Abstract
Background
Converging evidence suggests that cerebral metabolic and cellular homeostasis is altered in patients with recent-onset of schizophrenia. As a possible marker of metabolic changes that might link to altered neurotransmission, we used proton magnetic resonance spectroscopy (1H-MRS) to estimate brain temperature, and we evaluated its relationship to a relevant metabolite, glutamate, within this study population.
Methods
Twenty patients with recent-onset (≤24 months after first psychotic symptoms) of schizophrenia and 20 healthy controls were studied using 1H-MRS at 7 Tesla. We measured levels of N-acetylaspartate (NAA) and glutamate, and estimated brain temperature in a non-invasive manner.
Results
Healthy controls showed a significant negative correlation between glutamate and brain temperature in the anterior cingulate cortex. In contrast, the physiological correlation between glutamate and brain temperature was lost in patients with recent-onset of schizophrenia.
Conclusions
This study supports the hypothesized, disrupted relationship between brain metabolism and neurotransmission in patients with recent-onset of schizophrenia. The findings include mechanistic implications that are to be followed up in both preclinical and clinical studies.
Keywords: anterior cingulate cortex, proton magnetic resonance spectroscopy, brain temperature, schizophrenia, glutamate, imaging
Introduction
Schizophrenia (SZ) is a devastating brain disorder with complex, enigmatic pathology (1–3). Converging evidence suggests that seemingly diverse biological systems are altered in this disease. Involvement of oxidative stress and inflammation has been suggested by epidemiological studies and studies of tissues from patients (4–10), while altered glutamatergic neurotransmission has also been implicated in the underlying pathophysiology (11–15). These varied biological systems may be unified by the hypothesis of dysfunctional homeostatic regulation, with possible links to impaired cellular metabolism and dysfunctional neural networks (16).
Proton magnetic resonance spectroscopy (1H-MRS) may be a powerful approach to address this question, as this methodology allows us to estimate the levels of neurotransmitters and metabolites in the brains of living subjects, while also examining the effects of other clinical factors such as stage of illness and medication exposure (13–15). N-acetylasparatate (NAA) and glutamate are biochemically linked through reactions of the tricarboxylic acid (TCA) cycle. NAA is proposed to function as a physiological reservoir for glutamate in the healthy brain, among other roles such as acting as an osmolyte and as an acetate donor in myelination (17, 18).
Brain metabolites in health and disease are influenced by the effect of local cerebral energetics on enzymatically-driven, multi-step pathways. Brain temperature (BT) reflects the balance between heat produced by neural activity/metabolism and dissipation of this heat through blood flow (19–21). In preclinical studies, presynaptic BT gradients reportedly influence the release of neurotransmitters and neurotransmitter removal from the synapse (22, 23). In clinical investigations, an altered relationship between lateral ventricle temperature and cerebral blood flow, as well as the presence of higher occipital-frontal temperature gradient have been observed in SZ (24, 25). Thus, BT may be a useful and underused measure to study dysfunctional, metabolic homeostasis in SZ.
1H-MRS allows the non-invasive and quantitative estimation of human BT in vivo (26–29). 1H-MRS thermometry takes advantage of the linear dependence of the chemical shift of water on temperature and estimates temperature within a voxel based on the chemical shift differences between the water peak and NAA peak, the latter serving as a reference (26, 27). The precision of this method relies on the accuracy of resonance frequency determination, which is improved at higher magnetic field strength, such as 7 Tesla (7T), compared to that of lower magnetic field strengths (1.5T or 3T) (30, 31). Moreover, 1H-MRS can be used to examine the relationship between BT and co-localized, physiologically-linked brain metabolites, such as glutamate.
Since brain energy homeostasis affects biochemical cycling of glutamate pools and likely influences glutamate-related circuitry (32), in the present study we used 7T 1H-MRS to estimate glutamate, NAA, and BT in the anterior cingulate cortex (ACC). We then compared the relationship between glutamate and BT between healthy controls and patients with recent-onset of SZ, the latter defined as within 24 months of first psychotic symptoms. The ACC was selected based on evidence that altered neurotransmission in the ACC may be linked to cognitive deficits and negative symptoms in patients with SZ (33, 34).
