Abstract
The level of rescue of clock function in genetically arrhythmic Drosophila melanogaster hosts using interspecific clock gene transformation was used to study the putative intermolecular coevolution between interacting clock proteins. Among them PER and TIM are the two important negative regulators of the circadian clock feedback loop. We transformed either the D. pseudoobscura per or tim transgenes into the corresponding arrhythmic D. melanogaster mutant (per01 or tim01) and observed >50% rhythmicity but the period of activity rhythm was either longer (D. pseudoobscura-per) or shorter than 24 h (D. pseudoobscura-tim) compared to controls. By introducing both transgenes simultaneously into double mutants, we observed that the period of the activity rhythm was rescued by the pair of hemizygous transgenes (~24 h). These flies also showed a more optimal level of temperature compensation for the period. Under LD 12:12 these flies have a D. pseudoobscura like activity profile with the absence of morning anticipation as well as a very prominent earlier evening peak of activity rhythm. These observation are consistent with the view that TIM and PER form a heterospecific coevolved module at least for the circadian period of activity rhythms. However the strength of rhythmicity was reduced by having both transgenes present, so while evidence for a coevolution between PER and TIM is observed for some characters it is not for others.
Abbreviations: bp, base pair; CLK, clock; CRY, cryptochrome; CYC, cycle; D., Drosophila; D. mel, D. melanogaster; D. ps, D. pseudoobscura; DAM, Drosophila activity monitoring; DD, constant darkness; DD, continuous dark; DNA, deoxyribonucleic acid; E, evening; EP, evening peak; g, grams; h, hour; H, hour; L, litre; LD, light dark cycle; M, morning; min, minute; MP, morning peak; n.a., not available; ns, not significant; PAS, Per Ant Sim; PCR, polymerase chain reaction; per, period; Pers comm, personal communication; RNA, ribonucleic acid; SD, standard deviation; sec, second; SEM, standard error of the mean; tim, timeless; TTFL, transcriptional translational feedback loop; ZT, Zeitgeber time
Keywords: Drosophila, Circadian rhythms, Period, Timeless, Coevolution, D. pseudoobscura
Highlights
-
•
To understand the mechanism of intermolecular coevolution between period and its partner timeless, transgenes from D. pseudoobscura were introduced into D. melanogaster null mutants.
-
•
The single D. melanogaster transformants containing either per or tim from D. pseudoobscura displayed less than 50% rhythmicity and the period of locomotor activity rhythms was also either very long (26-31h for perps) or very short (21-22h for timps).
-
•
The two heterospecific transgenes together in the double mutant D. melanogaster flies could not show a significant increase in levels of rhythmicity however a significant improvement in the average period of locomotor activity was obtained (~24h).
-
•
Temperature compensation in the period of locomotor activity was also improved in these double tranformants. The Western blots and qPCR confirmed the expression of these transgenes.
1. Introduction
The molecular basis of the circadian clock has been extensively studied in several model species and has led to the idea that the general mechanism that underlies the clock is conserved. It consists of interlocked auto-regulatory feedback loops that function through the transcription/translation of positive and negative elements (Bell-Pedersen et al., 2005). In Drosophila the basic model for rhythm generation in the pacemaker cells involves several core genes, period (per), timeless (tim), Clock (Clk), cycle (cyc) and cryptochrome (cry). The interaction of the protein products of these genes with associated kinases and phosphatases leads to pace-setting of the clock by regulating the timing of nuclear entry and inter-molecular interactions (Peschel and Helfrich-Förster, 2011; Zheng and Sehgal, 2008).
D. melanogaster has been transformed with different species clock genes and the level of rescue of arrhythmic null mutants has been used as a tool to study interspecific functional conservation and species-specific characters (Petersen et al., 1988; Wheeler et al., n.d.; Peixoto et al., 1998; Tauber et al., 2003). PER and TIM are the two key clock proteins that mediate the negative limb of the circadian feedback loop. TIM binds to the PAS domain of PER (Hardin, 2011) and prevents its degradation (Kloss et al., 1998). Phylogenetic analysis of tim from D. virilis and D. hydei revealed that TIM, is more conserved than PER (Ousley et al., 1998). Ousley et al. (1998) also reported the first robust rescue of the tim01 mutant using a conspecific tim transgene and Nishinokubi et al. (2006) showed that the D. ananassae-tim transgene was also able to rescue behavioural rhythms of D. melanogaster tim01 mutants. In another study the same group induced the D. ananassae TIM protein in D. melanogaster tim01 transformants through heat shock and examined the behaviour of these flies (Nishinokubi et al., 2006). The level of TIM protein was increased initially by the application of heat shock and decreased after some time. Their results demonstrated that by applying this heat shock at different times of the day, these transgenic D. melanogaster tim01 flies became nocturnal, similar to wild-type D. ananassae. They also found that mating activity rhythms of the transformants were different from both parental species, suggesting that they are determined by different factors from those which control locomotor activity rhythms. Nevertheless, their results suggested that like per (Wheeler et al., n.d.; Tauber et al., 2003), tim might also play a role as a speciation gene and control some aspects of adaptive rhythmic behaviour.
While the transformation of interspecific clock genes into D. melanogaster hosts provides information on species-specific characteristics, the experimental paradigm usually involves introducing a single interspecific transgene. In one of these studies, phylogenetic analysis of the PER PAS interaction domain was correlated with the efficiency of per01 rescue (Piccin et al., 2000). Specifically, the Musca domestica per transgene gave better rescue that the D. pseudoobscura transgene even though Musca had a common ancestor with D. melanogaster much earlier than D. pseudoobscura, yet the phylogeny of the PER-PAS domain revealed that Musca lay closer to D. melanogaster than D. pseudoobscura (Piccin et al., 2000). As PER-PAS dimerises with TIM, these results may represent a coevolution of TIM with PER which might be reflected at the phylogenetic level in TIM sequences and functionally in rescue experiments. Consequently we asked whether the relatively poor rescue of D. melanogaster per01 rhythmicity by the D. pseudoobscura per transgene, which is about 50% but with longer periods of 27–28 h (Petersen et al., 1988; Peixoto et al., 1998), might be enhanced by performing a double interspecific transformation using per and tim transgenes from this species. Under a coevolution scenario we might expect an improved rescue if the PER-TIM interaction coevolves as a separate unit. However, introducing a second interspecific transgene could actually make rescue worse if the coevolving unit includes the positive regulators CLOCK and CYCLE, which also physically interact with PER-TIM (Lee et al., 1996).
Consequently, we co-inserted the D. pseudoobscura per and tim transgenes into the corresponding double mutant background per01; tim01 to test this coevolution scenario. In addition we also investigated whether any switching of D. melanogaster host circadian behavior to that of D. pseudoobscura occurred, as reported in D. pseudoobscura-per transformants for both species-specific locomotor and mating rhythms (Petersen et al., 1988; Tauber et al., 2003).
2. Materials and methods
2.1. The transgenic lines
The following D. melanogaster mutant lines were used 2A; per01 transformant strain carrying the 13.2 Kb D. melanogaster per transcription unit permel, (Citri et al., 1987) I26; per01 transformant strain carrying the D. pseudoobscura per coding sequence (perps) fused to the upstream non-coding melanogaster per sequences (Petersen et al., 1988).
t28s; tim01 transformant strain carrying the D. melanogaster tim transcription unit (timmel).
tim19, tim21, tim35; tim01 transformant strains carrying the D. pseudoobscura tim (timps) transcription unit with full length pseudoobscura TIM coding sequence attached to the melanogaster tim promoter. All of them were marked with w+.
