Abstract
Alterations in circulating thyroid hormone concentrations are associated with several psychological and behavioral disorders. In humans, behavioral disorders such as anxiety, depression, and attention-deficit hyperactivity disorder can be associated with thyroid disease. The Tpo-Cre;Prkar1aflox/flox;Epac1−/− (R1A-Epac1KO) mice, originally bred to investigate the role of exchange protein directly activated by cAMP (Epac1) in follicular thyroid cancer, displayed self-mutilating and aggressive behaviors during casual observation. To assess these atypical responses, behavioral testing was conducted with the R1A-Epac1KO mice, as well as their single knockout counterparts, the thyroid-specific Prkar1a−/− and global Epac1−/− mice. Mice of all three genotypes demonstrated increased aggressive behavior against an intruder mouse. In addition, Epac1−/− mice increased response to an auditory stimulus, and the Prkar1a−/− and R1A-Epac1KO mice increased swimming behavior in the Porsolt forced swim test. Interestingly, both Prkar1a−/− mice and R1A-Epac1KO mice have increased circulating thyroxine and corticosterone concentrations. Although hyperthyroidism has not been previously associated with aggression, increased thyroid hormone signaling might contribute to the increased aggressive response to the intruder mouse, as well as the increased swimming response. Mice with a genetic background of Tpo-Cre;Prkar1aflox/flox;Epac1−/− are aggressive, and both the thyroid-specific knockout of Prkar1a and global knockout of Epac1 likely contribute to this aggressive behavior. This study supports the hypothesis that altered thyroid signaling and aggressive behavior are linked.
Keywords: Hyperthyroidism, Aggression, Epac1, Behavioral phenotyping, Prkar1a
Introduction
Hyperthyroidism has long been associated with psychiatric symptoms. In humans with hyperthyroidism, comorbidity with affective disorders, including anxiety, depression, and attention-deficit hyperactivity disorder (ADHD) is often reported (Thomsen and Kessing, 2005; Grabe et al., 2005). Thyrotoxicosis induced by consumption of ground beef contaminated by bovine thyroid tissue induced aggression and fire-setting in a 4 year-old boy (Bhatara et al., 2009). Thyroid receptors are expressed throughout the adult brain, including regions involved in affective behavior such as the prefrontal cortex and the amygdala (Lechan et al., 1993). Therefore, alterations in thyroid hormone signaling are likely to affect behavior. Indeed, pharmacological induction of hyperthyroidism in rodents can cause an array of behavioral effects, including changes in locomotor activity, muscle strength, motor coordination, learning, memory, and affective behaviors (Yu et al., 2015; Bitiktas et al., 2016; Rakov et al., 2016). Behavioral effects differ based on the specific pharmacological or genetic induction of elevated thyroid function. Thyroid-specific deletion of Prkar1a causes hyperthyroidism in mice Pringle et al., 2012), but their behavior remains unspecified.
The thyroid-specific Prkar1a−/− mouse (R1A) was developed to investigate the tumorigenic role of PRKAR1A in the thyroid gland, a cAMP-responsive tissue (Pringle et al., 2012). PRKAR1A encodes the type1a regulatory subunit of protein kinase A (PKA), and mutations of this gene have been implicated in both inherited and sporadic cases of thyroid cancer (Sandrini et al., 2002). Over 40% of R1A mice develop follicular thyroid cancer (FTC) by one year of age, and R1A mice have hyperthyroidism (Pringle et al., 2012). Increased thyroid stimulating hormone (TSH) concentrations are associated with human thyroid cancer. PKA signals downstream of TSH through the production of cAMP (Hargadine et al., 1970; Haymart et al., 2008), however, because of the pervasive effects of cAMP, therapies attempting to target cAMP signaling can cause a large number of side effects (Saunders et al., 1997; Propper et al., 1999). Therefore, increasing focus has been on understanding downstream targets of cAMP signaling and targeting those pathways instead (Pringle et al., 2014).
Exchange protein directly activated by cAMP (Epac) presents a potential alternative therapeutic target. Epac is an intracellular sensor, in addition to PKA, that mediates the effects of cAMP to activate downstream Rap1 (Kawasaki et al., 1998; de Rooij et al., 1998), in many tissues including the thyroid (Ribeiro-Neto et al., 2004). Epac regulates Rap activity, both in concert with PKA and independently, and the effects, whether stimulatory or inhibitory, seem to be dependent on cell type and context, as well as the type of stimuli (Almahariq, et al. 2013; Cheng, et al. 2008; de Rooij et al. 1998; Tsygankova et al. 2001). Epac plays an important role in cell migration and invasion in other cancers (Grandoch et al., 2009). Epac1−/− mice are viable and fertile, with no obvious thyroid gland defects. Therefore, the R1A mice were crossed with Epac1−/− mice, generating Tpo-Cre;Prkar1aflox/flox;Epac1−/− (R1A-Epac1KO) mice to investigate the role of Epac1 in FTC.
Epac genes are expressed in both developing and adult brains; however, Epac1 is more highly expressed during development whereas Epac2 is more prevalent in adults (Kawasaki et al., 1998; Murray and Shewan, 2008). Developmental expression of EPAC proteins promotes cAMP-dependent axon growth and regeneration (Murray and Shewan, 2008), and are located throughout the hippocampus, the prefrontal cortex, cerebellum, and the suprachiasmatic nucleus of the hypothalamus (Nikolaev et al., 2004; Dwivedi et al., 2006; O’Neill et al., 2008). EPAC proteins have also been linked with behavioral changes. Epac2 deletion, but not Epac1 deletion, in mice altered anxiety-like behaviors, hyperactivity, and depressive-like responses (Zhou et al., 2016). Both Epac1−/− and Epac2−/− mice display normal cognitive functions such as LTP and spatial learning, whereas mice lacking both isoforms display deficits in these tasks (Yang et al., 2012), suggesting a redundant role for the proteins. Epac1 has been implicated in causing deficits in sensorimotor gating (Kelly et al., 2009), which is associated with many psychiatric disorders (Geyer, 2006).
Inadvertent behavioral alterations are often induced by genetic modifications of mice generated for other purposes. While working with the R1A-Epac1KO mice, behavioral abnormalities were observed that warranted further behavioral phenotypic investigation. This mouse model presents a unique opportunity to investigate the roles of Prkar1a and Epac1 in behavior, as well as the reciprocal effect of the double KO. Considering the Prkar1a knockout is thyroid-specific, behavioral alterations are likely the result of downstream effects of the deletion, such as alterations in thyroid hormone signaling. Here, we characterize the behavioral phenotype of all three genotypes, R1A, Epac1KO, and R1A-Epac1KO mice. We investigated sensorimotor function, memory, locomotor function, affective behaviors, and aggressive behavior to provide a relatively complete behavioral assessment. All three genotypes showed a dramatic increase in aggression towards an intruder mouse along with other alterations in affective behavior. This study is, to our knowledge, the first to implicate hyperthyroidism in murine aggressive-like behaviors, and provides insight into additional roles for Prkar1a and Epac1 in affective and aggressive behaviors.
