Abstract
Background
Malaria continues being a public health problem worldwide. Plasmodium vivax is the species causing the largest number of cases of malaria in Asia and South America. Due to the lack of a completely effective anti-malarial vaccine, controlling this disease has been based on transmission vector management, rapid diagnosis and suitable treatment. However, parasite resistance to anti-malarial drugs has become a major yet-to-be-overcome challenge. This study was thus aimed at determining pvmdr1, pvdhfr, pvdhps and pvcrt-o gene mutations and haplotypes from field samples obtained from an endemic area in the Colombian Amazonian region.
Methods
Fifty samples of parasite DNA infected by a single P. vivax strain from symptomatic patients from the Amazonas department in Colombia were analysed by PCR and the pvdhfr, pvdhps, pvmdr1 and pvcrt-o genes were sequenced. Diversity estimators were calculated from the sequences and the haplotypes circulating in the Colombian Amazonian region were obtained.
Conclusion
pvdhfr, pvdhps, pvmdr1 and pvcrt-o genes in the Colombian Amazonian region are characterized by low genetic diversity. Some resistance-associated mutations were found circulating in this population. New variants are also being reported. A selective sweep signal was located in pvdhfr and pvmdr1 genes, suggesting that these mutations (or some of them) could be providing an adaptive advantage.
Keywords: Malaria, Plasmodium vivax, Antimalarial drug resistance, Amazonian region, Colombia, pvdhfr, pvdhps, pvmdr1 and pvcrt-o
Background
Malaria continues being a worldwide problem; it is a vector-born disease (VBD) transmitted by parasites from the genus Plasmodium [1]. About 40% of the world’s population (around three billion people) are currently at risk of suffering from this disease. Malaria causes more than 200 million clinical cases and about 400,000 deaths each year [1]. The climatic and geographical conditions in Colombia enable the transmission of this infection [2], and both Plasmodium vivax and Plasmodium falciparum are prevalent.
There are three large Plasmodium endemic foci in Colombia: Urabá–lower Cauca–southern Córdoba, the Pacific coast and the Orinoquía–Amazonía transition region; such regions favour malarial incidence and dispersion due to their environmental conditions and social, political and cultural variables [1, 3]. The main difficulty in controlling P. vivax lies in the need for treating both blood stage and latent liver forms (hypnozoites), the latter causing relapses months or years after initial infection [4].
First-line treatment for uncomplicated P. vivax malaria in Colombia is currently based on a combination of chloroquine (CQ) for eliminating blood stages (schizonts) and primaquine (PQ) for liver stages (hypnozoites) [5]. In other countries, treatment is also based on the use of sulfadoxine–pyrimethamine (SP) [6]. Although these drugs remain effective overall, cases of resistance have been recorded during the last few years, making this one of the greatest problems in controlling and eliminating malaria. The first report of CQ-resistant P. vivax strains in Latin-America was published in 1989 (including Colombia and Brazil) [7]; however, resistance targets have not been clearly established. Regarding P. falciparum, parasite genes associated with resistance to anti-malarial drugs have been established with greater certainty. Simple, double or quadruple mutations in different genes enable the parasite to cope with anti-malarial drugs. Mutations in pfcrt, pfmdr1, pfdhfr and pfdhps genes have arisen/been fixed in several parasite populations and, since they confer drug resistance, this facilitates their dispersion.
There may be a clear association between pfcrt, pfmdr1, pfdhfr and pfdhps allele variants and drug resistance, but such association is not clear regarding P. vivax. Nevertheless, genetic studies of P. vivax drug susceptibility in different parts of the world [8–13] have identified point mutations in orthologous genes (pvcrt-o, pvmdr1, pvdhfr and pvdhps) to those associated with resistance in P. falciparum, providing potential markers of resistance to P. vivax anti-malarial drugs [14–16]. Molecular biology techniques provide a valid alternative for identifying resistance-associated mutations regarding anti-malarial drug therapy in different populations and could therefore be used as a tool for surveillance of P. vivax drug resistance.
Very few studies have been made in Colombia regarding the search for polymorphisms in these genes. A 2015 evaluation in Colombia’s Córdoba department [17] stated that the P. vivax population was more genetically diverse than it had been thought. However, some genome regions had low diversity due to a selective sweep associated with the PvDHPS A383G allele [17] which, in turn, has been associated with sulfadoxine resistance [18]. The region surrounding PvDHFR did not have a loss of genetic diversity, as with PvDHPS; nevertheless, two alleles (S58R and S117N) [17] were reported to be associated with SP resistance [11]. Five non-synonymous mutations have been reported in pvdmr1; the mutation triggering change Y976F has been associated with reduced sensitivity to CQ [19]. However, other studies have not found susceptibility to CQ, despite Y976F being present. This phenotype has been reported in the north of Colombia [17], although such marker’s role in resistance has not been completely clarified [16]. The Colombian Amazon region is another focus for malaria as the people inhabiting the area live far away from city centres (i.e. lacking basic services and sanitation), their insecticide use is infrequent and access to healthcare centres is limited, making them more vulnerable to acquiring the disease and putting them at greater risk of not receiving timely treatment. Bewilderingly, there have been no studies regarding the presence of genotypes associated with resistance to the anti-malarial drugs used against P. vivax in this region. This study was thus aimed at evaluating the genetic diversity of pvmdr1, pvdhfr, pvdhps and pvcrt-o fragments in P. vivax parasites from Colombia’s Amazonian region, thereby providing significant information for the molecular surveillance of drug-resistant P. vivax.
