Abstract
Background
Previous research examining the association between apolipoprotein E (APOE) gene polymorphism and risk for posttraumatic stress disorder (PTSD) has been inconsistent due to the use of small and select samples. This study examined the relation between APOE genotype and PTSD symptoms in two nationally representative samples of U.S. military veterans. The potential effect of cumulative trauma burden and social support in moderating this association was also evaluated.
Methods
The main sample consisted of 1,386 trauma-exposed European American (EA) veterans (mean age: 62–63 years) who participated in the National Health and Resilience in Veterans Study (NHRVS) in 2011. The independent replication sample consisted of 509 trauma-exposed EA veterans from the 2013 NHRVS.
Results
APOE ε4 allele carriers reported significantly greater severity of PTSD symptoms than non-carriers in the main, but not the replication, sample. In both samples, the interaction of APOE ε4 carrier status and cumulative trauma burden was associated with greater severity of PTSD symptoms (F range=2.53–8.09, all p’s <.01), particularly re-experiencing/intrusion symptoms (F range=3.59–4.24, p’s <.001). Greater social support was associated with lower severity of PTSD symptoms among APOE ε4 allele carriers with greater cumulative trauma burden (β range −0.27 to −0.60, p’s <.05).
Conclusion
U.S. military veterans who are APOE ε4 allele carriers and exposed to a high number of traumas may be at increased risk for developing PTSD symptoms than ε4 non-carriers. Greater social support may moderate this association, thereby highlighting the potential importance of social support-promoting interventions in mitigating the effect of ε4 × cumulative trauma burden on PTSD risk.
Keywords: Apolipoprotein, trauma, posttraumatic stress disorder, veterans, social support
Introduction
Posttraumatic stress disorder (PTSD) affects 6.4%–7.8% of trauma-exposed adults (Kessler, 2000; Pietrzak et al, 2011; Wisco et al, 2014). Myriad environmental and psychosocial risk and protective factors for PTSD have been identified in trauma-exposed populations, including military veterans (Andersen et al, 2014; Xue et al, 2015; Wisco et al, 2014). There is also increasing interest in identifying genetic risk factors for PTSD and how these markers interact with environmental factors to predict PTSD risk (e.g., Grabe et al, 2009; Kilpatrick et al, 2007). Examining the interactive effects of candidate genes and environmental factors on mental disorders, as opposed to investigating genetic or environmental influences independently, can substantially further our understanding of the etiology of these conditions (Dick et al, 2015). To date, candidate gene studies have identified possible risk alleles mapped to genes implicated in PTSD (Almli et al, 2014), and several genome-wide significant associations between genes such as AC068718 (rs10170218), RORA (rs8042149), ANKRD55 (rs159572), and ZNF626 (rs11085374) and PTSD (Guffanti et al, 2013; Logue et al, 2013; Stein et al, 2016).
While a growing body of research has evaluated whether polymorphisms in the apolipoprotein E (APOE) gene may contribute to PTSD risk (e.g., Kimbrel et al, 2015; Lyons et al, 2013), most studies have been small and focused on select samples of trauma survivors (e.g., Vietnam or Iraq/Afghanistan-era veterans). APOE is a protein-coding gene with three functionally different alleles (ε2, ε3, ε4), with ε3 being the most frequently occurring in the general population. The APOE gene encodes the APOE protein, which plays a role in neuronal repair through its involvement in cholesterol metabolism, and transportation of cholesterol and other lipids to neurons. The APOE ε4 allele, however, has been linked to hippocampal atrophy and memory impairment (Pievani et al, 2011; Small et al, 2004), reduced synaptic plasticity (Chen et al, 2010), and a more robust systemic and central nervous system inflammatory response (Lynch et al, 2003). It has also been associated with greater likelihood of developing several neurological and neuropsychiatric disorders (e.g., Skoog et al, 2015), especially Alzheimer’s disease (Bekris et al, 2010; Kim et al, 2009).
Studies examining the relation between APOE gene and PTSD have been mixed. In a sample of 172 Vietnam veterans, Lyons et al (2013) found that the presence of an APOE ε4 allele was associated with a greater number of PTSD symptoms, but not with PTSD diagnosis. They also observed that ε4 carriers with high combat exposure had greatest severity and risk for PTSD. More recently, a study of 859 non-Hispanic black veterans found that each additional APOE ε4 allele was associated with 60% greater likelihood of having a PTSD diagnosis and greater severity of PTSD symptoms, and that this finding was only observed among veterans with high levels of combat exposure; these effects were not observed in non-Hispanic white veterans (Kimbrel et al, 2015). APOE genotype was also unrelated to severity of post-deployment PTSD symptoms in 230 US army soldiers (Dretsch et al, 2015). Other studies have found the APOE ε2 allele, but not ε4, to be associated with memory impairment and re-experiencing symptoms of PTSD (Freeman et al, 2005), PTSD diagnosis (Kim, Chung et al, 2013), and PTSD symptom severity (Johnson et al, 2015), which is the opposite of the expectation based on the literature related to Alzheimer’s disease.
Gene × environment (G × E) effects of APOE ε4 and environmental exposures are also poorly understood. Aside from combat exposure severity (e.g., Kimbrel et al, 2015; Lyons et al, 2013), it remains unknown whether ε4 carrier status may interact with cumulative trauma burden to predict PTSD risk, as has been observed for other genetic polymorphisms (e.g., SLC6A4: Grabe et al, 2009). Further, no known study has evaluated potentially modifiable moderators of the APOE ε4 × trauma burden interaction, such as social support, a robust protective factor for PTSD (Ozer et al, 2003). A small number of studies have found low social support to interact with high trauma exposure and genetic risk factors to increase the likelihood of PTSD (Kilpatrick et al, 2007; Lian et al, 2014), suggesting that high levels of social support may buffer against genetic risk among trauma-affected individuals.
To address these gaps, we analyzed data from two nationally representative samples of trauma-exposed European American (EA) U.S. military veterans to evaluate two aims: (1) examine the relation between APOE ε4 carrier status, alone and interactively with cumulative trauma burden, and overall severity of PTSD symptoms and symptom clusters; and (2) determine whether levels of perceived social support may moderate any effects of APOE ε4 carrier status and the interaction of ε4 × trauma burden in predicting severity of PTSD symptoms.
