Abstract
Eating disorders and obesity adversely affect individuals both medically and psychologically, leading to reduced life expectancy and poor quality of life. While there exist a number of treatments for anorexia, morbid obesity and bulimia, many patients do not respond favorably to current behavioral, medical or bariatric surgical management. Neuromodulation has been postulated as a potential treatment for eating disorders and obesity. In particular, deep brain stimulation and transcranial non-invasive brain stimulation have been studied for these indications across a variety of brain targets. Here, we review the neurobiology behind eating and eating disorders as well as the current status of preclinical and clinical neuromodulation trials for eating disorders and obesity.
Keywords: anorexia nervosa, bulimia, deep brain stimulation, eating disorders, non-invasive brain stimulation, obesity, transcranial direct current stimulation, transcranial magnetic stimulation
Introduction
Eating disorders and obesity are characterized by abnormal and detrimental eating habits, and often result in significant medical and psychiatric comorbidities. They are associated with reduced quality of life and life expectancy.1–3 In many ways, morbid obesity and anorexia nervosa (AN) represent extremes on two ends of the eating spectrum. Morbid obesity is defined as a body mass index (BMI) greater than 40 kg/m2 (or greater than 35 kg/m2 in the presence of a significant obesity-related comorbid condition), while AN is defined by an extremely low BMI (<18.5 kg/m2) and concomitant anxiety and preoccupations related to weight and body image. Both diseases exert a significant individual and societal impact, albeit in different ways. Morbid obesity is a chronic, progressive disease with a prevalence of approximately 14%, and associations with comorbidities including cardiovascular disease, type 2 diabetes mellitus, osteoarthritis and various cancers.4,5 By contrast, only 0.7–3% of the population (with a 10:1 predominance in the female population) suffers from AN, but this disease is also associated with critical metabolic, endocrine and electrolyte imbalances, psychiatric comorbidities and an even higher risk of mortality due to suicide or medical complications (5–15% mortality rate).6–9 Bulimia nervosa (BN), a similar but distinct eating disorder characterized by recurrent episodes of binge-eating and inappropriate compensatory behaviors, is estimated to have an overall prevalence of 0.3% and may afflict as many as 1% of young women.10,11 It too is often functionally debilitating and accompanied by psychiatric comorbidities and linked to increased mortality.12
There is a need for additional treatment modalities in both obesity and eating disorders. Current treatments for obesity vary in efficacy and invasiveness, ranging from conservative measures (diet, exercise, cognitive behavioral therapy)13,14 to medications (e.g. benzphetamine, orlistat, rimonabant)15 and bariatric surgery (Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, sleeve gastrectomy, vertical band gastroplasty).16 Of these options, bariatric surgery is the most effective treatment for rapid weight loss but is still associated with an approximately 10–27% failure rate.17,18 Cognitive behavioral therapy (CBT), selective serotonin reuptake inhibitors (SSRIs) and neuroleptics constitute the mainstay treatments for AN at present. The efficacy of these treatments, however, is fairly poor and up to 30% of AN patients prove to be medically intractable.19 The situation is similar for BN; patients are typically treated with CBT20 and/or antidepressant pharmacotherapy,21 but the majority of patients remain symptomatic following therapy.22
Deep brain stimulation (DBS) is an invasive but non-lesional neurosurgical procedure that delivers electrical pulses to targeted brain structures via electrodes connected to an implantable pulse generator (IPG). It is well established as a safe and efficacious treatment for movement disorders such as Parkinson’s disease, dystonia and tremor,23 presenting a more flexible approach compared to lesioning treatments owing to its reversibility and modifiability. More recently, DBS has shown promise as a potential treatment for several circuit-based neuropsychiatric conditions, including obsessive-compulsive disorder (OCD),24,25 major depression,26,27 Tourette’s syndrome,28,29 and Alzheimer’s disease.30,31 It has also been explored for use in eating disorders such as morbid obesity and AN.
By contrast, non-invasive brain stimulation (NIBS) involves transcranial stimulation of cortical neural targets in a non-surgical manner. Of the many different NIBS modalities that exist, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) are among the most popular and frequently studied. Each differs in terms of its mechanism of stimulation; rTMS passes brief current pulses through a coil over the scalp in order to generate an electromagnetic field that inhibits (low frequency, <5 Hz rTMS) or activates (high frequency, >5 Hz rTMS) target neurons,32 while tDCS delivers weak electrical current to brain regions through electrodes placed on the scalp in order to either depolarize (anodal tDCS) or hyperpolarize (cathodal tDCS) resident neurons.33 Both rTMS and tDCS have been explored with varying success for a multitude of indications, including OCD,34,35 depression,36,37 anxiety,38,39 chronic pain,40,41 stroke rehabilitation42,43 and addiction.44,45 They too have been applied to eating disorders and obesity.
Here, we review the theoretical rationale and current results of DBS and NIBS for eating disorders and obesity.
Neurobiology of eating behavior: homeostatic and reward pathways
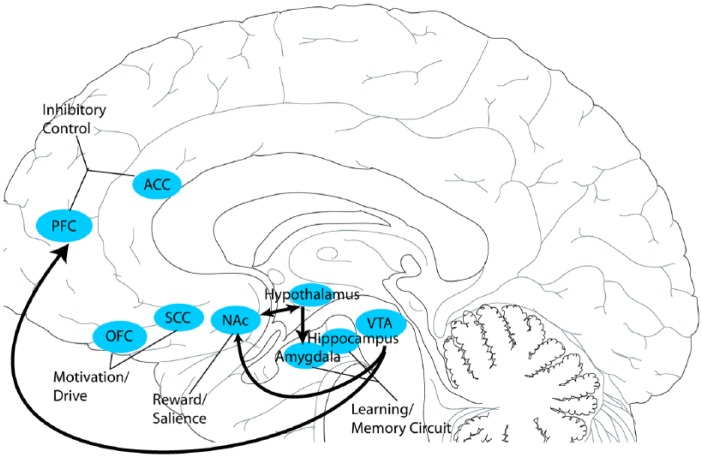
While the neural basis of eating behavior is not fully understood, it has been linked to an interaction between reward pathways (mesolimbic and mesocortical pathways; Figure 1) and homeostatic circuitry that regulates an individual’s perceived dietary energy needs. Certainly, the hypothalamus is well-recognized to play an integral role in maintaining homeostasis with regards to energy balance, constituting a key junction between the endocrine and nervous systems via its responsiveness to gut and adipocyte-derived hormones, integration of multimodal feeding-related sensory signals and close connections with brainstem regions (such as the nucleus of the solitary tract) involved in autonomic monitoring.46 The importance of hypothalamic control over eating behavior is further evinced by the consequences of lesioning this region, which variably include secondary anorexia47,48 and secondary obesity.49 Informed by rodent studies in the 1950s and 1960s, the hypothalamus was classically envisioned as regulating eating behavior through competing ‘feeding’ (lateral hypothalamus) and ‘satiety’ (ventromedial hypothalamus) centers that, when lesioned or stimulated, dramatically altered food intake patterns. Specifically, lateral hypothalamus lesions resulted in severe aphagia, food aversion and weight loss, while ventromedial lesions led to voracious, uncontrolled eating and weight gain.50,51–53
Figure 1.
Reward pathways and interactions. This schematic illustrates the hypothalamic–mesocorticolimbic pathways and potential anatomical targets involved with reward, cognitive control, motivation and the learning/memory circuits. Reward and saliency involve the ventral tegmental area, nucleus accumbens and caudate. The orbitofrontal cortex and subgenual cingulate cortex are thought to be involved in motivation and drive. The learning/memory circuit, which includes the amygdala, hippocampus and putamen, are also components in eating neurobiology. Inhibitory control has been known to involve the dorsolateral prefrontal cortex, ventromedial prefrontal cortex, and the anterior cingulate cortex. The arrows signify the direction of signal transmission.
ACC, anterior cingulate cortex; NAc, nucleus accumbens; OFC, orbitofrontal cortex; PFC, prefrontal cortex; SCC, subgenual cingulate cortex; VTA, ventral tegmental area.
