Abstract
STUDY QUESTION
How does a maternal diabetic hyperadiponectineamia affect signal transduction and lipid metabolism in rabbit preimplantation blastocysts?
SUMMARY ANSWER
In a diabetic pregnancy increased levels of adiponectin led to a switch in embryonic metabolism towards a fatty acid-dependent energy metabolism, mainly affecting genes that are responsible for fatty acid uptake and turnover.
WHAT IS KNOWN ALREADY
Although studies in cell culture experiments have shown that adiponectin is able to regulate lipid metabolism via 5′-AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor α (PPARα), data on the effects of adiponectin on embryonic lipid metabolism are not available. In a diabetic pregnancy in rabbits, maternal adiponectin levels are elevated fourfold and are accompanied by an increase in intracellular lipid droplets in blastocysts, implying consequences for the embryonic hormonal and metabolic environment.
STUDY DESIGN, SIZE, DURATION
Rabbit blastocysts were cultured in vitro with adiponectin (1 μg/ml) and with the specific AMPK-inhibitor Compound C for 15 min, 1 h and 4 h (N ≥ 3 independent experiments: for RNA analysis, n ≥ 4 blastocysts per treatment group; for protein analysis three blastocysts pooled per sample and three samples used per experiment). Adiponectin signalling was verified in blastocysts grown in vivo from diabetic rabbits with a hyperadiponectinaemia (N ≥ 3 independent experiments, n ≥ 4 samples per treatment group, eight blastocysts pooled per sample).
PARTICIPANTS/MATERIALS, SETTING, METHODS
In these blastocysts, expression of molecules involved in adiponectin signalling [adaptor protein 1 (APPL1), AMPK, acetyl-CoA carboxylase (ACC), p38 mitogen-activated protein kinases (p38 MAPK)], lipid metabolism [PPARα, cluster of differentiation 36 (CD36), fatty acid transport protein 4 (FATP4), fatty acid binding protein (FABP4), carnitine palmityl transferase 1 (CPT1), hormone-senstive lipase (HSL), lipoprotein lipase (LPL)] and members of the insulin/insulin-like growth factor (IGF)-system [IGF1, IGF2, insulin receptor (InsR), IGF1 receptor (IGF1R)] were analyzed by quantitative RT-PCR and western blot. Analyses were performed in both models, i.e. adiponectin stimulated blastocysts (in vitro) and in blastocysts grown in vivo under increased adiponectin levels caused by a maternal diabetes mellitus.
MAIN RESULTS AND THE ROLE OF CHANCE
In both in vitro and in vivo models adiponectin increased AMPK and ACC phosphorylation, followed by an activation of the transcription factor PPARα, and CPT1, the key enzyme of β-oxidation (all P < 0.05 versus control). Moreover, mRNA levels of the fatty acid transporters CD36, FATP4 and FABP4, and HSL were upregulated by adiponectin/AMPK signalling (all P < 0.05 versus control). Under diabetic developmental conditions the amount of p38 MAPK was upregulated (P < 0.01 versus non-diabetic), which was not observed in blastocysts cultured in vitro with adiponectin, indicating that the elevated p38 MAPK was not related to adiponectin. However, a second effect of adiponectin has to be noted: its intensification of insulin sensitivity, by regulating IGF availability and InsR/IGF1R expression.
LARGE SCALE DATA
Not applicable.
LIMITATIONS REASONS FOR CAUTION
There are two main limitations for our study. First, human and rabbit embryogenesis can only be compared during blastocyst development. Therefore, the inferences from our findings are limited to the embryonic stages investigated here. Second, the increased adiponectin levels and lack of maternal insulin is only typical for a diabetes mellitus type one model.
WIDER IMPLICATIONS OF THE FINDINGS
This is the first mechanistic study demonstrating a direct influence of adiponectin on lipid metabolism in preimplantation embryos. The numbers of young women with a diabetes mellitus type one are increasing steadily. We have shown that preimplantation embryos are able to adapt to changes in the uterine milieu, which is mediated by the adiponectin/AMPK signalling. A tightly hormonal control during pregnancy is essential for survival and proper development. In this control process, adiponectin plays a more important role than known so far.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the German Research Council (DFG RTG ProMoAge 2155), the EU (FP7 Epihealth No. 278418, FP7-EpiHealthNet N°317146), COST Action EpiConcept FA 1201 and SALAAM BM 1308. The authors have no conflict(s) of interest to disclose.
Keywords: adiponectin, 5′-AMP-activated protein kinase, peroxisome proliferator-activated receptor α, carnitine palmityl transferase 1, fatty acid uptake, lipid metabolism, preimplantation embryo
Introduction
Although adiponectin was once thought to be exclusively secreted from adipose tissue, it is now known that adiponectin and its receptors, adiponectin receptor 1 (AdipoR1) and 2 (AdipoR2), are expressed in multiple tissues, such as liver, skeletal muscle, placenta and in mammalian preimplantation embryos (Delaigle et al., 2004; Caminos et al., 2005; Jonsson et al., 2005; reviewed by Cikoš, 2012). This peptide hormone is involved in the regulation of glucose metabolism and fatty acid break down in adult organisms. It has been shown that adiponectin lowers intracellular lipid content in two ways. One way is by regulating circulating levels of insulin/insulin-like growth factor (IGF) and facilitating insulin/IGF sensitivity and action, thereby regulating fatty acid oxidation indirectly via the insulin/IGF-system (Combs et al., 2004; Gao et al., 2013).
The other way is direct stimulation of fatty acid oxidation and a reduction of fatty acid synthesis (for review see, Combs and Marliss 2014). Adiponectin regulates lipid metabolism primarily by phosphorylation and thus activation of 5′-AMP-activated protein kinase (AMPK) (Yamauchi et al., 2002). AMPK increases fatty acid oxidation in skeletal muscle by regulating acetyl-CoA carboxylase (ACC) through phosphorylation and thereby stimulating carnitine palmityl transferase 1 (CPT1), the key enzyme of β-oxidation (Carlson and Kim, 1973). Adiponectin influences peroxisome proliferator-activated receptor α (PPARα), key regulator of fatty acid metabolism controlling CPT1 and hormone-senstive lipase (HSL) expression (Staels et al., 1992; Yoon et al., 2006; Rakhshandehroo et al., 2010).
The adaptor protein (APPL1) is the ‘missing link’ in the adiponectin signalling cascade, transmitting signals from both adiponectin receptors, AdipoR1 and AdipoR2, to downstream targets (Mao et al., 2006). APPL1 is required for adiponectin-induced activation of AMPK and p38 mitogen-activated protein kinases (p38 MAPK) activation, which acts as an essential mediator in regulation of glucose uptake and fatty acid oxidation (Mao et al., 2006).
