Abstract
Mesenchymal stem cells (MSCs) have been widely studied with regard to their potential use in cell therapy protocols and regenerative medicine. However, a better comprehension about the factors and molecular mechanisms driving cell differentiation is now mandatory to improve our chance to manipulate MSC behavior and to benefit future applications. In this work, we aimed to study gene regulatory networks at an early step of osteogenic differentiation. Therefore, we analyzed both the total mRNA and the mRNA fraction associated with polysomes on human adipose tissue-derived stem cells (hASCs) at 24 h of osteogenesis induction. The RNA-seq results evidenced that hASC fate is not compromised with osteogenesis at this time and that 21 days of continuous cell culture stimuli are necessary for full osteogenic differentiation of hASCs. Furthermore, early stages of osteogenesis induction involved gene regulation that was linked to the management of cell behavior in culture, such as the control of cell adhesion and proliferation. In conclusion, although discrete initial gene regulation related to osteogenesis occur, the first 24 h of induction is not sufficient to trigger and drive in vitro osteogenic differentiation of hASCs.
Introduction
Mesenchymal stem cells (MSCs), including human adipose tissue-derived stem cells (hASCs), are undifferentiated cell populations characterized by the ability to undergo self-renewal and the capacity for multilineage differentiation1. hASCs can be found in adipose tissue in large amounts (millions to billions of cells), and they can be collected and harvested by a minimally invasive procedure. Due to their multipotent nature, when appropriately stimulated in vitro, they can differentiate into several tissue-specific lineages, including the chondrogenic, osteogenic and adipogenic lineages2. All these characteristics make these cells very valuable for cell-based medical therapies. Among clinical applications, the use of MSCs for bone regeneration has been gaining attention3. Understanding the biological pathways and transcription factors involved in determining the osteogenic fate is key to future advances and improvements of bone regeneration therapies.
Currently, there are many questions remaining about how to control the stem cell switch from self-renewal to differentiation in vitro. The fate and function of an in vitro MSC culture depend on many external factors, including the cell culture media and supplements (i.e., growth factors), mechano-electro stimuli, and the use of three-dimensional scaffolds4. Moreover, little is known about how cell fate determinants are regulated in functionally important gene regulatory networks, which remains a challenge. Gene expression analysis, both genome-wide and targeted at specific gene subsets, has played a key role in improving our understanding into the molecular pathways involved in hASCs self-renewal and differentiation5.
Considering the osteogenic differentiation of MSCs, there are a plethora of signals and molecular pathways described in the literature. Signaling pathways including TGFβ/BMP signaling, Wnt signaling, Hedgehogs, Notch, and FGFs have been involved (reviewed by Wu and colleagues, 20166). Recently, Yong and coworkers (2016) demonstrated the influence of ERK1/2 and PKA signaling pathways to the induction of osteogenesis on murine ASCs under an electromagnetic field (EMF) stimulus7. Accordingly, Fathi and Farahzadi (2017) confirmed Yong’s findings demonstrating that zinc sulphate, in the presence of EMF, induce the expression of osteogenic genes on murine ASCs via PKA, ERK1/2 and Wnt/β-catenin signaling pathways8.
The study of MSC differentiation and maturation has also been globally monitored by gene expression profiles that have historically focused on measuring total mRNA levels through high-throughput analyses. Using microarray expression profiling, van de Peppel and colleagues (2017) identified gene regulatory events during osteogenic and adipogenic lineage commitment of MSCs. Their data analysis revealed that expression levels of identified transcription factors did not always change and indicate additional post-transcriptional regulatory mechanisms during differentiation9. In fact, recent studies reveal that steady-state mRNA levels only loosely correspond to the composition of the proteome10,11, thereby indicating that post-transcriptional mechanisms play a major role in the regulation of gene expression. Until recently, precisely monitoring translation was far more challenging than measuring mRNA levels. However, this scenario has changed with the development of the polysome profiling approach12. The analysis of the mRNA fraction associated with polysomes has been used as a strategy to analyze posttranscriptional mechanisms involved in translational control as well as to quantitatively measure expression13. We previously demonstrated that, in a model of adipogenic differentiation, RNA-seq analysis of the mRNA fraction associated with polysomes showed a significant percentage of regulated mRNAs that were controlled at the translational level and/or by changes in their transcript levels12. Additionally, this previous work demonstrated that three days of in vitro cell differentiation induction with adipogenic stimulation medium are sufficient for the initiation of adipogenesis and the upregulation of its correlated gene networks.
In this work, we aimed to investigate genes and pathways involved in driving the initial trigger of osteogenic differentiation of hASCs. For this purpose, we focused on the early stages of osteogenic differentiation and analyzed both the total mRNA and the mRNA fraction associated with polysomes. Our question was whether gene network regulation as early as 24 h after induction could determine hASCs commitment to in vitro osteogenesis. Moreover, we aimed to assess how relevant the first differentiation signals could be to modulate hASC behavior in culture during osteogenesis induction.
Results and Discussion
Early stages of osteogenic differentiation display slight differences in gene regulation
The identity of the hASCs used in this work was confirmed by the expression of cell-surface antigens and by their capacity to undergo adipogenic and osteogenic differentiation. The immunophenotype profiles of the cells indicated that hASCs were uniformly positive for the membrane glycoprotein CD90, the endoglin receptor CD105 and the surface enzyme ecto-5′-nucleotidase CD73, confirming their canonical MSC characteristics (see Supplementary Fig. S1A). No detectable contamination by endothelial or hematopoietic cells was observed, as flow cytometry analysis was negative for CD31, CD19, CD34, CD117, CD11b, HLA-DR and CD45 markers (Supplementary Fig. S1A). The adipogenic and osteogenic differentiation potentials were verified by the presence of lipid-rich vacuoles stained with AdipoRed™ and by the presence of mineralized extracellular matrix stained with OsteoImage™ Mineralization, respectively (Supplementary Fig. S1B,C).
To understand the hASCs’ osteogenic differentiation, gene expression patterns were determined by studying the mRNA population associated with the translation machinery. In this work, we focused on the gene expression profile involved in the initial steps of osteogenesis. For this purpose, we allowed hASCs to differentiate in vitro in the presence of osteogenic induction media for 24 h. After this period of in vitro differentiation, it was not possible to observe any morphological differences or accumulation of mineralized extracellular matrix between the induced (IN) and non-induced cells (CT) (data not shown).
