Abstract
Study objectives
Plant-derived caffeine is regarded as a defensive compound produced to prevent herbivory. Caffeine is generally repellent to insects and often used to study the neurological basis for aversive responses in the model insect, Drosophila melanogaster. Caffeine is also studied for its stimulatory properties where sleep or drowsiness is suppressed across a range of species. Since limiting access to food also inhibits fly sleep—an effect known as starvation-induced sleep suppression—we tested whether aversion to caffeinated food results in reduced nutrient intake and assessed how this might influence fly studies on the stimulatory effects of caffeine.
Methods
We measured sleep and total consumption during the first 24 hours of exposure to caffeinated diets containing a range of sucrose concentrations to determine the relative influence of caffeine and nutrient ingestion on sleep. Experiments were replicated using three fly strains.
Results
Caffeine reduced total consumption and nighttime sleep, but only at intermediate sucrose concentrations. Although sleep can be modeled by an exponential dose response to nutrient intake, caffeine-mediated sleep loss cannot be explained by absolute caffeine or sucrose ingestion alone. Instead, reduced sleep strongly correlates with changes in total consumption due to caffeine. Other bitter compounds phenocopy the effect of caffeine on sleep and food intake.
Conclusions
Our results suggest that a major effect of dietary caffeine is on fly feeding behavior. Changes in feeding behavior may drive caffeine-mediated sleep loss. Future studies using psychoactive compounds should consider the potential impact of nutrition when investigating effects on sleep.
Keywords: Bitter tastant, caffeine, Drosophila, feeding behavior, food palatability, starvation-induced sleep suppression
Statement of Significance
It is thought that bitter taste sensation protects organisms from ingesting toxins. For example, most insects avoid feeding on plants that produce the bitter compound, caffeine. When laboratory Drosophila are exposed to caffeinated diets, flies show an aversion to feeding and also a reduction in sleep, which is generally assumed to be triggered by the psychostimulatory properties of caffeine observed across species. Since decreased nutritional intake can also reduce fly sleep, we assessed how caffeine-mediated changes to feeding behavior might contribute to the stimulatory effect of caffeine in Drosophila. This study is the first to measure how nutrition and food intake influence the stimulatory effect of caffeine. It is not yet known whether caffeine, as a tastant, influences behavior in other organisms.
INTRODUCTION
Caffeine is a secondary metabolite produced by plants and is generally bitter to insects.1–7 Studies in Drosophila have been fruitful in revealing some of the genetic mechanisms underlying caffeine aversion, including gustatory receptors such as Gr33a, Gr66a, and Gr93a, although the full extent and interaction of the molecular repertoire are unknown.8–10 Caffeine is also a popular psychostimulant that is often used to promote alertness after poor sleep.11 Due to the widespread use of caffeine as a remedy for sleepiness, a variety of animal systems, including flies, have been used to investigate its sleep-inhibiting effects, although these studies primarily focus on the acute effects of caffeine in well-rested animals.12–19 Flies exhibit many of the hallmarks of mammalian sleep, and Drosophila studies have uncovered sleep-regulatory mechanisms conserved between flies and mammals;12,14,15,20,21 yet, certain genetic and physiological responses to caffeine are not conserved—including the molecular pathways responsive to caffeine and the metabolites produced after caffeine ingestion17,22—suggesting the possibility that non-pharmacological effects might mediate some of the fly responses to caffeine.23
Limiting nutrient intake in flies also causes a stimulatory effect known as starvation-induced sleep suppression.24–29 Given the robust effect of nutrition on sleep, we hypothesized that aversion to caffeine might reduce total consumption, leading to the possibility that decreased nutrient intake contributes to the effect of caffeine on fly sleep. Past studies measuring the effects of caffeine on insect behavior have identified the importance of caffeine dosage, whereby low and high concentrations of caffeine produce different effects.23,30,31 Effective dietary caffeine levels are balanced between concentrations that are too low to consistently affect sleep (≤0.2 mg/mL caffeine) and concentrations that are toxic (≥1 mg/mL caffeine),13 although these dose responses vary slightly across studies.12,14,18 As a result, studies measuring the effects of caffeine on Drosophila sleep have most recently relied on using one concentration of caffeine on a specific diet (0.5 mg/mL caffeine in 5% sucrose).13,15,17 We measured sleep and total consumption on diets containing a range of sucrose concentrations to determine whether nutrition or changes in nutrient intake influence the reduction in nighttime sleep that is attributed to caffeine pharmacology.
METHODS
Flies
Fly lines used were Canton-S, w1118, iso31, Dahomey, Gr93a3 (Bloomington 27592, w1118;TI{GAL4}Gr93a3), and Gr33a1 (Bloomington 31427, w*;TI{TI}Gr33a1). Controls used for Gr93a3 and Gr33a1 flies were either w1118, Canton-S, or Dahomey. All flies were maintained at 25°C on a 12/12-hour light (zeitgeber time or ZT 0–12)/dark (ZT 12–24) cycle. Unless noted, female flies were used for all experiments. Flies were developed on standard Drosophila medium (recipe below), maintained in mixed-sex groups for 2–4 days following eclosion, and then sorted into single-sex groups under CO2 anesthesia. Single-sex groups were maintained on standard medium and transferred to fresh vials every 2–3 days until used for experiments.
Food
Unless noted otherwise, caffeine, papaverine, and quinine were used at concentrations of 0.5 mg/mL, 0.5 mg/mL, and 2 mM, respectively. Standard Drosophila medium was composed of 1.2% sucrose, 3.1% dry, active yeast, 5.8% cornmeal, and 0.7% agar (all w/v), supplemented with 1% (v/v) phosphoric acid and 1% (v/v) methylparaben mixture (22.2% methylparaben, w/v, in ethanol). Sucrose-only diets contained the indicated percentage of sucrose (w/v) with a 2% agar base. Starvation medium (0% sucrose) contained only the 2% agar base.
