Abstract
The mitochondrial membrane potential (ΔΨm) generated by proton pumps (Complexes I, III and IV) is an essential component in the process of energy storage during oxidative phosphorylation. Together with the proton gradient (ΔpH), ΔΨm forms the transmembrane potential of hydrogen ions which is harnessed to make ATP. The levels of ΔΨm and ATP in the cell are kept relatively stable although there are limited fluctuations of both these factors that can occur reflecting normal physiological activity. However, sustained changes in both factors may be deleterious. A long-lasting drop or rise of ΔΨmvs normal levels may induce unwanted loss of cell viability and be a cause of various pathologies. Among other factors, ΔΨm plays a key role in mitochondrial homeostasis through selective elimination of dysfunctional mitochondria. It is also a driving force for transport of ions (other than H+) and proteins which are necessary for healthy mitochondrial functioning. We propose additional potential mechanisms for which ΔΨm is essential for maintenance of cellular health and viability and provide recommendations how to accurately measure ΔΨm in a cell and discuss potential sources of artifacts.
Keywords: Mitochondria, Transmembrane potential, Heterogeneity, Quality control, Signaling, Mitophagy
Introduction
The mitochondrial membrane potential (ΔΨm) results from redox transformations associated with the activity of the Krebs cycle and serves as an intermediate form of energy storage which is used by ATP synthase to make ATP. These transformations generate not only an electrical potential (because of charge separation) but also a proton gradient, and together they form the transmembrane potential of hydrogen ions [1]. Available data indicate that signaling mechanisms driven by ATP and ΔΨm are different [2]. Normally, cells maintain stable levels of intracellular ATP and ΔΨm, and this stability is thought to be a requisite for normal cell functioning [3–5]. It also suggests that although these parameters can change due to the physiological activity, these changes should be transient, and that a prolonged perturbation of each factor may compromise the viability of the cells, leading to pathological consequences [6]. According to recent findings, ΔΨm can be used not only for ATP synthesis but it is also a factor determining viability of mitochondria participating in a process of elimination of disabled mitochondria. It is also a driving force for transport of charged compounds some of which are essential for mitochondrial viability. These non-energy-producing functions are often treated superficially in the literature. So, it is the purpose of this piece to discuss these other mechanisms which can play critical and even decisive roles in cell maintenance and viability. We will review the known roles of ΔΨm and give recommendations on its accurate measurements in a cell.
The significance of changes in ΔΨm and ATP levels for cellular activity. What is the main basis of the mitochondrial quality control mechanism?
In order not to have significant consequences to the viability of the cells, the concentration of ATP in the cell may vary only in some limited range [7]. When changes are sustained and fall below a threshold value, degenerative processes will result. Perhaps this is partially due to chemical reasons, since favoring ATPase over synthase activity leads to the deleterious net release of hydrogen ions. After a significant drop in the ATP levels, due to the energy supplydemand mismatch caused by insufficient ATP generation and a proportionally larger use of ATP, an intracellular acidosis is observed, accompanied by an essential depletion of intracellular pH buffers. We should note the unsuitability of the conventionally used term, “lactic acidosis” (i.e., excessive accumulation of lactate in the tissue) since the observed acidification in these circumstances in reality is highly dominated by the hydrolytic activity of the ATPase reaction rather than by the excess buildup of formed lactate (see explanations in Ref. [8]). The damaging effect of acidosis is explained at least partially by causing unwanted activation of proteases, nucleases and lipases [9–12], which leads to excessive degradation of cellular components. Given that even with a significant drop in ATP levels in the cell (from the normal low mM levels, to submillimolar concentrations) nearly all ATP-consuming enzymes remain saturated by ATP (because the available Michaelis constants for ATP of the ATP-consuming enzymes are normally orders lower, in the micromolar range), and possibly the products of ATP hydrolysis rather than ATP itself are those regulators that determine the viability of the cells. The idea as to why the cell keeps such a high level of ATP will be discussed later, but for now we note that the degradation of ATP leads not only to acidosis, but also to an increase in the levels of ADP and AMP, and the latter nucleotide is an important component of intracellular regulation due to the activation of AMP kinase which can initiate protective signaling cascades [13]. In any case, the homeostasis of ATP, although functioning across a relatively wide range, is a vital attribute of the cell. It has been hypothesized [6] that there is some sort of ATP-sensor in each kind of cell, responding to even brief but significant drops of ATP levels, potentially leading to cell death.
The direction of the membrane potential (negative inside) in the cell and mitochondria is such that the driving force is preferred for inward transport of cations and outward transport of anions. This property allows accumulation of cations of metals in the mitochondria exerted by intrinsic electrogenic transporters and depending on membrane potential. As examples, we would like to point out the highly reviewed topic of “Ca2+ transport” [14], and that of the much less discussed “transport of iron” (in ferrous (Fe2+) form), from the cytosol to mitochondria. Ca2+ is a well-recognized regulator of mitochondrial respiration [15] and intermediary metabolism [16]. In its turn, the ΔΨm-driven inward mitochondrial transport of Fe2+ together with the mitochondrial iron-sulfur (Fe-S) cluster processing machinery are required for the biogenesis of Fe-S clusters which are crucial cofactors for numerous proteins that play essential role in diverse cellular processes, including electron transport, enzyme catalysis, cofactor biosynthesis, ribosome biogenesis, DNA replication, DNA repair, transcription, and translation [17].
In addition, cations can be transported by antiporter transport systems [18–20] which formally do not depend on membrane potential (e.g., regulated by concentration gradient). As to anions - in particular, anionic substrates of respiration - they are mainly transported by antiporter systems not affected by the magnitude of the membrane potential. Possibly, the thermodynamic restriction for inward transport of anions in energized mitochondria might be evolutionarily determined to create a barrier for penetration of “extrinsic” polyanions (e.g., in the form of nucleic acids). It is known that in mitochondria there are systems for transport of nucleic acids which are necessary for the activities of the organelles [21–25]. The energetics of such inward transport of different nucleic acids is not very clear. Most data is available on the mechanism of tRNAs inward mitochondrial transport. Based on the transport of tRNALys in mitochondria of yeast, two modes of tRNA transport operation were suggested: direct import of a particular nucleic acid and its coimport with some proteinaceous partners. While for the first mode, solely ATP is needed, for the second mode both ATP and membrane potential are essential [26,27]. Albeit the transport of tRNAs was mostly observed in lower eukaryotes, the current suggestion that this mechanism is conserved in higher organisms offers the possibility of mitochondrial inward tRNAs transport across many species from animal kingdom including humans [26,28–30]. The mechanism of release of nucleic acids from mitochondria and cells [31], and particularly, of mitochondrial DNA [32], although justifiable in terms of thermodynamic principle, is quite speculative.
It is clear that mitochondrial proteins are important components of the mechanism involved in regulation of ΔΨm generation and execution of its roles in mitochondria. Now we will switch our focus toward two important proteins residing in mitochondria and playing important role in both formation and regulation of the mitochondrial membrane potential which is apparently relevant to the history of mitochondrial evolution.
To better evaluate the role of the mitochondrial membrane potential (for the mitochondrion and the host cell) it would be instructive to examine the theory of the bacterial origin of mitochondria [33], together with some of the differences in the energetics of mitochondria and their bacterial ancestors. In addition to some inherited bacterial features, mitochondria acquired the requisite synthesis of several proteins that play important role in their relationship with the host cell and the presence of which would have been useless and even harmful for a free-living bacterium in aqueous environment. One of these acquired proteins, is the adenine nucleotide transporter (ANT). The ANT exchanges ATP formed in the matrix for ADP generated by energy-consuming reactions in the cell [34]. Since one molecule of ATP, which has four negative charges (ATP4−), is exchanged for one molecule of ADP, which has three negative charges (ADP3−) it is important to point out that because of the net charge misbalance the equimolar exchange is driven by ΔΨm [35,36]. This exchange is reversible, and the direction of transport depends on the chemical energy of ADP and ATP gradient and ΔΨm. It has been suggested that the electrogenic exchange of ATP4− for ADP3− by ANT can potentially play role in maintaining ΔΨm, for example, in cells depleted of mitochondrial DNA (ρ°-cells), which use glycolysis as their only source of ATP [37].
Another important source of ΔΨm is the reverse operation of ATP synthase (i.e., under ATPase activity) which occurs when ΔΨm cannot be built up by normal operation of a mitochondrial respiratory chain. In order to prevent this reverse ATPase activity and to avoid the excessive consumption of cellular ATP, a remarkable mitochondrial protein, the ATPase inhibitory factor 1 (IF1), has evolved [38–40]. IF1 acts to hinder the counter-clockwise rotor gyration of the ATP synthase by interacting with F1 portion of the ATP synthase complex [41]. Perhaps, the evolution of this protein arose in opposition to the “selfish” behavior of the mitochondrion to use intracellular ATP to maintain ΔΨm. In agreement with this observation, it has been demonstrated that in the presence of IF1, ρ°-cells have a diminished ΔΨm while in the absence of IF1 they could maintain ΔΨm [42].
