Abstract
There is converging evidence that insulin plays a role in food-reward signaling in the brain and has effects on enhancing cognition. Little is known about how these effects are altered in individuals with insulin resistance. The present study was designed to identify the relationships between insulin resistance and functional brain connectivity following a meal. Eighteen healthy adults (7 male, 11 female, age: 41-57 years-old) completed a frequently-sampled intravenous glucose tolerance test to quantify insulin resistance. On separate days at least one week apart, a resting state functional magnetic resonance imaging scan was performed: once after a mixed-meal and once after a 12-hour fast. Seed-based resting state connectivity of the caudate nucleus and eigenvector centrality were used to identify relationships between insulin resistance and functional brain connectivity. Individuals with greater insulin resistance displayed stronger connectivity within reward networks following a meal suggesting insulin was less able to suppress reward. Insulin resistance was negatively associated with eigenvector centrality in the dorsal anterior cingulate cortex following a meal. These data suggest that individuals with less sensitivity to insulin may fail to shift brain networks away from reward and toward cognitive control following a meal. This altered feedback loop could promote overeating and obesity.
Keywords: fMRI, graph theory, imaging, prandial
In the brain, insulin serves as a key neuroregulatory protein (Ghasemi et al., 2013; Gray et al., 2014) signaling energy storage levels and regulating food intake. In addition to inhibiting feeding via the hypothalamus(Porte et al., 2005), insulin exerts direct effects on the mesolimbic reward system. In humans, increases in insulin are associated with decreases in activity in several regions, including the insula and striatum(Kroemer et al., 2013). Insulin resistance is a condition in which cells become less responsive to insulin, often connected to genetic background or acquired factors such as obesity, sedentary lifestyle, and inflammation. Insulin resistance typically develops in the liver, skeletal muscle, and adipose tissue, organs responsible for regulating fuel partitioning and utilization. Insulin resistance in these organs contributes to the development of hyperglycemia, dyslipidemia, and cardiometabolic disease. In contrast to its peripheral metabolic effects, the central effects of insulin on human brain cells are less well understood, largely due to the numerous barriers to studying insulin action in vivo in the human brain. As a result, the existence of insulin resistance in the brain and its physiological impact have not been fully elucidated. Some studies have suggested that abnormalities in insulin action in the brain can occur, particularly within striatal regions. Relative to healthy controls, insulin resistant individuals have blunted metabolic responses in the prefrontal cortex and the ventral striatum in response to peripheral infusions of insulin(Anthony et al., 2006). Studies have demonstrated an inverse relationship between insulin rise in response to a glucose challenge and brain responses to visual food stimuli in regions including the caudate nucleus, insular cortex, and orbitofrontal cortex(Heni et al., 2015, 2014; Kroemer et al., 2013). Functional brain responses to visual food stimuli were also reduced in participants who were insulin sensitive, particularly in corticolimbic regions involved in reward whereas insulin-resistant participants maintained heightened reactivity in these regions, suggesting that the ability of insulin to signal reward was inhibited(Alsaadi and Van Vugt, 2015). A separate line of research has identified a relationship between insulin and enhancement of cognitive function(Benedict et al., 2004; Ott et al., 2012) with insulin administration increasing activity in the prefrontal cortex(Del Parigi et al., 2002) potentially suggesting that insulin serves as a switch from reward based responding toward self-control(Kullmann et al., 2016). Here we examine the relationship between insulin resistance and brain functional connectivity in reward and cognitive control networks following a meal.
Resting state functional connectivity allows the quantification of functional associations between brain regions. When the brain is at rest (i.e. not performing an experimental task), brain activity exhibits low-frequency fluctuations (Park and Friston, 2013). These fluctuations can be correlated across brain regions to identify intrinsic connectivity networks(Fox et al., 2005). A variety of methods exist for examining resting state connectivity. These methods can be model-based, such as selecting a predefined brain region and examining correlations between that region and other regions. Recent development utilizing graph theory has proven to be particularly effective at identifying brain network dynamics(Bullmore and Bassett, 2011). Eigenvector centrality (EVC) is a graph theory measure that quantifies how connected each voxel of the brain is with all other voxels and indicates the “seniority” of a region among all voxels included in the analysis(Lohmann et al., 2010). Thus, a voxel with many connections to other voxels is assigned a high value, whereas those with a low number of connections receive a low score. These methods enable us to examine network dynamics, rather than changes in activity within isolated regions.
A limited number of studies have examined the relationship between prandial state and functional connectivity. A study of elderly adults mapped the effects of a mixed meal on nodes of regions involved in food intake and found that a mixed meal reduced connectivity between several regions of interest including the hippocampus, insula and anterior cingulate cortex(Paolini et al., 2014). Due to the region-based approach, connectivity of the striatal reward networks was not included in the analysis. A separate study of obese individuals found the effects of a meal on neural activity and connectivity are altered such that during fasting, obese individuals display enhanced connectivity in regions involved in reward and food intake, with increased connectivity between the hypothalamus and dorsal striatum relative to healthy individuals(Lips et al., 2014). In lean individuals, the consumption of a meal reduced hypothalamic connectivity, but the meal had no effect on connectivity in obese subjects. Although insulin resistance and obesity are correlated, not all individuals who are obese are insulin resistant, and not all insulin resistant individuals are obese(Jones et al., 2000; Zavaroni et al., 1994). Thus, studies are needed that examine the relationship between insulin resistance and brain connectivity independent of obesity.
The present study was designed to examine the effects of a meal on resting state functional connectivity and to identify the moderating effects of insulin sensitivity on reward and cognitive control networks independent of body mass index. To identify the relationship between insulin sensitivity and network architecture, we utilized both model-based and data-driven approaches. Given previous literature demonstrating altered activity in the head of the caudate in obesity(Stice et al., 2008) we selected a seed region in the head of the caudate nucleus from a meta-analysis on the neural basis of gustatory food receipt (Huerta et al., 2014). In addition to this model-based approach, we also utilized EVC to identify relationships between insulin sensitivity and brain network dynamics. We hypothesized that following a meal, insulin sensitive individuals with show reduced connectivity in reward networks, and a shift toward regions involved in cognitive control. This is the first study, to our knowledge, that has examined associations between insulin sensitivity, resting state network connectivity and the effects of a mixed meal independent of body mass index.
