Abstract
The white-striped longhorn beetle Batocera horsfieldi (Coleoptera: Cerambycidae) is a polyphagous wood-boring pest that causes substantial damage to the lumber industry. Moreover olfactory proteins are crucial components to function in related processes, but the B. horsfieldi genome is not readily available for olfactory proteins analysis. In the present study, developmental transcriptomes of larvae from the first instar to the prepupal stage, pupae, and adults (females and males) from emergence to mating were built by RNA sequencing to establish a genetic background that may help understand olfactory genes. Approximately 199 million clean reads were obtained and assembled into 171,664 transcripts, which were classified into 23,380, 26,511, 22,393, 30,270, and 87, 732 unigenes for larvae, pupae, females, males, and combined datasets, respectively. The unigenes were annotated against NCBI’s non-redundant nucleotide and protein sequences, Swiss-Prot, Gene Ontology (GO), Pfam, Clusters of Eukaryotic Orthologous Groups (KOG), and KEGG Orthology (KO) databases. A total of 43,197 unigenes were annotated into 55 sub-categories under the three main GO categories; 25,237 unigenes were classified into 26 functional KOG categories, and 25,814 unigenes were classified into five functional KEGG Pathway categories. RSEM software identified 2,983, 3,097, 870, 2,437, 5,161, and 2,882 genes that were differentially expressed between larvae and males, larvae and pupae, larvae and females, males and females, males and pupae, and females and pupae, respectively. Among them, genes encoding seven candidate odorant binding proteins (OBPs) and three chemosensory proteins (CSPs) were identified. RT-PCR and RT-qPCR analyses showed that BhorOBP3, BhorCSP2, and BhorOBPC1/C3/C4 were highly expressed in the antenna of males, indicating these genes may may play key roles in foraging and host-orientation in B. horsfieldi. Our results provide valuable molecular information about the olfactory system in B. horsfieldi and will help guide future functional studies on olfactory genes.
Introduction
The white-striped longhorn beetle, Batocera horsfieldi (Hope) (Coleoptera: Cerambycidae), is a polyphagous wood-boring pest that feeds on more than 20 plant species belonging to taxonomically distant plant families (Salicaceae, Juglandaceae, Fagaceae, Rosaceae, Caprifoliaceae, Betulaceae, Oleaceae, Moraceae, and Euphorbiaceae) [1–5]. B. horsfieldi is distributed mainly in southwest, southern, central south, and northern China, and in Vietnam, Japan, India, and Myanmar [6]. Its basic biology is similar to other members of subfamily Lamiinae and its life cycle is usually completed in 2–3 years [7]. Adults emerge in the early summer and feed mainly on branches of host plants until they are sexually mature [8].
B. horsfieldi is a pest with a quite complete protection mechanism. The crypticity of larvae towards damage makes their prevention and control difficult and traditional chemical pest control is rarely effective. Currently, research on chemoecology and behavior has laid the foundation for exploring new prevention and control means for B. horsfieldi. Liang et al. found that Viburnum awabuki and Betula luminifera could lure B. horsfieldi adults in need of extra nutrition [4]. Li et al. found through field investigation that B. horsfieldi concentrated on rosaceous plants for extra nutrition [2]. Yang et al. found that nutrition supplement of B. horsfieldi adults was related to changes in volatile components of the plants [9]. Yang et al. analyzed encounter and mating behaviors of B. horsfieldi adults through a video capture system. The study upon encountering behavior of B. horsfieldi provides the basis for studying calling mechanism and sex pheromone biosynthesis of B. horsfieldi as well as reproductive behavior of the adults [10]. Li et al. established a cDNA library for antennae of B. horsfieldi and conducted expression analysis for relevant olfactory genes [11].
During the long evolution process, sensitive smell of insects can help them recognize external volatile chemical substances so as to realize behaviors such as searching for food, mate, and spawning places [12–14]. The main olfactory sensors of insects are the antennae at the front of the head. There are many different varieties of receptors on the antennae. The receptors contain various functional proteins related to olfactory sensation; for example, odorant binding proteins (OBPs), chemosensory proteins (CSPs), and olfactory receptors. OBPs are acidic soluble proteins with low molecular weight (about 15 kD), which are distributed mainly in lymph in the olfactory receptors of insects [15]. The typical structure of OBPs comprises six conserved cysteines, which can form three disulfide bonds to support the 3D structure of OBPs [16]. So far, OBPs have been discovered in at least seven different orders of insects, namely Lepidoptera [17–23], Diptera [24, 25], Orthoptera [26], Hemiptera [27], Isoptera [28], Hymenoptera [29], and Coleoptera [30, 31].
While OBPs have been discovered in both insects and mammals, CSPs have been found only in insects. In 1994, Mckenna et al. discovered CSPs in antennae of Drosophila melanogaster for the first time by subtractive hybridization [32]. The molecular weight of CSPs is lower than that of OBPs (generally only 10–15 kD). CSPs have four conserved cysteine sites [33]. The sequence similarity of CSPs is higher than that of OBPs between different insects of the same and different species [34]. So far, CSPs have been discovered in insects such as Eurycantha calcarata [35], Aphis gossypii [36], Bombyx mori [37], Helicoverpa armigera [18], Agrotis ipsilon [38], Tomicus yunnanensis [39], Manduca sexta [40], Adelphocoris lineolatus [13], and Spodoptera littoralis [41].
In the present study, we used RNA sequencing to identify developmental stage-specific genes by building transcriptomes of larvae from the first instar to the prepupal stage, pupae, and adults (females and males) from emergence to mating (3-day-old). We identified differentially expressed genes among larvae, pupae, and female and male adults by comparative transcriptome analysis. We also screened B. horsfieldi candidate olfactory genes, including those encoding OBPs and CSPs, because the olfactory system is crucial for insects to locate hosts, oviposition sites, and food sources. Finally, we validated the differentially expressed candidate olfactory genes identified in the transcriptome data by RT-PCR and RT-qPCR.
Material and methods
Insect rearing and sample collection
Larvae, pupae and adults of B. horsfieldi were collected in June 2016 from in the Poplar Planting Base of Luojiang City, Sichuan Province, China (31.07°N, 104.08°E). The field studies did not involve endangered or protected species, and no specific permission was required for the research activity at this location. Adults were used just emergence and unmated. The characteristics used to identify mated B. horsfieldi were the villi on the abdomen of mated males and the obvious mating plaques on the backside of the mated females [42]. Female and male adults were placed on ice and quickly dissected into antenna, thorax (without thoracic legs), hind wing, and thoracic legs for RT-PCR analysis. RT-qPCR was performed using nucleic acids from male and female adult organism. All samples were immediately frozen in liquid nitrogen and stored at −80°C until use. Each sample contained either larvae, pupae, or male or female adult tissues from at least five insects. After pooling the tissues for each sample, three biological replicates were conducted for each treatment.
RNA extraction and sequencing
Mixed larvae from the first instar to the prepupal stage, pupae, and adults (females and males) were prepared for RNA extraction. Total RNA was isolated from homogenized sample in TRIzol reagent (Takara, Dalian, Liaoning, China) following the manufacturer’s protocols. The concentration of total RNA was quantified with a Qubit3.0 (Thermo Fisher Scientific, Waltham, MA, USA) and an Agilent2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). UV absorption values at 260 nm/280 nm was recorded to monitor the purity of the RNA products (Nanodrop2000, Thermo Fisher Scientific, Waltham, MA, USA). After RNA extraction, mRNAs were purified using the interaction of the poly (A) tails and magnetic oligo (dT) beads and collected using RNeasy RNA reagent. Mixed mRNAs were fragmented into 300–800 bp pieces using RNA fragment reagent (Illumina), and the pieces were collected using an RNeasy RNA cleaning kit (Qiagen). Subsequently, RNA fragments were copied to make first-strand cDNA using MMLV reverse transcriptase (Takara, Dalian, Liaoning, China) and random primers. Second-strand cDNA synthesis was performed using DNA Polymerase I and RNase H. The Illumina HiSeq2000 system and 125 paired-end reads were used for sequencing. Statistical analysis of the sequence lengths was performed to ensure sequence purity.
Assembly and functional annotation
Raw sequence data in fasta format were first processed through in-house Perl scripts [43]. In this step, clean data (clean reads) were obtained by removing reads containing adapter, poly-N, and low-quality reads from the raw sequence data [44, 45]. The Q20, Q30, GC content, and sequence duplication level of the clean data were calculated [44]. All downstream analyses were based on good-quality clean data.
The flow chart of transcriptome assembly described by Grabherr et al. [46] was used in the present analyses. A Perl pipeline described by Haas et al. [43] was used to analyze the sequence data. As suggested by Haas et al.[43], when multiple sequencing runs are conducted for a single experiment, the resultant reads can be concatenated into two files if paired-end sequencing is used. The left files (read 1 files) from all the samples were pooled into a single large left.fq file, and the right files (read 2 files) were pooled into a single large right.fq file. Transcriptome assembly was accomplished based on the left.fq and right.fq using Trinity (http://trinityrnaseq.github.io) with min_kmer_cov set to two by default and all other parameters set to default. The assembled unigenes were annotated by BLASTX searches and ESTScan against the NCBI non-redundant protein sequences (Nr), NCBI non-redundant nucleotide sequences (Nt), Swiss-Prot, Gene Ontology (GO), protein families (Pfam), Clusters of Eukaryotic Orthologous Groups (KOG), and KEGG Orthology (KO) databases (E <10−5), and the best annotations were selected [44, 45, 47]. Differentially expressed genes were selected based on a log2 fold change >1 and q value <0.005 using DESeq [48] (S1 Table). The simple sequence repeats (SSR) in the B. horsfieldi unigene sequences were screened with MISA software (http://pgrc.ipk-gatersleben.de/misa/misa.html). The expression level of genes were calculated based on FPKM method[49]. The nucleotide sequences of the identified olfactory gene are listed in S2 Table.
