2010 | Pregnant women enrolled between 2007 and 2008 | Southern Taiwan | 35 mother–child pairs | 47, 99, 100, 153, 154, 196, 197, 206, 207, 208, 209; sum of all eleven | Mean of BDE sum: lipid; | Breastmilk | BSID-III cognitive and language subscales assessed at 8–13 months |
| Note: No information provided on LOD for each congener or the % samples below LOD. For concentrations below the LOD, authors used the LOD/2. Confounders: maternal age, prepregnant BMI, infant’s gender, gestational age, infant’s age at assessment. | |||||||
| Gascon et al. 2011 (INMA Project) | Pregnant women enrolled between 1997 and 1998 | Island of Menorca, Spain | 78–240 mother–child pairs (depending on exposure matrix) | 47 | BDE-47 range: lipid (maternal); (child) | Cord blood/ child serum | MSCA total cognitive function score and ADHD-DSM-IV for attention deficit and hyperactivity assessed at 48 months |
| Note: LOD was . LOQ was . for BDE-47 was 51.1% in cord blood and 20.5% in child serum. Authors categorized exposures as “referents” () versus “exposed () and also modeled continuous exposures by replacing concentrations below the LOQ with LOQ/2. Confounders: sex, gestational age, age at delivery, evaluating psychologist, maternal age, fish consumption, weeks breastfeeding, parity, smoking, alcohol consumption, social class, maternal education, birth weight, prepregnancy BMI. | |||||||
| Shy et al. 2011 | Pregnant women enrolled between 2007 and 2008 | Southern Taiwan | 36 mother–child pairs | 15, 28, 47, 49, 99, 100, 153, 154, 183, 196, 197; sum of all 11 | BDE-47 range: lipid | Cord blood | BSID-III cognitive and language subscales assessed at 8–12 months |
| Note: No information provided on LOD for each congener. ranged from 55.5% (BDE-28) (BDE-47, 99, 153, 197). No information provided on how concentrations below LOD were handled. Confounders: maternal age, prepregnancy BMI, parity, maternal education, household income. | |||||||
| Gascon et al. 2012 (INMA Project) | Pregnant women enrolled between 2004 and 2008 | Gipuzkoa, Basque Country and Sabadell Catalonia, Spain | 290 mother–child pairs | 47, 99, 100, 153, 154, 183, 209; sum of all seven | BDE-47 range: lipid | Breastmilk | BSID mental score and psychomotor score assessed at 12–18 months |
| Note: LOD was calculated from blanks and defined as three times the SD of the blanks; LOQ was defined as five times the SD. Only congeners detected in samples were included in the total sum of seven PBDEs. ranged from 41.0–75.8% and was 63.1% for BDE-47. Authors used multiple imputation for concentrations below LOD or LOQ. Confounders: maternal age, social class, education, country of origin, smoking during pregnancy, parity, child care attendance, duration of predominant breastfeeding, maternal consumption of fish during pregnancy, prepregnancy BMI, child’s gestational age, child’s weight at birth. | |||||||
| Eskenazi et al. 2013 (CHAMACOS cohort) | Pregnant women enrolled between 1999 and 2000 | Salinas Valley, California, USA | 231– 256 mother–child pairs (depending on exposure matrix and outcome) | 17, 28, 47, 66, 85, 99, 100, 153, 154, 183; sum of 47, 99, 100, 153; sum of all ten | BDE-47 range: lipid (maternal); lipid (child). Sum of 47, 99, 100, 153 range: lipids (maternal); 5.8-1308.5 (child) | Maternal/ child serum | WPPSI-III performance IQ assessed at 5 y, WISC-IV FSIQ assessed at 7 y |
| Note: LOD for BDE-47 ranges from lipids for maternal samples and lipids for child samples. For all other congeners, LODs ranged between lipids for maternal samples and lipids for child samples. was 97.5% or greater for BDEs 47, 99, 100 and 153 in maternal and child serum. Authors used the machine-read value for values below the LOD if a signal was detected. Authors used imputation at random based on a log-normal probability distribution using maximum likelihood estimation for other samples below LOD. Confounders: maternal age, education, years in the United States, marital status, work outside the home, use of alcohol and tobacco during pregnancy, depression, parity, PPVT or TVIP score, housing density, household poverty, pregnancy exposure to environmental tobacco smoke, number of children in the home, father’s presence in the home, Home Observation for Measurement of the Environment (HOME) score, preschool and out-of-home child care attendance, psychometrician, location, and language of assessment, child sex, birth weight, and preterm delivery status. | |||||||
| Adgent et al. 2014 (PIN Babies study) | Pregnant women enrolled between 2004 and 2006 | Central North Carolina, USA | 184 mother–child pairs | 28, 47, 99, 100; 153 | BDE-47 range: lipid | Breastmilk | MSEL composite score assessed at 36 months |
| Note: LOD as follows (ng/g lipid): BDE 28: 0.3; BDE 47: 1.3; BDE 99: 1.1; BDE 100: 0.3; BDE 153: 0.3. was 90% or greater for all five congeners. For concentrations below the LOD, authors used the . Confounders: child sex, maternal age at start of pregnancy, parity, education, maternal race, breast-feeding duration, postpartum income, breast milk omega 3 fatty acid concentration, Home Observation for Measurement of the Environment (HOME) score, maternal stress. | |||||||
| Chen et al. 2014 (HOME study) | Pregnant women enrolled between 2003 and 2006 | Cincinnati Ohio, USA | 179–285 mother–child pairs (depending on age of assessment) | 47; sum of 47, 99, 100, 153 | BDE-47 10th-90th percentile range: lipid | Maternal serum | BSID-II MDI and PDI assessed at 12, 24, 36 months; WPPSI-III FSIQ assessed at 5 y |
| Note: LOD as follows (ng/g lipid): BDE 47: 4.2; BDE 99: 5.0; BDE 100: 1.4; BDE 153: 2.2. Among the 279 subjects, 6 had concentrations of BDEs 99, 100, or 153 below LOD, and all subjects had detectable concentrations of BDE-47. For concentrations below the LOD, authors used the . Confounders: maternal age at enrollment, maternal race/ethnicity, education, marital status, maternal serum cotinine concentrations at enrollment, maternal IQ, child sex, maternal depression, household income, Home Observation for Measurement of the Environment (HOME) score. | |||||||
| Zhang et al. 2016 (HOME study) | Pregnant women enrolled between 2003 and 2006 | Cincinnati, OH, USA | 231 mother–childpairs | 47, 99, 100, 153; sum of all four | BDE-sum: quartile 1: , quartile 4: lipid | Maternal serum | WISC-IV FSIQ and BASC-2 externalizing problems assessed at 8 y |
| Note: No information provided on LOD for each congener or the % samples below LOD. For concentrations below the LOD, authors used the . Confounders: maternal age, race, education, household income, parity, marital status, smoking status, maternal fish consumption, maternal depression, maternal IQ, child sex, Home Observation for Measurement of the Environment (HOME) score. | |||||||
| Cohort studya | |||||||
| Chao et al. 2011 | Pregnant women enrolled between 2007 and 2010 | Southern Taiwan | 70 mother–child pairs | 28, 47, 99, 100, 153, 154, 183, 196, 197, 203, 206, 207, 208, 209; sum of all 14 | BDE-47 range: lipid | Breastmilk | BSID-III cognitive and language subscales assessed at 8–12 months |
| Note: LODs were predetermined so that signal-to-noise . was 97 percent or greater for all congeners and was 100% for BDE-47. For concentrations below the LOD, authors used the LOD/2. Confounders: maternal age, prepregnancy BMI, parity, socioeconomic status, smoking and dietary habits, alcohol consumption, medical history, exposure to PBDEs from different sources, child’s gender, gestational age, infant age at time of assessment. | |||||||
| B. Studies measuring ADHD and attention-related behavioral outcomes | |||||||
|---|---|---|---|---|---|---|---|
| Study reference (Cohort, if applicable) | Study population | Location | Sample size | Congeners evaluated | PBDE range | Exposure matrix | ADHD-related outcomes |
| Prospective birth cohort | |||||||
| Roze et al. 2009 (GIC study) | Pregnant women enrolled between 2001 and 2002 | Northern provinces of Netherlands | 62 mother–child pairs | 47, 99, 100, 153, 154 | BDE-47 range: lipid | Maternal serum | CBCL attention sustained and attention selective subscale and parental ADHD questionnaire assessed at 5–6 y |
| Note: LOD ranged from serum. Mean levels of BDEs 47, 99, and 100 measured in blank samples were subtracted from values measured in study samples to correct for background levels (4.8, 1.9 and serum, respectively). Minimum was 95% for BDE-99 and 100 and was 97% for BDE-47. For concentrations below the LOD, authors used 0. Confounders: child sex, socioeconomic status, Home Observation for Measurement of the Environment (HOME) Inventory. | |||||||
| Gascon et al. 2011 (INMA Project) | Pregnant women enrolled between 1997 and 1998 | Island of Menorca, Spain | 77 to 220 mother–child pairs (depending on exposure matrix) | 47 | BDE-47 range: lipid (maternal); (child) | Cord blood/child serum | ADHD DSM-IV for attention deficit and hyperactivity assessed at 48 months |
| Note: LOD was . LOQ was . for BDE-47 was 51.1% in cord blood and 20.5% in child serum. Authors categorized exposures as “referents” () versus “exposed () and also modeled continuous exposures by replacing concentrations below the LOQ with LOQ/2. Confounders: sex, gestational age, age at delivery, evaluating psychologist, maternal age, birth weight, fish consumption, weeks breastfeeding, parity, smoking, alcohol consumption, social class, maternal education, prepregnancy BMI. | |||||||
