Abstract
17β-estradiol can rapidly modulate neuron function via membrane estrogen receptors (ERs) in a sex-specific manner. For example, female rat hippocampal neurons express palmitoylated versions of ERα and ERβ that associate with the plasma membrane. These membrane-associated ERs are organized by caveolin proteins into functional signaling microdomains with metabotropic glutamate receptors (mGluRs). ER/mGluR signaling mediates several sex-specific estradiol actions on hippocampal neuron function. An important unanswered question regards the mechanism by which sex-specific membrane-associated ER signaling is generated, especially since it has been previously demonstrated that mGluR action is not sex-specific. One possibility is that the genes necessary for the ER membrane complex are differentially expressed between males and females, including genes that encode ERα and β, caveolin 1 and 3, and/or the palmitoylacyltransferases DHHC-7 and -21. Thus we used qPCR to test the hypothesis that these genes show sex differences in expression in neonatal and adult rat hippocampus. As an additional control we tested the expression of the 20 other DHHC palmitoylacyltransferases with no known connections to ER. In neonatal hippocampus, no sex differences were detected in gene expression. In adult hippocampus, the genes that encode caveolin 1 and DHHC-7 showed decreased expression in females compared to males. Thus, select genes differ by sex at specific developmental stages, arguing for a more nuanced model than simple widespread perinatal emergence of sex differences in all genes enabling sex-specific estradiol action. These findings enable the generation of new hypotheses regarding the mechanisms by which sex differences in membrane-associated ER signaling are programmed.
Keywords: Caveolin, DHHC, palmitoylation, estrogen receptor, sex, hippocampus
1. Introduction
17β-estradiol (estradiol) is a potent modulator of neuron function across a broad temporal and contextual spectrum. At one end of the temporal spectrum are relatively slow, nuclear-initiated actions on gene expression. These typically occur via estrogen receptor (ER) dimerization, include concurrent interaction with nuclear transcription factors and co-activators, and then the ER complex binds to DNA, usually but not exclusively at estrogen response elements (EREs) [1]. These changes can be permanent. At the other end of the spectrum is rapid modulation of neuron function via membrane-initiated actions. More than forty years ago it was demonstrated that acute estradiol application changed the electrophysiological properties of preoptic/septal neurons within seconds [2]. This finding built upon the pioneering work of Szego and colleagues, who demonstrated that 17β-estradiol action outside of the nervous system can occur within seconds [3]. Since these seminal findings, work from many laboratories has shown that in a wide variety of organisms and neuron types that estradiol can rapidly modulate many aspects of neuron function, including but not limited to intrinsic and synaptic electrophysiological properties, intracellular signaling molecule initiation, non-ERE dependent changes in gene transcription, and anatomical properties [4–11].
The known receptors that enable rapid estradiol action include membrane-associated versions of ERα and ERβ, G-protein coupled receptors such as GPER-1, Gq-mER, and others [12–17]. Here we focus on membrane-associated ERα and ERβ, which are classical ERs that have received posttranscriptional palmitoylation by the palmitoylacyltransferase proteins DHHC-7 and DHHC-21 [18, 19]. Membrane-associated ERα and ERβ may exist in several splice variants [17, 20, 21], and in this study ERα and ERβ refer to all known variants due to primer design. Membrane-associated ERα and ERβ are typically coupled with metabotropic glutamate receptors (mGluRs) by caveolins throughout the nervous system, including the hippocampus [22–38]. This relationship is schematized in [12]. Given this widespread expression, it is not surprising that membrane-associated ER act through mGluRs to modulate multiple behaviors, ranging from cognitive tasks such as hippocampal-dependent memory consolidation to sex-specific behaviors such as lordosis [26, 27, 32, 33, 39–41].
Regarding the rat hippocampus, pyramidal neurons exhibit both membrane-associated ERα and ERβ. These ER can be coupled to either mGluR1a or mGluR2 via the organizing actions of caveolins 1 or 3 [18, 22–25]. These pathways mediate several estradiol actions that differ in incidence or mechanism by sex, including estradiol modulation of cAMP response element-binding protein (CREB) phosphorylation in vitro [18, 22–24], suppression of inhibitory synaptic transmission in vivo [25, 42], and hippocampal-dependent memory consolidation [39]. The basic underlying signaling pathways have been elucidated. There are at least two known pathways. In the first pathway, caveolin 1 couples membrane-associated ERα to mGluR1a and resulting second messenger cascade. This pathway is linked to inhibitory synaptic regulation, hippocampal-dependent memory consolidation, and how estradiol exposure alone phosphorylates the transcription factor CREB in hippocampal neurons. In this second pathway, caveolin 3 couples membrane-associated ERα and ERβ to mGluR2 and associated molecules. In hippocampal neurons, the second signaling pathway mediates how pre-exposure to estradiol attenuates CREB phosphorylation induced by the depolarizing action of 20 mM K+.