Homology analysis
A neighbor-joining (NJ) tree was constructed with MEGA version 5.0 and the Jones-Taylor-Thornton model [50]. The olfactory genes of other coleopteran species were obtained from the NCBI databases. Bootstrap support values were based on 1000 replicates. All the candidate olfactory genes were named according to the nomenclature system described previously [51, 52]. The olfactory genes from different species were marked with different colors and the phylogenetic tree was generated with iTOL software (http://itol.embl.de)
RT-PCR and RT-qPCR validation of differentially expressed candidate olfactory proteins
Seven OBPs and three CSPs that were predicted to be highly abundant in antenna or had complete ORFs were selected for further analysis. Specific primer pairs were derived from the transcriptome data, and primer pairs for each gene were designed to amplify 100–200 bp products, which were verified by sequencing. A semi-quantitative RT-PCR (Bio-Rad S1000, US) analysis was performed for each primer pair using rTaq DNA polymerase (Takara, Dalian, Liaoning, China) before the RT-qPCR analysis to ensure that the correct products were amplified and no primer dimers were present [53]. The RT-qPCR analysis was carried out using an Mx 3000P detection system (Agilent, Palo Alto, CA, USA) as described previously, with thermal cycler parameters of 2 min at 94°C, then 40 cycles of 20 s at 94°C, 20 s at 58°C, and 20 s at 72°C. The 18S gene was used as an internal control: 18S forward and reverse, 5’- GAGACTCTAGCCTGCTAACT-3’ and 5’-TGTTTGTACGCCGACAGT-3’. A standard curve was derived from 10-fold serial dilutions of plasmid containing the target DNA segment to determine the PCR efficiency and to quantify the amount of target mRNA. All primers tested gave amplification efficiencies of 90–100%. For each treatment, three biological replicates were conducted. RT-qPCR data were analyzed by the 2−ΔΔCT method [54]. The primers used in this experiment were designed with Primer premier 5.0 and Oligo 6.0 and are listed in S3 Table. A Chi-square test was using to compare the expression level of male and female adult. The RT-qPCR data were analyzed and output as PDF files using Graphpad 5.0.
Results
Illumina sequencing and assembly
This filtering resulted in a total of 50,028,651, 51,705,759, 49,935,243, and 47,402,329 clean reads in larvae, pupae, and females and males of B. horsfieldi, respectively. All the clean reads were assembled into transcripts by Trinity software; the longest copy of redundant transcripts was regarded as a unigene [43, 44, 46]. A total of 171,664 transcripts were obtained and assembled into 87,732 unigenes. Many unigenes exceeded 2000 bp in length, while approximately 21.13% unigenes exceeded 1000 bp, and 25.74% were 500–1000 bp (Table 1).
Table 1. Number and length of transcripts and unigenes.
| Larval | Pupal | Female | Male | |
|---|---|---|---|---|
| Raw reads | 51,907,500 | 53,393,179 | 51,993,763 | 49,026,747 |
| Clean reads | 50,028,651 | 51,705,759 | 49,935,243 | 47,402,329 |
| Clean bases | 7.48G | 7.76G | 7.49G | 7.11G |
| Q20% | 96.49 | 96.88 | 96.74 | 95.78 |
| Q30% | 91.29 | 92.04 | 91.8 | 89.35 |
| GC% | 42.88 | 44.99 | 42.35 | 40.24 |
| Transcripts | Unigenes | |||
| 200–500 bp | 98,529 | 16,827 | ||
| 500–1 k bp | 24,766 | 22,585 | ||
| 1 k-2 k bp | 18,588 | 18,539 | ||
| >2 k bp | 29,781 | 29,781 | ||
| Total number | 171,664 | 87,732 | ||
| Min length | 201 | 201 | ||
| Mean length | 1,188 | 2048 | ||
| Max length | 27,920 | 27,920 | ||
| N50 | 3,143 | 3669 | ||
| N90 | 360 | 855 | ||
| Total nucleotides | 203,893,683 | 179,705,476 | ||
| Number of Unigenes | Percentage% | |||
| Annotated in NR | 50,968 | 58.09 | ||
| Annotated in NT | 17,863 | 20.36 | ||
| Annotated in KO | 25,814 | 29.42 | ||
| Annotated in SwissProt | 40,700 | 46.39 | ||
| Annotated in PFAM | 42,320 | 48.23 | ||
| Annotated in GO | 43,197 | 49.23 | ||
| Annotated in KOG | 25,237 | 28.76 | ||
| Annotated in all databases | 8,275 | 9.43 | ||
| Annotated in at least one databases | 56,507 | 64.4 | ||
Annotation of the B. horsfieldi unigenes
The assembled unigenes were annotated against the Nr, Nt, Swiss-Prot, Pfam, GO, KOG/COG, and KO databases[53]. A total of 26,511 unigenes were annotated in B. horsfieldi pupae, 23,380 in larvae, 30,270 in males, and 22,393 in females. Among them, 549 were BP-specific, 595 were BL-specific, 515 were BF-specific, 922 were BM-specific, 11,012 were common among the groups, and 87,732 were in the BP-BL-BF-BM combined dataset (Table 2). The numbers and percentages of unigenes annotated in each of the databases were counted. The Nr database had the best matches against the unigenes in the BP-BL-BF-BM combined dataset (50,968, 58.10%) (Table 2) (S1–S12 Texts).
Table 2. Unigenes annotated in different databases.
| BP | BL | BM | BF | BP-specific | ||||||
| NO. | PCT(%) | NO. | PCT(%) | NO. | PCT(%) | NO. | PCT(%) | NO. | PCT(%) | |
| NR | 24906 | 93.95% | 22026 | 94.21% | 28423 | 93.90% | 21043 | 93.97% | 461 | 83.97% |
| NT | 8718 | 32.88% | 7735 | 33.08% | 8877 | 29.33% | 7595 | 33.92% | 160 | 29.14% |
| KO | 13092 | 49.38% | 11859 | 50.72% | 15030 | 49.65% | 11654 | 52.04% | 180 | 32.79% |
| Swissprot | 20419 | 77.02% | 18296 | 78.25% | 23089 | 76.28% | 17736 | 79.20% | 329 | 59.93% |
| PFAM | 20415 | 77.01% | 18186 | 77.78% | 23183 | 76.59% | 17535 | 78.31% | 389 | 70.86% |
| GO | 20801 | 78.46% | 17211 | 73.61% | 23586 | 77.92% | 18525 | 82.73% | 394 | 71.77% |
| KOG | 13798 | 52.05% | 11991 | 51.29% | 15260 | 50.41% | 11823 | 52.80% | 176 | 32.06% |
| Total NO. | 26511 | 23380 | 30270 | 22393 | 549 | |||||
| BL-specific | BM-specific | BF-specific | Common | BP-BL-BM-BF combined | ||||||
| NO. | PCT(%) | NO. | PCT(%) | NO. | PCT(%) | NO. | PCT(%) | NO. | PCT(%) | |
| NR | 476 | 80.00% | 784 | 85.03% | 397 | 77.09% | 10705 | 97.21% | 50968 | 58.10% |
| NT | 360 | 60.50% | 183 | 19.85% | 272 | 52.82% | 3810 | 34.60% | 17863 | 20.36% |
| KO | 271 | 45.55% | 342 | 37.09% | 218 | 42.33% | 6121 | 55.84% | 25806 | 29.41% |
| Swissprot | 431 | 72.44% | 563 | 61.06% | 360 | 69.90% | 9223 | 83.75% | 40700 | 46.39% |
| PFAM | 421 | 70.76% | 660 | 71.58% | 353 | 68.54% | 8804 | 79.95% | 42320 | 48.24% |
| GO | 435 | 73.11% | 563 | 61.06% | 360 | 69.90% | 8960 | 81.37% | 44535 | 50.76% |
| KOG | 225 | 37.82% | 326 | 35.36% | 174 | 33.79% | 6528 | 59.28% | 25237 | 28.77% |
| Total NO. | 595 | 922 | 515 | 11012 | 87732 | |||||
BP: Unigenes of Batocera horsfieldi pupae; BL: Unigenes of B. horsfieldi larvae; BM: Unigenes of B. horsfieldi males; BF: Unigenes of B. horsfieldi females; BP-specific: Specific unigenes of B. horsfieldi pupae; BL-specific: Specific unigenes of B. horsfieldi larvae; BM-specific: Specific unigenes of B. horsfieldi males; BF-specific: Specific unigenes of B. horsfieldi females; Common: Common unigenes of B. horsfieldi pupae, larvae, males, and females; BP-BL-BM-BF Combined: Total unigenes of B. horsfieldi pupae, larvae, males, and females. NO: number; PCT (%): percentage (%); NR: NCBI non-redundant protein sequences; NT: NCBI non-redundant nucleotide sequences; KO: KEGG Orthology; Swissprot: A manually annotated and reviewed protein sequence database; PFAM: Protein family; GO Gene Ontology; KOG: Clusters of Orthologous Groups of protein; Total NO: Total number of annotated unigenes.
