Abstract
Introduction
Articular cartilage is made up of hyaline tissue embodying chondrocytes, which arise from mesenchymal stromal cells (MSCs) and specialized extracellular matrix. Despite possessing resident progenitors in and around the joint primed for chondrogenesis, cartilage has limited intrinsic capacity of repair and cell turnover. Advances in isolation, culture, and characterization of these progenitors have raised the possibility for their use in cell-based cartilage repair. Chondroprogenitors (CPCs) have been classified as MSCs and have been postulated to play a vital role in injury response and are identified by their colony forming ability, proliferative potential, telomere dynamics, multipotency, and expression of stem cell markers. The combined presence of CPCs and chondrocytes within the same tissue compartments and the ability of chondrocytes to dedifferentiate and acquire stemness during culture expansion has obscured our ability to define and provide clear-cut differences between these 2 cell populations.
Objective
This review aims to evaluate and summarize the available literature on CPCs in terms of their origin, growth kinetics, molecular characteristics, and differential and therapeutic potential with emphasis on their difference from daughter chondrocytes.
Design
For this systematic review, a comprehensive electronic search was performed on PubMed and Google Scholar using relevant terms such as chondrocytes, chondroprogenitors, and surface marker expression.
Results and Conclusion
Our comparative analysis shows that there is an ill-defined distinction between CPCs and chondrocytes with respect to their cell surface expression (MSC markers and CPC-specific markers) and differentiation potential. Accumulating evidence indicates that the 2 subpopulations may be distinguished based on their growth kinetics and chondrogenic marker.
Keywords: chondrocytes, chondroprogenitors, cartilage repair
Introduction
Articular cartilage is made up of hyaline tissue embodying chondrocytes, which arise from mesenchymal stromal cells (MSCs) and specialized extracellular matrix.1 The anatomical complexity of mature cartilage contributes to its specialized viscoelastic properties and durability in daily weight-bearing functions. The lack of blood vessels, nerves, and lymphatics and the relative inability of chondrocytes to easily migrate through their surrounding extracellular matrix renders any form of cartilage injury above a critical size (~3 mm) difficult to repair. Currently, treatment strategies directed toward cartilage repair and restoration have progressed from marrow stimulation to gene therapy techniques with varying success.2 In the field of cell-based therapeutics, autologous chondrocyte implantation (ACI) and marrow stromal cell therapies have been the mainstay options. Follow-up studies have shown terminal differentiation, hypertrophy, and calcification postimplantation among the varied complications following treatment.3
Recently, the focus is toward the identification, characterization, and therapeutic potential of articular cartilage–derived stem/progenitor cells.4 The characteristics of these multipotent progenitors include self-renewal, differential adhesion to fibronectin with high colony forming efficiency, and high replicative potential expressing MSC markers.4,5 Since cartilage-derived chondroprogenitors (CPCs) and chondrocytes share the same anatomical location, and chondrocytes have the ability to dedifferentiate and acquire stemness-like characteristics in cultures, the ability to define and show clear-cut differences between the 2 cell populations is difficult.6 The objective of this systematic review is to evaluate and summarize the available literature on CPCs in terms of their origin, molecular characteristics, and therapeutic potential with emphasis on their difference from dedifferentiated full-depth chondrocytes.
Methods
Information Sources and Search Strategy
An electronic search was performed on PubMed and Google Scholar with the following keywords: “chondroprogenitors,” “cartilage mesenchymal progenitor,” “resident progenitors,” “cartilage stem cells,” “clonal chondroprogenitors,” “chondrogenic potential,” “chondroprogenitor surface markers,” “chondrocyte surface markers,” “chondrocyte differentiation,” “chondrocyte redifferentiation.” The above-mentioned text words and medical subject headings (MeSH) were entered depending on the characteristics of the databases. The reference lists from the obtained articles were also screened for additional relevant articles. Only publications in English were considered.
Discussion
Summary of Evidence
Continuum: Origin, Development, Distribution, and Cell Profile of Chondroprogenitors
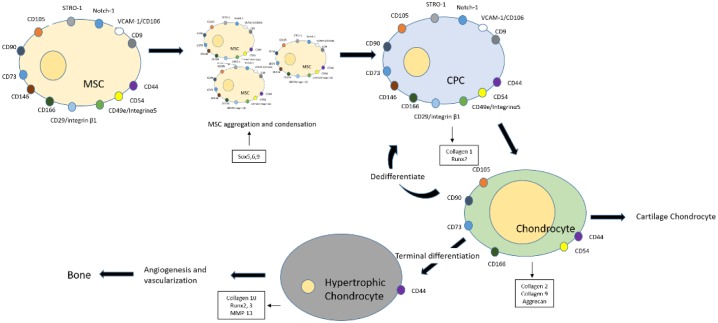
During embryonic development, chondrogenesis occurs in a coordinated manner, initiated by the condensation of prechondrogenic mesenchymal stem cells within the extracellular matrix of the limb buds.7 Condensed CPC cells undergo proliferation and aggregation in the presence of growth factors, cell adhesion, and intracellular signaling and eventually differentiate to form chondrocytes.8 Chondrocytes remain either as resting cells, which form the opposing cartilage, or proliferate to terminally differentiate, hypertrophy, and undergo endochondral ossification, which finally mineralizes to form bone7 ( Fig. 1 ). Lineage tracking studies have shown articular chondrocytes are matrilin-1 negative for gene and protein expression, whereas underlying epiphyseal chondrocytes are positive, indicating that bifurcation of cell fates is an early phenomenon probably regulated in part by expression of growth factor GDF5 in the developing joint.9
Figure 1.
Sequence of events during chondrogenesis. MSCs (mesenchymal stromal cells) undergo condensation and aggregation to form chondroprogenitors, which further differentiate to give rise to chondrocytes. The chondrocytes either remain as resting cells, which form the cartilage, or dedifferentiate to acquire stem cell–like potency or terminally differentiate to form hypertrophic chondrocytes. The hypertrophic cells undergo vascularization and endochondral ossification to form bone.
