Abstract
Recent findings suggest that the dorsolateral prefrontal cortex (DLPFC), a region consistently associated with impulse control, is vulnerable to transient suppression of its activity and attendant functions by excessive stress and/or cognitive demand. Using functional magnetic resonance imaging, we show that a capacity-exceeding cognitive challenge induced decreased DLPFC activity and correlated increases in the preference for immediately available rewards. Consistent with growing evidence of a link between working memory capacity and delay discounting, the effect was inversely proportional to baseline performance on a working memory task. Subjects who performed well on the working memory task had unchanged, or even decreased, delay discounting rates, suggesting that working memory ability may protect cognitive control from cognitive challenge.
Introduction
The subjective value of a reward has been shown to decrease as a function of delay to delivery, a phenomenon known as delay discounting (DD) (van den Bos and McClure, 2013). Delay discounting underlies the temptation to choose smaller, immediate rewards over larger, delayed rewards, and cognitive control has been posited to contribute to the ability to overcome such temptations (McClure et al., 2004; Berns et al., 2007; Peters and Büchel, 2011; van den Bos and McClure, 2013). In neuroimaging studies, cortical volume and activity in regions associated with cognitive control, such as dorsolateral prefrontal cortex (DLPFC) (Miller and Cohen, 2001), have been shown to predict selection of larger-later over smaller-sooner rewards (McClure et al., 2004; Bjork et al., 2009; Kim and Lee, 2011; Peters and Büchel, 2011; Gianotti et al., 2012; van den Bos and McClure, 2013). Moreover, disorders associated with impulsivity, such as gambling and addiction, have been associated with increased rates of delay discounting (Bickel et al., 2007) and impaired cognitive control (Goldstein and Volkow, 2011).
Converging lines of investigation have revealed that the DLPFC is vulnerable to transient suppression of its activity and attendant functions, including cognitive control, by excessive stress (Arnsten et al., 2012) or cognitive demand (Yun et al., 2010; Friese et al., 2013). Furthermore, it has been suggested that the capacity for self-control is limited and vulnerable to temporary depletion by excessive loads (Muraven and Baumeister, 2000). Studies investigating the effects of cognitive demand and stress on delay discounting have yielded mixed results (Hinson et al., 2003; Haushofer et al., 2013). Hinson and colleagues (2003) found that maintaining longer strings of digits in memory was associated with greater discounting of delayed rewards. However, a reanalysis of the data revealed that increased discount rates could be accounted for by increased random responding likely induced by the distraction of having to perform the memory task simultaneously (Franco-Watkins et al., 2006). Haushofer et al. (2013) found that neither subjective stress ratings, nor free cortisol levels assayed from salivary samples, were associated with changes in discount rates.
A fundamental cognitive function strongly associated with DLPFC activity is working memory (Braver et al., 1997; Yun et al., 2010) and a growing body of evidence has highlighted a potential link between working memory capacity and impulse control (Nichols and Wilson, 2015). Recent studies have demonstrated substantial overlap of prefrontal regions implicated in delay discounting and working memory (Wesley and Bickel, 2014), while working memory capacity has been shown to be inversely proportional to discount rates (Shamosh et al., 2008). Furthermore, Bickel and colleagues found that working memory training was associated with decreased impatience (Bickel et al., 2011).
Using functional magnetic resonance imaging (fMRI), we investigated the role of cognitive control in delay discounting using a task that has been shown to induce decreased DLPFC activity and function, in association with increased amygdala activity (Yun et al., 2010). The amygdala is thought to play an important role in mediating the effect of emotion on cognitive processing (Simpson et al., 2000; Dolcos and McCarthy, 2006). In previous work, we found that amygdala activity following cognitive challenge was associated with impaired cognitive performance (Yun et al., 2010). In the current study, participants completed blocks of delay discounting trials involving binary choices between monetary reward options that varied in magnitude and delay to delivery (Fig. 1). Half of the delay discounting blocks followed a high-load working memory block (4-back), while the other half followed a low-load block (1-back). The 4-back task was intended to exceed working memory capacity, leading to increased negative affect and stress, as well as impaired cognitive control. We measured discount rates and neural activity after exposure to high- and low-load working memory challenge. We hypothesized that the cognitive challenge imposed by the 4-back would transiently persist into subsequent delay discounting task blocks, increasing amygdala activity and decreasing DLPFC recruitment. Moreover, we hypothesized that the degree to which amygdala activity increased, and DLPFC activity decreased, following 4-back exposure, would reflect depletion of cognitive control, and hence, would be associated with greater discount rates following the 4-back task. Finally, we hypothesized that individual differences in baseline cognitive capacity, as measured by working memory task performance, would be reflected in individual differences in the effects of cognitive challenge on delay discounting.
