Mouse model of Clostridium difficile infection shows detrimental effect of aging on outcome associated with decreased initial innate immune response, which can be reversed by modifying intestinal microbiome.
Keywords: aging, Clostridium difficile infection, host response, innate immunity, microbiome
Abstract
Background
Clostridium difficile infection (CDI) is a serious threat for an aging population. Using an aged mouse model, we evaluated the effect of age and the roles of innate immunity and intestinal microbiota.
Methods
Aged (18 months) and young (8 weeks) mice were infected with C difficile, and disease severity, immune response, and intestinal microbiome were compared. The same experiment was repeated with intestinal microbiota exchange between aged and young mice before infection.
Results
Higher mortality was observed in aged mice with weaker neutrophilic mobilization in blood and intestinal tissue and depressed proinflammatory cytokines in early infection. Microbiota exchange improved survival and early immune response in aged mice. Microbiome analysis revealed that aged mice have significant deficiencies in Bacteroidetes phylum and, specifically, Bacteroides, Alistipes, and rc4-4 genera, which were replenished by cage switching.
Conclusions
Microbiota-dependent alteration in innate immune response early on during infection may explain poor outcome in aged host with CDI.
(See the editorial commentary by de Fischer and Relman, on pages 174–6.)
Clostridium difficile infection (CDI) occurs when there is disruption of normal colonic microbiota by antibiotics, leading to colonization with toxigenic C difficile bacteria, then production of toxin A (TcdA) and/or toxin B (TcdB), and mucosal injury and inflammation [1]. The Centers for Disease Control and Prevention has identified C difficile as 1 of the 3 bacteria in the “urgent threat” category of antimicrobial resistance threats in its report in 2013 (http://www.cdc.gov/drugresistance/threat- report-2013/). According to the report, approximately half of infections occur in people younger than 65, but more than 90% of deaths occur in people 65 and older. Advanced age is considered a risk factor for severe outcome in CDI [1]. Patients ages 70 and older were 3 times as likely to have severe disease compared with younger patients, in a study of 336 patients with CDI [2]. In another study of 161 patients, 30-day and 1-year mortality increased with age, especially above the age 75, even after controlling for comorbidity burden [3]. The association between aging and worse clinical outcome with CDI has been demonstrated in observational human studies [2, 3]. However, no study has investigated the potential mechanisms through which aging affects the CDI outcome. Using an aged mouse model developed in our laboratory [4], we first demonstrated the deleterious effect of aging on CDI outcome. In this study, we aim to further delineate the possible mechanisms by which aging leads to worse disease with CDI [1]. We measured key parameters of CDI in each step of pathogenesis including the intestinal microbiota composition, the C difficile bacterial load, toxin levels, and cellular and cytokine markers of immune responses. We then investigated the role of gut microbiota in determining the CDI outcome by observing the effects of modification of intestinal microbiota on inflammatory response and CDI outcome in young and aged mice.
METHODS
Murine Model of Clostridium difficile Infection
The infection model is a modification of the published protocol of Chen et al [5]. For aged mouse group, male wild-type C57BL/6 mice aged 18 months were provided by the National Institute of Aging (NIA). They were housed in the Charles River Laboratory. This study used male wild-type C57BL/6 mice aged 8 weeks (obtained from Charles River Laboratory) as controls. For a detailed description of the murine model of infection, please refer to the online Supplementary Methods.
Peripheral Blood
Peripheral blood (100 µL) was collected by cardiac puncture in EDTA tubes then analyzed using the Hemavet machine (Drew Scientific) at the Center for Comparative Medicine of University of Virginia. The peripheral blood total white blood cell (WBC) and differential counts were measured as cells/microliter.
Histopathology
Upon euthanasia, the mice were dissected and cecal and colonic tissues were harvested. The cecum was divided in half, one of which was used for flow cytometric analysis. The second half was divided into 3 pieces. One of these pieces was used for histopathology. For a detailed description of the histopathologic analysis, please refer to the online Supplementary Methods.
Flow Cytometric Analysis
With each mouse, one half of the cecum was harvested for flow cytometry. Single-cell leukocyte suspensions of epithelial and lamina propria cells were generated from mouse cecum as described by D’Auria et al [6]. The harvested epithelial and lamina propria cells were stained by using CD8-FITC, NK1.1-PE, B220-PercPCy5.5, CD4-PECy7, CD3ε-BV421, CD45-V500, Ly6G-APC, and CD11b-APCCy7. Leukocytes were gated using forward and side scatter. The number of tagged cells were measured per 100000 ungated events. For a detailed description of the preparation for flow cytometry analysis, please refer to the online Supplementary Methods.