MATERIALS AND METHODS
Subjects
From August 2014 through January 2016 participants were recruited from the Pittsburgh metropolitan area to participate in a study of brain responses to taste. Participants (40-60 years of age) provided written informed consent. As insulin sensitivity tends to decline with increasing age, the age range of 40-60 was selected to increase the likelihood of collecting a range of insulin sensitivity. Exclusion criteria were: being left handed, using any medications that could affect insulin sensitivity, use of corticosteroid inhaler or use of a steroid medication for more than one month, current use of antidepressants or psychoactive medications, chronic medical or neurological condition, surgery on the brain, heart or blood vessels, history of head injury that resulted in loss of consciousness for more than one minute, self-reported lifetime history of depression, anxiety or mental disorder, stroke, bypass surgery, convulsions, seizures, blood clots, diabetes, kidney or liver problems, pregnancy, presence of Hepatitis C antibodies, hematocrit less than 34, a history of being treated for mental health problems, claustrophobia, substance abuse (including alcohol), cancer, color blindness, or any other contraindication to MRI. All procedures were approved by the University of Pittsburgh Institutional Review Board and were in accordance with the principles set out in the Declaration of Helsinki.
Study visits
Participants underwent a screening visit for anthropometric measurements (height, weight, and calculation of body mass index utilizing a Tanita scale) and a blood draw following a 12-hour overnight fast. Blood samples were analyzed for serum glucose, insulin, hematocrit and presence of hepatitis C antibodies. Participants who met study criteria were scheduled for an intravenous glucose tolerance test and two MRI visits. Each visit was preceded by a 12-hour overnight fast. One MRI scan was conducted while the participant remained fasting and the alternate scan was conducted following the ingestion of a mixed meal (Ensure nutritional drink, Abbott Nutrition) 30-minutes prior to the beginning of the scan. Participants completed a visual analog scale at baseline and 30 minutes after the meal, as well as after the MRI scan. Self-report items included hunger, satisfaction, fullness, how much you think you can eat, and cravings for sweet, salty, savory, and fatty foods. All study visits were completed within four months, were at least one week apart, and meal condition was randomized and counter-balanced.
Measurement of insulin sensitivity
To measure systemic insulin sensitivity, participants underwent a frequently-sampled intravenous glucose tolerance test (IVGTT) protocol(Wasko et al., 2015). The study was conducted in the morning after a 12-hour fast. After fasting samples were collected, a 50% dextrose bolus (0.3 g/kg) was administered in less than one minute through a peripheral IV line followed by a 10ml normal saline flush. Twenty minutes later, insulin (Humulin-R 20 mU/kg) was injected through the same peripheral IV line followed by normal saline flushing. Blood samples were collected at minutes −10, −5, 0, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 through a dedicated peripheral line in the opposite arm. Glucose and insulin were measured at all time points and used to calculate insulin sensitivity (SI) using the Minimal Model (Bergman et al., 1979).
Magnetic Resonance Imaging
Scanning was performed using a 3T Siemens Trio TIM scanner using a 32-channel coil located at the Magnetic Resonance Center at the University of Pittsburgh. An axial whole brain high-resolution (1.0 mm isotropic) T1-weighted sequence (magnetization prepared rapid gradient echo, MPRAGE) was collected (TR=2300ms, TE=3.43ms, TI=900ms, FA=9°) with a field of view 256×224 with 176 slices. An axial whole brain T2*-weighted resting state blood oxygen level dependent (BOLD) acquisition using gradient-echo planar imaging (EPI) was also collected (TR=1500ms, TE=35ms, in-plane resolution=96×96, number of slices=17, multiband factor = 3, voxel size=2.3mm3 isocubic). A field map was collected to correct for spatial distortion in the resting state scan (TR=550ms, TE=4.92/7.38ms, in-plane resolution=96×96, number of slices=51). During the resting state scan, a fixation cross was presented on the screen. Participants were instructed to keep their eyes open and stay awake.
Neuroimaging preprocessing was performed utilizing statistical parametric mapping (SPM12) software. Using the field map, a spatial distortion correction and a motion correction were applied. The structural image was then linearly coregistered to the mean functional image and then segmented into 6 tissue classes (gray, white, CSF, skull, soft-tissue, and air). A resulting deformation map was used to normalize the functional images to the Montreal Neurological Institute (MNI) space. The functional images were then smoothed with an 8-mm full-width at half-maximum kernel.
Seed connectivity analysis
The region of interest for the caudate nucleus was selected from a meta-analysis on the neural basis of gustatory food receipt (Huerta et al., 2014). The time series for each voxel was regressed on time series for white matter, CSF and the six motion parameters (accounting for residual in-scanner motion), and then band-pass filtered using a Butterworth filter (0.01-0.1 Hz). The functional images were then smoothed with an 8-mm full-width at half-maximum kernel. Seed regions for the left and right head of the caudate (x, y, z = −8/+8, 22, 2; diameter = 4mm) were created in MarsBaR (http://marsbar.sourceforge.net/) and then limited to gray matter using standard MNI tissue probability maps in SPM12 with threshold probability greater than 0.6. The principal time-series of the region of interest was extracted using singular value composition and correlated against all other voxels for each session of each subject, covarying for white matter, cerebrospinal fluid, and motion parameters. This generated a single map of the correlation between the seed (left or right caudate) and the entire brain.
Eigenvector Centrality Analysis
To identify the relationship between SI, and network centrality, we utilized fast EVC mapping (Wink et al., 2012). Centrality is a graph theory metric that measures the connectedness of a node in a network (Joyce et al., 2010; Lohmann et al., 2010; Zuo et al., 2012). Eigenvector centrality uses principal components analysis to calculate the first principal component of the voxel-to-voxel correlation matrix. Each voxel is treated as a node in the network and voxels with greater connectedness, as measured by number of high correlation connections, are assigned a higher value. Areas with higher values are indicative of “hubs” of the brain that are central to the function of specific brain networks(van den Heuvel and Sporns, 2013). The result is an EVC spatial map, which identifies regions with more connectedness. A similar metric is known as mean centrality, which is a voxel’s average correlation with all other voxels. These EVC maps are, input into the group analyses described below. Based on our focus on the fronto-striatal networks, a mask was applied that included the frontal lobes, temporal lobes, and striatum.
Missing Data
Nineteen participants signed consent and completed the study. Upon review of the functional neuroimaging data, one participant did not have BOLD signal from the dorsal striatum and thus was removed from the dataset. One participant failed to complete the fasting visit, and a second participant did not have resting state BOLD data for the fed visit due to scanner failure. Two participants did not have plasma insulin data from the fed visit day and therefore were not included in correlation analyses between insulin rise and striatum connectivity during the fed visit. One participant was missing a baseline insulin value from a fed visit. The baseline value from the alternate MRI visit was instead utilized for analysis. Two participants were missing blood data from the final time point on the fed day.
