Abstract
Background and purpose
Liver disease, particularly non-alcoholic fatty liver disease (NAFLD), is a risk factor for cardiovascular disease, but little is known about its relationship with ischemic stroke.
Methods
In the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort of 30,239 American black and white adults, we assessed baseline NAFLD as fatty liver index (FLI) >60, and assessed liver biomarkers aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (GGT), and the AST/ALT ratio and risk of incident ischemic stroke over 5.8 years using a case-cohort study design.
Results
Considering 572 strokes and a 1,017-person cohort sample, NAFLD was inversely associated with stroke risk in men (HR: 0.50; 95% CI: 0.26, 0.96), as was being in the highest ALT quintile versus the lowest (HR: 0.39; 95% CI: 0.19, 0.78) and the highest versus lowest GGT quintile (HR: 0.45, 95% CI: 0.24, 0.85), but not in women. Conversely, FLI score above the 90th percentile was associated with increased stroke risk among women (HR: 2.26; 95% CI: 1.14–4.47), but not men. AST was not associated with stroke risk in either sex. AST/ALT ratio >2 was strongly associated with increased stroke risk in whites, but not blacks (HRs: 3.64; 95% CI: 1.42–9.35 and 0.97; 95% CI: 0.45–1.99, respectively; p for interaction = 0.03).
Conclusions
The relationships between NAFLD, liver biomarkers, and ischemic stroke are complex, and sex and race differences we observed require further study and confirmation.
Introduction
Liver dysfunction can contribute to thrombotic cardiovascular disease (CVD) through effects on synthesis of lipoproteins [1], coagulation proteins [2], and inflammation factors [3]. Many chronic liver diseases, such as hepatitis C and alcoholic liver disease are associated with increased cardiovascular risk [4, 5]. Non-alcoholic fatty liver disease (NAFLD), which is the accumulation of fat in the liver not caused by alcohol use, affects 10–46% of people in the United States [6], and is particularly prevalent in people with type 2 diabetes and obesity. It is considered the “hepatic manifestation of the metabolic syndrome” [7], and is associated with an increased risk of CVD [8–10].
Evaluating the association of liver dysfunction with CVD is challenging as there is no standard measure of liver function, and diagnosis of hepatocyte injury, structural liver disease, and hepatic dysfunction require a clinical diagnosis based on signs, symptoms, and biochemical measures of liver dysfunction. Levels of ALT, AST, and GGT are often increased in NAFLD and other liver diseases, and each has been associated with cardiovascular events including stroke in some cases [11–16]. Three prospective studies specifically investigated the association of imaging-defined NAFLD with stroke [9, 17, 18], with a meta-analysis of these, including only 100 strokes in total, reporting a 2-fold increased risk for stroke with NAFLD (95% CI: 1.46–2.98, p < 0.001) [19].
To extend literature on associations of liver biomarkers and NAFLD with stroke, we assessed the relationship between NAFLD, hepatic biomarkers and incident ischemic stroke in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. We assessed the biomarkers ALT, AST, GGT, the AST/ALT ratio, and the fatty liver index (FLI) [20] as a surrogate marker for NAFLD. We hypothesized that these biomarkers would be associated with increased risk of stroke, and that this risk might differ by race and by sex.
Methods
REGARDS is a population-based cohort study of 30,239 individuals aged 45 and above, designed to investigate racial and regional disparities in stroke in the contiguous United States [21]. The cohort was recruited from 2003–7. Participants were excluded from enrollment if their medical conditions precluded the expectation of long term participation. By design, 55% of the participants were female, 42% were black, and 56% lived in the stroke belt of the southeastern United States (North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas). At an initial telephone interview, participants gave verbal informed consent, during which demographic, socioeconomic, and medical history information was collected. At a subsequent at home visit, we obtained written informed consent, blood samples, anthropomorphic measurements, blood pressure, electrocardiogram (ECG), and medication inventory. All study procedures were reviewed and approved by the institutional review boards of the collaborating institutions (University of Alabama at Birmingham Institutional Review Board, University of Vermont Committee on Human Research, University of Cincinnati Institutional Review Board, Wake Forest University Institutional Review Board).