After 24 h of osteogenic induction, cells were subjected to isolation of total and polysomal RNA fractions by ultracentrifugation of cytoplasmic extracts into sucrose density gradients (10% to 50%). As previously demonstrated by our group12 and seen in this work, the hASCs had a polysome profile characteristic of cells with reduced levels of translational activity and with low amount of polysome complexes observed along the sucrose gradient (see Supplementary Fig. S2A).
Total and polysome-associated mRNA fractions were isolated and submitted to RNA-seq in an Illumina HiSeq platform. The total number of reads obtained for each sample is shown in Supplementary Table S1. The reads of all samples were mapped onto the reference genome (GRCh38), yielding a mean mapping percentage of approximately 80%, with approximately 17,000 genes detected.
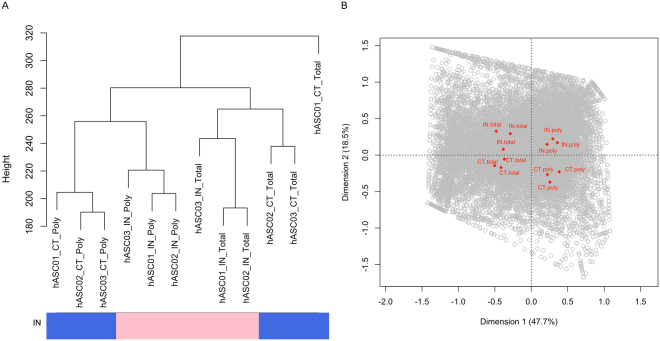
Analyzing the total number of transcripts, we observed that most of the transcripts (14,711; ~95%) were detected in both samples (polysomal and total RNA fractions). Despite this, the expression levels were variable and some mRNAs were identified in only one RNA fraction (Supplementary Fig. S2B). Hierarchical clustering and correspondence analysis (COA) showed that samples were grouped first according to RNA fraction (total vs. polysomal) and then according to condition (CT vs. IN), indicating that, in all conditions, samples grouped according to the RNA fraction regardless of donor origin (Fig. 1A,B). Supporting our previous report12, these results also evidenced that total and polysomal RNA populations are intrinsically more characteristic than donor “idiosyncrasy”.
Figure 1.
Results of hierarchical clustering and correspondence analysis showing the internal consistency of the data. (A) Dendrogram including all samples: CT and IN in the polysomal fraction and in total RNA (CT_Poly, IN_Poly and CT_Total, IN_Total, respectively). Log2 values of RPKM counts were used for the hierarchical clustering. The first branching event separates the fraction (polysomal from total) and the internal branching event separates the condition (CT and IN). This applies to all samples excluding TL01_CT_Total. (B) Correspondence analysis (COA) of samples. The first dimension (represented in the x-axis) separates RNA fraction (total vs. polysomal) and the second dimension (represented in the y-axis) separates the culture condition (CT vs IN).
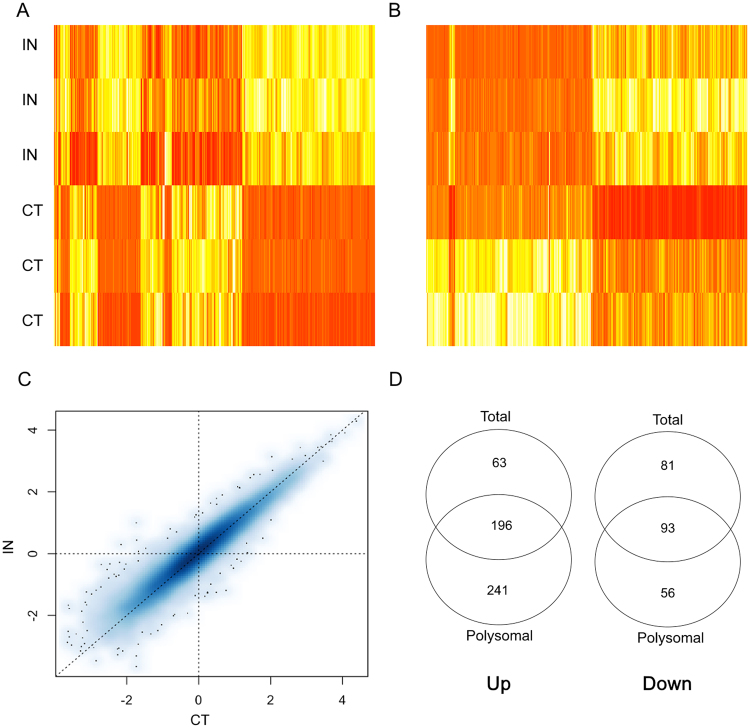
Differentially expressed genes (DEGs), considering an overall FDR ≤ 0.05 and a logFC ≥ ±2, were identified by paired comparisons between CT and IN conditions, and for polysomal and total RNA fractions (Supplementary Tables 2 and 3). Considering these results, representative heatmaps for total and polysomal RNA fractions allowed us to visualize the expression pattern of DEGs (Fig. 2A,B). Similar to previously reported12 and as expected, some differences in gene expression profile between induced and non-induced cells were noticed in both RNA fractions, but not between replicates. Also, RPKM values (log-10 reads per kilobase per million mapped reads) for polysomal RNA fraction in IN and CT conditions were illustrated in a scatter plot (Fig. 2C) showing that the expression levels of majority of genes are similar between conditions (blue central blur) and only some genes are differentially expressed (black points). Considering all analysis, we identified 586 DEGs in polysomal RNA fraction and 433 DEGs in total RNA fraction. Interestingly, comparing DEGs in total and polysomal RNA fractions, we observed differences in the quantity of up- and downregulated genes (Fig. 2D), with more upregulated subjects. In regard of this, Bionaz and coworkers (2015), evaluating differentiation of porcine ASCs, have described that osteogenesis is featured by a larger number of upregulated DEGs than downregulated ones at day 2 of in vitro differentiation. These data are compatible to that found in this work, but different from adipogenesis that display a large number of downregulated DEGs14.
Figure 2.
Diagrams for differentially expressed genes (DEGs) on osteogenic-induced (IN) and non-induced (CT) cells. Heatmaps of the DEGs are shown for the polysomal RNA fraction (A) and for the total RNA fraction (B). Data are displayed with different samples in the rows (three biological replicates for IN and for CT) and color intensities represent gene upregulation (red) and downregulation (yellow). (C) Scatterplot of log-10 RPKM values representing each gene in the polysomal fraction (median of reads per kilobase per million mapped reads from all patients for CT and IN samples). (D) Venn diagrams picturing set relationships between DEGs in the polysomal and total RNA fractions for upregulated genes (Up; left) and for downregulated genes (Down; right).
