Abstract
Aims
Recent guidelines recommend that patients with heart failure and left ventricular ejection fraction (LVEF) 40–49% should be managed similar to LVEF ≥ 50%. We investigated the effect of beta-blockers according to LVEF in double-blind, randomized, placebo-controlled trials.
Methods and results
Individual patient data meta-analysis of 11 trials, stratified by baseline LVEF and heart rhythm (Clinicaltrials.gov: NCT0083244; PROSPERO: CRD42014010012). Primary outcomes were all-cause mortality and cardiovascular death over 1.3 years median follow-up, with an intention-to-treat analysis. For 14 262 patients in sinus rhythm, median LVEF was 27% (interquartile range 21–33%), including 575 patients with LVEF 40–49% and 244 ≥ 50%. Beta-blockers reduced all-cause and cardiovascular mortality compared to placebo in sinus rhythm, an effect that was consistent across LVEF strata, except for those in the small subgroup with LVEF ≥ 50%. For LVEF 40–49%, death occurred in 21/292 [7.2%] randomized to beta-blockers compared to 35/283 [12.4%] with placebo; adjusted hazard ratio (HR) 0.59 [95% confidence interval (CI) 0.34–1.03]. Cardiovascular death occurred in 13/292 [4.5%] with beta-blockers and 26/283 [9.2%] with placebo; adjusted HR 0.48 (95% CI 0.24–0.97). Over a median of 1.0 years following randomization (n = 4601), LVEF increased with beta-blockers in all groups in sinus rhythm except LVEF ≥50%. For patients in atrial fibrillation at baseline (n = 3050), beta-blockers increased LVEF when < 50% at baseline, but did not improve prognosis.
Conclusion
Beta-blockers improve LVEF and prognosis for patients with heart failure in sinus rhythm with a reduced LVEF. The data are most robust for LVEF < 40%, but similar benefit was observed in the subgroup of patients with LVEF 40–49%.
Keywords: Heart failure, Ejection fraction, Beta-blockers, Mortality, Sinus rhythm, Atrial fibrillation
Introduction
Double-blind, randomized, placebo-controlled trials (RCTs) show that beta-blockers increase left ventricular ejection fraction (LVEF) and reduce morbidity and mortality for a broad range of patients with a reduced LVEF in sinus rhythm.1,2 Until recently, international guidelines on heart failure have recognized two left ventricular phenotypes; heart failure with reduced LVEF (HFrEF) or preserved LVEF (HFpEF).3,4 Values for LVEF are continuously distributed but measurement precision is imperfect; differences of up to 10% for an individual patient may be attributed to measurement error5 and therefore precise cut-points of LVEF cannot reliably differentiate between phenotypes. Recently, the European Society of Cardiology (ESC) suggested there should be a third intermediate phenotype, called mid-range ejection fraction (HFmrEF; 40–49%), thereby creating a clear separation between HFrEF (<40%) and HFpEF (≥50%).4 These guidelines suggest that until more information becomes available, patients with HFmrEF should be managed similarly to those with HFpEF, for which no therapy has been shown to improve mortality.4
The Beta-blockers in Heart Failure Collaborative Group (BB-meta-HF) was created to pool individual patient data (IPD) from the major heart failure RCTs comparing beta-blockers and placebo to address key issues in relevant patient subgroups.6 Most, but not all of these trials recruited patients with an LVEF ≤35% predominantly in sinus rhythm; IPD provides an opportunity to collate high-quality data from double-blind trials on the smaller number of patients with higher LVEF where the efficacy of beta-blockers is uncertain. Why beta-blockers appear ineffective in patients with heart failure and concomitant atrial fibrillation (AF),2,7,8 and whether this holds true regardless of LVEF is also unclear. In this paper, we investigate the effect of beta-blockers on LVEF and prognosis, stratified according to the baseline LVEF and heart rhythm.
Methods
The Beta-blockers in Heart Failure Collaborative Group (BB-meta-HF) includes the lead investigators from the relevant trials, with the support of the four pharmaceutical companies that conducted them (AstraZeneca, GlaxoSmithKline, Merck Serono and Menarini). This report was prepared according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) IPD guidance,9 and prospectively registered with Clinicaltrials.gov (NCT0083244) and the PROSPERO database of systematic reviews (CRD42014010012).10
Eligibility and search strategy
Detailed rationale and methods have previously been published.1,6,7 Only unconfounded placebo-controlled trials were eligible that recruited >300 patients, with a planned follow-up of >6 months and explicit reporting of mortality. All trials had appropriate ethical approval.
Eleven studies were included that account for 95.7% of eligible participants recruited in RCTs based on a systematic literature review: the Australia/New Zealand Heart Failure Study (ANZ),11 the Beta-Blocker Evaluation Survival Trial (BEST),12 the Carvedilol Post-Infarct Survival Control in LV Dysfunction Study (CAPRICORN),13 the Carvedilol Hibernating Reversible Ischaemia Trial: Marker of Success Study (CHRISTMAS),14 the Cardiac Insufficiency Bisoprolol Study (CIBIS I),15 the Cardiac Insufficiency Bisoprolol Study II (CIBIS-II),16 the Carvedilol Prospective Randomized Cumulative Survival Study (COPERNICUS),17 the Metoprolol in Idiopathic Dilated Cardiomyopathy Study (MDC),18 the Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF),19 the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS),20 and the U.S. Carvedilol Heart Failure Program (US-HF).21
All included studies had low risk of bias, as determined using the Cochrane Collaborations Risk of Bias Tool.22
Data collection and individual patient data integrity
A standardized data request form to obtain IPD from each trial has been published, along with search results and individual study demographics.6 IPD were obtained for all 11 trials identified in the systematic review, and data were extracted from original source files provided by the pharmaceutical companies and lead investigators. All data were cross-checked across different trial databases and compared with published reports. Discrepancies, inconsistencies, and incomplete data were checked against original case report forms and trial documentation to ensure IPD integrity. All 11 trial databases were then harmonized according to the standardized data request form to match patient characteristics and outcomes across all trials. Due to the small amount of missing data for relevant covariates, imputation was not performed.
Participants
We included all patients with baseline LVEF and an electrocardiogram (ECG) that showed either sinus rhythm or AF/atrial flutter (for the purposes of this report, reference to AF therefore includes atrial flutter). As we have already demonstrated an interaction of treatment effect with heart rhythm,7 patients with sinus rhythm and AF were analysed separately. Patients with heart block, or a paced rhythm at baseline were excluded.
