Abstract
BACKGROUND
The right atrium is densely innervated and provides sensory input to important cardiocirculatory reflexes controlling cardiac output and blood pressure. Its angiotensin (Ang) II-expressing innervation may release Ang II as a neuropeptide cotransmitter to modulate reflexes but has not yet been characterized.
METHODS
Intraoperative surgical biopsies from human right atria (n = 7) were immunocytologically stained for Ang II, tyrosine hydroxylase (TH), and synaptophysin (SYN). Tissue angiotensins were extracted and quantified by radioimmunoassay.
RESULTS
Angiotensinergic fibers were frequent in epicardial nerves and around vessels with variable TH co-localization (none to >50%/bundle). Fibers were also widely distributed between cardiomyocytes and in the endocardium where they were typically nonvaricose, TH/SYN-negative and usually accompanied by varicose catecholaminergic fibers. In the endocardium, some showed large varicosities and were partially TH or SYN-positive. A few endocardial regions showed scattered nonvaricose Ang fibers ending directly between endothelial cells. Occasional clusters of thin varicose terminals co-localizing SYN or TH were located underneath, or protruded into, the endothelium. Endocardial density of Ang and TH-positive fibers was 30–300 vs. 200–450/mm2. Atrial Ang II, III, and I concentrations were 67, 16, and 5 fmol/g (median) while Ang IV and V were mostly undetectable.
CONCLUSIONS
The human right atrium harbors an abundant angiotensinergic innervation and a novel potential source of atrial Ang II. Most peripheral fibers were noncatecholaminergic afferents or preterminal vagal efferents and a minority was presumably sympathetic. Neuronal Ang II release from these fibers may modulate cardiac and circulatory reflexes independently from plasma and tissue Ang II sources.
Keywords: angiotensin, blood pressure, cardiomyocyte, heart atria, human, hypertension, innervation, sympathetic
The heart is densely innervated by sympathetic and vagal efferent as well as afferent fibers and the intrinsic ganglionated plexus which together control cardiac function on a beat-to-beat basis. The corresponding autonomic cardiac reflexes serve to adjust cardiac output according to the variable needs of the circulation and thereby also control systemic blood pressure. They are organized into local intrinsic and superposed spinal and central nervous system levels that closely interact with each other. Sensory fibers originating in the right atrium provide key afferent input to these reflexes and play a crucial role for the adjustment of sympathetic and vagal output directed to the heart.1,2 Autonomic atrial fibers and their neurochemical signalling are therefore of particular interest to cardiac physiology and pharmacology.3
Neurons of the intrinsic and extrinsic cardiac nervous system express various neuropeptides such as neuropeptide Y (NPY) and other small signalling molecules like nitric oxide (NO) acting as neuromodulators or cotransmitters during sympathetic and parasympathetic neurotransmission.3–5 Angiotensin (Ang) II is the central effector peptide of the renin–angiotensin–aldosterone system and an important modulator of autonomic nervous function in the heart where it facilitates presynaptic noradrenaline (NA) release.6–8 Furthermore, it is an important neuropeptide cotransmitter in the central nervous system involved in the control of neuronal communication and central sympathetic nervous outflow.9
Recently, we and others found additional Ang II-positive neurons and fibers in dorsal root, trigeminal and coeliac ganglia of different species.10–13 We also provided evidence for an angiotensinergic co-phenotype of sympathetic fibers in the human kidney and heart where Ang II may serve as a neuropeptide cotransmitter.14–17 However, a detailed study of the distribution and characteristics of autonomic Ang II-expressing fibers in the human atria has not yet been performed. We hypothesized that neuronal Ang II expression is a relevant co-phenotype of the atrial autonomic innervation and particularly of sympathetic fibers. Neuronal Ang II release from these fibers could possibly modulate cardiac reflex activity independently from tissue or plasma-derived Ang II. In the present investigation, we therefore studied the angiotensinergic innervation in surgical specimens from human right atria immunocytologically and measured atrial Ang peptide concentrations.
METHODS
Immunocytology
Discarded right atrial pieces from 7 male patients aged 57 to 77 years who underwent cardiac bypass surgery were studied. Specimens (0.5–1.0 cm2) were obtained at the time of venous cannulation and immediately cooled (4 °C). One half was immersion fixed in phosphate buffered formaldehyde (2%) for 3 days while the other was shock frozen in liquid nitrogen and stored at −80 °C for Ang determinations. Transmural cryostat sections (20–25 µm) were prepared for immunocytological stainings and incubated with primary antibodies using a free-floating incubation technique.14 Detection of immunoreactive Ang II was with a mouse monoclonal IgG antibody that has been previously validated.10–12,14,15,18,19 These tests documented its specific binding to Ang II N-terminally linked to sepharose.10 It does not detect Ang I, Ang (1–7) or angiotensinogen but recognizes Ang (2–8) [Ang III], Ang (3–8) [Ang IV], Ang (4–8) [Ang V], and Ang (2–10).10 A rabbit monoclonal antibody (clone EP1533Y; Epitomics, Burlingame, CA, 2129-1; 1:200) was used for tyrosine 3-hydroxylase (TH), and mouse (SY38; Fitzgerald, Acton, MA, 10R-S124A; 1:600) and rabbit monoclonal antibodies (YE269; Epitomics, 1485-1; 1:400) for synaptophysin (SYN). Secondary labelling was with Cy3-tagged goat anti-mouse (Jackson ImmunoResearch, West Grove, PA, 115-165-146; 1:600) or Cy5-labeled donkey anti-rabbit IgG antibodies (Jackson ImmunoResearch, 711-175-152; 1:300).
