Abstract
Background
Our objective was to estimate the association between methadone and neonatal abstinence syndrome compared with buprenorphine using a probabilistic bias analysis to account for unmeasured confounding by severity of addiction.
Methods
We used a cohort of live-born infants exposed in utero to methadone or buprenorphine for maternal opioid maintenance therapy at Magee-Womens Hospital in Pittsburgh, PA from 2013‒2015 (n=716). We determined exposure and outcome status using pharmacy billing claims. We used log-binomial regression models to assess association of treatment with neonatal abstinence syndrome after adjusting for parity, maternal race, age, delivery year, employment, hepatitis c, smoking, marital, and insurance status. We implemented probabilistic bias analysis, informed by an internal validation study, to assess the impact of unmeasured confounding by severity of addiction.
Results
Infants exposed to methadone in utero were more likely to experience neonatal abstinence syndrome compared with those exposed to buprenorphine [RR: 1.3, 95% CI: 1.2, 1.5]. After adjustment, infants exposed to methadone were more likely (adjusted RR 1.3, 95% CI: 1.1, 1.5) than infants exposed to buprenorphine to have the syndrome. In the validation cohort (n=200), severe addiction was more common in methadone- versus buprenorphine-exposed deliveries (77% vs. 32%). However, adjustment for severe addiction in the bias analysis only slightly attenuated the association (RR 1.2, 95% CI: 1.0, 1.4), supporting conventional analysis.
Conclusions
Methadone is associated with increased risk of neonatal abstinence syndrome compared with buprenorphine in infants exposed in utero. This association is subject to minimal bias due to unmeasured confounding by severity of addiction.
Keywords: opioid maintenance therapy, methadone, buprenorphine, pregnancy, probabilistic bias analysis
INTRODUCTION
Pregnant women are not immune from the opioid epidemic in the U.S.1–4 The trend of rising opioid use in pregnancy parallels simultaneous increases in the number of cases of neonatal abstinence syndrome. Neonatal abstinence syndrome is a clinical condition in which the infants exposed to opioids in utero manifest symptoms of withdrawal from the drug postnatally.5–7 Neonatal abstinence is costly to treat8 and it has long term sequelae for the child, including neurocognitive and behavioral issues9 along with decreases in visual acuity.10 To reduce the risk of neonatal abstinence syndrome and a host of other poor maternal and child health outcomes, pregnant women with opioid use dependence are treated with either methadone or buprenorphine as opioid maintenance therapy.11 Literature has consistently shown that buprenorphine use is associated with lower risk of neonatal abstinence syndrome and shorter duration of neonatal treatment compared with the use of methadone.12–16 However, these findings may be biased because large databases often used for this research typically do not contain data on the severity of the mother’s addiction, a potential confounder.17, 18
In the U.S., women who suffer from more severe opioid addiction are often assigned to methadone treatment, while women with lower risk of relapse and drug diversion tend to be treated with buprenorphine. This prescribing preference exists in part because methadone and buprenorphine are delivered with different systems of care in the U.S. Women prescribed methadone must attend a clinic daily to obtain medication under direct observation, eliminating the chance of illegal distribution. Alternatively, women treated with buprenorphine are legally permitted a supply of medication for administration at home through outpatient providers.19 Therefore, it is critical to account for factors that determine this prescribing preference in comparative treatment studies.
Our objective was to estimate the association between methadone versus buprenorphine exposure as opioid maintenance therapy and neonatal abstinence syndrome after accounting for unmeasured confounding by severity of addiction.
METHODS
We used data on all singleton pregnancies delivered at 20 to 42 weeks of gestation with live-born infants exposed to in utero methadone or buprenorphine opioid maintenance therapy at Magee-Womens Hospital (MWH) in Pittsburgh, PA from 2013–2015. MWH delivers over 10,000 infants annually and cares for opioid-addicted mothers with treatment protocols similar to those at other U.S. institutions.14, 15, 20, 21 Buprenorphine is administered through prescription by a certified buprenorphine provider while methadone treatment requires daily visits to an opioid treatment clinic.22 The protocol is described in detail in the eAppendix 1.
International Classification of Diseases
Ninth (ICD-9) and Tenth Revision (ICD-10) codes in pharmacy billing claims were used to identify drug-dependent (ICD-9 64831) or drug-complicated deliveries (ICD-10 O99324). Billing claims that specifically documented exposure to methadone or buprenorphine as opioid maintenance therapy were then confirmed with dosing information extracted from the medical chart. Buprenorphine-exposed infants were those whose mothers were treated with Subutex® (buprenorphine, n=299) (Reckitt Benckiser Pharmaceuticals Inc., VA) or Suboxone® (buprenorphine + naloxone, n=10) (Reckitt Benckiser Pharmaceuticals Inc., VA). The exposure window of interest was the day of delivery because medication effect on neonatal abstinence syndrome is most influential closest to delivery23 and we lacked access to the entire treatment trajectories including treatment initiation dates.
