Abstract
The temperate bacteriophage lambda (λ) CII protein is a positive regulator of transcription from promoter pE, a component of the lysogenic response. The expression of cII was examined in vectors devoid of phage transcription-modulating elements. Their removal enabled evaluating if the expression of the small RNA OOP, on its own, could suppress CII activities, including complementing for a lysogenic response, cell toxicity and causing rapid cellular loss of ColE1 plasmids. The results confirm that OOP RNA expression from the genetic element pO-oop-to can prevent the ability of plasmid-encoded CII to complement for a lysogenic response, suggesting that it serves as a powerful regulatory pivot in λ development. Plasmids with a pO promoter sequence of 45 nucleotides (pO45), containing the −10 and −35 regions for oop, were non-functional; whereas, plasmids with pO94 prevented CII complementation, CII-dependent plasmid loss and suppressed CII toxicity, suggesting the pO promoter has an extended DNA sequence. All three CII activities were eliminated by the deletion of the COOH-terminal 20 amino acids of CII. Host mutations in the hflA locus, in pcnB and in rpoB influenced CII activities. These studies suggest that the COOH-terminal end of CII likely interacts with the β-subunit of RNA polymerase.
Keywords: bacteriophage lambda, CII regulation in lysogeny decision, OOP RNA
1. Introduction
The regulatory circuits for control of the lysis-lysogeny decision made by bacteriophage λ upon infecting an E. coli host and the key involvement and modulation of CII activity have been extensively reviewed [1,2,3,4,5,6,7,8,9]. The elevated expression of gene cII, which is rightward and downstream from promoter pR, appears essential for lysogeny. CII serves as a positive transcriptional regulator to stimulate leftward transcription antisense to cro and sense for cI from promoter pE (Figure 1A), which is partially embedded within the first eight codons of cII. The enhanced expression of cI, encoding the CI repressor, results in its binding to operator oL (preventing leftward transcription from pL) and to operator oR, thus shutting down sR (rightward mRNA start site)-cro-nutR-tR1-cII-O-P-ren…tR2-Q transcription arising from pR. CII drives a lysogenic outcome by stimulating the transcription of int from promoter pI and antisense transcription of Q from promoter pAQ. The cII gene is regulated at multiple levels: by repressors CI and Cro binding at oR and limiting transcription from pR and by nutR and the tRI terminator site between cro and cII [1]. CII is degraded by the host ATP-dependent membrane protease FtsH (HflB), which is essential for cell growth [10,11,12]. This activity is modulated by the HflK-HflC proteins encoded by the hflA locus, which form a complex with FtsH [13], and by HflD [14]. The C-terminal 16 residues of CII are necessary and sufficient for rapid degradation by FtsH but are not sufficient for the activation of transcription by CII [15]. Proteolytic degradation of CII by HflB(FtsH)-HflC-HflK is inhibited by λ gpCIII [16,17,18]. At some level, the small RNA OOP serves to target the cII mRNA for degradation [19,20], although, the cleavage site for RNaseIII-dependent (and -independent) degradation is outside (12 nucleotides downstream) of the coding sequence for cII [20]. While suggesting a mechanism for OOP activity, these important studies measured CII activity via its ability to stimulate galactokinase expression from plasmid encoded pE and did not directly measure CII complementation reflected as limiting a lytic response from infecting phages. We show that OOP synthesis prevents CII-dependent stimulation of pE expression from infecting heteroimmune imm434 phages, limiting them to a lytic, rather than lysogenic, response.
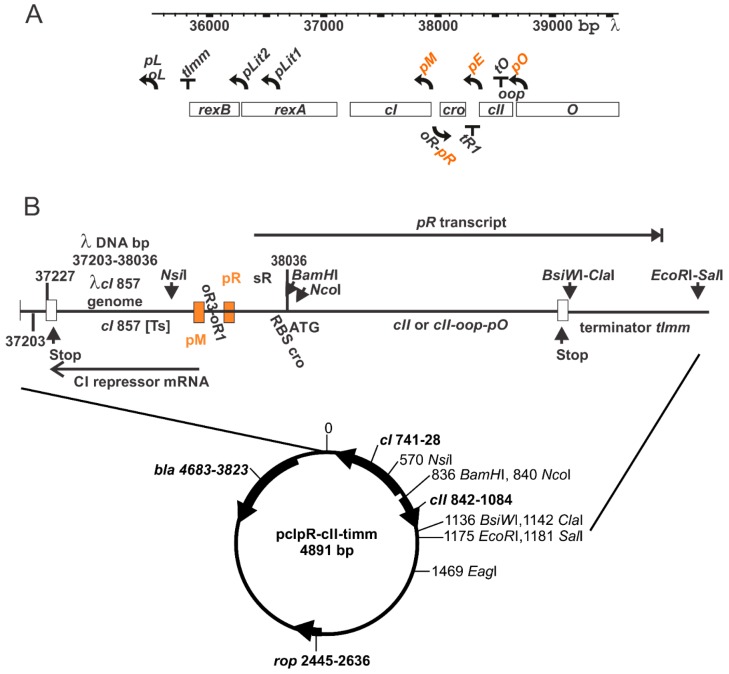
Figure 1.
λ imm-region map and synthetic cII plasmid expression vector. (A) Map of the imm-O region of λ showing immunity region genes rexB—cro, several promoters (those relevant are shown in orange) and terminators, tImm, tR1 and tO. The cI maintenance promoter, pM enables the expression of gene cI, encoding a repressor that binds to operators oL and oR and blocks transcription initiation from the major leftward and rightward promoters pL and pR. pO is required for transcription of the short OOP RNA [21]. The product of gene cII, transcribed from pR, stimulates repressor establishment transcription from promoter pE. The level of pE transcription can be 30- to 100-fold greater than the level of cI transcription from promoter pM upon thermal induction of a cro defective prophage [22,23,24] or 10- to 20-fold after λ infection [25]. tImm, terminates repressor establishment transcription from promoters pM and pE, preventing read-through into the oL/pR site. (B) Plasmid pcIpR-cII-timm (abbreviated herein as [cII] in tables) is a synthetic cII expression system (see Table S1 for related constructs) that eliminates gene cro, ninR and the tR1 terminator, as shown in the plasmid insert (not drawn to scale). Gene expression from the pR promoter is regulated by the encoded temperature sensitive CI [Ts857] repressor, via binding to the oR operator sequences. Gene cII is positioned immediately downstream of the WT ribosomal binding site (RBS) for cro, which perfectly matches/can base pair with the 3′ terminal AUUCCUCCA sequence of 16S rRNA [26]. Potential stem-loop configurations in pR mRNA ahead of cII for the various plasmid constructs used are shown in Figure 2. Sequence variations (1–3) of pcIpR-cII-timm enabling cII expression arrangements are shown in Figure S1. The overall organization of genetic elements on plasmid pcIpR-[…]-timm was described [27,28,29], where a synthetic version of tImm is inserted between the ClaI and EcoRI sites to prevent read-through of transcription arising from promoter pR.
Other aspects of CII function required further study. CII, as expressed from a multicopy plasmid, is highly toxic/lethal to E. coli [30] and this activity is ascribed to its inhibition of host cell DNA replication [31,32,33]. Localization of the toxic effect to sites within the CII protein have not been determined, nor has localization of the ability of CII to cause rapid loss of ColE1 plasmids, which is employed as an assay for replication inhibition. The influence of OOP RNA expression on CII toxicity has not been examined. The influence of OOP on CII complementation in trans, especially relative to OOP polyadenylation [34,35], required further examination. The influence of RNA polymerase mutations on CII activity and the impact of the high-frequency-lysogeny (Hfl) phenotype on CII complementation each required additional study. Finally, the genetics of cII expression is highly complex [36], with a large number of point mutations within and outside of the gene influencing its activity. Most of these mutations were rationalized based on the phenotype they conferred to an infecting λ phage and not independently evaluated synthetically. The effect of mRNA stem-loop formation immediately ahead of the ATG for cII (previously suggested to reduce ribosome attachment), as well as other mutations within and outside of cII, were examined and not surprisingly, mRNA structure ahead and within the N-terminal region of the cII transcript could influence cII expression. This work simultaneously assays for CII complementation, its cellular toxicity and the ability of CII to cause rapid loss of ColE1 plasmids. It examines the influence of cII linkage to oop, which amino acids of CII are required for complementation-toxicity-plasmid loss and the effect of host mutations in rpoB, the hflA locus and pcnB on CII activity. It explores the functional nucleotide length for pO activity and examines the effect of mRNA stem-loop formation ahead of the cII message and other mutations within and outside of cII on CII activities.
2. Materials and Methods
2.1. Strain Construction, Bacterial and Phage Strains Employed
Cellular growth medium, buffers, PCR amplification, DNA sequencing, transformation and P1 transduction methods were previously described [37], as were techniques for the construction of plasmid expression vectors for λ genes, gel analysis of plasmids, insertion localization and assays for plasmid loss and for cell viability resulting from the toxicity of plasmid-expressed λ genes [38]. E. coli strain employed: 594 [38], the rpoB mutants employed were isolated, mapped and sequenced in 594 [37]; pcnB::kan and hflA::kan alleles were moved by P1 from strains MG1655 pcnB::kan and X9368 hflA::kan (kindly provided by Drs. Grzegorz Węgrzyn and Susan Gottessman, respectively). Plasmids p27R and p27RpO− (the basis for the synthetic pO38683-87MH mutation: 38683–38687-ATTAT to CGCGC) were previously described [39]. The pO38683LK mutation was synthetically generated using information from [40]. Phage λcI72 DNA was used for amplifying λ gene segments for insertion into expression plasmids. Phages λimm434cII68 (38498:T–A) and λimm434cII2002 (38430:T–C) used for assaying CII complementation in trans were from the lab collection and are mutated in cII as reported [41]. The construction of pcIpR-D-GFPuv and visual assay for GFP expression was reported in [29].