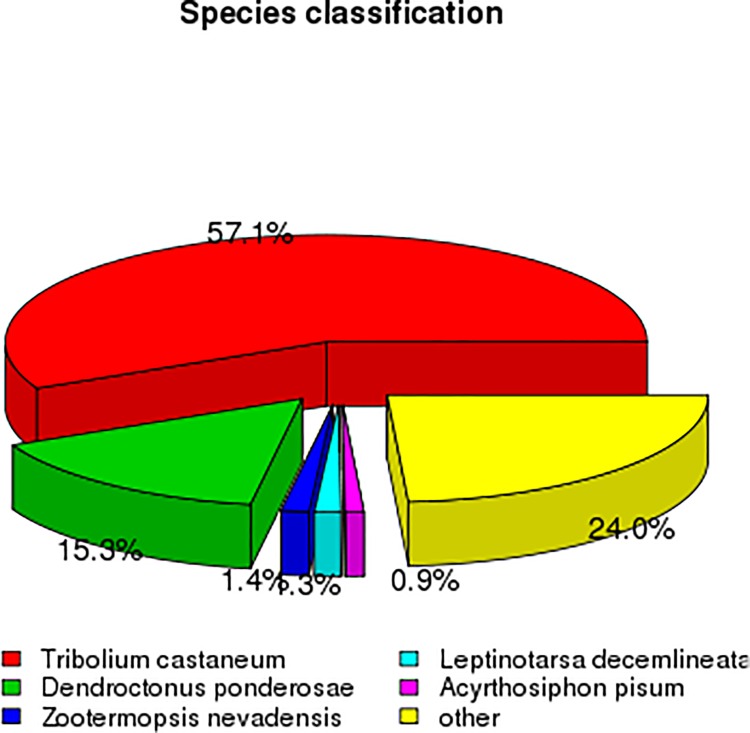
After functional annotation, the numbers of sequences from different species that matched the B. horsfieldi unigenes were calculated from the annotation results. The five most represented species (about 76% of all the species) were Tribolium castaneum (57.1% of the annotated sequences), Dendroctonus ponderosae (15.3%), Zootermopsis nevadensis (1.4%), Leptinotarsa decemlineata (1.3%), and Acyrthosiphon pisum (0.9%), as shown in Fig 1.
Fig 1. The five most represented species among the annotation results of the B. horsfieldi unigenes.
Functional annotation of the B. horsfieldi unigenes
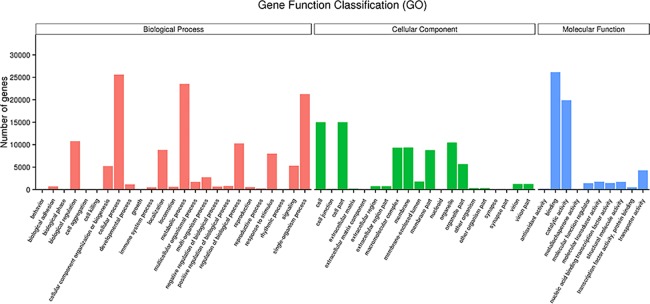
A total of 43,197 unigenes were annotated into 55 sub-categories under the three main GO categories: biological process, cellular component, and molecular function (Fig 2). There were 25 sub-categories under biological process, 20 under cellular component, and 10 under molecular function. The top 10 sub-categories were binding (26,156 unigenes), cellular process (25,616 unigenes), metabolic process (23,538 unigenes), single-organism process (21,263 unigenes), catalytic activity (19,882 unigenes), cell (14,998 unigenes), cell part (14,998 unigenes), biological regulation (10,787 unigenes), organelle (10,503 unigenes), and regulation of biological process (10,283 unigenes) (S13 Text).
Fig 2. Histogram of the gene ontology (GO) classification of the B. horsfieldi unigenes.
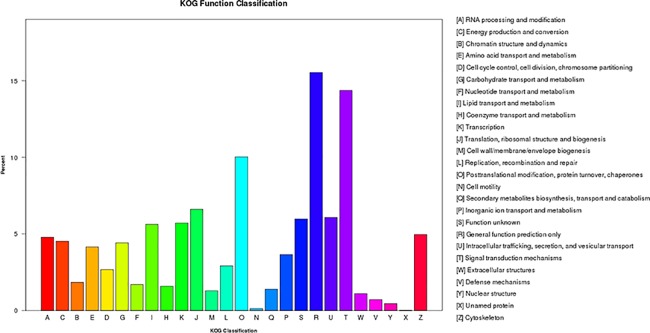
The KOG classification placed 25,237 unigenes into 26 functional categories (Fig 3). The ‘general function prediction only’ category was the largest (3,920 unigenes), followed by ‘signal transduction mechanisms’ (3,625 unigenes), and ‘posttranslational modification, protein turnover, chaperons’ (2,531 unigenes). The top three categories had 39.93% of the unigenes assigned to KOG categories (S14 Text).
Fig 3. Histogram of the clusters of eukaryotic orthologous groups (KOG) classification of the B. horsfieldi unigenes.
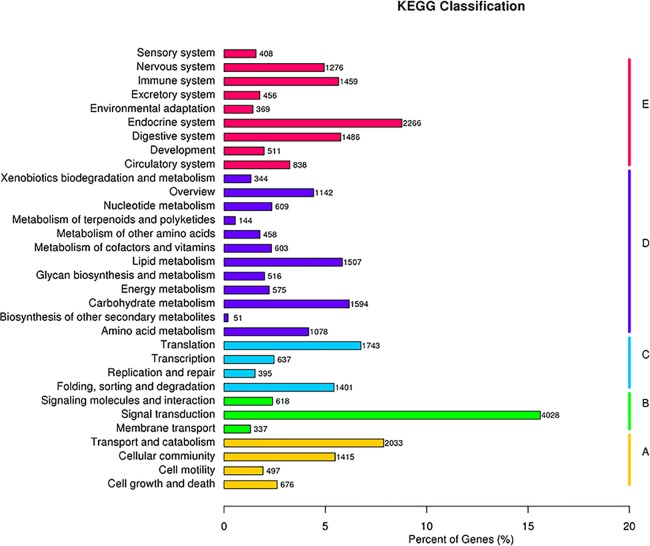
A total of 25,814 unigenes were classified into five KEGG Pathway functional categories (Fig 4): cellular process (4,621 unigenes), environmental information processing (4,983 unigenes), genetic information processing (4,176 unigenes), metabolism (8,621 unigenes), and organismal system (9,069 unigenes). The top three subcategories out of a total of 32 were ‘signal transduction’, ‘endocrine system’, and ‘transport and catabolism’ (S15 Text).
Fig 4. Histogram of the KEGG Pathway classification of the B. horsfieldi unigenes.
(A) Cellular processes, (B) Environmental information processing, (C) Genetic information processing, (D) Metabolism, (E) Organismal systems.
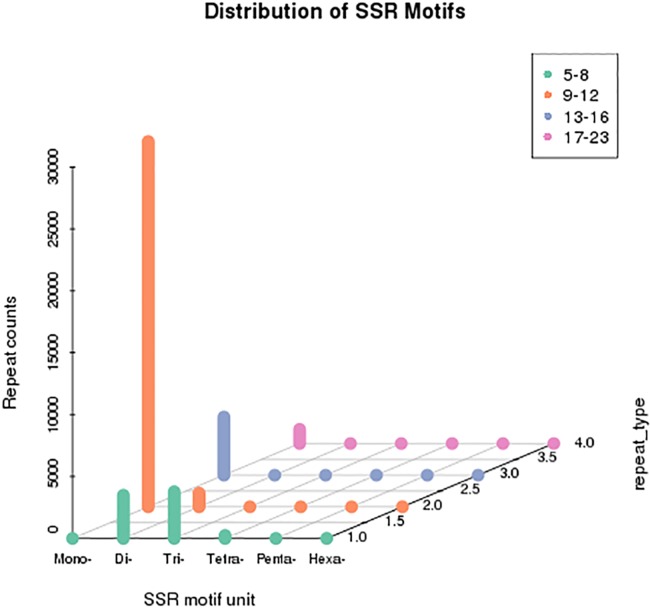
SSR analysis
We screened for SSRs in the B. horsfieldi unigene sequences using MISA software and designed primers with Primer 3 for the SSR markers (Fig 5). We identified 87,732 sequence segments with total length of 179,705,476 bp, among which 44,015 SSRs sequences were authenticated.
Fig 5. Scattergram of simple sequence repeats (SSRs) detected in the B. horsfieldi unigene sequences.
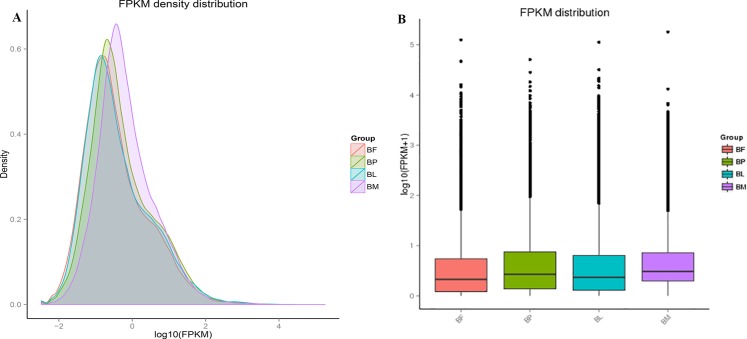
Expected number of fragments per kilobase of transcript sequence per millions base pairs sequenced (FPKM) analysis
We used the RSEM software to calculate statistics for the bowtie comparison results, and convert FPKM[55]. We obtained the number of read counts for each gene and conducted FPKM analysis accordingly. From the perspective of general distribution of expression quantity (Fig 6A) and discrete angle (Fig 6B), the gene expression quantity of different forms of B. horsfieldi are different.
Fig 6. Expression levels of the B. horsfieldi unigenes by FPKM analysis.
(A) The perspective of general distribution. (B) The dispersing perspective. BF: B. horsfieldi females, BP: B. horsfieldi pupae, BL: B. horsfieldi larvae, BM: B. horsfieldi males.
