Abstract
Possible target proteins of chloroplast thioredoxin (Trx) have been investigated in the stroma lysate of spinach chloroplasts. For that purpose, we immobilized a mutant of m-type Trx in which an internal cysteine at the active site was substituted with serine, on cyanogen bromide-activated resin. By using this resin, the target proteins in chloroplast were efficiently acquired when they formed the mixed-disulfide intermediates with the immobilized Trxs. We could acquire Rubisco activase (45 kDa) and 2-Cys-type peroxiredoxin (Prx), which were recently identified as targets of chloroplast Trxs. Glyceraldehyde-3-phosphate dehydrogenase and sedoheputulose 1,7-bisphosphatase, well-known thiol enzymes in the Calvin cycle, also were recognized among the collected proteins, suggesting the method is applicable for our purpose. Furthermore, four proteins were identified from a homology search of the NH2-terminal sequence of the acquired proteins: glutamine synthetase, a protein homologous to chloroplast cyclophilin, a homolog of Prx-Q, and the Rubisco small subunit. The Trx susceptibilities of the recombinant cyclophilin and Prx-Q of Arabidopsis thaliana were then examined. The method developed in the present study is thus applicable to investigate the various redox networks via Trxs and the related enzymes in the cell.
Thioredoxin (Trx) is a ubiquitous, disulfide oxidoreductase and regulates a number of phenomena in the cell by controlling the activity of enzymes through reduction of their disulfide bridge (1–3). In chloroplast of higher plants two isoforms of Trx are present: f-type (Trx-f) and m-type (Trx-m) (1, 4, 5). The active site sequence (-Trp-Cys-Gly-Pro-Cys-) of Trx is conserved regardless of the lower overall sequence homology between Trx-f and Trx-m. Recently, the crystal structures of both Trx-f and Trx-m from spinach were reported (6) and had basically the same structure as the other previously reported Trxs (7–9). The specificity of Trx for their targets in chloroplast therefore must be determined mainly by the surface charges around the active site (10).
The molecular mechanism for reduction of the target proteins by the reduced form of Trx already has been elucidated (11, 12). During the process to reduce the target, Trx forms a mixed-disulfide intermediate with the target. This disulfide bond then gets attacked by another cysteine residue at the active site of Trx, thus giving the reduced form of the target, whereas Trx itself becomes oxidized. In addition, we recently have clarified that Trx induces a conformational change in the target to enable reduction from the study of the activation process of chloroplast F1-ATPase by mutant Trx, in which one or both internal cysteines were substituted with serine (13).
Several chloroplast Trx target enzymes have been assigned after investigation of the change of the activity by reduction. Four enzymes in the Calvin cycle—glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (14), fructose 1,6-bisphosphatase (15), sedoheptulose 1,7-bisphosphatase (SBPase) (16), and phosphoribulokinase (17)—and CF1 from thylakoid membranes (18) are reported as targets for Trx-f. NADP-dependent malate dehydrogenase was assigned as a target for Trx-m (4). However, the whole network involved in the redox cascade in chloroplasts is as yet not well known, although a lot of the enzyme activities are obviously regulated by light and the accompanying electron transport. Within the past few years, acetyl CoA carboxyrase from Pisum sativum (19) and ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase from Arabidopsis thaliana (20) have been assigned as new targets. Additionally, the participation of chloroplast Trx on the function of 3′-phosphoadenosine 5′-phosphosulfate reductase, which is the key enzyme in the sulfate reduction pathway, also has been reported (21).
Recently Carlberg et al. (22) studied the phosphorylation of thylakoid proteins in the presence of either thiol reagent or thiol oxidant. They found that the level of thylakoid protein phosphorylation changed drastically depending on the redox state (23). Phosphorylation of light-harvesting complex II was strongly down-regulated under the thiol-reducing conditions, but the phosphorylation of the reaction center protein was stimulated 2-fold under the same conditions. They therefore concluded that multiple Trx-regulated protein kinases are working in the chloroplast.
In addition, there are several reports that the chloroplast Trx system also is involved in the antioxidative stress like Trx-h system in cytosol. Baier and Dietz (24) reported the physiological function of chloroplast 2-Cys type peroxiredoxin (Prx) against the cell-toxic alkyl hydroperoxides. Cheong et al. (25) reported that peroxidase activity of the recombinant 2-Cys Prx in the chloroplast of Chinese cabbage is induced by the yeast Trx system.
Nevertheless, to date there have been few attempts to capture the target proteins of Trx directly. During preparation of this manuscript, Yano, et al. (26) reported a new method to identify targets of Trx-h using subtraction display of the proteins in which cysteines were labeled with fluorescent dye before or after the incubation with Trx-DTT. They isolated more than 20 targets in peanut seeds on two-dimensional PAGE, and five of them were partially sequenced. Finally, they identified three allergens (Ara h2, Ara h3, and Ara h6) as the novel targets of Trx-h. Verdoucq et al. (27) observed in vivo formation of the Trx-target complex in yeast when a mutant Trx-h of A. thaliana, of which an internal cysteine at the active site was substituted with serine, was expressed in yeast. Their results suggest that the intermediate complex formed between a target and Trx can be stabilized when an internal cysteine of Trx is missing. They identified a new type of peroxidase in yeast cytosol as a counterpart for Trx.