Developmental studies using bromodeoxyuridine to identify growth zones in fetal articular cartilage identified 2 populations of positive cells, short-term labeled cells located interstitially and a second group of label retaining cells at the surface.10 These and other studies provided circumstantial evidence for the presence of stem/progenitor cells in the articular surface driving appositional growth and led Dowthwaite et al. devise a method to isolate CPCs.4 This specialized population was isolated in fetal calves using differential adhesion to fibronectin and was shown to exhibit phenotypic plasticity and high colony forming efficiency with expression of cell fate selector gene Notch-1. Subsequently, Alsalameh et al. confirmed the presence of CD105 (TGFβ endoglin) and CD166 (ALCAM) positive MSC-like progenitor cells from normal human cartilage.11 The percentage expression was doubled in osteoarthritic cartilage (OAC), but less than half the population was capable of adipogenic differentiation. Fickert et al. also identified and isolated cartilage resident progenitor cells from human OAC that showed CD9 (tetra span), CD90 (Thy-1), and CD166 triple positive staining and multipotency.12 Based on the Hoescht 33342 dye exclusion, the presence of progenitors (0.07%) was shown not only at the superficial zone of bovine cartilage but also in full-thickness human cartilage (0.14%) though devoid of adipogenic differential potential.13,14 Grogan et al. showed mesenchymal progenitors cells positive for STRO-1, Notch-1, and VCAM more at the mid-zone than the superficial and deep-zone of OAC.13 Another potential source of progenitor cells called migratory chondrogenic progenitor cells exhibiting STRO-1 and CD29 positivity with enhanced chondrogenic potential relative to osteogenic and adipogenic lineages was identified.15 These progenitor cells were found to migrate from the bone marrow through breaks in tidemark in late-stage OA.16 Existence of progenitor cell population from rheumatoid arthritis and late-stage OA cartilage exhibiting migratory potential with varying stem cell marker profile have also been reported.17 Besides the articular surface, cell proliferative marker Bromodeoxyuridine (BrdU) positivity was also demonstrated at the perichondrial groove of Ranvier in mature rabbit knee joints.18,19 In contrast with the results of Dowthwaite et al., the label retaining cells did not express Notch-1, Stro-1, and N-cadherin as compared to the cells at the superficial zone.4 Mesenchymal progenitor cells with desirable bias toward chondrogenic lineage have also been reported from other non-cartilage sources such as synovium, meniscus, and infrapatellar fat pad.15,20
The Archer group further identified, characterized, and evaluated CPCs and their role in cartilage repair. Their work shows CPCs isolated by fibronectin adhesion conform to the minimal criteria for classification as MSCs and that these cells also exhibit characteristics of hypo-immunogenicity with positive immunosuppressive properties.20-22 Clonal monolayer cultures taken up to 30 population doublings (PDs) label by immunofluorescence for stem cell markers STRO-1, CD90, Notch signaling proteins (Notch-1, Delta-1, and Jagged-1), collagen-1 (CI), and markers of the chondrogenic phenotype (collagen II [CII], aggrecan IGD, sox-9).23,24 A preclinical caprine cartilage repair study using CPCs and full depth chondrocytes showed CII-positivity.22 The therapeutic superiority of CPCs to chondrocytes, specifically dedifferentiated cells, is further highlighted by their 2.6-fold greater telomerase activity, exponential growth, and sox-9 expression in extended culture expansion.23 Comparative studies between equine bone marrow (BM)-MSCs and CPCs showed that the latter demonstrate superior capabilities for cartilage repair as they lacked the expression of hypertrophic markers Runx2 and typeX collagen.25
Use of CPCs for cartilage repair seems logical as these mesenchymal progenitor cells are primed for chondrogenesis and can be used as an alternative or in addition to mature chondrocytes when repairing larger lesions.
Differences and Similarities between Chondroprogenitors and Chondrocytes: Growth Kinetics, Cell Surface Marking Profile, and Differentiation Potential
The debate is whether progenitor-like cells within cartilage are the only cells expressing phenotypic plasticity and stemness or can dedifferentiated chondrocytes also exhibit similar traits? Monolayer expanded full-depth chondrocyte cultures have been known to lose their differentiated phenotype, reacquire mesenchymal-like phenotype, and redifferentiate in the presence of chondrogenic stimuli.24 Knowledge on what distinguishes the 2 populations and development of a minimum set of standard criteria for defining and characterizing the cartilage progenitors is vital. In this review, we aim to combine the relevant existing knowledge on CPCs and chondrocytes and compare the similarities and differences.
Growth Kinetics: Colony forming efficiency (CFE), Population Doublings (PD), and Senescence Assay (β-Galactosidase)
The CPCs are isolated by incubating digested cartilage explant cells in fibronectin-coated plates and this is followed by transfer of the formed discrete colonies (>32 cells/colony) for further expansion. The CFE is calculated based on the initial seeding density and the number of cells that adhere to the fibronectin-coated wells. The CPCs with a CFE ranging from 0.04% to 0.09% require 8 to 14 days to achieve a colony of 32 cells, an equivalent to 5PD.25 CPCs show high replicative potential reaching 60PD in 200 days, whereas unsorted primary chondrocyte monolayer cultures require around 60 days to reach 9.5PD.26 CPCs also exhibit an initial exponential growth over 20PD followed by a slower linear growth with evidence of replicative senescence in later passages.23 CPCs at PD greater than 30 continue to express positivity for CD90, STRO-1, Notch-1, and its ligands.22 Dedifferentiated chondrocytes by fifth monolayer passage lose their chondrogenic phenotype and their ability to redifferentiate following growth factor stimulation.27 Furthermore, immature bovine CPCs when compared to nonclonal dedifferentiated chondrocytes show 2.6-fold greater telomerase activity and maintain higher average telomere lengths of chromosomes.23 Comparison of human dedifferentiated chondrocytes and CPCs also show a similar trend with distinct high-molecular-weight bands denoting the presence of a subpopulation of stem-like cells visible only in cultures of expanded CPCs and absent in unsorted and dedifferentiated full depth chondrocyte populations.22 In the latter study, human CPCs were also shown to have higher telomerase activity compared to dedifferentiated chondrocytes.