Methods and Materials
Participants
Twenty patients diagnosed with recent-onset of SZ and twenty healthy controls were enrolled in this study. Patients were recruited from the Johns Hopkins Medical Institutions (outpatient psychiatric clinics), while controls were recruited from flyers in the Greater Baltimore area. All patients were clinically stable at the time of participation. Diagnosis was established and confirmed with the use of the Structured Clinical Interview for DSM-IV (SCID) (35), administered to all participants. Recent-onset was assessed using the SCID and was defined as within 24 months of the first psychotic symptoms. Psychopathology was assessed and quantified by clinical psychiatrists using the Scales for the Assessment of Positive and Negative Symptoms (SAPS and SANS) (36). Chlorpromazine (CP) equivalent dose was calculated (37). Participants were excluded for: (a) age below 18 years old or above 30 years old, (b) history of structural brain injury and/or history of traumatic brain injury with loss of consciousness, (c) any unstable medical condition, (d) history of vertigo, seizure disorder, or middle-ear disorder, (e) contraindication to magnetic resonance imaging (such as metal in the body or claustrophobia), (f) cannabis use in the past four months, (g) short acting benzodiazepine use in the past two weeks, (h) long acting benzodiazepine use in the past four weeks, or (i) pyrexia. All subjects provided written informed consent. The study was approved by a Johns Hopkins Institutional Review Board.
Neuropsychological Assessment
Participants completed a battery of neuropsychological tests (Table S1) to assess performance in domains of processing speed, attention/working memory, verbal memory, visual memory, ideational fluency, and executive function as previously described (38). Factor scores were calculated for each domain after controlling for age, sex, race, and premorbid ability using the Calibrated Neuropsychological Normative System (CNNS) (39, 40). Premorbid ability was estimated using the Hopkins Adult Reading Test (41). Composite neuropsychological performance, defined as the average of all domain scores, was calculated for each participant.
Magnetic resonance imaging (MRI) and spectroscopy acquisition
All scans were performed between noon and 2 pm. Participants were asked to wait 20 min in a standardized room prior to measurement of oral temperature, ensuring acclimatization. Core Body Temperature (CBT) was measured using a digital thermometer that was placed sublingually (42).
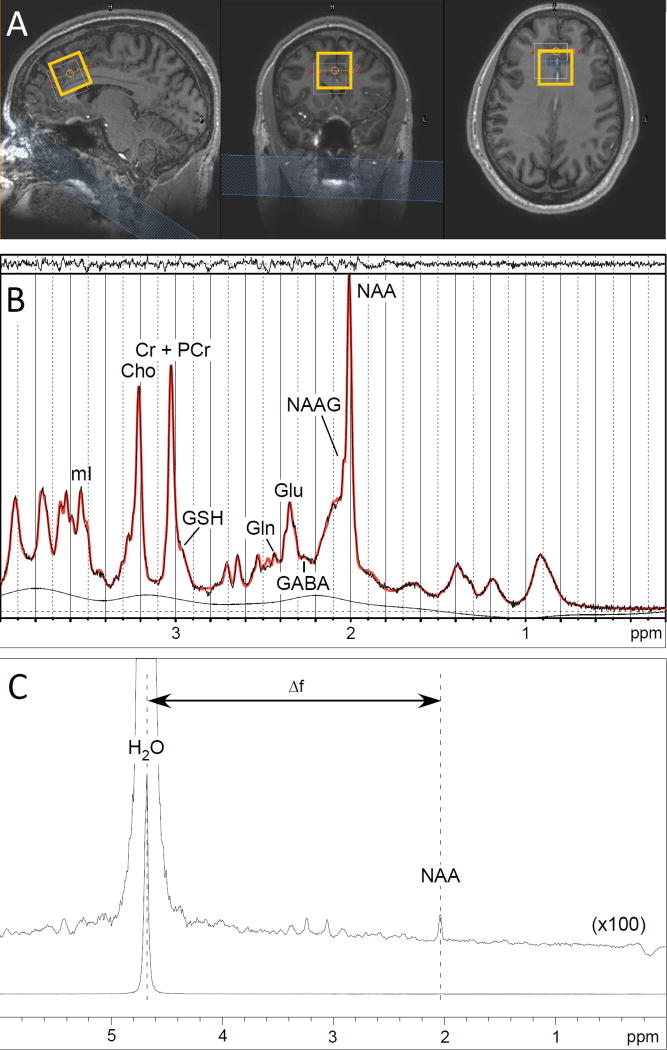
All investigations were performed on a 7T whole-body magnetic resonance scanner (Philips, Cleveland, USA) equipped with a birdcage transmit head coil in combination with a 32-channel receive coil (both Nova Medical Inc., Burlington, USA). Head movement was minimized with the use of foam padding. A T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (225 slices, 0.8 mm isotropic voxels, repetition time (TR) = 4.3 ms, echo time (TE) = 1.93 ms, flip angle = 7°, field of view = 220 × 220 × 180 mm3, 276 × 274 acquisition matrix, SENSE factor 4) was obtained for anatomical reference and tissue classification. The spectroscopic voxel (30 × 30 × 30 mm3) was positioned in the ACC, immediately superior and parallel to the genu of the corpus callosum (Figure 1A).
