Summary
Walking is the predominant locomotor behavior expressed by land-dwelling vertebrates, but it is unknown when the neural circuits essential for limb control first appeared. Certain fish species display walking-like behaviors, raising the possibility that the underlying circuitry originated in primitive marine vertebrates. We show that the neural substrates of bipedalism are present in the little skate Leucoraja erinacea, whose common ancestor with tetrapods existed ~420 million years ago. Leucoraja exhibits core features of tetrapod locomotor gaits, including left-right alternation and reciprocal extension-flexion of the pelvic fins. Leucoraja also deploys a remarkably conserved Hox transcription factor-dependent program essential for selective innervation of fin/limb muscle. This network encodes peripheral connectivity modules distinct from those used in axial muscle-based swimming, and has been apparently diminished in most modern fish. These findings indicate that the circuits essential for walking evolved through adaptation of a genetic regulatory network shared by all vertebrates with paired appendages.
In brief
The circuits involved in limb control were established in the common ancestor to all vertebrates with pair appendages millions of years before the first tetrapod walked on land
Introduction
The neural circuits controlling movement have a significant role in shaping the repertoires of behaviors expressed by animals. The ability to travel on land was a key step in the evolution of vertebrates, and many of the circuit elements necessary for limb-based locomotion are contained within the spinal cord (Arber, 2012; Grillner, 2006). It is thought that tetrapod locomotion evolved through the gradual transformation of a spinal network used to activate axial muscles during undulatory swimming behaviors, to a more localized circuitry dedicated to coordinating specific muscles within the limb (Grillner and Jessell, 2009; Jung and Dasen, 2015). This idea is supported by the observation that contemporary representatives of intermediate steps in tetrapod evolution, including amphibians and reptiles, often display locomotor behaviors that are a composite of these two fundamentally distinct motor strategies (Chevallier et al., 2008).
However, in certain fish species the spine remains relatively fixed during locomotion and forward propulsion is driven through oscillatory waves of muscle contraction within the pectoral fins (Rosenberger, 2001). This “rajiform” type of locomotion could involve fin-directed neural connections similar to those used in the tetrapod limb, allowing reciprocal activation and silencing of appendicular flexor and extensor muscles. Remarkably, in certain Rajiformes, including the little skate Leucoraja erinacea, the pelvic fins are employed in a walking-like behavior on the seafloor (Holst and Bone, 1993; Koester and Spirito, 2003). Skates accomplish this benthic form of ambulatory locomotion using specialized segments within the anterior pelvic fin called the crus (Lucifora and Vassallo, 2002; Macesic and Kajiura, 2010). Each crus is articulated through 6 muscle groups attached to the first pelvic radial (Figure 1A). Whether pelvic fin walking in Leucoraja relies on genetic programs and circuit components similar to those of tetrapods has not been investigated.
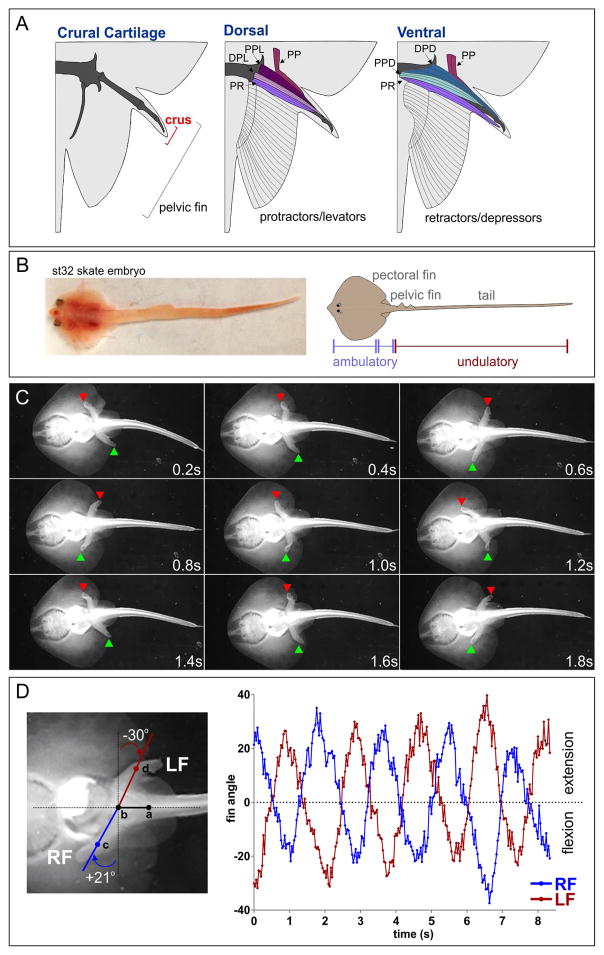
Figure 1. Locomotor Behaviors in Leucoraja erinacea.
(A) Schematics of cartilaginous and muscular elements in the pelvic crus. Protractor and levator (extensor) muscles are located predominantly on the dorsal surface, retractor and depressor (flexor) muscles ventrally. Abbreviations: PP, propterygium protractor; PPL, proximal propterygium levator; DPD, distal propterygium depressor; DPL, distal propterygium levator; PPD, proximal propterygium depressor; PR, propterygium retractor. Modified from (Macesic and Kajiura, 2010). (B) Dorsal view of a st32 skate embryo. Pectoral and pelvic fins mediate ambulatory locomotion, while the tail exhibits undulatory contractions at this stage. (C) Walking behavior in a Leucoraja hatchling. Video frames imaged from the ventral surface showing pelvic fin position (red and green arrowheads) during step cycles. (D) Quantification of fin angles relative to spine position during step cycles. Angles of the left fin (LF) and right fin (RF) were quantified by assigning four points to individual video frames, two along the spinal axis, and one on each fin. Fin extension is represented by positive-value deflection angles, flexion by negative values. Data are quantified from a single hatchling, but are representative of behaviors observed in n>3 animals. See also Figure S1.
Insights into the origins of limb-based locomotor circuits have emerged through comparative analyses of neuronal differentiation and wiring programs in aquatic and land-dwelling vertebrates (Fetcho, 1992; Murakami and Tanaka, 2011). In amniotes, limb-driven motor behaviors require a columnar group of motor neurons (MNs) generated selectively at brachial and lumbar levels of the spinal cord. These MN subtypes are contained within the lateral motor columns (LMCs) and are defined by expression of the transcription factor Foxp1 (Dasen et al., 2008; Rousso et al., 2008). In the absence of Foxp1, limb-level MNs revert to a hypaxial motor column (HMC) identity, a MN subtype thought to represent an ancestral population which facilitates locomotion in swimming vertebrates. Expression of Foxp1 in MNs is triggered by Hox genes expressed at limb-levels of the spinal cord, and the coordinate activities of Foxp1 and Hox proteins determine the identity of MN subtypes targeting individual limb muscles. At thoracic levels, the Hoxc9 gene is required to repress the limb-innervation program and ensure the registry between LMC and limb position (Jung et al., 2010). Interestingly, an expansion in the domain of Hoxc9 expression in snakes is associated with a loss of LMC neurons and a reversion to undulatory locomotion (Jung et al., 2014).
While alterations in Hox expression appear to underlie evolutionary changes in tetrapod MN organization, the origins of limb-based circuits are unclear. In all vertebrates that have been examined, the muscle activation sequences necessary to generate propulsive movement are controlled by central pattern generators (CPGs) within the spinal cord (Grillner, 2006). In swimming vertebrates, including lamprey and zebrafish, activation of axial MNs during undulatory locomotion is orchestrated by CPGs comprised of ipsilaterally projecting excitatory and commissural inhibitory interneurons (INs). This CPG coordinates MN firing across segments, and ensures reciprocal activation of MNs on opposite sides of the spinal cord. Although a similar circuit architecture is used for coordinating between left and right limbs during walking, tetrapods also require a CPG microcircuit necessary for control of appendicular flexor and extensor muscles (Zhang et al., 2014). The origins of this limb-specific CPG are unclear, although it is generally thought that the CPG and MN subtypes necessary for limb articulation evolved as tetrapods transitioned to land (Bagnall and McLean, 2014; Machado et al., 2015; Zhang et al., 2014). Given that in skates and rays alternation of fin extension and flexion provides the predominant propulsive force during swimming; spinal neuronal subtypes thought to be a tetrapod innovation may have appeared in early marine vertebrates.
In this study we investigated the molecular basis of pelvic fin walking in Leucoraja erinacea, a cartilaginous fish which provides a model system to infer the genetic programs present in the common ancestor of vertebrates with paired-appendages (Gillis and Shubin, 2009). Consistent with a basal origin of MN subtypes essential for walking, we find that Leucoraja and mammals share a conserved program required for the selective innervation and control of appendicular muscle. Through molecular profiling of skate pectoral MNs, we identify novel molecular features common to both fin and limb-innervating subtypes. We show that these genes are controlled through a Hox-dependent regulatory program, which has diverged in skates to accommodate a larger pectoral fin size. These findings indicate that the gene networks necessary for limb control were present in the ancestor to all vertebrates with paired-appendages, and that key elements of the neuromuscular system required for limb-based locomotion were established prior to terrestrialization.
Results
Bipedal Locomotor Behaviors in Leucoraja erinacea
To explore the developmental basis of pelvic fin walking in Leucoraja, we first characterized the emergence of spontaneous motor output between early embryonic and post-hatching stages. The earliest motility of Leucoraja embryos was displayed between stages (st) 17 and 24, prior to the appearance of fin buds at st25 (Maxwell et al., 2008). During these stages of development, Leucoraja embryos undulate along the entire length of the neuraxis (Movie S1). By late-gestation (stages 32–33), undulatory locomotion became restricted to the tail, while spinal segments adjacent to the fins remained relatively immobile (Figure 1B, Movie S2). At this phase, the pectoral fins levitated and depressed in bilaterally symmetrical sinusoidal waves extending rostrocaudally, similar to the pattern used in adult swimming. When embryos were removed from egg cases, these spontaneous bouts of pectoral fin contractions could generate propulsive force (Movie S2). The pelvic fins also exhibited episodes of rhythmic extension and flexion alternating between the left-right sides of the spinal cord (Movie S2).
By 2 weeks post-hatching, Leucoraja demonstrated locomotor behaviors bearing striking resemblance to tetrapod walking. When placed on a submerged solid substrate, Leucoraja hatchlings used the pelvic fins to walk along the surface of the tank (Figure 1C, Movie S3–S5). This behavior displayed hallmark features of ambulatory locomotion, including left-right alternation and reciprocal protraction-retraction of the pelvic fins. In some instances, Leucoraja initiated forward propulsion through bilateral synchronous retraction of both pelvic fins followed by left-right alternation of protraction and retraction (Movie S5). Unlike walking behaviors reported in other fish species (Flammang et al., 2016; Standen et al., 2014), bipedal locomotion in Leucoraja did not appear to be assisted through undulation of spinal segments. Measurement of the deflection angles of the left and right pelvic fins, relative to the spine, revealed symmetrically alternating and rhythmic walking-like gaits (Figure 1D).
If Leucoraja employs locomotor CPGs similar to tetrapods, the neuronal subtypes comprising this network might be present within the spinal cord. In mammals, coordination between left and right limbs depends on commissural INs (V0v and V0d subtypes), as well as ipsilaterally projecting excitatory (V2a) INs (Arber, 2012). CPG microcircuits coordinating limb flexor-extensor alternation require two classes of inhibitory INs (V1 and V2b) (Zhang et al., 2014). We detected expression of markers for each of these CPG IN types in Leucoraja embryos, which were present throughout the rostrocaudal extent of the spinal cord (Figure S1A–I). These observations indicate that Leucoraja contains the neuronal populations necessary for generating basic patterns of locomotor output. Consistent with this idea, rhythmic patterns of MN activity were recorded from pectoral nerves in decerebrated spinal preparations after application of CPG-inducing drugs (Figure S1J). Alternating bursts could also be recorded from the dorsal and ventral pectoral nerves, suggesting the presence of a flexor/extensor premotor CPG circuit (Figure S1K). Collectively, these observations indicate that locomotor circuits in Leucoraja develop during embryogenesis, and contain IN classes similar to those of tetrapod CPGs.