While these early experiments have since been deemed somewhat imprecise, this ‘dual center model’ has nonetheless held up well as a basic schema of hypothalamic control over appetite.54 The lateral hypothalamus is the only region in the brain containing neurons that produce orexins, a peptide implicated in hunger and arousal. Indeed, dysregulation or loss of orexin signaling has been associated with obesity.55 In contrast, the ventromedial hypothalamus contains a high number of leptin receptors, which are involved in satiety. In mice, high levels of leptin are usually associated with reduced feeding, while animals with low leptin levels tend to eat uncontrollably.56
Reward circuitry – in particular the mesolimbic and mesocortical pathways – also play a key role in governing eating behavior. The mesolimbic reward pathway is a dopaminergic pathway that runs within the medial forebrain bundle, connecting the ventral tegmental area (VTA) to the nucleus accumbens (NAc)57 within the ventral striatum. It has long been associated with immediate rewards, and along with other cortical reward areas is consistently activated by palatable foods, drugs of abuse, copulation and other rewarding stimuli.58–61 Moreover, mesolimbic dopamine release in response to rewarding stimuli is known both to track stimulus salience and novelty and reflect the influence of expectation and reinforcement, underlining its role in motivation, want and addiction.62,63 Unlike the hypothalamus, the NAc is believed to instantiate the value of stimuli like food, regardless of appetite or satiety.63,64 More specifically, rodent studies indicate the existence of a rostral ‘hotspot’ in the NAc shell where GABAergic and glutamatergic activation produces appetitive behaviors, while the same neurotransmitters administered in a more caudal ‘coldspot’ result in fearful or defensive behaviors.65,66 By contrast, the mesocortical pathway, which comprises dopaminergic output from the VTA to the prefrontal cortex (PFC), is heavily implicated in instantiation of delayed reward, cognitive control, motivation and regulation of emotional responses.67 Moreover, human imaging studies suggest that the PFC and mesocortical pathway are specifically involved in regulating food intake.68
It is important to note the close structural and functional interconnectivity between homeostatic circuitry and reward pathways in the brain. The mesolimbic pathway, for instance, is closely connected to the hypothalamic areas via the infero-medial branch of the medial forebrain bundle.69 It is also understood that orexins originating in the lateral hypothalamus modulate VTA and mesolimbic pathway activity.70,71 The relevance of this orexin regulation to behavior is borne out by rodent studies that revealed orexin receptor blockade reduces both interest in and NAc dopamine release response to drugs of abuse.72–74
Anatomical targets for obesity
When deciding on a neuromodulation target for obesity, there are multiple aspects of overeating that can potentially be treated. Different parts of the brain regulate motivation, volitional control, addiction, memory and reward. Moreover, it is important to determine if the goal is to increase metabolism, reduce the pleasure/addiction to eating and/or influence an individual’s decision-making and volitional control.
Hypothalamus
On the basis of aforementioned lesion studies in rodents, felines, canines, porcines and non-human primates,50–52,75–77 the hypothalamus has long been an attractive DBS target for obesity. Specifically, high-frequency stimulation (180–200 Hz) of the lateral hypothalamus has been shown to reduce feeding and produce sustained weight loss in rodent and primate DBS models.78,79 It also appears to result in increased metabolism in the hippocampus, amygdala and mammillary body.80 In contrast, low-frequency (50 Hz) lateral hypothalamus stimulation increased feeding.81–83 The opposite pattern occurred when targeting the ventromedial hypothalamus; here, high-frequency stimulation caused weight gain in rodent and primate models,84,85 while low-frequency stimulation promoted weight loss.77 Interestingly, as orexin deficiency is associated with obesity, driving this pathway may help to prevent and treat obesity.55
Nucleus accumbens
Obesity is a multifactorial disease that involves altered patterns of eating and satiety as well as dysfunctional reward and compulsive traits.86 Several models emphasize the role of dopaminergic reward circuits, with each proposing a different account of the specifics of the abnormality and how it relates to overeating.87 The reward surfeit model posits that those at risk for obesity have a greater reward responsivity in the gustatory and somatosensory cortex.88 Indeed, there is evidence that an elevated responsiveness of reward-related regions may portend a poor outcome in weight-loss programs.89 The incentive sensitization model suggests that repeated food intake leads to an elevated responsivity to foods due to conditioning.90 The reward deficit model posits there is lower brain dopamine activity in obese subjects, predisposing them to excessive eating.91
Animal models have demonstrated that lesioning the NAc eliminates food-hoarding behavior and promotes weight loss.92 High-frequency stimulation (160 Hz, 60 μs pulse width) of the lateral NAc shell in obese rats was noted to increase D2 receptor gene expression and DA levels and result in weight loss; however, there was no change in normal-weight rats.93 Interestingly, stimulation (130 Hz, 60 µs pulse width) of the medial NAc shell increased feeding.94 Furthermore, animal studies have demonstrated that bilateral high-frequency NAc stimulation reduces binge-eating.95
Other targets for obesity
There are many other potential DBS targets for obesity beyond the lateral hypothalamus and NAc. One study comparing fMRI BOLD activity in response to pictures of food between obese participants and healthy controls observed significantly greater activation in the obese group, not only in NAc/ventral striatum, but also in medial and lateral orbitofrontal cortex, medial PFC, insula, anterior cingulate cortex, amygdala, ventral pallidum, caudate, putamen and hippocampus. In contrast, there was decreased hypothalamic and dorsal anterior cingulate cortex activation in the obese cohort.96 Other work has found evidence of decreased PFC and striatum metabolism with correlating alterations in dopamine in obese individuals.59
Clinical DBS trials for obesity
Hypothalamus
Informed by promising preclinical data, lesioning experiments were performed on the lateral hypothalamus in the 1970s with the intent to break the reward cycle for eating. These studies demonstrated initial weight loss in obese patients; after 1 year, however, treated patients regained the weight.97,98 Nonetheless, this work provided further support for trials exploring neuromodulation of the homeostatic system.
In 2008, bilateral ventromedial hypothalamus stimulation for obesity was performed (Table 1). In this case report of a 50-year-old man with morbid obesity, high-frequency (130 Hz) stimulation did not alter the patient’s overall weight. However, low-frequency (50 Hz) stimulation resulted in initial weight loss followed by weight gain. These results were confounded by the patient turning off the stimulation at night in order to eat during the latter part of the trial, suggesting DBS may have affected the patient’s appetite but not overall desire to eat.30 High-frequency stimulation of ventromedial hypothalamus has resulted in interesting side effects other than weight loss. In one case report, stimulation led to memory benefits,30 while a second case report indicated that stimulation induced panic attacks.99 The failure of ventromedial hypothalamic stimulation may be due to redundant mechanisms in the hypothalamic-mediated regulation of feeding. Alternatively, stimulation may shut down both orexigenic and anorexigenic mechanisms via current spread to the neighboring lateral hypothalamus.100 This preliminary result was followed by a small, three-patient case series of bilateral lateral hypothalamic DBS in failed gastric bypass patients. High-frequency monopolar (185 Hz, 90 μs pulse width) stimulation was used with no adverse effects at 35 months. Resting metabolic rate was also measured using indirect calorimetry in a respiratory chamber, and was found to be augmented by DBS. While no improvement in weight was observed when DBS parameters were programmed to standard (movement disorder-informed) settings, stimulation programmed to optimize resting metabolic rate was associated with weight loss in two of three patients. Resting metabolic rate-optimized stimulation was also linked to subjective reports of decreased urge to eat and increased energy levels.101
Table 1.
DBS for morbid obesity patients. This table illustrates six reports of DBS for obesity. Four female patients and two male adult patients underwent DBS targeting either the hypothalamus or the nucleus accumbens. Four out of the six patients exhibited a reduction in their BMI after DBS.
| Study | Target | Stimulation parameters | Number of patients | Age | Gender | Initial BMI | BMI after DBS | Follow up (months) | Comorbidities |
|---|---|---|---|---|---|---|---|---|---|
| Hamani and colleagues30 | Ventral hypothalamus | 130, 50 Hz, 60 μs pw, 2.8 V | 1 | 50 | Male | 55 | – | 5 | DM2, HTN, OSA |
| Whiting and colleagues101 | Lateral hypothalamus | 185 Hz, 90 μs pw | 3 | 45–60 | 2 female, 1 male | 45–49 | (1) 0.9% decrease, (2) 12.3% decrease, (3) 16.4% decrease | 30–39 | (1) HTN; (2) OSA/DM2, HTN, migraine; (3) lower extremity edema |
| Mantione and colleagues102 | NAc | 185 Hz, 90 μs pw, 3.5 V | 1 | 47 | Female | 37 | 25 | 24 | OCD |
| Harat and colleagues103 | NAc | 130 Hz, 208 μs pw, 2 mA | 1 | 19 | Female | 53 | 48 | 14 | Cranipharyngioma |
DBS, deep brain stimulation; DM 2, type 2 diabetes mellitus; HTN, hypertension; NAc, nucleus accumbens; OSA, obstructive sleep apnea; pw, pulse width.
Nucleus accumbens
In addition to targeting the homeostatic center, clinical studies have also utilized DBS to stimulate the mesolimbic pathway – specifically the NAc. In two separate case reports, bilateral NAc DBS resulted in weight loss and a reduction in BMI.102,103 In the first case, a woman suffered from obesity and obsessive compulsive disorder. Bilateral NAc stimulation (185 Hz, 90 μs pulse width) resulted in a significant reduction in BMI and weight loss at 2 years after DBS implantation. In the second case, a woman suffered from hypothalamic obesity secondary to a craniopharyngioma status post resection. Chronic bilateral NAc stimulation (130 Hz, 208 µs pulse width) led to a reduction in BMI 14 months after surgery.
Anatomical targets for anorexia nervosa
Insula
AN is an eating disorder characterized by low body weight (<18.5 kg/m2), fear of gaining weight and a distorted perception of body and self-image. It is often comorbid with affective disorders such as depression or bipolar disorder. Moreover, it is frequently associated with reward processing abnormalities and obsessive habits resembling those seen in OCD behavior.104–106 Prior imaging studies suggest AN involves dysfunction within a number of neural pathways, including circuitry pertaining to self-awareness (parietal cortex, insula), visual and gustatory sensation (occipital cortex and insula) and reward (ventral striatum, anterior and subcallosal cingulate, dorsolateral PFC and ventromedial PFC).59,107–109 The insula, which subserves interoception (an individual’s self-observation of the body’s internal homeostasis), is implicated in many of these circuits and may particularly relate to distorted perceptions in AN.