Since the functionality of these pathways differs between female and male hippocampal neurons, this suggests the possibility that the mechanism responsible for programming these sex differences is the regulation of the expression of genes that encode the necessary signaling components. We systematically tested this hypothesis in neonatal (P8) and adult (P70) male and female rat hippocampus using qPCR. P8 was chosen given that this age occurs after the organizing influences of perinatal hormone action, a process sufficient to induce sex differences in estradiol-induced signaling to CREB [24]. P70 was chosen given that this date is past puberty and similar in age to the relevant investigations of sex-specific estradiol modulation of hippocampal neurons [25, 39, 42–46]. Previous experiments have already demonstrated that mGluR1a and mGluR2 action do not differ by sex or palmitolyation state [18, 22, 24], and that mGluR1 is not palmitoylated [47, 48]. Thus this study focused on genes associated with ER signaling. These genes include those that encode all known slice variants of membrane-associated ERα and ERβ, caveolin 1 and caveolin 3, and then DHHC-7 and DHHC-21. As a control, this study also examined the other 20 different DHHC palmitoylacyltransferases with no known connection to ER signaling.
2. Experimental Methods
2.1 Animals
All protocols were approved by the Animal Care and Use Committee at the University of Minnesota. Sprague-Dawley rats were born in the Mermelstein laboratory colony from dams purchased from Harlan Laboratories. Animals were housed in a room maintained at 20°C to 21°C, with a 12-hour light, 12-hour dark cycle and water available ad libitum. Animals were group housed with their dam until postnatal day 22 (P22). After P22 animals were group housed by sex. Multiple litters were used. Female estrous cycle was not monitored. Male and female animals were killed at P8 (5 males, 5 females were used in experiments regarding ERs and caveolins; 7 males, 7 females were used in experiments regarding DHHC-3; 4 males, 4 females were used in experiments regarding DHHC1-2, 4-23) and P70 (5 males, 5 females were used in experiments regarding ERs, caveolins, and DHHC-7; 7 males, 7 females were used in experiments regarding DHHC-21). Differing numbers of animals were used in experiments because an insufficient quantity of mRNA was extracted from a single animal to robustly analyze all target genes. Animals were anesthetized using isoflurane and decapitated. The brain was rapidly removed, blocked, and the hippocampus dissected from the caudal portion of the brain, following previously published techniques [18]. All dissections were made in ice-cold modified Hank’s balanced salt solution (HBSS) containing (in mM) 4.2 NaHCO3 and 1 HEPES (pH 7.35, 300 mOsm). After removal from the brain, the hippocampus was gently unrolled, and the dentate gyrus was removed. The remaining portion of the hippocampus was sliced into small pieces (≤ 0.5 cm in all dimensions). Tissue was immediately submerged in RNAlater (ThermoFisher Scientific), following the manufacturer’s recommendation of approximately 10 μl of RNAlater per 1 mg tissue. Tissue was stored at 4°C overnight and then frozen at −20°C until mRNA extraction.
2.2 PCR
Quantitative PCR (qPCR) was performed using previously published protocols [49]. mRNA was extracted and reverse transcribed from tissue using standard kits and following the manufacturer’s instructions for purification of RNA from animal tissues (RNAeasy Mini or Midi Kit; QauntiTect Reverse Transcription Kit; Qiagen, Valencia, CA, USA). Tissue was disrupted and homoegenized using a rotor-stator homogenizer. Residual DNA was removed via a gDNA Eliminator spin column and further DNAse digestion after RNA purification. qPCR amplification was performed using LightCycler 480 SYBR Green I Master Mix (Roche) on a LightCycler 480 II PCR machine (Roche). Threshold values were calculated using the Second Derivative Maximum method and standardized to the ribosome-related genes rpl13a and rps18 (LightCycler 480 Software 1.5, Roche). PCR for individual cDNA samples was performed in triplicate, and overall experiments were repeated at least twice. The thermal cycling program used was: a pre-incubation step at 95°C for 5 min, followed by at least 45 cycles consisting of a 10 s denaturing step at 95°C, annealing step for 10 s at 60°C, an extension step for 10 s at 72°C, and a measurement of fluorescent intensity. At the end of each cycling program, a melting curve was run. Upper and lower primer sequences were either developed for this study or previously published [18, 50] (Table 1). We note that the primers employed for ERα and ERβ were designed to detect all known splice variants.
Table 1.
Primer sequences.