We identified cases of neonatal abstinence syndrome from pharmacy billing codes indicating treatment with morphine after delivery. At MWH, all infants with known or suspected opioid exposure in utero are kept for neonatal abstinence syndrome observation for 5 to 7 days. Infants are scored using the Finnegan Scale every 3 to 4 hours.24 When the average of 3 consecutive scores is ≥8 on the Finnegan Scale, infants are given morphine treatment. In our cohort, morphine treatment was highly correlated with ICD code indicative of “Drug Withdrawal Syndrome in Newborn” (kappa>0.99).
Maternal characteristics and birth outcomes were obtained first from the MWH electronic pharmacy records comprised primarily of billing and ICD codes, and were informed with data provided by the birth record when data were missing. These data are a combination of self-report, clinical billing codes, and chart documentation by a health professional. Information on maternal race (Black, White, other), education level (less than high school, high school or equivalent, some college, college graduate), employment (yes, no), marital status (married, unmarried), insurance type (private, public), pre-pregnancy weight and height, parity, smoking during pregnancy (yes, no), and hepatitis c status (positive, negative) were available. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared and was categorized as underweight (<18.5), normal weight (BMI 18.5 to <25), overweight (BMI 25 to <30), or obese (BMI ≥30).25 Birth outcome data included gestational age at delivery, infant length of stay (days), birthweight, congenital anomalies (yes, no), admission into the neonatal intensive care unit (yes, no), and number of prenatal visits. Gestational age was determined using the best obstetric estimate in the chart from ultrasound or last menstrual period when ultrasound was not available. This study was approved by our Institutional Review Board.
Validation Cohort
Severity of addiction is a potential confounder that was unmeasured in our dataset. We therefore performed a validation study to collect indicators of addiction severity from medical chart abstraction on a random sample of 100 buprenorphine- and 100 methadone-treated women in our cohort. The study team identified four indicators of severity of addiction that were based on literature17, 18, 26, 27 and clinical expertise (details in eAppendix 2). One reviewer (LSL), who was blinded to outcome but not exposure status, performed the medical chart abstractions and entered data into an electronic database. The majority of information was abstracted from physicians’ notes and the social workers’ discharge plans.
We defined severe addiction as having any one of the four following indicators documented in the chart: 1) conversion to opioid maintenance therapy during pregnancy, 2) documented relapse during pregnancy, 3) use of illicit substances at delivery, and 4) use of benzodiazepines in pregnancy. When there was no documentation of conversion to opioid maintenance therapy in the chart, women were assumed to have conceived on the same treatment noted at delivery. All other lack of documentation was recorded as missing unless explicitly noted that the patient did not have the indicator (e.g. “patient did not relapse in this pregnancy”). Reconversion to therapy within one pregnancy was recorded as a relapse. Illicit substance use at the time of delivery included any of the following: marijuana, benzodiazepines, illicit buprenorphine, cocaine, nondescript intravenous drugs, heroin, or illicit opiate pills.
Statistical Analysis
Multivariable log-binomial regression models were used to estimate the independent association between neonatal abstinence syndrome and methadone compared with buprenorphine while accounting for clustering within each woman (25 who contributed multiple pregnancies).28 We calculated risk ratios (RR), risk differences (RD), and their 95% confidence intervals (CI). Risk differences were calculated using marginal standardization.29 We identified potential confounders a priori using theory-based conceptual models: maternal indication for opioid maintenance therapy, gestational age at opioid maintenance therapy initiation, duration of opioid dependence, maternal age, race, employment status, smoking status, marital status, insurance type, hepatitis c status, parity and year of delivery. The final model was limited to maternal age, race, employment status, smoking status, marital status, insurance type, hepatitis c status, parity and year of delivery, based on availability of data. We did not adjust for adequacy of prenatal care, total visits to the emergency room during the pregnancy, and gestational age because they are likely on the causal pathway.17
Probabilistic Bias Analysis
To quantify the extent to which unmeasured confounding by severity of addiction biased the association between opioid maintenance therapy and neonatal abstinence syndrome, we performed a probabilistic bias analysis. This approach is based on a set of methods developed and described in detail previously by Lash et al.31, 32 The parameters for this analysis were informed using data indicative of addiction severity from our internal validation study. We defined the limits of the relative risk due to confounding using the Flanders and Khoury method33. This method involved fitting two logistic regressions in the subcohort: the first modeling the odds of treatment type by severity of addiction, the second modeling the odds of neonatal abstinence syndrome by severity of addiction. The Flanders and Khoury method also incorporates the prevalence of severity in each treatment group (eAppendix 3). This information was used to determine the limits of the trapezoidal distribution used to parameterize the risk. We sampled the risk due to confounding from 100,000 simulated data sets using a Monte Carlo approach. Results were presented as bootstrapped point estimates with an interval defined as the 2.5th and 97.5th percentiles. This interval corresponds to the 95% confidence interval obtained in a conventional analysis but incorporates both systematic and random error. The results from the probabilistic bias analysis were then compared with the risk ratios and 95% confidence intervals from the conventional model.