Cytokine Assays
The remaining half of the cecum was divided and used for cytokine measurement. One of the pieces of cecal tissue was homogenized by bead beating with zirconium beads for 1 minute in ribonucleic acid (RNA) extraction lysis buffer (QIAGEN), then RNA was extracted using the QIAGEN RNA extraction kit. The RNA underwent reverse transcription using iCycler, and the complementary deoxyribonucleic acid (DNA) was used to perform quantitative polymerase chain reaction (qPCR) on KC, macrophage inflammatory protein (MIP-2), and interleukin (IL)-1β primers. For a detailed description of the primers and the qPCR methods, please refer to the online Supplementary Methods.
The second piece of cecal tissue was homogenized by bead beating for protein extraction and analyzed by Mouse 32 Multiplex Luminex assay (EMD Millipore, St. Charles, MO) at the Flow Cytometry Core at University of Virginia for levels of cytokines KC, MIP-2, IL-1β, tumor necrosis factor (TNF)-α, IL-6, IL-10, IL-17, granulocyte colony-stimulating factor (G-CSF), and granulocyte macrophage (GM)-CSF. For a detailed description of the preparation specimen and measurement of the cytokine protein levels, please refer to the online Supplementary Methods.
Quantitative Polymearse Chain Reaction of Extracted Stool Deoxyribonucleic Acid for Measurement of Clostridium difficile Burden and Microbiome Analysis
Stool was collected daily from mice. Genomic DNA was extracted from the stool samples using a modified protocol for QIAcube (QIAGEN). Quantitative PCR was performed using primers for C difficile TcdB (tcdB) gene for C difficile detection, primers for Bacteroidetes detection, 16S gene primers for universal bacterial detection, primers for Firmicutes detection, and primers for Enterobacteriaceae detection. For a detailed description of DNA extraction methods and primers, please refer to the online Supplementary Methods.
16S Sequencing for Microbiome Analysis
Using stool DNA extracted as described in the previous paragraph, the V1–V3 hypervariable regions of 16S rRNA gene from fecal DNA samples were amplified by PCR with broad-range primers and sequenced using MiSeq Reagent Kit v3. From the 16S rRNA sequences, bacteria present in each sample were identified and their relative abundance was quantified. For a detailed description of microbiome analysis, please refer to the online Supplementary Methods.
Stool Clostridium difficile Toxin
Clostridium difficile toxins were quantified using C difficile Tox A/B II enzyme-linked immunosorbent assay (ELISA) kit (TechLab, Blacksburg, VA) according to the manufacturer’s instructions. For a detailed description of protocol for C difficile toxin ELISA assay, please refer to the online Supplementary Methods.
Exchanging Intestinal Microbiota Using Cage Switching
In a succeeding experiment, cage switching was performed to encourage exchange and subsequent equalization of intestinal microbiota before infection. The NIA aged mice and young control mice were used. On arrival before starting antibiotic treatment, all mice were housed in single cages. Each aged mouse was paired with a young mouse, and their cages were exchanged every 2 days, resulting in 3 changes of the cages. The cages were not cleaned, leaving fecal pellets on the floor of the cage to be ingested during typical coprophagic behavior of the mice, thus leading to exchange of microbiota between young and aged mice. After 1 week, cage changes were stopped and the mice were placed on antibiotic water, to follow the CDI infection protocol outlined earlier. One group of infected mice (n = 10) were followed until day 14 to measure clinical outcome. Another group was divided into 2 groups based on day of euthanasia: day 2 (n = 5) and day 5 (n = 5).
Statistics
Statistical analyses were performed using GraphPad Prism (La Jolla, CA). Survival data were analyzed by log-rank (Mantel-Cox) survival analysis. Two-way analysis of variance with Bonferroni posttest analysis was used to compare between 2 groups across time points, and Student’s t test was used to compare groups at a single time point. A P < .05 was considered significant.
Study Approval
All experimental procedures were approved by the Center for Comparative Medicine and the University of Virginia Institutional Animal Care and Use Committee.