Ventral striatum/nucleus accumbens
One theory of AN pathogenesis suggests there exists an imbalance in serotonergic signaling within the ventral striatum, perhaps related to the aversion aspect of the disorder.110 Another theory is that there are perturbations in the reward pathways.111–113 Disrupting the dopamine system can affect the reward circuitry, leading to a dysphoric mood and anxiety.6,114 There is evidence of a dopamine imbalance in the ventral striatum.110 AN patients have been found to exhibit decreased ventral striatum activity as well as overactivity in the caudate.115 Furthermore, AN patients’ reward pathways are activated by disease-related stimuli, but are not necessarily activated with typical rewarding stimuli. For instance, there is overactivation of the ventral striatum in response to thin, underweight stimuli,116 unlike in morbid obesity where the ventral striatum is preferentially engaged by eating. Similarly, the ventromedial PFC is significantly more active in AN patients during exposure to high- and low-caloric food items.117
Other targets for anorexia
Evidence also exists for dysfunctional decision-making processes in AN patients. In fact, imaging work indicates there is reduced glucose perfusion in decision-making and motor action centers in patients with AN (superior frontal cortex, caudate, thalamus and putamen).118 Moreover, the anterior cingulate is implicated in AN by the finding that AN patients possess decreased cingulate volume compared to controls, with subsequent enlargement after recovery.119
Clinical DBS trials for anorexia nervosa
Ventral striatum/nucleus accumbens
Early case studies in the 1950s suggested that disrupting the reward pathways through limbic leucotomy,120,121 thalamotomy122 and capsulotomy123 could produce benefits in AN patients and prove useful as a last resort for intractable cases. However, the clinical outcomes have been inconclusive. Given evidence of reward pathway disruption in AN, the ventral striatum (and the NAc in particular)57 has been identified as a potential DBS target for this condition (Table 2). In one study, a cohort of AN patients with comorbid OCD, depression or anxiety underwent stereotactic radiofrequency ablation of the NAc (six patients) or bilateral NAc DBS (two patients; 135–185 Hz). In both treatment groups, BMI as well as comorbid psychiatric symptoms showed improvement after 1 year in conjunction with decreased psychoticism, neuroticism and lying tendencies, and improvements in memory/cognition at 6 months.124 Another trial examining bilateral NAc DBS (180 Hz) in four AN patients with comorbid OCD or generalized anxiety also demonstrated improvements in BMI.125 DBS targeting the bilateral ventral capsule/ventral striatum (120 Hz) in a patient with OCD, major depression and AN similarly produced gains in weight and BMI.119 Importantly, NAc DBS also appears to reverse a number of metabolic abnormalities seen in AN; according to one PET study, hypermetabolism in the frontal lobes, hippocampus and lentiform nucleus was decreased in six AN patients following NAc DBS.107
Table 2.
DBS for anorexia nervosa patients. This table illustrates 25 cases of DBS for anorexia nervosa. All 25 patients were adult female patients who underwent DBS targeting the NAc, SCC, VC/VS, or BNST. All of the studies, except for the VC/VS and BNST (no follow up for the VC/VS study, decrease in weight with the BNST study) studies, demonstrated an increase in BMI with DBS.
| Study | Target | Stimulation parameters | Number of patients | Age | Gender | Initial BMI | BMI after DBS | Follow up (months) | Comorbidities |
|---|---|---|---|---|---|---|---|---|---|
| Wang and colleagues124 | NAc | 135–185 Hz, 120–210 µs pw, 2.5–3.8 V | 2 | 18–28 | Female | 12.9–13.3 | 18–21 | 12 | (1) OCD, depression, anxiety; (2) depression, anxiety |
| Wu and colleagues125 | NAc | 180 Hz, 90 µs pw | 4 | 16–17 | Female | 10–13.3 | 17–22 | 9–50 | (1) OCD; (2) OCD; (3) OCD; (4) Anxiety disorder |
| Israel and colleagues126 | SCC | Right-sided: 130 Hz, 91 µs pw, 5 mA | 1 | 52 | Female | 14.4 | 19 | 30 | Depression |
| Lipsman and colleagues,127 Lipsman et al.128 | SCC | 130 Hz, 90 µs pw, 5–6 V | 16 | 34 | Female | 13.8 ± 1.5 | 17.3 ± 3.4 | 12 | |
| McLaughlin and colleagues 119 | VC/VS | 120 Hz, 120 µs pw, 7.5 V | 1 | 52 | Female | 18.5 | OCD, depression | ||
| Blomstedt and colleagues129 | BNST | 130 Hz, 120 µs pw, 4.3 V | 1 | 60 | Female | 15.2 | 14.3 | 12 |
BNST: bed nucleus of stria terminalis; NAc: nucleus accumbens; OCD: obsessive-compulsive disorder; pw: pulse width; SCC, subgenual cingulate cortex; VC/VS: ventral capsule/ventral striatum.
Subgenual cingulate cortex
Another potential target for AN DBS is the subgenual cingulate cortex (SCC), a region with cortical projections to the medial and orbitofrontal cortex and subcortical projections to NAc, which is known to play an important role in mood regulation.129 The SCC exhibits increased activity and decreased 5-HT2A binding in AN.130 It also exhibits altered connectivity, with predominance in left-sided abnormalities. More specifically, tractography work has identified increased left parieto-occipital, dorsolateral prefrontal and left cerebellum connectivity, as well as decreased lower thalamic, mid- and anterior cingulate, and left anterior temporal cortices connectivity within the SCC in AN patients. The most conspicuous differences were increased connectivity between the SCC and the ipsilateral parietal cortex and decreased connectivity between the SCC and bilateral thalami.129 These findings are consistent with dysfunctional affective circuitry in patients with AN. Greater pre-operative deficits in connectivity within the left fornix, inferior frontal occipital cortex and right anterior limb of the internal capsule have been associated with better post-operative DBS affective clinical outcomes.
In 2010, Israel and colleagues described a patient with depression and AN who underwent SCC stimulation that resulted in improvement in mood. Here, they implanted bilateral SCC electrodes; however, they only stimulated the right side chronically (130 Hz, 91 µs pulse width). This stimulation resulted in a sustained elevation in her BMI and improvement in depression.126 In 2013, Lipsman and colleagues completed a phase I clinical trial stimulating bilateral SCC (130 Hz, 90 µs pulse width) in six patients, finding improvement in mood, anxiety, anorexia-related obsessions and compulsions in two-thirds of the patients. More recently, Lipsman and colleagues published their open-label 1-year follow up on 16 patients (including the initial six patients) with SCC stimulation for AN. In this cohort, 14 of the 16 patients had mood disorders, anxiety disorders or both. Here, they reported sustained improvements in BMI and affective symptoms in some of the patients without significant side effects. Significant changes in cerebral glucose metabolism were also noted in AN-related regions (decreased metabolism: SCC, anterior cingulate; increased metabolism: parietal lobe).127,128 The most common adverse event was pain related to the incision site. Two of the 16 patients had their DBS system removed or deactivated due to poorly defined reasons.
Bed nucleus of stria terminalis
Yet another stimulation target currently under investigation for AN is the stria terminalis, a key output channel for the amygdala connecting to the hypothalamus. The bed nucleus of the stria terminalis (BNST) in particular is implicated in threat monitoring,132 and has been proposed to play a part in an anxiety-regulating network comprising the amygdala, hypothalamus, thalamus and orbitofrontal cortex133 that is closely modulated by serotonergic activity.134 A recent case report found that bilateral BNST stimulation (130 Hz, 120 µs pulse width) in a patient with major depressive disorder and AN resulted in improvement in depression and anxiety (including anxiety about food and eating) after 1 year, but without any concomitant BMI changes.129
Non-invasive brain stimulation for eating disorders
NIBS modalities such as rTMS and tDCS have also been explored for the treatment of obesity and eating disorders. Building on promising work in psychiatric indications such as depression and addiction,36–39,44,45,135 NIBS methods have typically targeted the PFC in the context of disorders of eating behavior. As a key node of the brain’s frontostriatal cognitive circuits, the PFC – particularly the dorsolateral PFC (dlPFC) – is known to underpin executive functions such as inhibitory cognitive control.136,137 Furthermore, its activity has been closely linked to self-control in a dietary context.68 The PFC is also implicated in higher-order reward processing as part of its involvement in the mesocortical dopaminergic pathway.138 Indeed, dopaminergic signaling within the PFC has been linked to regulation of food intake.139
NIBS for obesity
In line with this schema, studies examining the effect of single-session dlPFC NIBS in healthy participants who endorsed food cravings consistently found stimulation reduced cravings in the immediate aftermath of stimulation.140–144 However, a recent meta-analysis established that these single-session effects did not translate into a significant effect on food consumption.145 A total of eight trials have thus far explored the effect of dlPFC NIBS in overweight or obese participants (Table 3). An initial randomized, blinded trial explored the impact of single-session bilateral dlPFC (2 mA, 20 min, anodal right dlPFC stimulation and cathodal left dlPFC stimulation) tDCS in 19 participants, finding stimulation decreased food cravings but not food consumption during subsequent same-day testing.142 However, only 11 of 19 participants were overweight or obese, and no outcomes were reported for the overweight/obese cohort specifically. As such, the validity of these findings with respect to obese individuals is uncertain. In an ensuing randomized controlled trial of nine overweight participants, Montenegro and colleagues found that a single session of left dlPFC anodal tDCS (2 mA, 20 min) reduced appetite and desire to eat during immediate post-treatment testing.146 Subsequently, a double-blind, randomized, placebo-controlled crossover experiment found that three sessions of anodal tDCS (2 mA, 40 min) targeting left dlPFC led to reduced caloric consumption (as well as greater weight loss over the three-day study period) in nine overweight/obese participants following completion of stimulation. However, this finding only proved statistically significant when anodal stimulation was compared with inhibitory cathodal stimulation (not when anodal stimulation was compared to sham stimulation), limiting the interpretability of these results.147 Bravo and colleagues further probed the applicability of NIBS for obesity by examining the effect of five sessions of right dlPFC tDCS (2 mA, 30 min) in a double-blind, sham-controlled manner within two cohorts of interest, one composed of 11 obese participants and the other comprising 10 Prader-Willi syndrome (PWS) patients. In both groups, active stimulation was associated with decreased food cravings (as well as reduced compulsive eating behaviors in the PWS cohort) but no weight loss at 30 days post-treatment.148 Meanwhile, Burgess and colleagues conducted a blinded, sham-controlled trial of single-session dlPFC tDCS (2 mA, 20 min) in 30 overweight patients with binge-eating disorder using a right dlPFC anode/left dlPFC cathode montage.149 During immediate post-treatment testing, active stimulation decreased both food cravings and food intake. Furthermore, it was reported to reduce binge-eating desire but not binge-eating frequency in male patients only. Another blinded, sham-controlled study demonstrated diminished cravings in 13 overweight/obese individuals that was maintained 30 days after five sessions of anodal right dlPFC tDCS (2 mA, 40 min), constituting the first description of sustained NIBS outcomes in this population.150 It should be noted that the legitimacy of the blinding protocol has been questioned, however.151 More recently, Ray and colleagues reported a double-blind sham-controlled trial of single-session bilateral dlPFC tDCS (2 mA, 20 min) in 18 obese participants employing the same right dlPFC anode/left dlPFC cathode montage described by Burgess and colleagues. Here, active stimulation failed to produce significant effects in the cohort overall but did reduce immediate post-treatment food cravings and food consumption in certain participant subgroups when trait scores (e.g. impulsiveness, intent to restrict calories) were controlled.152
Table 3.