RESULTS
There were a total of 872 drug-dependent pregnancies in the study period. Of these, 745 (85%) received either methadone or buprenorphine as opioid maintenance therapy on the day of delivery and were eligible for this study (Figure 1). We excluded nine women with multi-fetal gestations (18 infants), six with a fetal death, and five who had stopped all medication due to relapse or weaning prior to delivery. Our final sample consisted of 716 pregnancies.
Figure 1.
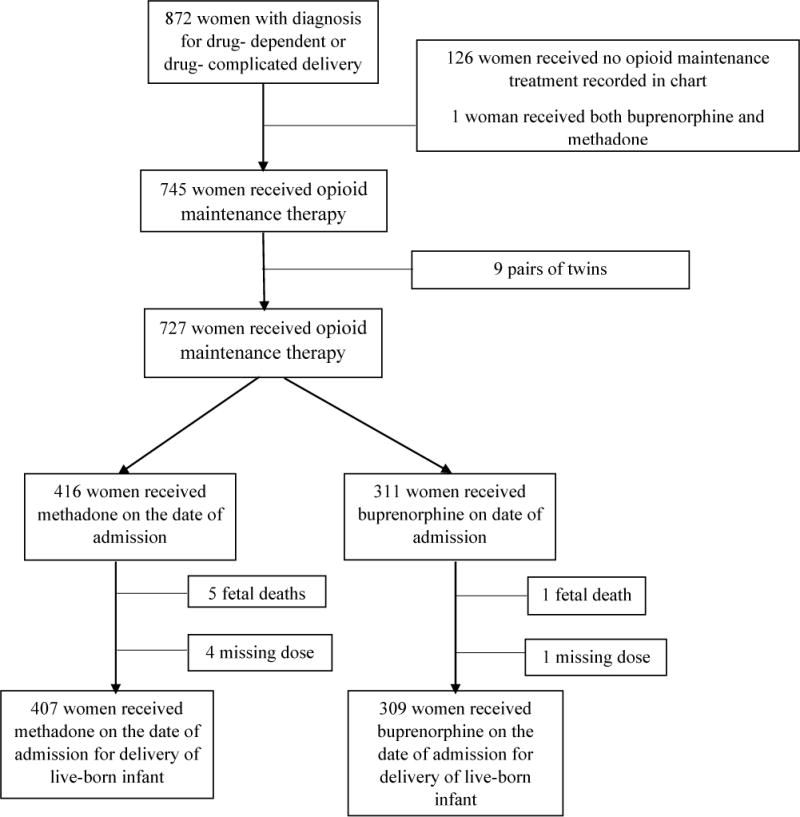
Flow diagram describing sample population (n=716, 2013–2015*Note: 25 women with 2 pregnancies).
Slightly more than half of pregnancies on opioid maintenance therapy were treated with methadone (57%) and the remaining with buprenorphine (43%). Women treated with methadone were more likely than their buprenorphine-treated counterparts to be unmarried, unemployed, hepatitis c positive, multiparous, and to have less than a high school education (Table 1). Methadone-treated pregnancies on average had shorter gestations and infants with lower birthweights. Race, age, pre-pregnancy BMI, and smoking status were not meaningfully different between treatment groups. These relationships were consistent in the validation cohort (Table 2).
Table 1.
Maternal Characteristics by Opioid Maintenance Treatment Type, Magee-Womens Hospital, 2013–2015 (n=716).
| Characteristic | Methadone N (%) n=407 |
Buprenorphine N (%) n=309 |
|---|---|---|
| Race | ||
| White | 381 (93.6) | 294 (95.1) |
| Black | 19 (4.7) | 8 (2.6) |
| Missing | 7 (1.5) | 7 (2.3) |
| Mother’s Education | ||
| Less than high school | 83 (20.4) | 45 (14.6) |
| High school graduate or GED completed | 165 (40.5) | 139 (45.0) |
| Some college credit | 78 (19.2) | 68 (22.0) |
| College graduate | 66 (16.2) | 54 (17.4) |
| Missing | 15 (3.7) | 3 (1.0) |
| BMI categorya | ||
| Underweight (<18.5kg/m2) | 11 (2.7) | 17 (5.5) |
| Normal weight | 116 (28.5) | 94 (30.4) |
| Overweight | 35 (8.6) | 30 (9.7) |
| Obese | 29 (7.1) | 24 (7.7) |
| Missing | 216 (53.1) | 144 (46.6) |
| Married | 35 (8.6) | 58 (18.8) |
| Employed | 139 (34.2) | 135 (43.7) |
| Smoked during pregnancy | 336 (82.6) | 250 (80.9) |
| Parity | ||
| Nulliparous | 118 (29.0) | 106 (34.3) |
| 1–2 previous pregnancies | 208 (51.1) | 151 (48.9) |
| Greater than 2 pregnancies | 81 (19.9) | 52 (16.8) |
| Infant with congenital anomaly | 50 (12.3) | 27 (8.7) |
| Hepatitis c positive | 61 (15.0) | 31 (10.0) |
| Mother’s age [Mean (SD)] | 29.1 (4.7) | 28.5 (4.9) |
| Prepregnancy BMI [Mean (SD)]b | 24.6 (5.3) | 24.2 (6.1) |
| Gestational age at delivery [Mean (SD)] | 37.4 (2.9) | 38.5 (2.5) |
| Birthweight [Mean (SD)] | 2734 (619.3) | 2999 (591.2) |
Prepregnancy BMI defined as underweight (<18.5 kg/m2), normal weight (18.5 to <25 kg/m2), overweight (25 to <30 kg/m2), obese (≥30 kg/m2).