For mitochondria, which occupy a large percentage of intracellular volume, the ATP/ADP exchange can easily saturate with ATP the limited intracellular volume in contrast to bacteria, living in a relatively unlimited extracellular space. Still, a reasonable question arises, why mitochondria export, and then maintain a relatively very high content of cellular ATP (mM), when most of the ATP-consuming systems, typically with Km only in µM levels, are in a state saturated by ATP which somehow may exclude ATP from being a factor regulating enzymatic activity.
ΔΨm provides the driving force for ATP synthesis in mitochondria. As noted above, the rotational direction of ATP synthase can lead to either the synthesis of ATP at the expense of ΔΨm (at clockwise rotation of ATP synthase complex) or to the hydrolysis of ATP, leading to the generation of ΔΨm (at counterclockwise rotation of ATP synthase complex) [43]. In addition, the membrane potential can be supported by the reverse operation of ANT importing cytosolic ATP into mitochondria in exchange for mitochondrial ADP [44,45]. We may conclude, the higher is the level of intracellular ATP, the more stable are the ΔΨm values, making ATP a compound buffering ΔΨm. The mitochondrion is successfully using this feature under conditions when oxidative phosphorylation is ceased, for example, during hypoxia, when the respiratory chain of mitochondria is stunned [46]. The maintenance of ΔΨm for the expense of the hydrolysis of cytoplasmic ATP potentially indicates a high degree of importance of mitochondrial ΔΨm homeostasis. On the other hand, for cells, such “theft” of ATP in the absence of oxidative ATP synthesis is eventually disadvantageous as this process can be seen as a quite selfish or parasitic manifestation on the part of mitochondria (rather than reflecting the usual symbiotic relationship between the host cell and mitochondria) reminiscent of their bacterial ancestry [47].
Additionally, it is necessary to consider the possible consequences of ΔΨm instability in mitochondria and what ΔΨm-dependent processes are essential for the functioning of mitochondria and the host cell. Such instabilities of ΔΨm have been described [48–51], and occasionally they are attributed to the oscillations of the mitochondrial permeability transition (MPT) [5]. As was suggested, due to depolarization and opening of a megachannel, mitochondria within a short time can release the accumulated unwanted substances, including cations. However, one critical factor is the time the mitochondrion stays in a state of depolarization. ΔΨm flickering (very brief episodes of depolarization) [5,52] may not lead to significant changes in mitochondrial functioning, while prolonged depolarization (the exact time threshold is difficult to establish) leads to a “point of no return” and the mitochondrion as a functional entity dies [53]. It is improbable that the death of a such single mitochondrion has fatal consequence for the cell. In neuron, the MPT induction of 15% of entire mitochondrial population was insufficient to trigger neuronal death [54] showing that under at least certain conditions somewhat massive mitochondria deaths might be required to reach the threshold to trigger cell death. Since the unitary MPT-induced mitochondrial death is associated with release of cytochrome C, AIF and other factors, apparently, the threshold for induction of the cells death is at least in part determined by levels of these deadly factors. Literally speaking, this threshold is the point of no return and depending on severity of the mitochondrial damage, it can induce cell death by apoptosis or, if the damage is extensive, by necrosis [55–59].
However, depolarization not accompanied by induction of MPT is also possible [52]. Particularly, partial depolarization at the transition from the state 4 (corresponding to approximately 180 mV measured in isolated mitochondria [60]) to state 3 (about 150 mV [60] in isolated mitochondria and 108 mV (with 158 mV in resting state) in live neurons [61]) is the normal acceptable physiological process, not accompanied by fatal changes. Durable, and maybe full, depolarization of mitochondria is monitored by the system of mitochondrial quality control with the participation of such players as the mitochondrial kinase PINK1 and the cytosolic E3 ubiquitin ligase Parkin [62]. This results in activation of the process of macroautophagy (mitophagy) where mitochondria are utilized/recycled without inducing cell death. This recycling process happens without the release of mitochondrial apoptotic factors, and, furthermore, such (solitary) events are not accompanied by the triggering of an irreversible cell death cascade.
Optimal values of ΔΨm
It is difficult to determine the “optimal” values of ΔΨm for the cells and mitochondria for the following reasons. On the one hand, the higher the ΔΨm, the higher the energy capacity of the inner mitochondrial membrane and the potentially higher the synthesis of ATP. On the other hand, while the inner membrane is an excellent electrical insulator, the high electric field is difficult (and energetically expensive) to maintain, given the presence of various membrane transport proteins accounting for a large portion of the membrane mass which are capable of transferring different solutes across the membrane.
The occurrence of inner membrane ion leaks could significantly compromise the magnitude of ΔΨm, with the leak being not simply proportional to, but exponentially dependent on ΔΨm [63]. Furthermore, at high ΔΨm the mitochondrial respiratory chain becomes a significant producer of reactive oxygen species (ROS) and the generation of ROS also depends exponentially on ΔΨm [64–67]. Given that excessive production of ROS could directly cause various pathologies [68,69], maintaining excessively high mitochondrial ΔΨm is potentially harmful to mitochondria and consequently to the cell [67]. On the other hand, sustained inappropriately low values of ΔΨ are also dangerous, not only because of the insufficient ability to produce ATP, but potentially also due to a low level of mitochondrial ROS production that could lead to an alternative state to that of oxidative stress, the so-called “reductive stress” [70], which could be as detrimental to homeostasis as oxidative stress.
The mechanism of ΔΨm control involves both the operation of the proton pumps, and the regulation of ΔΨm discharge. The latter can drive the synthesis of ATP coupled to a number of ATP hydrolysis-dependent endergonic reactions [23], or to producing heat resulting from controlled or uncontrolled ion leak across the inner mitochondrial membrane without energy being harnessed to do useful work [71]. If a minor decline in the ΔΨm could lead to a significant decrease in harmful levels of ROS production [65], it is reasonable to hypothesize that the optimal values of ΔΨm, might be achieved by application of “mild uncouplers” which lower ΔΨm to a level still allowing both production of required amounts of ATP, and also of lower ROS levels that would be relatively harmless to the cells [72]. Consequently, under specific cases of poorly regulated ΔΨm and ROS elevations “mild uncouplers” could potentially have beneficial properties in limiting the ravages of a number of diseases, including those associated with aging [73], obesity [74,75] and pathologies accompanied by oxidative stress, such as stroke and heart attack [76–78]. Therefore, an extensive search has been initiated among the compounds with various chemical structures for new drugs which could potentially be tested therapeutically as “mild uncouplers” of oxidative phosphorylation [79–84].
ΔΨm is a part of ΔµH+
The observed non-concerted oscillations of the mitochondrial membrane potential mean that at any given moment of time, the mitochondria in the cell can have different magnitudes of ΔΨm. Either single, or unified in clusters, mitochondrial structures were found to be equipotential [85]. However, even without oscillatory behavior, mitochondria may have different average ΔΨm within a cell or organ [86–88]. This may be a result of physiological or pathological processes whose mechanisms have not been deciphered yet. To understand whether the detected change in ΔΨ is real, physiologically determined, and accompanied by changes in energy status, it is necessary to consider all factors influencing the measured magnitudes of ΔΨm.
The mitochondrial membrane potential (ΔΨm) is only a part of the transmembrane potential energy of the hydrogen ion gradient (ΔµH+) on the inner mitochondrial membrane
where F = Faraday constant.
Just for the reader’s information the widely used term, “protonmotive force” (Δp, typically expressed in mV units) is a parameter directly proportional to the true free energy change, ΔµH+ (formally, ΔµH+ divided by -F (where F is Faraday constant)).
Although the total mitochondrial membrane energy capacity (ΔµH+) could be maintained after a possible increase of the concentration component (ΔpH), accompanied with the decrease in ΔΨm, the latter would cause the retardation of the inward mitochondrial transport of positively charged elements (cations of metals, as well as cationic peptides and other cationic compounds). Furthermore, this situation could result in inward transport of acidic compounds along the pH gradient causing further mitochondrial depolarization and the matrix acidification.