NIBS (rTMS and tDCS) for obesity patients. This table illustrates 31 cases of transcranial direct current stimulation and 13 cases of repetitive transcranial magnetic stimulation for obesity.
| Study | Modality | Target | Stimulation parameters | Stimulation timing | Number of patients | Age | Outcome | Follow up (effect duration) |
|---|---|---|---|---|---|---|---|---|
| Goldman and colleagues142 | tDCS | Bl dlPFC | 2 mA, anodal (R dlPFC), cathodal (L dlPFC) | Single 20-min session | 19* | 32.5 ± 11 | Decreased food cravings but not food intake | None (unknown effect duration) |
| Montenegro and colleagues146 | tDCS | L dlPFC | 2 mA, anodal | Single 20-min session | 9 | 20–32 | Decreased appetite/desire to eat | None (unknown effect duration) |
| Gluck and colleagues147 | tDCS | L dlPFC | 2 mA, anodal | 3 40-min sessions over 3 days | 9 | 42 ± 8 | Decreased caloric consumption and greater weight loss compared to cathodal tDCS | None (unknown effect duration) |
| Bravo and colleagues148 | tDCS | R dlPFC | 2 mA, anodal | 5 30-min sessions over 5 days | 11 | 47 ± 11 | Decreased food cravings but no weight loss | 30 days (effect observed at last follow up) |
| Luzi and colleagues153 | rTMS | Bl dlPFC | 18 Hz | 3 sessions per week for 5 weeks | 11 | – | Reduction in food cravings and weight loss | 9 weeks (effect observed at last follow up) |
| Burgess and colleagues149 | tDCS | Bl dlPFC | 2 mA, anodal (R dlPFC), cathodal (L dlPFC) | Single 20-min session | 30** | – | Decreased food cravings and food intake; decreased binge-eating desire in male participants only | None (unknown effect duration) |
| Ljubisavljevic and colleagues150 | tDCS | R dlPFC | 2 mA, anodal | 5 40-min sessions over 5 days | 13*** | 21 ± 2 | Decreased food cravings | 30 days (effect observed at last follow up) |
| Ray and colleagues152 | tDCS | Bl dlPFC | 2 mA, anodal (R dlPFC), cathodal (L dlPFC) | Single 20-min session | 18 | 23 ± 8 | No decrease in food cravings or intake overall; decreased cravings/consumption when trait scores were controlled | None (unknown effect duration) |
Bl, bilateral; dlPFC, dorsolateral prefrontal cortex; L, left; rTMS, repetitive transcranial magnetic stimulation; R, right; tDCS, transcranial direct current stimulation.
Only 11 of 19 participants were overweight or obese according to their BMI; **Burgess and colleagues’ participants were both overweight (BMI > 24.5 kg/m2) and met criteria for binge-eating disorder; ***Ljubisavljevic and colleagues also reported on 17 additional healthy participants with normal BMI.
Thus far, only a single study has looked at the effect of rTMS in obese participants. Luzi and colleagues found that 5 weeks of high-frequency (18 Hz) rTMS targeting bilateral dlPFC at a frequency of 3 sessions per week led to significant reductions in food cravings as well as weight loss in a cohort of 11 obese participants. Notably, this effect was maintained after 9 weeks and proved statistically significant in comparison to both inhibitory low-frequency rTMS and sham rTMS.153
NIBS for anorexia nervosa and bulimia nervosa
Thus far, five studies have investigated NIBS for AN (Table 4). Kamolz and colleagues first described a case report of left dlPFC rTMS in a patient with AN and comorbid depression (10 Hz, 2000 pulses, 41 sessions). According to this report, the patient’s depressive symptomology was significantly improved and her BMI increased from 12.4 to 16 after 3 months (29% increase).154 Two subsequent studies also yielded encouraging results with regard to AN and affective symptoms. Specifically, Van den Eynde and colleagues observed reduced perception of feeling fat, full and anxious in 10 AN patients immediately following left dlPFC rTMS (10 Hz, 1000 pulses, one session).155 Meanwhile, Khedr and colleagues reported a trial of tDCS for AN, finding that 10 sessions of left dlPFC anodal stimulation (2 mA, 25 min) improved AN and depressive symptoms in five of seven patients immediately post-treatment and in three of seven patients at 1 month follow up.156 More recently, McClelland and colleagues conducted a double-blind, sham-controlled trial examining the effects of a single session of left dlPFC rTMS (10 Hz, 1000 pulses) in 49 AN patients. This study demonstrated improved core AN symptoms and temporal discounting 24 h after treatment, lending support to earlier uncontrolled results.157 A five-patient case series by the same group investigated the effect of high-frequency left dlPFC rTMS (10 Hz, 1000 pulses, 20 sessions) on both eating disorder and affective symptoms over 12 months, finding eating disorder symptoms to be improved in three of five patients and affective symptoms to be improved in two of five. Despite this, all five patients lost weight over the follow-up period, indicating that NIBS’s impact on self-reported symptoms may not always translate to weight gain.158
Table 4.
NIBS (rTMS and tDCS) for AN patients. This table illustrates seven cases of tDCS and 65 cases of rTMS DBS for AN.
| Study | Modality | Indication | Target | Stimulation parameters | Stimulation timing | Number of patients | Age | Outcome | Follow up (effect duration) | Comorbidities |
|---|---|---|---|---|---|---|---|---|---|---|
| Kamolz and colleagues154 | rTMS | AN | L dlPFC | 10 Hz, 2000 pulses | 41 sessions | 1 | 24 | BMI increase from 12.4 to 16 (29%) | 3 months (effect observed at last follow up) |
Depression |
| Van den Eynde and colleagues155 | rTMS | AN | L dlPFC | 10 Hz, 1000 pulses | Single session | 10 | 18–44 | Reduced perception of feeling fat, full, anxious | None (unknown effect duration) | Not specified |
| Khedr and colleagues156 | tDCS | AN | L dlPFC | 2 mA, anodal | 10 25-min sessions over 10 days | 7 | 16–39 | Improved AN and depression symptoms in 5 patients immediately post-treatment, in 3 patients at 1 month post-treatment | 1 month (effect observed at last follow up) | Depression, anxiety |
| McClelland and colleagues158 | rTMS | AN | L dlPFC | 10 Hz, 1000 pulses | 20 20-min sessions | 5 | 23–52 | Improved eating disorder, affective symptoms; weight loss in all participants | 12 months (effect observed at last follow up) | Not specified |
| McClelland and colleagues157 | rTMS | AN | L dlPFC | 10 Hz, 1000 pulses | Single 20-min session | 49 | ~26±7.5 | Improved AN core symptoms and temporal discounting | 24 h (effect observed at last follow up) | Depression, anxiety |
AN, anorexia nervosa; Bl, bilateral; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; L, left; rTMS, repetitive transcranial magnetic stimulation; R, right; tDCS, transcranial direct current stimulation.