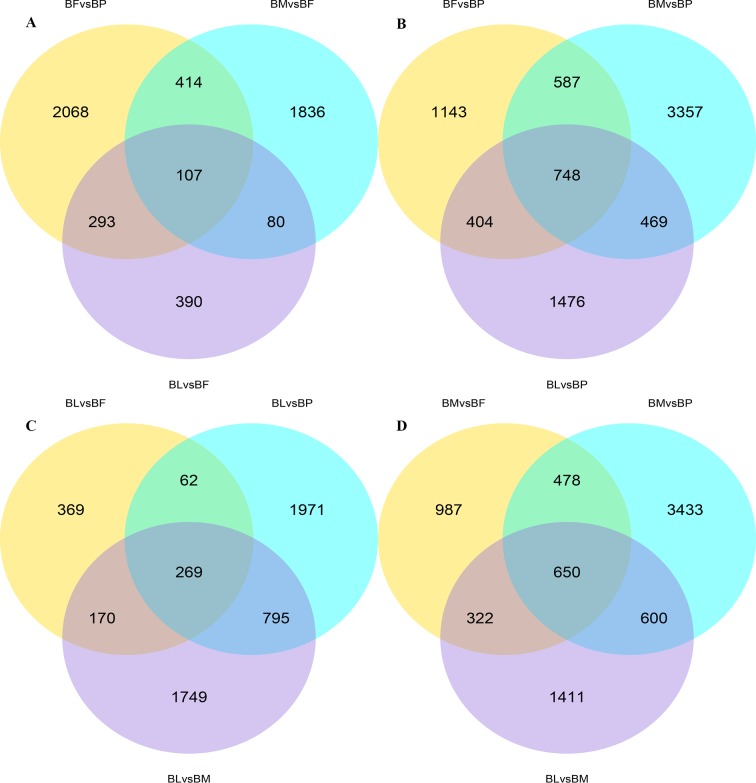
Differentially expressed genes
A total of 2,882, 2,437, and 870 genes were differentially expressed between female and pupae, female and male, female and larvae, respectively, and 107 of these genes were common to pupae, males, larvae, and females (Fig 7A). A total of 2,882, 5,161, and 3,097 genes were differentially expressed between pupae and females, pupae and males, pupae and larvae, respectively, and 748 of these genes were common to males, females, larvae, and pupae (Fig 7B). A total of 870, 3,097, and 2,983 genes were differentially expressed between larvae and female, larvae and pupae, and larvae and male, respectively, and 269 of these genes were common to females, males, pupae, and larvae (Fig 7C). A total of 2,437, 5,161, and 2,983 genes were differentially expressed between males and females, males and pupae, and males and larvae, respectively, and 650 of these genes were common to larvae, females, pupae, and males (Fig 7D) (S16–S21 Texts).
Fig 7. Venn diagram of the number of differentially expressed genes in males, females, larvae, and pupae.
BF: B. horsfieldi females, BP: B. horsfieldi pupae, BL: B. horsfieldi larvae, BM: B. horsfieldi males.
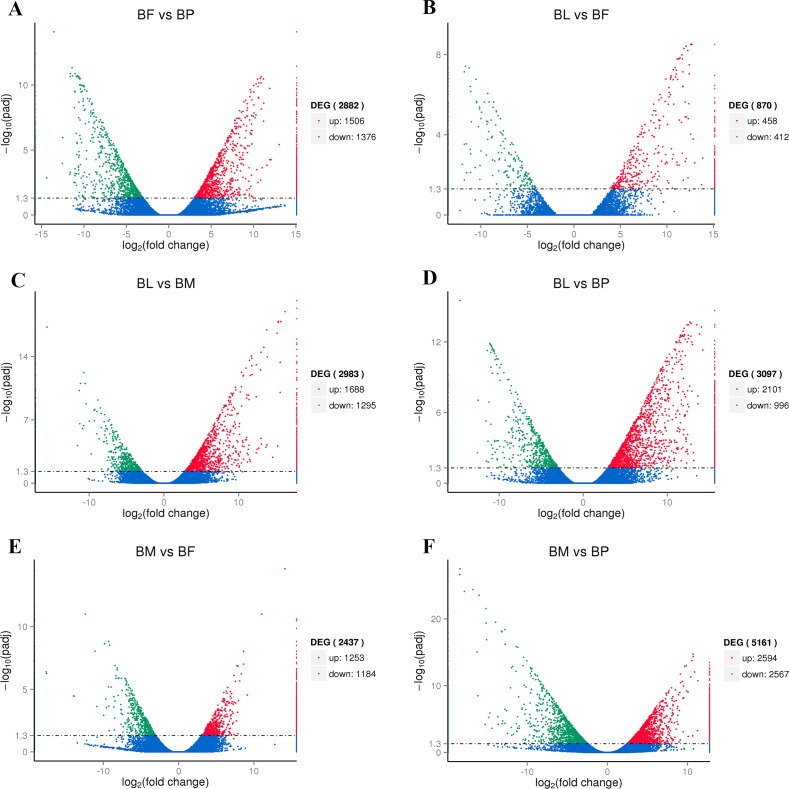
More genes were expressed in female than in pupae, in larvae than in female, in larvae than in male, in larvae than in pupae, in male than in female, and in male than in pupae (1,506, 458, 1688, 2,101, 1,253, and 2,594, respectively; Fig 8). Conversely, fewer genes were expressed in female than in pupae, in larvae than in female, in larvae than in male, in larvae than in pupae, in male than in female, and in male than in pupae (1,376, 412, 1,295, 996, 1,184, and 2,567, respectively; Fig 8).
Fig 8. Volcano plots of differentially expressed genes in larvae, pupae, males, and females.
Differentially expressed genes between (A) BF and BP, (B) BL and BF, (C) BL and BM, (D) BL and BP, (E) BM and BF and (F) BM and BP. Splashes represent different genes. Blue splashes indicate genes with no significant differential expression. Red splashes indicate significantly upregulated genes. Green splashes indicate significantly downregulated genes. BF: B. horsfieldi females, BP: B. horsfieldi pupae, BL: B. horsfieldi larvae, BM: B. horsfieldi males.
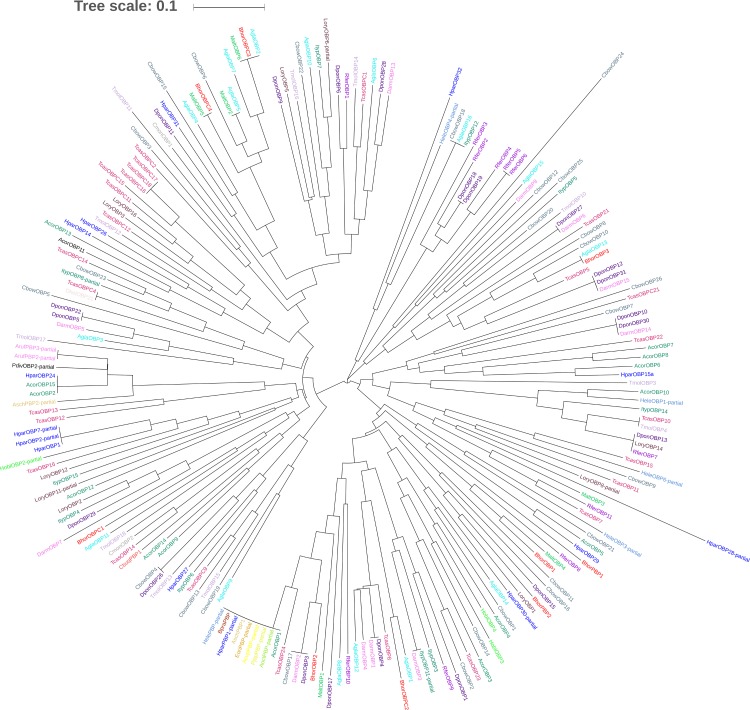
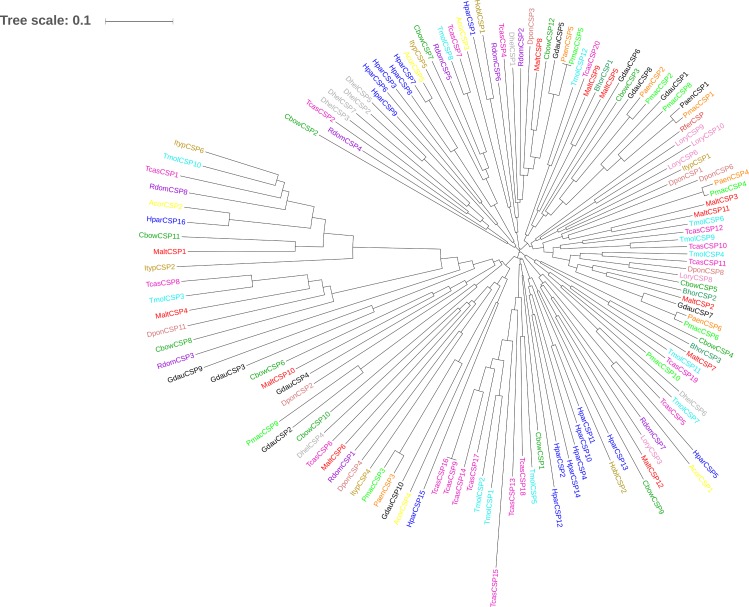
Phylogenetic analysis of candidate olfactory genes
We constructed two phylogenetic trees comparing BhorOBP1/2/3, BhorOBPC1/2/3/4 (minus-C OBP1/2/3/4) and the OBPs from 25 coleopteran insects, and BhorCSP1/2/3 and the CSPs from 16 coleopteran insects, respectively (Figs 9 and 10). Four minus-C OBPs from B. horsfieldi grouped together with the OBPs of other coleopteran species, whereas three OBPs of B. horsfieldi separated into different clades (Fig 9). The candidate CSPs, BhorCSP1, BhorCSP2, and BhorCSP3, separated into different clades (Fig 10).