Materials and Methods
Participants
Participants were recruited from a research panel of over 50,000 U.S. households developed and maintained by GfK Knowledge Networks, Inc. (Menlo Park, CA, USA), and representing approximately 98% of U.S. households. Participants in the research panel who answered affirmatively to the question, “Have you ever served on active duty in the U.S. Armed Forces, Military Reserves, or National Guard?” were eligible to participate in the National Health and Resilience in Veterans Study (NHRVS), a nationally representative study of U.S. veterans. The main sample consisted of 1,386 trauma-exposed EA U.S. military veterans from the NHRVS, conducted in 2011. Participants in the replication sample were an independent sample of 509 trauma-exposed EA U.S. military veterans from a second baseline cohort survey of the NHRVS conducted in 2013. Post-stratification weights were applied based on the demographic distribution of veterans (age, sex, education, race/ethnicity, metropoloitan area, and Census region) in the GfK Knowledge Networks survey panel and calibrated against U.S. Census data. The NHRVS was approved by the Veterans Affairs (VA) Connecticut Healthcare System and the VA Office of Research & Development.
Assessments
APOE genotyping
Participants provided saliva for DNA extraction. Saliva was collected using Oragene DNA (OG-250) kits. DNA was extracted using prepIT-L2P reagent (DNA Genotek, Ontario, Canada) according to manufacturer’s directions. Samples were genotyped with the PsychChip GWAS array. Genotypes were called using GenomeStudio software V2011.1 and genotyping module V1.8.4 (Illumina, San Diego, CA, USA). Ninety samples with missing genotyping rate >5% were excluded from analysis. The following criteria were used for including SNPs: minor allele frequency (MAF) > 0.01%, missing genotyping rate per SNP < 5% and Hardy-Weinberg equilibrium (HWE) p-value >10−5. This resulted in 423,415 autosomal SNPs and 2,737 samples. Duplicates were detected by estimating the genome-wide identity-by-descent (IBD) sharing for all pairwise samples in PLINK (Purcell et al, 2007) using 93,814 independent SNPs with MAF>0.01. Nine duplicate pairs and 12 additional pairs with a high level of IBD sharing (>0.1) were detected. We randomly removed one subject of the duplicate or related pairs, retaining 2,718 independent samples (2,270 EAs). We computed principal components (PC) for the GWAS data using EIGENSOFT (Price et al, 2006) based on a common set SNPs (64,219) with Hapmap3, which were in low linkage disequilibrium (LD) with one another and have a MAF>0.01. We detected and removed 95 outlier EA samples from the PC analysis, defined as samples whose ancestry was at least three standard deviations from the mean on one of the two largest PCs. We then imputed 1000 genomes variants into the EA samples following the best practice guidelines of IMPUTE2 (Howie et al, 2009). Pre-phasing was first performed with SHAPEIT (Delaneau et al, 2012) to infer haplotypes for the EA samples based on 295,837 autosomal SNPs with MAF > 0.01. Imputation was then carried out on pre-phased haplotypes using IMPUTE2 against reference data from the 1000 Genomes Phase III integrated variant set. After post-imputation QC (SNP missing rate <0.05, MAF >0.005, imputation quality score (info) >0.5, and HWE >10-6), 10,377,932 SNPs remained. We extracted two APOE SNPs—rs429358 and rs7412—from the imputed data of EA samples for a candidate gene analysis of APOE ε4 carrier status. These two SNPs define APOE status and were selected in accordance with previous literature (e.g., Kim et al, 2013; Kimbrel et al, 2015). One SNP (rs7412) was directly genotyped by the PsychChip array and the other (rs429358) was imputed with high quality score (Info = 0.99). To determine APOE ε4 carrier status, we made hard genotype calls of the imputed SNP by applying a posterior genotype probability threshold of 0.9. There was no evidence of deviation from Hardy-Weinberg expectations for the APOE genotype in either the main (p=0.14) or replication (p=0.74) sample. A dichotomous variable of 0 versus 1 or 2 APOE ε4 alleles was created.
Sociodemographic and Military Characteristics
Age, sex, household income, education, employment status, marital status, combat veteran status, and number of years of military service were assessed.
Cumulative Trauma Burden
The Trauma History Screen (THS) assessed lifetime exposure to 13 potentially traumatic events (Carlson et al, 2011), including child and adult physical and sexual assault, natural disaster, and unexpected loss of a loved one. Events were summed to yield a measure of cumulative trauma burden.
PTSD Symptoms
The PTSD Checklist (PCL) was used to assess lifetime and past month PTSD symptoms based on respondents’ worst reported traumatic event on the THS. The DSM-IV version (PCL-Specific Stressor [PCL-S]) was used in the main sample (α=.94) and the DSM-5 version (PCL-5) was administered in the replication sample (α=.95). Veterans were classified as having probable PTSD if their PCL score was ≥ 50 on the DSM-IV version (Weathers et al, 1993) and ≥ 38 on the DSM-5 version (Hoge et al, 2014). Comparability of the symptom clusters on the two PCL versions was achieved by using the 4-factor DSM-IV model of re-experiencing, avoidance, emotional numbing, and hyperarousal symptoms (King et al, 1998), and the DSM-5 model of intrusions, avoidance, negative cognitions and mood, and alterations in arousal and reactivity. Responses on items comprising each symptom cluster were summed to yield severity measures.
Social support
Social support was assessed using a 5-item version of the Medical Outcomes Study Social Support Scale (Amstadter et al, 2010); items assessed emotional and instrumental support (α=0.90 in main sample; α=0.87 in replication sample). Veterans were asked how often each kind of support was available when needed, and items included, “Someone to get together with for relaxation” and “Someone to love and make you feel wanted.”
Other Psychiatric Disorders
The Mini International Neuropsychiatric Interview adapted for self-report was used to assess lifetime DSM-IV diagnoses of major depressive, alcohol and drug use disorders (Lecrubier et al, 1997).