Methods
Study areas
The samples used in this study were taken from several settlements in the municipalities of Leticia and Puerto Nariño in Colombia’s Amazonas department after volunteers had signed informed consent forms. These municipalities are located along the Banks of the Amazonas and Loretoyacu rivers on the Amazonian frontier with Peru and Brazil. All sample taking-related procedures and managing patients were approved by the Universidad del Rosario’s School of Medicine and Health Sciences’ Research Ethics Committee (resolution CEI-ABN026-000161).
Extracting parasite DNA and confirming single Plasmodium vivax infection
Genomic DNA samples were extracted from FTA card-stored blood of Plasmodium spp. infected patients using a Pure Link Genomic DNA mini kit (Invitrogen), following the manufacturer’s specifications. The samples were eluted in 50 µL final volume of buffer containing 10 mM Tris–HCl, at pH 9.0 and 0.1 mM EDTA. P. vivax infection was confirmed, as described previously [20].
Samples positive for P. vivax were explored for selecting those which were only infected by a single strain. A pvmsp3α fragment was amplified and sequenced, as previously described [21, 22]. The resulting electropherograms were assembled and revised using CLC Genomics Workbench (v.3) [23]. Samples having overlapping peaks in electropherograms were discarded; only samples whose electropherograms showed single peaks were involved in analysis.
Amplifying, purifying and sequencing pvmdr1, pvdhfr, pvdhps and pvcrt-o
Fragments from each gene associated with resistance were amplified using 50 samples confirmed as being infected by just one P. vivax strain, using previously described primers (Table 1) and thermal profiles pvmdr1 [19], pvdhfr [24], pvdhps [25] and pvcrt-o [26]. PCR products were then purified using a Wizard SV Gel and PCR Clean-up System kit (Promega), following the manufacturer’s indications. Purified products were then bidirectionally sequenced using the ABI 3730 XL system (Macrogen, Seoul, South Korea).
Table 1.
Primers used for sequencing msp3-α, pvmdr1, pvdhfr, pvdhps and pvcrt-o
| Gene of interest | Primers used for sequencing | Size (bp) | |
|---|---|---|---|
| Primer | Sequence | ||
| msp3-α: [21] | P1 | 5′-CAGCAGACACCATTTAAGG-3′ | 2194 |
| P2 | 5′-CCGTTTGTTGATTAGTTGC-3′ | ||
| N1 | 5′-GACCAGTGTCATACCATTAACC-3′ | 1895 | |
| N2 | 5′-ATACTGGTTCTTCGTCTTCAGG-3′ | ||
| pvcrt-o: [26] | F | 5′-AAGAGCCGTCTAGCCATCC-3′ | 1186 |
| R | 5′-AGTTTCCCTCTACACCCG-3′ | ||
| pvdhps: [25] | PvDHPS-D | 5′-GGTTTATTTGTCGATCCTGTG-3′ | 1301 |
| PvDHPS-B | 5′-GAGATTACCCTAAGGTTGATGTATC-3′ | ||
| pvmdr1: [19] | 14-F | 5′-CCCTCTACATCTTAGTCATCG-3′ | 932 |
| 14-R | 5′-TGGTCTGGACAAGTATCTAAAA | ||
| 976-F | 5′-GGATAGTCATGCCCCAGGATTG-3′ | 604 | |
| 976-R | 5′-CATCAACTTCCCGGCGTAGC-3′ | ||
| pvdhfr: [24] | PvDA | 5′-ACCGCACCAGTTGATTCCTAC-3′ | 1018 |
| PvDB | 5′-TGTTAAAGCTGAAGTACACGAG-3′ | ||
| PvDF | 5′-ATGGAGGACCTTTCAGATGT-3′ | 785 | |
| PvDR | 5′-AACGCATTGCAGTTCTCCGA-3′ | ||
Analysing pvmdr1, pvdhfr, pvdhps and pvcrt sequences
The resulting electropherograms were corrected and assembled using CLC Genomics Workbench (v.3) [23] for obtaining a consensus sequence per sample and per gene. Consensus sequences were compared to the respective Salvador I (Sal-I) strain reference sequences having an antimalarial drug sensitivity profile (PlasmoDB access numbers: PVX_080100 for pvmdr1, PVX_089950 for pvdhfr, PVX_123230 for pvdhps and PVX_087980.1 for pvcrt-o). The respective amino acid (aa) sequence was inferred for each nucleotide sequence obtained from the Colombian Amazonian region, as well as Sal-I strain sequences and their orthologues in Plasmodium cynomolgi (a species phylogenetically-related to P. vivax; PlasmoDB access numbers: PCYB_101870 for pvmdr1, PCYB_053150 for pvdhfr, PCYB_143740 for pvdhps and PCYB_011610-t26_1 for pvcrt). The Muscle algorithm was then used for aligning them; this aa alignment was then taken as reference for inferring nucleotide alignment, using PAL2NAL software [27].
Genetic diversity and the effect of natural selection
DnaSP software (v.5.10.00) was used for calculating several genetic diversity estimators from pvmdr1, pvdhfr, pvdhps and pvcrt nucleotide alignment, such as the segregating sites number, the haplotypes number, nucleotide and haplotype diversity and nucleotide polymorphism [28]. The modified Nei–Gojobori method [29] was used with MEGA software (v.7) [30] for evaluating selective pressure by calculating synonymous (dS) and non-synonymous (dN) substitution rates. Tajima [31], Fu and Li [32], and Fay and Wu estimators [33] were used for evaluating deviations from the neutral model of molecular evolution.