Taken together, these RNA-seq results indicated that only 3–4% of the detected transcripts showed some degree of regulation in the first 24 h of differentiation. Our results suggest that most of this gene regulation is exerted through the control of transcript association with the translational machinery. Notwithstanding, the presence of DEGs when comparing control and induced conditions signalize the outset of both transcriptional and translational regulation in the initial steps of osteogenesis.
Osteogenic induction promotes limited gene expression modifications on the first day of induction
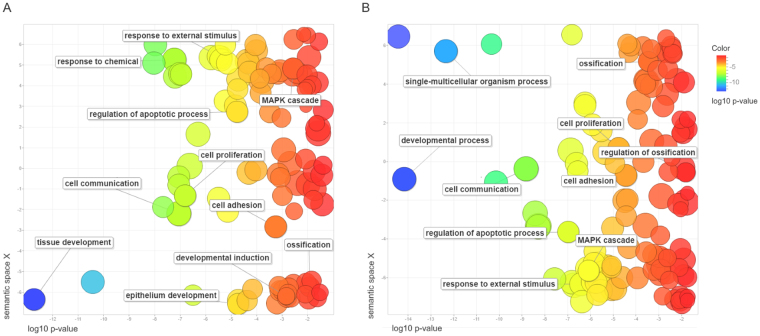
Gene ontology (GO) analysis for each set of DEGs from total and polysomal RNA fractions were conducted with g:Profiler (http://biit.cs.ut.ee/gprofiler/). The complete lists of GO analysis concerning cellular components, biological processes, molecular functions and signaling pathways (KEGG) could be found in the Supplementary Tables S4 and S5 (for total and polysomal RNA fractions, respectively). Although we have identified a smaller number of DEGs in the total fraction, the number of GO terms were higher than that found in the polysomal fraction (Supplementary Tables S4 and S5). In order to aid visualization of the GO terms found for biological processes and point out differences between RNA fractions analyzed, we summarized them (removing redundant GOs and grouping them by similarity) using REVIGO (http://revigo.irb.hr/)15. Most of the biological processes found were overrepresented in both fractions and are related to cell communication, cell proliferation and adhesion, response to external stimuli and development (Fig. 3). Biological processes such as regulation of cell cycle and cell adhesion have already been reported in other works regarding osteogenesis induction of MSCs, although their analyses have been performed with longer induction-differentiation periods16,17.
Figure 3.
Gene ontology (GO) analysis of biological processes regarding DEGs in polysomal (left chart) and total (right chart) RNA fractions. The figure shows a REVIGO scatterplot of the representative clusters of GO terms obtained with g:Profiler. In the two-dimensional space of the graph, the log10 p-value of each GO after REVIGO analyses is plotted on the x-axis, while the terms are scattered based on their semantic similarities on the y-axis. Bubble color indicates the provided p-value (legend in upper right-hand corner).
Based on our previous work showing the strong upregulation of adipogenesis-related genes after three days of differentiation induction12, we sought out the first steps of osteogenesis, searching for known markers of osteogenic differentiation. Interestingly, after only 24 h of osteogenic induction, we did not identify key transcriptional regulators of the osteogenesis such as Runt-related transcription factor 2 (RUNX2), a transcription factor closely related to osteogenesis that is responsible for activating a set of genes including osterix, osteocalcin, and type 1 collagen, among others18–20. Likewise, the study of cell-fate determination during osteogenic and adipogenic differentiation of MSCs made by van de Peppel and colleagues (2017) demonstrated that enriched biological processes and transcription factors that change expression are mostly identical between the lineages within the first four days of induction9. Nevertheless, our data on the polysomal and total RNA fractions showed GO clusters related to “ossification” or “regulation of ossification” (Fig. 3A,B) and for “bone mineralization”. In the upregulated gene set, we found important molecules previously related to osteogenesis, such as Bone Morphogenetic Protein 6 (BMP6), Forkhead box O1 (FOXO1), osteomodulin (OMD), Protein Tyrosine Kinase 2 or Focal Adhesion Kinase 1 (PTK2 or FAK), Neuronal Membrane Glycoprotein M6-B (GPM6B) and others.
BMPs are a class of TGFβ family growth factors comprising more than 20 members that act on various biological processes and signaling pathways, primarily through SMADs or MAPKs. Among its members, BMP2, BMP4, BMP6, BMP7 and BMP9 have been described to play an important role in bone formation21,22. Regarding BMP6, identified in our data, it has been shown that the overexpression of this gene in MSCs induces osteogenic differentiation in a more efficient manner than does BMP2 overexpression23. Furthermore, the increased level of BMP6 mRNA expression after 24 h of induction suggests that BMP6 may act as an early regulator for the commitment of the osteogenesis process.
FOXO1 is another transcription factor related to osteoblast differentiation. Its activity increases in murine MSCs after a few hours of induction with BMP2; however, RUNX2 appears only at later moments24, which is compatible with our observed data. However, when FOXO1 is silenced, the osteogenic markers decrease, as well as calcification24. Its influence on osteogenic differentiation was also verified in MC3T3-E1 cells, in which the interaction between FOXO1 and RUNX2 were demonstrated25. On the other hand, FOXO1 has also been described as essential for adipogenic differentiation, as knockdown of FOXO1 in mouse 3T3-L1 preadipocytes showed a decrease in lipid droplet formation when induced to adipocyte differentiation26. Thus, the data obtained in the present study and observed elsewhere showed a strong upregulation of the FOXO1 gene, suggesting that FOXO1 is an early marker of osteogenesis.
Other molecules, such as GPM6B, also drew our attention due to their relation with the osteogenic process. The absence of GPM6B decreased the expression of ALP mRNA and the mineralization capacity, proving to be an important protein in the process of osteogenic differentiation27.
Another interesting gene found in our data analysis is PTK2 or FAK. It is a cytoplasmic protein tyrosine kinase present during the development of most tissues. FAK has numerous roles that include influencing cell migration, proliferation, cell-cell and cell-ECM interactions (reviewed by28). However, FAK also plays an important role in osteogenic differentiation. Numerous studies have demonstrated that the absence of FAK leads to a decrease in bone mass or bone regeneration in vivo29,30. In addition, absence of FAK in bone marrow MSCs leads to less proliferation and osteoblast differentiation30,31. Another interesting fact is that FAK interacts with the ERK1/2 pathway, which is responsible for the phosphorylation and activation of RUNX2/CBFA-131.