RESULTS
Advanced Age in Mice Led to Worse Clinical Outcome With Clostridium difficile Infection and Poor Inflammatory Response, Independent of Infection Burden, in Early Infection
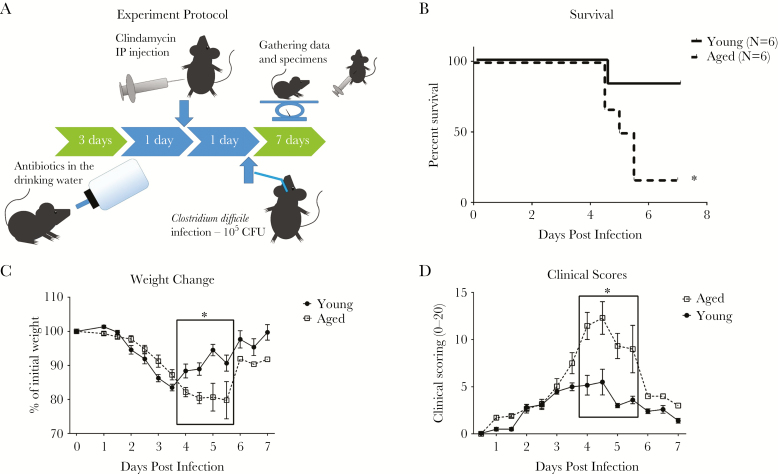
The mouse model of CDI is described in detail in Warren et al [7] and shown in Figure 1A. Young mice (8 weeks old) and aged mice (18 months old) were both infected with C difficile bacteria by oral gavage after exposure to antibiotics. Closely mimicking what is observed in humans, disease outcome in aged mice was strikingly worse than in young mice (Figure 1). Mortality was 83% in aged mice compared with 17% in young mice. Weight loss in aged mice was slower than in young mice but was persistent, whereas all of the young mice regained most of their original weight by day 7. Clinical scores (based on mouse appearance, activity, diarrhea, and weight loss as described in Pawlowski et al [8]) followed a similar pattern, with aged mice having slower increase but persistently higher scores.
Figure 1.
Disease outcome in aged and young mice. (A) Mouse model of Clostridium difficile infection (CDI). Mice are exposed to antibiotic cocktail of vancomycin, metronidazole, gentamicin, and colistin in the drinking water for 3 days, after which the mice are put back on regular water. Then the mice receive subcutaneous injection of clindamycin 1 day later. Another day later, the mice are orally gavaged with 105 colony-forming units (CFU) of vegetative cells of VPI10463, a highly toxigenic laboratory strain of C difficile. Young mice (8 weeks old) and aged mice (18 months old) were infected to measure outcome in relation to aging. Mortality (B) and weight changes (C) with CDI were measured and compared. (D) Clinical scores (based on mouse appearance, activity, diarrhea, and weight loss). Survival data were analyzed by log-rank (Mantel-Cox) survival analysis (*P < .05). Two-way analysis of variance with Bonferroni posttest analysis was used to compare weight and clinical scores between young and aged mice (*P < .0001). IP, intraperitoneal.
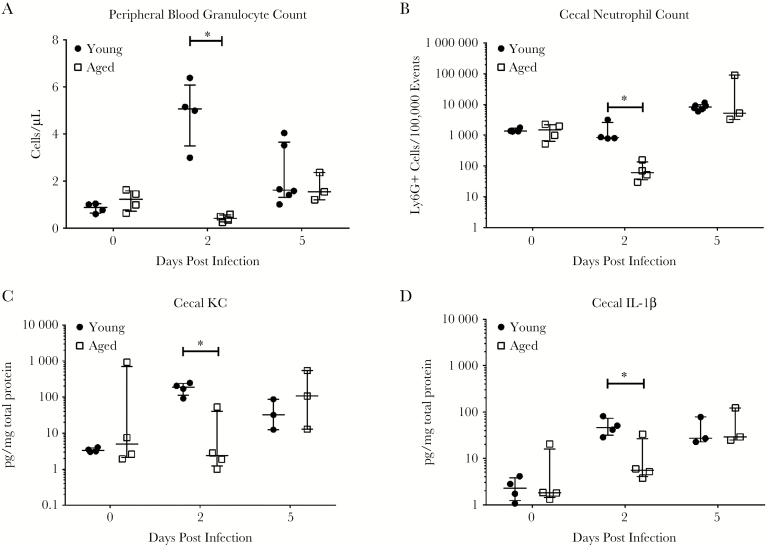
Next, we investigated whether there were differences in immune response associated with disease outcomes. Two time points were chosen for this study: day 2 (early response/before peak of infection) and day 5 postinfection (late response/peak of infection). Systemic and intestinal inflammatory responses as measured by neutrophil mobilization in circulation and in cecal tissues were lower in aged mice at day 2, whereas peak (day 5) immune responses were not significantly different when compared with young mice (Figure 2). At day 2, levels of proinflammatory cytokines (IL-1β, TNF-α), neutrophil chemokines (KC, MIP-2), and granulopoietic factors (G-CSF, GM-CSF) detected in the intestinal tissue were also significantly lower in the aged mice but did not show difference at day 5 (Figure 2, Supplementary Figure 1). At day 5, the messenger RNA (mRNA) expression showed significantly higher levels of KC and IL-1β in aged mice (Supplementary Figure 2). Levels of IL-6 and IL-10 were not significantly different between the groups at all time points (Supplementary Figure 1). Histopathology slides of cecum were scored by mucosal hypertrophy, vascular congestion, submucosal edema, epithelial disruption, and inflammatory cell infiltration as previously described [8]. The histopathology scores followed a similar trend as the immune response, with lower scores at day 2 but higher scores at day 5 in aged mice with pronounced intestinal tissue damage (Supplementary Figure 3).