MN Columnar Subtypes in Leucoraja Are Similar to Tetrapods
The ability of Leucoraja to exhibit tetrapod-like locomotor gaits might represent an example of convergent evolution, where independent genetic pathways give rise to neural circuits that encode similar patterns of motor output. Alternatively, they could be mediated by circuit connectivity programs that are shared between tetrapods and skates. Since all forms of locomotion are facilitated through activation of specific MN subtypes, we determined whether neuronal differentiation programs are similar between fin and limb MNs. We first examined determinants of basic molecular features of tetrapod MN fate in st32 Leucoraja embryos (Figure 2A). Leucoraja MNs were positioned in the ventral spinal cord and expressed markers of cholinergic neurotransmitter synthesis, including vesicular acetylcholine transporter (Vacht) (Figure 2B). Leucoraja also expressed transcription factors necessary for specifying MN identity in mice including Hb9, Isl1/2, and Lhx3 (Figure 2B, S2A–C). These factors were detected in MNs as early as st17 and continued to be produced until ~st30 (Figure S2A–C and data not shown).
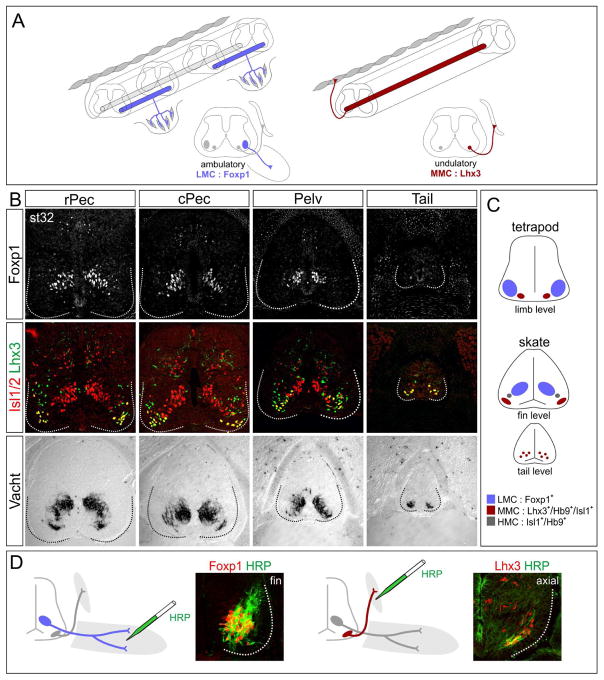
Figure 2. MN Columnar Organization in Leucoraja erinacea.
(A) Organization of MN columns in tetrapods. Limb-innervating LMC neurons express Foxp1. MMC neurons innervate axial muscles and express Lhx3. In fish, axial MNs drive undulatory locomotion. (B) Organization of MN columns in Leucoraja. A column of Foxp1+ MNs is found at pectoral and pelvic fin levels, but is absent from the tail. MMC neurons co-express Lhx3 and Isl1/2 and are found at all segmental levels. Vacht marks all spinal MN populations. Data are representative of n>3 embryos analyzed. Abbreviations: rPec, rostral pectoral fin; cPec, caudal pectoral fin; Pelv, pelvic fin level. (C) Summary of MN organization in Leucoraja and comparison to tetrapods. (D) HRP retrograde tracing showing pectoral MNs express Foxp1, while MNs projecting to epaxial muscles express Lhx3. See also Figure S2.
In tetrapods, MNs controlling limb muscle differentiate in a hierarchical manner, with each phase of their maturation governing a key aspect of peripheral innervation pattern. All MNs targeting the limb are contained within the LMC, a diverse neuronal group generated specifically at brachial and lumbar levels of the spinal cord. In amniotes, LMC neurons are defined by expression of Foxp1 and the retinoic acid synthetic enzyme Raldh2 (Jung et al., 2014). In contrast, MNs targeting dorsal epaxial muscles are present throughout the rostrocaudal extent of the spinal cord, are contained within the medial motor column (MMC), and coexpress the Lim homeodomain (HD) proteins Lhx3 and Isl1/2 (Figure 2A) (Tsuchida et al., 1994).
In Leucoraja, MNs expressing Foxp1 were detected as a single clustered and longitudinally arrayed column positioned in register with the pectoral and pelvic fins, but were absent from tail levels (Figure 2B, S2A). Raldh2 was detected in Foxp1+ MNs but was restricted to rostral pectoral segments (Figure S2D). Axial MMC-like neurons (Lhx3+;Isl1+) were also present, and localized to both fin and tail levels (Figure 2B). As in tetrapods, MMC-like and LMC-like neurons were clustered and segregated from each other at fin levels. In contrast, at tail levels MMC-like neurons did not appear to coalesce into a single column, similar to primary axial MNs in zebrafish (Appel et al., 1995). The position of Leucoraja MMC and LMC neurons was also inverted relative to that of tetrapods; with LMC-like neurons situated dorsomedially near the central canal, while MMC-like neurons settled in a ventrolateral position (Figure 2B,C). We also detected a minor population of MNs similar in molecular profile to ventrally projecting HMC neurons, which expressed Isl1 and Hb9, but lacked Foxp1 and Lhx3 (Figure S2E,F). These results indicate that Leucoraja expresses determinants of MN columnar subtypes similar to those of tetrapods.
In zebrafish and flies MNs express Lim and Mnx-class HD transcription factors, but these profiles do not predict a pattern of peripheral muscle connectivity comparable to that of tetrapods (Jung and Dasen, 2015). We therefore used retrograde tracing assays to determine the muscle target specificity of Leucoraja MNs expressing Foxp1 and Lhx3. We injected horseradish peroxidase (HRP) into muscles of st31 skates and assessed transcription factor profiles in labeled MNs. Injection of HRP into the pectoral and pelvic fins selectively labeled Foxp1+ MNs, indicating that Foxp1 expression defines both fin and limb-innervating subtypes (Figure 2D). In contrast injection into dorsal epaxial muscles labeled MNs that expressed Lhx3 (Figure 2D, S4C). These results indicate that the molecular codes defining motor columns targeting appendicular and epaxial muscle are shared by both amniotes and skates.
LMC Neurons Originated in Primitive Marine Vertebrates
Our analyses of MN differentiation programs in Leucoraja provide evidence that LMC neurons were present in the ancestor to skates and tetrapods, and therefore likely the common ancestor to all vertebrates with paired appendages. To further explore this hypothesis, we examined whether LMC neurons are present in other fish species. We assessed LMC determinants in two cartilaginous fish; the small-spotted catshark Scyliorhinus canicula, an elasmobranch, and the elephant shark Callorhinchus milii, a chimaerid. In both species Foxp1+ MNs were present at fin levels, but were absent or only weakly expressed in intervening non-fin and tail levels (Figure 3A,C). We also retrogradely traced pectoral MNs in catshark, and found that these MNs were Foxp1+ (Figure 3B). Surprisingly, we were unable to detect expression of Raldh2 in either Scyliorhinus or Callorhinchus, indicating expression of some LMC determinants have been lost in these species (Figure S3A).
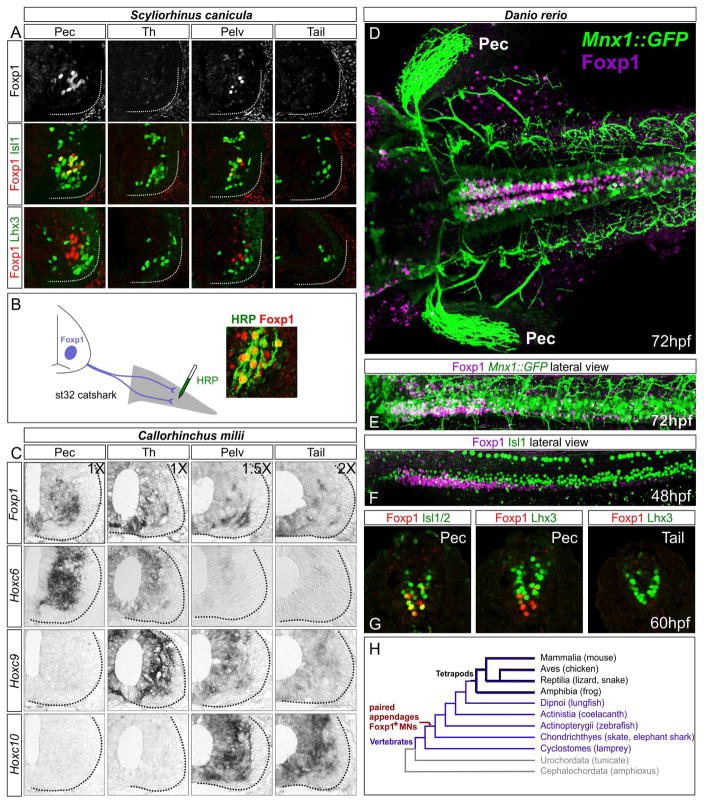
Figure 3. LMC Neurons are Present in Multiple Species of Aquatic Vertebrates.
(A) Expression of MN determinants in the small-spotted catshark Scyliorhinus canicula at st31. Foxp1+ MNs are detected at fin levels but are absent in interfin (“Th”) and tail levels. Lhx3+ MMC-like neurons are present at all levels and lack Foxp1. (B) Retrograde labeling of MNs after HRP injection into the pectoral fin in Scyliorhinus. Foxp1+ MNs co-label with HRP. (C) In situ hybridization showing expression of MN markers and Hox genes in Callorhinchus milii at st31. Foxp1 is detected at fin levels, and also at inter-fin and tail levels, possibly reflecting spinal INs. Hoxc6 is expressed at pectoral levels, Hoxc9 at inter-fin levels, and Hoxc10 at pelvic fin levels. Differences in relative image magnification are shown. (D–G) Expression of MN determinants in zebrafish. (D) Dorsal view of a 72hpf Mnx1::GFP embryo showing expression of Foxp1 at pectoral levels. GFP also labels projections to the pectoral (Pec) fin. Foxp1 is expressed in INs at more caudal segments. (E) Lateral view showing coexpression of GFP in a subset of Foxp1+ neurons. (E) Lateral view of 48hpf embryo showing Foxp1 and Isl1 expression. (G) Transverse sections showing Foxp1+ neurons lack Lhx3. (H) Simplified chordate phylogeny indicating presence of a limb/fin innervation program in the common ancestor to skates and tetrapods. See also Figure S3.
Although expression of LMC determinants has not been fully assessed in bony fish, the Lim HD code that defines the dorsoventral trajectory of motor axon entering the limb appears to be conserved (Jung and Dasen, 2015). We examined Foxp1 expression in zebrafish, and detected expression in pectoral fin MNs by 48 hours post fertilization (hpf) (Figure 3D–F, S3B–D). As in skates, all Foxp1+ MNs lacked Lhx3 expression in zebrafish and catsharks (Figure 3A, G). These results indicate that Foxp1+ MNs are present in multiple undulatory species, where presumably LMC neurons are utilized during fin-assisted postural stabilization and navigation. These results are consistent with an ancestral origin of a fin/limb innervation program in early marine vertebrates (Figure 3H).
LMC Neurons in Leucoraja Differentiate Into Flexor and Extensor Subtypes
Limb control relies on the ability of MNs to reciprocally activate functionally antagonistic pairs of muscle groups (e.g. extensors/flexors, abductors/adductors). This key feature of limb circuitry is thought to have evolved as vertebrates first transitioned to terrestrial habitats (Machado et al., 2015; Zhang et al., 2014). In tetrapods, MNs targeting flexor and extensor muscles can be defined by Lim HD protein expression within the LMC (Figure 4A) (Tsuchida et al., 1994). Laterally-positioned LMC neurons (LMCl) express Lhx1 and typically innervate dorsal extensor muscles, while flexor-innervating MNs are situated medially (LMCm) and express Isl1. Lhx1 promotes a dorsal trajectory of LMCl axons via induction of the EphA4 guidance receptor, which mediates axonal repulsion in response to ephrinA signaling from the ventral limb mesenchyme (Kao et al., 2012). Conversely, Isl1 promotes a ventral trajectory of motor axons via EphB1 expression in LMCm neurons.
Figure 4. MN Subtypes and Neuromuscular Connectivity in Leucoraja.
(A) Summary of Lim HD and Eph/ephrin expression in MNs and limb mesenchyme of tetrapods. (B) In Leucoraja, Foxp1+ LMC neurons segregate into a medial Isl1+ population (LMCm) and lateral Lhx1+ (LMCl) group at fin levels. (C) EphA4 is expressed by lateral (L) LMC neurons, while EphB1 is expressed medially (M). EphA4 is also detected in MMC neurons. (D and E) EphA4 is expressed in dorsal fin mesenchyme, while EphB1 is expressed ventrally. (F and G) Lmx1b is expressed in dorsal fin mesenchyme. (H) Dorsal view of a st29 Leucoraja embryo showing the pectoral and pelvic fins develop adjacent to each other. (I and J) Wholemount immunostaining of NFAP showing axon innervation pattern in the pectoral (I) and pelvic fins (J). (K, L) Vibratome cross-sections of st31 Leucoraja showing axonal projections in the pectoral fin by NFAP staining and dorsal and ventral muscle masses stained with MF20. (M,N) Projections and muscle in the pelvic fin. Panels K–N are composites of tiles made in Zen software. (O) HRP injections in dorsal muscles at st29, showing colocalization with Lhx1. See also Figure S4.