2.2. Fluorescence Assays for Expressed D-GFPuv and GFPuv*
Cultures were inoculated from single cfu of 594 into LB and 594[pcIpR-D-GFP-timm] into Luria-Bertani (LB) broth (10 g Bacto tryptone, 5 g Bacto yeast extract, 5 g NaCl per liter) made 50 µg/mL with ampicillin = LB + Amp50 and grown overnight (ON) at 30 °C. Larger parallel subcultures (150 mL LB) were made by inoculating 7 mL from each ON culture to LB and then shaken at 30 °C until A575 = 0.2, whereupon they were moved into shaking water baths at 37, 39, or 42 °C. Aliquots were withdrawn prior to transfer and at 20 through 180 min post-induction (3 mL to measure absorbance and 5 mL was transferred and held on ice). Following the last sample removal, all the aliquots were centrifuged at 8000 rpm for five min, decanted and the cell pellets were re-suspended in 5 mL of 1× phosphate buffered saline (PBS) solution (10× = 80 g NaCl, 2 g KCl, 14.4 g Na2HPO4·2H2O, 2.4 g KH2PO4 per liter distilled H2O, pH 7.4), pelleted and re-suspended in 1 mL PBS. Three aliquots (100 µL) of each of the re-suspended cell pellets were assayed for fluorescence using a Photon Technology International, Model LPS 100, spectrophotometer (Horiba Instruments, Inc., Edison, NJ, USA). The excitation wavelength was set at 395 nm and emission fluorescence measured at 509 nm. Triplicate assays for each time point were made and the results averaged. Values for cells with plasmid represent the average of triplicate assays subtracting the triplicate average for cultures of 594 transferred in parallel to 37, 39 or 42 °C. (We appreciate that the fluorescence of cells grown in minimal medium is lower but the fluorescence assays for cells grown in LB were undertaken to parallel the other assay procedures employed herein.) To prepare cell lysates the cell pellets in 1 mL PBS were pelleted at 4500 rpm for 10 min and the supernatant was decanted. The pellet was re-suspended in 0.15 mL lysing mix (minus EDTA) made by adding 1.5 mL 1 M Tris·HCl, pH 7.8, 45 mL 1 M sucrose, 44.7 mL of distilled H2O and 0.8 mL of 10 mg/mL solution of lysozyme made in 10 mM Tris·HCl, pH 7.8. The cell suspension was incubated on ice for 30 min and the spheroplasts pelleted at 4500 rpm for 10 min and supernatant decanted. The pellet was suspended in 1 mL of 0.01 M Tris·HCl, pH 7.8 and allowed to sit 15 min at ambient temperature (~21 °C). The cell lysate was transferred into another tube and three 100 µL aliquots were measured for green fluorescence protein (GFP) activity as for the intact cell preps. The recorded fluorescence of the cell lysates was about half that observed from whole cells.
2.3. Complementation Assay for CII
Overnight LB + Amp50 cultures of cells with plasmids were mixed with LB top agar, overlaid on LB agar plates (in triplicate), then were spotted with 10−6, 10−8, or 10−9 dilutions of cII defective phages λimm434 cII68 (lysate #433) and λimm434 cII2002 (lysate # 717) made in buffer (0.01 M Tris HCl, pH 7.8, 0.01 M NaCl) and incubated for 24 h at 30°, 37° and 39 °C.
2.4. Determination of Toxicity and Plasmid Loss
This was done as previously described [37]. In brief, a single colony of E. coli cells with plasmid was inoculated in 20 mL of LB + Amp50 broth and incubated ON at 30 °C. The saturated cells were diluted in buffer, spread on LB plates and incubated at 30, 37, 39 and 42 °C. The colony forming units (cfu) on the incubated LB plates were counted and the cell viability was assessed by dividing the cfu titer on the 37, 39 or 42 °C-incubated plates by the titer of cfu arising on the plates incubated at 30 °C. Simultaneously, plasmid loss was evaluated by picking cfu from the 30, 37, 39 and 42 °C incubated plates and then stabbing them to fresh LB and LB + Amp50 plates that were incubated ON at 30 °C. The picked cfu that had lost the plasmid grew on the LB but not LB + Amp50 plates.
2.5. Oligonucleotides Used for Plasmid Construction and Sequencing
The oligonucleotides used for plasmid construction are shown in Table S2. General sequencing: L37904 + 18 (37,904–37,922 λ bp in cI) from cI and R-153-19 from backbone of pcIpR-[]-timm to the right of timm. Plasmid construction: L-Bam-CII (λ bp in cII: 38,359–38,394) and R-ClaBsi-CII (λ bp in cII: 38,619–38,650). These primers were used for inserting wild type cII but with an ochre, not wild type (WT) UGA stop codon. R-Bsi-45poWT encodes the wild type sequence between the start of oop at 38,675 through 38,769 in gene O. The expressed portion of O between 38,686–38,769 is truncated with a synthetic ochre stop codon after λ bp 38,769. This normal O-fragment amber stop codon was introduced in primers R-Bsi-45po38383LK and R-Bsi-45po38684-88MH. R-Bsi-45po38383LK includes the pO− base change within “−10” region [40] at position 38,683, changing T to A. R-Bsi-45po38684-88MH changes the −10 sequence of pO as in [2] where bases 38,684–38,688 of the “−10” region sequence attatg/taatac (38,683–38,688) of pO were replaced by cgcgc/gcgcg. Primers for introducing COOH-terminal deletions within cII: R-Cla-Bsi-CII, R-Cla-Bsi-CIIΔ15, R-Cla-Bsi-CIIΔ30, R-Cla-Bsi-CIIΔ60, R-Cla-Bsi-CIIΔ90, R-Cla-Bsi-CIIΔ120 and R-Cla-Bsi-CIIΔ150. Other primers were used for introducing cII or cII-oop-po regions: L-BamXba38339 new was used to copy from λ WT sequence rightward from base 38,339–38,370 into pE(WT)-cII. R-noPo-Bsi-ClaI (38,618–38,653 bp λ), includes the same stop codon “TGA” as for WT cII, thus not altering the truncated oop sequence and was used to copy exactly cII, eliminating the portion of oop that does not overlap cII and the pO promoter region. R-Bsi-O-Po was used to add the 5′-end of oop sequence left of cII including the N-terminal end of O terminating with a synthetic ochre stop codon, including the pO sequence for oop with 39 bp homologous to the N-terminal end of gene O. Primers for introducing phage cII mutations 3638 and 3639 within the last six codons for cII, each with ochre cII termination codon introduced: R-Bsi-CII-3638 (methionine to valine, 4th amino acid encoded from end of cII), R-Bsi-CII-3639 (glutamine to arginine, 6th amino acid encoded from end of cII) and R-Bsi-3638 + 3639 (changes methionine to valine and glutamine to arginine in 4th and 6th amino acids from end of cII). Primers for introducing cy mutations (all silent): LXbaI-38339-cy3048, LXbaI-38339-cy2001, LXbaI-38339-cy42 and LXbaI-38339-cy3001. Primers LXbaI-38339-cy3048, LXbaI-38339-cy2001, LXbaI-38339-cy42 and LXbaI-38339-cy3001 were used with R-noPoBsiClaI (which eliminates oop) and R-Bsi-O-Po which includes oop-pO94. Primers used to make oop-pO-O containing plasmids for comparing their toxicity to cII-containing plasmids (see Table 7 in [5]): L-Bam-OOP#1, L-Bam-OOP#2, R-Cal-O, R-ClaI-36P and R-ClaI-63P.
2.6. Gene Expression Plasmid
Plasmid pcIpR, a precursor of pcIpR-[]-timm, was made by copying the cI857-pM-oR1-3-pR region of λcI857 DNA with primers L-Mfe-37203 + 19 and R-Bam-38036-19 (numbers represent corresponding base pairs of λ genome) and inserting the 833 bp fragment between the EcoRI and BamHI sites in pBR322. Inserting the MfeI end into EcoRI nullifies both sites. As an intermediate, a synthetic 710 BamH-SalI fragment including codon optimized (Dcoe) D-(GGSGAP spacer-AscI)-CAP-ClaI-timm-EcoRI-SalI fragment [29] on plasmid pSMART (synthesized by Integrated DNA Technologies, Inc. [IDT], Coraville, IA, USA) was inserted into pcIpR, eliminating its BamHI-SalI segment to make pcIpR-D-CAP-timm [27,28], which includes the exact sequence of λcI858 between bases 37,203 and 38,036 changing sequence and consensus RBS (underlined) left of the ATG for cro from λ38,029-TAAGGAGGT-TGT-ATG to 38,029-TAAGGAGGA-TCC-ATG, where ATG is the start of cII, not cro. In versions reported herein, cII expression units were cloned between BamHI and ClaI sites, eliminating the Dcoe-CAP-spacer segment.
3. Results
3.1. Thermally Inducible Gene Expression from pcIpR[]Timm Plasmid
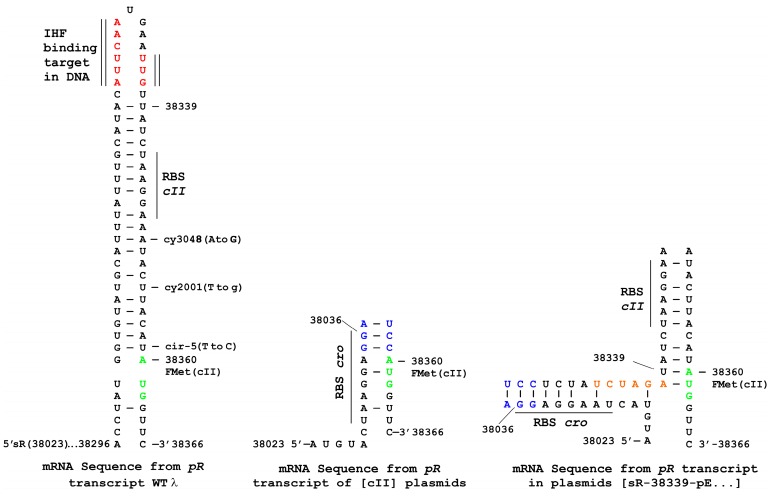
In this report, plasmid-based synthetic cII constructs are transcribed from λ rightward promoter pR, which is regulated by a cI [Ts] repressor binding to oR, Figure 1 and which fully limits expression from pR at 30 °C. This plasmid does not include the oL operator so that octamer looping between the operators and CI repressor complex binding to produce a higher stability repression complex [42,43,44,45] is not possible. Transcription-influencing factors (cro expression, nutR and tR1) mapping between pR and cII were eliminated. This included removing an IHF (integration host factor) binding site (Figure 2) ahead of the RBS for cII. This site was suggested both to stimulate cII transcription by suppressing transcriptional termination at tR1 [46] and to enhance cII mRNA translation by preventing the formation of a RNA:RNA duplex that includes the RBS in the message ahead of cII [47].