Chondroprogenitor Subpopulations
It is generally accepted that chondrocytes from OAC do not possess the same proliferative or differentiation capacity as cells from undiseased donors. A clear demonstration of the difference between the 2 cellular pools is the decline in average telomere length seen in osteoarthritic chondrocytes compared to undiseased counterparts. The same phenomenon is observed in monoclonal isolates of CPCs from healthy and OA donors, where there is an overall decline in average telomere length in OA populations, and this may be due to replicative exhaustion or the deleterious effect of free radicals generated through inflammatory episodes on telomere.28 CPCs in OAC have been further categorized into distinct subpopulations based on their proliferative and senescence profiles.29 High-resolution single telomere length analysis (STELA) shows that osteoarthritic CPCs exist as 2 subpopulations present at equal frequency and distinguishable based on telomere length and proliferative capacity. Thus, the unique growth kinetics between CPCs and chondrocytes as well as transcriptional changes could be used as phenotypic parameters to distinguish between these cell populations29 ( Table 1 ).
Table 1.
Proliferative and Senescence Potency of Equine Articular CPCs Cultured up to 100PD.30.
| Population Doubling (PD) | Days in Culture | Bromodeoxyuridine % Positivity (BrdU) | B-Galactosidase % |
|---|---|---|---|
| 22 | — | 96% | Negative |
| 44 | >50 | 88% | 15% |
| 75 | 120 | 79% | 11% |
Cell Surface Marking Profile
Classical MSC Markers: CD73, CD90, and CD105
CPCs isolated by fibronectin differential adhesion have been classified as MSC as they conform to the criteria set by the International Society of Cellular Therapy.21 They are positive (≥95%+) for CD105, CD73, and CD90 and negative (≤2%+) for hematopoietic stem cell markers CD34 and CD45, demonstrating multilineage potential via successful differentiation into chondrogenic, adipogenic, and osteogenic lineages (see Table 2 ).5 Besides bone, synovium-derived MSCs, and CPCs, migratory chondrogenic progenitors that can be isolated from OAC also are positive for classical MSC markers with the expression of CD73 higher than CD90 and CD105. Human articular chondrocytes(HAC) isolated by enzymatic digestion, 2 weeks in monolayer culture, showed upregulated expression of CD105 (95%) and CD90 (99.88%).31 Bernstein et al. also showed similar human BM-MSC and HAC have been shown to express greater levels of CD90 and lower levels of CD105.32
Table 2.
Summary of Potential Stem Cell Markers for Chondroprogenitors and Chondrocytes.
| Markers | Chondroprogenitors | Chondrocytes | IHC Cartilage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stem cell markers | CPC; articular cartilage derived progenitors isolated by fibronectin adhesion assay22 | Migratory progenitors from bone marrow with chondrogenic potential16 | Perichondrium chondroprogenitors18 | Based on FACS cell sorting/location and greater chondrogenic potential | Uncultured passage 026 | Monolayer 24 hours33 | Monolayer 2 weeks33 | Serial passages (P1-P6)22,31,32,34 | Zonal distribution |
| SZ/MZ/DZ4,13 | |||||||||
| CD73 | + | + | DU | DU | + | DU | DU | P3+ | DU |
| CD9013 | + | + | DU | + | + | 3.1% | 99.8% | P3+ | DU |
| CD90 low | |||||||||
| CD10532 | + | + | DU | − | + | 67.3% | 95% | P3 + | DU |
| P5—96.6% | |||||||||
| Stro-113,18,25 | + | 15% | + | − | DU | DU | DU | DU | + |
| SZ 81% | |||||||||
| MZ 52% | |||||||||
| DZ 38% | |||||||||
| Perichondrial groove of Ranvier | |||||||||
| Notch-14,13 | + | DU | BrdU label retaining cells | DU | DU | DU | DU | DU | + |
| − | SZ 86% | ||||||||
| MZ 10% | |||||||||
| DZ 34% | |||||||||
| VCAM-1/CD10613,35 | DU | DU | DU | DU | 56.6% | 95.3% | 62% | DU | + |
| SZ 84% | |||||||||
| MZ 41% | |||||||||
| DZ 34% | |||||||||
FACS expression values are expressed in mean %. − = negative expression; + = positive expression; DU = data unavailable (as far as our knowledge); P = passage; IHC = immunohistochemistry; SZ = superficial zone; MZ = mid-zone; DZ = deep-zone; BrdU = bromodeoxyuridine; PD = population doubling.
Stem Cell Markers: STRO-1, Notch1, and VCAM1 (CD106)
In normal cartilage almost half the cells are positive for STRO-1, Notch-1, and VCAM-1 antibody labeling, with labeling higher in the superficial zone,13 whereas in OAC, the expression is concentrated more at the midzone.4,13
STRO-1 is an MSC marker expressed by bone marrow stromal elements and endothelium.31 Immature bovine articular cartilage labels for STRO-1 positive cells in all zones, compared to mature cartilage explants, where expression is localized to the superficial zone.36 Perichondrial chondrogenic progenitors and CPCs displaying clonal behavior express positive STRO-1 immunolabeling in vitro.25 In FACS analysis of migratory chondrogenic progenitor only 15% of cells display positivity for STRO-1.16 Though no data on STRO-1 expression in isolated chondrocytes are available, experiments have shown monolayer expanded HAC cultures exhibit better chondrogenic potential in both scaffold and scaffold-free cultures as compared to STRO-1 immunoselected marrow stem cells.37 Notch1 is a known cell fate determination regulator in stem cells containing receptors for its ligands Delta4 and Jagged1.38 Dowthwaite et al. showed that 86 % of the cells at the superficial zone of calf cartilage expressed Notch1 as compared to 10% and 34% at mid-zone and deep-zone, respectively. These cells also showed progenitor features, namely, increased adhesion to fibronectin and high colony forming efficiency.4 Mature human cartilage shows restriction of Notch1 positivity to the superficial zone, but this is at odds with work in mice where the domain of Notch1 labeling expands throughout the depth of the cartilage with growth and development.13,39
HAC in early monolayer cultures showed 56% expression of the MSC marker VCAM-1, followed by its upregulation at about 2 weeks (95% expression). Downregulation was observed in extended cultures.31 No data on the expression of this stem cell marker in chondrogenic progenitors are available.