These results suggest that, although we saw a small number of DEGs between culture conditions (CT vs IN) and could not identify key transcriptional factors of the osteogenesis at the beginning of the differentiation (e.g., RUNX2), we found a set of genes related to the osteogenic differentiation and that interact with RUNX2. As such, it is possible to imagine that an initial molecular signaling begins after 24 h of induction and starts the process of differentiation of hASCs for an osteogenic fate.
The hASCs need 21 days of osteogenic induction to differentiate
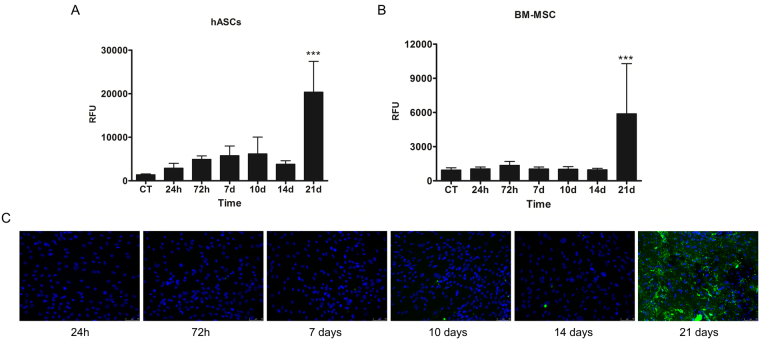
Considering that key transcriptional factors for osteogenesis were not identified in the first 24 h of osteogenic induction, we tested the induction time required to trigger cell differentiation to the osteogenic phenotype. Classic protocols are based on continuous or alternate induction, where the differentiation occurs in a period of 21 days with the medium being changed every 3–4 days. Thus, we performed a time course for differentiation induction, maintaining induction media in short periods of time (24 and 72 h) and longer periods of time (7, 10, 14 and 21 days). Our results showed that in cell cultures with less than 21 days of induction it was not possible to observe the presence of mineralized compartments, a definitive evidence of the osteogenic process (Fig. 4A,C). Nonetheless, it is important to point out that the evaluation of alkaline phosphatase (ALP) activity and/or expression of type I collagen, osteocalcin, BMP2 and RUNX2 would be early markers of osteogenic differentiation32. MAPK/ERK and cAMP/PKA signaling pathway have also been used for investigation of osteogenic induction since they have been implicated in the osteogenic differentiation process, being reported as regulators of proliferation and differentiation of bone cells during osteogenesis7,33. Fathi and Farahzadi (2017) provided evidence of osteogenic differentiation showing significant increasing in ALP activity after 16 days of murine ASC cultures treated with zinc sulfate and/or exposed to electromagnetic fields. However, cells treated only with osteogenic induction medium had low levels of ALP during the first days of induction, showing a slight increase at day 20 of the osteogenic differentiation process8. Compatible results were found in this study, since the presence of mineralized compartments was observed at the end of osteogenic induction, demonstrating that 21 days of induction were necessary for osteogenic differentiation of hASCs.
Figure 4.
hASCs need 21 days of in vitro induction to become osteoblasts. hASCs (A) or BM-MSC (B) were maintained in differentiation-inducing conditions for different periods of time and allowed to complete osteogenic differentiation (total of 21 days). The graphs represent the means (with standard deviations) of the relative fluorescence units (RFU) for OsteoImageTM staining in each time period. (C) Representative images of induced hASCs after 24 h to 21 days of differentiation period. Green fluorescence staining of the hydroxyapatite portion of the mineralized matrix on cell cultures after 21 days. ***denotes p < 0.001.
Interestingly, hASCs become committed within the first three days of adipogenic differentiation induction12, different to osteogenic differentiation. This could be a result of the tissue origin of the adult stem cells, where a cell niche effect could affect the differentiation capacity of the cells. However, when bone marrow stem cells were tested, again, 21 days of continued induction were needed to complete osteogenesis (Fig. 4B). These results indicated that cell commitment to osteogenic differentiation is slower than that for adipogenesis, with gradual changes over the induction time regardless of the origin of MSCs (adipose tissue or bone marrow; Fig. 4A,B).
IPA analyses reveal osteogenesis related signaling pathways
Complementary analysis of DEGs from the polysomal fraction was conducted with Ingenuity Pathway Analysis (IPA). In addition to GO terms, the IPA displayed cellular growth and proliferation with the highest score of representation, which reached a p-value of 6.67E-18 and 197 related molecules.
IPA showed a series of mRNAs related to signaling pathways among the polysomal DEGs. Among the 5 pathways with the highest p-values, two drew our attention: “Role of Osteoblasts, Osteoclasts and Chondrocytes in Rheumatoid Arthritis” and “p38 MAPK Signaling”. Interestingly, as observed in the diagrams presented using IPA (see Supplementary Fig. S3), the regulated genes in the osteo-related pathway were the ligands, cell receptors or the first intracellular signaling proteins of the cascade; this appears to be consistent with the applied protocol of 24 h of induction. Within this pathway, we found BMPs and activators/inhibitors of the Wnt pathway that were previously described as osteogenic regulators. Our analysis also identified Wnt2 and Wnt11 ligands, that are related to osteogenesis in hMSCs34. Furthermore, we have identified other regulated genes related to the Wnt pathway and osteogenesis, such as WNT1-induced Secreted Protein-1 (WISP1) and secreted Frizzled-related proteins (SFRPs or FRZB).
WISP-1 was the first gene discovered as a target for the Wnt pathway; however, further studies demonstrated that this molecule is highly expressed in bone and osteoblasts and influences osteogenesis both in vivo and in vitro through the interaction with BMP2 or Wnt35,36. The FRZB mRNA appeared in our data with a 6.5-fold change increase. The FRZB protein was demonstrated to be capable of modulating the Wnt pathway37,38 and improving osteogenic differentiation of MC3T3-E1 cells37. In addition, FAK (also regulated according to our experimental data) was able to influence the Wnt pathway during osteogenesis and deletion of FAK decreased Wnt/b-catenin signaling in BMSCs that were induced to undergo osteogenesis30.