Outcomes and effect measures
The primary outcomes for this analysis were all-cause mortality and cardiovascular death, which included additional deaths reported after the censor date for seven studies.19–21,23–26 Secondary outcomes were the first cardiovascular hospitalization and the composite of cardiovascular death and cardiovascular hospitalization (time to first event). All secondary outcomes were based on events from the study period only and do not include the MDC trial which did not collect this information. Three patients (one with sinus rhythm and two with AF) had missing event dates and were excluded from outcome analyses.
Most of the trials had limits for LVEF as inclusion or exclusion criteria, however these were typically defined preceding randomization (<25%,17 ≤35%,12,16,21 ≤40%,13,15,18,19 and <45%;11Supplementary material online, Figure S1). In this analysis, we used the baseline value of LVEF recorded in individual patient case report forms or core laboratory assessment, which in some patients was above the entry criterion according to that particular study. LVEF was analysed as a continuous variable to model interactions with outcomes, and classified as <20%, 20–25%, 26–34%, 35–39%, 40–49%, and≥50%, as well as <40%, 40–49%, ≥50% to align with guideline phenotypes.
Statistical analysis
A statistical analysis plan was generated and finalized by the Collaborative Group in advance of data analysis. Summary results are presented as percentages, or median and interquartile range (IQR; displayed as 25th–75th quartiles).
All analyses followed the principle of intention-to-treat. Patients were classified by heart rhythm and LVEF. Outcomes were analysed using a Cox proportional hazards regression model,27 stratified by study. This is a one-stage fixed effects approach and assumes that all trials are estimating a common treatment effect with baseline hazards that vary across studies. Fractional polynomials were used to find the best transformation,28 although a linear relationship with mortality was the best fit. Hazard ratios (HR) and 95% confidence intervals (CI) are presented, along with corresponding P-values. We pre-specified adjustment in Cox models for age, sex, systolic blood pressure, prior myocardial infarction, and baseline use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and diuretic therapy. Adjustments for treatment allocation and LVEF were also made where appropriate. Kaplan–Meier plots were used to graph the pooled, unadjusted trial data, with log-rank tests for comparison stratified by study. Only a minority of patients were followed for more than three years and therefore data were censored at 1200 days (3.3 years) from randomization. Pre-defined sensitivity analyses included additional multivariable adjustment [including diabetes, New York Heart Association (NYHA) class (I/II vs. III/IV), estimated glomerular filtration rate and digoxin]; data are not shown as these results did not differ with our main model. We performed a post hoc sensitivity analysis which excluded patients with an LVEF reported at exactly 40% from the 40–49% (mid-range) group. A post hoc analysis of cardiovascular hospitalization accounting for the competing risk of death was performed using the method of Fine and Gray23; results were similar to the results of the stratified Cox regression model.
We show the association between baseline LVEF and all cause-mortality by plotting the hazard of baseline LVEF relative to a baseline LVEF of 35%, fitted using an adjusted Cox proportional hazards model stratified by study. Follow-up LVEF was available in six trials.11,12,14,18,20,21 We used the last available result to calculate change in LVEF from baseline. As availability of follow-up LVEF is determined by survival, we chose not to perform any statistical hypothesis testing.
There was no evidence of violation of the proportional hazards assumption in any multivariable model as determined by Schoenfeld residuals.24 Effect modification was assessed using P-values from interaction terms fitted in the multivariable models.28,29 A two-tailed P-value of 0.05 was considered statistically significant. Analyses were performed on Stata Version 14.1 (StataCorp LP, TX, USA) and R Version 3.2.1 (R Core Team, Vienna).
Results
Individual patient data were obtained for 18 637 patients. Patients were excluded if they had a missing baseline ECG (n = 118), heart block (n = 510), paced rhythm (n = 616) or were missing their baseline LVEF (n = 91). The cohort included 14 262 patients in sinus rhythm and 3050 patients in AF (Supplementary material online, Figure S1), with a mean follow-up of 1.5 years (standard deviation 1.1) and median follow-up of 1.3 years (IQR 0.8–1.9). Median age was 65 (IQR 55–72) years, 24% were women and 66% had ischaemic heart disease (IHD) as the cause for heart failure. Median LVEF at baseline was 27% (21–33%) and was similar for patients in sinus rhythm (Table 1) and AF (Supplementary material online, Table S1). Combining both heart rhythms, 721 patients had an LVEF 40–49% and 317 had an LVEF ≥50%. Patients with a higher baseline LVEF were older, more likely to be women, have milder NYHA class, higher blood pressure, and were less likely to have heart failure due to IHD. There were no differences in patient characteristics between those assigned to beta-blockers or placebo (Supplementary material online, Table S2).
Table 1.