Data Analysis
Descriptive statistics were used to summarize sociodemographic, trauma, and clinical variables of trauma-exposed veterans. A series of univariate analyses of covariance (ANCOVAs) were conducted to evaluate the relation between APOE ε4 allele carrier status, trauma burden, and their interaction, and severity of lifetime and past-month PTSD symptoms in the main and replication samples. Covariates included age, sex, top 10 PCs from population stratification analysis, combat exposure (i.e., combat veteran vs. non-combat veteran), and nature of ‘worst’ traumatic event (i.e., assaultive vs. non-assaultive). To evaluate the role of social support in moderating the interaction between APOE ε4 allele carrier status × lifetime trauma burden, we incorporated an ε4 × trauma burden × social support interaction term into the ANCOVAs. To evaluate the relationship between APOE ε4 allele carrier status, cumulative trauma burden, and their interaction, and lifetime PTSD symptom clusters, we conducted a parallel series of univariate ANCOVAs with scores on each of the PTSD symptom clusters entered as dependent variables in separate analyses and other PTSD symptom clusters entered as additional covariates. A statistical significance threshold of α=0.01 was employed in analyses to reduce the likelihood of Type I error. These post-hoc analyses were limited to lifetime PTSD symptom clusters due to the low prevalence and variance of past-month PTSD symptom clusters. Reported raw frequencies are unweighted; means, percentages, and inferential statistics are post-stratification weighted to reflect the general population of U.S. veterans. Analyses were conducted using SPSS version 22.
Results
Table 1 shows sociodemographic, military, and clinical characteristics of trauma-exposed veterans in the main and replication samples. On average, participants in both samples were 62–63 years of age, predominantly male, some college or higher educated, married/cohabitating, retired, had household incomes <$60,000, were non-combat veterans, and spent an average of seven years in the military. On average, veterans in the samples reported experiencing 3.7–3.8 traumatic life events, with 7.1–10.0% screening positive for lifetime PTSD and 3.5–3.8% for past-month PTSD.
Table 1.
Sociodemographic, military, and trauma and clinical characteristics of the main and replication samples of trauma-exposed U.S. European-ancestry military veterans
| Main Sample (n=1,386) |
Replication Sample (n=509) |
|
|---|---|---|
|
| ||
| Weighted mean (SD) or n (weighted %) |
Weighted mean (SD) or n (weighted %) |
|
| Sociodemographic Characteristics | ||
| Age | 62.6 (14.3) | 62.4 (15.6) |
| Male sex | 1,260 (92.8%) | 457 (90.7%) |
| Some college or higher education | 1,183 (66.0%) | 428 (65.4%) |
| Married/living with partner | 1,084 (74.9%) | 379 (72.3%) |
| Currently employed | 536 (36.1%) | 152 (30.3%) |
| Household income ≥ $60,000/year | 728 (42.4%) | 255 (44.0%) |
| Military Characteristics | ||
| Combat veteran | 479 (32.7%) | 213 (42.0%) |
| Number of years in military | 6.8 (7.5) | 7.0 (7.2) |
| Trauma and Clinical Characteristics | ||
| Number of lifetime traumatic events | 3.8 (2.5) | 3.7 (2.5) |
| Index traumatic event | ||
| Sudden death of close family member of friend | 451 (34.4%) | 151 (32.7%) |
| Life-threatening illness or injury | 226 (16.6%) | 84 (14.2%) |
| Military-related trauma | 113 (7.9%) | 42 (9.5%) |
| Child physical or sexual abuse | 59 (3.2%) | 23 (5.4%) |
| Other traumatic event | 537 (37.9%) | 209 (38.2%) |
| Lifetime PCL score* | 28.4 (11.4) | 15.2 (15.2) |
| Positive screen for lifetime PTSD | 95 (7.1%) | 44 (10.0%) |
| Past-month PCL score* | 24.2 (10.1) | 9.4 (12.1) |
| Positive screen for past-month PTSD | 41 (3.8%) | 15 (3.5%) |
| Lifetime major depressive disorder | 244 (18.2%) | 60 (25.6%) |
| Lifetime alcohol use disorder | 659 (48.6%) | 212 (41.7%) |
| Lifetime drug use disorder | 200 (14.6%) | 63 (12.3%) |
| APOE ε4 allele carrier | 334 (24.1%) | 133 (25.6%) |
| 0 alleles | 1,052 (75.9%) | 376 (74.4%) |
| 1 allele | 318 (22.9%) | 124 (23.7%) |
| 2 alleles | 16 (1.2%) | 9 (1.9%) |
Note. PCL=PTSD Checklist; PTSD=posttraumatic stress disorder.
The DSM-IV version of the PCL was used in the main sample (score range=17–85) and the DSM-5 version of the PCL was used in the replication sample (score range=0–80).
Table 2 shows results of analyses evaluating the relation between APOE ε4 carrier status, trauma burden, and their interaction, and PTSD symptoms.
Table 2.
Results of analyses evaluating relation between APOE ε4 allele carrier status, cumulative trauma burden, and lifetime and past-month PTSD symptoms
| Main Sample (n=1,386) | ||||
|---|---|---|---|---|
| Lifetime PTSD symptoms |
Past-month PTSD symptoms |
|||
| F | p | F | p | |
| APOE ε4 carrier | 16.11 | <0.001 | 6.75 | 0.010 |
| Cumulative trauma burden | 28.31 | <0.001 | 22.48 | <0.001 |
| APOE ε4 × Cumulative trauma burden | 6.08 | <0.001 | 8.09 | <0.001 |
| Replication Sample (n=509) | ||||
| Lifetime PTSD symptoms |
Past-month PTSD symptoms |
|||
| F | p | F | p | |
| APOE ε4 carrier | 0.41 | 0.52 | 0.04 | 0.84 |
| Cumulative trauma burden | 11.97 | <0.001 | 8.66 | <0.001 |
| APOE ε4 × Cumulative trauma burden | 2.53 | 0.006 | 3.06 | 0.001 |
Note. PTSD=posttraumatic stress disorder; APOE ε4=apolipoprotein epsilon 4.
