By storing and releasing a multitude of compounds, vacuoles play a multifaceted role in the plant development and response to environment Al changes.
Abstract
Biochemical and electrophysiological studies on plant vacuolar transporters became feasible in the late 1970s and early 1980s, when methods to isolate large quantities of intact vacuoles and purified vacuolar membrane vesicles were established. However, with the exception of the H+-ATPase and H+-PPase, which could be followed due to their hydrolytic activities, attempts to purify tonoplast transporters were for a long time not successful. Heterologous complementation, T-DNA insertion mutants, and later proteomic studies allowed the next steps, starting from the 1990s. Nowadays, our knowledge about vacuolar transporters has increased greatly. Nevertheless, there are several transporters of central importance that have still to be identified at the molecular level or have even not been characterized biochemically. Furthermore, our knowledge about regulation of the vacuolar transporters is very limited, and much work is needed to get a holistic view about the interplay of the vacuolar transportome. The huge amount of information generated during the last 35 years cannot be summarized in such a review. Therefore, I decided to concentrate on some aspects where we were involved during my research on vacuolar transporters, for some our laboratories contributed more, while others contributed less.
HISTORY AND HOW I GOT IN TOUCH WITH VACUOLES
The term vacuole was first used by the French biologist Félix Dujardin (1801–1860) to name the empty space representing the contractile vacuole of protozoa (Dujardin, 1841). Such an empty space had been observed by plant biologists for many years; therefore, it was obvious that plant biologists adopted this term. In the early phase of vacuole research, methods were restricted to microscopic observations and staining procedures, such as staining with Neutral Red. During these early studies, De Vries (1885) performed experiments that led him to conclude that the vacuole is surrounded by a membrane, which he called the tonoplast. For a long time, the large size of leaf and root vacuoles hindered biochemical analyses of this organelle. This is reflected by the fact that the first review on vacuoles appeared only in 1978, published in the 29th volume of the Annual Review of Plant Physiology and Plant Molecular Biology series (Matile, 1978). When reading this article, it becomes apparent that the largest body of knowledge at the time came from work on yeast vacuoles. In yeast, biochemical characterization of vacuolar constituents as well as transport experiments, mainly for amino acids, had already been performed. By contrast, work with plant vacuoles at that time was limited to a few localization studies of vacuolar constituents. For example, it was shown that, in sugar beet (Beta vulgaris), Suc is present mostly in vacuoles and that phenolics and other secondary compounds are localized exclusively in vacuoles (Matile, 1978). At the time, the best described plant vacuolar system was the lutoids of Hevea brasiliensis (D’Auzac, 1975). Lutoids are small vacuole-like structures present in large quantities in the laticifers of H. brasiliensis. Collecting the latex and performing a centrifugation step was an easy way to obtain copious amounts of these vacuole-like structures, for which H+-ATPase and citrate transport activities could be demonstrated (D’Auzac, 1974, 1975). An important step toward the characterization of vacuolar transporters was achieved by establishing techniques for isolating vacuoles using protoplasts (Wagner and Siegelman, 1975; Buser-Suter et al., 1982). This technique allowed investigation of vacuoles from a great variety of plants and also allowed the analysis of their vacuolar contents. I joined Professor Philippe Matile’s laboratory at ETH Zurich for my Ph.D. in 1978, and I was the first student in his laboratory working on senescence and chlorophyll degradation, a topic on which he, and later his collaborator Stefan Hörtensteiner, were and are still very successful. The first goal of my work was to establish a protocol for chloroplast isolation from barley (Hordeum vulgare) leaves at different stages of senescence. To do this, my friend Urs Heck and I isolated mesophyll protoplasts from barley primary leaves and used a syringe to break the protoplasts gently (Martinoia et al., 1981). We observed that this procedure did not only release chloroplasts but also vacuoles. Therefore, it was obvious to us that, besides working on plant senescence, we would investigate the properties of barley mesophyll vacuoles. Compared with the widely used osmotic shock method, the release of vacuoles using a syringe has the advantage that it can be performed on ice and that the vacuolar membrane is not stretched. So, our new procedure minimalized the loss of vacuolar constituents, which allowed us to show that nitrate is strongly accumulated in vacuoles when present in high amounts (Martinoia et al., 1981). Around the time we published this research, the danger of high nitrate contents in food was heavily discussed, which was one reason that our article attracted a broad interest.
THE GOLDEN AGE OF VACUOLAR MEMBRANE TRANSPORT BIOCHEMISTRY
The newly established methods to isolate vacuoles was one reason why, in the late 1970s and 1980s, our knowledge about vacuolar transport processes progressed rapidly. Different protocols to perform transport experiments were established, and, thanks to the large size of the vacuole, slow and non-energy-dependent transport processes could be characterized. My starting point in this field was the visit of Georg Kaiser, a Ph.D. student of Professor Ulrich Heber. Ulrich Heber was professor in Würzburg and a world leader in photosynthesis, but he was also interested in how photosynthates end up in the vacuole. To answer this question, he needed a method where vacuoles could be isolated and purified very rapidly. Our method to isolate barley vacuoles was the most appropriate for this purpose. We combined this technique with a technique described for transport experiments of mitochondria and chloroplasts, where two aqueous layers are separated by a silicone oil layer, allowing rapid centrifugation to separate the organelles from the incubation medium. In this way, we succeeded in purifying vacuoles from 14CO2-fed protoplasts within less than 1 min (Kaiser et al., 1982). We were so enthusiastic about this collaboration that I decided to pursue my career as a postdoc in Ulrich Heber’s laboratory. There, we characterized the vacuolar transport properties for a large number of substrates.
The second reason why, in the late 1970s and 1980s, our knowledge about vacuolar transport processes progressed substantially was the establishment of methods to isolate purified vacuolar membrane vesicles from many different plants. Tonoplast vesicles could be isolated in larger amounts than vacuoles and in a way that they have a well-defined internal composition. Furthermore, tonoplast vesicles have the advantage that proton- and membrane potential-dependent transport can be measured efficiently using radiolabeled as well as nonradiolabeled substrates. The laboratories of Lincoln Taiz, Ron Poole, Heven Sze, and Masayoshi Maeshima, together with then-postdocs at the time Phil Rea and Eduardo Blumwald and additional collaborators, performed an enormous amount of work using vacuolar vesicles. These laboratories could show that the vacuole contains two proton pumps, an H+-ATPase (V-ATPase) and an H+-PPase (V-PPase). Both pumps are able to acidify the vacuolar lumen, and the scientists demonstrated that vacuoles possess sodium as well as calcium proton antiporters able to drive the uptake of these cations (Blumwald and Poole, 1985; Schumaker and Sze, 1986; Maeshima and Yoshida, 1989; for review, see Poole, 1978; Sze, 1985; Rea and Sanders, 1987; Nelson and Taiz, 1989). During these years, several attempts were made to purify vacuolar transporters, but to my knowledge, besides the proton pumps, only one approach was successful: the laboratories of Chrispeels and Maeshima purified the major membrane protein present in the vacuolar membrane fraction (Johnson et al., 1989; Maeshima, 1992). Analysis of this protein, called TIP for tonoplast intrinsic protein, revealed it to act as an aquaporin (for reviews on aquaporins, see Maurel et al., 2008, 2015). The vacuolar membrane contains only about 1% of the total cellular protein and, hence, transporters are present in only very low amounts. Furthermore, each purification step for a transporter requires its solubilization and reconstitution in proteoliposomes to test for transport activity. By contrast, the two vacuolar proton pumps, the V-ATPase and V-PPase, are prominent tonoplast proteins and could be purified by following their respective hydrolytic activities.
The characterization of the V-ATPase at the protein level was first achieved using yeast vacuoles (Schumacher and Krebs, 2011). The V-PPase was originally found in plants and only later in some microorganisms such as Acetubalaria or Rhodospirillum spp. (Maeshima, 2000). Subsequently, the purification and the determination of the amino acid composition of this proton pump were achieved using plant tonoplast vesicles and a cDNA from Arabidopsis (Sarafian et al., 1992; Maeshima, 2000). While the V-ATPase is a complex protein that is constituted by 13 subunits, the vacuolar H+-PPase is composed by only one subunit. The V-ATPase is stimulated by chloride and inhibited by bafilomycin (Schumacher and Krebs, 2010, 2011), while a potassium-stimulated and a potassium-independent V-PPase have been described (Segami et al., 2010). It is assumed that the K+-independent, type II V-PPase resides in the Golgi, while the K+-dependent pump is located in the vacuolar membrane (Segami et al., 2010). The V-PPase of Vigna radiata was recently crystalized and shown to contain 16 transmembrane domains. Six α-helices are responsible for proton translocation (Lin et al., 2012). Two studies have been published revealing different parts of the structure of a V-ATPase: Walker et al. (2013) succeeded in crystalizing the soluble part of this proton pump and, recently, using an electron cryomicroscopy approach, presented data for the membrane-embedded Vo motor of the V-ATPase (Mazhab-Jafari et al., 2016). Also using electron cryomicroscopy, a structure of the entire F-type ATPase from Pichia angusta mitochondria, which shares considerable homology to the V-ATPase, could be determined (Vinothkumar et al., 2016).
A further important breakthrough and extension of the established methods was the introduction of the patch-clamp technique (Neher and Sakmann, 1976) used to characterize plant ion channels (Schroeder et al., 1984). The first vacuolar channel identified was the slow vacuolar channel, which was later renamed TPC according to its animal homolog (Hedrich et al., 1986). This channel exhibits permeabilities for monovalent and divalent cations and requires Ca2+ for activation (Hedrich and Marten, 2011). The patch-clamp technique has the advantage that it can address questions at the single-vacuole level. Using this approach, Hedrich et al. (1989) could show that the V-ATPase and V-PPase are localized on the same vacuole and that the two proton pumps are not specific for distinct classes of vacuoles.
THE GOLDEN AGE OF VACUOLAR MEMBRANE TRANSPORTER IDENTIFICATION
As mentioned above, in contrast to work on mitochondria and chloroplasts, secondary active transporters or channels could not be identified from vacuolar membranes using biochemical approaches. Therefore, alternative methods had to be developed. Sentenac et al. (1992) showed that the expression of plant cDNA libraries in yeast mutants and identification of the cDNAs that complemented mutant phenotypes were a powerful tool to identify transport proteins of the plasma membrane. This tool could be used even when the transporter was not a structural homolog of the respective mutated yeast protein or yeast did not contain such a transporter (Riesmeier et al., 1992; Larsen et al., 2017). This approach opened a new avenue, and a large number of plasma membrane proteins have been identified since. The method also was quite successful regarding tonoplast transporters, but, in contrast to plasma membrane transporters, it was restricted mainly to the identification of plant transporters with homology to respective yeast transporters, such as for the calcium or sodium proton antiporters or where a clear-cut vacuolar phenotype for yeast was described (Hirschi et al., 1996; Gaxiola et al., 1999). For plant vacuolar transporters with no putative homologs in yeast, or for which the corresponding yeast transporter has not been identified, the prediction of a yeast phenotype for a specific substrate is, in many cases, very difficult. Furthermore, yeast does not possess all of the plant’s substrates and, accordingly, does not have a respective transporter. Screens of plant mutants exhibiting a specific phenotype are a powerful tool to identify new genes, and in some cases, such screens were used successfully to identify vacuolar transporters. With the aim to identify all enzymes involved in flavonoid biosynthesis, Caboche and collaborators searched for Arabidopsis seed mutants that displayed an altered color, naturally or after staining (Wisman et al., 1998). This screen revealed, among others, a vacuolar multidrug and toxin extrusion (MATE)-type transporter that is involved in flavonoid accumulation (Debeaujon et al., 2001; Marinova et al., 2007). In another screen, Quattrocchio and collaborators exploited the pH-dependent color change to search for vacuolar pH mutants looking at petunia (Petunia hybrida) flowers with altered color and identified a vacuolar P-type H+-ATPase that is expressed only in specific tissues (Verweij et al., 2008).
A big step forward in the identification of vacuolar transporters was the combination of the availability of the entire Arabidopsis (Arabidopsis thaliana) genome in the early 2000s with the development of high-resolution proteomic techniques and the application of these techniques to vacuolar membranes (Carter et al., 2004; Endler et al., 2006; Jaquinod et al., 2007). Many of the proteins identified in these investigations have homologs in other organisms and, hence, their function could be hypothesized. Because vacuolar membrane proteins constitute only a minor fraction of total membrane proteins, these fractions are prone to bear contaminations. Therefore, vacuolar localization has to be confirmed (e.g. by using a GFP-tagged version of the putative vacuolar protein). To get more information, expression in a heterologous system and analyses of plants that are mutated in the respective genes are required to verify whether the transporter of interest present in a vacuolar proteomic data set corresponds to the transporter with the expected localization and function. Furthermore, quantitative proteomic analysis under different environmental conditions was carried out as well as proteomic analysis for posttranslational modifications (Whiteman et al., 2008; Endler et al., 2009; Schneider et al., 2009; Schulze et al., 2012). All these data, together with gene expression data, can be combined to give new insights using correlation analysis. Recent advances in metabolomics allow a more general view of the compounds present in vacuoles. Such metabolomic analysis goes beyond initial experiments, where only one or a very restricted set of transporter substrates was investigated (Oikawa et al., 2011; Tohge et al., 2011). However, it should be mentioned that most vacuole isolation procedures are prone to cause a loss of vacuolar constituents; hence, the vacuolar proportion of a certain metabolite may not reflect the in vivo situation. A solution to this problem may be nonaqueous fractionation methods (Farré et al., 2001). Here, tissue is rapidly frozen and the different compartments are separated after homogenization to a fine powder and subsequent lyophilization in a density gradient of n-heptane and carbon tetrachloride. However, this technique has so far been used with tissues rather than specific cell types, such as, for example, mesophyll and epidermal cells. Since different cell types may differ in the accumulation of a specific compound, data from nonaqueous fractionation experiments may not always reflect the situation in a specific cell type.
TRANSPORTERS CONTROLLING THE VACUOLAR PH
The accumulation of Neutral Red was one of the earliest observations made with plant vacuoles, indicating that the vacuole is an acidic compartment. Acidification of the vacuole creates an electrochemical gradient that can be used to energize solute uptake or release by exploiting either the proton gradient (ΔpH) or the difference of the membrane potential (Δψ). Furthermore, the pH gradient can be used to trap weak bases, such as alkaloids that are positively charged by protonation in the acidic vacuole. It is often neglected that dicarboxylates and tricarboxylates also can be trapped within the vacuole, when, for example, the divalent ion form is specifically recognized by the transporter. Within the vacuole, the carboxylate may be converted to the monovalent form through protonation. As a consequence, the concentration difference for the transported form is reduced between cytosol and vacuole. Some compounds are trapped in the vacuole on the basis of acidic pH-induced conformational changes, as shown for apigenin 7(6-O-malonyl)glucoside or the trans-isomer of an o-coumaric acid glucoside, which is converted to the cis-form in the vacuole (Matern et al., 1983; Rataboul et al., 1985). For compounds whose properties do not change inside the vacuole, the ΔpH and/or the Δψ determine the concentration gradient that can be achieved for a certain compound. By contrast, the maximal concentration gradient of compounds that are protonated, deprotonated, or undergo conformational changes is more complex.
The Role of Proton Pumps
Investigations of the vacuolar pH revealed that the proton pumps play an important, but not an exclusive, role. In order to determine their respective roles in vacuolar acidification, the vacuolar pH was determined in knockout mutants of both the vacuolar H+-ATPase and H+-PPase. The vacuolar pH increased from 5.9 to 6.4 in the absence of the vacuolar V-ATPase and from 5.75 to 6 in the absence of the V-PPase, indicating that, even in the absence of both proton pumps, the vacuolar lumen may still be acidic (Krebs et al., 2010). This hypothesis was confirmed by a later work demonstrating that vacuoles missing both vacuolar ATPase and PPase still exhibit an acidic pH (Schumacher, 2014; Kriegel et al., 2015). Two possibilities could explain the remaining acidification: (1) the presence of an additional proton pump; or (2) the fusion of vesicles derived from the trans-Golgi network/early endosome (TGN/EE), which also bear a V-type H+-ATPase that differs in subunit composition from the vacuole-localized V-ATPase (Schumacher and Krebs, 2011). To date, no additional proton pump could be demonstrated in Arabidopsis mesophyll vacuoles; therefore, it is assumed that vesicle transport from the Golgi contributes to vacuolar acidification. Interestingly, depending on the developmental stage and environmental conditions, the role of the two proton pumps in acidification may differ (Schumacher and Krebs, 2010). It is worthwhile to mention that the vacuolar H+-PPase not only acts as a proton pump but its activity also is required to keep cytosolic PPi levels low. In the absence of the V-PPase, plants have defects in organ development due to the inhibition of gluconeogenesis as a consequence of inhibitory PPi concentrations (Ferjani et al., 2011). This indicates that, at least during germination, the vacuolar H+-PPase plays a more important role than soluble PPases in hydrolyzing cytosolic PPi.