Fig 9. Neighbor-joining phylogenetic tree of B. horsfieldi odorant binding proteins (BhorOBPs).
Values indicated at the nodes are bootstrap values based on 1000 replicates. Scale bar = 0.1. Bhor: Batocera horsfieldi; Tcas: Tribolium castaneum; Ityp: Ips typographus; Dpon: Dendroctonus ponderosae; Acor: Anomala corpulenta; Tmol: Tenebrio molitor; Malt: Monochamus alternatus; Pdiv: Phyllopertha diversa; Cmon: Cryptolaemus montrouzieri; Dhel: Dasrarcus helophoroides; Rfer: Rhynchophorus ferrugineus; Cbow: Colaphellus bowringi; Lory: Lissorhoptrus oryzophilus; Hobl: Holotrichia oblita; Agla: Anoplophora glabripennis; Hele: Hylamorpha elegans; Darm: Dendroctonus armandi; Hpar: Holotrichia parallela; Bpra: Brachysternus prasinus; Pjap: Popillia japonica; Eori: Exomala orientalis; Acup: Anomala cuprea; Asch: Anomala schonfeldti; Aoct: Anomala octiescostata; Aruf: Anomala rufocuprea; Cbuq: Cyrtotrachelus buqueti. The olfactory genes from different species are marked with different colors (S24 Text).
Fig 10. Neighbor-joining phylogenetic tree of B. horsfieldi chemosensory proteins (BhorCSPs).
Values indicated at the nodes are bootstrap values based on 1000 replicates. Scale bar = 0.1. Hpar: Holotrichia parallela; Tmol: Tenebrio molitor; Ityp: Ips typographus; Dpon: Dendroctonus ponderosae; Tcas: Tribolium castaneum; Acor: Anomala corpulenta; Bhor: Batocera horsfieldi; Gdau: Galeruca daurica; Paen: Pyrrhalta aenescens; Pmac: Pyrrhalta maculicollis; Dhel: Dasrarcus helophoroides; Cbow: Colaphellus bowringi; Malt: Monochamus alternatus; Rdom: Rhyzopertha dominica; Hobl: Holotrichia oblita; Lory: Lissorhoptrus oryzophilus; Rfer: Rhynchophorus ferrugineus. The olfactory genes from different species are marked with different colors (S25 Text).
Expression profiles of candidate olfactory genes
We identified 10 candidate CSPs and 16 candidate OBPs by searches against the Nr database (Deduced amino acid sequences are listed in S22 Text). Significant differences were detected in the expression profiles of the 10 candidate CSPs and 16 candidate OBPs in male and female adults (Tables 3 and 4, respectively).
Table 3. Differentially expressed CSP between males and females.
| Gene | Readcount-Male | Readcount-Female | log2Fold-change | q |
|---|---|---|---|---|
| Cluster-8309.12499 | 144.0953 | 7.6845 | 4.2280 | <0.005* |
| Cluster-8309.21386 | 283.1867 | 103.2319 | 1.4569 | >0.005 |
| Cluster-8309.22628 | 320.1960 | 200.2058 | 0.6775 | >0.005 |
| Cluster-8309.36051 | 1,276.0900 | 660.3976 | 0.9503 | >0.005 |
| Cluster-8309.36377 | 5,298.4300 | 6,861.21 | -0.3729 | >0.005 |
| Cluster-8309.36573 | 7,629.0900 | 8,266.90 | -0.1158 | >0.005 |
| Cluster-8309.41714 | 439.4133 | 44.2061 | 3.3133 | <0.005* |
| Cluster-8309.45810 | 527.3126 | 112.3486 | 2.2307 | >0.005 |
| Cluster-8309.52580 | 1,218.3300 | 186.9083 | 2.7045 | >0.005 |
| Cluster-8309.54261 | 545.3564 | 63.9106 | 3.0931 | <0.005* |
Q values were calculated according to the method of Anders et al., 2003.
*q < 0.005 is significantly different.
Table 4. Differentially expressed OBPs between males and females.
| Gene | Readcount-Male | Readcount-Female | log2Fold-change | q |
|---|---|---|---|---|
| Cluster-8309.12425 | 174.0043 | 102.9302 | 0.7575 | >0.005 |
| Cluster-8309.20864 | 122.1861 | 840.4307 | -2.8192 | <0.005* |
| Cluster-8309.27359 | 5,568.7170 | 720.3645 | 2.8177 | <0.005* |
| Cluster-8309.31830 | 8,752.9100 | 1,295.3270 | 2.7564 | <0.005* |
| Cluster-8309.32140 | 186.4977 | 61.9745 | 1.5894 | >0.005 |
| Cluster-8309.36213 | 2,129.661 | 44,723.7300 | -4.3923 | <0.005* |
| Cluster-8309.36426 | 57,197.9800 | 32,936.1900 | 0.7963 | >0.005 |
| Cluster-8309.37524 | 890.3307 | 208.1874 | 2.0965 | >0.005 |
| Cluster-8309.38445 | 5,044.1540 | 10,089.0100 | -1.0001 | >0.005 |
| Cluster-8309.39085 | 217.9598 | 163.1848 | 0.4176 | >0.005 |
| Cluster-8309.39777 | 682.6873 | 1416.6270 | -1.0532 | >0.005 |
| Cluster-8309.40165 | 399.8992 | 354.8279 | 0.1725 | >0.005 |
| Cluster-8309.40672 | 421.7275 | 641.6447 | -0.6055 | >0.005 |
| Cluster-8309.41624 | 3,1696.7900 | 494.2113 | 6.0031 | <0.005* |
| Cluster-8309.47478 | 4,136.7770 | 521.3067 | 2.7235 | <0.005* |
| Cluster-8309.59754 | 589.7868 | 60.3018 | 3.0721 | <0.005* |
Q values were calculated according to the method of Anders et al., 2003.
*q < 0.005 is significantly different.
To further explore the olfactory genes, a local BLAST search was performed against the B. horsfieldi unigene database using the known olfactory gene sequences of Lissorhoptrus oryzophilus, Monochamus alternatus, and Dendroctonus ponderosae as queries. The calculated expression values based on the FKPM method of all the candidate olfactory genes are listed in Table 5.
Table 5. Detailed information on the candidate olfactory genes of B. horsfieldi.
| Gene name | Unigene ID | Gene length | Status | FPKM (Pupae/Larvae/Male/Female) | BLASTx best hit | Gene ID |
|---|---|---|---|---|---|---|
| BhorOBP1 | Cluster-8309.27359 | 1699 | Complete ORF | 15.50/16.36/269.25/23.25 | odorant binding protein [Lissorhoptrus oryzophilus] | KC461118.1 |
| BhorOBP2 | Cluster-8309.40672 | 695 | Complete ORF | 30.37/311.36/66.44/59.51 | odorant binding protein 6 [Monochamus alternatus] | KC461116.1 |
| BhorOBP3 | Cluster-8309.41624 | 2778 | Complete ORF | 18.53/233.05/872.11/7.41 | odorant-binding protein 2 [Monochamus alternatus] | KC461117.1 |
| BhorOBP C1 | Cluster-8309.39777 | 739 | Complete ORF | 0.57/56.57/97.70/118.34 | minus-C odorant binding protein 1 [Batocera horsfieldi] | GU575294.1 |
| BhorOBP C2 | Cluster-8309.59754 | 943 | Complete ORF | 144.19/60.64/59.26/9.97 | minus-C odorant binding protein 2 [Batocera horsfieldi] | GU575295.1 |
| BhorOBP C3 | Cluster-8309.47478 | 574 | Complete ORF | 369.84/9.13/890.61/88.60 | minus-C odorant binding protein 4 [Batocera horsfieldi] | GU584933.1 |
| BhorOBP C4 | Cluster-8309.31830 | 1229 | Complete ORF | 130.68/44.54/623.72/51.82 | minus-C odorant binding protein 4 [Batocera horsfieldi] | GU584934.1 |
| BhorCSP1 | Cluster-8309.12499 | 888 | Complete ORF | 2.10/778.25/15.60/0.48 | chemosensory protein [Batocera horsfieldi] | HQ587040.1 |
| BhorCSP2 | Cluster-8309.41714 | 432 | Complete ORF | 15.04/536.49/167.20/11.72 | chemosensory protein 8 [Lissorhoptrus oryzophilus] | HQ587041.1 |
| BhorCSP3 | Cluster-8309.54261 | 2016 | Complete ORF | 13.50/7.44/21.38/1.38 | chemosensory protein 4 [Dendroctonus ponderosae] | HQ587042.1 |
Tissue- and sex-specific expressions of candidate olfactory genes
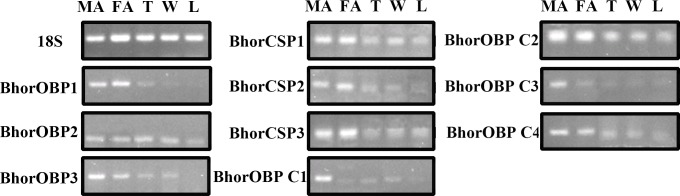
Semi-quantitative RT-PCR showed that all of the tested candidate olfactory genes were primarily male antenna specific (Fig 11) (S23 Text). BhorOBP2/C2 and BhorCSP1 showed olfactory and non-olfactory tissue expression, whereas BhorOBP1/3, BhorOBPC1/C3/C4, and BhorCSP2/3 showed olfactory tissue-specific expression. BhorOBP3 and BhorOBPC1/C3 were highly expressed in the antenna of males.
Fig 11. Tissue-specific expressions of candidate olfactory genes of B. horsfieldi by RT-PCR.