Figure 2.
Potential stem-loop configurations in pR mRNA ahead of cII drawn from λWT and synthetic cII expression plasmids. In each structure, the start site for pR-initiated mRNA is suggested as arising at λ sequence base 38,302 just downstream from pR [48]. The structure on left omits the cro-nutR-tR1 sequence and was inspired from a drawing in [36] showing the potential for stem loop mRNA secondary structure ahead of the translational start for cII that could impede translational initiation. The FMet codon for cII is green. An IHF (integration host factor) binding site in DNA [47] is shown in red which resembles the IHF consensus sequence AAAAAAnnnTTnnnWATCAAnnnTTR (where W is A or T, R is A or G and N is any nucleotide) but lacks the A-tract region about one helical turn from the consensus region [49]. The positions for some of the mutations [36] influencing cII that fall within the stem loop are shown. The structure in the middle includes the mRNA sequence produced from plasmid [CII]. This eliminates the stem-loop mRNA structure ahead of cII found in WT λ. It includes the WT λ sequence downstream from pR (bases 37,985–38,036 shown in Figure 1), the mRNA start site at 38,023 and the RBS for cro that is placed four bases ahead of the Fmet codon for cII (refer to Figure S1 and Materials and Methods). This arrangement included a base change equivalent to mutation cir-5 one base ahead of the FMet ATG starting at 38,360 of cII. The cir-5 mutation was suggested [36] to suppress the effect of mutation cII3086 at 38,360, which changes the ATG to GTG. The structure on the right includes plasmids that have the λ sequence for the 38,023 sR transcriptional start site downstream of pR and the WT λ DNA sequence downstream of base 38,339 (just downstream of the IHF binding site shown on figure at left), including the WT pE sequence. The bases for the BamHI (blue) and XbaI (pink) sites in middle and right drawings are shown as DNA sequences in Figure S1 (Plasmid groups 1 and 3, respectively).
The expression of a gene cloned downstream from the promoter pR within plasmid pcIpR-[]-timm is influenced by culture temperature, Table 1. Previously, cultures of 594[pcIpR-GFP-timm] and 594[pcIpR-D-GFP-timm] (where GFP was fused downstream from λ gene D) were used to show that both cell types formed fluorescent cfu (colony forming units) when grown up on spread plates incubated at 37, 39 or 42 °C, compared to colonies grown up at 30 °C which did not fluoresce [29]. In these GFP plasmids the RBS for cro was positioned as described in Figure 1 for [cII], as drawn in the middle structure, Figure 2. Table 1 shows the kinetics of D-GFP fusion protein expression from pR upon shifting the culture temperature of 594[pcIpR-D-GFP-timm] cells growing at 30 °C to 37, 39 or 42 °C. GFP expression in cells raised to 37 °C was not observed until about 60–120 min post induction. The level of gene expression for cells raised to 42 °C by 150–180 min post induction was 43–75X that over cells raised to 37 °C and 8–10-fold over cells raised to 39 °C. By analogy, it is assumed that when cII is cloned downstream from promoter pR, its expression will resemble that of the D-GFP fusion protein. The ability of the Ts CI857 repressor encoded by the plasmid to reduce transcription from pR at 37 °C is lost at 42 °C. Limited pR gene expression at both 36 and 37 °C was previously observed in studies where the gene for the highly toxic P protein of λ was cloned downstream of pR in pcIpR-P-timm [38].
Table 1.
Temperature dependence of thermally inducible intra-cellular D-GFP fusion expression from plasmid pcIpR-D-GFP-timm [fluorescence units (±SE) × 10−3].
| Induction Time (min) | Culture up-Shift from 30 °C ± SE a | ||
|---|---|---|---|
| 37 °C | 39 °C | 42 °C | |
| 0 | 0 (0.2) | 0 (4.0) | 3.2 (0.05) |
| 20 | 0 (0.2) | 1.4 (2.6) | 153.8 (0.5) |
| 40 | 0 (0.3) | 6.7 (2.0) | 415.4 (0.4) |
| 60 | 0.8 (0.3) | 23.2 (3.2) | 518.5 (0.5) |
| 90 | 0 (0.3) | 41.3 (0.3) | 624.6 (0.4) |
| 120 | 7.3 (14.9) | 30.0 (2.9) | 721.2 (0.4) |
| 150 | 10.8 (1.3) | 84.3 (3.4) | 808.4 (0.4) |
| 180 | 23.9 (2.6) | 136.9 (10.7) | 1039.5 (0.7) |
D-GFP is a fusion protein generated by fusing the DNA sequence for green fluorescence protein to DNA sequence for λ capsid decoration protein D. a All data represent triplicate determinations averaged. Then the triplicate average for up-shifted 594 cells (to 37, 39, or 42 °C for the indicated times) was subtracted from the triplicate averaged upshifted 594[pcIpR-D-GFP-timm] whole cells. The standard error (SE) values represent the average of SE values for determinations of 594 cells with or without a plasmid.
3.2. Untangling CII Activities: Cellular Toxicity and Promoting Plasmid Loss
The assay for CII toxicity measures if cells capable of expressing cII can survive and form cfu when grown in culture at 30 °C, then spread on plates incubated at 37, 39 or 42 °C. Per 1000 cfu of 594[cII] cells spread on plates incubated at 39° or 42 °C (Table 2), respectively, on average, only 30 or 1 cfu were observed. Cellular toxicity and plasmid loss is dependent on CII production. In CII1–92, deletion of the last five amino acids restores cell viability by 24-fold (0.73/0.03) at 39 °C. Deletion of the terminal 10 amino acids (in CII1–87) prevents plasmid loss at 42 °C. Deletion of the terminal 20 amino acids (in CII1–77) completely suppressed CII toxicity and its ability to stimulate plasmid loss. (Identical results were observed for CII1–67, CII1–57 and CII1–47 as for CII1–77.) Thus, the last 10 amino acids of CII are involved in plasmid loss. The last 5 amino acids significantly influence CII-dependent cellular toxicity.
Table 2.
Influence of C-terminal cII amino acids and host properties on cII complementation, toxicity and plasmid loss.
| Host [Plasmid] a | Intensity of CII-Activated CI434 Repression at 37–39 °C b | λimm434 cII− Plaque Formation at 37–39 °C c | Cell Viability, (±SE) and [% Plasmid Loss] Per Growth Temp. of Transformants e | |
|---|---|---|---|---|
| 39 °C | 42 °C | |||
| 594 [cII] = [cII1–97] | H (high) | − | 0.03 (0.001) [0] | <0.001 (0.0001) [100] |
| 594 [cII-oop-pO94] | 0 (none) | + d | 0.59 (0.03) [0] | 0.08 (0.03) [0] |
| 594 [cII1–92] | H | − | 0.73 (0.01) [0] | 0.005 (0.001) [100] |
| 594 [cII1–87] | H | − | 0.76 (0.02) [0] | 0.08 (0.03) [0] |
| 594 [cII1–77] | 0 | + d | 0.74 (0.10) [0] | 0.32 (0.10) [0] |
| 594 [cII-3638](38,642: A-G, M-V) | H | − | 0.62 (0.10) [0] | 0.002 (0.0004) [89] |
| 594 [cII-3639](38,634: A-G, Q-R) | H | − | 0.76 (0.02) [0] | <0.001 (<0.0001) [19] |
| 594 [cII-3638–3639] | H | − | 0.65 (0.04) [0] | <0.001 (<0.0001) [6] |
| 594 hflA::kan [cII] | S (slight) | + | 0.89 (0.01) [0] | 0.008 (<0.0001) [100] |
| 594 hflA::kan [cII1–92] | S | + | 0.69 (0.01) [0] | <0.001 (<0.0001) [100] |
| 594 hflA::kan [cII1–87] | S | + | 0.85 (0.02) [0] | 0.24 (<0.0001) [0] |
| 594 hflA::kan [cII1–77] | 0 | + d | 0.86 (0.10) [0] | 0.58 (0.01) [0] |
| 594 hflA::kan [cII-oop-pO94] | 0 | + d | 0.63 (<0.0001) [0] | 0.001 (0.0002) [100] |
| 594 pcnB::kan [cII] | 0 | + d | <0.001 (0.01) [67] | <0.001 (0.0001) [100] |
| 594 pcnB::kan [cII-oop-pO94] | 0 | + d | 0.46 (0.01) [64] | <0.001 (0.0003) [89] |
a All strains were made by transformation of an AmpR plasmid into strain 594, or into 594 cells that had been transduced with hflA::kan or pcnB::kan markers. All CII protein deletions were from the COOH-terminal end, where the WT CII is represented as cII1–97. b Intensity of CII-activated CI repression depends upon CII expression from the plasmid stimulating pE-cI434 transcription from an infecting λimm434cII− phage genome. “H” indicates a high level of CII complementation of the cII-defective infecting phage by the plasmid-encoded cII allele. The high level of CI434 expression from the phage genome represses lytic development, preventing the formation of individual plaques and phage lysis spots on overlay plates incubated at 37 and 39 °C. “0” indicates that no CII complementation was detected and the infecting phage plated, forming clear plaques, at an equivalent efficiency to that observed on overlaid 594 host cells without a plasmid. “S” indicates slight CII complementation, insufficient to block lytic phage growth and plaque formation and yielding weakly turbid plaques, suggesting the CII activity was not sufficient to drive establishment pE-cI434 transcription and CI434 repressor synthesis to a level that could prevent lytic growth of the infecting λimm434cII- phages. c Plaquing designations: “−” indicates that no individual plaques were observed in spots of 16 or 160 pfu and faint or no lysis was evident when 16,000 pfu were spotted. “+” indicates cell lysis when 16,000 pfu were spotted and individual pfu formation in each of the spot areas with applied 160 or 16 pfu. d Individual plaques were clear. e Measurements for plasmid loss: a minimum of 36 cfu per assay plate per plating temperature were screened, in triplicate. The assay was also conducted in parallel (for every experiment shown herein) at 30 °C, where there is no cII expression due to the fully active λ CI Ts857 repressor and at 37 °C; and for each assay, the viability was 1.0 with no plasmid loss.