Baseline characteristics for patients in sinus rhythm
| Characteristic | Left ventricular ejection fraction at baseline |
|||||
|---|---|---|---|---|---|---|
| <20% n = 2553 | 20–25% n = 3885 | 26–34% n = 5076 | 35–39% n = 1929 | 40–49% n = 575 | ≥50% n = 244 | |
| LVEF, median (IQR) | 0.15 (0.13–0.18) | 0.23 (0.21–0.24) | 0.30 (0.28–0.32) | 0.36 (0.35–0.38) | 0.40 (0.40–0.43) | 0.58 (0.53–0.65) |
| Age, median years (IQR) | 61 (51–69) | 63 (54–71) | 64 (55–71) | 64 (56–72) | 71 (61–75) | 75 (72–78) |
| Women, n (%) | 521 (20.4%) | 886 (22.8%) | 1272 (25.1%) | 518 (26.9%) | 198 (34.4%) | 129 (52.9%) |
| Years with HF diagnosis, median (IQR) | 3 (1–6) | 3 (1–6) | 2 (1–5) | 2 (1–5) | 2 (1–5) | 2 (1–5) |
| Ischaemic HF aetiology, n (%) | 1484 (58.1%) | 2572 (66.2%) | 3475 (68.5%) | 1562 (81.0%) | 522 (90.8%) | 209 (85.7%) |
| Prior myocardial infarction, n (%) | 1242 (48.7%) | 2187 (56.4%) | 2993 (59.2%) | 1374 (71.4%) | 412 (71.8%) | 88 (36.1%) |
| Diabetes mellitus, n (%) | 575 (25.1%) | 956 (26.0%) | 1153 (23.9%) | 409 (22.2%) | 135 (24.1%) | 71 (29.1%) |
| NYHA class III/IV, n (%) | 1624 (82.1%) | 2045 (77.6%) | 3265 (64.8%) | 721 (37.7%) | 136 (24.1%) | 64 (26.6%) |
| Heart rate, median bpm (IQR) | 84 (76–92) | 80 (72–90) | 78 (72–87) | 76 (70–84) | 76 (68–82) | 75 (68–83) |
| Systolic BP, median mmHg (IQR) | 114 (104–127) | 120 (110–136) | 127 (115–140) | 130 (116–140) | 131 (120–145) | 147 (132–160) |
| Diastolic BP, median mmHg (IQR) | 72 (66–80) | 77 (70–82) | 79 (70–83) | 80 (70–83) | 80 (70–85) | 82 (78–90) |
| Body mass index, median kg/m2 (IQR) | 27 (24–32) | 27 (24–31) | 27 (24–31) | 27 (25–30) | 27 (25–30) | 27 (24–31) |
| Estimated GFR, median mL/min (IQR) | 62 (50–76) | 61 (48–75) | 66 (53–80) | 65 (53–78) | 66 (53–78) | 69 (55–83) |
| Any diuretic therapy, n (%) | 2410 (94.4%) | 3547 (91.3%) | 4331 (85.3%) | 1273 (66.0%) | 376 (65.4%) | 199 (81.6%) |
| ACEi or ARB, n (%) | 2304 (94.8%) | 3490 (94.7%) | 4643 (94.8%) | 1774 (95.1%) | 508 (90.6%) | 203 (87.3%) |
| Aldosterone antagonists, n (%) | 207 (8.8%) | 381 (10.4%) | 360 (7.5%) | 85 (4.7%) | 31 (5.8%) | 27 (11.9%) |
| Digoxin, n (%) | 1833 (73.8%) | 2297 (60.4%) | 2475 (49.9%) | 555 (29.6%) | 138 (25.6%) | 48 (21.2%) |
Missing data report: n = 2828 for years with HF diagnosis; n = 30 for prior myocardial infarction; n = 809 for diabetes mellitus; n = 1504 for NYHA class; n = 62 for systolic BP; n = 67 for diastolic BP; n = 8 heart rate; n = 123 for body mass index; n = 664 for GFR; n = 918 for aldosterone antagonists; n = 376 for digoxin.
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; b.p.m., beats/minute; GFR, glomerular filtration rate; HF, heart failure; IQR, interquartile range; LVEF, left-ventricular ejection fraction; NYHA, New York Heart Association.
Association of LVEF with mortality
Left ventricular ejection fraction at baseline was inversely associated with all-cause mortality, with an adjusted HR of 1.16 for each 5% lower LVEF (95% CI 1.26–1.19; P < 0.0001). Figure 1 displays the hazard of all-cause mortality with LVEF 35% as the reference. The association between LVEF and prognosis was stronger for patients in sinus rhythm than AF (Supplementary material online, Table S3). Patients with LVEF ≥50% had the lowest mortality despite their older age (Supplementary material online, Figure S2); all-cause and cardiovascular mortality were 10.4% and 6.3% respectively for those with LVEF ≥50%, compared to 26.7% and 21.7% for those with LVEF <20%. Mortality was predominantly cardiovascular regardless of aetiology, both for patients in sinus rhythm (Supplementary material online, Table S4) and AF (Supplementary material online, Table S5), and mostly attributed to sudden death or worsening heart failure.
Figure 1.
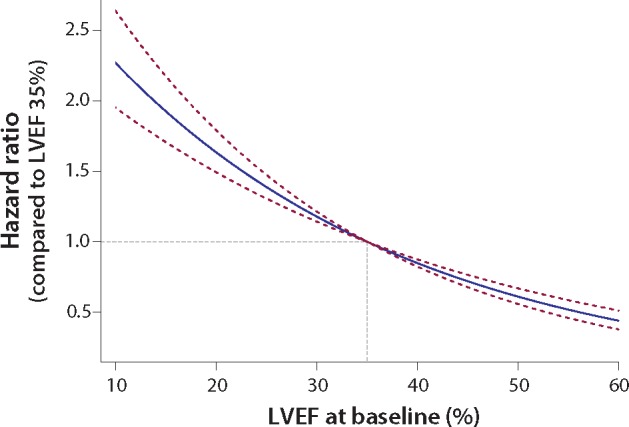
Hazard of all-cause mortality across the spectrum of LVEF. Hazard ratio and 95% confidence intervals for all-cause mortality according to baseline left ventricular ejection fraction (LVEF), relative to a patient with an LVEF of 35%. Hazard ratios are fitted using a Cox proportional hazards regression model, adjusted for treatment, age, gender, previous myocardial infarction, systolic blood pressure, heart rate, use of angiotensin inhibitors/receptor blockers and diuretics, and stratified by study.
Efficacy of beta-blockers
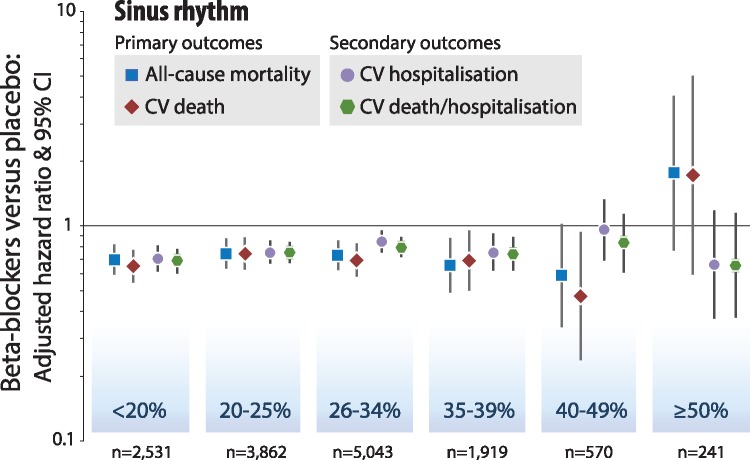
Beta-blockers were associated with reductions in all-cause and cardiovascular mortality compared to placebo for patients in sinus rhythm (interaction P > 0.5 for LVEF as a continuous measure). Beta-blockers were effective in all LVEF categories, except in the small subgroup where LVEF was ≥50% (Table 2, Figure 2). There was no evidence for a difference in benefit when LVEF was 40–49%; all-cause mortality occurred in 21/292 [7.2%] randomized to a beta-blockers compared to 35/283 [12.4%] assigned to placebo (adjusted HR 0.59, 95% CI 0.34–1.03), and cardiovascular death in 13/292 [4.5%] with beta-blockers and 26/283 [9.2%] with placebo; (adjusted HR 0.48, 95% CI 0.24–0.97) (Figure 3). Beta-blockers reduced both sudden death and deaths ascribed to heart failure for patients in sinus rhythm, but had no effect on non-cardiovascular mortality (Supplementary material online, Table S4). Secondary outcomes (cardiovascular hospitalization and the composite of cardiovascular death and cardiovascular hospitalization) were lower with beta-blockers in all LVEF categories for patients in sinus rhythm, but confidence intervals were wide when LVEF exceeded 40% (Table 2, Figure 2).