Sleep
Drosophila Activity Monitors (DAMs, Trikinetics) were used to collect sleep data. In all DAM experiments, at least n = 13 females were analyzed. For monitoring the effects of caffeine over several days, individual flies (4–6 days old) were placed into glass tubes (5 × 65 mm) for the DAMs after brief CO2 exposure. Approximately 0.4 mL of starvation or 5% sucrose medium, with or without 0.5 mg/mL caffeine, was provided in a 0.65 mL microcentrifuge tube affixed to the end of each fly enclosure, with the other end stoppered with a piece of foam. Flies were habituated to the DAM environment and the indicated diet at ZT 12 (lights off), and data collection started 12 hours later at ZT 0 (lights on). Previously, a similar approach used 1.5 days of habituation before data collection,13 but we required a shorter habituation period due to the use of a starvation medium. For Canton-S lifespan analyses in the DAMs, flies were placed in glass tubes at time = 0 and death was identified by the last hour of activity recorded for each fly. For surviving flies throughout the duration of the experiment, food was made fresh in 0.65 mL microcentrifuge tubes and replaced every 7 days.
To determine the effect of varied nutrition on responses to caffeine, flies (4–6 days old) were habituated in the DAMs starting at ZT 12 with standard Drosophila medium affixed to the end of each glass tube, as described above. After 12 hours (ZT 0), data collection was started for a 24-hour period to determine day 1 (baseline) behavior. Immediately before the end of the first 24-hour recording period, around ZT 0 on day 2, food was swapped for each fly by replacing the 0.65 mL microcentrifuge tubes containing standard medium with tubes containing the indicated sucrose diet, with or without caffeine. Data collection was subsequently continued for another 24 hours (day 2). The period in which food swapping occurred was removed from analyses. Experiments using papaverine or quinine were performed similarly.
Customized scripts in Matlab (Mathworks) were used to collect average sleep data and to plot sleep traces.32 Flies that exhibited no activity for a 12-hour period (typically not more than 1 fly per treatment) were considered dead and removed from analyses. Flies were scored as sleeping based on a period of 5 minutes of inactivity.12,14 Sleep is presented as minutes of sleep per hour, averaged over the indicated period (usually lights off, ZT 12–24). Sleep traces are plotted in 60-minute bins.
Total consumption
Feeding assays using radiolabeled medium were performed essentially as described33 using conditions to mimic the acute caffeine-exposure experiments in DAMs. Briefly, flies were placed on fresh vials of standard Drosophila medium for ~1–2 days before transfer at ZT 0 to vials containing experimental [α-32P]-dCTP labeled diets. After 24 hours, flies were collected and frozen. Total consumption in mg of medium was calculated from liquid scintillation counts, using an aliquot of radiolabeled medium as a calibration. Absolute sucrose ingestion was calculated based on dietary sucrose concentration and estimated medium density.
For assessment of excretion, flies were placed on fresh vials of standard Drosophila medium for ~1–2 days before transfer at ZT 0 to conical tubes (15 mL) containing [α-32P]-dCTP labeled diets and FD&C blue No. 1 to visualize excreta. Flies were frozen and collected after 24 hours, and excreta were washed from vials using PBS containing 0.01% Triton X-100. Scintillation counts for radiolabel absorbed by flies or in excretions were determined as described above.
Capillary feeder (CAFE) assays34 were performed as previously described with slight modifications.33 Briefly, flies were placed in CAFE chambers and provided with capillaries containing 5% sucrose and 5% yeast extract for 1.5 days of habituation. After the habituation period, flies were exposed to capillaries containing experimental diets at ZT 0 and total consumption was measured at 12-hour intervals over a 24-hour period. Fresh capillaries containing experimental diets were replaced at the 12-hour time point. Evaporation was controlled for by subtracting average evaporation from total feeding measures. Evaporation totaled <4% of total feeding. Dead flies were censored, and totaled ~1% of the experimental population.
Proboscis Extension Response
Flies were prepared and tested essentially as described.35 Briefly, flies were loaded into modified 200-µl pipette tips to allow access to the fly proboscis. Flies were then tested with positive (5% sucrose) and negative (water) controls, followed by alternating tastant solutions delivered 5 times each. For Gr93a3 experiments, diets used were 4% sucrose ± 1 mg/mL caffeine. For Gr33a1 experiments, diets used were 100 mM sucrose ± 1 mM quinine. To increase baseline PER, Gr93a3 and controls were starved for 24–48 hours on 1% agar prior to starting the assay. Proboscis extension was recorded as 0, 0.5 (partial), or 1 (full). After tastant testing was complete, flies were assessed again with positive and negative controls. Flies that did not respond to the positive control, or that responded to the negative control, were excluded from analyses.
Statistics
ANOVA (one- or two-way), unpaired two-tailed t tests, nonlinear regression using a one-phase association model, and Pearson’s correlation were performed using GraphPad Prism. Multiple comparisons were corrected using Sidak’s multiple comparisons test or Tukey’s multiple comparisons test. Independent trials were analyzed together. Post hoc analyses were run separately on independent trials.
RESULTS
Effect of Caffeine and Starvation on Sleep is not Additive
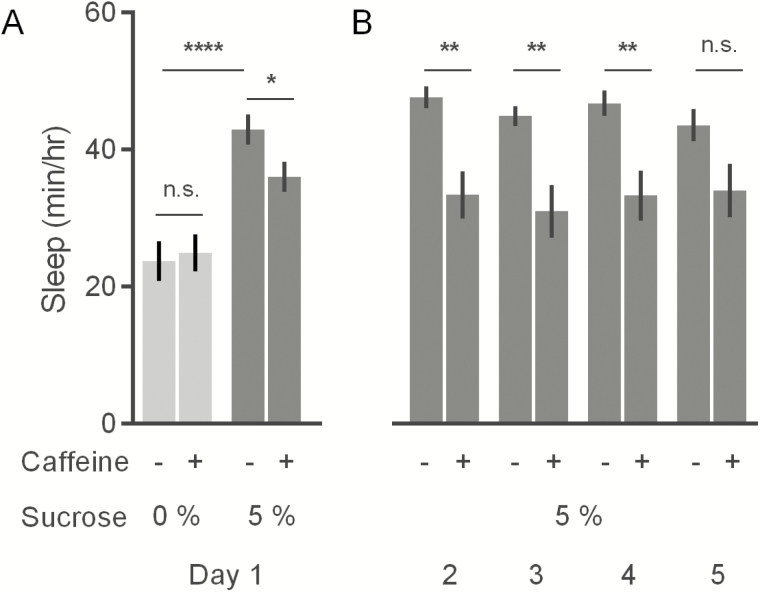
Recent studies have relied on diets containing 5% sucrose ± 0.5 mg/mL caffeine to reveal the most robust effects on nighttime sleep.13 Female flies were also shown to be more sensitive to caffeine-mediated effects.13 We replicated these studies to show that females on a caffeinated 5% sucrose medium indeed slept less during the 12-hour dark period, compared with flies on 5% sucrose diet without caffeine (p < .05; 5% sucrose = 42.9 ± 2.2 minutes/hour; 5% sucrose + caffeine = 36.0 ± 2.2 minutes/hour) (Figure 1A).