Statistical Analysis
MRI data analyses were performed in SPM12 using the statistical nonparametric mapping (SnPM) toolbox (http://warwick.ac.uk/snpm). First-level correlation/EVC maps for fasting and fed were entered into separate group analyses. The effect of the meal was analyzed using paired t-tests. Regressions included SI as the predictor of interest and body mass index as a nuisance covariate. Data were analyzed using permutation testing with cluster-level inference (5000 permutations, cluster forming threshold p = 0.005, FWE corrected p <.05). Metabolic data were analyzed using SPSS (v.23, IBM Corporation). Values are expressed as mean±SD unless otherwise stated. Effects of the meal on metabolic factors were examined using repeated-measures analysis of variance, or within-subject t-tests as appropriate. All analyses were two-tailed and a p < 0.05 was considered statistically significant.
RESULTS
Subject Characteristics (Table 1)
Table 1.
Participant Characteristics
| Variable | mean ± SD (Range, or Percentage) |
|---|---|
| Age (years) | 50.1 ± 4.9 (41 – 57) |
| Sex | 7 M/11 F |
| Ethnicity | |
| Caucasian, Non-Hispanic (n) | 11 (61%) |
| African-American (n) | 7 (39%) |
| Fasting glucose (mg/dL) | 92.0 ± 7.2 (81.0 – 106.0) |
| Fasting Insulin (μU/mL) | 7.1 ± 4.7 (2.0 – 16.0) |
| HOMA-IR2 | 1.6 ± 1.1 (0.4 – 3.9) |
| Insulin Sensitivity (10−4.mU−1.L.min−1) | 5.1 ± 2.1 (1.7 – 9.9) |
| Body Mass Index (kg/m2) | 26.6 ± 3.6 (21.2 – 36.1) |
| < 25 | n = 7 (39%) |
| 25-29.9 | n = 8 (44%) |
| > 30 | n = 3 (17%) |
| Fat Percent (%) | 30.34 ± 9.1 (18.2 – 47.3) |
| Fat Mass (kg) | 23.87 ± 8.9 (12.6 – 48.2) |
| Free-Fat Mass (kg) | 54.82 ± 12.41 (39.7 – 74.6) |
| HDL cholesterol (mg/dL) | 53.8 ± 15.5 (32.0 – 87.0) |
| LDL cholesterol (mg/dL) | 102.5 ± 30.9 (28.0 – 143.0) |
| Total Cholesterol (mg/dL) | 177.8 ± 29.2 (124.0 – 228.0) |
| Triglycerides (mg/dL) | 91.4 ± 58.8 (38.0 – 276.0) |
The 18 participants who were included in the data analysis included 7 males (39%) and 11 females (61%) with a mean age of 50.1±4.9 years of age. Eleven participants were White (non-Hispanic) and 7 participants were African-American. Female participants self-reported menopausal status. Of the eleven female participants, six (54%) were post-menopausal, three (27%) were pre-menopausal, and one was perimenopausal. One participant did not report her menopausal status, but did report hot flashes as a cause for waking at night. Additional data are displayed in Table 1. Fasting blood values are reported from the fed day baseline visit. As expected, SI and BMI were correlated (r (16) = −0.42, p = 0.09), thus BMI was included as a covariate in relevant analyses. The correlation is shown in supplementary figure S1. There was no significant correlation between SI and age (r (16) =.13, p =.61).
Effects of the meal on metabolic factors and ratings of hunger
In the fasting condition, there were no significant changes from baseline to post-scan in serum glucose, cholesterol (total, HDL, LDL, and VLDL), or triglycerides (Table 2). There was a significant decrease in plasma insulin levels from pre- to post-scan (t (15) = 2.63, p = 0.02). In the fed condition visit, the mixed meal increased plasma glucose (F (2, 28) = 19.1, p <.001) and insulin (F (2, 26) = 27.4, p <.001), decreased during the MRI scan, but remained significantly higher than baseline. The meal also increased VLDL-cholesterol levels such that levels at post-scan were higher than pre-scan but not significantly different from baseline. To compare the effects of the meal across visits, a paired t-test was performed for metabolites at the final time point. As expected, insulin levels were significantly higher on the fed day compared to the fasting day (14.5 vs 6.4 uU/ml, (t (13) = 3.47, p = 0.004). There was a trend toward higher total cholesterol on the fed day (t (13)=−2.01, p = 0.07), but all other metabolites were non-significant (p’s > 0.22).
Table 2. Effects of fasting and meal on metabolic factors.
During fasting, two measurements were made: baseline, and after completion of the MRI scan. On the meal day, three measurements were made: baseline, 30 minutes after consumption of the mixed meal, and after exiting the scanner. Pre-post measurements were compared using paired t-tests for the fasting day, and repeated-measures analysis of variance on the fed day. Means sharing the same superscript are not significantly different from each other (Fischer’s Least Significant Difference).
| Variable | Fasting | Fed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Scan | df | p | Baseline | Pre-Scan | Post-Scan | F | df | p | |
| Glucose | 80.5 (12.1) | 82.8 (12.8) | 15 | 0.43 | 75.2 (12.9)a | 103.4 (29.6)b | 84.0 (15.5)c | 19.1 | 2,28 | < .001 |
| Insulin | 8.0 (5.0) | 6.4 (5.1) | 15 | 0.02 | 7.2 (5.8)a | 35.2 (20.2)b | 14.5 (12.3)c | 27.4 | 2,26 | < .001 |
| HDL | 48.4 (13.9) | 48.0 (12.7) | 15 | 0.78 | 52.4 (16.1) | 52.8 (17.5) | 51.6 (15.7) | 0.08 | 2,28 | 0.92 |
| LDL | 100.9 (17.8) | 95.9 (29.0) | 14 | 0.35 | 102.5 (30.9) | 100.5 (32.0) | 100.6 (28.3) | 0.56 | 2,26 | 0.58 |
| VLDL | 17.9 (8.4) | 18.1 (9.5) | 14 | 0.86 | 18.3 (11.7)ab | 18.4 (13.4)a | 20.3 (14.7)b | 4.14 | 2,26 | 0.03 |
| Cholesterol | 169.8 (24.0) | 169.4 (21.0) | 15 | 0.93 | 179.7 (29.4) | 177.5 (30.1) | 179.9 (23.7) | 2.15 | 2,28 | 0.14 |
| Triglycerides | 119.1 (123.1) | 114.9 (105.5) | 15 | 0.58 | 113.6 (110.1) | 91.3 (66.7) | 100.9 (74.5) | 1.11 | 2,28 | 0.34 |
The meal significantly increased feelings of fullness (p =.02) and satisfaction (p =.01), and there was a marginally significant decrease in how much participants felt they could eat after the meal (p =.06). The meal did not have significant effects on the other ratings. There were no significant correlations between brain connectivity and subjective ratings (data not shown). All ratings are reported in Supplementary Table S1.