Table 2.
Beta-blockers vs. placebo according to left ventricular ejection fraction at baseline
| Baseline heart rhythm and LVEF category | All-cause mortality |
Cardiovascular death |
Cardiovascular hospitalization |
Composite of cardiovascular death | ||||
|---|---|---|---|---|---|---|---|---|
| or cardiovascular hospitalization | ||||||||
| Events/N | HR (95% CI); P-value | Events/N | HR (95% CI); P-value | Events/N | HR (95% CI); P-value | Events/N | HR (95% CI); P-value | |
| Sinus rhythm | ||||||||
| <20% | 623/2531 | 0.70 (0.60-0.83); P < 0.001 | 517/2531 | 0.67 (0.56-0.80); P < 0.001 | 762/2407 | 0.70 (0.60-0.81); P < 0.001 | 990/2407 | 0.68 (0.60-0.77); P < 0.001 |
| 20-25% | 619/3862 | 0.76 (0.65–0.89); P = 0.001 | 521/3862 | 0.78 (0.65–0.92); P = 0.004 | 1033/3807 | 0.75 (0.66–0.85); P < 0.001 | 1273/3807 | 0.75 (0.67–0.84); P < 0.001 |
| 26–34% | 631/5043 | 0.75 (0.64–0.88); P < 0.001 | 504/5042 | 0.73 (0.61–0.87); P < 0.001 | 1118/4972 | 0.84 (0.74–0.94); P = 0.003 | 1384/4972 | 0.80 (0.72–0.88); P < 0.001 |
| 35–39% | 189/1919 | 0.67 (0.50–0.90); P = 0.007 | 156/1919 | 0.72 (0.52–0.99); P = 0.041 | 401/1907 | 0.75 (0.61–0.91); P = 0.004 | 490/1907 | 0.74 (0.62–0.88); P = 0.001 |
| 40–49% | 55/570 | 0.59 (0.34–1.03); P = 0.066 | 38/570 | 0.48 (0.24–0.97); P = 0.040 | 144/566 | 0.95 (0.68–1.32); P = 0.76 | 164/566 | 0.83 (0.60–1.13); P = 0.23 |
| ≥50% | 24/241 | 1.79 (0.78–4.10); P = 0.17 | 15/241 | 1.77 (0.61–5.14); P = 0.29 | 50/241 | 0.66 (0.37–1.18); P = 0.16 | 54/241 | 0.66 (0.38–1.15); P = 0.14 |
| Atrial fibrillation | ||||||||
| <20% | 143/492 | 1.23 (0.88–1.74); P = 0.23 | 124/492 | 1.16 (0.80–1.67); P = 0.44 | 148/471 | 0.97 (0.69–1.35); P = 0.85 | 201/471 | 0.96 (0.72–1.28); P = 0.79 |
| 20–25% | 159/867 | 0.74 (0.54–1.02); P = 0.07 | 136/867 | 0.77 (0.54–1.08); P = 0.13 | 234/856 | 0.75 (0.58–0.98); P = 0.032 | 291/856 | 0.75 (0.59–0.95); P = 0.015 |
| 26–34% | 208/1093 | 0.98 (0.74–1.29); P = 0.87 | 166/1093 | 0.98 (0.72–1.34); P = 0.92 | 321/1083 | 1.01 (0.81–1.26); P = 0.92 | 390/1083 | 0.93 (0.76–1.13); P = 0.47 |
| 35–39% | 59/363 | 0.92 (0.53–1.58); P = 0.75 | 46/363 | 0.67 (0.35–1.25); P = 0.21 | 99/358 | 0.90 (0.60–1.36); P = 0.62 | 121/358 | 0.94 (0.65–1.37); P = 0.76 |
| 40–49% | 32/146 | 1.30 (0.63–2.67); P = 0.48 | 22/146 | 0.86 (0.36–2.03); P = 0.73 | 34/143 | 1.15 (0.57–2.32); P = 0.69 | 46/143 | 1.06 (0.58–1.94); P = 0.84 |
| ≥50% | 8/73 | 0.86 (0.19–3.94); P = 0.85 | 4/73 | 1.00 (0.10–9.91); P = 1.00 | 26/73 | 1.33 (0.56–3.16); P = 0.52 | 27/73 | 1.17 (0.51–2.71); P = 0.71 |
CI, confidence interval; HR, hazard ratio (adjusted for baseline characteristics and stratified by trial); n, number of individuals.
Figure 2.
Beta-blockers vs. placebo according to baseline LVEF in sinus rhythm. Intention to treat, one-stage Cox proportional hazards model in categories of left ventricular ejection fraction (LVEF) at baseline, adjusted for age, gender, previous myocardial infarction, systolic blood pressure, heart rate, and use of angiotensin inhibitors/receptor blockers, and diuretics. ‘n’ is the number of individual patients analysed from double-blind, randomized controlled trials for the primary outcomes with complete case data.
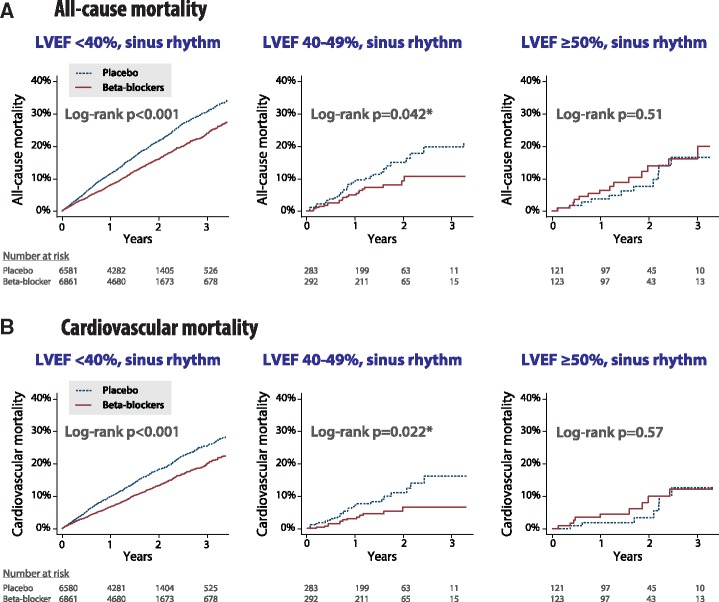
Figure 3.