These lines were genotyped using PCR for the presence of tim01, tim+, per+, per01 and also for the transgenes of timps and perps (Table 1). The location of the perps inserts was already known from Peixoto et al. (1998). Male flies carrying the timps transgenes, were crossed to double autosome balancer virgin females w; CyO/Sco; TM6b/MKRS to map the inserts, all of which were located on chromosome II. The transgenes; perps and timps were brought together following a series of crosses (see Supplementary Fig. 1) to obtain the homozygotes per01; tim01, timps; perps.
Table 1.
Primers used for the genotyping of the transgenic flies.
| No. | Primer name | Primer sequence | Annealing temperature |
|---|---|---|---|
| 1. | DmTim0 F | GCTCATCGCTTTTCATATGTT | 57 |
| 2. | DmTim R | AGGATGTGATTGGTAACCAC | 57 |
| 3. | DmPer0 F | TACCACCACGAGGACCTCTC | 57 |
| 4. | DmPer R | GATGGTGTCCGACGACAAAT | 57 |
| 5. | Pseudoobscura per F | ACCACCACGATGACCTCCCC | 59 |
| 6. | Pseudoobscura per R | TTGTTCTGCAACTCCTCCGCG | 59 |
| 7. | Pseudoobscura tim F | ACATACCGGAAACGCACGGG | 59 |
| 8. | Pseudoobscura tim R | CTTGTAGATCAGCGCGATCAAC | 59 |
2.2. Phylogenetic analysis
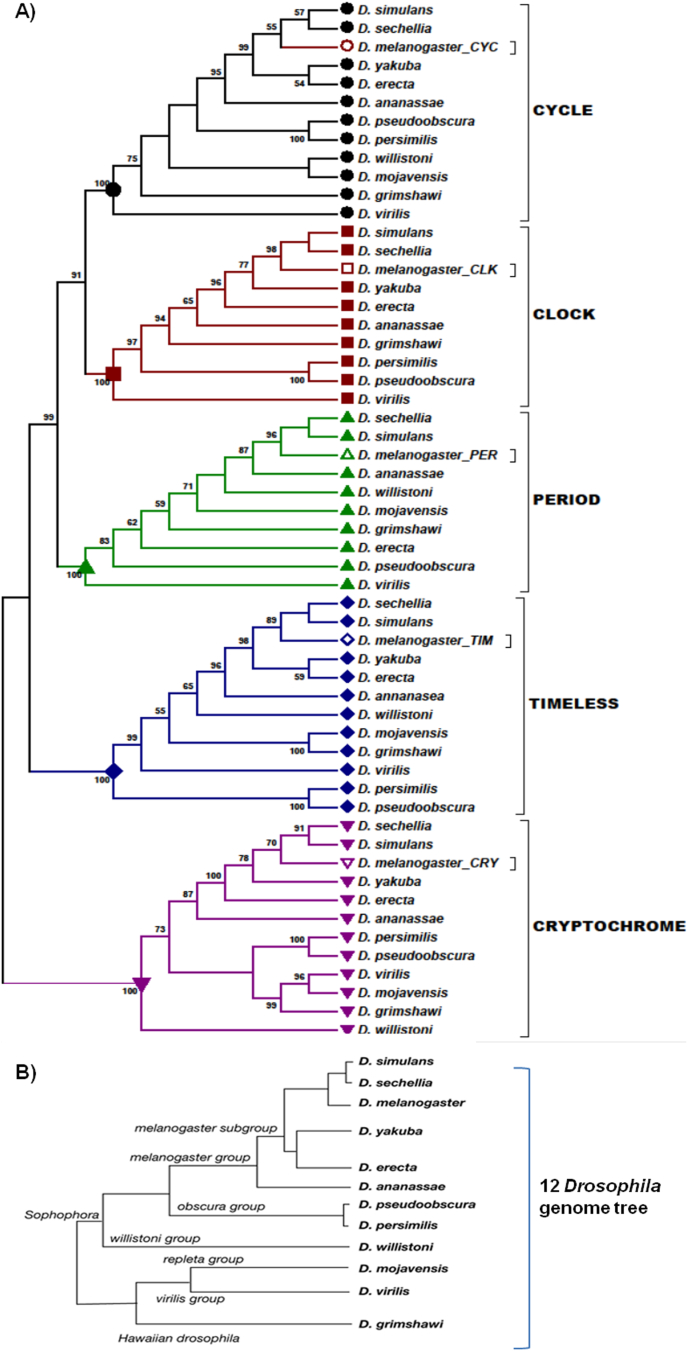
To infer the phylogenetic tree the protein sequences of PER, TIM, CLK, CYC and CRY of 12 Drosophila species (melanogaster, pseudoobscura, sechellia, virilis, simulans, yakuba, ananassae, willistoni, persimilis, erecta, mojavensis, grimshawi) were downloaded in FASTA format from FlyBase database (http://flybase.org/blast/checkJobStatus.html). Due to unavailability of complete Clock gene sequences in FlyBase, the sequences were collected from NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). To filter out the false positives, the resultant hits with >70%query coverage and identity were collected and analysed. To construct the phylogenetic tree, the downloaded sequences from the 5 genes were aligned in CLUSTALW multiple sequence alignments implemented in BioEdit (Hall, 1999). The nucleotide polymorphisms and variations were investigated in BioEdit. The phylogenetic analysis was performed by constructing the un-rooted Neighbor-Joining tree with 1000 bootstrap replicates implemented in MEGA5 (Tamura et al., 2011). The p-distance method was used to compute the evolutionary distances.
2.3. Behavioural analysis
Circadian locomotor activity of flies was recorded in Trikinetics monitors (Waltham, Ma, USA). Individual male flies were loaded into glass tubes containing sugar food. One end of the tube was closed with a cap and other end with cotton plug. Behavioural analysis was performed for all strain both at 25 °C and 18 °C. Initially the flies were kept for three to four days in 12:12 light/dark cycles (LD12:12) and in (constant darkness) DD for a further 7–10 days. The activity events were arranged into 30 min bins and CLEAN, a high resolution spectral analysis was used to obtain the free-running DD period, in addition to autocorrelation and actograms of individual flies (Rosato and Kyriacou, 2006). The average genotype locomotor activity profiles over 24 h were constructed in excel by using “Befly!” software (Allebrandt et al., 2013).Statistical analysis was performed using Statistics 5 and Oriana programs (Pegoraro et al., 2014). The activity profiles of the transgenic flies were compared at different temperatures as well as in comparison to each other for the morning (M) and evening (E) components of activity. Bins for those particular phases of activity were highlighted and statistical analysis was performed using the circular statistics package “Oriana” which implements the Watson-Williams F-Test.
2.4. Western blots
Western blots were performed for PER proteins using polyclonal anti-PER (a gift from Ralf Stanewsky). Flies were entrained under LD 12:12 on either 25 °C or 18 °C and collected in liquid nitrogen at different time intervals. Protein was extracted from the heads of about 50 flies. Polyacrylamide gels were prepared and proteins were loaded as per the protocol described in Pegoraro et al. (2014).