Prepregnancy BMI based on n=356.
GED=general education development, SD=standard deviation, BMI=body mass index
Table 2.
Maternal Characteristics by Opioid Maintenance Treatment Type in a Validation Subcohort, Magee-Womens Hospital, 2013–2015 (n=200).
| Characteristic | Methadone N (%) n=100 |
Buprenorphine N (%) n=100 |
|---|---|---|
| Race | ||
| White | 97 | 97 |
| Black | 3 | 2 |
| Missing | 0 | 1 |
| Mother’s Education | ||
| Less than high school | 19 | 9 |
| High school graduate or GED completed | 40 | 52 |
| Some college credit | 23 | 18 |
| College graduate | 14 | 19 |
| Missing | 4 | 2 |
| Married | 8 | 21 |
| Employed | 31 | 43 |
| Smoked during pregnancy | 84 | 80 |
| Parity | ||
| Nulliparous | 31 | 39 |
| 1–2 previous pregnancies | 47 | 46 |
| Greater than 2 pregnancies | 22 | 15 |
| Hepatitis c positive | 12 | 10 |
| Infant with congenital anomaly | 15 | 10 |
| Severe maternal addiction | 77 | 32 |
| Mother’s age [Mean (SD)] | 28.6 (5.1) | 28.2 (5.2) |
| Prepregnancy BMI [Mean (SD)]a | 24.6 (5.9) | 23.7 (5.3) |
| Gestational age at delivery [Mean (SD)] | 37.3 (3.2) | 39.1 (1.8) |
| Birthweight [Mean (SD)] | 2695 (631.6) | 3147 (472.4) |
Prepregnancy BMI based on n=43 in methadone treated women and n=54 in buprenorphine treated women.
GED=general education development, SD=standard deviation, BMI=body mass index
Neonatal abstinence syndrome occurred in 58% of the infants (n=415). Infants with treatment for the syndrome were more likely to be born to unmarried, unemployed, hepatitis c positive mothers with less than a high school education and a normal pre-pregnancy BMI (eTable 1). Infants diagnosed with neonatal abstinence syndrome were also more likely to be born at a later gestational age without a congenital anomaly compared with their counterparts not requiring treatment.
The incidence of neonatal abstinence syndrome was 65% in infants exposed in utero to methadone compared with 49% in infants exposed to buprenorphine. Infants exposed to methadone in utero were 30% more likely than infants exposed to buprenorphine to be treated for neonatal abstinence syndrome (unadjusted RR 1.3, 95% CI: 1.2, 1.5). After adjustment for parity, maternal race, employment status, hepatitis c status, age, year of delivery, smoking status, marital status, and insurance, the association did not change (adjusted RR 1.3, 95% CI: 1.1, 1.5). On the absolute scale, the adjusted RD was 0.14 (95% CI: 0.059, 0.22), indicating that methadone was associated with 14 excess cases of NAS for every 100 live-born infants born to mothers treated with methadone compared with buprenorphine (Table 3).
Table 3.
Results from conventional analyses of the risk of neonatal abstinence syndrome associated with methadone compared with buprenorphine as opioid maintenance therapy, at Magee-Womens Hospital, Pittsburgh, Pennsylvania (2013–2015).
| Opioid maintenance therapy | Events (n) |
Population at risk | Unadjusted risk per 100 livebirths | Unadjusted risk difference per 100 live-born infants (95% confidence interval) |
Adjusteda risk difference per 100 live-born infants (95% confidence interval) |
|---|---|---|---|---|---|
| Buprenorphine | 152 | 309 | 49 | Reference | Reference |
| Methadone | 263 | 407 | 65 | 15 (8.1, 23) | 14 (5.9, 22) |
|
| |||||
|
Unadjusted relative risk (95% confidence interval) |
Adjusteda
relative risk (95% confidence interval) |
||||
|
| |||||
| Buprenorphine | Reference | Reference | |||
| Methadone | 1.3 (1.2, 1.5) | 1.3 (1.1, 1.5) | |||
Adjusted for parity, maternal race, employment status, hepatitis c status, age, year of delivery, smoking status, marital status, and insurance.