Two cII missense mutations cII-3639 and cII-3638, that respectively change the 6th (cII mutation 38634 Gln to Arg) or 3rd (mutation 38642 Met to Val) amino acid from the COOH-terminal end [36], did not modulate CII toxicity but reduced plasmid loss at 42 °C. Combining the two mutations on one cII gene greatly reduced plasmid loss, i.e., 94% plasmid retention at 42 °C, suggesting an involvement of the six C-terminal amino acids of CII in plasmid loss.
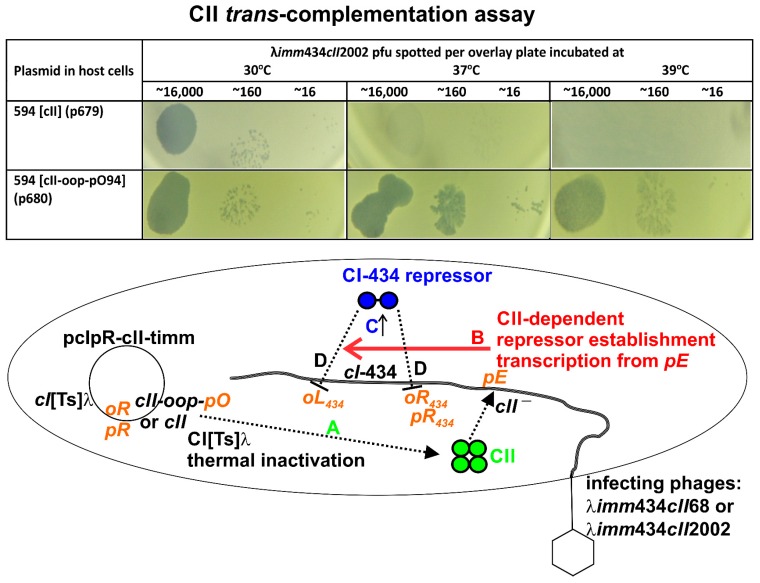
3.3. CII Complementation
The assay for CII complementation, Figure 3, measures the expression and ability of plasmid cloned cII to stimulate, in trans, repressor establishment transcription from promoter pE encoded on two infecting heteroimmune imm434 lambdoid phages mutated for cII (cII68, 38498: T-A, Trp to Arg; cII2002, 38430: T-C, Leu to Pro). Each hybrid phage includes the WT λ sequence rightward from λ base 38265 left of cII, including an identical IHF binding site, RBS for cII, the −10 and −35 sequences of pE and reported regions of extended dyad symmetry [50]. CII-stimulated, pE-dependent CI434 repressor establishment transcription from the infecting phage genome confers a high level of cI-434-repressor expression. The CI434 can bind to the imm434 oL and oR operators on the infecting phage. This blocks phage transcription from the imm434 pR promoter, lytic phage replication and growth and arrests plaque formation at 37 °C or 39 °C, effectively shunting the infecting cII-defective phage into a non-vegetative (pre-lysogenic) state. Even weak cII expression from [cII] at 37 °C was sufficient to prevent plaque formation by the infecting imm434 phages (Figure 3). Clear plaque forming units (pfu) arose in cell lawns incubated at 30 °C due to the repression of cII expression from [cII]. Plasmids deleted in cII for five or ten COOH-terminal amino acids (respectively cII1–92 and cII1–87), or those with cII mutations cII-3638, cII-3639 and cII-3638 + 3639 fully complemented for CII activation of repressor establishment transcription from pE (Table 2, Figure S2). Plasmid cII1–77 and those with larger terminal deletions failed to complement for CII activity. Thus, either the last 20 amino acids, or those 11 to 20 from the C-terminal end are required for CII activation of transcription from pE.
Figure 3.
CII trans-complementation assay. Top shows complementation results (additional examples are shown in Figures S2 and S3). The cartoon shows the sequential steps, A to D required for CII complementation. Each step is indicated by either dotted (steps A and D) or solid (steps B and C) lines. Steps: A cII expression occurs from plasmid pcIpR-cII-timm in 594 cells upon shifting cells grown at 30 °C to 37–42 °C. This inactivates the CIλ repressor, enabling transcription from plasmid promoter pR through gene cII. B CII protein encoded by plasmid stimulates repressor-establishment transcription from promoter pE on phage genome after 594 [pcIpE-cII-timm] cells are infected with phage λimm434cII2002. C High expression of the CI434 repressor protein occurs from CII-stimulated pE-mRNA transcribed from infecting phage genome. D CI434 repressor binds the oL434 and oR434 operator sites preventing leftward and rightward transcription and blocking lytic phage growth. The potential for OOP RNA expression from plasmid pcIpR-cII-oop-pO nullifies CII-activation of pE-dependent CI434 expression at 37–39 °C. The results for infections with λimm434cII68 were identical to infections with λimm434cII2002.
Contrary to an expectation that plaque formation would be inhibited, hflA cells with plasmids [cII], [cII1–92] and [cII1–87] allowed weak activation of transcription from pE but did not block phage growth, Table 2, Figure S2. Plasmid [cII1–77] was fully defective for CII complementation in hflA::kan cells as clear pfu were formed at 37–39 °C, Table 2. Significantly, the pcnB::kan mutation, shown to be a null allele for pcnB [51], completely abrogated CII complementation activity at 37 and 39 °C (Table 2, Figure S2). These results strongly implicate an involvement of poly (A) polymerase I in CII complementation that was not noted previously.
Cells with transduced rifampicin-resistant mutations in rpoB, encoding the β-subunit of E. coli RNA polymerase, were examined to determine if a CII-RpoB interaction was involved in CII complementation, cell toxicity, or plasmid loss. In the rpoB mutant BI (Table 3), CII expression from plasmids [cII] and [cII1–92] (but not [cII1–87]) yielded high level complementation, blocking lytic phage growth at 39 °C. The remaining rpoB mutants (B8, C1, C4, C10, D2, D6) interfered with CII complementation, Table 3. This result supports the conclusion that a CII-RpoB interaction participates in CII complementation. The loss of any CII complementation by [cII1–87] in five of seven rpoB mutants suggests that an interaction between the terminal ten amino acids of CII and RpoB is an essential component of CII-stimulated pE transcription.
Table 3.
Influence of rpoB mutations on CII complementation.
| Host(s) Strains a | CII-Construct Plasmid(s) with Thermally Inducible Expression of cII Allele | Intensity of CII-Activated CI434 Repression at 37–39 °C b | λimm434 cII− Plaque Formation at 37–39 °C b |
|---|---|---|---|
| 594 rpoB B1 | cII; cII1–92 | H | − |
| 594 rpoB B8, C1, C4, C10, D2, D6 | cII; cII1–92 | S | + |
| 594 rpoB B1, C1 | cII1–87 | S | + |
| 594 rpoB B8, C4, C10, D2, D6 | cII1–87 | 0 | + c |
a rpoB mutations in 594 host, PR: B1 Q148P, B8 L571Q, C4 Δ:GEV440-442V, D2 R451S, D6 G537C (each confer resistance to the toxic λ gpP protein); PS: C1 P564L, C10 R529H. PR and PS indicate which rpoB mutations confer resistance, or sensitivity to λ P protein lethality. b Designations as in Table 2. c Individual plaques were clear. The designations H, S, 0, for intensity of CII activated repression were as noted in Table 2.
3.4. Host Modulation of CII-Dependent Cellular Toxicity and Plasmid Retention
The host null mutation hflA::kan reduced CII toxicity at 39 °C (30-fold-compare viabilities of 0.03 and 0.89) and at 42 °C, Table 2. CII expression was highly toxic in 594 pcnB::kan cells, even at 39 °C (Table 2).
Several of the rpoB mutants, B1 (Q148P), B8 (L571Q), C4 (ΔGEV440-442V), D2 (R451S) and D6 (G537C) had been shown to confer cellular resistance to the highly toxic λ gpP, while C1 (P564L) and C10 (R529H) remained P-sensitive [37]. Except for B1, both PR and PS mutants, suppressed CII toxicity at 39 °C (cell viabilities: 594[cII], 0.03; rpoB[cII]: B1, 0.003; B8, 0.65; C1, 0.96; C4, 0.15; C10, 0.14; D2, 0.95; D6, 0.11) and 42 °C. Mutants C1 and D2 were highly protective. The PR mutants C4, D2 and D6 reduced or eliminated CII-dependent plasmid loss. These results suggest that CII:RpoB participates in cellular CII toxicity and plasmid loss at 42 °C (Table 4). The mutant C4 exhibited high toxicity without plasmid loss, suggesting CII toxicity and plasmid loss arise via different actions of CII.
Table 4.