Analyses are adjusted for age, sex, ancestral proportion scores, combat veteran status, and type of index trauma (assaultive vs. non-assaultive).
In the main sample, APOE ε4 carrier status, number of traumas, and their interaction were associated with greater severity of lifetime and past-month PTSD symptoms. Figure 1 illustrates the interaction of APOE ε4 and trauma burden on severity of lifetime PTSD symptoms in the main sample. Among ε4 carriers, those with a greater number of traumas had greater PTSD symptoms. Incorporation of an APOE ε4 × lifetime traumas × social support interaction term into this ANCOVA revealed a significant association with lifetime (3-way interaction, F=3.01, p<0.001) and past-month (3-way interaction, F=4.35, p<0.001) PTSD symptoms. Adjusted post-hoc analyses among ε4 carriers with greater cumulative trauma burden revealed that greater levels of social support were associated with significantly reduced likelihood of lifetime (β= −0.48, t=6.21, p=<0.001) and past-month (β= −0.60, t=7.89, p<0.001) PTSD symptoms. Among ε4 carriers with greater cumulative trauma burden (i.e., greater than the median number of 3 events, n=602), those with higher levels of perceived social support were less likely to screen positive for current PTSD (2.4% in highest tertile vs. 4.8% in middle tertile vs. 20.5% in lowest tertile; see Figure 2).
Figure 1.
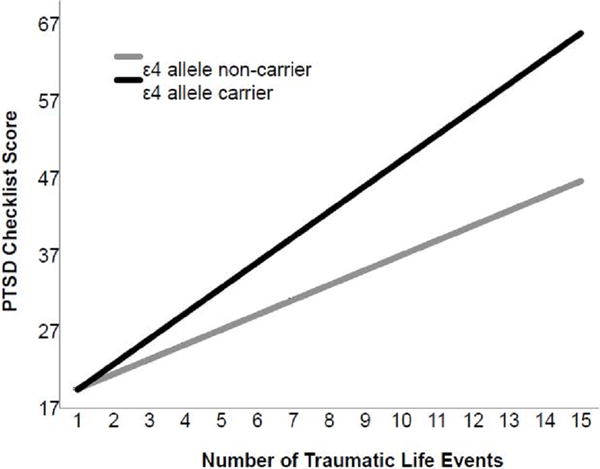
Interaction of APOE ε4 allele carrier status and cumulative trauma burden in predicting lifetime severity of PTSD symptoms in the main sample (n=1,386).
Note. PTSD=posttraumatic stress disorder; APOE ε4=apolipoprotein epsilon 4. PTSD Checklist score range=17-85. Lines represent fitted regression lines adjusted for age, sex, ancestral proportion scores, combat veteran status, and nature of index trauma (assaultive vs. non-assaultive). Slopes and 95% confidence intervals for ε4 allele non-carriers=1.93 (1.72–2.15) and 3.31 (2.96–3.66) for ε4 allele carriers.
Figure 2.
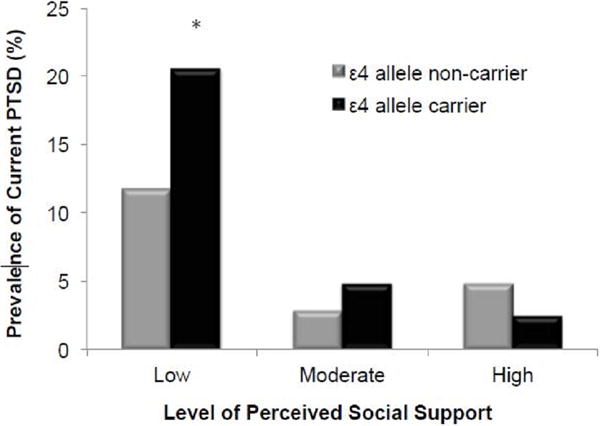
Prevalence of current PTSD in highly trauma-exposed veterans by ε4 carrier status and level of perceived social support in the main sample (n=602).
Note. Current PTSD was identified as a score ≥ 50 on the DSM-IV version of the PTSD Checklist. Low, moderate, and high levels of perceived social support reflect tertiles of total scores on the 5-item version of the Medical Outcomes Study Social Support Scale. *Statistically significantly greater prevalence than ε4 allele carriers with moderate and high levels of perceived social support, X2(2)=9.83, p=0.007.
In the replication sample, the interaction of APOE ε4 carrier status × cumulative trauma burden was significantly associated with lifetime and past-month PTSD symptoms; APOE ε4 carrier status was unrelated to these outcomes. Incorporation of an APOE ε4 × lifetime traumas × social support interaction term revealed a significant association with lifetime (F=3.86, p<0.001) and past-month (F=4.55, p<0.001) PTSD symptoms. Adjusted post-hoc multivariable linear regression analyses in ε4 carriers with greater cumulative trauma burden revealed that greater levels of social support were associated with significantly reduced likelihood of lifetime (β= −0.27, t=2.32, p=<0.024) and past-month (β= −0.37, t=3.07, p=0.003) PTSD symptoms. Among ε4 allele carriers with greater cumulative trauma burden, those with higher levels of social support were less likely to screen positive for current PTSD (0% in highest tertile vs. 8.3% in middle tertile vs. 11.8% in lowest tertile).
Adjustment for MDD and SUDs did not substantively change the results. In the main sample, the main and interactive effects of ε4 allele and ε4 allele × cumulative trauma burden remained significant for both lifetime PTSD symptoms: F=20.77, p<0.001 and F=36.13, p<0.001, respectively; and past-month PTSD symptoms: F=22.76, p<0.001; F=30.10, p<0.001, respectively. In the replication sample, the interaction of e4 allele × trauma burden also remained significant for past-month (F=2.78, p=0.003) but not for lifetime (F=1.30, p=0.23) PTSD symptoms.
Table 3 shows results of analyses examining the relationship between APOE ε4 carrier status, cumulative trauma burden, and the interaction of these variables, and lifetime PTSD symptom clusters. In the main sample, the interaction of APOE ε4 carrier status × lifetime traumas was significant for re-experiencing and avoidance symptoms. In the replication sample, ε4 carrier status was associated with greater severity of intrusions; and the interaction of APOE ε4 × cumulative trauma burden was significant for intrusion symptoms and alterations in arousal and reactivity.