| Hoffman et al. 2012 (PIN Babies study) | Pregnant women enrolled between 2001 and 2005 | Central NC, USA | 222 mother–child pairs | 28, 47, 99, 100, 153; sum of all five | BDE-47 range: lipid | Breastmilk | ITSEA activity/impulsivity and attention regulation subscales assessed at 24–36 months |
| Note: lipid for BDEs 28, 100, 153; lipid for BDE-99; and lipid for BDE-47. The five congeners (BDEs 28, 47, 99, 100, and 153) were detected in samples and was 100% for BDE-47 and 91-99% for BDEs 28, 100, 153. For concentrations below the LOD, authors used the . Confounders: child sex and age, household income, maternal age, race and education, parity, prenatal tobacco use, omega-3 fatty acid levels, duration of breastfeeding, Home Observation for Measurement of the Environment (HOME) Inventory. | |||||||
| Eskenazi et al. 2013 (CHAMACOS cohort) | Pregnant women enrolled between 1999 and 2000 | Salinas Valley, CA, USA | 285– 323 mother–child pairs (depending on outcome) | 17, 28, 47, 66, 85, 99, 100, 153, 154, 183; sum of 47, 99, 100, 153; sum of all ten | BDE-47 range: lipid (maternal); lipid (child). Sum of 47, 99, 100, 153 range: lipids (maternal); 5.8–1308.5 (child) | Maternal/child serum | CBCL attention problems, CBCL ADHD, K-CPT ADHD Confidence Index assessed at 5 y, CADS maternal report ADHD index, DSM-IV total scale with inattentive and hyperactivity/impulsivity subscales, BASC-2 maternal report hyperactivity scale and attention problems scale, CADS teacher report ADHD index and DSM-IV total scale with inattentive and hyperactivity/impulsivity subscales, BASC-2 teacher report hyperactivity scale and attention problems scale assessed at 7 y |
| Notes: LOD for BDE-47 ranges from lipids for maternal samples and lipids for child samples. For all other congeners, LODs ranged between lipids for maternal samples and lipids for child samples. was 97.5% or greater for BDEs 47, 99, 100 and 153 in maternal and child serum. Authors used the machine-read value for values below the LOD if a signal was detected. Authors used imputation at random based on a log-normal probability distribution using maximum likelihood estimation for other samples below LOD. Confounders: maternal age, education, years in the United States, marital status, work outside the home, use of alcohol and tobacco during pregnancy, depression, parity, PPVT or TVIP score, housing density, household poverty, pregnancy exposure to environmental tobacco smoke, number of children in the home, father’s presence in the home, Home Observation for Measurement of the Environment (HOME) Inventory, preschool and out-of-home child care attendance, psychometrician, location, and language of assessment, child sex, birth weight, and preterm delivery status. | |||||||
| Adgent et al. 2014 (PIN Babies study) | Pregnant women enrolled between 2004 and 2006 | Central NC, USA | 192 mother–child pairs | 28, 47, 99, 100, 153 | BDE-47 range: lipid | Breastmilk | BASC-2 attention and hyperactivity subscale assessed at 36 months |
| Note: LOD as follows (ng/g lipid): BDE 28: 0.3; BDE 47: 1.3; BDE 99: 1.1; BDE 100: 0.3; BDE 153: 0.3. was 90% or greater for all five congeners. For concentrations below the LOD, authors used the . Confounders: child sex, maternal age at start of pregnancy, parity, education, maternal race, breast-feeding duration, postpartum income, breast milk omega 3 fatty acid concentration, Home Observation for Measurement of the Environment (HOME) Inventory, maternal stress. | |||||||
| Chen et al. 2014 (HOME study) | Pregnant women enrolled between 2003 and 2006 | Cincinnati, OH, USA | 165–240 mother–child pairs (depending on age of assessment) | 47; sum of 47, 99, 100, 153 | BDE-47 10thh-90th percentile range: lipid | Maternal serum | BASC-2 attention and hyperactivity subscales assessed at 24, 36, 48, 60 months |
| Note: LOD as follows (ng/g lipid): BDE 28: 1.0; BDE 28: 0.8; BDE 47: 4.2; BDE 66: 1.0; BDE 85: 2.4; BDE 99: 5.0; BDE 100: 1.4; BDE 153: 2.2; BDE 154: 0.8; BDE 183: 1.7. Among the 279 subjects, 6 had concentrations of BDEs 99, 100, or 153 below LOD, and all subjects had detectable concentrations of BDE-47. For concentrations below the LOD, authors used the . Confounders: maternal age at enrollment, maternal race/ethnicity, education, marital status, maternal serum cotinine concentrations at enrollment, maternal IQ, child sex, maternal depression, household income, Home Observation for Measurement of the Environment (HOME) Inventory. | |||||||
| Cowell et al. 2015 | Women pregnant on September 11, 2001 | New York City, NY, USA | 107–109 mother–child pairs (depending on age of assessment) | 47, 99, 100, 153 | BDE-47 median 12.0, IQR lipid at 48 months and 11.3 IQR lipid at 72 months | Cord blood | CBCL attention problems assessed at age 48 and 72 months |
| Note: LOD was defined as the highest of (i) 3 times the standard deviation of the blank samples and (ii) the instrument detection limit. The median level detected in blank samples analyzed in parallel to the study samples was subtracted from all sample results. Authors focused statistical analyses on the four congeners detected in more than 50% of samples (BDEs 47, 99, 100, and 153). Among the 201 total cord plasma samples analyzed for PBDEs, at least 50% had detectable levels of BDE-47 (81.4%), BDE-99 (59.5%), BDE-100 (63.6%) and BDE-153 (49.8%). For concentrations below the LOD, authors used the . Confounders: age at assessment, sex of child, ethnicity, prenatal environmental tobacco smoke exposure in home, intelligence of mother, maternal demoralization, maternal age and marital status. | |||||||
| Sagiv et al. 2015 (CHAMACOS cohort) | Pregnant women enrolled between 1999 and 2000 and children enrolled between 2009 and 2011 | Salinas Valley, CA, USA | 622 mother–child pairs | 47, 99, 100, 153; sum of 47, 99, 100, 153 | BDE-47 range: lipid | Maternal/child serum | CPT II ADHD Confidence Index, CADS parent report ADHD index and DSM-IV inattentive and hyperactivity/impulsivity subscales assessed at 9 and 12 y, BASC-2 parent report hyperactivity scale and attention problems scale, BASC-2 youth self-report hyperactivity scale and attention problems scale assessed at 10.5 y |
| Notes: LOD for BDE-47 ranges lipids for maternal and lipids for child samples. For all other congeners, LODs ranged between lipids for maternal samples and lipids for child samples. BDE-47, 99, 100 and 153 sum had detection frequency ranging from 97.9–99.5%. Authors used imputation at random based on a log-normal probability distribution using maximum likelihood estimation for concentrations below LOD. Confounders: child sex, age at assessment, duration of breastfeeding, whether child attended preinventory, maternal age, education, parity, prenatal smoking status, verbal intelligence, depressive symptoms, family structure, Home Observation for Measurement of the Environment (HOME) inventory, average monthly income divided by number of household members supported during the study period, psychometrician who administered child-completed tasks or study interviewer who administered the maternal survey instrument, time of day assessment occurred, child video game usage. | |||||||
| Cross-sectional | |||||||
| Gump et al. 2014 | Children recruited from another study regarding lead effects | Oswego County, NY, USA | 43 children | 28, 47, 99, 100 | BDE-47 range: lipid | Child whole blood | Parental Strengths and Difficulties Questionnaire SDQ hyperactivity-inattention subscale assessed between 9–11 y |
| Notes: LOD ranged from 0.042 (BDE 47) to on a wet weight basis. Congeners 85, 153 and 154 were not detected in any samples so data were not reported in publication. Minimum was 76.74% for BDE 99 and was 86.05% for BDE-47 (). LOQ was wet weight. For concentrations below the LOQ, authors used the LOQ/2. Confounders: BMI percentile standing age and gender adjusted, socioeconomic status score, total blood lipid levels, age race. | |||||||
Note: Data was re-analyzed subsequent to collection from a prospective birth cohort. ADHD, attention deficit hyperactivity disorder; BASC, Behavior Assessment System for Children; BMI, body mass index; BSID, Bayley Scales of Infant and Toddler Development; CADS, Conners? ADHD virgule DSM-IV scales; CBCL, child behavior checklist; CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas; CPT, Conners? Continuous Performance Test; DSM, Diagnostic and Statistical Manual of Mental Disorders; FSIQ, full scale intelligent quotient; GIC, Groningen Infant COMPARE (Comparison of the Exposure-Effect Pathways to Improve the Assessment of Human Health Risks of Complex Environmental Mixtures of Organohalogens); HOME, Health Outcomes and Measures of the Environment; HOME study, Health Outcomes and Measures of the Environment; INMA, INfancia y Medio Ambiente (Environment and Childhood); IQR, interquartile range; ITSEA, infant-toddler social and emotional assessment; K-CPT, Kiddie Continuous Performance Test; LOD, limits of detection; LOQ, limits of quantification; MDI, Mental Development Index; MSCA, McCarthy Scales of Children's Abilities; MSEL, Mullen Scales of Early Learning; PDI, Psychomotor Development Index; PIN, pregnancy, infection, and nutrition; PPVT, Peabody Picture Vocabulary Test; TVIP, Test de Vocabulario en Imagenes Peabody; SD, standard deviation; SDQ, Strengths and Difficulties Questionnaire; WISC, Wechsler Intelligence Scale for Children; WPPSI-R, Wechsler Preschool and Primary Scale of Intelligence, Revised Edition.