In Leucoraja, pectoral and pelvic LMC neurons were segregated into medial Isl1+ and lateral Lhx1+ divisions (Figure 4B). In addition, EphA4 was detected in lateral LMC neurons whereas EphB1 was expressed medially (Figure 4C). In contrast, the guidance receptor Ret and the transcription factor Meis1, which contribute to dorsoventral target selection in mice (Kao et al., 2012), appeared to be expressed by all Leucoraja LMC neurons (Figure S4A,B). The fin bud mesenchyme was also compartmentalized similar to the limb. In mice, the Lim HD factor Lmx1b is expressed in the dorsal limb compartment and is essential for control of ephrin and Eph expression (Kao et al., 2012). In Leucoraja, Lmx1b and EphA4 were expressed in the dorsal fin bud while EphB1 was expressed ventrally (Figure 4D–G). These results indicate that the patterns of Lim HD, Eph, and ephrin gene expression are grossly conserved between tetrapod and Leucoraja MNs.
Establishing Neuromuscular Connectivity in Leucoraja
To determine whether the Lim HD/Eph/ephrin cascade defines a pattern of fin muscle innervation analogous to tetrapods, we examined the development of connections between LMC neurons and fin muscle. We used wholemount staining of neurofilament associated protein (NFAP) to assess how motor axons target the fin bud. Unlike tetrapods, where LMC axons converge at specific plexi prior to entering the limb, Leucoraja fin motor axons extend directly from each of the segmentally arrayed motor roots (Figure 4H–J). LMC-derived motor nerves subsequently bifurcate into dorsal and ventral rami between st27 and st29 (Figure 4K,M, S4D). Analysis of the pattern of muscle development, using an antibody recognizing myosin heavy chain (MF20), revealed that like in tetrapods, pectoral and pelvic fin muscles are partitioned into separate dorsal and ventral masses, and each mass is supplied by a dedicated motor nerve (Figure 4K–N). A similar fin innervation pattern and Lim HD code was observed in catshark (Figure S3D,E).
To assess whether Lim HD profiles define the targeting of specific muscle compartments in skates, we injected tracers into the fin buds and examined the molecular profile of retrogradely labeled MNs. Injection of HRP into the dorsal fin compartment labeled MNs that expressed Lhx1 and lacked Isl1 (Figure 4O). These results indicate the selective innervation of fin muscle groups is defined by a Lim HD code similar to that of tetrapods. Both the pectoral and pelvic fins contain a dorsal abductor/extensor and ventral adductor/flexor muscle mass, and the two divisions of LMC neurons innervate each group. Thus, the program governing the selection of flexor and extensor muscles by MNs are conserved between Leucoraja and tetrapods.
Molecular Profiling of Fin-Innervating MNs in Leucoraja
Our analyses of MN fate determinants in Leucoraja indicate that limb and fin subtypes are specified through a common gene network. To explore this in greater depth we performed an unbiased analysis of the Leucoraja MN transcriptome using RNAseq. Pectoral fin MNs of st31 embryos were retrogradely labeled, the spinal cord was enzymatically dissociated, and fluorescently labeled MNs were isolated and pooled (Figure 5A). To identify genes differentially expressed between LMC and non-LMC neurons we also isolated neural tissue from tail levels. Three pools of fin and non-fin neurons, each containing ~500 cells, were collected; RNA was extracted, and profiled by RNAseq. Transcripts were identified using de novo assembly and aligned to the zebrafish genome (Nakamura et al., 2015). This analysis identified a total of 5876 annotated genes, 592 of which displayed at least two-fold enrichment in pectoral MNs versus tail spinal cord (Figure 5B, Table S1).
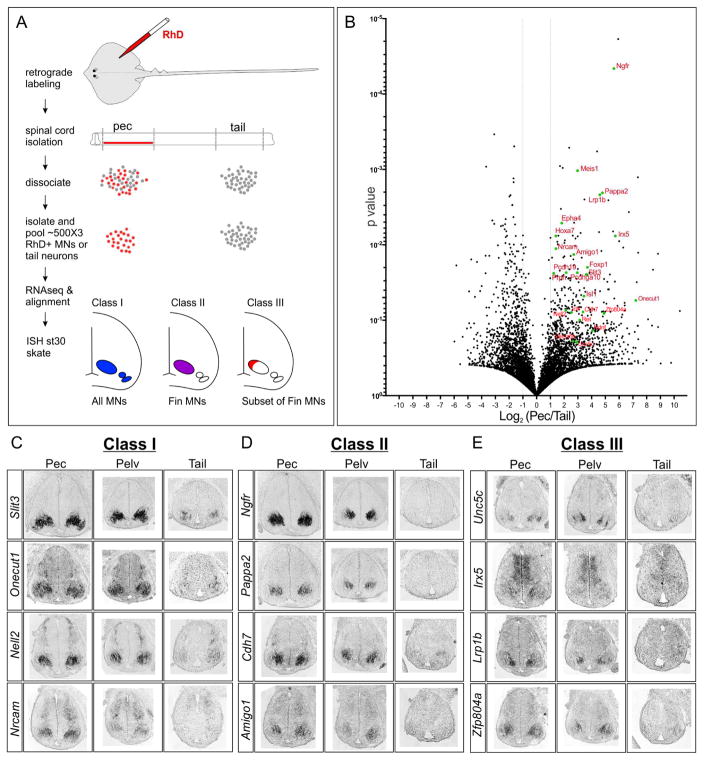
Figure 5. Analysis of the Leucoraja Fin MN Transcriptome.
(A) Strategy for labeling and purifying fin MNs in skate. Fin muscles were injected with rhodamine-dextran (RhD) and were individually picked and pooled for RNA-seq. Three distinct classes of expression patterns are indicated in bottom panels. (B) Volcano plot of genes differentially expressed between fin MNs and tail spinal cord. All genes that averaged 2-fold differences in expression values between fin MNs and tail spinal cord are shown. Both novel and known genes enriched in pectoral MNs are highlighted in red. Mouse gene nomenclature is used. (C) In situ hybridization of differentially expressed candidate genes in st30 skate spinal cord. Class I genes are expressed by all MNs, Class II are highly expressed in fin MNs, and Class III are expressed by subsets of fin-level MNs. See also Figure S5.
To confirm differential expression between the two samples, we selected 40 genes representing cell fate determinants and adhesion molecules for further analysis. We performed in situ hybridization at pectoral, pelvic, and tail levels of st31 skate embryos (Figure 5C–E, S5A). Expression of genes within this subset fell into one of three broad categories. The first class, represented by the Leucoraja homologs of the murine genes Slit3, Onecut1, Nell2, and Nrcam were expressed by all MNs along the rostrocaudal axis (Figure 5C). The second class, which included Ngfr, Pappa2, Cdh7, and Amigo1 homologs, were enriched in pectoral and pelvic fin MNs but were not detected or only weakly expressed at tail-levels (Figure 5D). A third class, including Unc5c, Irx5, Lrp1b, and Zfp804a homologs were expressed by subsets of LMC neurons (Figure 5E), likely representing pools of LMC neurons targeting specific fin regions.
We next assessed whether any of the novel genes identified in this screen display conserved expression in tetrapods. Analysis of Lrp1b and Ngfr in mouse and chick embryos showed restricted expression in LMC neurons, with transient expression of Lrp1b detected in thoracic PGC neurons of mice, while Ngfr was expressed in mouse thoracic HMC neurons (Figure S5B). Irx5 and Zfp804a were expressed by subsets of limb and thoracic-levels MNs, indicating these genes mark MN subpopulations (Figure S5B). In contrast, expression of Pappa2 and Amigo1 were not detected in either mouse or chick MNs, despite pronounced expression in sensory ganglia (Figure S5B). Collectively these results indicate that both tetrapods and skates deploy similar sets of LMC-restricted targets, but also diverge with respect to certain genes.
Hox Genes Define MN Topographic Organization in Leucoraja
Although tetrapods and elasmobranchs share common fin and limb-restricted genes there are marked differences in MN distribution between these groups. Leucoraja has relatively enlarged pectoral fins, extending along the length of body (~30 spinal segments), with the pelvic fins developing adjacent to the pectorals (Figure 4H). In mice, forelimb and hindlimb LMC populations each span ~6 segments, and are separated by 13 thoracic segments. Leucoraja therefore lacks an intervening region separating the two LMC populations. In addition, pectoral and pelvic fins are used for distinct behaviors (swimming versus walking), and likely require specialized LMC subtypes for these locomotor strategies.
In tetrapods, MN diversification and organization requires Hox transcription factors differentially expressed along the rostrocaudal axis (Philippidou and Dasen, 2013). MNs innervating forelimb muscle express Hox6 genes, while hindlimb LMC neurons express Hox10 genes. Hox proteins initiate LMC differentiation via induction of the Foxp1 gene, which is maintained through positive feed-forward autoregulation (Jung et al., 2014). At thoracic levels, Hoxc9 determines forelimb and hindlimb LMC position by suppressing Foxp1 autoregulation and extinguishing forelimb-associated Hox4-Hox8 genes. Interestingly, Leucoraja lacks the HoxC gene cluster in its entirety (King et al., 2011a). The absence of the Hoxc9 gene may have contributed to the caudally extended domain of pectoral LMC neurons in Leucoraja, and the presence of Foxp1+ MNs as a single continuous column spanning both pectoral and pelvic levels.
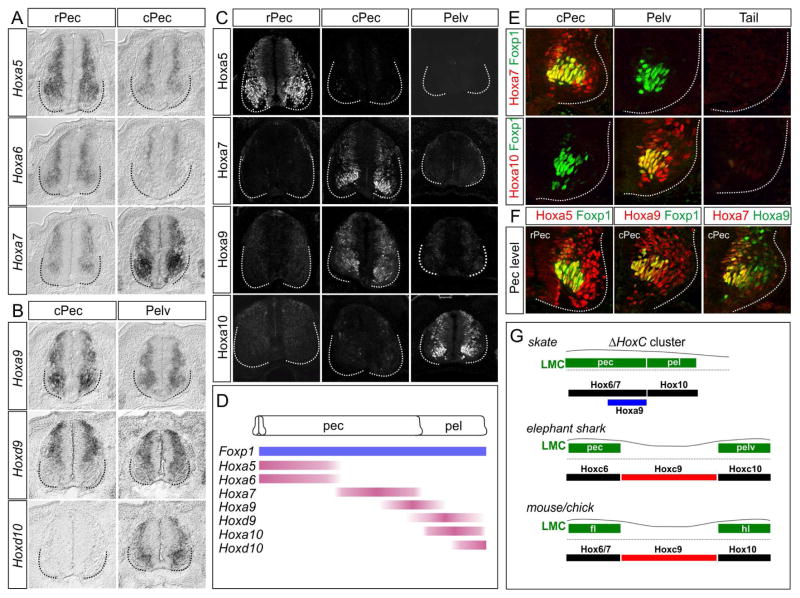
To explore whether Hox-dependent programs contribute to MN organization in Leucoraja, we examined the profile of Hox expression along the rostrocaudal axis. Because skates lack HoxC genes, and HoxB genes are typically excluded from MNs (Dasen et al., 2005), we focused on genes encoded by the HoxA and HoxD clusters. Analysis of HoxA and HoxD mRNA expression revealed patterns highly similar to those observed in mouse and chick. Hoxa5-Hoxa7 genes were expressed at pectoral-levels, whereas Hoxd9, Hoxd10, and Hoxa10 were restricted to pelvic levels (Figure 6A,B, S6A). To assess Hox expression in detail, we generated antisera that selectively labels Leucoraja Hox proteins (Figure 6C). Hoxa5 and Hoxa7 colocalized with Foxp1+ in pectoral MNs, while Hoxa10 was restricted to pelvic Foxp1+ MNs (Figure 6D,E, S6B). In pelvic LMC neurons we also detected expression of the MN subtype-restricted transcription factor Scip (Figure S4A,B) (Dasen et al., 2005), consistent with the idea that Leucoraja MNs acquire level-specific fates. Hoxa9 was also detected in Leucoraja MNs, but was coexpressed with pectoral LMC Hox genes, including Hoxa7 (Figure 6F). Interestingly, Hoxa9+ MNs lacked expression of the LMC determinant Raldh2 (Figure S6C), suggesting that Hoxa9 may contribute to restricting Raldh2 to rostral levels. These observations indicate that the profiles of Hox genes in Leucoraja are analogous to tetrapod forelimb and hindlimb MNs.