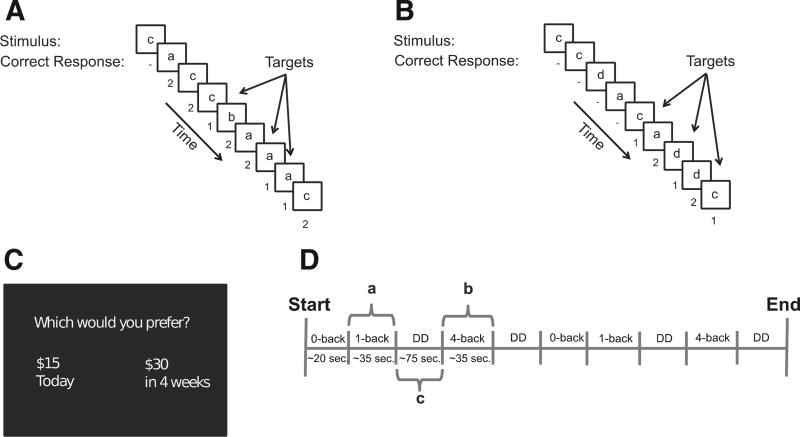
Fig. 1.
Experimental design. A) Sample 1-back task. B) Sample 4-back task. C) Sample delay discounting trial. D) Task structure. Participants completed 4 runs with counterbalanced order of 4-back and 1-back.
Materials and methods
Participants
Nineteen subjects were recruited to participate in the experiment, including 14 males. The mean age was 33.6 years (SD = 9.3). The Structured Clinical Interview for DSM-IV (SCID) (First et al., 2002) was used to confirm that subjects had no history of significant psychiatric illness. Informed consent was obtained using a consent form and procedures approved by the Institutional Review Board at the University of California, San Francisco. Subjects were recruited using an online bulletin board.
Delay discounting-working memory task
While undergoing fMRI scanning, participants performed a delay discounting-N-back working memory (DD-WM) task, which employed a mixed block and event-related design with 4 block types: 0-back, 1-back, 4-back, and delay discounting blocks (Fig. 1). The 0-back working memory condition was included as a low-demand condition to prevent participant fatigue. Participants completed four runs of the DD-WM task, with each run composed of 10 blocks (2 blocks of each working memory N-back load condition and 4 delay discounting blocks). The delay discounting blocks were presented directly after low (post-1-back) or high (post-4-back) N-back working memory loads. The order of blocks was counterbalanced across task runs with the constraint that delay discounting blocks always followed either 1-back or 4-back working memory blocks. We measured delay discounting behavior and neural activity after exposure to high (4-back) and low (1-back) working memory challenge.
The delay discounting trial items involved binary choices between monetary rewards varying by reward magnitude and time of delivery (Fig. 1C). The delay discounting trial items were constructed using an approach very similar to our previous studies (McClure et al., 2004, 2007). The magnitude of rewards was determined in the following manner: representative hyperbolic discounting parameter estimates from the literature were used to produce a range for constructing choices (0.0008–0.0714). A set of 32 candidate discounting parameter values was generated using an evenly spaced sequence across this range. The larger-later dollar amount was randomly drawn from a Gaussian distribution with mean $40 and standard deviation $10 that was clipped at $30 and $50. This value was used to calculate the smaller-sooner reward amount according to a discounting parameter (k) value randomly drawn (without replacement) from the set of candidate k values. The same set of candidate k values was used for both post-1-back and post-4-back delay discounting conditions and all participants faced the same 128 intertemporal choice pairs. The delay to the smaller-sooner reward was either “Today”, 2 weeks, or 4 weeks. The delay between the smaller-sooner and larger-later rewards was either 2 weeks or 4 weeks. In order to ensure incentive compatibility, one trial was randomly chosen following the experiment and paid to the participant, at the delay specified in the trial, in the form of a (post-dated) check.