In adults, adiponectin serum concentration is inversely associated with adipocyte mass. Maternal obesity elevates foetal adiponectin levels above the normal level and leads to increased foetal adipose tissue mass (Qiao et al., 2012). Diseases associated with abnormal adiponectin levels are pregestational diabetes, polycystic ovary syndrome and endometriosis, all of which are associated with subfertility (Imagawa et al., 2002; Carmina et al., 2008; Cheng et al., 2016).
Whereas the role of adiponectin in modulation of lipid metabolism and insulin responsiveness in adult tissues is well established, its role in regulating lipid utilization and insulin sensitivity in preimplantation embryos is largely unknown. Adiponectin and its receptors are expressed in rabbit and mouse preimplantation embryos and influences mammalian embryo development and glucose metabolism (reviewed by Cikoš, 2012).
In previous studies, we have shown that the rabbit blastocyst is not able to produce insulin (Ramin et al., 2010) and that it compensates for the maternal insulin deficiency by upregulation of IGF1 and IGF2 synthesis (Thieme et al., 2012). Furthermore, we have demonstrated that an experimentally induced maternal diabetes mellitus type 1 in rabbits correlates with an increased level of adiponectin in maternal serum and uterine tissue. This increase was accompanied by a strong increase in lipid vesicles in blastocysts (Schindler et al., 2013, 2014). Mammalian preimplantation embryos, i.e. human, bovine, mouse, pig and rabbit, contain lipid droplets in various amounts (reviewed by Sturmey et al., 2009). The stored lipids serve as an energy source and influence cell–cell interactions, cell proliferation and intracellular transport mechanisms (for review see Stubbs and Smith, 1984). However, excess lipid accumulation above the normal level is linked with impaired embryo quality due to cellular dysfunction and/or cell death caused by increased lipid peroxidation and mitochondrial dysfunction (Abe et al., 2002; Jeong et al., 2009). In the current study, we have focused on the potential role of adiponectin in embryonic lipid metabolism, to clarify whether maternal diabetes during early pregnancy affects the embryonic lipid metabolism via adiponectin.
Materials and Methods
Embryo recovery
Embryos were collected from sexually mature rabbits (outbred ZIKA-hybrid New Zealand White), stimulated with 110 IU pregnant mare's serum gonadotropin s.c. (Intervet, Germany) 3 days before mating. After mating, 75 IU hCG had been injected i.v. (Intervet, Germany) to ensure ovulation. Mating and embryo recovery were performed as described previously (Schindler et al., 2013). For in vivo and in vitro analysis, gastrulation stages 1 and 2 were used.
For RNA isolation, blastocysts were washed three times in phosphate-buffered saline (PBS) containing 0.05% polyvinyl alcohol (PVA). The extracellular coverings were removed mechanically and samples were stored in PBS at −80°C until RNA isolation.
For western blot analysis, blastocysts were washed 3 times in PBS and the extracellular coverings were removed mechanically in 0.05% PVA/PBS containing protease and phosphatase inhibitors (Roche, Germany). Samples were stored in radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors at −80°C until further processing.
Embryo in vitro culture
To study the effects of adiponectin on the expression of adiponectin signalling molecules [APPL1, AMPK, ACC, p38 MAPK, lipogenic molecules (PPARα, CPT1, cluster of differentiation 36 (CD36), fatty acid transport protein 4 (FATP4), fatty acid binding protein 4 (FABP4), HSL and lipoprotein lipase (LPL)) and the insulin/IGF-system (IGF1, IGF2, IGF receptor (IGF1R) and insulin receptor (InsR))], day 6 blastocysts were cultured in groups of three to five at 37°C in a water saturated atmosphere of 5% O2, 5% CO2, 90% N2 for one or 4 h with 1 μg/ml adiponectin (Biovendor, Germany) for RNA analysis. Control embryos were cultured without adiponectin. To prove AMPK-dependency, blastocysts were pre-cultured with Compound C, a specific AMPK inhibitor (10 μM, Sigma Germany) dissolved in dimethylsulfoxid (DMSO) for 30 min and subsequently cultured with 1 μg/ml adiponectin for 1 h. A DMSO group was included as solvent control for Compound C. For protein analysis, blastocysts were cultured with 1 μg/ml adiponectin for 15 min or 4 h in groups of six to nine. Incubation periods of adiponectin for protein and RNA analysis are based on our pervious study (Fischer et al., 2010) and the literature (Yoon et al., 2006). After culture, blastocysts were processed as describe above and stored at −80°C.
Alloxan treatment
Experimental insulin-dependent diabetes (diabetic) was induced in mature 18–20-week-old female rabbits by alloxan (Sigma-Aldrich, Germany) treatment 10–12 days before mating, as described (Ramin et al., 2010). Surgery was performed under Ketanest (Pfizer, Germany) and Dorbene (Zoetis, Germany) anaesthesia. Rabbits were held in a diabetic condition with permanent blood glucose concentrations of >14 mmol/l by regular insulin supplementation three times per day (Huminsulin basal (NPH), Lilly Deutschland GmbH, Germany), starting on the second day after alloxan treatment. The blood glucose level was monitored as described previously (Schindler et al., 2014) and routinely measured during the whole experiment and at the time of death (Supplementary Table S1). Insulin and adiponectin levels have been measured and published previously and are summarized in Supplementary Table S1. All animal experiments were performed in accordance with the principles of laboratory animal care and the experimental protocol had been approved by the local ethics committee (Landesverwaltungsamt Dessau; reference number: 42502-2-812).
RNA isolation and cDNA synthesis
Messenger RNA of single blastocysts was extracted with Dynabeads® Oligo (dT)25 (Invitrogen, Germany) and subsequently used for cDNA synthesis. All procedures were carried out according to the manufacturer's instructions, but with modifications described previously (Schindler et al., 2014). The final volume of cDNA reaction was adjusted with water to 100 μl.
Real time PCR analyses
Real time quantitative PCR analyses (RT-qPCR) were performed in duplicate using the Applied Biosystems StepOnePlus™ System (Applied Biosystems, Germany) with a no-template control for each primer set with SYBR Green detection. The nucleotide sequences of the primers used are listed in Supplementary Table SII. The PCR products were sequenced and analyzed as described (Schindler et al., 2013). GAPDH, which is unaffected by the treatment (Thieme et al., 2012), was simultaneously quantified as endogenous control and target gene expression was normalized to that of GAPDH in each sample. In each RT-qPCR, a calibration curve was included from serial dilutions (107−103 copies) of primer-specific DNA plasmid standards. Results were calculated as amounts of target RNA in molecules per molecule GAPDH RNA, and expressed as relative amounts as a percentage or fold change of control samples.