Methods
1. Animals
Male adult mice comprising four genotypes were maintained in a sterile environment under a 12-hour light/dark cycle with access to ad libitum food (Harlan Teklad 8640; Madison, WI, USA) and filtered tap water, except when noted below. Prior to behavioral testing, all mice were group housed with littermates. Mice selected for behavioral testing were then separated and single-housed, allowing 3 weeks to acclimate prior to the initiation of the first test. Cages were kept on ventilated racks in a temperature- and humidity-controlled vivarium at The Ohio State University. TPO-cre; Prkar1aloxP/loxP (Pringle et al., 2012) were mated with Epac1 KO mice (Suzuki et al., 2010), provided kindly by the Ishikawa group at Yokohama City University, Japan, to produce a double knockout, R1A-Epac1KO. All experiments were performed in a C57BL/6 CBA and FVB mixed background. All experimental mice were derived from the same parents of origin and inbred to produce each of the four genetic models: TPO-cre; Prkar1aloxp/loxp (R1A), TPO-cre; Prkar1aloxp/loxp; Epac1−/− (R1A-Epac1KO), Prkar1aloxp/loxp; Epac1−/− (Epac1KO), and Prkar1aloxp;loxp (WT Control). Twelve animals from each of the four genotypes were included in the behavioral study. Animals were between 6–10 months of age at the onset of testing, and were coded with random experimental numbers to ensure the experimenter remained uninformed of the experimental groups. All behavioral testing was conducted during the animal’s light phase, between the hours of 1000 and 1800 h EST, and mice were acclimatized to the room for 30 minutes prior to testing. The rooms used for behavioral testing and acclimatization were at approximately the same light level as in the animal housing room. Behavioral testing was conducted in the order of least to most stressful over 40 days, with at least one day between tests (see Table 1). For tests which required multiple days, mice were divided into two groups, with each genotype equally represented in both groups. Testing was not conducted on days in which cage changes occurred. All experiments were conducted with approval from the Ohio State University Institutional Animals Care and Use Committee and were consistent with the NIH regulations.
Table 1.
Order and timing of behavioral tests.
| Day | Behavioral Test |
|---|---|
| 1 | Initial Assessment |
| 3 | Visual Placing, Contact Placing, Limb Clasping, and Auditory Function Tests, Grip Strength Test |
| 5–6 | Find the Hidden Cookie Test |
| 8 | Open Field and Elevated Plus Maze |
| 11–12 | Marble Burying Test |
| 17 | Rotorod Test |
| 19–23 | Sucrose Anhedonia Test |
| 25 | Y-maze |
| 28 | Digging Test |
| 30 | Porsolt Forced Swim Test |
| 33–34 | Aggression Against and Intruder Test |
| 39 | Hot Plate Test |
2. Initial Assessment
An initial assessment of each mouse was conducted to ensure behavioral differences did not arise from gross deficits. Body weight was measured and length was measured from the tip of the nose to the base of the tail. Vibrissae were visually assessed and assigned a score from 0–4; mice with all whiskers present and un-barbered were assigned a score of 0, mice with no whiskers were given a score of 4, and scores of 1, 2, and 3 were assigned for mice with conditions between the two extremes. Eye appearance was assessed visually for discrepancies in size, shape, and condition, with a score of 0 assigned for normal, a score of 1 for mild abnormalities, and a score of 2 was given for mice with severe abnormalities. Muscle tone was assessed by handling each animal, allowing him to climb on the examiner’s fingers, using the 0–2 scale described for eye appearance. Ear appearance was scored using a 0–2 scale where 0 indicated both ears were normal, 1 indicated one ear pinna was malformed or missing, and a 2 indicated both ear pinna were malformed or missing.
3. Sensorimotor Tests
Several sensorimotor tests were conducted to detect any deficits that could potentially affect other behaviors. The visual placing test was used to detect visual impairment. Each mouse was held by the tail and lowered slowly towards the edge of the table. A positive score was assigned when the mouse extended its forepaws prior to touching the table on at least 2 of 3 trials. Contact placing and orientation to touch of vibrissae was conducted to detect deficits in sensory function of vibrissae. In a dimly lit room (3 lux of red light), each mouse with vibrissae was lowered slowly towards the edge of a table until its vibrissae touched the edge. The reaction to touch was evaluated and scored on a scale of 1–3. A score of 1 indicated no response, and a score of 3 indicated maximal response. The average of three trials was used for analysis. A mouse lacking vibrissae was assigned a score of 0. Limb clasping was assessed for each mouse by suspending the mouse by its tail about 30 cm above a table for 5 seconds. A score of 0 was given if all of the limbs were held against the body (clasped), a score of 3 was assigned if all of the limbs were extended out. Scores of 1 and 2 were assigned for stages between the two extremes. The limb-clasping score was the sum of three trials.
The time to find a hidden cookie in their home cage was tracked to test olfactory senses. A miniature peanut butter sandwich cookie (2–3g) was placed in the corner of each mouse’s cage and covered with 3–6 cm of bedding. The mouse was replaced into his cage, and the time required to locate the cookie was recorded. The test was terminated if the cookie was not located within 10 minutes. Auditory function was assessed by sounding a clicker 15 cm behind the mouse’s head. A positive score was recorded when the animal turned its head towards the sound on 2 of 3 trials. Each mouse’s forelimb grip strength was measured using a grip strength meter (Columbus Instruments). Each mouse was placed onto the pull bars, gently pulled, and the peak force was recorded by the meter. Three trials were averaged for each mouse. The rotating rod test was used to assess each mouse’s motor function. Each mouse was placed onto a rota-rod treadmill (Med Associates Inc. #ENV-577M) at 4 rpm. The rod slowly increased its turning rate to 40 rpm over a 5 minute interval. Each animal was tested three times, with at least 5 minutes between trials. The time until the mouse fell off the rod or failed to remain on the top of the rod for two revolutions was recorded and averaged for the three trials.
4. Memory Tests
Y-Maze spontaneous alternation test was used to measure spatial working memory. Spontaneous alternation is the tendency to choose a different option than the one previously chosen. Mice tend to explore the arms of the Y-maze that they have explored less than others (Deacon and Rawlins, 2006). Therefore, this task is a sensitive measure of spatial memory (Wolf et a., 2016). Each mouse was placed in the center of the Y-maze and recorded for 5 minutes. The maze was cleaned with 70% ethanol between each mouse. The number of alternating three choice sequences over the total chances to alternate between arms was scored to provide the percentage of alternations.
5. Affective Behavioral Tests
The open field test was used to assess anxiety-like behaviors and locomotor activity in a novel environment. Each mouse was placed into an open arena and tested for 20 minutes as previously described (Fonken et al., 2009). Central tendency was measured for anxiety-like responses by calculating the proportion of time spent in the center of the open field. We assessed central tendency during the first 5 min of testing. as well as the total duration of testing. Locomotor activity was measured separately as the total number of beam breaks during the duration of testing.