2.5. Quantitative real-time PCR
qPCR was performed to check the expression of tim. Flies were entrained under LD 12:12 at 25 °C for 3 days and were collected in liquid nitrogen at ZT12. RNA was extracted from the heads and qPCR by using trizol. The following primer pair FTIMpseud1 (GATCTGCTGGGATGGACGAT) and RTIMpseud1 (GCCACCTCGTTGTCACACTC) was used for the amplification of cDNA. They were designed against the variable regions between the two species genes.
3. Results
3.1. Protein sequence analysis
The protein sequences encoded by the clock genes per, tim, Clock, cyc and cry were compared using the genomes from the 12 Drosophila species (Clark et al., 2007) particularly focusing on D. melanogaster and D. pseudoobscura. Protein sequences of TIM were aligned and the level of similarity obtained (Fig. 1). The similarity between D. melanogaster and D. pseudoobscura TIM was 75%. The D. pseudoobscura/persimilis group was clustered further away from D. melanogaster than all other Drosophila species investigated. For PER, the position of D. pseudoobscura was further from other Drosophila species except D. virilis (Fig. 1).
Fig. 1.
Phylogenetic analysis of circadian genes in 12 Drosophila species. The tree was generated by Neighbor-Joining method in Mega5. The p-distance model was used to calculate the genetic distance. Numbers at the bases of branches refer to bootstrap values (%). B) Reference phylogram from 12 Drosophila genome project created using pair wise genomic mutation distances.
For CLK the position of D. pseudoobscura was further from D. melanogaster than D. ananassae and D. grimshawi, while D. virilis was the most distant species. The phylogenetic analysis of CYC showed D. pseudoobscura closer to D. melanogaster than D. mojavensis, D. grimshawi and D. virilis. The CYC protein sequence similarity was 70% between D. melanogaster and D. pseudoobscura. The sequence similarity for CRY was 81% between D. melanogaster and D. pseudoobscura but the topology of the tree is rather different from those of the previous clock proteins. In particular, D. pseudoobscura and D. persimilis CRY is relatively closer to D. melanogaster than D. virilis and D. willistoni (Fig. 1a). Of all the trees, this one resonates with the accepted phylogenetic positions in the 12 Drosophila genome project (Fig. 1b). Considering the evolutionary distance between all Drosophila species, the position of the D. pseudoobscura/persimilis clade is anomalous for PER, CLK and TIM but not for CRY and CYC.
3.2. Locomotor activity rhythms
Behavioural analysis was performed on all the transgenic (hemizygous and homozygous) and control lines at 25 °C and 18 °C. Flies were entrained under LD12:12 for 3–4 days and then placed in DD for 7–10 days.
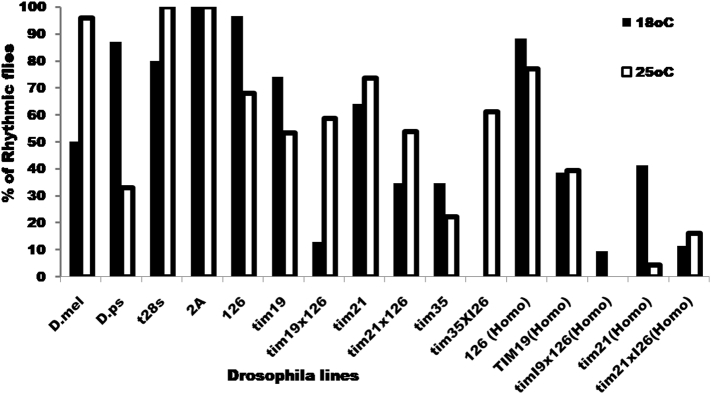
The parental D. melanogaster and D. pseudoobscura strains showed interesting temperature-dependent circadian phenotypes, with D. pseudoobscura showing higher levels of rhythmicity at lower temperatures (18 °C), with D. melanogaster showing the opposite phenotype (Fig. 2, Table 2). This species-specific phenotype was partially reflected in the corresponding transformants D. melanogaster lines carrying timmel (melt28s) which generated higher levels of rhythmicity at 25 °C whereas permel (mel2A) showed 100% rhythmicity at both temperatures. Similarly, the D. melanogaster perps transformant lines I26 and tim19 revealed much higher levels of rhythmicity at the colder temperatures (Fig. 2, Table 2) whereas D. melanogaster timps line tim21 revealed a similarly good rescue at both temperatures. However, when the timps transgenes were co-expressed with the perps (I26) transgene, a similar level of rescue was observed compared to the single transgenes at 25 °C but this was significantly reduced at lower temperatures. One other hand D. melanogaster per-tim double transformant (from the cross I26 × tim35) generated low levels of rescue at both temperatures (Table 2).
Fig. 2.
Locomotor rhythms detected in hemizygous and homozygous transgenic flies at 18 and 25 °C and percent rhythmic flies innes under 18 and experimental and control lines.
Table 2.
Results of the activity analysis for the hemizygous transgenic and control flies used in this study under 25 °C and 18 °C. Chi-square test was performed to compare the rhythmicity of the flies under two temperature conditions. (N = total number of flies, Narr = no. of arrhythmic flies, Nr = no. of rhythmic flies, %r = percentage of rhythmic flies, D. melanogaster Canton-S, I26 × tim19 and I26 × tim21 show more rhythmicity on 25 °C while D. pseudoobscura and I26 lines showed preference for 18 °C.
| 25 °C |
18 °C |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | N | Narr | Nr | %r | Period | SEM | N | Narr | Nr | %r | Period | SEM | Chi sq. | Pref | Period |
| D. melanogaster Canton-S | 25 | 1 | 24 | 96 | 23.95 | 0.13 | 22 | 11 | 11 | 50.00 | 23.64 | 0.08 | * | 25 °C | |
| D. pseudoobscura Flagstaff | 30 | 20 | 10 | 33 | 23.70 | 0.16 | 31 | 4 | 27 | 87.00 | 22.67 | 0.05 | * | 18 °C | * |
| w/Y; tim01timmel/tim01 (t28s) | 23 | 0 | 23 | 100 | 23.20 | 0.16 | 28 | 6 | 22 | 80.00 | 23.00 | 0.16 | |||
| per01/Y, w; permel/+ (2A) | 30 | 0 | 30 | 100 | 24.87 | 0.06 | 11 | 0 | 11 | 100.00 | 24.01 | 0.07 | 25 °C | ||
| per01/Y; tim+; perps/+ (I26) | 28 | 9 | 19 | 67.85 | 31.23 | 0.46 | 30 | 1 | 29 | 96.60 | 26.77 | 0.07 | * | 18 °C | * |
| w/Y; tim01, timps/tim01 (tim19) | 30 | 14 | 16 | 53.3 | 20.82 | 0.39 | 35 | 9 | 26 | 74.20 | 21.09 | 0.21 | 18 °C | ||
| per01/Y; tim01, timps/tim01; perps/+ (19 × 126) | 46 | 19 | 27 | 58.69 | 24.51 | 0.79 | 32 | 28 | 4 | 12.95 | 24.89 | 0.23 | * | 25 °C | |
| w/Y; tim01, timps/tim01 (tim21) | 34 | 9 | 25 | 73.5 | 20.09 | 0.83 | 55 | 22 | 33 | 63.93 | 21.10 | 0.15 | 18 °C | ||
| per01/Y; tim01, timps/tim01; perps/+ (21 × 126) | 41 | 19 | 22 | 53.65 | 26.54 | 0.63 | 63 | 43 | 20 | 34.60 | 24.77 | 0.15 | * | 25 °C | |
| per01/Y; tim01, timps/tim01; perps/+ (I26 × 35) | 27 | 21 | 6 | 22.22 | 23.99 | 0.18 | 26 | 17 | 9 | 34.60 | 25.16 | 0.43 | |||
| w/Y; tim01, timps/tim01 (tim35) | 32 | 12 | 20 | 61.2 | 22.32 | 0.15 | |||||||||
| per01/Y; +; perps/perps (I26) | 26 | 6 | 20 | 76.92 | 29.60 | 0.48 | 34 | 4 | 30 | 88.20 | 26.08 | 0.09 | * | ||
| w/Y; tim01, timps/tim01, timps (19) | 28 | 17 | 11 | 39.2 | 26.16 | 0.23 | 31 | 19 | 12 | 38.70 | 24.39 | 0.38 | 18 °C | ||
| per01/Y; timpstim01/timpstim01; perps/perps (19 × 126) | 47 | 46 | 1 | 0.00 | 21 | 19 | 2 | 9.50 | 22.68 | 0.17 | na | ||||
| w/Y; tim01, timps/tim01 (21) | 53 | 30 | 23 | 4.35 | 25.79 | 0.50 | 29 | 17 | 12 | 41.30 | 22.75 | 0.13 | 18 °C | ||
| per01/Y; timpstimo1/timpstim01; perps/perps (21 × 126) | 31 | 26 | 5 | 16.1 | 21.00 | 0.72 | 29 | 17 | 12 | 11.40 | 22.39 | 0.12 | |||
All these results described above were obtained with hemizygous single copies of the relevant transgenes. The homozygous perps (I26) line showed very high levels of rescue at both temperatures but in combination with homozygous timps transgenes, the rescue was extremely poor (Fig. 2, Table 2). Consequently, the results so far suggest that having both heterospecific per and tim transgenes in a melanogaster host compromises the normal and species-specific functioning of the clock and thus do not support any kind of coevolutionary scenario.