Figure 2.
Immune response to Clostridium difficile infection in aged and young mice. Blood and cecal specimens were harvested from uninfected mice and C difficile-infected mice at postinfection day 2 (before peak of infection) and day 5 (around peak of infection). There were no baseline differences before infection between aged and young mice. Circulating granulocyte count (A), cecal tissue neutrophil count (B), and cytokine responses (C and D) were measured. The cytokines measured include neutrophil chemoattractant chemokines (KC [C], macrophage inflammatory protein-2), proinflammatory cytokines (interleukin [IL]-1β [D], tumor necrosis factor-α), and granulocyte hematopoietic factors (granulocyte colony-stimulating factor [CSF], granulocyte macrophage-CSF), IL-6 and IL-10. (The rest of the results are shown in Supplementary Figure 1.) Student’s t test was used to compare young and aged mice at each time point (*P < .05).
To determine whether the lower immune response in aged mice early on in the infection led to an uncontrolled burden of infection later resulting in higher mortality, infection burden was measured. Stool samples were analyzed using qPCR to measure the copy number of TcdB (tcdB) gene and ELISA to measure levels of TcdA and TcdB (Supplementary Figure 4). There were no differences early on in the infection burden on days 1–3, with slightly higher C difficile load by qPCR in aged mice on day 4 but no difference in levels of toxin.
Cage Switching Leads to Improvement in Clinical Outcome in Aged Mice and Abolished the Differences in Immune Responses Between Aged and Young Mice
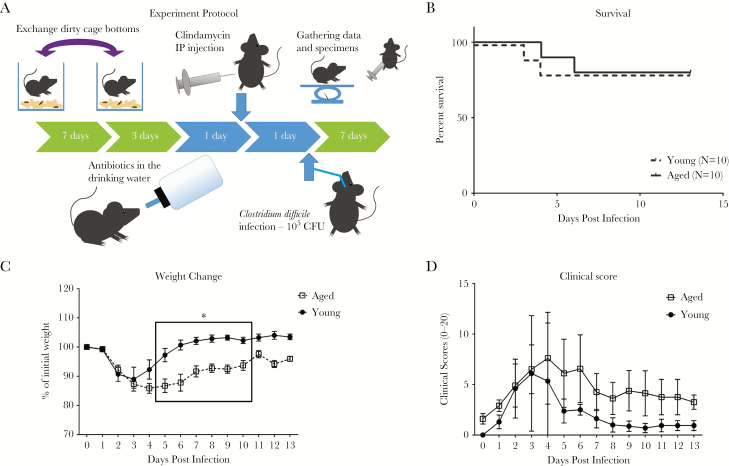
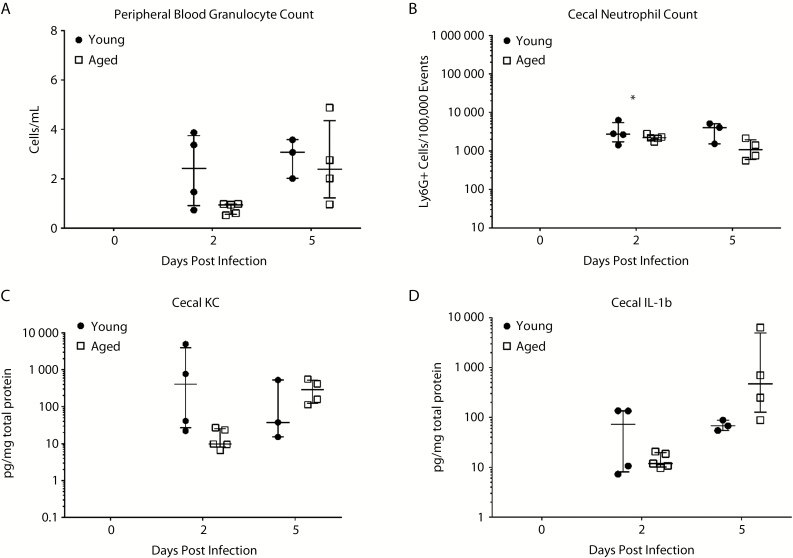
To test the impact of intestinal microbiota on innate immunity and outcomes, intestinal microbiota was exchanged by cage switching (Figure 3A). For 1 week before antibiotic exposure, dirty cage bottoms of young and aged mice were exchanged every 2 days to induce, by way of the natural coprophagic behavior, exchange of the intestinal microbiota. Afterward, the young and aged mice were exposed to broad-spectrum antibiotics then infected with C difficile as described previously. Outcome was dramatically better in the aged mice, whereas there were no changes in the young mice with cage bottom switching (Figure 3). Mortality was now 20% for both young and aged mice. This finding implies that the cage switching corrected an abnormality (possibly replenished what was previously lacking) in aged mice while not transmitting a detrimental factor to young mice. On the other hand, weight loss and clinical score showed persistent differences even with cage switching, suggesting that there were persistent differences in host factors affecting disease outcome as well. After cage switching, there was a decrease in the differences in both systemic and intestinal inflammation between aged and young mice previously noted on day 2 (early infection). The neutrophil count in aged mice became similar to young mice in peripheral blood and in cecal tissue, although there was still a trend for lower numbers in aged mice (Figure 4). Cytokine production of KC, MIP-2, IL-1β, TNF-α, G-CSF, and GM-CSF all showed a trend toward lower numbers in aged mice, but they were now not statistically significant different between aged and young mice (Figure 4, Supplementary Figure 5). Overall, these data indicate that interchanging microbiota corrected previous deficiencies in inflammatory response in aged mice without detrimental changes in young mice.