Role of ΔΨm in mitochondrial protein transport and retrograde signaling
Most mitochondrial proteins are encoded in the nucleus, synthesized in cytosol and imported into the mitochondria in a complex fashion that in many cases requires ΔΨm. When ΔΨm is nulled, the transport of proteins into mitochondria can be limited or even suspended. In the earlier work from the lab of Lan Bo Chen, it has been shown that the mitochondrial energetics in some cultured cells is represented mainly by ΔpH [86]; the question then arises if the import of necessary proteins into mitochondria is feasible under these conditions. It had been originally proposed that ~50% of proteins transported into mitochondria do not have a cleavable canonical mitochondrial targeting sequence (MTS; a signal sequence of 12–70 or more amino acids residues at N-terminus with a net positive charge [89]) (e.g., cytochrome c or ATP/ADP translocator [90,91]). A recent systematic N-proteome analysis of yeast mitochondria indicated that up to 70% of mitochondrial proteins are in fact synthesized with a pre-sequence, suggesting that the cleavable pre-sequences are significantly more abundant than previously assessed and thus the pre-sequence pathway could be by far the major pathway of mitochondrial protein import [92].
For import, mitochondrial proteins first use translocases of the outer membrane (TOMs). Some proteins are inserted into the outer membrane while others (e.g., mitochondrial carrier proteins, subunits of the membrane-bound complexes) are either transported into the matrix and processed there or inserted into the inner membrane using TIM translocases, ΔΨm, and ATP for processing the pre-sequence by peptidase, and finally the export of the processed inner membrane protein from matrix to inner membrane requiring ΔpH [93,94], reviewed in Ref. [95]. Thus, for partial reactions of the protein translocation into mitochondria both energy components of the transmembrane potential (ΔΨm and ΔpH) are required.
It is possible that it is the ΔpH which is involved in part of retrograde signaling through release of mitochondrial proteins which then appear in the nucleus [96]. Mitochondrial retrograde signaling represents communication from mitochondria to the nucleus under both normal and pathological conditions and it serves mitochondria to signal their status to the nucleus and cell. Changes in both ΔΨm and ΔpH were shown to trigger the retrograde response although the detailed mechanisms are not known [97]. Perhaps the fluctuation of ΔpH and ΔΨ in time (and ΔΨm oscillations at the expense of its transformation to ΔpH) could trigger or contribute to the efficacy of retrograde signaling, even though fluctuations of ΔΨm do not always reflect the changes in global energetics of mitochondria. To evaluate the total energy capacity of mitochondria it is necessary to evaluate not only ΔΨm, but also ΔpH, which will be discussed later.
Heterogeneity of ΔΨm as a basis for the incidence of pathologies
There is a close relationship between the functional and morphological (ultrastructural) state of the mitochondria [98]. The ultrastructural diversity of mitochondria within an organism, tissue or single cell is striking (reviewed in Ref. [47]). Within a single cell, this diversity is noticeably larger after the onset of certain pathologies [99–101]. Consequently, functional diversity of mitochondria under certain pathological conditions is also larger than under physiological conditions, which was observed at the ultrastructural level (e.g., electron-microscopic patterns of cytochrome oxidase activity in different mitochondria of myopathic cell are very different [102]).
The levels of mitochondrial heterogeneity reflect the different functional state of mitochondria and can be assessed by the magnitude of ΔΨm in mitochondrial suspension [88], and among mitochondria within a single cell ([88,103,104], reviewed in Ref. [105]) and in the tissue [87], or by the redox status, measured by fluorescence of flavin nucleotides [103,106]. Under certain pathological conditions described, the population of mitochondria in the tissue with decreased ΔΨ becomes more pronounced [88]. The latter observation suggests that a population analysis of mitochondria by a distribution of ΔΨm could be examined as potential prognostic factor which may assess the degree of tissue dysfunction or damage in pathologies and aging.
The emergence and increase of heterogeneity of the mitochondrial functional state, estimated by the values of ΔΨm, could result from many factors involved in the development of pathological process. First, we note that a significant portion of pathogenic factors is associated with oxidative stress, which can cause fragmentation (fission) of the mitochondrial network. This asymmetric division process could result in the formation of at least two subpopulations of mitochondria [107,108]. This process of asymmetric distribution of damaged and intact internal contents of mitochondria followed by division of mitochondria themselves is reminiscent of that seen in yeast and bacteria [109–112]. Strong evidence supports the notion that an intrinsic quality control mechanism is triggered that ensures that the population of the low-potential mitochondria will undergo degradation via mitophagy using the previously mentioned mechanism [62]. The appearance of the low-potential mitochondria in the general population of mitochondria in the cell implies that either the mitochondrial quality control system has failed, or the process damaging mitochondria exceeds the capacity to fix/eliminate damaged ones, or there could be a defect in the mechanism of degradation of mitochondria. Some additional, as yet unknown reasons cannot be excluded.
Additionally to the pivotal role played in the elimination of low-potential mitochondria, the mitochondrial quality control system also encounters high-potential (hyperpolarized) mitochondria that could present a serious burden due to their excessive production of ROS. However, the risk occurs only when hyperpolarization is prolonged [113], while short-term hyperpolarization (but not ΔΨm flickering if not accompanied by generation of excessive ROS [52]) can carry a signal function, which can be useful since a short-term small burst of ROS generation can prime (precondition) the system to mitigate the damage from a subsequent significant burst of ROS. However, the correct interpretation of “mitochondrial hyperpolarization” is complex and susceptible to artifacts due to difficulties inherent in measurements of the membrane potential that are usually performed by using fluorescent probes [52]. Under these conditions, these probes are theoretically distributed by the Nernst potential.
where Fin and Fout – the concentration of permeable cations inside and outside of mitochondria, correspondingly, but significant realworld artifacts can and do occur when, e.g., practical dye concentrations exceed certain limits.
In the next chapter, we will discuss the potential drawbacks in available techniques to evaluate the mitochondrial membrane potential.
Measurements of mitochondrial membrane potential: facts and artifacts
Measurement of ΔΨm, values of which are frequently given in the literature, are either made in the suspension of mitochondria or in cells in situ. For suspensions, most often the principle of redistribution of penetrating cations (for mitochondria) or anions (for submitochondrial particles) is used. When energization of these structures is changed, it results in changes of the probe levels in the incubation medium. Measurements are often performed using selective electrodes (e.g., a TPP+-sensitive electrode), or spectral methods, for example, in suspensions of mitochondria by fluorescence changes of probes carrying a delocalized positive charge [114]. Conventionally, high concentrations of fluorophore are used, resulting in a scenario where fully energized mitochondria promote the accumulation of the probe to levels that can result in the selfquenching of the probe's fluorescence [115,116]. Under these conditions, deenergization associated with the release of these fluorescent probes from mitochondria will increase mitochondrial fluorescence due to the process of dequenching of the intramitochondrial localized fluorescence of the residual probe pool. Therefore, in a practical sense, measurements of ΔΨm can be done under non-quenching and quenching conditions depending on the concentration of the fluorescent probe used [117].
The total fluorescence of the probes accumulated in the mitochondria of the cell is determined by many factors. Firstly, these probes are typically more hydrophobic than amphiphilic substances; consequently, in addition to transportation into the mitochondrial matrix, they can non-specifically bind to phospholipid membranes, particularly mitochondrial membranes. This nonspecific binding can be quite high and strongly depending on membrane composition, which may vary [115,118], suggesting an advantage in using the less hydrophobic probes to avoid influence by such artifacts.
Secondly, the fluorescence properties of the probe itself in the mitochondria can be also affected by the cellular membrane potential that should be considered and which can also vary.
Thirdly, there may be an active pumping of different positivelycharged and hydrophobic substances exerted by P-glycoproteins. This process represents the action of the multiple drug resistance (MDR) pumps, affecting the cytosolic content of these substances (including fluorescent probes many of which are good substrates for MDR pumps [119]) which may significantly depend on the activity of these pumps. MDR contribution to the distribution and uptake of these dyes may vary greatly and could be difficult to quantify [120]. MDR activity due to multiplicity of MDR forms and the uncertainty of their operation may significantly hinder the adequate assessment of the level of the membrane potential. The known inhibitors of MDR pumps, such as verapamil, cyclosporine A, or progesterone could be too toxic and/or result in adverse effects on the cell function, so caution is urged regarding their use.
Fourthly, as we have pointed out, variations in the mitochondrial ΔpH make difficult to evaluate the entire transmembrane potential of hydrogen ions, which could be corrected by converting ΔpH into ΔΨm using, for example, nigericin. However, nigericin should be carefully titrated due to potential undesirable artifacts associated with the activation of electrogenic transport of potassium ions into the mitochondria, which together with electroneutral K/H exchange will lead to uncoupling.