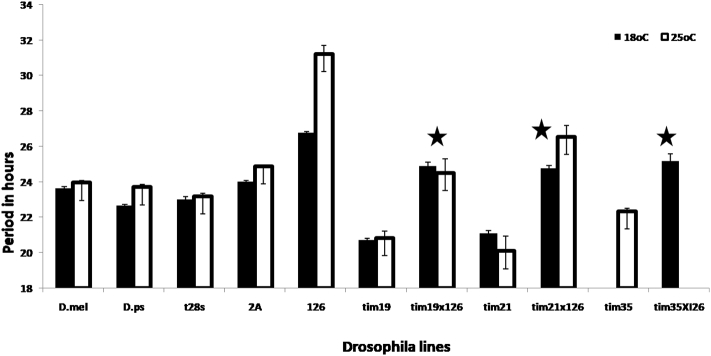
The average period of locomotor activity rhythm for the perps transgenic strains I26 and I20 fell between 30–32 h whereas timps (lines tim19, tim21 and tim35) had shorter mean periods of 20–22 h (Table 2, Fig. 3). The doubly transgenic lines (timps-tim19, 21 and 35 crossed with perps I26) (Table 2) showed periods that were very close to 24 h in two of the crosses involving timps 21 and 35. However, the timps 19/perps I26 transformants gave a period of 26 h which is almost exactly intermediate between the values of the two parental lines, as did the corresponding transformants with timps21. Consequently two of the crosses show results consistent with PER-TIM coevolution whereas the others may simply reflect an averaging of the two parental values.
Fig. 3.
Mean period (±sem) of locomotor activity of the different lines under 18 and 25 °C. The lines indicated with stars are the hemizygous double transgenics (carrying per and tim transgenes from pseudoobscura).
There is a significant effect of temperature on the period of activity rhythms for D. pseudoobscura, as well as for the D. melanogaster perps transgenic lines (I26) (F(1,26) = 26.7, p = 2.15e−05 and F(1,35) = 68.9, p = 8.79e−10 respectively) (Fig. 3). The two timps transgenic lines tested at both temperatures did not have defective temperature compensation. When timps was combined with perps the double transgenic per01/Y; tim01, timps/tim01; perps/+ also showed good temperature compensation (Fig. 3, Table 2). Thus combining the two transgenes from D. pseudoobscura not only yielded an optimisation in the average period but also produced more optimal temperature compensation.
3.3. Locomotor activity profiles under LD 12:12 conditions
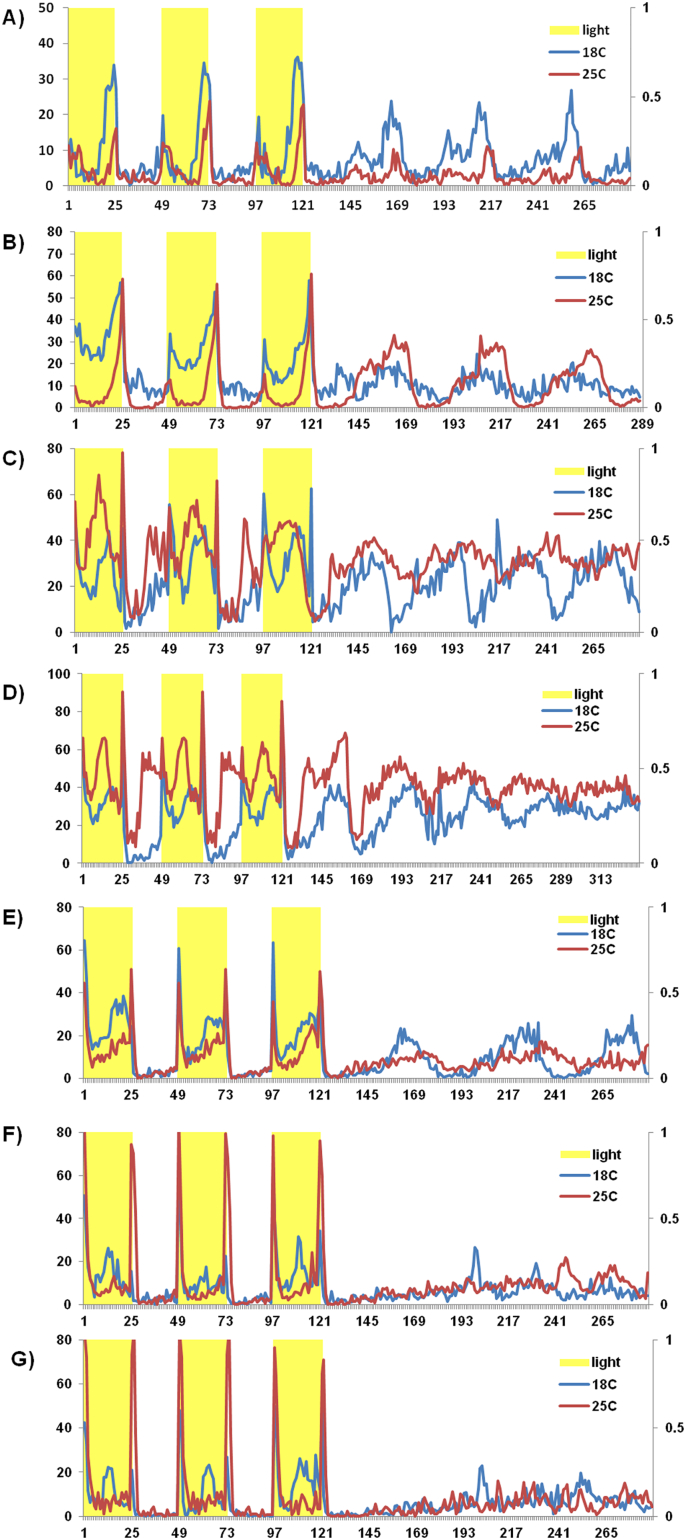
Fig. 4 shows the activity of flies under LD12:12 cycles and for the first few days of DD. D. pseudoobscura (Fig. 4A) and D. melanogaster both show generally higher levels of activity under 18 °C compared to 25 °C under LD12:12 Interestingly in DD this is reversed in D. melanogaster but not D. pseudoobscura. The timps transformants (tim19 and tim21, Fig. 4C and D respectively) show considerable nocturnal activity under LD12:12 and, very surprisingly, an ‘afternoon’ peak at 25 °C in addition to the usual morning (M) and evening (E) peaks. In DD the cycles of activity appear to damp quite quickly at the higher temperature. The perps I26 transformant shows higher levels of DD activity at the colder temperature, mimicking D. pseudoobscura (compare panel 4E to 4A) and the doubly transgenic flies show a strong afternoon peak at the cold temperature and less so at the warmer one. In DD the damping is immediate (Fig. 4F and G).