Figure 1.
Effect of caffeine and starvation on nighttime sleep of Canton-S females. (A) Nighttime sleep on starvation or 5% sucrose medium ± 0.5 mg/mL caffeine during the first day of exposure. (B) Nighttime sleep on 5% sucrose diet ± 0.5 mg/mL caffeine during days 2 through 5 of exposure. Values represent average ± SEM. n = 15–16 flies per diet. * p ≤ .05, ** p ≤ .01, **** p ≤ .0001.
We next quantified the individual and combined effects of caffeine supplementation and starvation—when flies are on an agar-only medium—to determine the relative influence of each on nighttime sleep. Consistent with previous studies on starvation-mediated sleep suppression,24–29 starved flies showed significantly reduced sleep (p < .0001; 0% sucrose = 23.8 ± 2.9 minutes/hour; 5% sucrose = 42.9 ± 2.2 minutes/hour). Caffeine supplementation did not decrease sleep further, indicating that there is no additive effect of caffeine on sleep under starvation conditions (p = .78; 0% sucrose = 23.8 ± 2.9 minutes/hour; 0% sucrose + caffeine = 24.9 ± 2.7 minutes/hour) (Figure 1A). Although this is consistent with the idea that caffeine and nutrient deprivation affect sleep by overlapping mechanisms, sleep under starvation may already be at a minimum level, making it difficult to generate or measure further decreases in sleep.
Since life is shortened under starvation, animals on agar-only medium were only monitored for 1 day. However, we measured the extended effect of caffeine on nighttime sleep over 5 days on 5% sucrose medium. In agreement with previous reports,13 flies slept less on caffeinated compared with caffeine-free medium (F = 36.3, p < .0001) (Figure 1B). Additionally, the sleep trends from days 2–5 of caffeine exposure did not differ (F = 0.30, p = .82) and post hoc tests revealed that caffeine supplementation significantly decreased nighttime sleep on days 2–4 (Figure 1B).
Dietary Sucrose Concentration Influences Caffeine-Mediated Effects on Sleep
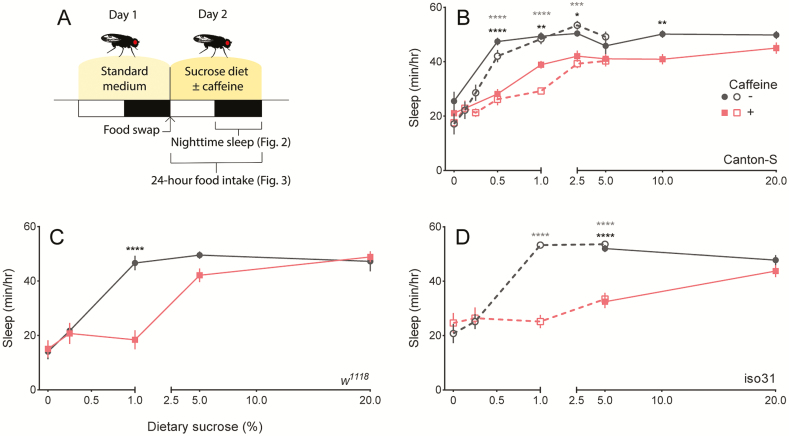
To investigate the potential interaction between nutrition and caffeine, we next measured the effect of 0.5 mg/mL caffeine on nighttime sleep using diets ranging from 0 to 20% sucrose. To account for the variable effects of caffeine on nighttime sleep across genotypes,13 we tested females of three fly strains (Canton-S, w1118, and iso31). To avoid death on low nutrient diets within our experimental time frame, we measured the acute effect of caffeine on fly sleep over 24 hours. Flies were exposed to caffeine starting at ZT 0 or when the “lights on” period began (Figure 2A). This experimental design is sufficient to reveal nutritional- or caffeine-driven effects on nighttime sleep.13,24
Figure 2.
Effect of sucrose concentration and caffeine on nighttime sleep. (A) Graphic of experimental design used to habituate flies to the DAM environment. White and black rectangles denote the light (ZT 0–12) and dark (ZT 12–24) periods, respectively. Food was swapped from the standard Drosophila medium to sucrose diets at the end of day 1. The effect of the various sucrose diets ± 0.5 mg/mL caffeine on nighttime sleep is shown for (B) Canton-S, (C) w1118, and (D) iso31 females. Values represent average ± SEM. n = 15–17 flies per diet. When applicable, independent trials are shown separately using open or closed symbols. Significance of trials denoted with open or closed symbols is represented by gray or black asterisks, respectively. *p ≤ .05, **p ≤ .01, ***p ≤ .001, ****p ≤ .0001. ZT, zeitgeber time.
Flies showed consistent baseline behavior before exposure to caffeine (day 1, Figure S1). After caffeine exposure, there was a significant interaction between diet and caffeine on nighttime sleep in all three strains, demonstrating that the impact of caffeine on sleep depends on diet (Figure 2B–D, Table S1). Post hoc analyses revealed the nature of the diet-dependent effect—caffeine supplementation resulted in decreased nighttime sleep on intermediate sucrose concentrations. Caffeine had no effect on nighttime sleep on sucrose-rich (20%) diet and, in agreement with our previous findings (Figure 1A), there was no additive effect of caffeine on starvation medium or on diets with low (≤0.25%) sucrose (Figure 2B–D).