Results
pvmdr1, pvdhfr, pvdhps and pvcrt-o polymorphism
Fifty samples infected by a single population of P. vivax were used for evaluating the polymorphism of several fragments from genes associated with resistance. Five polymorphisms were observed for pvdmr1 (Fig. 1), four being non-synonymous and just one being synonymous; this gene’s nucleotide diversity (π) was 0.0013 and no substitution producing a change in aa Y976F (one of the first chemoresistance molecular marker candidates) [8] was found in samples from the Colombian Amazonian region. However, 62% of samples evaluated had T958M polymorphism and variant D994E was observed in 54% of the samples (Table 2 and Fig. 2). F983L and L1022 polymorphisms, encoding singleton sites, were observed as well as phenylalanine being replaced by leucine in position 1070 (Table 2 and Fig. 2). Concerning DNA, the 5 polymorphisms produced 5 different haplotypes (Fig. 1), haplotype 3 occurring most frequently (53%), followed by haplotype 2 (39%). The synonymous substitution of a cytosine for a thymine was found in position 3064, located in position 1 of the codon triplet (2% frequency).
Fig. 1.
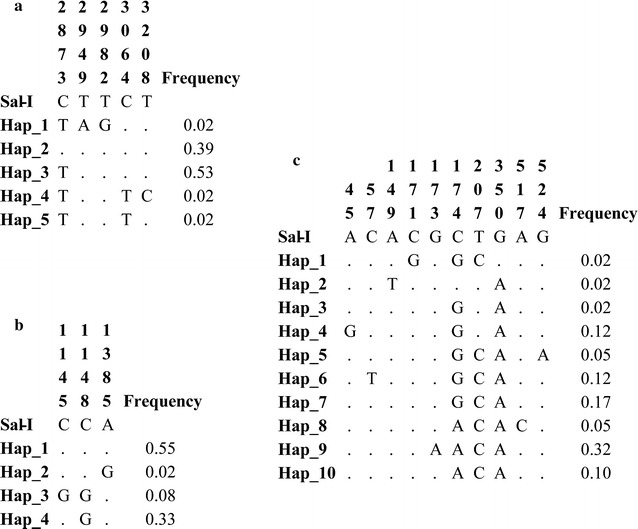
DNA haplotypes found in the Colombian Amazon region. a Aligning pvmdr1 gene non-conserved residues. b Aligning pvdhps non-conserved residues. c Aligning pvdhfr non-conserved residues. Dots indicate conserved residues. Nucleotide positions are indicated according to the P. vivax Sal-I strain numbering (PlasmoDB accession PVX_089950 for pvmdr1, PVX_123230 for pvdhps and PVX_080100 for pvdhfr)
Table 2.
P. vivax resistance-associated molecular marker prevalence
| Codon | % of mutations | Mutation | Protein change |
|---|---|---|---|
| mdr1 | |||
| 2873 | 62 | ACG>ATG | T958M |
| 2949 | 38 | TTT>TTA | F983L |
| 2982 | 54 | TTC>CTC | D994E |
| 3064 | 2 | CTA>TTA | L1022 |
| 3208 | 2 | TTC>CTC | F1070L |
| dhfr | |||
| 45 | 12.2 | GCA>GCG | A15 |
| 57 | 12.2 | GTC>GTT | V19 |
| 149 | 2.44 | AAC>ATC | N50I |
| 171 | 2.44 | TTC>TTG | F57L |
| 173 | 31.7 | AGC>AAG | S58K |
| 174 | 97.5 | AGC>AGG/AGA | S58R |
| 207 | 82.9 | TAT>TAC | Y69 |
| 350 | 97.6 | AGC>AAC | S117N |
| 517 | 4.88 | ATT>CTT | I173L |
| 524 | 4.88 | GGA>GAA | G175E |
| dhps | |||
| 1145 | 8.3 | TCC>TGC | S382C |
| 1148 | 41.6 | GCC>GGC | A383G |
| 1385 | 2 | AAG>AGAG | K462R |
Fig. 2.
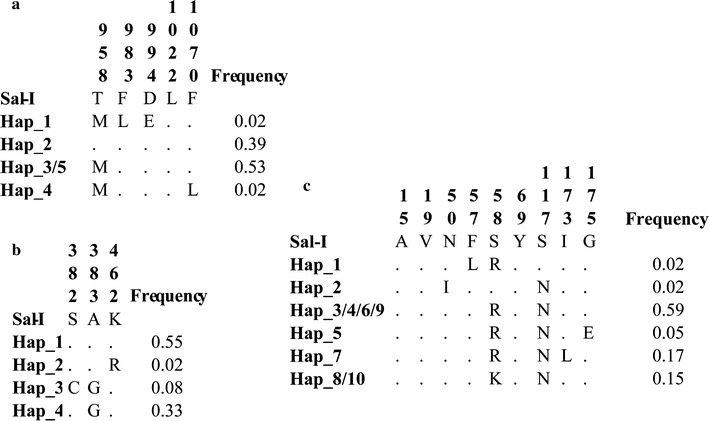
Amino acid haplotypes found in the Colombian Amazon region. a PvMDR1 alignment. b PvDHPS alignment. c PvDHFR alignment. Dots indicate conserved residues. Amino acid positions are indicated according to P. vivax Sal-I strain numbering (PlasmoDB accession PVX_089950 for PvMDR1, PVX_123230 for PvDHPS and PVX_080100 for PvDHFR)
pvdhfr fragments could only be obtained in 41 of the 50 samples; 10 polymorphisms were observed (Fig. 1), with a π = 0.0037 and 10 different haplotypes (Fig. 1). Some polymorphisms observed coincided with those reported in other populations; 97.6% of the samples evaluated here had S117N substitutions, Y69 had 82.9% prevalence and S58K 31.7%, whilst the S58R polymorphism occurred in 97.5% of the samples (Table 2 and Fig. 2). Regarding DNA haplotypes, haplotype 9 occurred with the greatest frequency (32%) compared to haplotypes 1, 2 and 3 (Fig. 1). Synonymous substitutions were observed in changes A15 and V19 (12.2%).