Study design
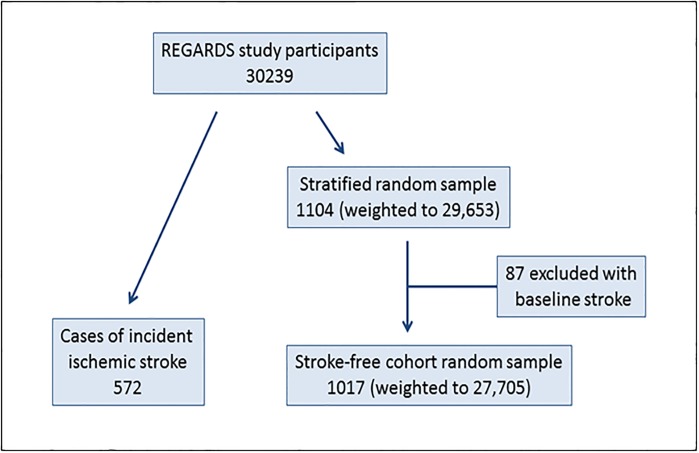
To minimize assay cost and improve efficiency, we conducted a case-cohort study within REGARDS consisting of 572 cases of incident ischemic stroke and a stratified cohort random sample (CRS) of 1,104 participants (Fig 1), as previously described [22]. Briefly, cases were ascertained through September 1, 2011, with a median follow-up time of 5.8 years. REGARDS participants or their proxies were telephoned every 6 months for health status updates. In cases of death, occurrence of stroke symptoms, or suspected cerebrovascular event, medical records were obtained and reviewed. Strokes events were validated by ≥2 physicians, and classified as hemorrhagic or ischemic. Selection of the cohort sample was stratified on age, race and sex. We excluded 87 CRS participants who had prebaseline stroke.
Fig 1. Case-cohort study design.
Covariates
Race, prebaseline stroke, and alcohol drinks per week were established by participant self-report. Data on statin and warfarin use were collected by self-report. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or self-reported use of anti-hypertensive medications. Diabetes was defined as fasting glucose ≥126 mg/dL, non-fasting glucose ≥200mg/dL, self-reported physician diagnosis of diabetes, or self-reported use of diabetes medication. Dyslipidemia was defined as total cholesterol ≥240 mg/dL, low density lipoprotein ≥160 mg/dL, high density lipoprotein ≤40 mg/dL, or use of cholesterol-lowering medications. Atrial fibrillation was established by self-report or presence on baseline electrocardiogram. Left ventricular hypertrophy was determined by baseline electrocardiogram [23]. Baseline CVD was defined as electrocardiogram evidence of myocardial infarction or self-reports of myocardial infarction, coronary artery bypass, stent, angioplasty, or peripheral artery disease.
Laboratory methods
Fasting blood samples drawn at in-home visits were centrifuged locally and shipped on ice overnight to the study laboratory at the University of Vermont, where they were recentrifuged and stored at -80°C [24]. AST, ALT, and GGT were measured in serum using the Roche Elecsys 2010 analyzer (Roche Diagnostics Indianapolis, IN). Laboratory analytical inter-assay CV ranges were 1.0%-5.4% for ALT, 1.8%-6.8% for AST, and 0.7%-3.0% for GGT.
Fatty liver index
NALFD status was determined using the FLI, a surrogate marker for non-alcoholic fatty liver disease developed by Bedogni et. al.[20]. The FLI was designed for use in studies where diagnosis by biopsy or imaging modalities is not feasible. It compares well with ultrasound [25–27] and proton magnetic resonance spectroscopy [28] determinations of hepatic steatosis, though it does not quantitatively predict liver fat. FLI is calculated from body mass index (BMI), GGT, waist circumference, and triglycerides according to the formula:
Based on previously established cutpoints [29], we defined likely NAFLD as FLI >60, while a score of <20 ruled out NAFLD, and scores of 20 to 60 were indeterminate. Compared to 1H-magnetic resonance spectroscopy, an FLI >60 is 91% specific and has a positive likelihood ratio of 5.10, such that individuals with NAFLD are 5 times more likely to score >60, while the negative likelihood ratio for a score <20 is 0.27, indicating that individuals without NAFLD are about 4 times more likely to score below 20 [28]. Individuals who consumed excessive amounts of alcohol (≥14 drinks/week for men, ≥7 drinks/week for women) represented < 5% of the participants, were considered more likely to have alcoholic fatty liver disease than NAFLD, so were excluded from analysis of FLI and stroke risk.
Statistical analyses
Statistical analyses were performed using SAS 9.3 (Cary, NC). Demographic and stroke risk factor correlates of FLI categories and liver enzyme quintiles were assessed by univariate analyses using the Rao-Scott χ2 statistic for categorical variables or linear regression for continuous variables, with weighting to account for stratified sample selection.
Hazard ratios for liver markers and ischemic stroke risk were calculated using weighted Cox proportional hazards models, with 95% confidence intervals (CI) calculated with robust sandwich estimators [30]. Model 1 either adjusted for or was stratified by age, sex, and race. All models also adjusted for an age by race interaction term, due to the known interaction of these factors for stroke risk [31]. Model 2 was additionally adjusted for the Framingham stroke risk factors: systolic blood pressure (SBP), left ventricular hypertrophy (LVH), smoking, prevalent CVD, atrial fibrillation, diabetes, and hypertension medication use. We did not adjust for alcohol use as it was not associated with stroke risk (data not shown).