In the present study, we report an efficient comprehensive survey of the proteins that can interact with chloroplast Trx. To favor the formation of the disulfide intermediate, we also used mutant Trx, which lacks an internal Cys at the active site. After the formation of the mixed-disulfide intermediates between candidate proteins and the immobilized Trx mutant [Trx-mC41S (13)], the acquired proteins were eluted from the resin by reduction. In this report we identify four proteins as a potential target for Trx-m in spinach chloroplast.
Materials and Methods
Materials.
Cyanogen bromide-activated Sepharose 4B and Percoll were purchased from Amersham Pharmacia. The Bradford protein assay system was from Bio-Rad. DTT and Escherichia coli NADPH-Trx reductase were from Sigma. 4-Acetamido-4′-maleimidyl-stilbene-2,2′-disulfonate (AMS) was from Molecular Probes. Other chemicals were of the highest grade commercially available.
Preparation of the Recombinant Trx-m and the Mutants.
The recombinant Trx-m and the mutants, Trx-mC41S and Trx-mC37S/C41S, were expressed in E. coli and purified as described (13). The protein concentration of Trx was calculated from A278 with the published molecular absorption coefficient value for Trx-m of 20,500 M−1⋅cm−1 (28).
Chloroplast Stroma Lysate.
The chloroplasts were prepared from spinach leaves and purified by a 30–65% (vol/vol) Percoll discontinuous gradient. After centrifugation, the green band between the 30% and 65% layers was collected as the intact chloroplast. This preparation was subsequently washed to remove Percoll and suspended in 50 mM Tricine-KOH (pH 8.0) at a chlorophyll concentration of 1 mg/ml and incubated for 15 min at 4°C. The stroma lysate protein was obtained as the supernatant of this suspension after centrifugation for 1 h at 100,000 × g.
Preparation of the Trx-Immobilized Resin.
Trx-m mutants in 100 mM sodium carbonate buffer (pH 9.5) containing 0.5 M NaCl was incubated with cyanogen bromide-activated Sepharose 4B resin that had been swelled with the same solution for 2 h at room temperature according to the manufacturer's instructions. After termination of the coupling reaction by centrifugation, the unreacted side chains on the resin were blocked by incubation with 50 mM Tris⋅HCl (pH 8.0) for 12 h at 4°C. The amounts of the immobilized Trx on the gel were calculated based on the amounts of Trx used and the amount remaining in the solution after the coupling reaction. In our case, more than 90% of Trx was immobilized.
Collection of the Target Proteins by Immobilized Trx Mutant.
The chloroplast stroma lysate (5 mg protein) was incubated with 1 ml of Trx-immobilized resin (a total of 2 mg of Trx was immobilized) at 25°C for 1 h under gentle stirring. Then the resin was washed with 50 mM Tris⋅HCl (pH 8.0) and 200 mM NaCl to remove the nonspecifically bound proteins. The washing was repeated until the absorbance of the wash buffer at 280 nm became almost zero. Finally, the resin was suspended in 50 mM Tris⋅HCl (pH 8.0), 200 mM NaCl, and 10 mM DTT and was incubated for 30 min at 25°C. The eluted proteins were separated from the resin by centrifugation. SDS/PAGE was performed with 15% (wt/vol) acrylamide gel (29), and the separated protein bands were visualized by Coomassie brilliant blue R-250.
NH2-Terminal Sequence.
Each protein separated by SDS/PAGE was transferred onto poly(vinylidene difluoride) membrane, and the NH2-terminal sequence was determined by the Edman degradation method with a peptide sequencer model PPSQ21 (Shimadzu).
Cloning, Expression, and Purification of A. thaliana Chloroplast Prx-Q (Prx-QAt) and Cyclophilin (CyPAt).
Total RNA from total tissues of 14-day A. thaliana was prepared by the method described in ref. 30. The genes for chloroplast Prx-Q (Prx-QAt) (31) and cyclophilin (ChCyPAt) (32), without the transit sequences, were obtained by reverse transcriptase-PCR using the oligonucleotides as follows: for Prx-QAt, 5′-gaagatcttcatatgaaggttaacaagggtcag-3′ (NdeI) and 5′-gctctagactcgagtcaagcagctttgagaaac-3′ (XhoI); for ChCyPAt, 5′-aactgcagcatatggctgctgaggaagaaga-3′ (NdeI) and 5′-cggaattcaagcatctaacgggagct-3′ (EcoRI). The restriction sites for the enzyme shown in parentheses are underlined. The amplified DNA fragments were cloned into the NdeI and XhoI sites or NdeI and EcoRI sites of pET-23c (Novagen), and the DNA sequences were confirmed by DNA sequencing (Prism 310, Applied Biosystems).
The obtained plasmids, Prx-QAt-pET23c and ChCyPAt-pET23c, were transformed into the expression host E. coli BL21(DE3), and the desired proteins were overexpressed.
Overexpressed Prx-QAt was purified as follows. E. coli cells were suspended in 20 mM Tris⋅HCl (pH 7.5) and disrupted by a French pressure cell (5501-M, Ohtake Works, Tokyo) at 4°C. The disrupt cells were centrifuged at 100,000 × g for 40 min, and the supernatant (crude extract) was applied to a DEAE-TOYOPEAL 650 M column (Tosoh, Tokyo), which was equilibrated with 20 mM Tris⋅HCl (pH 7.5) previously. Proteins were eluted with a 0–150 mM linear gradient of NaCl in 20 mM Tris⋅HCl (pH 7.5). The peak fractions containing Prx-QAt were collected and stored in a 3.6 M ammonium sulfate suspension at 4°C.