Simultaneous stainings were performed to detect co-localization of Ang II with TH or SYN. Nuclear DNA was visualized with 4′,6′-diamidino-2-phenylindole (DAPI). Test stainings with a polyclonal Ang II-antibody provided identical results (not shown). Furthermore, control assays without primary or secondary antibodies showed no specific staining. Leica DM6000B confocal fluorescent light and SP2 laser scanning microscopes were used for optical analysis by indirect immunofluorescence and Leica imaging software. Endocardial fiber density per mm2 was determined by counting all visible fibers in the 2D optical plane multiplied by the thickness of the section. All procedures were in accordance with the Declaration of Helsinki and its amendments and performed with due permission from local authorities.
Angiotensin determinations
Atrial concentrations of Ang I to V were determined according to a previously published protocol in all except one specimens too small for the procedure.14 The peptides were extracted from tissue homogenates and separated by high-pressure liquid chromatography before quantification by radioimmunoassay.20 Concentrations were expressed as fmol/gww. Detection levels were 0.24 and 0.48 fmol/gww for Ang II and I, respectively, and ~2 to 5 fmol/gww for the other peptides. Peptide recovery rates were >67%. Mean values with SDs were calculated and median values.
RESULTS
Immunoreactivity for Ang II was detected in atrial autonomic nerve fibers and also in cardiomyocytes (Figures 1 and 2). All specimens showed comparable and homogenous Ang II staining intensity in this respect. Ang II-positive fibers were present in all epicardial nerves and fiber bundles together with TH-positive catecholaminergic fibers (Figure 2). The larger epicardial nerves further divided into smaller bundles and fascicles on their way to the periphery and deeper atrial layers thereby following preformed anatomical septa (Figure 1a) or small arteries where they formed an adventitial network. In the epicardial nerves and large proximal bundles, Ang II-positive fibers were typically nonvaricose and their number usually comparable to that of TH-positive fibers (Figure 2). Co-localization of Ang II and TH immunoreactivity was variable between none to over 50% in a bundle and particularly frequent in epicardial nerves. It was occasionally present in fibers of the vascular adventitia and virtually absent in smaller bundles innervating the myocardium. The epicardial epithelium and subepithelium were not innervated by Ang II-positive fibers.
Figure 1.
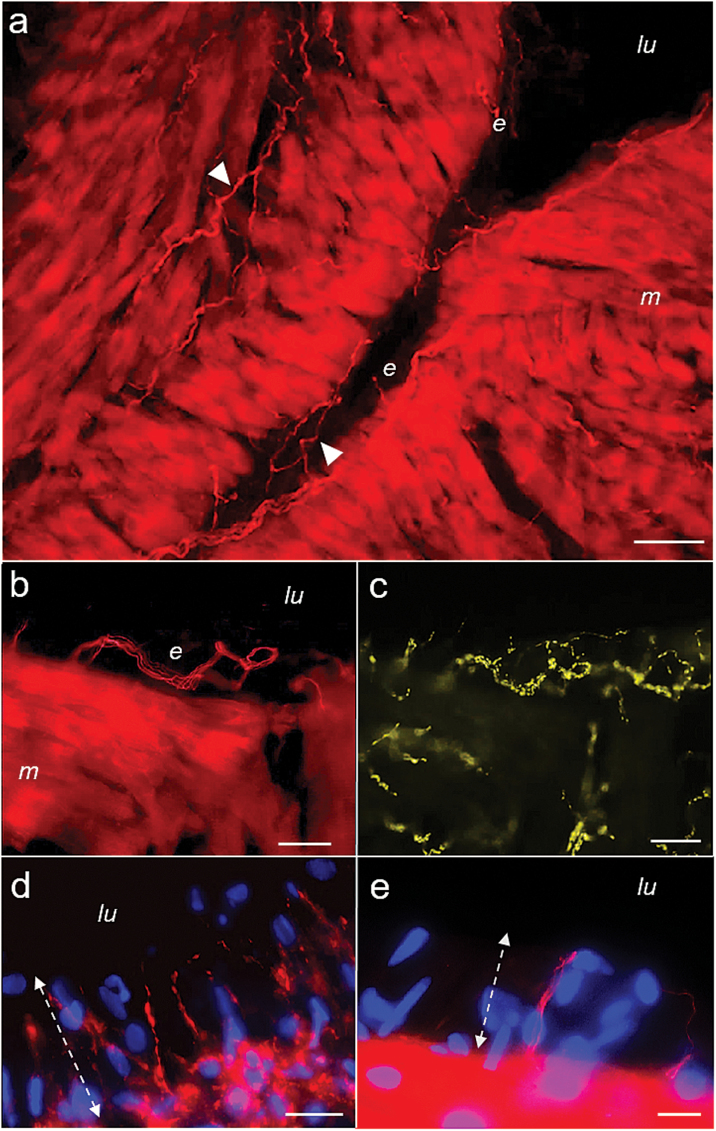
Sections from right atrial biopsies stained for angiotensin (Ang) II (red). (a) An overview picture showing atrial trabeculation with intensive Ang II-immunoreactivity in atrial cardiomyocytes (m) and in numerous nonvaricose fibers running in small bundles within the myocardium and the endocardium (e, closed arrowheads). (b) and (c) The endocardium at higher magnification after co-staining for Ang II (b) and synaptophysin (SYN; light green; c). A bundle of nonvaricose Ang II-positive fibers can be seen forming a loop in the subendothelial space (b). The fibers were accompanied by numerous thin and highly varicose SYN-positive fibers that can also be seen between cardiomyocytes (c). SYN immunoreactivity did not co-localize to the nonvaricose Ang-positive fibers. (d) Thin and varicose Ang-positive terminals protruding vertically into the endothelium that were additionally tyrosine hydroxylase (TH) positive (not shown). (e) Nonvaricose Ang II-positive yet TH and SYN-negative terminals interspersed between endothelial cells that could be found in some endocardial areas (overview and additional pictures in Supplementray Material). Dual channel confocal light microscopy. DNA staining with 4′,6′-diamidino-2-phenylindole (blue). Scale bars represent 50 µm (a), 25 µm (b, c, d), and 10 µm (e); lu, atrial lumen; dotted lines (d,e) indicate endothelial zone and luminal border.