Data analysis
Behavioral dependent variables of interest were i) 4-back performance accuracy, defined as the overall percentage of targets that were correctly identified, and ii) the discount rate. Within-subjects estimates of the discount rate were obtained using maximum likelihood estimation to fit participants' choice behavior with the hyperbolic discounting function, which assumes that the subjective value (V) of a reward of magnitude r available after delay t is given by
| (1) |
where k is the individually determined discounting parameter. Larger values of k indicate greater rates of delay discounting (i.e., greater impatience). To model trial-by-trial behavior, we employed a logistic (softmax) choice rule, which computes the probability (PSS) of choosing the smaller-sooner (SS) option on any given trial as a function of the difference between VSS and VLL:
| (2) |
where the sensitivity parameter m > 0 quantifies the extent to which the model's choice is determined by the difference in subjective value for options SS and LL. When m = 0, choice behavior is random. Increasing values of m indicate increasingly consistent, less “noisy” responding. This approach allowed us to test for evidence of an increase in noisiness of responding following cognitive challenge. In our study, there was no significant difference between average estimates of m for post-4-back DD vs. post-1-back DD (paired t-test, p = 0.34). This is of particular relevance because a reanalysis of the results of a highly cited behavioral study that investigated the role of working memory load on delay discounting with a different paradigm (Hinson et al., 2003) revealed that increased estimates of discounting could be accounted for by increased random responding likely induced by the distraction of having to perform the secondary task (Franco-Watkins et al., 2006). We followed the convention in the literature of normalizing the k estimates using the natural logarithm (Kirby, 1997). Five subjects chose only one of the options on all or nearly all trials. These ceiling/floor results prevented estimation of the relevant parameter for these subjects and, as a result, these subjects were excluded from analyses involving the parameter estimates. Estimation of the discounting parameter was carried out with custom MatLab code. Changes in delay discounting following cognitive challenge were calculated as post-4-back log(k) − post-1-back log(k).
MR data acquisition and processing
fMRI data were acquired at the University of California, San Francisco Neuroscience Imaging Center on a Siemens 3 Tesla TIM Trio scanner with a 12-channel head coil. Functional images were collected along an oblique axial plane defined by the anterior commissure–posterior commissure line with the following echoplanar sequence: TR = 2 sec, TE = 29 msec, flip angle = 75°, FOV = 240 mm, 64 × 64 matrix, 32 slices acquired in a sequential ascending sequence with a 1 mm interslice gap. Acquired voxel dimensions were 3.75 × 3.75 × 3.50 mm. To mitigate non-equilibrium effects, images corresponding to the first 4 TRs of each run were discarded from analysis. High-resolution T1-weighted structural scans were acquired in the sagittal plane using an MP-RAGE sequence with the following parameters: TR = 2.3 sec, TE = 2.95 ms, flip angle = 9°, FOV = 256 mm, voxel dimensions were 1.0 × 1.0 × 1.2 mm, for 160 slices acquired.
Image processing was performed with Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Image preprocessing included motion correction (INRIAlign; http://www.fil.ion.ucl.ac.uk/spm/ext/#INRIAlign) via affine registration of all runs, where the first image of each run was realigned to the first image of the first run, and then re-alignment proceeded within each run. Images were then slice-time corrected with respect to the middle slice. The Artifact Detection Tools (ART) toolbox (http://www.nitrc.org/projects/artifact_detect) was then used to identify outlying volumes in the time series based on global image intensity values (>Z = 3) and head motion (>2 mm translational movement in x, y, or z plane or >0.02 degree rotation in yaw, pitch, or roll).
Next, aCompCor (Behzadi et al., 2007), a principal components-based approach to noise reduction of BOLD time series data, was applied. ACompCor is based on a spatial principal components analysis (PCA) of BOLD time series data from noise regions of interest (ROIs), defined from spatially eroded masks of the participant's white matter and cerebrospinal fluid (CSF) tissue compartments. The white matter and CSF masks were derived by segmentation of the participant's high-resolution T1-weighted structural scan using SPM8. In order to minimize partial voluming of these noise ROIs with gray matter, the white matter mask was eroded by first applying a >.99 threshold followed by erosion by 2 voxels in X, Y, and Z directions, and subsequent further exclusion of gray matter voxels by subtracting out any voxels falling within a gray matter binary mask. The gray matter binary mask was derived from the Talairach–Daemon-based Wake Forest University PickAtlas (Maldjian et al., 2003) and was reverse normalized into the subject's native space using the inverse of the transformation matrix generated by normalization of the participant's high-resolution T1 scan to the MNI 152 brain template. The CSF mask was eroded by first applying a >0.90 threshold, then applying a 3-dimensional 2-voxel nearest-neighbor criterion that dropped CSF voxels with less than 2 CSF nearest neighbors. A binary union mask of these noise ROIs was generated and co-registered to the mean functional scan. Before proceeding with a principal component analysis (PCA) of the functional time series data of voxels contained in this white matter/CSF noise ROI mask, voxels in the mask that showed even weak relationship with the task regressors (p <0.2) were excluded. Time series data for the remaining voxels in the noise ROI mask were then subjected to a PCA, and a number of noise components comprising weighted averages of white matter and CSF voxel time series were identified. PCA components were saved for use as nuisance regressors in the 1st level statistical model if their associated eigenvalues were greater than the eigenvalues generated by 200 PCA iterations on random normal data of equal size and rank to the noise ROI data matrix (p <.05). This process (PCA estimation and Monte Carlo simulation to determine “significant” noise components) was done separately for each run. Data were then spatially smoothed with a 6 mm FWHM Gaussian filter.