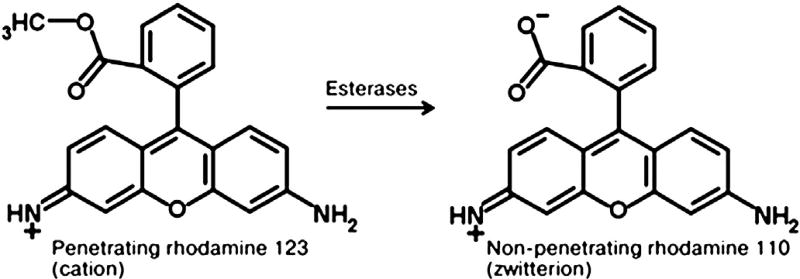
Fifthly, it is necessary to consider the possibility that these membrane potential sensitive probes undergo intracellular modifications, significantly altering the ratio of the fluorescence of the initial probe and its product. This can occur, for example, with lipophilic, cationic rhodamine derivatives, which are frequently represented by ester-derivatives. For intracellular and intramitochondrial esterases, these probes can be suitable substrates, and their deesterification could result in the transformation of a cation into zwitterion which is also the case for number of other permeable dyes used in the form of AM (Fura-AM, Indo-AM, etc.), which after deesterification afford much lower permeability than the original product [121]. As a representative example of these probes Fig. 1 shows rhodamine 123.
Fig. 1.
Possible transition of a permeable rhodamine 123 into impermeable rhodamine 110.
In this case, because of esterase activity, rhodamine 110 might be formed, which is a zwitterion that can be trapped in the cell and the mitochondria due to its low permeability. However, the fluorescence properties of rhodamine 110 (zwitterion) and the rhodamine 123 (cation) are similar. It will lead to an increase in fluorescence in the mitochondria, because rhodamine 123 is accumulated in accordance with the magnitude of ΔΨm, giving a certain level of fluorescence, while the deesterified product (rhodamine 110) could contribute to that fluorescence independently on the membrane potential. Consequently, deesterification could result in the often-observed artifact of a lack of complete release of the probe after mitochondrial deenergization by uncouplers or inhibitors. This can occur if the uncoupler is added after the probe, and not vice versa and could explain the old observation made in the laboratory of Lan Bo Chen on a long retention of fluorescence (attributed that time to the fluorescence of rhodamine 123 only) in the mitochondria of tumor and muscle cells [122]. Although the mechanism of this retention has been studied extensively, it was originally concluded (incorrectly) that these cells and their mitochondria hold elevated levels of membrane potential [123,124]. However, this assumption was flawed since the potential cannot contribute to the permanent binding of the probe to the mitochondria. In this scenario, the extra fluorescence contribution (the “hyperpolarization”) derives from non-Nernstian behavior of the rhodamine 110. Consequently, the data on mechanisms of retention of the cationic dyes must be carefully evaluated, as they require the state of mitochondrial hyperpolarization in every case to be clearly proved. Additionally, the retention of different dyes, including those used in photodynamic therapy (e.g., protoporhyrins derivatives) may also be due to similar intracellular reactions, resulting in that a permeable form after entering the cell (or the mitochondria) producing a non-permeable form, which could be trapped by system [121].
Sixthly, the formed mitochondrial membrane potential could be influenced by mitochondrial ATP production required to support cellular ATP-consuming reactions (endergenic reactions) and other cellular and mitochondrial functions, e.g., activities of respiratory complexes, O2 consumption/respiration, etc. These all need to be carefully evaluated to ensure the correct interpretation of the apparent membrane potential levels obtained [125,126]. For example, supplementation with a mitochondrial ATPase inhibitor, e.g., oligomycin, could eliminate the influence of mitochondrial ATP hydrolysis on the drop of ΔΨm (reviewed in Ref. [127]).
Application and assessment of commonly used fluorescent probes for ΔΨm measurements
We will make a brief overview and assessment of probes most frequently used for evaluation of ΔΨm. The interested reader is recommended to see several excellent reviews and research papers for a more in-depth and critical analysis of different mitochondrial probes [61,126,128–130]. One of the more commonly used types of probes are so called “slow cationic dyes” which, upon being added to cells or isolated mitochondria, become rather slowly distributed between the external and internal mitochondrial space, but this distribution does not always obey the Nernst law. They also cannot quickly respond to fast changes in the membrane potential, but they are reliable for the steady state measurements or for tracking slow changes of ΔΨm. Among them: DiOC6(3), Rhodamine 123 (Rh123), Tetramethylrhodamine ethyl (TMRE) or methyl (TMRM) ester, Nonylacridine orange (NAO), Saphranine O, Merocyanine 540, JC-1 or JC-9 and many others.
From the first glance, JC-1 [131] looks very attractive due to its ability to discriminate low and high membrane potential in mitochondria [132] (where in high-potential mitochondria, the dye forms red-fluorescent J-aggregates, whereas low potential mitochondria possess green fluorescence). However, specialists point out its limited applicability because of the significant and frequent episodes of paradoxically uninterpretable experimental data (e.g., see Ref. [126]). Among its adverse effects are: the changes of fluorescence independent of ΔΨm (including those observed in the presence of H2O2 [133]), the inconvenient necessity to use it as a ratiometric dye which requires special technique, and the high dependence of the results on the peculiarities of the loading protocol. In addition, it is a very efficient photosensitizer which makes not easily interpretable results on the appearance in a single mitochondrial filament of regions with J-aggregates [131] which could be a result of a photodamage associated with mitochondrial segregation and fission [108]. However, most, if not all mitochondrial fluorescent probes suffer from the same critique of being unwanted photosensitizers which sometimes can be exploited [49,134]. Therefore, these probes must be used carefully with the minimal light exposure to minimize photo-generated ROS toxicity.
Although NAO demonstrates a strong ΔΨm-dependency, but its practical use can be limited due to its high toxicity and the fact that it stains both native and fixed samples through an ΔΨm-independent interaction with cardiolipin of the inner mitochondrial membranes (which makes it more usable for determination the mitochondrial mass rather than the membrane potential [135]). But even its use as a marker of cardiolipin or mitochondrial mass has been criticized [136].
Rosamine based MitoTracker dyes (such as MitoTracker Orange CMTMRos and MitoTracker Red CMXRos) also behave similarly to NAO. However, they undergo a multi step conversion starting from non-fluorescent compounds, and after oxidation, they acquire a positive charge and sequestrate inside the mitochondria where they bind to intramitochondrial components. This latter property of these dyes, which is conventionally exploited to track mitochondria in fixed samples, obviously makes them poor reporters of changing mitochondrial ΔΨm. In general, ΔΨm–independent binding of dyes to cellular components is a problem for interpretation of dyes when used as ΔΨm reporter (which requires ΔΨm-unrelated binding of the probe to be considered [115,118]). A significant disadvantage of DiOC6(3) lies in its very high level of non-specific bindings [137]. Some degree of non-specific binding also exists, although to a lesser extent, for rhodamine-based probes in the order TMRE > Rh123 > TMRM [115].
In light of this brief critical review, we suggest that TMRM may represent a reasonable set of compromises and can provide a valid estimation of ΔΨm under carefully controlled conditions.
Conclusions and perspectives
At first glance, it seems that the conflict between the hypothesis that the quality control system of mitochondria is based on the evaluation of the magnitude of the membrane potential and the data that some proteins can be transported without membrane potential may yield a conclusion that it is not a protein transport which is a factor that requires homeostasis of ΔΨm. However, it should be noted that the ΔΨm threshold for transport of proteins into mitochondria is low and 20–40 mV is sufficient to motivate this process [138]; these magnitudes of ΔΨm, in principle can persist even when using uncouplers that may not dissipate the entire ΔΨm, although such low values are very difficult to measure [139]. It is generally assumed that the vectorial protein import is ceased in the presence of uncouplers in the system, although binding of the immature preprotein to the TOM complex does not require energy of ATP or ΔΨm [140], and in the absence of energy, translocation of proteins is stunned at this stage [141]. This property is successfully utilized by the machinery of the mitochondrial quality control system, the Pink1-Parkin system. PINK1 is typically imported into energized mitochondria and subsequently degraded. However, when the membrane potential drops, PINK1 remains bound to the TOM complex thus recruiting Parkin. Parkindependent ubiquitination induces the organelle specific degradation by mitophagy [62].