Data was re-analyzed subsequent to collection from a prospective birth cohort.
Studies of Intelligence
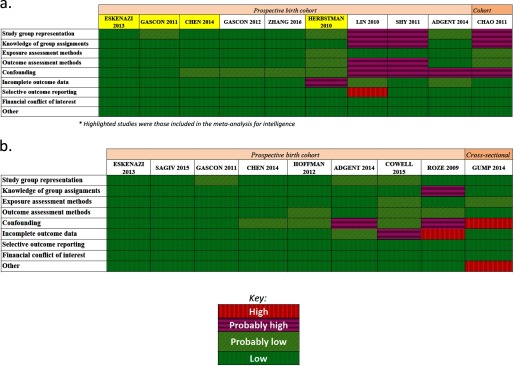
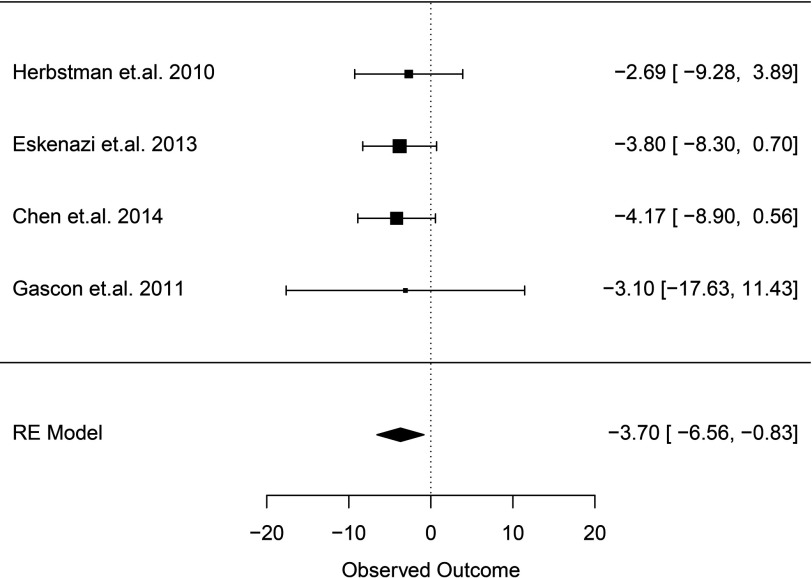
Nine of 10 studies that evaluated intelligence were prospective birth cohorts, and one was a cohort study that reanalyzed data previously collected from a prospective birth cohort (Chao et al. 2011). Seven studies conducted assessments using BSID (Chao et al. 2011; Chen et al. 2014; Gascon et al. 2012; Herbstman et al. 2010; Lin et al. 2010; Shy et al. 2011) or Mullen Scales of Early Learning (MSEL) (Adgent et al. 2014) at ages up to 36 months Five studies assessed FSIQ at ages 4 to 8 y (Chen et al. 2014; Eskenazi et al. 2013; Herbstman et al. 2010; Zhang et al. 2016) or MSCA total cognitive score at age 4 y (Gascon et al. 2011). Studies measured PBDE exposure in maternal serum or cord blood (), both maternal and child blood/serum (), or breast milk () (Table 3a). One included study relevant to ADHD outcomes (Roze et al. 2009) also reported measuring outcomes related to intelligence [Total and Performance Intelligence levels assessed using a short form of the Wechsler Preschool and Primary Scale of Intelligence, Revised Edition (WPPSI-R)] but did not report estimates of association in the publication, and the authors did not respond to requests for these data. Risk of bias generally differed for studies evaluating IQ at a later age and those evaluating children at younger ages. Studies of FSIQ at a later age were consistently rated as “low” or “probably low” risk of bias across domains. The only exception to this was Herbstman et al. (2010), which received a rating of “probably high” risk of bias for incomplete outcome reporting because of concerns regarding missing data. In contrast, many of the studies conducted only at younger ages utilizing the BSID were rated as “probably high” risk of bias in one or more domains (Figure 2a and Tables S4–S18). Four studies measuring BDE-47 in maternal serum during gestation or at birth or cord blood at birth and assessing FSIQ or MSCA in children 4–7 y old were amenable to a meta-analysis (Chen et al. 2014; Eskenazi et al. 2013; Gascon et al. 2011; Herbstman et al. 2010) (Table 4). The meta-analysis reported an overall decrement of 3.70 IQ points (95% CI: 0.83, 6.56; ; Figure 3) per 10-fold increase (in other words, times 10) in lipid-adjusted PBDE concentration (PBDE concentration range: lipid). Our updated search on September 27, 2016, identified a newer study (Zhang et al. 2016) assessing the same cohort of children as Chen et al. (2014) but at a later time point (8 y old instead of 5 y old). However, because Zhang et al. (2016) assessed children at an older time point than the other three studies included in our meta-analysis did (4, 6, and 7 y), we decided to keep Chen et al. (2014) in the meta-analysis to stay within the age range at assessment. We performed a sensitivity analysis replacing the Chen et al. (2014) with Zhang et al. (2016) and found that the overall estimate changed minimally from to (Figure S1).
Figure 2.
Summary of risk of bias judgments (low, probably low, probably high, high) for the human studies included in our systematic review of PBDE exposure and a) IQ or b) ADHD outcome. Risk of bias designations for individual studies are assigned according to criteria provided in Supplemental Material, “Instructions for Making Risk of Bias Determinations” and the justification for each study is provided in Tables S4–S18.
Table 4.
Human studies included in the meta-analysis of developmental exposure to PBDEs and IQ in children.
| Study reference | Study population details | Meta-analysis estimate [95% CI] | Relevant details |
|---|---|---|---|
| Herbstman et al. 2010 | New York (urban) | (95% CI: , 3.89) | BDE-47 measured in cord blood at birth. FSIQ assessed for 96 children at 6 y. Adjusted for age at testing, race/ethnicity, IQ of mother, sex of child, gestational age at birth, maternal age, environmental tobacco smoke exposure, maternal education, material hardship, breastfeeding, language and location of interview. |
| Maternal high school completion rate: 81.5% | Estimate from publication was (95% CI: , 1.69), from Table 3: change in FSIQ per ln-unit increase. We converted from natural log to log 10 by multiplying by a factor of ln (10). | ||
| Race/ethnicity: 40.4% white, 28.0% Chinese, 6.4% Asian (non-Chinese), 15.2% Black, 10.0% Other | |||
| Gascon et al. 2011 | Spain (small island population) | (95% CI: , 11.43) | BDE-47 in cord blood at birth. McCarthy Scales of Children’s Abilities (total cognitive score) assessed for 78 children at 48 months. Adjusted for sex, age of the child, preterm, evaluating psychologist, maternal age, social class, education, parity, smoking during pregnancy, alcohol consumption, prepregnancy BMI. |
| Maternal secondary school completion rate: 41.6% | Estimate from publication was (95% CI: , 6.5), from Table 4: regression estimate comparing “exposed” group () with “referent” group (), . Study authors provided additional data re-analyzing with continuous linear regression using log10-transformed exposures. | ||
| Race/ethnicity: not reported | |||
| Eskenazi et al. 2013 | California (rural/agricultural) | (95% CI: , 0.70) | BDE-47 measured in maternal serum during pregnancy or at delivery. FSIQ assessed for 231 children at 7 y. Adjusted for child’s age, sex, HOME Inventory at 6-months visit, language of assessment, and maternal y living in United States before giving birth. |
| Maternal high school completion rate: 20.5% | From publication, Table S6 | ||
| Race/ethnicity: “predominantly Mexican-American” | |||
| Chen et al. 2014 | Ohio (urban) | (95% CI: , 0.56) | BDE-47 measured in maternal serum during gestation. FSIQ assessed in 190 children at 5 y. Adjusted for maternal age at enrollment, race, education, marital status, maternal serum cotinine concentrations at enrollment, maternal IQ, child sex, maternal depression, household income, and HOME inventory. |
| Maternal high school completion rate: | From publication, Table S5 | ||
| Race/ethnicity: 67% non-Hispanic white |
Note: FSIQ, Full Scale Intelligence Quotient; HOME, Health Outcomes and Measures of the Environment.
Figure 3.
