Structural MRI abnormalities are inconsistently reported in epilepsy. In the largest neuroimaging study to date, Whelan et al. report robust structural alterations across and within epilepsy syndromes, including shared volume loss in the thalamus, and widespread cortical thickness differences. The resulting neuroanatomical map will guide prospective studies of disease progression.
Keywords: epilepsy, MRI, thalamus, precentral gyrus
Abstract
Progressive functional decline in the epilepsies is largely unexplained. We formed the ENIGMA-Epilepsy consortium to understand factors that influence brain measures in epilepsy, pooling data from 24 research centres in 14 countries across Europe, North and South America, Asia, and Australia. Structural brain measures were extracted from MRI brain scans across 2149 individuals with epilepsy, divided into four epilepsy subgroups including idiopathic generalized epilepsies (n =367), mesial temporal lobe epilepsies with hippocampal sclerosis (MTLE; left, n = 415; right, n = 339), and all other epilepsies in aggregate (n = 1026), and compared to 1727 matched healthy controls. We ranked brain structures in order of greatest differences between patients and controls, by meta-analysing effect sizes across 16 subcortical and 68 cortical brain regions. We also tested effects of duration of disease, age at onset, and age-by-diagnosis interactions on structural measures. We observed widespread patterns of altered subcortical volume and reduced cortical grey matter thickness. Compared to controls, all epilepsy groups showed lower volume in the right thalamus (Cohen’s d = −0.24 to −0.73; P < 1.49 × 10−4), and lower thickness in the precentral gyri bilaterally (d = −0.34 to −0.52; P < 4.31 × 10−6). Both MTLE subgroups showed profound volume reduction in the ipsilateral hippocampus (d = −1.73 to −1.91, P < 1.4 × 10−19), and lower thickness in extrahippocampal cortical regions, including the precentral and paracentral gyri, compared to controls (d = −0.36 to −0.52; P < 1.49 × 10−4). Thickness differences of the ipsilateral temporopolar, parahippocampal, entorhinal, and fusiform gyri, contralateral pars triangularis, and bilateral precuneus, superior frontal and caudal middle frontal gyri were observed in left, but not right, MTLE (d = −0.29 to −0.54; P < 1.49 × 10−4). Contrastingly, thickness differences of the ipsilateral pars opercularis, and contralateral transverse temporal gyrus, were observed in right, but not left, MTLE (d = −0.27 to −0.51; P < 1.49 × 10−4). Lower subcortical volume and cortical thickness associated with a longer duration of epilepsy in the all-epilepsies, all-other-epilepsies, and right MTLE groups (beta, b < −0.0018; P < 1.49 × 10−4). In the largest neuroimaging study of epilepsy to date, we provide information on the common epilepsies that could not be realistically acquired in any other way. Our study provides a robust ranking of brain measures that can be further targeted for study in genetic and neuropathological studies. This worldwide initiative identifies patterns of shared grey matter reduction across epilepsy syndromes, and distinctive abnormalities between epilepsy syndromes, which inform our understanding of epilepsy as a network disorder, and indicate that certain epilepsy syndromes involve more widespread structural compromise than previously assumed.
Introduction
Epilepsy is a prevalent neurological disorder, comprising many different syndromes and conditions, affecting 0.6–1.5% of the population worldwide (Bell et al., 2014). Approximately one-third of affected individuals do not respond to antiepileptic drug therapy (French, 2007). Alternative treatment options may not be appropriate (Englot et al., 2011), and are not always effective (Téllez-Zenteno et al., 2005; Englot et al., 2011). The identification of shared biological disease pathways may help elucidate diagnostic and prognostic biomarkers and therapeutic targets, which, in turn, could help to optimize individual treatment (Pitkänen et al., 2016). However, disease biology remains unexplained for most cases—especially in commonly occurring epilepsies.
Epilepsy is a network disorder typically involving widespread structural alterations beyond the putative epileptic focus (Bernhardt et al., 2015; Vaughan et al., 2016). Hippocampal sclerosis is a common pathological substrate of mesial temporal lobe epilepsy (MTLE), but extrahippocampal abnormalities are also frequently observed in MTLE, notably in the thalamus (Keller and Roberts, 2008; Coan et al., 2014; Alvim et al., 2016) and neocortex (Keller and Roberts, 2008; Bernhardt et al., 2009b, 2010; Blanc et al., 2011; Labate et al., 2011; Vaughan et al., 2016). Neocortical abnormalities are also reported in idiopathic generalized epilepsies (IGE) (Bernhardt et al., 2009a), and many childhood syndromes (O’Muircheartaigh et al., 2011; Vollmar et al., 2011; Ronan et al., 2012; Overvliet et al., 2013). Thus, common epilepsies may be characterized by shared disturbances in distributed cortico-subcortical brain networks (Berg et al., 2010), but the pattern, consistency and cause of these disturbances, and how they relate to functional decline (Vlooswijk et al., 2010; Bernasconi, 2016; Nickels et al., 2016), are largely unknown.
Currently, we lack reliable data from large cross-sectional neuroimaging, brain tissue, or biomarker studies in the common epilepsies. Brain tissue is not available from large cohorts of patients: common forms of epilepsy are often unsuitable for surgical treatment, so biopsied tissues are simply unavailable in sufficient numbers for research into disease biology. Brain-wide post-mortem studies also require extensive effort for comprehensive analysis. MRI offers detailed information on brain structure, but MRI measures from groups of individuals with and without epilepsy are not always consistent. For example, MTLE is associated with hippocampal sclerosis in up to 70% of brain MRI scans (Blümcke et al., 2013). However, the effects of laterality, and the extent of extrahippocampal grey matter loss are inconsistently reported in studies of left versus right MTLE (Kemmotsu et al., 2011; Liu et al., 2016). Similarly, abnormalities of the basal ganglia, hippocampus, lateral ventricles, and neocortex have all been reported in IGE (Betting et al., 2006), but most alterations are non-specific, and visual inspection of clinical MRI in IGE is typically normal (Woermann et al., 1998). Genome-wide association studies (GWAS) have identified genetic variants associated with complex epilepsies by ‘lumping’ different epilepsy types together (International League Against Epilepsy Consortium on Complex Epilepsies, 2014), but MRI studies are typically of smaller scale, and have not widely explored whether distinct epilepsy syndromes share common structural abnormalities.
There are many sources of inconsistency in previously reported MRI findings. First, epileptic seizures and syndromes are diverse; classifications are often revised and contested (Berg et al., 2010; Scheffer et al., 2017). Second, most cross-sectional brain imaging studies are based on small samples (typically <50 cases), limiting the power to detect subtle group differences (Button et al., 2013). Third, variability in scanning protocols, image processing, and statistical analysis may affect the sensitivity of brain measures across studies.
The Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) Consortium was formed to address these issues (Bearden and Thompson, 2017). ENIGMA is a global initiative, combining large samples with coordinated image processing, and integrating genomic and MRI data across hundreds of research centres worldwide. Prior ENIGMA studies have identified genetic variants associated with variations in brain structure (Stein et al., 2012; Hibar et al., 2015, 2017a; Adams et al., 2016), and have reliably characterized patterns of brain abnormalities in schizophrenia (van Erp et al., 2016), major depression (Schmaal et al., 2016), obsessive compulsive disorder (Boedhoe et al., 2017), attention deficit hyperactivity disorder (Hoogman et al., 2017), and many other brain illnesses (Thompson et al., 2017). Large-scale, collaborative initiatives such as ENIGMA may improve our understanding of epilepsy, helping clinicians make more informed decisions and provide personalized treatment strategies (Ben-Menachem, 2016). Thus, we formed the Epilepsy Working Group of ENIGMA (‘ENIGMA-Epilepsy’) to apply coordinated, well-powered studies of imaging and genetic data in epilepsy.
Here, in the largest analysis of structural brain abnormalities in epilepsy to date, we ranked effect sizes for 16 subcortical and 68 cortical brain regions in 2149 individuals with epilepsy and 1727 healthy controls, using harmonized image processing, quality control, and meta-analysis. First, we grouped all epilepsies together, to determine whether biologically distinct syndromes show robust, common structural deficits. Second, we assessed a well-characterized form of epilepsy: MTLE with hippocampal sclerosis, analysing patients with left- and right-sided hippocampal sclerosis as independent groups. Third, we examined another major set of epilepsy syndromes: IGE. Finally, we studied all remaining epilepsies as a combined subgroup, to understand the relative contributions of IGE, MTLE-L, MTLE-R, and all other syndromes on shared patterns of structural compromise. We tested how age at scan, age of onset, and epilepsy duration affected brain structural measures. Based on existing neuroimaging (Gotman et al., 2005; Bernhardt et al., 2009a; Liu et al., 2016), neurophysiological (Gotman et al., 2005), neuropathological (Thom et al., 2009), and genetic data (International League Against Epilepsy Consortium on Complex Epilepsies, 2014), we predicted that (i) biologically distinct epilepsy syndromes would exhibit shared patterns of structural abnormalities; (ii) MTLEs with left or right hippocampal sclerosis would show distinct patterns of hippocampal and extrahippocampal structural deficits; and (iii) IGEs would also display subcortical volume and cortical thickness differences, compared to healthy controls.
Materials and methods
Each centre received approval from their local institutional review board or ethics committee. Written informed consent was provided according to local requirements (Supplementary Table 1).
Experimental design
Participants
Twenty-four cross-sectional samples from 14 countries were included in the study, totalling 2149 people with epilepsy and 1727 research centre-matched healthy control subjects (Fig. 1 and Table 1). The locations, dates, and periods of participant recruitment are provided in Supplementary Table 1. An epilepsy specialist assessed seizure and syndrome classifications at each centre, using International League Against Epilepsy terminology (Berg et al., 2010). Participants were aged 18–55.
Figure 1.
Study flowchart. ILAE = International League Against Epilepsy; MOU = memorandum of understanding.
Table 1.