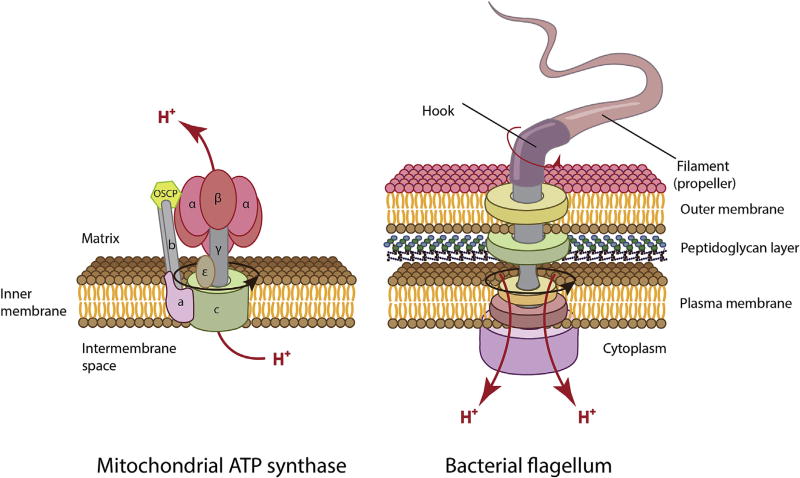
The current level of knowledge about the systems that are harnessing ΔΨm and processes that are controlled by ΔΨm in mitochondria highlights two aspects that are of prime importance. First is the vectorial transport of charged compounds (inward transport of positive compounds and outward transport of negative ones), including electrogenic transport of cations (e.g., Ca2+, which can serve as a powerful bioenergetic regulator of mitochondrial bioenergetics [15]). The second is the harnessing of ΔΨm to create torque used for ATP synthesis [43]. This latter aspect is an important component of bioenergetics. Bacteria are using membrane potential not only to generate rotary torque required for ATP synthesis [142] but also for mechanical translocation via the bacterial flagellum [143] while mitochondria are using ΔΨm to power only the rotor in ATP synthase machinery [43,144] without harnessing it for motility. The flagellar motor couples ion flow across the cytoplasmic membrane to rotation and this flow is driven by both the membrane potential and the transmembrane ion concentration gradient. Interestingly, it has been suggested that the flagellar motor of archaeon Halobacterium salinarum is driven directly by ATP and is not coupled to the proton gradient or the ion motive force of the cell [145]. For comparison, both mechanisms supported by ΔΨ are shown in Fig. 2. In evolutionary terms, the presence of electrochemical potential of hydrogen ions, and, in particular, ΔΨm as an intermediate of ATP generation provides an additional control function over transmembrane transport. This may give some advantages over purely chemical processes of energy generation (e.g., substrate-level phosphorylation, where protonmotive force is irrelevant). Given this, it seems that the vital necessity of preserving the energy components of mitochondria, primarily ΔΨm and associated with it ΔpH is their involvement in the ATP synthesis and as a driving force of the import of the cations and proteins. ΔΨm is implicated in many additional functions, adding further complexity and diversity to the role of mitochondria in cellular homeostasis, including the mitochondrial quality control, ROS generation, MPT pore stabilization and maintenance of the retrograde signaling through mitochondrial export of negatively charged molecules including DNA [146]. Furthermore, ΔΨm impacts directly or secondarily various cellular processes, e.g., production of heat, control of redox and pH microenvironments, proliferation, cell death, etc., where the mechanism of ΔΨm involvement remains rather obscure and is beyond the scope of this review.
Fig. 2.
Transformation of ΔΨ into energy of a rotary motion using a rotor of ATP synthase (left) and bacterial flagellum (right).
Acknowledgments
The study was supported by the Russian Science Foundation (14-15-00147) and in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Abbreviations
- ΔΨm
mitochondrial membrane potential
- ROS
reactive oxygen species
- MDR
multiple drug resistance
- MPT
mitochondrial permeability transition.
Footnotes
The authors claim absence of conflict of interests.
References
- 1.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. Camb. Philosophical Soc. 1966;41:445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 2.Glagolev AN, Skulachev VP. The proton pump is a molecular engine of motile bacteria. Nature. 1978;272:280–282. doi: 10.1038/272280a0. [DOI] [PubMed] [Google Scholar]
- 3.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaniv Y, Juhaszova M, Nuss HB, Wang S, Zorov DB, Lakatta EG, Sollott SJ. Matching ATP supply and demand in mammalian heart: in vivo, in vitro, and in silico perspectives. Ann. N. Y. Acad. Sci. 2010;1188:133–142. doi: 10.1111/j.1749-6632.2009.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izyumov DS, Avetisyan AV, Pletjushkina OY, Sakharov DV, Wirtz KW, Chernyak BV, Skulachev VP. “Wages of fear”: transient threefold decrease in intracellular ATP level imposes apoptosis. Biochimica Biophysica Acta. 2004;1658:141–147. doi: 10.1016/j.bbabio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Ataullakhanov FI, Vitvitsky VM. What determines the intracellular ATP concentration. Biosci. Rep. 2002;22:501–511. doi: 10.1023/a:1022069718709. [DOI] [PubMed] [Google Scholar]
- 8.Silachev DN, Gulyaev MV, Zorova LD, Khailova LS, Gubsky LV, Pirogov YA, Plotnikov EY, Sukhikh GT, Zorov DB. Magnetic resonance spectroscopy of the ischemic brain under lithium treatment. Link to mitochondrial disorders under stroke. Chemico-Biological Interact. 2015;237:175–182. doi: 10.1016/j.cbi.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Rizack MA. An epinephrine-sensitive lipolytic activity in adipose tissue. J. Biol. Chem. 1964;236:657–662. [PubMed] [Google Scholar]
- 10.Eastman A. Deoxyribonuclease II in apoptosis and the significance of intracellular acidification. Cell Death Differ. 1994;1:7–9. [PubMed] [Google Scholar]
- 11.Gottlieb RA, Giesing HA, Zhu JY, Engler RL, Babior BM. Cell acidification in apoptosis: granulocyte colony-stimulating factor delays programmed cell death in neutrophils by up-regulating the vacuolar H(+)-ATPase. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5965–5968. doi: 10.1073/pnas.92.13.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morana SJ, Wolf CM, Li J, Reynolds JE, Brown MK, Eastman A. The involvement of protein phosphatases in the activation of ICE/CED-3 protease, intracellular acidification, DNA digestion, and apoptosis. J. Biol. Chem. 1996;271:18263–18271. doi: 10.1074/jbc.271.30.18263. [DOI] [PubMed] [Google Scholar]
- 13.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem. Sci. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 14.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 15.Hansford RG, Zorov D. Role of mitochondrial calcium transport in the control of substrate oxidation. Mol. Cell. Biochem. 1998;184:359–369. [PubMed] [Google Scholar]
- 16.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 17.Zorov DB, Isaev NK, Plotnikov EY, Zorova LD, Stelmashook EV, Vasileva AK, Arkhangelskaya AA, Khrjapenkova TG. The mitochondrion as janus bifrons. Biochem. Biokhimiia. 2007;72:1115–1126. doi: 10.1134/s0006297907100094. [DOI] [PubMed] [Google Scholar]
- 18.Dordick RS, Brierley GP, Garlid KD. On the mechanism of A23187-induced potassium efflux in rat liver mitochondria. J. Biol. Chem. 1980;255:10299–10305. [PubMed] [Google Scholar]
- 19.Shi GY, Jung DW, Brierley GP. Induction of Na+/K+ exchange in swollen heart mitochondria. J. Bioenergetics Biomembr. 1980;12:233–247. doi: 10.1007/BF00744686. [DOI] [PubMed] [Google Scholar]
- 20.Gunter KK, Gunter TE. Transport of calcium by mitochondria. J. Bioenergetics Biomembr. 1994;26:471–485. doi: 10.1007/BF00762732. [DOI] [PubMed] [Google Scholar]
- 21.Nagley P. Trafficking in small mitochondrial RNA molecules. Trends Genet. TIG. 1989;5:67–69. doi: 10.1016/0168-9525(89)90028-0. [DOI] [PubMed] [Google Scholar]
- 22.Topper JN, Bennett JL, Clayton DA. A role for RNAase MRP in mitochondrial RNA processing. Cell. 1992;70:16–20. doi: 10.1016/0092-8674(92)90529-l. [DOI] [PubMed] [Google Scholar]
- 23.Zorov DB. Mitochondrial damage as a source of diseases and aging: a strategy of how to fight these. Biochimica Biophysica Acta. 1996;1275:10–15. doi: 10.1016/0005-2728(96)00042-4. [DOI] [PubMed] [Google Scholar]
- 24.Entelis NS, Kolesnikova OA, Martin RP, Tarassov IA. RNA delivery into mitochondria. Adv. Drug Deliv. Rev. 2001;49:199–215. doi: 10.1016/s0169-409x(01)00135-1. [DOI] [PubMed] [Google Scholar]
- 25.Koulintchenko M, Konstantinov Y, Dietrich A. Plant mitochondria actively import DNA via the permeability transition pore complex. EMBO J. 2003;22:1245–1254. doi: 10.1093/emboj/cdg128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider A. Mitochondrial tRNA import and its consequences for mitochondrial translation. Annu. Rev. Biochem. 2011;80:1033–1053. doi: 10.1146/annurev-biochem-060109-092838. [DOI] [PubMed] [Google Scholar]
- 27.Tarassov I, Entelis N, Martin RP. An intact protein translocating machinery is required for mitochondrial import of a yeast cytoplasmic tRNA. J. Mol. Biol. 1995;245:315–323. doi: 10.1006/jmbi.1994.0026. [DOI] [PubMed] [Google Scholar]