Meta-analysis of human studies ( studies, 595 children) for PBDE exposure (represented as congener BDE-47, lipid-adjusted) measured in cord blood or maternal serum during gestation or at birth for IQ outcome (FSIQ or McCarthy Scale) assessed in children between 48–84 months: reported effect estimates [95% confidence interval (CI)] from individual studies (inverse-variance weighted, represented by size of rectangle) and overall pooled estimate from random effects (RE) model per 10-fold increase (in other words, times 10) in PBDE exposure. Heterogeneity statistics: Cochran’s ; ; . Estimates were adjusted as follows: Herbstman et al. 2010: age at testing, race/ethnicity, IQ of mother, sex of child, gestational age at birth, maternal age, environmental tobacco smoke exposure, maternal education, material hardship, breastfeeding, language and location of interview; Gascon et al. 2011: sex, age of the child, preterm, evaluating psychologist, maternal age, social class, education, parity, smoking during pregnancy, alcohol consumption, prepregnancy BMI; Eskenazi et al. 2013: child’s age, sex, HOME score at 6-months visit, language of assessment, and maternal years living in United States before giving birth; Chen et al. 2014: maternal age at enrollment, race, education, marital status, maternal serum cotinine concentrations at enrollment, maternal IQ, child sex, maternal depression, household income, and HOME (Home Observation for Measurement of the Environment) inventory.
Estimates of association from studies using the BSID were extracted but could not be combined in a meta-analysis or visually displayed collectively in a figure because estimates were reported on different scales and used different association metrics (Table 5). Based on comparison of the body of evidence to prespecified criteria, we concluded that the quality of the overall body of evidence for the intelligence outcome was “moderate” (Table 1a); i.e., the evidence did not warrant downgrading or upgrading.
Table 5.
Reported association estimates for BSID outcome and 95% confidence interval (CI) or p-value, as available from individual studies.
| Study reference | Child age at assessment | Exposure/matrix | Association measure | Association estimate (95% CI) (or p-value) & data source | |
|---|---|---|---|---|---|
| Chen et al. 2014 | 220 | 36 months | Lipid-adjusted BDE-47 in maternal serum | Adjusted beta (log10) | 0.58 (, 5.53) |
| Negative estimate indicates higher exposures associated with poorer outcomes | Supplemental Material, Table S2 | ||||
| Gascon et al. 2012 | 290 | 12–18 months | Lipid-adjusted BDE-47 in maternal colostrum | (, 1.06) | |
| Table 3a | |||||
| Herbstman et al. 2010 | 114 | 36 months | Lipid-adjusted BDE-47 in cord blood | (, 2.90) | |
| Table 3a | |||||
| Chao et al. 2011 | 70 | 8–12 months | Lipid-adjusted BDE-47 in breastmilk | Spearman rho correlation | 0.065 (0.591) |
| Positive estimate indicates high exposures associated with poorer outcomes | Table 3 | ||||
| Shy et al. 2011 | 36 | 8–12 months | Lipid-adjusted BDE-47 in cord blood | Adjusted odds ratio | 1.04 |
| indicates high exposures associated with poorer outcomes | Table 4b |
Association estimates were originally reported on natural log scale; estimates were transformed to base 10 scale by multiplying by ln(10).
95% CI not reported; p-value not reported but authors noted .
We found some evidence of a dose–response gradient in several studies. Eskenazi et al. (2013) reported a significant dose-response trend across quartiles of the sum of BDE-47, BDE-99, BDE-100, and BDE-153 in maternal serum in decreasing WISC verbal comprehension evaluated in children at age 7 y (). Adgent et al. (2014) investigated the relationship across quartiles of BDE-28, BDE-47, BDE-99, BDE-100, and BDE-153 in breastmilk and reported similar small and imprecise estimates that were generally in a positive direction for MSEL composite scores. Herbstman et al. (2010) reported significant differences for BDE-47 measured in maternal serum comparing the 25th to 75th percentile ( lipid) for FSIQ at when children were assessed at 48 months, but not at 72 months. A dose–response relationship was also supported by the results of our meta-analysis (Figure 3) that demonstrated a statistically significant decrement in intelligence with increased PBDE exposures, assuming a linear relationship. However, Zhang et al. (2016) evaluated trends for FSIQ across quartiles of prenatal exposures to the sum of BDE-47, BDE-99, BDE-100, and BDE-153 and reported significant differences comparing the third with first quartile, but no overall trend (). We judged these collective findings to be not consistent or strong enough to warrant upgrading the overall quality of evidence for dose response.
We rated the overall strength of the evidence as “sufficient” for intelligence (Table 1a) based on: a) “moderate” quality of the body of evidence; b) direction of the association (i.e., consistent evidence of an inverse association between PBDEs exposure with intelligence across studies and among the combination of similar studies in the meta-analysis); c) confidence in the association with multiple well-conducted studies (i.e., most studies) (all, for those included in the meta-analysis) were prospective cohort studies and were of “low” or “probably low” risk of bias overall; the cohorts as a group represented geographically and socioeconomically diverse populations (Tables 3 and 4); and a statistically significant overall estimate of association from the combination of similar studies in a meta-analysis (Figure 3).
We agreed that it was not possible to eliminate the possibility of publication bias, particularly because we did not find enough studies to perform a formal statistical analysis for publication bias; however, we judged that the potential for risk of publication bias was not enough to alter our conclusions. Our rationale for this judgment was based on a) having conducted a comprehensive search that included the gray literature to identify government reports, conference abstracts, theses, and dissertations that may not have been subsequently published, in an attempt to capture a comprehensive collection of studies; and b) the results of our quantitative evaluation of the association estimate that an unpublished study would have to have to change our confidence in the estimate of our meta-analysis for intelligence. Our analysis reported that to enlarge the CI of our meta-analysis association estimate such that it would overlap zero, a new or unpublished study would have to report 0.69 (95% CI: , 5.20) increased IQ points per 10-fold increase in (in other words, times 10) PBDE exposure (Figure S2a). We judged the unpublished existence of a well-conducted study with such a result to be unlikely, given that this association estimate was in the opposite direction of all the other studies (including the four prospective cohort studies included in our meta-analysis) and would indicate that an increase in PBDE exposure would be associated with an increase in IQ, which we thought, based on current human and animal evidence, to be highly unlikely. Further, this central estimate (0.69) represents an association 3.38 IQ points [per 10-fold increase (in other words, times 10) in PBDE exposure] higher than the smallest association estimate reported by studies included in our meta-analysis [ from Herbstman et al. (2010)], and we judged it to be unlikely that an unpublished study would report such a finding.
To shift our meta-analysis to have an overall association estimate of zero would require a new study reporting an estimate of 12.0 (95% CI: 7.49, 16.51) increased IQ points per 10-fold increase (in other words, times 10) in PBDE exposure (Figure S1). We concluded this to be highly unlikely, and as such, collectively, these results increased confidence in our final rating of “sufficient” evidence that PBDE exposure diminishes intelligence even if the potential for publication bias could not be entirely ruled out.
Studies of ADHD and Attention-Related Behaviors
Eight of nine studies that evaluated ADHD and attention-related behaviors were prospective birth cohorts; the remaining study (Gump et al. 2014) was a cross-sectional study that we decided met our inclusion criteria because exposure was assessed a week prior to evaluating the outcomes. Assessments of ADHD and attention-related behaviors included Behavior Assessment System for Children (BASC) (Adgent et al. 2014; Chen et al. 2014; Eskenazi et al. 2013; Sagiv et al. 2015), CADS (Eskenazi et al. 2013; Sagiv et al. 2015), CBCL (Cowell et al. 2015; Eskenazi et al. 2013; Roze et al. 2009), Conners’ Continuous Performance Test (CPT) (Sagiv et al. 2015), DSM of Mental Disorders (Gascon et al. 2011; Sagiv et al. 2015), Infant-Toddler Social and Emotional Assessment (ITSEA) (Hoffman et al. 2012), Kiddie Continuous Performance Test (K-CPT) (Eskenazi et al. 2013), parental ADHD questionnaire (Roze et al. 2009), or Parental SDQ (Gump et al. 2014) at a wide range of ages (2–11 y). Studies measured PBDE exposure in maternal serum or cord blood (), both maternal and child blood/serum (), child whole blood (), or breast milk ().
We rated most risk-of-bias domains as “low” or “probably low” across all nine studies of ADHD and attention-related behavioral conditions (Figure 2b and Tables S4–S18). The most prevalent instances of “high” or “probably high” ratings in the body of evidence were for confounding and/or incomplete outcome reporting (Adgent et al. 2014; Cowell et al. 2015; Gump et al. 2014; Roze et al. 2009). For example, Roze et al. (2009) received a “high” risk-of-bias rating for incomplete outcome data because they reported only statistically significant results, whereas Cowell et al. (2015) received a “probably high” rating for this domain because reviewers had concern about missing outcome data that could not definitively be ruled out as related to participant’s exposure levels. Roze et al. (2009) also received “probably high” ratings for the blinding domain because the authors did not discuss blinding of outcome assessor to the exposure of participants. Roze et al. (2009), Gump et al. (2014), and Adgent et al. (2014) received “high” or “probably high” risk-of-bias ratings for the confounding domain because they did not adjust for all the important confounders that we determined beforehand, and in particular lacked adjustment for maternal characteristics (maternal age, education, marital status, exposure to alcohol/smoking during pregnancy, etc.). Furthermore, Gump et al. (2014) received a “high” rating for the “Other” category because PBDE exposures were measured the week prior to assessing ADHD outcomes, technically satisfying our inclusion criteria of assessing exposures prior to outcome but raising some concerns regarding whether the exposure truly preceded the outcome.