Figure 1. 7T Proton Magnetic Resonance Spectroscopy (1H-MRS).
A. Voxel placement. From left to right: sagittal view, coronal view, and axial view of voxel placement in the anterior cingulate cortex (ACC). The thin line shows the chemical shift displacement effect of water relative to NAA (bold line).
B. Representative 1H-MRS spectrum. An example of a spectrum (black line) acquired from voxel placement in the ACC, with LCModel-fitted spectrum (bold red line). Above the spectrum the residual signal after fitting is displayed. The baseline is displayed below the spectrum.
C. Representative spectrum from 1H-MRS thermometry. An example of a spectrum acquired from voxel placement in the ACC using a semi-localized by adiabatic refocusing sequence (sLASER).
Spectra were acquired using a STimulated Echo Acquisition Mode sequence (STEAM; TR = 3000 ms, TE = 15 ms, mixing time (TM) = 25 ms, 64 averages with water suppression and two averages without water suppression). Water suppression was achieved using variable pulse powers and optimized relaxation delays (VAPOR) presaturation pulses.
STEAM spectra were analyzed using LCModel version 6.3 (43). The basis set was simulated using VeSPA density matrix simulation program (44), and consisted of 20 metabolites, including glutamate, NAA, creatine (Cr), and phosphocreatine (PCr) with spin systems taken from literature (45) (Figure 1B). The default macromolecule spectra along with the spline baseline from LCModel were used to fit the baseline with an added constraint of 0.2 ppm knot spacing for the baseline. There were no metabolite concentration uncertainties provided by LCModel that exceeded a Cramer-Rao Lower Bound (CRLB) of 2% for glutamate or NAA. Signal-to-noise values were high and full-width half maximum measures were low in all acquired spectra (mean ± standard deviation = 97.0 ± 17.7 and 11.9 ±2.6 Hz, respectively). Glutamate and NAA values from all participants were therefore considered high quality and were then normalized to the value of total creatine (Cr+PCr = tCr) for further analyses. Glutamate/tCr and NAA/tCr are here forward referred to as ‘glutamate ratio’ and ‘NAA ratio’ respectively. Fractional tissue composition of grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) in the 1H-MRS voxel were calculated using the ‘Gannet’ program (46).
For in vivo 1H-MRS thermometry a semi-localized by adiabatic refocusing sequence (sLASER; TR = 3000 ms, TE = 120 ms, 2048 points, 16 averages) was used without water suppression. BT was estimated using the water chemical shift relative to NAA, which was measured using the program ‘csx3’ (47). The water-NAA chemical shift differences were used to calculate BT using the formula given by BT(Celsius)=282.0-92.2(δwater−δNAA) (28).
Statistical analysis
All statistical analyses were performed using Stata 13.1 software (STATA, College Station, Texas, USA). We first tested the homogeneity of the sample by using χ2 test or Fisher’s exact test for dichotomous variables and t-test for continuous variables. Differences between groups were tested using two sample t-tests, with the exception of testing differences in BT and CBT where a paired t-test was employed. Pearson’s correlation or Spearman’s correlation analysis was used depending on the normality of the data. Normality of the data was judged by the skewness and kurtosis normality test. Partial correlation analysis was performed when accounting for CP equivalent dose. Results are presented as mean ± Standard Deviation (SD). The threshold for statistical significance in all analyses was set as P < 0.05, with further correction for multiple comparisons when noted.
Results
Study participants and neuropsychological performance
20 patients and 20 healthy controls completed 7T 1H-MRS acquisition. There were no differences between patients and controls in age, gender, race, and years of education (Table 1). Among patients, the duration of illness ranged from 1–24 months (median 12 months). Six patients and two healthy control participants were smokers. Four patients were not taking antipsychotic medication at the time of participation. Two patients were taking typical antipsychotic medication, and the other 14 patients were taking second-generation antipsychotic medication, the latter of which included two patients taking clozapine). The range of CP equivalents within the study population was 0–400. While the majority of patients were prescribed antipsychotic monotherapy, three patients were prescribed a selective serotonin reuptake inhibitor medication and one was taking lithium.
Table 1.