Beta-blockers vs. placebo in sinus rhythm according to heart failure phenotype. Kaplan Meier plots for unadjusted (A) all-cause mortality and (B) cardiovascular mortality according to baseline left ventricular ejection fraction (LVEF). * Similar results in post hoc analysis when excluding patients with an LVEF reported as exactly 40% from the 40–49% group: (A) log-rank P = 0.030 and (B) log-rank P = 0.039; n = 147 placebo, and n = 143 beta-blockers.
Patients with AF at baseline demonstrated no consistent benefit on clinical outcomes with beta-blockers, regardless of LVEF (Figure 4). Fewer patients and events reduced the power to identify or refute modest differences in outcome.
Figure 4.
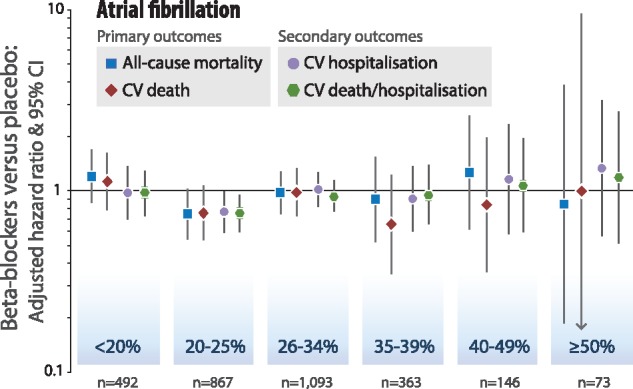
Beta-blockers vs. placebo according to baseline LVEF in atrial fibrillation. Intention to treat, one-stage Cox proportional hazards model in categories of left ventricular ejection fraction (LVEF) at baseline, adjusted for age, gender, previous myocardial infarction, systolic blood pressure, heart rate, and use of angiotensin inhibitors/receptor blockers, and diuretics. ‘n’ is the number of individual patients analysed from double-blind, randomized controlled trials for the primary outcomes with complete case data.
Change in LVEF
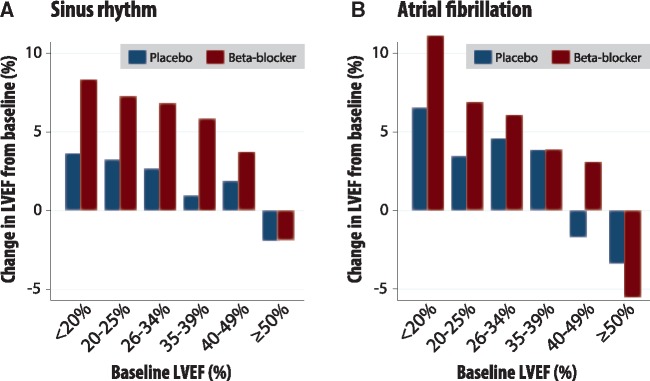
Change in LVEF was measured in 4601 patients in sinus rhythm and 996 patients in AF who survived to a follow-up assessment (median 1.0 years after baseline; IQR 0.3–2.0) (Supplementary material online, Figure S3). In sinus rhythm, LVEF increased more in patients randomized to beta-blockers than placebo, unless LVEF was ≥50% at baseline (Table 3, Figure 5). Increases in LVEF with beta-blockers were smaller for patients with IHD as the cause for heart failure compared to non-ischaemic cardiomyopathy (Supplementary material online, Table S6). Beta-blockers also increased LVEF for patients in AF in most LVEF categories except ≥50% (Table 3, Figure 5).
Table 3.
Absolute mortality difference and observed change in left ventricular ejection fraction
| Classification | ‘Reduced’ LVEF |
‘Mid–range’ LVEF | ‘Preserved’ LVEF | |||
|---|---|---|---|---|---|---|
| LVEF at baseline | <20% | 20–25% | 26–34% | 35–39% | 40–49% | ≥50% |
| Sinus rhythm: all aetiologya | ||||||
| Change in absolute mortality; beta-blockers vs. placebo (95% CI)b | n = 2552 | n = 3885 | n = 5076 | n = 1929 | n = 575 | n = 244 |
| –6.9% (–10.3% to –3.5%) | –3.9% (–6.3% to –1.6%) | –3.2% (–5.1% to –1.4%) | –3.4% (–6.1% to –0.7%) | –5.2% (–10.0% to –0.3%) | ||
| +2.3% (–5.3% to + 9.9%) | ||||||
| Change in LVEF from baseline to follow-up; mean difference (SE) beta-blockers vs. placeboc | n = 1106 | n = 1068 | n = 1600 | n = 375 | n = 251 | n = 201 |
| +4.7% (0.5%) | +4.0% (0.5%) | +4.2% (0.5%) | +4.9% (0.9%) | +1.9% (1.1%) | +0.1% (1.2%) | |
| Atrial fibrillation: all aetiology | ||||||
| Change in absolute mortality; beta-blockers vs. placebo (95% CI)a | n = 494 | n = 867 | n = 1101 | n = 367 | n = 146 | n = 73 |
| +2.8% (–5.3% to + 10.9%) | –4.1% (–9.3% to + 1.1%) | –0.8% (–5.5% to + 3.9%) | –3.2% (–10.7% to + 4.3%) | +3.2% (–10.4% to + 16.7%) | +0.3% (–14.0% to + 14.6%) | |
| Change in LVEF from baseline to follow-up; mean difference (SE) beta-blockers vs. placebob | n = 177 | n = 200 | n = 369 | n = 98 | n = 93 | n = 59 |
| +4.6% (1.7%) | +3.4% (1.2%) | +1.5% (1.0%) | +0.1% (1.9%) | +4.8% (1.9%) | –2.2% (3.0%) | |
CI, confidence interval; LVEF, left ventricular ejection fraction; SE, standard error of the mean difference; IQR, interquartile range.
See Supplementary material online, Table S6 for data according to ischaemic/non-ischaemic aetiology in sinus rhythm.
Median follow-up of 1.3 years (IQR 0.8–1.9).
Median 1.0 years after baseline assessment (IQR 0.3–2.0).
Figure 5.