Meta-analyses were not feasible because there were not enough combinable studies. We assessed association estimates related to ADHD outcomes (BASC-2, CADS, CBCL, DSM-IV, K-CPT) by evaluating linear regression estimates from the fully adjusted models for lipid-adjusted BDE-47 exposures measured in cord blood, maternal serum, or breastmilk when available and dichotomous/categorical/correlation estimates when continuous estimates were not available (Table 6). We saw positive associations between PBDE exposures and ADHD or attention-related behavioral effects generally, although data were limited and CIs generally overlapped the null (Table 6). We agreed that the possibility of publication bias could not be confidently eliminated, as there were not enough studies to combine in a meta-analysis (and thus quantify the size of the association estimate needed to change our confidence in the meta-analysis estimate, as above for IQ) or to perform a formal statistical analysis for publication bias. However, we identified findings from the gray literature through our comprehensive search, and many studies reported findings that were not statistically significant. As such, we judged that there was insufficient evidence to warrant downgrading the body of evidence for publication bias.
Table 6.
Reported association estimates for ADHD outcome and 95% CI or p-value, as available from individual studies.
| Study reference | Assessment (child age) | Exposure and matrix | Association measure and interpretation | Association estimate (95% CI) (or p-value), data source, confounders | |
|---|---|---|---|---|---|
| Chen et al. 2014 | 183 | BASC-2, Hyperactivity (5 y) | Lipid-adjusted BDE-47 in maternal serum | Adjusted beta (log10) | 3.29 (0.3, 6.27) |
| Positive estimate indicates higher exposures associated with nonoptimal behavior | Plotted in Figure 2 in manuscript; authors provided data | ||||
| Adjusted for maternal age at enrollment, race, education, marital status, maternal serum cotinine concentrations at enrollment, maternal IQ, child sex, maternal depression, household income, HOME inventory | |||||
| Adgent et al. 2014 | 192 | BASC-2, Hyperactivity (3 y) | Lipid-adjusted BDE-47 in breastmilk | 0.3 (, 3.3) | |
| Table S1 | |||||
| Adjusted for sex, parity, maternal education, maternal race, breastfeeding duration, income maternal age, fatty acids, and fatty acid analysis batch | |||||
| BASC-2, Attention (3 y) | (, 2.2) | ||||
| Table S1 | |||||
| Adjusted for same confounders as above | |||||
| Roze et al. 2009 | 60 | CBCL, Attention sustained (5–6 y) | Lipid-adjusted BDE-47 in maternal serum | Adjusted correlation coefficient | () |
| Table 4 | |||||
| Negative estimate indicates higher exposures associated with poorer outcomes | |||||
| Adjusted for: socioeconomic status, HOME inventory score, child sex | |||||
| Eskenazi et al. 2013 | 233 | K-CPT, ADHD Conf. Index (5 y) | Lipid-adjusted BDE-47 in maternal serum | Adjusted odds ratio (log10) | 6.2 (1.1, 11.4) |
| indicates higher exposures associated with poorer outcomes | Table S6 | ||||
| Adjusted for child age, at assessment, sex, maternal education, number of children in the home, psychometrician | |||||
| 266 | Maternal-reported CADS, ADHD Index (7 y) | 2.6 (0.4, 4.8) | |||
| Table S6 | |||||
| Adjusted for same confounders as above | |||||
| 266 | Maternal-Reported CADS, DSM-IV ADHD (7 y) | 2.2 (0.0, 4.5) | |||
| Table S6 | |||||
| Adjusted for same confounders as above | |||||
| Gascon et al. 2011 | 77 | Teacher-Reported ADHD DSM-IV (4 y) | Lipid-adjusted BDE-47 in cord blood | Adjusted relative risk | 0.4 (0.1, 1.7) |
| indicates higher exposures associated with poorer outcomes | Table 4 | ||||
| Adjusted for sex, age of child, preterm, maternal age, prepregnancy BMI, fish consumption, duration of breastfeeding | |||||
| Cowell et al. 2015 | 107 | CBCL (6 y) | Lipid-adjusted BDE-47 in cord blood | Adjusted incidence rate ratio | 0.91 (0.75, 1.10) |
| Table 2 | |||||
| indicates higher exposures associated with poorer outcomes | |||||
| Adjusted for age at exam, sex, ethnicity, environmental tobacco smoke, maternal intelligence, maternal age, marital status, maternal demoralization at exam |
Note: ADHD, attention deficit hyperactivity disorder; BASC, Behavior Assessment System for Children; CADS, Conners’ ADHD virgule DSM-IV Scales; CBCL, Child Behavior Checklist; CI, confidence interval; DSM, Diagnostic and Statistical Manual of Mental Disorders; HOME, Health Outcomes and Measures of the Environment; K-CPT, Kiddie Continuous Performance Test.
The overall strength of the evidence was “limited” for the outcome of ADHD and attention-related behaviors (Table 1b) based on: 1) “moderate” quality of the body of evidence; 2) direction of the effect, i.e., evidence of an increasing adverse effect with increasing exposure to PBDEs existed, but it was not consistent over all studies; and 3) confidence in the effect (multiple well-conducted studies). Generally, given the limitations of the body of evidence, overall chance, bias, and confounding could not be ruled out with reasonable confidence.
Discussion
Understanding what puts children at risk for neurological disorders is critical to preventing harm. To our knowledge, this study was the first systematic review and meta-analysis of developmental exposure to PBDEs. Our review found “sufficient evidence of toxicity” based on diminished intelligence associated with increased exposure to PBDEs, and “limited evidence of toxicity” based on increases in ADHD and attention-related behaviors with increased exposure to PBDEs. We identified nine reviews of this topic published between 2003 and 2016 (Berghuis et al. 2015; Brandt 2012; Chao et al. 2014; De Cock et al. 2012; Kim et al. 2014; Muir 2003; Pinson et al. 2016; Roth and Wilks 2014; Vrijheid et al. 2016), none of which conducted a meta-analysis or consistently applied all nine components of a systematic literature review as described in the Literature Review Appraisal Toolkit (LRAT), a tool derived from a number of standard practice appraisals of the methodological quality of the literature reviews conducted in the medical sciences (see http://policyfromscience.com/lrat/) (Ades et al. 2012; Garg et al. 2008; Higgins and Green 2011; Moher et al. 2009; Mulrow 1987; Oxman et al. 1994; Schulz et al. 2010; Shea et al. 2007). Broadly, our results that PBDEs are associated with adverse neurodevelopment (either directly or indirectly, e.g, as a thyroid hormone disruptor), were generally consistent with the findings of all but one of the nine reviews (Roth and Wilks 2014). That review concluded that the available evidence “raises questions” but does not “support a strong causal association” between PBDEs and adverse neurodevelopmental and neurobehavioral outcomes in infants and children (Roth and Wilks 2014). The authors of that study also did not specify a definition of “strong causal association,” so it is not possible to directly compare their findings with the findings from our review. Possible explanations for the different conclusions are that we performed a meta-analysis, which strengthened our capacity to detect an association beyond individual study findings or that Roth and Wilks (2014) did not include the Chen et al. (2014) study that was included in our review (Chen et al. 2014).
We found an association of 3.7-point reduction in IQ per 10-fold increase (in other words, times 10) in PBDE exposure when combining results from four prospective birth cohort studies investigating PBDE exposures within the range lipid. In comparison, for the well-studied adverse effects of lead on IQ, it has been estimated in a pooled analysis of 1,333 children participating in seven international population-based longitudinal cohort studies followed from birth or infancy until 5–10 y of age that there is a 7-point reduction in IQ per approximately 10-fold increase (in other words, times 10) in child blood lead levels (6.9-point decrement in IQ associated with blood lead level increase from 2.4 to ) (Lanphear et al. 2005). Even mild decrements in individual IQ can result in serious consequences at the societal level (Bellinger 2012), and as such, these neurological health effects are of great concern to public health. These results underscore the importance of strengthening efforts to prevent the widespread entry of potential neurotoxicants into the environment and to remove PBDEs and other toxic industrial chemicals that have become ubiquitous in our environment. Although public health efforts to reduce lead exposures in the U.S. have significantly reduced childhood blood lead levels (Needleman and Gee 2013), strong policies and regulations are still needed to eliminate lead exposures that persist in communities (Bellinger 2016) and in workplaces (Hipkins et al. 2004) and to reduce other environmental chemical exposures also associated with adverse neurodevelopmental risks (Bennett et al. 2016).
Preventing the entry of toxic environmental chemicals into the marketplace is critical: Once chemicals are released into commerce, exposures can persist long after the chemicals have been “recalled.” Since 2003, several restrictions, phase-outs, and bans on PBDEs have been implemented in the U.S., Canada, and the European Union, reducing use of PBDEs (CDC 2013; Environment Canada 2015; Council of the European Union, European Parliament 2003; U.S. EPA 2014). However, human exposure to PBDEs remains ongoing and widespread because PBDEs were in commerce for over 40 y, and they persist in the environment (Besis and Samara 2012; Fromme et al. 2016; Hites 2004; Law et al. 2008; Sjodin et al. 2008). Notably, PBDEs were introduced as a substitute for polybrominated biphenyls (PBBs), compounds that had been banned (Birnbaum and Staskal 2004), also underscoring the need for policies that can ensure that “safer” substitutes are less toxic than the replacement chemicals.