A pvdhps fragment was obtained in 48 of the 50 samples; three polymorphisms and 4 haplotypes were observed in this gene (Fig. 1), the π was 0.0011, all polymorphisms being non-synonymous. The A383G substitution occurred in 41.6% of samples, whilst polymorphism A382C was only observed in 8.3% of them (Table 2 and Fig. 2). Haplotype 1 occurred in 55% of samples compared to haplotype 4 in 33% of them (Fig. 1). Finally, there was no polymorphism in pvcrt-o, showing that all isolates from the Colombian Amazonian region analysed here had 100% identity with the Sal-1 reference strain.
Natural selection in genes associated with resistance
Several tests based on the neutral molecular evolution model were performed to determine whether fragments from genes associated with resistance were under some selective pressure; dN–dS ratio values had no significant values (Table 3). Tajima, and Fu and Li tests revealed gave negative values; however, they were not significant. Nevertheless, Fay and Wu H estimator gave significant P. vivax pvdhfr and pvdmr1 negative values for the population in the south of the Colombian Amazonian region (Table 3).
Table 3.
Genetic diversity estimators and neutral tests
| n | Gene | Sites | Ss | H | Hd | θ | π | dN–dS | Fay and Wu |
|---|---|---|---|---|---|---|---|---|---|
| H | |||||||||
| 41 | dhfr | 558 | 10 | 10 | 0.84 | 0.0040 | 0.0037 | − 0.006* | − 3.64† |
| 48 | dhps | 648 | 3 | 4 | 0.56 | 0.0010 | 0.0011 | 0.002 | 0.32 |
| 50 | mdr1 | 522 | 5 | 5 | 0.55 | 0.0021 | 0.0013 | 0.001 | − 1.50† |
| 33 | crt-o | 375 | 0 | 0 | 0.00 | 0.0000 | 0.0000 | – | – |
n total sequences used for analysis, Sites total sites used to rule out gap sites, Ss amount of segregating sites, H amount of haplotypes, Hd haplotype diversity, θ nucleotide polymorphism per site, π nucleotide diversity per site, dN–dS ratio of non-synonymous to synonymous nucleotide polymorphism
* p = 0.038
†p < 0.01
Discussion
Plasmodium vivax genetic diversity is an important challenge which must be overcome in attempts to control this infection [34]. Such diversity could enable the parasite to evade host immune responses and confer an adaptive advantage for resisting anti-malarial drugs [35].
The transmission of Plasmodium species in Colombia varies according to each geographical area, enabling several of them to cohabit in the same area. Plasmodium falciparum/P. vivax multiple infections could go unnoticed during diagnosis [6, 20] and thus the attending doctor cannot prescribe suitable treatment for patients; in turn, this could exercise selective pressure on parasite populations. Choosing the right treatment is thus crucial for preventing the appearance and propagation of resistance [1]. The samples analysed here came from a tri-border region involving Colombia, Peru and Brazil. First-line treatment for uncomplicated P. vivax infections is similar for the three countries, where a combination of CQ and PQ is used [5, 36, 37]. Previous studies have reported polymorphisms associated with resistance to anti-malarial drugs in South and Central America [7, 10].
Polymorphisms have been found in Central America in pvmdr1 and pvdhfr which may be related to a loss of P. vivax susceptibility to anti-malarial drugs [10]. Nevertheless, no in vitro susceptibility studies have been reported so far to support such association. Cases of CQ therapeutic failure concerning treating P. vivax malaria have been documented in South America. Around 12% of therapeutic failure due to CQ-resistant parasites and 6.4% due to mefloquine-resistant parasites have been reported in Brazil’s Amazon region [38]. Taking into account that the sampling site in this study was near the tri-border area, the interchange of parasite strains carrying resistance mutations was likely and such haplotypes would become dispersed throughout neighbouring endemic areas. Although, P. vivax CQ resistance mechanisms have not been fully elucidated, they could involve various genetic loci. Plasmodium falciparum pfcrt-o and pfmdr1 variants have been identified as being associated with CQ resistance; these genes’ orthologues in P. vivax have thus been evaluated for determining whether they might be associated with resistance [39].
Previous studies have found that PvMDR1 Y976F and F1076L variants seem to have been associated with CQ resistance [40]. One such polymorphism (variant Y976F) has been found to occur frequently in the east of the Brazilian Amazonian region [7]. Nevertheless, the role of Y976F in CQ resistance is not yet clear [16]. Neither Y976F nor F1076L have been observed in the Colombian Amazonian region’s population. Even so, other PvMDR1 polymorphisms were found in this population which have not been reported previously and could thus be substitutions in the strains circulating in the Colombian Amazon region. This suggested that haplotypes segregating in the Brazilian Amazonian region are different to those segregating in the Colombian equivalent and, therefore, gene flow between the Brazilian and Colombian Amazon regions seems to be limited or restricted. Further analysis is required to corroborate whether additional substitutions found in Colombia are related to clinical-resistance phenotypes.