Primary analysis of FLI was based on the cut points above for fatty liver (<20 and >60), with secondary analyses using FLI as a continuous variable and dichotomized at the 90th percentile. We chose the 90th percentile to identify individuals with a high likelihood of hepatic steatosis [20]. Levels of AST, ALT, and GGT were analyzed in sex-specific quintiles, based on their distribution in the CRS, and as continuous variables, with log-transformation for the latter to correct for skewed distributions. We also assessed AST/ALT ratio as a risk factor, since ratios >2 are associated with alcoholic hepatitis and predict long-term complications from chronic liver disease [32]. Interactions of the liver markers with race and sex were tested using cross-product terms with p<0.10 for the interaction term considered significant.
Results
In the CRS the mean age was 64.7 years, 55% were women, 41% were black and 39% had a BMI >30 kg/m2. Among cases and the CRS, FLI was missing in 133 participants, AST in 117, ALT in 111, and GGT in 100. In Table 1, NAFLD was present in 44% of the CRS based on FLI criteria and only 19% had a low FLI (<20). There was no difference in NAFLD by region of residence or alcohol use, and the strongest correlates of NAFLD were male sex, components of the metabolic syndrome, and LVH, which was 5 times more prevalent in those with NAFLD (Table 1).
Table 1. Baseline characteristics by FLI score in the cohort random sample.
| Low FLI (<20) | Intermediate FLI (20–60) | High FLI (>60) | p* | FLI ≥90th Percentile (≥91.4) | p † | |
|---|---|---|---|---|---|---|
| Participants | 19% | 36% | 44% | |||
| Age, mean (SD) | 64.5 (11) | 66.1 (9) | 64.0 (8) | 0.11 | 61 (8) | <0.001 |
| Women | 73% | 53% | 49% | <0.001 | 56% | 0.85 |
| Black | 35% | 38% | 46% | 0.05 | 47% | 0.34 |
| Stroke belt | 36% | 38% | 30% | 0.39 | 33% | 0.78 |
| BMI, mean (SD) | 23.1 (2.5) | 27.2 (3.0) | 33.7 (5.6) | <0.001 | 40.0 (6.4) | <0.001 |
| Waist (cm), mean (SD) | 78 (8) | 91 (7) | 107 (12) | <0.001 | 119 (12) | <0.001 |
| SBP (mmHg), mean (SD) | 121 (15) | 126 (16) | 131 (17) | <0.001 | 131 (16) | 0.04 |
| Current smoking | 13% | 11% | 15% | 0.43 | 15% | 0.66 |
| Hypertension | 37% | 55% | 68% | <0.001 | 75% | 0.002 |
| Dyslipidemia | 37% | 60% | 69% | <0.001 | 64% | 0.24 |
| CVD | 12% | 16% | 18% | 0.25 | 12% | 0.26 |
| Diabetes | 8% | 14% | 34% | <0.001 | 40% | <0.001 |
| Atrial fibrillation | 10% | 7% | 10% | 0.51 | 4% | 0.06 |
| LVH | 2% | 8% | 10% | 0.003 | 10% | 0.43 |
| Alcohol drinks/wk, mean (SD) | 3.9 (15.8) | 2.6 (7.9) | 1.7 (4.0) | 0.15 | 1.0 (3.3) | 0.006 |
| Statin use | 23% | 34% | 35% | 0.04 | 32% | 0.86 |
BMI: body mass index, SBP: systolic blood pressure, CVD: cardiovascular disease, LVH: left ventricular hypertrophy
* p values for differences between the groups, by Rao-Scott χ2 statistic (categorical variables) or linear regression (continuous variables)
† p values for ≥90th percentile compared with all others, by Rao-Scott χ2 statistic (categorical variables) or linear regression (continuous variables)
In the fully adjusted model, NAFLD was associated with reduced stroke risk and there was a significant interaction between NAFLD and sex (p = 0.09), but not race (p = 0.9). In men, NAFLD was inversely associated with stroke risk, but there was no association in women (Table 2). In secondary analyses the same pattern was seen for FLI as a continuous variable. By contrast, women in the top 10% of FLI score were at increased risk of stroke compared to those with lower values, while among men the association remained inverse, but not statistically significantly (and the sex interaction term was not significant). To assure that this inverse association in men was not due to use of vasculoprotective medications in high-risk subjects with NAFLD, we tested the effect of adding baseline statin and warfarin use to the fully-adjusted model, but this did not change the nature of the association (HR for NAFLD in men: 0.43; 95% CI: 0.22, 0.84).