We also tested anxiety-like behaviors using the elevated-plus maze and assessing the time spent in the open arms vs. time in the enclosed arms. Thirty minutes after open field testing, each mouse was placed in the center of the elevated plus maze with two enclosed arms (50cm × 10cm × 40cm) and two open arms (50cm × 10cm). The maze was elevated 40 cm off the ground. Mice were allowed to freely explore for 5 minutes and recorded on video. The recorded behavior was then scored using Observer software (Noldus, Leesburg, VA) for the number of entries in the open arms and the percentage of time spent in the open arms.
We tested for depressive-like behaviors using two tests, the Porsolt forced-swim test and the sucrose preference test. In the Porsolt test, the time spent floating, or immobile is interpreted as behavioral despair and is reversible with antidepressant treatment (Porsolt et al., 1978; Crawley, 1999). Each mouse was placed into a cylindrical beaker (diameter = 24 cm, height = 53 cm) filled with room temperature (22 ± 2°C) water approximately 17 cm deep. Each mouse was recorded for 5 minutes, removed from the water, dried, and returned to his cage. The video was subsequently scored using Observer software for latency to first float, the number of floating bouts, and the total time floating.
Rodents typically prefer sweetened water, and a lack of preference indicates anhedonic behavior (Willner et al., 1987). Mice were acclimated to the removal of their water and the addition of two water bottles in their cage for three days with access to water in their new water bottles ad libitum. The bottles were weighed and refilled twice per day, within 1 hour of the onset of dark phase, and within 2 hours after the light phase. On the fourth day, one water bottle was randomly switched to one containing a 3% sucrose solution for two days. The bottles were rotated on day 2 to control for side preference. The total percent preference was calculated by dividing the volume of sucrose consumed by the volume of water consumed over the two days of testing.
6. Compulsive Behavior Tests
The digging test and marble burying test were used to assess compulsive behaviors. The digging test was conducted over a 3 minute duration by filling a cage with approximately 5 cm of corncob bedding, which was lightly tamped down to make a flat, even surface. The latency to first digging, total number of digging bouts, and total duration of digging was recorded. In the marble burying test, home cages were filled with approximately 1.5 inches of corncob bedding and lightly tamped down. Sixteen marbles were placed on top of the bedding, and the mouse was placed into the cage for 20 minutes. The number of marbles buried to 100% depth, 50% depth, and the total number of marbles buried was recorded. The marbles were cleaned with soapy water, rinsed with 70% ethanol, and dried between animals.
7. Pain Tolerance Test
The hot plate test was used to measure pain tolerance in mice. Each mouse was habituated to the hot plate (Life Sciences Series 8 Model PE34) surface at room temperature for 30 seconds prior to testing. The surface of the plate and surrounding walls were wiped with 70% ethanol between animals. Each mouse was then placed onto the heated plate (55°C) and the latency to a reaction was recorded. Reaction behaviors included lifting the front or rear paws, licking the paws, or jumping. The mouse was removed as soon as a response was observed.
8. Aggression Tests
The offensive aggression against an intruder in the home cage paradigm was used to test for aggressive behaviors (Miczek, 1987; Hilakivi-Clarke and Lister, 1992). The home cage was not changed within one week prior to testing. A stimulus male mouse (8-week old male Swiss-Webster) was introduced into each mouse’s home cage. The latency to the first attack by the resident mouse was recorded. In addition, the number of attacks, bites, pursuits, and tail rattles displayed by the resident mouse were also noted. The animals were recorded, and the recordings were scored for total duration of fighting. The stimulus mouse was removed from the cage after 10 minutes, or if visibly injured. Stimulus mice were used only one time per day of testing, and testing was conducted over two consecutive days. Although intruder mice were not age- or size-matched to the resident mice, the intruder mice were all of similar size and age.
9. Corticosterone and Thyroid Hormone Assays
Blood was collected from a separate cohort of mice to assess serum corticosterone concentration. Blood was obtained via cardiac puncture from 10 animals per genotype and centrifuged for 20 minutes at 4°C to separate the serum from whole blood. Serum corticosterone concentration was measured using a Corticosterone Enzyme Immunoassay Kit (Arbor Assays) according to the manufacturer’s protocol. The intra-assay coefficient of variation for this immunoassay is 5.175%; the inter-assay variability is 7.93%. Cross reactivity of Desoxycorticosterone has been reported as 12.3% cross reactivity, while no other related steroid compounds exceeded 1% cross reactivity. Thyroxine (T4) concentration was assessed using blood from an additional cohort of mice (n=10). Serum from 12 mice per genotype was collected as above and shipped on dry ice to the Hormone Assay and Analytical Services Core at Vanderbilt University Medical Center for analysis using double antibody radioimmunoassay (RIA). The T4 RIA was developed in the laboratories of the Division of the Diabetes, Endocrinology and Metabolism, Department of Medicine and Vanderbilt University Medical Center. Cross reactivity to diiodo-L-tyrosine and moniodo-L-tyrosine <0.01%, D-Thyoxine 100% and 3,3,5-Triiodo-L-thyronine (T3) 3%. The inter-assay variability for this assay is 5.9%. The intra-assay coefficient of variation for this assay is 2.65%; the inter-assay variability is 8.47%. The primary anti-L thyroxine antibody was developed in rabbit and purchased from Sigma Aldrich (T2652), and the T4 I125 and T4 standards were purchased from MP Biomedicals. The sensitivity of the assay is 0.5 ng/mL.
10. Data Analyses
All parametric pairwise comparisons were conducted using a 1-way ANOVA with genotype as the independent variable. Significant ANOVA results were followed with a multiple comparison Dunnett’s test with all comparisons to the wild-type control group. We also estimated the effect size using eta2 for ANOVA results and Cohen’s d for the results of the post hoc multiple comparisons between each genotype relative to the WT group. Data failing to meet the assumptions of parametric testing were first transformed. If assumptions remained unmet, then the nonparametric Kruskall-Wallis test was used. A significant result was followed by Dunn’s test, comparing each group to the wild-type control group. Categorical data was compared using Fisher’s exact test, followed by pairwise multiple comparisons with Bonferroni correction.
Results
1. Initial Assessment
Statistically significant differences in body weights were observed between groups (F3,48=3.35, p<0.03, η=0.186). R1A-Epac1KO mice weighed significantly less (29.71±1.17g) than WT animals (33.55±0.58g, p<0.02, d=1.606) (Figure 1a). Body length also differed between groups (F3,48=3.01, p=0.04, η=0.168). Body lengths of R1A-Epac1KO mice were significantly shorter (9.74±0.13cm) than WT mice (10.09±0.10cm, p=0.049, d=0.833) (Figure 1b). Initial assessments of vibrissae, eye appearance, muscle tone, and ear appearance showed no differences between groups (p>0.05).
Figure 1.