Chondrogenic and Osteogenic Markers: CollagenII, Sox9, CollagenI, and Runx2
Chondrogenic differentiation of MSCs is regulated by several signaling and transcription factors (see Table 3 ).40 SRY-related box transcription9 (Sox9) is the principal factor in mesenchymal condensation and differentiation toward the chondrogenic lineage.41,42 Sox9 specifically binds with Sox5 and Sox6 activating promoter elements to initiate the production of the major extracellular components of the cartilaginous matrix CII, collagen9, and aggrecan.43 CPCs and dedifferentiated chondrocytes (22PD) showed similar mRNA and protein expression of sox9. But freshly isolated chondrocytes showed greater sox9 expression as compared to CPCs.23 Migratory chondrogenic progenitor cells and clonal CPCs showed positive CI and sox9 expression with negative CII expression.27 Monolayer HAC cultures have been reported to express only CII in early cultures with a switch to CI around the third week, probably due to dedifferentiation. Downregulation of CII with the upregulation of CI represents the differentiation status of chondrocytes in monolayer cultures.6 Early CPC cultures thus seem to show a CI+/CII− expression, whereas early chondrocyte cultures a CII+/CI− expression with a switch to CI+/CII− with prolonged cultures.44 The CI–CII ratio between the CPCs and HAC isolated from the same cartilage subjected to similar culture conditions over specific time intervals could give us valuable information in discerning the 2 populations.
Table 3.
Summary of Chondrogenic and Osteogenic Markers for Chondroprogenitors and Chondrocytes.
| Markers | Chondroprogenitors | Chondrocytes | IHC Cartilage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Chondrogenic and osteogenic markers | CPC; articular cartilage derived progenitors isolated by fibronectin adhesion assay22 | Migratory progenitors from bone marrow with chondrogenic potential16 | Perichondrium chondroprogenitors18 | Based on FACS cell sorting/location and greater chondrogenic potential | Uncultured passage 026 | Monolayer 24 hours33 | Monolayer 2 weeks33 | Serial passages (P1-P6)22,31,32,34 | Zonal distribution |
| SZ/MZ/DZ4,13 | |||||||||
| Collagen I6,27,34 | + | + | DU | DU | − | − | + | P5—high | - |
| Third week | |||||||||
| Collagen II6,27,34 | - | - | DU | DU | + | + | − | P5—low | + |
| Third week | |||||||||
| Sox-923 | + | + | DU | DU | + | DU | DU | + | + |
| Up to 50PD | 22PD | SZ 68% | |||||||
| MZ 48% | |||||||||
| DZ 38% | |||||||||
| Runx216,45 | + | + | DU | − | P—unknown | OA cartilage | |||
| Osteogenic lineage | |||||||||
FACS expression values are expressed in mean %. − = negative expression; + = positive expression; DU = data unavailable (as far as our knowledge); P = passage; IHC = immunohistochemistry; SZ = superficial zone; MZ = mid-zone; DZ = deep-zone; BrdU = bromodeoxyuridine; PD = population doubling.
Runt-related transcription factor-2 (Runx2) has been described to be a major transcription factor for differentiation of MSCs to osteoblastic lineage.46 CPCs and migratory chondrogenic progenitor cells being MSCs with multipotency also showed positive Runx2 levels as compared to very low expression by chondrocytes.16 The upregulation of Sox9 and CII with knockdown of Runx2 has been postulated to play an important role in the enhancement of the chondrogenic potential of CPCs.44
Chondroprogenitor Surface Markers: CD9 (Tetraspan), CD29 (Integrin β1), CD44, CD49c (Integrin α3), CD49e (Integrin α5), CD54, CD146, CD151, and CD166
A recent review states that CD49e, CD166, CD90, and CD105 can be used to discern the 2 populations, namely, CPCs and HAC,47 since a large percentage of chondrocytes do not express the aforementioned markers (see Table 4 ). But the ability of chondrocytes to dedifferentiate and acquire stemness raises questions about the use of antibodies to these proteins as biomarkers for CPC subpopulations.
Table 4.
Summary of Potential Chondroprogenitor-Specific Markers.
| Markers | Chondroprogenitors | Chondrocytes | IHC cartilage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Chondroprogenitor specific markers | CPC; articular cartilage derived progenitors isolated by fibronectin adhesion assay22 | Migratory progenitors from bone marrow with chondrogenic potential16 | Perichondrium chondroprogenitors18 | Based on FACS cell sorting/location and greater chondrogenic potential | Uncultured passage 026 | Monolayer 24 hours33 | Monolayer 2 weeks33 | Serial passages (P1-P6)22,31,32,34 | Zonal distribution |
| SZ/MZ/DZ4,13 | |||||||||
| CD9 (tetraspan family)13 | DU | DU | DU | + | 5% | Monolayer culture; 18-fold increase | DU | DU | |
| CD29 (Integrin β1)48 | DU | + | DU | DU | + | P5—95.2% | + | ||
| Cartilage matrix | |||||||||
| CD49c (Integrin α3)31,48 | DU | + | DU | DU | DU | 66.5% | 84% | P1 to P6—2.4-fold upregulation | + |
| Cartilage matrix | |||||||||
| CD49e (Integrin α5)16,49 | P2—99% | + | DU | DU | +/− | 94.2% | 99.9% | P5—0.7% | DU |
| CD4450 | DU | + | DU | DU | + | 97.6% | 91.8% | P5—95.6% | Chondrocytes SZ |
| CD54 (ICAM-1)35 | DU | DU | DU | + | 98.8% | 88.5% | 78.4% | P1-P6—2.6-fold upregulation | + |
| All zones | |||||||||
| CD14651 | DU | DU | DU | + | − | − | DU | + | − |
| P2 ≃ 44% | |||||||||
| P5 ≃ 90% | |||||||||
| CD1515,31 | DU | DU | DU | DU | + | 94% | 84.6% | P1 to P6—maintained expression | DU |
| CD16622,33,52 | DU | DU | DU | + | + | 35.2% | 92.3% | P5—96.6% | + |
| SZ and MZ (coexpress CD105) | |||||||||
FACS expression values are expressed in mean %. − = negative expression; + = positive expression; DU = data unavailable (as far as our knowledge); P = passage; IHC = immunohistochemistry; SZ = superficial zone; MZ = mid-zone; DZ = deep-zone; BrdU = bromodeoxyuridine; PD = population doubling.