ENIGMA - Epilepsy Working Group demographics, including age (in years), mean age at onset of epilepsy (in years), mean duration of illness (in years), sex, and case-control breakdown for participating sites
| Site name | Age controls (Mean ± SD) | Age cases (Mean ± SD) | Age of onset (Mean ± SD) | Duration of illness (Mean ± years) | Female controls | Female cases | Total controls | Total cases | MTLE-L cases | MTLE-R cases | IGE cases | ‘Other’ cases | Total n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bern | 32.5 ± 9.39 | 30.48 ± 10.13 | - | - | 41 | 28 | 78 | 56 | 10 | 8 | 12 | 26 | 134 |
| Bonn | 40.11 ± 13.4 | 39.68 ± 13.4 | 16.86 ± 11.96 | 22.82 ± 14.18 | 40 | 60 | 77 | 108 | 71 | 37 | 0 | 0 | 185 |
| BRI | 34.73 ± 10.61 | 33.28 ± 10.59 | 17.9 ± 11.49 | 17.9 ± 12.93 | 49 | 46 | 112 | 79 | 10 | 13 | 18 | 38 | 191 |
| Brussels | 26.64 ± 4.34 | 33.79 ± 9.9 | 14.46 ± 10.13 | 19.02 ± 12.77 | 24 | 49 | 44 | 83 | 11 | 0 (4) | 8 | 60 | 127 |
| CUBRIC | 28.04 ± 8.16 | 28.42 ± 8.06 | 13.56 ± 5.18 | 14.81 ± 9.91 | 34 | 34 | 48 | 48 | 0 | 0 | 44 | 0 (4) | 96 |
| EKUT_A | 34.82 ± 11.38 | 33.58 ± 11.07 | 17.04 ± 11.09 | 16.84 ± 13.18 | 30 | 28 | 49 | 47 | 6 | 0 | 5 | 36 | 96 |
| EKUT_B | 35.33 ± 12.27 | 31.13 ± 10.74 | 17.32 ± 10.8 | 14.45 ± 11.14 | 9 | 18 | 18 | 24 | 0 | 0 | 16 | 8 | 42 |
| EPICZ | 30.48 ± 9.39 | 30.42 ± 10.13 | - | - | 59 | 71 | 116 | 113 | 19 | 27 | 0 | 67 | 229 |
| EPIGEN_3.0 | 34.75 ± 9.36 | 36.2 ± 9.97 | 17.03 ± 13.7 | 18.93 ± 10.88 | 30 | 37 | 70 | 60 | 8 | 5 | 0 | 47 | 130 |
| EPIGEN_1.5 | 31.7 ± 9.24 | 37.46 ± 10.69 | 14.51 ± 11.8 | 22.68 ± 14.28 | 24 | 35 | 47 | 52 | 27 | 25 | 0 | 0 | 99 |
| Florence | 35.29 ± 8.48 | 28 ± 7.77 | 12.69 ± 8.02 | 14.27 ± 8.06 | 8 | 12 | 14 | 31 | 0 (1) | 0 | 5 | 25 | 45 |
| Greifswald | 42.26 ± 14.97 | 26.23 ± 7.49 | 28.12 ± 17.86 | 14.13 ± 12.81 | 60 | 21 | 99 | 39 | 0 | 0 | 39 | 0 | 138 |
| IDIBAPS-HCP | 33.13 ± 5.99 | 36.77 ± 9.52 | 18.07 ± 11.72 | 17.64 ± 10.51 | 29 | 67 | 52 | 115 | 17 | 36 | 0 (3) | 59 | 167 |
| KCL_CNS | 31.68 ± 8.4 | 33.2 ± 8.9 | 13.22 ± 8.2 | 20.67 ± 11.23 | 54 | 50 | 101 | 96 | 5 | 0 (4) | 32 | 55 | 197 |
| KCL_CRF | 28.73 ± 8.29 | 31.47 ± 11.33 | 23.13 ± 7.55 | 8.33 ± 9.99 | 16 | 7 | 26 | 15 | 0 (3) | 0 (2) | 0 (4) | 6 | 41 |
| Kuopio | 25.16 ± 1.55 | 33.35 ± 11.21 | 24 ± 13.22 | 9.35 ± 11.23 | 33 | 135 | 67 | 240 | 0 | 9 | 36 | 195 | 307 |
| MNI | 30.74 ± 7.38 | 32.53 ± 9.92 | 16.48 ± 9.72 | 16.05 ± 11.32 | 21 | 71 | 46 | 128 | 45 | 38 | 0 | 45 | 174 |
| NYU | 30.1 ± 10.36 | 33.23 ± 9.66 | 16.96 ± 11.27 | 16.43 ± 12.7 | 62 | 93 | 118 | 159 | 8 | 11 | 36 | 104 | 277 |
| RMH | 39.35 ± 20.26 | 38.08 ± 15.91 | 28.23 ± 17.98 | 10.18 ± 12.65 | 12 | 70 | 28 | 146 | 22 | 13 | 25 | 86 | 174 |
| UCSD | 36.89 ± 15.1 | 37.67 ± 11.79 | 19.32 ± 14.77 | 18.8 ± 15.36 | 16 | 22 | 37 | 43 | 14 | 8 | 0 | 21 | 80 |
| UNAM | 33.2 ± 12.29 | 31.47 ± 11.81 | 16.26 ± 11.33 | 15.03 ± 12.53 | 25 | 24 | 35 | 36 | 10 | 10 | 0 | 16 | 71 |
| UNICAMP | 34.39 ± 10.45 | 39.98 ± 10.25 | 12.07 ± 9.52 | 27.96 ± 12.54 | 249 | 183 | 398 | 291 | 107 | 84 | 40 | 60 | 689 |
| UNIMORE | 28.47 ± 5.25 | 28.36 ± 10.26 | 12.58 ± 8.13 | 14.34 ± 10.94 | 20 | 47 | 34 | 82 | 0 (3) | 0 (2) | 40 | 37 | 116 |
| XMU | 31.54 ± 6.99 | 28.79 ± 9.06 | 17.04 ± 12.2 | 11.76 ± 8.78 | 4 | 20 | 13 | 58 | 25 | 15 | 11 | 7 | 71 |
| Combined | 33.31 ± 9.91 | 34.36 ± 10.65 | 17.63 ± 11.47 | 17.42 ± 11.99 | 949 | 1228 | 1727 | 2149 | 415 | 339 | 367 | 1028 | 3876 |
Also provided is the total number of MTLE cases with left hippocampal sclerosis, MTLE cases with right hippocampal sclerosis, IGE and all-other-epilepsies (‘other’) cases per site. Research centres with fewer than five participants for a given phenotype are marked as ‘0’ for that phenotype, with the original sample size noted in parentheses.
SD = standard deviation.
To test for shared and syndrome-specific structural alterations, analyses included one group combining all epilepsies (‘all-epilepsies’; n = 2149), and four stratified subgroups: (i) left MTLE with left hippocampal sclerosis (MTLE-L; n = 415); (ii) right MTLE with right hippocampal sclerosis (MTLE-R; n = 339); (iii) IGE (n = 367); and (iv) all other epilepsies (n = 1028). Supplementary Table 2 lists all syndromic diagnoses included in the aggregate ‘all-epilepsies’ group. For the MTLE subgroups, we included anyone with the typical electroclinical constellation (Berg et al., 2010), and a neuroradiologically-confirmed diagnosis of unilateral hippocampal sclerosis on clinical MRI. Participants were included in the IGE subgroup if they presented with tonic-clonic, absence or myoclonic seizures with generalized spike-wave discharges on EEG. Participants were included in the ‘all-other-epilepsies’ subgroup if they were diagnosed with non-lesional MTLE (43.3%), occipital (1.67%), frontal (8.78%), or parietal lobe epilepsy (0.84%), focal epilepsies not otherwise specified (37.03%), or another unclassified syndrome (8.37%; Supplementary Table 2). We excluded participants with a progressive disease (e.g. Rasmussen’s encephalitis), malformations of cortical development, tumours or previous neurosurgery.
MRI data collection and processing
Structural T1-weighted MRI brain scans were collected at the 24 participating centres. Scanning details are provided in Supplementary Table 3. T1-weighted images from cases and controls were analysed at each site using FreeSurfer 5.3.0, for automated analysis of brain structure (Fischl, 2012). Volumetric measures were extracted for 12 subcortical grey matter regions (six left and six right, including the amygdala, caudate, nucleus accumbens, pallidum, putamen, and thalamus), the left and right hippocampi, and the left and right lateral ventricles. Cortical thickness measures were extracted for 34 left-hemispheric grey matter regions, and 34 right-hemispheric grey matter regions (68 total; Supplementary Table 4). Visual inspections of subcortical and cortical segmentations were conducted following standardized ENIGMA protocols (http://enigma.usc.edu), used in prior genetic studies of brain structure (Stein et al., 2012; Hibar et al., 2015, 2017a; Adams et al., 2016), and large-scale case-control studies of neuropsychiatric illnesses (Schmaal et al., 2015, 2016; Hibar et al., 2016; van Erp et al., 2016; Boedhoe et al., 2017). Analysts were blind to participants’ diagnoses. Each analyst was instructed to execute a series of standardized bash scripts, identifying participants with volumetric or thickness measures greater or less than 1.5 times the interquartile range as outliers. Outlier data were then visually inspected, by overlaying the participant’s cortical segmentations on their whole-brain anatomical images. If the blinded local analyst judged any structure as inaccurately segmented, that structure was omitted from the analysis. The Supplementary material provides further information.
Statistical analysis
Participant demographics
All research centres tested for differences in age between individuals with epilepsy and controls using an unpaired, two-tailed t-test in the R statistics package (https://www.r-project.org). Each centre also tested for sex differences between individuals with epilepsy and controls using a chi-squared test in SPSS Statistics package (IBM Corp., Version 21.0).
Meta-analytical group comparisons
Each research centre tested for case-versus-control differences using multiple linear regressions (via the lm function implemented in R), where a binary indicator of diagnosis (0 = healthy control, 1 = person with epilepsy) was the predictor of interest, and the volume or thickness of a specified brain region was the outcome measure. We calculated effect size estimates across all brain regions using Cohen’s d, adjusting for age, sex and intracranial volume (ICV). ICV is a reliable, indirect measure of head size (Hansen et al., 2015), used as a covariate in other large-scale ENIGMA collaborations (Schmaal et al., 2015, 2016; Hibar et al., 2016; van Erp et al., 2016; Boedhoe et al., 2017). Cohen’s d effect sizes and regression beta coefficients were pooled across centres using a random-effects, restricted maximum likelihood method of meta-analysis via the R package, metafor (Viechtbauer, 2010). The Supplementary material provides additional details.
Meta-analytical regression with clinical variables
Each centre conducted a series of linear regressions, testing the association between subcortical volume or cortical thickness, and: (i) age at onset of epilepsy; and (ii) duration of epilepsy. All centres tested for interactions between diagnosis of epilepsy (including syndrome groups) and age at time of scan. Beta values representing the unstandardized slopes of each regression were extracted for each analysis. Sex and ICV were included as covariates in all secondary analyses.
Correction for multiple comparisons
We conducted four independent regressions (one case versus control regression, and three regressions with clinical variables) across 84 regions of interest, adjusting the statistical significance threshold to Pthresh < 1.49 × 10−4 to correct for 336 comparisons. To account for correlations between tests, we also applied a less conservative adjustment for false discovery rate (FDR), using the Benjamini and Hochberg method (Benjamini and Hochberg, 1995). For clarity, we report only P-values significant after stringent Bonferroni correction; FDR-adjusted P-values are summarized in the Supplementary material.
Power analyses
Across all regions of interest, we calculated the sample sizes necessary to achieve 80% power to detect case-control differences, given the observed effect sizes at each region of interest, based on two-tailed t-tests, using G*Power Version 3.1. For each region of interest, we also estimated N80: the total number of samples required, per group, to achieve 80% power to detect group differences using a t-test at the threshold of P < 0.05 (two-tailed).