Protein preparation and immunoblotting
Protein preparation, quantification and western blot were performed with 8–10 blastocysts as described (Schindler et al., 2013). For detection of CPT1, activating transcription factor 2 (ATF2), p38 MAPK and β-Actin, membranes were blocked in Tris-buffered saline containing 0.1% Tween with 3% (wt/vol) nonfat dry milk at room temperature for at least 1 h. For phosphorylated ACC (pACC), ACC2, phosphorylated AMPK (pAMPK), AMPK and PPARα detection, membranes were blocked in Tris-buffered saline containing 0.1% Tween with 3% (wt/vol) bovine serum albumin for at least 1 h. The primary antibody was incubated at 4°C overnight. The antibodies used are summarized in Supplementary Table SIII. Protein amount was calculated as the ratio of band intensities (pACC, ACC2, p38 MAPK, AMPK, ATF2 and CPT1 protein versus β-actin protein, and pAMPK protein versus AMPK protein (pAMPK/AMPK), respectively) in the same blot to correct for differences in protein loading.
Statistics
Data are expressed as mean ± SEM. The levels of significance between groups were calculated using Student's t-test after proving normal distribution. Multiple comparisons were made by factorial variance analysis (ANOVA) adjusted according to Bonferroni (SigmaPlot v. 12.0, Germany). Statistical significance is indicated as follows *P < 0.05, **P < 0.01 and ***P < 0.001 or by different letters in Figs 2 and 4A and B (P < 0.05). All experiments were repeated at least three times.
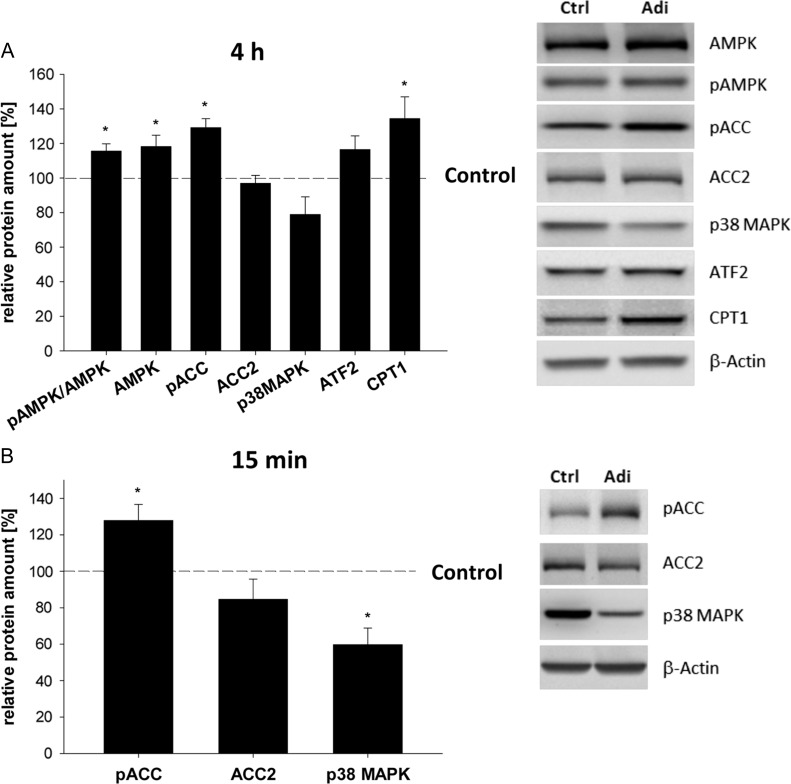
Figure 1.
Relative amounts of phosphorylated acetyl-CoA carboxylase, p38 mitogen-activated protein kinase and acetyl-CoA carboxylase 2 protein in blastocysts cultured in vitro with 1 μg/ml adiponectin. (A) In vitro culture of blastocysts for 4 h with 1 μg/ml adiponectin led to significantly higher protein level of AMPK and carnitine palmityl transferase 1 (CPT1) compared to treatment control (set 100%) and are indicated with the dashed line (*P < 0.05, Student's t-test). Furthermore, phosphorylation of AMPK (ratio pAMPK/AMPK) and ACC (pACC) were increased. No significant changes were observed for acetyl-CoA carboxylase 2 (ACC2), p38 mitogen-activated protein kinase (p38 MAPK) and activating transcription factor 2 (ATF2) compared to the treatment control (set 100%, indicated with ---). Culture of blastocysts was performed in groups of at least three blastocysts. Per sample three blastocysts were pooled. The number of samples used per treatment group in each independent experiment was at least 3 (n ≥ 3,) with N = 3 (number of individual and independent experimental replicates). Protein levels were analyzed by western blot and results are shown as mean ± SEM. A representative western blot is shown. (B) pACC, ACC2 and p38 MAPK protein expression was quantified in blastocysts cultured for 15 min with adiponectin by western blot and calculated in relation to β-actin (mean ± SEM, N = 3 (number of independent experiments), n ≥ 3 (number of samples used per treatment group in each independent experiment, per sample three blastocysts have been pooled)). Phosphorylation of ACC was increased, while no changes in amount of ACC2 protein was observed after 15 min incubation with adiponectin. Total p38 MAPK protein amount was significantly decreased by adiponectin (*P < 0.05 Student's t-test).
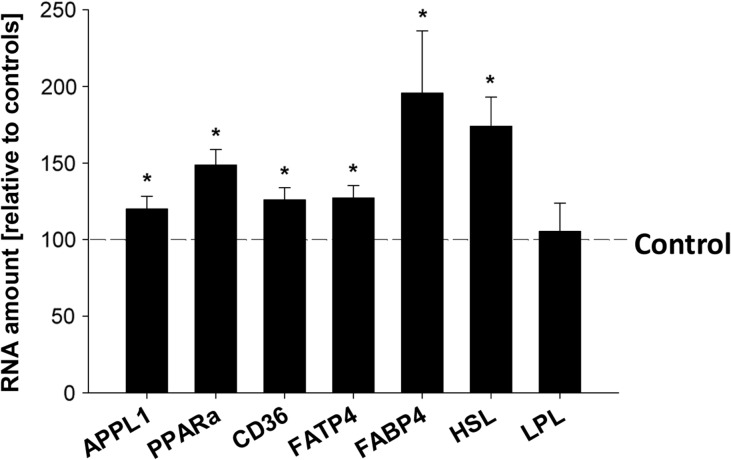
Figure 3.
Relative amounts of adiponectin target genes in blastocysts stimulated in vitro with adiponectin for 4 h. Transcription analyses of adaptor protein (APPL1), peroxisome proliferator-activated receptor alpha (PPARα), CD36, fatty acid transporter 4 (FATP4), fatty acid binding protein 4 (FABP4), hormone sensitive lipase (HSL) and lipoprotein lipase (LPL) were performed by RT-qPCR in blastocysts cultured in vitro with adiponectin (1 μg/ml) for 4 h. Culture was performed in groups of at least 4 and repeated in three independent replicates (N = 3 (number of individual and independent experiments); n ≥ 4 (number of samples used per treatment group in each independent experiment, per sample four blastocysts have been pooled)). Values are expressed as fold change relative to the treatment control (set 100% and indicated with the dashed line) (*P < 0.05, Student's t-test).