Fig. 4.
Locomotor activity profile of different fly lines under 18 and 25 °C in three days of LD followed by 3 days in DD (yellow boxes shows photoperiod in LD 12:12). Level of activity (y-axis) plotted against bins of activity (x-axis). A) D. pseudoobscura (Flagstaff), B) D. melanogaster Canton-S, C) w; tim01, timps/tim01 (tim19), D) w; tim01, timps/tim01 (tim21), E) per01; tim+; perps/+ (I26), F) per01/Y; tim01, timps/tim01; perps/+ (19), G) per01/Y; tim01, timps/tim01; perps/+ (21). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Expression studies
We wished to examine the levels of expression of the various transgenes. Expression of PER was compared by using Western blotting. The analysis was performed by collecting the fly heads at 25 °C. Unlike the D. melanogaster PER antibodies, the corresponding TIM reagents did not recognise D. pseudoobscura TIM on the blots (Fig. 5A). We therefore compared the single perps transgenic (I26) line and the double transgenic timps19 × perps I26 and w; per01 was used as the negative control (Fig. 5). The usual cycling PER pattern with peaks of expression around ZT24/0 were observed even though the double transgenic flies were largely behaviourally arrhythmic in DD (Fig. 5A).
Fig. 5.
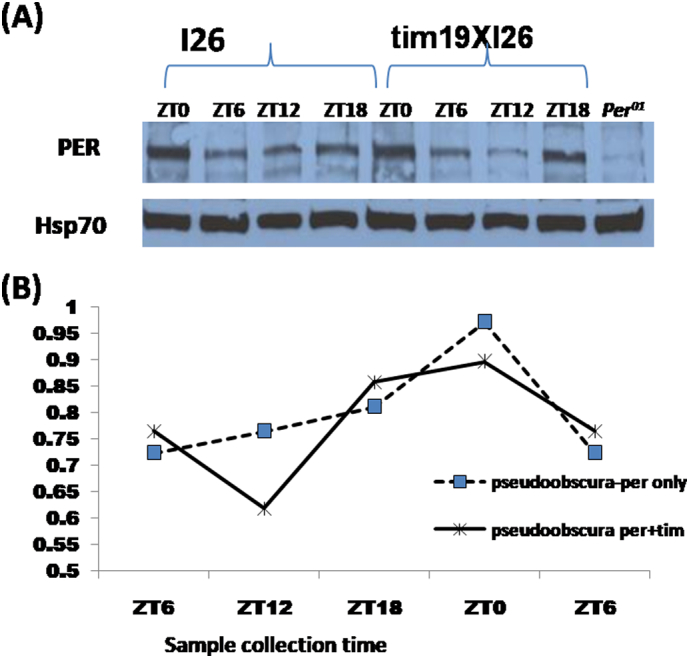
Comparison of PER protein oscillation against HSP70 in per01/Y; tim+; perps/+ (I26) line and per01/Y; tim01, timps/tim01; perps/+ (19) lines under LD12:12 A) Western blot indicating PER protein expression (Hsp70 was used as a loading control), B) Graph representing level of protein plotted against the time of sample collection.
As the TIM antibodies were not recognising the D. pseudoobscura protein the expression of the timps transgene was investigated with Real-Time PCR on transgenic timps 19 and timps 21, the double transgenic timps19 × perps I26 and timps21 × perps I26 and D. pseudoobscura and D. melanogaster. Fly heads were collected at ZT12 from individuals maintained at 25 °C. Three samples of each fly line were collected at ZT12 and three technical replicates were used for each. The fly line w; tim01 was used as the negative control. The analysis showed that the level of tim in D. pseudoobscura is far higher than all the other lines as was tim01, which is expected as the negative auto-regulation by TIM is removed in the mutant (Fig. 6A). A statistical analysis was performed by excluding D. pseudoobscura. Results revealed that only timmel (t28s) has a significantly higher level of tim mRNA compared to all other transgenes(F(5,47) = 3.51, p = .009, Fig. 6B). However the very low relative levels of tim transcript are clearly sufficient to drive circadian rhythmicity, both in the wild-type and transgenics.
Fig. 6.
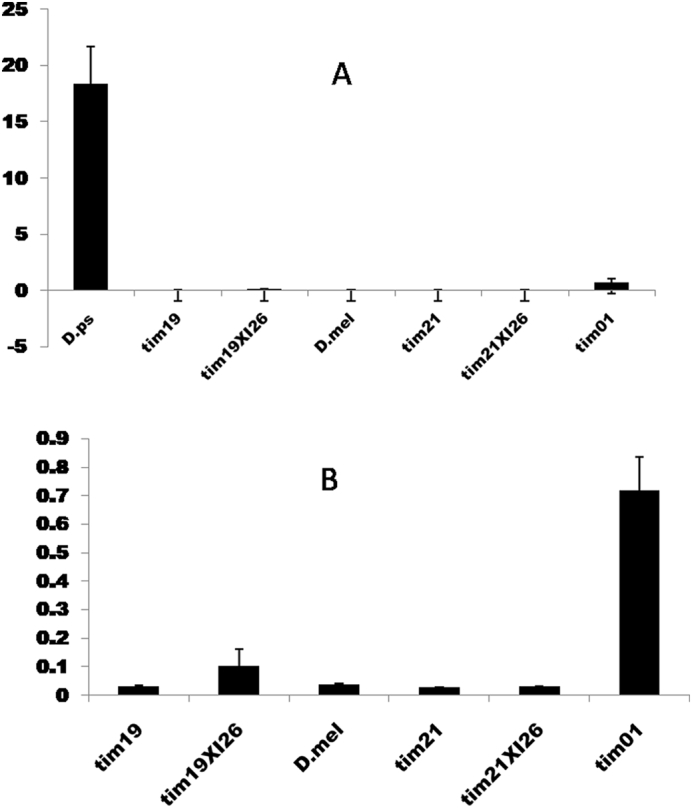
Comparison of mean tim mRNA level (±sem) among the different fly lines with and without D. pseudoobscura.