Results
Participant demographics
The sample size-weighted mean age across all epilepsy samples was 34.4 (range: 26.2–40) years, and the weighted mean age of healthy controls was 33.3 (range: 25.2–42.3) years. The weighted mean age at onset of epilepsy and duration of epilepsy were 17.6 (range: 12.1–28.2) years and 17.4 (range: 8.3–28) years, respectively. Females comprised 57% of the total epilepsy sample (range: 34–75% by individual sample), and 53% of the controls (range: 31–71% by individual sample). Case-control differences in age were observed at 8 of 24 research centres, and case-control differences in sex were observed at 2 of 24 research centres (Supplementary Table 5); hence, age and sex were included as covariates in all group comparisons.
Volumetric findings
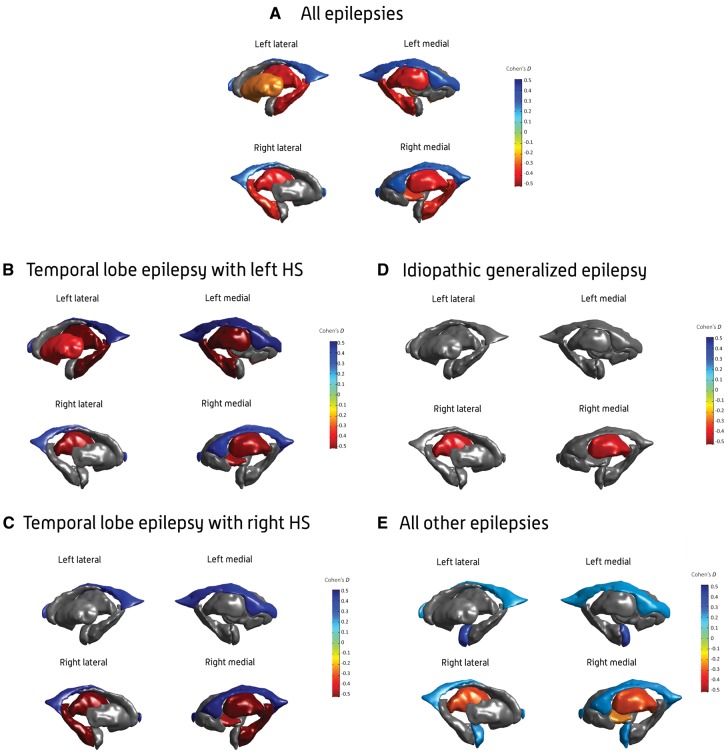
Compared to controls, the aggregate all-epilepsies group exhibited lower volumes in the left (d = −0.36; P = 1.31 × 10−6) and right thalamus (d = −0.37; P = 7.67 × 10−14), left (d = −0.35; P = 3.04 × 10−7) and right hippocampus (d = −0.34; P = 6.63 × 10−10), and the right pallidum (d = −0.32; P = 8.32 × 10−9). Conversely, the left (d = 0.29; P = 2.14 × 10−12) and right (d = 0.27; P = 3.73 × 10−15) lateral ventricles were enlarged across all epilepsies when compared to controls (Table 2 and Fig. 2A). A supplementary analysis of all-epilepsies, excluding individuals with hippocampal sclerosis or other lesions, revealed similar patterns of volume loss in the right thalamus and pallidum, and bilaterally enlarged ventricles; however, volume differences were not observed in the hippocampus (Supplementary Table 6).
Table 2.
Effect size differences between epilepsy cases and healthy controls (Cohen’s d) for the mean volume of subcortical structures, controlling for age, sex and intracranial volume
| Structure | Phenotype | Cohen’s d | SE | Z score | 95% CI | P-value | I2 | N80 | Number of controls | Number of cases |
|---|---|---|---|---|---|---|---|---|---|---|
| Amygdala (LH) | All-other-epilepsies | 0.327 | 0.065 | 5.024 | 0.199–0.455 | 5.05 x 10−7 | 45.470 | 148 | 1448 | 998 |
| Amygdala (RH) | All-other-epilepsies | 0.218 | 0.057 | 3.799 | 0.106–0.33 | 1.46 x 10−4 | 31.256 | 335 | 1422 | 989 |
| Hippocampus (LH) | MTLE-L | −1.728 | 0.191 | −9.056 | −2.102 to −1.354 | 1.35 x 10−19 | 85.532 | 7 | 1412 | 410 |
| All epilepsies | −0.353 | 0.069 | −5.121 | −0.488 to −0.217 | 3.04 x 10−7 | 71.845 | 127 | 1707 | 2125 | |
| Hippocampus (RH) | MTLE-R | −1.906 | 0.15 | −12.694 | −2.2 to −1.611 | 6.36 x 10−37 | 72.476 | 6 | 1286 | 336 |
| All epilepsies | −0.336 | 0.054 | −6.175 | −0.443 to −0.229 | 6.63 x 10−10 | 54.801 | 141 | 1719 | 2129 | |
| Lateral ventricle (LH) | MTLE-L | 0.465 | 0.089 | 5.203 | 0.289–0.640 | 1.96 x 10−7 | 43.124 | 74 | 1417 | 414 |
| MTLE-R | 0.39 | 0.081 | 4.808 | 0.231–0.549 | 1.52 x 10−6 | 26.750 | 105 | 1291 | 338 | |
| All epilepsies | 0.288 | 0.041 | 7.025 | 0.207–0.368 | 2.14 x 10−12 | 23.338 | 191 | 1722 | 2135 | |
| All-other-epilepsies | 0.198 | 0.045 | 4.373 | 0.109–0.287 | 1.23 x 10−5 | 0.218 | 402 | 1452 | 996 | |
| Lateral ventricle (RH) | MTLE-R | 0.444 | 0.065 | 6.867 | 0.317−0.57 | 6.57 x 10−12 | 0.003 | 81 | 1292 | 338 |
| MTLE-L | 0.363 | 0.093 | 3.917 | 0.1814−0.544 | 8.95 x 10−5 | 47.227 | 121 | 1418 | 414 | |
| All epilepsies | 0.268 | 0.034 | 7.864 | 0.2−0.334 | 3.73 x 10−15 | 0 | 220 | 1722 | 2137 | |
| All-other-epilepsies | 0.212 | 0.046 | 4.581 | 0.122−0.303 | 4.62 x 10−6 | 3.528 | 350 | 1453 | 996 | |
| Pallidum (RH) | MTLE-L | −0.452 | 0.09 | −5.009 | −0.628 to −0.275 | 5.48 x 10−7 | 43.985 | 78 | 1406 | 414 |
| MTLE-R | −0.451 | 0.089 | −5.071 | −0.624 to −0.276 | 3.96 x 10−7 | 36.432 | 79 | 1278 | 332 | |
| All epilepsies | −0.316 | 0.055 | −5.762 | −0.424 to −0.208 | 8.32 x 10−9 | 55.575 | 159 | 1710 | 2112 | |
| All-other-epilepsies | −0.235 | 0.060 | −3.942 | −0.352 to −0.118 | 8.07 x 10−5 | 36.141 | 286 | 1440 | 976 | |
| Putamen (LH) | MTLE-L | −0.385 | 0.079 | −4.878 | −0.539 to −0.23 | 1.07 x 10−6 | 28.474 | 107 | 1352 | 410 |
| Thalamus (LH) | MTLE-L | −0.843 | 0.126 | −6.693 | −1.089 to −0.595 | 2.19 x 10−11 | 70.462 | 24 | 1384 | 408 |
| All epilepsies | −0.358 | 0.074 | −4.839 | −0.503 to −0.213 | 1.31 x 10−6 | 75.649 | 124 | 1687 | 2104 | |
| Thalamus (RH) | MTLE-R | −0.727 | 0.103 | −7.066 | −0.928 to −0.525 | 1.60 x 10−12 | 51.499 | 31 | 1285 | 335 |
| MTLE-L | −0.462 | 0.117 | −3.941 | −0.691 to −0.232 | 8.12 x 10−5 | 67.376 | 75 | 1412 | 414 | |
| IGE | −0.403 | 0.087 | −4.633 | −0.574 to −0.233 | 3.60 x 10−6 | 39.715 | 98 | 1210 | 363 | |
| All epilepsies | −0.368 | 0.049 | −7.476 | −0.464 to −0.271 | 7.67 x 10−14 | 44.822 | 117 | 1716 | 2137 | |
| All-other-epilepsies | −0.305 | 0.047 | −6.502 | −0.397 to −0.213 | 7.92 x 10−11 | 4.985 | 170 | 1446 | 998 |
CI = confidence interval; LH = left hemisphere; RH = right hemisphere; SE = standard error; I2 = heterogeneity index; N80 = number of subjects required in each group to yield 80% power to detect significant group differences (P < 0.05, two-tailed). Uncorrected P-values are reported. Subcortical structures that failed to survive Bonferroni correction (P < 1.49 x 10−4) are not reported (see ‘Materials and methods’ section for statistical threshold determination). See Supplementary material for a full list of volume differences with adjustment for false discovery rate (FDR).
Figure 2.
Subcortical volume findings. Cohen’s d effect size estimates for case-control differences in subcortical volume, across the (A) all-epilepsies, (B) mesial temporal lobe epilepsies with left hippocampal sclerosis (HS; MTLE-L), (C) mesial temporal lobe epilepsies with right hippocampal sclerosis (MTLE-R), (D) idiopathic generalized epilepsies (IGE), and (E) all-other-epilepsies groups. Cohen’s d effect sizes were extracted using multiple linear regressions, and pooled across research centres using random-effects meta-analysis. Subcortical structures with P-values < 1.49 × 10−4 are shown in heatmap colours; strength of heat map is determined by the size of the Cohen’s d (d < 0 = blue, d > 0 = yellow/red). Image generated using MATLAB, with annotations added using Adobe Photoshop. An interactive version of this figure is available online, via ‘ENIGMA-Viewer’: http://enigma-viewer.org/ENIGMA_epilepsy_subcortical.html. See Supplementary material for guidelines on how to use the interactive visualization.
The MTLE-L subgroup showed lower volumes in the left hippocampus (d = −1.73; P = 1.35 × 10−19), left (d = P = 2.19 × 10−11) and right thalamus (d = −0.46; P = 8.12 × 10−5), left putamen (d = −0.39; P = 1.07 × 10−6), and right pallidum (d = −0.45; P = 5.48 × 10−7). As in the overall group comparison, we observed larger left (d = 0.47; P = 1.96 × 10−7) and right lateral ventricles (d = 0.36; P = 8.95 × 10−5) in MTLE-L patients relative to controls (Table 2 and Fig. 2B).
The MTLE-R subgroup showed lower volumes across a number of regions in the right hemisphere only, including the hippocampus (d = −1.91; P = 6.36 × 10−37), thalamus (d = −0.73; P = 1.6 × 10−12), and pallidum (d = −0.45; P = 3.96 × 10−7), together with increased volumes of the left (d = 0.39; P = 1.52 × 10−6) and right lateral ventricles (d = 0.44; P = 6.57 × 10−12) compared to controls (Table 2 and Fig. 2C).