Results
AMPK signalling by adiponectin in rabbit preimplantation embryos in vitro
In rabbit blastocysts adiponectin increased phosphorylation and total protein amount of AMPK (Fig. 1A). Phosphorylation of ACC was elevated, whereas no significant changes were observed for ACC2 and p38 MAPK protein amounts after 4 h of adiponectin stimulation (Fig. 1A). A short stimulus (15 min) increased ACC phosphorylation and reduced p38 MAPK amount, while ACC2 amount was not changed (Fig. 1B).
Adiponectin-dependent expression of lipogenic genes in rabbit preimplantation embryos in vitro
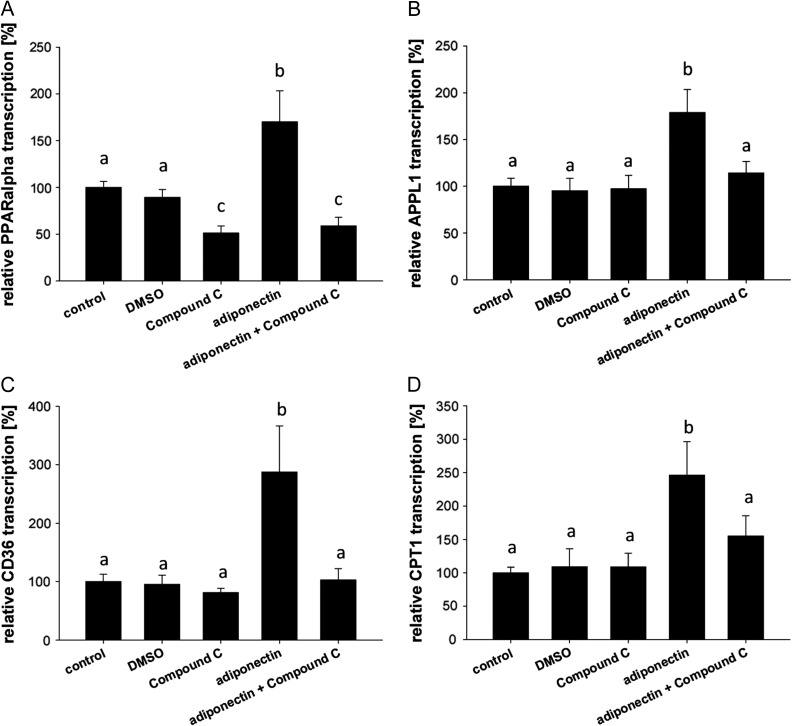
After 1 h of stimulation with adiponectin, the quantification of PPARα, APPL1, CPT1 and CD36 transcripts revealed an increased expression of all markers in cultured blastocysts (Fig. 2). A simultaneous incubation with Compound C, a specific AMPK inhibitor, blocked this effect completely. Surprisingly, Compound C blocked PPARα transcription compared to the solvent control DMSO indicating that, even without external stimuli in blastocysts, PPARα transcription is AMPK-dependent.
Figure 2.
Changes in mRNAs in blastocysts cultured in vitro with adiponectin and/or Compound C for 1 h. Quantitative RT-PCR (RT-qPCR) of peroxisome proliferator-activated receptor alpha (PPARα; A), adaptor protein (APPL1; B), cluster of differentiation 36 (CD36; C) and CPT1 (D) after in vitro culture (for 1 h) of blastocysts with 1 μg/ml adiponectin or without (control; set 100%) showed an increased expression pattern. Inhibition of AMPK by Compound C blocked the adiponectin mediated increased transcription. In vitro culture was performed in groups of at least four blastocysts in each treatment and repeated in three independent replicates (N = 3 (number of individual and independent experiments); n ≥ 4 (number of blastocysts used per treatment group in each independent experiment)). Results are shown as mean ± SEM and control was set 100%. Means with different letters are significantly different (P < 0.05, Multiple comparisons by factorial variance analysis (ANOVA)).
After 4 h stimulation, expression of APPL1, PPARα, CD36, FATP4, FABP4 and CPT1 remained increased (Figs 1A and 3). Transcript levels of HSL were increased, whereas the expression of LPL was not affected (Fig. 3), which closely reflects the regulation pattern observed in vivo (Table I).
Table I.
Adiponectin target gene expression in blastocysts from non-diabetic and diabetic rabbits.
| Gene | RNA amounts [molecules/per 100 molecules GAPDH] | P value | |
|---|---|---|---|
| Non-diabetic | Diabetic | ||
| APPL1 | 15.57 [1.22] | 20.76 [1.53] | 0.030 |
| PPARa | 1.00 [0.13] | 1.39 [0.16] | 0.084 |
| CD36 | 0.54 [0.07] | 0.54 [0.06] | 0.965 |
| HSL | 0.40 [0.09] | 1.03 [0.13] | 0.004 |
| LPL | 2.15 [0.34] | 2.27 [0.80] | 0.906 |
| ATF2 | 0.86 [0.13] | 5.58 [0.93] | 0.002 |
RNA target genes amounts were quantified by RT-PCR in 10 to 15 blastocysts in each group. Values are expressed per 100 molecules GAPDH [mean value ± SEM]. The levels of significance between groups were calculated using Student's t-test after confirming a normal distribution. APPL1, adaptor protein 1; PPARa, peroxisome proliferator-activated receptor alpha; CD36, cluster of differentiation 36; HSL, hormone sensitive lipase; LPL, lipoprotein lipase; ATF2, activating transcription factor 2.