Figure 6. Hox gene Expression Defines LMC Organization in Leucoraja.
(A, B) Expression of indicated HoxA and HoxD genes at pectoral and pelvic fin levels in Leucoraja at st29. Hoxa5, Hoxa6, Hoxa7, and Hoxa9 are expressed by MNs at pectoral fin levels. Hoxd9 and Hoxd10 are expressed at pelvic fin levels. (C) Expression of Hoxa5, Hoxa7, Hoxa9, and Hoxa10 proteins in Leucoraja spinal cord at st30. HoxA proteins display collinear patterns along the rostrocaudal axis, with Hoxa5 expressed rostrally and Hoxa10 caudally. (D) Summary of Hox and Foxp1 expression patterns in the skate spinal cord. Foxp1 expression is detected as continuous column adjacent to pectoral and pelvic fins. (E) Hox protein expression at caudal pectoral/pelvic fin levels. Hoxa7 colocalizes with Foxp1 in caudal pectoral LMC neurons, while Hoxa10 colocalizes with Foxp1 in pelvic LMC neurons. (F) Hox expression patterns at pectoral fin levels. Hoxa5 is coexpressed with Foxp1 in rostral pectoral LMC neurons. Hoxa9 is coexpressed with Foxp1 in caudal pectoral LMC and colocalizes with Hoxa7. (G) Summary of Hox expression and LMC organization in skate and elephant shark, relative to the fins; and chick and mouse, relative to the forelimbs (fl) and hindlimbs (hl). See also Figure S6.
Conserved Hox Activities in Fin and Limb MN Specification
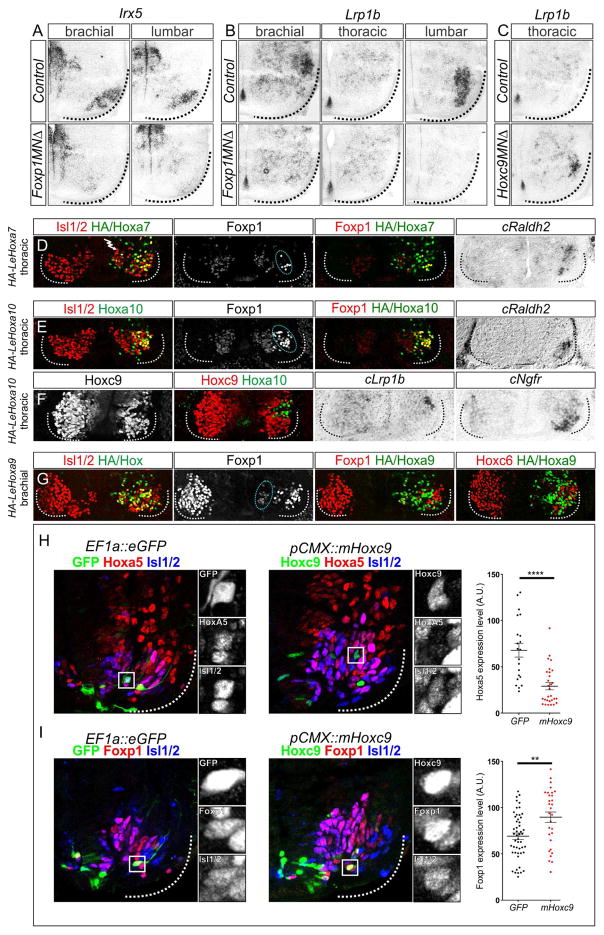
If Hox proteins have a conserved role in determining the organization and identity of fin/limb MNs, their functions are likely to be conserved across species. We therefore performed a cross-species comparison of LMC gene regulation by Hox proteins and the LMC determinant Foxp1. We first asked if any of the fin-level restricted genes identified in our RNAseq analysis are regulated by Hox genes in mice, by examining expression in mutants where LMC specification is disrupted. In mice mutant for the Hox accessory factor Foxp1, LMC differentiation is eroded (Dasen et al., 2008; Rousso et al., 2008). We found that expression of Lrp1b and Irx5 were markedly reduced at limb levels in Foxp1 mutants (Figure 7A,B). By contrast, in Hoxc9 mutants, where thoracic MNs are transformed to an LMC identity, Lrp1b was ectopically expressed in thoracic segments (Figure 7C). These results are consistent with the idea that both fin and limb-innervating MNs are specified through a common Hox-dependent network.
Figure 7. Conserved Hox Activities Govern Limb and Fin MN Differentiation.
(A,B) Loss of Irx5 and Lrp1b expression at brachial and lumbar levels in Foxp1 mutants at e13.5. (C) Ectopic expression of Lrp1b at thoracic levels in Hoxc9 mutants at e13.5. (D) Misexpression of LeHoxa7 at thoracic levels in chick elevates expression of Foxp1 and induces Raldh2, indicating a conversion to an LMC fate. (E) Misexpression of LeHoxa10 at thoracic levels in chick also induces Foxp1 and Raldh2 expression. (F) LeHoxa10 represses Hoxc9 and induces expression of Lrp1b and Ngfr. (G) LeHoxa9 misexpression at brachial levels attenuates Foxp1 expression and represses Hoxc6, indicating a conversion to a thoracic preganglionic motor column (PGC) fate. For each chick electroporation experiment, n>3 embryos were analyzed. (H) Misexpression of Hoxc9 in skate rostral pectoral spinal cord suppresses Hoxa5 expression. Graph on right shows quantification of nuclear pixel intensities of neurons electroporated with Hoxc9 and GFP control constructs. ****p<0.0001 (Wilcoxon–Mann–Whitney test). (I) Effects of Hoxc9 misexpression on Foxp1 at pectoral levels. Foxp1 levels are slightly increased. **p<0.01 (two-tailed Student’s t test). See also Figure S7.
We next tested whether Leucoraja (Le) Hox genes could promote specific fates when expressed in MNs of chick embryos. We cloned LeHoxa5, LeHoxa7, LeHoxa10, and LeHoxa9 cDNAs into pCAGGs expression vectors and assessed their activities via in ovo neural tube electroporation. We examined the ability of LeHoxa5, LeHoxa7 and LeHoxa10 to transform thoracic MNs to an LMC fate. Similar to their tetrapod orthologs, LeHoxa7 and LeHoxa10 induced an LMC identity, as assessed by induction of ectopic Foxp1 and Raldh2 expression (Figure 7D,E) (Lacombe et al., 2013). Misexpression of LeHoxa10 in thoracic chick MNs also induced expression of the LMC-restricted genes Ngfr and Lrp1b (Figure 7F). In contrast, LeHoxa5 only weakly induced expression of Foxp1 in thoracic MNs (Figure S7A), similar to murine Hoxa5. These results indicate that LeHoxa7 and LeHoxa10 can induce multiple features of limb MN fate, and are functionally similar to their amniote orthologs.
Divergent Hox9 Activity in MN Organization
Leucoraja lacks a thoracic-like spinal domain, raising the question of whether this unique organizational pattern reflects the absence of the Hoxc9 gene, or activity differences that evolved between Hox9 gene paralogs. Murine Hoxa9 and Hoxc9 proteins are equivalent with respect to repression of LMC differentiation when ectopically expressed in chick brachial MNs (Jung et al., 2014). In addition, Hoxc9 orthologs derived from coelacanth, pufferfish, zebrafish, and elephant shark show similar activities, suggesting deep functional conservation of Hox9 genes (Jung et al., 2014). Consistent with this idea, expression of MN columnar subtype-associated Hox genes, relative to fin MNs, is conserved in elephant sharks, which retain the HoxC cluster (Figure 3C, 6G). Moreover, a Hox-dependent cis- regulatory element critical for establishing the profile of Foxp1 expression in MNs is conserved among vertebrate classes (Figure S7C–E) (Jung et al., 2014). These observations suggest the marked differences in columnar organization between tetrapods and skates are not due to changes in intrinsic Hox9 activities or Foxp1 regulatory sequences.
To test for functional differences between Leucoraja and tetrapod Hox9 proteins, we expressed LeHoxa9 in chick brachial MNs and examined whether it displays a repressive activity towards LMC differentiation. Misexpression of LeHoxa9 attenuated Foxp1 expression, repressed brachial Hox genes, and induced expression of thoracic MN determinants (Figure 7G, S7B); activities identical to that of mouse Hoxc9 and Hoxa9 (Jung et al., 2014). These results indicate that the absence of a thoracic-like domain in Leucoraja is not due to intrinsic differences between elasmobranch and tetrapod Hox9 proteins, but rather suggests an overall reduced Hoxa9 activity in skates.
To further address this hypothesis we examined the effect of misexpressing Hox9 proteins in Leucoraja. We electroporated a mouse Hoxc9 expression construct in the spinal cord of st27 skate embryos, and assessed MN differentiation after 11 days of incubation. We chose Hoxc9 for this analysis, because it is functionally equivalent to Hoxa9, and allowed us to discriminate electroporated neurons from endogenous Hoxa9+ MNs. Consistent with a conserved activity of Hox9 proteins in repressing more rostral Hox genes, we observed that MNs expressing Hoxc9 showed markedly reduced levels of Hoxa5 (Figure 7H). Interestingly, in Hoxc9 electroporated MNs Foxp1 levels were slightly elevated (Figure 7I), suggesting that Hox9 proteins have lost the ability to repress LMC specification in skates. Together, these results provide evidence that the organization and identity of LMC neurons in Leucoraja are achieved through Hox-dependent mechanisms, and that diminished Hoxa9/Hoxc9 activity contributed to their expanded domain of pectoral LMC neurons.
Discussion
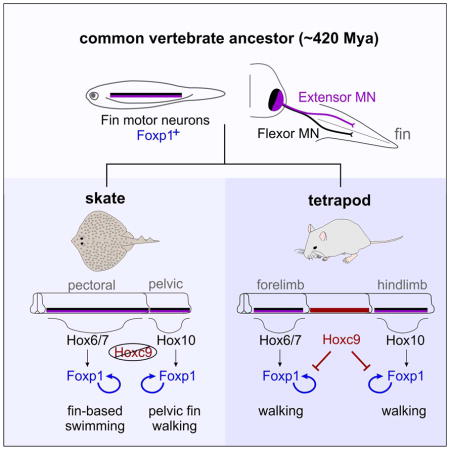
The ability to travel on land was a hallmark step in the evolution of vertebrates, but when the neuronal subtypes essential for limb control appeared is unknown. We found that skates deploy many of the same genetic and circuit connectivity programs essential for walking in tetrapods. Our studies show that both fin and limb MNs are defined by a conserved Hox-dependent gene network, necessary for reciprocal control of appendicular muscle, and that this program was likely present in the common ancestor of skates and tetrapods. These findings could provide a foundation for defining the evolutionary origins of limb-based motor behaviors, and resolving the underlying developmental programs that contribute to the assembly of locomotor circuits.
Limb and Fin Innervating MNs Share a Common Origin
The pelvic fins of Leucoraja display patterns of motor output bearing striking resemblance to the tetrapod limb, including left-right alternation, reciprocal extension-flexion, and joint-like movements along the proximal-distal axis. Although walking-like gaits have been described in both lobed- and ray-fin fish (Flammang et al., 2016; King et al., 2011b; Standen et al., 2014), whether these behaviors are facilitated through a common genetic program has not been examined. In tetrapods, control of limb muscle requires diverse subtypes of MNs that are specified through a common transcription factor, Foxp1. Deletion of Foxp1 from MNs in mice leads to an erosion of extensor-flexor alternation, and severe defects in locomotor coordination (Machado et al., 2015; Surmeli et al., 2011). We found that Foxp1+ MNs are present in multiple species of fish, including the little skate, catshark, elephant shark, and zebrafish. Fin MNs in skate also share several organizational features of limb MNs, including the coalescence into columnar groups, the partitioning into extensor and flexor subtypes, and segregation from axially-projecting MN populations. These observations indicate that the establishment of an LMC molecular program preceded vertebrate terrestrialization.
Our studies also indicate that appendage-derived guidance cues acting along the dorsoventral axis are highly conserved between skates and tetrapods. These results are consistent with the view that patterning of appendages was established early in the vertebrate lineage, including Hox-dependent programs essential for the proximal-distal organization and rostrocaudal limits of fin position (Nakamura et al., 2016; Nakamura et al., 2015; Woltering et al., 2014). An implication of our analyses is that early neuromuscular gene networks were also critical in determining functional properties common to all paired appendages, including the ability to reciprocally control muscle antagonists. The emergence of a genetic system allowing independent activation of fin muscles likely had a significant impact on enabling the subsequent diversification of limb-based motor behaviors.