Additionally, the MAPK signaling cascade was highlighted in our IPA (Supplementary Fig. S3) and g:Profiler analyses. This signaling pathway is vital in the control of cellular behavior by transducing external stimuli. MAPKs are related to osteoblast commitment predominantly through ERK and p38 activities. Interestingly, the GO term “regulation of ERK1 and ERK2 cascade” were also identified and includes some known genes that activate this pathway (Supplementary Tables S4 and S5). The relevant role of ERK1/2 signaling pathway in osteogenic differentiation have been demonstrated previously with different methods of osteogenic induction7,8,39. Also, p38 MAPK is another well-known essential pathway in bone formation and differentiation40,41. In both cases, kinases are responsible for phosphorylation and activation of genes such as RUNX2 (reviewed by42).
Osteogenic induction modulates hASC adhesion and proliferation
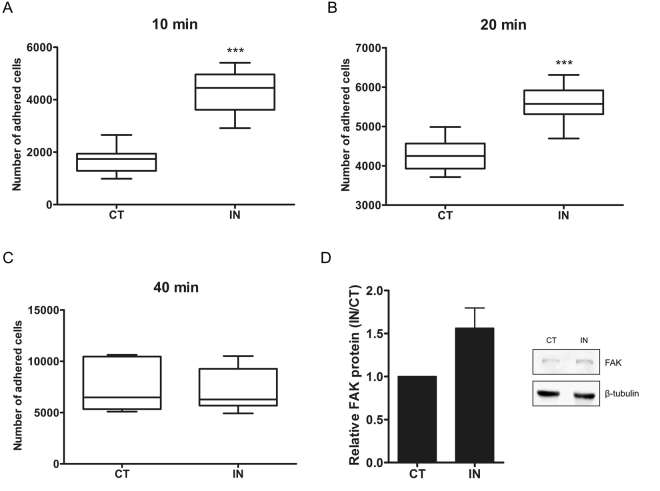
It is known that mesenchymal stem cells have the ability to adhere to surfaces such as plastics43, as well as contain a number of cell surface molecules that promote adhesion44. Our GO analysis grouped a series of upregulated genes related to cell adhesion. To understand and confirm these data, we tested the immediate adherence performance of hASCs, evaluating 10, 20 and 40 min after cell seeding. Control and 24 h induced hASCs with osteogenic medium were evaluated. The number of adherent cells was determined by nuclei counting with the support of a high content imaging system. As shown in Fig. 5A–C, induced hASCs were significantly more adhesive (about 2 times more) after the first 10 and 20 min than noninduced cells. This difference was not maintained after 40 min of adhesion, which showed a similar number of adherent cells in control and induced conditions.
Figure 5.
Induced hASCs have improved adhesion capacity. The graphs in A, B and C depict the number of adherent cells obtained by the counting of the nuclei between control and induced conditions. (A) Adherent cells after 10 min of culture; (B) Adherent cells after 20 min of culture; and (C) Adherent cells after 40 min of culture. (D) Western Blot analysis and quantification of FAK protein. β-tubulin was used to normalize the amount of FAK under control and induced conditions. Representative images of a cropped Western Blot gel are displayed to evidence the bands, though uncropped images of blots are shown in Supplementary Fig. S4. ***denotes p < 0.001.
As discussed above, FAK protein is related to cell-substrate adhesion and was therefore identified in our analysis among the genes related to adhesion. To confirm the presence of FAK, we performed a western blot assay and compared the amounts of FAK protein between control and induced conditions. After 24 h of osteogenic induction FAK expression was increased in comparison to noninduced cells (Fig. 5D and Supplementary Fig. S4).
Considering the influence of cell shape on MSCs differentiation45 and that osteogenic differentiation is favored when the cells are adhered, flattened and spread46, our hypothesis is that the increased adhesive capacity of hASCs after 24 h of induction is preparing cells for osteogenic differentiation.
Another GO term that we observed in our results that called our attention was related to cell proliferation. Although proliferation and differentiation are independent cell behaviors, there are a number of studies showing that, in many cases, cells need to reduce or stop proliferation to begin the process of differentiation47. As such, we investigated the proliferative profile of the hASCs that were induced to undergo osteogenesis at different time points. The proliferation of induced cells was compared with that of noninduced cells by evaluating nuclei and Ki67 staining. After 10 and 14 days of culture, the induced condition showed significantly higher number of cells than control condition (see Supplementary Fig. S5A–C). The results also indicated that, after 7 and 10 days, induced cells had higher proliferation rates compared to control cells (~10% more cells incorporated Ki67 on the seventh day) (Supplementary Fig. S5D–F). Thus, these results demonstrated that, even under the stimulus of osteogenesis induction, the hASCs continue to proliferate until at least the 14th day of culture.
Investigating the genes described in cell proliferation we found that the “Cell cycle regulator” (RGCC) and “zinc finger and BTB domain containing 16” (ZBTB16) had an increased fold change in their transcript levels in total and polysomal RNA fractions of induced cells. These genes have already been described as being involved in the process of cell proliferation and cell cycle regulation48–50. Some studies noticed that during osteogenic differentiation there was an increase in cell number when compared, for example, to adipogenic differentiation17,46. Thus, an increase in cell number appears to be a natural feature of the in vitro osteogenic differentiation process.
Conclusion
In summary, the present study showed that, with only 24 h of osteogenesis induction, hASCs were not fully committed to the osteogenic phenotype. The analysis of the polysome-associated transcripts showed that most differentially expressed mRNAs are regulated at this level. Among the upregulated genes, we could not identify canonical pathways or classical transcription factors related to the osteogenic process. Nevertheless, the data revealed other signals and proteins that are related to the initial regulation of osteogenic differentiation, such as BMP6 and FOXO1. An interesting difference between induced and noninduced hASCs was related to cell proliferation and adhesion, in which induced cells displayed an improved capacity compared with control cells. Taken together, our results suggest that a 24 h induction period is not sufficient to trigger and drive in vitro osteogenic differentiation of hASCs and that cells need a prolonged stimulus period to be committed to differentiation.