Suppression of CII toxicity and plasmid loss by oop expression in rpoB mutants.
| Host Strains with rpoB Alleles | Cell Viability (±SE) [% Plasmid Loss] Per Growth of Transformant at 42 °C | |
|---|---|---|
| [cII] a | [cII-oop-pO94] b | |
| 594 c | <0.001 (0.001) [100] | 0.08 (0.03) [0] |
| 594 rpoB B1 | <0.001 (<0.0001) [100] | 0.07 (0.01) [0] |
| 594 rpoB B8 | 0.01 (<0.0001) [78] | 0.13 (0.03) [0] |
| 594 rpoB C1 | 0.007 (0.10) [100] | 0.36 (<0.0001) [0] |
| 594 rpoB C4 | 0.003 (0.010) [0] | 0.07 (<0.0001) [0] |
| 594 rpoB C10 | 0.004 (0.001) [100] | 1.00 (0.01) [0] |
| 594 rpoB D2 | 0.38 (<0.0001) [0] | 0.10 (<0.0001) [81] |
| 594 rpoB D6 | 0.02 (0.01) [6] | 0.03 (0.01) [86] |
a Plasmid includes precise sequence (ATG to TTC) for cII cloned into pcIpR-cII-timm in the same position in plasmid, relative to pR and the sR start site for rightward transcription as is gene cro in λWT, including its RBS sequence, except for substitution of a BamHI site cloning site incorporated into the RBS, eliminating the natural DNA sequence leftward (upstream) of cII, which includes a stem-loop structure [36] that could influence cII expression. The cII sequence in [cII] terminates with an ochre rather than TGA stop codon, Figure S1. b Plasmid includes the same sequence as in [cII] but with natural amber termination signal for cII, plus the natural partially cII-overlapping oop sequence, plus 94 nucleotides rightward of the start point (38685) for 5′ end of oop transcription, that includes the pO promoter for oop, through base 38769 in gene O. This encodes 28 amino acids from the N-terminal end of O, terminating with an inserted ochre stop codon, Figure S1 plasmids 2. c Data from Table 2 repeated here for comparison.
3.5. Influence of oop RNA Expression on cII-Dependent Cellular Toxicity and Plasmid Loss
The oop RNA gene is expressed leftward from promoter pO and terminates at the hairpin formed at the end of oop, designated the tO terminator (Figure 1). 52-bases of the oop sequence overlap the C-terminal region of cII (Figure S1). Plasmid loss: oop expression from [cII-oop-pO94] eliminated CII-dependent plasmid loss at 42 °C in WT 594 cells (Table 2) and in rpoB mutants B1, B8, C1 and C10 (Table 4). It was unable to do so in 594 cells with null mutations hflA, pcnB (Table 2) or the rpoB mutants D2 and D6 (Table 4). CII-toxicity: oop expression suppressed CII toxicity at 39 and 42 °C in strain 594 (Table 2) and in the rpoB mutants B1, B8, C4 and by >50–250 fold in rpoB mutants C1 and C10, respectively (Table 4).
3.6. Influence of Terminally Overlapping Divergent cII and oop Transcription on CII Complementation
Several [cII-oop-pO] plasmid constructs were examined herein. oop expression from [cII-oop-pO94], abrogated CII complementation in 594 WT and the 594 hflA cells at 39 °C (Table 2). In [cII-oop-pO94] the promoter for oop is within the 94 bp to the right of the 5′end of the OOP RNA. The putative sequence for pO was predicted in the original sequence determined by Scherer et al. [52] to include a −10 region between 8 and 13 nucleotides rightward from base 38,675 encoding the 5′ end of the OOP RNA and a −35 region (Figure S1, plasmids 2). An attempt was made to establish a minimal sequence encoding pO and to assess the effect of introduced mutations within the −10 for pO. Plasmids were constructed that included the predicted WT −10 and −35 regions ahead of the actual start of oop transcription, i.e., variations of [cII-oop-pO45] and versions that included the −10 mutations LK (A to T at 38,683) [40] or MH (i.e., changing bases 38,683–38,687, ATTAT to CGCGC) [39]. The WT pO45 (p763) and both the pO45 −10 mutant plasmids (p759, p762) fully complemented for CII-activated CI434 expression at 37–39 °C (Table 5, Figure S3), suggesting there was no interference of CII complementation and little if any oop expression by the pO45 plasmids. The −10 position for pO suggested by Scherer et al. [52] appears correct since introduction of the MH mutation into the pO94 plasmid (p681) allowed for full CII complementation, i.e., for CII-activated CI434 expression at 37–39 °C. Thus, the −10 mutation MH nullified oop expression, at least in comparison to that from WT [cII-oop-pO94] (Table 2). These data suggest that unrecognized upstream nucleotides between 45 and 94 from the 5′ end of oop contribute to the function of pO.
Table 5.
Expression variations for cII in 594 host.
| CII-Construct Plasmids with Thermally Inducible Expression of cII Allele | Intensity of CII-Activated CI434 Expression at 37–39 °C a | λimm434 cII− Plaque Formation at 37–39 °C a | Cell Viability (±SE) [% Plasmid Loss] Per Growth Temp. of Transformants | |
|---|---|---|---|---|
| 39 °C | 42 °C | |||
| sR-38339-pE-cII (p747) | H | − | 0.62 (0.10) [0] | <0.001 (<0.004) [94] |
| sR-38339-pE-cII-oop-pO94 (p748) | 0 | + d | 0.82 (0.02) [0] | 0.001 (<0.0001) [94] |
| sR-38339-pE-cII-cy3048 in −10 pE (p767) | 0-S b | + | 0.89 (0.01) [0] | 0.08 (<0.0001) [0] |
| sR-38339-pE-cII-cy2001 in −10 pE (p765) | 0-S b | + | 0.69 (<0.0001) [0] | 0.01 (<0.0001) [0] |
| sR-38339-pE-cII-cy42 in −35 pE (p764) | 0-S b | + | 1.00 (0.020) [0] | 1.00 (<0.0001) [42] |
| sR-38339-pE-cII-cy3001 in −35 pE (p766) | H | − | 0.72 (<0.0001) [0] | <0.001 (0.001) [8] |
| cII-oop-pO45WT (p763) | H | − | 0.72 (0.10) [0] | 0.07 (<0.0001) [0] |
| cII-oop-pO45-38,683-87MH2 in −10 pO (p759) | H | − | 0.67 (0.10) [0] | <0.001 (0.002) [100] |
| cII-oop-pO45-38683LK in −10 pO (p762) | H | − | 0.78 (0.20) [0] | <0.001 (<0.0001) [50] |
| cII-oop-pO94-38683-87MH (p681) c | H | − | 0.71 (<0.0001) [0] | <0.001 (<0.0001) [86] |
a Designations as in Table 2. b 0-S = Phage lysis spots/plaque areas were weak-turbid at 39 °C but well-formed clear plaques were observed at 37 °C. c Sequence change determined in putative −10 region of pO recovered from p27RpO− [39] was attatG to cgcgcG and was identical to the synthetic change introduced into p759, see Figure S1, plasmids 2. d Individual plaques were clear.
3.7. Plasmid Construction Variations and Influence on cII Expression
The WT pR mRNA can form a stem-loop structure ahead of the amino-terminal region of cII (Figure 2) [36,53]. It was proposed [36] that this stem-loop structure reduces the rate of translation initiation, possibly by restricting the access of ribosomes to the initiation region and that the binding of IHF to this region was essential for the translation of cII mRNA [47]. Several plasmid variants were generated (Figure S1, plasmids 3), including ones with upstream WT bases 38,339 through the cII mRNA start codon. These encoded the potential for mRNA stem-loop formation occluding the ribosomal binding site (RBS bases 38,343-38,348) ahead of AUG for cII (Figure 2) but not including the IHF binding site, nor most of the region of dyad symmetry present in the WT cII mRNA [47]. These plasmids encoded the −10 and −35 regions of pE (respectively, bases 38,350–38,355 outside of cII and the dual CGTT sequences encoded by bases 38,369–38372 and 38,379–38,382 within the amino terminal end of cII). Some of them included the pO94 sequence, or four point mutations believed to inactivate pE, i.e., mutations cy3048 or cy2001 within −10 and cy42 or cy3001 within each of the CGTT sequences in −35.
Several differences were apparent when comparing data for plasmids with/without the 38,339–38,360 sequence that could form a stem-loop structure. Plasmid [cII] was 21-fold more toxic in cells raised to 39 °C than [sR-38339-pE-cII] (Table 2 and Table 5), suggesting the formation of a stem-loop can reduce cII expression. In contrast, the ability of oop expression from plasmid [cII-oop-pO94] to reduce CII toxicity at 42 °C (Table 2) decreased by 8-fold for [sR-38339-pE-cII-oop-pO94] (Table 5) and suppression of plasmid loss at 42 °C no longer occurred. This suggests stem-loop formation ahead of the cII message can influence activities involving pO94.
Adding −10 region mutations cy3048 or cy2001 (Figure S1) to [sR-38,339-pE-cII] reduced CII toxicity by 10 to 80-fold, and, even without oop, they completely suppressed CII-dependent plasmid loss at 42 °C (Table 5). The −35 region mutation cy3001 reduced plasmid loss and cy42 eliminated CII toxicity at 42 °C. The plasmid [sR-38,339-pE-cII] with added cy mutations 2001, 42, or 3048 poorly complemented in trans for CII (Table 5), permitting clear plaque formation by the infecting imm434cII phages at 37 °C and weak- turbid pfu at 39 °C. (The cy3048 mutation was included in plasmids described in [47]). Plasmid [sR-38339-pE-cII] with cy3001 complemented better, suppressing plaque formation at 39 °C, yet supporting individual turbid plaque formation at 37 °C.
It was assumed that neither the point mutations (cy3048, cy2001) introduced ahead of cII in the −10 for pE nor the silent mutations (cy42 and cy3001) within cII in the −35 of pE would influence CII complementation in trans. This assumption proved wrong, as CII complementation by [sR-38339-pE-cII] was altered by the putatively cis acting cy mutations.
4. Discussion
The studies reported here both confirm and extend several of the observations made by Kobiler et al. [15], who demonstrated CII1–82, deleted for the terminal 15 amino acids, was degraded very slowly by FtsH, compared to CII WT, suggesting the terminal amino acids of the CII monomer represent an important target for FtsH proteolysis and thus CII protein stability within the cell. But they concluded that the terminal 16 amino acids were not required for CII activity, i.e., CII1–81 was functional, CII1–80 was non-functional. These results parallel our finding a CII1–77 protein deleted for the COOH-terminal 20 amino acids was unable to complement for CII. However, we found that the terminal amino acids of WT CII1–97 can influence its ability to complement, its toxicity and its effect on plasmid stability in a WT host. Whereas, CII1–92 and CII1–87 were as active as CII1–97 for CII complementation, CII toxicity was reduced by 20-fold in CII1–92 and plasmid loss was fully suppressed in CII1–87. Thus, the C-terminal end of CII is not without activity in a WT host. Deletion of the C-terminal 10 residues in CII1–87 eliminated complementation in hosts with five distinct point mutations in rpoB, each conferring rifampicin resistance, suggesting that these residues influence CII complementation.