CD9
CD9 is a part of a family of cellular glycoproteins otherwise known as tetraspanins, which are known to interact with integrins and play a vital role in cell development, growth, and motility.53 CD9 has been reported as a marker for CPCs isolated from OAC.47 No data on its expression level by CPCs from normal cartilage exist. Dedifferentiated chondrocytes could have accounted for CD9 positive subpopulation in the latter study as the cultures used were unsorted chondrocytes, which showed 5% labeling, with a subsequent 18-fold increase following monolayer culture expansion.12 Other studies on HAC have also shown CD9+ levels in one third of the dedifferentiated population.32
Integrin markers
Integrins are membrane receptors involved in cell adhesion and recognition of a number of vital processes. CD29 (integrin β1) is the most abundant beta integrin expressed by stem cells, namely, migratory chondrogenic progenitor cells, and also by chondrocytes.16 CD105+ BM-MSCs with high CD29 expression show a greater chondrogenic differentiating capacity than low CD29 BM-MSCs.54 Chondrocytes harvested from full-depth cartilage show 95% expression of CD2922 by the fifth passage. Human MSCs have been reported positive for CD49c (integrin α3) and CD49e (integrin α5) with upregulation in continuous culture.27 Various reports show that CPCs, migratory chondrogenic progenitor cells, and chondrocytes express CD49c.16,31
CD49e encoded by the ITGA5 gene with integrin β1 forms a heterodimeric fibronectin receptor and plays an important role in extracellular mediated signaling. CD49e has been considered as a CPC-specific marker as a large percentage of chondrocytes do not express it.22,47 Uncultured rat chondrocytes were CD49e− and in culture showed upregulation followed by a downregulation with passages.55 Williams et al. showed a distinct subpopulation of cells (0.7%) within the full depth chondrocytes at fifth passage expressing CD49e. CPCs isolated by fibronectin adhesion assay showed 100% CD49e expression.22 On the contrary, chondrocytes isolated from tibial plateau and condyles showed high levels of CD49e at early cultures (P0).26 Monolayer HAC cultures showed high expression of CD49e at 24 hours with 99.9% expression at 2 weeks. Koelling et al. also showed migratory chondrogenic progenitor cells and HAC expressing high levels of CD49e.16 The role of CD49e as a definitive biomarker for CPCs seems to be inconclusive given its highly variable and context-dependent expression.
Other CPC markers
CD44 and CD54 (intracellular adhesion molecule-1) are both hyaluronan receptors and have been shown to modulate chondrocyte metabolism.56 CD44 plays an important role in hyaluronan endocytosis, cell migration, and proliferation. These receptors form the critical link between the chondrocyte cell surface and hyaluronan proteoglycan aggregates contributing to the highly hydrated nature of the cartilage.57,58 Early HAC cultures showed high expression of ICAM-1 (CD54).35 Gene and surface marker expression studies on HAC monolayer cultures over passages have shown upregulation of CD44 with downregulation of CD54 probably due to the spreading out of chondrocytes and increased cell-to-cell contact.6 The CII/CI ratio correlated well with CD54/CD44 ratio as the chondrocytes acquired a dedifferentiated phenotype. Similarly, CD44 expression has also been reported in migratory chondrogenic progenitor cells and CD54 in CPCs isolated from OAC.16,47
CD146, melanoma cell adhesion molecule (MCAM, muc18), is expressed by early mesenchymal lineage stem cells.59 In comparison with unsorted chondrocytes and adipose-derived MSCs, CD146+ FACS sorted chondrocytes showed efficient colony-forming capacity with greater chondrogenic potential.51 Similar results were shown in another study where immunostaining of the cartilage exhibited CII-rich matrices with nil CD146 expression. Monolayer cultures of chondrocytes over passages exhibited a gradual transition of CII+/CD146− cells becoming CII+/CD146+ and finally CII−/CD146+.60 It is suggested that the appearance and enrichment of CD146+ cells in culture is probably due to the ability of dedifferentiated chondrocytes to acquire a mesenchymal phenotype—termed a “reserve stem cell” pool—during dedifferentiation following monolayer expansion.
CD151 (tetraspanin family) is a membrane protein that forms functional complexes activating proenzyme of matrix-metalloproteinase 7, which leads to cartilage destruction.61,62 Reverse transcriptase–polymerase chain reaction analysis of CD151 mRNA showed overexpression in OAC, whereas in normal cartilage the expression was only 30%. HAC monolayer cultures showed upregulation of CD151 with better expression by chondrocytes with chondrogenic capacity.6,31,63
CD166 (activated leucocyte cell adhesion molecule), biomarker for mesenchymal progenitor cells within human cartilage, exhibited stronger chondrogenic potential.64 The superficial and middle zone of the cartilage showed exclusive CD166+ cells that coexpressed CD105.53 CD166+ enriched cells showed greater chondrogenic potential, thus has been suggested as a biomarker to identify and localize CPCs. Monolayer chondrocyte cultures have shown a 3-fold upregulation of CD166 as an index of their dedifferentiated status.6
Differentiation Potential
Chondroprogenitors isolated by the fibronectin assay have been classified as MSCs as they not only adhere to plastic and express the relevant surface markers but also demonstrate trilineage differentiation into adipogenic, osteogenic, and chondrogenic lineage.22,25 Migratory chondrogenic progenitors have also demonstrated multipotency when cultured in established differentiation conditions and additionally showed differentiation in 3D alginate cultures without chondrogenic supplementation.16 CD9/CD90/CD166 triple positive cells sorted by FACS from OAC showed trilineage multipotency characteristic of mesenchymal progenitor cells.12 CD146+ FACS sorted chondrocytes in comparison to unsorted chondrocytes and adipose-derived MSCs showed efficient colony-forming capacity with greater chondrogenic potential.51,60 Similarly, CD166+ immunomagnetic separated cells from OAC showed stronger expression of chondrogenic phenotype.52 Chondrocytes isolated from OAC also showed differentiation into adipogenic, osteogenic, and chondrogenic lineage comparable to MSCs, with chondrocytes showing higher chondrogenic potential than MSCs.37,65 Another comparative differentiation study between expanded clonal CPCs (CPCs > 30PD) and full-depth chondrocytes showed similar adipogenic profile but restricted osteogenic potential for CPCs.22 Both CPC and chondrocyte pellets post-chondrogenic differentiation showed smooth surface with positive staining for glycosaminoglycans and CII.25,37
Thus, CPCS, migratory chondrogenic progenitors, and sorted and unsorted chondrocytes all showed multipotency following directed differentiation. Analysis of chondrocytes behavior with expansion show that they not only undergo hypertrophy but also a population of cells dedifferentiate to acquire mesenchymal-like progenitor properties capable of redifferentiation with appropriate differentiation stimuli.24,33,66
Limitations of Existing Treatment Options Including the Use of Chondroprogenitors
Among the current cell-based therapeutics in the treatment of cartilage defects, ACI, stem cell transplantation, and marrow stromal studies are currently the mainstay treatment options. ACI besides being expensive and requiring multiple surgical interventions also has other drawbacks like limited availability, the requirement for extensive cell expansion, dedifferentiation, progressive loss of redifferentiation capacity, and expression of hypertrophic differentiation markers after implantation. On the other hand, although MSCs satisfy the need of a high cell yield, the disadvantage with MSCs are their reduced chondrogenic activity in advanced osteoarthritis.67 Current research on BM-MSCs for cartilage regeneration demonstrates extracellular matrix calcification and terminal differentiation in vitro on chondrogenic induction, ultimately resulting in failure of transplantation.67 A recent comparative study between equine BM-MSCs and CPCs showed that the latter have superior capability for cartilage repair as they lack expression of hypertrophic markers (Runx2 and collagenX).67,68
The ability of chondrocytes to dedifferentiate in culture and exhibit stem cell markers mandates the need to uncover a unique marker for CPCs. The lack of specific biomarkers for CPCs has hindered the identification and tracking of these cells in in vivo and in vitro.