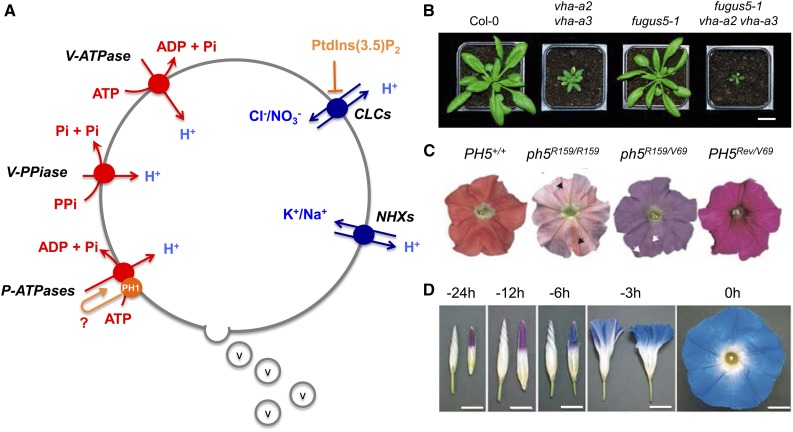
In an elegant approach using the color of petunia flowers to screen for vacuolar pH mutants, Verweij et al. (2008) identified a P-type H+-ATPase, named PH5, that contributes to vacuolar acidification in petunia flowers. However, although the effect on vacuolar pH was clear, overexpression of PH5 did not rescue the pH effect observed in a mutant deficient in PH3, a transcription factor implicated in pH regulation (Fig. 1). Interestingly, a homolog of PH5, AHA10, is expressed in vacuoles of the seed coat endothelium of Arabidopsis and is required for proanthocyanin accumulation (Baxter et al., 2005; Appelhagen et al., 2015). This shows that, besides the V-ATPase and V-PPase, an additional proton pump contributes to vacuolar acidification in some specific tissues. In a further screen, the laboratory of Francesca Quattrocchio identified a second P-type H+-ATPase, PH1, that affects vacuolar acidification and interacts with PH5. This pump exhibits some homology to bacterial Mg2+ transporters (Faraco et al., 2014). However, it is still mysterious how PH1 can function, since it bears an Asn at position 782, while all other P-type ATPases have an Asp at this position, an amino acid absolutely required for functionality (Eisenach et al., 2014). Interaction of PH1 with PH5, therefore, may result in a stimulation of proton-pumping activity. When both P-type H+-ATPases were overexpressed, the petunia ph3 mutant phenotype was rescued.
Figure 1.
Transporters involved in vacuolar pH regulation. A, Vacuolar pumps (in red) and transporters (blue) known to be implicated in establishing the vacuolar pH. In orange are factors involved in the regulation of pumps and transporters. PH1 has been shown to interact with the P-ATPase PH5 by stimulating the pump activity of PH5; for PH1, no pump activity could be demonstrated. Vesicles (v) from the secretory pathway participate in acidifying the vacuole. B, Phenotypes of double mutants of the two V-ATPases and V-PPase (fugus) and the corresponding triple knockout (Kriegel et al., 2015). C, Colors of ph5 mutants and complementation lines of petunia flowers (Verweij et al., 2008). The different colors indicate different pH levels. D, Color change in morning glory flowers during flower opening. The transition from pink to blue is correlated with an increase in expression of a vacuolar HNX and reflects the more alkaline pH (Yoshida et al., 2009).
Proton Antiporters and Other Factors Modulate the Vacuolar pH
Besides the proton pumps, proton-dependent transporters as well as compounds stored in the vacuole play an important role for the vacuolar pH. The vacuolar sap often contains high concentrations of phosphate, malate, citrate, as well as Asp and Glu that can strongly buffer protons between pH 3 and 7 (Martinoia et al., 2007). Carboxylate concentrations fluctuate strongly between day and night, which may result in alterations of the vacuolar pH if no regulatory mechanisms were involved. Furthermore, vacuolar nitrate is exported from the vacuole during the day and reduced to ammonium to be incorporated into amino acids. This process is linked with the alkalinization of the cell. However, to the best of my knowledge, not much is known about whether the vacuolar pH fluctuates diurnally and whether the buffer capacity underlies control mechanisms. The observations by Hurth et al. (2005) that a mutant deficient in the vacuolar malate transporter AttDT has much lower vacuolar and total malate contents and is more affected in its photosynthetic capacity when submitted to acid stress indicate that, indeed, the buffering capacity may play a role in optimizing plant metabolism at least under some stress conditions. An exception regarding our knowledge about vacuolar pH fluctuations and regulation involves guard cells, where experimental evidence showed that stomatal closure leads to an acidification of the guard cell vacuole (Bak et al., 2013).
Proton antiporters have an important impact on the vacuolar pH. The first demonstration that cation (Na+/K+) proton antiporters (NHXs) indeed modify the vacuolar pH was provided by cloning the gene responsible for the color change of morning glory (Ipomoea tricolor) during flower opening. The initial purple color of the flower turns to bright blue, and this is accompanied by a pH shift from 6.6 to 7.7 (Yoshida et al., 2005). This pH shift was shown to be correlated with the expression of an NHX gene. Later studies using ratiometric methods showed that the absence of NHX1 and NHX2 in Arabidopsis causes an acidification of the vacuolar pH in roots as well as in the hypocotyl (Bassil et al., 2011b). Similarly, an nhx5 nhx6 double mutant exhibited a more acidic pH in root vacuoles and in leaf sap (Wang et al., 2015). Since, in contrast to the vacuolar NHX1 to NHX4 proteins, NHX5 and NHX6 localize to the endosome (Bassil et al., 2011a), this result further supports the hypothesis that vacuolar acidification is partly due to vesicle transport within the TGN/EE network. However, it cannot be excluded that NHX5/6 mutants are impaired in the delivery of certain vacuolar proteins that also are implicated in vacuolar pH regulation. The calcium proton antiporters of the CAX family also are localized to the vacuolar membrane (for review, see Pittman, 2011). In an early work, Guern et al. (1989) observed vacuolar acidification by the addition of 1 mm Ca2+. However, this concentration is beyond physiological conditions; therefore, it is unlikely that CAX proteins play a significant role in regulating the vacuolar pH. Nevertheless, and since Ca2+ plays a central role in many regulatory processes, it also may exert an indirect effect on vacuolar and cytosolic pH (for review, see Pittman, 2012).
CLCa and CLCc are localized in the vacuolar membrane and exchange protons for NO3− and Cl−, respectively. CLCb also is present in the tonoplast and has been shown to exchange nitrate with protons (von der Fecht-Bartenbach et al., 2010). Both NO3− and Cl− can be present at high concentrations and, hence, their uptake is accompanied by considerable proton fluxes (Barbier-Brygoo et al., 2011). It is likely that nitrate-dependent fluxes are larger than those for chloride, since vacuolar nitrate exhibits diurnal variations. In Arabidopsis, the absence of phosphatidylinositol-3-phosphate 5-kinase was shown to slow down stomatal closure. Furthermore, a corresponding mutant in Vicia faba exhibited reduced abscisic acid (ABA)-induced guard cell closure and acidification (Bak et al., 2013). Although the first results indicated that the product of this kinase reaction, phosphatidylinositol-3,5-bisphosphate, would act directly on proton pumps, very recent results convincingly show that it interacts with CLCa and inhibits NO3−/H+ antiport, resulting in a stronger acidification (Carpaneto et al., 2017). Thus, CLCs indeed play an important role in regulating vacuolar pH. However, it remains to be established under which circumstances their activity is regulated. In yeast, the CLC homolog Gef1 is localized in late Golgi and prevacuolar compartments. Replacement of native Gef1 by a nonfunctional form resulted in the acidification of the lumen of Gef1-containing compartments. Therefore, it would be interesting to see whether, in Arabidopsis, the Golgi-localized CLCd also has an impact on the vacuolar pH. Also, proton-independent channels and transporters may have an impact on vacuolar pH, since, for instance, anions would reduce the Δψ and allow a higher accumulation of protons. A comparison of ancient melon species with sweet melon (Cucumis melo) identified a transporter, called PH, that is mutated in sweet melon and exhibits some similarities to the PIN and PIN-like auxin/H+ transporters (Cohen et al., 2014). The gene is expressed in fruits of many different plants, and knockdown in cucumber (Cucumis sativus) or tomato (Solanum lycopersicum) resulted in much less acidic fruits. PH was localized to the endoplasmic reticulum, again indicating that vesicle transport to the vacuole has a great impact on the vacuolar pH, which determines fruit pH. Fruits mutated in PH contain less citrate and more nucleosides; however, the function of PH is still unclear.
In conclusion, the regulation of the vacuolar pH is very complex. Several players have been identified; however, how they interact and how they are regulated remain largely elusive. Furthermore, the exact role of the transcription factor PH3 identified in petunia needs to be elucidated. It also remains to be established whether additional factors play a role in regulating vacuolar pH in fruits. Finally, the role of carboxylate transporters and CHX has not been investigated in sufficient detail so far.
SECONDARY COMPOUNDS AND XENOBIOTICS: ABC AND MATE TRANSPORTERS EMERGE
Detoxification and Localization
Secondary metabolites were among the first compounds that were localized in the vacuole (Matile 1982). This fact led to attributing a role to the vacuole as a cellular rubbish bin. Plants produce toxic secondary compounds to fight against pathogens and insects as well as to withstand abiotic stresses, such as high light or UV. Plants also are exposed to toxic compounds produced either by other organisms or from anthropological sources. It could be shown that plant-specific secondary compounds, toxins from diverse sources as well as xenobiotics, are modified by plants using a similar set of enzymes to that described in animals, such as cytochrome P450 monooxygenases and diverse transferases (Klein et al., 1996). In all cases, the goal of these modifications is to produce a hydrophilic compound that can be either excreted, which is the case in animals, or stored within the vacuole. Inside the vacuole, they cannot harm the metabolic processes of the cytosol. For plant secondary compounds, glycosylation is the most common modification to fulfill this role, but glucuronidation, sulfation, amino acid conjugation, and glutathionation also have been observed (Martinoia et al., 2007, 2012; Shitan and Yazaki, 2013). Modified xenobiotics can be either glycosylated or glutathionated (Klein et al., 1996). While secondary compounds produced to fight against harmful environmental conditions are mostly stable compounds, many secondary compounds produced to fight against pathogens and herbivores, such as cyanogenic glucosides or glucosinolates, are hydrolyzed when a cell is damaged and converted to toxic compounds, protecting plants from their enemies (Fig. 2; Matile, 1980; Martinoia et al., 2007; Shitan and Yazaki, 2013).
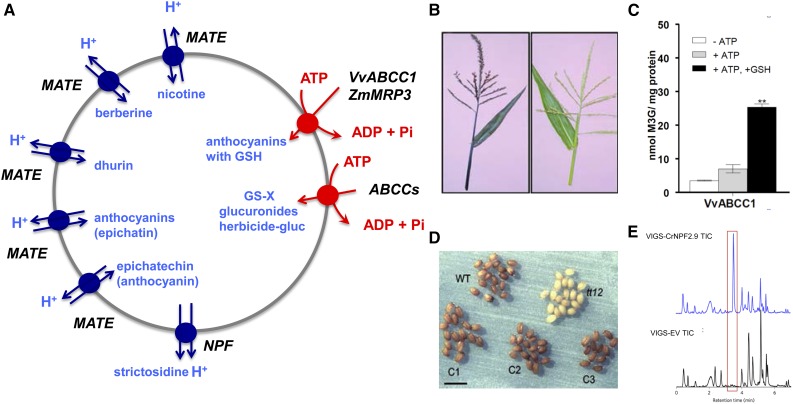
Figure 2.
Vacuolar transport of secondary products and xenobiotics. A, Vacuolar pumps (red) and transporters (blue) known to be implicated in delivering or releasing secondary plant products and xenobiotics. B, Phenotypes of a wild-type (left) and a mutant (right) maize in the putative anthocyanin transporter ZmMRP3 (Goodman et al., 2004). C, Transport of anthocyanins requires glutathione (Francisco et al., 2013). D, Color of wild-type (WT), tt12 mutant, corresponding to a mutation in a MATE transporter, and complemented Arabidopsis seeds (Debeaujon et al., 2001). E, Silencing of the vacuolar NPF in Catharathus roseus plants leads to a high accumulation of strictosidine (peak framed in red; Payne et al., 2017).
Early experiments on secondary compounds focused mainly on how alkaloids are taken up by vacuoles. The laboratory of Guern (Renaudin and Guern, 1987) and others claimed that, at a cytosolic pH of 7.5, a considerable proportion of most alkaloids is present in the unprotonated form that can diffuse through a lipid bilayer of the tonoplast and be trapped in the acidic vacuole by protonation. By contrast, the laboratory of Zenk published data in favor of a transport-mediated process (Deus-Neumann and Zenk, 1986). Nowadays, it is generally accepted that vacuolar alkaloid accumulation occurs through transport-mediated processes (for review, see Martinoia et al., 2007; Shitan and Yazaki, 2013).
A Localization Study Paved the Way for the Discovery of Vacuolar ABC Transporters
In 1982, Schmitt and Sandermann (1982) published a study that, 10 years later, was to have a great impact on vacuolar transport research. They showed that glutathionated xenobiotics end up in the vacuole. At that time, just after my postdoc in Würzburg, I was Oberassistent in the laboratory of Professor Nikolaus Amrhein at ETH Zurich. With him and Erwin Grill, we frequently discussed this puzzling fact: that compounds released at the beginning of industrialization or compounds developed as pesticides or herbicides could be recognized by a plant transporter. However, looking at the animal literature, we realized that animals produce many endogenous glutathione conjugates and that these compounds are excreted by an ATPase that was unknown at the time. Therefore, we produced our own 14C-labeled glutathione derivative by conjugating N-ethylmaleimide and glutathione. Using this molecule as a model compound, we could show that vacuoles possess a glutathione conjugate transporter that is energized directly by ATP (Martinoia et al., 1993), indicating that plants have functional ABC transporters. This was the starting point to investigate which compounds are transported into the vacuole, either by ABC-type transporters or by using the proton-motive force (Martinoia et al., 2007, 2012). A distinction is easy for vacuolar transport processes, because, in contrast to the V-ATPase, which is inhibited by bafilomycin, ABC transporters can be inhibited efficiently by vanadate, which also inhibits P-type ATPases. At that time, I became a professor at Poitiers University and moved to France. Thanks to Professor Amrhein, I could keep a small research group at the ETH in Zurich, and I was very lucky that Markus Klein, an outstanding Ph.D. student from the University of Cologne, proposed to collaborate with our group in Poitiers. This allowed us to continue our investigation into the energization mechanism used by the different substrates.
The first three vacuolar ABC transporters (i.e. members of the C subfamily), also named MRPs at the time, were cloned and characterized by the laboratory of Phil Rea and our group in 1997/1998 (Li et al., 1997; Lu et al., 1998; Tommasini et al., 1998). They exhibit similar properties: all of them transport glutathione conjugates and glucuronide conjugates. For AtMRP2/AtABCC2 and AtMRP3/AtABCC3, it could be shown that they also transport chlorophyll catabolites. Later studies showed that AtABCC2 transports even more substrates (see below). However, there was no evidence that these ABC transporters were involved in the transfer of endogenously produced secondary compounds into the vacuole.
Vacuoles Transport a Large Variety of Secondary Metabolic Compounds
Endogenously produced flavonoids were shown to require the proton gradient across the vacuolar membrane as an energy source for uptake. By contrast, it was observed that these barley flavonoids were not taken up by a proton antiporter by Arabidopsis vacuoles but by an ABC-type transporter in barley vacuoles. Similarly, glucosylated xenobiotics are transported into barley vacuoles by an ABC transporter (Klein et al., 1996; Frangne et al., 2002). By contrast, the vacuolar uptake of glucosylated chlorsulfuron in red beet occurred by a proton antiporter (Bartholomew et al., 2002). Whether glycosylated xenobiotics are transported by proton antiporters or ABC transporters likely depends on the plant species and the substrates. A second class of transporters shown to be implicated in detoxification in bacteria, fungi, animals, and plants are the MATE transporters. In plants, it was shown that a plasma membrane-localized MATE, ALF5, is required to protect roots against toxic compounds (Diener et al., 2001). The first indication that MATE transporters can function as vacuolar flavonoid transporters came from a screen for flavonoid biosynthesis genes, as mentioned above. Meanwhile, many secondary compounds, such as alkaloids (berberine and nicotine), cyanogenic glucosides (dhurrin), and some flavonoids (e.g. epichatechins), have been demonstrated to be transported by MATEs (Goossens et al., 2003; Shoji et al., 2009; Shitan and Yazaki, 2013; Takanashi et al., 2017). Identification of these transporters was obtained using different approaches and methods. In the case of dhurrin, the authors could take advantage of the fact that, in some cases, genes encoding the biosynthetic enzymes of secondary metabolites are organized in gene clusters (Darbani et al., 2016). Interestingly, the dhurrin transporter of sorghum (Sorghum bicolor) is a member of such a cluster (Darbani et al., 2016). However, so far, this appears to be an exception. In the case of nicotine, a gene coding for a MATE transporter, Nt-JAT1, putatively involved in vacuolar nicotine transport, was found by analyzing genes induced after jasmonic acid treatment of tobacco (Nicotiana tabacum), which induces nicotine synthesis (Goossens et al., 2003). Subsequent analysis showed that the corresponding gene product localized to the tonoplast and was required for nicotine accumulation (Morita et al., 2009). At the same time as the function of Nt-JAT1 was established, two other MATE transporters, NtMATE1 and NtMATE2, acting as vacuolar nicotine transporters, were identified (Shoji et al., 2009). While the two latter MATEs are expressed specifically in roots, Nt-JAT1 is expressed in roots, stems, and leaves (Morita et al., 2009). Hence, taking into account the results also obtained for anthocyanins (see below), it appears that, for most secondary compounds, MATEs are responsible for their vacuolar delivery. Interestingly, despite the intensive and excellent work on glucosinolates, no vacuolar transporter for this class of secondary compounds has been found to date.