- 28.Salinas-Giege T, Giege R, Giege P. tRNA biology in mitochondria. Int. J. Mol. Sci. 2015;16:4518–4559. doi: 10.3390/ijms16034518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahata B, Mukherjee S, Mishra S, Bandyopadhyay A, Adhya S. Functional delivery of a cytosolic tRNA into mutant mitochondria of human cells. Science. 2006;3141:471–474. doi: 10.1126/science.1129754. [DOI] [PubMed] [Google Scholar]
- 30.Rubio MA, Rinehart JJ, Krett B, Duvezin-Caubet S, Reichert AS, Soll D, Alfonzo JD. Mammalian mitochondria have the innate ability to import tRNAs by a mechanism distinct from protein import. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9186–9191. doi: 10.1073/pnas.0804283105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 32.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 33.Andersson SG, Kurland CG. Origins of mitochondria and hydrogenosomes. Curr. Opin. Microbiol. 1999;2:535–541. doi: 10.1016/s1369-5274(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 34.Weidemann MJ, Erdelt H, Klingenberg M. Adenine nucleotide translocation of mitochondria. Identification of carrier sites. Eur. J. Biochem. 1970;16:313–335. doi: 10.1111/j.1432-1033.1970.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 35.Gropp T, Brustovetsky N, Klingenberg M, Muller V, Fendler K, Bamberg E. Kinetics of electrogenic transport by the ADP/ATP carrier. Biophysical J. 1999;77:714–726. doi: 10.1016/S0006-3495(99)76926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaNoue K, Mizani SM, Klingenberg M. Electrical imbalance of adenine nucleotide transport across the mitochondrial membrane. J. Biol. Chem. 1978;253:191–198. [PubMed] [Google Scholar]
- 37.Appleby RD, Porteous WK, Hughes G, James AM, Shannon D, Wei YH, Murphy MP. Quantitation and origin of the mitochondrial membrane potential in human cells lacking mitochondrial DNA. Eur. J. Biochem. 1999;262:108–116. doi: 10.1046/j.1432-1327.1999.00350.x. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Bermudez J, Cuezva JM. The ATPase Inhibitory Factor 1 (IF1): a master regulator of energy metabolism and of cell survival. Biochimica Biophysica Acta. 2016;1857:1167–1182. doi: 10.1016/j.bbabio.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Pullman ME, Monroy GC. A naturally occurring inhibitor of mitochondrial adenosine triphosphatase. J. Biol. Chem. 1963;238:3762–3769. [PubMed] [Google Scholar]
- 40.Hashimoto T, Yoshida Y, Tagawa K. Regulatory proteins of F1F0-ATPase: role of ATPase inhibitor. J. Bioenergetics Biomembr. 1990;22:27–38. doi: 10.1007/BF00762843. [DOI] [PubMed] [Google Scholar]
- 41.Gledhill JR, Montgomery MG, Leslie AG, Walker JE. How the regulatory protein, IF(1)inhibits F(1)-ATPase from bovine mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15671–15676. doi: 10.1073/pnas.0707326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez-Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, Mehta MM, Wang T, Santos JH, Woychik R, Dufour E, Spelbrink JN, Weinberg SE, Zhao Y, DeBerardinis RJ, Chandel NS. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol. Cell. 2016;61:199–209. doi: 10.1016/j.molcel.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker JE. The ATP synthase: the understood, the uncertain and the unknown. Biochem. Soc. Trans. 2013;41:1–16. doi: 10.1042/BST20110773. [DOI] [PubMed] [Google Scholar]
- 44.Chinopoulos C. Mitochondrial consumption of cytosolic ATP: not so fast. FEBS Lett. 2011;585:1255–1259. doi: 10.1016/j.febslet.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Chinopoulos C. The“B space” of mitochondrial phosphorylation. J. Neurosci. Res. 2011;89:1897–1904. doi: 10.1002/jnr.22659. [DOI] [PubMed] [Google Scholar]
- 46.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J. Physiol. 1995;486(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zorov DB, Plotnikov EY, Silachev DN, Zorova LD, Pevzner IB, Zorov SD, Babenko VA, Jankauskas SS, Popkov VA, Savina PS. Microbiota and mitobiota. Putting an equal sign between mitochondria and bacteria. Biochem. Biokhimiia. 2014;79:1017–1031. doi: 10.1134/S0006297914100046. [DOI] [PubMed] [Google Scholar]
- 48.Huser J, Rechenmacher CE, Blatter LA. Imaging the permeability pore transition in single mitochondria. Biophysical J. 1998;74:2129–2137. doi: 10.1016/S0006-3495(98)77920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slodzinski MK, Aon MA, O'Rourke B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. J. Mol. Cell. Cardiol. 2008;45:650–660. doi: 10.1016/j.yjmcc.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krippeit-Drews P, Dufer M, Drews G. Parallel oscillations of intracellular calcium activity and mitochondrial membrane potential in mouse pancreatic B-cells. Biochem. Biophysical Res. Commun. 2000;267:179–183. doi: 10.1006/bbrc.1999.1921. [DOI] [PubMed] [Google Scholar]
- 52.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochimica Biophysica Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 53.Zorov DB, Kinnally KW, Tedeschi H. Voltage activation of heart inner mitochondrial membrane channels. J. Bioenergetics Biomembr. 1992;24:119–124. doi: 10.1007/BF00769538. [DOI] [PubMed] [Google Scholar]
- 54.Khodjakov A, Rieder C, Mannella CA, Kinnally KW. Laser micro-irradiation of mitochondria: is there an amplified mitochondrial death signal in neural cells? Mitochondrion. 2004;3:217–227. doi: 10.1016/j.mito.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Kroemer G, Petit P, Zamzami N, Vayssiere JL, Mignotte B. The biochemistry of programmed cell death. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995;9:1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- 56.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc. Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 57.Zamzami N, Larochette N, Kroemer G. Mitochondrial permeability transition in apoptosis and necrosis. Cell death Differ. 2005;12(Suppl 2):1478–1480. doi: 10.1038/sj.cdd.4401682. [DOI] [PubMed] [Google Scholar]
- 58.Baines CP. How and when do myocytes die during ischemia and reperfusion: the late phase. J. Cardiovasc. Pharmacol. Ther. 2011;16:239–243. doi: 10.1177/1074248411407769. [DOI] [PubMed] [Google Scholar]
- 59.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochimica Biophysica Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 60.Kamo N, Muratsugu M, Hongoh R, Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J. Membr. Biol. 1979;49:105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- 61.Gerencser AA, Chinopoulos C, Birket MJ, Jastroch M, Vitelli C, Nicholls DG, Brand MD. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. J. Physiol. 2012;590:2845–2871. doi: 10.1113/jphysiol.2012.228387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brand MD, Chien LF, Ainscow EK, Rolfe DF, Porter RK. The causes and functions of mitochondrial proton leak. Biochimica Biophysica Acta. 1994;1187:132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 64.Liu SS, Huang JP. Co-existence of “reactive oxygen species” with Q-cycle and proton cycle in respiratory chain of mitochondria. In: Parker L, Traber MG, Xin WJ, Champaighn Il, editors. Proceedings of the International Symposium on Natural Antioxidants. Molecular Mechanisms and Health Effects. AOCS; 1996. pp. 511–529. [Google Scholar]
- 65.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 66.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 67.Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophysics. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- 68.Zorov DB, Bannikova SY, Belousov VV, Vyssokikh MY, Zorova LD, Isaev NK, Krasnikov BF, Plotnikov EY. Reactive oxygen and nitrogen species: friends or foes? Biochem. Biokhimiia. 2005;70:215–221. doi: 10.1007/s10541-005-0103-6. [DOI] [PubMed] [Google Scholar]
- 69.Zorov DB, Isaev NK, Plotnikov EY, Silachev DN, Zorova LD, Pevzner IB, Morosanova MA, Jankauskas SS, Zorov SD, Babenko VA. Perspectives of mitochondrial medicine. Biochem. Biokhimiia. 2013;78:979–990. doi: 10.1134/S0006297913090034. [DOI] [PubMed] [Google Scholar]
- 70.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human alpha B-crystallin mutation causes oxidoreductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Divakaruni AS, Brand MD. The regulation and physiology of mitochondrial proton leak. Physiology. 2011;26:192–205. doi: 10.1152/physiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 72.Cunha FM, Caldeira da Silva CC, Cerqueira FM, Kowaltowski AJ. Mild mitochondrial uncoupling as a therapeutic strategy. Curr. Drug Targets. 2011;12:783–789. doi: 10.2174/138945011795528778. [DOI] [PubMed] [Google Scholar]