ChCyPAt expressed in E. coli was purified as follows. The crude extract was obtained as described above and applied to a DEAE-TOYOPEAL 650 M column, then eluted with a 0–200 mM linear gradient of NaCl in 20 mM Tris⋅HCl (pH 7.5). The peak fraction containing ChCyPAt was stored in a 2.8 M ammonium sulfate suspension at 4°C.
In Vitro Reduction of Prx-QAt and ChCyPAt Assisted by Trx-m.
Prx-QAt (3 μM) in 35 mM Hepes-NaOH (pH 7.3) was incubated for 1 h at 25°C with 50 μM CuCl2 or various concentrations of DTT in the presence or absence of Trx-m. Then the redox states of the proteins were determined as described below.
To oxidize ChCyPAt, 10 μM of the protein in 25 mM Pipes-NaOH (pH 6.9) was incubated with 50 μM CuCl2 for 2 h at 25°C, and then dialyzed against 25 mM Tris⋅HCl (pH 8.1). The obtained oxidized form ChCyPAt (3.0 μM) was incubated with CuCl2 or DTT in the presence or absence of Trx-m for 1 h at 25°C, and their redox states were determined.
The redox states of Prx-QAt and ChCyPAt were assessed by the method described (33). Briefly, proteins were precipitated with 5% trichloroacetic acid (final concentration) and collected by centrifugation. The obtained protein precipitates then were washed with acetone and dissolved in a freshly prepared solution containing 1% SDS, 50 mM Tris⋅HCl (pH 7.5), and 10 mM AMS. Labeled proteins then were separated by nonreducing SDS/PAGE (15%).
Measurement of Peroxidase Activity of Prx-Q.
Trx-m-dependent peroxidase activity was measured indirectly by the coupled oxidation of NADPH at 25°C (31). The standard reaction mixture contained 50 mM Hepes-NaOH (pH 7.3), 1.0 μM Trx-m, 1.0 μM E. coli Trx-reductase, 0.18 mM NADPH, and 1.0 μM Prx-QAt. The reaction was initiated by the addition of H2O2 at the final concentration of 0.2 mM, and the absorbance at 340 nm was monitored.
Other Methods.
The protein (34) and chlorophyll (35) concentrations were determined by previously described methods.
Results
Mutant Trx-m Immobilized Resin.
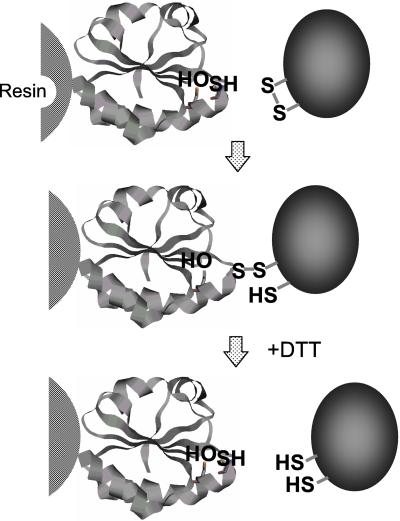
The reduced forms of Trx and the target proteins form mixed-disulfide intermediates during the reduction pathway (11, 12). Then Trx itself forms an internal disulfide bridge, thus releasing the reduced target protein. This intermediate state should be stabilized when Trx lacks the second cysteine residue (Fig. 1, second step; see ref. 27). In this case, the intermediate will dissociate only after incubation with DTT (Fig. 1, third step).
Figure 1.
Model of the process to capture the target proteins by Trx mutant. The internal cystein at the active site of Trx was substituted with serine and immobilized on the resin. The mixed-disulfide intermediate should be broken by the addition of DTT, thus eluting the target proteins.
We here developed a method based on this reaction mechanism. Mutant Trx-mC41S was immobilized on a resin, and a crude protein preparation from spinach chloroplast stroma was first incubated with this resin for 30 min at 25°C to ensure the formation of the mixed-disulfide intermediate. After washing with NaCl, the trapped proteins on the resin were eluted by a 30-min incubation with 10 mM DTT (Fig. 2). As the amount of proteins eluted from the Trx-mC41S resin conjugate was much more than that from the Trx-fC40S resin conjugate, we used only the Trx-m resin conjugate for further experiments. We could not trap any proteins by using a similar resin with Trx-mC37S/C41S (data not shown). Hence, the role of Cys37 on Trx-m for the interaction with the target is important.
Figure 2.
Elution of proteins from the Trx-immobilized resin with DTT. The target proteins in the chloroplast stroma lysate were initially retained on the resin and subsequently eluted by the addition of DTT. Each of the protein bands was assigned by NH2-terminal sequencing. White triangles show the proteins for which an NH2-terminal sequence was determined and assigned in the database. Black triangles show the mutant Trx-m that was released from resin. The bars on the right side of the gel indicate the positions of the molecular markers. The protein bands shown with gray triangles remained unassigned based on their NH2-terminal sequences. SBPase, sedoheptulose 1,7-bis phosphatase; PPIase, peptide-propyl cis-trans isomerase.
Analysis of the Target Proteins for Trx.
More than 14 bands of eluted proteins from the Trx-immobilized resin were clearly recognizable on the Coomassie brilliant blue R-250-stained gel (Fig. 2). Within these bands, we could determine the NH2-terminal sequences of 10 different protein bands, and finally 13 different NH2-terminal sequences were successfully analyzed (Table 1).
Table 1.