| Gene Name | GenBank Accession Number | Upper and Lower Primer Sequences |
|---|---|---|
| cav1 (caveolin 1) | NM_031556 | 5′-GCAGTTGTACCGTGCATCAAGAG-3′ and 5′-CGGATATTGCTGAATATCTTGCC-3′ |
| cav3 (caveolin 3) | NM_019155.2 | 5′-TGGAGGCACGGATCATCAAG -3′ and 5′-ACACGCCATCGAAGCTGTAA -3′; |
| esr1 (estrogen receptor α) | NM_012689.1 | 5′-TTTCTTTAAGAGAAGCATTCAAGGA-3′ and 5′-TTATCGATGGTGCATTGGTTT -3′ |
| esr2 (estrogen receptor β) | NM_012754.1 | 5′-ATGTACCCCTTGGCTTCTGC -3′ and 5′-ACTGCTGCTGGGAGGAGATA-3′ |
| rpl13a (ribosomal protein L13a) | NM_173340 | 5′-TGCTGCCGCACAAGACCAAA-3′ and 5′-AACTTTCTGGTAGGCTTCAGCCGC-3′ |
| rps18 (ribosomal protein S18) | NM_213557.1 | 5′-AAAATCCGAGCCCATAGAGG-3′ and 5′-TCTTCTTGGACACACCCACA-3′ |
| zhhdc1 (DHHC-1) | NM_001039099.1 | 5′-GCAGCAAGCCTTAGGATGAT -3′ and 5′-TCAGGGCCAGGATGACAG -3′; |
| zhhdc2 (DHHC-2) | NM_145096.2 | 5′-GCCACCTCCTTACGGATTCT-3′ and 5′-GCAGGGTTGCTCATACCG-3′ |
| zhhdc3 (DHHC-3) | AY886522.1 | 5′-TGCTTTGAAGAAGACTGGACAA-3′ and 5′-AAGAGCAGGGCCTCAAAAC-3′ |
| zhhdc4 (DHHC-4) | NM_001013123.1 | 5′-CATCAGCTCTTCCACACACG-3′ and 5′-TGTATTCCGCGTAAACTAGCC-3′ |
| zhhdc5 (DHHC-5) | NM_001039338.1 | 5′-TACACAGGGCTTCGAACACA-3′ and 5′-TGCCCAAGAGACTGCTATCC-3′ |
| zhhdc6 (DHHC-6) | NM_001037652.1 | 5′-GAACCATGCGTCCTTCACA-3′ and 5′-AAAGCAGCATGGGTGCAG-3′ |
| zdhhc7 (DHHC-7) | NM_133394.1 | 5′-CAATATGCAATGACGAAACTGAG-3′ and 5′-GAAGACAGCTTCATCCCTTCC-3′ |
| zhhdc8 (DHHC-8) | AY871204.1 | 5′-CCAGCACCCTCTTCTTCGTA-3′ and 5′-GAGGATGCCATTGTAGACAGG-3′ |
| zhhdc9 (DHHC-9) | NM_001039016.2 | 5′-ACACTCTTCTTTGCCTTCGAGT-3′ and 5′-AGCAGCAAACACAGGGATG-3′ |
| zdhhc11 (DHHC-11) | NM_001039342.2 | 5′-AACAACTTGACTTGGCCTACG -3′ and 5′-TGGCGAAAGAGTAGACAGCA -3′ |
| zhhdc12 (DHHC-12) | NM_001013239.1 | 5′-CTGACCTGGGGAATCACG-3′ and 5′-CTTGCTCTTCCCATTGACG-3′ |
| zhhdc13 (DHHC-13) | NM_001039037.1 | 5′-CTGGGCCATCCGACAAGGGC -3′ and 5′-CAGAGTGGGGTCTGCACCATGC -3′ |
| zhhdc14 (DHHC-14) | NM_001039343.1 | 5′-CCGGCAGACCGGCGTTTTCT -3′ and 5′-CAGGATGCCACCGACCACGG -3′ |
| zhhdc15 (DHHC-15) | NM_001039101.1 | 5′-CGCCGGGTACTGTCCTGGGT -3′ and 5′-GGTTGGGCTGCTGTGGGAGTG -3′ |
| zhhdc16 (DHHC-16) | NM_001039346.1 | 5′-CTACCGGCGTCGATGCCCAC -3′ and 5′-GAGCAGGGAGCGCAGGCAAA -3′ |
| zhhdc17 (DHHC-17) | NM_001039340.1 | 5′-ACCGAAACGGGCTGTGTGCC -3′ and 5′-TCCGCCCAAGAGGCTCACCAT -3′ |
| zhhdc18 (DHHC-18) | NM_001039339.1 | 5′-AGCCTGATCGACCGGAGGGG -3′ and 5′-CTGGCGTCTGGCTTGGCTCC -3′ |
| zhhdc19 (DHHC-19) | NM_001039259.1 | 5′-CCTAATTCACACGAGCCATCT -3′ and 5′-GGAAGAGTGGAATCAGGAAGC -3′ |
| zhhdc20 (DHHC-20) | NM_001039336.1 | 5′-GCGTAGTGGGCTGGGTTCCG -3′ and 5′-CACGCACAGCTCCACCACGTA -3′ |
| zhhdc21 (DHHC-21) | AY886536.1 | 5′-GATGGGAGCGCTTCGGCCTC -3′ and 5′-CCACATGCAGAGCGGGAGCTG-3′ |
| zhhdc22 (DHHC-22) | NM_001039325.1 | 5′-GATCAGGGTTGCGTCTGG -3′ and 5′-GCCAGCATCCTCGATTACAT -3′ |
| zhhdc23 (DHHC-23) | NM_213627.2 | 5′-TCGGCCGGAGACGTGTGAGA -3′ and 5′-AAGCCACGCGGAGCAGAACC -3′ |
There is no DHHC-10. Abbreviations: Domain-Containing Cysteine-Rich (DHHC)
2.3 Statistics
Statistical analysis followed previously published methods [51]. Briefly, data were analyzed with 2-tailed Mann-Whitney U tests (Prism 6.07; GraphPad Software). Probability values ≤ 0.05 were considered a priori significant. Data are presented as mean ± SEM.