If LMC neurons were present in the ancestor of skates and tetrapods, how were they utilized in locomotion? Although the exact configuration and usage of fins in the common ancestor is unclear, one model suggests that early fin-bearing vertebrates contained a single fin extending the length of the trunk (Tanaka and Onimaru, 2012). It is plausible that early vertebrates employed a form of locomotion similar to that exhibited by pectoral fins of skates and rays, generating propulsive force through sinusoidal waves of fin extension and flexion. In later vertebrates, this elongate fin and its LMC subtypes were separated into pectoral and pelvic groups, allowing independent evolution of fin-specific motor output. This segregation appears to result from a Hox-dependent repression of fin/limb programs in both the mesoderm and spinal cord at thoracic levels. In the lateral plate mesoderm of mice, Hoxc9 has been shown to represses the forelimb identity determinant Tbx5, while in MNs, Hoxc9 is essential to repress Foxp1 (Jung et al., 2014; Nishimoto et al., 2014). The coordination of limb mesodermal and neural patterning therefore appears to be mediated by a common Hox-dependent system acting on tissue-restricted determinants of regional identity.
Hox Genes and MN Organizational Diversity in Vertebrates
Our studies show that each of the intrinsic signaling pathways essential for tetrapod MN diversification is present within Leucoraja. Through analysis of transcripts expressed by skate fin MNs, we also identified novel genes shared by LMC neurons of skate, mouse, and chick. Expression of Irx5 and Lrp1b are lost in mouse mutants where MN specification is disrupted, while Foxp1, Ngfr and Lrp1b can be induced by skate Hox proteins in non-limb MNs of chick. These observations demonstrate that the LMC-promoting activities of Hox factors are functionally conserved across species. Notable species-specific differences include the loss of Raldh2 expression from pelvic MNs in skates, and the absence of Pappa2 and Amigo2 expression from chick and mouse MNs. Although the functional consequences of these changes are unclear, they may have contributed to differences in the relative settling position and connectivity of skate and tetrapod LMC neurons.
A significant difference between skates and other fin/limb-bearing vertebrate is the absence of a thoracic-like spinal region. In skates, pectoral LMC neurons span over twenty-five segments and are positioned adjacent to the pelvic fin motor column. This expansion in LMC distribution appears to be partially due to the absence of Hoxc9, as a consequence of HoxC cluster deletion. In agreement with this model, targeted mutation of the HoxC cluster in mice causes LMC determinants to be ectopically expressed in thoracic segments (Jung et al., 2014). The loss of Hoxc9 is unlikely to be the sole mechanism underlying the expanded domain of pectoral MNs in skates. In catsharks, which also lack HoxC genes (King et al., 2011a), LMC organization is similar to tetrapods, with a clear separation of pectoral and pelvic populations. Presumably, in other elasmobranch species loss of Hoxc9 is compensated by the activities of functionally equivalent Hox genes, which may include Hoxa9 or other paralogs. Alternatively, skates may lack accessory factors essential for the ability of Hox9 proteins to attenuate Foxp1 expression, which could account for the inability of Hoxc9 misexpression to downregulate Foxp1, as well as the pronounced co-expression of Hoxa9 and Foxp1 in caudal pectoral MNs.
The Evolution of Spinal Locomotor Circuits
While our findings reveal that MN subtypes essential for fin/limb control appeared in early vertebrates, the precise origin of ambulatory locomotion in Leucoraja is less clear. Although pelvic fin walking employs many of the same Hox-dependent circuit components used by mammals, it seems improbable that a bipedal locomotor gait was present in their common ancestor. Leucoraja and tetrapods likely evolved pedalism independently, building off circuit features that were initially used for fin-based locomotion.
If a fin-based propulsion system was present in the common ancestor of skates and tetrapods, later vertebrates displaying undulatory behaviors would have had to revert to an axial muscle-based locomotor system; possibly through repression of the Hox-dependent fin/limb circuit program. In agreement with this hypothesis, an expanded domain of Hoxc9 expression in snakes is associated with repression of the limb-level Hox/Foxp1 network, and a reversion to axial muscle-based locomotion (Jung et al., 2014). These observations are consistent with the idea that modification in a small number of regulatory factors, including Hox genes, can facilitate evolutionary transitions in basic forms of locomotion (Dixit et al., 2008).
Limb-based locomotion requires the activities of spinal networks to coordinate the activation of specific MN subtypes. We found that Leucoraja and tetrapods share highly conserved classes of spinal INs, essential for CPGs to orchestrate patterns of MN firing. However, it remains to be determined whether Leucoraja and tetrapod CPG networks exhibit the same functional conservation as MN subtypes. While the mechanisms that determine how CPGs select a particular MN are unknown, recent evidence indicate that MNs can play an instructive role in determining the pattern of connectivity and output of motor circuits (Baek et al., 2017; Goetz et al., 2015; Hinckley et al., 2015; Machado et al., 2015; Song et al., 2016). If similar MN-dependent mechanisms operate in Leucoraja, their relatively simple fin musculature may provide an attractive system to resolve the principles through which locomotor circuits are constructed.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jeremy Dasen (jeremy.dasen@nyumc.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal Work
Hoxc9 and Foxp1 conditional mutants and Mnx1::GFP zebrafish have been described previously (Baek et al., 2017; Flanagan-Steet et al., 2005; Surmeli et al., 2011). Animal procedures were performed in accordance with the US National Institutes of Health Animal Protection Guidelines and approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine. Animal ages are indicated in the main text and figure legends. No gender-specific differences in reported phenotypes are expected, but were not formally tested.
Leucoraja Manipulations
Leucoraja erinacea embryos of the desired stages were obtained from the Marine Resources Department of Woods Hole Marine Biology Laboratory (MBL). Animals at MBL are maintained in accordance with procedures approved by the MBL Institutional Animal Care and Use Committee (IACUC) following standards established by the NIH. Prior to use, egg cases were maintained in reconstituted Instant Ocean (Aquarium Systems) at 16°C with a standard aquarium filtration system. Before manipulation, embryos were anesthetized with MS-222 (0.17 g/L; Sigma-Aldrich) at room temperature for 10 min (Dahn et al., 2007).
METHODS DETAILS
Video Imaging of Leucoraja Embryos and Hatchlings
Skates were placed in a 25 cm square glass tank and imaged from the ventral surface using a CMOS camera (Basler Ace a1920–155) with an 8 mm lens (Computar M0814-MP2). The bottom of the tank was covered with a thin (2 mm) Sylgard layer that was prepared by curing on a sheet of Glad Press’n Seal to create a textured surface. The tank was partially surrounded by dark infrared transmitting plastic (CYRO ACRYLITE® IR acrylic 11460) and illuminated obliquely and from below with multiple infrared (850 nm) illuminators (CMVIsion-IR200). Images were 8-bit, 960x600 pixels at 60 frames/sec. Data was analyzed in real-time with a custom program written in LabView 2014 (National Instruments). To estimate movement, we computed the number of pixels that changed above baseline noise (8/255) from frame to frame. Empirically, frames with changes greater than 0.7% of their pixels corresponded to movement of the skate’s body. These frames were timestamped and saved, uncompressed, for as long as the skate moved. Cessation of movement and video recording occurred once 150 consecutive frames had elapsed with < 0.7% pixel change.
Retrograde Labeling
Retrograde tracing of MNs was performed through intramuscular injection of horseradish peroxidase (HRP, Sigma-Aldrich). Prior to injection, embryos were removed from egg cases with the yolk sac attached and placed in reconstituted Instant Ocean at room temperature. Retrograde labeling was performed by injection of 1–2 μl of a 10% HRP solution into the desired muscle of anesthetized skate embryos. Embryos were then removed from anesthetic and incubated for 6–8 hours at room temperature in aerated Instant Ocean. After incubation, skates were euthanized by rabid decapitation and processed for immunohistochemistry as described below. For each experiment, injections were performed on 4–6 embryos and data shown in figures are representative of results in which the labeling efficiency was >50% in the targeted MN subtypes.
In ovo Chick Electroporations
In ovo electroporation of Hox genes was performed on Hamburger Hamilton (HH) stage 13–15 chick embryos and analyzed at HH stage 26–27. Fertilized chicken eggs (Charles River) were incubated in a humidified incubator at 39°C for 40–48 hours until they reached HH12–15. The top of the egg shell was removed and a 1 mg/ml DNA solution containing ~0.02% Fast green was injected into the central canal of the neural tube using a sharpened glass capillary tube. Electrodes (Platinum/Iridium (80%/20%), 250 μm diameter, UEPMGBVNXNND, FHC Inc.) were placed on both sides of the neural tube (4 mm separation) and DNA was electroporated using an ECM 830 electroporator (ECM 830, BTX; 25V, 4 pulses, 50 ms duration, 1 second interval).
In each electroporation, Hox expression constructs (pCAGGs) were used in the range of 0.1–0.2 mg/ml using pBKS as carrier DNA (0.8–0.9 mg/ml). Leucoraja Hox genes were isolated by RT-PCR, cloned into pCAGGs expression vectors, and confirmed by DNA sequencing. Sequence information on Leucoraja HoxA genes was obtained from GenBank (accession number FJ944024) (Mulley et al., 2009).
In ovo Skate Electroporations
Stage 27–28 Leucoraja embryos were used for electroporation. Embryos were removed from egg cases and kept in 35 mm culture dishes containing Instant Ocean. A raised platform was made using glass slides and playdough. Embryo yolk sacs were kept in the bottom of the dish and the embryo was placed on the glass slides. DNA solutions (1–2 mg/ml) containing ~0.02% Fast green were injected into the central canal of the neural tube and directly into spinal tissue using sharpened glass capillary tubes. After DNA injection, the embryos were placed between microelectrodes. Electric pulses (ECM 830 electroporator, BTX; 20–25V, 5–8 pulses, 50 ms duration, 1 second interval) were given to the embryos in the anesthetic solution. After electroporation, embryos were returned to Instant Ocean; incubated for 11 days at room temperature; and analyzed at ~st30.
In Situ Hybridization
Embryos were fixed in 4% PFA for 1.5–2 hours at 4°C. Embryos were washed 5–6 times in cold PBS, 15–30 minutes for each wash, and incubated overnight in 30% sucrose. For skate tissue, the spinal cord was removed prior to sucrose treatment. Tissue was embedded in OCT, frozen in dry ice, and sectioned at 16 μm on a cryostat.
Antisense riboprobes were generated using the Digoxigenin-dUTP (SP6/T7) labeling kit (Sigma-Aldrich). To generate antisense riboprobes for Leucoraja genes, sequence information was obtained from SkateBase (http://skatebase.org) (Wyffels et al., 2014). RNA was extracted from st30 skate embryos using TRIzol (Invitrogen) and cDNA was generated using SuperScript II Reverse Transcriptase (Invitrogen). Riboprobes were prepared by PCR using cDNA templates, incorporating a T7 polymerase promoter sequence in the antisense oligo. Oligonucleotide sequences used to generate in situ probes are listed in Table S2.
For in situ hybridization sections were first dried for 10–15 minutes at room temperature, placed in 4% PFA, and fixed for 10 minutes at room temperature. Slides were then washed three times for 3 minutes each in PBS, and then placed in Proteinase K solution (1 μg/ml) for 5 minutes at room temperature. After an additional PFA fixation and washing step, slides were treated in triethanolamine for 10 minutes, to block positive charges in tissue. Slides were then washed three times in PBS and blocked for 2–3 hours in hybridization solution (50% formamide, 5X SSC, 5X Denhardt’s solution, 0.2 mg/ml yeast RNA, 0.1 mg/ml salmon sperm DNA). Prehybridization solution was removed, and replaced with 100 μl of hybridization solution containing 100 ng of DIG-labeled antisense probe. Slides were then incubated overnight (12–16 hours) at 72°C.