Adiponectin-dependent expression of the insulin/IGF-system in rabbit preimplantation embryos in vitro
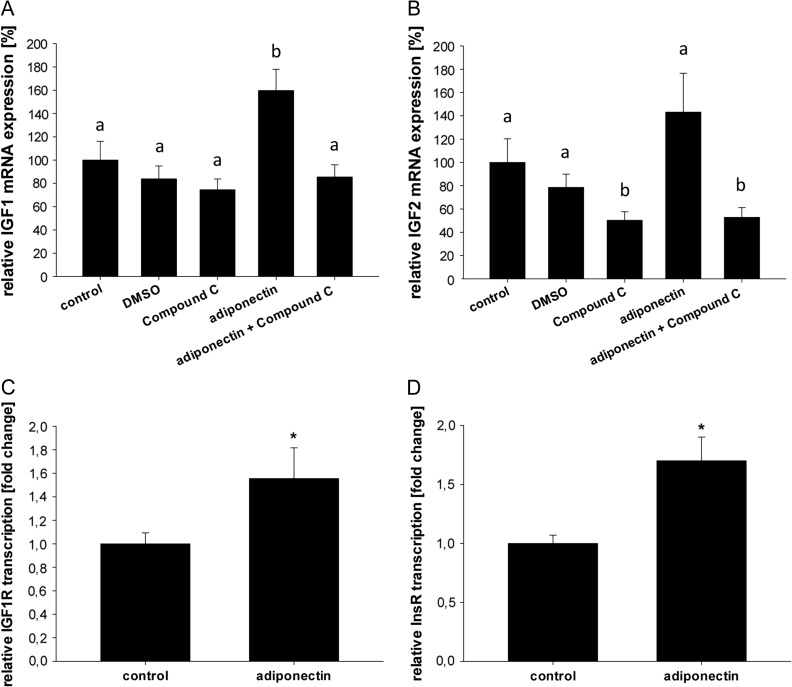
Adiponectin stimulation of rabbit blastocysts for 1 h led to an increased expression of IGF1 (Fig. 4A). This effect could be blocked by Compound C, indicating that adiponectin regulates IGF1 expression in an AMPK-dependent manner. IGF2 transcription was not significantly upregulated after adiponectin stimulation. However, AMPK inhibition by Compound C decreased IGF2 mRNA amount, indicating an AMPK-dependent transcription (Fig. 4B). Messenger RNA amounts of IGF1R and InsR (Fig. 4C, D) were elevated in blastocyst cultured with adiponectin. These data demonstrate a clear insulin/IGF/adiponectin interaction with an increased insulin and IGF sensitivity due to adiponectin.
Figure 4.
Transcription of insulin/insulin-like growth factor system in blastocysts cultured in vitro with adiponectin. (A) Analysis of insulin-like growth factor 1 (IGF1) mRNA amounts by RT-qPCR revealed an increased expression in blastocysts after in vitro culture with 1 μg/ml adiponectin or without [control, set 100%] after 1 h incubation. Inhibition of AMPK by Compound C blocked adiponectin mediated increased mRNA. (B) In vitro culture with 1 μg/ml adiponectin did not significantly influence insulin-like growth factor 2 (IGF2) mRNA level after 1 h incubation compared to treatment control [control, set 100%]. However, co-culture with Compound C reduces IGF2 mRNA compared to the solvent control, DMSO. In vitro culture was performed in groups of at least four blastocysts in each treatment and repeated in three independent replicates (N = 3 (number of individual and independent experiments); n ≥ 4 (number of blastocysts used per treatment group in each independent experiment)). Results are shown as mean ± SEM. Means with different letters are significantly different (P < 0.05, Multiple comparisons by factorial variance analysis (ANOVA)). In blastocysts cultured for 4 h with adiponectin mRNA levels of IGF1 receptor (IGF1R; C) and insulin receptor (InsR; D) were significantly increased compared to control, which was set 100% (*P < 0.05, Student's t-test). In vitro culture was performed in groups of at least four blastocysts in each treatment and repeated in three independent replicates (N = 3 (number of individual and independent experiments); n ≥ 4 (number of samples used per treatment group in each independent experiment)). Results are shown as mean ± SEM.
Adiponectin signalling in preimplantation embryos from diabetic rabbits
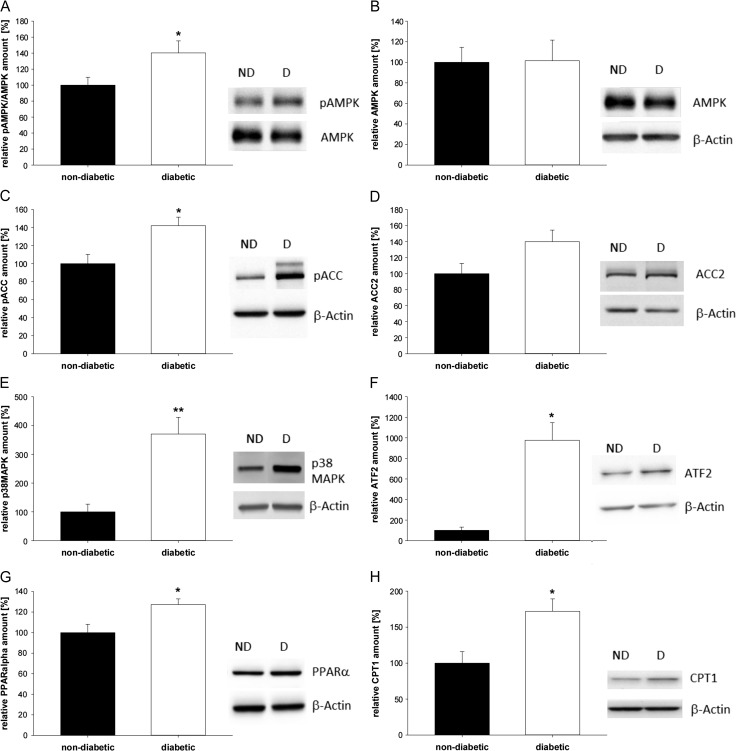
In blastocysts from diabetic rabbits, the phosphorylation of AMPK was significantly higher than in non-diabetic (ND) rabbits (Fig. 5A). The total AMPK protein amount was not affected (Fig. 5B). Phosphorylation of ACC was significantly increased in blastocysts from diabetic rabbits (Fig. 5C). No significant changes of the ACC2 protein amount were observed (Fig. 5D). Protein amount of p38 MAPK was upregulated under diabetic developmental conditions (Fig. 5E). Protein (Fig. 5F) and mRNA (Table I) of ATF2, essential for p38 MAPK-mediated suppression of lipid deposition, were significantly increased in blastocysts from diabetic rabbits.
Figure 5.
Western blot and densitometric quantification of proteins in blastocysts from diabetic rabbits. Phosphorylation and protein levels of phospho-AMPK (A), AMPK (B), phospho-ACC (C), ACC2 (D), p38 MAPK (E), ATF2 (F), PPARα (G) and CPT1 (H) were quantified in 6 day old blastocysts from diabetic [D] and non-diabetic rabbits [ND]. Rabbits were made diabetic before pregnancy. For all analyzed proteins a representative western blot is shown. The western blot is representative of more than three independent experiments with similar results (N ≥ 3 (number of individual and independent experiments), n ≥ 4 (number of samples used per treatment group in each independent experiment, per sample eight blastocysts have been pooled)). The relative amounts are shown in diagrams after normalization for the levels of β-actin. Values are expressed as mean ± SEM in % of non-diabetic controls (*P < 0.05, **P < 0.01, Student's t-test).
Expression of lipogenic signal molecules in the preimplantation embryo from diabetic rabbits
Transcription of PPARα was slightly elevated in blastocysts from diabetic rabbits (Table I). Western blot analysis showed a significantly higher protein amount of PPARα (Fig. 5G). The protein expression of CPT1 was increased in blastocysts from diabetic rabbits (Fig. 5H, Supplementary Fig. S1). Transcription of CD36 and LPL was not changed under diabetic developmental conditions in blastocysts, whereas the mRNA amount of HSL was increased (Table I).