4. Discussion
4.1. Protein sequence alignment
D. pseudoobscura separated from the D. melanogaster group between 25 (Russo et al., 1995) and 30 Mya (Schlötterer et al., 1994). However for both PER and TIM, the position of the D. pseudoobscura-persimilis clade is further away than is D. virilis, which itself diverged from D. melanogaster 40–60 Mya (Powell and DeSalle, 1995) (Fig. 1A, B). The position of D. willistoni is also not in accordance with the reference sequence. This may suggest some kind of non-neutral evolution of these proteins, and provides an additional rational for further exploring the coevolutionary idea.
For D. pseudoobscura the highest level of similarity was obtained as expected with its sibling North American species D. persimilis. Two PER interaction domains have been identified on TIM, PER1 and PER2 (Saez and Young, 1996). Piccin et al. (2000) in their phylogeny, reported the canonical phylogenetic positions when using the full PER protein sequence but by comparing only the PAS interaction domains of PER, M. domestica was grouped closer to D. melanogaster than D. pseudoobscura. This corresponded with the enhanced rescue of Musca PER compared to D. pseudoobscura PER in D. melanogaster per0 hosts. The same was observed with the comparison of the PAS region of Ceratitis capitata PER (Mazzotta et al., 2005). PAS is the protein-protein interaction domain important for signalling and sensory function. In Drosophila its function is to promote the interactions of PER with TIM, and CLK with CYC (Mazzotta et al., 2005). However on PER there is a CLOCK-CYCLE Domain (CCID) for interaction with CLK (Chang and Reppert, 2003).
The unusual phylogenetic position of PAS may be due to the amino acids in this region being under selective constraints so it does not evolve independently. It may coevolve in concert with the dimerization domains of its conspecific molecular partners (Mazzotta et al., 2005). Perhaps the PER interaction domain of TIM should also reflect the unusual phylogeny of the PAS domain of its partner PER. However in the present study the results of the phylogenetic analysis using the amino acid sequence of only PER1 and PER2 interacting domains of TIM gave the same position for D. pseudoobscura as that of the full TIM sequence.
4.2. Coevolution
Coevolution is an evolutionary process in which a heritable change in one entity establishes selective pressure for a change in another entity. These entities can range from nucleotides to amino acids to protein to entire organism and perhaps the whole ecosystem. The well-studied example of coevolution involves physically interacting proteins in which precise, complementary structural conformations of interacting partners are needed to maintain a functional interaction (Fraser et al., 2004). The basic aim of the current study was to investigate whether PER and TIM form a coevolved module that can interact more efficiently with other clock proteins compared to the situation where only one of these negative regulators was heterospecific. For this purpose transgenes of per and tim from D. pseudoobscura were introduced into D. melanogaster host.
The comparison of the level of rescue in the rhythmic behaviour of hemizygous/homozygous transgenics with single per or tim or both was performed. Previous studies using perps transformants showed that perps cannot rescue rhythmic behaviour efficiently. It has low penetrance (30–50%) and flies have very long average periods (Petersen et al., 1988; Peixoto et al., 1998). The same observations were confirmed in the present study. The level of rescue for the timps transgenic flies was also <50% under 25 °C but increased at 18 °C. The period of these lines was short (21–22 h). These results suggested that the two pseudoobscura proteins are unable to interact fully with their melanogaster partner molecules. The rescue observed in tim01 flies using D. virilis (Ousley et al., 1998) and D. ananassae tim transgenes (Nishinokubi et al., 2006) corresponds to the position of these flies with respect to D. melanogaster in the phylogenetic analysis.
When both heterospecific transgenes were studied simultaneously in double mutant hosts, no significant increase in levels of rhythmicity was obtained, indeed it was reduced suggesting that adding additional heterospecific clock proteins or even increasing the dose of one interspecific molecule, was disruptive for the host clock – the opposite of coevolution. However a significant and dramatic improvement in the average period of the hemizygous double transgenics was observed. The average period ranged from 24 to 26.5 h for all three lines (tim19, tim21 and tim35), While this might initially appear to fit with a coevolution hypothesis, it was also noticeable how some of the double transgenic periods appeared intermediate to the parental single transgenic values. Consequently, we cannot securely state that these results are reflecting a more optimal PER-TIM interaction of the heterospecific clock proteins.
The activity profiles of the transgenic flies showed some aspects of the species-specific behaviour controlled by the clock genes. For example, the perps I26 line showed higher levels of activity in the DD phase at colder temperatures, similar to D. pseudoobscura. The most interesting novel phenotype was the afternoon peak of locomotor activity in LD12:12 observed in the single 8b transformants. This is reminiscent of the same afternoon component that is observed in wild-type when exposed to naturally cycling warm summer temperatures but is not generally seen under laboratory square wave lighting conditions at constant temperature (Vanin et al., 2012; Green et al., 2015). However, given the generally shorter endogenous period of the transformants, the apparent afternoon peak may represent an earlier phased E peak that is followed by a startle response when the lights go off, which would superficially resemble an E peak.
The bimodal activity profile of D. melanogaster is under the control of several sets of neurons (Helfrich-Förster, 2005). Under LD conditions in the laboratory, the PDF-positive sLNvs have been implicated in control of the morning locomotor activity forming a neuronal basis for the morning (M) oscillator, while the fifth PDF-negative sLNv and LNds seem to be responsible for the evening activity and hence termed the evening (E) oscillator (Grima et al., 2004; Nitabach and Taghert, 2008; Stoleru et al., 2004). The activity profile of D. pseudoobscura did not show any morning anticipation of activity in LD. This may be an adaptive response of this species where the morning activity lost its selective advantage due to the long summer days of more northern climatic zones (Hermann et al., 2013). This type of activity profile has also been reported from high latitude species D. montana (Kauranen et al., 2012), D. virilis (Dubruille and Emery, 2008), D. ezoana and D. littoralis (Menegazzi et al., 2017). These species do not express PDF in the sLNv's (or express at very low levels), (Kauranen et al., 2012) and the latter two species do not express CRY in the lLNvs (Menegazzi et al., 2017). Hermann et al., 2013 reported some apparent differences in the PER expression in the clock neurons between Sophophora (to which D. pseudoobscura belongs) and Drosophila subgenuses. Indeed, they reported that D. pseudoobscura also showed reduced PDF immunostaining in the sLNvs which might be expected to generate weaker rhythms, given the prominent role of these neurons as the pacemaker cells in DD (Nitabach and Taghert, 2008).
4.3. Effect of temperature
The analysis of locomotor activity of the different fly strains under two different temperature conditions gave some interesting results. In D. melanogaster the locomotor peak of activity is temperature modulated so that with a rise in temperature the E (evening) peak of activity moves later in the day, generating a mid-day siesta allowing the fly to avoid the desiccating effect of the hottest part of the day (Majercak et al., 1999; Collins et al., 2004; Low et al., 2008). D. pseudoobscura clearly favoured colder temperatures for expressing rhythmic behaviour while D. melanogaster flies were more rhythmic under higher temperature conditions. Hennessy (1999) also reported similar observation with D. melanogaster transgenes and perps transgenes favouring warmer and colder conditions respectively. In the current study, the timmel (t28s) behaves like Canton-S but the timps transgenic line tim19 favoured colder conditions. This suggested that in restoring wild-type clock function, the timps transgene takes on a dominant effect. Such dominant effects of pseudoobscura transgenes in mating rhythms have been seen before with perps in per+ backgrounds (Petersen et al., 1988; Tauber et al., 2003). These observations reveal that restoring TIM from D. pseudoobscura in tim-null mutants appears to generate pseudoobscura like colder temperature characteristics.