Clinical and demographic characteristics
| Characteristics | SZ (N=20) | Control (N=20) | P |
|---|---|---|---|
| Age (Years) | 24.25 ± 4.22 | 23.10 ± 2.99 | 0.326 |
| Gender (Male/Female) | 13/7 | 13/7 | 1.000a |
| Race (African American/Caucasian/Asian) | 16/4/0 | 14/4/2 | 0.606b |
| Years of Education (Years) | 12.83 ± 2.20 | 13.60 ± 1.96 | 0.246 |
| Smoking (Yes/No) | 6/14 | 2/18 | 0.235b |
| CP Equivalent Dose (mg) | 188.00 ± 130.59 | NA | |
| Neuropsychological Performancec | |||
| Composite | 88.19 ± 10.64 | 100.92 ± 9.10 | <0.001* |
| Processing Speed | 86.70 ± 15.84 | 101.65 ± 16.99 | 0.007* |
| Attention/Working Memory | 85.60 ± 17.48 | 104.53 ± 16.86 | 0.001* |
| Verbal Memory | 84.60 ±14.12 | 96.70 ± 15.80 | 0.015 |
| Visual Memory | 85.25 ± 16.59 | 96.45 ± 14.99 | 0.031 |
| Ideational Fluency | 92.85 ± 14.57 | 107.85 ± 12.23 | 0.001* |
| Executive Function | 94.15 ± 18.65 | 98.85 ± 14.02 | 0.373 |
| SAPS | 13.55 ± 16.53 | NA | |
| SANS | 17.85 ± 11.99 | NA |
Data are displayed as mean ± standard deviation with significance (P values) from two sample t-tests unless otherwise specified.
χ2 test
Fisher’s exact test
The threshold for significance in comparisons within each of the six cognitive domains was set as P < 0.0083, accounting for multiple comparisons. The threshold for significance in all other comparisons displayed was set as P < 0.05.
Indicates significance.
Not applicable, NA.
Patients with recent-onset of SZ had lower composite neuropsychological scores (P < 0.001) and lower scores in the domains of processing speed, attention/working memory, and ideational fluency compared to controls (P < 0.008, Table 1).
7T 1H-MRS: basic characterization and relationship with neuropsychological performance
There were no differences between patients with recent-onset of SZ and controls in segmentation fractions (white matter, grey matter, cerebrospinal fluid) or in CRLB values, a marker of data quality, for glutamate and NAA (Table S2). The ratio of tCr to water in the voxel was not different between patients with SZ and controls (P = 0.36), and there was no correlation found between the ratio of tCr to water and BT, supporting use of tCr for normalization of the glutamate and NAA spectra in this study population. There was no correlation between BT and white matter, grey matter, or cerebrospinal fluid when assessed across the whole study population or within either cohort (patients, controls).
We observed no difference in glutamate or NAA ratios between patients with recent-onset of SZ and controls (glutamate ratio: SZ 1.29 ± 0.10, Control 1.33 ± 0.14, P = 0.30; NAA ratio: SZ 1.29 ± 0.07, Control 1.32 ± 0.07, P = 0.15). There was also no difference in glutamate or NAA measures relative to water between patients with recent-onset of SZ and controls before or after correction for CSF within the voxel (glutamateuncorrected: SZ 7.56 ± 1.18, Control 7.56 ± 1.34, P = 0.16; NAAuncorrected: SZ 7.56 ± 1.34, Control 8.33 ± 2.45, P = 0.23; glutamateCSF_corrected: SZ 8.38 ± 1.24, Control 9.03 ± 1.94, P = 0.22; NAACSF_corrected: SZ 8.38 ± 1.42, Control 9.09 ± 2.55, P = 0.29. Using data from the total study population and controlling for cohort, glutamate ratio did not correlate with neurocognitive performance in any cognitive domain. There was also no correlation between glutamate ratio and neuropsychological performance within the control group. Within the patients, glutamate ratio showed a negative correlation with executive function (r = −0.620, P = 0.004) (Table 2), but no correlation with other domains of neuropsychological performance. Among patients, glutamate ratio was not correlated with total score on the SAPS (r = 0.37, P = 0.10) or SANS (r = −0.16, P = 0.50).
Table 2.
Relationship between glutamate and neuropsychological performance in the anterior cingulate cortex within patients with recent-onset of SZ.
| Clinical Characteristics | Correlation Coefficienta | Pb |
|---|---|---|
| Neuropsychological performance | ||
| Processing Speed | 0.093 | 0.695 |
| Attention/Working Memory | 0.190 | 0.422 |
| Verbal Memory | 0.220 | 0.351 |
| Visual Memory | 0.102 | 0.669 |
| Ideational Fluency | 0.135 | 0.570 |
| Executive Function | −0.620 | 0.004* |
Pearson’s correlation analysis was applied to data from 20 patients with recent-onset of SZ.
The threshold for significance in comparisons within each of the six cognitive domains was set as P < 0.0083, accounting for multiple comparisons.