FA: female antenna; MA: male antenna; T: thorax; W: hind wing; L: leg. 18S RNA was used as an internal control.
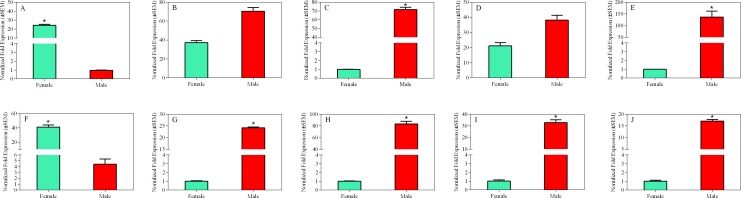
The RT-qPCR results were mostly consistent with the RT-PCR results; BhorOBP2 and BhorCSP1 showed high non-sex-specific expression, whereas BhorOBP1/3, BhorCSP2/3, and BhorOBPC1/C2/C3/C4 showed high sex-specific expression (Fig 12).
Fig 12. Sex-specific expressions of candidate olfactory genes of B. horsfieldi by RT-qPCR.
The expression levels of male and female adults are indicated by red and green bars, respectively. Expression levels were calculated by the 2−ΔΔCT method with three biological repeats. Asterisk shows a significant expression level (Chi-square test, p<0.05). (A–J) Sex-specific differentially expressed genes between male and female adults. (A) BhorOBP1, (B) BhorOBP2, (C) BhorOBP3, (D) BhorCSP1, (E) BhorCSP2, (F) BhorCSP3, (G) BhorOBPC1, (H) BhorOBPC2, (I) BhorOBPC3, (J) BhorOBPC4.
Discussion
Establishing insect transcription libraries for high-throughput sequencing is an important approach for molecular biology studies of insects. Transcriptome data can be used to detect new genes, transcription sites, and differentially expressed genes, as well as obtain functional gene information and transcription expression abundance, and the data can be used for molecular marker development, gene expression analysis, and small RNA analysis. [56–59]. Transcriptome for many insects has been established and analyzed, including Ericerus pela [60], M. alternatus [61], Timema cristinae [62], Cyrtotrachelus buqueti [53], Drosophila melanogaster [63], Bombyx mori [64], and Bombus terrestris [65].
In the present study, developmental transcriptomes were established for B. horsfieldi at various stages, mixed-age larvae, pupae, and male and female adults, and a relatively comprehensive gene pool was obtained. The alignments against the Nr database showed that 57.1% and 15.3% of the B. horsfieldi unigenes were similar to T. castaneum and D. ponderosae sequences, respectively. Thousands of differentially expressed genes were identified, facilitating developmental and evolutionary studies of B. horsfieldi, and contribute to future work in B. horsfieldi comparative genomics. Insects can sense changes in odorant substances in the environment through olfactory receptors, which transform chemical signals from odorant substances into electrophysiology signals that can generate various kinds of behaviors [11]. Proteins are required for odorant substances to interact with insect olfactory receptors, and for turning the chemical signals into electrophysiology signals [66]. These proteins are related to the olfactory sensation of insects and participate in the transmission of a series of signals in the insect olfactory system [67]. Moreover, olfactory proteins have been shown to act in insect nutrient uptake, life span, and behavior changes during developmental stages [67–69]. The developmental transcriptomes of B. horsfieldi provide an opportunity to understand the relationship between olfactory proteins and development. The evolution analysis of the OBPs of B. horsfieldi and 25 Coleoptera species and the CSPs of B. horsfieldi and 16 Coleoptera species showed that the OBPs of B. horsfieldi were quite similar to those of Anoplophora glabripennis and the CSPs of B. horsfieldi were quite similar to those of Monochamus alternates. Therefore, we speculated that these genes may have evolved from the same ancestral gene, but differentiated by adaptation to different types of environmental chemical factors during evolution, and perform the same or similar functions among different species [53].
Seven candidate OBPs and three candidate CSPs in male and female adults, showed significant differences in expression. To further explore the significant differences among these genes, a local BLAST was performed on the B. horsfieldi unigene database based on the known olfactory sequences of L. oryzophilus, M. alternatus, D. ponderosae, and B. horsfieldi. BhorOBP1/2/3/C1/C2/C3/C4 were annotated as encoding odorant binding protein domains, while BhorCSP1/2/3 were annotated as encoding chemosensory protein domains. The cloning and functional analysis of olfactory genes will be the focus of the next research.
Combining RT-PCR and RT-qPCR data, most of the candidate olfactory genes were shown to have male-specific expression patterns in B. horsfieldi, suggesting that the olfactory system is highly developed in male and that olfactory detection plays a relatively important role in males. This result supports the existence of a contact sex pheromone that is produced by B. horsfieldi female, as previously shown on the molecular level [70]. Additionally, components of the female-produced sex pheromone have been identified in other longhorn beetles, such as Prionus californicus [71], Migdolus fryanus [72], Vesperus xatarti [73], and Ortholeptura valida [74].
BhorOBP1/3, BhorOBPC1/C3/C4, and BhorCSP2/3 showed olfactory-specific expression, suggesting that these candidate olfactory genes may play key roles in foraging and host-orientation in B. horsfieldi. A comprehensive, good-quality sequence resource from the developmental transcriptomes of B. horsfieldi larvae, pupae, female and male adults was constructed in this study. This resource enriches what is known about B. horsfieldi genomics, thus facilitating our understanding of metamorphosis, development, and fitness to environmental change. Several potential functional olfactory genes were identified. Future studies aimed at exploring the functions of these genes are the next logical step.
Supporting information
(DOCX)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLS)
(DOCX)
(DOCX)
Acknowledgments
We thank Novogene (Beijing, China) for assistance in original data processing and related bioinformatics analysis. We thank the members of our laboratory for the collection of materials. The materials used in this work were supported by the Sichuan Provincial Key Laboratory of Ecological Forestry Engineering, Sichuan Agricultural University, Chengdu, China. We thank Margaret Biswas, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China: 31270694 to Wei Yang. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gao RT, Wang HQ, Xu BX, Zheng SK, Wang XQ, Gong YH. Study on the habit of absorbing replenishing nutrition of Batocera horsfieldi and its relation with the host trees. Forest Research. 1995;8(6):619–23. [Google Scholar]
- 2.Li J, Wang MQ, Zhang ZC, Chen JY, Zhang GA. Behavioral response of Batocera horsfieldi adults to plant volatiles. Scientia Silvae Sinicae. 2008;44(6):168–70. [Google Scholar]
- 3.Li JQ, Yang ZQ, Mei ZX, Zhang YL. Pest risk analysis and control countermeasure of Batocera horsfieldi. Forest Research. 2009;22(1):148–53. [Google Scholar]
- 4.Liang XY, Yang W, Yang YL, Yang CP, Yang Y. Preference of Batocera horsfieldi for adults to feeding plants. Chinese Bulletion of Entomology. 2008;45(1):78–82. [Google Scholar]
- 5.Sun QY, Zhao ZC. A preliminary study on Batocera horsfieldi. Journal of Jiangsu Forestry Science and Technology. 1991;2(1):22–5. [Google Scholar]
- 6.Yang H, Yang W, Yang CP, Zhu TH, Huang Q, Han S, et al. Electrophysiological and behavioral responses of the whitestriped longhorned beetle, Batocera lineolata, to the diurnal rhythm of host plant volatiles of holly, Viburnum awabuki. J Insect Sci. 2013;13(6):85–101. Epub 2013/11/16. doi: 10.1673/031.013.8501 ; PubMed Central PMCID: PMC3835053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan AJ, Ji BZ, Qian FJ, Chen JY, Ye ZY. A study on Batocera horsfieldi (Hope). Nanjiang Forestry University. 1997;21(1):1–6. [Google Scholar]
- 8.Zhuge PP, Luo SL, Wang MQ, Zhang G. Electrophysiological responses of Batocera horsfieldi (Hope) adults to plant volatiles. Journal of Applied Entomology. 2010;134(7):600–7. [Google Scholar]
- 9.Yang H, Yang W, Yang MF, Yang CP, Zhu TH, Huang Q. Effects of plant volatiles on the EAG and behavioral responses of Batocera horsfieldi Hope (Coleoptera: Cerambycidae). Journal of Agricultural and Urban Entomology. 2010;27(1):20–32. [Google Scholar]
- 10.Yang H, Yang W, Yang CP, Yang MF, Wang BX, Zhu TH, et al. Behavioral responses of encountering adults of the white-striped longhorn beetle, Batocera lineolata (Coleoptera: Cerambycidae), in the laboratory conditions. Acta Entomol Sin. 2012;55(1):70–6. [Google Scholar]
- 11.Li H, Zhang G, Wang MQ. Chemosensory protein genes of Batocera horsfieldi (Hope): identification and expression pattern. Journal of Applied Entomology. 2012;136(10):781–92. [Google Scholar]
- 12.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–14. Epub 2004/09/02. doi: 10.1016/j.neuron.2004.08.019 S0896627304005264 [pii]. . [DOI] [PubMed] [Google Scholar]