Table 3.
Results of analyses evaluating relation between APOE ε4 allele carrier status, cumulative trauma burden, and lifetime PTSD symptom clusters
| Main Sample (n=1,386) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Re-experiencing | Avoidance | Emotional Numbing | Hyperarousal | |||||
| F | p | F | p | F | p | F | p | |
| APOE ε4 carrier | 4.12 | 0.043 | 5.98 | 0.015 | 2.32 | 0.13 | 4.11 | 0.043 |
| Cumulative trauma burden | 9.08 | <0.001 | 3.30 | <0.001 | 4.05 | <0.001 | 3.09 | <0.001 |
| APOE ε4 × Cumulative trauma burden | 4.24 | <0.001 | 3.01 | 0.001 | 1.63 | 0.085 | 2.14 | 0.016 |
| Replication Sample (n=509) | ||||||||
| Intrusions | Avoidance | Negative Cognitions and Mood | Alterations in Arousal and Reactivity | |||||
| F | p | F | p | F | p | F | p | |
| APOE ε4 carrier | 14.99 | <0.001 | 0.22 | 0.64 | 5.29 | 0.022 | 1.96 | 0.16 |
| Cumulative trauma burden | 3.89 | <0.001 | 0.74 | 0.71 | 1.12 | 0.34 | 2.96 | 0.001 |
| APOE ε4 × Cumulative trauma burden | 3.59 | <0.001 | 0.86 | 0.57 | 1.47 | 0.15 | 5.30 | <0.001 |
Note. PTSD=posttraumatic stress disorder; APOE ε4=apolipoprotein epsilon 4.
Analyses are adjusted for age, sex, ancestral proportion scores, combat veteran status, nature of index trauma (assaultive vs. non-assaultive), and other PTSD symptom clusters.
The DSM-IV version of the PCL was used in the main sample and the DSM-5 version of the PCL was used in the replication sample.
Discussion
This study examined the association between APOE genotype, trauma exposure, and PTSD symptoms in two contemporary, nationally representative cohorts of EA military veterans. APOE ε4 allele carriers reported greater lifetime and past-month PTSD symptoms than non-carriers in the main sample, however, this association was not significant in our replication sample. This finding aligns with a recent meta-analysis supporting an association between ε4 carrier status and higher risk for combat-related PTSD (Roby, 2017). It is possible that the lack of a main effect in the replication sample was due to small sample size, and differences in sociodemographic factors and environmental exposures across samples may also partly account for the inconsistent findings. It is also possible that the discrepancy in findings between the main and replication samples may be attributable in part to the different PCL versions (DSM-IV vs. DSM-5) used; however, after recomputing a PCL-5 summary score by removing the new DSM-5 PTSD symptoms from the PCL-5 such that it more closely aligned with the PCL-S (DSM-IV), the main effect of ε4 allele carrier status remained non-significant for both lifetime (F=0.44, p=0.51) and past-month (F=0.01, p=0.92) symptoms. Further evaluation of a possible association between APOE ε4 status and PTSD symptoms is needed in different trauma-exposed samples.
In both samples, APOE ε4 carriers with greater trauma burden reported greater lifetime and past month PTSD symptoms. This finding is consistent with previous work linking ε4 allele carrier status to PTSD among veterans with high combat exposure (Lyons et al, 2013; Kimbrel et al, 2015), and extends it to demonstrate that cumulative trauma burden may also moderate the effect of ε4 allele carriage on PTSD symptoms. The APOE ε4 allele may hinder the neuronal repair and recovery that is necessary after injury related to extensive stress exposure (Lyons et al, 2013; Kimbrel et al, 2015). Trauma exposure across the lifespan can deleteriously affect brain structure and function, including reductions in hippocampal volume (Bremner, 2006; Woon et al, 2010) and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (Bremner, 2006; Carpenter et al, 2007; Elzinga et al, 2008), which can increase risk for developing PTSD symptoms (Gilbertson et al, 2002; Pitman et al, 2012). APOE ε4 allele carriers are similarly at increased risk of several deficits relevant to PTSD, including hippocampal volume loss and amygdala atrophy (den Heijer et al, 2002; Goni et al, 2012) and greater cortisol levels (Gill-Bea et al, 2010), possibly further exacerbating PTSD vulnerability. Given that individuals with PTSD are at higher risk of dementia (Yaffe et al, 2010), it is also possible that the ε4 allele contributes to a shared mechanistic pathway for the development of both conditions, or that the development of dementia symptoms plays a role in the ε4-PTSD association. For example, a recent study found that Alzheimer’s model mice displayed more exaggerated and frequent responses to a PTSD-like induction than control mice (Justice et al, 2015). Additional research utilizing prospective designs is needed to elucidate possible mechanisms linking ε4, PTSD, and cognitive decline and dementia in trauma-affected individuals.
Analyses of PTSD symptom clusters revealed that the APOE ε4 × trauma burden association was linked to greater re-experiencing and avoidance symptoms in the main sample, and greater intrusion symptoms and alterations in arousal and reactivity in the replication sample. In light of prior studies showing higher levels of cerebrospinal fluid cortisol among APOE ε4 carriers (Gill-Bea et al, 2010; Peskind et al, 2001), it is possible that greater HPA axis reactivity among ε4 carriers may exacerbate hyperarousal symptoms among highly trauma-exposed individuals, which may in turn trigger re-experiencing/intrusion symptoms. Greater hippocampal atrophy and volume loss in ε4 carriers may also contribute to deficits in memory for fear extinction (den Heijer et al, 2002; Fanselow, 2000; Pievani et al, 2011), which can maintain re-experiencing/intrusion symptoms. Villasana and colleagues (2016) found that mice carrying the ε4 allele had higher levels of heme oxygenase-1, a marker of stress, in the hippocampus, and also exhibited greater anxiety-related behaviors and conditioned fear responding. These findings suggest a possible mechanism linking ε4 carriage and intrusive symptoms among highly trauma-exposed veterans. Clearly, more research is needed to elucidate mechanisms underlying this association.