Effects of the meal on brain connectivity
A mixed-meal had significant effects on caudate nucleus connectivity. In the fasting state, the left caudate nucleus displayed stronger connectivity with the precuneus during the fasting condition relative to fed condition (Figure 1a). The head of the right caudate nucleus displayed significantly stronger functional connectivity during fasting (relative to fed) with several regions in the ventral default mode network including the precuneus, parahippocampus, anterior insula, and medial prefrontal cortex (Figure 1b). Cluster information is displayed in Table 3. There were no regions that displayed stronger connectivity in the fed condition relative to fasting for either caudate seed. There were no differences between conditions for EVC.
Figure 1.
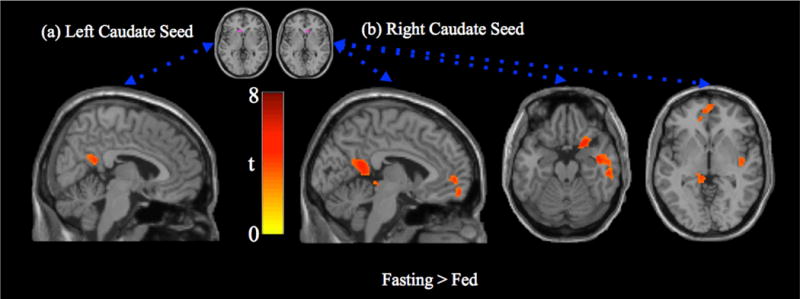
Paired t-test (fasting > fed) for caudate seed region connectivity. (a) Left caudate seed region connectivity is more strongly associated with activity in the posterior cingulate/precuneus in the fasting state (tmin = 2.9, tmax = 4.9). (b) Right caudate seed region connectivity is more strongly associated with the posterior cingulate cortex, medial prefrontal cortex, anterior insula and parahippocampus in the fasting state (tmin = 2.9, tmax = 6.2). There were no regions with stronger connectivity in the fed state.
Table 3. Significant Clusters in Statistical Analyses.
The table displays anatomical regions (Talairach atlas), Brodmann Areas, and Montreal Neurological Imaging (x, y, z) coordinates, as well as cluster size (k) and peak activation (tpeak). (a) Paired t-test (fasting > fed) for right and left caudate seed regions. (b) Regression of seed regions (right caudate, left caudate) and eigenvector centrality (EVC) on insulin sensitivity (SI) covarying for body mass index. (c) Regression of dorsal anterior cingulate cluster connectivity on SI during fasting and fed states.
| Hemisphere | Region | Brodmann Area | x | y | z | k | tpeak | |
|---|---|---|---|---|---|---|---|---|
| (a) t-test | ||||||||
| Right Caudate | R | Inferior Frontal Gyrus, Anterior Insula | 47, 38, 28 | 28 | 16 | −22 | 350 | 6.17 |
| R | Medial Temporal Gyrus, Superior Temporal Gyrus | 20, 22, 13, 38 | 54 | 8 | −14 | 614 | 5.43 | |
| L | Medial Frontal Gyrus, Anterior Cingulate Cortex | 10, 11, 32 | −8 | 60 | −14 | 306 | 4.66 | |
| L | Posterior Cingulate Cortex, Precuneus, Hippocampus, Parahippocampus | 31, 30, 23, 29, 7 | −16 | −32 | −8 | 827 | 5.89 | |
| Left Caudate | R | Posterior Cingulate Cortex, Precuneus | 30, 23 | 2 | −48 | 20 | 152 | 4.9 |
| (b) Regression | ||||||||
| Right Caudate | L | Putamen | −24 | −2 | 4 | 300 | 4.31 | |
| Left Caudate | L | Putamen | −18 | 0 | 4 | 406 | 8.17 | |
| R | Putamen | 18 | 2 | 0 | 399 | 7.64 | ||
| EVC | Bilateral | Anterior Cigulate, Medial Frontal Cortex | 32, 6, 8, 24 | 26 | 2 | 46 | 852 | 5.77 |
| (c) dACC | ||||||||
| Connectivity | ||||||||
| Fasting | R | Middle Temporal Gyrus, Cuneus, Middle Occipital Gyrus | 39, 30, 19, 37, 22 | 36 | −70 | 10 | 1166 | 6.46 |
| L | Middle Occipital Gyrus, Cuneus, Middle Temporal Gyrus | 30, 17 | −26 | −64 | 2 | 674 | 7.02 | |
| Fed | L | Superior Frontal Gyrus, Medial Frontal Gyrus | 9, 10, 32 | −20 | 52 | 26 | 628 | −5.60 |
| R | Postcentral Gyrus, Supramarginal Gyrus | 1, 2, 3, 40 | 44 | −34 | 44 | 769 | 8.24 |
Associations between insulin sensitivity and functional connectivity of the caudate nucleus
In the fasting state, there were no significant associations between SI and functional connectivity for either the left or right caudate seed. However, in the fed condition, SI was predictive of caudate connectivity (Figure 2) such that higher SI was associated with lower connectivity between the left caudate and bilateral putamen (R: tmax(14) = 7.64, p < 0.05, x, y, z = 18, 2, 0, k = 399; and L: tmax(14)= 8.17, p < 0.05, x, y, z = −18, 0, 4, k = 406). The connectivity between the right caudate seed and left putamen trended toward significance (tmax(14)= 4.31, p < 0.05, x, y, z = −24, −2, 4, k = 300, whole brain p < 0.10). All analyses included BMI as a nuisance covariate. To examine the effects of age on the effects, we performed a hierarchical regression. Cluster data (extracted eigenvariate) was regressed on age (step 1) and insulin sensitivity (step 2). Age was not a significant predictor of activity in either cluster (p >.28), and covarying for age did not eliminate the association between insulin sensitivity and either cluster (p’s <.001).
Figure 2.
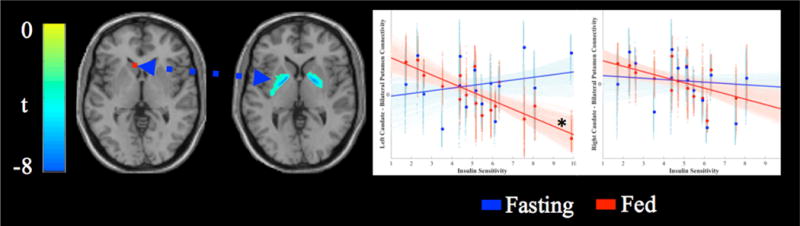
Association between insulin sensitivity and caudate seed connectivity. Activated voxels range from blue (t = −3.01) to green (left peak t = −8.17, right peak t = −7.64). Scatterplots display the association between insulin sensitivity (x-axis) and connectivity between the seed region and bilateral putamen clusters after adjusting for BMI. Solid lines display the best fit line for the mean connectivity of the cluster, faint lines display best fit lines for each voxel within the cluster. Fasting data are presented in blue, data from the fed state are presented in red. The left caudate seed was significantly associated with insulin sensitivity in the fed state (*).