A key challenge to our review was that many of the included studies were not combinable in a meta-analysis. Included ADHD studies generally reported association estimates on different scales or based on categories of exposures using different ranges, or they evaluated the health outcome at different life stages and with different assessment tools, leading us to conclude the data were too heterogeneous to be combined. Thus, we found limited evidence to determine whether there is a consistent relationship between PBDEs and ADHD. Due to differences in the timing and nature of exposure and outcome assessment, many of the intelligence studies also were not combinable in the meta-analysis. Having additional studies would have increased our statistical power and the precision of our association estimate.
The four studies combinable in a meta-analysis were selected based on similarities in study design, timing of exposure and outcome measurement, and intelligence assessment method, but other aspects of these studies may have differed and could have impacted study comparability. For instance, studies did not all adjust for the same confounding variables, which could potentially influence the comparability of association estimates across studies. However, each of the studies in the meta-analysis was rated as having either “low” or “probably low” risk of bias for confounding, as each study adjusted for all or nearly all of the key confounders identified in our protocol. Thus, we considered differences across studies in the confounders that were adjusted for to be minor and unlikely to have influenced the meta-analysis findings. Reviewed studies also showed heterogeneity in the assessment age, exposure matrix, and assessment tool used in studies to derive the summary estimate of association in the meta-analysis. However, we selected association estimates from studies assessing children at similar ages using similar assessment tools for intelligence and utilized lipid-adjusted measures that could be combined even in measured in different exposure matrices and therefore concluded that this heterogeneity was anticipated to be minimal and that scientific rationale existed for combining these estimates. Furthermore, there was minimal statistical heterogeneity, supporting the appropriateness of combining these studies in a meta-analysis.
The inability to combine studies in a meta-analysis due to lack of reporting in published studies is a challenge for systematic reviews in environmental health. The meta-analysis reported for IQ would not have been possible without the cooperation of study authors and their willingness and ability to provide additional data and information. To advance the capacity to conduct robust systematic reviews in environmental health, key data should be requested by journals when manuscripts are submitted for publication. Several high-impact journals have adopted checklists for the reporting of elements necessary to describe studies comprehensively and transparently, such as the ARRIVE guidelines for experimental animal studies (http://www.nc3rs.org.uk/ARRIVE/) (Kilkenny et al. 2010) or Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for strengthening the reporting of observational human studies (http://www.strobe-statement.org/) (von Elm et al. 2008), which can help to ensure that these details are available for incorporation into future reviews.
Conclusion
We found an association of PBDEs with decrements on IQ [3.7-point reduction in IQ per 10-fold increase (in other words, times 10) in PBDE exposure] and concluded that there was “sufficient” evidence supporting an association between developmental PBDE exposure and IQ reduction. Our findings suggest that preventing exposure to PBDEs could help prevent loss of human intelligence and, potentially, prevent other neurodevelopmental disorders in children.
Supplemental Material
Acknowledgments
The authors acknowledge the work of H. Tesoro in data extraction, L. Rosman, in literature search, and P. I. Johnson, in risk-of-bias rating.
We gratefully acknowledge the following corresponding study authors for providing additional information and data for our analysis: A. Chen, B. Gump, J. Daniels, B. Eskenazi, M. Gascon, and J. Herbstman.
This study was supported by John Merck Foundation, U.S. EPA (award number A124617-01), U.S. EPA (award number 83543301), National Institutes of Health/National Institute of Environmental Health Sciences (award number PO1ES022841).
References
- Ades AE, Caldwell DM, Reken S, Welton NJ, Sutton AJ, Dias S. 2012. NICE DSU technical support document 7: Evidence synthesis of treatment efficacy in decision making: a reviewer's checklist. The Decision Support Unit. London UK:National Institute for Health and Care Excellence, PMID: 27905719 [PubMed] [Google Scholar]
- Adgent MA, Hoffman K, Goldman BD, Sjödin A, Daniels JL. 2014. Brominated flame retardants in breast milk and behavioural and cognitive development at 36 months. Paediatr Perinat Epidemiol 28:48–57, PMID: 24313667, 10.1111/ppe.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2004. Toxicological profile for polybrominated biphenyls and polybrominated diphenyl ethers. https://www.atsdr.cdc.gov/toxprofiles/tp68.pdf [accessed 20 March 2017].
- Bagwell CL, Molina BSG, Pelham WE Jr. , Hoza B. 2001. Attention-deficit hyperactivity disorder and problems in peer relations: predictions from childhood to adolescence. J Am Acad Child Adolesc Psychiatry 40(11):1285–1292, 10.1097/00004583-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. . 2011. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64(4):401–406, PMID: 21208779, 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Barkley RA. 2002. Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. J Clin Psychiatry 63 (Suppl 12):10–15. [PubMed] [Google Scholar]
- Batty GD, Deary IJ, Gottfredson LS. 2007a. Premorbid (early life) IQ and later mortality risk: systematic review. Ann Epidemiol 17(4):278–288, PMID: 17174570, 10.1016/j.annepidem.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Batty GD, Deary IJ, Schoon I, Gale CR. 2007b. Childhood mental ability in relation to food intake and physical activity in adulthood: the 1970 British Cohort Study. Pediatrics 119:e38–e45, PMID: 17200256, 10.2105/AJPH.2007.109488. [DOI] [PubMed] [Google Scholar]
- Batty GD, Wennerstad KM, Smith GD, Gunnell D, Deary IJ, Tynelius P, et al. . 2009. IQ in early adulthood and mortality by middle age: cohort study of 1 million Swedish men. Epidemiology 20(1):100–109, PMID: 19234402, 10.1097/EDE.0b013e31818ba076. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. 2012. A strategy for comparing the contributions of environmental chemicals and other risk factors to neurodevelopment of children. Environ Health Perspect 120(4):501–507, PMID: 22182676, 10.1289/ehp.1104170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. 2012. Comparing the population neurodevelopmental burdens associated with children's exposures to environmental chemicals and other risk factors. Neurotoxicology 33(4):641–643, PMID: 22525934, 10.1016/j.neuro.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. 2016. Lead contamination in Flint—an abject failure to protect public health. N Engl J Med 374:1101–1103, 10.1056/NEJMp1601013. [DOI] [PubMed] [Google Scholar]
- Bennett D, Bellinger DC, Birnbaum LS, Bradman A, Chen A, Cory-Slechta DA, et al. . 2016. Project TENDR: targeting environmental neurodevelopmental risks the TENDR consensus statement. Environ Health Perspect 124(7):A118–A122, PMID: 27479987, 10.1289/EHP358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis SA, Bos AF, Sauer PJJ, Roze E. 2015. Developmental neurotoxicity of persistent organic pollutants: an update on childhood outcome. Arch Toxicol 89:687–709, 10.1007/s00204-015-1463-3. [DOI] [PubMed] [Google Scholar]
- Besis A, Samara C. 2012. Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments–a review on occurrence and human exposure. Environ Pollut 169:217–229, PMID: 22578798, 10.1016/j.envpol.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. 2004. Brominated flame retardants: cause for concern?. Environ Health Perspect 112(1):9–17, PMID: 14698924, 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. . 2011. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 127(6):1034–1042, PMID: 21606152, 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- Brandt BL. 2012. Household environmental toxins and neurodevelopment in children: University of Montana. [Master’s Thesis]. http://scholarworks.montana.edu/xmlui/bitstream/handle/1/966/BrandtB0512.pdf;jsessionid=EE64166ACD74D28485E2B896AFE8D9FE?sequence=1 [accessed 20 March 2017].
- CDC (Centers for Disease Control and Prevention). 2013. Biomonitoring summary: Polybrominated diphenyl ethers and 2,2',4,4',5,5'-hexabromobiphenyl (bb-153). http://www.cdc.gov/biomonitoring/PBDEs_BiomonitoringSummary.html [accessed 20 March 2017].
- Chao HR, Huang H-L, Hsu Y-C, Lin C-W, Lin D-Y, Gou Y-Y, et al. . 2014. Impact of brominated POPs on the neurodevelopment and thyroid hormones of young children in an indoor environment—a review. Aerosol Air Quality Research 14:1320–1332, 10.4209/aaqr.2013.05.0156. [DOI] [Google Scholar]
- Chao HR, Tsou TC, Huang HL, Chang-Chien GP. 2011. Levels of breast milk PBDEs from southern Taiwan and their potential impact on neurodevelopment. Pediatr Res 70(6):596–600, PMID: 21857391, 10.1203/PDR.0b013e3182320b9b. [DOI] [PubMed] [Google Scholar]
- Chao HR, Wang SL, Lee WJ, Wang YF, Päpke O. 2007. Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ Int 33:239–245, PMID: 17079016, 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjödin A, et al. . 2014. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: the HOME study. Environ Health Perspect 122(8):856–862, PMID: 24870060, 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. 2001. Conners' rating scales revised. Multi-Health Systems, Incorporated.
- Costa LG, Giordano G. 2007. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 28(6):1047–1067, PMID: 17904639, 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. . 2015. Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3-7 years. Neurotoxicol Teratol 52:143–150, PMID: 26344673, 10.1016/j.ntt.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council of the European Union, European Parliament, 2003. Directive 2003/11/EC of the European Parliament and of the Council of 6 February 2003 amending for the 24th time Council Directive 76/769/ EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (pentabromodiphenyl ether, octabromodiphenyl ether). http://publications.europa.eu/en/publication-detail/-/publication/0a566c4b-75ab-459b-8546-dbae21493f1b/language-en [accessed 11 July 2017].