A special case are anthocyanins and other flavonoids, where both MATE and ABC transporters have been shown to be involved. Gomez et al. (2009) observed that the expression pattern of two MATEs strongly correlated with the induction of anthocyanin formation in grapevine (Vitis vinifera). By heterologous expression in yeast, the authors showed that the identified MATEs could transport acylated anthocyanins but not malvidine- or cyanidine-3-glucosides. These glucosides are minor compounds in Arabidopsis but very prominent anthocyanins in many grapevine accessions and in maize (Zea mays). By contrast, two Medicago truncatula MATEs, MATE1 and MATE2, transport glucosylated anthocyanins. But both have a strong preference for other compounds as well, such as epicatechin, transported by MATE1, which is expressed in the seed coat, like Arabidopsis TT12, and malonylated flavonoids, transported by MATE2 (Zhao and Dixon, 2009; Zhao et al., 2011). Similar to the M. truncatula MATEs, the Arabidopsis MATE transporter TT12 is able to transport anthocyanin glucosides but has, as mentioned above, a strong preference for epicatechin (Marinova et al., 2007).
Genetic and biochemical experiments provided evidence that also vacuolar ABCC transporters are implicated in the transport of glucosylated anthocyanins (Goodman et al., 2004; Francisco et al., 2013). This transport is strictly dependent on glutathione, which is transferred to the vacuolar lumen in conjunction with anthocyanins. Whether anthocyanin and glutathione are just cotransported or build a temporary glutathione conjugate due to their proximity in the cavity of the transporter remains unknown. MATEs are up-regulated when their corresponding substrates are synthesized; by contrast, such a correlation was not observed for ABCC transporters. The reason might be that the latter transporters recognize a multitude of substrates and, hence, have to be expressed continuously.
Much less is known about the export of secondary compounds from the vacuole. An example are flavonoid glucuronides, which accumulate only transiently in the vacuoles of primary rye (Secale cereale) leaves, demonstrating that secondary compounds stored in vacuoles can be metabolized or released on demand (Strack et al., 1982). Another example is the synthesis of complex alkaloids, which can occur in different cell types of a plant, and for which transient vacuolar accumulation of intermediates is postulated (Miettinen et al., 2014). Very recently, a first transporter catalyzing the export of a secondary metabolite was identified. In an attempt to obtain a better view on the fluxes of intermediates of monoterpene indole alkaloids that lead to the synthesis of vinblastine and vincristine in C. roseus, Payne et al. (2017) identified an NPF transporter responsible for the vacuolar efflux of strictosidine, a central intermediate of the pathway produced within the vacuole from secologanin and tryptamine.
HEAVY METALS AND METALOIDS: ABOUT OVERLAPPING SPECIFICITY AND TOXICITY
All living organisms need several heavy metals, so-called micronutrients, as cofactors of enzymes. Plants have the tendency to take up more of these micronutrients than required to build up a reserve for times of starvation. However, all heavy metals are potentially toxic, if present in excess in the cytosol, and, thus, need to be stored safely in the vacuole. Depending on the nutritional status of the plant, they may be either reexported from the vacuole to support the growth of new organs, including seeds, or they may be stored throughout the lifetime of the plant. In addition to micronutrients, soils may contain other heavy metal(oids), such as Cd2+, Pb2+, Hg2+, Al3+, or arsenate. Due to their toxicity, most plants try to exclude these nonessential heavy metal(oids). However, a certain proportion is always taken up by transporters that cannot completely discriminate between beneficial and toxic heavy metals (for review, see Krämer and Clemens, 2006).
Heavy Metal Transporters Exhibit Often Overlapping Specificities
Many publications on essential vacuolar heavy metal transporters reveal that, in different plants, their homologs often exhibit different specificities. This is not (or must not be) a contradiction, since it has been shown that few amino acid changes can considerably shift the substrate specificity of ion transporters. For example, the wild-type zinc transporter of rice (Oryza sativa; OsMTP1) can efficiently complement a zinc-sensitive yeast mutant and, to some degree, also yeast mutants that are sensitive for Co2+ and Fe2+. Exchanging Leu-82 to Phe strongly decreased the capacity of OsMTP1 to confer zinc tolerance but considerably increased Fe2+ and Co2+ tolerance (Menguer et al., 2013). Therefore, the exact role of a given transporter needs to be established in planta. A holistic view is required to understand the specific role of a given transporter. Exact expression analysis and tissue-specific, or even better, cell-specific, ionics can give further information. Furthermore, posttranslational modifications, such as phosphorylation or heteromerization, may change substrate affinities. Therefore, in this section, I will present some general paradigms (Fig. 3).
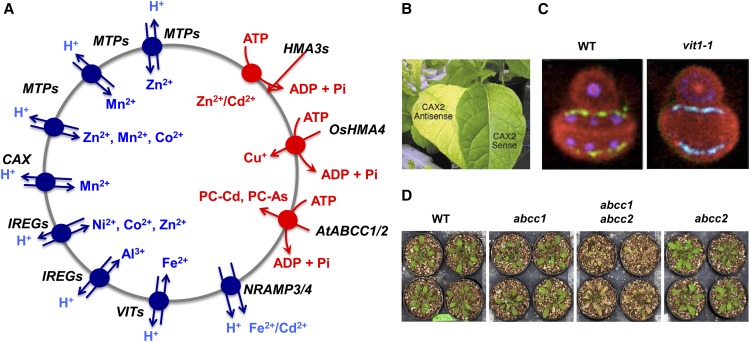
Figure 3.
Vacuolar transport of essential and toxic heavy metals. A, Vacuolar pumps (in red) and transporters (blue) known to be implicated in delivering or releasing heavy metals. B, Expression of CAX2 in the antisense or sense orientation results in plants that are more sensitive or more tolerant to manganese (Hirschi, 2001). C, Iron distribution, but not iron total content, is altered in seeds of Arabidopsis mutants for the vacuolar iron transporter VIT1 (Kim et al., 2006). D, In Arabidopsis, the double knockout abcc1 × abcc2 is hypersensitive to arsenic (Song et al., 2010). WT, Wild type.
Iron storage in seeds is an example that demonstrates how different heavy metals may be stored. Two major subcellular iron stores are found in all plant cells: chloroplasts, where iron is present as ferritin, and vacuoles. Seeds are an important iron store for plants, and they also are relevant for human nutrition. In Arabidopsis seeds, at least 95% of the iron is stored in vacuoles, but in other seeds, such as peas (Pisum sativum), the vacuolar proportion of iron may be much lower (Kim et al., 2006; Briat et al., 2007; Curie and Mari, 2017). Within the Arabidopsis seed, iron is located mainly in the endodermis that surrounds the provascular strands of the embryo. Considerable amounts also are found in subepidermal cells. The uptake of iron into vacuoles of endodermal cells is catalyzed by AtVIT1, an iron-specific transporter (Kim et al., 2006). AtVIT1 is a homolog of the yeast CCC1 protein (Li et al., 2001). To the best of my knowledge, the transporter that delivers iron to the vacuoles of subepidermal cells has not been characterized. Seeds of vit1 mutants show dispersed iron localization, while the localization of Zn2+ and Mn2+ is not altered, confirming the iron specificity of this transporter. Interestingly, however, the total amount of iron is not altered in the mutant. Nevertheless, vit1 seedlings grow only poorly on alkaline soils, demonstrating that, not only the amount, but also the correct deposition, of iron is important for plant survival. Phytate is the major phosphorous store in seeds and forms very strong complexes with iron that are not bioavailable (Shi et al., 2007; Nagy et al., 2009). Iron, as well as manganese, are colocalized largely with phosphorous, suggesting that both elements are complexed with phytate and that phytate is present in different cell types. The latter was deduced from metal-imaging experiments that showed a distinct localization of these two heavy metals (Kim et al., 2006). Phytate accumulates in vacuoles through ABCC transporters such as ABCC5 in Arabidopsis (Shi et al., 2007; Nagy et al., 2009). Further VIT1 and VIT1-like (VTL) proteins from Arabidopsis and other species have been characterized (Momonoi et al., 2009; Zhang et al., 2012; Gollhofer et al., 2014). Arabidopsis encodes five VTL proteins, and the fact that, for three of them, overexpression caused increased iron content in seeds suggests that all five VTLs can transport iron. It is worthwhile to mention that VIT-type iron transporters are also required to stabilize anthocyanin and, hence, alter flower color. In tulip (Tulipa gesneriana) flowers, regions expressing a VIT homolog appear blue, while those regions where the vacuolar iron transporter is not present are purple (Momonoi et al., 2009). Rice has two VIT homologs, OsVIT1 and OsVIT2, which are expressed mainly in the flag leaf (Zhang et al., 2012). OsVIT2 but not OsVIT1 expression is strongly down-regulated in roots and shoots under iron deficiency and up-regulated under high-iron conditions, indicating that OsVIT2 plays a major role in regulating cytosolic iron homeostasis. In contrast to AtVIT1, the rice homologs have been shown to transport also manganese, indicating that they may act as transporters for different divalent cations.
Two types of structurally unrelated transporters have been shown to be able to deliver Mn2+ to the vacuole: CAXs and MTPs. By complementation of a corresponding yeast mutant, CAX transporters were originally identified as Ca2+ proton antiporters. CAX transporters are present in plants, fungi, bacteria, and most animals, but not in mammals and insects. Later studies showed that some CAX members transport not only Ca2+ but also other divalent cations, such as Mn2+ and Cd2+ (Hirschi et al., 2000; Shigaki et al., 2003). It was shown that CAX2 suppresses the Mn sensitivity of a corresponding yeast mutant and that Arabidopsis cax2 mutants were more sensitive to manganese stress. These mutants also had higher Mn2+ contents in seeds. However, this effect was observed not only for Mn2+ but also for Zn2+ and Fe2+. The substrate specificity of many CAX proteins was shown to be determined by just one or two amino acid residues. Exchanging this/these specific residue(s) can convert a Ca2+/Mn2+ transporter to a Ca2+-specific transporter or a Ca2+-specific transporter to an Mn2+ transporter (Pittman, 2005, 2011; Delhaize et al., 2007; Mei et al., 2009).
The second class of Mn2+ transporters responsible for the delivery of Mn2+ into the vacuole was identified by expressing a cDNA library from Stylosanthes hamate in a manganese-sensitive yeast mutant (Delhaize et al., 2003). S. hamate can grow on acid soils and tolerates high manganese concentrations. The so-identified protein, ShMTP1, belongs to the MTP family, a subfamily of the cation diffusion facilitator family, and confers Mn tolerance when expressed in Arabidopsis. ShMTP1 and the three ShMTP homologs form a separate clade within the MTP family. Like S. hamate, Arabidopsis contains four genes in this clade. To my knowledge, only one member, AtMTP11, has been analyzed in detail, because its expression is much higher than that of the other MTPs (Delhaize et al., 2007). AtMTP11 has been localized to the prevacuolar compartment, indicating that the majority of Mn2+ present in the large central vacuole is delivered by the TGN/EE pathway. Growth of the mtp11 mutant was strongly affected by increased Mn2+ concentrations in the medium, but surprisingly, mtp11 did not exhibit reduced Mn2+ uptake. Apparently, at least in Arabidopsis cv Columbia, a feedback mechanism to govern Mn2+ uptake, as known for Fe2+ or Zn2+, does not seem to exist. By contrast, the rice homolog OsMTP8.1.1 is localized in the tonoplast, and disruption of OsMTP8.1 not only resulted in lower chlorophyll content and impaired growth but also in reduced Mn2+ uptake. No differences were observed for other divalent cations, indicating that OsMTP8.1 is an Mn2+-specific transporter (Chen et al., 2013).
MTPs also are responsible for the delivery of zinc into the vacuole. AtMTP1 and AtMTP3 are probably the best characterized zinc transporters. Deletion of either of them results in plants that are sensitive to high zinc exposure, probably due to their different and complementary expression patterns in shoots and roots (Krämer and Clemens, 2006; Sinclair and Krämer, 2012). AtMTP3 is strongly up-regulated under high-zinc conditions and under iron starvation. Under high-zinc conditions, AtAMTP3 is probably responsible for reducing cytosolic zinc in order to prevent the replacement of iron as a cofactor in iron-containing enzymes. Interestingly, AtMTP1 bears a long His-rich loop that slows down zinc transport, since deletion of this loop results in a hyperactive zinc transporter. This loop, therefore, could function as a buffer or sensor for cytosolic zinc (Tanaka et al., 2015). In addition to MTPs, a P-type ATPase, HMA3 in Arabidopsis, also acts as a zinc and cadmium transporter (Morel et al., 2009). However, this ATPase differs from species to species with respect to substrate specificity and expression pattern. When expressed in yeast, Arabidopsis HMA3 also transports cobalt, cadmium, and lead. In Arabidopsis, HMA3 is expressed mainly in guard cells, hydathodes, and vascular tissues. Initial observations indicated that it acts mainly as a zinc transporter (Morel et al., 2009); however, a study using a genome-wide association study (GWAS) provided evidence that AtHMA3 is a main factor controlling leaf cadmium concentrations (Chao et al., 2012). Indeed, athma3 mutant plants are more sensitive to Zn2+ and Cd2+. The predominant expression in the root may indicate that AtHMA3 is responsible for the translocation of cadmium from the root. This may explain the observation that, in mesophyll cells of Arabidopsis plants mutated in the two major phytochelatin (PC) transporters, only very low amounts of vacuolar cadmium could be detected, indicating that the transport of nonconjugated Cd2+ is minor in Arabidopsis mesophyll cell vacuoles (Park et al., 2012). By contrast, in planta experiments in rice indicate that OsHMA3 is involved mainly in the delivery of cadmium to the vacuole, playing a central and predominant role in Cd2+ detoxification. A rice cultivar that transfers only low amounts of cadmium to the shoot possesses a highly active OsHMA3, while a rice cultivar transferring high amounts of cadmium to the shoot has an HMA3 with impaired function (Ueno et al., 2010; Miyadate et al., 2011).
Hyperaccumulators are plants that tolerate and take up much more of one or several heavy metals than normal plants (Krämer and Clemens, 2006). Besides an increased translocation from root to shoot, hyperaccumulators need very efficient vacuolar storage capacities. In the zinc/cadmium hyperaccumulator Arabidopsis halleri, AhMTP1 as well as AhHMA3 are highly expressed. For AhMTP1, it has been proposed that this is partly due to gene duplication or triplication (Dräger et al., 2004; Elbaz et al., 2006). A higher expression of HMA3 also was observed in other hyperaccumulators such as Noccaea caerulescens, which is due mainly to an increased copy number of the HMA3 gene (Ueno et al., 2011).
As for other heavy metals, copper homeostasis is controlled mainly by uptake, root-to-shoot translocation, and finally by balanced uptake and release from the vacuole that allows delivering defined amounts of copper to the cytosol. Using rice accessions that exhibit different seed copper contents, Huang et al. (2016) mapped a quantitative trait locus explaining 43% of the difference to a P-type ATPase, OsHMA4. OsHMA4 is a vacuolar Cu+ transporter. Loss-of-function mutants retained less Cu+ in the root and delivered higher amounts to the shoots, which also was reflected by higher copper contents in seeds.
Many heavy metal transporters exhibit a rather broad substrate specificity, and plants exposed to soils containing high amounts of a certain heavy metal are forced to counterbalance the excessive uptake of certain heavy metals. Schaaf et al. (2006) have shown that AtIREG2 is a vacuolar nickel transporter that is coregulated with the major iron transporter of the plasma membrane, IRT1. ireg2 mutants are more sensitive to nickel exposure, and this is more pronounced under iron deficiency, which demonstrates the important role of IREG2 for iron homeostasis. While in roots of ireg2, all other heavy metals behaved as in the wild type, IREG2 overexpression plants contained more manganese, zinc, and cobalt. This suggested that also this transporter may exhibit a rather broad substrate specificity. Indeed, Morrissey et al. (2009) showed that AtIREG2 exhibits very similar characteristics for Co2+ as described for Ni2+. Next-generation sequencing of Psychotria gabriellae, an Ni2+ hyperaccumulator, and expression studies in yeast and Arabidopsis allowed the identification of PgIREG2 as an Ni2+ transporter (Merlot et al., 2014). Similar to the A. halleri zinc transporters, the expression of PgIREG2 in P. gabriellae is much higher than in the close relative Psychotria semperflorens, which is no Ni2+ hyperaccumulator.
Considering the facts that only a few amino acid changes convert the specificity of many heavy metal transporters and that many of them are not absolutely specific for a given heavy metal, it is not surprising that nonessential, toxic heavy metals also are taken up by plants. They can be delivered to the vacuole by one of the heavy metal transporters recognizing also a nonessential heavy metal, such as CAX2. As mentioned above, HMA3 can act preferentially as a zinc or cadmium transporter, hence either playing a major role in the storage of an essential heavy metal, as in Arabidopsis, or being involved in the detoxification of a toxic heavy metal, as in rice.