Observed change in LVEF in survivors. Change in left ventricular ejection fraction (LVEF) from baseline in patients who survived to follow-up, with median time between measurements of 1.0 years (interquartile range 0.3–2.0 years). Those with follow-up LVEF were older in age compared to those without follow-up LVEF [67 (IQR 56–74) vs. 64 (55–71) years, respectively], but with similar baseline LVEF [27% (20–33) vs. 27% (21–33)] and frequency of ischaemic cardiomyopathy (65% vs. 67%). The variance for each category of change in LVEF (beta-blockers vs. placebo) is presented in Table 3. (A) Sinus rhythm; n = 4, 601 patients. (B) Atrial fibrillation; n = 996.
Discussion
This analysis suggests that for patients with heart failure in sinus rhythm, the effect of beta-blockers on mortality in patients with LVEF 40–49% is similar to that observed with LVEF < 40%. Consistent with the outcome data, LVEF increased with beta-blockers in all groups, except those with LVEF ≥50%. Only the SENIORS trial20 intentionally enrolled patients with any LVEF, but despite showing efficacy for beta-blockers in those with LVEF > 35%,25 there were too few patients and events to draw any conclusions in patients with more preserved LVEF. The lower the LVEF, the higher the rate of adverse outcomes and therefore the benefit of beta-blockers might be expected to be greatest in those with lower LVEF, as seen in a subgroup analysis of the MERIT-HF trial.26 However, we demonstrate a substantial 4.7% absolute reduction in cardiovascular mortality in patients with LVEF 40–49% and sinus rhythm (number needed to treat to prevent one cardiovascular death = 21 during a median follow-up of 1.3 years). This finding was statistically significant despite the relatively low number of trial patients studied in this LVEF category. Our preference in statistical analysis is always to report the interaction of treatment effect across continuous variables such as LVEF, instead of relying on efficacy in subgroups. However in this case, the data are dominated by patients with LVEF < 40% and interaction tests are known to have low power.30 Similar improvements in LVEF were seen for those in AF, but this did not translate into better outcomes with beta-blockers for patients in AF.
The mechanisms by which beta-blockers exert benefit are uncertain.2 Blocking adrenergic receptors has direct effects on cardiomyocytes, reduces heart rate, alters vascular function, and modifies the neuro-endocrine response to heart failure.31 The importance of these mechanisms may vary by aetiology, left ventricular phenotype, heart rhythm and clinical indication. For example, beta-blockers are recommended for the treatment of ventricular tachycardia and prevention of ventricular fibrillation in the context of an acute coronary syndrome,32 but may have deleterious effects compared to other therapy in hypertension or non-cardiac surgery.33 An improvement in LVEF is usually considered evidence of therapeutic benefit, but this analysis suggests we should be cautious about making such assumptions. The increase in LVEF with beta-blockers was smaller for patients with IHD, but the benefit on mortality was similar to those with a non-ischaemic cause for heart failure. The increase in LVEF with beta-blockers was similar for patients in sinus rhythm and AF, yet those with AF obtained no benefit on morbidity or mortality. The underlying reasons for this discrepancy remains a subject of discussion,4,8 and the increase in both incidence and prevalence of AF34 highlights a growing unmet clinical and research need.
Recent guidelines from the ESC suggest that left ventricular dysfunction should be classified as HFrEF when LVEF is <40%, HFmrEF when 40–49% and HFpEF only when LVEF is 50% or greater.4 The guideline points out that trials have, until recently, mostly used an LVEF of 40% or 45% to define HFpEF and none have identified an intervention that reduced morbidity or mortality for such patients.4 Accordingly, the guideline recommends that patients with HFmrEF be managed in the same way as HFpEF until new evidence becomes available. Interestingly, a post hoc analysis of the Treatment of Preserved cardiac function heart failure with an Aldosterone antagonist Trial (TOPCAT) also suggested a reduction in cardiovascular mortality with spironolactone in patients with an investigator-recorded LVEF 45–49%, but not when LVEF was greater than this.35 Initial data from the Candesartan in Heart failure—Assessment of moRtality and Morbidity (CHARM) program of trials suggests that angiotensin inhibition has a similar benefit in patients with LVEF 40–49% as with < 40%.36 In line with our data, it is possible that future guideline recommendations for patients with this intermediate phenotype should be more similar to those for HFrEF than HFpEF, and that the threshold for differences in heart failure therapy should be at, or around, an LVEF of 50%.
This analysis has limitations, with varied design and objectives of the component trials and relatively sparse outcome data for patients with LVEF >40%. The distribution of LVEF was not normal due to the inclusion criteria of the component RCTs; although the 40–49% group was weighted towards the lower end of mid-range LVEF, we found that primary outcomes were reduced in this group in sinus rhythm even when excluding those with an LVEF of 40%. In any trial, there is concern about whether the patients enrolled reflect the population encountered in clinical practice due to selection criteria, and this analysis is no different. However, our data represent the vast majority of patients enrolled in double-blind RCTs of beta-blockers.
Our use of individual-patient baseline LVEF, rather than the screening LVEF that qualified for inclusion, meant that most trials contributed some data to the LVEF 40–49% group. Although the SENIORS trial, with a distinct type of beta-blocker, was the only RCT to specifically recruit patients with higher LVEF, it only accounted for 44% of patients in this category. In trials of HFrEF, LVEF measured in a core echocardiography laboratory will exceed the LVEF inclusion criterion in 20–40% of patients.37–40 Some of the differences between the core laboratory and investigators may be explained by measurement error, but there also appears to be a bias on the part of investigators, conscious or unconscious, towards measuring an LVEF that allows for patient inclusion. Regression towards the mean will also result in repeat measures being less extreme; thus our approach of using double-blind data will have reduced, but not eliminated measurement bias and inadvertent misclassification. Both in research trials and clinical practice, measurements such as LVEF have inherent variability that requires clinical review and oversight.
Reported measurements such as blood pressure and LVEF are prone to digit preference (e.g. 40% rather than 39%) and variability in timing, technique, and quantification. The impact of this can be lessened by including a large amount of raw data (see Supplementary material online, Figure S3) or by using, where available, software generated LVEF (e.g. by Teichholz or Simpson’s biplane method) rather than an ‘eyeball’ assessment. Patients who died had no follow-up LVEF and therefore this could have introduced bias in measured changes in LVEF.
Determination of LVEF may be less accurate for patients in AF due to variability in cardiac cycle length.41 The smaller number of patients with AF, although large in comparison to many published interventional trials,42 limits our ability to make detailed comparisons to patients in sinus rhythm. Finally, data on natriuretic peptides, diastolic ventricular filling dynamics and atrial structure and function were lacking, which often help to describe different heart failure phenotypes.