The CII complementation assay employed here assumed that cII expression from a plasmid would stimulate, in trans, pE transcription from infecting imm434 heteroimmune phages, each with a nullifying cII mutation. No complementation is observed at 30 °C, since there is no cII expression. But, expressing a high level of CI434 repressor will shut down imm434 pL and pR transcription from the infecting phage, blocking plaque formation (lytic phage growth), without influencing cII expression encoded on the immλ plasmid. This assay measured two components of CII activity. Trace levels of cII expression were expected at 37 °C (and hence turbid plaque formation but not inhibition of lytic growth). Intermediate levels of cII expression were expected at 39 °C (compared to 42 °C with fully constitutive cII expression), turbid plaque formation and perhaps plaque inhibition. It was found: (i) constructs exhibiting weak CII complementation supported turbid or weak-turbid plaque formation at 37 and 39 °C; (ii) cells defective for CII complementation gave clear pfu at 37 and 39 °C; and (iii) cells with full CII complementation prevented plaque formation at both 37 and 39 °C. Kobilier et al. [15] suggested that CII inhibits lytic phage functions perhaps through an effect on phage DNA replication and/or Q gene expression. The results for high CII complementation, do not distinguish between (i) CII action to stimulate very high CI434 expression that can shut down phage transcription, from (ii) an activity of CII that independently and perhaps non-specifically, inhibits lytic growth, e.g., by inhibiting replication, or by any other mechanism. There are some hints to tease apart the two potential actions of CII, each manifested by the inhibition of phage plating and to make sense of mutations arising within and outside of cII that influence CII activity.
Extrinsic factors modulate the link between high CII complementation and high CII toxicity. Early studies identified host hflA and hflB mutations (conferring high frequency of lysogeny phenotype) that enable λ+ and λcIII mutants to lysogenize these cells at high frequencies [54], suggesting higher CII activity in these cells. What seems undisputed is that the FtsH (HflB) ATP-dependent membrane protease degrades CII [15] and that the HflK-HflC proteins encoded by the hflA locus can individually inhibit the FtsH-mediated proteolysis of CII in vitro [16]. When it was examined whether an hflA::kan null mutant would support enhanced CII degradation, 30-fold less CII toxicity was observed at 39 °C. This suggested that inactivating the hflA locus reduces CII toxicity and its interference with lytic phage growth, which is more in keeping with its effect on FtsH and that CII toxicity can be a factor in λ development. We propose that by reducing some form of CII toxicity, e.g., via enhanced CII degradation, the frequency of λ lysogeny is increased. This is an alternative explanation for the HflA phenotype to one based on enhancing CII activity.
What is responsible for CII toxicity? It was shown that the single base substitution G537C responsible for rpoB D2 [37] can completely suppress CII toxicity and plasmid loss at the highest level of cII expression at 42 °C (with cell viability of 0.95 at 39 °C and no plasmid loss), while reducing but not eliminating CII complementation at 39 °C. This suggests that both CII toxicity and plasmid loss depend upon a binding interaction between CII and E. coli RNA polymerase holoenzyme, or a direct interaction with the β-subunit encoded by rpoB. The rpoB D2 mutation also confers resistance to cell killing by the highly toxic λ gpP. Even trace expression of P significantly exceeds the replication-inhibition and plasmid curing levels conferred by CII. For example, minimally expressed P from plasmid pcIpR-P-timm in cells grown at 36 °C [38] cured the cells of ColE1 plasmids. Hammer et al. previously isolated E. coli rpoB mutants S531F and P564L resistant to killing by CII protein [55]. One mutant used, rpoB C1 (P564L), shared an identical mutation as reported by Hanmer et al. [55] but was not nearly so resistant to CII as were the rpoB D2 cells.
Kedzierska et al. [31] found that E. coli growth inhibition was independent of mutations in CII (A30T) [56] or in rpoA (L271E) [57] that abolish transcriptional activation by CII [58] at several positively regulated promoters [59]. They suggested that CII toxicity is independent of its transcription activation function, even given the potential for 294 CII binding sites noted to be present in the E. coli genome [31]. They rationalized, because of the almost immediate effect of CII overproduction, that its inhibitory effect to both E. coli and λ replication was linked to interference with the DNA replication process. Herein, an assay for cellular loss of ColE1 plasmids was employed as an indicator for replication inhibition. The rpoB mutations D2 (R451S) and C4 (Δ:GEV440-442V) completely abolished plasmid loss when cII was constitutively expressed at 42 °C and mutation D6 (G537C) was almost as effective. These results suggest that the inhibitory effect of CII on plasmid maintenance involves its interaction and interference at a stage of RNA polymerase participation in replication.
RNA transcripts terminated by the oop terminator, tO are reported very labile, compared to transcripts from other terminators [60]. The influence of polyadenylation on cII and cII-oop expression was examined, since it was suggested that polyadenylation by E. coli poly (A) polymerase I (PAP I) leads to destabilization of RNA in bacterial cells [61,62]. Reports suggest that OOP RNA is polyadenylated by PAP I, that this is impaired in a pcnB mutant [35] and that polyadenylated OOP RNA is rapidly degraded [34] in comparison to non-polyadenylated OOP. CII activity in cells with a pcnB null mutation was examined. [Note that cII-mRNA expression terminates at tImm from [cII] missing oop-pO]. Surprisingly, all CII complementation activity was suppressed, the imm434cII phages plated efficiently (suggesting any CII made was not inhibiting phage growth) and yet high-level cellular CII toxicity and plasmid loss were evident. It would appear, that cellular capacity for polyadenylation is essential for some aspect of CII complementation. Coupling cII-oop expression could reverse cellular toxicity at 39 °C but not plasmid loss. This was a first indication that the state of cII-mRNA can influence CII complementation.
The results illustrate a profound nullifying effect of oop expression from promoter pO on CII complementation, toxicity and plasmid loss, suggesting emphatically that OOP is a regulatory pivot in λ development and that oop transcription can abrogate CII activity and drive the lytic response. This effect occurred in the lexA+ cells employed, where a LexA repressor binding sequence within the oop-pO sequence [63] can potentially limit oop expression. RNaseIII-dependent and independent cleavage of a cII-mRNA:OOP RNA hybrid was suggested as a mechanism by which OOP RNA could modulate CII-mRNA expression. Both cleavage sites are outside of cII [20] and thus secondary nucleolytic degradation of the cII-mRNA would be required to inactivate the message, for which the efficiency has not been reported.
The −10 and −35 regions of pO were suggested from the original DNA sequence of this region [52] and are fully contained within pO45 that includes 45 nucleotides upstream from the 5′-end of oop sequence. It can be argued from CII toxicity and plasmid loss data at 42 °C that there is some oop expression from pO45, since mutations at LK (38,683) and MH (38,683-87) in −10 suppressed this but the plasmid cII-oop-pO45WT still retained a high level of CII complementation. CII complementation was completely defective in cells with the cII-oop-pO94 plasmid but was restored in cells with plasmid cII-oop-pO94MH with an altered −10 region of pO. This data supports, both that the −10 region is correctly identified and that extending pO an additional 49 bases rightward into the O sequence from pO45 to pO94 profoundly influences cII expression/complementation, all other factors being equivalent. The tO-oop-pO transcriptional unit was identified from thermally induced defective prophage [21] deleted for all cell lysis and morphogenesis genes rightward from genes O-P-ren. The level of oop RNA synthesis increased 15+ fold following de-repression of replication competent prophage but remained static after de-repression of eight strains defective in λ replication initiation [64], including prophage with oriλ defects. These and other studies suggested there was no direct correspondence between the level of OOP RNA synthesis and the number of λ DNA copies per cell. Because the oop sequence was found to be dispensable for λ growth [65] (as is cII), questions relating to whether oop expression is regulated, or is merely gene dosage dependent have remained unsolved. It is unknown if the OOP RNA made from a multicopy plasmid equates to that made from an induced prophage. None of the plasmids employed herein were capable of oriλ-dependent replication initiation. Thus, the difference between oop expression from pO45 and pO94 most likely depends upon a transcriptional up-regulation signal for oop located in the additional O gene sequence present in pO94.
Based on these studies, it is suggested that assays measuring the global cellular effect of CII should include a functional cII-oop-pO construct. In this report, plasmid-expressed CII complemented, in trans, pE transcription initiation from infecting cII-defective imm434 phages. An opposite assay was employed to assess cII expression from infecting phages, where the CII made stimulated, in trans, pE transcription from a plasmid devoid of oop and apparently a promoter for cII. In these assays [8,66] the phage encoded CII activity stimulated pE dependent GFP or mCherry expression from ColE1 plasmid pSA11 with λ coordinates 38,336–38,653 [67]. The λ segment on the plasmid was deleted for the IHF binding site ahead of cII but included the stem-loop structure ahead of cII up through five amino acids from its COOH-terminal end, eliminating the 5′ end of oop and its pO promoter. It was assumed there was little/no cII expression from the plasmid borne λ segment, only from the infecting phages. The possible influence of pO transcription on pE expression from the plasmid remained unexamined.
In this work, plasmids were made that could support the potential for stem-loop formation, Figure 2 (structure on right) ahead of cII and they conferred reduced CII toxicity and likely reduced cII expression. The potential for stem-loop formation ahead of cII reversed some of the activities involving oop expression from promoter pO94. Accordingly, point mutations were introduced within the stem-loop but outside of cII. They also reduced CII toxicity and suppressed its effect on plasmid loss. Amazingly, the single silent mutation, cy42 in codon four of cII, within pE, completely suppressed CII toxicity at 42 °C. These observations require further study to gain understanding but strongly suggest that the hitherto un-explored state of cII-mRNA structure (excluding the IHF binding site and regions of dyad symmetry ahead of cII) remain an important component in cII expression.