Therapeutic Potential of Chondroprogenitors: Cartilage Injury, Osteoarthritis, and Animal Studies
In response to cartilage injury by enzymatic digestion, resident progenitor cells showed effortless migration to the site with healing of the cartilage.69 Similarly, migratory progenitor cells with chondrogenic potential have been identified in late-stage osteoarthritis and may contribute to repair mechanisms.16 Several studies from OAC show that progenitor cells with chondrogenic potential increase in frequency and play an important role in intrinsic repair of articular cartilage. The expression of CD105+/CD166+ cells were doubled in OAC as compared to normal cartilage.11 Similarly, STRO-1/Notch-1/VCAM-1 positive cell expression was increased at the mid-zone of OA cartilage.13 With regard to cartilage repair, an in vivo caprine study showed that CPC-seeded membrane integrated seamlessly with surrounding tissue. When examined the tissue showed positivity for CII hinting at repair.22 Autologous CPCs seeded on scaffold also showed significant results in treatment of focal cartilage defects.70
Whether CPCs exhibit phenotypic stability has been tested by injection intramuscularly into SCID mice. Even though cells stained positively for glycosaminoglycans, they failed to form a functional matrix at the ectopic site.71 In HAC studies, 2 of the 12 clonal cell lines at 31PD subjected for cytogenetic analysis showed an abnormal karyotype pattern, thus necessitating caution and need for karyotyping prior to clinical application.22
Limitation
In this systematic review, though we have taken measures to summate and present all the data available with reference to comparison of the 2 populations, some limitations were encountered. Our search strategy only covered articles that were published in English. Few publications were excluded as they were not referenceable and a few because they were yet unpublished. Since the discovery of CPCs has been quite recent, several gaps exist in the current literature and the amount of research done, thus limiting us in providing a complete picture. The terminology chondroprogenitors in the literature has also been used to label other cell populations residing around the joint, which exhibit chondrogenic potential, and this review also includes comparison of these cells with chondrocytes.
Conclusion
A large body of information indicates that stem cell-like progenitor cells with significant chondrogenic potential exist within and surrounding articular cartilage. These CPCs have been postulated to play a vital role in injury response and are identified by their colony forming ability, proliferative potential, telomere dynamics, multipotency, and expression of stem cell markers. However, full-depth chondrocytes dedifferentiated following monolayer culture expansion also demonstrate important elements of stem cell–like properties and potency. Our comparative analysis shows there is an ill-defined distinction between CPCs and chondrocytes with respect to their cell surface expression and differentiation potential. Accumulating evidence indicates that the 2 subpopulations may be distinguished based on their growth kinetics, CI, CII, and Runx2 expression. Additional studies are necessary to distinguish the CPCs from chondrocytes, ideally obtained from the same source subject to similar culture conditions to identify the most suitable combination of surface markers. Whether it is unsorted cartilage cultures exhibiting mesenchymal phenotype due to their reserve stem cell characteristics or CPCs having high proliferative potential outgrowing chondrocytes needs further probing using techniques to fluorescently label and track specific cell populations. Despite the opaque nature of our knowledge of cartilage-derived progenitor cell characteristics, CPCs show superiority over chondrocytes as a cell-type for cell-based cartilage repair. To expand the use of these mesenchymal progenitors that are primed for chondrogenesis, growth and development for regenerative medicine applications mandates further investigation to uncover more unequivocal biomarkers. These studies would allow us to follow the specific fates of cartilage-derived progenitor cells to an unprecedented degree, allowing lineage tracking during growth and development as well through injury and disease to give a better understanding.