Detoxification of Toxic Heavy Metals and Metalloids
However, the majority of plants rely on the complexation of toxic heavy metals to the glutathione derivative PCs, which ultimately are delivered to the vacuole. Cadmium, arsenic, and other heavy metal(oids) induce the formation of PCs by the activation of PC synthase (Mendoza-Cózatl et al., 2011). Early experiments expressing vacuolar ABC transporters in yeast and observations in Saccharomyces cerevisiae and Schizosaccharomyces pombe indicated that ABC transporters play a role in the transport of heavy metal conjugates (Szczypka et al., 1994; Ortiz et al., 1995). However, for a long period and despite big efforts, the molecular identity of the PC transporter remained elusive. Together with the laboratory of Professor Youngsook Lee, we screened all known ABCCs, since they were and still are the only class of putative vacuolar ABC transporters. A screen with Cd2+ did not reveal a clear result, probably because the Cd-PC complex is relatively stable at cytosolic pH. At this pH, the arsenic-PC complex is less stable; consequently, using arsenic and arsenic-based herbicides allowed us to observe a slight arsenic-sensitive phenotype for two ABCC mutants, atabcc1 and atabcc2. This led us to produce the corresponding abcc1 abcc2 double mutant, which was very sensitive to small concentrations of arsenic. Using a wheat (Triticum aestivum) PC synthase-expressing yeast strain, and performing experiments with yeast vacuoles isolated from empty vector controls and yeast expressing AtABCC1 or AtABCC2, it could be shown that these two transporters act as apo-PC as well as PC-heavy metal complex transporters (Song et al., 2010). Further experiments using vacuoles isolated from wild-type and double mutant plants confirmed that AtABCC1 and AtABCC2 are the main PC transporters in Arabidopsis. A later study showed that these transporters also are responsible for the vacuolar import of PC-Cd and PC-Hg (Park et al., 2012). The corresponding double mutant, therefore, also is sensitive when exposed to cadmium or mercury. It should be mentioned that, besides AtABCC1 and AtABCC2, also AtABCC3 was shown to play a similar, although minor, role (Brunetti et al., 2015). Rice possesses only one homolog to AtABCC1 and AtABCC2, OsABCC1. OsABCC1 also acts as a vacuolar PC transporter, and rice knockout plants for this transporter are sensitive to arsenic but not to cadmium, highlighting the importance of OsHMA3 in cadmium tolerance. At the reproductive stage, osabcc1 plants stored less arsenic in the nodes, a tissue that acts as a transfer site for nutrients and where OsABCC1 is strongly expressed, and transferred more arsenic to the grains (Song et al., 2014). Combining the knowledge about OsABCC1 and about the synthesis of glutathione and PCs, and using appropriate promoters, may be a strategy to reduce arsenic allocation to rice seeds and, hence, ameliorate food quality in regions with high soil arsenic contents.
A special case is the fern Pteris vittata, an arsenic hyperaccumulator. This plant preferentially takes up arsenate and accumulates arsenite in the vacuole. Two homologs of the yeast plasma membrane arsenic exporter ACR have been identified in this fern. In contrast to yeast, the two P. vittata ACRs are localized in the vacuolar membrane (Indriolo et al., 2010). However, only the down-regulation of ACR3, but not of ACR3;1, results in an arsenic-sensitive phenotype, indicating that ACR3 is the major factor conferring arsenic tolerance. ACRs occur in mosses, lycophytes, other ferns, and gymnosperms but not in angiosperms.
Aluminum is partially present as Al3+ in acidic soils and very toxic in this form. However, some plants, such as buckwheat (Fagopyrum esculentum) or rice, are tolerant to aluminum. While certain plants such as rice mostly rely on external detoxification of Al3+, others take up Al3+ and store it in the vacuole (Yokosho et al., 2016). As mentioned above, anthocyanin color is strongly dependent on heavy metals. In the case of the popular garden plant Hydrangea macrophylla, the blue color of its petals is due to the accumulation of aluminum in the vacuole (Yokosho et al., 2016). While we know a lot about external detoxification of Al3+, internal detoxification is not well understood. Nevertheless, two classes of vacuolar transporters associated with aluminum tolerance have been reported. One is an ABC transporter, ALS3 in Arabidopsis and OsALS1 in rice, which are related to the human TAP family that act as peptide transporters (Larsen et al., 2005; Huang et al., 2012). The corresponding rice knockout mutants are hypersensitive to aluminum. The second, FeREG1 from buckwheat, is highly expressed in roots and highly up-regulated when plants were exposed to aluminum (Yokosho et al., 2016). Overexpression of FeREG1 in Arabidopsis conferred increased aluminum tolerance. An effect on other heavy metals could not be observed. By contrast, no effect on aluminum could be observed in mutants of AtREG2, the Arabidopsis homolog of FeREG1. In brief, two different transporters required for internal aluminum tolerance are known. However, how and in which form they transport aluminum into the vacuole remain to be established.
How Is the Specific Release of Vacuole-Localized Heavy Metals Achieved?
Much less is known about the export mechanism of heavy metals from the vacuole. Two transporters, NRAMP3 and NRAMP4, have been shown to act as vacuolar iron exporters and to be required for embryo development under iron-limiting conditions (Lanquar et al., 2005). There is evidence that these transporters also are responsible for the export of manganese, and NRAMP4, but not NRAMP3, exhibits a zinc-related phenotype in yeast, indicating that Zn2+ export relies on this NRAMP, at least partially. Thus, it remains an open question how plants specifically regulate vacuolar Fe, Mn, and Zn export. The fact that nramp3 nramp4 double mutants are more tolerant when plants are exposed to cadmium indicates that this heavy metal also is released by these transporters.
Two reports convincingly show that the copper transporter COPT5 acts as a copper exporter. In one case, COPT5 was localized to the prevacuolar compartment, while other work showed its localization to the large, central vacuole. Vacuoles of a copt5 mutant contain considerably higher copper contents, and plant growth is more impaired in the mutant than in the wild type under copper-limiting conditions (Garcia-Molina et al., 2011; Klaumann et al., 2011).
In many cases, the heavy metal gradient between the vacuolar lumen and the cytosol is rather large, posing the risk of heavy metal leakage from the vacuole into the cytosol. To limit this risk, most heavy metals are bound to chelators within the vacuole. The importance of such complexing compounds, for instance, is demonstrated in the case of nicotianamine, which is transported into the vacuole by the ZIF transporter that belongs to the so-called MFS family (Haydon and Cobbett, 2007; Haydon et al., 2012). In the absence of ZIF, plants are similarly zinc sensitive as the zinc transporter mutant mtp1. For N. caerulescens, a zinc hyperaccumulator, it has been shown that zinc is quantitatively chelated to nicotianamine in the mesophyll, while in the epidermis, zinc is associated mainly with malate and citrate (Schneider et al., 2013). The synthesis of nicotianamine, which forms more stable complexes with zinc than malate or citrate, indicates that the plant invests more energy to protect the metabolically active mesophyll cells than the metabolically less active epidermal cells. This result also demonstrates that, depending on the plant species, the cell type, and possibly also the nutritional state, heavy metals are complexed with different compounds. This diversity of metal-complexing compounds was reviewed recently (Flis et al., 2016). In seeds, iron and other divalent cations are complexed to phytate, while PCs are thought to play a major role for nonessential heavy metals and metalloids such as cadmium and arsenic. A question that remains for most essential heavy metals, however, is how the complex is dissolved once the plant is in need of a specific heavy metal. So far, this has been clarified only for iron, at least in seeds. The first step, release of iron from phytate, is catalyzed by phytases. Since iron is present in the Fe3+ form within the vacuole, it has to be reduced to Fe2+ in a second step. There is evidence that this is mediated by an influx of ascorbate into the vacuole through a so-far unknown transporter (Grillet et al., 2014; Curie and Mari, 2017). Fe2+ is finally exported from the vacuole by NRAMP3 and NRAMP4 (Lanquar et al., 2005).
SWEET AND SOUR: VACUOLAR CARBOXYLATE AND SUGAR TRANSPORT
Localization studies in the late 1970s and early 1980s showed that the vacuole contains soluble carbohydrates, organic acids, and amino acids (Matile, 1982; Martinoia et al., 2007, 2012; Shitan and Yazaki, 2013). As mentioned above, our 14CO2-labeling experiment with barley protoplasts revealed that mainly carboxylates and sugars are very rapidly transferred into the vacuole (Kaiser et al., 1982). I was particularly interested to analyze vacuolar malate transport. One reason was the observation that in vivo malate transfer into vacuoles preceded all other photosynthates. The second was the importance and central role of malate in plant metabolism (Fernie and Martinoia, 2009).
Vacuolar Malate and Citrate Transport
Hence, after my move as a postdoc to Würzburg University, I started my career in the transport field with a detailed biochemical analysis of vacuolar malate transport in barley mesophyll vacuoles. The results showed that transport was stimulated by the vacuolar ATPase, and inhibition studies indicated that the same transporter was responsible for the vacuolar uptake of all carboxylates tested, including citrate (Martinoia et al., 1985). Similar results were obtained by other groups (Marigo et al., 1988), and interestingly, electrophysiologists could demonstrate that vacuoles bear a malate channel (Cheffings et al., 1997; Hafke et al., 2003). This raised the question of whether the malate fluxes observed with radioisotope labeling and the malate currents measured by electrophysiology were catalyzed by the same protein. During my time as an Oberassistent at ETH Zurich, I had two major goals: one was to get more information about vacuolar ABC transporters and detoxification; the other was to learn more about malate and citrate transport. Biochemical studies showed that citrate uptake worked in a way similar to malate uptake, but the affinity for citrate was considerably higher than that for malate (Oleski et al., 1987; Rentsch and Martinoia, 1991). However, despite a huge effort, we did not succeed in purifying the malate transporter (Martinoia et al., 1991). I had given up the hope of identifying the vacuolar malate transporter when, in 2002, Ekkehard Neuhaus from Technische Universität Kaiserslautern told me that he probably had identified the vacuolar malate transporter and offered me to collaborate on its characterization. The identified gene exhibited a high similarity to the renal dicarboxylate transporter and, indeed, encoded for a vacuolar malate transporter called AttDT (Emmerlich et al., 2003). AttDT mutants contain much less malate but more citrate (Hurth et al., 2005). A similar behavior was observed recently for tomato fruits in which the corresponding gene was silenced, while overexpression led to increased fruit malate content and decreased citrate content (Liu et al., 2017; Ye et al., 2017). Transport experiments with Arabidopsis vacuoles showed that, while malate uptake was strongly reduced, citrate uptake was comparable to that of the wild type.
In view of the flux analysis performed in different laboratories, this result was surprising. It was also surprising that Arabidopsis knockout mutants grown under control conditions did not exhibit any phenotype; only a slightly higher sensitivity to acid stress and a higher respiratory coefficient could be observed, indicating higher malate respiration, because the plants cannot store malate in the vacuole. The discovery of AttDT paved the way to address the longstanding question of whether the malate transport activity observed in flux experiments was catalyzed by the same transporter as the channel activity described using the patch-clamp technique. Hurth et al. (2005) showed that the channel activity was still present in the transporter mutant and, hence, the observed channel activity was not derived from AttDT. Considering that not all members of a membrane protein family have to be localized on the same membrane, Kovermann et al. (2007) posed the question of whether a member of the ALMT family, which had been shown to confer aluminum tolerance by exuding malate at the plasma membrane, could act as a vacuolar malate channel. Arabidopsis encodes for 13 ALMTs that can be subdivided into four clades (Dreyer et al., 2012). Indeed, AtALMT9, a member of clade II of these channels, was shown to be localized on the vacuolar membrane and to exhibit malate channel activity (Kovermann et al., 2007). A follow-up study that concentrated on chloride flux analysis showed that AtALMT9 also acts as a malate-activated chloride channel (De Angeli et al., 2013). The malate concentrations required for this activation (i.e. for an increased channel open probability) are in the range of the free malate concentration reported to occur in the cytosol. This result attributed a new role to the many tasks already described for malate: that of a channel modulator. Electrophysiological and biochemical studies indicate that ALMTs most probably assemble as tetramers (De Angeli et al., 2013). A second vacuolar ALMT, AtALMT6, was analyzed in detail. It is activated by Ca2+ and catalyzes the efflux of anions. However, despite the fact that AtALMT6 is expressed nearly exclusively in guard cells, so far no guard cell- or stomata-related phenotype could be observed for atalmt6 mutants (Meyer et al., 2011). The low cytosolic malate concentration combined with the low affinity of these channels poses the question of whether vacuolar ALMTs are indeed important for malate accumulation. Clade II consists of five members, and most likely all of them are localized on the vacuolar membrane. Therefore, only multiple mutants can answer the question about their role in malate accumulation. However, since vacuoles of knockout mutants in AttDT still contain about 30% of the vacuolar malate observed in the wild type (Hurth et al., 2005), it is tempting to speculate that ALMTs play a role in both malate and chloride accumulation. In line with this hypothesis, very recent work, using GWAS to find factors impacting malate contents, revealed that a homolog of ALMT9 plays a major role in malate accumulation in tomato fruits (Ye et al., 2017). A vacuolar ALMT of grapevine was shown to facilitate the transport of malate as well as tartrate, a major carboxylate in grapevine berries (Zhang et al., 2013). Although no direct correlation was shown, the high expression of this ALMT may indicate that, also in grapevine, ALMTs exhibit an important function in malate accumulation.
Vacuolar Sugar Transporters
Several of the proteomics studies cited above paved the way to identify sugar transporters because they highlighted a large number of putative sugar transporters. However, this also raised the question of why the vacuole contains so many sugar transporters and about their specific functions. A complete overview and description of all transporters and findings would be beyond the scope of this review; therefore, I limit this part to the description of the different classes of vacuolar carbohydrate transporters and some general findings, which should be regarded as a personal choice. Five classes of carbohydrate transporters have been identified so far: TMTs, VGTs, ERD-L, Sweets, and AtSuc4 and its homologs (for details, see Hedrich et al., 2015).
TMTs (Wormit et al., 2006) and VGT1 (Aluri and Büttner, 2007) are both hexose importers (Fig. 4). VGT1 is expressed in all aboveground parts of the plant, but mainly in flowers, and here most prominently in pollen. When expressed in yeast, the transport activity of VGT1 for Glc was twice that observed for Fru. Arabidopsis vgt1 mutants flowered later than the corresponding wild type, and seed germination was impaired. Interestingly, the two other members of the VGT family in Arabidopsis were shown to preferentially transport Xyl after expression in yeast, but they were not analyzed in planta (Hector et al., 2008). TMTs are the main vacuolar hexose transporters, at least in leaves. Under cold stress conditions, when plants accumulate large amounts of sugars, leaf Glc and Fru contents are reduced to less than 10% in a tmt triple mutant compared with the wild type. Interestingly, under these conditions, the Suc content also was reduced by approximately 30%, in line with recent results showing that TMTs also transport Suc and may exert a dual function (Schulz et al., 2011). A very important recent finding was the identification of the sugar beet Suc transporter BvTST2, a TMT homolog that exhibits the highest identity (70%) to AtTMT2 (now also called AtTST2; Jung et al., 2015). Quantitative proteomics data revealed that this transporter is one of the most prominent tonoplast proteins in sugar beet. Based on these findings, the authors concentrated their investigation on this transporter and could show that BvTST2 acts almost exclusively as a Suc proton antiporter. Together with the observation that Suc accumulation correlated with BvTST2 transcript levels, the authors concluded BvTST2 to be the major Suc transporter involved in vacuolar Suc accumulation in sugar beet. Future work might address the question of whether specific amino acid residues play a role in determining substrate specificity in this class of transporters, as is the case for many other ion transporters.
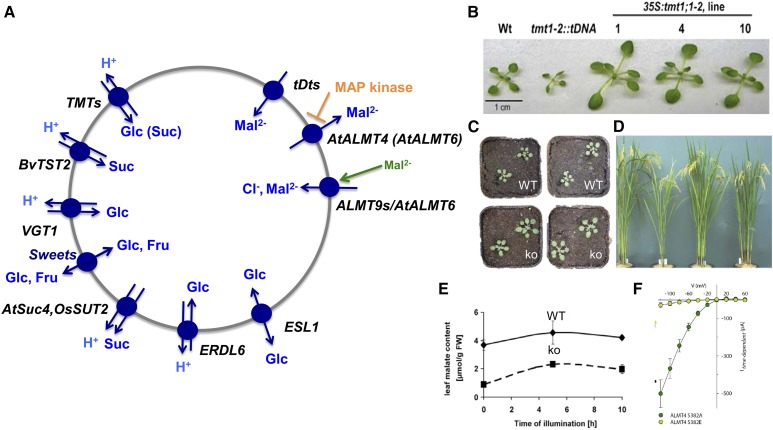
Figure 4.
The vacuole as temporary store for sugars and carboxylates. A, Vacuolar transporters known to be implicated in delivering or releasing sugars and carboxylates. For ALMT4, refer to the stomata section. B, Knockout and overexpression of the Glc transporter TMT1 has a large effect on seedling growth (Wingenter et al., 2010). C, Overexpression of the vacuolar SWEET16 results in Arabidopsis plants that grow faster on soil (Klemens et al., 2013). D, Rice plants deficient in the vacuolar Suc exporter OsSUT2 exhibit retarded growth (Eom et al., 2011). E, Arabidopsis plants missing the vacuolar malate transporter AttDT have strongly reduced malate content (Emmerlich et al., 2003). F, The activity of the malate channel ALMT4 is completely inhibited by phosphorylation (Eisenach et al., 2017). FW, Fresh weight; ko, knockout; WT, wild type.