Though there were expected differences comparing the study cohort of opioid dependent mothers to all births at MWH from 2013–2014 (eTable 2), the validation subsample was similar to the full study cohort (eTable 3). In the validation subsample, methadone-treated women were more likely than buprenorphine-treated women to have converted to opioid maintenance treatment during pregnancy (58% vs 12%; median gestational age at conversion: 12 weeks vs before conception), relapsed in pregnancy (23% vs 4%), used any illicit substance at delivery (24% vs 15%), or used benzodiazepines during pregnancy (28% vs 8%) (Table 4). Prevalence of having any one of the indicators of severe addiction was higher in the methadone group compared with buprenorphine (77% vs. 32%). This composite of addiction severity was associated with a slightly higher risk of neonatal abstinence syndrome (odds ratio 1.2, 95% CI: 0.7, 2.1). These results were robust to removal of each individual factor included in the severity index (data not shown).
Table 4.
Characteristics of a subsample of opioid use dependent singleton pregnancies with severity of addiction indicators abstracted from medical charts at Magee-Womens Hospital in Pittsburgh, 2013–2015 (n=200).
| Characteristic | Methadone n=100 |
Buprenorphine n=100 |
|---|---|---|
| Converted to opioid maintenance therapy in pregnancy | ||
| Yes | 58 | 12 |
| No | 18 | 30 |
| Missing | 24 | 58 |
| Gestational age at conversion (Median, IQR), weeks | 12 (5, 22) | Prior to conception (prior, 4) |
| Relapse in pregnancy | ||
| Yes | 23 | 4 |
| No | 9 | 24 |
| Missing | 68 | 72 |
| Using illicit substance at time of delivery | ||
| Yes | 24 | 15 |
| No | 34 | 71 |
| Missing | 42 | 14 |
| Used benzodiazepines in pregnancy | ||
| Yes | 28 | 8 |
| No | 33 | 22 |
| Missing | 39 | 70 |
| Neonatal abstinence syndrome | ||
| Yes | 61 | 54 |
| No | 39 | 46 |
IQR=interquartile range
There was a large amount of missing data in the validation cohort that varied by treatment (eTable 4). Women treated with buprenorphine were more likely than methadone-treated women to have missing data for more than one indicator of severity. Despite the difference in rate of missing data, women treated with buprenorphine also had documentation indicating less severity (e.g. “patient did not relapse in pregnancy”) more often than methadone-treated women. This is true for each severity indicator excluding benzodiazepine use (Table 4).
After accounting for unmeasured confounding by severity of addiction in the probabilistic bias analysis, the association between methadone and risk of neonatal abstinence syndrome was slightly attenuated from the conventional results [point estimate 1.2 (95% simulation interval: 1.0, 1.4; Table 5)]. The bootstrapped 5th and 95th percentiles in the bias analysis were slightly wider than the conventional confidence intervals as they accounted for both systematic and random error.
Table 5.
Comparison of results from adjusted conventional and probabilistic bias analyses accounting for unmeasured confounding by severity of addiction on the risk of neonatal abstinence syndrome associated with methadone compared with buprenorphine as opioid maintenance therapy, at Magee-Womens Hospital, Pittsburgh, Pennsylvania (2013–2015).
| Opioid maintenance therapy | Conventional analysis: Adjusteda relative risk (95% confidence interval) |
Bias Analysis 1: Adjusteda point estimate (95% bootstrapped simulation interval)b |
|---|---|---|
| Buprenorphine | Reference | Reference |
| Methadone | 1.3 (1.1, 1.5) | 1.2 (1.0, 1.4) |
Adjusted for parity, maternal race, employment status, hepatitis c status, age, year of delivery, smoking status, marital status, and private vs. public insurance.
minimum RRC=1.0, mode 1=1.02, mode 2=1.11, maximum RRC=1.13
RRc=relative risk due to confounding
DISCUSSION
There is agreement in the literature that buprenorphine confers benefits over methadone for opioid maintenance therapy in pregnancy, including decreased risk of neonatal abstinence syndrome in the exposed infants.14–16, 18 Nonetheless, there is a potential for these findings to be biased due to unmeasured confounding.17, 18 Our conventional analysis results suggested that the risk of neonatal abstinence syndrome in infants exposed to in-utero methadone was 30 percent higher compared with buprenorphine-exposed infants. The results from the probabilistic bias analysis suggest that unmeasured confounding by severity of addiction only slightly biased the conventional results away from the null. Although we found that women receiving methadone had more indicators of severe addiction than women receiving buprenorphine, the relatively weak relationship between addiction severity and neonatal abstinence syndrome reduced the potential for prescribing differences to confound the primary association.