Material and Methods
Isolation, characterization, culture and differentiation of hASCs
Human stem cells were obtained from adipose tissue derived from lipoaspirate samples from four female healthy donors aged 27, 32, 44 and 56 years old. Samples were obtained after signed informed consent was provided by the donors or their legal representatives, in accordance with guidelines for research involving human subjects and with the approval of the Ethics Committee of Fundação Oswaldo Cruz, Brazil (approval number 419/07). hASCs were isolated and expanded as previously described elsewhere51 with modifications. Briefly, 200 ml of adipose tissue were digested with 1 mg/ml collagenase type I (GIBCO) solution for 30 min at 37 °C, 5% CO2 under constant shaking. The cell suspension obtained was centrifuged and treated with hemolysis buffer to remove contaminating erythrocytes. The cells were washed and plated at a density of 1 × 105 cells/cm2 in T75 culture flasks in DMEM supplemented with 10% FBS, penicillin (100 units/ml) and streptomycin (100 µg/ml). Flasks were incubated in a humidified incubator at 37 °C with 5% CO2. The culture medium was changed every 3–4 days until the cells reached 80% to 90% confluence. All tests were performed with cell cultures at passages 4 to 6. Cell characterization was performed according to the minimal criteria for defining MSCs as established by the International Society for Cellular Therapy43. Flow cytometry analysis were conducted as previously described elsewhere52 with modifications. At brief, cells were trypsinized and incubated with bovine serum albumin (BSA) 1% diluted in PBS, at 4 °C, for one hour. Then the cells were incubated for another one hour, at 4 °C in the dark, with the following antibodies: FITC-conjugated anti-human CD90, CD34, CD31 and CD19; APC-conjugated anti-human CD73; PE-conjugated anti-human CD45, HLA-DR, CD117 and CD11b. Mouse IgG antibodies (FITC, APC, PE) were used as negative controls. After incubation, the cells were washed for data acquisition at FACSCanto II (Becton Dickinson). At least 10,000 events, for each sample, were collected and analyzed with FlowJo® v.10 software (Flowjo, LLC).
For adipogenic and osteogenic differentiation, hASCs were treated with hMSC Adipogenic Differentiation Medium (hMSC Adipogenic BulletKit, Lonza) or with hMSC Osteogenic Differentiation Medium (hMSC Osteogenic BulletKit, Lonza) respectively, in accordance with the manufacturer’s instructions. Briefly, adipogenic differentiation was induced by cycles of induction/maintenance over 28 days. Induction medium consisted of adipogenic inducers indomethacin, insulin, dexamethasone and IBMX. Osteogenic differentiation was induced with medium containing β-glycerophosphate, ascorbic acid and dexamethasone over 21 days. The medium was replaced every 3–4 days.
Efficiency of adipogenic and osteogenic differentiation was determined by assessing the cytoplasmic accumulation of triglycerides by AdipoRed™ Assay Reagent (Lonza) or by assessing the mineralized extracellular matrix through OsteoImage™ Mineralization Assay (Lonza), respectively.
Sucrose density gradient separation and RNA purification
Polysomal fractions were prepared with a modified version of the procedure described previously12. At brief, hASC cultures at 60 to 70% confluence were induced to osteogenic differentiation (as described in section 2.1) for 24 h, and then the cultures were treated with 0.1 mg/ml cycloheximide (Sigma-Aldrich) for 10 min at 37 °C, followed by trypsinization. The resulting cell pellets were resuspended in 0.1 mg/ml cycloheximide in PBS and was centrifuged (2000 × g for 5 min); this step was repeated twice. The cells were lysed by incubation for 10 min on ice with polysome buffer (15 mM Tris-HCl pH 7.4, 1% Triton X-100, 15 mM MgCl2, 0.3 M NaCl, 0.1 μg/ml cycloheximide). The cell lysates were centrifuged at 12000 × g for 10 min at 4 °C. The supernatants were carefully isolated, loaded onto 10% to 50% sucrose gradients (BioComp model 108 Gradient Master ver. 5.3) and centrifuged at 150000 × g (SW40 rotor, HIMAC CP80WX HITACHI) for 160 min at 4 °C. The sucrose gradient was fractionated with the ISCO gradient fractionation system (ISCO Model 160 Gradient Former Foxy Jr. Fraction Collector), connected to a UV detector to monitor absorbance at 275 nm, and the polysome profile was recorded. The total and polysomal RNA fractions were extracted using the Direct-zolTM RNA MiniPrep (Zymo Research) according to the manufacturer’s instructions.
cDNA library construction and RNA sequencing (RNA-seq)
We used 1 µg of total and polysome-associated RNA fractions for RNA-seq from three independent biological sample replicates. The cDNA libraries were prepared with the TruSeq Stranded Total RNA Sample Preparation kit (Illumina, Inc.), following the manufacturer instructions, and the purified products were evaluated with an Agilent Bioanalyzer (Agilent). The RNA-seq was performed in an Illumina HiSeq platform using RNA-seq kit according to the manufacturer’s recommendation (Illumina, Inc.).
Data analysis
Mapping and counting was performed with the R package Rsubread53 against the new version of the human genome GRCh38. Parameters were set for unique mapping of the reads. For the determination of differential expression between differentiating cells (IN) and undifferentiated stem cells (CT), only those genes with counts of more than 1 per million in at least three conditions were considered. Differentially expressed genes (DEGs) were identified with edgeR Bioconductor package54. Correction for multiple testing was performed with FDR (false discovery rate). RPKM values (reads per kilobase per million mapped reads, an expression measure) for each sample were also determined in order to compare the expression of the samples in both conditions. We performed correspondence analysis (COA), a dimension reduction method of the matrix of counts, to explore associations between variables. In COA, it is possible to simultaneously visualize samples and genes, revealing associations between them. Genes, or samples, lying close to each other tend to behave similarly.
Based on initial results of DEGs we used a stringent analysis using a false discovery rate (FDR) threshold of 0.05 and a log2Fold change (logFC). Genes with a logFC > 2 were considered upregulated, and genes with a logFC < −2 were considered downregulated. An enrichment analysis of this set of genes was performed using g:Profiler55 (http://biit.cs.ut.ee/gprofiler/) and REVIGO15 (http://revigo.irb.hr/) consortium database. Complement analysis was performed with Ingenuity Pathways Analysis (IPA) software, data version 26127183 (Qiagen, Valencia CA, USA).
Adhesion assay
After induction of osteogenic differentiation for 24 h, cells were trypsinized, plated on 24-well plates (2,5 × 104 cells/well) and kept for 10, 20 and 40 min in a humidified incubator at 37 °C with 5% CO2 as previously described56. The non-adherent cells were removed by washing twice with PBS. Adherent cells were fixed with 4% paraformaldehyde followed by DAPI staining for counting. The quantitative analysis was performed using an Operetta HTS imaging system (PerkinElmer, Waltham MA, USA) at 100× magnification. Sixty five fields were analyzed and photographed for counting labeled cells with Harmony 3.5.2 software (PerkinElmer).