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. 2001. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect 109 (suppl 1):49–68, PMID: 11250805, 10.2307/3434846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cock M, Maas YG, van de Bor M. 2012. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review. Acta Paediatr 101(8):811–818, PMID: 22458970, 10.1111/j.1651-2227.2012.02693.x. [DOI] [PubMed] [Google Scholar]
- Der G, Batty GD, Deary IJ. 2009. The association between IQ in adolescence and a range of health outcomes at 40 in the 1979 US national longitudinal study of youth. Intelligence 37(6):573–580, 10.1016/j.intell.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188, PMID: 3802833. [DOI] [PubMed] [Google Scholar]
- Environment Canada. 2015. Polybrominated diphenyl ethers regulations (SOR/SOR/2008-218). http://www.ec.gc.ca/lcpe-cepa/eng/regulations/detailReg.cfm?intReg=108 [accessed 20 March 2017].
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, et al. . 2013. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect 121(2):257–262, PMID: 23154064, 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubig PA, Aguiar A, Schantz SL. 2010. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ Health Perspect 118(12):1654, PMID: 20829149, 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Monuteaux MC, Doyle AE, Seidman LJ. 2001. A psychometric measure of learning disability predicts educational failure four years later in boys with attention-deficit/hyperactivity disorder. J Atten Disord 4:220–230, 10.1177/108705470100400404. [DOI] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. 2009. Human internal and external exposure to PBDEs–a review of levels and sources. Int J Hyg Environ Health 212(2):109–134, PMID: 18554980, 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Fromme H, Becher G, Hilger B, Völkel W. 2016. Brominated flame retardants–exposure and risk assessment for the general population. Int J Hygiene and Environ Health 219(1):1–23, PMID: 26412400, 10.1016/j.ijheh.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Garg AX, Hackam D, Tonelli M. 2008. Systematic review and meta-analysis: when one study is just not enough. Clin J Am Soc Nephrol 3(1):253–260, PMID: 18178786, 10.2215/CJN.01430307. [DOI] [PubMed] [Google Scholar]
- Gascon M, Fort M, Martínez D, Carsin AE, Forns J, Grimalt JO, et al. . 2012. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ Health Perspect 120:1760–1765, PMID: 23052368, 10.1289/ehp.1205266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Vrijheid M, Martínez D, Forns J, Grimalt JO, Torrent M, et al. . 2011. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ Int 37(3):605–611, PMID: 21237513, 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gill U, Chu I, Ryan JJ, Feeley M. 2004. Polybrominated diphenyl ethers: human tissue levels and toxicology. Rev Environ Contam Toxicol 183:55–97, PMID: 15369322, 10.1007/978-1-4419-9100-3_3. [DOI] [PubMed] [Google Scholar]
- Goodman R. 1997. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry 38(5):581–586, PMID: 9255702, 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Bellinger D, Bergman Å, Cordier S, Davey-Smith G, Eskenazi B, et al. . 2008. The faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol 102:73–75, PMID: 18226057, 10.1111/j.1742-7843.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. 2006. Developmental neurotoxicity of industrial chemicals. Lancet 368(9553):2167–2178, PMID: 17174709, 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Gudjonsson GH, Sigurdsson JF, Sigfusdottir ID, Young S. 2012. An epidemiological study of ADHD symptoms among young persons and the relationship with cigarette smoking, alcohol consumption and illicit drug use. J Child Psychol Psychiatry 53(3):304–312, PMID: 22066497, 10.1111/j.1469-7610.2011.02489.x. [DOI] [PubMed] [Google Scholar]
- Gump BB, Yun S, Kannan K. 2014. Polybrominated diphenyl ether (PBDE) exposure in children: possible associations with cardiovascular and psychological functions. Environ Res 132:244–250, PMID: 24834818, 10.1016/j.envres.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. . 2008. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926, PMID: 18436948, 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpin VA. 2005. The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Arch Dis Child 90(1):i2–i7, PMID: 15665153, 10.1136/adc.2004.059006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. . 2010. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect 118(5):712–719, PMID: 20056561, 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández Weldon R, LaKind JS. 2016. Biomonitoring of dioxins and furans: levels and trends in humans. In: Special Volume in Honor of Otto Hutzinger. Alaee ME, ed New York, NY:Springer International Publishing. [Google Scholar]
- Hertz-Picciotto I, Delwiche L. 2009. The rise in autism and the role of age at diagnosis. Epidemiology 20(1):84–90, PMID: 19234401, 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S. 2011. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. www.cochrane-handbook.org [accessed 20 March 2017].
- Hipkins KL, Materna BL, Payne SF, Kirsch LC. 2004. Family lead poisoning associated with occupational exposure. Clin Pediatr (Phila) 43(9):845–849, PMID: 15583781, 10.1177/000992280404300909. [DOI] [PubMed] [Google Scholar]
- Hites RA. 2004. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol 38(4):945–956, PMID: 14998004. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Adgent M, Goldman BD, Sjodin A, Daniels JL. 2012. Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers. Environ Health Perspect 120(10):1438–1442, PMID: 22814209, 10.1289/ehp.1205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). 2006. IARC monographs on the evaluation of carcinogenic risks to humans: preamble (amended January 2006). http://monographs.iarc.fr/ENG/Preamble/index.php [accessed 20 March 2017].
- Institute of Medicine. 1981. Costs of environment-related health effects: A plan for continuing study. Washington, DC. https://www.nap.edu/catalog/812/cost-of-environmental-related-health-effects-a-plan-for-continuing [accessed 20 March 2017].
- Johnston C, Mash EJ. 2001. Families of children with ADHD: review and recommendations for future research. Clin Child Fam Psychol Rev 4:183–207, PMID: 11783738. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Koustas E, Vesterinen HM, Sutton P, Atchley DS, Kim AN, et al. . 2016. Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environment international 92:716–728, 10.1016/j.envint.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, et al. . 2014. The Navigation Guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect 122:1028–1039, PMID: 24968388, 10.1289/ehp.1307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, et al. . 2012. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect 120(6):799–806, PMID: 22275730, 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. 2010. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160(7):1577–1579, PMID: 20649561, 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YR, Harden FA, Toms LML, Norman RE. 2014. Health consequences of exposure to brominated flame retardants: a systematic review. Chemosphere 106:1–19, PMID: 24529398, 10.1016/j.chemosphere.2013.12.064. [DOI] [PubMed] [Google Scholar]
- Koustas E, Lam J, Sutton P, Johnson PI, Atchley DS, Sen S, et al. . 2014. The Navigation Guide - evidence-based medicine meets environmental health: systematic review of nonhuman evidence for PFOA effects on fetal growth. Environ Health Perspect 122(10):1015–1027, PMID: 24968374, 10.1289/ehp.1307177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, et al. . 2014. The Navigation Guide - evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect 122(10):1040–1051, PMID: 24968389, 10.1289/ehp.1307923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Sutton P, Kalkbrenner A, Windham G, Halladay A, et al. . 2016. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PloS ONE 11(9):e0161851, PMID: 27653281, 10.1371/journal.pone.0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Sutton P, Padula AM, Cabana MD, Koustas E, Vesterinen H, et al. . 2015a. Applying the navigation guide systematic review methodology. Case study #5: association between developmental exposures to PBDEs and human neurodevelopment. York, UK:PROSPERO Centre for Reviews and Dissemination, http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015019753 [accessed 11 July 2017]. [Google Scholar]
- Lam J, Sutton P, Padula AM, Cabana MD, Koustas E, Vesterinen H, et al. . 2015b. Instructions for grading the quality and strength of evidence. In: “Association Between Formaldehyde Exposure and Asthma, A Systematic Review of the Evidence Protocol.” San Francisco, CA:University of California, San Francisco, 96–108, http://www.crd.york.ac.uk/PROSPEROFILES/19753_PROTOCOL_20150322.pdf [accessed 11 July 2015]. [Google Scholar]
- Law RJ, Herzke D, Harrad S, Morris S, Bersuder P, Allchin CR. 2008. Levels and trends of HBCD and BDEs in the European and Asian environments, with some information for other BFRs. Chemosphere 73(2):223–241, PMID: 18472134, 10.1016/j.chemosphere.2008.02.066. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Lambertini L, Birnbaum LS. 2012. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect 120(7):a258–a260, PMID: 22543002, 10.1289/ehp.1104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. . 2005. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect 113(7):894–899, PMID: 16002379, 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. 2011. McCarthy scales of children’s abilities. In: Encyclopedia of child behavior and development. Goldstein S, Naglieri JA, eds Boston, MA: Springer, 928–929. [Google Scholar]
- Lin DY, Chao HR, Gou YY, Huang CY. Infants ingesting high breast milk levels of polybrominated diphenyl ethers may have negative impact on their neurodevelopment. In: Proceedings of the 2010 International Conference, 2010, 325–327. [Google Scholar]