Correlations between the insulin rise after a meal and caudate-putamen connectivity
To explore the relationship between the insulin rise following a meal and striatum connectivity, the insulin change from baseline to 30 minutes post-meal ingestion was calculated. Connectivity values for left and right putamen were regressed on the change score while adjusting for SI and BMI. The change in insulin following meal ingestion predicted left caudate connectivity with the right putamen (Fchange (1,11) = 7.24, p = 0.02, R2change = 0.14) such that a larger rise in insulin following the meal predicted lower connectivity between the left caudate and right putamen (B = −0.46, t (11) = −2.69, p < 0.05). The relationships between glucose rise and caudate connectivity were nonsignificant after adjusting for BMI and SI (p’s > 0.38).
Eigenvector Centrality
In the fasting state, there were no significant associations between SI and EVC. However, in the fed condition, SI was predictive of EVC (Figure 3) such that higher SI was associated with stronger centrality of the dorsal anterior cingulate cortex (tmax(15) = 5.77, p < 0.05, x, y, z = 26, 2, 46, k = 852). Both analyses included BMI as a nuisance covariate.
Figure 3.
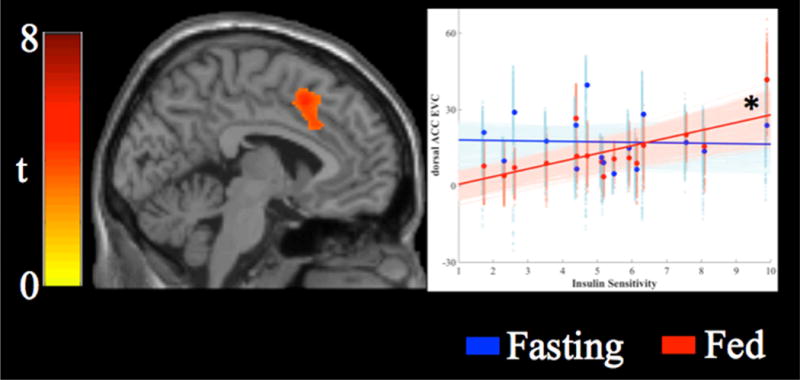
Association between insulin sensitivity and eigenvector centrality. Insulin sensitivity was associated with increased eigenvector centrality in the dorsal anterior cingulate cortex (tmin = 3.0, tmax = 5.8). Scatterplots display the association between insulin sensitivity (x-axis) and connectivity between the seed region and each cluster. Solid lines display the best fit line for the mean connectivity of the cluster, faint lines display best fit lines for each voxel within the cluster. Fasting data are presented in blue, data from the fed state are presented in red. Eigenvector centrality in the dorsal anterior cingulate cortex was significantly associated with insulin sensitivity in the fed state (*).
Associations between insulin sensitivity and functional connectivity of the dorsal anterior cingulate cortex
To further examine the effects of a meal on functional connectivity, we extracted the activity of the dACC cluster and used it as a seed region. In the fasting state, individuals with higher SI had stronger connectivity between the dACC and bilateral clusters including the middle temporal gyrus, posterior cingulate cortex and occipital cortex (Figure 4a; R: tmax(14) = 7.02, p < 0.05, x, y, z =−26, −64, 2, k = 674; L: tmax(14) = 6.46, p < 0.05, x, y, z = 36, −70, 10, k = 1166). In the fed state, individuals with higher SI had lower connectivity between the dACC and a region in the left superior/medial frontal gyrus (Figure 4b; tmax(14) = 5.60, p < 0.05, x, y, z = −20, 52, 26, k = 628), but stronger connectivity between the dACC and the right postcentral gyrus (Figure 4c; tmax(14) = 8.24, p < 0.05, x, y, z = 44, −34, 44, k = 769).
Figure 4.
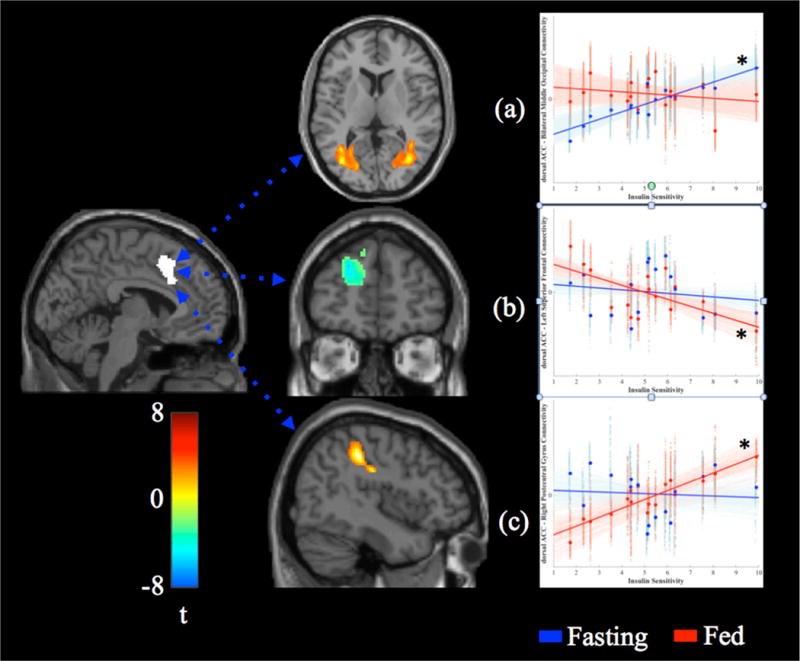
Associations between insulin sensitivity (SI) and dorsal anterior cingulate (dACC) connectivity in the fasting and fed state. In the fasting state (a), higher SI was associated with stronger connectivity between the dACC and bilateral middle temporal gyrus/occipital cortex. In the fed state, higher SI was associated with weaker connectivity between the dACC and left superior/medial frontal gyrus (b), but stronger connectivity between the dACC and right postcentral gyrus (c). Solid lines display the best-fit line for the mean connectivity of the cluster, faint lines display best fit lines for each voxel within the cluster. Fasting data are presented in blue, data from the fed state are presented in red. Significant associations are marked with an asterisk (*).