Cell proliferation assay
Ki67 and DAPI staining were used to evaluate proliferation of hASCs in the Operetta HTS imaging system. Nine fields of view at 100× magnification were used to perform quantitative analysis in the Harmony 3.5.2 software. Briefly, hASC were seeded at 96-well plates (103 cells/well) and incubated at 37 °C, 5% CO2 with DMEM 10% FBS until reached 80% of confluence. Then, osteogenic induction medium was added and maintained in culture for 1, 3, 7, 10 and 14 days. After each period of incubation, cells were fixed with 4% paraformaldehyde, permeabilized with Triton X-100 0,5% (30 min), blocked with BSA 1%, diluted in PBS and stained with Ki67 antibody (Abcam).
Western blot analysis
Cell extracts were prepared by adding lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM MgCl2, 0.5% Nonidet-40, 1 mM dithiothreitol) supplemented with a protease inhibitor cocktail (Sigma-Aldrich). Cells were removed with a cell scraper, incubated with lysis buffer at 4 °C for 2 h and then centrifuged at 16100 × g. Supernatants were collected and treated with SDS sample buffer. Western blot analyses were performed with rabbit anti-FAK (1:1000, Cell Signaling) and rabbit anti-tubulin (1:2000, Cell Signaling) antibodies. The peroxidase-conjugated anti-rabbit IgG was used as secondary antibody. ImageJ software (https://imagej.nih.gov/ij/) was used for the quantitative analysis and different gel images were quantified using linear signal ranges.
Statistical analysis
Statistical differences were determined by one-way ANOVA followed by Tukey’s test and, for grouped analysis, two-way ANOVA followed by Bonferroni post hoc test. Analyses were performed using GraphPad Prism 5 software. Values of p ≤ 0.05 were considered significant. Data are expressed as the mean ± standard deviation. All experiments were performed with three biological replicates.
Data availability
The RNA-seq raw data are deposited in the ArrayExpress repository under the number E-MTAB-6298. Analyzed datasets are available in this article and its supplementary files.
Electronic supplementary material
Acknowledgements
We would like to thank all the staff of the Carlos Chagas Institute (Fiocruz-PR) for the laboratory and administrative support; the staff of the Bioinformatics Unit (Institut Pasteur Montevideo) for RNA-seq data analysis; and the staff of Cirurgia Plástica Dr. Paulo Bettes for tissue collection. We thank the Program for Technological Development in Tools for Health-PDTIS-FIOCRUZ for use of its facilities. This study was supported by Fundação Araucária and CNPq.
Author Contributions
A.W.R. and A.B.B.A. contributed equally to this work, conceived, designed, and performed the experiments, analyzed the data, and drafted the paper; L.S. and H.N. analyzed the raw data, assisted in data interpretation and drafted the paper; I.T.P. and P.S. performed experiments and analyzed the data; P.S.L.B. procured and processed patient samples; A.C., B.D., and M.A.S., conceived the experimental setup, contributed to data analysis and drafted the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Anny Waloski Robert and Addeli Bez Batti Angulski contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22991-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- 2.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived Stem Cells: Isolation, Expansion and Differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao J, Zhang W, Yang T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol. Res. 2015;48:1–7. doi: 10.1186/s40659-015-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sisakhtnezhad S, Alimoradi E, Akrami H. External factors influencing mesenchymal stem cell fate in vitro. Eur. J. Cell Biol. 2017;96:13–33. doi: 10.1016/j.ejcb.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y, Russell I, Walworth C, Chen C. Gene Expression in Stem Cells. Crit. Rev. Eukaryot. Gene Expr. 2009;19:289–300. doi: 10.1615/CritRevEukarGeneExpr.v19.i4.30. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen PK, Rhee J-W, Wu JC. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol. 2016;1:831–841. doi: 10.1001/jamacardio.2016.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yong Y, Ming ZD, Feng L, Chun ZW, Hua W. Electromagnetic fields promote osteogenesis of rat mesenchymal stem cells through the PKA and ERK1/2 pathways. J. Tissue Eng. Regen. Med. 2016;10:E537–E545. doi: 10.1002/term.1864. [DOI] [PubMed] [Google Scholar]
- 8.Fathi E, Farahzadi R. Enhancement of osteogenic differentiation of rat adipose tissue-derived mesenchymal stem cells by zinc sulphate under electromagnetic field via the PKA, ERK1/2 and Wnt/β-catenin signaling pathways. PLoS One. 2017;12:1–19. doi: 10.1371/journal.pone.0173877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Peppel J, et al. Identification of Three Early Phases of Cell-Fate Determination during Osteogenic and Adipogenic Differentiation by Transcription Factor Dynamics. Stem Cell Reports. 2017;8:947–960. doi: 10.1016/j.stemcr.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Sousa Abreu, R., Penalva, L. O., Marcotte, E. M. & Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 10.1039/b908315d (2009). [DOI] [PMC free article] [PubMed]
- 11.Maier T, Güell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Spangenberg L, et al. Polysome profiling shows extensive posttranscriptional regulation during human adipocyte stem cell differentiation into adipocytes. Stem Cell Res. 2013;11:902–912. doi: 10.1016/j.scr.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Fromm-Dornieden C, et al. Novel polysome messages and changes in translational activity appear after induction of adipogenesis in 3T3-L1 cells. BMC Mol. Biol. 2012;13:9. doi: 10.1186/1471-2199-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bionaz M, Monaco E, Wheeler MB. Transcription adaptation during in vitro adipogenesis and osteogenesis of porcine mesenchymal stem cells: Dynamics of pathways, biological processes, up-stream regulators, and gene networks. PLoS One. 2015;10:1–35. doi: 10.1371/journal.pone.0137644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Supek, F., Bošnjak, M., Škunca, N. & Šmuc, T. Revigo summarizes and visualizes long lists of gene ontology terms. PLoS One6 (2011). [DOI] [PMC free article] [PubMed]
- 16.Kulterer B, et al. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics. 2007;8:1–15. doi: 10.1186/1471-2164-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monaco, E., Bionaz, M., Rodriguez-Zas, S., Hurley, W. L. & Wheeler, M. B. Transcriptomics comparison between porcine adipose and bone marrow mesenchymal stem cells during in vitro osteogenic and adipogenic differentiation. PLoS One7 (2012). [DOI] [PMC free article] [PubMed]
- 18.Komori T, et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 19.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell. 1997;89:747–754. doi: 10.1016/S0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 20.Bruderer M, Richards RG, Alini M, Stoddart MJ. Role and regulation of runx2 in osteogenesis. Eur. Cells Mater. 2014;28:269–286. doi: 10.22203/eCM.v028a19. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, et al. Bone morphogenetic proteins: Relationship between molecular structure and their osteogenic activity. Food Sci. Hum. Wellness. 2014;3:127–135. doi: 10.1016/j.fshw.2014.12.002. [DOI] [Google Scholar]