After hybridization, slides were transferred to a container with 400 ml of 5X SSC and incubated at 72°C for 20 minutes. During this step, coverslips were removed using forceps. Slides were then washed in 400 ml of 0.2X SSC for 1 hour at 72°C. Slides were transferred to buffer B1 (0.1 M Tris pH 7.5, 150 mM NaCl) and incubated for 5 minutes at room temperature. Slides were then transferred to staining trays and blocked in 0.75 ml/slide of B1 containing 10% heat inactivated goat serum. The blocking solution was removed and replaced with antibody solution containing 1% heat inactivated goat serum and a 1:5000 dilution of anti-DIG-AP antibody (Roche). Slides were then incubated overnight at 4°C in a humidified chamber. The following day, slides were washed 3 times, 5 minutes each, with 0.75 ml/slide of buffer B1. Slides were then transferred to buffer B3 (0.1 M Tris pH 9.5, 100 mM NaCl, 50 mM MgCl2) and incubated for 5 minutes. Slides were then developed in 0.75 ml/slide of B3 solution containing 3.5 μl/ml BCIP and 3.5 μl/ml NBT for 12–48 hours. After color development, slides were washed in ddH20 and coverslipped in Glycergel (Agilent). A more detailed in situ hybridization protocol is available on our lab website (http://www.med.nyu.edu/dasenlab).
Immunohistochemistry
For antibody staining of sections, slides were first placed in PBS for 5 minutes to remove OCT. Sections were then transferred to humidified trays and blocked for 20–30 minutes in 0.75 ml/slide of PBT (PBS with 0.1% Triton) containing 1% Bovine serum albumin (BSA). The blocking solution was replaced with primary staining solution containing antibodies diluted in PBT with 0.1% BSA. Primary antibody staining was performed overnight at 4°C. Slides were then washed three times for 5 minutes each in PBT. Fluorophore-conjugated secondary antibodies were diluted 1:500–1:1000 in PBT and filtered through a 0.2 μm syringe filter. Secondary antibody solution was added to slides (0.75 ml/slide) and incubated at room temperature for 1 hour. Slides were washed three times in PBT, followed by a final wash in PBS. Coverslips were placed on slides using 110 μl of Vectashield (Vector Laboratories).
For vibratome sectioning, embryos were embedded in 4% low melting point agarose and sectioned at 200 μm using a Leica VT1200 vibratome. Sections were individually collected and placed in multi-well plates containing PBS. For staining, sections were first blocked in PBT containing 1% BSA for 1 hour. Primary antibodies were diluted in PBT containing 0.1% BSA, 0.1% sodium azide, and sections were incubated overnight at room temperature. Sections were then washed 4–5 times in PBT; 20–30 minutes each wash. Secondary antibodies were diluted in PBT containing 0.1% BSA, 0.1% sodium azide, and incubated overnight at room temperature. Sections were then washed 4–5 times in PBT, 20–30 minutes each wash, followed by a final wash in PBS for 10 minutes. Sections were mounted between two coverslips containing 80–100 μl of Vectashield.
For wholemount immunohistochemistry PFA fixed embryos were bleached for 24 hours at 4°C in a 10% H2O2, 10% DMSO solution prepared in methanol. Embryos were washed three times for 10 minutes each in methanol, followed by five washes for 10 minutes in PBS. Primary antibodies were diluted in staining solution (5% BSA, 20% DMSO in PBS) and specimens were incubated in staining solution on a rotator overnight at room temperature. Samples were then washed three times for 5 minutes each in PBS, followed by four 1 hour washes in PBS. Specimens were then incubated in secondary antibodies diluted in staining solution overnight at room temperature. Samples were then washed three times for 5 minutes each in PBS, followed by four 1 hour washes in PBS, a single 10 min wash in 50% methanol, and three 20 minute washes in 100% methanol. Samples were transferred to glass depression slides and tissue was cleared by incubating samples in BABB solution (1-part benzyl alcohol: 2-parts benzyl benzoate). Confocal images of embryos were obtained from Z-stacks using Zen software (Zeiss). Further details of immunohistochemistry protocols are available on our lab website: (https://med.nyu.edu/dasenlab/protocols.html).
Antibodies
Antibodies against Hox proteins, LIM HD proteins, and other proteins were used as described (Dasen et al., 2005; Jung et al., 2010; Tsuchida et al., 1994). Additional antibodies include monoclonal anti-HA (1:16K; Covance) and antibodies obtained from the Developmental Studies Hybridoma Bank (DSHB). Antibodies against Leucoraja Hox proteins were generated in rabbits and guinea pigs at Covance using the following peptide sequences, with an additional cysteine (C) residue incorporated for conjugation: LeHoxa5, DSATMHSSRYGYGYNC; LeHoxa7, TAGTSVFQNTSGFSEATSC; LeHoxa9, ENDDLLASRYASGPLAQASRC; LeRaldh2, CERAQRRTVGNPFDPATE. Antibody specificity was evaluated by comparison of immunoreactivity with Hox mRNA expression patterns in skate spinal cord.
MN Isolation, RNA Extraction, Library Preparation, RNA Sequencing and Bioinformatics
Stage 30 skate embryos were removed from egg cases and kept in 35 mm culture dishes. Pectoral fins were injected multiple times with Dextran Rhodamine (RhD) (15% solution in ddH20; 3k MW lysine fixable, Molecular Probes, D-3308). During injection procedures embryos were kept in the anesthetic solution. After injections embryos were returned to reconstituted Instant Ocean and incubated for 1–3 days at room temperature. MN dissociation was performed as described (Hempel et al., 2007) with a few modifications: 1. Before pronase incubation, isolated spinal cords were cut along the dorsal midline to make open-book preparations. 2. Spinal cords were incubated in pronase solution for 30 min. at room temperature. 3. Trituration was performed in 100 μl of ACSF containing 1% FBS.
RNA was extracted from purified MNs, using the Arcturus Picopure RNA isolation kit. RNA quality and quantity were measured with an Agilent Picochip using a Bioanalyzer. Library preps for RNA-Seq were made from 500 pg of total RNA, as follows: cDNA was amplified using the Nugen Ovation RNA-Seq System V2 kit (Part 7102) and 100 ng of amplified cDNA were used to prepare Illumina libraries, using the Nugen Ovation Ultralow Library system (Parts No 0303-05 and 0330-31), and amplified by 7 cycles of PCR. The samples were mixed into one pool and run in one 50-nucleotide paired-end lanes on the HiSeq 2500 sequencer, using v4 chemistry.
Because a high-quality annotated genome is not available for Leucoraja, we used a de novo assembly method to build a transcriptome and quantify gene expression with RNA-seq data. RNA-seq data from pectoral fin neurons and from tail neurons were cleaned with Trimmomatic (v0.33) (Bolger et al., 2014), then merged and assembled with Trinity (r2013-02-25) (Haas et al., 2013). The resulting transcriptome assembly contained 82,429 contigs, of which 19,244 were similar to annotated zebrafish genes (BLAST e-value < 10e-4). We then quantified gene expression in each RNA-seq data file (3 replicates from pectoral fin and 3 replicates from tail) by quasi-mapping to the set of transcriptome contigs with Salmon (v0.8.2) (Patro et al., 2017). The gene expression values were tested for differential expression using the Bioconductor package edgeR (v3.18.1) (Robinson et al., 2010).
Sequence Comparisons
DNA sequences from vertebrate species were aligned using Multiz alignment in UCSC genome browser. A previously identified Hox binding element in the Foxp1 locus (chr6:99,190,051-99,190,212 in mm10 assembly) (Jung et al., 2014) was subjected to BLAT in UCSC genome browser and SkateBLAST to retrieve homologous genomic sequences from elephant shark (C. milii) and little skate (L. erinacea), respectively: elephant shark, KI635892, 4582601- 4582762 (calMil1 assembly); little skate, LSb2-ctg161924 (Little skate Genomic Contigs Build 2). Identified genomic sequences were then re-subjected to BLAT to mouse genome assembly (mm10) for the sequence comparisons and manually added into the alignments.
Extracellular Ventral Root Recordings
Late st32 skate embryos were removed from egg cases and anesthetized with MS-222 solution. Animals were eviscerated in the anesthetic solution while the bathing solution was oxygenated with a 95% O2, 5% CO2 gas mixture. Further dissections were performed in the Elasmobranch dissecting solution (250 mM NaCl, 6 mM KCl, 0.2 mM NaH2PO4, 20 mM NaHCO3, 360 mM Urea, 3 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 5 mM HEPES). Pectoral fin nerves were exposed at mid-pectoral fin levels on one side by removing skin and underlying muscles, and tissues on contralateral side were completely removed to expose the spinal cord. Dissected spinal cord samples were transferred to a recording chamber (a Sylgard plate with a capacity of 8 ml) and incubated for 30 minutes in Elasmobranch recording solution (250 mM NaCl, 6 mM KCl, 0.2 mM NaH2PO4, 20 mM NaHCO3, 360 mM Urea, 1 mM MgCl2, 4 mM CaCl2, 10 mM glucose, and 5 mM HEPES) by perfusion at a speed of 5 ml/min.
Fire polished glass electrodes were made from capillary tubes with 1mm outer diameter/0.5mm inner diameter (Sutter Instrument). NMDA stock solution (20 mM in dH2O, Sigma-Aldrich) was made fresh and added directly into the recording solution via bath perfusion system with a flow rate of 5 ml/min. Extracellular signals were recorded from the dorsal and ventral nerves of the pectoral fin (Differential AC Amplifier 1700, A-M Systems, LLC.; amplified 1000X and acquired with 100 Hz low pass and 1 kHz high pass filters) and fed to an A/D interface (Digidata 1550 digitizer, Molecular Devices; sampled at 10 kHz). Data was analyzed with Clampfit (v10, Molecular Devices).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were carried out using Prism 7 software with two-tailed Student’s t tests when two cases were compared. When data did not meet normality criteria, equivalent non-parametric (Wilcoxon–Mann–Whitney) tests were used to compare differences between two groups. Differences were considered to be significant when p-values were below 0.05. No blinding, stratification, or randomization of data was performed. The ages and number of animals analyzed in each experimental manipulation can be found in the figure legends and methods details. Specific quantification and animal exclusion parameters are described below.
Chick electroporation
Chick electroporation data shown in figures are representative of results obtained from n>3 chick embryos, from at least 2 independent electroporation experiments. Typically n>40 chick eggs were electroporated in each experiment, of which 5–8 embryos survived after 3 days of incubation. Among surviving embryos, 2–3 embryos typically expressed the gene of interest in MNs, while other surviving embryos showed inefficient electroporation or more dorsal expression. Embryos with low electroporation efficiency in MNs (less than ~10%) were excluded from further analyses. Results shown in figures are representative of embryos in which the electroporation efficiency was >40% of MNs.
Skate electroporation
Quantification of electroporated neurons was performed by pooling data from multiple embryo electroporated with the same construct. For each experiment, a GFP control sample was electroporated in parallel. For each experiment the number of animals and neurons quantified are as follows: Hoxa5 analysis: 21 neurons from 4 control eGFP electroporated embryos, 29 neurons from 5 Hoxc9-pCMX embryos (p-value<0.0001; Wilcoxon–Mann–Whitney test); Foxp1 analysis: 50 neurons from 4 control eGFP electroporated embryos, 30 neurons from 5 Hoxc9-pCMX embryos (p-value=0.0016; two-tailed Student’s t test).
Skate walking behaviors
Quantification of fin angle deflections were performed by assigning four points to individual video frames, two along the spinal axis, and one on each fin. Video frames were manually assigned points by visual inspection. Data shown in figure are taken from a single skate hatchling during a single walking bout, but are representative of behaviors observed in three animals.
DATA AND SOFTWARE AVAILABILITY
Leucoraja RNAseq data and analyses were deposited into the GEO repository under accession number GSE105156.
Supplementary Material
(A) Summary of transcription factor profiles of spinal locomotor IN classes in tetrapods. (B–H) Sections of pectoral fin level spinal cord in st32 embryos showing expression of indicated IN markers. (B) Evx1 labels V0v IN subtypes, as well as V0c, and V0g INs. Pax2 labels V0d and other IN classes. (C) En1 labels most V1 INs while Foxp2 labels a subset of V1 in mice. Few V1 INs coexpress Foxp2 and En1 in skate at this stage. (D) Foxp2+ INs express Lhx1. (E) Lhx3+ neurons lacking Isl1/2 define V2a INs. (F) Chx10 defines V2a INs. (G and H) Gata2 and Gata3 are expressed by V2b INs. (I) Temporal and spatial profile of indicated IN class markers in Leucoraja spinal cord between st30 and 32. Expression is shown at pectoral fin, pelvic fin, and tail levels. Scale bar represents 50μm. (J) In vitro preparation of skate spinal cord recorded from motor nerves via suction electrodes. To measure spinal CPG activity, the spinal cord was isolated and dorsal and ventral nerve roots were individually dissected. Extracellular recordings from the dorsal pectoral nerve after exposure to locomotor CPG inducing drug NMDA is shown. (K) At high concentrations of NMDA, alternating activity patterns were recorded from the dorsal and ventral pectoral nerves, suggesting presence of an extensor-flexor CPG.