NH2-terminal sequences of the acquired proteins by immobilized Trx resin
| Sequences* | Molecular† mass (kDa) [origin] | Predicted proteins | Interaction with Trx | Ref. | Accession number‡ |
|---|---|---|---|---|---|
| AENEEKNTDKWAHLAKDFS | 45.0 | Rubisco activase | Yes | ||
| AENEEKNTDKWAHLAKDFS | 45.7 [Spiol] | 45 kDa | 20 | S45033 | |
| SLQSDHGTVNRVEQLLNLDVTPYT | 44.0 | GS | This study§ | ||
| ALQSDNSTVNRVETLLNLDTKPYS | 41.9 [Arth] | 45 | S69727 | ||
| AENEQKNTDK | 40.5 | Rubisco activase | This study | ||
| AENEEKNTDK | 41.8 [Spiol] | 41 kDa | 20 | S45033 | |
| ALGVAINGFGAIGRNFL | 40.0 | GAPDH | Yes | ||
| KLKVAINGFGRIGRNFL | 36.2 [Spiol] | 14 | X15189 | ||
| ELGDSLNEALAIATTDEGLI | 40.0 | Sedoheptulose 1,7-bisphosphatase (SBPase) | Yes | ||
| ELGDSLEEFLAKATTDKGLI | 34.9 [Spiol] | 37 | L76556 | ||
| SAELPLVGNVAPDFEAGRVF | 25.7 | 2-Cys Prx | Yes | ||
| ADDLPLVGNKAPDFEAEAVF | 21.9 [Spiol] | 25 | X94219 | ||
| STETDVQDVQAEVTSKVFFDVDIGGE | 21.6 | Cyclophilin (PPIase) | This study | ||
| AEEEEVIEPQAKVTNKVYFDVEIGGE | 19.7 [Arth] | 32 | L14845 | ||
| KVKEGTLAPAFSLKXQ | 18.6 | Prx-Q-like protein | Yes | ||
| KVNKGQAAPDFTLKDQ | 17.0 [Arth] | 31 | AB023041 | ||
| PLKAGDIAPKFSLPDQ | 17.4 [Ecoli] | BCP | 51 | AE005477 | |
| MKVXPTQNMVRYETLXYLPP | 14.3 | Rubisco small subunit (rbs2) | This study | X97600 | |
| MKVWPTQNMKRYETLSYLPP | 14.3 [Spiol] |
The upper lane is the obtained sequence and the lower sequence is that of the most homologous protein obtained from the databases.
The upper value is calculated from SDS/PAGE and the lower one is calculated from the sequence of the indicated protein. The abbreviations used in the brackets were: Spiol, S. oleracea; Arth, A. thaliana; E coli, E. coli.
GenBank accession numbers are indicated.
The homologous proteins of the obtained NH2-terminal sequences were surveyed by using the program fasta (36). We could assign four proteins that already were reported as the target of the chloroplast Trx: Rubisco activase 45 kDa (20), GAPDH (14), sedoheptulose 1,7-bisphosphatase (16, 37), and 2-Cys Prx (25). Additionally the known proteins, glutamine synthetase (GS) (monomer molecular mass 44 kDa), Rubisco activase (41 kDa), and Rubisco small subunit were obtained.
The 21.6-kDa protein was identified as a homolog of cyclophilin because the sequence of this protein showed the highest homology with the sequence from the position of the processing site of the precursor of cyclophilin reported for A. thaliana (32), and the molecular size of the obtained protein was almost same as the mature size of the reported enzyme. The 18.6-kDa protein was predicted to be a homolog of bacterioferritin comigratory protein (BCP) from E. coli. Kong et al. (31) recently reported a homologous protein of BCP obtained from Sedum lineare as a new type of Prx, Prx-Q (31), and our 18.6-kDa protein showed high similarity in the NH2-terminal sequence. The 14.3-kDa protein was the small subunit of Rubisco. The NH2-terminal sequences of the additional three proteins (43 kDa, 32 kDa, and 27.5 kDa; sequence data not shown) did not have homology to any data registered in the databases.
Biochemical Assay of Prx.
To confirm whether a newly identified protein really can interact with the chloroplast Trx, a biochemical characterization is necessary. For 2-Cys Prx from Chinese cabbage chloroplast, Trx and NADPH-Trx reductase were required for the function (25). The novel Prx of S. lineare (Prx-Q) also can reduce the H2O2 assisted by E. coli TrxR and Trx (31).
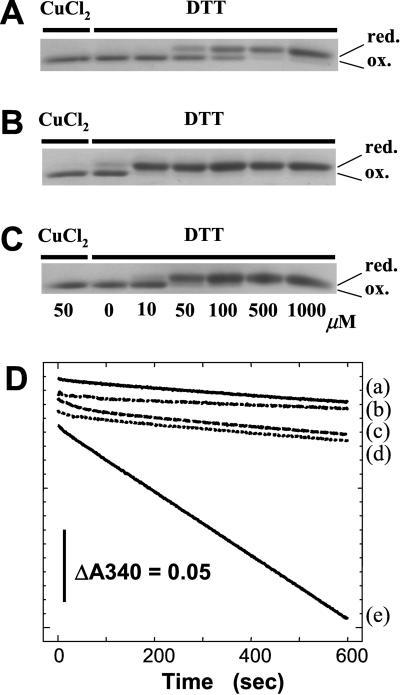
In the present study, we therefore prepared the recombinant Prx-Q of A. thaliana (Prx-QAt) to confirm that this Prx interacts with Trx-m. The oxidized form of Prx-QAt was partially reduced by incubation with 50–100 μM DTT, and 500 μM DTT was required for complete reduction (Fig. 3A). The concentration of DTT required for reduction was drastically decreased when stoichiometric amounts of Trx-m were supplemented (Fig. 3B). Even when the substoichiometric amounts of Trx-m (0.04 μM) were used, Prx-QAt was completely reduced at lower concentrations of DTT (50 μM) (Fig. 3C).