For individual participant (first-level) analyses, SPM's canonical hemodynamic response function (HRF; a double gamma function, with a temporal derivative term) was convolved with task stimulus vectors denoting the onsets and durations of delay discounting trials, modeled as event epochs, and N-back working memory blocks, modeled as block epochs, yielding first-level task regressors and their temporal derivatives. Temporal derivatives of the task regressors were included in the first-level model to account for small latency variation in the hemodynamic response and its fit to the canonical HRF. Seven motion parameters, calculated via the ART toolbox, consisting of the temporal derivatives of the 6 motion parameters as well as a summary measure of total motion, were included as regressors to remove fluctuations in BOLD signal attributable to participant head movement. To further ensure that the data were optimally cleaned of noise, regressors were also included for i) data points identified by the ART toolbox as outliers and ii) the aCompCor noise components. The spatially smoothed BOLD image time series were modeled with the aforementioned regressors. Parameters (i.e., beta coefficients) representing the fit of each regressor to a voxel's time series were estimated using the general linear model after applying a high pass temporal filter (128 s cut-off) to remove low-frequency noise. Mean beta images were calculated across the task runs and contrast images for key task comparisons were derived by subtracting the corresponding beta images. The mean functional image, derived from the motion correction preprocessing step, was normalized to a standard neuroanatomical space (Montreal Neurological Institute's MNI-EPI template; http://www.bic.mni.mcgill.ca), resulting in 3 mm3 isotropic voxel dimensions, and the normalization parameters were applied to first-level beta and contrast images. Second-level analyses were then conducted on the primary fMRI contrasts of interest, 4-back − 1-back, post-4-back DD − post-1-back DD.
The next three sets of analyses were undertaken to examine fMRI activations during the DD-WM task. First, fMRI activations were contrasted between high (4-back) and low (1-back) working memory loads in order to demonstrate activation of the well-described prefrontal-parietal working memory network (Braver et al., 1997) by the high working memory load in our sample. In order to address study hypotheses regarding the impact of the cognitive challenge on DLPFC and amygdala functioning, a priori anatomical ROIs were generated in MNI space, using the Talairach-Daemon-based Wake Forest University PickAtlas (Maldjian et al., 2003). The DLPFC ROI was defined by combining binary masks of Brodmann Area (BA) 9 + BA 46, after removing medial regions of BA 9, to isolate the dorsolateral prefrontal cortex (DLPFC). For each participant, post-4-back DD − post-1-back DD fMRI mean contrast values were extracted separately from left and right DLPFC and amygdala ROIs for the regression analysis described below. The PickAtlas anatomical amygdala mask was also used for small-volume correction in the statistical testing of the 4-back − 1-back contrast. Type I error was controlled in working memory activation analyses via a cluster-level family-wise error (FWE) corrected threshold of p < .001. Next, the effects of the high working memory load cognitive challenge on delay discounting were examined by contrasting activations to post-4-back DD vs. post-1-back DD via both voxel-wise and ROI-based analyses. Type I error was controlled in delay discounting voxel-wise analyses via a 2-step process: selection of an initial “cluster forming threshold” at the voxel-level, which in our case was an uncorrected voxel-wise p value = .005, followed by correction for multiple comparisons at the cluster level using an FWE-corrected p value = .001. We also performed a small-volume correction using an anatomically defined bilateral amygdala mask (see above) and a voxel-wise FWE-corrected threshold of p <.05, in order to detect differences in amygdala activation during post-4-back delay discounting relative to post-1-back delay discounting. We tested for laterality of the post-4-back DD-post-1-back DD effect on DLPFC using contrast values extracted from the anatomically defined left and right DLPFC ROIs, via a paired t-test (alpha = .05). Lastly, change in log(k) was regressed on anatomically defined right DLPFC and bilateral amygdala ROI post-4-back DD—post-1-back DD contrast values, with 4-back performance accuracy included as a regressor to control for variation in working memory performance (alpha=.05). Given that least-squares regression is sensitive to outliers (Fox, 1997), we subsequently fit the same model using the robust regression function lmrob available in R version 3.0.0 (The R Foundation for Statistical Computing, http://www.R-project.org); the results did not change appreciably.