Finally, it is unclear if CII can stimulate pE transcription from every DNA template, e.g., can it express pE from a template where cII is being transcribed? In discussing a voting rule for lysogeny, Zeng et al. [66] found there was no pE activity in cells defective for dnaJ, which is required for λ replication. Long ago, an enormous difference was reported [23] between CII- and CIII-dependent pE expression following the induction of a λcI857cro27 prophage in a WT host that supported the replication of an induced λ prophage, compared to the induction of the same prophage in a dnaB[Ts] host that prevented λ replication and from which there was no detectable pE transcription. For the WT cells, there was a 10- to 15-fold increase in DNA copies of the λcI857cro27 prophage by about 25 min post induction [22]. It remains unclear if the increased number of λ templates were responsible for the large difference in pE expression between replication competent and deficient conditions.
5. Conclusions
The results confirm that OOP RNA expression from the genetic element pO-oop-to can suppress high CII activity and that OOP RNA likely serves as a powerful regulatory pivot in temperate lambdoid phage development.
Plasmids with a pO94, comprising 94 bases rightward from oop, prevented CII complementation, CII-dependent plasmid loss and suppressed CII toxicity, suggesting that the active pO promoter to produce OOP requires an extended DNA sequence, beyond that required to encode the −10 and −35 regions.
All three CII activities were eliminated by the deletion of its COOH-terminal 20 amino acids.
E. coli mutations were shown to influence CII activities. (a) Inactivating the hflA locus encoding HflK-HflC proteins that modulate the FtsH ATP-dependent membrane protease significantly reduced CII trans-complementation and toxicity; (b) A null allele of pcnB, encoding poly (A) polymerase I, eliminated CII complementation and increased CII toxicity; (c) Five of six rpoB point mutations significantly reduced CII trans-complementation; (d) The CII1–87 mutant, deleted for the terminal 10 amino acids, lost its ability to complement in five rpoB mutant cells.
The results suggest that the terminal end of CII likely interacts with the β-subunit of RNA polymerase.
Acknowledgments
Funding: This study was funded by NSERC Canada Discovery grant 138296 to Sidney Hayes. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Connie Hayes for construction and sequence characterization of several plasmids described herein.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1999-4915/10/3/115/s1.
Author Contributions
Conceived and designed the experiments, analyzed the data and wrote the paper: Sidney Hayes. Performed the experiments and helped analyze the data: Karthic Rajamanickam.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Court D.L., Oppenheim A.B., Adhya S.L. A new look at bacteriophage lambda genetic networks. J. Bacteriol. 2007;189:298–304. doi: 10.1128/JB.01215-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casjens S.R., Hendrix R.W. Bacteriophage lambda: Early pioneer and still relevant. Virology. 2015;479–480:310–330. doi: 10.1016/j.virol.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendrix R., Casjens S. Bacteriophage lambda and its genetic neighborhood. In: Calendar R., editor. The Bacteriophages. 2nd ed. Oxford University Press; Oxford, UK: 2006. pp. 409–447. [Google Scholar]
- 4.Dodd I.B., Shearwin K.E., Egan J.B. Revisited gene regulation in bacteriophage lambda. Curr. Opin. Genet. Dev. 2005;15:145–152. doi: 10.1016/j.gde.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Herskowitz I., Hagen D. The lysis-lysogeny decision of phage lambda: Explicit programming and responsiveness. Annu. Rev. Genet. 1980;14:399–445. doi: 10.1146/annurev.ge.14.120180.002151. [DOI] [PubMed] [Google Scholar]
- 6.Little J.W. Lysogeny, prophage induction and lysogenic conversion. In: Waldor M.K., Friedman D.I., Adhya S.L., editors. Phages: Their Role in Bacterial Pathogenesis and Biotechnology. ASM Press; Washington, DC, USA: 2005. pp. 37–54. [Google Scholar]
- 7.Kobiler O., Oppenheim A.B., Herman C. Recruitment of host ATP-dependent proteases by bacteriophage lambda. J. Struct. Biol. 2004;146:72–78. doi: 10.1016/j.jsb.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Kobiler O., Rokney A., Friedman N., Court D.L., Stavans J., Oppenheim A.B. Quantitative kinetic analysis of the bacteriophage lambda genetic network. Proc. Natl. Acad. Sci. USA. 2005;102:4470–4475. doi: 10.1073/pnas.0500670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oppenheim A.B., Kobiler O., Stavans J., Court D.L., Adhya S. Switches in bacteriophage lambda development. Annu. Rev. Genet. 2005;39:409–429. doi: 10.1146/annurev.genet.39.073003.113656. [DOI] [PubMed] [Google Scholar]
- 10.Herman C., Thevenet D., D’Ari R., Bouloc P. The HflB protease of Escherichia coli degrades its inhibitor lambda cIII. J. Bacteriol. 1997;179:358–363. doi: 10.1128/jb.179.2.358-363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kihara A., Akiyama Y., Ito K. Host regulation of lysogenic decision in bacteriophage lambda: Transmembrane modulation of ftsh (HflB), the cii degrading protease, by hflkc (HflA) Proc. Natl. Acad. Sci. USA. 1997;94:5544–5549. doi: 10.1073/pnas.94.11.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shotland Y., Koby S., Teff D., Mansur N., Oren D.A., Tatematsu K., Tomoyasu T., Kessel M., Bukau B., Ogura T., et al. Proteolysis of the phage lambda cII regulatory protein by ftsh (HflB) of Escherichia coli. Mol. Microbiol. 1997;24:1303–1310. doi: 10.1046/j.1365-2958.1997.4231796.x. [DOI] [PubMed] [Google Scholar]
- 13.Kihara A., Akiyama Y., Ito K. A protease complex in the escherichia coli plasma membrane: Hflkc (HflA) forms a complex with ftsh (HflB), regulating its proteolytic activity against secy. EMBO J. 1996;15:6122–6131. [PMC free article] [PubMed] [Google Scholar]
- 14.Kihara A., Akiyama Y., Ito K. Revisiting the lysogenization control of bacteriophage lambda. Identification and characterization of a new host component, HflD. J. Biol. Chem. 2001;276:13695–13700. doi: 10.1074/jbc.M011699200. [DOI] [PubMed] [Google Scholar]
- 15.Kobiler O., Koby S., Teff D., Court D., Oppenheim A.B. The phage lambda cII transcriptional activator carries a C-terminal domain signaling for rapid proteolysis. Proc. Natl. Acad. Sci. USA. 2002;99:14964–14969. doi: 10.1073/pnas.222172499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandyopadhyay K., Parua P.K., Datta A.B., Parrack P. Escherichia coli HflK and HflC can individually inhibit the HflB (ftsh)-mediated proteolysis of lambdacii in vitro. Arch. Biochem. Biophys. 2010;501:239–243. doi: 10.1016/j.abb.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Kobiler O., Rokney A., Oppenheim A.B. Phage lambda cIII: A protease inhibitor regulating the lysis-lysogeny decision. PLoS ONE. 2007;2:e363. doi: 10.1371/journal.pone.0000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyt M.A., Knight D.M., Das A., Miller H.I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: Role of the viral cIII and host HFLA, HIMA and HIMD genes. Cell. 1982;31:565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- 19.Krinke L., Wulff D.L. Oop RNA, produced from multicopy plasmids, inhibits lambda cII gene expression through an rnase III-dependent mechanism. Genes Dev. 1987;1:1005–1013. doi: 10.1101/gad.1.9.1005. [DOI] [PubMed] [Google Scholar]
- 20.Krinke L., Wulff D.L. Rnase III-dependent hydrolysis of lambda cII-O gene mRNA mediated by lambda oop antisense RNA. Genes Dev. 1990;4:2223–2233. doi: 10.1101/gad.4.12a.2223. [DOI] [PubMed] [Google Scholar]
- 21.Hayes S., Szybalski W. Control of short leftward transcripts from the immunity and ORI regions in induced coliphage lambda. Mol. Gen. Genet. 1973;126:275–290. doi: 10.1007/BF00269438. [DOI] [PubMed] [Google Scholar]
- 22.Hayes S., Hayes C. Control of lambda repressor prophage and establishment transcription by the product of gene TOF. Mol. Gen. Genet. 1978;164:63–76. doi: 10.1007/BF00267600. [DOI] [PubMed] [Google Scholar]
- 23.Hayes S., Hayes C. Control of bacteriophage lambda repressor establishment transcription: Kinetics of l-strand transcription from the y-cII-oop-O-P region. Mol. Gen. Genet. 1979;170:75–88. [PubMed] [Google Scholar]
- 24.Hayes S., Slavcev R.A. Polarity within pM and pE promoted phage lambda cI-rexA-rexB transcription and its suppression. Can. J. Microbiol. 2005;51:37–49. doi: 10.1139/w04-115. [DOI] [PubMed] [Google Scholar]
- 25.Reichardt L., Kaiser A.D. Control of lambda repressor synthesis. Proc. Natl. Acad. Sci. USA. 1971;68:2185–2189. doi: 10.1073/pnas.68.9.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salis H.M., Mirsky E.A., Voigt C.A. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 2009;27:946–950. doi: 10.1038/nbt.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes S. Phage Lambda Display Constructions. 8663913 b 1. U.S. Patent. 2014 Mar 4;
- 28.Hayes S. Phage Lambda Display Constructions. 2761105. Canadian Patent. 2014 Feb 10;
- 29.Hayes S., Gamage L.N., Hayes C. Dual expression system for assembling phage lambda display particle (LDP) vaccine to porcine circovirus 2 (PCV2) Vaccine. 2010;28:6789–6799. doi: 10.1016/j.vaccine.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 30.Simatake H., Rosenberg M. Purified lambda regulatory protein cII positively activates promoters for lysogenic development. Nature. 1981;292:128–132. doi: 10.1038/292128a0. [DOI] [PubMed] [Google Scholar]