Various mutations in pvdhfr and pvdhps have been associated with resistance to SP [41]. SP in Colombia and Brazil is not currently used as first-line treatment for P. vivax infection, even though having been included in Colombian Ministry of Health guidelines towards the end of the 1990s [5]. However, SP is used as the drug of choice for treating P. falciparum in Colombia [37, 42] and in northern Perú [43]. Diagnosis in the three countries is by thick smear; however, several reports have highlighted under-recording of cases by this method and a high rate of coinfection on the Colombia–Brazil–Perú frontier [20, 44]. This diagnostic technique’s inherent limitations and the large amount of misdiagnosed co-infections could affect selecting the proper treatment, thereby creating selective pressure on the parasite, leading to the fixing of SP resistance in P. vivax strains [45], even if SP may not be being used for treating vivax malaria. Polymorphisms found in PvDHFR (F57L/I, S58R, T61M and S117T/N) usually appear in patients having therapeutic failure [46]. Parasites having double substitutions (F57L–S117N), triple substitutions (5F7L–5S8R–S117N) and quadruple substitutions (F57L–S58R–T61M–S117T) have thus been associated with SP resistance [10, 47, 48]. Ten different polymorphisms were found in the Amazon region evaluated in this work; double substitutions (S58R–S117N) were observed in 59% of them. It has been proposed that the current use of SP in Colombia has exercised selective pressure on both P. falciparum and P. vivax [49]; validation follow-up is thus recommended to observe its efficacy as treatment. A triple mutation haplotype was observed (S58R–S117N–I173L) in 17% of samples; however, it is not clear whether this haplotype could be associated with resistance.
PvDHPS polymorphisms in aa 382, 383, 512, 553 and 585 (homologues for P. falciparum positions 436, 437, 540, 581 and 613, respectively) [26] seem to be involved in resistance to sulfadoxine. A high S382C mutation prevalence in chloroquine and mefloquine-resistant parasites has been observed in Brazil’s Amazon region [38, 50]. A383G substitution prevalence was 41.6%, the most prevalent in this study, unlike S382C and K462R mutations; this could have been related to changes in aa position 382 and 383 which are frequent in regions having high SP use [51]. In addition to the aforementioned polymorphisms, new pvdhfr and pvdhps variants and haplotypes were found in P. vivax strains from the Colombian Amazonian region.
pvcrt is an orthologue of pfcrt whose association with resistance to CQ is controversial [52]; CRT has shown no contribution whatsoever to resistance to chloroquine regarding P. vivax, even though sensitive or resistant P. vivax parasites cause substitution 76K [53]. Concerning the Colombian Amazonian region this gene fragment was seen to be highly conserved, having 100% identity with the P. vivax Sal-1 strain which is susceptible to anti-malarial drugs.
Selective sweep could be taking place, fixing advantageous variants
Point mutations or determined haplotypes in P. falciparum that alter dhfr, dhps, mdr1 and crt-o protein products, enable the parasite to resist several drugs. Such mutations are usually fixed rapidly in populations due to a selective advantage causing DNA regions containing such genes to have low genetic diversity (such effect is known as selective sweep). The pvdhfr, pvdhps, pvmdr1 and pvcrt-o fragments analysed in the population from Colombia’s southern Amazon region had low genetic diversity; such polymorphisms seemed to be neutral according to Tajima, Fu and Li and dN–dS tests. However, the Fay and Wu H estimator gave significant pvdhfr and pvmdr1 values, suggesting a selective sweep. A candidate-resistant gene (pvdhps) having this pattern has been recorded previously in Colombia’s Córdoba department [17] according to information regarding the complete genome of P. vivax clinical isolates. Some mutations found in these P. vivax genes could have been fixed by conferring an advantage, in turn fixing neutral variants and thus reducing genetic diversity. As Fay and Wu tests gave statistically significant values, the southern Colombian Amazonian region’s parasite population might be under selective pressure, probably exerted by anti-malarial drugs; a selective sweep might thereby be fixing mutations enabling the appearance of resistant phenotypes. However, genes involved in selective sweep were different between Tierra Alta and the Colombian Amazonian region, probably due to differential treatment in these populations or populations in Colombia being structured with low gene flow between them, as seen in previous studies [54–56]. Further analysis is required to assess the selective sweep hypothesis regarding these genes in the Colombian Amazonian region.
Conclusions
Even though studies in malaria-endemic regions have focused on the search for alternatives for improving the available treatment against malaria, the impact of these strategies on the evolution of P. vivax populations must be evaluated, due to the appearance and dispersion of resistance in such regions. Although, resistance is not fully understood concerning P. vivax and resistance-markers have not been clearly defined yet [16], dhfr, dhps, mdr1 and crt-o genes have mutations which (in some cases) seem to provide resistance or at least reduced susceptibility to anti-malarial drugs [57]. Even though not all four genes have confirmed roles regarding P. vivax drug susceptibility, they are potential targets for P. vivax resistance. These four genes have low genetic diversity in the Colombian Amazon region. However, some of the mutations found were associated with resistance, i.e. the F1070L mutation in PvMDR1, S117N and S58R/K in PvDHFR and A383G and A382C in PvDHPS, whilst other new variants were also found in these genes. pvmdr1 and pvdhfr fragments’ diversity pattern could be the consequence of a selective sweep, indicating that these genes’ variants might be becoming fixed in the population due to an adaptive advantage; these mutations could endow the parasite population with lower sensitivity to anti-malarial drugs. However, additional sequence analysis and in vitro assays are needed to confirm whether these variants are associated with resistance and/or therapeutic failures. The results reported here could be taken as a base tool for advising on how malaria control measures are implemented in Colombia and avoid the dispersion of haplotypes associated with resistance in this region.