The ideal approach to eliminate unmeasured confounding is to conduct a randomized controlled trial. However, the largest double-blinded, flexible-dosing, randomized controlled trial comparing methadone and buprenorphine use in pregnancy (Maternal Opioid Treatment: Human Experimental Research trial) was plagued with the same biases faced in observational research.12 Analyzing only women who remained on randomized treatment, Jones et al.12 found no difference in percent of infants requiring treatment for NAS between treatment groups, though more morphine (mean dose 10.4 vs. 1.1 mg) and longer hospital stays (17.5 vs. 10.0 days) were needed for infants exposed to methadone in utero. Importantly, investigators found that 33% of women randomized to buprenorphine discontinued treatment, with 71% of them reporting “dissatisfaction” with treatment. This is in stark contrast to only 18% of methadone patients discontinuing treatment, of whom only 13% reported “dissatisfaction” with treatment. Only those women who continued allocated treatment were included in the final analyses. Furthermore, despite randomization, women who remained on methadone treatment had longer cumulative lifetime drug use. Together, these findings demonstrate a similar bias to unmeasured confounding as addiction severity may have influenced treatment choice and continuation regardless of randomization.
Our results are consistent with a large meta-analysis of 11 studies including 855 methadone-treated women and 515 buprenorphine-treated women for opioid dependence and risk of neonatal abstinence syndrome.18 These authors described a summary estimate of 1.11 (95% CI: 1.02, 1.23) reported as an increased risk of neonatal abstinence syndrome by 10% in infants exposed to methadone compared with buprenorphine in utero. The authors conducted a sensitivity analysis for unmeasured confounding by indication applying the VanderWeele and Arah34 approach for unmeasured confounding. Unlike our analysis, which was informed by an internal validation study, these authors used bias parameters informed by the extant literature. They found that after accounting for unmeasured confounding by indication, the risk of neonatal abstinence syndrome associated with methadone treatment in the conventional analysis was biased away from the null (50th percentile adjusted RR 1.01, 95% CI: 0.92, 1.11). Consistent with our conceptual model, bias parameters reflected values for unmeasured confounding that conferred increased risk for poor neonatal outcomes in the methadone-treated women [RR of confounder-NAS association (RRCD) 1.05–1.25] that was reversed in the buprenorphine patients (RRCD 0.80–0.95). Prevalence of unmeasured confounding by indication was assumed to be 40% in both treatment groups. Inputs for this bias analysis have been previously questioned as the assumptions informing these are subjective and results vary by slight changes in their inputs.35 Our findings extend this work by using an internal validation study to inform the bias parameters and draw conclusions from one study center limiting heterogeneity in treatment practices. Using more conservative bias parameters informed from the validation cohort slightly weakened the impact of unmeasured confounding on our results by comparison.
In our probabilistic bias analysis, informed from the validation cohort, the RR for neonatal abstinence syndrome associated with methadone compared with buprenorphine marginally decreased from 1.3 (95% CI: 1.1, 1.5) to 1.2 (95% CI: 1.0, 1.4) when limits were defined by the Flanders and Khoury method.33 We therefore maintain that the risk of neonatal abstinence syndrome associated with methadone treatment even after accounting for severity, may not be fully explained by unmeasured confounding.
It was surprising that accounting for severity of addiction did not further attenuate the association between methadone treatment and neonatal abstinence syndrome compared with buprenorphine. However, the impact of addiction severity on the association between opioid maintenance therapy and neonatal abstinence syndrome is likely limited by the weak relationship between addiction severity and the syndrome. Of note, infants born to women actively abusing heroin during pregnancy have a lower risk of neonatal abstinence syndrome compared with those women receiving methadone as replacement therapy.16, 36 Therefore, behaviors associated with more severe addiction such as relapse and later conversion to opioid maintenance therapy may not increase the risk of neonatal abstinence syndrome. It is important to note that the lower risk of neonatal abstinence with active abuse does not negate other potential risks such as reduced prenatal care. Opioid maintenance therapy should remain the standard care in accordance with recommendations from the American Congress of Obstetricians and Gynecologists.11, 37
Of note, the rate of congenital birth anomalies was relatively high in this cohort (11%). Though this variable has not been validated, nor can it differentiate minor from major defects, it did demonstrate a clustering which may be of interest for future study. An estimated 26% of all anomalies affected the heart or circulation, 22% were classified as genitourinary, and 14% were classified as orofacial anomalies. Furthermore, the relationship between prematurity and neonatal abstinence syndrome is deserving of future study. Infants born preterm (<37 weeks), had lower rates of the syndrome compared with those born at full term. Currently, we cannot be certain if the association between preterm delivery and neonatal abstinence syndrome is causal as the pathophysiology of is unknown. Though a commonly accepted plausibility is immature opioid receptor development in the neonate (and therefore decreased risk of dependence and subsequent neonatal abstinence syndrome), it could also be an artifact as the Finnegan Scale was developed to assess neonatal abstinence syndrome in term infants.