Figure 3.
(A–C) Reduction levels of Prx-QAt under the redox conditions were determined by AMS labeling. The purified Prx-QAt was in the oxidized form. A total of 3 μM of Prx-QAt was incubated with 50 μM CuCl2 or the indicated concentration of DTT in the absence (A) or presence of 4 μM (B) or 0.04 μM (C) Trx-m. Then the reduction level of Prx-QAt was determined by the incorporation of AMS and the change of the mobility during SDS/PAGE. (D) Trx-m dependent peroxidase activity of Prx-QAt was measured with H2O2 as a substrate and NADPH as a reductant. The decrease in the absorbance at 340 nm was monitored (trace e). In traces a–c, Trx-m, Trx reductase, or Prx-QAt was omitted from the reaction mixture, respectively. In trace d, Trx-mC41S was used instead of Trx-m.
The peroxidase activity of the recombinant Prx-QAt was examined next. Prx-QAt showed the peroxidase activity in the presence of Trx-m when H2O2 was used as a substrate (Fig. 3D, trace e). This activity was not observed when one of the requisite components, Trx-m, NADPH-Trx-reductase from E. coli, or Prx-QAt was omitted (Fig. 3D, traces a–c). NADPH oxidation was not observed with Trx-mC41S (Fig. 3D, trace d).
Biochemical Assay of Cyclophilin.
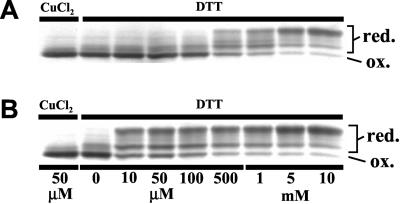
To date, there is no information available on the redox regulation of the peptidyl-prolyl cis-trans isomerase (PPIase) activity of cyclophilin. We therefore first examined whether the recombinant cyclophilin possesses a redox-sensitive disulfide bond in the molecule. Recombinant ChCyPAt was first oxidized completely in the presence of CuCl2. When the oxidized ChCyPAt was reduced by the incubation with 10 μM to 10 mM DTT, multiple bands were observed after the AMS labeling, suggesting that the oxidized form ChCyPAt gradually became reduced. Again the concentration of DTT required for this transition was decreased drastically when Trx-m was added (Fig. 4B). These results strongly suggest that ChCyPAt has disulfide bond(s), of which reduction is assisted by Trx-m.
Figure 4.
Reduction levels of ChCyPAt under the redox conditions were determined by AMS labeling. The oxidized form ChCyPAt (3 μM) was incubated with 50 μM CuCl2 or the indicated concentration of DTT in the absence (A) or presence of 3 μM (B) Trx-m. Then the reduction level of ChCyPAt was determined by the incorporation of AMS and the change of the mobility in SDS/PAGE.
Discussion
The Target Enzymes for Trx in the Chloroplasts.
The formation of the mixed-disulfide intermediates between Trx and the target proteins during the reduction process was clarified from the study of E. coli Trx (11). Then a number of studies focused on solving the structure of this intermediate state (9, 38). A mutant Trx, in which an internal cysteine was substituted with serine, was used for these studies. In 1999, Verdoucq et al. (27) introduced the Cys-mutant of cytosolic Trx-h of A. thaliana into yeast cells. And they identified a new type of Prx, YLR109 in Saccharomyces cerevisiae, which formed a mixed-disulfide intermediate with mutant Trx and presented Trx-dependent Prx activity. This work is a solid confirmation of our strategy in vivo.
In contrast, little information on the target enzymes of chloroplast Trx has accumulated (4, 5). To obtain comprehensive information on the Trx target proteins we used an immobilized internal cysteine mutant Trx as a functional probe and successfully isolated some known target proteins (Table 1). Within them, sedoheptulose 1,7-bisphosphatase and GAPDH are well-known Trx target enzymes in the Calvin-Benson cycle (4). This result implies that the specificity between the immobilized Trx and their targets is conserved in our system. On the other hand, it should be noted that the well-known thiol enzymes in chloroplast like glucose 6-phosphate dehydrogenase (39) and NADP-malate dehydrogenase were not captured. Therefore our method must improve to capture additional undiscovered targets.
Rubsico Activase and Rubisco Small Subunit.
Rubisco activase is one of the key enzymes that regulates the activity of the entire Calvin-Benson cycle via regulation of the activity of Rubisco. Zhang and Portis (20) recently reported that Rubisco activase is a target for chloroplast Trx. From the analysis of the redox-sensitive activity of the C-terminus deletion mutant of the larger isoform of Rubisco activase (46 kDa), they have concluded that the activity of the 46-kDa isoform is regulated by Trx-f but the smaller isoform (43 kDa) is not, and that two cysteines that are close to the C termini (Cys392 and Cys411) must be the targets for Trx. In addition, they reported cooperative behavior between these two isoforms. Both Rubisco activase isoforms (45 kDa and 41 kDa) of spinach chloroplast, which correspond to the 46-kDa and 43-kDa proteins from A. thaliana, were trapped by our immobilized mutant Trx (Fig. 2). This result suggests that these isoforms form a heterodimer complex in the stroma, although a possibility that Trx-m interacts with both isoforms independently under our experimental conditions is not completely impossible. Additional biochemical assessments on the possible heterodimer are necessary. As we obtained these isoforms from resins with either Trx-f or Trx-m (data not shown), we could not specify which type of chloroplast Trx mainly interacts with the activase, although Zhang and Portis (20) have suggested that Trx-f was more efficient. This discrepancy might be attributable to the differences between the two methods, the complete reaction mediated by Trx, and the partial reaction catalyzed by our mutant.