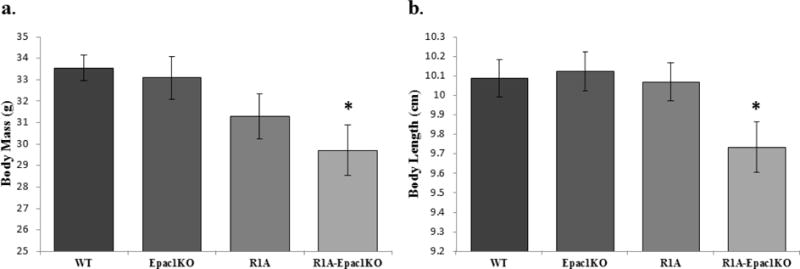
Initial morphological and behavioral assessment of Epac1−/− (Epac1KO), Prkar1a−/− (R1A), Tpo-Cre;Prkar1aflox/flox;Epac1−/− (R1A-Epac1KO) mice. R1A-Epac1KO mice weigh less (F=3.35, p=0.03) (a) and are shorter in length than wild-type (WT) mice (F=2.99, p=0.04) (b). Data are presented as mean ± SEM, n=12/group, * p<0.05.
2. Sensorimotor Tests
All of the groups exhibited similar visual ability; all mice were able to extend their paws to the same degree when lowered toward the edge of the table (p = 0.10). No significant differences were observed when visual cues were removed by lowering the animals toward the table in dim red light, indicating similar ability in sensing using vibrissae (p = 0.08). Assessment of olfactory function revealed no significant differences between genotypes (p = 0.82); all groups required a similar amount of time to find a cookie hidden beneath their bedding.
Tests of limb function indicated that all genotypes have similar limb function as shown in their ability to clasp their limbs when suspended in the air (p = 0.70). However, grip strength was significantly different among genotypes (H=13.699, p = 0.003), but the post hoc test did not show any group mean was significantly different than WT (Table S1). Overall locomotor ability did not differ between genotypes (p = 0.40, Table S1); all groups remained on a rotating rod for a similar length of time.
Auditory function was assessed by observing a mouse’s response to a clicking noise behind his head on at least 2 of 3 trials. Genotypes differed in this task (p < 0.001) such that Epac1KO (p = 0.001) and R1A-Epac1KO mice (p = 0.003) turned toward the click more frequently when compared with WT mice (Figure 2a).
Figure 2.

Affective behavior in Epac1−/− (Epac1KO), Prkar1a−/− (R1A), Tpo-Cre;Prkar1aflox/flox;Epac1−/− (R1A-Epac1KO) mice. a) Mice of Epac1KO and R1A-Epac1KO genotypes turned toward an auditory stimulus more frequently than WT mice (Fisher’s exact test, p<0.001) b) R1A-Epac1KO mice floated less than WT mice in the Porsolt forced swim test (F3,42=3.66, p=0.02). c) Total locomotor activity, measured by total beam breaks in the open field test, was not significantly different between genotypes (F3,47=1.00, p=0.40). Data are presented as mean ± SEM, n=8–12/group, * p<0.05.
3. Memory Tests
Deficits in spatial memory tested using the Y-maze were not detected for any genotype when compared with wild-type control mice. There were neither differences detected in the percent of alternation (p=0.21, Table S1), nor in the number of total alternations (p=0.31, Table S1).
4. Affective Behavioral Tests
Anxiety-like behavioral responses were tested using the elevated-plus maze and the open field test. Mice did not exhibit anxiety-like behavior in either test. In the elevated plus maze, mice from each genotype spent a similar duration of time in the open arm (p=0.60, Table S1) and made an equivalent number of entries into the open arm (p=0.74, Table S1) throughout the duration of the test. Likewise, mice of each genotype spent a similar amount of time in the center of the open field both in the first 5 minutes (p=0.84, Table S1) and throughout the full 20 minute duration of testing (0.99, Table S1). In addition, mice of each genotype reared a similar number of times throughout testing (p=0.20, Table S1).
Depressive-like behaviors were tested using the sucrose preference test and Porsolt forced swim test. None of the genotypes displayed a difference in the total percent preference of sucrose (p=0.20, Table S1). In the forced swim test, there were no differences in the number of floating bouts (p=0.64), however there was a significant difference in the duration of floating (F3,42=3.66, p=0.02, η=0.224, Figure 2b). Dunnett’s test shows the R1A-Epac1KO genotype (52.63±13.79 sec) floats significantly less than WT mice (100.17±11.26 sec, p=0.03, d=1.075).The Epac1KO (p=0.94, d=0.034) and R1A (p=0.057, d=0.866) genotypes were not significantly different from the WT group. Total locomotor activity was measured in the open field test to determine whether the difference in floating duration might be due to a difference in overall locomotion. There were no significant differences between genotypes in total locomotor activity as measured in the open field test (F3,47=1.00, p=0.40, Figure 2c).
5. Compulsive Behavior Tests
Compulsive behaviors were tested with a digging and marble burying test. Neither test revealed a significant difference between genotypes. Mice of each genotype dug for a similar duration (p=0.97, Table S1), a similar number of bouts (p=0.50), and the latency to dig was also similar (p=0.33). In the marble burying test, the number of marbles buried to 100% coverage was not different between genotypes (p=0.79, Table S1), but the number buried to just 50% depth was significantly different between groups (F3,48=3.01, p=0.04, d=0.173,Table S1). Dunnett’s post hoc test, comparing each genotype with WT, does not show a significant difference. Visual inspection of the data indicates the Epac1KO may bury more marbles than the R1A group, however this comparison was not included in the post hoc analysis. The total number of marbles buried, including those buried to 50% and 100% depth, did not differ among genotypes (p=0.09, Table S1).
6. Allodynia Tests
Allodynia was measured using the hot plate test. The duration of time until the mouse began lifting or licking his paws was not significantly different among genotypes (p=0.10, Table S1).
7. Aggression Tests
Aggressive behaviors were observed using the intruder in a home-cage paradigm. The latency to the first aggressive encounter was significantly different between genotypes (F3,41=3.71, p=0.02, η=0.316). Mice of R1A (58.94±1.65 sec, p=0.050, d=1.130), Epac1KO (52.07±1.61 sec, p=0.02, d=1.446), and R1A-Epac1KO (52.61±1.52 sec, p=0.04, d=1.374) genotypes all displayed a significantly shorter duration of time until the initiation of an attack compared with WT mice (159.78±2.11sec) mice (Figure 3a). The total number of aggressive encounters, including attacks, bites, pursuits, and tail rattles, was significantly different between genotypes (H=7.83, p=0.0497, Figure 3b). Mice of the R1A (45.20±8.01) genotype had a significantly greater number of aggressive encounters compared with WT mice (20.75±3.59, p=0.04). When the specific aggressive behaviors are separated, the number of attack behaviors was significantly different between genotypes (F3,41=4.29, p=0.01, η=0.258). R1A (23.74±1.27, p=0.01, d=1.264), Epac1KO (18.87±1.22, p=0.04, d=0.920), and R1A-Epac1KO (21.74±1.26, p=0.04, d=1.086) all displayed a greater number of attack behaviors compared with WT (9.53±0.88) mice (Figure 3c). However, the number of bites (p=0.13), pursuits (p=0.25), and tail rattles (p=0.87) were not significantly different. The total duration of aggressive encounters was also significantly different between genotypes (F3,41=4.757, p=0.01, η =0.284, Figure 3d). Again, mice of the R1A genotype (53.73±9.50 sec) had a longer duration of aggression compared with WT mice (20.34±3.54 sec, p=0.01, d=1.392).