Of note, APOE ε2 carriage has been found to be associated with memory impairment, more re-experiencing symptoms, and greater overall PTSD symptoms in small samples of humans (Freeman et al, 2005; Johnson et al, 2015), while stress-exposed mice carrying the ε2 allele have been found to have impairments in fear memory extinction (Johnson et al, 2015; Olsen et al, 2012). However, a meta-analysis did not find support for an effect of the e2 variant on risk for combat-related PTSD (Roby, 2017). Further research in larger samples is needed to help resolve these inconsistent findings regarding APOE genotype and PTSD (Johnson et al, 2015; Rogers and Weeber, 2008).
Higher levels of social support were found to be associated with lower PTSD symptoms among APOE ε4 allele carriers with greater cumulative trauma burden. This finding adds to a small literature demonstrating an effect of social support in moderating the association between other genetic polymorphisms (Kilpatrick et al, 2007; Lian et al, 2014) and PTSD, depressive symptoms, and suicidal ideation (Chen, Kumsta et al, 2011; Kilpatrick et al, 2007; Kim, Stewart et al, 2014). Social support has been linked with lower cortisol levels (McQuaid et al, 2016; Rosal et al, 2004), improved cardiovascular and immune functioning (Uchino, 2006), and less threatening appraisal of stressful events (Sippel et al, 2015), suggesting a potential role in buffering the impact of trauma-related stress responses. It is also possible, however, that veterans with more PTSD symptoms engage in behaviors that lead to less social support (e.g., avoidance, social isolation) or may inaccurately perceive their social support (e.g., Platt et al, 2016). Nonetheless, assuming that social support may help buffer against the development of PTSD symptoms, facilitating the enhancement of social support networks (e.g., one-to-one mentorship programs, peer support groups, social/relationship skills interventions, Vet-to-Vet programs) among highly trauma-exposed veterans at elevated genetic risk for PTSD may be an important aspect of prevention initiatives (e.g., Hogan et al, 2002; Pietrzak et al, 2009; Resnick and Rosenheck, 2008; Williams et al, 2012).
The current findings should be considered in light of several limitations. First, PTSD symptoms were assessed via self-report, and it is unclear whether results would be comparable if PTSD symptoms were assessed using a structured clinical interview such as the Clinician Administered PTSD Scale (CAPS). However, statistically significant and large magnitude associations have been observed between total scores on the PCL and on the CAPS (Macdonald et al, 2013; Monson et al, 2008). Second, the main sample was larger than the replication sample and different versions of the PCL (i.e., DSM-IV vs. DSM-5) were used in these samples; thus, it is unclear whether differences in patterns of associations between the samples may be related to reduced statistical power or versions of the PCL. Third, the sample was comprised predominantly of male EA veterans. Further research is needed to examine the role of APOE ε4 carrier status on risk for PTSD among more diverse samples of veterans and other trauma-affected populations. Fourth, given the strong association between APOE ε4 carrier status and poorer cognitive functioning and dementia (e.g., Engelborghs et al, 2003; Wisdom et al, 2011), it remains to be determined whether cognitive impairment might influence the association between ε4 and PTSD, as formal neuropsychological testing was not conducted in this cohort of veterans. Fifth, there have been a number of limitations raised with respect to G × E research with candidate genes, including the lack of replication of some findings, publication bias, and the frequent use of underpowered samples (e.g., Dick et al, 2015). However, the current study incorporated a number of recommendations made for improving the rigor of G × E studies, including having an adequately powered main sample, an independent replication sample, and a priori selection of candidate genes (i.e., APOE) and environmental factors (i.e., trauma exposure) based on theoretical rationale and existing literature (Dick et al, 2015). A recent review also recommended additional research examining the effect of positive exposures (i.e., social support) on psychopathology (Leighton et al, 2017).
Conclusion
Results of the present study suggest that APOE ε4 carrier status interacts with cumulative trauma burden to predict severity of PTSD symptoms, particularly re-experiencing/intrusive symptoms, in U.S. military veterans. Further, greater social support is associated with lower severity of PTSD symptoms in ε4 allele carriers with greater cumulative trauma burden. Further research is needed to examine the complex relationship and mechanistic pathways between APOE genotype, PTSD, and cognitive decline and dementia (Lee et al, 2008; Meziab et al, 2014; Peavy et al, 2007; Yaffe et al, 2010); evaluate how APOE and other gene polymorphisms are linked to transdiagnostic aspects of trauma-related psychopathology (e.g., Mota et al, 2015); and examine the efficacy of interventions to enhance social support in mitigating PTSD symptoms among at-risk trauma survivors.
Acknowledgments
Funding
The U.S. Department of Veterans Affairs supported this work through its support of the VA National Center for PTSD and the Consortium to Alleviate PTSD. Dr. Krystal received additional support from a National Institute on Alcohol Abuse and Alcoholism grant (2P50AA012870-14) and National Center for Advancing Translational Science grants (UL1RR024139, principal investigator: R. Sherwin; UH2TR000960, principal investigator: Dr.Krystal).
Footnotes
This work took place at the Clinical Neurosciences Division, U.S. Department of Veterans Affairs National Center for PTSD, VA Connecticut Healthcare System, West Haven, CT, USA.
Disclosure
No author has any relevant conflict of interest to disclose.