Video taken from the dorsal surface of a st17 skate embryo showing rhythmic undulation along the rostrocaudal axis. Large white oval structure attached to the embryo is the yolk sac.
Video is composite of three movies of the ventral surface of st33 skate embryos. The first clip shows rhythmic undulation of the tail. The second clip shows sinusoidal waves of pectoral fin contractions, which can generate propulsion. The third clip shows that the pelvic fins display both left-right alternation and reciprocal patterns of protraction and retraction. Large white oval structure attached to the embryo is the yolk sac.
Video taken from the ventral surface of a 1-week old hatchling displaying walking behavior. Pelvic fins display left-right alternation and reciprocal protraction and retraction.
List of pectoral fin MN enriched genes in Leucoraja, Related to Figure 5.
(A–C) Transverse sections of Maxwell stages 25–32 spinal cord showing the temporal profile of MN subtype determinants in Leucoraja. Sections are from pectoral fin levels. (A) Foxp1 staining detects LMC neurons starting at st27. (B) Lhx3 and Isl1 coexpression in the ventrolateral spinal cord defines MMC neurons and is detected by st29. Lhx3 is also expressed by ventromedial MN precursors as early as st25, prior to expression in mature MMC neurons. (C) Isl1 and Hb9 staining. Hb9 is detected in medial MNs between st25 and st29. As in tetrapods, Hb9 becomes restricted within the LMC to lateral populations (LMCl) and MMC neurons. Isl1 expression is maintained at relatively high levels by medial LMC neurons after st29. Isl1 and Lhx3 antibodies also detect ventral spinal IN populations. (D) Raldh2 expression is detected in Foxp1+ LMC neurons at rostral pectoral fin levels, but is absent from caudal pectoral and pelvic LMC neurons at st29. (E) Expression of Lhx3, Isl1, and Hb9 showing HMC-like MNs (Isl1+; Hb9+; Foxp1−, Lhx3−) at st31. HMC-like neurons are characterized by coexpression of Hb9 and Isl1 and were detected at both pectoral and pelvic fin levels. Summary of MN columnar organization in mice and skates, showing the position of molecularly defined MN subtypes. In mice, a population of autonomic preganglionic MNs is also present at thoracic levels, which we are unable to detect in Leucoraja, likely due to lack of appropriate molecular markers.
(A) Raldh2 expression is not detected in fin-level MNs of catshark (Scyliorhinus canicula) or elephant shark (Callorhinchus milii). In both species, expression of Raldh2 is detected in the tissue surrounding the spinal cord, similar to the pattern observed in mice. (B) Dorsal view of 48hpf zebrafish embryo showing Foxp1 expression at pectoral (pec) levels. Isl1 staining labels a subset of MNs as well as larger diameter sensory neurons. Foxp1 expression in the ventral spinal cord extends from the hindbrain to the rostral spinal cord, adjacent to myotomes m2-m5, consistent with previous studies (Ma et al., 2010). (C,D) High magnification images of Foxp1 protein expression at pectoral levels in zebrafish, showing colocalization with Mnx1::GFP (at 72 hpf) and Isl1 (at 48 hpf). In zebrafish a subset of Foxp1+ neurons express Isl1 at relatively weak levels. (E) Wholemount NFAP staining showing projection of motor axons toward the pectoral and pelvic fin in catshark. (F) Sections of catshark embryos showing a subset of Foxp1+ MNs express Isl1 or Lhx1. In catshark LMCm and LMCl neurons appear to be intermixed.
(A) Expression of Ret, Meis1, and Scip in Leucoraja embryos at st32. Ret expression is detected in medially positioned neurons in an area overlapping the position of Foxp1+ LMC neurons. Ret is detected in MNs at both pectoral and pelvic fin levels. Meis1 also shows an LMC-restricted expression pattern. Scip expression is detected at pelvic fin levels in medially positioned neurons overlapping the area where ventrally-projecting LMCm neurons are generated. (B) Schematic illustrating expression patterns of Ret, Meis1, and Scip within the skate developing spinal cord. (C) MF20 staining showing position of axial muscles in st29 Leucoraja at the indicated rostrocaudal level. (D) Ontogeny of fin innervation in Leucoraja erinacea. Sections show expression of indicated markers at st25, early (e) st27, late (l) st27, and st29. Pectoral fin level motor axons bifurcate into dorsal (d) and ventral (v) branches at late st27; pelvic fin level axons at st29. Foxp1 staining is shown to indicate state of LMC differentiation.
(A) Expression of additional candidate genes in st31 skate at pectoral, pelvic and tail levels of the spinal cord. Nomenclature for murine genes is used. (B) Expression of indicated genes identified in skate in the spinal cord of chick and mouse embryos.
(A) Expression of HoxA and HoxD genes at st32. At these stages, expression of Hox genes extends to more dorsal regions of the spinal cord. (B) Expression of Hox proteins in MNs at st30. Panels on left show colocalization of indicated Hox proteins with the LMC determinant Foxp1. Panel on right show colocalization with Isl1/2. (C) Expression of Hoxa9 is mutually exclusive with Raldh2 at pectoral levels in skate.
(A) Hoxa5 induces a small number of Foxp1+ MNs at thoracic levels. (B) Misexpression of LeHoxa9 induces expression of Bmp5 at brachial levels, a marker for thoracic preganglionic column (PGC) neurons. (C) Location of Hox binding sites within the Foxp1 locus. Chip-seq signal maps and binding motif for Hoxc9 are shown for comparison. A Foxp1 BAC-GFP reporter (RP23-430H20) contains Hox binding regions (reg) 3a and 4c, and these sites are necessary for reporter expression in LMC neurons in mice. Data are modified from previous analyses (Jung et al., 2010; Jung et al., 2014) (D) Evolutionary conservation of the Foxp1 cis-element reg4c among selected vertebrates. Reg4c is conserved across species while reg3a is not conserved in zebrafish or skate. (E) Sequences of Hox binding motif among indicated vertebrate species; showing that the core Hox binding sequence TGATTTAT is conserved.
Video taken from the ventral surface of a 1-week old hatchling displaying walking behavior. In this example the skate also “punts”, using synchronous activation of both pelvic fins to initiate movement.
Video taken from the ventral surface of a 1-week old hatchling displaying walking behavior. In this example the skate walks and punts. The pelvic fins flex across the proximal-distal axis during walking, showing functional similarities to joints present in the tetrapod hindlimb.
Highlights.
The little skate Leucoraja erinacea exhibits bipedal walking-like behaviors
Neuronal subtypes essential for walking originated in primitive jawed fish
Fin and limb motor neurons share a common Hox-dependent gene network
Modulation of Hox patterning facilitates evolutionary changes in MN organization
Acknowledgments
We thank Claude Desplan, Gord Fishell, Michael Halassa, Tom Jessell, and Dasen lab members for feedback; Sylvie Mazan for providing Scyliorhinus embryos, and David Lee and Helen Kim for technical assistance. We are indebted to Andrew Gillis and Martin Cohn for advice on Leucoraja manipulations. We thank the NYU Genome Technology Center and Applied Bioinformatics Laboratories for their expert assistance with the analysis and interpretation of data. This work was supported by the Biomedical Research Council of A*STAR to BV, Cancer Center Support Grant P30CA016087 to AH, Australian Research Council Discovery grants DP1096002 and DP160104427 to PDC, the Human Frontiers Science Program LT000130/2009L to CAB, National Institute on Deafness and Communication Disorders R00 DC012775 to DS, and the National Institute of Neurological Disorders and Stroke (NINDS) R21 NS099933, R01 NS062822, and HHMI to JD.
Footnotes
Author Contributions
HJ, MB, and JD devised the project, designed and performed experiments, and wrote the paper. KD analyzed zebrafish embryos and skate innervation patterns. CB and PC raised and collected elephant shark embryos. BT and BV isolated elephant shark genes. SB and AH assisted with RNAseq experiments and bioinformatics. DS, JD, and HJ established methods for analyzing skate behaviors. All authors read the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, Eisen JS. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125. doi: 10.1242/dev.121.12.4117. [DOI] [PubMed] [Google Scholar]
- Arber S. Motor circuits in action: specification, connectivity, and function. Neuron. 2012;74:975–989. doi: 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Baek M, Pivetta C, Liu JP, Arber S, Dasen JS. Columnar-Intrinsic Cues Shape Premotor Input Specificity in Locomotor Circuits. Cell Rep. 2017;21:867–877. doi: 10.1016/j.celrep.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall MW, McLean DL. Modular organization of axial microcircuits in zebrafish. Science. 2014;343:197–200. doi: 10.1126/science.1245629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier S, Jan Ijspeert A, Ryczko D, Nagy F, Cabelguen JM. Organisation of the spinal central pattern generators for locomotion in the salamander: biology and modelling. Brain research reviews. 2008;57:147–161. doi: 10.1016/j.brainresrev.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Dahn RD, Davis MC, Pappano WN, Shubin NH. Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature. 2007;445:311–314. doi: 10.1038/nature05436. [DOI] [PubMed] [Google Scholar]
- Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Dixit R, Vijayraghavan K, Bate M. Hox genes and the regulation of movement in Drosophila. Developmental neurobiology. 2008;68:309–316. doi: 10.1002/dneu.20589. [DOI] [PubMed] [Google Scholar]
- Fetcho JR. The spinal motor system in early vertebrates and some of its evolutionary changes. Brain, behavior and evolution. 1992;40:82–97. doi: 10.1159/000113905. [DOI] [PubMed] [Google Scholar]
- Flammang BE, Suvarnaraksha A, Markiewicz J, Soares D. Tetrapod-like pelvic girdle in a walking cavefish. Sci Rep. 2016;6:23711. doi: 10.1038/srep23711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development. 2005;132:4471–4481. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- Gillis JA, Shubin NH. The Evolution of Gnathostome Development: Insight From Chondrichthyan Embryology. Genesis. 2009;47:825–841. doi: 10.1002/dvg.20567. [DOI] [PubMed] [Google Scholar]
- Goetz C, Pivetta C, Arber S. Distinct limb and trunk premotor circuits establish laterality in the spinal cord. Neuron. 2015;85:131–144. doi: 10.1016/j.neuron.2014.11.024. [DOI] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Current opinion in neurobiology. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel CM, Sugino K, Nelson SB. A manual method for the purification of fluorescently labeled neurons from the mammalian brain. Nat Protoc. 2007;2:2924–2929. doi: 10.1038/nprot.2007.416. [DOI] [PubMed] [Google Scholar]
- Hinckley CA, Alaynick WA, Gallarda BW, Hayashi M, Hilde KL, Driscoll SP, Dekker JD, Tucker HO, Sharpee TO, Pfaff SL. Spinal Locomotor Circuits Develop Using Hierarchical Rules Based on Motorneuron Position and Identity. Neuron. 2015;87:1008–1021. doi: 10.1016/j.neuron.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst RJ, Bone Q. On Bipedalism in Skates and Rays. Philos T R Soc B. 1993;339:105–108. [Google Scholar]
- Jung H, Dasen JS. Evolution of patterning systems and circuit elements for locomotion. Developmental cell. 2015;32:408–422. doi: 10.1016/j.devcel.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Lacombe J, Mazzoni EO, Liem KF, Jr, Grinstein J, Mahony S, Mukhopadhyay D, Gifford DK, Young RA, Anderson KV, et al. Global control of motor neuron topography mediated by the repressive actions of a single hox gene. Neuron. 2010;67:781–796. doi: 10.1016/j.neuron.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Mazzoni EO, Soshnikova N, Hanley O, Venkatesh B, Duboule D, Dasen JS. Evolving hox activity profiles govern diversity in locomotor systems. Developmental cell. 2014;29:171–187. doi: 10.1016/j.devcel.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao TJ, Law C, Kania A. Eph and ephrin signaling: lessons learned from spinal motor neurons. Seminars in cell & developmental biology. 2012;23:83–91. doi: 10.1016/j.semcdb.2011.10.016. [DOI] [PubMed] [Google Scholar]
- King BL, Gillis JA, Carlisle HR, Dahn RD. A natural deletion of the HoxC cluster in elasmobranch fishes. Science. 2011a;334:1517. doi: 10.1126/science.1210912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HM, Shubin NH, Coates MI, Hale ME. Behavioral evidence for the evolution of walking and bounding before terrestriality in sarcopterygian fishes. Proceedings of the National Academy of Sciences of the United States of America. 2011b;108:21146–21151. doi: 10.1073/pnas.1118669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester DM, Spirito CP. Punting: An unusual mode of locomotion in the little skate, Leucoraja erinacea (Chondrichthyes : Rajidae) Copeia. 2003:553–561. [Google Scholar]