Figure 2.
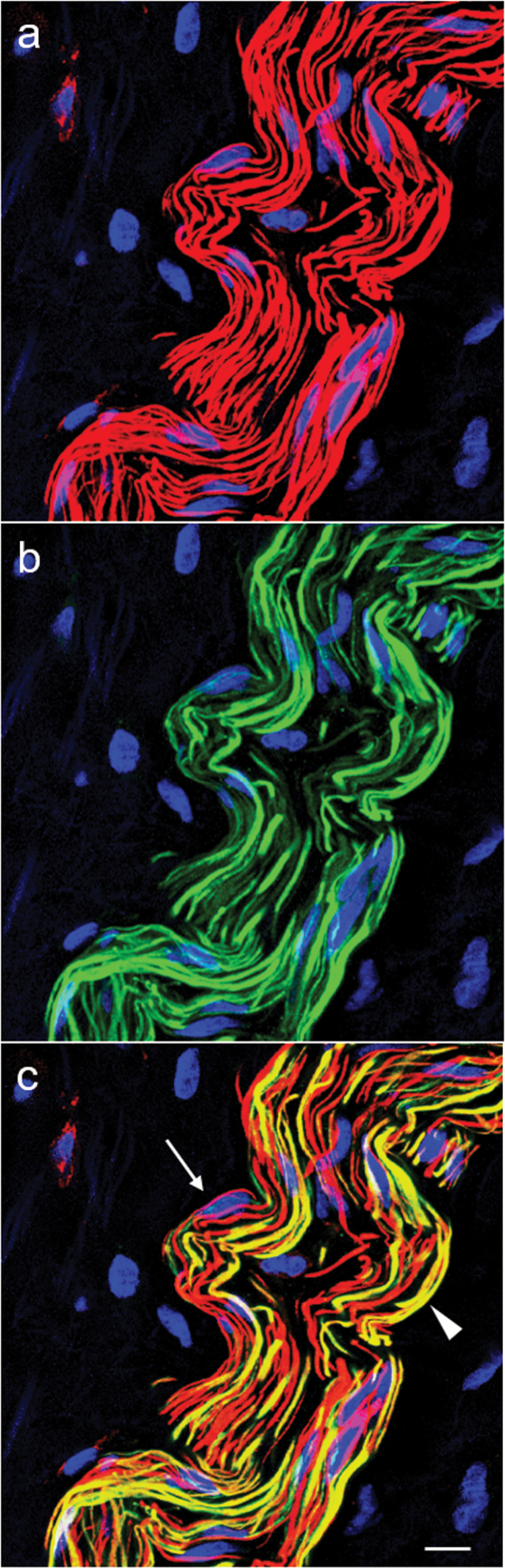
Epicardial fiber bundle consisting of nonvaricose angiotensin (Ang) II-positive (red, a) and tyrosine hydroxylase (TH)-positive fibers (green, b). (c) An overlay picture with co-localization of Ang II and TH immunoreactivity in numerous fibers (yellow). The arrow points to an exclusively Ang II-positive fiber next to adjacent exclusively TH-positive fibers. The arrowhead indicates a fiber with co-localization of Ang II and TH. There are Schwann cell nuclei visible between the fibers. Dual channel laser scanning microscopy. Staining of nuclear DNA was with 4′,6′-diamidino-2-phenylindole (blue). Scale bar represents 10 µm.
In the myocardial layer, Ang II-positive fibers were ubiquitously present between cardiomyocytes where they continued to be typically nonvaricose and negative for TH and SYN (Figure 3). They were usually seen in small groups of 2 to 6 individuals and accompanied by up to 3 times as many exclusively TH-positive fibers with a thinner and highly varicose shape (Figure 3d–f). Despite a comprehensive analysis of all specimens, no Ang II-positive varicose endings could be detected between cardiomyocytes. They were rarely visible in the adventitia of local vessels. No close synaptic contacts between nerve fibers or with other cells were observed. SYN-positive and TH-positive fibers showed both a varicose morphology and comparable anatomical distribution while TH-positive fibers were generally SYN-positive. Finally, also cardiomyocytes showed intense Ang II staining (Figures 1a, b and 3a).
Figure 3.