Authors’ contributions
JRC conceived and designed the experiments. JRC, PAC-A, CHN, AO-O, EO-C and MFO-D performed the experiments. DG-O performed the evolutionary analysis. JRC, CHN, PAC-A, DG-O, MAP analysed the data. JRC, DG-O, PAC-A, CHN, MEP, MAP wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank the Amazonian region Governor’s office for providing resources through Colombia’s General Royalties System and Colombia’s Science, Technology and Innovation Fund’s (Project BPIN-266) special agreement (020) for financing this project. We would like to express our gratitude to Jason Garry for translating and revising this manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The DNA sequences and the data related were deposited in the GenBank database with Accession Numbers: MG100841–MG100859.
Ethics approval and consent to participate
The P. vivax-infected patients from whom parasite genomic DNA was obtained gave their informed consent after having been notified about the research’s purpose. All procedures were approved by the Fundación Instituto de Inmunología de Colombia and the Universidad del Rosario’s School of Medicine and Health Sciences’ research ethics committee (Resolution CEI-ABN026-000161).
Funding
This project was funded by the Colombian Royalties System as approved by the body overseeing the Colombian Science, Technology and Innovation Fund, Project BPIN-266, special agreement (020).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- Pvmdr1
P. vivax multidrug resistance 1 gene
- Pvdhfr
P. vivax dihydrofolate reductase
- Pvdhps
P. vivax dihydropteroate synthase
- Pvcrt-o
P. vivax chloroquine resistance transporter
- CQ
chloroquine
- PQ
primaquine
- SP
sulfadoxine–pyrimethamine
References
- 1.WHO . World malaria report 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 2.Rodriguez JC, Uribe GA, Araujo RM, Narvaez PC, Valencia SH. Epidemiology and control of malaria in Colombia. Mem Instituto Oswaldo Cruz. 2011;106(Suppl 1):114–122. doi: 10.1590/S0074-02762011000900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santa Maria-Salamanca M, Londoño-Soto B, Urquijo-Velasquez LE, Díaz-Gómez A, Padilla-Rodríguez JC. Gestión para la vigilancia entomológica y control de la transmisión de malaria. Colombia: Ministerio de la protección social; 2010. pp. 2–130. [Google Scholar]
- 4.Chu CS, White N. Management of relapsing Plasmodium vivax malaria. Expert Rev Anti Infect Ther. 2016;14:885–900. doi: 10.1080/14787210.2016.1220304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PAHO . Guia de atención clínica de malaria 2010 (Documento actualizado de versión convenio 256/09) República de Colombia: Ministerio de la protección social; 2010. p. 2010. [Google Scholar]
- 6.Das S, Banik A, Hati AK, Roy S. Low prevalence of dihydro folate reductase (dhfr) and dihydropteroate synthase (dhps) quadruple and quintuple mutant alleles associated with SP resistance in Plasmodium vivax isolates of West Bengal, India. Malar J. 2016;15:395. doi: 10.1186/s12936-016-1445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goncalves LA, Cravo P, Ferreira MU. Emerging Plasmodium vivax resistance to chloroquine in South America: an overview. Mem Instituto Oswaldo Cruz. 2014;109:534–539. doi: 10.1590/0074-0276130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gama BE, Oliveira NK, Souza JM, Daniel-Ribeiro CT, Ferreira-da-Cruz Mde F. Characterisation of pvmdr1 and pvdhfr genes associated with chemoresistance in Brazilian Plasmodium vivax isolates. Mem Instituto Oswaldo Cruz. 2009;104:1009–1011. doi: 10.1590/S0074-02762009000700012. [DOI] [PubMed] [Google Scholar]
- 9.Grigg MJ, William T, Menon J, Barber BE, Wilkes CS, Rajahram GS, et al. Efficacy of artesunate-mefloquine for chloroquine-resistant Plasmodium vivax malaria in malaysia: an open-label, randomized, controlled trial. Clin Infect Dis. 2016;62:1403–1411. doi: 10.1093/cid/ciw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jovel IT, Mejia RE, Banegas E, Piedade R, Alger J, Fontecha G, et al. Drug resistance associated genetic polymorphisms in Plasmodium falciparum and Plasmodium vivax collected in Honduras, Central America. Malar J. 2011;10:376. doi: 10.1186/1475-2875-10-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirahmadi S, Talha BA, Nour BY, Zakeri S. Prevalence of mutations in the antifolates resistance-associated genes (dhfr and dhps) in Plasmodium vivax parasites from Eastern and Central Sudan. Infect Genet Evol. 2014;26:153–159. doi: 10.1016/j.meegid.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Ruebush TK, 2nd, Zegarra J, Cairo J, Andersen EM, Green M, Pillai DR, et al. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am J Trop Med Hyg. 2003;69:548–552. [PubMed] [Google Scholar]