- Lacombe J, Hanley O, Jung H, Philippidou P, Surmeli G, Grinstein J, Dasen JS. Genetic and functional modularity of Hox activities in the specification of limb-innervating motor neurons. PLoS genetics. 2013;9:e1003184. doi: 10.1371/journal.pgen.1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucifora LO, Vassallo AI. Walking in skates (Chondrichthyes, Rajidae): anatomy, behaviour and analogies to tetrapod locomotion. Biol J Linn Soc. 2002;77:35–41. [Google Scholar]
- Ma LH, Gilland E, Bass AH, Baker R. Ancestry of motor innervation to pectoral fin and forelimb. Nature communications. 2010;1:49. doi: 10.1038/ncomms1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macesic LJ, Kajiura SM. Comparative Punting Kinematics and Pelvic Fin Musculature of Benthic Batoids. Journal of morphology. 2010;271:1219–1228. doi: 10.1002/jmor.10865. [DOI] [PubMed] [Google Scholar]
- Machado TA, Pnevmatikakis E, Paninski L, Jessell TM, Miri A. Primacy of Flexor Locomotor Pattern Revealed by Ancestral Reversion of Motor Neuron Identity. Cell. 2015;162:338–350. doi: 10.1016/j.cell.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell EE, Frobisch NB, Heppleston AC. Variability and conservation in late chondrichthyan development: ontogeny of the winter skate (Leucoraja ocellata) Anatomical record. 2008;291:1079–1087. doi: 10.1002/ar.20719. [DOI] [PubMed] [Google Scholar]
- Mulley JF, Zhong YF, Holland PWH. Comparative genomics of chondrichthyan Hoxa clusters. Bmc Evol Biol. 2009:9. doi: 10.1186/1471-2148-9-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Tanaka M. Evolution of motor innervation to vertebrate fins and limbs. Developmental biology. 2011;355:164–172. doi: 10.1016/j.ydbio.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Gehrke AR, Lemberg J, Szymaszek J, Shubin NH. Digits and fin rays share common developmental histories. Nature. 2016;537:225–228. doi: 10.1038/nature19322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Klomp J, Pieretti J, Schneider I, Gehrke AR, Shubin NH. Molecular mechanisms underlying the exceptional adaptations of batoid fins. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:15940–15945. doi: 10.1073/pnas.1521818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto S, Minguillon C, Wood S, Logan MP. A combination of activation and repression by a colinear Hox code controls forelimb-restricted expression of Tbx5 and reveals Hox protein specificity. PLoS genetics. 2014;10:e1004245. doi: 10.1371/journal.pgen.1004245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417-+. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidou P, Dasen JS. Hox genes: choreographers in neural development, architects of circuit organization. Neuron. 2013;80:12–34. doi: 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger LJ. Pectoral fin locomotion in batoid fishes: undulation versus oscillation. The Journal of experimental biology. 2001;204:379–394. doi: 10.1242/jeb.204.2.379. [DOI] [PubMed] [Google Scholar]
- Rousso DL, Gaber ZB, Wellik D, Morrisey EE, Novitch BG. Coordinated actions of the forkhead protein Foxp1 and Hox proteins in the columnar organization of spinal motor neurons. Neuron. 2008;59:226–240. doi: 10.1016/j.neuron.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Ampatzis K, Bjornfors ER, El Manira A. Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature. 2016;529:399–402. doi: 10.1038/nature16497. [DOI] [PubMed] [Google Scholar]
- Standen EM, Du TY, Larsson HC. Developmental plasticity and the origin of tetrapods. Nature. 2014;513:54–58. doi: 10.1038/nature13708. [DOI] [PubMed] [Google Scholar]
- Surmeli G, Akay T, Ippolito GC, Tucker PW, Jessell TM. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell. 2011;147:653–665. doi: 10.1016/j.cell.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Onimaru K. Acquisition of the paired fins: a view from the sequential evolution of the lateral plate mesoderm. Evolution & development. 2012;14:412–420. doi: 10.1111/j.1525-142X.2012.00561.x. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Woltering JM, Noordermeer D, Leleu M, Duboule D. Conservation and Divergence of Regulatory Strategies at Hox Loci and the Origin of Tetrapod Digits. PLoS biology. 2014:12. doi: 10.1371/journal.pbio.1001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyffels J, King BL, Vincent J, Chen C, Wu CH, Polson SW. SkateBase, an elasmobranch genome project and collection of molecular resources for chondrichthyan fishes. F1000Res. 2014;3:191. doi: 10.12688/f1000research.4996.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lanuza GM, Britz O, Wang Z, Siembab VC, Zhang Y, Velasquez T, Alvarez FJ, Frank E, Goulding M. V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron. 2014;82:138–150. doi: 10.1016/j.neuron.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Summary of transcription factor profiles of spinal locomotor IN classes in tetrapods. (B–H) Sections of pectoral fin level spinal cord in st32 embryos showing expression of indicated IN markers. (B) Evx1 labels V0v IN subtypes, as well as V0c, and V0g INs. Pax2 labels V0d and other IN classes. (C) En1 labels most V1 INs while Foxp2 labels a subset of V1 in mice. Few V1 INs coexpress Foxp2 and En1 in skate at this stage. (D) Foxp2+ INs express Lhx1. (E) Lhx3+ neurons lacking Isl1/2 define V2a INs. (F) Chx10 defines V2a INs. (G and H) Gata2 and Gata3 are expressed by V2b INs. (I) Temporal and spatial profile of indicated IN class markers in Leucoraja spinal cord between st30 and 32. Expression is shown at pectoral fin, pelvic fin, and tail levels. Scale bar represents 50μm. (J) In vitro preparation of skate spinal cord recorded from motor nerves via suction electrodes. To measure spinal CPG activity, the spinal cord was isolated and dorsal and ventral nerve roots were individually dissected. Extracellular recordings from the dorsal pectoral nerve after exposure to locomotor CPG inducing drug NMDA is shown. (K) At high concentrations of NMDA, alternating activity patterns were recorded from the dorsal and ventral pectoral nerves, suggesting presence of an extensor-flexor CPG.
Video taken from the dorsal surface of a st17 skate embryo showing rhythmic undulation along the rostrocaudal axis. Large white oval structure attached to the embryo is the yolk sac.
Video is composite of three movies of the ventral surface of st33 skate embryos. The first clip shows rhythmic undulation of the tail. The second clip shows sinusoidal waves of pectoral fin contractions, which can generate propulsion. The third clip shows that the pelvic fins display both left-right alternation and reciprocal patterns of protraction and retraction. Large white oval structure attached to the embryo is the yolk sac.
Video taken from the ventral surface of a 1-week old hatchling displaying walking behavior. Pelvic fins display left-right alternation and reciprocal protraction and retraction.
List of pectoral fin MN enriched genes in Leucoraja, Related to Figure 5.
(A–C) Transverse sections of Maxwell stages 25–32 spinal cord showing the temporal profile of MN subtype determinants in Leucoraja. Sections are from pectoral fin levels. (A) Foxp1 staining detects LMC neurons starting at st27. (B) Lhx3 and Isl1 coexpression in the ventrolateral spinal cord defines MMC neurons and is detected by st29. Lhx3 is also expressed by ventromedial MN precursors as early as st25, prior to expression in mature MMC neurons. (C) Isl1 and Hb9 staining. Hb9 is detected in medial MNs between st25 and st29. As in tetrapods, Hb9 becomes restricted within the LMC to lateral populations (LMCl) and MMC neurons. Isl1 expression is maintained at relatively high levels by medial LMC neurons after st29. Isl1 and Lhx3 antibodies also detect ventral spinal IN populations. (D) Raldh2 expression is detected in Foxp1+ LMC neurons at rostral pectoral fin levels, but is absent from caudal pectoral and pelvic LMC neurons at st29. (E) Expression of Lhx3, Isl1, and Hb9 showing HMC-like MNs (Isl1+; Hb9+; Foxp1−, Lhx3−) at st31. HMC-like neurons are characterized by coexpression of Hb9 and Isl1 and were detected at both pectoral and pelvic fin levels. Summary of MN columnar organization in mice and skates, showing the position of molecularly defined MN subtypes. In mice, a population of autonomic preganglionic MNs is also present at thoracic levels, which we are unable to detect in Leucoraja, likely due to lack of appropriate molecular markers.
(A) Raldh2 expression is not detected in fin-level MNs of catshark (Scyliorhinus canicula) or elephant shark (Callorhinchus milii). In both species, expression of Raldh2 is detected in the tissue surrounding the spinal cord, similar to the pattern observed in mice. (B) Dorsal view of 48hpf zebrafish embryo showing Foxp1 expression at pectoral (pec) levels. Isl1 staining labels a subset of MNs as well as larger diameter sensory neurons. Foxp1 expression in the ventral spinal cord extends from the hindbrain to the rostral spinal cord, adjacent to myotomes m2-m5, consistent with previous studies (Ma et al., 2010). (C,D) High magnification images of Foxp1 protein expression at pectoral levels in zebrafish, showing colocalization with Mnx1::GFP (at 72 hpf) and Isl1 (at 48 hpf). In zebrafish a subset of Foxp1+ neurons express Isl1 at relatively weak levels. (E) Wholemount NFAP staining showing projection of motor axons toward the pectoral and pelvic fin in catshark. (F) Sections of catshark embryos showing a subset of Foxp1+ MNs express Isl1 or Lhx1. In catshark LMCm and LMCl neurons appear to be intermixed.
(A) Expression of Ret, Meis1, and Scip in Leucoraja embryos at st32. Ret expression is detected in medially positioned neurons in an area overlapping the position of Foxp1+ LMC neurons. Ret is detected in MNs at both pectoral and pelvic fin levels. Meis1 also shows an LMC-restricted expression pattern. Scip expression is detected at pelvic fin levels in medially positioned neurons overlapping the area where ventrally-projecting LMCm neurons are generated. (B) Schematic illustrating expression patterns of Ret, Meis1, and Scip within the skate developing spinal cord. (C) MF20 staining showing position of axial muscles in st29 Leucoraja at the indicated rostrocaudal level. (D) Ontogeny of fin innervation in Leucoraja erinacea. Sections show expression of indicated markers at st25, early (e) st27, late (l) st27, and st29. Pectoral fin level motor axons bifurcate into dorsal (d) and ventral (v) branches at late st27; pelvic fin level axons at st29. Foxp1 staining is shown to indicate state of LMC differentiation.
(A) Expression of additional candidate genes in st31 skate at pectoral, pelvic and tail levels of the spinal cord. Nomenclature for murine genes is used. (B) Expression of indicated genes identified in skate in the spinal cord of chick and mouse embryos.
(A) Expression of HoxA and HoxD genes at st32. At these stages, expression of Hox genes extends to more dorsal regions of the spinal cord. (B) Expression of Hox proteins in MNs at st30. Panels on left show colocalization of indicated Hox proteins with the LMC determinant Foxp1. Panel on right show colocalization with Isl1/2. (C) Expression of Hoxa9 is mutually exclusive with Raldh2 at pectoral levels in skate.
(A) Hoxa5 induces a small number of Foxp1+ MNs at thoracic levels. (B) Misexpression of LeHoxa9 induces expression of Bmp5 at brachial levels, a marker for thoracic preganglionic column (PGC) neurons. (C) Location of Hox binding sites within the Foxp1 locus. Chip-seq signal maps and binding motif for Hoxc9 are shown for comparison. A Foxp1 BAC-GFP reporter (RP23-430H20) contains Hox binding regions (reg) 3a and 4c, and these sites are necessary for reporter expression in LMC neurons in mice. Data are modified from previous analyses (Jung et al., 2010; Jung et al., 2014) (D) Evolutionary conservation of the Foxp1 cis-element reg4c among selected vertebrates. Reg4c is conserved across species while reg3a is not conserved in zebrafish or skate. (E) Sequences of Hox binding motif among indicated vertebrate species; showing that the core Hox binding sequence TGATTTAT is conserved.
Video taken from the ventral surface of a 1-week old hatchling displaying walking behavior. In this example the skate also “punts”, using synchronous activation of both pelvic fins to initiate movement.
Video taken from the ventral surface of a 1-week old hatchling displaying walking behavior. In this example the skate walks and punts. The pelvic fins flex across the proximal-distal axis during walking, showing functional similarities to joints present in the tetrapod hindlimb.