Table 2. Hazard ratio (95% confidence interval) of ischemic stroke by FLI*.
| Model† | NAFLD (FLI >60) vs FLI <20 | FLI >90th Percentile vs Lower | Per 10 unit increment of FLI Score |
|---|---|---|---|
| Model 1 | |||
| All | 1.00 (0.69, 1.43) | 1.59 (1.02, 2.46) | 1.02 (0.97, 1.07) |
| Men | 0.67 (0.39, 1.17) | 1.26 (0.69, 2.30) | 0.96 (0.90, 1.03) |
| Women | 1.55 (0.98, 2.46) | 2.10 (1.15, 3.85) | 1.09 (1.02, 1.16) |
| p for interaction | 0.06 | 0.47 | 0.10 |
| Model 2 | |||
| All | 0.65 (0.43, 1.00) | 1.18 (0.68, 2.06) | 0.95 (0.90, 1.01) |
| Men | 0.50 (0.26, 0.96) | 0.75 (0.35, 1.60) | 0.90 (0.83, 0.97) |
| Women | 1.03 (0.58, 1.82) | 2.26 (1.14, 4.47) | 1.03 (0.95, 1.12) |
| p for interaction | 0.09 | 0.11 | 0.14 |
* Hazard ratios shown in bold were statistically significant based on the confidence interval
† Model 1: adjusted for age, race, and age*race
Model 2: additionally adjusted for the Framingham stroke risk factors
We then examined the individual liver biomarkers AST, ALT, and GGT by sex-specific quintiles (S1–S3 Tables). Age and race were significantly associated with all three biomarkers. In particular, black participants were more likely to be in the lower quintiles of ALT, but the higher quintiles of GGT. Younger age was associated with higher levels of all three biomarkers. While all of the liver biomarkers were significantly associated with waist circumference and BMI, for AST these were inverse relationships, while ALT and GGT had positive associations. Higher GGT and ALT, but not AST, were associated with higher alcohol consumption. High GGT was associated with several other cardiovascular risk factors, including SBP, current smoking, dyslipidemia and LVH. Associations were also seen between ALT and atrial fibrillation, and between AST and diabetes.
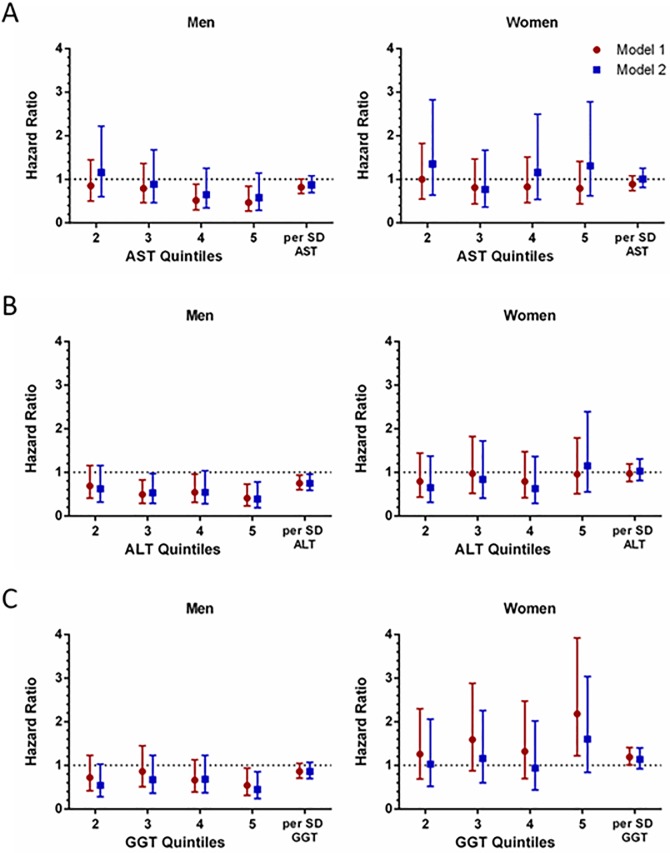
Fig 2 shows the hazard ratio of stroke by quintiles of each biomarker, compared with the 1st quintile, and the hazard ratio per SD of each log-transformed marker. Individuals with an AST/ALT ratio > 2 were excluded from these analyses, as this can be an indicator of alcoholic liver disease. GGT quintiles, but not AST or ALT quintiles, showed significant interaction with sex as continuous variables in the fully adjusted model (p = 0.06, 0.53, and 0.41, respectively), so we performed separate analyses for men and women. There were no significant interactions by race.
Fig 2. Hazard ratio of ischemic stroke by (A) AST, (B) ALT, and (C) GGT.
Model 1: Cox proportional hazards model adjusted for age, race, and age*race
Model 2: Additionally adjusted for the Framingham stroke risk factors
Percentile cut-off values (U/L) for 20th, 40th, 60th, 80th for men: AST (15.4, 18.5, 21.3, 25.5), ALT (12.1, 15.1, 18.9, 24.2), GGT (17.0 21.6, 28.1, 41.8). For women: AST (14.1, 16.4, 19.4, 23.1), ALT (9.3, 12.5, 14.9, 19.2), GGT (12.8, 16.9, 22.6, 31.2).