Footnotes
Acknowledgments and Funding: The authors gratefully acknowledge Dr. Ilyas Khan, Swansea University Medical School, for reading and making valuable suggestions to this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Hu JC, Athanasiou KA. Structure and function of articular cartilage. In: An YH, Martin KL, eds. Handbook of histology methods for bone and cartilage. Berlin: Springer; 2003. p. 73-95. [Google Scholar]
- 2. Camp CL, Stuart MJ, Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014;6:265-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pietschmann MF, Niethammer TR, Horng A, Gulecyuz MF, Feist-Pagenstert I, Jansson V, et al. The incidence and clinical relevance of graft hypertrophy after matrix-based autologous chondrocyte implantation. Am J Sports Med. 2012;40:68-74. [DOI] [PubMed] [Google Scholar]
- 4. Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889-97. [DOI] [PubMed] [Google Scholar]
- 5. Archer CW, Williams R, Nelson L, Khan IM. Articular cartilage-derived stem cells: identification, characterisation and their role in spontaneous repair. Rheumatol Curr Res. 2012;S3:005. doi: 10.4172/2161-1149.S3-005. [DOI] [Google Scholar]
- 6. Hamada T, Sakai T, Hiraiwa H, Nakashima M, Ono Y, Mitsuyama H, et al. Surface markers and gene expression to characterize the differentiation of monolayer expanded human articular chondrocytes. Nagoya J Med Sci. 2013;75:101-11. [PMC free article] [PubMed] [Google Scholar]
- 7. Shimizu H, Yokoyama S, Asahara H. Growth and differentiation of the developing limb bud from the perspective of chondrogenesis. Dev Growth Differ. 2007;49:449-54. [DOI] [PubMed] [Google Scholar]
- 8. Karamboulas K, Dranse HJ, Underhill TM. Regulation of BMP-dependent chondrogenesis in early limb mesenchyme by TGFbeta signals. J Cell Sci. 2010;123:2068-76. [DOI] [PubMed] [Google Scholar]
- 9. Hyde G, Dover S, Aszodi A, Wallis GA, Boot-Handford RP. Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol. 2007;304:825-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl). 2001;203:469-79. [DOI] [PubMed] [Google Scholar]
- 11. Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522-32. [DOI] [PubMed] [Google Scholar]
- 12. Fickert S, Fiedler J, Brenner RE. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 2004;6:R422-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun. 2007;358:99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muhammad H, Schminke B, Bode C, Roth M, Albert J, von der Heyde S, et al. Human migratory meniscus progenitor cells are controlled via the TGF-beta pathway. Stem Cell Rep. 2014;3:789-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4:324-35. [DOI] [PubMed] [Google Scholar]
- 17. Schminke B, Miosge N. Cartilage repair in vivo: the role of migratory progenitor cells. Curr Rheumatol Rep. 2014;16:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karlsson C, Stenhamre H, Sandstedt J, Lindahl A. Neither Notch1 expression nor cellular size correlate with mesenchymal stem cell properties of adult articular chondrocytes. Cells Tissues Organs. 2008;187:275-85. [DOI] [PubMed] [Google Scholar]
- 19. Karlsson C, Thornemo M, Henriksson HB, Lindahl A. Identification of a stem cell niche in the zone of Ranvier within the knee joint. J Anat. 2009;215:355-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee SY, Nakagawa T, Reddi AH. Mesenchymal progenitor cells derived from synovium and infrapatellar fat pad as a source for superficial zone cartilage tissue engineering: analysis of superficial zone protein/lubricin expression. Tissue Eng Part A. 2010;16:317-25. [DOI] [PubMed] [Google Scholar]
- 21. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-7. [DOI] [PubMed] [Google Scholar]
- 22. Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5:e13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan IM, Bishop JC, Gilbert S, Archer CW. Clonal chondroprogenitors maintain telomerase activity and Sox9 expression during extended monolayer culture and retain chondrogenic potential. Osteoarthritis Cartilage. 2009;17:518-28. [DOI] [PubMed] [Google Scholar]
- 24. Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315-25. [DOI] [PubMed] [Google Scholar]
- 25. Nelson L, McCarthy HE, Fairclough J, Williams R, Archer CW. Evidence of a viable pool of stem cells within human osteoarthritic cartilage. Cartilage. 2014;5:203-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benz K, Stippich C, Freudigmann C, Mollenhauer JA, Aicher WK. Maintenance of “stem cell” features of cartilage cell sub-populations during in vitro propagation. J Transl Med. 2013;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, et al. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20:1170-8. [DOI] [PubMed] [Google Scholar]
- 28. Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B172-9. [DOI] [PubMed] [Google Scholar]
- 29. Fellows CR, Williams R, Davies IR, Gohil K, Baird DM, Fairclough J, et al. Characterisation of a divergent progenitor cell sub-populations in human osteoarthritic cartilage: the role of telomere erosion and replicative senescence. Sci Rep. 2017;7:41421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCarthy H, Archer C. Articular cartilage progenitor cells exhibit delayed senescence and retain their chondrogenic potential following extensive in vitro expansion. 2014. [Google Scholar]
- 31. Diaz-Romero J, Nesic D, Grogan SP, Heini P, Mainil-Varlet P. Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor cells. J Cell Physiol. 2008;214:75-83. [DOI] [PubMed] [Google Scholar]
- 32. Bernstein P, Sperling I, Corbeil D, Hempel U, Fickert S. Progenitor cells from cartilage—no osteoarthritis-grade-specific differences in stem cell marker expression. Biotechnol Prog. 2013;29:206-12. [DOI] [PubMed] [Google Scholar]
- 33. Lee HJ, Choi BH, Min BH, Park SR. Changes in surface markers of human mesenchymal stem cells during the chondrogenic differentiation and dedifferentiation processes in vitro. Arthritis Rheum. 2009;60:2325-32. [DOI] [PubMed] [Google Scholar]
- 34. Horton WA, Dwyer C, Goering R, Dean DC. Immunohistochemistry of types I and II collagen in undecalcified skeletal tissues. J Histochem Cytochem. 1983;31:417-25. [DOI] [PubMed] [Google Scholar]
- 35. Kienzle G, von Kempis J. Vascular cell adhesion molecule 1 (CD106) on primary human articular chondrocytes: functional regulation of expression by cytokines and comparison with intercellular adhesion molecule 1 (CD54) and very late activation antigen 2. Arthritis Rheum. 1998;41:1296-305. [DOI] [PubMed] [Google Scholar]