Caffeine and Dietary Sucrose Concentration Influence Total Consumption
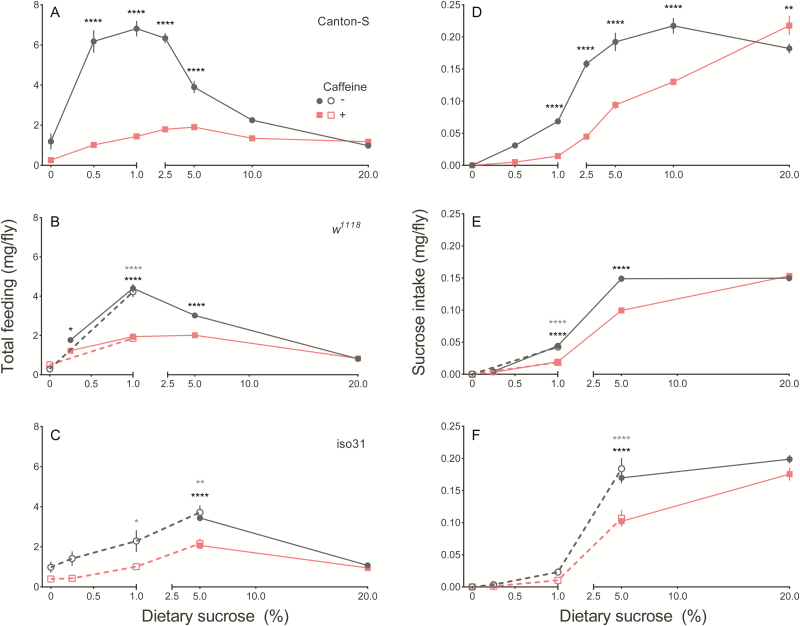
Since caffeine-mediated effects on sleep depend on diet, we next tested whether caffeine also alters food intake. We measured total consumption over 24 hours on diets containing 5% sucrose plus a range of caffeine from 0 to 2 mg/mL and found significantly decreased consumption with increasing concentrations of caffeine (Figure S2). We next tested for dietary influences on caffeine intake by measuring total consumption of diets ranging from 0 to 20% sucrose ± 0.5 mg/mL caffeine. For these measures, we again tested females of three fly strains to reveal potential strain-specific effects.
We found a significant interaction between diet and caffeine on total consumption for all strains tested (Figure 3A–C, Table S2), suggesting that consumption of caffeinated diets was influenced by sucrose concentration. Post hoc analyses revealed a trend similar to the nighttime sleep studies—caffeine significantly decreased consumption of diets containing intermediate sucrose levels, but not of starvation or high (20%) sucrose medium (Figure 3A–C). Radioisotope-labeling of fly food to measure total consumption relies on efficient absorption of the consumed radiolabel. Since caffeine can act as a diuretic,36 we tested whether caffeine consumption influenced our feeding measures through increased tracer excretion. We found that the tracer was absorbed at ≥95% across all tested diets, suggesting that our radiolabel-based method accurately reflects total consumption (Figure S3A). Nonetheless, we also used the CAFE assay to directly measure food intake.34 In the CAFE, flies feed on liquid food and the volume consumed is directly measured. Similar to our previous findings, flies consumed significantly less diet containing caffeine (Figure S3B).
Figure 3.
Effect of sucrose concentration and caffeine on total consumption and absolute sucrose ingestion. Consumption of the indicated diet over 24 hours is shown for (A) Canton-S, (B) w1118, and (C) iso31 females. Sucrose intake on the indicated diets is calculated from total consumption for (D) Canton-S, E) w1118, and (F) iso31 females. Values represent average ± SEM. n = 7–12 measures per diet. When applicable, independent trials are shown separately using open or closed symbols. Significance of trials denoted with open or closed symbols is represented by gray or black asterisks, respectively. *p ≤ .05, **p ≤ .01, ****p ≤ .0001.
Total consumption in Figure 3A–C is a proxy for absolute caffeine intake, since we used a single concentration of caffeine for these experiments—however, diets varied in sucrose content. Similar to our analyses on total consumption, we found a significant interaction between diet and caffeine on absolute sucrose ingestion (Figure 3D–F, Table S3). Post hoc comparisons demonstrated that caffeine decreased sucrose ingestion in most mid-range sucrose diets tested. Caffeine did not significantly decrease sucrose ingestion on low-sucrose diets (≤0.25%, Figure 3D–F), although intake is expected to be low given the dietary composition. On non-caffeinated diets, compensatory feeding, whereby an animal regulates consumption to achieve a target dietary intake,33,37,38 was evident at ≥5% sucrose. At lower sucrose concentrations, under-compensation occurred, possibly due to a limit on how much flies can eat daily. Compensatory feeding was clearly disrupted by caffeine supplementation (Figure 3D–F).
Effect of Caffeine can be Mimicked by Bitter Tastants
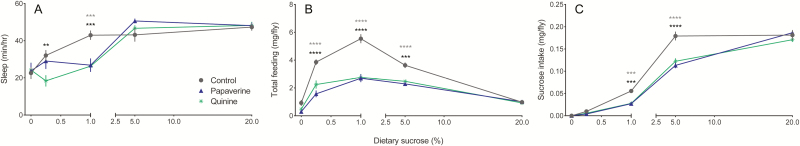
Our results suggest that decreased food intake is an additional factor under conditions in which caffeine reduces nighttime sleep. If reduced consumption plays an important role in caffeine-mediated effects on sleep, we hypothesized that supplementing the fly diet with other aversive or bitter compounds would mimic the effect of caffeine on sleep and food intake. We first quantified the influence of two bitter tastants, papaverine and quinine, on Canton-S nighttime sleep.3,8–10 These analyses were similar to our caffeine experiments; we measured sleep and feeding upon acute exposure to diets ranging from 0 to 20% sucrose ± 0.5 mg/mL papaverine or 2 mM quinine, which represent concentrations used in previous studies that affect proboscis extension responses or other food-related behaviors.3,10
Similar to our analyses of caffeinated diets, we found a significant interaction between diet and bitter additive on nighttime sleep (Figure 4A, Table S4A), demonstrating that the effect of papaverine and quinine is influenced by diet. Post hoc tests revealed a significant decrease in nighttime sleep on papaverine diets containing 1% sucrose, and on quinine diets containing 0.25% and 1% sucrose, but there was no significant effect of either compound on starvation medium or on higher sucrose diets (Figure 4A).