Sweet transporters are localized on both the plasma and vacuolar membranes and mediate the facilitated diffusion of hexoses and Suc with low affinity (Chen et al., 2015). Two Sweets, Sweet16 and Sweet17, have been localized to the vacuolar membrane. Although Sweet17 can facilitate the uptake and release of Fru (Klemens et al., 2013; Guo et al., 2014), it was shown, using a GWAS approach, that in Arabidopsis leaves Sweet17 is involved mainly in releasing Fru from vacuoles (Chardon et al., 2013). sweet17 mutant plants contain more Fru but less Glc. By contrast, Sweet16 acts mainly as a Glc facilitator, but it also transports Fru and Suc, with transport activities in oocytes being about 40% and 20%, respectively, compared with Glc transport activity (Chardon et al., 2013).
AtSuc4 is the sole member of the SUT/Suc family that is localized to the vacuolar membrane in Arabidopsis (Endler et al., 2006), and vacuolar localization of Suc4 homologs has been shown for other plant species (Eom et al., 2011; Payyavula et al., 2011). AtSuc and its homologs act as Suc proton symporters that export Suc from the vacuole and play important roles in plant development. Eom et al. (2011) showed that rice plants deficient in the corresponding transporter, OsSUT2, had retarded growth, reduced tiller number, reduced 1,000-grain weight, and reduced root dry weight when compared with the wild type. Some members of the Early Responsive to Dehydration (ERD) family, a very diverse class of proteins coding for nonrelated proteins, localize to the vacuolar membrane. Evidence for carbohydrate transport has been provided for four members, but only one has been investigated in detail. Thirteen further genes of this class putatively encode carbohydrate transporters, but their in planta roles and localization await further analysis. ERDL6 acts as a Glc proton symporter, delivering Glc to the cytosol, in line with the fact that the corresponding erdl6 mutant contains more Glc. This increased Glc content phenotype is accentuated under conditions requiring carbohydrate delivery to the cytosol. Accordingly, ERDL6 is strongly down-regulated when the plant’s survival depends on the accumulation of carbohydrates inside the vacuole, such as during cold stress (Poschet et al., 2011). A further member of the ERD family, ESL1 (Erd six like1), in contrast to ERDL6, has been suggested to act as a Glc facilitator (Kiyosue et al., 1998; Yamada et al., 2010). However, in planta experiments and the physiological role of this transporter have not been reported so far.
Several investigations focused on an overexpression of vacuolar carbohydrate transporters (Fig. 4). Although there is the risk that some of the observed effects are due to transporter expression in cell types where these transporters are not normally present, some results gave new insights or confirmed hypotheses gained from transporter mutant analysis. All cases showed how tightly vacuolar carbohydrate uptake and release must be regulated to allow for an optimal function (Chardon et al., 2013; Guo et al., 2014). Probably the most surprising result was that the overexpression of AtTMT1 resulted in higher seed yield and larger seeds, which were reflected by increased amounts of lipids and proteins (Wingenter et al., 2010). This result may indicate that vacuolar loading of carbohydrates during the photosynthetic period may be a limiting factor for plant productivity.
The fact that the vacuole contains so many sugar transporters is puzzling, and it can be postulated that their activity needs to be tightly regulated in order to coordinate plant global sugar transport. TMTs contain a large cytosolic loop, which attracted the attention of Ekkehard Neuhaus and his collaborators. In a series of elegant experiments, they searched for potential interacting partners and identified a member of the mitogen-activated protein3 kinases as a kinase that phosphorylates and activates AtTMT1 (Wingenter et al., 2011). Further studies are now needed to understand these complex regulatory networks.
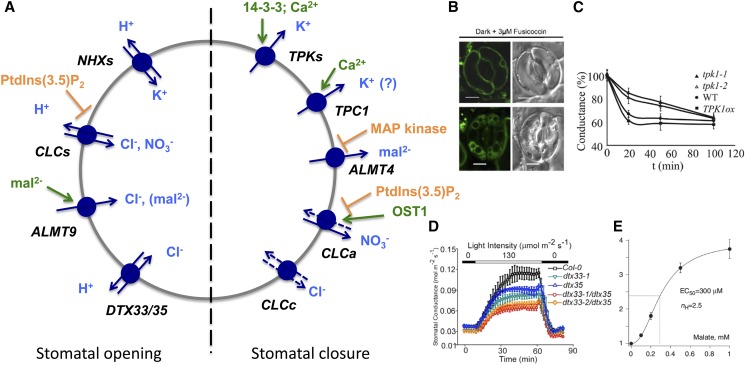
THE ROLE OF THE VACUOLE IN STOMATAL MOVEMENT
While channels and transporters of the guard cell plasma membrane have attracted much attention for many years, and while they have been described extensively, the characterization of vacuolar channels and transporters and their role in guard cell volume regulation only started a few years ago. A recent review nicely summarizes our current knowledge (Eisenach and De Angeli, 2017); however, due to the important role of the vacuole in stomata movement, some aspects also will be discussed in this article. Stomatal opening is induced by guard cell swelling. Due to the action of the vacuolar proton pumps, the membrane potential of the vacuolar lumen is slightly positive. Hence, the accumulation of potassium, the major osmolyte in most guard cells, is catalyzed by NHX cation proton antiporters. NHXs have been shown to transport both Na+ and K+. However, in guard cells, their major role is the delivery of K+ during stomatal opening (Barragán et al., 2012; Andrés et al., 2014). nhx1 nhx2 double mutants exhibited the expected defect in stomatal opening, but interestingly, not only stomatal opening was impaired but also ABA-dependent closure. Since the cytosolic K+ concentrations have been reported to be at least 50 to 100 mm, it is thermodynamically unlikely that these transporters can both import and export potassium. It is more likely that the impaired potassium proton antiport activity of nhx1 nhx2 and the resulting stronger acidification lead to alterations in the membrane potential that cause some secondary effects. Candidates for potassium export are the TPKs. For the tpk1 mutant, a slight delay in ABA-mediated stomatal closure was reported, indicating that there is some redundancy with other TPKs (Gobert et al., 2007; Latz et al., 2007). The absence of TPC1 has no effect on ABA-mediated stomatal closure; however, tpc1 mutants do not close efficiently in the presence of Ca2+ (Peiter et al., 2005). There is contrasting evidence about the role of TPC1 in releasing Ca2+ and mediating calcium signals. Choi et al. (2014) showed that Ca2+ waves are strongly impaired in Arabidopsis tpc1 mutant roots in response to a salt stress. Similarly, leaves of Arabidopsis mutants that are exposed to the green peach aphid (Myzus persicae) respond with reduced local elevation of cytosolic calcium (Vincent et al., 2017). By contrast, in a careful study on guard cell calcium waves, Islam et al. (2010) could not observe altered Ca2+ oscillations in guard cells of tcp1 mutant plants. The difference observed by Peiter et al. (2005) between wild-type and mutant plants could be a secondary effect (e.g. to a change of the membrane potential but also to the fact that, in the absence of TPC1, Ca2+ does not activate TPC1-mediated release of K+ or Na+). Interestingly, the TPC1-dependent current density is much higher in guard cells than in mesophyll vacuoles, and in guard cells, this channel is activated at lower Ca2+ concentrations (Rienmüller et al., 2010). These results may suggest that TPC1 plays a role in stomatal movement, but the physiological function has to be established (Fig. 5).
Figure 5.
The roles of vacuolar transporters in stomatal movement. A, Vacuolar transporters and channels known to be implicated in stomatal opening (left side) and closure (right side). The proton pumps generating the electrochemical potential are not indicated. Activating factors (green) and inhibiting factors (orange) are shown. B, NHX1 and NHX2 are required for stomata opening (Andrés et al., 2014). Mutants in the vacuolar K+ channel TPK1 have delayed stomatal closure (Gobert et al., 2007). C, Mutants in the vacuolar K+ channel TPK1 have a delayed stomatal closure (Gobert et al.,2007). D, The MATE transporters dtx33 and dtx35 act as vacuolar chloride channels and are required for stomatal opening (Zhang et al., 2017). E, The chloride channel activity of AtALMT9 is strongly stimulated by malate at physiological concentrations (De Angeli et al., 2013). WT, Wild type.
Chloride, nitrate, and malate are the major anions that compensate for the positive charges of potassium. Their ratio strongly depends on the nutritional state of the plant as well as on the plant species. Some plants accumulate large amounts of malate, while others prefer to use chloride as a counteranion (Eisenach and De Angeli, 2017).Two CLCs, CLCa, which is an NO3− proton antiporter, and CLCc, which, based on structural and physiological data, acts as a Cl− proton antiporter, are responsible for the vacuolar delivery of the respective anions to guard cell vacuoles (Jossier et al., 2010; Barbier-Brygoo et al., 2011; Wege et al., 2014). Interestingly, both CLCs also are involved in stomatal closure. A reason for this dual function was proposed for CLCa. This channel can be phosphorylated by AtOST1, a kinase playing a central role in ABA-mediated stomatal closure (Wege et al., 2014). Phosphorylation was found to stimulate NO3− currents at membrane potentials below 0 mV and, hence, was able to mediate NO3− efflux into the cytosol in vivo.
ALMTs act as malate and chloride channels, and at least AtALMT9 plays an important role in stomata regulation (De Angeli et al., 2013). As described above, AtALMT9 is a malate channel as well as a malate-activated chloride channel. In guard cells, it has been proposed to mainly play a role as a chloride channel. atalmt9 plants exhibit an impaired stomatal aperture and have slightly retarded growth and reduced water loss. By contrast, for mutants in AtALMT6, which has a highly specific guard cell expression and requires Ca2+ to activate anion currents, no phenotype has been described so far (Meyer et al., 2011). Nevertheless, its specific guard cell expression suggests that this ALMT plays a role under specific conditions. The determined ion currents would be compatible with a role of ALMT6 in anion release and, hence, in guard cell closure. Surprisingly, the MATE transporters DTX33 and DTX35 have been described to function as chloride uptake channels, although a slight permeability also was observed for nitrate but not for organic anions (Zhang et al., 2017). These MATEs are highly expressed in guard cells. Epidermal strip experiments performed in the presence of 30 mm chloride revealed that stomatal apertures were reduced in the single mutants and even more in the double mutant. At 5 mm chloride in the solution, a difference could only be observed for the double mutant, underlining the role of these MATEs for chloride accumulation. In line with these results, stomatal conductance as well as water loss were reduced in dtx33 and dtx35 plants, with an additive effect in the double mutant. This work also revealed how broad the substrate specificity for MATEs is, ranging from secondary compounds of different classes to citrate and inorganic anions.
Vacuolar malate transport is a potentially important factor for stomatal movement. Malate can act as an osmotic agent and counterion to potassium. The ratio between inorganic anions and malate depends on the plant species. A malate transporter, AttDT, was identified several years ago (Emmerlich et al., 2003); however, so far, no effect on stomata movement has been described in attdt, although the expression of this transporter in guard cells is high. Whether it does not play a role or whether there is a redundancy with ALMTs will have to be investigated. Very recently, a first channel releasing malate from guard cell vacuoles was identified. Mutants of AtALMT4, another member of the ALMT family, do not close stomata efficiently in response to ABA, resulting in increased wilting in response to drought (Eisenach et al., 2017). The channel activity is largely regulated by a phosphorylatable Ser at the C terminus. Dephosphomimetic mutants exhibited a constitutive open channel activity, which, at the whole-plant level, resulted in impaired stomatal opening and closure and strongly reduced growth. The amino acids in proximity to the phosphorylatable Ser indicated that a MAP kinase could be responsible for the phosphorylation, a hypothesis that could be verified in vitro (Fig. 5).
Recently, a long-neglected issue in stomata physiology, the role of starch and sugars, has been reinvestigated, and the importance of starch and sugars for stomatal control has been highlighted (Santelia and Lawson, 2016). Hence, the importance of vacuolar sugar transporters in relation to stomatal function is emerging. All vacuolar sugar transporters characterized so far are expressed in guard cells, but none of them has an expression ratio between guard cells and mesophyll that is higher than 2-fold.
Finally, guard cell vacuoles also play a role in signaling events. CAX1 and CAX3 probably play a role in the interaction between Ca2+ and auxin (Cho et al., 2012), and also the vacuolar pH is likely to affect the Ca2+ waves of guard cells (Allen et al., 2000). Phytate or inositolhexakisphosphate (InsP6), which is not only a phosphate store but also an important signaling molecule, has been reported to catalyze Ca2+ release from vacuoles (Lemtiri-Chlieh et al., 2003). Despite all this information, the channel releasing Ca2+ from vacuoles is still elusive. AtABCC5, which is responsible for vacuolar phytate transport in seeds, is highly expressed in guard cells, and in the absence of this transporter, stomatal movement in response to ABA, Ca2+, and auxin is deregulated (Klein et al., 2003). However, it is unclear whether the observed phenotype is due directly to the role of InsP6 as a signaling molecule or to impaired Ca2+ signaling as a consequence of the absence of InsP6 transport, which very probably affects cytosolic InsP6 concentrations.
THE ROLE OF VACUOLES IN HORMONE HOMEOSTASIS
Phytohormones are signaling molecules rarely associated with vacuoles. Nevertheless, vacuoles and the corresponding transporters play an important role in hormone homeostasis. Besides being present in their active form, most hormones also are present as conjugates that are inactive but may be hydrolyzed to release the active hormone. Although not proven for most of them, it is generally assumed that the largest amount of hormone conjugates is stored within the vacuole. The best investigated example is ABA glucoside. In Arabidopsis, the endoplasmic reticulum and the vacuole contain the β-glucosidases BG1 and BG2, respectively. These enzymes are activated under stress conditions and hydrolyze the ABA conjugate to release ABA (Lee et al., 2006; Xu et al., 2012). These β-glucosidases are important to protect plants against drought and salt stress. Uptake experiments using labeled ABA glucoside showed that both ABC-type transporters and antiporters are involved in ABA glucoside uptake into mesophyll vacuoles (Burla et al., 2013). Both ABCC1 and ABCC2 were able to catalyze transport, but the antiporter(s) responsible for vacuolar uptake has not been identified. The low affinity observed for the overall vacuolar transport of ABA glucoside indicates that, at least with regard to the vacuole, transport itself is not part of the signaling event. Since the vacuole is an acidic compartment, a considerable proportion of the released ABA will be protonated and, hence, can easily diffuse to the cytoplasm. However, it cannot be ruled out that an ABA exporter exists at the tonoplast that would allow for a better control of cytosolic free ABA.
Auxin forms a multitude of conjugates with sugars, amino acids, and peptides (Ludwig-Müller, 2011). To date, vacuolar transport of these conjugates has not been addressed. However, it was shown that a vacuolar auxin exporter is required for correct plant development: WAT1, which encodes a transport protein, has a strong impact on the thickness of cell walls (Ranocha et al., 2013). Transport experiments using vacuoles, yeast cells, and oocytes provided strong evidence that WAT1 mediates the export of auxin from vacuoles. The fact that local application of auxin restored secondary wall thickness was a further indication that the phenotype observed in a wat1 mutant was due to disturbed auxin homeostasis. These results show that the efflux of auxin from vacuoles plays an important role in plant development. Vacuolar auxin can either be generated by hydrolysis of auxin conjugates inside the vacuole or by import from the cytosol, most probably by diffusion of the protonated form. Although, for the latter case, the vacuole is more acidic than the cytosol and, consequently, at equilibrium, the vacuolar concentration is lower than in the cytosol, the amount of auxin within the vacuole may be considerable. Assuming a pH difference of 1 between the cytosol and the vacuole, and a vacuolar volume of 80%, vacuoles would contain approximately 40% of the total cellular auxin. Small fluctuations in the vacuolar pH, therefore, would result in considerable concentration changes in the cytosol.
ACC, the precursor in ethylene biosynthesis, has been shown to be localized at 60% to 70% in the vacuoles of barley and wheat, similar to other neutral amino acids (Bouzayen et al., 1989; Tophof et al., 1989; Vanderstraeten and Van Der Straeten, 2017). It is likely that ACC can be exported to the cytosol when required to support ethylene synthesis. As for hormones, this precursor can be conjugated to several compounds. The most prominent and best investigated is malonyl-ACC, which accumulates during water stress (Vanderstraeten and Van Der Straeten, 2017). Two other conjugates, jasmonyl-ACC and γ-glutamyl-ACC, also have been described. It is still unclear whether and to which extend these conjugates can act as ACC pools. Vacuolar, proton-motive force-driven transport activity has been demonstrated for malonyl-ACC. Identifying the corresponding vacuolar transporter may elucidate whether malonyl-ACC plays a role in ethylene signaling events.