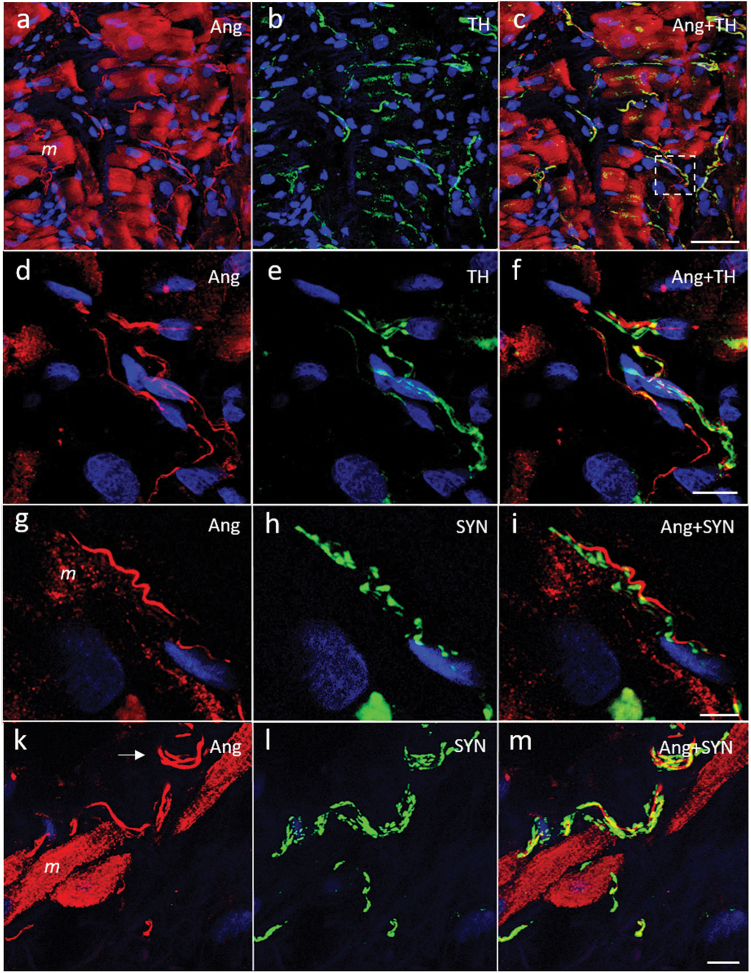
Laser scanning micrographs of atrial myocardium stained for angiotensin (Ang) II (red, a, d, g, k) and co-stained for tyrosine hydroxylase (TH, green; b, e) or synaptophysin (SYN, green; h, l). The overlay pictures are shown in panels c, f, i, and m (co-localization yellow). (a–c) An overview picture. Numerous nonvaricose and exclusively Ang II-positive fibers can be seen between cardiomyocytes (m). Myocytes equally show intense Ang II staining (a). The fibers are accompanied by thin varicose and exclusively TH-positive fibers (b). (d–f) A magnification of the boxed area in panel c. (g–m) Ang II-positive fibers (arrow) next to cardiomyocytes that were accompanied by thin and highly varicose SYN-positive and presumably efferent fibers. DNA staining with 4′,6′-diamidino-2-phenylindole (blue). Scale bars represent 50 µm (a–c), 10 µm (d–f, k–m), and 5 µm (g–i).
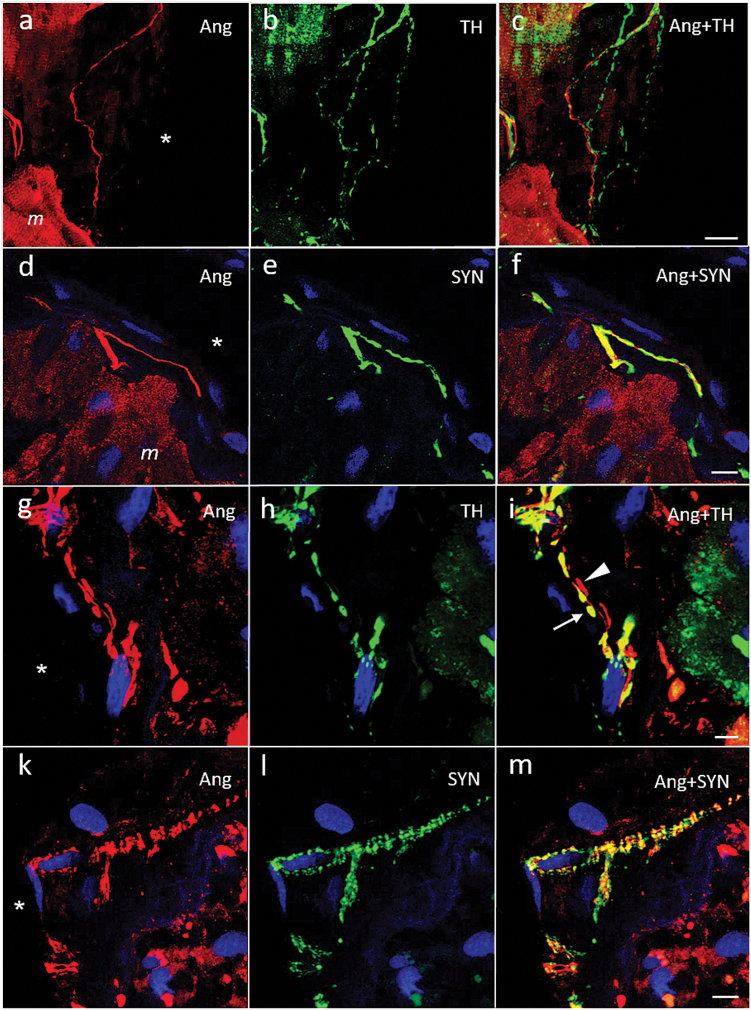
In the endocardium, several subtypes of Ang II-positive fibers could be distinguished (Figures 1b, d, e and 4). The first and by far most frequent type was seen in regions with flat endothelial cells where Ang fibers were nonvaricose, did not co-localize TH or SYN and ran in parallel with the endocardial surface (Figures 1b and 4a, d; Supplementary Figure 1). They were observed singularly or in small groups and usually accompanied by highly varicose catecholaminergic fibers (Figures 1c and 4c, f). Local clusters and loops could be observed (Figure 1b). These fibers always remained at a distance from the endothelial cell line. Another type was less frequent and characterized by large and spindle-shaped varicosities. Such fibers were either TH-positive or TH-negative (Figure 4g–i). Occasionally, groups of multiple, very thin, and closely apposed Ang II-positive fibers exhibiting countless small varicosities with a characteristic flower spray or arachnoid appearance were observed that were also positive for SYN (Figure 4k–m). Still another type was found in areas with apparently high prismatic endothelial cells. These exclusively Ang II-positive nonvaricose endings protruded vertically between the endothelial cells while their tips attained the atrial luminal border (Figure 1e). Accompanying exclusively double TH/SYN-positive varicose terminals were seen sticking out similarly into the endothelium (Supplementary Figures 2 and 3). Finally, in some areas, densely packed varicose Ang II-positive terminals co-localizing TH protruded vertically into the endothelium (Figure 1d). The average density of endocardial Ang-positive fibers was estimated between 30 and 300/mm2 and that of catecholaminergic fibers between 200 and 450/mm2, with great local variability.