DISCUSSION
The aim of the current study was to examine whether there was an association between systemic insulin sensitivity and the functional connectivity of the brain following a meal covarying for BMI. We utilized both a model-based approach including a seed region in the caudate nucleus, as well as a data-driven approach using EVC. We found that the caudate nucleus was more strongly connected to several regions in the fasting state relative to the fed state. These regions included anterior insula, hippocampus, as well as anterior and posterior cingulate. In the fasting state, there were no associations between SI and the functional connectivity of the caudate nucleus or EVC. However, following a meal, an association emerged such that SI was inversely associated with the functional connectivity between the caudate and putamen after adjusting for BMI. Conversely, SI was positively associated with EVC in the dACC – a region central to cognitive control. These data suggest that after a meal, individuals who are more sensitive to insulin show a reduction in reward network functional connectivity, but an increase in cognitive control networks. This finding could help explain the neural network changes that underlie overeating, or failure to stop eating that could lead to the further development of obesity.
A primary focus of the present study was examining the connectivity of the head of the caudate nucleus - a region involved in taste processing and reward(Huerta et al., 2014; Stice et al., 2013) that shows altered reactivity to taste stimuli in obese individuals(Nummenmaa et al., 2012; E. Stice et al., 2008). Utilizing a seed-based method, we found that in the fasting state the head of the caudate was more strongly connected to regions involved in interoception and the ventral default mode network relative to in the fed condition. These data are in line with previous findings that a meal replacement reduces connectivity in regions in what has been termed the “brain network for appetite”(Paolini et al., 2014). The insula is involved in interoception(Craig, 2009) as well as in gustatory processing(Veldhuizen et al., 2011) and autonomic control(Ryan et al., 2012). Although the insula is not included in the traditional “loop” models of striatal organization (Alexander et al., 1990), several studies of functional connectivity of the caudate have identified insula-caudate connectivity(Postuma and Dagher, 2006; Robinson et al., 2012) as well as alterations in caudate-insula connectivity in obese individuals during anticipatory reward processing(Nummenmaa et al., 2012). Additionally, in a meta analysis of the neural bases of food perception, the insula emerged as a significant region associated with taste receipt, likely related to its role as primary gustatory cortex(Huerta et al., 2014). In addition to its role in taste processing, the insula has been identified as a hub of the salience network, which is central to the integration of sensory and cognitive information(Menon and Uddin, 2010; Seeley et al., 2007). Combined, these data suggest that in the fasting state (relative to after a meal), interoceptive and self-referential networks are more closely tied to reward regions – perhaps suggesting that individuals would be more tuned toward reward receipt relative to after a meal.
Following the mixed-meal, resting state functional connectivity of the striatum differed based upon an individual’s level of insulin sensitivity. Specifically, individuals who were more sensitive to insulin had lower connectivity between the caudate nucleus and putamen relative to individuals who were less sensitive to insulin. These associations were not present in the fasting state, suggesting that the meal has the effect of reducing connectivity within the striatum, but this effect is stronger in individuals who are more sensitive to insulin. In a recent study of overweight Hispanic girls, insulin sensitivity was negatively associated with neural reactivity to high-calorie food pictures in the insula, putamen and ACC (Adam et al., 2015). The present results extend these findings by suggesting that the relationship between insulin sensitivity and the brain extends beyond discrete regions and is associated with brain network architecture, even when at rest. The putamen is a central component of the striatum, subserving reward, cognition, and movement(Alexander et al., 1990). Activity in the putamen has been linked to a shift from goal-directed to habit-learned behavior, specifically in the context of food satiation(Tricomi et al., 2009). A study of the functional connectivity of the putamen has mapped somatomotor specific regions within the striatum(Choi et al., 2012). Notably, the area of the putamen that we found to show altered connectivity with the caudate seed region overlaps with the region of the putamen functionally linked to the tongue. The processing of taste is transduced through the brainstem, thalamus and taste cortices (insula/operculum and orbitofrontal cortex) prior to reaching the striatum, which then guides habit and behavior(Rolls, 2012). Thus, by understanding alterations in the striatum we can potentially understand a crucial link between the sensations involved in food intake and the behavior and habits that may promote food seeking. Although the present study only examined resting state functional connectivity, these findings may have relevance for other studies involving the anticipation and receipt of palatable tastes.
In the fed state, systemic insulin sensitivity (SI) was predictive of EVC in the dACC. The dACC is a key network hub involved in the monitoring of actions, and shifting behavior when appropriate(Shenhav et al., 2016). In our data, higher SI was associated with stronger EVC in the dACC consistent with a model of insulin sensitive individuals “switching” from reward to cognitive control networks after a meal. This association was not present in the fasting state suggesting that it is the effects of the post-prandial state that are altering network architecture. Recent work has demonstrated that intranasal insulin administration can improve executive functioning(Krug et al., 2010), potentially via alterations in reward and executive functioning regions(Kullmann et al., 2013). Future studies that selectively manipulate circulating insulin are needed to confirm the role of insulin in modulating cognitive control networks, as well as how these relationships are altered in insulin resistant individuals.
In order to extend our understanding of the significance of the dACC centrality and relationship to SI, we performed a functional connectivity analysis. The analysis found that, when fasting, individuals with higher SI had stronger connectivity between the dACC and clusters in the middle temporal gyrus extending into the occipital lobe. Previous studies have found differences in responses to food stimuli in occipito-temporal regions with greater reactivity when in the fasted state relative to after a meal (Frank et al., 2010). The current data would support a model in which healthy individuals (i.e., those with higher SI) have a strengthened connectivity between occipito-temporal regions and the salience network, but after a meal these connections are weakened. In the fed state, however, individuals with higher SI exhibited stronger connectivity between the dACC and postcentral gyrus, but weaker connectivity between the dACC and the superior/middle frontal gyrus. The postcentral gyrus is a hub of the sensorimotor network that is consistently activated in food-stimuli paradigms (Huerta et al., 2014), and future research will be needed to more fully understand the behavioral implications of these changes in connectivity. However, these data do support a model in which healthier individuals show a shift from differences in connectivity in ventral brain regions towards more dorsal regions following a meal, possibly reflecting a shift from reward/affect toward more executive/self-control states.
A limitation of the current study is that the mixed meal has effects on numerous hormones (e.g. insulin, ghrelin, etc.), so we are unable to conclude that this effect is directly due to insulin signaling. However, future studies that involve more controlled manipulations of insulin levels (e.g. hyperinsulinemic euglycemic clamp) may be able to provide more mechanistic insight into what is driving the association. In the current study, we investigated the relationship between the insulin rise following the meal and caudate-putamen connectivity while statistically covarying for SI. Notably, a larger insulin rise was predictive of lower caudate-putamen connectivity whereas glucose rise was not. These data suggest that the changes in functional connectivity are more strongly related to changes in insulin levels, rather than changes in blood glucose.