Indicates significance.
Brain temperature (BT)
BT, as measured by 1H-MRS, was higher than CBT in both patients with recent-onset of SZ (P < 0.001) and healthy controls (P <0.001), consistent with previous reports (19, 48). Patients with SZ and controls did not differ in BT or CBT (Table S3). There was no correlation found between BT or CBT and sex or age. While ΔT, defined as ΔT=BT-CBT, is higher in some patient populations with robust excitotoxicity, oxidative stress, or inflammatory processes (49–52), in our study population ΔT in controls (0.68 ± 0.72 °C) did not differ from that of patients with recent-onset of SZ (0.84 ± 0.50 °C) (P = 0.43). CBT and BT values were not correlated within controls (r = 0.182, P = 0.443) or within the patients (r = 0.231, P = 0.327).
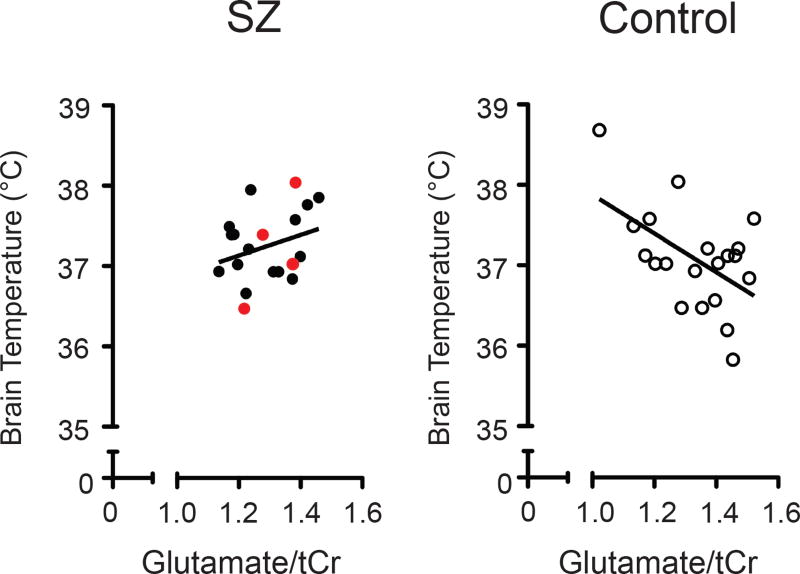
A negative correlation was found between glutamate ratio and BT in controls (r = −0.527, P = 0.017) (Figure 2, Table S4A), but was not found in patients with recent-onset of SZ (r = 0.294, P = 0.209), even after correction for CP equivalent dose (r = 0.301, P = 0.210) or after exclusion of those four patients who were not taking antipsychotic medication (r = 0.206, P = 0.445). The difference in the correlation coefficients between the two cohorts (controls, patients) was significant (Fisher's z = −2.5916, P = 0.0096). No correlation was observed in either cohort between a) NAA ratio and BT or b) CBT and either metabolite ratio (glutamate, NAA) (Table S4A). Secondary analyses exploring the relationship between BT, CBT, and other metabolites measured using the LCModel revealed a negative correlation between BT and the combined measure of glutamate and glutamine relative to tCr in controls (r = −0.568, P = 0.009) (Table S4A), but this relationship was not found in patients with recent-onset of SZ (r = 0.362, P = 0.117), and there were no significant correlations between BT and glutamine/tCr in either cohort. BT did not correlate with neurocognitive performance, or with clinical characteristics (total score on the SAPS or SANS, CP equivalent dose) (Table S4B).
Figure 2. Correlations between brain temperature and glutamate in patients with recent-onset of schizophrenia (SZ) and healthy controls in anterior cingulate cortex.
Glutamate values are normalized to total creatine (tCr). Data points from the patient group are indicated with closed circles and those from controls are marked with open circles. The four patients who were not taking antipsychotic medication have data points shown in red. For each group, the solid line represents the linear regression fit. Pearson’s correlation coefficient in SZ (r = 0.294, P = 0.209) and controls (r = −0.527, P = 0.017). Degrees Celsius, °C.
Discussion
Here we report a negative relationship between BT and glutamate ratio in the ACC of the healthy subjects. Interestingly, the correlation between these variables is not observed in patients with recent-onset of SZ. Our results also support the promise of this technique in exploring further the relationship between local BT and metabolite pools.