Higher AST showed a borderline inverse association with stroke risk for men in model 1, but this was not significant after adjustment for stroke risk factors, and there was no association of AST with stroke risk in women. There was a strong inverse association of ALT with ischemic stroke in men, but no association in women in models 1 and 2. For women, higher GGT was associated with stroke in model 1 only, while for men, higher GGT was associated with reduced stroke risk in the fully adjusted model (HR: 0.45 for top quintile; 95% CI: 0.24–0.85).
When the liver enzymes were analyzed as continuous variables (Fig 1), higher ALT again emerged as a marker of lower stroke risk in men (HR: 0.75 per SD; 95% CI: 0.60–0.94), but not in women (HR: 1.03 per SD; 95% CI: 0.81–1.31), after adjustment for stroke risk factors (model 2). GGT, analyzed as a continuous variable, was associated with stroke in women in model 1 (HR: 1.19 per SD; 95% CI: 1.01–1.41), but it was not significantly associated with stroke in men or women after adjustment for stroke risk factors.
An AST/ALT ratio >2 was associated with lower alcohol intake in univariate analysis (S2 Table), but this was not significant after adjustment for age, sex, and race (p = 0.8). Blacks were more likely to have a ratio >2 than whites. Unlike the other measures, there was no sex interaction of AST/ALT >2 with stroke (p = 0.3), but there was a significant race interaction in the fully adjusted model (p = 0.03), so analyses were stratified by race. A ratio >2 was significantly associated with risk of stroke in whites but not blacks, with hazard ratios of 2.74 (95% CI: 1.37–5.48) for whites and 1.29 (95% CI: 0.70–2.40) for blacks, adjusted for age and sex. Adding the Framingham stroke risk factors increased the point estimate in whites but not blacks (HRs: 3.64; 95% CI, 1.42–9.35 and 0.97; 95% CI: 0.47–1.99 in whites and blacks, respectively). In a sensitivity analysis excluding heavy drinkers, results were similar (HRs: 3.64 for whites and 0.95 for blacks).
Discussion
Our study revealed complex associations between liver biomarkers and stroke risk, with differences by race and sex. NAFLD by FLI and higher ALT and GGT were inversely associated with stroke risk in men, while higher GGT and FLI ≥90th percentile were positively associated with stroke risk among women. An AST/ALT ratio >2, often linked to alcoholic liver disease, was not associated with greater self-reported alcohol use in this cohort, and its relationship with stroke risk differed by race, but not sex, with a strong positive association with stroke in whites but not blacks.
Inverse associations of NAFLD and other liver biomarkers with stroke risk were unexpected, as NAFLD is closely associated with many CVD risk factors including obesity, hypertension, dyslipidemia, and insulin resistance, and it is linked to atherosclerosis progression [4]. Of note, one study addressing risk of pooled cardiovascular endpoints including stroke suggested an inverse association of NAFLD assessed using FLI in persons older than age 50, and a positive association in younger people [33], which is consistent with our findings from our predominantly older cohort. In that study, stroke was not separately analyzed. It is also possible that FLI didn’t adequately detect NAFLD in our study compared to imaging based classification used in other studies [19]. However, the FLI has been linked as might be predicted to risk of incident hypertension [34] and diabetes [35] by others. Despite inverse associations of NAFLD with stroke risk, we also observed positive associations of NAFLD with cardiovascular risk factors using the FLI classification we employed.
The current study is the largest by far that we are aware of to address NAFLD and ischemic stroke risk. While many studies reported positive associations NAFLD and pooled cardiovascular events [9, 17, 19], only three prior studies investigated ischemic stroke separately, with only 100 stroke events considered in totality [15, 17, 18]. These small studies reported mixed results, they did not all adjust for confounders, yet a meta-analysis of these limited studies reported a 2-fold increased risk [19]. Though ischemic stroke and coronary heart disease have many risk factors in common, the fact that they are distinct entities with differing risk profiles [36] may underlie our findings.
Measures of AST, ALT, and GGT have differing specificity for the presence versus absence of liver disease. The aminotransferases AST and ALT are found in the liver, serum, and other tissues, but are primarily indicators of liver damage, with ALT considered to be a better marker of liver injury and steatosis [37]. GGT is less specific to the liver and has been used as a marker of biliary disease and alcohol intake [38]. While increased ALT is often observed with fatty liver and predicts the development of diabetes and the metabolic syndrome [39], its relationship with stroke remains unclear. Similar to our findings, the British Women’s Heart and Health Study saw no association between ALT and stroke in women [13]. In a prospective study of Korean men, ALT was a risk factor for intracerebral hemorrhage, but not ischemic stroke [40]. A German study found no relationship between ALT and overall stroke in middle-aged men and women, but noted an inverse association with ischemic stroke [41], as we observed for men. Unlike our study, they saw no significant interaction between ALT and sex for stroke risk. Interestingly, a meta-analysis found that low ALT predicted cardiovascular mortality, particularly in older people [42]. The researchers speculated that low ALT may be an indicator of poor nutrition, reduced liver cell turnover, or low skeletal muscle mass.