Figure 3.
Disease outcome in aged and young mice after cage switching. Young and aged mice were infected with Clostridium difficile after dirty cage bottoms were exchanged between the groups for 7 days to mix the intestinal microbiota (A). Mortality (B), weight changes (C), and clinical scores (D) were compared. Survival data were analyzed by log-rank (Mantel-Cox) survival analysis. Two-way analysis of variance with Bonferroni posttest analysis was used to compare weight and clinical scores between young and aged mice (*P < .0001). CFU, colony-forming units.
Figure 4.
Immune response after cage switching in aged and young mice. Granulocyte count in peripheral blood (A), numbers of neutrophils recruited to the cecal tissue (B), and cecal cytokines—KC (C) and interleukin (IL)-1β (D)—were measured. (The rest of the cytokine responses are shown in Supplementary Figure 5.) Student’s t test was used to compare young and aged mice at each time point (*P < .05).
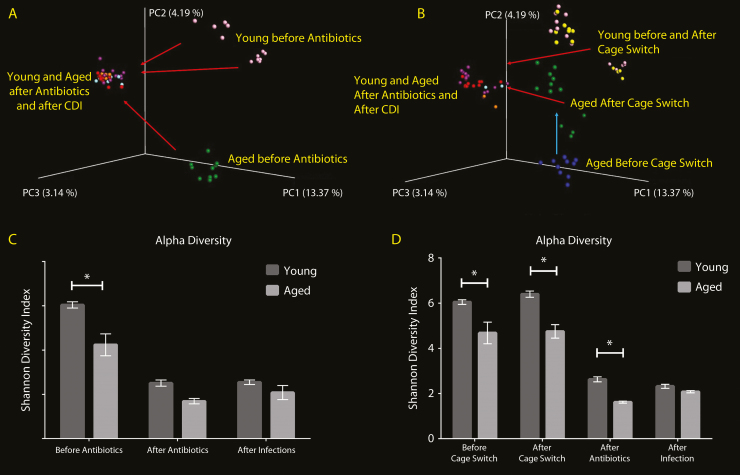
Aged Mouse Intestinal Microbiome Is Significantly Different From Young Mouse Microbiome at Baseline, Which is Corrected With Cage Switching
For baseline intestinal microbiota comparison, DNA was extracted from stool collected before antibiotic exposure. Principal coordinate analysis (PCoA) to assess overall microbiome difference between individuals revealed that young and aged mice have very distinct microbiome at baseline (Figure 5A). There seems to be variation in young mice with each shipment as shown by 2 distinct groups, whereas the aged mice were all grouped together. Broad-spectrum antibiotic exposure and subsequent infection with C difficile seem to have the biggest effect on the microbiota, with young and aged mice microbiota converging in the coordinate space and becoming more similar. Alpha diversity as measured by Shannon Diversity Index, a measure of diversity of the bacterial population within an individual, was significantly lower in aged mice compared with young mice at baseline, although after antibiotic exposure there was no significant difference (Figure 5C). With cage switching experiments, intestinal microbiota was first analyzed at baseline, then after cage switching before antibiotic exposure, and then with antibiotics and with infection (Figure 5B). Cage switching caused microbiota from aged mice to shift significantly towards young mice, whereas young mice did not have any significant changes. It is interesting to note that antibiotic exposure and subsequent infection caused the biggest shift in microbiome again, and young and aged mice microbiota became more similar on PCoA. These findings suggest a persistent effect of the intestinal microbiota on CDI outcome, which could be due to a priming effect of the microbiota on innate immune system even before antibiotic exposure and infection. Cage switching did not improve alpha diversity in aged mice (Figure 5D), which suggests that overall diversity was not significant in providing protection against CDI in aged mice with cage switching.
Figure 5.