Discussion
Adiponectin plays an important role in regulation of cellular lipid metabolism and systemic adipose deposits. Adiponectin levels were found to be closely related to the level of insulin. Whereas a maternal diabetes mellitus type 2 is associated with a decreased adiponectin level, type 1 diabetes led to an increase in adiponectin level (Imagawa et al., 2002; Stefan et al., 2003). In the diabetic rabbit model, adiponectin levels were increased in serum (3.7-fold) and the endometrium (2.2-fold) (Schindler et al., 2013). However, in the Fallopian and uterine secretions adiponectin amounts are still unknown because of the small sample volumes and resulting methodical limitations. Two main signalling pathways of adiponectin regulating lipid metabolism are known: the direct AMPK- and/or p38 MAPK-dependent activation of fatty acid uptake and β-oxidation, and the indirect way via the insulin/IGF-system (Yoon et al., 2006). In the current study, we found that both metabolic pathways are activated due to hyperadiponectinaemia (summarized in Fig. 6) with profound consequences for early development: a strong stimulation of lipid metabolism in a vulnerable stage of embryo development.
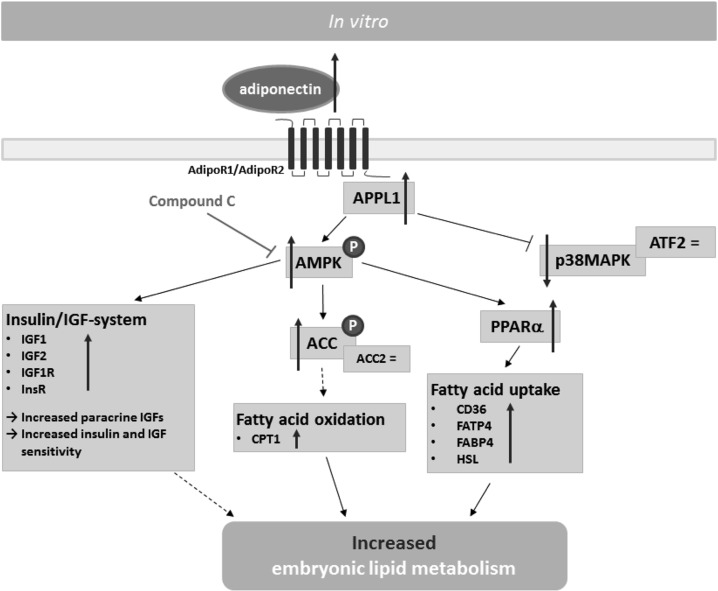
Figure 6.
Model for adiponectin signalling in rabbit blastocysts stimulated in vitro with adiponectin. After binding of adiponectin to its receptors, AMPK is activated via APPL1. AMPK regulates β-oxidation by ACC phosphorylation, leading to an increased protein amount of CPT1. Furthermore, adiponectin increases PPARα expression and thereby regulating fatty acid uptake. However, the amount of p38 MAPK, a downstream target of APPL1, was decreased in blastocysts cultured in vitro with adiponectin. Besides its ability to regulate embryonic lipid metabolism directly, adiponectin increases paracrine amount of IGFs and InsR and IGF1R transcription in rabbit blastocysts via AMPK, which may indirectly influence embryonic lipid metabolism ( Direct activation,
Direct activation,  Direct inhibition,
Direct inhibition,  Indirect regulation, = no change,
Indirect regulation, = no change,  increase).
increase).
Adiponectin signalling starts with ligand binding to AdipoRs. In blastocysts from diabetic rabbits only AdipoR1 is upregulated (Schindler et al., 2013). We have already shown that adiponectin stimulation (for 15 min) in vitro led to an increased activation of AMPK in rabbit blastocysts (Fischer et al., 2010). Here, we show that AMPK phosphorylation is also elevated in blastocysts from diabetic rabbits. From mouse knockout studies, it is known that AdipoR1 is more involved in the activation of AMPK by adiponectin than AdipoR2 (Yamauchi et al., 2007). The increased activation of AMPK correlates well with the upregulation of AdipoR1 expression in blastocysts from diabetic rabbits and is strong experimental evidence that blastocysts from diabetic mothers are more sensitive to adiponectin.
In contrast to mice studies (Yamauchi et al., 2007), we have shown an AMPK-dependent PPARα expression. PPARα regulates promotor activity of lipogenic genes in mice, such as CD36, a fatty acid transporter, sterol regulating binding protein 1 (SREBP1) and CPT1 (Awazawa et al., 2009). In blastocysts from diabetic rabbits SREBP1 activity is increased (Schindler et al., 2014). In the present study, CPT1 expression was adiponectin-dependently upregulated. The increased CPT1 amount in blastocysts from diabetic rabbits indicates that embryonic metabolism is shifted towards a metabolism gaining energy from β-oxidation of fatty acids. The importance of β-oxidation has been proven in bovine and mice studies. By using a specific inhibitor for CPT1, it has been shown in mice that β-oxidation is essential for oocyte maturation, zygote cleavage and blastocyst development (Ferguson and Leese, 2006; Dunning et al., 2010; Paczkowski et al., 2014). In mouse adipocytes adiponectin suppresses lipolysis via AdipoR1 by altering activation of HSL (Qiao et al., 2011). Both adiponectin stimulation in vitro and an increased adiponectin level in vivo led to an increased transcription of HSL and may contribute to the increased accumulation of intracellular lipids in rabbit blastocysts (Schindler et al., 2014).
The other signalling pathway by which adiponectin may alter directly embryonic lipid metabolism is via p38 MAPK. p38 MAPK regulates fatty acid and glucose metabolism in response to adiponectin (Yamauchi et al., 2003; Aouadi et al., 2006). To our surprise in vitro stimulation with adiponectin led to a decreased amount of p38 MAPK. p38 MAPK signalling is required to promote development to the blastocysts stage in mice by regulating glucose consumption, increasing apoptosis and disrupting tight junction integrity (Bell and Watson, 2013; Sozen et al., 2015). However, in blastocysts from diabetic rabbits phosphorylation of p38 MAPK was increased. For suppression of lipid deposition by p38 MAPK, the activation of ATF2 is necessary (Yan et al., 2013). In blastocysts from diabetic rabbits ATF2 transcription was increased, supporting our findings of an increased expression of p38 MAPK. No significant changes of ATF2 expression were observed after adiponectin supplementation in vitro, which is in accordance with the observed decreased amount of p38 MAPK. Therefore, we can conclude that under in vivo conditions a ligand other than adiponectin is responsible for activating the p38 MAPK/ATF2 pathway.