NIBS has also been studied in BN. Thus far, two case reports, one case series and three randomized control trials have studied the effects of PFC rTMS for this indication (Table 5).155,159–164 In both case studies, patients with comorbid BN and depression were treated with multiple sessions of rTMS and subsequently demonstrated improvement in depressive symptoms along with complete recovery from binging and purging symptoms in the short term. These results were observed immediately following 10 days of left dlPFC rTMS (20 Hz),159 and up to 2 months after 20 days of bilateral dorsomedial PFC rTMS (10 Hz, 3000 pulses).164 Another case series compared the effect of single-session left dlPFC rTMS (10 Hz, 1000 pulses) on 14 right-handed patients and 7 left-handed patients, finding that the two groups exhibited decreased cravings immediately post-treatment but had opposing mood changes (improved mood in right-handed patients, worsened mood in left-handed patients).162 However, results from randomized sham-controlled trials have been mixed. Van den Eynde and colleagues reported favorable results in their 38-patient trial of single-session left dlPFC rTMS (10 Hz, 1000 pulses), observing a significantly decreased urge to eat and fewer binge-eating episodes at 24 h post-treatment following stimulation compared to sham stimulation.163 By contrast, an earlier study subjected a 14-patient cohort to 15 days of left dlPFC rTMS (20 Hz, 2000 pulses), but did not find any significant difference in binge symptoms between active and sham rTMS.161 A similarly null result was obtained in a more recent 47-patient trial by Gay and colleagues, who found no difference in the rates of binging or purging symptoms over 15 days following a 10-day course of left dlPFC rTMS (10 Hz, 1000 pulses, 20 min).160 The impact of tDCS on BN has been examined only once, in a randomized double-blind, sham-controlled trial in 39 patients. Interestingly, Kekic and colleagues found that both right and left dlPFC anodal tDCS (2 mA, 20 min) were associated with a decreased urge to binge-eat and improved temporal discounting 24 h post-treatment, but only right dlPFC was followed by improved mood.165
Table 5.
NIBS (rTMS and tDCS) for BN patients. This table illustrates 39 cases of tDCS and 122 cases of rTMS DBS for BN.
| Study | Modality | Indication | Target | Stimulation parameters | Stimulation timing | Number of patients | Age | Outcome | Follow up (effect duration) | Comorbidities |
|---|---|---|---|---|---|---|---|---|---|---|
| Hausmann and colleagues159 | rTMS | BN | L dlPFC | 20 Hz | 10 sessions over 10 days | 1 | 28 | Complete recovery from binging/purging episodes; ~50% depression improvement | None (unknown effect duration) | Depresion |
| Walpoth and colleagues161 | rTMS | BN | L dlPFC | 20 Hz, 2000 pulses | 15 sessions over 15 days | 14 | ~25 ± 7 | No significant improvement in binge symptoms between sham and real rTMS | None (unknown effect duration) | Depression |
| Van den Eynde and colleagues163 | rTMS | BN | L dlPFC | 10 Hz, 1000 pulses | Single 20-min session | 38 | ~30.5 ± 11 | Decreased urge to eat, fewer binge-eating episodes | 24 h (effect observed at last follow up) | – |
| Van den Eynde and colleagues162 | rTMS | BN | L dlPFC | 10 Hz, 1000 pulses | Single 20-min session | 21 (7 left-handed) | ~25 ± 13 | Decreased craving; worsened mood in left-handed patients but improved mood in right-handed patients | None (unknown effect duration) | – |
| Downar and colleagues164 | rTMS | BN | Bl dmPFC | 10 Hz, 3000 pulses (per hemisphere) | 20 sessions over 20 days | 1 | 43 | Full remission from binging/purging episodes, depression | 2 months (effect observed at last follow up) | Depression |
| Gay and colleagues160 | rTMS | BN | L dlPFC | 10 Hz, 1000 pulses | 10 20-min sessions over 10 days | 47 | 19–40 | No significant improvement in binging/purging symptoms between sham and real rTMS | 15 days (effect observed at last follow up) | Depression, anxiety, alcohol/substance dependence |
| Kekic and colleagues165 | tDCS | BN | L and R dlPFC (separate sessions) |
2 mA, anodal | 20 min sessions | 39 | 18–48 | Decreased urge to binge-eat, improved temporal discounting with both L and R dlPFC anodal tDCS; improved mood only following R dlPFC anodal tDCS | 24 h (effect observed at last follow up) | Depression, anxiety |
Bl, bilateral; BN, bulimia nervosa; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; L, left; mA, milliamps; rTMS, repetitive transcranial magnetic stimulation; R, right; tDCS, transcranial direct current stimulation.
Conclusion
Obesity and eating disorders remain a significant burden on individuals and society. They are complex conditions that are difficult to treat given the underlying interplay between body homeostasis, reward pathways and affective/limbic circuitry. Despite aggressive medical and surgical management, there remain many refractory patients.
DBS offers a unique, long-lasting, modifiable and targeted treatment for obesity and eating disorders. Its applicability to these conditions is supported by both preclinical and clinical work. However, a number of further studies – including randomized controlled trials – are necessary to better determine DBS’s effectiveness in this field. Furthermore, the exact stimulation parameters, anatomical targets, patient selection, timing and indications for DBS procedures need to be refined. While no significant side effects from any of the DBS targets has been noted to date, future trials should continue to include screening for depression, anxiety and changes in cognition in these weight- and affective-related disorders. In addition, it is worth noting that AN and morbidly obese patients are at increased risk of DBS complications such as infection and hardware erosion due to their poor nutritional status,166 necessitating special surgical care in treating them.
NIBS has also shown promise for the treatment of obesity, anorexia and bulimia. A small number of randomized, sham-controlled trials have already explored the impact of NIBS for these indications, but further work is necessary to clarify equivocal results, delineate the long-term effects of treatment and better capture weight change-related outcomes in addition to cognitive and affective symptoms. NIBS represents a potentially important treatment option for obesity and eating disorders, given its excellent safety–feasibility profile and lack of serious side effects. However, key limitations of NIBS should be noted, including the need for continued recurrent dosing in order to avoid the wearing off of beneficial effects, the variability of targeting methods and uncertainty over the ideal stimulation parameters, and the variability of effects of different NIBS modalities in terms of their effect on target tissue.151 Additional studies must address these shortcomings if NIBS is to become a reliable and effective tool for obesity and eating disorders. Taken together, these findings suggest a potential role for both DBS and NIBS in the treatment of morbid obesity and eating disorders.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Darrin J. Lee, Division of Neurosurgery, Toronto Western Hospital, Department of Surgery, University of Toronto, Toronto, ON, Canada
Gavin J.B. Elias, Division of Neurosurgery, Toronto Western Hospital, Department of Surgery, University of Toronto, Toronto, ON, Canada
Andres M. Lozano, Division of Neurosurgery, Toronto Western Hospital, Department of Surgery, University of Toronto, 399 Bathurst St., West Wing 4-431, Toronto, ON M5T 2S8, Canada.