Results
Imaging results
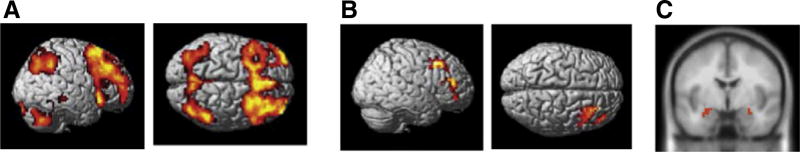
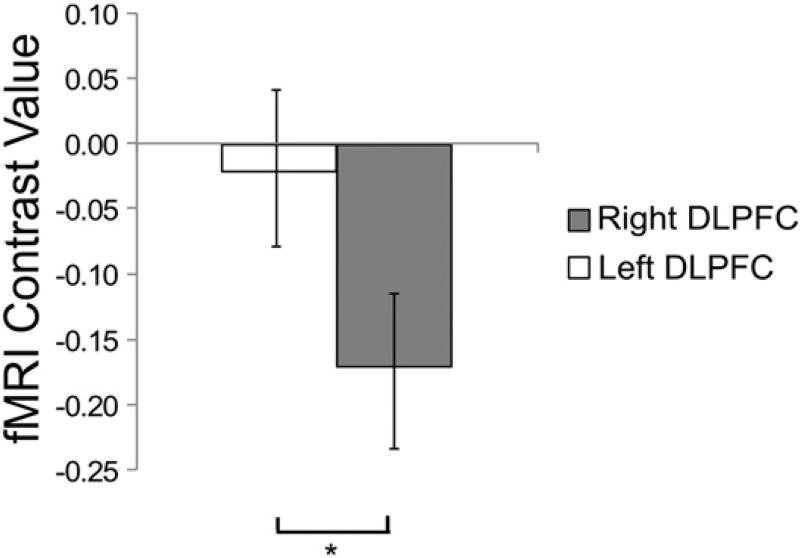
Consistent with prior literature (Braver et al., 1997; Yun et al., 2010), DLPFC and regions in parietal cortex implicated in working memory were more strongly activated when participants were engaged in the 4-back task than the 1-back task (Fig. 2A), indicating that the cognitive challenge recruited the prefrontal target areas as intended. We next measured the impact of the cognitive challenge on subsequent activity. Consistent with our hypothesis, post-4-back delay discounting blocks were associated with decreased activation in right DLPFC (rDLPFC; Fig. 2B) and increased activation in bilateral amygdala, relative to post-1-back delay discounting blocks (Fig. 2C). Our analysis did not reveal an impact of cognitive challenge on activity in the left DLPFC (Fig. 3).
Fig. 2.
fMRI Results. A) Increased activity in DLPFC and lateral parietal cortex during 4-back relative to 1-back (p <0.001, cluster-level FWE-corrected). B) Contrast of post-1-back DD> post-4-back DD showing decreased DD-related rDLPFC activity following 4-back exposure (p < 0.001, cluster-level FWE-corrected). C) Contrast of post-4-back DD > post-1-back DD shoing increased DD-related bilateral amygdala activity following 4-back exposure (p < 0.05, small volume corrected).
Fig. 3.
Hemispheric lateralization of change in DLPFC activation for post-4-back DD contrast. Figure shows mean fMRI contrast values for left and right DLPFC (anatomically defined ROIs) with 95% confidence intervals (*p < 0.05).
Our primary interest was in measuring the effect of DLPFC suppression on delay discounting behavior. We did this by testing whether post-4-back activity in rDLPFC and/or amygdala predicted changes in discount rates following cognitive challenge (post-4-back log(k) − post-1-back log(k)). Mean contrast values (post-4-back DD−post-1-back DD) were extracted from anatomically defined rDLPFC and amygdala ROIs for each subject and were included as predictors in a multivariate linear regression. As mentioned above, since we anticipated that individual variation in cognitive capacity would influence measured effects of cognitive challenge, we included 4-back performance as a predictor to control for individual differences in working memory capacity. As shown in Table 1, greater rDLPFC activation decreases and amygdala activation increases were independently associated with larger increases in discount rates from post-1-back to post-4-back DD blocks, controlling for 4-back performance.
Table 1.
Linear regression model predicting change in delay discounting following cognitive challenge. For each change measure, change is calculated as post-4-back DD − post-1-back DD. Change in delay discounting = post-4-back log(k) − post-1-back log(k). rDLPFC and amygdala ROIs were defined anatomically and mean contrast values were used as regressors.
| Predictors | Standardized beta coefficients |
|---|---|
| Change in DLPFC (right) activation | −0.412* |
| Change in amygdala (bilateral) activation | 0.675** |
| 4-back accuracy | −0.669** |
| — | — |
| Adjusted R2 | 0.696*** |
p < 0.05 significance.
p < 0.01 significance.
p < 0.005 significance.