The IGE subgroup showed lower volumes in the right thalamus (d = −0.4; P = 3.6 × 10−6) compared to controls (Table 2 and Fig. 2D).
The all-other-epilepsies subgroup showed lower volumes in the right thalamus (d = −0.31; P = 7.9 × 10−11) and the right pallidum (d = −0.24; P = 8.1 × 10−5) compared to controls. The all-other-epilepsies subgroup also showed significant enlargements of the left (d = 0.33; P = 5.1 × 10−7) and right amygdala (d = 0.22; P = 1.46 × 10−4), and the left (d = 0.2; P = 1.2 × 10−5) and right lateral ventricles (d = 0.21; P = 4.62 × 10−6) compared to controls (Table 2 and Fig. 2E).
All volume differences can be visualized using the interactive ENIGMA-Viewer tool (Zhang et al., 2017), at http://enigma-viewer.org/ENIGMA_epilepsy_subcortical.html (Supplementary material). Volume differences significant after FDR adjustment can also be visualized at http://enigma-viewer.org/ENIGMA_epilepsy_subcortical_fdr.html (Supplementary Tables 26–30).
Cortical thickness findings
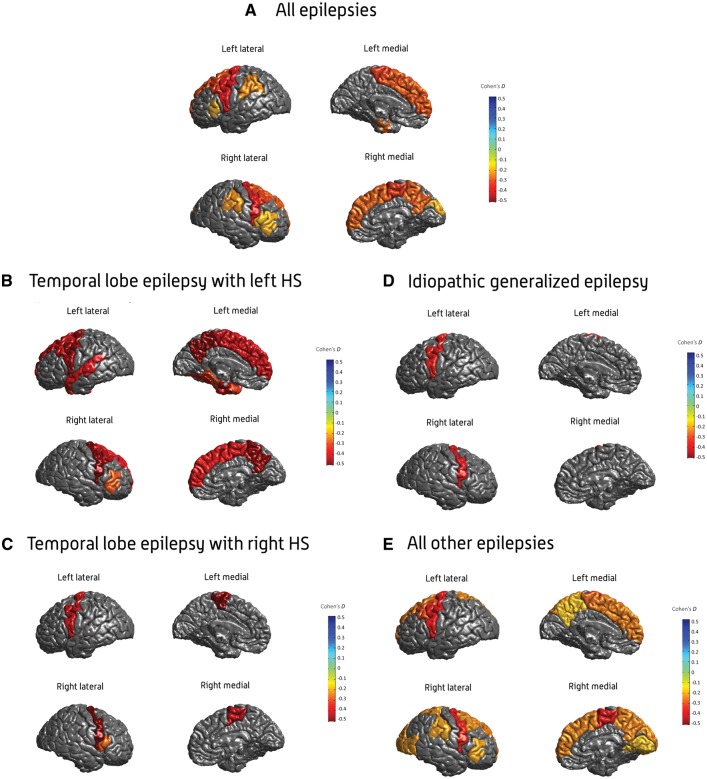
The all-epilepsies group showed reduced thickness of cortical grey matter across seven regions bilaterally, including the left (d = −0.38; P = 1.82 × 10−18) and right precentral gyri (d = −0.4; P = 8.85 × 10−20), left (d = −0.32; P = 2.11 × 10−15) and right caudal middle frontal gyri (d = −0.31; P = 2.09 × 10−9), left (d = −0.31; P = 2.05 × 10−6) and right paracentral gyri (d = −0.32; P = 2.19 × 10−9), left (d = −0.19; P = 1.29 × 10−4) and right pars triangularis (d = −0.2; P = 4.25 × 10−8), left (d = −0.28; P = 1.51 × 10−7) and right superior frontal gyri (d = −0.27; P = 4.49 × 10−6), left (d = −0.19; P = 1.05 × 10−5) and right transverse temporal gyri (d = −0.18; P = 2.81 × 10−5), and left (d = −0.23; P = 9.87 × 10−5) and right supramarginal gyri (d = −0.22; P = 5.24 × 10−5). The all-epilepsies group also showed unilaterally thinner right cuneus (d = −0.2; P = 9.68 × 10−8), right pars opercularis (d = −0.18; P = 6.48 × 10−7), right precuneus (d = −0.28; P = 2.7 × 10−5), and left entorhinal gyrus (d = −0.26; P = 2.04 × 10−5), compared to healthy controls (Table 3 and Fig. 3A). Supplementary analysis in a non-lesional epilepsy subgroup revealed a similar pattern of cortical thickness differences compared to controls, suggesting that the changes observed in our main analysis were not driven by the inclusion of patients with hippocampal sclerosis or other common lesions (Supplementary Table 7).
Table 3.
Effect size differences between epilepsy cases and healthy controls (Cohen’s d) for the mean thickness of cortical structures, controlling for age, sex and intracranial volume
| Structure | Phenotype | Cohen’s d | SE | Z score | 95% CI | P-value | I2 | N80 | Number of controls | Number of cases |
|---|---|---|---|---|---|---|---|---|---|---|
| Caudal middle frontal gyrus (LH) | MTLE-L | −0.403 | 0.07 | −5.789 | −0.538 to −0.2663 | 7.07 x 10−9 | 13.807 | 98 | 1344 | 412 |
| All epilepsies | −0.319 | 0.04 | −7.935 | −0.397 to −0.24 | 2.11 x 10−15 | 17.112 | 156 | 1650 | 2061 | |
| All other epilepsies | −0.291 | 0.045 | −6.425 | −0.38 to −0.202 | 1.32 x 10−10 | 0 | 197 | 1447 | 1000 | |
| Caudal middle frontal gyrus (RH) | MTLE-L | −0.441 | 0.087 | −5.089 | −0.611 to −0.271 | 3.61 x 10−7 | 39.444 | 82 | 1348 | 412 |
| All epilepsies | −0.307 | 0.051 | −5.991 | −0.407 to −0.206 | 2.09 x 10−9 | 46.443 | 168 | 1653 | 2059 | |
| All other epilepsies | −0.212 | 0.045 | −4.699 | −0.301 to −0.124 | 2.62 x 10−6 | 0 | 350 | 1451 | 998 | |
| Cuneus (RH) | All other epilepsies | −0.234 | 0.045 | −5.186 | −0.323 to −0.146 | 2.15 x 10−7 | 0 | 288 | 1449 | 996 |
| All epilepsies | −0.204 | 0.038 | −5.333 | −0.279 to −0.129 | 9.68 x10−8 | 11.423 | 379 | 1651 | 2057 | |
| Entorhinal gyrus (LH) | MTLE-L | −0.445 | 0.072 | −6.158 | −0.5865 to −0.303 | 7.35 x 10−10 | 0 | 81 | 1102 | 303 |
| All epilepsies | −0.264 | 0.062 | −4.261 | −0.385 to −0.142 | 2.04 x 10−5 | 56.648 | 227 | 1402 | 1724 | |
| Fusiform gyrus (LH) | MTLE-L | −0.359 | 0.069 | −5.183 | −0.494 to −0.223 | 2.19 x 10−7 | 13.465 | 123 | 1339 | 412 |
| Lateral occipital gyrus (RH) | All other epilepsies | −0.211 | 0.045 | −4.659 | −0.299 to −0.122 | 3.18 x 10−6 | 2.50 x 10−3 | 354 | 1450 | 997 |
| Lingual gyrus (RH) | All other epilepsies | −0.180 | 0.045 | −3.972 | −0.268 to −0.091 | 7.12 x 10−5 | 1.25 x 10−2 | 491 | 1450 | 996 |
| Paracentral gyrus (LH) | MTLE-R | −0.505 | 0.102 | −4.944 | −0.705 to −0.305 | 7.67 x 10−7 | 52.283 | 63 | 1292 | 338 |
| MTLE-L | −0.426 | 0.099 | −4.313 | −0.62 to −0.232 | 1.61 x 10−5 | 53.165 | 88 | 1344 | 412 | |
| All epilepsies | −0.311 | 0.065 | −4.748 | −0.439 to −0.182 | 2.05 x 10−6 | 67.476 | 164 | 1650 | 2061 | |
| All other epilepsies | −0.257 | 0.045 | −5.680 | −0.346 to −0.168 | 1.34 x 10−8 | 0 | 239 | 1447 | 1000 | |
| Paracentral gyrus (RH) | MTLE-R | −0.421 | 0.064 | −6.538 | −0.548 to −0.295 | 6.24 x 10−11 | 0.407 | 90 | 1296 | 338 |
| MTLE-L | −0.378 | 0.075 | −5.021 | −0.526 to −0.231 | 5.14 x 10−7 | 23.536 | 111 | 1348 | 412 | |
| All other epilepsies | −0.351 | 0.045 | −7.733 | −0.44 to −0.262 | 1.05 x 10−14 | 3.43 x 10−3 | 129 | 1451 | 998 | |
| All epilepsies | −0.315 | 0.053 | −5.983 | −0.418 to −0.212 | 2.19 x 10−9 | 49.261 | 160 | 1654 | 2059 | |
| Parahippocampal gyrus (LH) | MTLE-L | −0.3 | 0.073 | −4.11 | −0.444 to −0.1572 | 3.95 x 10−5 | 19.366 | 176 | 1335 | 410 |
| Pars opercularis (RH) | MTLE-R | −0.271 | 0.071 | −3.8 | −0.411 to −0.131 | 1.45 x 10−4 | 12.105 | 215 | 1295 | 338 |
| All epilepsies | −0.177 | 0.036 | −4.976 | −0.247 to −0.107 | 6.48 x 10−7 | 2.624 | 503 | 1652 | 2059 | |
| Pars triangularis (LH) | All epilepsies | −0.192 | 0.05 | −3.828 | −0.2897 to −0.094 | 1.29 x 10−4 | 44.414 | 427 | 1650 | 2060 |
| Pars triangularis (RH) | MTLE-L | −0.285 | 0.06 | −4.738 | −0.403 to −0.167 | 2.16 x 10−6 | 0 | 195 | 1346 | 412 |
| All epilepsies | −0.199 | 0.036 | −5.48 | −0.27 to −0.128 | 4.25 x 10−8 | 4.66 | 398 | 1652 | 2058 | |
| All other epilepsies | −0.210 | 0.045 | −4.650 | −0.299 to −0.122 | 3.32 x 10−6 | 2.58 x 10−3 | 357 | 1449 | 998 | |
| Precentral gyrus (LH) | MTLE-L | −0.466 | 0.081 | −5.755 | −0.625 to −0.307 | 8.64 x 10−9 | 31.602 | 74 | 1339 | 412 |
| MTLE-R | −0.415 | 0.09 | −4.596 | −0.592 to −0.238 | 4.31 x 10−6 | 40.044 | 93 | 1287 | 338 | |
| All epilepsies | −0.384 | 0.044 | −8.768 | −0.469 to −0.298 | 1.82 x 10−18 | 27.649 | 108 | 1645 | 2058 | |
| All other epilepsies | −0.375 | 0.046 | −8.237 | −0.464 to −0.286 | 1.76 x 10−16 | 5.59 x 10−3 | 113 | 1442 | 997 | |
| IGE | −0.342 | 0.071 | −4.78 | −0.482 to −0.201 | 1.75 x 10−6 | 0.003 | 136 | 1043 | 297 | |
| Precentral gyrus (RH) | MTLE-R | −0.52 | 0.086 | −6.073 | −0.687 to −0.352 | 1.25 x 10−9 | 33.288 | 60 | 1293 | 337 |
| MTLE-L | −0.492 | 0.078 | −6.335 | −0.6436 to −0.339 | 2.37 x 10−10 | 26.33 | 66 | 1345 | 412 | |
| All epilepsies | −0.399 | 0.044 | −9.102 | −0.485 to −0.313 | 8.85 x 10−20 | 27.929 | 100 | 1649 | 2054 | |
| IGE | −0.39 | 0.072 | −5.442 | −0.531 to −0.25 | 5.27 x 10−8 | 0.005 | 105 | 1044 | 295 | |
| All other epilepsies | −0.348 | 0.045 | −7.672 | −0.437 to −0.259 | 1.70 x 10−14 | 0 | 131 | 1448 | 996 | |
| Precuneus (LH) | MTLE-L | −0.536 | 0.135 | −3.965 | −0.801 to −0.271 | 7.35 x 10−5 | 75.18 | 56 | 1343 | 412 |
| All other epilepsies | −0.178 | 0.047 | −3.819 | −0.27 to −0.087 | 1.34 x 10−4 | 4.474 | 497 | 1446 | 998 | |