References
- 1. de la Rie SM, Noordenbos G, van Furth EF. Quality of life and eating disorders. Qual Life Res 2005; 14: 1511–1522. [DOI] [PubMed] [Google Scholar]
- 2. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry 2006; 19: 389–394. [DOI] [PubMed] [Google Scholar]
- 4. Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012; 307: 491–497. [DOI] [PubMed] [Google Scholar]
- 5. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA 1999; 282: 1523–1529. [DOI] [PubMed] [Google Scholar]
- 6. Kaye WH, Wagner A, Fudge JL, et al. Neurocircuity of eating disorders. Curr Top Behav Neurosci 2011; 6: 37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polivy J, Herman CP. Causes of eating disorders. Annu Rev Psychol 2002; 53: 187–213. [DOI] [PubMed] [Google Scholar]
- 8. Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003; 34: 383–396. [DOI] [PubMed] [Google Scholar]
- 9. Zipfel S, Lowe B, Reas DL, et al. Long-term prognosis in anorexia nervosa: lessons from a 21-year follow-up study. Lancet 2000; 355: 721–722. [DOI] [PubMed] [Google Scholar]
- 10. Fairburn CG, Beglin SJ. Studies of the epidemiology of bulimia nervosa. Am J Psychiatry 1990; 147: 401–408. [DOI] [PubMed] [Google Scholar]
- 11. Hudson JI, Hiripi E, Pope HG, Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 2007; 61: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry 2011; 68: 724–731. [DOI] [PubMed] [Google Scholar]
- 13. Collazo-Clavell ML. Safe and effective management of the obese patient. Mayo Clin Proc 1999; 74: 1255–1259; quiz 1259–1260. [DOI] [PubMed] [Google Scholar]
- 14. Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE – a randomized controlled study. Arch Intern Med 2004; 164: 31–39. [DOI] [PubMed] [Google Scholar]
- 15. Kang JG, Park CY. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J 2012; 36: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brethauer SA, Chand B, Schauer PR. Risks and benefits of bariatric surgery: current evidence. Cleve Clin J Med 2006; 73: 993–1007. [DOI] [PubMed] [Google Scholar]
- 17. Axer S, Szabo E, Naslund I. Weight loss and alterations in co-morbidities after revisional gastric bypass: a case-matched study from the Scandinavian Obesity Surgery Registry. Surg Obes Relat Dis 2017; 13: 796–800. [DOI] [PubMed] [Google Scholar]
- 18. Wijngaarden LH, Jonker FHW, van den Berg JW, et al. Impact of initial response of laparoscopic adjustable gastric banding on outcomes of revisional laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Obes Relat Dis 2017; 13: 594–599. [DOI] [PubMed] [Google Scholar]
- 19. Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry 2002; 159: 1284–1293. [DOI] [PubMed] [Google Scholar]
- 20. National Collaborating Centre for Mental Health. Eating disorders: core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. Leicester: British Psychological Society, 2004. [PubMed] [Google Scholar]
- 21. McElroy SL, Guerdjikova AI, Mori N, et al. Current pharmacotherapy options for bulimia nervosa and binge eating disorder. Expert Opin Pharmacother 2012; 13: 2015–2026. [DOI] [PubMed] [Google Scholar]
- 22. Poulsen S, Lunn S, Daniel SI, et al. A randomized controlled trial of psychoanalytic psychotherapy or cognitive-behavioral therapy for bulimia nervosa. Am J Psychiatry 2014; 171: 109–116. [DOI] [PubMed] [Google Scholar]
- 23. Anderson TR, Hu B, Iremonger K, et al. Selective attenuation of afferent synaptic transmission as a mechanism of thalamic deep brain stimulation-induced tremor arrest. J Neurosci 2006; 26: 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology 2006; 31: 2384–2393. [DOI] [PubMed] [Google Scholar]
- 25. Roh D, Chang WS, Chang JW, et al. Long-term follow-up of deep brain stimulation for refractory obsessive-compulsive disorder. Psychiatry Res 2012; 200: 1067–1070. [DOI] [PubMed] [Google Scholar]
- 26. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry 2011; 168: 502–510. [DOI] [PubMed] [Google Scholar]
- 27. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005; 45: 651–660. [DOI] [PubMed] [Google Scholar]
- 28. Ackermans L, Duits A, van der Linden C, et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain 2011; 134: 832–844. [DOI] [PubMed] [Google Scholar]
- 29. Vandewalle V, van der Linden C, Groenewegen HJ, et al. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet 1999; 353: 724. [DOI] [PubMed] [Google Scholar]
- 30. Hamani C, McAndrews MP, Cohn M, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol 2008; 63: 119–123. [DOI] [PubMed] [Google Scholar]
- 31. Laxton AW, Tang-Wai DF, McAndrews MP, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol 2010; 68: 521–534. [DOI] [PubMed] [Google Scholar]
- 32. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985; 1: 1106–1107. [DOI] [PubMed] [Google Scholar]
- 33. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul 2008; 1: 206–223. [DOI] [PubMed] [Google Scholar]
- 34. Palm U, Leitner B, Kirsch B, et al. Prefrontal tDCS and sertraline in obsessive compulsive disorder: a case report and review of the literature. Neurocase 2017; 23: 173–177. [DOI] [PubMed] [Google Scholar]
- 35. Lee YJ, Koo BH, Seo WS, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in treatment-resistant obsessive-compulsive disorder: an open-label pilot study. J Clin Neurosci 2017; 44: 264–268. [DOI] [PubMed] [Google Scholar]
- 36. Brunoni AR, Moffa AH, Sampaio-Junior B, et al. Trial of electrical direct-current therapy versus escitalopram for depression. N Engl J Med 2017; 376: 2523–2533. [DOI] [PubMed] [Google Scholar]
- 37. Wang YM, Li N, Yang LL, et al. Randomized controlled trial of repetitive transcranial magnetic stimulation combined with paroxetine for the treatment of patients with first-episode major depressive disorder. Psychiatry Res 2017; 254: 18–23. [DOI] [PubMed] [Google Scholar]
- 38. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry 2017; 78: 61–65. [DOI] [PubMed] [Google Scholar]
- 39. Heeren A, Billieux J, Philippot P, et al. Impact of transcranial direct current stimulation on attentional bias for threat: a proof-of-concept study among individuals with social anxiety disorder. Soc Cogn Affect Neurosci 2017; 12: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saitoh Y. Repetitive transcranial magnetic stimulation for intractable pain. Brain Nerve 2017; 69: 207–218. [DOI] [PubMed] [Google Scholar]
- 41. Khedr EM, Omran EAH, Ismail NM, et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: a double blinded, randomized clinical trial. Brain Stimul 2017; 10: 893–901. [DOI] [PubMed] [Google Scholar]
- 42. Hao Z, Wang D, Zeng Y, et al. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev 2013; 5: CD008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 2006; 5: 708–712. [DOI] [PubMed] [Google Scholar]
- 44. Boggio PS, Sultani N, Fecteau S, et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 2008; 92: 55–60. [DOI] [PubMed] [Google Scholar]
- 45. Camprodon JA, Martinez-Raga J, Alonso-Alonso M, et al. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend 2007; 86: 91–94. [DOI] [PubMed] [Google Scholar]
- 46. Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am 2008; 37: 811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Climo LH. Anorexia nervosa associated with hypothalamic tumor: the search for clinical-pathological correlations. Psychiatr J Univ Ott 1982; 7: 20–25. [PubMed] [Google Scholar]
- 48. McClean P, Redmond AO. Hypothalamic tumour presenting as anorexia nervosa. Ulster Med J 1988; 57: 224–227. [PMC free article] [PubMed] [Google Scholar]
- 49. Bauer HG. Endocrine and other clinical manifestations of hypothalamic disease; a survey of 60 cases, with autopsies. J Clin Endocrinol Metab 1954; 14: 13–31. [DOI] [PubMed] [Google Scholar]
- 50. Anand BK, Brobeck JR. Localization of a ‘feeding center’ in the hypothalamus of the rat. Proc Soc Exp Biol Med 1951; 77: 323–324. [DOI] [PubMed] [Google Scholar]
- 51. Stenger J, Fournier T, Bielajew C. The effects of chronic ventromedial hypothalamic stimulation on weight gain in rats. Physiol Behav 1991; 50: 1209–1213. [DOI] [PubMed] [Google Scholar]
- 52. Brown FD, Fessler RG, Rachlin JR, et al. Changes in food intake with electrical stimulation of the ventromedial hypothalamus in dogs. J Neurosurg 1984; 60: 1253–1257. [DOI] [PubMed] [Google Scholar]
- 53. Hervey GR. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol 1959; 145: 336–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 1999; 22: 221–232. [DOI] [PubMed] [Google Scholar]
- 55. Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 1998; 18: 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cohen P, Zhao C, Cai X, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 2001; 108: 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bonacasa B, Sanchez ML, Rodriguez F, et al. 2-methoxyestradiol attenuates hypertension and coronary vascular remodeling in spontaneously hypertensive rats. Maturitas 2008; 61: 310–316. [DOI] [PubMed] [Google Scholar]
- 58. Iozzo P. Metabolic imaging in obesity: underlying mechanisms and consequences in the whole body. Ann N Y Acad Sci 2015; 1353: 21–40. [DOI] [PubMed] [Google Scholar]
- 59. Val-Laillet D, Aarts E, Weber B, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin 2015; 8: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav 1988; 44: 599–606. [DOI] [PubMed] [Google Scholar]
- 61. Radhakishun FS, Westerink BH, van Ree JM. Dopamine release in the nucleus accumbens of freely moving rats determined by on-line dialysis: effects of apomorphine and the neuroleptic-like peptide desenkephalin-gamma-endorphin. Neurosci Lett 1988; 89: 328–334. [DOI] [PubMed] [Google Scholar]
- 62. Freeman AS, Bunney BS. Chronic neuroleptic effects on dopamine neuron activity: a model for predicting therapeutic efficacy and side effects? Psychopharmacol Ser 1987; 3: 225–235. [DOI] [PubMed] [Google Scholar]
- 63. Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol 1989; 40: 191–225. [DOI] [PubMed] [Google Scholar]