Figure 3.
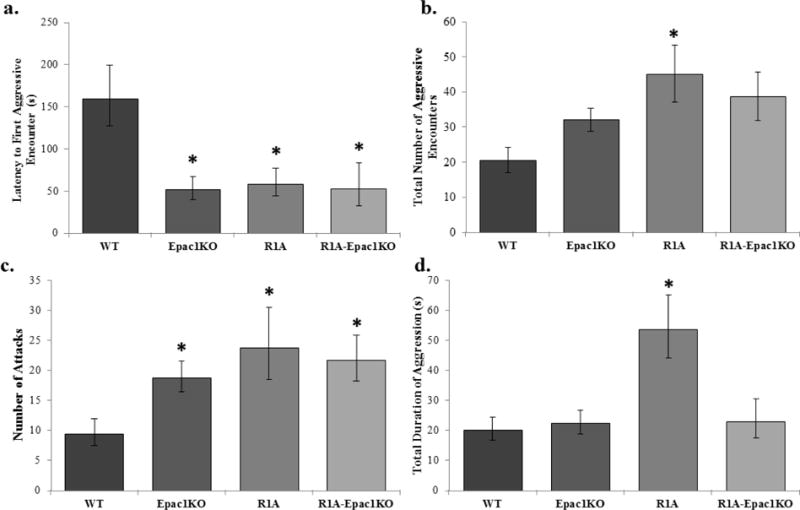
Aggression against an intruder mouse in the home cage. a) Mice of Epac1−/− (Epac1KO), Prkar1a−/− (R1A), Tpo-Cre;Prkar1aflox/flox;Epac1−/− (R1A-Epac1KO) genotypes had a shorter latency to attack an intruder mouse when compared with WT mice (F=3.71, p=0.02). b) R1A mice had a greater number of aggressive encounters with the intruder mouse than WT mice (Mann-Whitney, χ2=7.83, p=0.0497). c) Epac1KO, R1A, and R1A-Epac1KO mice had a greater number of attacks against the intruder mouse compared with WT mice (F=4.29, p=0.01). d) R1A mice displayed aggression for a greater duration of the 10 minute test compared with WT mice (F=4.76, p=0.01). Data are presented as mean ± SEM, n=8–12/group, * p<0.05.
8. Corticosterone and Thyroid Hormone Assays
Serum corticosterone was measured as a proxy for stress levels. Corticosterone concentrations were significantly different (F3,40=4.74, p=0.01, η=0.253). Mice of both the R1A (111.59±37.21 ng/ml, p=0.03, d=1.622) and the R1A-Epac1KO (168.23±52.39 ng/ml, p=0.01, d=22.50) genotype had higher concentrations of corticosterone than WT mice (28.88±9.63 ng/ml) (Figure 4a). Plasma T4 concentration was measured as an indicator of hyperthyroidism. T4 concentrations were significantly different between groups (H=15.63, p=0.001). Mice of both the R1A (181.45±37.84 ng/ml, p=0.04) and R1A-Epac1KO (202.33±35.12 ng/ml, p=0.005) genotypes had higher concentration of T4 than WT mice (47.72±3.74 ng/ml) (Figure 4b).
Figure 4.
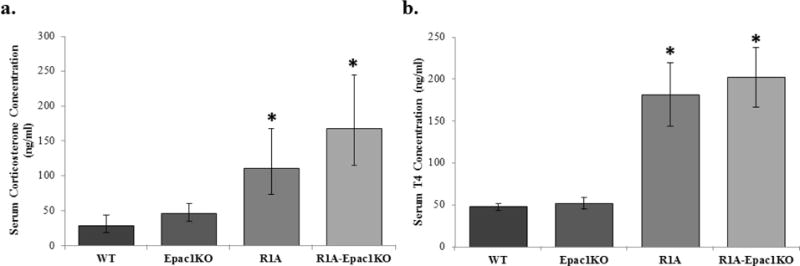
Serum corticosterone and thyroxine (T4) concentrations in Epac1−/− (Epac1KO), Prkar1a−/− (R1A), Tpo-Cre;Prkar1aflox/flox;Epac1−/− (R1A-Epac1KO) mice. a) Serum corticosterone was elevated in R1A and R1A-EpacKO mice compared with WT mice (F=4.74, p=0.01). b) T4 is also higher in R1A and R1A-EpacKO mice compared with WT mice (Mann-Whitney, p=0.001). Data are presented as mean ± SEM, n=10/group, * p<0.05.
Discussion
R1A-Epac1KO mice were hyperthyroid and had a significant increase in aggression compared with their counterpart control mice. These mice were generated to better understand molecular mechanisms involved in FTC. However, atypical behaviors were observed which presented the opportunity to investigate behavioral alterations induced by these genetic modifications. To verify that the increase in aggressiveness did not represent nonspecific traits such as sensorimotor skills, a complete behavioral assessment was conducted.
In addition to aggression, the R1A-Epac1KO mice were shorter in length and weighed less than WT mice, whereas mice of the R1A or Epac1KO genotype did not differ from WT in morphology (Fig. 1a and b). The reduced weight of the R1A-Epac1KO mice is likely to be due, at least in part, to their hyperthyroid state. None of the genotypes had abnormalities in their eyes, ears, or vibrissae; therefore, variation in gross sensory anatomy does not contribute to behavioral differences.
Several sensorimotor tasks were assessed, and none of the genotypes had impaired visual, locomotor, vibrissae, olfactory, or limb function. Grip strength differed between groups, but the Dunnett’s post hoc test, which compares each group with WT, did not reveal any group as different from WT mice. Visual inspection of the data indicates the Epac1KO mice might differ from the R1A-Epac1KO mice, but this contrast was not included in the post hoc comparisons (Table S1). R1A-Epac1KO mice weigh slightly less, which could contribute to a decrease in musculature and therefore grip strength. In a test for auditory function, the Epac1KO and R1A-Epac1KO mice turned toward a click sounded behind their heads more often than WT mice (Fig. 1d). Although this could indicate heightened auditory processing, it is more likely an indicator of an increased startle response, or hyper-responsiveness to the stimulus. In this test, positive scores were given when a mouse turned his head toward the click on at least 2 of 3 trials. Each mouse turned toward at least one of the clicks; therefore WT mice did not have impaired hearing. Instead, it is possible that the WT mice became accustomed to the noise, and no longer responded after the first click, whereas the Epac1KO and R1A-Epac1KO mice remained responsive. One consequence of reduced cAMP signaling through Epac is deficits in sensorimotor gating (Kelly et al., 2009). Reduced Epac1 in these mice could cause a reduced ability to modify their startle reflex during successive auditory stimuli.