| Precuneus (RH) | MTLE-L | −0.473 | 0.104 | −4.558 | −0.676 to −0.27 | 5.16 x 10−6 | 57.498 | 72 | 1348 | 412 |
| All epilepsies | −0.275 | 0.066 | −4.197 | −0.404 to −0.147 | 2.70 x 10−5 | 67.608 | 209 | 1654 | 2055 | |
| All other epilepsies | −0.238 | 0.053 | −4.471 | −0.343 to −0.134 | 7.78 x 10−6 | 22.378 | 279 | 1451 | 994 | |
| Superior frontal gyrus (LH) | MTLE-L | −0.411 | 0.06 | −6.804 | −0.529 to −0.292 | 1.02 x 10−11 | 0 | 94 | 1343 | 412 |
| All epilepsies | −0.283 | 0.054 | −5.251 | −0.389 to −0.177 | 1.51 x 10−7 | 51.773 | 197 | 1649 | 2059 | |
| All other epilepsies | −0.243 | 0.059 | −4.138 | −0.358 to −0.128 | 3.51 x 10−5 | 34.545 | 267 | 1446 | 999 | |
| Superior frontal gyrus (RH) | MTLE-L | −0.365 | 0.06 | −6.051 | −0.483 to −0.246 | 1.44 x 10−9 | 0 | 119 | 1345 | 412 |
| All epilepsies | −0.269 | 0.059 | −4.588 | −0.385 to −0.154 | 4.49 x 10−6 | 59.483 | 218 | 1650 | 2058 | |
| All other epilepsies | −0.235 | 0.052 | −4.489 | −0.337 to −0.132 | 7.15 x 10−6 | 20.049 | 286 | 1448 | 997 | |
| Superior parietal gyrus (LH) | All other epilepsies | −0.224 | 0.045 | −4.954 | −0.313 to −0.136 | 7.27 x 10−7 | 0.001 | 314 | 1444 | 996 |
| Superior parietal gyrus (RH) | All other epilepsies | −0.220 | 0.045 | −4.864 | −0.309 to −0.131 | 1.15 x 10−6 | 0.002 | 326 | 1450 | 997 |
| Supramarginal gyrus (LH) | All epilepsies | −0.232 | 0.06 | −3.894 | −0.348 to −0.115 | 9.87 x 10−5 | 59.391 | 293 | 1606 | 1965 |
| Supramarginal gyrus (RH) | All epilepsies | −0.223 | 0.055 | −4.045 | −0.331 to −0.115 | 5.24 x 10−5 | 52.895 | 317 | 1597 | 1971 |
| All other epilepsies | −0.206 | 0.047 | −4.418 | −0.297 to −0.115 | 9.95 x 10−6 | 0 | 371 | 1395 | 961 | |
| Temporal pole (LH) | MTLE-L | −0.315 | 0.068 | −4.649 | −0.447 to −0.182 | 3.33 x 10−6 | 10.901 | 160 | 1341 | 410 |
| Transverse temporal gyrus (LH) | MTLE-R | −0.312 | 0.073 | −4.249 | −0.456 to −0.168 | 2.15 x 10−5 | 15.614 | 163 | 1289 | 338 |
| All epilepsies | −0.192 | 0.044 | −4.406 | −0.278 to −0.107 | 1.05 x 10−5 | 28.178 | 427 | 1647 | 2061 | |
| Transverse temporal gyrus (RH) | All epilepsies | −0.182 | 0.044 | −4.188 | −0.267 to −0.097 | 2.81 x 10−5 | 27.918 | 475 | 1654 | 2059 |
| All other epilepsies | −0.18 | 0.045 | −3.982 | −0.269 to −0.091 | 6.84 x 10−5 | 0.012 | 486 | 1451 | 998 |
CI = confidence interval; LH = left hemisphere; RH = right hemisphere; SE = standard error; I2 = heterogeneity index; N80 = number of subjects required in each group to yield 80% power to detect significant group differences (P < 0.05, two-tailed). Uncorrected P-values are reported. Cortical regions that failed to survive Bonferroni correction (P < 1.49 x 10−4) are not reported (see ‘Materials and methods’ section for statistical threshold determination). See Supplementary material for a full list of cortical differences with adjustment for false discovery rate (FDR).
Figure 3.
Cortical thickness findings. Cohen’s d effect size estimates for case-control differences in cortical thickness, across the (A) all-epilepsies, (B) mesial temporal lobe epilepsies with left hippocampal sclerosis (MTLE-L), (C) mesial temporal lobe epilepsies with right hippocampal sclerosis (MTLE-R), (D) idiopathic generalized epilepsies (IGE), and (E) all-other-epilepsies groups. Cohen’s d effect sizes were extracted using multiple linear regressions, and pooled across research centres using random-effects meta-analysis. Cortical structures with P-values < 1.49 × 10−4 are shown in heatmap colours; strength of heat map is determined by the size of the Cohen’s d (d < 0 = blue, d > 0 = yellow/red). Image generated using MATLAB with annotations added using Adobe Photoshop. An interactive version of this figure is available online, via ‘ENIGMA-Viewer’: http://enigma-viewer.org/ENIGMA_epilepsy_cortical.html. See Supplementary material for guidelines on how to use the interactive visualization. HS = hippocampal sclerosis.
The MTLE-L and MTLE-R subgroups showed distinct patterns of cortical thickness reductions when compared to healthy controls (Table 3, Fig. 3B and C). In MTLE-R, lower cortical thickness was reported across four motor regions, including the left (d = −0.51; P = 7.67 × 10−7) and right paracentral gyri (d = −0.42; P = 6.24 × 10−11), and the left (d = −0.42; P = 4.31 × 10−6) and right precentral gyri (d = −0.52; P = 1.25 × 10−9). The MTLE-R subgroup also showed thickness changes in the left transverse temporal gyrus (d = −0.31; P = 2.15 × 10−5), and right pars opercularis (d = −0.27; P = 1.45 × 10−4) (Table 3 and Fig. 3C). By contrast, in MTLE-L, lower thickness was observed across six regions of the motor cortex, including the left (d = −0.43; P = 1.61 × 10−5) and right paracentral gyri (d = −0.38; P = 5.14 × 10−7), left (d = −0.47; P = 8.64 × 10−9) and right precentral gyri (d = −0.49; P = 2.37 × 10−10), and left (d = −0.54; P = 7.35 × 10−5) and right precuneus (d = −0.47; P = 5.16 × 10−6). The MTLE-L group also showed thickness changes across five regions of the frontal cortex, including the left (d = −0.41; P = 1.02 × 10−11) and right superior frontal gyri (d = −0.37; P = 1.44 × 10−9), left (d = −0.4; P = 7.07 × 10−9) and right caudal middle frontal gyri (d = −0.44; P = 3.61 × 10−7), and the right pars triangularis (d = −0.29; P = 2.16 × 10−6). In MTLE-L, thickness alterations were also observed in four regions of the temporal cortex, including the left temporopolar cortex (d = −0.32; P = 3.33 × 10−6), left parahippocampal gyrus (d = −0.3; P = 3.95 × 10−5), left entorhinal gyrus (d = −0.45; P = 7.35 × 10−10), and left fusiform gyrus (d = −0.36; P = 2.19 × 10−7) (Table 3 and Fig. 3B).
The IGE subgroup showed reduced thickness in the left (d = −0.34; P = 1.75 × 10−6) and right precentral gyri (d = −0.39; P = 5.27 × 10−8), when compared to healthy controls (Table 3 and Fig. 3D).
The all-other-epilepsies subgroup showed lower thickness across six cortical regions bilaterally, including the left (d = −0.38; P = 1.76 × 10−16) and right precentral gyri (d = −0.35; P = 1.7 × 10−14), left (d = −0.26; P = 1.34 × 10−8) and right paracentral gyri (d = −0.35; P = 1.1 × 10−14), left (d = −0.29; P = 1.32 × 10−10) and right caudal middle frontal gyri (d = −0.21; P = 2.62 × 10−6), left (d = −0.22; P = 7.27 × 10−7) and right superior parietal gyri (d = −0.22; P = 1.15 × 10−6), left (d = −0.24; P = 3.51 × 10−5) and right superior frontal gyri (d = −0.23; P = 7.15 × 10−6), and the left (d = −0.18; P = 1.34 × 10−4) and right precuneus (d = −0.24; P = 7.78 × 10−6) compared to controls. The all-other-epilepsies group also showed unilaterally reduced thickness in six right hemispheric regions, including the cuneus (d = −0.23; P = 2.15 × 10−7), lateral occipital gyrus (d = −0.21; P = 3.18 × 10−6), pars triangularis (d = −0.21; P = 3.32 × 10−6), supramarginal gyrus (d = −0.21; P = 9.95 × 10−6), transverse temporal gyrus (d = −0.18; P = 6.84 × 10−5), and lingual gyrus (d = −0.18; P = 7.12 × 10−5), compared to controls (Table 3 and Fig. 3E).
An interactive 3D visualization of these results is available via the ENIGMA-Viewer tool (Zhang et al., 2017), at http://enigma-viewer.org/ENIGMA_epilepsy_cortical.html (Supplementary material). Cortical thickness differences significant after FDR adjustment can also be visualized at http://enigma-viewer.org/ENIGMA_epilepsy_cortical_fdr.html (Supplementary Tables 31–35).