References
- Almli LM, Fani N, Smith AK, Ressler KJ. Genetic approaches to understanding post-traumatic stress disorder. International Journal of Neuropsychopharmacology. 2014;17(2):355–370. doi: 10.1017/S1461145713001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Begle AM, Cisler JM, Hernandez MA, Muzzy W, Acierno R. Prevalence and correlates of poor self-rated health in the United States: The national elder mistreatment study. Am J Geriatr Psychiatry. 2010;18(7):615–623. doi: 10.1097/JGP.0b013e3181ca7ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SB, Karstoft KI, Bertelsen M, Madsen T. Latent trajectories of trauma symptoms and resilience: The 3-year longitudinal prospective USPER study of Danish veterans deployed in Afghanistan. J Clin Psychiatry. 2014;75(9):1001–1008. doi: 10.4088/JCP.13m08914. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Yu CE, Bird TD, Tsuang DW. Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol. 2010;23(4):213–227. doi: 10.1177/0891988710383571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues in Clinical Neuroscience. 2006;8(4):445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EB, Smith SR, Palmieri PA, et al. Development and validation of a brief self-report measure of trauma exposure: the Trauma History Screen. Psychol Assess. 2011;23(2):463–77. doi: 10.1037/a0022294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci. 2010;107(26):12011–12016. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc Natl Acad Sci. 2011;108(50):19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2012;9(2):179–81. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, Van Duijn CM, Hofman A, Breteler MMB. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59(5):746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, et al. Candidate gene-environment interaction research: Reflections and recommendations. Perspect Psychol Sci. 2015;10(1):37–59. doi: 10.1177/1745691614556682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretsch MN, Williams K, Emmerich T, Crynen G, Ait-Ghezala G, Chaytow H, … Iverson GL. Brain-derived neurotropic factor polymorphisms, traumatic stress, mild traumatic brain injury, and combat exposure contribute to postdeployment traumatic stress. Brain and Behavior. 2015;6(1):e00392. doi: 10.1002/brb3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: a study among healthy young subjects. Psychoneuroendocrinology. 2008;33(2):227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Engelborghs S, Dermaut B, Goeman J, Saerens J, Marien P, Pickut BA, et al. Prospective Belgian study of neurodegenerative and vascular dementia: APOE genotype effects. J Neurol Neurosurg Psychiatry. 2003;74:1148–1151. doi: 10.1136/jnnp.74.8.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Freeman T, Roca V, Guggenheim F, Kimbrell T, Griffin WST. Neuropsychiatric associations of apolipoprotein E alleles in subjects with combat-related posttraumatic stress disorder. J Neuropsychiatry Clin neurosci. 2005;17(4):541–543. doi: 10.1176/jnp.17.4.541. [DOI] [PubMed] [Google Scholar]
- Gil-Bea FJ, Aisa B, Solomon A, Solas M, del Carmen Mugueta M, Winblad B, et al. HPA axis dysregulation associated to apolipoprotein E4 genotype in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2010;22(3):829–838. doi: 10.3233/JAD-2010-100663. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni J, Cervantes S, Arrondo G, Lamet I, Pastor P, Pastor MA. Selective brain gray matter atrophy associated with APOE ε4 and MAPT H1 in subjects with mild cognitive impairment. Journal of Alzheimer’s Disease. 2013;33(4):1009–1019. doi: 10.3233/JAD-2012-121174. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. Am J Psychiatry. 2009;166(8):926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- Guffanti G, Galea S, Yan L, Roberts AL, Solovieff N, Aiello AE, et al. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718. 1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology. 2013;38(12):3029–3038. doi: 10.1016/j.psyneuen.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BE, Linden W, Najarian B. Social support interventions: Do they work? Clinical Psychol Rev. 2002;22(3):381–440. doi: 10.1016/s0272-7358(01)00102-7. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 2014;1(4):269–77. doi: 10.1016/S2215-0366(14)70235-4. [DOI] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Zuloaga DG, Bidiman E, Marzulla T, Weber S, Wahbeh H, et al. ApoE2 exaggerates PTSD-related behavioral, cognitive, and neuroendocrine alterations. Neuropsychopharmacology. 2015;40(10):2443–2453. doi: 10.1038/npp.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Huang L, Tian JB, Cole A, Pruski M, Hunt AJ, … Zheng H. Posttraumatic stress disorder-like induction elevates β-amyloid levels, which directly activates corticotropin-releasing factor neurons to exacerbate stress responses. Journal of Neuroscience. 2015;35(6):2612–2623. doi: 10.1523/JNEUROSCI.3333-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61:4–14. [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63(3):287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TY, Chung HG, Shin HS, Kim SJ, Choi JH, Chung MY, et al. Apolipoprotein E gene polymorphism, alcohol use, and their interactions in combat‐related posttraumatic stress disorder. Depress Anxiety. 2013;30(12):1194–1201. doi: 10.1002/da.22138. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Kang HJ, Kim SY, Lee JY, et al. Interactions between a serotonin transporter gene, life events and social support on suicidal ideation in Korean elders. J Affect Disord. 2014;160:14–20. doi: 10.1016/j.jad.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Kimbrel NA, Hauser MA, Garrett M, Ashley‐Koch A, Liu Y, Dennis MF, et al. Effect of the APOE ε4 allele and combat exposure on PTSD among Iraq/Afghanistan-era veterans. Depress Anxiety. 2015;32(5):307–315. doi: 10.1002/da.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DW, Leskin GA, King LA, Weathers FW. Confirmatory factor analysis of the clinician-administered PTSD Scale: Evidence for the dimensionality of posttraumatic stress disorder. Psychol Assess. 1998;10(2):90–96. [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European Psychiatry. 1997;12(5):224–231. [Google Scholar]