Activation of p38 MAPK has been observed in relation to inflammatory response, oxygen tension and oxygen radical metabolites (reviewed by Ono and Han, 2000). Since diabetic pregnancies are associated with inflammation and oxygen radical metabolites (reviewed by Zhao and Reece, 2013), it is tempting to speculate that both factors are responsible for the observed increased amount of p38 MAPK. Furthermore, excess lipid accumulation in non-adipose tissue leads to cell dysfunction caused by an increased lipid peroxidation and mitochondrial dysfunction. This observation is known as lipotoxicity (for review see Brookheart et al., 2009). The lipotoxity may contribute to the observed increased apoptosis rate, higher embryo loss and developmental delay in diabetic and/or obese animals (Jungheim et al., 2011; Wu et al., 2015; Desmet et al., 2016). Facts supporting that the ‘lipotoxicity hypothesis’ in embryos is also applicable for the diabetic model, is the increased apoptosis and higher expression of ATF4, an endoplasmic stress marker, in blastocysts from diabetic rabbits (Ramin et al., 2010; Schindler et al., 2014).
Adiponectin influences fatty metabolism also indirectly via the insulin/IGF-system. In rabbit preimplantation embryos insulin is not expressed, but its family members IGF1 and IGF2 and both receptors, InsR and IGF1R, are (Ramin et al., 2010). It was shown that blastocysts compensate for the maternal insulin deficiency by upregulation of IGFs synthesis (Thieme et al., 2012). Interestingly, not only IGF1 and IGF2, but also IGF1R and InsR are upregulated after incubation with adiponectin in non-diabetic blastocysts. These results imply an increased insulin/IGFs availability and sensitivity, due to an increased receptor expression in response to adiponectin, which is already known from adult tissues (Combs et al., 2004; Gao et al., 2013).
Taken together, we show that adiponectin regulates embryonic lipid metabolism by AMPK signalling (Fig. 6). Adiponectin directly influences fatty acid utilization, by regulating lipolysis, fatty acid transport and β-oxidation. In our model of an experimentally induced diabetes mellitus type 1, the preimplantation embryo switches its metabolism towards a fatty acid-dependent energy metabolism, which is mediated by the adiponectin/adipoR1/AMPK network. Contrary to cell models (Yoon et al., 2006; Xin et al., 2011), adiponectin does not directly increase the amount of p38 MAPK in embryos, indicating a divergent adiponectin pathway in adult tissues and embryonic cells. Furthermore, we propose and show another potential mechanism: the simultaneous stimulation of insulin/IGF-system by adiponectin. The current study shows that preimplantation embryos are able to adapt to changes in the surrounding milieu and that adiponectin is an important mediator in this adaptation process.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Acknowledgement
We thank Sabine Schrötter and Michaela Kirstein for excellent technical assistance.
Authors’ roles
M.S. performed experiments, analyzed the data and wrote the manuscript. A.N.S., M.P., K.J.G., T.S. and J.G. performed the experiments and edited the paper. BF and ANS conceived and designed the study and edited the paper.
Funding
This work was supported by the German Research Council (DFG RTG ProMoAge 2155), the EU (FP7 Epihealth No. 278418, FP7-EpiHealthNet N°317146), COST Action EpiConcept FA 1201 and SALAAM BM 1308.
Conflict of interest
None declared.
References
- Abe H, Yamashita S, Satoh T, Hoshi H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol Reprod Dev 2002;61:57–66. doi:10.1002/mrd.1131. [DOI] [PubMed] [Google Scholar]
- Aouadi M, Laurent K, Prot M, Le Marchand-Brustel Y, Binétruy B, Bost F. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes 2006;55:281–289. [DOI] [PubMed] [Google Scholar]
- Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun 2009;382:51–56. doi:10.1016/j.bbrc.2009.02.131. [DOI] [PubMed] [Google Scholar]
- Bell C, Watson AJ. p38 MAPK regulates cavitation and tight junction function in the mouse blastocyst. PLOS ONE 2013;8:e59528 doi:10.1371/journal.pone.0059528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookheart RT, Michel CI, Schaffer JE. As a matter of fat. Cell Metab 2009;10:9–12. doi:10.1016/j.cmet.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminos JE, Nogueiras R, Gallego R, Bravo S, Tovar S, García-Caballero T, Casanueva FF, Diéguez C. Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab 2005;90:4276–4286. doi:10.1210/jc.2004-0930. [DOI] [PubMed] [Google Scholar]
- Carlson CA, Kim KH. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem 1973;248:378–380. [PubMed] [Google Scholar]
- Carmina E, Chu MC, Moran Ca, Tortoriello D, Vardhana P, Tena G, Preciado R, Lobo R. Subcutaneous and omental fat expression of adiponectin and leptin in women with polycystic ovary syndrome. Fertil Steril 2008;89:642–648. doi:10.1016/j.fertnstert.2007.03.085. [DOI] [PubMed] [Google Scholar]
- Cheng L, Shi H, Jin Y, Li X, Pan J, Lai Y, Lin Y et al. Adiponectin deficiency leads to female subfertility and ovarian dysfunctions in mice. Endocrinology 2016:en.2015–2080. doi:10.1210/en.2015-2080. [DOI] [PubMed] [Google Scholar]
- Cikoš S. Adiponectin and its receptors in preimplantation embryo development. Vitam Horm 2012;90:211–238. doi:10.1016/B978-0-12-398313-8.00009-9. [DOI] [PubMed] [Google Scholar]
- Čikoš Š, Burkuš J, Bukovská A, Fabian D, Rehák P, Koppel J. Expression of adiponectin receptors and effects of adiponectin isoforms in mouse preimplantation embryos. Hum Reprod 2010;25:2247–2255. doi:10.1093/humrep/deq193. [DOI] [PubMed] [Google Scholar]
- Combs TP, Marliss EB. Adiponectin signaling in the liver. Rev Endocr Metab Disord 2014;15:137–147. doi:10.1007/s11154-013-9280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 2004;145:367–383. doi:10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology 2004;145:5589–5597. doi:10.1210/en.2004-0503. [DOI] [PubMed] [Google Scholar]
- Desmet KLJ, Van Hoeck V, Gagné D, Fournier E, Thakur A, O'Doherty AM, Walsh CP, Sirard MA, Bols PEJ, Leroy JLMR. Exposure of bovine oocytes and embryos to elevated non-esterified fatty acid concentrations: integration of epigenetic and transcriptomic signatures in resultant blastocysts. BMC Genomics 2016;17:1004 doi:10.1186/s12864-016-3366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biology of Reproduction 2010;83:909–918. doi:10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- Ferguson EM, Leese HJ. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol Reprod Dev 2006;73:1195–1201. doi:10.1002/mrd.20494. [DOI] [PubMed] [Google Scholar]
- Fischer S, Santos AN, Thieme R, Ramin N, Fischer B. Adiponectin stimulates glucose uptake in rabbit blastocysts. Biol Reprod 2010;83:859–865. doi:10.1095/biolreprod.110.084665. [DOI] [PubMed] [Google Scholar]