3. Results
3.1 Estrogen receptor expression does not vary by sex in rat hippocampus
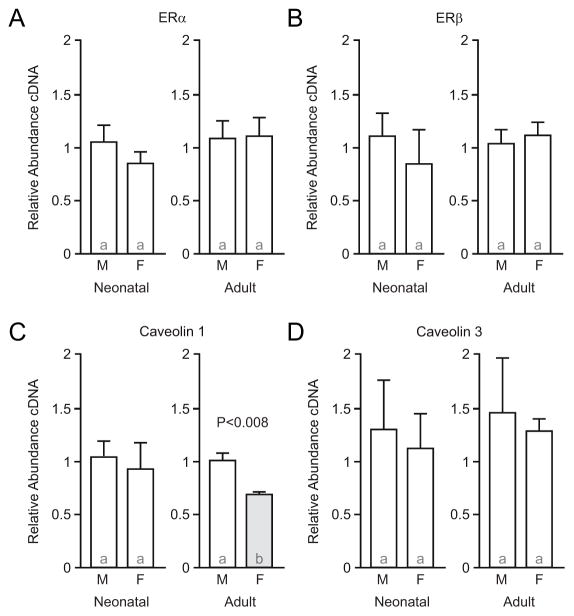
In the first experiment, we tested if ERα and ERβ show differential expression by sex in the hippocampus. Membrane-associated ERα and ERβ are encoded by the genes esr1 and esr2, respectively. These are the same genes that also encode for nuclear-expressed ERα and ERβ. We found no evidence that the expression of esr1, the gene that encodes membrane-associated ERα, differed by sex in either neonatal or adult hippocampus (Figure 1A; neonatal: U=7, P=0.31; adult: U=11, P=0.84). Similarly, esr2, the gene that encodes membrane-associated ERβ, did not differ by sex (Figure 1B; neonatal: U=7, P=0.56; adult: U=8, P=0.73). These results support the conclusion that overall gene expression of ERα and ERβ do not differ by sex in rat hippocampus. This finding serves as an important control as it verifies the results of previous studies from other laboratories that analyzed ER mRNA, protein, and immunocytochemical expression [52–55], and helps interpret how measurements at the foundational level of gene expression relate to those made across transcription.
Figure 1.
Caveolin 1 expression is decreased in adult female compared to male hippocampus, with no sex differences detected in estrogen receptor α, β, or caveolin 3 expression. A, qPCR analysis of estrogen receptor α (ERα) expression in neonatal (P8) and adult (P60) male (M) and female (F) rat hippocampus. B, estrogen receptor β (ERβ). C, caveolin 1. D, caveolin 3. Bar color and letters indicate statistically significantly different groups.
3.2 Caveolin 1 expression is decreased in adult female compared to male hippocampus
Caveolins are necessary for coupling membrane-associated ERα and ERβ to specific mGluRs [23]. The next experiments tested whether caveolin 1 and caveolin 3 expression differed by sex. Expression of cav1, which encodes caveolin 1, did not differ by sex in neonatal hippocampus (Figure 1C; U=11, P=0.84). In adult hippocampus, expression of cav1 was decreased in females compared to males (Figure 1C; U=0, P=0.0079). Expression of cav3, which encodes caveolin 3, did not differ by sex at either developmental time point (Figure 1D; neonatal: U=10, P>0.99; adult: U=12, P>0.99). These data indicate that expression of caveolin 1, but not caveolin 3, may be developmentally regulated in a sex-specific manner.
3.3 DHHC-7 expression is decreased in adult female compared to male hippocampus
Membrane-associated ERα and ERβ must be palmitoylated in order to properly signal. The palmitoylacyltransferase proteins DHHC-7 and DHHC-21 are necessary for ER palmitoylation and membrane signaling in hippocampal neurons and cancer cells [18, 19]. In neonatal hippocampus, no sex differences were detected in the expression of any gene encoding a known DHHC palmitoylacyltransferase (Table 2), regardless of whether the DHHC is known to be linked to ER signaling. This finding includes the genes that encode DHHC-7 and DHHC-21, zdhhc7 and zdhhc21, respectfully (Figure 2). In adult hippocampus, we targeted the genes that encode DHHC-7 and DHHC-21. Expression of zdhhc7, which encodes DHHC-7, was decreased in adult females compared to males (Figure 2A; U=2, P=0.0317). Expression of zdhhc21, which encodes DHHC-21, did not differ by sex (Figure 2B; U=23.5, P=0.92). As a control, we also measured expression of the gene that encodes DHHC-11, which has no known connection to ER palmitoylation in either neuronal or nonneuronal cells [18, 19]. Expression of zdhhc11, which encodes DHHC-11, did not differ by sex in adult hippocampus (Male: 1.10 ± 0.22, Female: 1.12 ± 0.24; U=11.5, P=0.89). This data indicate that the expression of DHHC-7, but not DHHC-21, may be developmentally regulated in a sex-specific manner, similar to caveolin 1 expression.