Comparison of intestinal microbiota between young and aged mice. To compare intestinal microbiota between young and aged mice, the microbiome was analyzed from stool specimens collected at baseline, after exposure to antibiotics, and after of Clostridium difficile infection (CDI). Composition of the intestinal microbiota was analyzed using Illumina Miseq Sequencing. Principal coordinate analysis (PCoA) to evaluate beta diversity between individuals was used for comparing young and aged mice (A). The alpha diversity, referring to diversity of flora within an individual mouse, was measured using Shannon Diversity Index (C). Dirty cage bottoms of young and aged mice were exchanged between young and aged mice to mix the intestinal microbiota. Stool specimens were obtained before cage exchange, after cage exchange, after exposure to antibiotics, and after CDI, to follow the changes associated with each factor. The PCoA showed baseline differences in microbiome between young (yellow) and aged (blue) mice (B). The Shannon Diversity Index demonstrated a significantly lower diversity in aged mice that did not change with cage switching, antibiotic exposure, or infection (D). Two-way analysis of variance with Bonferroni posttest analysis was used to compare alpha diversity between young and aged mice at each time point (*P < .05).
Aged Mouse Microbiota Is Deficient in Specific Bacteria, Which Are Replenished From Young Mice With Cage Switching
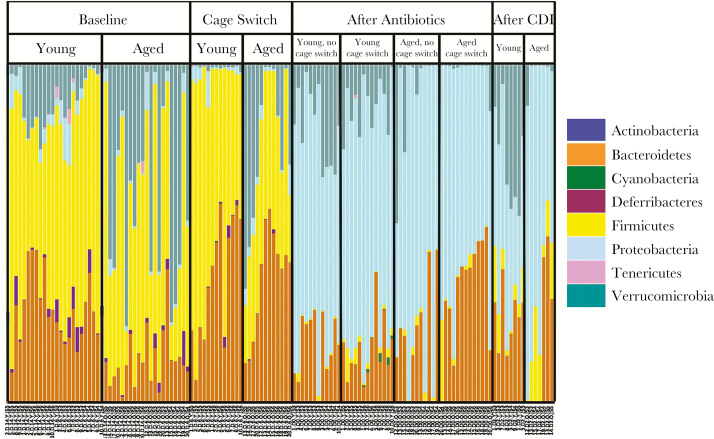
Analysis of the microbiota composition was done to provide additional insight. Normal human and mouse intestinal flora at baseline mainly consists of 2 major phyla, Firmicutes and Bacteroidetes, and to a lesser extent, other bacteria including Proteobacteria. At the baseline, aged mice had a significantly lower proportion of Bacteroidetes compared with young mice (13.0 ± 6.96% vs 29.8 ± 5.51%, P < .05) (Figure 6) as analyzed by 16S sequencing. Quantitative PCR with primers specific for the Bacteroidetes phylum also showed a significant decrease in the absolute abundance of Bacteroidetes in aged mice (9.376 × 106 ± 4.854 × 106 colony-forming units [CFUs] aged vs 5.147 × 107 ± 2.693 × 107 CFUs young, P < .0001) (Supplementary Figure 6). Antibiotic treatment led to a dramatic shift in composition of the microbiota, with a switch from Firmicutes and Bacteroidetes to Proteobacteria as the predominant phylum in the microbiota in both young and aged mice (Figure 6). Cage switching increased Bacteroidetes in aged mice, whereas young mice did not change significantly toward aged mice (Figure 6). It is interesting to note that aged mice appeared to have retained much of the Bacteroidetes gained even after antibiotic exposure.
Figure 6.
Phylum level composition of the intestinal microbiota in young and aged mice. Composition of the intestinal microbiota at the phylum level was analyzed using Illumina Miseq Sequencing. CDI, Clostridium difficile infection.
Random Forest Analysis was done to determine the most important bacteria accounting for the microbial differences. The top 3 signature genera in differentiating young and aged mice at baseline were also the top 3 genera that were significantly changed after cage switch in the aged mice (Tables 1 and 2). Bacteroides and Alistipes, which are Bacteroidetes, and rc4-4, which belongs to Firmicutes, were the signature genera identified.
Table 1.
Signature Genera Deficient in Aged Mouse Microbiome Compared to Young Micea
| Aged vs Young Mice At Baseline | ||
|---|---|---|
| Genus (Phylum) | Mean %Increase in Error on Removal (SD) | Relative Abundance in Young/ Aged Mice (P Value)b |
| Bacteroides (Bacteroidetes) | 8.03 (0.98) | 15.4%/0.3% (<.0001) |
| rc4-4 (Firmicutes) | 6.26 (0.89) | 0.7%/0.0% (<.0001) |
| Alistipes (Bacteroidetes) | 4.11 (0.68) | 0.2%/0.0% (<.0001) |
Abbreviations: CDI, Clostridium difficile infection; SD, standard deviation.
aTo investigate which components of the microbiota may be important in affecting CDI outcome, we identified signature genera that are the most important in determining the difference between young and age mice microbiota by random forest analysis.
bWilcoxon rank-sum test.