- 64. Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron 2015; 86: 646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci 2001; 21: 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste ‘liking’/‘disliking’ reactions, place preference/avoidance, and fear. J Neurosci 2002; 22: 7308–7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McClure SM, Laibson DI, Loewenstein G, et al. Separate neural systems value immediate and delayed monetary rewards. Science 2004; 306: 503–507. [DOI] [PubMed] [Google Scholar]
- 68. Lowe CJ, Hall PA, Staines WR. The effects of continuous theta burst stimulation to the left dorsolateral prefrontal cortex on executive function, food cravings, and snack food consumption. Psychosom Med 2014; 76: 503–511. [DOI] [PubMed] [Google Scholar]
- 69. Coenen VA, Panksepp J, Hurwitz TA, et al. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci 2012; 24: 223–236. [DOI] [PubMed] [Google Scholar]
- 70. Hrabovszky E, Molnar CS, Borsay BA, et al. Orexinergic input to dopaminergic neurons of the human ventral tegmental area. PLoS One 2013; 8: e83029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Valdivia S, Patrone A, Reynaldo M, et al. Acute high fat diet consumption activates the mesolimbic circuit and requires orexin signaling in a mouse model. PLoS One 2014; 9: e87478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lawrence AJ, Cowen MS, Yang HJ, et al. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 2006; 148: 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lei K, Wegner SA, Yu JH, et al. Orexin-1 receptor blockade suppresses compulsive-like alcohol drinking in mice. Neuropharmacology 2016; 110: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Prince CD, Rau AR, Yorgason JT, et al. Hypocretin/orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci 2015; 6: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rolls ET, Sanghera MK, Roper-Hall A. The latency of activation of neurones in the lateral hypothalamus and substantia innominata during feeding in the monkey. Brain Res 1979; 164: 121–135. [DOI] [PubMed] [Google Scholar]
- 76. Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med 1951; 24: 123–140. [PMC free article] [PubMed] [Google Scholar]
- 77. Melega WP, Lacan G, Gorgulho AA, et al. Hypothalamic deep brain stimulation reduces weight gain in an obesity-animal model. PLoS One 2012; 7: e30672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Takaki A, Aou S, Oomura Y, et al. Feeding suppression elicited by electrical and chemical stimulations of monkey hypothalamus. Am J Physiol 1992; 262: R586–R594. [DOI] [PubMed] [Google Scholar]
- 79. Sani S, Jobe K, Smith A, et al. Deep brain stimulation for treatment of obesity in rats. J Neurosurg 2007; 107: 809–813. [DOI] [PubMed] [Google Scholar]
- 80. Soto-Montenegro ML, Pascau J, Desco M. Response to deep brain stimulation in the lateral hypothalamic area in a rat model of obesity: in vivo assessment of brain glucose metabolism. Mol Imaging Biol 2014; 16: 830–837. [DOI] [PubMed] [Google Scholar]
- 81. Herberg LJ, Blundell JE. Lateral hypothalamus: hoarding behavior elicited by electrical stimulation. Science 1967; 155: 349–350. [DOI] [PubMed] [Google Scholar]
- 82. Folkow B, Rubinstein EH. Behavioural and autonomic patterns evoked by stimulation of the lateral hypothalamic area in the cat. Acta Physiol Scand 1965; 65: 292–299. [DOI] [PubMed] [Google Scholar]
- 83. Delgado JM, Anand BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am J Physiol 1953; 172: 162–168. [DOI] [PubMed] [Google Scholar]
- 84. Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev 1946; 26: 541–559. [DOI] [PubMed] [Google Scholar]
- 85. Lacan G, De Salles AA, Gorgulho AA, et al. Modulation of food intake following deep brain stimulation of the ventromedial hypothalamus in the vervet monkey: laboratory investigation. J Neurosurg 2008; 108: 336–342. [DOI] [PubMed] [Google Scholar]
- 86. Haslam DW, James WP. Obesity. Lancet 2005; 366: 1197–1209. [DOI] [PubMed] [Google Scholar]
- 87. Volkow ND, Wang GJ, Fowler JS, et al. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays 2010; 32: 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stice E, Spoor S, Bohon C, et al. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008; 117: 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Murdaugh DL, Cox JE, Cook EW, 3rd, et al. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage 2012; 59: 2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Berridge KC, Ho CY, Richard JM, et al. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 2010; 1350: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets 2002; 6: 601–609. [DOI] [PubMed] [Google Scholar]
- 92. Kelley AE, Stinus L. Disappearance of hoarding behavior after 6-hydroxydopamine lesions of the mesolimbic dopamine neurons and its reinstatement with L-dopa. Behav Neurosci 1985; 99: 531–545. [DOI] [PubMed] [Google Scholar]
- 93. Halpern CH, Tekriwal A, Santollo J, et al. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. J Neurosci 2013; 33: 7122–7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. van der Plasse G, Schrama R, van Seters SP, et al. Deep brain stimulation reveals a dissociation of consummatory and motivated behaviour in the medial and lateral nucleus accumbens shell of the rat. PLoS One 2012; 7: e33455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Doucette WT, Khokhar JY, Green AI. Nucleus accumbens deep brain stimulation in a rat model of binge eating. Transl Psychiatry 2015; 5: e695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Stoeckel LE, Weller RE, Cook EW, 3rd, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008; 41: 636–647. [DOI] [PubMed] [Google Scholar]
- 97. Quaade F. Letter: stereotaxy for obesity. Lancet 1974; 1: 267. [DOI] [PubMed] [Google Scholar]
- 98. Quaade F, Vaernet K, Larsson S. Stereotaxic stimulation and electrocoagulation of the lateral hypothalamus in obese humans. Acta Neurochir (Wien) 1974; 30: 111–117. [DOI] [PubMed] [Google Scholar]
- 99. Wilent WB, Oh MY, Buetefisch CM, et al. Induction of panic attack by stimulation of the ventromedial hypothalamus. J Neurosurg 2010; 112: 1295–1298. [DOI] [PubMed] [Google Scholar]
- 100. Taghva A, Corrigan JD, Rezai AR. Obesity and brain addiction circuitry: implications for deep brain stimulation. Neurosurgery 2012; 71: 224–238. [DOI] [PubMed] [Google Scholar]
- 101. Whiting DM, Tomycz ND, Bailes J, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg 2013; 119: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mantione M, van de Brink W, Schuurman PR, et al. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery 2010; 66: E218; discussion E218. [DOI] [PubMed] [Google Scholar]
- 103. Harat M, Rudas M, Zielinski P, et al. Nucleus accumbens stimulation in pathological obesity. Neurol Neurochir Pol 2016; 50: 207–210. [DOI] [PubMed] [Google Scholar]
- 104. Halmi KA, Agras WS, Crow S, et al. Predictors of treatment acceptance and completion in anorexia nervosa: implications for future study designs. Arch Gen Psychiatry 2005; 62: 776–781. [DOI] [PubMed] [Google Scholar]
- 105. Keating C. Theoretical perspective on anorexia nervosa: the conflict of reward. Neurosci Biobehav Rev 2010; 34: 73–79. [DOI] [PubMed] [Google Scholar]
- 106. Strober M, Freeman R, Lampert C, et al. The association of anxiety disorders and obsessive compulsive personality disorder with anorexia nervosa: evidence from a family study with discussion of nosological and neurodevelopmental implications. Int J Eat Disord 2007; 40(Suppl.): S46–S51. [DOI] [PubMed] [Google Scholar]
- 107. Zhang HW, Li DY, Zhao J, et al. Metabolic imaging of deep brain stimulation in anorexia nervosa: a 18F-FDG PET/CT study. Clin Nucl Med 2013; 38: 943–948. [DOI] [PubMed] [Google Scholar]
- 108. Delvenne V, Goldman S, De Maertelaer V, et al. Brain glucose metabolism in anorexia nervosa and affective disorders: influence of weight loss or depressive symptomatology. Psychiatry Res 1997; 74: 83–92. [DOI] [PubMed] [Google Scholar]
- 109. Ellison Z, Foong J, Howard R, et al. Functional anatomy of calorie fear in anorexia nervosa. Lancet 1998; 352: 1192. [DOI] [PubMed] [Google Scholar]
- 110. Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw 2002; 15: 603–616. [DOI] [PubMed] [Google Scholar]
- 111. Kaye WH, Bailer UF. Understanding the neural circuitry of appetitive regulation in eating disorders. Biol Psychiatry 2011; 70: 704–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kaye WH, Wierenga CE, Bailer UF, et al. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci 2013; 36: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kaye WH, Wierenga CE, Bailer UF, et al. Does a shared neurobiology for foods and drugs of abuse contribute to extremes of food ingestion in anorexia and bulimia nervosa? Biol Psychiatry 2013; 73: 836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci 2009; 10: 573–584. [DOI] [PubMed] [Google Scholar]
- 115. Wagner A, Aizenstein H, Venkatraman VK, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry 2007; 164: 1842–1849. [DOI] [PubMed] [Google Scholar]
- 116. Fladung AK, Gron G, Grammer K, et al. A neural signature of anorexia nervosa in the ventral striatal reward system. Am J Psychiatry 2010; 167: 206–212. [DOI] [PubMed] [Google Scholar]
- 117. Uher R, Murphy T, Brammer MJ, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry 2004; 161: 1238–1246. [DOI] [PubMed] [Google Scholar]
- 118. Delvenne V, Goldman S, De Maertelaer V, et al. Brain glucose metabolism in eating disorders assessed by positron emission tomography. Int J Eat Disord 1999; 25: 29–37. [DOI] [PubMed] [Google Scholar]
- 119. McLaughlin NC, Didie ER, Machado AG, et al. Improvements in anorexia symptoms after deep brain stimulation for intractable obsessive-compulsive disorder. Biol Psychiatry 2013; 73: e29–e31. [DOI] [PubMed] [Google Scholar]