WE LEARNED A LOT ABOUT VACUOLES BUT WE NEED TO LEARN A LOT MORE
In 1982, Matile wrote a review article with the title “Vacuoles Come of Age” (Matile, 1982). At that time, the basic functions of vacuoles were known, it had been shown that they contain hydrolytic enzymes and store many primary and secondary compounds, but only very few transport studies were published. Thirty-five years later, we know much more. Vacuolar proteomics and metabolomics studies have been carried out, transport studies using biochemical and electrophysiological approaches have been performed for a large number of substrates, and a large number of genes encoding transporters and channels have been identified from many different plants. Knockout and overexpression plants for these transporters have taught us a lot about transporter function and physiological roles. Nevertheless, we still need to identify many more transporters. This is particularly true for secondary compounds, but also for transporters of central interest for the primary metabolism, such as sulfate, citrate, or large peptide importers, which have all been characterized biochemically (Martinoia et al., 2012). Suc uptake can be catalyzed by a proton antiporter (Jung et al., 2015) as well as by facilitated diffusion (Kaiser and Heber, 1984). While the proton-dependent transporter has been identified (Jung et al., 2015), the protein responsible for facilitated diffusion, which plays a central role for fructan synthesis and is the predominant vacuolar Suc transporter in many plants, remains to be identified. A further intriguing aspect is salt tolerance. We know that NHXs transport both K+ and Na+ into the vacuole, but we do not know how (or if) they discriminate between the two cations in response to nutritional and salt stress states. Are posttranslational modifications or protein-protein interactions responsible? Or does a so far unknown transporter exist that can confer Na+ tolerance? A further question concerns the finding of high DNase and RNase activities within the vacuole. Do small RNA or DNA chains enter the vacuole through a transporter, or are they delivered to the vacuole through vesicles? The demonstration of a nucleotide transporter strongly indicates that RNA and DNA are degraded within the vacuole and that nucleotides are exported to the cytosol for further use (Wormit et al., 2004; Bernard et al., 2011).
Furthermore, our knowledge of import processes is generally better than that of the export of solutes and nutrients from the vacuole. This is particularly true for kinetic parameters that are difficult to measure for exporters. We also do not know enough about the specific role of a given transporter in a specific tissue or cell type. An example is the pollen tube, where the vacuole plays a central role for fast elongation. Very recently, a first anion transporter implicated in pollen tube growth was discovered (Zhang et al., 2017), but we still miss a holistic view. We may reconsider early results with mutants, since at that time the gold standard we have nowadays was not established. Many mutants of a given transporter exhibit a phenotype that very often fits our expectations. Nevertheless, we have to be aware that phenotypes of constitutive mutants may not only reflect directly the phenotype linked to a given gene product but be the result of secondary effects that are consequences of this mutation and, hence, correspond to secondary effects. In some cases, careful controls can give us the answer whether primary observations are indeed due to the gene of interest; in other cases, this is much more difficult. If a transporter has an impact on the membrane potential or pH, it may affect other transporters, and the observed phenotype will consequently be the sum of the different effects of altered presence and function of a whole set of proteins. The long-lasting discussion about the Ca2+ permeability of TPC1 is such an example. Recent results strongly argue that this channel indeed exhibits Ca2+ permeability; however, it cannot be excluded that the efflux of Na+ and K+ through this channel does affect the membrane potential, which, in turn, activates other Ca2+ channels. Also, it cannot be excluded, or, in some cases, it is even likely, that the regulation of vacuolar activities occurs through vesicle trafficking.
Another open question concerns the specificity of transporters in planta. This is particularly true for divalent cations and flavonoids. As described in this review, many vacuolar transporters for heavy metals can transport several divalent cations, and many members show an overlapping expression. How plants regulate their activity and whether a specific property is dominating in planta still need to be elucidated. Similarly, we still lack knowledge about pH regulation. How stable is the pH in a given cell? And how do the proton pumps interact with each other and with other transport proteins to establish a given pH under changing environmental and metabolic conditions? We know even less about the membrane potential, where our knowledge is based on some measurements with microelectrodes, but there are no data about the likely dynamics of the vacuolar membrane potential.
Also, our knowledge of how the activity of vacuolar transporters is regulated by posttranslational regulation, by transporter interaction, or by interaction with cytosolic proteins, such as kinases, phosphatases, and scaffold proteins, is still very scarce. All this knowledge is absolutely required to get a global view on how ion and metabolite fluxes across the vacuolar membrane affect plant growth. Modeling studies where specific conditions are anticipated may help to design experiments more efficiently.
Looking further ahead, there are many biotechnological approaches where vacuoles can be exploited. The potential role of the vacuole in phytoremediation already has been shown but can and must be improved if this technology is to become successful (Song et al., 2003; Shim et al., 2013). The large space of vacuoles allows them to accumulate large amounts of valuable secondary products. Here, we can still learn from idioblasts accumulating huge concentrations of secondary products such as flavonoids, glucosinolates, or vinblastine/vincristine. The example of anthocyanin-producing tomato has demonstrated that this is possible and, thus, is an excellent proof of concept (Butelli et al., 2008).
The interest of Philippe Matile was mainly in comparing animal and plant lytic compartments, (i.e. the lysosome and the vacuole, respectively) and to learn more about the ontogeny of plant vacuoles and their constituents. The main topic of my Ph.D. was chloroplast senescence and chlorophyll degradation. Although we published several articles on vacuoles, this was a side project at that time. However, for me, it was as often happens when going out to eat: I find the starter more interesting than the main course; and so, I was more attracted to continue to work on vacuoles. I think I was lucky that we started to work on this topic at the right time. Very little was known about vacuolar transporters, but many methods had already been established in the early 1980s. Soon after, powerful molecular techniques and mutant plants became available, allowing us to undertake the next steps in characterizing vacuolar transporters and searching for their physiological roles. Using a mix of logical thinking, intuition, fruitful discussions, collaborations with friends and good colleagues, and sometimes a pinch of luck, we often found solutions to the challenges we posed for ourselves. The community that works on vacuolar transporters made huge progress in shedding light on the role of diverse vacuolar transporters and channels and their role for the different aspects of plant development and response to the environment. While at the beginning of the era of vacuolar research, only single aspects could be described due to the limited tools available, nowadays, we can integrate our research in complex, yet by far not complete, networks. As mentioned above, there is still much missing and to be discovered, but I am confident that our view of the vacuole will gradually evolve to a global view, and then the vacuole can be regarded as a real adult.
Acknowledgments
I thank my three tutors, Philippe Matile, Ulrich Heber, and Nikolaus Amrhein, for their great support, which was not restricted to the period I worked in their laboratories. I also thank all my postdocs, Ph.D., and Master students working on vacuolar projects, which were often very laborious and also frustrating. Finally, among all the excellent collaborations I had, I would like to highlight two persons: Professor Ekkehard Neuhaus, without whom I would not have been able to come back to my favorite topic, malate transport; and Professor Youngsook Lee, for without her, our work on heavy metals never would have developed in such a successful way. I also thank Stefan Hörtensteiner and Cornelia Eisenach for critical reading and their great help in correcting this article. Thanks also to Lorenzo Borghi for help in preparing the figures.
Footnotes
Articles can be viewed without a subscription.
References
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al. (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 [DOI] [PubMed] [Google Scholar]
- Aluri S, Büttner M (2007) Identification and functional expression of the Arabidopsis thaliana vacuolar glucose transporter 1 and its role in seed germination and flowering. Proc Natl Acad Sci USA 104: 2537–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés Z, Pérez-Hormaeche J, Leidi EO, Schlücking K, Steinhorst L, McLachlan DH, Schumacher K, Hetherington AM, Kudla J, Cubero B, et al. (2014) Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc Natl Acad Sci USA 111: E1806–E1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhagen I, Nordholt N, Seidel T, Spelt K, Koes R, Quattrocchio F, Sagasser M, Weisshaar B (2015) TRANSPARENT TESTA 13 is a tonoplast P3A-ATPase required for vacuolar deposition of proanthocyanidins in Arabidopsis thaliana seeds. Plant J 82: 840–849 [DOI] [PubMed] [Google Scholar]
- Bak G, Lee EJ, Lee Y, Kato M, Segami S, Sze H, Maeshima M, Hwang JU, Lee Y (2013) Rapid structural changes and acidification of guard cell vacuoles during stomatal closure require phosphatidylinositol 3,5-bisphosphate. Plant Cell 25: 2202–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Brygoo H, De Angeli A, Filleur S, Frachisse JM, Gambale F, Thomine S, Wege S (2011) Anion channels/transporters in plants: from molecular bases to regulatory networks. Annu Rev Plant Biol 62: 25–51 [DOI] [PubMed] [Google Scholar]
- Barragán V, Leidi EO, Andrés Z, Rubio L, De Luca A, Fernández JA, Cubero B, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24: 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew DM, Van Dyk DE, Lau SMC, O’Keefe DP, Rea PA, Viitanen PV (2002) Alternate energy-dependent pathways for the vacuolar uptake of glucose and glutathione conjugates. Plant Physiol 130: 1562–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Ohto MA, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E (2011a) The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 23: 224–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E (2011b) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23: 3482–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter IR, Young JC, Armstrong G, Foster N, Bogenschutz N, Cordova T, Peer WA, Hazen SP, Murphy AS, Harper JF (2005) A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc Natl Acad Sci USA 102: 2649–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Traub M, Kunz HH, Hach S, Trentmann O, Möhlmann T (2011) Equilibrative nucleoside transporter 1 (ENT1) is critical for pollen germination and vegetative growth in Arabidopsis. J Exp Bot 62: 4627–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E, Poole RJ (1985) Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol 78: 163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzayen M, Latché A, Pech JC, Marigo G (1989) Carrier-mediated uptake of 1-(malonylamino)cyclopropane-1-carboxylic acid in vacuoles isolated from Catharanthus roseus cells. Plant Physiol 91: 1317–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat JF, Curie C, Gaymard F (2007) Iron utilization and metabolism in plants. Curr Opin Plant Biol 10: 276–282 [DOI] [PubMed] [Google Scholar]
- Brunetti P, Zanella L, De Paolis A, Di Litta D, Cecchetti V, Falasca G, Barbieri M, Altamura MM, Costantino P, Cardarelli M (2015) Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J Exp Bot 66: 3815–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burla B, Pfrunder S, Nagy R, Francisco RM, Lee Y, Martinoia E (2013) Vacuolar transport of abscisic acid glucosyl ester is mediated by ATP-binding cassette and proton-antiport mechanisms in Arabidopsis. Plant Physiol 163: 1446–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser-Suter C, Wiemken A, Matile P (1982) A malic acid permease in isolated vacuoles of a Crassulacean acid metabolism plant. Plant Physiol 69: 456–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EG, Hall RD, Bovy AG, Luo J, et al. (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26: 1301–1308 [DOI] [PubMed] [Google Scholar]
- Carpaneto A, Boccaccio A, Lagostena L, Di Zanni E, Scholz-Starke J (2017) The signaling lipid phosphatidylinositol-3,5-bisphosphate targets plant CLC-a anion/H+ exchange activity. EMBO Rep 18: 1100–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C, Pan S, Zouhar J, Avila EL, Girke T, Raikhel NV (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16: 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao DY, Silva A, Baxter I, Huang YS, Nordborg M, Danku J, Lahner B, Yakubova E, Salt DE (2012) Genome-wide association studies identify heavy metal ATPase3 as the primary determinant of natural variation in leaf cadmium in Arabidopsis thaliana. PLoS Genet 8: e1002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardon F, Bedu M, Calenge F, Klemens PAW, Spinner L, Clement G, Chietera G, Léran S, Ferrand M, Lacombe B, et al. (2013) Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Curr Biol 23: 697–702 [DOI] [PubMed] [Google Scholar]
- Cheffings CM, Pantoja O, Ashcroft FM, Smith JA (1997) Malate transport and vacuolar ion channels in CAM plants. J Exp Bot 48: 623–631 [DOI] [PubMed] [Google Scholar]
- Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB (2015) Transport of sugars. Annu Rev Biochem 84: 865–894 [DOI] [PubMed] [Google Scholar]
- Chen Z, Fujii Y, Yamaji N, Masuda S, Takemoto Y, Kamiya T, Yusuyin Y, Iwasaki K, Kato S, Maeshima M, et al. (2013) Mn tolerance in rice is mediated by MTP8.1, a member of the cation diffusion facilitator family. J Exp Bot 64: 4375–4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D, Villiers F, Kroniewicz L, Lee S, Seo YJ, Hirschi KD, Leonhardt N, Kwak JM (2012) Vacuolar CAX1 and CAX3 influence auxin transport in guard cells via regulation of apoplastic pH. Plant Physiol 160: 1293–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111: 6497–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Itkin M, Yeselson Y, Tzuri G, Portnoy V, Harel-Baja R, Lev S, Sa’ar U, Davidovitz-Rikanati R, Baranes N, et al. (2014) The PH gene determines fruit acidity and contributes to the evolution of sweet melons. Nat Commun 5: 4026. [DOI] [PubMed] [Google Scholar]
- Curie C, Mari S (2017) New routes for plant iron mining. New Phytol 214: 521–525 [DOI] [PubMed] [Google Scholar]
- Darbani B, Motawia MS, Olsen CE, Nour-Eldin HH, Møller BL, Rook F (2016) The biosynthetic gene cluster for the cyanogenic glucoside dhurrin in Sorghum bicolor contains its co-expressed vacuolar MATE transporter. Sci Rep 6: 37079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Auzac J. (1974) Mise en evidence d’un mechanism d’accumulation du citrate dans les lutoides du latex d’ Hevea brasiliensis. Physiol Veg 12: 617–635 [Google Scholar]
- D’Auzac J. (1975) Caracterization d’une ATPase membranaire en presence d’une phosphatase acide dans les lutoides d’ Hevea brasiliensis. Phytochemistry 14: 671–675 [Google Scholar]
- De Angeli A, Zhang J, Meyer S, Martinoia E (2013) AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat Commun 4: 1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Peeters AJM, Léon-Kloosterziel KM, Koornneef M (2001) The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13: 853–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Pittman JK, White RG, Leung H, Miao Y, Jiang L, Ryan PR, Richardson AE (2007) A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. Plant J 51: 198–210 [DOI] [PubMed] [Google Scholar]
- Delhaize E, Kataoka T, Hebb DM, White RG, Ryan PR (2003) Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 15: 1131–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deus-Neumann B, Zenk MH (1986) Accumulation of alkaloids in plant vacuoles does not involve an ion-trap mechanism. Planta 167: 44–53 [DOI] [PubMed] [Google Scholar]
- De Vries H. (1885) Plasmolytische Studien über die Wand der Vakuolen. Jarb Wiss Bot 16: 465–598 [Google Scholar]
- Diener AC, Gaxiola RA, Fink GR (2001) Arabidopsis ALF5, a multidrug efflux transporter gene family member, confers resistance to toxins. Plant Cell 13: 1625–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dräger DB, Desbrosses-Fonrouge AG, Krach C, Chardonnens AN, Meyer RC, Saumitou-Laprade P, Krämer U (2004) Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. Plant J 39: 425–439 [DOI] [PubMed] [Google Scholar]
- Dreyer I, Gomez-Porras JL, Riaño-Pachón DM, Hedrich R, Geiger D (2012) Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front Plant Sci 3: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin F. (1841) Histoire naturelle des zoophytes: Infusoires. Librairie Encyclopédique de Roret, Paris
- Eisenach C, Baetz U, Huck NV, Zhang J, De Angeli A, Beckers GJM, Martinoia E (2017) ABA-induced stomatal closure involves ALMT4, a phosphorylation-dependent vacuolar anion channel of Arabidopsis. Plant Cell 29: 2552–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach C, Baetz U, Martinoia E (2014) Vacuolar proton pumping: more than the sum of its parts? Trends Plant Sci 19: 344–346 [DOI] [PubMed] [Google Scholar]