Table 2.
DHHC expression in neonatal male and female rat hippocampus.
| DHHC Name | Expression | Statistics (U, P) |
|---|---|---|
| DHHC-1 | M: 1.01 ± 0.05; F: 0.79 ± 0.13 | 4, 0.34 |
| DHHC-2 | M: 1.02 ± 0.11; F: 0.93 ± 0.07 | 6, 0.60 |
| DHHC-3 | M: 1.02 ± 0.08; F: 1.12 ± 0.15 | 21, 0.71 |
| DHHC-4 | M: 1.01 ± 0.07; F: 0.90 ± 0.05 | 4, 0.34 |
| DHHC-5 | M: 1.01 ± 0.06; F: 1.22 ± 0.16 | 5, 0.49 |
| DHHC-6 | M: 1.04 ± 0.16; F: 1.06 ± 0.03 | 4, 0.34 |
| DHHC-7 | M: 1.02 ± 0.11; F: 0.93 ± 0.07 | 6, 0.60 |
| DHHC-8 | M: 1.01 ± 0.07; F: 1.01 ± 0.16 | 7, 0.89 |
| DHHC-9 | M: 1.01 ± 0.08; F: 0.97 ± 0.10 | 8, 0.99 |
| DHHC-11 | M: 1.01 ± 0.10; F: 1.32 ± 0.11 | 2, 0.11 |
| DHHC-12 | M: 1.03 ± 0.14; F: 1.27 ± 0.11 | 2, 0.11 |
| DHHC-13 | M: 1.01 ± 0.05; F: 0.83 ± 0.12 | 4, 0.34 |
| DHHC-14 | M: 1.01 ± 0.09; F: 1.11 ± 0.16 | 7, 0.89 |
| DHHC-15 | M: 1.00 ± 0.04; F: 1.13 ± 0.05 | 3, 0.14 |
| DHHC-16 | M: 1.00 ± 0.05; F: 0.71 ± 0.13 | 3, 0.20 |
| DHHC-17 | M: 1.02 ± 0.10; F: 1.25 ± 0.19 | 4, 0.34 |
| DHHC-18 | M: 1.01 ± 0.10; F: 1.15 ± 0.13 | 5, 0.49 |
| DHHC-19 | M: 1.09 ± 0.21; F: 0.89 ± 0.30 | 6, 0.69 |
| DHHC-20 | M: 1.01 ± 0.06; F: 1.21 ± 0.14 | 4, 0.34 |
| DHHC-21 | M: 1.05 ± 0.17; F: 0.87 ± 0.19 | 5, 0.49 |
| DHHC-22 | M: 1.03 ± 0.14; F: 0.78 ± 0.07 | 3, 0.20 |
| DHHC-23 | M: 1.10 ± 0.27; F: 0.68 ± 0.18 | 4, 0.34 |
No significant differences were detected between neonatal males and females. Values are mean ± SEM. Values are relative gene expression normalized to males, and are unitless. Abbreviations: M, male; F, female; DHHC, Domain-Containing Cysteine-Rich
Figure 2.
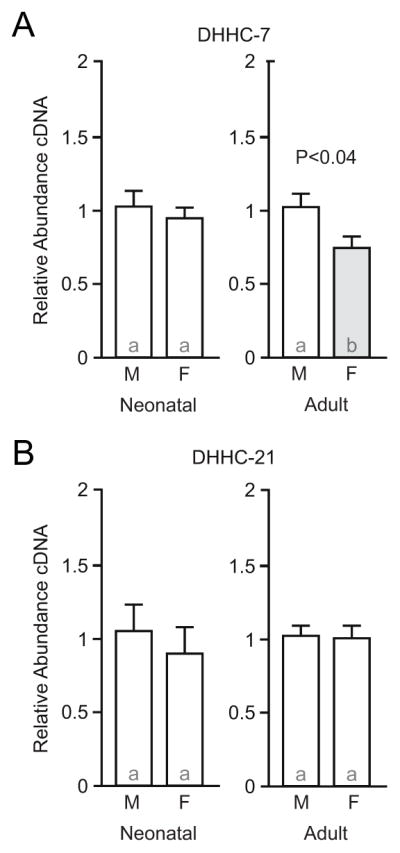
DHHC-7 expression is decreased in adult female compared to male hippocampus, with no sex differences detected in DHHC-21 expression. A, qPCR analysis of DHHC-7 expression in neonatal (P8) and adult (P60) male (M) and female (F) rat hippocampus. B, DHHC-21. Bar color and letters indicate statistically significantly different groups. Measurements of other DHHC genes are found in Table 2.