Figure 4.
The influence of bitter tastants on Canton-S female behavior. (A) Nighttime sleep, (B) total consumption, and (C) sucrose intake on a range of sucrose diets ± 0.5 mg/mL papaverine or 2 mM quinine. Values represent average ± SEM. n = 13–16 flies (nighttime sleep) or 10–12 measures (feeding) per diet. Significant differences between control and papaverine or between control and quinine are denoted by gray or black asterisks, respectively. **p ≤ .01, ***p ≤ .001, ****p ≤ .0001.
We next measured total feeding across these diets to determine whether dietary papaverine and quinine also decreased food intake. We detected a significant interaction between diet and compound on total consumption (Figure 4B, Table S4B), again demonstrating that the effect of papaverine and quinine is influenced by diet. Both compounds significantly decreased feeding on diets between 0.25–5% sucrose, but not on starvation or sucrose-rich (20%) medium (Figure 4B). Finally, we determined the effect of papaverine and quinine on absolute sucrose intake and found results similar to that for caffeine, whereby there was a significant interaction between compound and diet (Figure 4C). Post hoc analyses again showed a significant reduction in sucrose ingestion only on intermediate-concentration diets (1 and 5% sucrose) containing either compound (Figure 4C).
Sucrose and Total Intake Modulate the Influence of Caffeine on Sleep
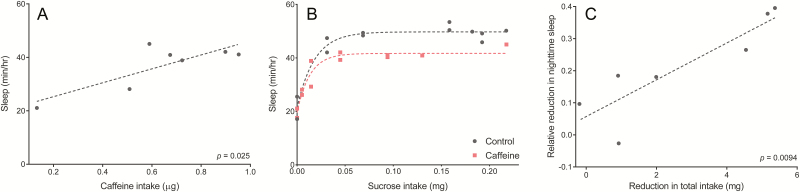
Differences in total consumption—and therefore, in absolute caffeine ingestion—between diets allowed us to assess whether nighttime sleep is described by a dose response to caffeine. Surprisingly, there was a positive relationship between absolute caffeine intake and nighttime sleep (Pearson’s correlation coefficient, r = 0.82, p = .025) (Figure 5A). These results clearly show the importance of considering nutrient ingestion in sleep studies, since reduced sleep is not associated with increased caffeine intake alone—and in fact demonstrates the opposite trend—although the range of caffeine ingestion is limited (0.1 to 1 µg per fly).
Figure 5.
Correlates of nighttime sleep in Canton-S females. (A) Relationship between nighttime sleep and absolute caffeine ingestion on sucrose diets containing caffeine. (B) Relationship between nighttime sleep and absolute sucrose ingestion for caffeinated versus non-caffeinated diets. (C) Relationship between nighttime sleep loss and reduction in food intake resulting from caffeine. Each point represents the effect of caffeine on a single sucrose concentration diet. For (A) and (C), a linear fit and Pearson’s correlation p-value are shown. For (B), a one-phase association was used to fit a nonlinear trendline.
We next compared absolute sucrose intake with nighttime sleep for caffeinated and control diets separately. On non-caffeinated diets, nighttime sleep is reasonably described by an exponential response to absolute sucrose ingestion (Figure 5B). On caffeinated diets, Canton-S flies showed less nighttime sleep compared with that of controls, although this effect is diminished at lower sucrose ingestion (Figure 5B), and the difference is not evident at high sucrose intake in w1118 and iso31 flies (Figure S4A).
Previous reports focused on nighttime sleep to highlight the most robust effects of caffeine,13 which indeed more greatly impacted nighttime compared with daytime sleep (Figure S1). Daytime sleep during the first 12 hours of caffeine exposure was rarely decreased—and actually increased in Canton-S on some diets (Figure S5). Although accounting for total, 24-hour sleep in w1118 and iso31 flies produced similar results to nighttime sleep comparisons, caffeine had no effect on total sleep in Canton-S across sucrose intake levels (Figure S4). Overall, these findings indicate that when absolute sucrose intake is accounted for, caffeine reduces only nighttime sleep in Canton-S and both nighttime and total sleep in w1118 and iso31 flies at intermediate amounts of sucrose ingestion.
Since caffeine had an effect on nighttime sleep that was not totally explained by the amount of sucrose ingested, we next determined whether absolute changes in food intake might predict sleep loss on caffeinated diets. For each sucrose concentration, we plotted the relative reduction in nighttime sleep against the decrease in food intake induced by caffeine (Figure 5C). Indeed, nighttime sleep loss was strongly correlated to reduced consumption (Pearson’s correlation coefficient, r = 0.88, p = .0094). Overall, these results show that caffeine supplementation is associated with reduced nighttime sleep only when feeding is decreased.