Duration of illness, age at onset, and age-by-diagnosis effects on brain abnormalities
A secondary analysis identified significant associations between duration of epilepsy and several affected brain regions in the all-epilepsies, MTLE-R, and all-other-epilepsies groups. In the all-epilepsies group, duration of epilepsy negatively associated with volume measures in the left hippocampus (b = −8.32; P = 8.16 × 10−13), left (b = −13.58; P = 3.52 × 10−15), and right thalamus (b = −12.25; P = 1.58 × 10−13), and right pallidum (b = −2.67; P = 1.78 × 10−7), in addition to bilateral thickness measures in the left (b = −0.003; P = 2.99 × 10−11) and right pars triangularis (b = −0.002; P = 4.24 × 10−9), left (b = −0.003; P = 1.61 × 10−15) and right caudal middle frontal gyri (b = −0.003; P = 1.65 × 10−17), left (b = −0.003; P = 1.77 × 10−13) and right supramarginal gyri (b = −0.003; P = 2.58 × 10−19), left (b = −0.003; P = 5.84 × 10− 12) and right precentral gyri (b = −0.003; P = 2.54 × 10−24), left (b = −0.004; P = 1.94 × 10−12) and right superior frontal gyri (b = −0.003; P = 4.65 × 10−11), left (b = −0.004; P = 1.05 × 10−10) and right transverse temporal gyri (b = −0.003; P = 8.24 × 10−10), and left (b = −0.002; P = 5.22 × 10−6) and right paracentral gyri (b = −0.002; P = 5.63 × 10−6). Duration of epilepsy also negatively associated with unilateral thickness measures in the right precuneus (b = −0.003; P = 6.03 × 10−21), right pars opercularis (b = −0.003; P = 5.59 × 10−13), and right cuneus (b = −0.002; P = 1.1 × 10−9; Supplementary Table 8). In the MTLE-R subgroup, duration of epilepsy negatively associated with volume measures in the right hippocampus (b = −22.42; P = 1.1 × 10−7), and the right thalamus (b = −18.11; P = 1.84 × 10−5), and thickness measures in the left transverse temporal gyrus (b = −0.007; P = 8.39 × 10−5; Supplementary Table 8). In the all-other-epilepsies subgroup, duration of epilepsy negatively associated with bilateral thickness measures in the left (b = −0.003; P = 3.39 × 10−7) and right caudal middle frontal gyri (b = −0.003; P = 6.91 × 10−8), left (b = −0.003; P = 1.36 × 10−9) and right superior frontal gyri (b = −0.003; P = 3.16 × 10−7), and the left (b = −0.003; P = 3.17 × 10−5) and right precuneus (b = −0.003; P = 5.01 × 10−9), in addition to unilateral thickness measures in the right precentral gyrus (b = −0.004; P = 1.16 × 10−12), right cuneus (b = −0.003; P = 8.57 × 10−8), right pars triangularis (b = −0.003; P = 5.16 × 10−7), and right supramarginal gyrus (b = −0.003; P = 2.24 × 10−7). Duration of epilepsy also showed a positive association with the size of the left lateral ventricle in the all-other-epilepsies group (b = 13.6; P = 1.17 × 10−5).
In the all-epilepsies group, age at onset of epilepsy negatively associated with thickness measures in the left (b = −0.003; P = 2.66 × 10−15) and right superior frontal gyri (b = −0.003; P = 9.77 × 10−10), left (b = −0.003; P = 2.78 × 10−9) and right pars triangularis (b = −0.003; P = 6.51 × 10−7), right pars opercularis (b = −0.003; P = 5.4 × 10−14), left transverse temporal gyrus (b = −0.003; P = 1.03 × 10−8), and right cuneus (b = −0.001; P = 4.9 × 10−6). In the all-other-epilepsies subgroup, age at onset negatively correlated with thickness measures in the left (b = −0.003; P = 3.21 × 10−8) and right superior frontal gyri (b = −0.002; P = 1.18 × 10−4), left (b = −0.002; P = 8.42 × 10−6) and right precuneus (b = −0.002; P = 7.23 × 10−5), right pars triangularis (b = −0.003; P = 2.53 × 10−5), and right supramarginal gyrus (b = −0.002; P = 2.38 × 10−6). Age at onset also positively associated with the size of the right lateral ventricle in the all-other-epilepsies subgroup (b = 57.73; P = 1.62 × 10−7).
Age at onset negatively associated with other regional volumetric and thickness measures in the all-epilepsies, IGE, MTLE-L, MTLE-R, and all-other-epilepsies groups, but these associated areas showed no significant structural differences in the primary case-control analysis (Table 1 and Supplementary Table 8).
There were no interaction effects between age and syndromic diagnosis in the all-epilepsies, MTLE-L, MTLE-R, IGE, or all-other-epilepsies groups.
Power analyses for detection of case-control differences
In our sample of 2149 individuals with epilepsy and 1727 healthy controls, we had 80% power to detect Cohen’s d effect sizes as small as d = 0.091 at the standard alpha level of P < 0.05 (two-tailed), and 80% power to detect Cohen’s d effect sizes as small as d = 0.149 at the study’s stringent Bonferroni-corrected threshold of P < 1.49 × 10−4.
N80, the number of cases and controls required to achieve 80% power to detect group differences using a two-tailed t-test at P < 0.05, ranged from N80 = 6, to detect group effects in the right hippocampus in our MTLE-R group, to N80 = 503, to detect group effects in the right pars opercularis in our ‘all epilepsies’ group (Tables 2 and 3).
Discussion
In the largest coordinated neuroimaging study of epilepsy to date, we identified a series of quantitative imaging signatures—some shared across common epilepsy syndromes, and others characteristic of selected, specific epilepsy syndromes. Our sample of 2149 individuals with epilepsy and 1727 controls provided 80% power to detect differences as small as d = 0.091 (P < 0.05, two-tailed), allowing us to identify subtle, consistent brain abnormalities that are typically undetectable on visual inspection, or overlooked using smaller case-control designs. This international collaboration addresses prior inconsistencies in the field of epilepsy neuroimaging, providing a robust, in vivo map of structural aberrations, upon which future studies of disease mechanisms may expand.
In the first of five cross-sectional MRI analyses, we investigated a diverse aggregation of epilepsy syndromes, putative causes, and durations of disease. This all-epilepsies group exhibited shared, diffuse brain structural differences across several regions including the thalamus, pallidum, precentral, paracentral, and superior frontal cortices. With the exception of hippocampal volume and entorhinal thickness differences (Supplementary material), these structural alterations were not driven by any specific syndrome or dataset (Supplementary Figs 3 and 7). Our findings suggest a common neuroanatomical signature of epilepsy across a wide spectrum of disease types, complementing recent evidence for shared genetic susceptibility to a wide spectrum of epilepsies (International League Against Epilepsy Consortium on Complex Epilepsies, 2014). Some structural and genetic pathways may be shared across syndromes, despite the heterogeneity of epilepsy and seizure types. This shared MRI signature underpins the contemporary shift towards the study of epilepsies as network phenomena (Caciagli et al., 2014).
In MTLE, as expected, we observed hippocampal volume abnormalities ipsilateral to the patient’s side of seizure onset. Neither MTLE-L nor MTLE-R showed significant contralateral hippocampal volume reductions, confirming that sporadic, unilateral MTLE is not routinely underpinned by bilateral hippocampal damage (Blümcke et al., 2013). Both MTLE groups showed extrahippocampal abnormalities in the ipsilateral thalamus and pallidum, with widespread reductions in cortical thickness, supporting a growing body of literature indicating that MTLE, as an example of a specific disease constellation in the epilepsies, is also a network disease, extending beyond the mesial temporal regions (Keller et al., 2014; de Campos et al., 2016). Disruption of this network, notably in the thalamus (Keller et al., 2015; He et al., 2017) and thalamo-temporal white matter tracts (Keller et al., 2015, 2017), may be associated with postoperative seizure outcome in MTLE.
Patients with left and right MTLE showed distinct patterns of structural abnormalities when compared to controls, resolving conflicting findings from smaller studies, some reporting an equal distribution of structural differences (Liu et al., 2016), and others indicating more diffuse abnormalities, either in left MTLE (Keller et al., 2002, 2012; Bonilha et al., 2007; Kemmotsu et al., 2011; de Campos et al., 2016) or in right MTLE (Pail et al., 2009). The structural differences observed in the present study may reflect a younger age at onset of epilepsy in left MTLE, which occurred, on average, 1.2 years earlier than those with right MTLE (Supplementary Table 20). Independent, large-scale studies of MTLE patients have confirmed a significantly earlier age at onset in left, compared to right, MTLE (Blümcke et al., 2017). Duration-related effects were also observed in right, but not left, MTLE, pointing to possible biological distinctions between the two.
In IGE, a clinically and biologically distinct group of epilepsies typically associated with ‘normal’ MRI on clinical inspection (Woermann et al., 1998), we identified reduced volume of the right thalamus, and thinner precentral gyri in both hemispheres, supporting prior reports of structural (Bernhardt et al., 2009a), electroencephalographic, and functional (Gotman et al., 2005) abnormalities in IGE. These IGE cases were considered typical by reviewing neurologists, suggesting that this common type of epilepsy is also associated with quantifiable structural brain abnormalities.
The precentral gyri, site of the primary motor cortex, showed bilateral structural deficits across all epilepsy groups (all-epilepsies, IGE, MTLE-L, MTLE-R, and all-other-epilepsies), without detectable inter-cohort or between-disease heterogeneity (Supplementary Figs 3–12). Atrophy of the motor cortex has been linked to seizure frequency and duration of epilepsy in MTLE (Coan et al., 2014); here, we observed a negative correlation between precentral (and postcentral) grey matter thickness and duration of epilepsy in the aggregate all-epilepsies group.
The right thalamus also showed evidence of structural compromise across all epilepsy cohorts, re-emphasizing the importance of the thalamus as a major hub in the epilepsy network (He et al., 2017; Jobst and Cascino, 2017). Loss of feed-forward inhibition between the thalamus and its neocortical connections may be epileptogenic (Paz and Huguenard, 2015), and thalamocortical abnormalities have previously been reported in IGE (Gotman et al., 2005; Bernhardt et al., 2009a; O’Muircheartaigh et al., 2012) and MTLE (Mueller et al., 2010; Bernhardt et al., 2012). These findings support prior ‘system epilepsies’ hypotheses of pathophysiology (Avanzini et al., 2012), suggesting that a broad range of common epilepsies share vulnerability within a thalamocortical structural pathway involved in, and likely affected by, seizures (Liu et al., 2003; Bernhardt et al., 2013). Given this study’s cross-sectional design, we cannot determine if these are causative changes, consequences of recurrent seizures, prolonged drug treatment, or a combination of factors. The epilepsies, as a broad group, may involve progressive structural change (Caciagli et al., 2017), indicating the need for large-scale longitudinal studies.