- 31.Kedzierska B., Glinkowska M., Iwanicki A., Obuchowski M., Sojka P., Thomas M.S., Wegrzyn G. Toxicity of the bacteriophage lambda cII gene product to Escherichia coli arises from inhibition of host cell DNA replication. Virology. 2003;313:622–628. doi: 10.1016/S0042-6822(03)00376-3. [DOI] [PubMed] [Google Scholar]
- 32.Kourilsky P., Gros D. Lysogenization by bacteriophage lambda IV inhibition of phage DNA synthesis by the products of genes cII and cIII. Biochimie. 1976;58:1321–1327. doi: 10.1016/S0300-9084(77)80015-1. [DOI] [PubMed] [Google Scholar]
- 33.McMacken R., Mantei N., Butler B., Joyner A., Echols H. Effect of mutations in the C2 and C3 genes of bacteriophage lambda on macromolecular synthesis in infected cells. J. Mol. Biol. 1970;49:639–655. doi: 10.1016/0022-2836(70)90288-3. [DOI] [PubMed] [Google Scholar]
- 34.Szalewska-Palasz A., Wrobel B., Wegrzyn G. Rapid degradation of polyadenylated oop RNA. FEBS Lett. 1998;432:70–72. doi: 10.1016/S0014-5793(98)00834-5. [DOI] [PubMed] [Google Scholar]
- 35.Wrobel B., Herman-Antosiewicz A., Szalewska-Palasz S., Wegrzyn G. Polyadenylation of oop RNA in the regulation of bacteriophage lambda development. Gene. 1998;212:57–65. doi: 10.1016/S0378-1119(98)00127-9. [DOI] [PubMed] [Google Scholar]
- 36.Wulff D.L., Rosenberg M.R. Establishment of repressor synthesis. In: Hendrix R.W., Roberts J.W., Stahl F.W., Weisberg R.A., editors. Lambda II. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 1983. pp. 519–684. [Google Scholar]
- 37.Hayes S., Wang W., Rajamanickam K., Chu A., Banerjee A., Hayes C. Lambda gpP-DnaB helicase sequestration and gpP-RpoB associated effects: On screens for auxotrophs, selection for Rif(R), toxicity, mutagenicity, plasmid curing. Viruses. 2016;8:172. doi: 10.3390/v8060172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes S., Erker C., Horbay M.A., Marciniuk K., Wang W., Hayes C. Phage lambda P protein: Trans-activation, inhibition phenotypes and their suppression. Viruses. 2013;5:619–653. doi: 10.3390/v5020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayes S., Horbay M.A., Hayes C. A CI-independent form of replicative inhibition: Turn off of early replication of bacteriophage lambda. PLoS ONE. 2012;7:e36498. doi: 10.1371/journal.pone.0036498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krinke L., Mahoney M., Wulff D.L. The role of the oop antisense RNA in Coliphage lambda development. Mol. Microbiol. 1991;5:1265–1272. doi: 10.1111/j.1365-2958.1991.tb01900.x. [DOI] [PubMed] [Google Scholar]
- 41.Daniels D.L., Schroeder J.L., Szybalski W., Sanger F., Blattner F.R. Appendix I. A molecular map of coliphage lambda. In: Hendrix R.W., Roberts J.W., Stahl F., Weisberg R.A., editors. Lambda II. Cold Spring Harbor Laboratory; New York, NY, USA: 1983. pp. 469–517. [Google Scholar]
- 42.Dodd I.B., Perkins A.J., Tsemitsidis D., Egan J.B. Octamerization of lambda CI repressor is needed for effective repression of P(RM) and efficient switching from lysogeny. Genes Dev. 2001;15:3013–3022. doi: 10.1101/gad.937301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dodd I.B., Shearwin K.E., Perkins A.J., Burr T., Hochschild A., Egan J.B. Cooperativity in long-range gene regulation by the lambda cI repressor. Genes Dev. 2004;18:344–354. doi: 10.1101/gad.1167904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Revet B., von Wilcken-Bergmann B., Bessert H., Barker A., Muller-Hill B. Four dimers of lambda repressor bound to two suitably spaced pairs of lambda operators form octamers and DNA loops over large distances. Curr. Biol. 1999;9:151–154. doi: 10.1016/S0960-9822(99)80069-4. [DOI] [PubMed] [Google Scholar]
- 45.Svenningsen S.L., Costantino N., Court D.L., Adhya S. On the role of CRO in lambda prophage induction. Proc. Natl. Acad. Sci. USA. 2005;102:4465–4469. doi: 10.1073/pnas.0409839102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peacock S., Weissbach H., Nash H.A. In vitro regulation of phage lambda cII gene expression by Escherichia coli integration host factor. Proc. Natl. Acad. Sci. USA. 1984;81:6009–6013. doi: 10.1073/pnas.81.19.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahajna J., Oppenheim A.B., Rattray A., Gottesman M. Translation initiation of bacteriophage lambda gene cII requires integration host factor. J. Bacteriol. 1986;165:167–174. doi: 10.1128/jb.165.1.167-174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniels D.L., Schroeder J.L., Szybalski W., Sanger F., Coulson A.R., Hong G.F., Hill D.F., Petersen G.B., Blattner F.R. Appendix II. Complete annotated lambda sequence. In: Hendrix R.W., Roberts J.W., Stahl F., Weisberg R.A., editors. Lambda II. Cold Spring Harbor Laboratory; New York, NY, USA: 1983. pp. 519–676. [Google Scholar]
- 49.Nunez J.K., Bai L., Harrington L.B., Hinder T.L., Doudna J.A. CRISPR immunological memory requires a host factor for specificity. Mol. Cell. 2016;62:824–833. doi: 10.1016/j.molcel.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 50.Grosschedl R., Schwarz E. Nucleotide sequence of the cro-cII-oop region of bacteriophage 434 DNA. Nucleic Acids Res. 1979;6:867–881. doi: 10.1093/nar/6.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jasiecki J., Wegrzyn G. Growth-rate dependent RNA polyadenylation in Escherichia coli. EMBO Rep. 2003;4:172–177. doi: 10.1038/sj.embor.embor733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherer G., Hobom G., Kossel H. DNA base sequence of the Po promoter region of phage lamdba. Nature. 1977;265:117–121. doi: 10.1038/265117a0. [DOI] [PubMed] [Google Scholar]
- 53.Rosenberg M., Court D., Shimatake H., Brady C., Wulff D.L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978;272:414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- 54.Belfort M., Wulff D.L. Genetic and biochemical investigation of the Escherichia coli mutant hfl-1 which is lysogenized at high frequency by bacteriophage lambda. J. Bacteriol. 1973;115:299–306. doi: 10.1128/jb.115.1.299-306.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hammer K., Jensen K.F., Poulsen P., Oppenheim A.B., Gottesman M. Isolation of Escherichia coli rpob mutants resistant to killing by lambda cII protein and altered in pyre gene attenuation. J. Bacteriol. 1987;169:5289–5297. doi: 10.1128/jb.169.11.5289-5297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho Y.S., Mahoney M.E., Wulff D.L., Rosenberg M. Identification of the DNA binding domain of the phage lambda cII transcriptional activator and the direct correlation of CII protein stability with its oligomeric forms. Genes Dev. 1988;2:184–195. doi: 10.1101/gad.2.2.184. [DOI] [PubMed] [Google Scholar]
- 57.Thomas M.S., Glass R.E. Escherichia coli RPOA mutation which impairs transcription of positively regulated systems. Mol. Microbiol. 1991;5:2719–2725. doi: 10.1111/j.1365-2958.1991.tb01980.x. [DOI] [PubMed] [Google Scholar]
- 58.Obuchowski M., Giladi H., Koby S., Szalewska-Palasz A., Wegrzyn A., Oppenheim A.B., Thomas M.S., Wegrzyn G. Impaired lysogenisation of the Escherichia coli rpoa341 mutant by bacteriophage lambda is due to the inability of cII to act as a transcriptional activator. Mol. Gen. Genet. 1997;254:304–311. doi: 10.1007/s004380050420. [DOI] [PubMed] [Google Scholar]
- 59.Giffard P.M., Booth I.R. The rpoa341 allele of Escherichia coli specifically impairs the transcription of a group of positively-regulated operons. Mol. Gen. Genet. 1988;214:148–152. doi: 10.1007/BF00340193. [DOI] [PubMed] [Google Scholar]
- 60.Gross G., Hollatz I. Coliphage lambda to terminator lowers the stability of messenger RNA in Escherichia coli hosts. Gene. 1988;72:119–128. doi: 10.1016/0378-1119(88)90133-3. [DOI] [PubMed] [Google Scholar]
- 61.Ingle C.A., Kushner S.R. Development of an in vitro mRNA decay system for Escherichia coli: Poly(A) polymerase I is necessary to trigger degradation. Proc. Natl. Acad. Sci. USA. 1996;93:12926–12931. doi: 10.1073/pnas.93.23.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarkar N. Polyadenylation of mRNA in bacteria. Pt 11Microbiology. 1996;142:3125–3133. doi: 10.1099/13500872-142-11-3125. [DOI] [PubMed] [Google Scholar]
- 63.Lewis L.K., Harlow G.R., Gregg-Jolly L.A., Mount D.W. Identification of high affinity binding sites for lexa which define new DNA damage-inducible genes in Escherichia coli. J. Mol. Biol. 1994;241:507–523. doi: 10.1006/jmbi.1994.1528. [DOI] [PubMed] [Google Scholar]
- 64.Hayes S. Initiation of coliphage lambda replication, lit, oop rna synthesis and effect of gene dosage on transcription from promoters PL, PR and PR. Virology. 1979;97:415–438. doi: 10.1016/0042-6822(79)90352-0. [DOI] [PubMed] [Google Scholar]
- 65.Oppenheim A.B., Rattray A.J., Bubunenko M., Thomason L.C., Court D.L. In vivo recombineering of bacteriophage lambda by PCR fragments and single-strand oligonucleotides. Virology. 2004;319:185–189. doi: 10.1016/j.virol.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 66.Zeng L., Skinner S.O., Zong C., Sippy J., Feiss M., Golding I. Decision making at a subcellular level determines the outcome of bacteriophage infection. Cell. 2010;141:682–691. doi: 10.1016/j.cell.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlosser-Silverman E., Elgrably-Weiss M., Rosenshine I., Kohen R., Altuvia S. Characterization of Escherichia coli DNA lesions generated within j774 macrophages. J. Bacteriol. 2000;182:5225–5230. doi: 10.1128/JB.182.18.5225-5230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.