- Lee BK, Glass TA, Wand GS, McAtee MJ, Bandeen-Roche K, Bolla KI, Schwartz BS. Apolipoprotein e genotype, cortisol, and cognitive function in community-dwelling older adults. Am J Psychiatry. 2008;165(11):1456–1464. doi: 10.1176/appi.ajp.2008.07091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton C, Botto A, Silva JR, Jimenez JP, Luyten P. Vulnerability or sensitivity to the environment? Methodological issues, trends, and recommendations in gene–environment interactions research in human behavior. Frontiers in Psychiatry. 2017;8:106. doi: 10.3389/fpsyt.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Y, Xiao J, Wang Q, Ning L, Guan S, Ge H, et al. The relationship between glucocorticoid receptor polymorphisms, stressful life events, social support, and post-traumatic stress disorder. BMC Psychiatry. 2014;14(1):232. doi: 10.1186/s12888-014-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol Psychiatry. 2013;18(8):937–942. doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, et al. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278(49):48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Genderson M, Grant MD, Logue M, Zink T, McKenzie R, et al. Gene‐environment interaction of ApoE genotype and combat exposure on PTSD. Am J Med Genet. 2013;162(7):762–769. doi: 10.1002/ajmg.b.32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald A, Greene CJ, Torres JG, Frueh BC, Morland LA. Concordance between clinician-assessed and self-reported symptoms of posttraumatic stress disorder across three ethnoracial groups. Psychological Trauma: Theory, Research, Practice, and Policy. 2013;5(3):201–208. [Google Scholar]
- McQuaid RJ, McInnis OA, Paric A, Al-Yawer F, Matheson K, Anisman H. Relations between plasma oxytocin and cortisol: The stress buffering role of social support. Neurobiology of Stress. 2016;3:52–60. doi: 10.1016/j.ynstr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimer’s & Dementia. 2014;10(3):S236–S241. doi: 10.1016/j.jalz.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Monson CM, Gradus JL, Young-Xu Y, Schnurr PP, Price JL, Schumm JA. Change in pottraumatic stress disorder symptoms: Do clinicians and patients agree? Psychological Assessment. 2008;20(2):131–138. doi: 10.1037/1040-3590.20.2.131. [DOI] [PubMed] [Google Scholar]
- Mota N, Sumner JA, Lowe SR, Neumeister A, Uddin M, Aiello AE, et al. The rs1049353 polymorphism in the CNR1 gene interacts with childhood abuse to predict posttraumatic threat symptoms. J Clin Psychiatry. 2015;76(12):e1622–e1623. doi: 10.4088/JCP.15l10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RH, Agam M, Davis MJ, Raber J. ApoE isoform‐dependent deficits in extinction of contextual fear conditioning. Genes, Brain and Behavior. 2012;11(7):806–812. doi: 10.1111/j.1601-183X.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Peavy GM, Lange KL, Salmon DP, Patterson TL, Goldman S, Gamst AC, et al. The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biol Psychiatry. 2007;62(5):472–478. doi: 10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56(8):1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Medical comorbidity of full and partial posttraumatic stress disorder in United States adults: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2011;73(8):697–707. doi: 10.1097/PSY.0b013e3182303775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Southwick SM. Psychological resilience and postdeployment social support protect against traumatic stress and depressive symptoms in soldiers returning from Operations Enduring Freedom and Iraqi Freedom. Depress Anxiety. 2009;26(8):745–751. doi: 10.1002/da.20558. [DOI] [PubMed] [Google Scholar]
- Pievani M, Galluzzi S, Thompson PM, Rasser PE, Bonetti M, Frisoni GB. APOE4 is associated with greater atrophy of the hippocampal formation in Alzheimer’s disease. Neuroimage. 2011;55(3):909–919. doi: 10.1016/j.neuroimage.2010.12.081. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt JM, Lowe SR, Galea S, Norris FH, Koenen KC. A longitudinal study of the bidirectional relationship between social support and posttraumatic stress following a natural disaster. J Trauma Stress. 2016;29(3):205–213. doi: 10.1002/jts.22092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SG, Rosenheck RA. Integrating peer-provided services: a quasi-experimental study of recovery orientation, confidence, and empowerment. Psychiatr Serv. 2008;59(11):1307–1314. doi: 10.1176/ps.2008.59.11.1307. [DOI] [PubMed] [Google Scholar]
- Roby Y. Apolipoprotein E variants and genetic susceptibility to combat-related post-traumatic stress disorder: A meta-analysis. Psychiatric Genetics. 2017;27(4):121–130. doi: 10.1097/YPG.0000000000000174. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Weeber EJ. Reelin and apoE actions on signal transduction, synaptic function and memory formation. Neuron Glia Biology. 2008;4(03):259–270. doi: 10.1017/S1740925X09990184. [DOI] [PubMed] [Google Scholar]
- Rosal MC, King J, Ma Y, Reed GW. Stress, social support, and cortisol: Inverse associations? Behav Med. 2004;30(1):11–22. doi: 10.3200/BMED.30.1.11-22. [DOI] [PubMed] [Google Scholar]
- Sippel LM, Pietrzak RH, Charney DS, Mayes LC, Southwick SM. How does social support enhance resilience in the trauma-exposed individual? Ecology & Society. 2015;20(4):136–145. [Google Scholar]
- Skoog I, Waern M, Duberstein P, Blennow K, Zetterberg H, Börjesson-Hanson A, et al. A 9-year prospective population-based study on the association between the APOE* E4 allele and late-life depression in Sweden. Biol Psychiatry. 2015;78(10):730–736. doi: 10.1016/j.biopsych.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004;19(4):592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Stein MB, Chen CY, Ursano RJ, Cai T, Gelernter J, Heeringa SG, et al. Genome-wide association studies of posttraumatic stress disorder in 2 cohorts of US Army soldiers. JAMA Psychiatry. 2016;73(7):695–704. doi: 10.1001/jamapsychiatry.2016.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behavioral Med. 2006;29(4):377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Villasana LE, Weber S, Akinyeke T, Raber J. Genotype differences in anxiety and fear learning and memory of WT and ApoE4 mice associated with enhanced generation of hippocampal reactive oxygen species. J Neurochem. 2016;138(6):896–908. doi: 10.1111/jnc.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Vol. 462 Annual convention of the international society for traumatic stress studies; San Antonio, TX: 1993. [Google Scholar]
- Williams RM, Bambara J, Turner AP. A scoping study of one-to-one peer mentorship interventions and recommendations for application with veterans with postdeployment syndrome. Journal of Head Trauma Rehabilitation. 2012;27(4):261–273. doi: 10.1097/HTR.0b013e3182585cb6. [DOI] [PubMed] [Google Scholar]
- Wisco BE, Marx BP, Wolf EJ, Miller MW, Southwick SM, Pietrzak RH. Posttraumatic stress disorder in the US veteran population: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2014;75(12):1338–1346. doi: 10.4088/JCP.14m09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiology of aging. 2011;32(1):63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Xue C, Ge Y, Tang B, Liu Y, Kang P, Wang M, Zhang L. A meta-analysis of risk factors for combat-related PTSD among military personnel and veterans. PloS one. 2015;10(3):e0120270. doi: 10.1371/journal.pone.0120270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]