- 120. Morgan JF, Crisp AH. Use of leucotomy for intractable anorexia nervosa: a long-term follow-up study. Int J Eat Disord 2000; 27: 249–258. [DOI] [PubMed] [Google Scholar]
- 121. Mitchell-Heggs N, Kelly D, Richardson A. Stereotactic limbic leucotomy: a follow-up at 16 months. Br J Psychiatry 1976; 128: 226–240. [DOI] [PubMed] [Google Scholar]
- 122. Zamboni R, Larach V, Poblete M, et al. Dorsomedial thalamotomy as a treatment for terminal anorexia: a report of two cases. Acta Neurochir Suppl (Wien) 1993; 58: 34–35. [DOI] [PubMed] [Google Scholar]
- 123. Barbier J, Gabriels L, van Laere K, et al. Successful anterior capsulotomy in comorbid anorexia nervosa and obsessive-compulsive disorder: case report. Neurosurgery 2011; 69: E745–E751; discussion E751. [DOI] [PubMed] [Google Scholar]
- 124. Wang J, Chang C, Geng N, et al. Treatment of intractable anorexia nervosa with inactivation of the nucleus accumbens using stereotactic surgery. Stereotact Funct Neurosurg 2013; 91: 364–372. [DOI] [PubMed] [Google Scholar]
- 125. Wu H, Van Dyck-Lippens PJ, Santegoeds R, et al. Deep-brain stimulation for anorexia nervosa. World Neurosurg 2013; 80: S29 e21–10. [DOI] [PubMed] [Google Scholar]
- 126. Israel M, Steiger H, Kolivakis T, et al. Deep brain stimulation in the subgenual cingulate cortex for an intractable eating disorder. Biol Psychiatry 2010; 67: e53–e54. [DOI] [PubMed] [Google Scholar]
- 127. Lipsman N, Woodside DB, Giacobbe P, et al. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet 2013; 381: 1361–1370. [DOI] [PubMed] [Google Scholar]
- 128. Lipsman N, Lam E, Volpini M, et al. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial. Lancet Psychiatry 2017; 4: 285–294. [DOI] [PubMed] [Google Scholar]
- 129. Blomstedt P, Naesstrom M, Bodlund O. Deep brain stimulation in the bed nucleus of the stria terminalis and medial forebrain bundle in a patient with major depressive disorder and anorexia nervosa. Clin Case Rep 2017; 5: 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hayes DJ, Lipsman N, Chen DQ, et al. Subcallosal cingulate connectivity in anorexia nervosa patients differs from healthy controls: a multi-tensor tractography study. Brain Stimul 2015; 8: 758–768. [DOI] [PubMed] [Google Scholar]
- 131. Bailer UF, Price JC, Meltzer CC, et al. Altered 5-HT(2A) receptor binding after recovery from bulimia-type anorexia nervosa: relationships to harm avoidance and drive for thinness. Neuropsychopharmacology 2004; 29: 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry 2010; 68: 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Luyten L, Hendrickx S, Raymaekers S, et al. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry 2016; 21: 1272–1280. [DOI] [PubMed] [Google Scholar]
- 134. Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry 2016; 21: 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Fregni F, Orsati F, Pedrosa W, et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 2008; 51: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science 2009; 324: 646–648. [DOI] [PubMed] [Google Scholar]
- 137. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24: 167–202. [DOI] [PubMed] [Google Scholar]
- 138. Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry 2011; 2: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Doherty JM, Schier CJ, Vena AA, et al. Medial prefrontal cortical dopamine responses during operant self-administration of sweetened ethanol. Alcohol Clin Exp Res 2016; 40: 1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Uher R, Yoganathan D, Mogg A, et al. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biol Psychiatry 2005; 58: 840–842. [DOI] [PubMed] [Google Scholar]
- 141. Barth KS, Rydin-Gray S, Kose S, et al. Food cravings and the effects of left prefrontal repetitive transcranial magnetic stimulation using an improved sham condition. Front Psychiatry 2011; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Goldman RL, Borckardt JJ, Frohman HA, et al. Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite 2011; 56: 741–746. [DOI] [PubMed] [Google Scholar]
- 143. Kekic M, McClelland J, Campbell I, et al. The effects of prefrontal cortex transcranial direct current stimulation (tDCS) on food craving and temporal discounting in women with frequent food cravings. Appetite 2014; 78: 55–62. [DOI] [PubMed] [Google Scholar]
- 144. Lapenta OM, Sierve KD, de Macedo EC, et al. Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite 2014; 83: 42–48. [DOI] [PubMed] [Google Scholar]
- 145. Lowe CJ, Vincent C, Hall PA. Effects of noninvasive brain stimulation on food cravings and consumption: a meta-analytic review. Psychosom Med 2017; 79: 2–13. [DOI] [PubMed] [Google Scholar]
- 146. Montenegro RA, Okano AH, Cunha FA, et al. Prefrontal cortex transcranial direct current stimulation associated with aerobic exercise change aspects of appetite sensation in overweight adults. Appetite 2012; 58: 333–338. [DOI] [PubMed] [Google Scholar]
- 147. Gluck ME, Alonso-Alonso M, Piaggi P, et al. Neuromodulation targeted to the prefrontal cortex induces changes in energy intake and weight loss in obesity. Obesity (Silver Spring) 2015; 23: 2149–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Bravo GL, Poje AB, Perissinotti I, et al. Transcranial direct current stimulation reduces food-craving and measures of hyperphagia behavior in participants with Prader-Willi syndrome. Am J Med Genet B Neuropsychiatr Genet 2016; 171B: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Burgess EE, Sylvester MD, Morse KE, et al. Effects of transcranial direct current stimulation (tDCS) on binge eating disorder. Int J Eat Disord 2016; 49: 930–936. [DOI] [PubMed] [Google Scholar]
- 150. Ljubisavljevic M, Maxood K, Bjekic J, et al. Long-term effects of repeated prefrontal cortex transcranial direct current stimulation (tDCS) on food craving in normal and overweight young adults. Brain Stimul 2016; 9: 826–833. [DOI] [PubMed] [Google Scholar]
- 151. Hall PA, Vincent CM, Burhan AM. Non-invasive brain stimulation for food cravings, consumption, and disorders of eating: a review of methods, findings and controversies. Appetite 2017. pii: S0195-6663(16)30685-7. [DOI] [PubMed] [Google Scholar]
- 152. Ray MK, Sylvester MD, Osborn L, et al. The critical role of cognitive-based trait differences in transcranial direct current stimulation (tDCS) suppression of food craving and eating in frank obesity. Appetite 2017; 116: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Luzi L, Ferrulli A, Adamo M, et al. Efficacy of deep transcranial magnetic stimulation on satiety and body weight. Presented at the Annual Meeting of the American Diabetes Association, 12 June 2016. New Orleans, Louisiana. [Google Scholar]
- 154. Kamolz S, Richter MM, Schmidtke A, et al. Transcranial magnetic stimulation for comorbid depression in anorexia. Nervenarzt 2008; 79: 1071–1073. [DOI] [PubMed] [Google Scholar]
- 155. Van den Eynde F, Guillaume S, Broadbent H, et al. Repetitive transcranial magnetic stimulation in anorexia nervosa: a pilot study. Eur Psychiatry 2013; 28: 98–101. [DOI] [PubMed] [Google Scholar]
- 156. Khedr EM, Elfetoh NA, Ali AM, et al. Anodal transcranial direct current stimulation over the dorsolateral prefrontal cortex improves anorexia nervosa: a pilot study. Restor Neurol Neurosci 2014; 32: 789–797. [DOI] [PubMed] [Google Scholar]
- 157. McClelland J, Kekic M, Bozhilova N, et al. A randomised controlled trial of neuronavigated repetitive transcranial magnetic stimulation (rTMS) in anorexia nervosa. PLoS One 2016; 11: e0148606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. McClelland J, Kekic M, Campbell IC, et al. Repetitive transcranial magnetic stimulation (rTMS) treatment in enduring anorexia nervosa: a case series. Eur Eat Disord Rev 2016; 24: 157–163. [DOI] [PubMed] [Google Scholar]
- 159. Hausmann A, Mangweth B, Walpoth M, et al. Repetitive transcranial magnetic stimulation (rTMS) in the double-blind treatment of a depressed patient suffering from bulimia nervosa: a case report. Int J Neuropsychopharmacol 2004; 7: 371–373. [DOI] [PubMed] [Google Scholar]
- 160. Gay A, Jaussent I, Sigaud T, et al. A lack of clinical effect of high-frequency rTMS to dorsolateral prefrontal cortex on bulimic symptoms: a randomised, double-blind trial. Eur Eat Disord Rev 2016; 24: 474–481. [DOI] [PubMed] [Google Scholar]
- 161. Walpoth M, Hoertnagl C, Mangweth-Matzek B, et al. Repetitive transcranial magnetic stimulation in bulimia nervosa: preliminary results of a single-centre, randomised, double-blind, sham-controlled trial in female outpatients. Psychother Psychosom 2008; 77: 57–60. [DOI] [PubMed] [Google Scholar]
- 162. Van den Eynde F, Broadbent H, Guillaume S, et al. Handedness, repetitive transcranial magnetic stimulation and bulimic disorders. Eur Psychiatry 2012; 27: 290–293. [DOI] [PubMed] [Google Scholar]
- 163. Van den Eynde F, Claudino AM, Mogg A, et al. Repetitive transcranial magnetic stimulation reduces cue-induced food craving in bulimic disorders. Biol Psychiatry 2010; 67: 793–795. [DOI] [PubMed] [Google Scholar]
- 164. Downar J, Sankar A, Giacobbe P, et al. Unanticipated rapid remission of refractory bulimia nervosa, during high-dose repetitive transcranial magnetic stimulation of the dorsomedial prefrontal cortex: a case report. Front Psychiatry 2012; 3: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kekic M, McClelland J, Bartholdy S, et al. Single-session transcranial direct current stimulation temporarily improves symptoms, mood, and self-regulatory control in bulimia nervosa: a randomised controlled trial. PLoS One 2017; 12: e0167606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Hamani C, Richter E, Schwalb JM, et al. Bilateral subthalamic nucleus stimulation for Parkinson’s disease: a systematic review of the clinical literature. Neurosurgery 2005; 56: 1313–1321; discussion 1321–1314. [DOI] [PubMed] [Google Scholar]