- Makey CM, McClean MD, Sjodin A, Weinberg J, Carignan CC, Webster TF. 2014. Temporal variability of polybrominated diphenyl ether (PBDE) serum concentrations over one year. Environ Sci Technol 48(24):14642–14649, PMID: 25383963, 10.1021/es5026118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalec D. 2011. Bayley scales of infant development: Third edition In: Encyclopedia of child behavior and development. Goldstein S, Naglieri JA, eds Boston, MA:Springer, 215–215. [Google Scholar]
- Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Elmi AF, Tilly MK, et al. . 2016. Consumer product chemicals in indoor dust: a quantitative meta-analysis of US studies. Environ Sci Technol 50(19):10661–10672, PMID: 27623734, 10.1021/acs.est.6b02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS MED 6(7):e1000097, PMID: 19621072, 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R, Cushing LJ, Jesdale BM, Schwartz JM, Guo W, Guo T, et al. . 2016. Environmental chemicals in an urban population of pregnant women and their newborns from San Francisco. EnvironSciTechnol, 50(22):12464–12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir T. 2003. Is there a relationship between the rise in thyroid and neurodevelopmental health effects in North America and the rise in concentrations of PBDEs in the environment? Organohalogen Compounds 61:195–198. [Google Scholar]
- Mulrow CD. 1987. The medical review article: state of the science. Ann Intern Med 106(3):485–488, PMID: 3813259. [DOI] [PubMed] [Google Scholar]
- Needleman H, Gee D. 2013. Lead in petrol 'makes the mind give way'. In: Late lessons from early warnings: Science, precaution, innovation, Section 3 46–75. Copenhagen, Denmark:European Environment Agency. [Google Scholar]
- Neisser U, Boodoo G, Bouchard Jr TJ, Boykin AW, Brody N, Ceci SJ, et al. . 1996. Intelligence: knowns and unknowns. Am Psychol 51(2):77–101, 10.1037//0003-066X.51.2.77. [DOI] [Google Scholar]
- Newschaffer CJ, Falb MD, Gurney JG. 2005. National autism prevalence trends from United States special education data. Pediatrics 115:e277–e282, PMID: 15741352, 10.1542/peds.2004-1958. [DOI] [PubMed] [Google Scholar]
- Nijmeijer JS, Minderaa RB, Buitelaar JK, Mulligan A, Hartman CA, Hoekstra PJ. 2008. Attention-deficit/hyperactivity disorder and social dysfunctioning. Clinal Psychol Rev 28:692–708, PMID: 18036711, 10.1016/j.cpr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Norstrom RJ, Simon M, Moisey J, Wakeford B, Weseloh DV. 2002. Geographical distribution (2000) and temporal trends (1981-2000) of brominated diphenyl ethers in Great Lakes hewing gull eggs. Environ Sci Technol 36(22):4783–4789, 10.1021/es025831e. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council). 2000. Scientific frontiers in developmental toxicology and risk assessment. Washington, DC:National Academies Press. [PubMed] [Google Scholar]
- NTP (National Toxicology Program). 2015. Handbook for conducting a literature-based health assessment using OHAT approach for systematic review and evidence integration. U.S. Department of Health and Human Services. Office of Health Assessment and Translation. https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf [accessed 20 March 2017].
- Oxman AD, Cook DJ, Guyatt GH, Bass E, Brill-Edwards P, Browman G, et al. . 1994. Users' guides to the medical literature: VI. How to use an overview. JAMA 272(17):1367–1371, PMID: 7933399. [DOI] [PubMed] [Google Scholar]
- Pinson A, Bourguignon JP, Parent AS. 2016. Exposure to endocrine disrupting chemicals and neurodevelopmental alterations. Andrology 4(4):706–722, PMID: 27285165, 10.1111/andr.12211. [DOI] [PubMed] [Google Scholar]
- Prior M. 2003. Is there an increase in the prevalence of autism spectrum disorders? J Paediatr Child Health 39(2):81–82, PMID: 12603792, 10.1046/j.1440-1754.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. . 2006. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 118(6):e1845–e1859, PMID: 17116700, 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. 2014. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 122:711–718, PMID: 24755067, 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N, Wilks MF. 2014. Neurodevelopmental and neurobehavioural effects of polybrominated and perfluorinated chemicals: a systematic review of the epidemiological literature using a quality assessment scheme. Toxicol Lett 230(2):271–281, PMID: 24583043, 10.1016/j.toxlet.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF. 2009. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect 117(12):1953–1958, PMID: 20049217, 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. 2005. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr 94:2–15, PMID: 15858952. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Kogut K, Gaspar FW, Gunier RB, Harley KG, Parra K, et al. . 2015. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9-12 years of age. Neurotoxicol Teratol 52:151–161, PMID: 26271888, 10.1016/j.ntt.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkever DS. 2014. Assessing the IQ-earnings link in environmental lead impacts on children: have hazard effects been overstated? Environ Res 131:219–230, PMID: 24814698, 10.1016/j.envres.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Sawaya GF, Guirguis-Blake J, LeFevre M, Harris R, Petitti D. 2007. Update on methods: estimating certainty and magnitude of net benefit. http://www.uspreventiveservicestaskforce.org/uspstf07/methods/benefit.htm#copyright [accessed 20 March 2017]. [DOI] [PubMed]
- Schulz KF, Altman DG, Moher D. 2010. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 8:18, PMID: 20334633, 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. . 2007. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7:10, PMID: 17302989, 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy CG, Huang HL, Chang-Chien GP, Chao HR, Tsou TC. 2011. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers. Bull Environ Contam Toxicol 87(6):643–648, PMID: 21953308, 10.1007/s00128-011-0422-9. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, Patterson DG. 2004. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers and polybrominated and polychlorinated biphenyls in breast milk. Anal Chem 76(15):4508–4514, PMID: 15283595, 10.1021/ac0495384. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, et al. . 2008. Serum concentrations of polybrominated diphenyl ethers (pbdes) and polybrominated biphenyl (PBB) in the United States population: 2003-2004. Environ Sci Technol 42(4):1377–1384, 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Faraone SV, Tarko L, McDermott K, Biederman J. 2014. Attention-deficit/hyperactivity disorder and adverse health outcomes in adults. J Nerv Ment Dis 202(10):725–731, PMID: 25211634, 10.1097/NMD.0000000000000191. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 1991. “ Guidelines for developmental toxicity risk assessment.” http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=23162#Download [accessed 20 March 2017].
- U.S. EPA. 1996. “Guidelines for reproductive toxicity risk assessment.”http://www.epa.gov/raf/publications/pdfs/REPRO51.PDF [accessed 20 March 2017].
- U.S. EPA. 2013. “America’s children and the environment.” 3rd ed. https://www.epa.gov/ace/americas-children-and-environment-third-edition [accessed 20 March 2017].
- U.S. EPA. 2014. “Polybrominated diphenyl ethers (PBDEs) action plan summary.” http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/pbde.html [accessed 20 March 2017].
- Vesterinen HM, Johnson PI, Atchley DS, Sutton P, Lam J, Zlatnik MG, et al. . 2014. Fetal growth and maternal glomerular filtration rate: asystematic review. J Matern Fetal Neonatal Med:1–6, PMID: 25382561, 10.3109/14767058.2014.980809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SN, Bitsko RH, Danielson ML, Perou R, Blumberg SJ. 2010. Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children—United States, 2003 and 2007. MMWR, 59:1439–1443. [PubMed] [Google Scholar]
- Viswanathan M, Ansari M, Berkman N, Chang S, Hartling L, McPheeters L, et al. . 2012. “Assessing the risk of bias of individual studies in systematic reviews of health care interventions.” (Agency for Healthcare Research and Quality, Methods Guide for Comparative Effectiveness Reviews) AHRQ Publication No. 12-EHC047-EF; www.effectivehealthcare.ahrq.gov/ [accessed 29 June 2017]. [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. 2008. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61(4):344–349, PMID: 18313558, 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. 2016. Environmental pollutants and child health-a review of recent concerns. Int J Hyg Environ Health 219(4–5):331–342, PMID: 27216159, 10.1016/j.ijheh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Sakai S. 2003. Environmental release and behavior of brominated flame retardants. Environ Int 29(6):665–682, PMID: 12850086, 10.1016/S0160-4120(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Webster TF. 2013. Associations between PBDEs in office air, dust, and surface wipes. Environ Int 59:124–132, PMID: 23797055, 10.1016/j.envint.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeier PM, Schacht A, Barkley RA. 2010. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health 46(3):209–217, PMID: 20159496, 10.1016/j.jadohealth.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Weiss G, Hechtman L. 1993. Hyperactive children grow up: ADHD in children, adolescents and adults. New York, NY:Guildford. [Google Scholar]
- Woodruff TJ, Sutton P, The Navigation Guide Work Group. 2011a. An evidence-based medicine methodology to bridge the gap between clinical and environmental health sciences. Health Aff 30:931–937, PMID: 21555477, 10.1377/hlthaff.2010.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. 2011b. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect 119:878–885, PMID: 21233055, 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Sutton P. 2014. The Navigation Guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ Health Perspect 122(10):1007–1014, PMID: 24968373, 10.1289/ehp.1307175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 1994. Polybrominated diphenyl ethers. Geneva, Switzerland:IPCS, Environmental Health Criteria. [Google Scholar]
- Zhang H, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. . 2016. Prenatal PBDE and PCB exposures and reading, cognition, and externalizing behavior in children. Environ Health Perspect, 125(4):746–752, PMID: 27385187, 10.1289/ehp478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.