In healthy individuals, intranasal insulin suppresses brain responses to food stimuli(Guthoff et al., 2010) and has been shown to alter resting state connectivity in reward regions(Kullmann et al., 2012). The present study builds upon these findings to show that SI is an independent predictor of resting state caudate connectivity following a meal, independent of BMI. These associations were not present in the fasting state, suggesting that post-prandial signaling between the periphery and central nervous system is altered in insulin resistant individuals. Although SI is associated with a larger rise in insulin following a meal, when we statistically adjusted for SI, post-prandial insulin rise was a significant predictor of caudate connectivity lending support to the role of insulin.
The influence of insulin in reward signaling within the striatum has been well documented in animal models(Figlewicz and Benoit, 2009). In addition to signaling in the brainstem via the ventral tegmental area(Figlewicz et al., 1994), recent work has demonstrated that insulin exerts direct effects on neurons in the nucleus accumbens and increases dopamine release, thereby serving as a reward signal(Stouffer et al., 2015). Alterations in dopamine receptors and signaling have been documented in obese individuals(Wang et al., 2001) suggesting that alterations in food reward may occur in obese individuals – similar to neural alterations that occur in other chemical addictions(Volkow et al., 2011). The current study extends these by demonstrating insulin resistance, independent of obesity, may alter the ability of insulin to serve as a feedback signal following a meal. Future studies using dopamine receptor ligands would provide significant insight into the role of dopamine as a mediator between insulin resistance, insulin signaling, and dopamine following meal intake. An additional limitation of the current data is the broad concept of “reward” – a concept that has many aspects including motivation, hedonic state, and reinforcement. The present study was not designed to measure which aspects of reward are altered in insulin resistance, but future studies are needed to explicitly identify which aspects of reward may be altered.
Although changes in plasma glucose and insulin levels were monitored, this study did not measure circulating hormones that affect appetite and have neurobiological targets. Future studies are needed to fully characterize the specific effects of insulin independent of other hormones, potentially through the use of euglycemic-hyperinsulinemic clamps. A second limitation is the range of adiposity in the study population. Although we statistically adjusted for BMI in all analyses, future studies limited to normal weight, overweight or obese individuals would be better suited to identify the interaction between insulin resistance and obesity on brain network connectivity. The present study also utilized a relatively small sample size. In order to account for this in the neuroimaging analyses, statistical non-parametric mapping was utilized, as it does not assume a normal distribution – a common problem in smaller sample sizes. Participants completed limited self-report on the experience of the meal thereby limiting the ability to infer relationships between brain connectivity and subjective experience of reward.
In conclusion, this is the first study to examine associations between SI and resting state brain connectivity both during fasting and after a meal while covarying for BMI. We found that resting state functional connectivity between the caudate nucleus and putamen was decreased following a meal. However, this effect was blunted in individuals with less insulin sensitivity. The change in connectivity was associated with the rise in insulin after a meal, but not in glucose suggesting insulin signaling is responsible for the change in connectivity. Individuals with higher SI had stronger EVC in the dACC following a meal, suggesting a strengthening of cognitive control networks. Future studies are needed to specifically manipulate circulating insulin (e.g. hyperinsulinemic clamp) and identify the behavioral effects (e.g. craving, self control) of these changes in connectivity. These studies could provide key insights into how changes in insulin sensitivity affect brain structures involved in food reward.
Supplementary Material
Acknowledgments
The authors thank the staff members of the University of Pittsburgh Montefiore Clinical Translational Research Center and the Department of Radiology for their assistance in conducting this study. J.R. designed the study, collected and analyzed data and wrote the manuscript, H.K. performed data preprocessing and analyses, H.A. assisted with experimental design and analysis, N.H. performed all glucose tolerance testing, F.G.S.T. designed the IVGTT studies, collected and analyzed the data, and edited the manuscript. All authors provided written feedback on the manuscript. J.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity and accuracy of the data analysis.
FUNDING
This work was supported by the National Institutes of Health (grant numbers UL1 RR024153, UL1 TR000005, K01 DK095759, R03 DK108976, and R21 DK082878 (FGST).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
References
- Adam TC, Tsao S, Page KA, Hu H, Hasson RE, Goran MI. Insulin sensitivity and brain reward activation in overweight Hispanic girls: a pilot study. Pediatr Obes. 2015;10:30–36. doi: 10.1111/j.2047-6310.2013.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Alsaadi HM, Van Vugt DA. Insulin sensitivity affects corticolimbic brain responses to visual food cues in polycystic ovary syndrome patients. Horm Mol Biol Clin Investig. 2015;24:101–115. doi: 10.1515/hmbci-2015-0048. [DOI] [PubMed] [Google Scholar]
- Anthony K, Reed LJ, Dunn JT, Bingham E, Hopkins D, Marsden PK, Amiel SA. Attenuation of Insulin-Evoked Responses in Brain Networks Controlling Appetite and Reward in Insulin Resistance. Diabetes. 2006;55:2986–2992. doi: 10.2337/db06-0376. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig ADB. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Del Parigi A, Gautier JF, Chen K, Salbe AD, Ravussin E, Reiman E, Tataranni PA. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci. 2002;967:389–397. [PubMed] [Google Scholar]
- Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol. 2009;296:R9–R19. doi: 10.1152/ajpregu.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Szot P, Chavez M, Woods SC, Veith RC. Intraventricular insulin increases dopamine transporter mRNA in rat VTA/substantia nigra. Brain Res. 1994;644:331–334. doi: 10.1016/0006-8993(94)91698-5. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, Fritsche A, Preissl H. Processing of food pictures: Influence of hunger, gender and calorie content. Brain Res, Neural Mechanisms of Ingestive Behaviour and Obesity. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A. Insulin in the brain: sources, localization and functions. Mol Neurobiol. 2013;47:145–171. doi: 10.1007/s12035-012-8339-9. [DOI] [PubMed] [Google Scholar]