- 73.Padalko VI. Uncoupler of oxidative phosphorylation prolongs the lifespan of Drosophila. Biochem. Biokhimiia. 2005;70:986–989. doi: 10.1007/s10541-005-0213-1. [DOI] [PubMed] [Google Scholar]
- 74.Kalinovich AV, Shabalina IG. Novel mitochondrial cationic uncoupler C4R1 is an effective treatment for combating obesity in mice. Biochem. Biokhimiia. 2015;80:620–628. doi: 10.1134/S0006297915050156. [DOI] [PubMed] [Google Scholar]
- 75.Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul. Toxicol. Pharmacol. RTP. 2007;48:115–117. doi: 10.1016/j.yrtph.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 76.Plotnikov EY, Silachev DN, Jankauskas SS, Rokitskaya TI, Chupyrkina AA, Pevzner IB, Zorova LD, Isaev NK, Antonenko YN, Skulachev VP, Zorov DB. Mild uncoupling of respiration and phosphorylation as a mechanism providing nephro- and neuroprotective effects of penetrating cations of the SkQ family. Biochem. Biokhimiia. 2012;77:1029–1037. doi: 10.1134/S0006297912090106. [DOI] [PubMed] [Google Scholar]
- 77.De Felice FG, Ferreira ST. Novel neuroprotective, neuritogenic and antiamyloidogenic properties of 2,4-dinitrophenol: the gentle face of Janus. IUBMB life. 2006;58:185–191. doi: 10.1080/15216540600702198. [DOI] [PubMed] [Google Scholar]
- 78.Holmuhamedov EL, Jahangir A, Oberlin A, Komarov A, Colombini M, Terzic A. Potassium channel openers are uncoupling protonophores: implication in cardioprotection. FEBS Lett. 2004;568:167–170. doi: 10.1016/j.febslet.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 79.Antonenko YN, Denisov SS, Silachev DN, Khailova LS, Jankauskas SS, Rokitskaya TI, Danilina TI, Kotova EA, Korshunova GA, Plotnikov EY, Zorov DB. A long-linker conjugate of fluorescein and triphenylphosphonium as mitochondria-targeted uncoupler and fluorescent neuro- and nephroprotector. Biochimica Biophysica Acta. 2016;1860:2463–2473. doi: 10.1016/j.bbagen.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 80.Rokitskaya TI, Antonenko YN. Fullerenol C60(OH)24 increases ion permeability of lipid membranes in a pH-dependent manner. Biochimica Biophysica Acta. 2016;1858:1165–1174. doi: 10.1016/j.bbamem.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 81.Antonenko YN, Nechaeva NL, Baksheeva VE, Rokitskaya TI, Plotnikov EY, Kotova EA, Zorov DB. Intramitochondrial accumulation of cationic Atto520-biotin proceeds via voltage-dependent slow permeation through lipid membrane. Biochimica Biophysica Acta. 2015;1848:1277–1284. doi: 10.1016/j.bbamem.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 82.Denisov SS, Kotova EA, Plotnikov EY, Tikhonov AA, Zorov DB, Korshunova GA, Antonenko YN. A mitochondria-targeted protonophoric uncoupler derived from fluorescein. Chem. Commun. 2014;50:15366–15369. doi: 10.1039/c4cc04996a. [DOI] [PubMed] [Google Scholar]
- 83.Silachev DN, Khailova LS, Babenko VA, Gulyaev MV, Kovalchuk SI, Zorova LD, Plotnikov EY, Antonenko YN, Zorov DB. Neuroprotective effect of glutamate-substituted analog of gramicidin A is mediated by the uncoupling of mitochondria. Biochimica Biophysica Acta. 2014;1840:3434–3442. doi: 10.1016/j.bbagen.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Khailova LS, Silachev DN, Rokitskaya TI, Avetisyan AV, Lyamsaev KG, Severina II, Il'yasova TM, Gulyaev MV, Dedukhova VI, Trendeleva TA, Plotnikov EY, Zvyagilskaya RA, Chernyak BV, Zorov DB, Antonenko YN, Skulachev VP. A short-chain alkyl derivative of Rhodamine 19 acts as a mild uncoupler of mitochondria and a neuroprotector. Biochimica Bbiophysica Acta. 2014;1837:1739–1747. doi: 10.1016/j.bbabio.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Amchenkova AA, Bakeeva LE, Chentsov YS, Skulachev VP, Zorov DB. Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. J. Cell Biol. 1988;107:481–495. doi: 10.1083/jcb.107.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson LV, Walsh ML, Bockus BJ, Chen LB. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J. Cell Biol. 1981;88:526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, Kirpatovsky VI, Zorov DB. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007;72:1493–1502. doi: 10.1038/sj.ki.5002568. [DOI] [PubMed] [Google Scholar]
- 88.Popkov VA, Plotnikov EY, Lyamzaev KG, Silachev DN, Zorova LD, Pevzner IB, Jankauskas SS, Zorov SD, Babenko VA, Zorov DB. Mitodiversity. Biochem. Biokhimiia. 2015;80:532–541. doi: 10.1134/S000629791505003X. [DOI] [PubMed] [Google Scholar]
- 89.Attardi G, Schatz G. Biogenesis of mitochondria. Annu. Rev. Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- 90.Zimmerman R, Paluch U, Sprinzl M, Neupert W. Cell-free synthesis of the mitochondrial ADP/ATP carrier protein of Neurospora crassa. Eur. J. Biochem. 1979;99:247–252. doi: 10.1111/j.1432-1033.1979.tb13251.x. [DOI] [PubMed] [Google Scholar]
- 91.Zimmermann R, Paluch U, Neupert W. Cell-free synthesis of cytochrome c. FEBS Lett. 1979;108:141–146. doi: 10.1016/0014-5793(79)81196-5. [DOI] [PubMed] [Google Scholar]
- 92.Vogtle FN, Wortelkamp S, Zahedi RP, Becker D, Leidhold C, Gevaert K, Kellermann J, Voos W, Sickmann A, Pfanner N, Meisinger C. Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell. 2009;139:428–439. doi: 10.1016/j.cell.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 93.Rojo EE, Stuart RA, Neupert W. Conservative sorting of F0-ATPase subunit 9: export from matrix requires delta pH across inner membrane and matrix ATP. EMBO J. 1995;14:3445–3451. doi: 10.1002/j.1460-2075.1995.tb07350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 95.Kulawiak B, Hopker J, Gebert M, Guiard B, Wiedemann N, Gebert N. The mitochondrial protein import machinery has multiple connections to the respiratory chain. Biochimica Biophysica Acta. 2013;1827:612–626. doi: 10.1016/j.bbabio.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Monaghan RM, Whitmarsh AJ. Mitochondrial proteins moonlighting in the nucleus. Trends Biochem. Sci. 2015;40:728–735. doi: 10.1016/j.tibs.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 97.Kleine T, Leister D. Retrograde signaling: organelles go networking. Biochimica Biophysica Acta. 2016;1857:1313–1325. doi: 10.1016/j.bbabio.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 98.Hackenbrock CR. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J. Cell Biol. 1966;30:269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vital A, Vital C. Mitochondria and peripheral neuropathies. J. Neuropathol. Exp. neurology. 2012;71:1036–1046. doi: 10.1097/NEN.0b013e3182764d47. [DOI] [PubMed] [Google Scholar]
- 100.Sarnat HB, Flores-Sarnat L, Casey R, Scott P, Khan A. Endothelial ultrastructural alterations of intramuscular capillaries in infantile mitochondrial cytopathies: “mitochondrial angiopathy”. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2012;32:617–627. doi: 10.1111/j.1440-1789.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 101.Behbehani AW, Goebel H, Osse G, Gabriel M, Langenbeck U, Berden J, Berger R, Schutgens RB. Mitochondrial myopathy with lactic acidosis and deficient activity of muscle succinate cytochrome-c-oxidoreductase. Eur. J. Pediatr. 1984;143:67–71. doi: 10.1007/BF00442753. [DOI] [PubMed] [Google Scholar]
- 102.van Ekeren GJ, Stadhouders AM, Egberink GJ, Sengers RC, Daniels O, Kubat K. Hereditary mitochondrial hypertrophic cardiomyopathy with mitochondrial myopathy of skeletal muscle, congenital cataract and lactic acidosis, Virchows Archiv. Pathological Anat. Histopathol. 1987;412:47–52. doi: 10.1007/BF00750730. [DOI] [PubMed] [Google Scholar]
- 103.Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 2002;21:1616–1627. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuznetsov AV, Troppmair J, Sucher R, Hermann M, Saks V, Margreiter R. Mitochondrial subpopulations and heterogeneity revealed by confocal imaging: possible physiological role? Biochimica Biophysica Acta. 2006;1757:686–691. doi: 10.1016/j.bbabio.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 105.Kuznetsov AV, Margreiter R. Heterogeneity of mitochondria and mitochondrial function within cells as another level of mitochondrial complexity. Int. J. Mol. Sci. 2009;10:1911–1929. doi: 10.3390/ijms10041911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Romashko DN, Marban E, O'Rourke B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1618–1623. doi: 10.1073/pnas.95.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vorobjev IA, Zorov DB. Diazepam inhibits cell respiration and induces fragmentation of mitochondrial reticulum. FEBS Lett. 1983;163:311–314. doi: 10.1016/0014-5793(83)80842-4. [DOI] [PubMed] [Google Scholar]