Physiological fluctuations in BT are increasingly recognized as mechanistically related to co-localized cell function (53). There is accumulating evidence indicating that diverse cellular machinery underlying neurotransmitter release and synaptic transmission are sensitive to local temperature (54, 55). Furthermore both dopamine release and its removal from the synapse dynamically change over the range of physiologic BT (22, 23). Nonetheless, such preclinical knowledge has not translated to clinical studies yet. Though more human studies are needed, the present data suggest that, in health, the total glutamate pool in the ACC may diminish with increased BT, whereas such physiological correlation is lost in patients with recent-onset of SZ. Related, integrative studies with both preclinical and clinical components are expected to elucidate further the pathophysiological relationship of these measures relevant to SZ.
This study benefits from several technical and methodological strengths: we used a high-field strength scanner at 7T, allowing for better signal-to-noise ratio, and increased spectral resolution. These methodological characteristics allow precise BT measurement and improved ability to quantify glutamate and NAA compared with lower field strengths (31). In our study, we followed strict exclusion criteria, including recent cannabis and benzodiazepine use that could potentially affect the final results (56). Furthermore, we obtained past medical history and systematically assessed neuropsychological performance and symptoms across the population in order to probe the relationship between BT and clinical characteristics.
We also acknowledge the limitations of the present study. First, even with the use of the vendor-supplied macromolecule (MM) basis set in the LCModel, macromolecular resonances may still effect estimations of glutamate concentrations (57). Nevertheless there is no reason to expect any systematic differences in MM resonances between cohorts, and therefore this factor is unlikely to alter the conclusions of this study. Second, the sample size was relatively small. Larger sample size is needed to examine further the relationship between clinical characteristics such as smoking status or medication use and these findings within and between cohorts. Third, spatial coverage was limited to the ACC and we focused on glutamate while other brain regions and other brain metabolites are also relevant to pathophysiology of this disease. Finally, the cross-sectional study design supports descriptive, but not causal interpretations.
Ota et al. recently estimated BT in chronic SZ using diffusion-weighted imaging with 3T MRI (24). In this intriguing study, BT was measured inside the lateral ventricle and then tested for correlation with cerebral blood flow in several brain regions (24). A separate study of patients with SZ also examined BT using 3T 1H-MRS, but without references to metabolite concentrations (25). In contrast, as far as we are aware, this study is the first application of 1H-MRS-based BT measurement in psychiatric research at 7T, with the higher magnetic field strength allowing more precise BT measurement due to separation of the NAA peak from that of NAAG. We also uniquely examined the relationship between BT and glutamate in the same brain region, to which measurement of co-localized blood flow (58) could be added in further studies.
Together, the present study highlights the potential utility of 1H-MRS to evaluate altered relationships between BT and metabolite pools that may be affected in parallel in SZ. Loss of these physiological relationships may reflect SZ-associated imbalance between glutamatergic signaling and metabolic cascades. Our findings include mechanistic implications that should be pursued in both preclinical and clinical studies.
Supplementary Material
Acknowledgments
This work was supported by the following USPHS grants: MH-084018 (A.S.), MH-094268 Silvio O. Conte center (A.S.), MH-069853 (A.S.), MH-085226 (A.S.), MH-088753 (A.S.), MH-092443 (A.S.), MH-103789 (A.S), RUSK/S-R foundations (A.S.), NARSAD (A.S., J.C.), and the Alexander Wilson Schweizer Fellowship (J.C.) In addition, a fund from Mitsubishi Tanabe Pharma Corporation was partly utilized for limited expenses related to patient recruitment. We thank Ms. Yukiko Lema for helping to edit the figures and manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science (New York, NY) 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- 2.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakhshi K, Chance SA. The neuropathology of schizophrenia: A selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neuroscience. 2015;303:82–102. doi: 10.1016/j.neuroscience.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Coughlin JM, Ishizuka K, Kano SI, Edwards JA, Seifuddin FT, Shimano MA, et al. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Molecular psychiatry. 2013;18:10–11. doi: 10.1038/mp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes LN, Severance EG, Leek JT, Gressitt KL, Rohleder C, Coughlin JM, et al. Inflammatory molecular signature associated with infectious agents in psychosis. Schizophrenia bulletin. 2014;40:963–972. doi: 10.1093/schbul/sbu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kano S, Colantuoni C, Han F, Zhou Z, Yuan Q, Wilson A, et al. Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Molecular psychiatry. 2013;18:740–742. doi: 10.1038/mp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emiliani FE, Sedlak TW, Sawa A. Oxidative stress and schizophrenia: recent breakthroughs from an old story. Current opinion in psychiatry. 2014;27:185–190. doi: 10.1097/YCO.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulak A, Steullet P, Cabungcal JH, Werge T, Ingason A, Cuenod M, et al. Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: insights from animal models. Antioxidants & redox signaling. 2013;18:1428–1443. doi: 10.1089/ars.2012.4858. [DOI] [PubMed] [Google Scholar]