Table 2.
Signature Genera Replenished in Aged Mouse Microbiome After Cage Switching With Young Micea
| Aged Mice Before vs After Cage Switching | ||
|---|---|---|
| Genus (Phylum) | Mean %Increase in Error on Removal (SD) | Relative Abundance Before/ After Cage Switch (P Value)b |
| rc4-4 (Firmicutes) | 6.86 (0.81) | 0.0%/1.7% (<.0001) |
| Alistipes (Bacteroidetes) | 2.99 (1.02) | 0.0%/0.2% (<.0001) |
| Bacteroides (Bacteroidetes) | 2.28 (0.50) | 0.3%/27.2% (<.0034) |
Abbreviations: SD, standard deviation.
aWe used random forest analysis to identify the key signature genera that determines the difference between baseline aged mouse microbiome and aged mouse microbiome replenished by switching cages with young mice.
bWilcoxon rank-sum test.
DISCUSSION
Elucidating mechanisms underlying susceptibility of the aged host is difficult because of multiple factors associated with aging [9]. We were able to model the increased severity of disease in older patients using our mouse model of CDI, with higher mortality and persistent weight loss. The model enabled us to evaluate the possible mechanisms underlying differences in outcome. Pathogenesis of CDI involved 3 key steps: (1) colonization with toxigenic C difficile, (2) production of TcdA and/or TcdB, and (3) mucosal injury and inflammation [1]. We investigated each step of pathogenesis as potential key elements in CDI in the elderly, including C difficile bacterial load, toxin levels, and cellular and cytokine markers of immune responses. Our results showed that the difference in mortality with aging does not seem to result from differences in infection burden or toxin production. However, there were significant differences in early innate immune response between young and aged mice. Aged mice had lower levels of total WBC count, total neutrophil count, intestinal neutrophil count, and intestinal cytokine levels. These results appear to contradict what is observed from human studies that have shown higher inflammation associated with worse outcome [10–13]. However, the weaker inflammatory response in aged mice in our study was observed before the peak of infection, a time point that may have been missed in human studies. Later on in the infection, aged mice had higher inflammation as measured by tissue cytokine mRNA expression, neutrophil infiltration, and histopathology.
Animal studies of CDI have demonstrated the importance of innate immune response. Blocking signaling pathways in the innate immune response resulted in significantly worse clinical outcome in CDI [14, 15]. Knockout of MyD88 or Nod1 genes, key players in innate immune response, resulted in reduced recruitment of neutrophils in the intestinal tissue and increased lethality [14, 15]. These studies support the beneficial role of innate immune response, possibly as a part of the early defense against CDI.
Intestinal microbiota is considered an important defense against CDI pathogenesis by inhibiting colonization with C difficile [16] because CDI is almost always preceded by disruption of the intestinal microbiota [17]. As expected, the baseline microbiota was significantly different between young and aged mice, but with antibiotic exposure the microbiota of the young and aged mice converged into a similar state by PCoA. However, cage switching led to a dramatic improvement in mortality of aged mice along with a shift in intestinal microbiome. Although the mechanism still needs to be elucidated, these findings present compelling evidence of a role for intestinal microbiota in modifying CDI outcome in the aged host. The effect of microbiota on outcome was not affected by antibiotic exposure and was accompanied by an increase in early innate immune response, especially neutrophil recruitment. A possible explanation is a priming effect on the innate immune system by the microbiota. Research studies into the effect of intestinal microbiota on the innate immune system have been done on infections other than CDI [18, 19]. Mice with depleted intestinal microbiota have decreased immune response in clearing bacteria from the lungs in pneumonia [18] or in phagocytic killing of bacteria by neutrophils [19], which can be reversed by introducing normal flora or by administering bacterial cell wall components. In addition, Bacteroides [20–22] and Alistipes [23] genera, both identified as key genera in our analysis, have both been associated with protection against CDI in human studies and modulate immune response for protection against experimental colitis in mouse models [24, 25].