Figure 4.
Laser scanning micrographs of atrial endocardium stained for angiotensin (Ang) II (red, a, d, g, k) and co-stained for tyrosine hydroxylase (TH, green; b, h) or synaptophysin (SYN, green; e, l). (c, f, i, and m) Overlay pictures, respectively (co-localization yellow). (a–c) and (d–f) show nonvaricose and exclusively Ang II-positive fibers in the subendothelial space that were accompanied by varicose TH and SYN-positive fibers (see also Supplementary Figure 1). Ang II-positive cardiomyocytes (m) can bee seen in the vicinity. (g–i) Ang II-positive endocardial fibers characterized by large spindle-shaped varicosities that were either TH-positive (arrow) or negative (arrowhead). (k–m) A site with multiple thin and highly varicose Angiotensinergic fibers in a brush-like or arachnoid arrangement underneath the endothelium. The fibers were SYN-positive. SYN immunoreactivity was more extensive than that for Ang II. DNA staining with 4′,6′-diamidino-2-phenylindole (blue); atrial lumen indicated by ‘asterisk’. Scale bars represent 20 µm (a–c), 10 µm (g–i, k–m), and 5 µm (d–f).
Atrial Ang peptide concentrations are shown in Table 1. Ang II concentrations were highest followed by Ang III and Ang I with notable interindividual variability. On average, Ang III concentrations were ~1/3 of the Ang II concentrations (median). Ang I concentrations were comparably low and Ang II/I and Ang II/III always >1. Ang IV and Ang V concentrations were mostly below the detection limit.
Table 1.
Atrial angiotensin concentrations [fmol/gww] and ratios (n = 6)
| Samplea | Ang I (Ang 1–10) | Ang II (Ang 1–8) | Ang III (Ang 2–8) | Ang IV (Ang 3–8) | Ang V (Ang 4–8) | Ang II/I | Ang II/III |
|---|---|---|---|---|---|---|---|
| 1 | <5.5 | 441 | 9.3 | 2.8 | 5.1 | >80 | 47.4 |
| 2 | 11 | 74 | 36 | <2.1 | <2.1 | 7 | 2.1 |
| 3 | <3.5 | 46 | 15 | <1.8 | <1.8 | >13 | 3.1 |
| 4 | 4.2 | 203 | 81 | <1.6 | <1.6 | 48 | 2.5 |
| 5 | 2.6 | 59 | 17 | <0.82 | <0.82 | 23 | 3.5 |
| 6 | 6.6 | 18 | 3.5 | <2.9 | <2.9 | 3 | 5.1 |
| Mean ± SDb | 6 ± 3 | 140 ± 160 | 27 ± 29 | ND | ND | 29 ± 30 | 11 ± 18 |
| Medianb | 5 | 67 | 16 | ND | ND | 18 | 3 |
Abbreviation: Ang, angiotensin; ND, not determined.
aThe detection limit is given where concentrations were unmeasurable.
bValues were calculated using the detection limits if applicable.
DISCUSSION
We found an abundant angiotensinergic innervation of the human right atrium with a widespread presence of Ang-positive fibers in epicardial nerves and the myocardium and endocardium. In the epicardial nerves, TH and Ang co-expression was frequent. Peripheral Ang-positive fibers in the myocardium and endocardium, instead, were mainly nonvaricose, noncatecholaminergic, and also SYN-negative with important exceptions notably in the endocardium and vessels. Next, Ang II could also be demonstrated intracellularly in cardiomyocytes. Atrial tissue Ang II and Ang III concentrations were high with respect to the other peptides including Ang I. Functionally, neuronal Ang II release from these angiotensinergic fibers has implications as an independent source of atrial Ang II and a modulator of autonomic neurotransmission.
We relied on a highly sensitive monoclonal antibody and confocal or laser scanning microscopy to detect Ang II in tissue sections at high resolution. This allowed us to differentiate closely apposed nerve fibers and their Ang II staining reliably. Previously, we and others have already detected angiotensinergic neurons and fibers in rat and human spinal as well as sympathetic ganglia.10–13 Furthermore, angiotensinergic neurons and fibers were found in human and rat cardiac autonomic ganglia and ventricles and finally also in the kidneys while showing a partial catecholaminergic co-phenotype.11,14,16,17 Ventricular Ang II-positive fibers were infrequent. In contrast, we now document a comparably rich angiotensinergic innervation of the human right atrium with local differences concerning its catecholaminergic co-phenotype.
Studies of human cardiac transplants and in experimental animal have consistently demonstrated that the majority of the cardiac innervation is of extrinsic origin leaving only a comparably limited innervation attributable to the intrinsic ganglionated plexus.21,22 Similar observations have been made in biopsies from human right atria and cardiac autopsy specimens where TH and NPY-positive intrinsic ganglionic neurons were usually rare.5,23–25 As a vagal relay station, intrinsic cardiac neurons provide most of the postganglionic parasympathetic innervation with a typically cholinergic phenotype. Additionally, there are also numerous neurons with an afferent or interconnecting function.1,5,21,24 Some of the exclusively Ang II-positive atrial fibers in our study may thus have belonged to these intrinsic neurons. Nevertheless, most of the Ang II-positive and catecholaminergic innervation identified in our specimens was probably extrinsic to the heart.