Affective behaviors were tested using the open field test, the elevated plus maze, sucrose preference test, and the Porsolt forced swim test. None of the genotypes tested displayed anxiety-like or depressive-like behaviors compared with WT mice. Although Epac1 was previously associated with anxiety and depression in human twin studies (Middeldorp et al., 2010), experimental studies indicate that Epac2KO, but not Epac1KO, is associated with an increase in anxiety- and depressive-like behaviors (Zhou et al., 2016). Mice of the R1A-Epac1KO genotype and, marginally, the R1A genotype, floated less, and conversely swam more, than WT mice in the Porsolt forced swim test (Fig. 2a). Total locomotor activity assessed in the open field test did not differ between genotypes, and therefore increased swimming is not simply a difference in overall locomotion. This increase in swimming is instead likely represents a hyper-vigilant state.
Excessive grooming and barbering were observed while working with the mice, specifically the R1A-Epac1KO genotype, and the behavior was proposed to be due to a compulsive-like behavior. Barbering can represent a compulsive behavior (Garner et al., 2004), or result as a response to stress (Van den Broek et al., 1993). The Epac1KO mice had a marginal increase in marble-burying behavior compared with WT, which might indicate slight compulsion. An additional explanation for the behavior arose from Epac proteins’ role in hyperalgesia (Eijkelkamp et al., 2010); knockout of Epac could decrease sensitivity to pain. As a result, the lack of Epac1 could be contributing to the excessive barbering in which the mice did not cease barbering when it became painful. However, there were no differences in pain tolerance among the three genotypes. It is likely the observed barbering was less a compulsive behavior and more a response to stress.
Epac proteins are important for memory consolidation and retrieval (Ouyang et al., 2008; Ma et al., 2009; Yang et al., 2012), however, the majority of studies do not separate the effects of Epac1 and Epac2. Memory was assessed using the spontaneous alternation Y-maze test. When placed in a Y-maze, mice have a strong tendency to explore each arm alternately. Spontaneous alternation behavior relies on limbic and non-limbic pathways of memory (Lalonde, 2002). This test is therefore a simple measure of memory retention. The Epac1KO mice tested here, as well as the R1A and R1A-Epac1KO mice, did not show memory deficits in the Y-maze test of spontaneous alternation. Enhanced Epac signaling improves memory formation (Ma et al., 2009); deletion of both Epac1 and Epac2 impairs memory (Yang et al., 2012). However, knockout of Epac1 or Epac2 individually does not alter memory retrieval (Yang et al., 2012). Therefore, the role of Epac1 and Epac2 in memory appears redundant, and therefore Epac2 likely preserved memory function in these mice.
These mice were observed to not only self-harm, but also, littermate males had a tendency to fight if group housed. The intruder mouse in the home cage paradigm was used to assess aggressive behavior. Previous studies have suggested that differences in aggressive behavior are associated with differences in body weight. However, studies vary in this conclusion. Wild house mice selected specifically for their increased aggressive behavior did not differ in body weight from mice selected as less aggressive (Veenema et al., 2003). Other studies indicate that resident mice that are smaller than the intruder mouse are less aggressive (Hilakivi-Clarke and Lister, 1992). Epac1KO and R1A mice did not differ in body weight from WT, and therefore body weight does not explain differences in aggression between these two genotypes and WT mice. The R1A-Epac1KO mice were slightly smaller than WT mice, which could potentially indicate these mice would be less aggressive than the WT mice. However, here, mice of all three genotypes were more aggressive than WT mice. Mice of all three genotypes initiated aggression towards the intruder mouse more quickly and initiated more attacks compared with WT mice (Fig. 3a and c). Specific aggressive behaviors including attacks, bites, pursuits, and tail rattles were counted, and mice of the R1A genotype had a greater number of total aggressive behaviors than WT mice (Fig. 3b). R1A mice also displayed aggressive behavior for a greater duration of the 10 minute testing period than WT mice (Fig. 3d); some of these mice attacked nearly continuously for the entire testing period.
The aggressive behavior displayed by Epac1KO mice could be a consequence of the mice being hyper-responsive to stimuli. Many studies indicate a role for cAMP pathways in neuronal function, but the canonical pathway via PKA has been of focus. However, reduced cAMP signaling decreases signaling to Epac, and one behavioral outcome is a deficit in sensorimotor gating (Kelly et al., 2009), which is associated with numerous psychiatric disorders including schizophrenia (Geyer, 2006). Both Epac1KO and R1A-Epac1KO mice had an increased response to auditory stimulus, which could indicate deficits in sensorimotor gating. The stimulus of the intruder mouse could induce a hyper-responsive aggressive reaction. Therefore, the elimination of Epac1signaling likely contributes to increased aggression.
The R1A mice are also significantly more aggressive than WT mice. R1A mice might be more aggressive than Epa1KO and R1A-Epac1KO mice since R1A mice have a higher total number of aggressive encounters and longer duration of aggression compared with WT mice. The number of aggressive encounters included tail rattles, bites, and pursuits along with the number of attacks. The R1A mice displayed all of these behaviors more frequently than WT mice, whereas Epac1KO and R1A-Epac1KO mice only attacked more frequently than WT. Tail rattles and pursuits take more time than a short attack while passing the intruder mouse, which could contribute to the increase in total duration of aggression.
R1A mice have previously been identified as having hyperthyroidism (Pringle et al., 2012), and indeed, both the R1A and R1A-Epac1KO mice were confirmed as having elevated T4 levels (Fig. 4b). Hyperthyroidism can affect many behavioral responses including anxiety, depression, hyperactivity, impaired learning, and spatial memory (Yu et al., 2015; Bitiktas et al., 2016; Rakov et al., 2016). However, hyperthyroidism has not been definitively associated with aggression. One study noted observations of increased aggression in hyperthyroid pigs (Noszczyk-Nowak et al., 2007), but this was not substantiated with systematic behavioral testing. Additionally, a few case studies of thyrotoxicosis and hyperthyroidism in humans have associated the increase in thyroid hormones with aggressive behaviors (Bhatara et al., 2009). R1A and R1A-Epac1KO mice increased swimming behavior in the Porsolt forced swim test compared with wild type mice, which indicates an increased responsiveness. An overactive thyroid can also activate the HPA axis, increasing circulating corticosterone (Steinetz and Beach, 1963; Kamilaris et al., 1991; Nikolopoulou et al., 2015). Serum corticosterone concentrations were elevated in both R1A and R1A-Epac1KO mice, suggesting that indeed, the HPA axis is activated in these genotypes. Corticosterone is important for the regulation of social and emotional behavior (Condren et al., 2002; Van Honk et al., 2010; Montoya et al., 2012), and either low or high activity of the HPA can induce aggression (Walker et al., 2016). Therefore, HPA activity is a second potential mechanism behind the increased aggressive behavior in R1A and R1A-Epac1KO mice.