Conclusion
For patients with heart failure in sinus rhythm and LVEF <40%, beta-blockers improve left ventricular systolic function and reduce cardiovascular morbidity and mortality. These benefits also apply to patients with LVEF 40–49%, a group in which beta-blocker therapy seems more likely to help than to harm. No benefit was seen in patients with LVEF ≥50%, but too few patients have been studied in double-blind RCTs to draw firm conclusions on the efficacy or safety of beta-blockers for HFpEF. No consistent evidence of prognostic benefit was observed for patients with heart failure and concomitant AF.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We are indebted to the other members of the Beta-blockers in Heart Failure Collaborative Group for database access and extraction support (for full list, please see design paper6), the steering committees of the included trials (in particular representatives of the MERIT-HF trial), as well as the late Philip Poole-Wilson (1943–2009; Imperial College, London, UK). This work is dedicated to the memory of Henry Krum (1958–2015; Monash University Melbourne, Australia), one of the founding members of the Collaborative Group. This project was only possible with the support of the pharmaceutical companies that have marketed beta-blockers in heart failure, and the group wishes to extend their gratitude to AstraZeneca, GlaxoSmithKline, Menarini Farmaceutica and Merck Serono for full access to trial data. We gratefully acknowledge incorporation of data from the BEST trial through the National Heart Lung and Blood Institute BioLINCC programme.
Authors’ contributions
J.G.F.C., K.V.B., and D.K. drafted the manuscript. D.K. also participated in the design of the study, leads the collaborative group and performed data management and statistical analysis. J.H. and D.G.A. performed independent statistical analyses and also manuscript revision. M.D.F. participated in the design and coordination of the study, and manuscript revision. All other named authors read, revised, and approved the final manuscript.
Funding
Menarini Farmaceutica Internazionale provided an unrestricted research grant for administrative costs; GlaxoSmithKline provided data extraction support; and IRCCS San Raffaele a collaborative research grant. DK is funded through a National Institute for Health Research (NIHR) Career Development Fellowship (CDF-2015-08-074) that also supports K.V.B. The opinions expressed are those of the authors and do not represent the NIHR or the UK Department of Health.
Conflict of interest: All authors have completed the ICMJE uniform disclosure form (www.icmje.org/coi_disclosure.pdf) and declare: J.G.F.C. reports grants and personal fees from Amgen, grants and personal fees from Novartis and Stealth Biotherapeutics, non-financial support from Medtronic and Boston Scientific, all outside the submitted work. K.V.B. has nothing to disclose. M.D.F. reports grants from Novarts and personal fees from AstraZeneca, all outside the submitted work. D.G.A. has nothing to disclose. J.H. has nothing to disclose. A.J.S.C. reports grants and personal fees from Menarini, during the conduct of the study and personal fees from Servier, Lone Star, Vifor and Respicardia, all outside the submitted work. L.M. has nothing to disclose. J.J.V.M. reports payments for trial-related activities to the University of Glasgow from Novartis, Cardiorentis, Amgen, Oxford University/Bayer, GlaxoSmithKline, Theracos, Abbvie, DalCor, Pfizer, Merck, AstraZeneca, Bristol Myers Squibb, and Kidney Research UK (KRUK)/Kings College Hospital, London/Vifor-Fresenius Pharma, all outside the submitted work. F.R. reports grants or personal fees from SJM, Servier, Zoll, AstraZeneca, Sanofi, Cardiorentis, Amgen, BMS, Pfizer, Fresenius, Vifor, Roche, Bayer, Abbott and Novartis, all outside the submitted work. D.J.V. has nothing to disclose. T.G.L. reports personal fees from Novartis, St Jude Medical, and Vifor, all outside the submitted work. M.B. reports personal fees from Boehringer-Ingelheim, Medtronic, Servier, Abbot, Astra-Zeneca and BMS, all outside the submitted work. B.A. has nothing to disclose. J.K. has nothing to disclose. M.P. reports personal fees from Amgen, Boehringer Ingelheim, Cardiorentis and Sanofi, all outside the submitted work. A.S.R. has nothing to disclose. G.R. has nothing to disclose. H.W. reports personal fees from AstraZeneca during the conduct of the study. A.H. has nothing to disclose. J.W. reports grants and personal fees from AstraZeneca Sweden, during the conduct of the study; and previously a Medical Adviser on cardiovascular research for AstraZeneca, Sweden. D.K. reports grants from Menarini, during the conduct of the study (unrestricted grant for administration of the Beta-Blockers in Heart Failure Collaborative Group); professional development support from Daiichi Sankyo and personal fees from Atricure, both outside the submitted work; and Chief Investigator of the RAte control Therapy Evaluation in permanent Atrial Fibrillation trial (RATE-AF; NCT02391337).
Statement
The Steering Committee Lead (Dr Kotecha) and the Centre for Statistics in Medicine, Oxford, UK (Prof. Altman and Dr Holmes), had full access to all the data and had joint responsibility for the decision to submit for publication after discussion with all the named authors.
References
- 1. Kotecha D, Manzano L, Krum H, Rosano G, Holmes J, Altman DG, Collins PD, Packer M, Wikstrand J, Coats AJ, Cleland JG, Kirchhof P, von Lueder TG, Rigby AS, Andersson B, Lip GY, van Veldhuisen DJ, Shibata MC, Wedel H, Bohm M, Flather MD.. Beta-Blockers in Heart Failure Collaborative G. Effect of age and sex on efficacy and tolerability of beta blockers in patients with heart failure with reduced ejection fraction: individual patient data meta-analysis. BMJ 2016;353:i1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kotecha D, Flather MD, Altman DG, Holmes J, Rosano G, Wikstrand J, Packer M, Coats AJS, Manzano L, Bohm M, van Veldhuisen DJ, Andersson B, Wedel H, von Lueder TG, Rigby AS, Hjalmarson A, Kjekshus J, Cleland JGF; Beta-Blockers in Heart Failure Collaborative G. Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol 2017;69:2885–2896. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL.. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240–e319. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 5. McGowan JH, Cleland JG.. Reliability of reporting left ventricular systolic function by echocardiography: a systematic review of 3 methods. Am Heart J 2003;146:388–397. [DOI] [PubMed] [Google Scholar]
- 6. Kotecha D, Manzano L, Altman DG, Krum H, Erdem G, Williams N, Flather MD; Beta-Blockers in Heart Failure Collaborative Group. Individual patient data meta-analysis of beta-blockers in heart failure: rationale and design. Syst Rev 2013;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, von Lueder TG, Wedel H, Rosano G, Shibata MC, Rigby A, Flather MD; Beta-Blockers in Heart Failure Collaborative Group. Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet 2014;384:2235–2243. [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 9. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF.. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD Statement. JAMA 2015;313:1657–1665. [DOI] [PubMed] [Google Scholar]
- 10. Kotecha D, Manzano L, Krum H, Altman DG, Holmes J, Flather M; The Beta-Blockers in Heart Failure Collaborative Group. Individual patient data meta-analysis. PROSPERO register. 2014. https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014010012 (20 September 2017).