CONCLUSIONS
A limitation of our study is the potential differences in the housing conditions of the mice before shipment. Aged mice were from the NIA aged mouse colony, which are housed in Charles River Laboratories. Young mice are not provided by NIA and were purchased from Charles River Laboratories separately, but it is possible that the mice were not housed in the same conditions. This raises the possibility that the difference in microbiota in aged mice represents an effect of environmental conditions and not aging itself. It is worth noting, however, that similar changes in microbiome, especially a decrease in Bacteroides species, are seen with aging in human studies [20, 26]. In addition, the dramatic effect of cage switching on clinical outcomes validate the critical influence of the intestinal microbiota in CDI. There is a dearth of publications focusing on the effects of aging on CDI. This is the first study to look at the interplay of microbiota and immune response in this setting using an animal model. Our findings shed new light on the role of innate immune response and present a potential mechanism by which intestinal microbiota, apart from providing colonization resistance, affect CDI severity and outcome [16, 27]. Further research is warranted to dissect the dynamic interaction between the microbiome and the host immune response with aging.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Presented in part: ID Week 2016 (Abstract number 2227), New Orleans, Louisiana.
Notes
Acknowledgments. We are grateful to the Universit of Virginia Research Histology Core, Flow Cytometry Core, and Genomics Core for technical assistance and to Dr. Melanie Rutkowski for thoughtful review of the manuscript.
Financial support. This study was partially funded by National Institutes of Health/National Institute of Allergy and Infectious Diseases grants U01 AI075526 and T32 5T32AI007046-39.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Disclaimer. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
References
- 1. Cohen SH, Gerding DN, Johnson S et al. . Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 2. Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis 2009; 15:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 2005; 173:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Opstal E, Kolling GL, Moore JH et al. . Vancomycin treatment alters humoral immunity and intestinal microbiota in an aged mouse model of Clostridium difficile infection. J Infect Dis 2016; 214:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen X, Katchar K, Goldsmith JD et al. . A mouse model of Clostridium difficile-associated disease. Gastroenterology 2008; 135:1984–92. [DOI] [PubMed] [Google Scholar]
- 6. D’Auria KM, Kolling GL, Donato GM et al. . In vivo physiological and transcriptional profiling reveals host responses to Clostridium difficile toxin A and toxin B. Infect Immun 2013; 81:3814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warren CA, van Opstal EJ, Riggins MS et al. . Vancomycin treatment’s association with delayed intestinal tissue injury, clostridial overgrowth, and recurrence of Clostridium difficile infection in mice. Antimicrob Agents Chemother 2013; 57:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pawlowski SW, Calabrese G, Kolling GL et al. . Murine model of Clostridium difficile infection with aged gnotobiotic C57BL/6 mice and a BI/NAP1 strain. J Infect Dis 2010; 202:1708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shin JH, High KP, Warren CA. Older is not wiser, immunologically speaking: effect of aging on host response to Clostridium difficile infections. J Gerontol A Biol Sci Med Sci 2016; 71:916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steiner TS, Flores CA, Pizarro TT, Guerrant RL. Fecal lactoferrin, interleukin-1beta, and interleukin-8 are elevated in patients with severe Clostridium difficile colitis. Clin Diagn Lab Immunol 1997; 4:719–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Feghaly RE, Stauber JL, Deych E et al. . Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. Clin Infect Dis 2013; 56:1713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamontagne F, Labbé AC, Haeck O et al. . Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg 2007; 245:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loo VG, Poirier L, Miller MA et al. . A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005; 353:2442–9. [DOI] [PubMed] [Google Scholar]
- 14. Hasegawa M, Yamazaki T, Kamada N et al. . Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J Immunol 2011; 186:4872–80. [DOI] [PubMed] [Google Scholar]
- 15. Jarchum I, Liu M, Shi C et al. . Critical role for MyD88-mediated neutrophil recruitment during Clostridium difficile colitis. Infect Immun 2012; 80:2989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borriello SP, Barclay FE. An in-vitro model of colonisation resistance to Clostridium difficile infection. J Med Microbiol 1986; 21:299–309. [DOI] [PubMed] [Google Scholar]
- 17. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372:1539–48. [DOI] [PubMed] [Google Scholar]
- 18. Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun 2014; 82:4596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clarke TB, Davis KM, Lysenko ES et al. . Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 2010; 16:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol 2002; 51:448–54. [DOI] [PubMed] [Google Scholar]
- 21. Manges AR, Labbe A, Loo VG et al. . Comparative metagenomic study of alterations to the intestinal microbiota and risk of nosocomial Clostridum difficile-associated disease. J Infect Dis 2010; 202:1877–84. [DOI] [PubMed] [Google Scholar]
- 22. Schubert AM, Rogers MA, Ring C et al. . Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. MBio 2014; 5:e01021–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamilton MJ, Weingarden AR, Unno T et al. . High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes 2013; 4:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neff CP, Rhodes ME, Arnolds KL et al. . Diverse intestinal bacteria contain putative zwitterionic capsular polysaccharides with anti-inflammatory properties. Cell Host Microbe 2016; 20:535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dziarski R, Park SY, Kashyap DR et al. . Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. PLoS One 2016; 11:e0146162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woodmansey EJ, McMurdo ME, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol 2004; 70:6113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buffie CG, Bucci V, Stein RR et al. . Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015; 517:205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.