Behavioral results
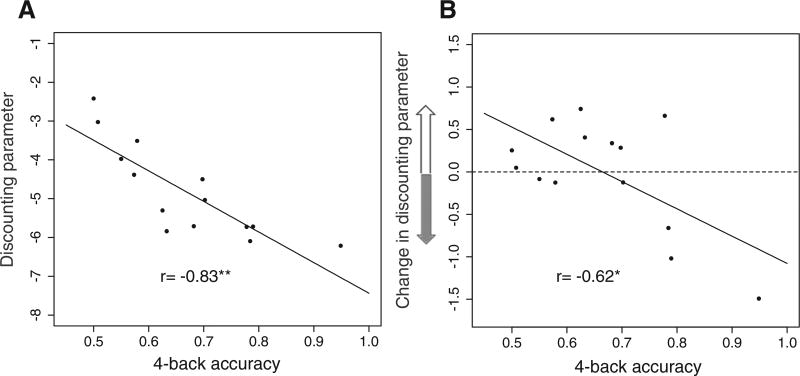
Paired t-tests confirmed that average 0-back accuracy (81%) was significantly greater than 1-back accuracy (74%, p < 0.0001), which was greater than 4-back accuracies (67%, p = 0.0012). We measured delay discounting preferences at the individual level using the single-parameter hyperbolic discounting function; higher values of the parameter indicate greater impatience. 4-back accuracy was negatively correlated with impatience (post-1-back log(k); Pearson's r = −0.83, p < 0.0005; Fig. 4A).
Fig. 4.
Relationship between 4-back performance and delay discounting. A) Correlation between 4-back accuracy and discount rate (post-1-back log(k)). B) Correlation between 4-back accuracy and change in discount rate (post-4-back log(k) − post-1-back log(k)) (*p < 0.05; **p < 0.001).
With respect to the effect of cognitive challenge on discount rate, we first tested for a simple mean difference in discount rate (log(k)) from post-1-back to post-4-back delay discounting blocks and found no evidence for an effect across all subjects (paired t-test, p = 0.98). In fact, several participants' responses were consistent with decreased impatience following cognitive challenge (Fig. 4B). We next analyzed the influence of individual variation in working memory capacity on the impact of cognitive challenge by measuring the correlation between 4-back performance and change in discount rate from post-1-back to post-4-back delay discounting blocks. Here we saw a significant effect, with poorer 4-back performance significantly correlated with greater increases in discount rate from post-1-back to post-4-back delay discounting blocks (Pearson's r = −0.62, p = 0.02; Fig. 4B). As can be seen in Fig. 4B, the participants with decreased discount rates following cognitive challenge were more likely to have performed well on the 4-back than those with increased discount rates. Taken together, these results suggest that the effect of a cognitive challenge on the discount rate depends crucially on individual-level variation in cognitive capacity.
Discussion
Previous research on delay discounting has led to the hypothesis that the ability to delay gratification in the face of immediate temptation relies on DLPFC function (McClure et al., 2004, 2007; Figner et al., 2010; Kim and Lee, 2011; Peters and Büchel, 2011; van den Bos and McClure, 2013), and DLPFC function appears to be vulnerable to suppression by excessive stress and/or cognitive demand (Yun et al., 2010; Arnsten et al., 2012; Friese et al., 2013). Furthermore, it has been suggested that the capacity for impulse control is a limited resource vulnerable to temporary depletion (Muraven and Baumeister, 2000), though results from the limited number of studies that have tested the impact of cognitive challenge on delay discounting behavior have led to mixed results (Hinson et al., 2003; Haushofer et al., 2013). Taken together, our results support the hypothesis that prefrontally mediated cognitive control is fundamental for delay discounting and provides further evidence for the role of rDLPFC in self-control (Kerns et al., 2004; Knoch and Fehr, 2007; Aron, 2011; van den Bos and McClure, 2013). We find evidence that cognitive challenge can lead to impaired self-control in delay discounting, but the effect of a given cognitive challenge is likely moderated by baseline cognitive capacity. We believe the novel contributions of this study include (1) that the effect of cognitive challenge on discount rates was correlated with the effects on DLPFC and amygdala activity. We found that DLPFC suppression and increased amygdala activity following cognitive challenge predicted greater increases in discount rates. (2) The impact of cognitive challenge on discount rates depended on working memory ability. Lower accuracy on the N-back was associated with greater increases in discount rates following cognitive challenge.
Although the cognitive challenge posed by the 4-back task was clearly sufficient to induce increases in amygdala activity and reduced DLFPC recruitment, it did not lead to a significant mean increase in the discount rate frompost-1-back to post-4 back delay discounting blocks. However, there were substantial individual differences in working memory capacity across participants that likely moderated the impact of the 4-back working memory challenge on discount rates. As mentioned above, working memory capacity has been shown to predict individual differences in discount rates (Shamosh et al., 2008), while working memory training has been demonstrated to decrease discounting (Bickel et al., 2011). Furthermore, working memory capacity has been correlated with other aspects of impulsive choice, such as response inhibition (Nichols and Wilson, 2015). Using 4-back performance accuracy as a measure of individual working memory capacity, we observed a similar relationship in the current study. Specifically, lower 4-back accuracy was associated with higher discount rates in the post-1-back delay discounting control condition (Fig. 4A). Moreover, poorer performance on the 4-back task predicted greater increases in log(k) following 4-back exposure, suggesting that the 4-back task had a greater subsequent impact on delay discounting in those participants for whom it was most challenging (Fig. 4B). These findings are consistent with recent evidence suggesting that greater working memory capacity may “protect” DLPFC function from acute stress (Otto et al., 2013) and may help explain why past studies have yielded mixed results. One method for clarifying this phenomenon further would be to tailor the cognitive challenge to the individual participant's abilities using adaptive experimental design optimization (Cavagnaro et al., 2009).