Although neuronal Ang II is presumably derived from cellular angiotensinogen expression coexisting with cathepsin D and converting enzyme expression to allow its generation and then transported to the periphery like other neuropeptides, neuronal Ang II uptake via AT1-receptors remains a theoretical possibility.4,10–12,15 Kushiku et al.26 reported the presence of angiotensinogen messenger RNA expression in the canine inferior cervical and stellate ganglia that provide the main sympathetic efferent innervation of the heart. Expression of angiotensinogen messenger RNA and cellular Ang II immunoreactivity have also been described in the dorsal motor nucleus of the vagus suggesting that vagal motor fibers innervating the heart may equally express Ang II and contribute to its angiotensinergic innervation.27,28
Surprisingly, the vast majority of Ang II-positive fibers in the myocardium and endocardium did not co-localize TH and SYN suggesting that they were either afferents or possibly preterminal cholinergic efferents. Sensory fibers are usually negative for SYN, a marker of presynaptic vesicles, unless they are varicose. Such varicose terminals expressing SYN, calcitonin gene-related peptide or substance P have been detected in the human endocardium but were infrequent.29–32 In contrast, we could not identify varicose Ang II-expressing endings between cardiomyocytes despite a comprehensive search suggesting that they were rare at the most. Instead, catecholaminergic fibers co-localizing Ang II were a frequent feature of epicardial nerves. They could have mainly belonged to interganglionic connections or possibly had non-atrial targets such as the large vessels or the ventricles to explain their relative paucity within the atrial myocardium and endocardium.
Detailed studies in humans and experimental animals have previously documented an extensive network of sympathetic and vagal efferent as well as sensory fibers covering the entire endocardium. These fibers may branch out and express a host of different neuropeptides.29–31,33 Moreover, they have been reported to keep at a distance from the endothelial cell line.30 We now report nonvaricose exclusively Ang II-positive and also varicose exclusively TH-positive fiber terminals projecting into the endothelium itself. These endings were visible only on our cross sections of the atrial wall but may have been undetectable on typical whole-mount preparations with a plane view.21,29–31,33 The endings probably belonged to Ang-expressing afferents and catecholaminergic sympathetic efferents and may release Ang II or monoamines locally for yet unknown purposes. Ang II-positive endings may furthermore display chemosensitive properties because of their proximity to the blood compartment where they could readily sense molecules and gases diffusing into the paracellular space. Indeed, most atrial afferent fibers have been characterized to be chemosensitive.34
We also observed highly varicose Ang II-positive endocardial terminals with a catecholaminergic co-phenotype producing dense vertical palisades between endothelial cells with probably similar chemosensitive properties. Next, there were occasional Ang II-positive fibers in the endocardium forming varicose and closely apposed SYN-positive flower spray endings. In rats, such fibers have been identified as terminal arborizations of vagal afferents.33 Finally, some angiotensinergic fibers with large, spindle-shaped varicosities were either TH-positive and probably sympathetic or TH-negative and then possibly vagal motor fibers. In general, the endocardial density of Ang II-positive fibers was lower than that of TH-positive sympathetic fibers. We could not distinguish collaterals and many fibers may have finally belonged to a much smaller number of original feeders. Our data therefore provide only preliminary estimates and cannot be compared unconditionally with others.30
Angiotensinergic fibers may release Ang II as a neuropeptide that would then hypothetically compete with Ang II of tissue origin for Ang receptor binding.4,15,35 Angiotensin receptors (AT) have been documented on cardiac sympathetic and vagal fibers, intrinsic neurons, and also sensory fibers.7,8,36 Our immunocytological findings lend support to a new pathophysiological concept where cardiac autonomic fibers are not only a target of Ang II but may also act as an independent source of Ang II during neurochemical signalling.15 The close apposition of angiotensinergic and catecholaminergic fibers in the atrial myocardium and endocardium was highly suggestive of Ang-mediated cross-talk. Cardiac sympathetic and vagal motor fibers may control their activity mutually by neurochemical NPY and NO signalling.3 Our observations strongly suggest, that the sympathovagal balance may be additionally modulated by neuronal Ang II. Such neurochemical fine tuning could help optimize the activity of intrinsic neuronal circuits or reset the long-term activity of higher nervous centers controlling cardiac function and circulatory homeostasis.2,3,34
Cardiac angiotensins are compartmentalized at the interstitial and intracellular level and depend on locally produced Ang II.35,37 Our determinations of human atrial Ang I, II, and III concentrations were comparable to published animal data.14,37 The downstream metabolites Ang IV and V were mostly undetectable. Interestingly, atrial Ang II concentrations were highest and Ang II/I ratios consistently >1 similar to the Ang profile in the kidneys.19 The high-atrial Ang II concentrations may be explained by an active Ang II-generating pathway with accumulation in cardiomyocytes via AT1 receptor-mediated uptake thus protecting it from degradation.35,37 In contrast, efficient angiotensinase activity and tissue clearance into the blood stream may explain the low Ang IV and V concentrations. Neuronal Ang II could possibly influence the local interstitial Ang III/II ratio and the balance between cellular AT1 and AT2 receptor activation.6 Moreover, the immunocytological detection of Ang II in cardiac tissue has thus far been methodologically difficult.38 We now provide clear experimental evidence for its presence within cardiomyocytes. The greater than usual thickness of the tissue slices and the free-floating incubation technique facilitated its detection.17
Of note, NPY concentrations were found to decrease by up to 50% and NA concentrations by >90% after experimental cardiac sympathectomy.39 Day et al.40 measured cardiac somatostatin concentrations in human hearts. They were reported to depend on the somatostatin-positive fiber density. The authors concluded that somatostatin release by the cardiac autonomic innervation could generate biologically relevant tissue concentrations. Therefore, it would not be surprising, if there were also a significant contribution of neuronal Ang II to its total amount in the heart. Enhanced neuronal Ang II spillover with increased cardiac Ang II exposure could become relevant in situations with pathologic neuronal overactivity.2
Some limitations of our study need to be addressed. Due to the paucity of material, we were unable to analyze the origins and phenotypes of Ang II-positive fibers in greater detail using additional immunocytological markers. Neither could we determine other relevant Ang peptides like Ang 1–7, a ligand of AT2 and mas receptors, or tissue enzymes.6 The presence of immunocytological Ang II, however, could be confirmed by our determinations of tissue Ang concentrations. The Ang II-antibody showed cross-reactivity with Ang III to V. This effect, however, would not change fiber classifications. Ang IV and V concentrations were extremely low and therefore unlikely to have interfered. The variable content of muscle tissue in the specimens was another possible source of bias during Ang quantification. The biopsies were from random sites and no inference can therefore be made with respect to atrial anatomy. Finally, clinical diagnoses and medical treatments could have influenced the neural phenotype or individual Ang concentrations. Nevertheless, our findings were consistent across all biopsies lending overall validity to our conclusions.