- 13.Schousboe ML, Ranjitkar S, Rajakaruna RS, Amerasinghe PH, Morales F, Pearce R, et al. Multiple origins of mutations in the mdr1 gene—a putative marker of chloroquine resistance in P. vivax. PLoS Negl Trop Dis. 2015;9:e0004196. doi: 10.1371/journal.pntd.0004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orjuela-Sanchez P, de Santana Filho FS, Machado-Lima A, Chehuan YF, Costa MR, Alecrim M, et al. Analysis of single-nucleotide polymorphisms in the crt-o and mdr1 genes of Plasmodium vivax among chloroquine-resistant isolates from the Brazilian Amazon region. Antimicrob Agents Chemother. 2009;53:3561–3564. doi: 10.1128/AAC.00004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunyarataphan S, Leartsakulpanich U, Taweechai S, Tarnchompoo B, Kamchonwongpaisan S, Yuthavong Y. Evaluation of the activities of pyrimethamine analogs against Plasmodium vivax and Plasmodium falciparum dihydrofolate reductase-thymidylate synthase using in vitro enzyme inhibition and bacterial complementation assays. Antimicrob Agents Chemother. 2006;50:3631–3637. doi: 10.1128/AAC.00448-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price RN, Auburn S, Marfurt J, Cheng Q. Phenotypic and genotypic characterisation of drug-resistant Plasmodium vivax. Trends Parasitol. 2012;28:522–529. doi: 10.1016/j.pt.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winter DJ, Pacheco MA, Vallejo AF, Schwartz RS, Arevalo-Herrera M, Herrera S, et al. Whole genome sequencing of field isolates reveals extensive genetic diversity in Plasmodium vivax from Colombia. PLoS Negl Trop Dis. 2015;9:e0004252. doi: 10.1371/journal.pntd.0004252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pornthanakasem W, Riangrungroj P, Chitnumsub P, Ittarat W, Kongkasuriyachai D, Uthaipibull C, et al. Role of Plasmodium vivax dihydropteroate synthase polymorphisms in sulfa drug resistance. Antimicrob Agents Chemother. 2016;60:4453–4463. doi: 10.1128/AAC.01835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suwanarusk R, Chavchich M, Russell B, Jaidee A, Chalfein F, Barends M, et al. Amplification of pvmdr1 associated with multidrug-resistant Plasmodium vivax. J Infect Dis. 2008;198:1558–1564. doi: 10.1086/592451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camargo-Ayala PA, Cubides JR, Nino CH, Camargo M, Rodriguez-Celis CA, Quinones T, et al. High Plasmodium malariae prevalence in an endemic area of the Colombian Amazon region. PLoS ONE. 2016;11:e0159968. doi: 10.1371/journal.pone.0159968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce MC, Galinski MR, Barnwell JW, Snounou G, Day KP. Polymorphism at the merozoite surface protein-3alpha locus of Plasmodium vivax: global and local diversity. Am J Trop Med Hyg. 1999;61:518–525. doi: 10.4269/ajtmh.1999.61.518. [DOI] [PubMed] [Google Scholar]
- 22.Rungsihirunrat K, Chaijaroenkul W, Siripoon N, Seugorn A, Na-Bangchang K. Genotyping of polymorphic marker (MSP3alpha and MSP3beta) genes of Plasmodium vivax field isolates from malaria endemic of Thailand. Trop Med Int Health. 2011;16:794–801. doi: 10.1111/j.1365-3156.2011.02771.x. [DOI] [PubMed] [Google Scholar]
- 23.Qiagen: QIAGEN Bioinformatics. 2017.
- 24.Alam MT, Bora H, Bharti PK, Saifi MA, Das MK, Dev V, et al. Similar trends of pyrimethamine resistance-associated mutations in Plasmodium vivax and P. falciparum. Antimicrob Agents Chemother. 2007;51:857–863. doi: 10.1128/AAC.01200-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korsinczky M, Fischer K, Chen N, Baker J, Rieckmann K, Cheng Q. Sulfadoxine resistance in Plasmodium vivax is associated with a specific amino acid in dihydropteroate synthase at the putative sulfadoxine-binding site. Antimicrob Agents Chemother. 2004;48:2214–2222. doi: 10.1128/AAC.48.6.2214-2222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu F, Wang B, Cao J, Sattabongkot J, Zhou H, Zhu G, et al. Prevalence of drug resistance-associated gene mutations in Plasmodium vivax in Central China. Korean J Parasitol. 2012;50:379–384. doi: 10.3347/kjp.2012.50.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34:W609–W612. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics (Oxford, England) 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Rosenberg HF, Nei M. Positive Darwinian selection after gene duplication in primate ribonuclease genes. Proc Natl Acad Sci USA. 1998;95:3708–3713. doi: 10.1073/pnas.95.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu YX, Li WH. Statistical tests of neutrality of mutations. Genetics. 1993;133:693–709. doi: 10.1093/genetics/133.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fay JC, Wu CI. Hitchhiking under positive Darwinian selection. Genetics. 2000;155:1405–1413. doi: 10.1093/genetics/155.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neafsey DE, Galinsky K, Jiang RH, Young L, Sykes SM, Saif S, et al. The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nat Genet. 2012;44:1046–1050. doi: 10.1038/ng.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zambrano-Villa S, Rosales-Borjas D, Carrero JC, Ortiz-Ortiz L. How protozoan parasites evade the immune response. Trends Parasitol. 2002;18:272–278. doi: 10.1016/S1471-4922(02)02289-4. [DOI] [PubMed] [Google Scholar]
- 36.Ministério-da-Saúde, Secretaria-de-Vigilância-em-Saúde . Guia prático de tratamento da malária no Brasil. Brasília: Departamento de Vigilância Epidemiológica, Ministério da Saúde; 2010. p. 36. [Google Scholar]
- 37.MINSA. Norma Técnica de Salud para la atención de la Malaria y Malaria Grave en el Perú. 2007.