Our results may shed light on the mechanism by which excessive cognitive challenge may increase impatience. As has been suggested previously, high levels of catecholamine release during exposure to stress may disrupt DLPFC function and cognitive control (Arnsten et al., 2012). Given the well-established link between amygdala activity and the stress response (Sprengelmeyer et al., 1999; Hurlemann et al., 2009; Feinstein et al., 2011), the significant relationship between increased amygdala activity following 4-back exposure and impatience in our experiment is consistent with this mechanism, though we are precluded from making strong claims given that we did not directly assay stress levels. It is worth noting that an inverse relationship between activity in amygdala and DLPFC has been a frequent finding in emotion regulation studies (Ochsner et al., 2002; Heatherton and Wagner, 2011). Haushofer et al. (2013) tested the hypothesis that increased stress induces impatience using a social stress test and both self-report and physiological stress measurements. Utilizing a variety of delay discounting measures, they found no evidence of an effect. Given our finding that cognitive challenge may affect discounting by interfering with prefontally mediated cognitive control, it may be the case that Haushofer et al. did not find evidence for an effect because the particular stressor they employed did not suppress cognitive control adequately.
As noted above, one reason for an absence of mean increase in discount rate following cognitive challenge was that a number of subjects who performed well on the 4-back showed decreased impatience following cognitive challenge. This finding is consistent with the growing body of evidence supporting a “priming” effect of sub-capacity demands on DLPFC, in contrast to the “depleting” effect of excessive demands. Based on nonhuman primate research, Amy Arnsten and colleagues have proposed an “inverted-U” effect of catecholamines on DLPFC function (Arnsten, 2010; Arnsten et al., 2012; Arnsten and Jin, 2014). According to this model, moderate levels of catecholamine release during alert waking states may strengthen DLPFC function, while excessive catecholamine release during stress may impair DLPFC function. They suggest that this occurs because varying levels of catecholamine release stimulate different receptor types, with lower levels of norepinephrine (NE) engaging higher affinity alpha-2 adrenergic receptors, while higher levels of NE release engage lower affinity alpha-1 and beta adrenergic receptors. Consistent with this model, a recent primate study found that animals that received guanfacine, an alpha-2 adrenergic receptor agonist, showed decreased impatience in a delay discounting experiment (Kim et al., 2011). For our purposes, the key finding is that cognitive control varies following cognitive challenge in a manner that is reflected in DLPFC activity and rates of delay discounting.
Of note, a limitation of our study is the relatively small sample size, which may have interfered with the detection of relationships with small effect sizes, and increases the value of future replication of the study findings. In addition, the absence of self-report or physiological stress measures means that uncertainty remains regarding the true emotional impact of our cognitive challenge.
In conclusion, we found that a cognitive challenge led to decreased rDLPFC activity and correlated increases in impatience on a delay discounting task, controlling for working memory performance. Taken together, these results support the hypothesis that prefrontally mediated cognitive control is fundamental for delay discounting, and provide further evidence for the role of right prefrontal cortex in self-control (Kerns et al., 2004; Knoch and Fehr, 2007; Aron, 2011). Increased amygdala activity following cognitive challenge was also associated with increased impatience, consistent with a role of increased levels of stress causing impaired prefrontally mediated cognitive control. Contrary to expectations, a number of subjects who performed well on the working memory task showed decreased impatience following cognitive challenge, which may reflect a “priming” effect of sub-capacity cognitive challenge.
Acknowledgments
This work was supported by grants NIMH R25MH060482 (to G.J. Aranovich), NSF 1358507 (to S.M. McClure), VA CSR&D: and IK2 CX001028 (S. Fryer), and NIMH R01 MH076989 (to D.H. Mathalon).