Our findings must be interpreted within the bounds of their limitations. We used a large administrative database that lacked detailed information on treatment and addiction histories. Without information on the initiation, timing, and duration of exposure to medication, we were unable to appropriately assess how these factors influence the development of neonatal abstinence syndrome. We relied on the dose and medication treatment on the day of delivery as a relatively crude measure of exposure, as it is thought that treatment closest to the time of delivery has the strongest impact on neonatal abstinence syndrome.23 Though using this approach allows for misclassification of exposure, this is unlikely to affect our findings as only six of 200 women in our validation cohort had documentation of ever changing treatment (including prior to pregnancy). The lack of information on addiction history contributes considerably to the unmeasured confounding remaining in the analysis. Furthermore, by using treatment for neonatal abstinence syndrome as our outcome measurement, we restricted our analysis to only the more severe cases of the syndrome. Though having a gradient of Finnegan scores or morphine dose may be informative, those receiving treatment incur the largest costs and this approach is subject to less misclassification due to the subjectivity of the Finnegan Scale.
The lack of adjustment for prescribing preferences by severity of addiction, which is typically unmeasured, is one of the greatest shortcomings in the current literature. Our probabilistic bias analysis aimed to minimize this limitation using information from our internal validation cohort. As was expected due to the nature and sensitivity of this topic, upon chart review there was a substantial amount of missing data in the validation cohort with a missingness that differed by opioid maintenance type. Differential missingness was likely driven by more buprenorphine-treated patients entering into pregnancy on treatment and potentially having less interaction with the healthcare system due to an overall superior health profile. Both may contribute to less documentation in their charts. Though to our knowledge we are the first to use an internal validation cohort to derive information on severity to adjust for unmeasured confounding, our findings are subject to the limitations of the data available to us and to the parameterization the severity index. Future research with the aim of developing a robust severity index is warranted. Nevertheless, this approach is preferable to deriving effect estimates exclusively from the literature.
Prescribing preferences for opioid maintenance therapy are often warranted as many women benefit from the different methods of delivery of care in the U.S. However, in many places in the U.S. patients do not have access to both treatment options due to both a lack of clinics and licensed providers along with limitations imposed by insurance. Lack of treatment options can result in structural confounding in other studies. In our study population, it is unlikely that this impacted our results as women had access to both treatment options and both were covered under Pennsylvania Medicaid, the primary insurer of this population.
As both observational studies and randomized trials are subject to the biases inherent in opioid maintenance treatment choices, it is imperative to account for this unmeasured confounding when comparing methadone with buprenorphine exposures in pregnancy to advocate for availability of both options if one is superior. Our results suggest that the previous findings that buprenorphine is associated with lower risk of neonatal abstinence syndrome compared with methadone in infants exposed in utero are subject to minimal bias from unmeasured confounding. Applying similar bias analyses to the association of these treatments with other neonatal outcomes is necessary to fully inform treatment decisions.
Supplementary Material
Acknowledgments
Neggin Mokhtari, MD & Allison Serra, MD
Sources of Funding: This work was supported, in part, by Grant # HD047905 from the NICHD and Obstetric-Fetal Pharmacology Research Center. Lara S. Lemon is also a Ruth Kirschstein T-32 grant recipient. Robert W. Platt holds the Albert Boehringer I Chair at McGill University, and a Chercheur-national (National Scholar) award from the Fonds de Recherche du Québec – Santé.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest
The statistical code is available from Dr. Lemon upon request; authors must obtain permission from our Institutional Review Board to analyze the data directly
References
- 1.Dart RC, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–8. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 2.Williams AR, Bisaga A. From AIDS to Opioids - How to Combat an Epidemic. N Engl J Med. 2016;375(9):813–5. doi: 10.1056/NEJMp1604223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salihu HM, et al. National trends in maternal use of opioid drugs among pregnancy-related hospitalizations in the United States, 1998 to 2009. Am J Perinatol. 2015;32(3):289–98. doi: 10.1055/s-0034-1384642. [DOI] [PubMed] [Google Scholar]
- 4.Bateman BT, et al. Patterns of opioid utilization in pregnancy in a large cohort of commercial insurance beneficiaries in the United States. Anesthesiology. 2014;120(5):1216–24. doi: 10.1097/ALN.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudak ML, Tan RC. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540–60. doi: 10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- 6.Tolia VN, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118–26. doi: 10.1056/NEJMsa1500439. [DOI] [PubMed] [Google Scholar]