Reduced Total Consumption of Caffeinated Medium is not Dependent on Taste
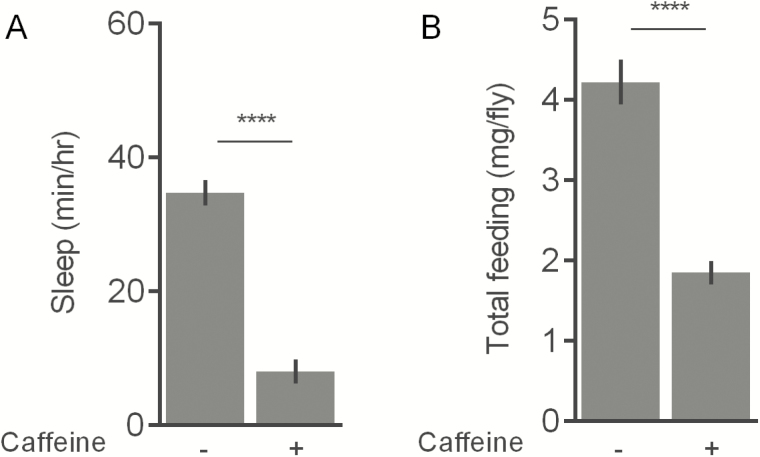
We next hypothesized that bitter taste mutants that are insensitive to caffeine would also show unaltered food consumption with caffeine supplementation. This would allow us to decouple the effects of caffeine on food intake and sleep. In agreement with previous studies,9 we found that a Gr93a mutant (Gr93a3) was not responsive to caffeine in short-term taste assays (Figure S6). We then measured nighttime sleep and feeding under conditions that supported the greatest effects of caffeine throughout our study (1% sucrose diet ± 0.5 mg/mL caffeine). Gr93a3 showed a significant reduction in nighttime sleep with caffeine (p < .0001; 1% sucrose = 34.8 ± 1.9 minutes/hour; 1% sucrose + caffeine = 8.1 ± 1.8 minutes/hour) (Figure 6A). However, while Gr93a3 flies were insensitive to the taste of caffeine in proboscis extension response assays, caffeine still significantly reduced 24-hour total consumption (p < .0001; 1% sucrose = 4.2 ± 0.28 mg/fly; 1% sucrose + caffeine = 1.85 ± 0.14 mg/fly) (Figure 6B). Similar findings have been reported in Gr66a mutants, which are not initially responsive to caffeine8 but show reduced total consumption.3
Figure 6.
Effect of caffeine on sleep and total consumption in Gr93a3 females. (A) Nighttime sleep and (B) 24-hour food intake of Gr93a3 flies on 1% sucrose diet ± 0.5 mg/mL caffeine. Note that complementary experiments performed at the same time using the control strain, w1118, were reported in Figures 2C and 3B. Values represent average ± SEM. n = 16 flies per diet. ****p ≤ .0001.
Interestingly, Gr33a1 mutants were not sensitive to quinine in short-term assays (Figure S7A),10 but also showed reduced 24-hour total consumption on diets containing quinine (Figure S7B). Overall, these results suggest that postingestive responses likely mediate the effects of caffeine and other bitter tastants on long-term feeding. While the caffeine concentrations we used did not result in fly deaths over the 24-hour assay period, these conditions shorten life over long-term exposure (Figure S8), suggesting that toxicity might also contribute to Drosophila behavioral responses on caffeinated diets.
DISCUSSION
Given the known influence of nutrition on Drosophila sleep24–29,39, we hypothesized that aversion to caffeine would reduce food intake and that this decrease in nutrient ingestion might contribute to the sleep-suppressing properties of caffeine in flies. By using high-resolution measurements of food intake,33 we modeled the relationship between absolute sucrose ingestion and nighttime sleep as an exponential association. This model describes a baseline sleep on starvation medium, a plateau showing maximum sleep with sufficient sucrose ingestion, and a rate constant that defines the sensitivity to sucrose. Starvation-mediated sleep suppression has generally not been studied in conjunction with quantitative nutrient intake measurements, and it will be of interest to determine how genetic manipulations that affect the response to starvation24–29 modify model parameters. Since our diets contained only sucrose—and other nutrients such as protein are known to affect sleep40–42—future studies might also use our model to quantitatively dissect the contribution of various food components, either individually or synergistically, to fly sleep and determine whether sleep is indeed accurately described by a dose-response to nutrients.
Consistent with our hypothesis, caffeine reduced food intake using two different feeding assays33,34 and nutrition played a strong role in modifying the effect of caffeine on nighttime sleep in three fly strains. Intermediate sucrose intake supported the greatest effects of caffeine on Drosophila behavior and high sucrose ingestion abrogated caffeine-mediated sleep loss. It is not straightforward to interpret the absence of an effect on nighttime sleep during starvation—sleep may already be suppressed to a minimal level such that an additional effect of caffeine cannot be measured. Additionally, absolute caffeine ingestion is minimized on agar-only medium.
Accounting for the effect of caffeine on absolute sucrose ingestion did not explain changes in nighttime sleep, suggesting that there is minimal overlap between the mechanisms underlying caffeine-mediated sleep loss and starvation-mediated sleep suppression. Hence, total nutrient ingestion is a modifier of the sleep-suppressing effect of caffeine but is not predictive of sleep loss. Instead, changes in sleep are correlated with changes in feeding behavior—larger mismatches between target and actual intake due to caffeine lead to a greater sleep loss. We speculate that food-related behaviors beyond absolute nutrient ingestion, such as meal timing or size, may be the important factors that perturb sleep.43,44 Although it is not known if excretion influences Drosophila sleep, we detected a trend suggesting excretion is increased ~3% on caffeinated diets. Although this trend was small, total excretion may be a feeding-related behavior of interest in future studies.
Given that the aversive tastants quinine and papaverine act similarly to caffeine by interacting with diet to reduce fly sleep, we hypothesized that bitter taste sensation mediates the effect of these compounds. Surprisingly, a gustatory receptor mutant (Gr93a3) that is insensitive to caffeine in electrophysiological tests and short-term avoidance assays9 still shows a significant nighttime sleep loss and decreased consumption on caffeinated diets. This is consistent with another report showing decreased consumption of caffeinated food in Gr66a mutants3 and our results demonstrating reduced intake of quinine-containing medium in a Gr33a mutant, despite previous studies showing these flies are unresponsive to caffeine8 or quinine,10 respectively, in short-term taste tests. These results suggest that postingestive mechanisms might regulate long-term food intake in response to bitter substances. Since caffeine and other plant-derived compounds are commonly toxic to insects to prevent herbivory,45 postingestional malaise to aversive compounds might underlie long-term changes in behavior.46 While the concentration of caffeine used throughout our experiments caused no deaths during the 24-hour test period, 0.5 mg/mL caffeine was toxic compared with drug-free controls over a lifetime of exposure. Consequently, early effects of caffeine toxicity may be evident in our behavioral measures.