- 11. Australia/New Zealand Heart Failure Research Collaborative Group. Randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Lancet 1997;349:375–380. [PubMed] [Google Scholar]
- 12. Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659–1667. [DOI] [PubMed] [Google Scholar]
- 13. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385–1390. [DOI] [PubMed] [Google Scholar]
- 14. Cleland JG, Pennell DJ, Ray SG, Coats AJ, Macfarlane PW, Murray GD, Mule JD, Vered Z, Lahiri A.. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet 2003;362:14–21. [DOI] [PubMed] [Google Scholar]
- 15. CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure. The cardiac insufficiency bisoprolol study (CIBIS). Circulation 1994;90:1765–1773. [DOI] [PubMed] [Google Scholar]
- 16. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 17. Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL.. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 18. Waagstein F, Bristow MR, Swedberg K, Camerini F, Fowler MB, Silver MA, Gilbert EM, Johnson MR, Goss FG, Hjalmarson A.. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in dilated cardiomyopathy (MDC) trial study group. Lancet 1993;342:1441–1446. [DOI] [PubMed] [Google Scholar]
- 19. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 20. Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Bohm M, Anker SD, Thompson SG, Poole-Wilson PA.. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2004;26:215–225. [DOI] [PubMed] [Google Scholar]
- 21. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH.. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JPT, Altman DG, Sterne JAC.. Chapter 8: Assessing risk of bias in included studies In Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chichester, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- 23. Fine J, Gray R.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 24. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–241. [Google Scholar]
- 25. van Veldhuisen DJ, Cohen-Solal A, Bohm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole-Wilson PA, Flather MD.. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data from SENIORS (Study of effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure). J Am Coll Cardiol 2009;53:2150–2158. [DOI] [PubMed] [Google Scholar]
- 26. Goldstein S, Fagerberg B, Hjalmarson A, Kjekshus J, Waagstein F, Wedel H, Wikstrand J.. Metoprolol controlled release/extended release in patients with severe heart failure: analysis of the experience in the MERIT-HF study. J Am Coll Cardiol 2001;38:932–938. [DOI] [PubMed] [Google Scholar]
- 27. Tudur Smith C, Williamson PR.. A comparison of methods for fixed effects meta-analysis of individual patient data with time to event outcomes. Clin Trials 2007;4:621–630. [DOI] [PubMed] [Google Scholar]
- 28. Sauerbrei W, Royston P.. Building multivariable prognostic and diagnostic models: transformation of the predictors by using fractional polynomials. J Roy Stat Soc 1999;162:71–94. [Google Scholar]
- 29. Royston P, Sauerbrei W.. A new approach to modelling interactions between treatment and continuous covariates in clinical trials by using fractional polynomials. Stat Med 2004;23:2509–2525. [DOI] [PubMed] [Google Scholar]
- 30. Altman DG, Bland JM.. Interaction revisited: the difference between two estimates. BMJ 2003;326:219.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. von Lueder TG, Kotecha D, Atar D, Hopper I.. Neurohormonal blockade in heart failure. Cardiac Fail Rev 2017;3:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ.. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the european society of cardiology (ESC). Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 33. Ziff OJ, Samra M, Bromage DI, Howard JP, Francis DP, Kotecha D.. [Late-breaking conference report] Marked variation in the efficacy of beta-blockers across cardiovascular health: a global systematic assessment of mortality, myocardial infarction and stroke. European Society of Cardiology Congress 2017. http://spo.escardio.org/SessionDetails.aspx? eevtid=1220&sessId=22276 (28 August 2017). [Google Scholar]
- 34. Lane DA, Skjoth F, Lip GYH, Larsen TB, Kotecha D.. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc 2017;6:e005155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA.. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016;37:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lund LH. [Late-breaking conference report] Heart failure with mid ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire EF spectrum. Heart Fail 2017. https://www.escardio.org/Congresses-&-Events/Heart-Failure/Congress-resources/candesartan-provides-similar-benefit-for-patients-with-mid-range-ejection-fracti (30 April 2017). [DOI] [PubMed] [Google Scholar]
- 37. Oh JK, Pellikka PA, Panza JA, Biernat J, Attisano T, Manahan BG, Wiste HJ, Lin G, Lee K, Miller FA Jr, Stevens S, Sopko G, She L, Velazquez EJ.. Core lab analysis of baseline echocardiographic studies in the STICH trial and recommendation for use of echocardiography in future clinical trials. J Am Soc Echocardiogr 2012;25:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kutyifa V, Kloppe A, Zareba W, Solomon SD, McNitt S, Polonsky S, Barsheshet A, Merkely B, Lemke B, Nagy VK, Moss AJ, Goldenberg I.. The influence of left ventricular ejection fraction on the effectiveness of cardiac resynchronization therapy: MADIT-CRT (Multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy). J Am Coll Cardiol 2013;61:936–944. [DOI] [PubMed] [Google Scholar]
- 39. Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD.. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail 2014;7:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chung ES, Katra RP, Ghio S, Bax J, Gerritse B, Hilpisch K, Peterson BJ, Feldman DS, Abraham WT.. Cardiac resynchronization therapy may benefit patients with left ventricular ejection fraction >35%: a PROSPECT trial substudy. Eur J Heart Fail 2010;12:581–587. [DOI] [PubMed] [Google Scholar]
- 41. Kotecha D, Mohamed M, Shantsila E, Popescu BA, Steeds RP.. Is echocardiography valid and reproducible in patients with atrial fibrillation? A systematic review. Europace 2017;19:1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kotecha D, Calvert M, Deeks JJ, Griffith M, Kirchhof P, Lip GYH, Mehta S, Slinn G, Stanbury M, Steeds RP, Townend JNA.. Review of rate control in atrial fibrillation, and the rationale and protocol for the RATE-AF trial. BMJ Open 2017;7:e015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.