To our knowledge, there have been no reports on the interactions between Rubisco and chloroplast Trx. The small subunit of spinach Rubisco encoded by the rbs2 gene has four cysteines in the molecule (Cys41, Cys77, Cys90, and Cys112), and the distances within these cysteines were too far from each other (11–25 Å) to form a disulfide bond (40). The putative interaction between Trx and the Rubisco small subunit therefore should be investigated further to conclude whether the suggested interaction occurs under physiological conditions.
GS.
GS is a well-characterized enzyme, the activity of which decreases when oxidized (41). In chloroplast this enzyme is a key in photorespiration and catalyzes the rate-limiting step in the pathway (42). This enzyme is already known to be activated by Trx in green algae (43, 44). Lichter and Häberlein (45) also found Trx-dependent activation of Chlorella GS but not spinach GS, although the data for this Trx insensitivity of spinach GS was ambiguous. Recently, Choi et al. (46) reported that the enzyme activity of plastidic GS cloned from Canavalia lineata was significantly activated by DTT. Taken together, it is plausible that the chloroplast GS is a target of chloroplast Trx. From the mutational analysis, Choi et al. suggested that Cys306 and Cys371 are important residues for this thiol regulation (figure 3 of ref. 46). However, the corresponding cysteine residues in the three-dimensional structure of S. typhimurium GS (Cys270 and Ala341) are nearly inaccessible from the outside of the molecule (47). As the dodecameric structure of bacterial GS has already been elucidated (48) and as GS from higher plants is expected to be octameric (49), there might be intrinsic differences between their monomer structures.
Prxs.
2-Cys-type Prx in chloroplasts was first reported by Baier and Dietz (50). From the analysis of the 2-Cys-type Prx-deficient mutant A. thaliana, the physiological role of this Prx was assigned as an antioxidant that protects the photosynthetic apparatus from oxidative stress (24). Recently, the biochemical properties of recombinant 2-Cys-type Prx from Chinese cabbage were investigated, and the H2O2 reduction activity of this enzyme coupled with NADPH reduction via Trx reductase and Trx was shown in vitro (25). Our result therefore supports the possible reduction of 2-Cys Prx by Trx-m in the chloroplast.
The 18.6-kDa protein we obtained was originally recognized as having the highest homology to BCP from E. coli. Jeong et al. (51) recently reported that this BCP from E. coli has a Trx-dependent thiol peroxidase activity. In addition, Kong et al. (31) have reported Prx activity for the protein obtained from the Crassulacean acid metabolism type plant S. lineare, which is a homolog of BCP and apparently the same protein as our 18.6-kDa protein, designated Prx-Q. They showed electron transfer from NADPH to H2O2 via the E. coli Trx-Trx reductase system plus Prx-Q. Here we could clearly show that this novel Prx is present in the stroma and certainly interacts with chlroplast Trx-m (Fig. 3 A–C) and that the reduced form Trx-m can reduce Prx-Q (Fig. 3D). We have demonstrated that chloroplast Trx cooperates with Prx in the chloroplast to reduce H2O2.
Chloroplast Cyclophilin.
Gasser et al. (52) first reported the existence of the PPIases in plants, which are the family of enzymes that catalyze or facilitate protein folding. In 1992, Breiman et al. (53) found that cyclosporin A-sensitive PPIase (cyclophilin) is predominantly localized on the thylakoids. This chloroplast cyclophilin was encoded by a nuclear gene and transported posttranslationally into the chloroplast (32). The four amino acids at the NH2 termini corresponding to cyclophilin from A. thaliana are missing from the NH2-terminal sequence of our 20-kDa protein, nevertheless, their overall homology between the two was remarkably high. Although the redox regulation of the PPIase activity of the recombinant ChCyPAt was somewhat ambiguous (data not shown), the AMS labeling clearly showed that ChCyPAt has multiple internal disulfide bonds, and that DTT reduction of the disulfides is assisted by Trx-m (Fig. 4). The disulfide bond formation in the cyclophilin molecule of C. elegans has previously been suggested by Dornan et al. (54). Recently, Lee et al. (55) have reported that a mammal cyclophilin, CyP-A, binds to 1-Cys-type Prx and supports the peroxidase activity as an electron donor. This finding allowed proposing the cascade from Trx to 1-Cys Prx via cyclophilin in the cell. Further biochemical studies on the redox regulation of PPIase activity of the chloroplast cylclophilin will provide important information on the physiological role of this protein.
Conclusions.