In conclusion, we provide immunocytological evidence for an abundant angiotensinergic innervation of the human right atrium that was mostly noncatecholaminergic. Angiotensinergic fibers may serve as an independent neuronal Ang II source and possibly contribute to tissue Ang concentrations. Furthermore, neuronal Ang II may modulate the cardiac sympathovagal balance and adjust autonomic cardiac reflex activity with important implications for cardiac function and circulatory homeostasis. The exact functional role of angiotensinergic fibers in the heart, however, remains unclear and merits further experimental clarification.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
The technical assistance of Catherine Amstutz and Françoise Nicoud is gratefully acknowledged.
REFERENCES
- 1. Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 1997; 247:289–298. [DOI] [PubMed] [Google Scholar]
- 2. Ardell JL, Andresen MC, Armour JA, Billman GE, Chen PS, Foreman RD, Herring N, O’Leary DS, Sabbah HN, Schultz HD, Sunagawa K, Zucker IH. Translational neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol 2016; 594:3877–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol 2009; 94:46–53. [DOI] [PubMed] [Google Scholar]
- 4. Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res 2006; 326:583–598. [DOI] [PubMed] [Google Scholar]
- 5. Wake E, Brack K. Characterization of the intrinsic cardiac nervous system. Auton Neurosci 2016; 199:3–16. [DOI] [PubMed] [Google Scholar]
- 6. Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res 2015; 116:960–975. [DOI] [PubMed] [Google Scholar]
- 7. Trendelenburg AU, Meyer A, Klebroff W, Guimarães S, Starke K. Crosstalk between presynaptic angiotensin receptors, bradykinin receptors and alpha 2-autoreceptors in sympathetic neurons: a study in alpha 2-adrenoceptor-deficient mice. Br J Pharmacol 2003; 138:1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horackova M, Armour JA. ANG II modifies cardiomyocyte function via extracardiac and intracardiac neurons: in situ and in vitro studies. Am J Physiol 1997; 272:R766–R775. [DOI] [PubMed] [Google Scholar]
- 9. O’Callaghan EL, Choong YT, Jancovski N, Allen AM. Central angiotensinergic mechanisms associated with hypertension. Auton Neurosci 2013; 175:85–92. [DOI] [PubMed] [Google Scholar]
- 10. Imboden H, Patil J, Nussberger J, Nicoud F, Hess B, Ahmed N, Schaffner T, Wellner M, Müller D, Inagami T, Senbonmatsu T, Pavel J, Saavedra JM. Endogenous angiotensinergic system in neurons of rat and human trigeminal ganglia. Regul Pept 2009; 154:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patil J, Heiniger E, Schaffner T, Mühlemann O, Imboden H. Angiotensinergic neurons in sympathetic coeliac ganglia innervating rat and human mesenteric resistance blood vessels. Regul Pept 2008; 147:82–87. [DOI] [PubMed] [Google Scholar]
- 12. Patil J, Schwab A, Nussberger J, Schaffner T, Saavedra JM, Imboden H. Intraneuronal angiotensinergic system in rat and human dorsal root ganglia. Regul Pept 2010; 162:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anand U, Yiangou Y, Sinisi M, Fox M, MacQuillan A, Quick T, Korchev YE, Bountra C, McCarthy T, Anand P. Mechanisms underlying clinical efficacy of Angiotensin II type 2 receptor (AT2R) antagonist EMA401 in neuropathic pain: clinical tissue and in vitro studies. Mol Pain 2015;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patil J, Stucki S, Nussberger J, Schaffner T, Gygax S, Bohlender J, Imboden H. Angiotensinergic and noradrenergic neurons in the rat and human heart. Regul Pept 2011; 167:31–41. [DOI] [PubMed] [Google Scholar]
- 15. Bohlender J, Imboden H. Angiotensinergic neurotransmission in the peripheral autonomic nervous system. Front Biosci (Landmark Ed) 2012; 17:2419–2432. [DOI] [PubMed] [Google Scholar]
- 16. Bohlender J, Pfarrer B, Patil J, Nussberger J, Thalmann GN, Imboden H. Angiotensinergic innervation of the kidney: Localization and relationship with catecholaminergic postganglionic and sensory nerve fibers. Histol Histopathol 2012; 27:1413–1428. [DOI] [PubMed] [Google Scholar]
- 17. Bohlender J, Nussberger J, Tevaearai H, Imboden H. [Autonomic angiotensinergic fibres in the human heart with an efferent sympathetic cophenotype]. Ann Cardiol Angeiol (Paris) 2015; 64:175–179. [DOI] [PubMed] [Google Scholar]
- 18. Frei N, Weissenberger J, Beck-Sickinger AG, Höfliger M, Weis J, Imboden H. Immunocytochemical localization of angiotensin II receptor subtypes and angiotensin II with monoclonal antibodies in the rat adrenal gland. Regul Pept 2001; 101:149–155. [DOI] [PubMed] [Google Scholar]
- 19. Bohlender JM, Nussberger J, Birkhäuser F, Grouzmann E, Thalmann GN, Imboden H. Resetting of renal tissular renin-angiotensin and bradykinin-kallikrein systems after unilateral kidney denervation in rats. Histochem Cell Biol 2017; 147:585–593. [DOI] [PubMed] [Google Scholar]