- 9.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biological psychiatry. 2013;74:400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biological psychiatry. 2013;73:951–966. doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu W, MacDonald ML, Elswick DE, Sweet RA. The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Annals of the New York Academy of Sciences. 2015;1338:38–57. doi: 10.1111/nyas.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophrenia bulletin. 2013;39:120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poels EM, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA, et al. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Molecular psychiatry. 2014;19:20–29. doi: 10.1038/mp.2013.136. [DOI] [PubMed] [Google Scholar]
- 15.Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of Glutamate Alterations in Schizophrenia: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. JAMA psychiatry. 2016;73:665–674. doi: 10.1001/jamapsychiatry.2016.0442. [DOI] [PubMed] [Google Scholar]
- 16.Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Molecular psychiatry. 2016;21:10–28. doi: 10.1038/mp.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ, et al. N-acetylaspartate as a reservoir for glutamate. Medical hypotheses. 2006;67:506–512. doi: 10.1016/j.mehy.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 18.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Progress in neurobiology. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Frontiers in bioscience (Landmark edition) 2010;15:73–92. doi: 10.2741/3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du F, Cooper AJ, Thida T, Sehovic S, Lukas SE, Cohen BM, et al. In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA psychiatry. 2014;71:19–27. doi: 10.1001/jamapsychiatry.2013.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyatkin EA, Brown PL, Wise RA. Brain temperature fluctuation: a reflection of functional neural activation. The European journal of neuroscience. 2002;16:164–168. doi: 10.1046/j.1460-9568.2002.02066.x. [DOI] [PubMed] [Google Scholar]
- 22.Guatteo E, Chung KK, Bowala TK, Bernardi G, Mercuri NB, Lipski J. Temperature sensitivity of dopaminergic neurons of the substantia nigra pars compacta: involvement of transient receptor potential channels. Journal of neurophysiology. 2005;94:3069–3080. doi: 10.1152/jn.00066.2005. [DOI] [PubMed] [Google Scholar]
- 23.Xie T, McCann UD, Kim S, Yuan J, Ricaurte GA. Effect of temperature on dopamine transporter function and intracellular accumulation of methamphetamine: implications for methamphetamine-induced dopaminergic neurotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7838–7845. doi: 10.1523/JNEUROSCI.20-20-07838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ota M, Sato N, Sakai K, Okazaki M, Maikusa N, Hattori K, et al. Altered coupling of regional cerebral blood flow and brain temperature in schizophrenia compared with bipolar disorder and healthy subjects. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:1868–1872. doi: 10.1038/jcbfm.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiloh R, Kushnir T, Gilat Y, Gross-Isseroff R, Hermesh H, Munitz H, et al. In vivo occipital-frontal temperature-gradient in schizophrenia patients and its possible association with psychopathology: a magnetic resonance spectroscopy study. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2008;18:557–564. doi: 10.1016/j.euroneuro.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Cady EB, D'Souza PC, Penrice J, Lorek A. The estimation of local brain temperature by in vivo 1H magnetic resonance spectroscopy. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;33:862–867. doi: 10.1002/mrm.1910330620. [DOI] [PubMed] [Google Scholar]
- 27.Corbett RJ, Laptook AR, Tollefsbol G, Kim B. Validation of a noninvasive method to measure brain temperature in vivo using 1H NMR spectroscopy. Journal of neurochemistry. 1995;64:1224–1230. doi: 10.1046/j.1471-4159.1995.64031224.x. [DOI] [PubMed] [Google Scholar]
- 28.Cady EB, Penrice J, Robertson NJ. Improved reproducibility of MRS regional brain thermometry by 'amplitude-weighted combination'. NMR in biomedicine. 2011;24:865–872. doi: 10.1002/nbm.1634. [DOI] [PubMed] [Google Scholar]
- 29.Rieke V, Butts Pauly K. MR thermometry. Journal of magnetic resonance imaging : JMRI. 2008;27:376–390. doi: 10.1002/jmri.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephenson MC, Gunner F, Napolitano A, Greenhaff PL, Macdonald IA, Saeed N, et al. Applications of multi-nuclear magnetic resonance spectroscopy at 7T. World journal of radiology. 2011;3:105–113. doi: 10.4329/wjr.v3.i4.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi C, Dimitrov IE, Douglas D, Patel A, Kaiser LG, Amezcua CA, et al. Improvement of resolution for brain coupled metabolites by optimized (1)H MRS at 7T. NMR in biomedicine. 2010;23:1044–1052. doi: 10.1002/nbm.1529. [DOI] [PubMed] [Google Scholar]