- Eisenach C, De Angeli A (2017) Ion transport at the vacuole during stomatal movements. Plant Physiol 174: 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz B, Shoshani-Knaani N, David-Assael O, Mizrachy-Dagri T, Mizrahi K, Saul H, Brook E, Berezin I, Shaul O (2006) High expression in leaves of the zinc hyperaccumulator Arabidopsis halleri of AhMHX, a homolog of an Arabidopsis thaliana vacuolar metal/proton exchanger. Plant Cell Environ 29: 1179–1190 [DOI] [PubMed] [Google Scholar]
- Emmerlich V, Linka N, Reinhold T, Hurth MA, Traub M, Martinoia E, Neuhaus HE (2003) The plant homolog to the human sodium/dicarboxylic cotransporter is the vacuolar malate carrier. Proc Natl Acad Sci USA 100: 11122–11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, Keller F, Baginsky S, Martinoia E, Schmidt UG (2006) Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol 141: 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler A, Reiland S, Gerrits B, Schmidt UG, Baginsky S, Martinoia E (2009) In vivo phosphorylation sites of barley tonoplast proteins identified by a phosphoproteomic approach. Proteomics 9: 310–321 [DOI] [PubMed] [Google Scholar]
- Eom JS, Cho JI, Reinders A, Lee SW, Yoo Y, Tuan PQ, Choi SB, Bang G, Park YI, Cho MH, et al. (2011) Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol 157: 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco M, Spelt C, Bliek M, Verweij W, Hoshino A, Espen L, Prinsi B, Jaarsma R, Tarhan E, de Boer AH, et al. (2014) Hyperacidification of vacuoles by the combined action of two different P-ATPases in the tonoplast determines flower color. Cell Rep 6: 32–43 [DOI] [PubMed] [Google Scholar]
- Farré EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L (2001) Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol 127: 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferjani A, Segami S, Horiguchi G, Muto Y, Maeshima M, Tsukaya H (2011) Keep an eye on PPi: the vacuolar-type H+-pyrophosphatase regulates postgerminative development in Arabidopsis. Plant Cell 23: 2895–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Martinoia E (2009) Malate: jack of all trades or master of a few? Phytochemistry 70: 828–832 [DOI] [PubMed] [Google Scholar]
- Flis P, Ouerdane L, Grillet L, Curie C, Mari S, Lobinski R (2016) Inventory of metal complexes circulating in plant fluids: a reliable method based on HPLC coupled with dual elemental and high-resolution molecular mass spectrometric detection. New Phytol 211: 1129–1141 [DOI] [PubMed] [Google Scholar]
- Francisco RM, Regalado A, Ageorges A, Burla BJ, Bassin B, Eisenach C, Zarrouk O, Vialet S, Marlin T, Chaves MM, et al. (2013) ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3-O-glucosides. Plant Cell 25: 1840–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangne N, Eggmann T, Koblischke C, Weissenböck G, Martinoia E, Klein M (2002) Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles: energization occurs by H+-antiport and ATP-binding cassette-type mechanisms. Plant Physiol 128: 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Molina A, Andrés-Colás N, Perea-García A, Del Valle-Tascón S, Peñarrubia L, Puig S (2011) The intracellular Arabidopsis COPT5 transport protein is required for photosynthetic electron transport under severe copper deficiency. Plant J 65: 848–860 [DOI] [PubMed] [Google Scholar]
- Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA 96: 1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJM (2007) The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc Natl Acad Sci USA 104: 10726–10731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollhofer J, Timofeev R, Lan P, Schmidt W, Buckhout TJ (2014) Vacuolar-Iron-Transporter1-Like proteins mediate iron homeostasis in Arabidopsis. PLoS ONE 9: e110468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, Terrier N, Torregrosa L, Vialet S, Fournier-Level A, Verriès C, Souquet JM, Mazauric JP, Klein M, Cheynier V, et al. (2009) Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol 150: 402–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CD, Casati P, Walbot V (2004) A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. Plant Cell 16: 1812–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens A, Häkkinen ST, Laakso I, Seppänen-Laakso T, Biondi S, De Sutter V, Lammertyn F, Nuutila AM, Söderlund H, Zabeau M, et al. (2003) A functional genomics approach toward the understanding of secondary metabolism in plant cells. Proc Natl Acad Sci USA 100: 8595–8600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet L, Ouerdane L, Flis P, Hoang MTT, Isaure MP, Lobinski R, Curie C, Mari S (2014) Ascorbate efflux as a new strategy for iron reduction and transport in plants. J Biol Chem 289: 2515–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J, Mathieu Y, Kurkdjian A, Manigault P, Manigault J, Gillet B, Beloeil JC, Lallemand JY (1989) Regulation of vacuolar pH of plant cells. II. A 31P NMR study of the modifications of vacuolar pH in isolated vacuoles induced by proton pumping and cation/H+ exchanges. Plant Physiol 89: 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WJ, Nagy R, Chen HY, Pfrunder S, Yu YC, Santelia D, Frommer WB, Martinoia E (2014) SWEET17, a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiol 164: 777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafke JB, Hafke Y, Smith JAC, Lüttge U, Thiel G (2003) Vacuolar malate uptake is mediated by an anion-selective inward rectifier. Plant J 35: 116–128 [DOI] [PubMed] [Google Scholar]
- Haydon MJ, Cobbett CS (2007) A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in Arabidopsis. Plant Physiol 143: 1705–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon MJ, Kawachi M, Wirtz M, Hillmer S, Hell R, Krämer U (2012) Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. Plant Cell 24: 724–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector RE, Qureshi N, Hughes SR, Cotta MA (2008) Expression of a heterologous xylose transporter in a Saccharomyces cerevisiae strain engineered to utilize xylose improves aerobic xylose consumption. Appl Microbiol Biotechnol 80: 675–684 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Flügge UI, Fernandez JM (1986) Patch clamp studies of ion transport in isolated plant vacuoles. FEBS Lett 204: 228–232 [Google Scholar]
- Hedrich R, Kurkdjian A, Guern J, Flügge UI (1989) Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar-lysosomal compartment. EMBO J 8: 2835–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Marten I (2011) TPC1-SV channels gain shape. Mol Plant 4: 428–441 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Sauer N, Neuhaus HE (2015) Sugar transport across the plant vacuolar membrane: nature and regulation of carrier proteins. Curr Opin Plant Biol 25: 63–70 [DOI] [PubMed] [Google Scholar]
- Hirschi K. (2001) Vacuolar H+/Ca2+ transport: who’s directing the traffic? Trends Plant Sci 6: 100–104 [DOI] [PubMed] [Google Scholar]
- Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ (2000) Expression of Arabidopsis CAX2 in tobacco: altered metal accumulation and increased manganese tolerance. Plant Physiol 124: 125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Zhen RG, Cunningham KW, Rea PA, Fink GR (1996) CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA 93: 8782–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Yamaji N, Chen Z, Ma JF (2012) A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J 69: 857–867 [DOI] [PubMed] [Google Scholar]
- Huang XY, Deng F, Yamaji N, Pinson SRM, Fujii-Kashino M, Danku J, Douglas A, Guerinot ML, Salt DE, Ma JF (2016) A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat Commun 7: 12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurth MA, Suh SJ, Kretzschmar T, Geis T, Bregante M, Gambale F, Martinoia E, Neuhaus HE (2005) Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiol 137: 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriolo E, Na G, Ellis D, Salt DE, Banks JA (2010) A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell 22: 2045–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y (2010) Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant Cell Physiol 51: 302–311 [DOI] [PubMed] [Google Scholar]
- Jaquinod M, Villiers F, Kieffer-Jaquinod S, Hugouvieux V, Bruley C, Garin J, Bourguignon J (2007) A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol Cell Proteomics 6: 394–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KD, Herman EM, Chrispeels MJ (1989) An abundant, highly conserved tonoplast protein in seeds. Plant Physiol 91: 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossier M, Kroniewicz L, Dalmas F, Le Thiec D, Ephritikhine G, Thomine S, Barbier-Brygoo H, Vavasseur A, Filleur S, Leonhardt N (2010) The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J 64: 563–576 [DOI] [PubMed] [Google Scholar]
- Jung B, Ludewig F, Schulz A, Meißner G, Wöstefeld N, Flügge UI, Pommerrenig B, Wirsching P, Sauer N, Koch W, et al. (2015) Identification of the transporter responsible for sucrose accumulation in sugar beet taproots. Nat Plants 1: 14001. [DOI] [PubMed] [Google Scholar]
- Kaiser G, Heber U (1984) Sucrose transport into vacuoles isolated from barley mesophyll protoplasts. Planta 161: 562–568 [DOI] [PubMed] [Google Scholar]
- Kaiser G, Martinoia E, Wiemken A (1982) Rapid appearance of photosynthetic products in the vacuoles isolated from barley mesophyll protoplasts by a new fast method. Z Pflanzenphysiol 107: 103–113 [Google Scholar]
- Kim SA, Punshon T, Lanzirotti A, Li L, Alonso JM, Ecker JR, Kaplan J, Guerinot ML (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314: 1295–1298 [DOI] [PubMed] [Google Scholar]
- Kiyosue T, Abe H, Yamaguchi-Shinozaki K, Shinozaki K (1998) ERD6, a cDNA clone for an early dehydration-induced gene of Arabidopsis, encodes a putative sugar transporter. Biochim Biophys Acta 1370: 187–191 [DOI] [PubMed] [Google Scholar]
- Klaumann S, Nickolaus SD, Fürst SH, Starck S, Schneider S, Neuhaus HE, Trentmann O (2011) The tonoplast copper transporter COPT5 acts as an exporter and is required for interorgan allocation of copper in Arabidopsis thaliana. New Phytol 192: 393–404 [DOI] [PubMed] [Google Scholar]
- Klein M, Perfus-Barbeoch L, Frelet A, Gaedeke N, Reinhardt D, Mueller-Roeber B, Martinoia E, Forestier C (2003) The plant multidrug resistance ABC transporter AtMRP5 is involved in guard cell hormonal signalling and water use. Plant J 33: 119–129 [DOI] [PubMed] [Google Scholar]
- Klein M, Weissenböck G, Dufaud A, Gaillard C, Kreuz K, Martinoia E (1996) Different energization mechanisms drive the vacuolar uptake of a flavonoid glucoside and a herbicide glucoside. J Biol Chem 271: 29666–29671 [DOI] [PubMed] [Google Scholar]
- Klemens PAW, Patzke K, Deitmer J, Spinner L, Le Hir R, Bellini C, Bedu M, Chardon F, Krapp A, Neuhaus HE (2013) Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol 163: 1338–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovermann P, Meyer S, Hörtensteiner S, Picco C, Scholz-Starke J, Ravera S, Lee Y, Martinoia E (2007) The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J 52: 1169–1180 [DOI] [PubMed] [Google Scholar]
- Krämer U, Clemens S (2006) Functions and homeostasis of zinc, copper, and nickel in plants. In Tamás M, Martinoia E, eds, Molecular Biology of Metal Homeostasis and Detoxification: From Microbes to Man. Springer, Berlin, pp 215–272 [Google Scholar]
- Krebs M, Beyhl D, Görlich E, Al-Rasheid KAS, Marten I, Stierhof YD, Hedrich R, Schumacher K (2010) Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc Natl Acad Sci USA 107: 3251–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel A, Andrés Z, Medzihradszky A, Krüger F, Scholl S, Delang S, Patir-Nebioglu MG, Gute G, Yang H, Murphy AS, et al. (2015) Job sharing in the endomembrane system: vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell 27: 3383–3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquar V, Lelièvre F, Bolte S, Hamès C, Alcon C, Neumann D, Vansuyt G, Curie C, Schröder A, Krämer U, et al. (2005) Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J 24: 4041–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Xu D, Halkier BA, Nour-Eldin HH (2017) Advances in methods for identification and characterization of plant transporter function. J Exp Bot 68: 4045–4056 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Geisler MJ, Jones CA, Williams KM, Cancel JD (2005) ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J 41: 353–363 [DOI] [PubMed] [Google Scholar]
- Latz A, Becker D, Hekman M, Müller T, Beyhl D, Marten I, Eing C, Fischer A, Dunkel M, Bertl A, et al. (2007) TPK1, a Ca2+-regulated Arabidopsis vacuole two-pore K+ channel is activated by 14-3-3 proteins. Plant J 52: 449–459 [DOI] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126: 1109–1120 [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EAC, Webb AAR, Manison NF, Brownlee C, Skepper JN, Chen J, Prestwich GD, Brearley CA (2003) Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc Natl Acad Sci USA 100: 10091–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen OS, McVey Ward D, Kaplan J (2001) CCC1 is a transporter that mediates vacuolar iron storage in yeast. J Biol Chem 276: 29515–29519 [DOI] [PubMed] [Google Scholar]
- Li ZS, Lu YP, Zhen RG, Szczypka M, Thiele DJ, Rea PA (1997) A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc Natl Acad Sci USA 94: 42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SM, Tsai JY, Hsiao CD, Huang YT, Chiu CL, Liu MH, Tung JY, Liu TH, Pan RL, Sun YJ (2012) Crystal structure of a membrane-embedded H+-translocating pyrophosphatase. Nature 484: 399–403 [DOI] [PubMed] [Google Scholar]
- Liu R, Li B, Qin G, Zhang Z, Tian S (2017) Identification and functional characterization of a tonoplast dicarboxylate transporter in tomato (Solanum lycopersicum). Front Plant Sci 8: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YP, Li ZS, Drozdowicz YM, Hörtensteiner S, Martinoia E, Rea PA (1998) AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with Atmrp1. Plant Cell 10: 267–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J. (2011) Auxin conjugates: their role for plant development and in the evolution of land plants. J Exp Bot 62: 1757–1773 [DOI] [PubMed] [Google Scholar]
- Maeshima M. (1992) Characterization of the major integral protein of vacuolar membrane. Plant Physiol 98: 1248–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima M. (2000) Vacuolar H+-pyrophosphatase. Biochim Biophys Acta 1465: 37–51 [DOI] [PubMed] [Google Scholar]
- Maeshima M, Yoshida S (1989) Purification and properties of vacuolar membrane proton-translocating inorganic pyrophosphatase from mung bean. J Biol Chem 264: 20068–20073 [PubMed] [Google Scholar]
- Marigo G, Bouyssou H, Laborie D (1988) Evidence for a malate transport into vacuoles isolated from Catharanthus roseus cells. Bot Acta 101: 187–191 [Google Scholar]
- Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Routaboul JM, Debeaujon I, Klein M (2007) The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 19: 2023–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Flügge UI, Kaiser G, Heber U, Heldt HW (1985) Energy-dependent uptake of malate into vacuoles isolated from barley mesophyll protoplasts. Biochim Biophys Acta 806: 311–319 [Google Scholar]
- Martinoia E, Grill E, Tommasini R, Kreuz K, Amrhein N (1993) ATP-dependent glutathione S-conjugate export pump in the vacuolar membrane of plants. Nature 364: 247–249 [Google Scholar]
- Martinoia E, Heck U, Wiemken A (1981) Vacuoles as storage compartments for nitrate in barley leaves. Nature 289: 292–294 [Google Scholar]
- Martinoia E, Maeshima M, Neuhaus HE (2007) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58: 83–102 [DOI] [PubMed] [Google Scholar]
- Martinoia E, Meyer S, De Angeli A, Nagy R (2012) Vacuolar transporters in their physiological context. Annu Rev Plant Biol 63: 183–213 [DOI] [PubMed] [Google Scholar]
- Martinoia E, Vogt E, Rentsch D, Amrhein N (1991) Functional reconstitution of the malate carrier of barley mesophyll vacuoles in liposomes. Biochim Biophys Acta 1062: 271–278 [DOI] [PubMed] [Google Scholar]
- Matern U, Heller W, Himmelspach K (1983) Conformational changes of apigenin 7-O-(6-O-malonylglucoside), a vacuolar pigment from parsley, with solvent composition and proton concentration. Eur J Biochem 133: 439–448 [DOI] [PubMed] [Google Scholar]
- Matile P. (1978) Biochemistry and function of vacuoles. Annu Rev Plant Physiol Plant Mol Biol 29: 193–213 [Google Scholar]
- Matile P. (1980) The mustard oil bomb: compartmentation of the myrosinase system. Biochem Physiol Pflanz 175: 722–731 [Google Scholar]
- Matile P. (1982) Vacuoles come of age. Physiol Veg 20: 303–310 [Google Scholar]
- Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L (2015) Aquaporins in plants. Physiol Rev 95: 1321–1358 [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59: 595–624 [DOI] [PubMed] [Google Scholar]
- Mazhab-Jafari MT, Rohou A, Schmidt C, Bueler SA, Benlekbir S, Robinson CV, Rubinstein JL (2016) Atomic model for the membrane-embedded VO motor of a eukaryotic V-ATPase. Nature 539: 118–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, Cheng NH, Zhao J, Park S, Escareno RA, Pittman JK, Hirschi KD (2009) Root development under metal stress in Arabidopsis thaliana requires the H+/cation antiporter CAX4. New Phytol 183: 95–105 [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Jobe TO, Hauser F, Schroeder JI (2011) Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr Opin Plant Biol 14: 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menguer PK, Farthing E, Peaston KA, Ricachenevsky FK, Fett JP, Williams LE (2013) Functional analysis of the rice vacuolar zinc transporter OsMTP1. J Exp Bot 64: 2871–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Hannibal L, Martins S, Martinelli L, Amir H, Lebrun M, Thomine S (2014) The metal transporter PgIREG1 from the hyperaccumulator Psychotria gabriellae is a candidate gene for nickel tolerance and accumulation. J Exp Bot 65: 1551–1564 [DOI] [PubMed] [Google Scholar]
- Meyer S, Scholz-Starke J, De Angeli A, Kovermann P, Burla B, Gambale F, Martinoia E (2011) Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J 67: 247–257 [DOI] [PubMed] [Google Scholar]
- Miettinen K, Dong L, Navrot N, Schneider T, Burlat V, Pollier J, Woittiez L, van der Krol S, Lugan R, Ilc T, et al. (2014) The seco-iridoid pathway from Catharanthus roseus. Nat Commun 5: 3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyadate H, Adachi S, Hiraizumi A, Tezuka K, Nakazawa N, Kawamoto T, Katou K, Kodama I, Sakurai K, Takahashi H, et al. (2011) OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol 189: 190–199 [DOI] [PubMed] [Google Scholar]