- Gray SM, Meijer RI, Barrett EJ. Insulin regulates brain function, but how does it get there? Diabetes. 2014;63:3992–3997. doi: 10.2337/db14-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthoff M, Grichisch Y, Canova C, Tschritter O, Veit R, Hallschmid M, Häring HU, Preissl H, Hennige AM, Fritsche A. Insulin modulates food-related activity in the central nervous system. J Clin Endocrinol Metab. 2010;95:748–755. doi: 10.1210/jc.2009-1677. [DOI] [PubMed] [Google Scholar]
- Heni M, Kullmann S, Gallwitz B, Häring HU, Preissl H, Fritsche A. Dissociation of GLP-1 and insulin association with food processing in the brain: GLP-1 sensitivity despite insulin resistance in obese humans. Mol Metab. 2015;4:971–976. doi: 10.1016/j.molmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heni M, Kullmann S, Ketterer C, Guthoff M, Bayer M, Staiger H, Machicao F, Häring HU, Preissl H, Veit R, Fritsche A. Differential effect of glucose ingestion on the neural processing of food stimuli in lean and overweight adults. Hum Brain Mapp. 2014;35:918–928. doi: 10.1002/hbm.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta CI, Sarkar PR, Duong TQ, Laird AR, Fox PT. Neural bases of food perception: coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obes Silver Spring Md. 2014;22:1439–1446. doi: 10.1002/oby.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CN, Abbasi F, Carantoni M, Polonsky KS, Reaven GM. Roles of insulin resistance and obesity in regulation of plasma insulin concentrations. Am J Physiol Endocrinol Metab. 2000;278:E501–508. doi: 10.1152/ajpendo.2000.278.3.E501. [DOI] [PubMed] [Google Scholar]
- Joyce KE, Laurienti PJ, Burdette JH, Hayasaka S. A new measure of centrality for brain networks. PloS One. 2010;5:e12200. doi: 10.1371/journal.pone.0012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer NB, Krebs L, Kobiella A, Grimm O, Vollstädt-Klein S, Wolfensteller U, Kling R, Bidlingmaier M, Zimmermann US, Smolka MN. (Still) longing for food: insulin reactivity modulates response to food pictures. Hum Brain Mapp. 2013;34:2367–2380. doi: 10.1002/hbm.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R, Benedict C, Born J, Hallschmid M. Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory. J Clin Endocrinol Metab. 2010;95:E468–472. doi: 10.1210/jc.2010-0744. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Frank S, Heni M, Ketterer C, Veit R, Häring HU, Fritsche A, Preissl H. Intranasal insulin modulates intrinsic reward and prefrontal circuitry of the human brain in lean women. Neuroendocrinology. 2013;97:176–182. doi: 10.1159/000341406. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Frank S, Heni M, Ketterer C, Veit R, Häring HU, Fritsche A, Preissl H. Intranasal Insulin Modulates Intrinsic Reward and Prefrontal Circuitry of the Human Brain in Lean Women. Neuroendocrinology. 2012 doi: 10.1159/000341406. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol Rev. 2016;96:1169–1209. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- Lips MA, Wijngaarden MA, van der Grond J, van Buchem MA, de Groot GH, Rombouts SARB, Pijl H, Veer IM. Resting-state functional connectivity of brain regions involved in cognitive control, motivation, and reward is enhanced in obese females. Am J Clin Nutr. 2014;100:524–531. doi: 10.3945/ajcn.113.080671. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Margulies DS, Horstmann A, Pleger B, Lepsien J, Goldhahn D, Schloegl H, Stumvoll M, Villringer A, Turner R. Eigenvector Centrality Mapping for Analyzing Connectivity Patterns in fMRI Data of the Human Brain. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, Nuutila P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PloS One. 2012;7:e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott V, Benedict C, Schultes B, Born J, Hallschmid M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes Obes Metab. 2012;14:214–221. doi: 10.1111/j.1463-1326.2011.01490.x. [DOI] [PubMed] [Google Scholar]
- Paolini B, Laurienti PJ, Norris J, Rejeski WJ. Meal replacement: calming the hot-state brain network of appetite. Eat Behav. 2014;5:249. doi: 10.3389/fpsyg.2014.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Porte D, Baskin DG, Schwartz MW. Insulin Signaling in the Central Nervous System A Critical Role in Metabolic Homeostasis and Disease From C. elegans to Humans. Diabetes. 2005;54:1264–1276. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal Ganglia Functional Connectivity Based on a Meta-Analysis of 126 Positron Emission Tomography and Functional Magnetic Resonance Imaging Publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PM, Uecker A, Friehs G, Young KA, Griffin JL, Lovallo WR, Fox PT. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60:117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Taste, olfactory and food texture reward processing in the brain and the control of appetite. Proc Nutr Soc. 2012:1–14. doi: 10.1017/S0029665112000821. [DOI] [PubMed] [Google Scholar]
- Ryan JP, Sheu LK, Critchley HD, Gianaros PJ. A neural circuitry linking insulin resistance to depressed mood. Psychosom Med. 2012;74:476–482. doi: 10.1097/PSY.0b013e31824d0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci Off J Soc Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. 2016;19:1286–1291. doi: 10.1038/nn.4384. [DOI] [PubMed] [Google Scholar]
- Stice E, Burger KS, Yokum S. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am J Clin Nutr. 2013;98:1377–1384. doi: 10.3945/ajcn.113.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation Between Obesity and Blunted Striatal Response to Food Is Moderated by TaqIA A1 Allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, de Vaca SC, Reith MEA, Carr KD, Rice ME. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 2015;6:8543. doi: 10.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O’Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundström JN. Identification of Human Gustatory Cortex by Activation Likelihood Estimation. Hum Brain Mapp. 2011;32:2256–2266. doi: 10.1002/hbm.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and Drug Reward: Overlapping Circuits in Human Obesity and Addiction. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusll N, Fowler JS. Brain dopamine and obesity. The Lancet. 2001;357:354–357. doi: 10.1016/S0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wasko MCM, McClure CK, Kelsey SF, Huber K, Orchard T, Toledo FGS. Antidiabetogenic effects of hydroxychloroquine on insulin sensitivity and beta cell function: a randomised trial. Diabetologia. 2015;58:2336–2343. doi: 10.1007/s00125-015-3689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink AM, de Munck JC, van der Werf YD, van den Heuvel OA, Barkhof F. Fast eigenvector centrality mapping of voxel-wise connectivity in functional magnetic resonance imaging: implementation, validation, and interpretation. Brain Connect. 2012;2:265–274. doi: 10.1089/brain.2012.0087. [DOI] [PubMed] [Google Scholar]
- Zavaroni I, Bonini L, Fantuzzi M, Dall’Aglio E, Passeri M, Reaven GM. Hyperinsulinaemia, obesity, and syndrome X. J Intern Med. 1994;235:51–56. doi: 10.1111/j.1365-2796.1994.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, Milham MP. Network centrality in the human functional connectome. Cereb Cortex N Y N 1991. 2012;22:1862–1875. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