The reduction/oxidation cascade via Trx in chloroplast appears to be the most important regulation network for the metabolic pathways in chloroplast. Historically identifying these Trx-regulated enzymes in chloroplast has been time-consuming. The strategy to identify targets of Trx reported by Yano et al. (26) was one comprehensive way. The method we reported here has an advantage that we can specifically concentrate the Trx target proteins from the stroma lysate (Fig. 2). On one hand, this strategy is still somewhat incomplete as we were unable to acquire some well-known chloroplastic thiol enzymes like glucose 6-phosphate dehydrogenase and NADP-malate dehydrogenase. And we could not discriminate the targets for Trx-m and Trx-f by using this method. Despite these limitations, we will be able to add more information on the target enzymes of Trx, including our unassigned proteins, using the combination of this Trx-resin chromatography method and two-dimensional PAGE analysis or mass spectrometry.
The method reported here is one of the most rapid means of elucidating the whole cascade controlled by Trx. In addition, the method is also applicable to the target proteins for Trx on the thylakoid membrane, the targets for multiple kinds of Trx-h in cytosol, and related proteins involved in disulfide bond-dependent reduction/oxidation in plants, and in mammals and bacteria as well.
Acknowledgments
We thank H. Taguchi, H. Noji, T. Suzuki, Y. Kato-Yamada, K. Sugiyama, N. Kawakami, H. Konno, and P. Kroth for their valuable suggestions and helpful discussions. We also thank J. Suzuki, N. Hosoya-Matsuda, and D. Yamazaki for their technical assistance. Thanks to Dr. J. Hardy for critically reading the manuscript. Special thanks to Prof. M. Yoshida for his continuous encouragement and helpful discussion. This work was supported by grants-in-aid for science research on priority areas (A) Molecular basis for organization of photosynthesis through plant body to T.H. (nos. 11151209 and 12025207) from the Ministry of Education, Science, Sports, Culture, and Technology of Japan.
Abbreviations
- Trx
thioredoxin
- Prx
peroxiredoxin
- AMS
4-acetamido-4′-maleimidyl-stilbene-2,2′-disulfonate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GS
glutamine synthetase
- BCP
bacterioferritin comigratory protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Buchanan B B. Annu Rev Plant Physiol. 1980;31:341–374. [Google Scholar]
- 2.Buchanan B B. Arch Biochem Biophys. 1981;33:147–162. [Google Scholar]
- 3.Holmgren A. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 4.Jacquot J-P, Lancelin J-M, Meyer Y. New Phytol. 1997;136:543–570. doi: 10.1046/j.1469-8137.1997.00784.x. [DOI] [PubMed] [Google Scholar]
- 5.Ruelland E, Miginiac-Maslow M. Trends Plant Sci. 1999;4:136–141. doi: 10.1016/s1360-1385(99)01391-6. [DOI] [PubMed] [Google Scholar]
- 6.Capitani G, Markovic-Housley Z, DelVal G, Morris M, Jansonius J N, Schürmann P. J Mol Biol. 2000;302:135–154. doi: 10.1006/jmbi.2000.4006. [DOI] [PubMed] [Google Scholar]
- 7.Eklund H, Cambillau C, Sjoeberg B-M, Holmgren A, Joernvall H, Hoeoeg J-O, Braenden C-I. EMBO J. 1984;3:1443–1449. doi: 10.1002/j.1460-2075.1984.tb01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katti S K, Le Master D M, Eklund H. J Mol Biol. 1990;212:167–184. doi: 10.1016/0022-2836(90)90313-B. [DOI] [PubMed] [Google Scholar]
- 9.Qin J, Clore G M, Gronenborn A M. Structure (London) 1994;15:503–522. doi: 10.1016/s0969-2126(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 10.Mora-Garcia S, Rodriguez-Suarez R, Wolosiuk R A. J Biol Chem. 1998;273:16273–16280. doi: 10.1074/jbc.273.26.16273. [DOI] [PubMed] [Google Scholar]
- 11.Holmgren A. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 12.Holmgren A. Structure (London) 1995;15:239–243. doi: 10.1016/s0969-2126(01)00153-8. [DOI] [PubMed] [Google Scholar]
- 13.Stumpp M T, Motohashi K, Hisabori T. Biochem J. 1999;341:157–163. [PMC free article] [PubMed] [Google Scholar]
- 14.Baalmann E, Backhausen J E, Rak C, Vetter S, Scheibe R. Arch Biochem Biophys. 1995;324:201–208. doi: 10.1006/abbi.1995.0031. [DOI] [PubMed] [Google Scholar]
- 15.Clancey C J, Gilbert H F. J Biol Chem. 1987;262:13545–13549. [PubMed] [Google Scholar]
- 16.Cadet F, Meunier J C, Ferte N. Biochem J. 1987;241:71–74. doi: 10.1042/bj2410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolosiuk R A, Buchanan B B. Arch Biochem Biophys. 1978;189:97–101. doi: 10.1016/0003-9861(78)90119-4. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz O, Schürmann P, Strotmann H. J Biol Chem. 1997;272:16924–16927. doi: 10.1074/jbc.272.27.16924. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki Y, Kozaki A, Hatano M. Proc Natl Acad Sci USA. 1997;94:11908–11101. doi: 10.1073/pnas.94.20.11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang N, Portis R., Jr Proc Natl Acad Sci USA. 1999;96:9438–9443. doi: 10.1073/pnas.96.16.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setya A, Murillo M, Leustek T. Proc Natl Acad Sci USA. 1996;93:13383–13388. doi: 10.1073/pnas.93.23.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlberg I, Rintamaeki E, Aro E-M, Andersson B. Biochemistry. 1999;38:3197–3204. doi: 10.1021/bi982506o. [DOI] [PubMed] [Google Scholar]