4. Discussion
Here we tested the hypothesis that the expression of genes necessary for membrane-associated ER signaling complexes differ by sex in the rat hippocampus. There are three principle findings of this study. First, no sex differences were detected in the expression of any gene in neonatal hippocampus. Second, the genes that encode caveolin 1 and DHHC-7 were decreased in adult hippocampus. Third, the other genes analyzed, including those that encode for all known DHHCs, did not show sex differences in expression in adult hippocampus. This study demonstrates that caveolin 1 and DHHC-7 represent a promising route for future experiments targeting the mechanisms underlying sex specific membrane-associated ER signaling.
A priori, there were three possible broad outcomes for this experiment. The first possible outcome was that all the genes necessary for membrane-associated ER signaling showed sex differences in expression. This result was unlikely given the pleotropic actions of membrane-associated ER on hippocampal neurons, and the lack of detected sex differences in autoradiographic measurements of estradiol binding, ERα and ERβ mRNA, protein, and immunocytochemical expression in rat hippocampus [52–56]. This demonstrated consistency in ER expression across translation, along with both our and other laboratory’s previous studies showing strong relationships between ERα, caveolin 1 and 3, and DHHC-22 mRNA and protein expression [18, 19, 23], validates the approach of this study to assess sex differences at the foundational level of gene expression. Nevertheless, it is important to acknowledge an important limitation of this study: that change in mRNA expression does not necessarily directly translate to changes in protein expression, or once created, that a protein is trafficked to the plasma membrane versus other destinations within the neuron. Indeed, ERα and ERβ must be modified post-transcription in order to be trafficked to the membrane, so mRNA measurements of esr1 and esr2 assess both the nuclear and membrane versions of ERα and ERβ. We also did not measure the expression of gper1, which encodes G-Protein Coupled Estrogen Receptor 1, a membrane estrogen receptor distinct from ERα and ERβ [16, 57]. Future experiments should directly assess sex differences in ERα, ERβ, and GPER-1 availability at the plasma membrane, as it is possible that this may be part of the mechanism generating sex-specific estradiol signaling. Other limitations which should also be considered when interpreting these data include that the dissection employed does not distinguish between different cell types or the various hippocampal regions (other than the dentate gyrus, which was removed), and that the estrous cycle in females was not monitored.
The second possible broad outcome of this experiment was that none of the assessed genes showed sex differences in expression. A priori, we found this outcome unlikely, given the robust sex differences and estradiol-sensitivity displayed by the hippocampus and its component cells in many metrics, including gene expression in a number of different contexts [12, 43, 44, 58–63]. This sensitivity to gonadal sex is not limited to the hippocampus. Sex differences in gene expression are widespread across the brain, even in regions not directly involved with sex specific behaviors such as reproduction [64, 65].
This leads us to the third broad possible outcome, that select genes showed sex differences in expression. A priori, we considered this outcome to be the most likely and potentially insightful. For example, if only genes that encode caveolins showed a sex difference, then that would indicate that regulation of caveolin expression played a significant role in generate sex-specific ER signaling. Another possibility was that genes linked to a specific membrane-associated ER pathway showed sex differences in expression. Indeed, this is the principal finding of this study. Both caveolin 1 and DHHC-7 showed sex differences in expression in adult hippocampus. It is highly significant that both caveolin 1 and DHHC-7 showed sex differences in expression. Caveolin 1 couples membrane-associated ERα to mGluR1a and the resulting second messenger signaling cascade, and DHHC-7 is necessary for ERα to signal from the membrane in both cultured and adult hippocampal neurons [18, 19].
An interesting point about the findings of this study is the direction of effect in adult animals. Namely, that caveolin 1 and DHHC-7 were decreased in expression in adult female hippocampus compared to male hippocampus. While we have only observed action of the ERα/mGluR/caveolin 1 pathway in female hippocampal neurons in the context of signaling to CREB [22, 24], similar interactions occur in adult male hippocampus neurons in other contexts. In particular, this study’s findings strongly resonate with the known actions of membrane- associated ERα on glutamatergic transmission in adult hippocampal neurons. Oberlander and colleagues showed that a specific estradiol-sensitive receptor in each sex exclusively mediates how estradiol rapidly potentiates glutamatergic neurotransmission in both male and female hippocampus [44]. Activation of ERα modulated glutamatergic transmission in males, not females. Given that caveolin 1 is necessary for organizing the ERα/mGluR pathway [23], it is possible that the increased expression of caveolin 1 and DHHC-7 in males is responsible for enabling estradiol-induce potentiation of glutamatergic signaling by enabling the trafficking of ERα. Consistent with this speculation, caveolin 1 expression has been implicated with several forms of synaptic plasticity [66–68], and in males downregulation of caveolin 1 in the hippocampus is correlated with deficits in hippocampus-dependent learning tasks [69]. Much less is known about the role of DHHC-7 and links to synaptic plasticity, however it has been implicated in the palmitoylation of other membrane receptors, the G protein alpha subunit, regulation of GABAergic synapse function and molecules such as NCAM and PDE10A that regulate synaptic plasticity [70–76].