A heterogeneous subgroup of individuals without confirmed diagnoses of IGE or MTLE with hippocampal sclerosis showed similar patterns of structural alterations to those observed in the aggregate all-epilepsies cohort. The findings included enlarged ventricles, smaller right pallidum and right thalamus, and reduced thickness across the motor and frontal cortices. Hippocampal abnormalities were not observed in this subgroup, suggesting that the patterns of reduced hippocampal grey matter observed in the aggregate group were driven by the inclusion of MTLEs with hippocampal sclerosis. Unlike the IGE, MTLE, and aggregate epilepsy cohorts, this subgroup also showed bilateral enlargement of the amygdala—a phenomenon previously reported in non-lesional localization-related epilepsies (Reyes et al., 2017) and non-lesional MTLEs (Takaya et al., 2012; Coan et al., 2013). Non-lesional MTLEs formed a large proportion of this ‘all-other-epilepsies’ cohort (43.3%; 445 individuals), but the subgroup included many other focal and unclassified syndromes, potentially obscuring specific biological interpretations. Future, sufficiently powered studies will stratify this cohort into finer-grained subtypes to delineate syndrome-specific effects.
Despite its international scale, our study has limitations. All results were derived from cross-sectional data: we cannot distinguish between historical acute damage and progressive abnormalities. We cannot disentangle the relative contributions of environmental and treatment-related factors, including antiepileptic medications, seizure types and frequencies, disease severity, language dominance, and other initial precipitating factors. On average, duration of epilepsy was at least 10 years; longitudinal investigations of new-onset and paediatric epilepsies will provide a more comprehensive understanding. Despite using standardized image processing protocols, quality control, and statistical techniques, some brain measures showed a wide distribution of effect sizes across research centres, which may reflect sample heterogeneity and differences in scanning protocols (Supplementary material).
We observed modest thickness differences across the majority of cortical regions; Cohen’s d effect sizes ranged from small to moderate (d = 0.2–0.5), with some very small effects (d < 0.2) noted in the right pars opercularis, bilateral pars triangularis, and bilateral transverse temporal gyri of the aggregate all-epilepsies group. Other large-scale ENIGMA studies have reported similarly modest (albeit less widespread) cortical abnormalities in psychiatric illnesses including major depression (Schmaal et al., 2016) and bipolar disorder (Hibar et al., 2017b). Although epilepsy is characterized by an enduring predisposition to generate abnormal excessive or synchronous neuronal activity in the brain (Fisher et al., 2014), our findings indicate that common epilepsies are associated with widespread, but relatively subtle, structural alterations of the neocortex. Replication in independent MRI cohorts, complemented by advanced imaging modalities and large-scale gene expression datasets, will help elucidate how these cortical abnormalities relate to underlying disease processes.
Overall, in the largest neuroimaging analysis of epilepsy to date, we demonstrate a pattern of robust brain structural abnormalities within and between syndromes. Specific functional interpretations cannot be inferred from grey matter differences, but lower volume and thickness measures may reflect tissue loss, supporting recent observations that the common epilepsies cannot always be considered benign (Gaitatzis et al., 2004; Bell et al., 2016; Devinsky et al., 2017). The study provides a macroscopic neuroanatomical map upon which neuropathological work, animal models, and further gene expression studies, can expand. Our consortium plans to investigate more specific neuroanatomical traits and epilepsy phenotypes, explore sophisticated shape and sulcal measures, and eventually conduct genome-wide association analysis of brain measures, to improve our understanding and treatment of the epilepsies.
Web resources
All image processing, quality assurance, and statistical analysis protocols for this study can be downloaded from the ENIGMA website, at: http://enigma.usc.edu/ongoing/enigma-epilepsy/enigma-epilepsy-protocols/.
Supplementary Material
Acknowledgements
We thank Dr Costin Leu, Dr Sinéad Kelly, Dr Michael Nagle, and Dr Craig Hyde for helpful discussions. The RCSI EPIGEN centre thanks Professor James F. Meaney, Dr Andrew J. Fagan, Dr Jason McMorrow and Dr Gerard Boyle for designing MR acquisition protocols and assisting in the acquisition of MRI data. The IDIBAPS-HCP centre thanks Dr Mar Carreño for providing clinical data. The NYU centre thanks Dr Heath Pardoe for designing MR acquisition protocols, and Xiuyuan Wang for conducting image quality inspection and analysis. The Bern centre thanks Prof. Kaspar Schindler, epilepsy surgery program PI, for providing clinical input, Dr Christian Weisstanner for supporting neuroradiological quality assessment, Dr. Andrea Seiler for collecting clinical information, and Dr Heinz Krestel for supporting data collection. The Tübingen centre thanks Dr Silke Klamer for recruitment of the EKUT_B cohort. The Brussels site thanks Dr Xavier De Tiège for making the control scans available. We thank the International League Against Epilepsy Consortium on Complex Epilepsies for advertising the ENIGMA-Epilepsy project amongst its members. New groups are welcome to join the consortium at http://enigma.usc.edu
Funding
This study was supported in part by a Center grant (U54 EB020403) from the National Institutes of Health as part of the 2014 Big Data to Knowledge (BD2K) Initiative. The work was partly undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. We are grateful to the Wolfson Trust and the Epilepsy Society for supporting the Epilepsy Society MRI scanner. The UNICAMP research centre was funded by FAPESP (São Paulo Research Foundation); Contract grant number: 2013/07559-3. The BRI at the Florey Institute of Neuroscience and Mental Health acknowledges funding from the National Health and Medical Research Council of Australia (NHMRC Project Grant 628952, Practitioner Fellowship 1060312). The UCSD research centre acknowledges support from the U.S. National Institute of Neurological Disorders and Stroke (NIH/NINDS, grant no. R01NS065838). The UNAM centre was funded by grants UNAM-DGAPA IB201712 and Conacyt 181508 RRC Graduate Fellowship Conacyt 329866. UNIMORE acknowledges funding from the Carismo Foundation (grant number: A.010@FCRMO RINT@MELFONINFO) and the Italian Ministry of Health, Emilia-Romagna Region (N. PRUA1GR-2013-00000120). Work conducted at Kuopio University Hospital was funded by Government Grant 5772810. Work at the University of Eastern Finland was funded by Vaajasalo Foundation and Saastamoinen Foundation. Funding sources for the King’s College London research centre include: National Institute for Health Research Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust; Medical Research Council (grants G0701310 and MR/K013998/1); Epilepsy Research UK. Work conducted at the University of Liverpool was funded by the UK Medical Research Council (grant MR/K023152/1). The Cardiff University centre acknowledges funding from Cardiff University Brain Research Imaging Centre, Cardiff and Vale University Health Board, Epilepsy Research UK and Health and Care Research Wales, Wales Government. Montreal Neurological Institute funding sources include the Canadian Institutes of Health Research (CIHR MOP-57840 and CIHR MOP-123520). Dr. Bernhardt acknowledges funding through NSERC Discovery, CIHR Foundation, SickKids New Investigator, and FRQS Junior 1. NYU funding includes: Finding a Cure for Epilepsy and Seizures (FACES); The Morris and Alma Schapiro Fund; Epilepsy Foundation. The Royal Melbourne Hospital group received funding from The Royal Melbourne Hospital Neurosciences Foundation. The Bern research centre was funded by Swiss National Science Foundation, grants no. 124089, 140332 and 320030-163398. The NYU School of Medicine site acknowledges support from Finding A Cure for Epilepsy and Seizures (FACES). Dr. Chen at the Ohio State University was partially sponsored by the National Science Foundation IIS-1302755, DBI-1260795, DBI-1062057, and CNS-1531491. At the Florence research centre, Dr. Blümcke and Dr. Haaker received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no: Health-Fs-602531-2013 (see DESIRE, http://epilepsydesireproject.eu/, for more information). The Xiamen University group was partly supported by the National Nature Science Foundation of China (No. 61772440), and the Open Project Program of the State Key Lab of CAD&CG (No. A1706). Dr Altmann holds an MRC eMedLab Medical Bioinformatics Career Development Fellowship. This work was partly supported by the Medical Research Council [grant number MR/L016311/1], and supported by the MRC through the MRC Sudden Death Brain Bank (C.S.) and by a Project Grant (G0901254 to J.H. and M.W.) and Training Fellowship (G0802462 to M.R.).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- ENIGMA
Enhancing Neuro Imaging Genetics through Meta-Analysis
- IGE
idiopathic generalized epilepsy
- MTLE-L/R
mesial temporal lobe epilepsy with left/right hippocampal sclerosis
References
- Adams HH, Hibar DP, Chouraki V, Stein JL, Nyquist PA, Rentería ME, et al. Novel genetic loci underlying human intracranial volume identified through genome-wide association. Nat Neurosci 2016; 19: 1569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvim MK, Coan AC, Campos BM, Yasuda CL, Oliveira MC, Morita ME, Cendes F. Progression of gray matter atrophy in seizure-free patients with temporal lobe epilepsy. Epilepsia 2016; 57; 621–9. [DOI] [PubMed] [Google Scholar]
- Avanzini G, Manganotti P, Meletti S, Moshé SL, Panzica F, Wolf P, et al. The system epilepsies: a pathophysiological hypothesis. Epilepsia 2012; 53: 771–8. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM. Emerging global initiatives in neurogenetics: the Enhancing Neuroimaging Genetics Through Meta-Analysis (ENIGMA) Consortium. Neuron 2017; 94: 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GS, Neligan A, Giavasi C, Keezer MR, Novy J, Peacock JL, et al. Outcome of seizures in the general population after 25 years: a prospective follow-up, observational cohort study. J Neurol Neurosurg Psychiatry 2016; 87: 843–50. [DOI] [PubMed] [Google Scholar]
- Bell GS, Neligan A, Sander JW. An unknown quantity—the worldwide prevalence of epilepsy. Epilepsia 2014; 55: 958–62. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995; 57: 289–300. [Google Scholar]
- Ben-Menachem E. Epilepsy in 2015: the year of collaborations for big data. Lancet Neurol 2016; 15: 6–7. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 2010; 51: 676–85. [DOI] [PubMed] [Google Scholar]
- Bernasconi N. Is epilepsy a curable neurodegenerative disease? Brain 2016; 139: 2336–7. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology 2010; 74: 1776–84. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi N, Kim H, Bernasconi A. Mapping thalamocortical network pathology in temporal lobe epilepsy. Neurology 2012; 78: 129–36. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bonilha L, Gross DW. Network analysis for a network disorder: the emerging role of graph theory in the study of epilepsy. Epilepsy Behav 2015; 50: 162–70. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Kim H, Bernasconi N. Patterns of subregional mesiotemporal disease progression in temporal lobe epilepsy. Neurology 2013; 81: 1840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Rozen DA, Worsley KJ, Evans AC, Bernasconi N, Bernasconi A. Thalamo–cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. Neuroimage 2009a; 46: 373–81. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology 2009b; 72: 1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betting LE, Mory SB, Lopes-Cendes I, Li LM, Guerreiro MM, Guerreiro CAM, et al. MRI reveals structural abnormalities in patients with idiopathic generalized epilepsy. Neurology 2006; 67: 848–52. [DOI] [PubMed] [Google Scholar]