Previous studies have identified genetic and neuronal manipulations that modulate caffeine responses, and it will be insightful to test whether these mechanisms are similarly influenced by nutritional factors. The stimulatory effect of caffeine in flies requires the vesicular monoamine transporter (DVMAT),13 which is needed for synaptic release of dopamine, and the D1 dopamine receptor.19 Silencing the paired anterior medial (PAM) neurons, a subset of dopaminergic neurons activated by caffeine, prevents caffeine-induced sleep loss.13 Dopaminergic transmission also has ties to sleep- and nutrition-related signaling in the absence of caffeine. Potentiation of dopamine signaling with a dopamine transporter (DAT) mutant decreases sleep,47 and activating either the tyrosine hydroxylase (TH) or PAM neurons causes sleep loss.13,48,49 PAM and other neurons mediate food- or water-related signals during learning,50–53 and sucrose ingestion can both activate and inhibit different dopaminergic neurons54 and suppress a class of PAM neurons.55 As such, a fine-scale analysis of PAM neuronal classes might help us to determine whether there is an overlap in responses to nutrient and caffeine ingestion. Future work testing how genes and circuits that respond to caffeine also respond to nutritional factors could establish a causal link to the stimulatory effects of caffeine in flies and may also provide insight into previously identified genetic and metabolic differences between mammalian and fly responses to caffeine.17,22
In summary, studies on the stimulatory effects of caffeine or other drugs in Drosophila should carefully consider the impact of feeding behavior. The measure of total consumption provides absolute drug ingestion levels and reveals potential changes in nutrient intake. Testing multiple diets will also determine the nutritional base that supports the most robust effects on sleep. This approach has been useful in previous studies examining the effect of various additives on food palatability and lifespan.56,57 The optimal diet might be strain-specific and depend on numerous factors, including innate feeding behavior, the nutritional levels suitable for sustaining basal sleep, and palatability of the diet or additives. Interestingly, the iso31 strain has been featured for its robust circadian rhythm58 and preferentially used to show a robust decrease in nighttime sleep on caffeinated diets.13 Although it is not clear what makes iso31 unique, maximal feeding occurs at higher sugar concentrations for iso31 than other lines. Our results are consistent with the idea that the greatest sensitivity to caffeine would occur on the diet where food intake was maximal. Consequently, a careful choice of conditions for each genotype might be instrumental in determining Drosophila sleep responses to nutrition or drugs.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
DISCLOSURE STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alex Keene (Florida Atlantic University) and the Bloomington Drosophila Stock Center (NIH P40OD018537) for fly lines, Margaux Ehrlich for technical assistance, Seth Tomchik for technical expertise, and Amita Sehgal for comments on the manuscript. This work was supported by the National Institutes of Health (R01AG045036, W.W.J.).
Ryuichi Yamada is now at Graduate School of Agricultural and Life Sciences, The University of Tokyo, Japan.
REFERENCES
- 1. Detzel A, Wink M. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology. 1993; 4(1):8–18. [Google Scholar]
- 2. Pontes G, Minoli S, Insaurralde IO, de Brito Sanchez MG, Barrozo RB. Bitter stimuli modulate the feeding decision of a blood-sucking insect via two sensory inputs. J Exp Biol. 2014; 217(Pt 20): 3708–3717. [DOI] [PubMed] [Google Scholar]
- 3. Sellier MJ, Reeb P, Marion-Poll F. Consumption of bitter alkaloids in Drosophila melanogaster in multiple-choice test conditions. Chem Senses. 2011; 36(4): 323–334. [DOI] [PubMed] [Google Scholar]
- 4. Wada-Katsumata A, Silverman J, Schal C. Changes in taste neurons support the emergence of an adaptive behavior in cockroaches. Science. 2013; 340(6135): 972–975. [DOI] [PubMed] [Google Scholar]
- 5. Nathanson JA. Caffeine and related methylxanthines: possible naturally occurring pesticides. Science. 1984; 226(4671): 184–187. [DOI] [PubMed] [Google Scholar]
- 6. Glendinning JI, Brown H, Capoor M, Davis A, Gbedemah A, Long E. A peripheral mechanism for behavioral adaptation to specific “bitter” taste stimuli in an insect. J Neurosci. 2001; 21(10): 3688–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glendinning JI, Tarre M, Asaoka K. Contribution of different bitter-sensitive taste cells to feeding inhibition in a caterpillar (Manduca sexta). Behav Neurosci. 1999; 113(4): 840–854. [PubMed] [Google Scholar]
- 8. Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006; 16(18): 1812–1817. [DOI] [PubMed] [Google Scholar]
- 9. Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci USA. 2009; 106(11): 4495–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009; 19(19): 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malinauskas BM, Aeby VG, Overton RF, Carpenter-Aeby T, Barber-Heidal K. A survey of energy drink consumption patterns among college students. Nutr J. 2007; 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hendricks JC, Finn SM, Panckeri KA et al. . Rest in Drosophila is a sleep-like state. Neuron. 2000; 25(1): 129–138. [DOI] [PubMed] [Google Scholar]
- 13. Nall AH, Shakhmantsir I, Cichewicz K, Birman S, Hirsh J, Sehgal A. Caffeine promotes wakefulness via dopamine signaling in Drosophila. Sci Rep. 2016; 6: 20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000; 287(5459): 1834–1837. [DOI] [PubMed] [Google Scholar]
- 15. Ho KS, Sehgal A. Drosophila melanogaster: an insect model for fundamental studies of sleep. Methods Enzymol. 2005; 393: 772–793. [DOI] [PubMed] [Google Scholar]
- 16. Ko CH, Koon CM, Yu SL et al. . Hypnotic effects of a novel anti-insomnia formula on Drosophila insomnia model. Chin J Integr Med. 2016; 22(5): 335–343. [DOI] [PubMed] [Google Scholar]
- 17. Wu MN, Ho K, Crocker A, Yue Z, Koh K, Sehgal A. The effects of caffeine on sleep in Drosophila require PKA activity, but not the adenosine receptor. J Neurosci. 2009; 29(35): 11029–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin FJ, Pierce MM, Sehgal A, Wu T, Skipper DC, Chabba R. Effect of taurine and caffeine on sleep-wake activity in Drosophila melanogaster. Nat Sci Sleep. 2010; 2: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andretic R, Kim YC, Jones FS, Han KA, Greenspan RJ. Drosophila D1 dopamine receptor mediates caffeine-induced arousal. Proc Natl Acad Sci USA. 2008; 105(51): 20392–20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cirelli C, Bushey D. Sleep and wakefulness in Drosophila melanogaster. Ann N Y Acad Sci. 2008; 1129: 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hendricks JC, Sehgal A, Pack AI. The need for a simple animal model to understand sleep. Prog Neurobiol. 2000; 61(4): 339–351. [DOI] [PubMed] [Google Scholar]