In contrast to ALT, the data linking GGT and AST with stroke risk is more consistent, though in the case of AST, very little research has been published. While AST may be elevated during acute stroke [43], we are aware of only one prospective study on AST and stroke risk, which showed an association between higher AST and intracerebral hemorrhage in Korean men, but no association with other stroke types [40]. Our finding of no association with ischemic stroke is consistent with this. Several studies reported direct associations of GGT with stroke risk [41, 44–47]. Of note, a study in Japan found that GGT was associated with higher risk of ischemic stroke in women but not in men [48], similar to our results.
One purpose of our study was to investigate racial differences in associations between liver biomarkers and ischemic stroke. In the United States, blacks have a lower prevalence of NAFLD [49], but an increased risk of stroke, especially at younger ages [31]. Although black participants had higher FLI scores and GGT than white participants, we did not see significant differences by race in the relationships of liver markers with stroke risk, with the exception of the AST/ALT ratio >2. An elevated AST/ALT ratio can be an indicator of alcoholic hepatitis and of fibrosis and cirrhosis in chronic liver disease [32], and we found an association with stroke risk in whites only.
The strengths of this study include the large, well-characterized, prospective cohort of geographically dispersed blacks and whites. We relied on physician-adjudicated stroke outcomes to minimize misclassification. The major limitation is the use of a surrogate marker for NAFLD. The gold standard for clinical diagnosis of NAFLD is liver biopsy, which is not appropriate for epidemiological studies. While the FLI compares well with ultrasound determination of fatty liver, it is less accurate than liver biopsy or magnetic resonance imaging for identification and grading of liver steatosis [50]. In support of its use, we saw strong correlations between FLI and components of the metabolic syndrome, and a higher prevalence of NAFLD in men compared to women, similar to findings using imaging-based NAFLD assessment [29, 51]. Also, the association of NAFLD by FLI with stroke showed a similar pattern to that of ALT, which is also used as a surrogate marker for NAFLD. As the FLI has not been validated in a black population, and we observed unexpectedly higher, not lower prevalence of NAFLD in blacks, it is possible that the FLI misclassified NAFLD in blacks. This misclassification would bias results to the null hypothesis yet we observed the same association of NAFLD with stroke risk in blacks and whites, supporting the validity of the FLI in blacks. We did not have information on viral infections to determine any impact on the findings. We were also unable to investigate the role of alcohol use in more depth, due to low self-reported alcohol consumption in this cohort.
Conclusions
High levels of several of the liver disease biomarkers, especially those related to NAFLD, were associated with lower stroke risk in men, but with higher risk or no association in women. Particularly in the case of GGT and ALT, men in the lowest quintile were at increased stroke risk. Low levels of these biomarkers are not considered to be indicators of any disease process, and it is unclear what this relationship represents. While liver disease is gaining appreciation as a risk factor for atherosclerotic CVD, there is currently little known about differences in the relationship between liver disease and CVD by sex [52] or of the association of liver biomarkers with stroke. Further work is needed to confirm our results and identify the mechanisms and implications of these findings.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Data Availability
The data underlying the findings include potentially identifying participant information, and cannot be made publicly available due to ethical/legal restrictions. However, data including statistical code from this manuscript are available to researchers who meet the criteria for access to confidential data. Data can be obtained upon request through the University of Alabama at Birmingham at regardsadmin@uab.edu.
Funding Statement
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional support was provided by the training grant T32HL-007894 from the National Heart, Lung, and Blood Institute, National Institutes of Health.