- 38.Fernandez D, Segura C, Arboleda M, Garavito G, Blair S, Pabon A. In vitro susceptibility of Plasmodium vivax to antimalarials in Colombia. Antimicrob Agents Chemother. 2014;58:6354–6359. doi: 10.1128/AAC.03191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguiar AC, Pereira DB, Amaral NS, De Marco L, Krettli AU. Plasmodium vivax and Plasmodium falciparum ex vivo susceptibility to anti-malarials and gene characterization in Rondonia, West Amazon, Brazil. Malar J. 2014;13:73. doi: 10.1186/1475-2875-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mekonnen SK, Aseffa A, Berhe N, Teklehaymanot T, Clouse RM, Gebru T, et al. Return of chloroquine-sensitive Plasmodium falciparum parasites and emergence of chloroquine-resistant Plasmodium vivax in Ethiopia. Malar J. 2014;13:244. doi: 10.1186/1475-2875-13-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sastu UR, Abdullah NR, Norahmad NA, Saat MN, Muniandy PK, Jelip J, et al. Mutations of pvdhfr and pvdhps genes in vivax endemic-malaria areas in Kota Marudu and Kalabakan, Sabah. Malar J. 2016;15:63. doi: 10.1186/s12936-016-1109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCollum AM, Mueller K, Villegas L, Udhayakumar V, Escalante AA. Common origin and fixation of Plasmodium falciparum dhfr and dhps mutations associated with sulfadoxine–pyrimethamine resistance in a low-transmission area in South America. Antimicrob Agents Chemother. 2007;51:2085–2091. doi: 10.1128/AAC.01228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams HA, Vincent-Mark A, Herrera Y, Chang OJ. A retrospective analysis of the change in anti-malarial treatment policy: Peru. Malar J. 2009;8:85. doi: 10.1186/1475-2875-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nino CH, Cubides JR, Camargo-Ayala PA, Rodriguez-Celis CA, Quinones T, Cortes-Castillo MT, et al. Plasmodium malariae in the Colombian Amazon region: you don’t diagnose what you don’t suspect. Malar J. 2016;15:576. doi: 10.1186/s12936-016-1629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans AG, Wellems TE. Coevolutionary genetics of Plasmodium malaria parasites and their human hosts. Integr Comp Biol. 2002;42:401–407. doi: 10.1093/icb/42.2.401. [DOI] [PubMed] [Google Scholar]
- 46.Prajapati SK, Joshi H, Dev V, Dua VK. Molecular epidemiology of Plasmodium vivax anti-folate resistance in India. Malar J. 2011;10:102. doi: 10.1186/1475-2875-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tjitra E, Baker J, Suprianto S, Cheng Q, Anstey NM. Therapeutic efficacies of artesunate-sulfadoxine–pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations. Antimicrob Agents Chemother. 2002;46:3947–3953. doi: 10.1128/AAC.46.12.3947-3953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Auliff A, Wilson DW, Russell B, Gao Q, Chen N, le Anh N, et al. Amino acid mutations in Plasmodium vivax DHFR and DHPS from several geographical regions and susceptibility to antifolate drugs. Am J Trop Med Hyg. 2006;75:617–621. [PubMed] [Google Scholar]
- 49.Saralamba N, Nakeesathit S, Mayxay M, Newton PN, Osorio L, Kim JR, et al. Geographic distribution of amino acid mutations in DHFR and DHPS in Plasmodium vivax isolates from Lao PDR, India and Colombia. Malar J. 2016;15:484. doi: 10.1186/s12936-016-1543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chehuan YF, Costa MR, Costa JS, Alecrim MG, Nogueira F, Silveira H, et al. In vitro chloroquine resistance for Plasmodium vivax isolates from the Western Brazilian Amazon. Malar J. 2013;12:226. doi: 10.1186/1475-2875-12-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rungsihirunrat K, Sibley CH, Mungthin M, Na-Bangchang K. Geographical distribution of amino acid mutations in Plasmodium vivax DHFR and DHPS from malaria endemic areas of Thailand. Am J Trop Med Hyg. 2008;78:462–467. [PubMed] [Google Scholar]
- 52.Pava Z, Handayuni I, Wirjanata G, To S, Trianty L, Noviyanti R, et al. Expression of Plasmodium vivax crt-o is related to parasite stage but not ex vivo chloroquine susceptibility. Antimicrob Agents Chemother. 2015;60:361–367. doi: 10.1128/AAC.02207-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen I, Eastman R, Lanzer M. Drug-resistant malaria: molecular mechanisms and implications for public health. FEBS Lett. 2011;585:1551–1562. doi: 10.1016/j.febslet.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 54.Buitrago SP, Garzon-Ospina D, Patarroyo MA. Size polymorphism and low sequence diversity in the locus encoding the Plasmodium vivax rhoptry neck protein 4 (PvRON4) in Colombian isolates. Malar J. 2016;15:501. doi: 10.1186/s12936-016-1563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forero-Rodriguez J, Garzon-Ospina D, Patarroyo MA. Low genetic diversity in the locus encoding the Plasmodium vivax P41 protein in Colombia’s parasite population. Malar J. 2014;13:388. doi: 10.1186/1475-2875-13-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forero-Rodriguez J, Garzon-Ospina D, Patarroyo MA. Low genetic diversity and functional constraint in loci encoding Plasmodium vivax P12 and P38 proteins in the Colombian population. Malar J. 2014;13:58. doi: 10.1186/1475-2875-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mint Lekweiry K, Ould Mohamed Salem Boukhary A, Gaillard T, Wurtz N, Bogreau H, Hafid JE, et al. Molecular surveillance of drug-resistant Plasmodium vivax using pvdhfr, pvdhps and pvmdr1 markers in Nouakchott, Mauritania. J Antimicrob Chemother. 2012;67:367–374. doi: 10.1093/jac/dkr464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The DNA sequences and the data related were deposited in the GenBank database with Accession Numbers: MG100841–MG100859.