Conclusions
Overall, mice of Epac1KO, R1A, and R1A-Epac1KO genotypes are hyper-responsive and aggressive. However, although we do not definitively determine the mechanism of increased aggression in each genotype, it is likely different in the Epac1KO and R1A mice, because the single-knockout mice performed differently in some behavioral tests and circulating hormone concentrations are different between these genotypes. A deficit in sensorimotor gating is a potential source of these behavioral abnormalities in Epac1KO mice based on both our results and previous studies, whereas the overactive thyroid and resulting activation of the HPA are potential culprits in R1A mice. The R1A-Epac1KO mice carry both mutations, and thus have a behavioral phenotype induced by both. Very limited investigation of hyperthyroidism and aggressive behavior exists, and this may be an interesting area of research requiring further study.
Supplementary Material
Highlights.
Thyroid-specific knockout of Prkar1a and global Epac1 knockout induce aggression.
Prkar1a knockout causes hyperthyroidism, leading to hyper-responsiveness to stimuli.
Epac1−/− also causes hyper-responsiveness, likely due to sensorimotor gating deficits.
Both mutations contribute to aggression in Tpo-Cre;Prkar1aflox/flox;Epac1−/− mice.
Acknowledgments
The research present here was supported by the National Institutes of Health R21CA202745 (RJN), R01NS092388 (RJN), R01CA170249 (LSK), and P01CA124570 (LSK); as well as a Pelotonia Postdoctoral Fellowship award (DH). The VUMC Hormone Assay and Analytical Services Core is supported by NIH grants DK059637 and DK020593. The authors thank Yoshihiro Ishikawa for sharing the Epac1−/− mouse model and Dana Shaw and Alexa Magner for their technical contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none.
References
- Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F, Cheng X. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol. 2013;83:122–128. doi: 10.1124/mol.112.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatara VS, McMillin JM, Kummer M. Aggressive behavior and fire-setting in a 4-year-old boy associated with ingestion of ground beef contaminated with bovine thyroid tissue: A case report and review of neuropsychiatrie findings in pediatric thyrotoxicosis. J Child Adolesc Psychopharmacol. 2009;5:255–271. [Google Scholar]
- Bitiktas S, Kandemir B, Tan B, Kavraal S, Liman N, Dursun N, Donmez-Altuntas H, Aksan-Kurnaz I, Suer C. Adult-onset hyperthyroidism impairs spatial learning: possible involvement of mitogen-activated protein kinase signaling pathways. Neuroreport. 2016;27:802–808. doi: 10.1097/WNR.0000000000000612. [DOI] [PubMed] [Google Scholar]
- Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condren RM, O’Neill A, Ryan MCM, Barrett P, Thakore JH. HPA axis response to a psychological stressor in generalized social phobia. Psychoneuroendocrinol. 2002;27:693–704. doi: 10.1016/s0306-4530(01)00070-1. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: Experimental design evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nature Protocols. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Mondal AC, Rizavi HS, Faludi G, Palkovits M, Sarosi A, Conley RR, Pandey GN. Differential and brain region-specific regulation of Rap-1 and Epac in depressed suicide victims. JAMA Psychiatry. 2006;63:639–648. doi: 10.1001/archpsyc.63.6.639. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N, Wang H, Garza-Carbajal A, Willemen HLDM, Zwartkruis FJ, Wood JN, Dantzer R, Kelley KW, Heijnen CJ, Kavelaars A. Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, Protein Kinase Cε, and MEK/ERK. J Neurosci. 2010;30:12806–12815. doi: 10.1523/JNEUROSCI.3142-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, Nelson RJ. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205:349–354. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Garner JP, Weisker SM, Dufour B, Mench JA. Barbering (fur and whisker trimming) by laboratory mice as a model of human trichotillomania and obsessive-compulsive spectrum disorders. Comp Med. 2004;54:216–224. [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res. 2006;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabe HJ, Volzke H, Ludemann J, Wolff B, Schwahn C, John U, Meng W, Freyberger HJ. Mental and physical complaints in thyroid disorders in the general population. Acta Psychiatr Scand. 2005;112:286–293. doi: 10.1111/j.1600-0447.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- Grandoch M, Rose A, ter Braak M, Jendrossek V, Rubben H, Fischer JW, Schmidt M, Weber AA. Epac inhibits migration and proliferation of human prostate carcinoma cells. Br J Cancer. 2009;101:2038–2042. doi: 10.1038/sj.bjc.6605439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargadine HR, Lowenstein JM, Greenspan FS. Elevated serum TSH in human thyroid cancer. Oncology. 1970;24:172–180. doi: 10.1159/000224517. [DOI] [PubMed] [Google Scholar]
- Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen J. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–814. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke LA, Lister RG. The role of body weight in resident-intruder aggression. Aggressive Behavior. 1992;18:281–287. [Google Scholar]
- Kamilaris TC, DeBold CR, Johnson EO, Mamalaki E, Listwak SJ, Calogero AE, Kalogeras KT, Gold PW, Orth DN. Effects of short and long duration hypothyroidism and hyperthyroidism on the plasma adrenocorticotropin and corticosterone responses to ovine corticotropin-releasing hormone in rats. Endocrinol. 1991;128:2567–2576. doi: 10.1210/endo-128-5-2567. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Stein JM, Vecsey CG, Favilla C, Yang X, Bizily SF, Esposito MF, Wand G, Kanes SJ, Abel T. Developmental etiology for neuroanatomical and cognitive deficits in mice overexpressing Galphas, a G-protein subunit genetically linked to schizophrenia. Mol Psychiatry. 2009;14:398–415. doi: 10.1038/mp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Qi Y, Berrodin TJ, Davis KD, Schwartz HL, Strait KA, Oppenheimer JH, Lazar MA. Immunocytochemical delineation of thyroid hormone receptor beta 2-like immunoreactivity in the rat central nervous system. Endocrinol. 1993;132:2461–2469. doi: 10.1210/endo.132.6.7684976. [DOI] [PubMed] [Google Scholar]
- Ma N, Abel T, Hernandez PJ. Exchange protein activated by cAMP enhances long-term memory formation independent of protein kinase A. Learn Mem. 2009;16:367–370. doi: 10.1101/lm.1231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA. The psychopharmacology of aggression. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology. Vol. 19. New York: Plenum Press; 1987. pp. 183–328. [Google Scholar]