In general, caveolins and DHHC palmitoylacyltransferases play crucial roles in both trafficking and organizing a wide range of plasma membrane-initiated signaling cascades. In the nervous system, caveolins are implicated in intracellular trafficking and with physically organizing receptors and other signaling molecules with lipid rafts on or near the plasma membrane, including mGluRs [67, 77–79]. In the hippocampus the expression of all three caveolin isoforms have been documented [23, 66]. Beyond mGluRs and membrane-associated ERs [23, 80–82], caveolins are also involved with endocytosis, trafficking, and organizing a diverse multitude of relevant molecules such as dopamine receptors, NMDA and AMPA receptors, M1 muscarinic receptors, receptor tyrosine kinases and cAMP signaling pathway components both in and outside the nervous system [83–87]. Similar to caveolins, DHHC palmitoylacyltransferases play crucial roles in intracellular trafficking. DHHC palmitoylacyltransferases perform S-palmitoylation, which is a reversible post-translational modification involving attaching a 16-carbon fatty acid palmitate to cysteine residues embedded within a specific peptide sequence on target proteins [88]. This palmitate group serves the dual function of being a trafficking signal and a lipophilic anchor. There are 22 known DHHCs, which show differing levels of substrate specificity and individual function [89, 90]. mRNA for all of these DHHCs are present in hippocampal neurons, and the expression of all DHHC genes is examined in this study (Table 2). DHHC palmitoylacyltransferases regulate molecules necessary for synaptic function and are sensitive to synaptic plasticity, and their internal distribution can be dynamically regulated [91–94]. Given the diversity and sheer range of processes modulated by the members of the DHHC family, we do not find it unusual that a specific DHHC enzyme previously implicated in membrane-associated ER function such as DHHC-7 shows differential expression by sex.
5. Summary
Here we have presented evidence that the expression of genes that encode caveolin 1 and DHHC-7 are decreased in adult female compared to male hippocampus. There were no sex differences detected in gene expression in neonatal animals. In adult animals, no sex differences in gene expression were detected for estrogen receptor α and β, Caveolin 3, DHHC-21. Overall, this body of data is useful for generating new hypotheses regarding the mechanisms by which sex differences in membrane-associated ER signaling are programmed.
Highlights.
Estrogen receptor α and β expression does not vary by sex in neonatal or adult rat hippocampus.
Caveolin 1 expression is decreased in adult female compared to male hippocampus, but not in neonatal rat hippocampus.
Caveolin 3 expression does not vary by sex in neonatal or adult rat hippocampus.
DHHC-7 expression is decreased in adult female compared to male hippocampus, but not in neonatal rat hippocampus.
DHHC-21 expression does not vary by sex in neonatal or adult rat hippocampus.
Acknowledgments
We thank Dr. Valerie Hedges for assistance with extracting RNA from adult hippocampus and Dr. Scott Belcher for statistical consultation. Funding: This work was supported by DA035008 (P.G.M), DA041808 (P.G.M.), NIH F32 DA030828 (J.M.), and R01 MH109471 (J.M.).
Footnotes
Conflicts of Interest
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Charlier TD, Cornil CA, Ball GF, Balthazart J. Diversity of mechanisms involved in aromatase regulation and estrogen action in the brain. Biochimica et biophysica acta. 2010;1800(10):1094–105. doi: 10.1016/j.bbagen.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114(1):152–7. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 3.Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967;58(4):1711–8. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29(5):241–9. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32(4):532–49. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–80. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 7.Remage-Healey L. Brain estrogen signaling effects acute modulation of acoustic communication behaviors: A working hypothesis. Bioessays. 2012;34:1009–16. doi: 10.1002/bies.201200081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micevych P, Christensen A. Membrane-initiated estradiol actions mediate structural plasticity and reproduction. Frontiers in neuroendocrinology. 2012;33(4):331–41. doi: 10.1016/j.yfrne.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mani SK, Mermelstein PG, Tetel MJ, Anesetti G. Convergence of multiple mechanisms of steroid hormone action. Horm Metab Res. 2012;44(8):569–76. doi: 10.1055/s-0032-1306343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph LM, Cornil CA, Mittelman-Smith MA, Rainville JR, Remage-Healey L, Sinchak K, Micevych PE. Actions of Steroids: New Neurotransmitters. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36(45):11449–11458. doi: 10.1523/JNEUROSCI.2473-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu P, Liu J, Yasrebi A, Gotthardt JD, Bello NT, Pang ZP, Roepke TA. Gq Protein-Coupled Membrane-Initiated Estrogen Signaling Rapidly Excites Corticotropin-Releasing Hormone Neurons in the Hypothalamic Paraventricular Nucleus in Female Mice. Endocrinology. 2016;157(9):3604–20. doi: 10.1210/en.2016-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011;42:236–41. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22(19):8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145(7):3055–61. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 15.Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Frontiers in bioscience (Landmark edition) 2011;16:1560–73. doi: 10.2741/3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava DP, Evans PD. G-protein oestrogen receptor 1: trials and tribulations of a membrane oestrogen receptor. Journal of neuroendocrinology. 