References
- 1.Dixon JL, Ginsberg HN. Hepatic synthesis of lipoproteins and apolipoproteins. Semin Liver Dis. 1992;12:364–372. doi: 10.1055/s-2008-1040406 [DOI] [PubMed] [Google Scholar]
- 2.Lisman T, Porte RJ. Pathogenesis, prevention and management of bleeding and thrombosis in patients with liver disease. Res Pract Thromb Haemost. 2017;1 doi: 10.1002/rth2.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–66. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- 4.Gaudio E, Nobili V, Franchitto A, Onori P, Carpino G. Nonalcoholic fatty liver disease and atherosclerosis. Intern Emerg Med. 2012;7 Suppl 3:S297–305. [DOI] [PubMed] [Google Scholar]
- 5.Adinolfi LE, Zampino R, Restivo L, Lonardo A, Guerrera B, Marrone A, et al. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol. 2014;20:3410–3417. doi: 10.3748/wjg.v20.i13.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;126(2):137–415. [DOI] [PubMed] [Google Scholar]
- 7.Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005;16:421–427. [DOI] [PubMed] [Google Scholar]
- 8.Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44:1236–1243. doi: 10.1080/00365520903171284 [DOI] [PubMed] [Google Scholar]
- 9.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pisto P, Santaniemi M, Bloigu R, Ukkola O, Kesaniemi YA. Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open. 2014;4:e004973 doi: 10.1136/bmjopen-2014-004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol. 1995;142:699–708. [DOI] [PubMed] [Google Scholar]
- 12.Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191:391–396 doi: 10.1016/j.atherosclerosis.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 13.Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women’s Heart and Health Study and meta-analysis. Arterioscler Thromb Vasc Biol. 2007;27:2729–2735. doi: 10.1161/ATVBAHA.107.152298 [DOI] [PubMed] [Google Scholar]
- 14.Monami M, Bardini G, Lamanna C, Pala L, Cresci B, Francesconi P, et al. Liver enzymes and risk of diabetes and cardiovascular disease: results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism. 2008;57:387–392. doi: 10.1016/j.metabol.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 15.Moshayedi H, Ahrabi R, Mardani A, Sadigetegad S, Farhudi M. Association between non-alcoholic fatty liver disease and ischemic stroke. Iran J Neurol. 2014;13:144–148. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XW, Li M, Hou WS, Li K, Zhou JR, Tang ZY. Association between Gamma-Glutamyltransferase Level and Risk of Stroke: A Systematic Review and Meta-analysis of Prospective Studies. J Stroke Cerebrovasc Dis. 2015;24:2816–2823. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 17.El Azeem HA, Khalek el SA, El-Akabawy H, Naeim H, Khalik HA, Alfifi AA. Association between nonalcoholic fatty liver disease and the incidence of cardiovascular and renal events. J Saudi Heart Assoc. 2013;25:239–246. doi: 10.1016/j.jsha.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fracanzani AL, Tiraboschi S, Pisano G, Consonni D, Baragetti A, Bertelli C, et al. Progression of carotid vascular damage and cardiovascular events in non-alcoholic fatty liver disease patients compared to the general population during 10 years of follow-up. Atherosclerosis. 2016;246:208–213. doi: 10.1016/j.atherosclerosis.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 19.Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab Syndr. 2016. doi: 10.1016/j.dsx.2016.12.033 [DOI] [PubMed] [Google Scholar]
- 20.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33 doi: 10.1186/1471-230X-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The REasons for Geographic And Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 22.Zakai NA, Judd SE, Alexander K, McClure LA, Kissela BM, Howard G, et al. ABO blood type and stroke risk: the REasons for Geographic And Racial Differences in Stroke Study. J Thromb Haemost. 2014;12:564–570. doi: 10.1111/jth.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soliman EZ, Howard G, Prineas RJ, McClure LA, Howard VJ. Calculating Cornell voltage from nonstandard chest electrode recording site in the Reasons for Geographic And Racial Differences in Stroke study. J Electrocardiol. 2010;43:209–14. doi: 10.1016/j.jelectrocard.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS, Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47:243–246. doi: 10.1016/j.clinbiochem.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalhana S, Leitao J, Alves AC, Bourbon M, Cortez-Pinto H. How good is controlled attenuation parameter and fatty liver index for assessing liver steatosis in general population: correlation with ultrasound. Liver Int. 2014;34:e111–117. doi: 10.1111/liv.12305 [DOI] [PubMed] [Google Scholar]
- 26.Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11:1201–1204. doi: 10.1016/j.cgh.2012.12.031 [DOI] [PubMed] [Google Scholar]
- 27.Zelber-Sagi S, Webb M, Assy N, Blendis L, Yeshua H, Leshno M, et al. Comparison of fatty liver index with noninvasive methods for steatosis detection and quantification. World J Gastroenterol. 2013;19(1):57–64. doi: 10.3748/wjg.v19.i1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuthbertson DJ, Weickert MO, Lythgoe D, Sprung VS, Dobson R, Shoajee-Moradie F, et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol. 2014;171:561–569. doi: 10.1530/EJE-14-0112 [DOI] [PubMed] [Google Scholar]
- 29.Gastaldelli A, Kozakova M, Hojlund K, Flyvbjerg A, Favuzzi A, Mitrakou A, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49:1537–1544. doi: 10.1002/hep.22845 [DOI] [PubMed] [Google Scholar]