- 20. Nussberger J, Brunner DB, Waeber B, Brunner HR. Specific measurement of angiotensin metabolites and in vitro generated angiotensin II in plasma. Hypertension 1986; 8:476–482. [DOI] [PubMed] [Google Scholar]
- 21. Wharton J, Polak JM, Gordon L, Banner NR, Springall DR, Rose M, Khagani A, Wallwork J, Yacoub MH. Immunohistochemical demonstration of human cardiac innervation before and after transplantation. Circ Res 1990; 66:900–912. [DOI] [PubMed] [Google Scholar]
- 22. Steele PA, Gibbins IL, Morris JL. Projections of intrinsic cardiac neurons to different targets in the guinea-pig heart. J Auton Nerv Syst 1996; 56:191–200. [DOI] [PubMed] [Google Scholar]
- 23. Franco-Cereceda A, Lundberg JM, Hökfelt T. Somatostatin: an inhibitory parasympathetic transmitter in the human heart?Eur J Pharmacol 1986; 132:101–102. [DOI] [PubMed] [Google Scholar]
- 24. Hoover DB, Isaacs ER, Jacques F, Hoard JL, Pagé P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience 2009; 164:1170–1179. [DOI] [PubMed] [Google Scholar]
- 25. Singh S, Johnson PI, Javed A, Gray TS, Lonchyna VA, Wurster RD. Monoamine- and histamine-synthesizing enzymes and neurotransmitters within neurons of adult human cardiac ganglia. Circulation 1999; 99:411–419. [DOI] [PubMed] [Google Scholar]
- 26. Kushiku K, Yamada H, Shibata K, Tokunaga R, Katsuragi T, Furukawa T. Upregulation of immunoreactive angiotensin II release and angiotensinogen mRNA expression by high-frequency preganglionic stimulation at the canine cardiac sympathetic ganglia. Circ Res 2001; 88:110–116. [DOI] [PubMed] [Google Scholar]
- 27. Aguirre JA, Coveñas R, Croix D, Alonso JR, Narváez JA, Tramu G, González-Barón S. Immunocytochemical study of angiotensin-II fibres and cell bodies in the brainstem respiratory areas of the cat. Brain Res 1989; 489:311–317. [DOI] [PubMed] [Google Scholar]
- 28. Sangaleti CT, Crescenzi A, Michelini LC. Endogenous angiotensin and pressure modulate brain angiotensinogen and AT1A mRNA expression. Hypertension 2004; 43:317–323. [DOI] [PubMed] [Google Scholar]
- 29. Wharton J, Gulbenkian S, Merighi A, Kuhn DM, Jahn R, Taylor KM, Polak JM. Immunohistochemical and ultrastructural localisation of peptide-containing nerves and myocardial cells in the human atrial appendage. Cell Tissue Res 1988; 254:155–166. [DOI] [PubMed] [Google Scholar]
- 30. Marron K, Wharton J, Sheppard MN, Gulbenkian S, Royston D, Yacoub MH, Anderson RH, Polak JM. Human endocardial innervation and its relationship to the endothelium: an immunohistochemical, histochemical, and quantitative study. Cardiovasc Res 1994; 28:1490–1499. [DOI] [PubMed] [Google Scholar]
- 31. Marron K, Wharton J, Sheppard MN, Fagan D, Royston D, Kuhn DM, de Leval MR, Whitehead BF, Anderson RH, Polak JM. Distribution, morphology, and neurochemistry of endocardial and epicardial nerve terminal arborizations in the human heart. Circulation 1995; 92:2343–2351. [DOI] [PubMed] [Google Scholar]
- 32. Luff SE, Young SB, McLachlan EM. Ultrastructure of substance P-immunoreactive terminals and their relation to vascular smooth muscle cells of rat small mesenteric arteries. J Comp Neurol 2000; 416:277–290. [PubMed] [Google Scholar]
- 33. Cheng Z, Powley TL, Schwaber JS, Doyle FJ 3rd. Vagal afferent innervation of the atria of the rat heart reconstructed with confocal microscopy. J Comp Neurol 1997; 381:1–17. [DOI] [PubMed] [Google Scholar]
- 34. Waldmann M, Thompson GW, Kember GC, Ardell JL, Armour JA. Stochastic behavior of atrial and ventricular intrinsic cardiac neurons. J Appl Physiol (1985) 2006; 101:413–419. [DOI] [PubMed] [Google Scholar]
- 35. Danser AH. Cardiac angiotensin II: does it have a function?Am J Physiol Heart Circ Physiol 2010; 299:H1304–H1306. [DOI] [PubMed] [Google Scholar]
- 36. Diz DI, Barnes KL, Ferrario CM. Contribution of the vagus nerve to angiotensin II binding sites in the canine medulla. Brain Res Bull 1986; 17:497–505. [DOI] [PubMed] [Google Scholar]
- 37. Danser AH, van Kats JP, Admiraal PJ, Derkx FH, Lamers JM, Verdouw PD, Saxena PR, Schalekamp MA. Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension 1994; 24:37–48. [DOI] [PubMed] [Google Scholar]
- 38. Serneri GG, Boddi M, Cecioni I, Vanni S, Coppo M, Papa ML, Bandinelli B, Bertolozzi I, Polidori G, Toscano T, Maccherini M, Modesti PA. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res 2001; 88:961–968. [DOI] [PubMed] [Google Scholar]
- 39. Maccarrone C, Jarrott B. Differential effects of surgical sympathectomy on rat heart concentrations of neuropeptide Y-immunoreactivity and noradrenaline. J Auton Nerv Syst 1987; 21:101–107. [DOI] [PubMed] [Google Scholar]
- 40. Day SM, Gu J, Polak JM, Bloom SR. Somatostatin in the human heart and comparison with guinea pig and rat heart. Br Heart J 1985; 53:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.