An overall trend toward shortening of period under 18 °C was seen in the controls (both natural and transgenic) and perps transformant lines. The same trend was reported in previous studies using these fly lines (Piccin et al., 2000; Hennessy, 1999; Peixoto et al., 1998). However the difference in the average period for the perps flies was large and >4 h. Compared to the perps transgenics, the temperature compensation was much better in single timps and double perps and timps transgenics. These results suggested that temperature compensation is disturbed more by heterospecific PER molecules than TIM molecules. This would fit with the general view that PER is more important for thermal adaptation of the clock (Majercak et al., 2004; Sawyer, 1997) than TIM, the latter being more relevant for light sensitivity of the clock (Zeng et al., 1996) and associated photoperiodic phenotypes such as diapause (Tauber et al., 2007).
The phase analysis of the locomotor activity in the transgenic flies in LD12:12 also showed some very interesting results. Bywalez et al. (2012) found that M and E components of locomotor activity do not occur at a fixed time and respond differently to day length and temperature. They suggested that the two underlying oscillators have different sensitivities and the phase of evening activity is more sensitive to high temperature, resulting in a delay. The phase of the morning peak of activity was compared for the transgenic lines and it was revealed that timps transgenics are active earlier under 25 °C than 18 °C which is the normal heat avoiding response by D. melanogaster flies, controlled at least partially through the reduced 3′ splicing of the per transcript (Majercak et al., 1999; Low et al., 2008). This splicing also delays the E peak in hot days, generating the siesta. However the single timps transformants flies cannot adjust their evening peak according to the temperature conditions and showed an earlier E peak on hotter rather than colder temperatures. Combining timps with perps, produced a later evening peak at 25 °C than at 18 °C so the normal hot day response requires both heterospecific PER and TIM partners, revealing a possible example of coevolution.
In summary, our results provide limited support for a coevolution of PER and TIM within species, and certainly not for maintaining robust levels of rhythmicity. Some positive evidence was found for the free-running period of activity rhythm and there were some other sporadic examples where having PER and TIM from the same species gave a more optimal circadian phenotype in our transgenics. There were also some examples of apparent species-specific behaviour associated with the corresponding transgenes. However in the main, we have not showed any compelling evidence for PER-TIM representing a co-evolved module as initially hinted at by the phylogenetic and functional analyses of PER by Piccin et al. (2000).
The following is the supplementary data related to this article.
Genetic crosses showing A) the lines carrying D. pseudoobscura-tim on chromosome II were crossed to the double balancers (P) to combine the transgenes with markers on chromosome III. B) The lines carrying D. pseudoobscura-per on chromosome III were crossed to the double balancers (P) to combine the transgenes with markers on chromosome II. C) The final strains obtained from cross A and B were crossed with each other to combine the two transgenes of D. pseudoobscura per and tim in the double mutant background with only male flies. D) Final cross to obtain the homozygous transformant female flies having two copies of D. pseudoobscura-tim and per in the per0; tim0 background. The triple balancer FM7a/per01; CyO/Sco; MKRS/+ females were crossed to the w/Y; tim01, timps/tim01, timps; perps/perps males.
Acknowledgement
We are pleased to acknowledge BBSRC grant BB/G02085X/1 to Eran Tauber. We also acknowledge the Higher Education Commission of Pakistan and University of Peshawar, Pakistan for funding the research at University of Leicester, UK.
Conflicts of interest
The authors confirmed no conflicts of interests.
References
- Allebrandt K.V., Amin N., Müller-Myhsok B., Esko T., Teder-Laving M., Azevedo R.V.D.M.…Roenneberg T. A KATP channel gene effect on sleep duration: from genome-wide association studies to function in Drosophila. Mol. Psychiatry. 2013;18(1):122–132. doi: 10.1038/mp.2011.142. https://doi.org/10.1038/mp.2011.142 [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D., Cassone V.M., Earnest D.J., Golden S.S., Hardin P.E., Thomas T.L., Zoran M.J. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat. Rev. Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywalez W., Menegazzi P., Rieger D., Schmid B., Helfrich-Förster C., Yoshii T. The dual-oscillator system of Drosophila melanogaster under natural-like temperature cycles. Chronobiol. Int. 2012;29:395–407. doi: 10.3109/07420528.2012.668505. [DOI] [PubMed] [Google Scholar]
- Chang D.C., Reppert S.M. A novel C-terminal domain of Drosophila PERIOD inhibits dCLOCK:CYCLE-mediated transcription. Curr. Biol. 2003;13:758–762. doi: 10.1016/s0960-9822(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Citri Y., Colot H.V., Jacquier A.C. A family of unusually spliced biologically active transcripts encoded by a Drosophila clock gene. Nature. 1987;326:42–47. doi: 10.1038/326042a0. [DOI] [PubMed] [Google Scholar]
- Clark A.G. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450(7167):203–218. doi: 10.1038/nature06341. https://doi.org/10.1038/nature06341 Nov 8. [DOI] [PubMed] [Google Scholar]
- Collins B.H., Rosato E., Kyriacou C.P. Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock, and phospholipase C. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1945–1950. doi: 10.1073/pnas.0308240100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubruille R., Emery P. A plastic clock: how circadian rhythms respond to environmental cues in Drosophila. Mol. Neurobiol. 2008;38:129–145. doi: 10.1007/s12035-008-8035-y. [DOI] [PubMed] [Google Scholar]
- Fraser H.B., Hirsh A.E., Wall D.P., Eisen M.B. Coevolution of gene expression among interacting proteins. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9033–9038. doi: 10.1073/pnas.0402591101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E.W., O'Callaghan E.K., Hansen C.N., Bastianello S., Bhutani S., Vanin S.…Kyriacou C.P. Drosophila circadian rhythms in seminatural environments: summer afternoon component is not an artifact and requires TrpA1 channels. Proc. Natl. Acad. Sci. 2015;112(28):8702–8707. doi: 10.1073/pnas.1506093112. https://doi.org/10.1073/pnas.1506093112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B., Chélot E., Xia R., Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. 1999;41:95–98. [Google Scholar]
- Hardin P.E. 1st ed. Vol. 74. 2011. Molecular genetic analysis of circadian timekeeping in Drosophila; pp. 141–173. (Adv. Genet.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C. Neurobiology of the fruit fly's circadian clock. Genes Brain Behav. 2005;4:65–76. doi: 10.1111/j.1601-183X.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- Hennessy J.M. University of Leicester, UK; Phd Dissertation: 1999. Functional Analysis of a Drosophila Clock Gene. [Google Scholar]
- Hermann C., Saccon R., Senthilan P.R. The circadian clock network in the brain of different Drosophila species. J. Comp. Neurol. 2013;521:367–388. doi: 10.1002/cne.23178. [DOI] [PubMed] [Google Scholar]