References
- Arnsten AF. The use ofα-2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert. Rev. Neurother. 2010;10:1595–1605. doi: 10.1586/ern.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Jin LE. Molecular influences on working memory circuits in dorsolateral prefrontal cortex. Prog. Mol. Biol. Transl. Sci. 2014;122:211–231. doi: 10.1016/B978-0-12-420170-5.00008-8. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Laibson D, Loewenstein G. Intertemporal choice-toward an integrative framework. Trends Cogn. Sci. 2007;11:482–488. doi: 10.1016/j.tics.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol. Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW. Delay discounting correlates with proportional lateral frontal cortex volumes. Biol. Psychiatry. 2009;65:710–713. doi: 10.1016/j.biopsych.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Cavagnaro DR, Myung JI, Pitt MA, Kujala JV. Adaptive design optimization: a mutual information-based approach to model discrimination in cognitive science. Neural Comput. 2009;22:887–905. doi: 10.1162/neco.2009.02-09-959. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J. Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Curr. Biol. 2011;21:34–38. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nat. Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- First Michael B, Spitzer Robert L, Gibbon Miriam, Williams Janet BW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) [Google Scholar]
- Fox J. Applied Regression Analysis, Linear Models, and Related Methods. Sage Publications, Inc.; Thousand Oaks, CA, US: 1997. [Google Scholar]
- Franco-Watkins AM, Pashler H, Rickard TC. Does working memory load lead to greater impulsivity? Commentary on Hinson, Jameson, and Whitney (2003) J. Exp. Psychol. Learn. Mem. Cogn. 2006;32:443–447. doi: 10.1037/0278-7393.32.2.443. [DOI] [PubMed] [Google Scholar]
- Friese M, Binder J, Luechinger R, Boesiger P, Rasch B. Suppressing emotions impairs subsequent stroop performance and reduces prefrontal brain activation. PLoS One. 2013;8:e60385. doi: 10.1371/journal.pone.0060385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti LRR, Figner B, Ebstein RP, Knoch D. Why some people discount more than others: baseline activation in the dorsal PFC mediates the link between COMT genotype and impatient choice. Front. Neurosci. 2012;6:54. doi: 10.3389/fnins.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haushofer J, Cornelisse S, Seinstra M, Fehr E, Joëls M, Kalenscher T. No effects of psychosocial stress on intertemporal choice. PLoS One. 2013;8:e78597. doi: 10.1371/journal.pone.0078597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn. Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J. Exp. Psychol. Learn. Mem. Cogn. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Schlaepfer TE, Matusch A, Reich H, Shah NJ, Zilles K, Maier W, Bauer A. Reduced 5-HT(2A) receptor signaling following selective bilateral amygdala damage. Soc. Cogn. Affect. Neurosci. 2009;4:79–84. doi: 10.1093/scan/nsn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee D. Prefrontal cortex and impulsive decision making. Biol. Psychiatry. 2011;69:1140–1146. doi: 10.1016/j.biopsych.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Bobeica I, Gamo NJ, Arnsten AFT, Lee D. Effects of α-2A adrenergic receptor agonist on time and risk preference in primates. Psychopharmacology (Berl) 2011;219:363–375. doi: 10.1007/s00213-011-2520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN. Bidding on the future: evidence against normative discounting of delayed rewards. J. Exp. Psychol. Gen. 1997;126:54–70. [Google Scholar]
- Knoch D, Fehr E. Resisting the power of temptations. Ann. N. Y. Acad. Sci. 2007;1104:123–134. doi: 10.1196/annals.1390.004. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J. Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychol. Bull. 2000;126:247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Nichols TT, Wilson SJ. Working memory functioning and addictive behavior: insights from cognitive neuroscience. In: Wilson SJ, editor. The Wiley Handbook on the Cognitive Neuroscience of Addiction. Wiley-Blackwell: 2015. pp. 55–76. [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Otto AR, Raio CM, Chiang A, Phelps EA, Daw ND. Working-memory capacity protects model-based learning from stress. Proc. Natl. Acad. Sci. 2013;110:20941–20946. doi: 10.1073/pnas.1312011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn. Sci. 2011;15:227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway ARA, Engle RW, Braver TS, Gray JR. Individual differences in delay discounting relation to intelligence, working memory, and anterior prefrontal cortex. Psychol. Sci. 2008;19:904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Öngür D, Akbudak E, Conturo TE, Ollinger JM, Snyder AZ, Gusnard DA, Raichle ME. The emotional modulation of cognitive processing: an fMRI study. J. Cogn. Neurosci. 2000;12:157–170. doi: 10.1162/089892900564019. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Schroeder U, Grossenbacher PG, Federlein J, Büttner T, Przuntek H. Knowing no fear. Proc. Biol. Sci. 1999;266:2451–2456. doi: 10.1098/rspb.1999.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bos W, McClure SM. Towards a general model of temporal discounting. J. Exp. Anal. Behav. 2013;99:58–73. doi: 10.1002/jeab.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol. Psychiatry. 2014;75:435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun RJ, Krystal JH, Mathalon DH. Working memory overload: fronto-limbic interactions and effects on subsequent working memory function. Brain Imaging Behav. 2010;4:96–108. doi: 10.1007/s11682-010-9089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]