- 23.Rintamäki E, Martinsuo P, Pursiheimo S, Aro E-M. Proc Natl Acad Sci USA. 2000;97:11644–11649. doi: 10.1073/pnas.180054297. . (First Published September 26, 2000; 10.1073/pnas.180054297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baier M, Dietz K-J. Plant Physiol. 1999;119:1407–1414. doi: 10.1104/pp.119.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheong N E, Choi Y O, Lee K O, Kim W Y, Jung B G, Chi H Y, Jeong J S, Kim K, Cho M J, Lee S Y. Plant Mol Biol. 1999;40:825–834. doi: 10.1023/a:1006271823973. [DOI] [PubMed] [Google Scholar]
- 26.Yano H, Wong J H, Lee Y M, Cho M-J, Buchanan B B. Proc Natl Acad Sci USA. 2001;98:4794–4799. doi: 10.1073/pnas.071041998. . (First Published March 27, 2001; 10.1073/pnas.071041998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdoucq L, Vignols F, Jacquot J-P, Chartier Y, Meyer Y. J Biol Chem. 1999;274:19714–19722. doi: 10.1074/jbc.274.28.19714. [DOI] [PubMed] [Google Scholar]
- 28.Schürmann P. Methods Enzymol. 1995;252:274–283. doi: 10.1016/0076-6879(95)52030-9. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Kawakami N, Kawabata C, Noda K. Plant Cell Physiol. 1992;33:511–517. [Google Scholar]
- 31.Kong W, Shiota S, Shi Y, Nakayama H, Nakayama K. Biochem J. 2000;351:107–114. doi: 10.1042/0264-6021:3510107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippuner V, Chou I T, Scott S V, Ettinger W F, Theg S M, Gasser C S. J Biol Chem. 1994;269:7863–7868. [PubMed] [Google Scholar]
- 33.Kobayashi T, Kishigami S, Sone M, Inokuchi H, Mogi T, Ito K. Proc Natl Acad Sci USA. 1997;94:11857–11862. doi: 10.1073/pnas.94.22.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradford M M. Anal Biochem. 1975;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Arnon D I. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson W R, Lipman D J. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin W, Mustafa A Z, Henze K, Schnarrenberger C. Plant Mol Biol. 1996;32:485–491. doi: 10.1007/BF00019100. [DOI] [PubMed] [Google Scholar]
- 38.Jeng M F, Reymond M T, Tennant L L, Holmgren A, Dyson H J. Eur J Biochem. 1998;257:299–308. doi: 10.1046/j.1432-1327.1998.2570299.x. [DOI] [PubMed] [Google Scholar]
- 39.Scheibe R, Anderson L E. Biochim Biophys Acta. 1981;636:58–64. doi: 10.1016/0005-2728(81)90075-x. [DOI] [PubMed] [Google Scholar]
- 40.Taylor T C, Andersson I. Biochemistry. 1997;36:4041–4046. doi: 10.1021/bi962818w. [DOI] [PubMed] [Google Scholar]
- 41.Levine R L, Oliver C N, Fulks R M, Stadtman E R. Proc Natl Acad Sci USA. 1981;78:2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozaki A, Takeba G. Nature (London) 1996;384:557–560. [Google Scholar]
- 43.Tischner R, Schmidt A. Plant Physiol. 1982;70:113–116. doi: 10.1104/pp.70.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Florencio F J, Gadal P, Buchanan B B. Plant Physiol Biochem. 1993;31:649–655. [Google Scholar]
- 45.Lichter A, Häberlein I. J Plant Physiol. 1998;153:83–90. [Google Scholar]
- 46.Choi Y A, Kim S G, Kwon Y M. Plant Sci. 1999;149:175–182. [Google Scholar]
- 47.Gill H S, Eisenberg D. Biochemistry. 2001;40:1903–1912. doi: 10.1021/bi002438h. [DOI] [PubMed] [Google Scholar]
- 48.Almassy R J, Janson C A, Hamlin R, Xuong N-H, Eisenberg D. Nature (London) 1986;323:304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- 49.McParland R H, Guevara J G, Becker R R, Evans H J. Biochem J. 1976;153:597–606. doi: 10.1042/bj1530597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baier M, Dietz K-J. Plant J. 1997;12:179–190. doi: 10.1046/j.1365-313x.1997.12010179.x. [DOI] [PubMed] [Google Scholar]
- 51.Jeong W, Cha M-K, Kim I-H. J Biol Chem. 2000;275:2924–2930. doi: 10.1074/jbc.275.4.2924. [DOI] [PubMed] [Google Scholar]
- 52.Gasser C S, Gunning D A, Budelier K A, Brown S M. Proc Natl Acad Sci USA. 1990;87:9519–9523. doi: 10.1073/pnas.87.24.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breiman A, Fawcett T W, Ghirardi M L, Mattoo A K. J Biol Chem. 1992;267:21293–21296. [PubMed] [Google Scholar]
- 54.Dornan J, Page A P, Taylor P, Wu S-Y, Winter A D, Husi H, Walkinshaw M D. J Biol Chem. 1999;274:34877–34883. doi: 10.1074/jbc.274.49.34877. [DOI] [PubMed] [Google Scholar]
- 55.Lee S P, Hwang Y S, Kim Y J, Kwon K-S, Kim H J, Kim K, Chae H Z. J Biol Chem. 2001;276:29826–29832. doi: 10.1074/jbc.M101822200. [DOI] [PubMed] [Google Scholar]