- 13.Gu SH, Zhang XY, Zhang YJ, Wu KM, Guo YY. Cloning and expression pattern analysis of an odorant binding protein gene AlinOBP1 in the lucerne plant bug, Adelphocoris lineolatus (Goeze) (Hemiptera: Miridae). Acta Entomol Sin. 2010;53(5):487–96. [Google Scholar]
- 14.Gu SH, Wang SP, Zhang XY, Wu KM, Guo YY, Zhou JJ, et al. Identification and tissue distribution of odorant binding protein genes in the lucerne plant bug Adelphocoris lineolatus (Goeze). Insect Biochem Mol Biol. 2011;41(4):254–63. Epub 2011/01/15. doi: S0965-1748(11)00004-X [pii] doi: 10.1016/j.ibmb.2011.01.002 . [DOI] [PubMed] [Google Scholar]
- 15.Ji P, Liu JT, Gu SH, Zhu XQ, Zhang YJ, Guo YY. Expression and binding specificity analysis of odorant binding protein AlucOBP7 from Apolygus lucorum (Hemiptera: Miridae). Acta Entomol Sin. 2013;56(6):575–83. [Google Scholar]
- 16.Pelosi P, Zhou JJ, Ban LP, Calvello M. Soluble proteins in insect chemical communication. Cell Mol Life Sci. 2006;63(14):1658–76. Epub 2006/06/21. doi: 10.1007/s00018-005-5607-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campanacci V, Krieger J, Bette S, Sturgis JN, Lartigue A, Cambillau C, et al. Revisiting the specificity of Mamestra brassicae and Antheraea polyphemus pheromone-binding proteins with a fluorescence binding assay. J Biol Chem. 2001;276(23):20078–84. Epub 2001/03/29. doi: 10.1074/jbc.M100713200 M100713200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Gu S, Zhang Y, Guo Y, Wang G. Candidate olfaction genes identified within the Helicoverpa armigera Antennal Transcriptome. PLoS One. 2012;7(10):e48260 Epub 2012/10/31. doi: 10.1371/journal.pone.0048260 PONE-D-12-23655 [pii]. ; PubMed Central PMCID: PMC3482190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maida R, Ziegelberger G, Kaissling KE. Ligand binding to six recombinant pheromone-binding proteins of Antheraea polyphemus and Antheraea pernyi. J Comp Physiol B. 2003;173(7):565–73. Epub 2003/07/25. doi: 10.1007/s00360-003-0366-4 . [DOI] [PubMed] [Google Scholar]
- 20.Maleszka R, Stange G. Molecular cloning, by a novel approach, of a cDNA encoding a putative olfactory protein in the labial palps of the moth Cactoblastis cactorum. Gene. 1997;202(1–2):39–43. Epub 1998/01/14. doi: S0378-1119(97)00448-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 21.Vogt RG, Prestwich GD, Lerner MR. Odorant-binding-protein subfamilies associate with distinct classes of olfactory receptor neurons in insects. J Neurobiol. 1991;22(1):74–84. Epub 1991/01/01. doi: 10.1002/neu.480220108 . [DOI] [PubMed] [Google Scholar]
- 22.Wang GR, Wu KM, Guo YY. Cloning, expression and immunocytochemical localization of a general odorant-binding protein gene from Helicoverpa armigera (Hübner). Insect Biochem Mol Biol. 2003;33(1):115–24. Epub 2002/12/03. doi: S0965174802001820 [pii]. . [DOI] [PubMed] [Google Scholar]
- 23.Xiu WM, Dong SL. Molecular characterization of two pheromone binding proteins and quantitative analysis of their expression in the beet armyworm, Spodoptera exigua Hübner. J Chem Ecol. 2007;33(5):947–61. Epub 2007/03/30. doi: 10.1007/s10886-007-9277-2 . [DOI] [PubMed] [Google Scholar]
- 24.Biessmann H, Walter MF, Dimitratos S, Woods D. Isolation of cDNA clones encoding putative odourant binding proteins from the antennae of the malaria-transmitting mosquito, Anopheles gambiae. Insect Mol Biol. 2002;11(2):123–32. Epub 2002/04/23. doi: 316 [pii]. . [DOI] [PubMed] [Google Scholar]
- 25.Ozaki M, Wada-Katsumata A, Fujikawa K, Iwasaki M, Yokohari F, Satoji Y, et al. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science. 2005;309(5732):311–4. Epub 2005/06/11. doi: 1105244 [pii] doi: 10.1126/science.1105244 . [DOI] [PubMed] [Google Scholar]
- 26.Ban L, Scaloni A, D'Ambrosio C, Zhang L, Yahn Y, Pelosi P. Biochemical characterization and bacterial expression of an odorant-binding protein from Locusta migratoria. Cell Mol Life Sci. 2003;60(2):390–400. Epub 2003/04/08. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickens JC, Callahan FE, Wergin WP, Erbe EF. Olfaction in a hemimetabolous insect: Antennal-specific protein in adult Lygus lineolaris (Heteroptera: Miridae). Journal of Insect Physiology. 1995;41(10):857–67. [Google Scholar]
- 28.Krieger MJ, Ross KG. Identification of a major gene regulating complex social behavior. Science. 2002;295(5553):328–32. Epub 2001/11/17. doi: 10.1126/science.1065247 1065247 [pii]. . [DOI] [PubMed] [Google Scholar]
- 29.Lu D, Li X, Liu X, Zhang Q. Identification and molecular cloning of putative odorant-binding proteins and chemosensory protein from the bethylid wasp, Scleroderma guani Xiao et Wu. J Chem Ecol. 2007;33(7):1359–75. Epub 2007/06/15. doi: 10.1007/s10886-007-9310-5 . [DOI] [PubMed] [Google Scholar]
- 30.Graham LA, Brewer D, Lajoie G, Davies PL. Characterization of a subfamily of beetle odorant-binding proteins found in hemolymph. Mol Cell Proteomics. 2003;2(8):541–9. Epub 2003/07/29. doi: 10.1074/mcp.M300018-MCP200 M300018-MCP200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 31.Nagnan-Le Meillour P, Francois MC, Jacquin-Joly E. Identification and molecular cloning of putative odorant-binding proteins from the American palm weevil, Rhynchophorus palmarum L. J Chem Ecol. 2004;30(6):1213–23. Epub 2004/08/12. . [DOI] [PubMed] [Google Scholar]
- 32.McKenna MP, Hekmat-Scafe DS, Gaines P, Carlson JR. Putative Drosophila pheromone-binding proteins expressed in a subregion of the olfactory system. J Biol Chem. 1994;269(23):16340–7. Epub 1994/06/10. . [PubMed] [Google Scholar]
- 33.Picimbon JF, Dietrich K, Breer H, Krieger J. Chemosensory proteins of Locusta migratoria (Orthoptera: Acrididae). Insect Biochem Mol Biol. 2000;30(3):233–41. Epub 2000/03/25. doi: S0965174899001216 [pii]. . [DOI] [PubMed] [Google Scholar]
- 34.Wanner KW, Willis LG, Theilmann DA, Isman MB, Feng Q, Plettner E. Analysis of the insect os-d-like gene family. J Chem Ecol. 2004;30(5):889–911. Epub 2004/07/28. . [DOI] [PubMed] [Google Scholar]
- 35.Marchese S, Angeli S, Andolfo A, Scaloni A, Brandazza A, Mazza M, et al. Soluble proteins from chemosensory organs of Eurycantha calcarata (Insects, Phasmatodea). Insect Biochem Mol Biol. 2000;30(11):1091–8. Epub 2000/09/16. doi: S0965174800000849 [pii]. . [DOI] [PubMed] [Google Scholar]
- 36.Gu SH, Wu KM, Guo YY, Field LM, Pickett JA, Zhang YJ, et al. Identification and expression profiling of odorant binding proteins and chemosensory proteins between two wingless morphs and a winged morph of the cotton aphid Aphis gossypii glover. PLoS One. 2013;8(9):e73524 Epub 2013/09/28. doi: 10.1371/journal.pone.0073524 PONE-D-13-14003 [pii]. ; PubMed Central PMCID: PMC3779235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong DP, Zhang HJ, Zhao P, Lin Y, Xia QY, Xiang ZH. Identification and expression pattern of the chemosensory protein gene family in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2007;37(3):266–77. Epub 2007/02/14. doi: S0965-1748(06)00250-5 [pii] doi: 10.1016/j.ibmb.2006.11.012 . [DOI] [PubMed] [Google Scholar]
- 38.Gu SH, Sun L, Yang RN, Wu KM, Guo YY, Li XC, et al. Molecular characterization and differential expression of olfactory genes in the antennae of the black cutworm moth Agrotis ipsilon. PLoS One. 2014;9(8):e103420 Epub 2014/08/02. doi: 10.1371/journal.pone.0103420 PONE-D-14-12668 [pii]. ; PubMed Central PMCID: PMC4118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu JY, Zhao N, Yang B. Global Transcriptional Analysis of Olfactory Genes in the Head of Pine Shoot Beetle, Tomicus yunnanensis. Comp Funct Genomics. 2012;2012(5828):491748 Epub 2012/07/05. doi: 10.1155/2012/491748 ; PubMed Central PMCID: PMC3385610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grosse-Wilde E, Kuebler LS, Bucks S, Vogel H, Wicher D, Hansson BS. Antennal transcriptome of Manduca sexta. Proc Natl Acad Sci U S A. 2011;108(18):7449–54. Epub 2011/04/19. doi: 1017963108 [pii] doi: 10.1073/pnas.1017963108 ; PubMed Central PMCID: PMC3088587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legeai F, Malpel S, Montagne N, Monsempes C, Cousserans F, Merlin C, et al. An Expressed Sequence Tag collection from the male antennae of the Noctuid moth Spodoptera littoralis: a resource for olfactory and pheromone detection research. BMC Genomics. 2011;12(1):86. Epub 2011/02/01. doi: 1471-2164-12-86 [pii] doi: 10.1186/1471-2164-12-86 ; PubMed Central PMCID: PMC3045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji BZ, Qian FJ, Yan AJ. Improvement of method of Batocera horsfieldi (Hope). Forest Pest and Disease. 1996;1(1):45–6. [Google Scholar]