- 7.Brown JD, et al. Rates of Neonatal Abstinence Syndrome Amid Efforts to Combat the Opioid Abuse Epidemic. JAMA Pediatr. 2016 doi: 10.1001/jamapediatrics.2016.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roussos-Ross K, et al. Opioid use in pregnant women and the increase in neonatal abstinence syndrome: what is the cost? J Addict Med. 2015;9(3):222–5. doi: 10.1097/ADM.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 9.Maguire DJ, et al. Long-Term Outcomes of Infants with Neonatal Abstinence Syndrome. Neonatal Netw. 2016;35(5):277–86. doi: 10.1891/0730-0832.35.5.277. [DOI] [PubMed] [Google Scholar]
- 10.Walhovd KB, et al. Child neuroanatomical, neurocognitive, and visual acuity outcomes with maternal opioid and polysubstance detoxification. Pediatr Neurol. 2015;52(3):326–32.e1. 3. doi: 10.1016/j.pediatrneurol.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070–6. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- 12.Jones HE, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–31. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacroix I, et al. Buprenorphine versus methadone in pregnant opioid-dependent women: a prospective multicenter study. Eur J Clin Pharmacol. 2011;67(10):1053–9. doi: 10.1007/s00228-011-1049-9. [DOI] [PubMed] [Google Scholar]
- 14.Wiegand SL, et al. Buprenorphine and naloxone compared with methadone treatment in pregnancy. Obstet Gynecol. 2015;125(2):363–8. doi: 10.1097/AOG.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 15.Meyer MC, et al. Methadone and buprenorphine for opioid dependence during pregnancy: a retrospective cohort study. J Addict Med. 2015;9(2):81–6. doi: 10.1097/ADM.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norgaard M, Nielsson MS, Heide-Jorgensen U. Birth and Neonatal Outcomes Following Opioid Use in Pregnancy: A Danish Population-Based Study. Subst Abuse. 2015;9(Suppl 2):5–11. doi: 10.4137/SART.S23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brogly SB, et al. Confounding of the Comparative Safety of Prenatal Opioid Agonist Therapy. J Addict Res Ther. 2015;6(4) doi: 10.4172/2155-6105.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brogly SB, et al. Prenatal buprenorphine versus methadone exposure and neonatal outcomes: systematic review and meta-analysis. Am J Epidemiol. 2014;180(7):673–86. doi: 10.1093/aje/kwu190. [DOI] [PubMed] [Google Scholar]
- 19.Kraus ML, et al. Statement of the American Society Of Addiction Medicine Consensus Panel on the use of buprenorphine in office-based treatment of opioid addiction. J Addict Med. 2011;5(4):254–63. doi: 10.1097/ADM.0b013e3182312983. [DOI] [PubMed] [Google Scholar]
- 20.Wachman EM, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. Jama. 2013;309(17):1821–7. doi: 10.1001/jama.2013.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritham UA, Paul JA, Hayes MJ. Opioid dependency in pregnancy and length of stay for neonatal abstinence syndrome. J Obstet Gynecol Neonatal Nurs. 2012;41(2):180–90. doi: 10.1111/j.1552-6909.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin FR, et al. A review of a national training initiative to increase provider use of MAT to address the opioid epidemic. Am J Addict. 2016;25(8):603–609. doi: 10.1111/ajad.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai RJ, et al. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. Bmj. 2015;350:h2102. doi: 10.1136/bmj.h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finnegan LP, et al. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2(1–2):141–58. [PubMed] [Google Scholar]
- 25.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 26.Metz V, et al. Impact of treatment approach on maternal and neonatal outcome in pregnant opioid-maintained women. Hum Psychopharmacol. 2011;26(6):412–21. doi: 10.1002/hup.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krans EE, et al. Factors associated with buprenorphine versus methadone use in pregnancy. Subst Abus. 2016;37(4):550–557. doi: 10.1080/08897077.2016.1146649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 29.Localio AR, Margolis DJ, Berlin JA. Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. J Clin Epidemiol. 2007;60(9):874–82. doi: 10.1016/j.jclinepi.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lash TL, Fink AK. Semi-automated sensitivity analysis to assess systematic errors in observational data. Epidemiology. 2003;14(4):451–8. doi: 10.1097/01.EDE.0000071419.41011.cf. [DOI] [PubMed] [Google Scholar]
- 32.Lash TL, Abrams B, Bodnar LM. Comparison of bias analysis strategies applied to a large data set. Epidemiology. 2014;25(4):576–82. doi: 10.1097/EDE.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flanders WD, Khoury MJ. Indirect assessment of confounding: graphic description and limits on effect of adjusting for covariates. Epidemiology. 1990;1(3):239–46. doi: 10.1097/00001648-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Vanderweele TJ, Arah OA. Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments, and confounders. Epidemiology. 2011;22(1):42–52. doi: 10.1097/EDE.0b013e3181f74493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joyce AR, et al. Response to Smith and Brogly et al. commentaries on Zedler et al. Addiction. 2016;111(12):2131–2133. doi: 10.1111/add.13608. [DOI] [PubMed] [Google Scholar]
- 36.Binder T, Vavrinkova B. Prospective randomised comparative study of the effect of buprenorphine, methadone and heroin on the course of pregnancy, birthweight of newborns, early postpartum adaptation and course of the neonatal abstinence syndrome (NAS) in women followed up in the outpatient department. Neuro Endocrinol Lett. 2008;29(1):80–6. [PubMed] [Google Scholar]
- 37.Wilder CM, Winhusen T. Pharmacological Management of Opioid Use Disorder in Pregnant Women. CNS Drugs. 2015;29(8):625–36. doi: 10.1007/s40263-015-0273-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