- Middeldorp CM, Vink JM, Hettema JM, de Geus EJ, Kendler KS, Willemsen G, Neale MC, Boomsma DI, Chen X. An association between Epac-1 gene variants and anxiety and depression in two independent samples. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:214–219. doi: 10.1002/ajmg.b.30976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya ER, Terburg D, Bos PA, van Honk J. Testosterone, cortisol, and serotonin as key regulators of social aggression: A review and theoretical perspective. Motiv Emot. 2012;36:65–73. doi: 10.1007/s11031-011-9264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Shewan DA. Epac mediates cyclic AMP-dependent axon growth, guidance, and regeneration. Mol Cell Neurosci. 2008;38:578–588. doi: 10.1016/j.mcn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Nikolopoulou E, Mytilinaios D, Calogero AE, Kamilaris TC, Troupis T, Chrousos GP, Johnson EO. Modulation of central glucocorticoid receptors in short- and long-term experimental hyperthyroidism. Endocrine. 2015;49:828–841. doi: 10.1007/s12020-015-0528-7. [DOI] [PubMed] [Google Scholar]
- Noszczyk-Nowak A, Pasławska U, Skrzypczak P, Nicpoń J, Gajek J, Zysko D. Symptoms, biochemical and hematological blood parameters in pigs in the course of experimental hyperthyroidism. Medycyna Weterynaryjna. 2007;63:219–223. [Google Scholar]
- O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hasings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Zhang L, Zhu JJ, Schwede F, Thomas SA. Epac signaling is required for hippocampus-dependent memory retrieval. Proc Natl Acad Sci USA. 2008;105:11993–11997. doi: 10.1073/pnas.0804172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioral despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–381. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Pringle DR, Yin Z, Lee AA, Manchanda PK, Yu L, Parlow AF, Jarjoura D, La Perle KMD, Kirschner LS. Thyroid specific ablation of the Carney Complex gene, PRKAR1A, results in hyperthyroidism and follicular thyroid cancer. Endocr-Relat Cancer. 2012;19:435–446. doi: 10.1530/ERC-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle DR, Vasko VV, Yu L, Manchanda PK, Lee AA, Zhang X, Kirschner JM, Parlow AF, Saji M, Jarjoura D, Ringel MD, La Perle KMD, Kirschner LS. Follicular thyroid cancers demonstrate dual activation of PKA and mTOR as modeled by thyroid-specific deletion of Prkar1a and Pten in mice. J Clin Endocrinol Metab. 2014;99:E804–E812. doi: 10.1210/jc.2013-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper DJ, Saunders MP, Salisbury AJ, Long L, O’Byrne KJ, Braybrooke JP, Dowsett M, Taylor M, Talbot DC, Ganesan TS, Harris AL. Phase I study of the novel cyclic AMP (cAMP) analogue 8-Chloro-cAMP in patients with cancer: Toxicity, hormonal, and immunological effects. Clin Cancer Res. 1999;5:1682–1689. [PubMed] [Google Scholar]
- Rakov H, Engels K, Hones GS, Strucksberg KH, Moeller LC, Kohrle J, Zwanziger D, Fuhrer D. Sex-specific phenotypes of hyperthyroidism and hypothyroidism in mice. Biol Sex Differ. 2016;7:36. doi: 10.1186/s13293-016-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Neto F, Leon A, Urbani-Brocard J, Lou L, Nyska A, Altschuler DL. cAMP-dependent oncogenic action of Rap1b in the thyroid gland. J Biol Chem. 2004;279:46868–46875. doi: 10.1074/jbc.M406858200. [DOI] [PubMed] [Google Scholar]
- Sandrini F, Matyakhina L, Sarlis NJ, Kirschner LS, Farmakidis C, Gimm O, Stratakis CA. Regulatory subunit type I-α of protein kinase A (PRKAR1A): A tumor-suppressor gene for sporadic thyroid cancer. Gene Chromosomes Cancer. 2002;35:182–192. doi: 10.1002/gcc.10112. [DOI] [PubMed] [Google Scholar]
- Saunders MP, Salisbury AJ, O’Byrne KJ, Long L, Whitehouse RM, Talbot DC, Mawer B, Harris AL. A novel cyclic adenosine monophosphate analog induces hypercalcemia via production of 1,25-dihydroxyvitamin D in patients with solid tumors. J Clin Endocrinol Metab. 1997;82:4044–4048. doi: 10.1210/jcem.82.12.4410. [DOI] [PubMed] [Google Scholar]
- Steinetz BG, Beach VL. Some influences of thyroid on the pituitary-adrenal axis. Endocrinol. 1963;72:45–58. doi: 10.1210/endo-72-1-45. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Yokoyama U, Abe T, Kiyonari H, Yamashita N, Kato Y, Kurotani R, Sato M, Okumura S, Ishikawa Y. Differential roles of EPAC in regulating cell death in neuronal and myocardial cells. J Biol Chem. 2010;285:24248–24259. doi: 10.1074/jbc.M109.094581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen AF, Kessing LV. Increased risk of hyperthyroidism among patients hospitalized with bipolar disorder. Bipolar Disord. 2005;7:351–357. doi: 10.1111/j.1399-5618.2005.00205.x. [DOI] [PubMed] [Google Scholar]
- Tsygankova OM, Saavedra A, Rebhun JF, Quilliam LA, Meinkoth JL. Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol Cell Biol. 2001;21:1921–1929. doi: 10.1128/MCB.21.6.1921-1929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broek FAR, Omzight CM, Beynen AC. Whisker trimming behavior in A2G mice is not prevented by offering means of withdrawal from it. Lab Anim. 1993;27:270–272. doi: 10.1258/002367793780745462. [DOI] [PubMed] [Google Scholar]
- Van Honk J, Harmon-Jones E, Morgan BE, Schutter DJ. Socially explosive minds: the triple imbalance hypothesis of reactive aggression. J Pers. 2010;78:67–94. doi: 10.1111/j.1467-6494.2009.00609.x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Meijer OC, de Kloet ER, Koolhaas JM, Bohus BG. Differences in basal and stress-induced HPA regulation of wild house mice selected for high and low aggression. Hormones and Behavior. 2003;43:197–204. doi: 10.1016/s0018-506x(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Walker SE, Papilloud A, Huzard D, Sandi C. The link between aberrant hypothalamic-pituitary-adrenal axis activity during development and the emergence of aggression – Animal studies. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacol. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wolf A, Bauer B, Abner EL, Ashkenazy-frolinger T, Hartz AMS. A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLOS One. 2016;11:e0147733. doi: 10.1371/journal.pone.0147733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shu X, Liu D, Pei L, Zhu L, Tian Q, Wang J, Lu Y. EPAC null mutation impairs learning and social interactions via aberrant regulation of miR-124 and Zif268 translation. Neuron. 2012;73:774–788. doi: 10.1016/j.neuron.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Zhou H, Yang Y, Jiang Y, Wang T, Lv L, Zhou Q, Yang Y, Dong X, He J, Huang X, Chen J, Wu K, Xu L, Mao R. The bidirectional effects of hypothyroidism and hyperthyroidism on anxiety- and depression-like behaviors in rats. Horm Behav. 2015;69:106–115. doi: 10.1016/j.yhbeh.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ma SL, Yeung PKK, Wong YH, Tsim KWK, So KF, Lam LCW, Chung SK. Anxiety and depression with neurogenesis defects in exchange protein directly activated by cAMP 2-deficient mice are ameliorated by a selective serotonin reuptake inhibitor. Prozac Transl Psychiatry. 2016;6:e881. doi: 10.1038/tp.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