- Momonoi K, Yoshida K, Mano S, Takahashi H, Nakamori C, Shoji K, Nitta A, Nishimura M (2009) A vacuolar iron transporter in tulip, TgVit1, is responsible for blue coloration in petal cells through iron accumulation. Plant J 59: 437–447 [DOI] [PubMed] [Google Scholar]
- Morel M, Crouzet J, Gravot A, Auroy P, Leonhardt N, Vavasseur A, Richaud P (2009) AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol 149: 894–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Shitan N, Sawada K, Van Montagu MC, Inze D, Rischer H, Goossens A, Oksman-Caldentey KM, Moriyama Y, Yazaki K (2009) Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc Natl Acad Sci USA 106: 2447–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J, Baxter IR, Lee J, Li L, Lahner B, Grotz N, Kaplan J, Salt DE, Guerinot ML (2009) The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell 21: 3326–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy R, Grob H, Weder B, Green P, Klein M, Frelet-Barrand A, Schjoerring JK, Brearley C, Martinoia E (2009) The Arabidopsis ATP-binding cassette protein AtMRP5/AtABCC5 is a high affinity inositol hexakisphosphate transporter involved in guard cell signaling and phytate storage. J Biol Chem 284: 33614–33622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakmann B (1976) Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260: 799–802 [DOI] [PubMed] [Google Scholar]
- Nelson N, Taiz L (1989) The evolution of H+-ATPases. Trends Biochem Sci 14: 113–116 [DOI] [PubMed] [Google Scholar]
- Oikawa A, Matsuda F, Kikuyama M, Mimura T, Saito K (2011) Metabolomics of a single vacuole reveals metabolic dynamism in an alga Chara australis. Plant Physiol 157: 544–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleski N, Mahdavi P, Bennett AB (1987) Transport properties of the tomato fruit tonoplast. II. Citrate transport. Plant Physiol 84: 997–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz DF, Ruscitti T, McCue KF, Ow DW (1995) Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J Biol Chem 270: 4721–4728 [DOI] [PubMed] [Google Scholar]
- Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee TG, Martinoia E, Lee Y (2012) The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J 69: 278–288 [DOI] [PubMed] [Google Scholar]
- Payne RME, Xu D, Foureau E, Teto Carqueijeiro MI, Oudin A, Bernonville TD, Novak V, Burow M, Olsen CE, Jones DM, et al. (2017) An NPF transporter exports a central monoterpene indole alkaloid intermediate from the vacuole. Nat Plants 3: 16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payyavula RS, Tay KH, Tsai CJ, Harding SA (2011) The sucrose transporter family in Populus: the importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. Plant J 65: 757–770 [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434: 404–408 [DOI] [PubMed] [Google Scholar]
- Pittman JK. (2005) Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytol 167: 733–742 [DOI] [PubMed] [Google Scholar]
- Pittman JK. (2011) Vacuolar Ca2+ uptake. Cell Calcium 50: 139–146 [DOI] [PubMed] [Google Scholar]
- Pittman JK. (2012) Multiple transport pathways for mediating intracellular pH homeostasis: the contribution of H+/ion exchangers. Front Plant Sci 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RJ. (1978) Energy coupling for membrane transport. Annu Rev Plant Physiol Plant Mol Biol 29: 437–460 [Google Scholar]
- Poschet G, Hannich B, Raab S, Jungkunz I, Klemens PAW, Krueger S, Wic S, Neuhaus HE, Büttner M (2011) A novel Arabidopsis vacuolar glucose exporter is involved in cellular sugar homeostasis and affects the composition of seed storage compounds. Plant Physiol 157: 1664–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranocha P, Dima O, Nagy R, Felten J, Corratgé-Faillie C, Novák O, Morreel K, Lacombe B, Martinez Y, Pfrunder S, et al. (2013) Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat Commun 4: 2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rataboul P, Alibert G, Boller T, Boudet AM (1985) Intracellular transport and vacuolar accumulation of O-coumaric acid glucoside in Melitolus alba mesophyll cell protoplasts. Biochim Biophys Acta 816: 25–36 [Google Scholar]
- Rea PA, Sanders D (1987) Tonoplast energization: 2 H+ pumps, one membrane. Physiol Plant 71: 131–141 [Google Scholar]
- Renaudin JP, Guern J (1987) Ajmalicine transport into vacuoles isolated from Catharanthus cells. In Marin B, ed, Plant Vacuoles. Springer, New York, pp 339–347 [Google Scholar]
- Rentsch D, Martinoia E (1991) Citrate transport into barley mesophyll vacuoles: comparison with malate-uptake activity. Planta 184: 532–537 [DOI] [PubMed] [Google Scholar]
- Rienmüller F, Beyhl D, Lautner S, Fromm J, Al-Rasheid KAS, Ache P, Farmer EE, Marten I, Hedrich R (2010) Guard cell-specific calcium sensitivity of high density and activity SV/TPC1 channels. Plant Cell Physiol 51: 1548–1554 [DOI] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1992) Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J 11: 4705–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D, Lawson T (2016) Rethinking guard cell metabolism. Plant Physiol 172: 1371–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian V, Kim Y, Poole RJ, Rea PA (1992) Molecular cloning and sequence of cDNA encoding the pyrophosphate-energized vacuolar membrane proton pump of Arabidopsis thaliana. Proc Natl Acad Sci USA 89: 1775–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G, Honsbein A, Meda AR, Kirchner S, Wipf D, von Wirén N (2006) AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J Biol Chem 281: 25532–25540 [DOI] [PubMed] [Google Scholar]
- Schmitt R, Sandermann H (1982) Specific localization of b-D-glucoside conjugates of 2,4-dichlorophenoxyacetic acid in soybean vacuoles. Z Naturforsch C 37: 772–777 [Google Scholar]
- Schneider T, Persson DP, Husted S, Schellenberg M, Gehrig P, Lee Y, Martinoia E, Schjoerring JK, Meyer S (2013) A proteomics approach to investigate the process of Zn hyperaccumulation in Noccaea caerulescens (J & C. Presl) F.K. Meyer. Plant J 73: 131–142 [DOI] [PubMed] [Google Scholar]
- Schneider T, Schellenberg M, Meyer S, Keller F, Gehrig P, Riedel K, Lee Y, Eberl L, Martinoia E (2009) Quantitative detection of changes in the leaf-mesophyll tonoplast proteome in dependency of a cadmium exposure of barley (Hordeum vulgare L.) plants. Proteomics 9: 2668–2677 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hedrich R, Fernandez JM (1984) Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature 312: 361–362 [Google Scholar]
- Schulz A, Beyhl D, Marten I, Wormit A, Neuhaus E, Poschet G, Büttner M, Schneider S, Sauer N, Hedrich R (2011) Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J 68: 129–136 [DOI] [PubMed] [Google Scholar]
- Schulze WX, Schneider T, Starck S, Martinoia E, Trentmann O (2012) Cold acclimation induces changes in Arabidopsis tonoplast protein abundance and activity and alters phosphorylation of tonoplast monosaccharide transporters. Plant J 69: 529–541 [DOI] [PubMed] [Google Scholar]
- Schumacher K. (2014) pH in the plant endomembrane system: an import and export business. Curr Opin Plant Biol 22: 71–76 [DOI] [PubMed] [Google Scholar]
- Schumacher K, Krebs M (2010) The V-ATPase: small cargo, large effects. Curr Opin Plant Biol 13: 724–730 [DOI] [PubMed] [Google Scholar]
- Schumacher K, Krebs M (2011) V-ATPases: rotary engines for transport and traffic. In Geisler M, Venema K, eds, Transporters and Pumps in Plant Signaling. Signaling and Communication in Plants, Vol 7 Springer, Berlin, pp 293–312 [Google Scholar]
- Schumaker KS, Sze H (1986) Calcium transport into the vacuole of oat roots: characterization of H+/Ca2+ exchange activity. J Biol Chem 261: 12172–12178 [PubMed] [Google Scholar]
- Segami S, Nakanishi Y, Sato MH, Maeshima M (2010) Quantification, organ-specific accumulation and intracellular localization of type II H+-pyrophosphatase in Arabidopsis thaliana. Plant Cell Physiol 51: 1350–1360 [DOI] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C (1992) Cloning and expression in yeast of a plant potassium ion transport system. Science 256: 663–665 [DOI] [PubMed] [Google Scholar]
- Shi J, Wang H, Schellin K, Li B, Faller M, Stoop JM, Meeley RB, Ertl DS, Ranch JP, Glassman K (2007) Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nat Biotechnol 25: 930–937 [DOI] [PubMed] [Google Scholar]
- Shigaki T, Pittman JK, Hirschi KD (2003) Manganese specificity determinants in the Arabidopsis metal/H+ antiporter CAX2. J Biol Chem 278: 6610–6617 [DOI] [PubMed] [Google Scholar]
- Shim D, Kim S, Choi YI, Song WY, Park J, Youk ES, Jeong SC, Martinoia E, Noh EW, Lee Y (2013) Transgenic poplar trees expressing yeast cadmium factor 1 exhibit the characteristics necessary for the phytoremediation of mine tailing soil. Chemosphere 90: 1478–1486 [DOI] [PubMed] [Google Scholar]
- Shitan N, Yazaki K (2013) New insights into the transport mechanisms in plant vacuoles. Int Rev Cell Mol Biol 305: 383–433 [DOI] [PubMed] [Google Scholar]
- Shoji T, Inai K, Yazaki Y, Sato Y, Takase H, Shitan N, Yazaki K, Goto Y, Toyooka K, Matsuoka K, et al. (2009) Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant Physiol 149: 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair SA, Krämer U (2012) The zinc homeostasis network of land plants. Biochim Biophys Acta 1823: 1553–1567 [DOI] [PubMed] [Google Scholar]
- Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, et al. (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA 107: 21187–21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WY, Sohn EJ, Martinoia E, Lee YJ, Yang YY, Jasinski M, Forestier C, Hwang I, Lee Y (2003) Engineering tolerance and accumulation of lead and cadmium in transgenic plants. Nat Biotechnol 21: 914–919 [DOI] [PubMed] [Google Scholar]
- Song WY, Yamaki T, Yamaji N, Ko D, Jung KH, Fujii-Kashino M, An G, Martinoia E, Lee Y, Ma JF (2014) A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci USA 111: 15699–15704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack D, Meurer B, Weissenbock G (1982) Tissue-specific kinetics of flavonoid accumulation in primary leaves of rye (Secale cereale L). Z Pflanzenphysiol 108: 131–141 [Google Scholar]
- Szczypka MS, Wemmie JA, Moye-Rowley WS, Thiele DJ (1994) A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J Biol Chem 269: 22853–22857 [PubMed] [Google Scholar]
- Sze H. (1985) H+-translocating ATPases: advances using membrane vesicles. Annu Rev Plant Physiol Plant Mol Biol 36: 175–208 [Google Scholar]
- Takanashi K, Yamada Y, Sasaki T, Yamamoto Y, Sato F, Yazaki K (2017) A multidrug and toxic compound extrusion transporter mediates berberine accumulation into vacuoles in Coptis japonica. Phytochemistry 138: 76–82 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Fujiwara T, Tomioka R, Krämer U, Kawachi M, Maeshima M (2015) Characterization of the histidine-rich loop of Arabidopsis vacuolar membrane zinc transporter AtMTP1 as a sensor of zinc level in the cytosol. Plant Cell Physiol 56: 510–519 [DOI] [PubMed] [Google Scholar]
- Tohge T, Ramos MS, Nunes-Nesi A, Mutwil M, Giavalisco P, Steinhauser D, Schellenberg M, Willmitzer L, Persson S, Martinoia E, et al. (2011) Toward the storage metabolome: profiling the barley vacuole. Plant Physiol 157: 1469–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasini R, Vogt E, Fromenteau M, Hörtensteiner S, Matile P, Amrhein N, Martinoia E (1998) An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J 13: 773–780 [DOI] [PubMed] [Google Scholar]
- Tophof S, Martinoia E, Kaiser G, Hartung W, Amrhein N (1989) Compartmentation and transport of 1-aminocyclopropane-1-carboxylic acid and N-malonyl-1-aminocyclopropane-1-carboxylic acid in barley and wheat mesophyll cells and protoplasts. Physiol Plant 75: 333–339 [Google Scholar]
- Ueno D, Milner MJ, Yamaji N, Yokosho K, Koyama E, Clemencia Zambrano M, Kaskie M, Ebbs S, Kochian LV, Ma JF (2011) Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J 66: 852–862 [DOI] [PubMed] [Google Scholar]
- Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF (2010) Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci USA 107: 16500–16505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderstraeten L, Van Der Straeten D (2017) Accumulation and transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: current status, considerations for future research and agronomic applications. Front Plant Sci 8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij W, Spelt C, Di Sansebastiano GP, Vermeer J, Reale L, Ferranti F, Koes R, Quattrocchio F (2008) An H+ P-ATPase on the tonoplast determines vacuolar pH and flower colour. Nat Cell Biol 10: 1456–1462 [DOI] [PubMed] [Google Scholar]
- Vincent TR, Avramova M, Canham J, Higgins P, Bilkey N, Mugford ST, Pitino M, Toyota M, Gilroy S, Miller AJ, et al. (2017) Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell 29: 1460–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinothkumar KR, Montgomery MG, Liu S, Walker JE (2016) Structure of the mitochondrial ATP synthase from Pichia angusta determined by electron cryo-microscopy. Proc Natl Acad Sci USA 113: 12709–12714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Fecht-Bartenbach J, Bogner M, Dynowski M, Ludewig U (2010) CLC-b-mediated NO−3/H+ exchange across the tonoplast of Arabidopsis vacuoles. Plant Cell Physiol 51: 960–968 [DOI] [PubMed] [Google Scholar]
- Wagner GJ, Siegelman HW (1975) Large-scale isolation of intact vacuoles and isolation of chloroplasts from protoplasts of mature plant tissues. Science 190: 1298–1299 [Google Scholar]
- Walker JE. (2013) The ATP synthase: The understood, the unceritude and the unknown. Biochim Soc Trans. 41: 1–16 [DOI] [PubMed] [Google Scholar]
- Wang L, Wu X, Liu Y, Qiu QS (2015) AtNHX5 and AtNHX6 control cellular K+ and pH homeostasis in Arabidopsis: three conserved acidic residues are essential for K+ transport. PLoS ONE 10: e0144716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege S, De Angeli A, Droillard MJ, Kroniewicz L, Merlot S, Cornu D, Gambale F, Martinoia E, Barbier-Brygoo H, Thomine S, et al. (2014) Phosphorylation of the vacuolar anion exchanger AtCLCa is required for the stomatal response to abscisic acid. Sci Signal 7: ra65. [DOI] [PubMed] [Google Scholar]
- Whiteman SA, Serazetdinova L, Jones AM, Sanders D, Rathjen J, Peck SC, Maathuis FJ (2008) Identification of novel proteins and phosphorylation sites in a tonoplast enriched membrane fraction of Arabidopsis thaliana. Proteomics 8: 3536–3547 [DOI] [PubMed] [Google Scholar]
- Wingenter K, Schulz A, Wormit A, Wic S, Trentmann O, Hoermiller II, Heyer AG, Marten I, Hedrich R, Neuhaus HE (2010) Increased activity of the vacuolar monosaccharide transporter TMT1 alters cellular sugar partitioning, sugar signaling, and seed yield in Arabidopsis. Plant Physiol 154: 665–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenter K, Trentmann O, Winschuh I, Hörmiller II, Heyer AG, Reinders J, Schulz A, Geiger D, Hedrich R, Neuhaus HE (2011) A member of the mitogen-activated protein 3-kinase family is involved in the regulation of plant vacuolar glucose uptake. Plant J 68: 890–900 [DOI] [PubMed] [Google Scholar]
- Wisman E, Hartmann U, Sagasser M, Baumann E, Palme K, Hahlbrock K, Saedler H, Weisshaar B (1998) Knock-out mutants from an En-1 mutagenized Arabidopsis thaliana population generate phenylpropanoid biosynthesis phenotypes. Proc Natl Acad Sci USA 95: 12432–12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormit A, Traub M, Flörchinger M, Neuhaus HE, Möhlmann T (2004) Characterization of three novel members of the Arabidopsis thaliana equilibrative nucleoside transporter (ENT) family. Biochem J 383: 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormit A, Trentmann O, Feifer I, Lohr C, Tjaden J, Meyer S, Schmidt U, Martinoia E, Neuhaus HE (2006) Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. Plant Cell 18: 3476–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZY, Lee KH, Dong T, Jeong JC, Jin JB, Kanno Y, Kim DH, Kim SY, Seo M, Bressan RA, et al. (2012) A vacuolar β-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell 24: 2184–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Osakabe Y, Mizoi J, Nakashima K, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K (2010) Functional analysis of an Arabidopsis thaliana abiotic stress-inducible facilitated diffusion transporter for monosaccharides. J Biol Chem 285: 1138–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Wang X, Hu T, Zhang F, Wang B, Li C, Yang T, Li H, Lu Y, Giovannoni JJ, et al. (2017) An InDel in the promoter of Al-ACTIVATED MALATE TRANSPORTER9 selected during tomato domestication determines fruit malate contents and aluminum tolerance. Plant Cell 29: 2249–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosho K, Yamaji N, Mitani-Ueno N, Shen RF, Ma JF (2016) An aluminum-inducible IREG gene is required for internal detoxification of aluminum in buckwheat. Plant Cell Physiol 57: 1169–1178 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kawachi M, Mori M, Maeshima M, Kondo M, Nishimura M, Kondo T (2005) The involvement of tonoplast proton pumps and Na+(K+)/H+ exchangers in the change of petal color during flower opening of morning glory, Ipomoea tricolor cv. Heavenly Blue. Plant Cell Physiol 46: 407–415 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Miki N, Mononoi K, Kawachi M, Katou K, Okazaki Y, Uozumi N, Maeshima M, Kondo T (2009) Synchrony between flower opening and petal-color change from red to blue in morning glory, Ipomoea tricolor cv. Heavenly Blue. Proc Jpn Acad Ser B Phys Biol Sci 85: 187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao FG, Tang RJ, Yu Y, Song J, Wang Y, Li L, Luan S (2017) Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc Natl Acad Sci USA 114: E2036–E2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Baetz U, Krügel U, Martinoia E, De Angeli A (2013) Identification of a probable pore-forming domain in the multimeric vacuolar anion channel AtALMT9. Plant Physiol 163: 830–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu YH, Yi HY, Gong JM (2012) Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J 72: 400–410 [DOI] [PubMed] [Google Scholar]
- Zhao J, Dixon RA (2009) MATE transporters facilitate vacuolar uptake of epicatechin 3′-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 21: 2323–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Huhman D, Shadle G, He XZ, Sumner LW, Tang Y, Dixon RA (2011) MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula. Plant Cell 23: 1536–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]