- 22.Luu HH, et al. Distinct Roles of Bone Morphogenetic Proteins in Osteogenic Differentiation of MesenchymalStem Cells. J. Orthop. Reseach. 2007;25:665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 23.Mizrahi, O. et al. BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther. 370–377. 10.1038/gt.2012.45 (2012) [DOI] [PubMed]
- 24.Teixeira CC, et al. Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. J. Biol. Chem. 2010;285:31055–31065. doi: 10.1074/jbc.M109.079962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siqueira MF, et al. FOXO1 modulates osteoblast differentiation. Bone. 2011;48:1043–1051. doi: 10.1016/j.bone.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munekata K, Sakamoto K. Forkhead transcription factor Foxo1 is essential for adipocyte differentiation. Vitr. Cell. Dev. Biol. - Anim. 2009;45:642–651. doi: 10.1007/s11626-009-9230-5. [DOI] [PubMed] [Google Scholar]
- 27.Drabek K, V D Peppel J, Eijken M, Van Leeuwen JPTM. GPM6B regulates osteoblast function and induction of mineralization by controlling cytoskeleton and matrix vesicle release. J. Bone Miner. Res. 2011;26:2045–2051. doi: 10.1002/jbmr.435. [DOI] [PubMed] [Google Scholar]
- 28.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J. Cell Sci. 2010;123:1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 29.Kim J-B, et al. Reconciling the roles of FAK in osteoblast differentiation, osteoclast remodeling, and bone regeneration. Bone. 2007;41:39–51. doi: 10.1016/j.bone.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun C, et al. FAK Promotes Osteoblast Progenitor Cell Proliferation and Differentiation by Enhancing Wnt Signaling. J. Bone Miner. Res. 2016;31:2227–2238. doi: 10.1002/jbmr.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp. Cell Res. 2007;313:22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutkovskiy A, Stensløkken K-O, Vaage IJ. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016;22:95–106. doi: 10.12659/MSMBR.901142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddappa R, et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc. Natl. Acad. Sci. 2008;105:7281–6. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, et al. Wnt11 plays an important role in the osteogenesis of human mesenchymal stem cells in a PHA/FN/ALG composite scaffold: possible treatment for infected bone defect. Stem Cell Res. Ther. 2016;7:18. doi: 10.1186/s13287-016-0277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ono M, Inkson CA, Kilts TM, Young MF. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J. Bone Miner. Res. 2011;26:193–208. doi: 10.1002/jbmr.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda A, et al. WNT1-Induced Secreted Protein-1 (WISP1), a novel regulator of bone turnover and Wnt signaling. J. Biol. Chem. 2015;290:14004–14018. doi: 10.1074/jbc.M114.628818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thysen S, Cailotto F, Lories R. Osteogenesis induced by frizzled-related protein (FRZB) is linked to the netrin-like domain. Lab. Investig. 2016;96:570–580. doi: 10.1038/labinvest.2016.38. [DOI] [PubMed] [Google Scholar]
- 38.Uren A, et al. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J. Biol. Chem. 2000;275:4374–82. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- 39.Zhang P, Wu Y, Jiang Z, Jiang L, Fang B. Osteogenic response of mesenchymal stem cells to continuous mechanical strain is dependent on ERK1/2-Runx2 signaling. Int. J. Mol. Med. 2012;29:1083–1089. doi: 10.3892/ijmm.2012.885. [DOI] [PubMed] [Google Scholar]
- 40.Greenblatt M, Shim J. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J. Clin. Invest. 2010;120:2457–2473. doi: 10.1172/JCI42285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aenlle KK, Curtis KM, Roos BA, Howard GA. Hepatocyte Growth Factor and p38 Promote Osteogenic Differentiation of Human Mesenchymal Stem Cells. Mol. Endocrinol. 2014;28:722–730. doi: 10.1210/me.2013-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez-Carballo E, Gámez B, Ventura F. p38 MAPK Signaling in OsteoblastDifferentiation. Front. Cell Dev. Biol. 2016;4:1–20. doi: 10.3389/fcell.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 44.Chamberlain G, Fox J, Ashton B, Middleton J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 45.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stemm cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 47.Ruijtenberg S, van den Heuvel S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle. 2016;15:196–212. doi: 10.1080/15384101.2015.1120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fosbrink M, et al. Response gene to complement 32 is required for C5b-9 induced cell cycle activation in endothelial cells. Exp. Mol. Pathol. 2009;86:87–94. doi: 10.1016/j.yexmp.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tegla CA, et al. RGC-32 is a novel regulator of the T-lymphocyte cell cycle. Exp. Mol. Pathol. 2015;98:328–337. doi: 10.1016/j.yexmp.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi W-I, et al. Role of promyelocytic leukemia zinc finger (PLZF) in cell proliferation and cyclin-dependent kinase inhibitor 1A (p21WAF/CDKN1A) gene repression. J. Biol. Chem. 2014;289:18625–40. doi: 10.1074/jbc.M113.538751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farahzadi R, Fathi E, Mesbah-Namin SA, Zarghami N. Zinc sulfate contributes to promote telomere length extension via increasing telomerase gene expression, telomerase activity and change in the TERT gene promoter CpG island methylation status of human adipose-derived mesenchymal stem cells. PLoS One. 2017;12:1–25. doi: 10.1371/journal.pone.0188052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Fathi E, Farahzadi R, Charoudeh HN. L-carnitine contributes to enhancement of neurogenesis from mesenchymal stem cells through Wnt/β-catenin and PKA pathway. Exp. Biol. Med. 2017;242:482–486. doi: 10.1177/1535370216685432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao, Y., Smyth, G. K. & Shi, W. The Subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41 (2013). [DOI] [PMC free article] [PubMed]
- 54.Robinson MD, McCarthy DJ, Smyth G. K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reimand J, et al. g:Profiler—a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Res. 2016;44:W83–W89. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zych J, et al. Polysome profiling shows the identity of human adipose-derived stromal/stem cells in detail and clearly distinguishes them from dermal fibroblasts. Stem Cells Dev. 2014;23:2791–802. doi: 10.1089/scd.2013.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq raw data are deposited in the ArrayExpress repository under the number E-MTAB-6298. Analyzed datasets are available in this article and its supplementary files.