- 43.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013;8(8):1494–512. Epub 2013/07/13. doi: nprot.2013.084 [pii] doi: 10.1038/nprot.2013.084 ; PubMed Central PMCID: PMC3875132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Xiong M, Lei C, Zhu F. The developmental transcriptome of the synanthropic fly Chrysomya megacephala and insights into olfactory proteins. BMC Genomics. 2015;16(1):1–12. Epub 2015/01/24. doi: 10.1186/s12864-014-1200-y s12864-014-1200-y [pii]. ; PubMed Central PMCID: PMC4311427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pei MS, Niu JX, Li CJ, Cao FJ, Quan SW. Identification and expression analysis of genes related to calyx persistence in Korla fragrant pear. BMC Genomics. 2016;17(1):132–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52. Epub 2011/05/17. doi: nbt.1883 [pii] doi: 10.1038/nbt.1883 ; PubMed Central PMCID: PMC3571712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang LW, Ming R, Zhang JS, Tao AF, Fang PP, Qi JM. De novo transcriptome sequence and identification of major bast-related genes involved in cellulose biosynthesis in jute (Corchorus capsularis L.). BMC Genomics. 2015;1(16):1062–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. Epub 2010/10/29. doi: gb-2010-11-10-r106 [pii] doi: 10.1186/gb-2010-11-10-r106 ; PubMed Central PMCID: PMC3218662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–5. Epub 2010/05/04. doi: nbt.1621 [pii] doi: 10.1038/nbt.1621 ; PubMed Central PMCID: PMC3146043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–9. Epub 2011/05/07. doi: msr121 [pii] doi: 10.1093/molbev/msr121 ; PubMed Central PMCID: PMC3203626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang YL, Chen Q, Zhao HB, Ren BZ. Identification and comparison of candidate olfactory genes in the olfactory and non-olfactory organs of elm pest Ambrostoma quadriimpressum (Coleoptera: Chrysomelidae) based on transcriptome analysis. PLoS One. 2016;11(1–28). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glusman G, Bahar A, Sharon D, Pilpel Y, White J, Lancet D. The olfactory receptor gene superfamily: data mining, classification, and nomenclature. Mamm Genome. 2000;11(11):1016–23. Epub 2000/11/04. . [DOI] [PubMed] [Google Scholar]
- 53.Yang H, Su T, Yang W, Yang C, Lu L, Chen Z. The developmental transcriptome of the bamboo snout beetle Cyrtotrachelus buqueti and insights into candidate pheromone-binding proteins. PLoS One. 2017;12(6):e0179807 Epub 2017/07/01. doi: 10.1371/journal.pone.0179807 PONE-D-17-00530 [pii]. ; PubMed Central PMCID: PMC5491049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. Epub 2002/02/16. doi: 10.1006/meth.2001.1262 S1046-2023(01)91262-9 [pii]. . [DOI] [PubMed] [Google Scholar]
- 55.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2010;12:323. Epub 2011/08/06. doi: 1471-2105-12-323 [pii] doi: 10.1186/1471-2105-12-323 ; PubMed Central PMCID: PMC3163565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anholt RR, Dilda CL, Chang S, Fanara JJ, Kulkarni NH, Ganguly I, et al. The genetic architecture of odor-guided behavior in Drosophila: epistasis and the transcriptome. Nat Genet. 2003;35(2):180–4. Epub 2003/09/06. doi: 10.1038/ng1240 ng1240 [pii]. . [DOI] [PubMed] [Google Scholar]
- 57.Etebari K, Palfreyman RW, Schlipalius D, Nielsen LK, Glatz RV, Asgari S. Deep sequencing-based transcriptome analysis of Plutella xylostella larvae parasitized by Diadegma semiclausum. BMC Genomics. 2011;12:446. Epub 2011/09/13. doi: 1471-2164-12-446 [pii] doi: 10.1186/1471-2164-12-446 ; PubMed Central PMCID: PMC3184118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y, Zhou W, Zhou Y, Wu J, Zhou X. Transcriptome and comparative gene expression analysis of Sogatella furcifera (Horvath) in response to southern rice black-streaked dwarf virus. PLoS One. 2012;7(4):e36238 Epub 2012/05/05. doi: 10.1371/journal.pone.0036238 PONE-D-12-04233 [pii]. ; PubMed Central PMCID: PMC3338671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Jiang R, Wu H, Liu P, Xie J, He Y, et al. Next-generation sequencing-based transcriptome analysis of Cryptolaemus montrouzieri under insecticide stress reveals resistance-relevant genes in ladybirds. Genomics. 2012;100(1):35–41. Epub 2012/05/16. doi: S0888-7543(12)00079-1 [pii] doi: 10.1016/j.ygeno.2012.05.002 . [DOI] [PubMed] [Google Scholar]
- 60.Yang P, Zhu JY, Gong ZJ, Xu DL, Chen XM, Liu WW, et al. Transcriptome analysis of the Chinese white wax scale Ericerus pela with focus on genes involved in wax biosynthesis. PLoS One. 2012;7(4):e35719 Epub 2012/04/27. doi: 10.1371/journal.pone.0035719 PONE-D-12-03919 [pii]. ; PubMed Central PMCID: PMC3334986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, Li DZ, Min SF, Mi F, Zhou SS, Wang MQ. Analysis of chemosensory gene families in the beetle Monochamus alternatus and its parasitoid Dastarcus helophoroides. Comp Biochem Physiol Part D Genomics Proteomics. 2014;11:1–8. Epub 2014/06/04. doi: S1744-117X(14)00021-5 [pii] doi: 10.1016/j.cbd.2014.05.001 . [DOI] [PubMed] [Google Scholar]
- 62.Comeault AA, Sommers M, Schwander T, Buerkle CA, Farkas TE, Nosil P, et al. De novo characterization of the Timema cristinae transcriptome facilitates marker discovery and inference of genetic divergence. Mol Ecol Resour. 2012;12(3):549–61. Epub 2012/02/22. doi: 10.1111/j.1755-0998.2012.03121.x . [DOI] [PubMed] [Google Scholar]
- 63.Hou D, Ruiz M, Andrulis ED. The ribonucleaes Dis3 is an essential regulator of the developmental transciptome. BMC Genomics. 2012;13(1):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shao W, Zhao QY, Wang XY, Xu XY, Tang Q, Li M, et al. Alternative splicing and trans-splicing events revealed by analysis of the Bombyx mori transcriptome. RNA. 2012;18(7):1395–407. Epub 2012/05/26. doi: rna.029751.111 [pii] doi: 10.1261/rna.029751.111 ; PubMed Central PMCID: PMC3383970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colgan TJ, Carolan JC, Bridgett SJ, Sumner S, Blaxter ML, Brown MJ. Polyphenism in social insects: insights from a transcriptome-wide analysis of gene expression in the life stages of the key pollinator, Bombus terrestris. BMC Genomics. 2011;12(1):623. Epub 2011/12/22. doi: 1471-2164-12-623 [pii] doi: 10.1186/1471-2164-12-623 ; PubMed Central PMCID: PMC3276680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogt RG, Callahan FE, Rogers ME, Dickens JC. Odorant binding protein diversity and distribution among the insect orders, as indicated by LAP, an OBP-related protein of the true bug Lygus lineolaris (Hemiptera, Heteroptera). Chem Senses. 1999;24(5):481–95. Epub 1999/11/27. . [DOI] [PubMed] [Google Scholar]
- 67.Vogt RG. Odorant binding protein homologues of the malaria mosquito Anopheles gambiae; possible orthologues of the OS-E and OS-F OBPs of Drosophila melanogaster. J Chem Ecol. 2002;28(11):2371–6. Epub 2003/01/14. . [DOI] [PubMed] [Google Scholar]
- 68.Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315(5815):1133–7. Epub 2007/02/03. doi: 1136610 [pii] doi: 10.1126/science.1136610 . [DOI] [PubMed] [Google Scholar]
- 69.Arya GH, Weber AL, Wang P, Magwire MM, Negron YL, Mackay TF, et al. Natural variation, functional pleiotropy and transcriptional contexts of odorant binding protein genes in Drosophila melanogaster. Genetics. 2010;186(4):1475–85. Epub 2010/09/28. doi: genetics.110.123166 [pii] doi: 10.1534/genetics.110.123166 ; PubMed Central PMCID: PMC2998325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang H, Yang W, Yang MF, Yang CP, Pan WQ, Feng B. Mating and oviposition behavior of Batocera horsfieldi. Scientia Silvae Sinicae. 2011;47(6):88–92. [Google Scholar]
- 71.Rodstein J, Millar JG, Barbour JD, McElfresh JS, Wright IM, Barbour KS, et al. Determination of the relative and absolute configurations of the female-produced sex pheromone of the cerambycid beetle Prionus californicus. J Chem Ecol. 2011;37(1):114–24. Epub 2010/12/04. doi: 10.1007/s10886-010-9890-3 ; PubMed Central PMCID: PMC3030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leal WS, Bento JMS, Vilela EF, Lucia D. Female sex pheromone of the longhorn beetle Migdolus fryanus Westwood: N-(2' S)-methylbutanoyl 2-methylbutlyamine. Experientia. 1994;50(9):853–6. [Google Scholar]
- 73.Boyer FD, Malosse C, Zagatti P, Einhorn J. Identification and synthesis of vesperal, the female sex pheromone of the longhorn beetle Vesperus xatarti. Cheminform. 1998;29(26):757–64. [Google Scholar]
- 74.Ray AM, Zunic A, Alten RL, McElfresh JS, Hanks LM, Millar JG. cis-Vaccenyl acetate, a female-produced sex pheromone component of Ortholeptura valida, a longhorned beetle in the subfamily Lepturinae. J Chem Ecol. 2011;37(2):173–8. Epub 2011/01/29. doi: 10.1007/s10886-011-9908-5 ; PubMed Central PMCID: PMC3043240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLS)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.