- 36. Otsuki S, Grogan SP, Miyaki S, Kinoshita M, Asahara H, Lotz MK. Tissue neogenesis and STRO-1 expression in immature and mature articular cartilage. J Orthop Res. 2010;28:96-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li S, Sengers BG, Oreffo RO, Tare RS. Chondrogenic potential of human articular chondrocytes and skeletal stem cells: a comparative study. J Biomater Appl. 2015;29:824-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427-30. [DOI] [PubMed] [Google Scholar]
- 39. Hayes AJ, Dowthwaite GP, Webster SV, Archer CW. The distribution of Notch receptors and their ligands during articular cartilage development. J Anat. 2003;202:495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang KG, Saris DB, Geuze RE, Helm YJ, Rijen MH, Verbout AJ, et al. Impact of expansion and redifferentiation conditions on chondrogenic capacity of cultured chondrocytes. Tissue Eng. 2006;12:2435-47. [DOI] [PubMed] [Google Scholar]
- 41. Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giuliani N, Lisignoli G, Magnani M, Racano C, Bolzoni M, Dalla Palma B, et al. New insights into osteogenic and chondrogenic differentiation of human bone marrow mesenchymal stem cells and their potential clinical applications for bone regeneration in pediatric orthopaedics. Stem Cells Int. 2013;2013:312501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muhammad H, Schminke B, Miosge N. Current concepts in stem cell therapy for articular cartilage repair. Expert Opin Biol Ther. 2013;13:541-8. [DOI] [PubMed] [Google Scholar]
- 45. Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12:963-73. [DOI] [PubMed] [Google Scholar]
- 46. Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol. 2010;658:43-9. [DOI] [PubMed] [Google Scholar]
- 47. Jayasuriya CT, Chen Q. Potential benefits and limitations of utilizing chondroprogenitors in cell-based cartilage therapy. Connect Tissue Res. 2015;56:265-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shakibaei M, Merker HJ. Beta1-integrins in the cartilage matrix. Cell Tissue Res. 1999;296:565-73. [DOI] [PubMed] [Google Scholar]
- 49. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ostergaard K, Salter DM, Andersen CB, Petersen J, Bendtzen K. CD44 expression is up-regulated in the deep zone of osteoarthritic cartilage from human femoral heads. Histopathology. 1997;31:451-9. [DOI] [PubMed] [Google Scholar]
- 51. Su X, Zuo W, Wu Z, Chen J, Wu N, Ma P, et al. CD146 as a new marker for an increased chondroprogenitor cell sub-population in the later stages of osteoarthritis. J Orthop Res. 2015;33:84-91. [DOI] [PubMed] [Google Scholar]
- 52. Pretzel D, Linss S, Rochler S, Endres M, Kaps C, Alsalameh S, et al. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther. 2011;13:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hadjiargyrou M, Kaprielian Z, Kato N, Patterson PH. Association of the tetraspan protein CD9 with integrins on the surface of S-16 Schwann cells. J Neurochem. 1996;67:2505-13. [DOI] [PubMed] [Google Scholar]
- 54. Cicione C, Diaz-Prado S, Muinos-Lopez E, Hermida-Gomez T, Blanco FJ. Molecular profile and cellular characterization of human bone marrow mesenchymal stem cells: donor influence on chondrogenesis. Differentiation. 2010;80:155-65. [DOI] [PubMed] [Google Scholar]
- 55. Tran A, Truong M, Choi B, Park S, Min B. Identification and characterization of novel stem/progenitor cells in rat adult articular cartilage. Osteoarthritis Cartilage. 2014;22:S442. [Google Scholar]
- 56. Knudson CB, Knudson W. Hyaluronan and CD44: modulators of chondrocyte metabolism. Clin Orthop Relat Res. 2004;(427 Suppl):S152-62. [PubMed] [Google Scholar]
- 57. Fox AJS, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cell Mater. 2006;12:92-101. [DOI] [PubMed] [Google Scholar]
- 59. Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23:934-43. [DOI] [PubMed] [Google Scholar]
- 60. Jiang Y, Cai Y, Zhang W, Yin Z, Hu C, Tong T, et al. Human cartilage-derived progenitor cells from committed chondrocytes for efficient cartilage repair and regeneration. Stem Cells Transl Med. 2016;5:733-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yanez-Mo M, Tejedor R, Rousselle P, Sanchez -Madrid F. Tetraspanins in intercellular adhesion of polarized epithelial cells: spatial and functional relationship to integrins and cadherins. J Cell Sci. 2001;114:577-87. [DOI] [PubMed] [Google Scholar]
- 62. Fujita Y, Shiomi T, Yanagimoto S, Matsumoto H, Toyama Y, Okada Y. Tetraspanin CD151 is expressed in osteoarthritic cartilage and is involved in pericellular activation of pro-matrix metalloproteinase 7 in osteoarthritic chondrocytes. Arthritis Rheum. 2006;54:3233-43. [DOI] [PubMed] [Google Scholar]
- 63. Grogan SP, Barbero A, Diaz-Romero J, Cleton-Jansen AM, Soeder S, Whiteside R, et al. Identification of markers to characterize and sort human articular chondrocytes with enhanced in vitro chondrogenic capacity. Arthritis Rheum. 2007;56:586-95. [DOI] [PubMed] [Google Scholar]
- 64. Swart GW. Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol. 2002;81:313-21. [DOI] [PubMed] [Google Scholar]
- 65. Oda T, Sakai T, Hiraiwa H, Hamada T, Ono Y, Nakashima M, et al. Osteoarthritis-derived chondrocytes are a potential source of multipotent progenitor cells for cartilage tissue engineering. Biochem Biophys Res Commun. 2016;479:469-75. [DOI] [PubMed] [Google Scholar]
- 66. van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223-32. [DOI] [PubMed] [Google Scholar]
- 67. Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704-13. [DOI] [PubMed] [Google Scholar]
- 68. McCarthy HE, Bara JJ, Brakspear K, Singhrao SK, Archer CW. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J. 2012;192:345-51. [DOI] [PubMed] [Google Scholar]
- 69. Seol D, Yu Y, Choe H, Jang K, Brouillette MJ, Zheng H, et al. Effect of short-term enzymatic treatment on cell migration and cartilage regeneration: in vitro organ culture of bovine articular cartilage. Tissue Eng Part A. 2014;20:1807-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Frisbie DD, McCarthy HE, Archer CW, Barrett MF, McIlwraith CW. Evaluation of articular cartilage progenitor cells for the repair of articular defects in an equine model. J Bone Joint Surg Am. 2015;97:484-93. [DOI] [PubMed] [Google Scholar]
- 71. Marcus P, De Bari C, Dell’Accio F, Archer CW. Articular chondroprogenitor cells maintain chondrogenic potential but fail to form a functional matrix when implanted into muscles of SCID mice. Cartilage. 2014;5:231-40. [DOI] [PMC free article] [PubMed] [Google Scholar]