- 22. Coelho A, Fraichard S, Le Goff G et al. . Cytochrome P450-dependent metabolism of caffeine in Drosophila melanogaster. PLoS One. 2015; 10(2): e0117328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mustard JA. The buzz on caffeine in invertebrates: effects on behavior and molecular mechanisms. Cell Mol Life Sci. 2014; 71(8): 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keene AC, Duboué ER, McDonald DM et al. . Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr Biol. 2010; 20(13): 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murakami K, Yurgel ME, Stahl BA et al. . translin is required for metabolic regulation of sleep. Curr Biol. 2016; 26(7): 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mattaliano MD, Montana ES, Parisky KM, Littleton JT, Griffith LC. The Drosophila ARC homolog regulates behavioral responses to starvation. Mol Cell Neurosci. 2007; 36(2): 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004; 167(1): 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Z, Yu Y, Zhang V, Tian Y, Qi W, Wang L. Octopamine mediates starvation-induced hyperactivity in adult Drosophila. Proc Natl Acad Sci USA. 2015; 112(16): 5219–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thimgan MS, Suzuki Y, Seugnet L, Gottschalk L, Shaw PJ. The Perilipin homologue, Lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. PLoS Biol. 2010; 8(8): e1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kretschmar JA, Baumann TW. Caffeine in citrus flowers. Phytochemistry. 1999; 52(1): 19–23. [Google Scholar]
- 31. Singaravelan N, Nee’man G, Inbar M, Izhaki I. Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectars. J Chem Ecol. 2005; 31(12): 2791–2804. [DOI] [PubMed] [Google Scholar]
- 32. King LB, Koch M, Murphy KR, Velazquez Y, Ja WW, Tomchik SM. Neurofibromin loss of function drives excessive grooming in Drosophila. G3 (Bethesda). 2016; 6(4): 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deshpande SA, Carvalho GB, Amador A et al. . Quantifying Drosophila food intake: comparative analysis of current methodology. Nat Methods. 2014; 11(5): 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ja WW, Carvalho GB, Mak EM et al. . Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007; 104(20): 8253–8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shiraiwa T, Carlson JR. Proboscis extension response (PER) assay in Drosophila. J Vis Exp. 2007;. (3):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Coca A, Casa DJ, Antonio J, Green JM, Bishop PA. Caffeine and diuresis during rest and exercise: a meta-analysis. J Sci Med Sport. 2015; 18(5): 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J Exp Biol. 1994; 197: 215–235. [DOI] [PubMed] [Google Scholar]
- 38. Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Methods. 2005; 2(11): 813–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Linford NJ, Chan TP, Pletcher SD. Re-patterning sleep architecture in Drosophila through gustatory perception and nutritional quality. PLoS Genet. 2012; 8(5): e1002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Q, Tabuchi M, Liu S et al. . Branch-specific plasticity of a bifunctional dopamine circuit encodes protein hunger. Science. 2017; 356(6337): 534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Catterson JH, Knowles-Barley S, James K, Heck MM, Harmar AJ, Hartley PS. Dietary modulation of Drosophila sleep-wake behaviour. PLoS One. 2010; 5(8): e12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murphy KR, Deshpande SA, Yurgel ME et al. . Postprandial sleep mechanics in Drosophila. Elife. 2016; 5: e19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Afaghi A, O’Connor H, Chow CM. High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr. 2007; 85(2): 426–430. [DOI] [PubMed] [Google Scholar]
- 44. Kant AK, Graubard BI. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005-2010. Am J Clin Nutr. 2014; 100(3): 938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wink M. Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor Appl Genet. 1988; 75(2): 225–233. [Google Scholar]
- 46. Ayestaran A, Giurfa M, de Brito Sanchez MG. Toxic but drank: gustatory aversive compounds induce post-ingestional malaise in harnessed honeybees. PLoS One. 2010; 5(10): e15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005; 25(32): 7377–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seidner G, Robinson JE, Wu M et al. . Identification of neurons with a privileged role in sleep homeostasis in Drosophila melanogaster. Curr Biol. 2015; 25(22): 2928–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sitaraman D, Aso Y, Rubin GM, Nitabach MN. Control of sleep by dopaminergic inputs to the Drosophila mushroom body. Front Neural Circuits. 2015; 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin S, Owald D, Chandra V, Talbot C, Huetteroth W, Waddell S. Neural correlates of water reward in thirsty Drosophila. Nat Neurosci. 2014; 17(11): 1536–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huetteroth W, Perisse E, Lin S, Klappenbach M, Burke C, Waddell S. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr Biol. 2015; 25(6): 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamagata N, Ichinose T, Aso Y et al. . Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proc Natl Acad Sci USA. 2015; 112(2): 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu C, Plaçais PY, Yamagata N et al. . A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012; 488(7412): 512–516. [DOI] [PubMed] [Google Scholar]
- 54. Cohn R, Morantte I, Ruta V. Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell. 2015; 163(7): 1742–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamagata N, Hiroi M, Kondo S, Abe A, Tanimoto H. Suppression of dopamine neurons mediates reward. PLoS Biol. 2016; 14(12): e1002586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yamada R, Deshpande SA, Keebaugh ES, Ehrlich MR, Soto Obando A, Ja WW. Mifepristone reduces food palatability and affects Drosophila feeding and lifespan. J Gerontol A Biol Sci Med Sci. 2017; 72(2): 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Deshpande SA, Yamada R, Mak CM et al. . Acidic food pH increases palatability and consumption and extends Drosophila lifespan. J Nutr. 2015; 145(12): 2789–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ryder E, Blows F, Ashburner M et al. . The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics. 2004; 167(2): 797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.