- 108.Zorov DB, Popkov VA, Zorova LD, Vorobjev IA, Pevzner IB, Silachev DN, Zorov SD, Jankauskas SS, Babenko VA, Plotnikov EY. Mitochondrial aging; is there a mitochondiral clock? J. Gerontol. A. Biol. Sci. Med. Sci. 2017 doi: 10.1093/gerona/glw184. http://dx.doi.org/10.1093/gerona/glw184. [DOI] [PubMed]
- 109.Shapiro L, McAdams HH, Losick R. Generating and exploiting polarity in bacteria. Science. 2002;298:1942–1946. doi: 10.1126/science.1072163. [DOI] [PubMed] [Google Scholar]
- 110.Barker MG, Walmsley RM. Replicative ageing in the fission yeast Schizosaccharomyces pombe. Yeast. 1999;15:1511–1518. doi: 10.1002/(sici)1097-0061(199910)15:14<1511::aid-yea482>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 111.Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 112.Katajisto P, Dohla J, Chaffer CL, Pentinmikko N, Marjanovic N, Iqbal S, Zoncu R, Chen W, Weinberg RA, Sabatini DM. Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science. 2015;348:340–343. doi: 10.1126/science.1260384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gergely P, Jr, Niland B, Gonchoroff N, Pullmann R, Jr, Phillips PE, Perl A. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J. Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature. 1969;222:1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 115.Scaduto RC, Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophysical J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O'Reilly CM, Fogarty KE, Drummond RM, Tuft RA, Walsh JV., Jr Quantitative analysis of spontaneous mitochondrial depolarizations. Biophysical J. 2003;85:3350–3357. doi: 10.1016/S0006-3495(03)74754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nicholls DG, Ward MW. Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci. 2000;23:166–174. doi: 10.1016/s0166-2236(99)01534-9. [DOI] [PubMed] [Google Scholar]
- 118.Brauner T, Hulser DF, Strasser RJ. Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes. Biochimica Biophysica Acta. 1984;771:208–216. doi: 10.1016/0005-2736(84)90535-2. [DOI] [PubMed] [Google Scholar]
- 119.Forster S, Thumser AE, Hood SR, Plant N. Characterization of rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PloS One. 2012;7:e33253. doi: 10.1371/journal.pone.0033253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Diaz G, Diana A, Falchi AM, Gremo F, Pani A, Batetta B, Dessi S, Isola R. Intra- and intercellular distribution of mitochondrial probes and changes after treatment with MDR modulators. IUBMB life. 2001;51:121–126. doi: 10.1080/15216540119470. [DOI] [PubMed] [Google Scholar]
- 121.Fugit KD, Anderson BD. The role of pH and ring-opening hydrolysis kinetics on liposomal release of topotecan. J. Control. Release Off. J. Control. Release Soc. 2014;174:88–97. doi: 10.1016/j.jconrel.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Summerhayes IC, Lampidis TJ, Bernal SD, Nadakavukaren JJ, Nadakavukaren KK, Shepherd EL, Chen LB. Unusual retention of rhodamine 123 by mitochondria in muscle and carcinoma cells. Proc. Natl. Acad. Sci. U. S. A. 1982;79:5292–5296. doi: 10.1073/pnas.79.17.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Davis S, Weiss MJ, Wong JR, Lampidis TJ, Chen LB. Mitochondrial and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by human breast adenocarcinoma-derived MCF-7 cells. J. Biol. Chem. 1985;260:13844–13850. [PubMed] [Google Scholar]
- 124.Modica-Napolitano JS, Aprille JR. Basis for the selective cytotoxicity of rhodamine 123. Cancer Res. 1987;47:4361–4365. [PubMed] [Google Scholar]
- 125.Lemasters JJ, Ramshesh VK. Imaging of mitochondrial polarization and depolarization with cationic fluorophores. Methods Cell Biol. 2007;80:283–295. doi: 10.1016/S0091-679X(06)80014-2. [DOI] [PubMed] [Google Scholar]
- 126.Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. BioTechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davidson SM, Yellon D, Duchen MR. Assessing mitochondrial potential, calcium, and redox state in isolated mammalian cells using confocal microscopy. Methods Mol. Biol. 2007;372:421–430. doi: 10.1007/978-1-59745-365-3_30. [DOI] [PubMed] [Google Scholar]
- 129.Solaini G, Sgarbi G, Lenaz G, Baracca A. Evaluating mitochondrial membrane potential in cells. Biosci. Rep. 2007;27:11–21. doi: 10.1007/s10540-007-9033-4. [DOI] [PubMed] [Google Scholar]
- 130.Metivier D, Dallaporta B, Zamzami N, Larochette N, Susin SA, Marzo I, Kroemer G. Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1-triggered apoptosis of Jurkat T lymphoma cells. Comparison of seven mitochondrion-specific fluorochromes. Immunol. Lett. 1998;61:157–163. doi: 10.1016/s0165-2478(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 131.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- 133.Chinopoulos C, Tretter L, Adam-Vizi V. Depolarization of in situ mitochondria due to hydrogen peroxide-induced oxidative stress in nerve terminals: inhibition of alpha-ketoglutarate dehydrogenase. J. Neurochem. 1999;73:220–228. doi: 10.1046/j.1471-4159.1999.0730220.x. [DOI] [PubMed] [Google Scholar]
- 134.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Investigation. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maftah A, Petit JM, Ratinaud MH, Julien R. 10-N nonyl-acridine orange: a fluorescent probe which stains mitochondria independently of their energetic state. Biochem. Biophysical Res. Commun. 1989;164:185–190. doi: 10.1016/0006-291x(89)91700-2. [DOI] [PubMed] [Google Scholar]
- 136.Jacobson J, Duchen MR, Heales SJ. Intracellular distribution of the fluorescent dye nonyl acridine orange responds to the mitochondrial membrane potential: implications for assays of cardiolipin and mitochondrial mass. J. Neurochem. 2002;82:224–233. doi: 10.1046/j.1471-4159.2002.00945.x. [DOI] [PubMed] [Google Scholar]
- 137.Terasaki M, Song J, Wong JR, Weiss MJ, Chen LB. Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell. 1984;38:101–108. doi: 10.1016/0092-8674(84)90530-0. [DOI] [PubMed] [Google Scholar]
- 138.Pfanner N, Neupert W. Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. EMBO J. 1985;4:2819–2825. doi: 10.1002/j.1460-2075.1985.tb04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holoubek A, Vecer J, Opekarova M, Sigler K. Ratiometric fluorescence measurements of membrane potential generated by yeast plasma membrane H(+)-ATPase reconstituted into vesicles. Biochimica Biophysica Acta. 2003;1609:71–79. doi: 10.1016/s0005-2736(02)00656-9. [DOI] [PubMed] [Google Scholar]
- 140.Mokranjac D, Neupert W. Energetics of protein translocation into mitochondria. Biochimica Biophysica Acta. 2008;1777:758–762. doi: 10.1016/j.bbabio.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 141.Zwizinski C, Schleyer M, Neupert W. Transfer of proteins into mitochondria. Precursor to the ADP/ATP carrier binds to receptor sites on isolated mitochondria. J. Biol. Chem. 1983;258:4071–4074. [PubMed] [Google Scholar]
- 142.Noji H, Yasuda R, Yoshida M, Kinosita K., Jr Direct observation of the rotation of F1-ATPase. Nature. 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- 143.Sowa Y, Rowe AD, Leake MC, Yakushi T, Homma M, Ishijima A, Berry RM. Direct observation of steps in rotation of the bacterial flagellar motor. Nature. 2005;437:916–919. doi: 10.1038/nature04003. [DOI] [PubMed] [Google Scholar]
- 144.Itoh H, Takahashi A, Adachi K, Noji H, Yasuda R, Yoshida M, Kinosita K. Mechanically driven ATP synthesis by F1-ATPase. Nature. 2004;427:465–468. doi: 10.1038/nature02212. [DOI] [PubMed] [Google Scholar]
- 145.Streif S, Staudinger WF, Marwan W, Oesterhelt D. Flagellar rotation in the archaeon Halobacterium salinarum depends on ATP. J. Mol. Biol. 2008;384:1–8. doi: 10.1016/j.jmb.2008.08.057. [DOI] [PubMed] [Google Scholar]
- 146.Zullo S, Sieu LC, Slightom JL, Hadler HI, Eisenstadt JM. Mitochondrial Dloop sequences are integrated in the rat nuclear genome. J. Mol. Biol. 1991;221:1223–1235. [PubMed] [Google Scholar]