- 30.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–1172. [DOI] [PubMed] [Google Scholar]
- 31.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. doi: 10.1002/ana.22385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botros M, Sikaris KA. The De Ritis ratio: the test of time. Clin Biochem Rev. 2013;34:117–130. [PMC free article] [PubMed] [Google Scholar]
- 33.Kunutsor SK, Bakker SJL, Blokzijl H, Dullaart RPF. Associations of the fatty liver and hepatic steatosis indices with risk of cardiovascular disease: Interrelationship with age. Clin Chim Acta. 2017;466:54–60. doi: 10.1016/j.cca.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 34.Bonnet F, Gastaldelli A, Pihan-Le Bars F, Natali A, Roussel R, Petrie J, et al. Gamma-glutamyltransferase, fatty liver index and hepatic insulin resistance are associated with incident hypertension in two longitudinal studies. Journal of hypertension. 2017;35:493–500. doi: 10.1097/HJH.0000000000001204 [DOI] [PubMed] [Google Scholar]
- 35.Yadav D, Choi E, Ahn SV, Koh SB, Sung KC, Kim JY, et al. Fatty liver index as a simple predictor of incident diabetes from the KoGES-ARIRANG study. Medicine (Baltimore). 2016;95:e4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons LA, Simons J, Friedlander Y, McCallum J. A comparison of risk factors for coronary heart disease and ischaemic stroke: the Dubbo Study of Australian Elderly. Heart Lung Circ. 2009;18:330–333. doi: 10.1016/j.hlc.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 37.Kunutsor SK, Apekey TA, Walley J. Liver aminotransferases and risk of incident type 2 diabetes: a systematic review and meta-analysis. Am J Epidemiol. 2013;178:159–171. doi: 10.1093/aje/kws469 [DOI] [PubMed] [Google Scholar]
- 38.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227 [DOI] [PubMed] [Google Scholar]
- 39.Goessling W, Massaro JM, Vasan RS, D’Agostino RB Sr, Ellison RC, Fox CS. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology. 2008;135:1935–1944. doi: 10.1053/j.gastro.2008.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HC, Kang DR, Nam CM, Hur NW, Shim JS, Jee SH, et al. Elevated serum aminotransferase level as a predictor of intracerebral hemorrhage: Korea Medical Insurance Corporation Study. Stroke. 2005;36:1642–1647. doi: 10.1161/01.STR.0000173404.37692.9b [DOI] [PubMed] [Google Scholar]
- 41.Weikert C, Drogan D, di Giuseppe R, Fritsche A, Buijsse B, Nothlings U, et al. Liver enzymes and stroke risk in middle-aged German adults. Atherosclerosis. 2013;228:508–514. doi: 10.1016/j.atherosclerosis.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Ning H, Que S, Wang L, Qin X, Peng T. Complex association between alanine aminotransferase activity and mortality in general population: a systematic review and meta-analysis of prospective studies. PLoS One. 2014;9:e91410 doi: 10.1371/journal.pone.0091410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parakh N, Gupta HL, Jain A. Evaluation of enzymes in serum and cerebrospinal fluid in cases of stroke. Neurol India. 2002;50(4):518–519. [PubMed] [Google Scholar]
- 44.Wannamethee SG, Lennon L, Shaper AG. The value of gamma-glutamyltransferase in cardiovascular risk prediction in men without diagnosed cardiovascular disease or diabetes. Atherosclerosis. 2008;201:168–175. doi: 10.1016/j.atherosclerosis.2008.01.019 [DOI] [PubMed] [Google Scholar]
- 45.Korantzopoulos P, Tzimas P, Kalantzi K, Kostapanos M, Vemmos K, Goudevenos J, et al. Association between serum gamma-glutamyltransferase and acute ischemic nonembolic stroke in elderly subjects. Arch Med Res. 2009;40:582–589. doi: 10.1016/j.arcmed.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 46.Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H, et al. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112:2130–2137. doi: 10.1161/CIRCULATIONAHA.105.552547 [DOI] [PubMed] [Google Scholar]
- 47.Targher G. Elevated serum gamma-glutamyltransferase activity is associated with increased risk of mortality, incident type 2 diabetes, cardiovascular events, chronic kidney disease and cancer—a narrative review. Clin Chem Lab Med. 2010;48:147–157. doi: 10.1515/CCLM.2010.031 [DOI] [PubMed] [Google Scholar]
- 48.Shimizu Y, Imano H, Ohira T, Kitamura A, Kiyama M, Okada T, et al. Gamma-Glutamyltranspeptidase and incident stroke among Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Stroke. 2010;41:385–388. doi: 10.1161/STROKEAHA.109.569061 [DOI] [PubMed] [Google Scholar]
- 49.Schneider AL, Lazo M, Selvin E, Clark JM. Racial differences in nonalcoholic fatty liver disease in the U.S. population. Obesity (Silver Spring). 2014;22:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58:1007–1019. doi: 10.1016/j.jhep.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 51.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978 [DOI] [PubMed] [Google Scholar]
- 52.Baggio G, Corsini A, Floreani A, Giannini S, Zagonel V. Gender medicine: a task for the third millennium. Clin Chem Lab Med. 2013;51(4):713–727. doi: 10.1515/cclm-2012-0849 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The data underlying the findings include potentially identifying participant information, and cannot be made publicly available due to ethical/legal restrictions. However, data including statistical code from this manuscript are available to researchers who meet the criteria for access to confidential data. Data can be obtained upon request through the University of Alabama at Birmingham at regardsadmin@uab.edu.