- 32.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends in neurosciences. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Roberts RC, Barksdale KA, Roche JK, Lahti AC. Decreased synaptic and mitochondrial density in the postmortem anterior cingulate cortex in schizophrenia. Schizophrenia research. 2015;168:543–553. doi: 10.1016/j.schres.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, McGuire PK, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2515–2521. doi: 10.1038/npp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.First MBSR, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department, New Yok State Psychiatric Institute; 2002. [Google Scholar]
- 36.Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Archives of general psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 37.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biological psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojeda N, Pena J, Schretlen DJ, Sanchez P, Aretouli E, Elizagarate E, et al. Hierarchical structure of the cognitive processes in schizophrenia: the fundamental role of processing speed. Schizophrenia research. 2012;135:72–78. doi: 10.1016/j.schres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Parmenter BA, Testa SM, Schretlen DJ, Weinstock-Guttman B, Benedict RH. The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) Journal of the International Neuropsychological Society : JINS. 2010;16:6–16. doi: 10.1017/S1355617709990750. [DOI] [PubMed] [Google Scholar]
- 40.Testa SM, Winicki JM, Pearlson GD, Gordon B, Schretlen DJ. Accounting for estimated IQ in neuropsychological test performance with regression-based techniques. Journal of the International Neuropsychological Society : JINS. 2009;15:1012–1022. doi: 10.1017/S1355617709990713. [DOI] [PubMed] [Google Scholar]
- 41.Schretlen DJ, Winicki JM, Meyer SM, Testa SM, Pearlson GD, Gordon B. Development, psychometric properties, and validity of the hopkins adult reading test (HART) The Clinical neuropsychologist. 2009;23:926–943. doi: 10.1080/13854040802603684. [DOI] [PubMed] [Google Scholar]
- 42.Hooper VD, Andrews JO. Accuracy of noninvasive core temperature measurement in acutely ill adults: the state of the science. Biological research for nursing. 2006;8:24–34. doi: 10.1177/1099800406289151. [DOI] [PubMed] [Google Scholar]
- 43.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 44.Soher B, Semanchuk P, Todd D, Steinberg J, Young K. VeSPA: integrated applications for RF pulse design, spectral simulation and MRS data analysis. 2011 doi: 10.1002/mrm.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR in biomedicine. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 46.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. Journal of magnetic resonance imaging : JMRI. 2014;40:1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soher BJ, van Zijl PC, Duyn JH, Barker PB. Quantitative proton MR spectroscopic imaging of the human brain. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1996;35:356–363. doi: 10.1002/mrm.1910350313. [DOI] [PubMed] [Google Scholar]
- 48.McIlvoy L. Comparison of brain temperature to core temperature: a review of the literature. The Journal of neuroscience nursing : journal of the American Association of Neuroscience Nurses. 2004;36:23–31. doi: 10.1097/01376517-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Campos F, Blanco M, Barral D, Agulla J, Ramos-Cabrer P, Castillo J. Influence of temperature on ischemic brain: basic and clinical principles. Neurochemistry international. 2012;60:495–505. doi: 10.1016/j.neuint.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 51.Karaszewski B, Wardlaw JM, Marshall I, Cvoro V, Wartolowska K, Haga K, et al. Measurement of brain temperature with magnetic resonance spectroscopy in acute ischemic stroke. Annals of neurology. 2006;60:438–446. doi: 10.1002/ana.20957. [DOI] [PubMed] [Google Scholar]
- 52.Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiology of disease. 2003;12:163–173. doi: 10.1016/s0969-9961(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Wang B, Normoyle KP, Jackson K, Spitler K, Sharrock MF, et al. Brain temperature and its fundamental properties: a review for clinical neuroscientists. Frontiers in neuroscience. 2014;8:307. doi: 10.3389/fnins.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao Y, Zorman S, Gundersen G, Xi Z, Ma L, Sirinakis G, et al. Single reconstituted neuronal SNARE complexes zipper in three distinct stages. Science (New York, NY) 2012;337:1340–1343. doi: 10.1126/science.1224492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nature reviews Neuroscience. 2005;6:829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- 56.Prescot AP, Renshaw PF, Yurgelun-Todd DA. gamma-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug and alcohol dependence. 2013;129:232–239. doi: 10.1016/j.drugalcdep.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Kim M, Normoyle KP, Llano D. Thermal Regulation of the Brain-An Anatomical and Physiological Review for Clinical Neuroscientists. Frontiers in neuroscience. 2015;9:528. doi: 10.3389/fnins.2015.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.