- Blanc F, Martinian L, Liagkouras I, Catarino C, Sisodiya SM, Thom M. Investigation of widespread neocortical pathology associated with hippocampal sclerosis in epilepsy: a postmortem study. Epilepsia 2011; 52: 10–21. [DOI] [PubMed] [Google Scholar]
- Blümcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, Pfäfflin M, et al. Histopathological findings in tissue obtained from epilepsy surgery. N Engl J Med 2017; 377: 1648–56. [DOI] [PubMed] [Google Scholar]
- Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a task force report from the ILAE Commission on Diagnostic Methods. Epilepsia 2013; 54: 1315–29. [DOI] [PubMed] [Google Scholar]
- Boedhoe PSW, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, Benedetti F, et al. Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta- and mega-analysis. AJP 2017; 174: 60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Halford JJ, Eckert M, Appenzeller S, et al. Asymmetrical extra-hippocampal grey matter loss related to hippocampal atrophy in patients with medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2007; 78: 286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14: 365–76. [DOI] [PubMed] [Google Scholar]
- Caciagli L, Bernhardt BC, Hong S-J, Bernasconi A, Bernasconi N. Functional network alterations and their structural substrate in drug-resistant epilepsy. Front Neurosci 2014; 8: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caciagli L, Bernasconi A, Wiebe S, Koepp MJ, Bernasconi N, Bernhardt BC. A meta-analysis on progressive atrophy in intractable temporal lobe epilepsy: time is brain? Neurology 2017; 89: 506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan AC, Campos BM, Yasuda CL, Kubota BY, Bergo FP, Guerreiro CA, et al. Frequent seizures are associated with a network of gray matter atrophy in temporal lobe epilepsy with or without hippocampal sclerosis. PLoS One 2014; 9: e85843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan AC, Morita ME, de Campos BM, Yasuda C, Cendes F. Amygdala enlargement in patients with mesial temporal lobe epilepsy without hippocampal sclerosis. Front Neurol 2013; 4: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos B, Coan A, Yasuda C, Casseb R, Cendes F. Large-scale brain networks are distinctly affected in right and left mesial temporal lobe epilepsy. Hum Brain Mapp 2016; 37: 3137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Friedman D, Cheng JY, Moffatt E, Kim A, Tseng ZH. Underestimation of sudden deaths among patients with seizures and epilepsy. Neurology 2017; 89: 886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg 2011; 115: 1248–55. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage 2012; 62: 774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55: 475–82. [DOI] [PubMed] [Google Scholar]
- French JA. Refractory epilepsy: clinical overview. Epilepsia 2007; 48: 3–7. [DOI] [PubMed] [Google Scholar]
- Gaitatzis A, Johnson AL, Chadwick DW, Shorvon SD, Sander JW. Life expectancy in people with newly diagnosed epilepsy. Brain 2004; 127: 2427–32. [DOI] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA 2005; 102: 15236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TI, Brezova V, Eikenes L, Haberg A, Vangberg TR. How does the accuracy of intracranial volume measurements affect normalized brain volumes? Sample size estimates based on 966 subjects from the HUNT MRI cohort. AJNR Am J Neuroradiol 2015; 36: 1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Doucet GE, Pustina D, Sperling MR, Sharan AD, Tracy JI. Presurgical thalamic “hubness” predicts surgical outcome in temporal lobe epilepsy. Neurology 2017; 88: 2285–93. [DOI] [PubMed] [Google Scholar]
- Hibar DP, Adams HHH, Jahanshad N, Chauhan G, Stein JL, Hofer E, et al. Novel genetic loci associated with hippocampal volume. Nat Commun 2017a; 8: 13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Stein JL, Rentería ME, Arias-Vasquez A, Desrivières S, Jahanshad N, et al. Common genetic variants influence human subcortical brain structures. Nature 2015; 520: 224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA bipolar disorder working group. Mol Psychiatry 2017b, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Westlye LT, van Erp TGM, Rasmussen J, Leonardo CD, Faskowitz J, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry 2016; 21: 1710–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 2017; 4: 310–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International League Against Epilepsy Consortium on Complex Epilepsies. Genetic determinants of common epilepsies: a meta-analysis of genome-wide association studies. Lancet Neurol 2014; 13: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst BC, Cascino GD. Thalamus as a “hub” to predict outcome after epilepsy surgery. Neurology 2017; 88: 2246–7. [DOI] [PubMed] [Google Scholar]
- Keller SS, Glenn GR, Weber B, Kreilkamp BAK, Jensen JH, et al. Preoperative automated fibre quantification predicts postoperative seizure outcome in temporal lobe epilepsy. Brain 2017; 140: 68–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Mackay CE, Barrick TR, Wieschmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage 2002; 16: 23–31. [DOI] [PubMed] [Google Scholar]
- Keller SS, O’Muircheartaigh J, Traynor C, Towgood K, Barker G, Richardson MP. Thalamotemporal impairment in temporal lobe epilepsy: a combined MRI analysis of structure, integrity, and connectivity. Epilepsia 2014; 55: 306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Richardson MP, Schoene-Bake J, O’Muircheartaigh J, et al. Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Ann Neurol 2015; 77: 760–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 2008; 49; 741–57. [DOI] [PubMed] [Google Scholar]
- Keller SS, Schoene-Bake S, Gerdes JS, Weber B, Deppe M. Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: cross-sectional evidence for progressive neurologic injury. PLoS One 2012; 7; e46791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmotsu N, Girard HM, Bernhardt BC, Bonilha B, Lin JJ, Tecoma ES, et al. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia 2011; 52: 2257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labate A, Cerasa A, Aguglia U, Mumoli L, Quattrone A, Gambardella A. Neocortical thinning in ‘benign’ mesial temporal lobe epilepsy. Epilepsia 2011; 52: 712–17. [DOI] [PubMed] [Google Scholar]
- Liu M, Bernhardt BC, Bernasconi A, Bernasconi N. Gray matter structural compromise is equally distributed in left and right temporal lobe epilepsy. Human Brain Mapping 2016; 37: 515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RSN, Lemieux L, Bell GS, Hammers A, Sisodiya SM, Bartlett PA, et al. Progressive neocortical damage in epilepsy. Ann Neurol 2003; 53: 312–24. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Finlay D, Garcia P, et al. Involvement of the thalamocortical network in TLE with and without mesiotemporal sclerosis. Epilepsia 2010; 51: 1436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels KC, Zaccariello MJ, Hamiwka LD, Wirrell EC. Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat Rev Neurol 2016; 12: 465–76. [DOI] [PubMed] [Google Scholar]
- O’Muircheartaigh J, Vollmar C, Barker GJ, Kumari V, Symms MR, et al. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology 2011; 76: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Muircheartaigh J, Vollmar C, Barker GJ, Kumari V, Symms MR, et al. Abnormal thalamocortical structural and functional connectivity in juvenile myoclonic epilepsy. Brain 2012; 135: 3635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvliet GM, Besseling RMH, Jansen JFA, van der Kruijs SJM, Vles JSH, Hofman PAM, et al. Early onset of cortical thinning in children with rolandic epilepsy. Neuroimage Clin 2013; 2: 434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pail M, Brázdil M, Mareček R, Mikl M. An optimized voxel-based morphometric study of gray matter changes in patients with left-sided and right-sided mesial temporal lobe epilepsy and hippocampal sclerosis (MTLE/HS). Epilepsia 2009; 51: 511–18. [DOI] [PubMed] [Google Scholar]
- Paz JT, Huguenard JR. Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat Neurosci 2015; 18: 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Löscher W, Vezzani A, Becker AJ, Simonato M, Lukasiuk K, et al. Advances in the development of biomarkers for epilepsy. Lancet Neurol 2016; 15: 843–56. [DOI] [PubMed] [Google Scholar]
- Reyes A, Thesen T, Kuzniecky R, Devinsky O, McDonald CR, et al. Amygdala enlargement: temporal lobe epilepsy subtype or nonspecific finding? Epilepsy Res 2017; 132: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan L, Alhusaini S, Scanlon C, Doherty CP, Delanty N, Fitzsimons M. Widespread cortical morphologic changes in juvenile myoclonic epilepsy: evidence from structural MRI. Epilepsia 2012; 53: 651–8. [DOI] [PubMed] [Google Scholar]
- Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, et al. ILAE Classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017; 58: 512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2016; 22: 900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TGM, Sämann PG, Frodl T, Jahanshad N, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2015; 21: 806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet 2012; 44: 552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya S, Ikeda A, Takahiro M-O, Matsumoto R, Inouchi M, et al. Temporal lobe epilepsy with amygdala enlargement: a morphological and functional study. J Neuroimaging 2012; 24: 54–62. [DOI] [PubMed] [Google Scholar]
- Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 2005; 128: 1188–98. [DOI] [PubMed] [Google Scholar]
- Thom M, Eriksson S, Martinian L, Caboclo LO, McEvoy AW, Duncan JS, et al. Temporal lobe sclerosis associated with hippocampal sclerosis in temporal lobe epilepsy: neuropathological features. J Neuropathol Exp Neurol 2009; 68: 928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Andreassen OA, Arias-Vasquez A, Bearden CE, Boedhoe PS, Brouwer RM, et al. ENIGMA and the individual: predicting factors that affect the brain in 35 countries worldwide. Neuroimage 2017; 145: 389–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D, Rayner G, Tailby C, Jackson GD. MRI-negative temporal lobe epilepsy: a network disorder of neocortical connectivity. Neurology 2016; 87: 1934–42. [DOI] [PubMed] [Google Scholar]
- van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 2016; 21: 547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft 2010; 36. [Google Scholar]
- Vlooswijk MC, Jansen JF, de Krom MC, Majoie HM, Hofman PA, Backes WH, et al. Functional MRI in chronic epilepsy: associations with cognitive impairment. Lancet Neurol 2010; 9: 1018–27. [DOI] [PubMed] [Google Scholar]
- Vollmar CV, O’Muircheartaigh J, Barker GJ, Symms MR, Thompson P, et al. Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain 2011; 134: 1710–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woermann FG, Sisodiya SM, Free SL, Duncan JS. Quantitative MRI in patients with idiopathic generalized epilepsy. Evidence of widespread cerebral structural changes. Brain 1998; 121: 1661–7. [DOI] [PubMed] [Google Scholar]
- Zhang G, Kochunov P, Hong E, Kelly S, Whelan CD, Jahanshad N, et al. ENIGMA-viewer: interactive visualization strategies for conveying effect sizes in meta-analysis. BMC Bioinformatics 2017; 18 (Suppl 6): 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.