- Gao H, Fall T, van Dam RM, Flyvbjerg A, Zethelius B, Ingelsson E, Hägg S. Evidence of a causal relationship between adiponectin levels and insulin sensitivity. Diabetes 2013;62:1338–1344. doi:10.2337/db12-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson LC, Martin KL, Leese HJ. Effects of metabolic inhibitors on mouse preimplantation embryo development and the energy metabolism of isolated inner cell masses. Mol Reprod Dev 1996;43:323–330. doi:10.1002/(SICI)1098-2795(199603)43:3<323::AID-MRD6>3.0.CO;2-S.. [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in Type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000;20:1595–1599. [DOI] [PubMed] [Google Scholar]
- Imagawa A, Funahashi T, Nakamura T, Moriwaki M, Tanaka S, Nishizawa H, Sayama K et al. Elevated serum concentration of adipose-derived factor, adiponectin, in patients with Type 1 diabetes. Diabetes Care 2002;25:1665–1666. [DOI] [PubMed] [Google Scholar]
- Jeong WJ, Cho SJ, Lee HS, Deb GK, Lee YS, Kwon TH, Kong IK. Effect of cytoplasmic lipid content on in vitro developmental efficiency of bovine IVP embryos. Theriogenology 2009;72:584–589. doi:10.1016/j.theriogenology.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Jonsson JR, Moschen AR, Hickman IJ, Richardson MM, Kaser S, Clouston AD, Powell EE, Tilg H. Adiponectin and its receptors in patients with chronic hepatitis C. J Hepatol 2005;43:929–936. doi:10.1016/j.jhep.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Jungheim ES, Macones GA, Odem RR, Patterson BW, Moley KH. Elevated serum α-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil Steril 2011;96:880–883. doi:10.1016/j.fertnstert.2011.07.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 2006;8:516–523. doi:10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- Natale DR, Paliga AJM, Beier F, D'Souza SJA, Watson. AJ. p38 MAPK signaling during murine preimplantation development. Dev Biol 2004;268:76–88. doi:10.1016/j.ydbio.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal 2000;12:1–13. [DOI] [PubMed] [Google Scholar]
- Paczkowski M, Schoolcraft WB, Krisher RL. Fatty acid metabolism during maturation affects glucose uptake and is essential to oocyte competence. Reproduction 2014;148:429–439. doi:10.1530/REP-14-0015. [DOI] [PubMed] [Google Scholar]
- Qiao L, Kinney B, Schaack J, Shao J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes 2011;60:1519–1527. doi:10.2337/db10-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Liping, Yoo HS, Madon Alysha, Kinney Brice, Hay WW, Shao Jianhua. Adiponectin enhances mouse fetal fat deposition. Diabetes 2012;61:3199–3207. doi:10.2337/db12-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhshandehroo M, Knoch B, Müller Mi, Kersten. S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res 2010;2010 doi:10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhshandehroo M, Sanderson LM, Matilainen M, Stienstra R, Carlberg C, de Groot P, Müller M, Kersten S. Comprehensive analysis of PPARalpha-dependent regulation of hepatic lipid metabolism by expression profiling. PPAR Res 2007;2007:26839 doi:10.1155/2007/26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramin N, Thieme R, Fischer S, Schindler M, Schmidt T, Fischer B, Navarrete Santos A. Maternal diabetes impairs gastrulation and insulin and IGF-I receptor expression in rabbit blastocysts. Endocrinology 2010;151:4158–4167. doi:10.1210/en.2010-0187. [DOI] [PubMed] [Google Scholar]
- Schindler M, Fischer S, Thieme R, Fischer B, Navarrete Santos A. cAMP-responsive element binding protein: a vital link in embryonic hormonal adaptation. Endocrinology 2013;154:2208–2221. doi:10.1210/en.2012-2096. [DOI] [PubMed] [Google Scholar]
- Schindler M, Pendzialek M, Navarrete Santos A, Plösch T, Seyring S, Gürke J, Haucke E, Knelangen JM, Fischer B, Navarrete Santos. A. Maternal diabetes leads to unphysiological high lipid accumulation in rabbit preimplantation embryos. Endocrinology 2014;155:1498–1509. doi:10.1210/en.2013-1760. [DOI] [PubMed] [Google Scholar]
- Sozen B, Ozturk S, Yaba A, Demir N. The p38 MAPK signalling pathway is required for glucose metabolism, lineage specification and embryo survival during mouse preimplantation development. Mech Dev 2015;138:375–398. doi:10.1016/j.mod.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Staels B, Peinado-Onsurbe J, Auwerx J. Down-regulation of hepatic lipase gene expression and activity by fenofibrate. Biochim Biophys Acta 1992;1123:227–230. [DOI] [PubMed] [Google Scholar]
- Stefan N, Stumvoll M, Vozarova B, Weyer C, Funahashi T, Matsuzawa Y, Bogardus C, Tataranni. PA. Plasma adiponectin and endogenous glucose production in humans. Diabetes Care 2003;26:3315–3319. [DOI] [PubMed] [Google Scholar]
- Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta 1984;779:89–137. [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Reis A, Leese HJ, McEvoy TG. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod Domest Anim 2009;44:50–58. doi:10.1111/j.1439-0531.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- Thieme R, Schindler M, Ramin N, Fischer S, Mühleck B, Fischer B, Navarrete Santos A. Insulin growth factor adjustment in preimplantation rabbit blastocysts and uterine tissues in response to maternal Type 1 diabetes. Mol Cell Endocrinol 2012;358:96–103. doi:10.1016/j.mce.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Wu LL, Russell DL, Wong SL, Chen Miaoxin, Tsai TS, St John JC, Norman RJ, Febbraio MA, Carroll John, Robker RL. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 2015;142:681–691. doi:10.1242/dev.114850. [DOI] [PubMed] [Google Scholar]
- Xin X, Zhou L, Reyes CM, Liu F, Dong LQ. APPL1 mediates adiponectin-stimulated p38 MAPK activation by scaffolding the TAK1-MKK3-p38 MAPK pathway. Am J Physiol Endocrinol Metab 2011;300:E103–E110. doi:10.1152/ajpendo.00427.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–1295. doi:10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003;423:762–769. doi:10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 2007;13:332–339. doi:10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- Yan J, Gan L, Qi R, Sun C. Adiponectin decreases lipids deposition by p38 MAPK/ATF2 signaling pathway in muscle of broilers. Mol Biol Rep 2013;40:7017–7025. doi:10.1007/s11033-013-2821-y. [DOI] [PubMed] [Google Scholar]
- Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 2006;55:2562–2570. doi:10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Reece EA. New concepts in diabetic embryopathy. Clin Lab Med 2013;33:207–233. doi:10.1016/j.cll.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Sun X, Jin L, Stringfield T, Lin L, Chen Y. Expression profiles of adiponectin receptors in mouse embryos. Gene Expr Patterns 2005;5:711–715. doi:10.1016/j.modgep.2005.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.