- Kauranen H., Menegazzi P., Costa R. Flies in the north: locomotor behavior and clock neuron organization of Drosophila montana. J. Biol. Rhythm. 2012;27:377–387. doi: 10.1177/0748730412455916. [DOI] [PubMed] [Google Scholar]
- Kloss B., Price J.L., Saez L. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- Lee C., Parikh V., Itsukaichi T. Resetting the Drosophila clock by photic regulation of PER and a PER-TIM complex. Science. 1996;271:1740–1744. doi: 10.1126/science.271.5256.1740. [DOI] [PubMed] [Google Scholar]
- Low K.H., Lim C., Ko H.W., Edery I. Natural variation in the splice site strength of a clock gene and species-specific thermal adaptation. Neuron. 2008;60:1054–1067. doi: 10.1016/j.neuron.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majercak J., Sidote D., Hardin P.E., Edery I. How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron. 1999;24:219–230. doi: 10.1016/s0896-6273(00)80834-x. https://doi.org/10.1016/S0896-6273(00)80834-X [DOI] [PubMed] [Google Scholar]
- Majercak J., Chen W.-F., Edery I. Splicing of the period gene 3′-terminal intron is regulated by light, circadian clock factors, and phospholipase C. Mol. Cell. Biol. 2004;24(8):3359–3372. doi: 10.1128/MCB.24.8.3359-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzotta G.M., Sandrelli F., Zordan M.A., Mason M., Benna C., Cisotto P., Rosato E., Kyriacou C.P., Costa R. The clock gene period in the medfly Ceratitis capitata. Genet. Res. 2005;86:13–30. doi: 10.1017/S0016672305007664. [DOI] [PubMed] [Google Scholar]
- Menegazzi P., Dalla Benetta E., Beauchamp M., Schlichting M., Steffan-Dewenter I., Helfrich-Förster C. Adaptation of circadian neuronal network to photoperiod in high-latitude European Drosophilids. Curr. Biol. 2017;27(6):833–839. doi: 10.1016/j.cub.2017.01.036. https://doi.org/10.1016/j.cub.2017.01.036 [DOI] [PubMed] [Google Scholar]
- Nishinokubi I., Shimoda M., Ishida N. Mating rhythms of Drosophila: rescue of tim01 mutants by D. ananassae timeless. J. Circadian Rhythms. 2006;4:4. doi: 10.1186/1740-3391-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach M.N., Taghert P.H. Organization of the Drosophila circadian control circuit. Curr. Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Ousley A., Zafarullah K., Chen Y., Emerson M., Hickman L., Sehgal A. Conserved regions of the timeless (tim) clock gene in Drosophila analyzed through phylogenetic and functional studies. Genetics. 1998;148:815–825. doi: 10.1093/genetics/148.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro M., Noreen S., Bhutani S., Tsolou A., Schmid R. Molecular evolution of a pervasive natural amino-acid substitution in Drosophila cryptochrome. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0086483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto A.A., Hennessy J.M., Townson I., Hasan G., Rosbash M., Costa R., Kyriacou C.P. Molecular coevolution within a Drosophila clock gene. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4475–4480. doi: 10.1073/pnas.95.8.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel N., Helfrich-Förster C. Setting the clock—by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 2011;585:1435–1442. doi: 10.1016/j.febslet.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Petersen G., Hall J.C., Rosbash M. The period gene of Drosophila carries species-specific behavioral instructions. EMBO J. 1988;7:3939–3947. doi: 10.1002/j.1460-2075.1988.tb03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccin A., Couchman M., Clayton J.D., Chalmers D., Costa R., Kyriacou C.P. The clock gene period of the housefly, Musca domestica, rescues behavioral rhythmicity in Drosophila melanogaster. Evidence for intermolecular coevolution? Genetics. 2000;154:747–758. doi: 10.1093/genetics/154.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.R., DeSalle R. Drosophila molecular phylogenies and their uses. In: Hecht M.K., Macintyre R.J., Clegg M.T., editors. Evolutionary Biology. vol. 28. Springer; Boston, MA: 1995. pp. 87–138. (Evolutionary Biology). [Google Scholar]
- Rosato E., Kyriacou C.P. Analysis of locomotor activity rhythms in Drosophila. Nat. Protoc. 2006;1:559–568. doi: 10.1038/nprot.2006.79. [DOI] [PubMed] [Google Scholar]
- Russo C.A., Takezaki N., Nei M. Molecular phylogeny and divergence times of drosophilid species. Mol. Biol. Evol. 1995;12:391–404. doi: 10.1093/oxfordjournals.molbev.a040214. [DOI] [PubMed] [Google Scholar]
- Saez L., Young M.W. Regulation of nuclear entry of the Drosophila clock proteins period and timeless. Neuron. 1996;17:911–920. doi: 10.1016/s0896-6273(00)80222-6. [DOI] [PubMed] [Google Scholar]
- Sawyer L.A. Natural variation in a Drosophila clock gene and temperature compensation. Science. 1997;278:2117–2120. doi: 10.1126/science.278.5346.2117. [DOI] [PubMed] [Google Scholar]
- Schlötterer C., Hauser M.T., Von Haeseler A., Tautz D. Comparative evolutionary analysis of rDNA ITS regions in Drosophila. Mol. Biol. Evol. 1994;11:513–522. doi: 10.1093/oxfordjournals.molbev.a040131. [DOI] [PubMed] [Google Scholar]
- Stoleru D., Peng Y., Agosto J., Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber E., Roe H., Costa R., Hennessy J.M., Kyriacou C.P. Temporal mating isolation driven by a behavioral gene in Drosophila. Curr. Biol. 2003;13:140–145. doi: 10.1016/s0960-9822(03)00004-6. [DOI] [PubMed] [Google Scholar]
- Tauber E., Zordan M., Sandrelli F., Pegoraro M., Osterwalder N., Breda C., Daga A., Selmin A., Monger K., Benna C., Rosato E., Kyriacou C.P., Costa R. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science. 2007;316:1895–1898. doi: 10.1126/science.1138412. [DOI] [PubMed] [Google Scholar]
- Vanin S., Bhutani S., Montelli S. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature. 2012;484:371–375. doi: 10.1038/nature10991. [DOI] [PubMed] [Google Scholar]
- Wheeler, D.A., Kyriacou, C.P., Greenacre, N.L., Yu, Q., Rutila, J.E., Rosbash, M., Hall, J., (n.d.). Molecular transfer of a species-specific behavior from Drosophila simulans to Drosophila melanogaster. Science, 251(4997), 1082–1085. [DOI] [PubMed]
- Zeng H., Qian Z., Myers M.P., Rosbash M. A light-entrainment mechanism for the Drosophila circadian clock. Nature. 1996;380:129–135. doi: 10.1038/380129a0. [DOI] [PubMed] [Google Scholar]
- Zheng X., Sehgal A. Probing the relative importance of molecular oscillations in the circadian clock. Genetics. 2008;178:1147–1155. doi: 10.1534/genetics.107.088658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genetic crosses showing A) the lines carrying D. pseudoobscura-tim on chromosome II were crossed to the double balancers (P) to combine the transgenes with markers on chromosome III. B) The lines carrying D. pseudoobscura-per on chromosome III were crossed to the double balancers (P) to combine the transgenes with markers on chromosome II. C) The final strains obtained from cross A and B were crossed with each other to combine the two transgenes of D. pseudoobscura per and tim in the double mutant background with only male flies. D) Final cross to obtain the homozygous transformant female flies having two copies of D. pseudoobscura-tim and per in the per0; tim0 background. The triple balancer FM7a/per01; CyO/Sco; MKRS/+ females were crossed to the w/Y; tim01, timps/tim01, timps; perps/perps males.