2013;25(11):1219–30. doi: 10.1111/jne.12071. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhri RA, Schwartz N, Elbaradie K, Schwartz Z, Boyan BD. Role of ERalpha36 in membrane-associated signaling by estrogen. Steroids. 2014;81:74–80. doi: 10.1016/j.steroids.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, Britson KA, Mermelstein PG. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology. 2013;154:4293–304. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedram A, Razandi M, Deschenes RJ, Levin ER. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Molecular biology of the cell. 2012;23(1):188–99. doi: 10.1091/mbc.E11-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishii H, Hattori Y, Munetomo A, Watanabe H, Sakuma Y, Ozawa H. Characterization of rodent constitutively active estrogen receptor alpha variants and their constitutive transactivation mechanisms. General and comparative endocrinology. 2017;248:16–26. doi: 10.1016/j.ygcen.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Kim CK, Torcaso A, Asimes A, Chung WCJ, Pak TR. Structural and functional characteristics of estrogen receptor beta (ERbeta) splice variants: implications for the aging brain. Journal of neuroendocrinology. 2017 doi: 10.1111/jne.12488. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 22.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25(20):5066–78. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27(37):9941–50. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meitzen J, Grove DD, Mermelstein PG. The Organizational and Aromatization Hypotheses Apply to Rapid, Nonclassical Hormone Action: Neonatal Masculinization Eliminates Rapid Estradiol Action in Female Hippocampal Neurons. Endocrinology. 2012;153(10):4616–21. doi: 10.1210/en.2012-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang GZ, Woolley CS. Estradiol Acutely Suppresses Inhibition in the Hippocampus through a Sex-Specific Endocannabinoid and mGluR-Dependent Mechanism. Neuron. 2012;74(5):801–8. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaban V, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. Journal of neuroscience research. 2011;89(11):1707–10. doi: 10.1002/jnr.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27(35):9294–300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–55. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo J, Hamid N, Bondar G, Dewing P, Clarkson J, Micevych P. Sex differences in hypothalamic astrocyte response to estradiol stimulation. Biology of sex differences. 2010;1(1):7. doi: 10.1186/2042-6410-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30(39):12950–7. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150(3):1369–76. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, Foster Olive M, Carroll ME, Meisel RL, Mermelstein PG. Estradiol Facilitation of Cocaine Self-Administration in Female Rats Requires Activation of mGluR5. eNeuro. 2016;3(5) doi: 10.1523/ENEURO.0140-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez LA, Peterson BM, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behavioural brain research. 2014;271:39–42. doi: 10.1016/j.bbr.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson BM, Martinez LA, Meisel RL, Mermelstein PG. Estradiol impacts the endocannabinoid system in female rats to influence behavioral and structural responses to cocaine. Neuropharmacology. 2016;110:118–24. doi: 10.1016/j.neuropharm.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain structure & function. 2015;(220):2415–22. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spampinato SF, Merlo S, Molinaro G, Battaglia G, Bruno V, Nicoletti F, Sortino MA. Dual Effect of 17beta-Estradiol on NMDA-Induced Neuronal Death: Involvement of Metabotropic Glutamate Receptor 1. Endocrinology. 2012;153:5940–5948. doi: 10.1210/en.2012-1799. [DOI] [PubMed] [Google Scholar]
- 37.Spampinato SF, Molinaro G, Merlo S, Iacovelli L, Caraci F, Battaglia G, Nicoletti F, Bruno V, Sortino MA. Estrogen receptors and type 1 metabotropic glutamate receptors are interdependent in protecting cortical neurons against beta-amyloid toxicity. Mol Pharmacol. 2012;81(1):12–20. doi: 10.1124/mol.111.074021. [DOI] [PubMed] [Google Scholar]
- 38.Mahavongtrakul M, Kanjiya MP, Maciel M, Kanjiya S, Sinchak K. Estradiol dose-dependent regulation of membrane estrogen receptor-alpha, metabotropic glutamate receptor-1a, and their complexes in the arcuate nucleus of the hypothalamus in female rats. Endocrinology. 2013;154(9):3251–60. doi: 10.1210/en.2013-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(38):15184–94. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Jiang Q, Yu L, Lu ZY, Meng SP, Su D, Burnstock G, Ma B. 17beta-estradiol rapidly attenuates P2X3 receptor-mediated peripheral pain signal transduction via ERalpha and GPR30. Endocrinology. 2013;154(7):2421–33. doi: 10.1210/en.2012-2119. [DOI] [PubMed] [Google Scholar]
- 41.Seredynski AL, Balthazart J, Ball GF, Cornil CA. Estrogen Receptor beta Activation Rapidly Modulates Male Sexual Motivation through the Transactivation of Metabotropic Glutamate Receptor 1a. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(38):13110–23. doi: 10.1523/JNEUROSCI.2056-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabatadze N, Huang G, May RM, Jain A, Woolley CS. Sex Differences in Molecular Signaling at Inhibitory Synapses in the Hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(32):11252–65. doi: 10.1523/JNEUROSCI.1067-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scharfman HE, MacLusky NJ. Sex differences in hippocampal area CA3 pyramidal cells. Journal of neuroscience research. 2017;95(1–2):563–575. doi: 10.1002/jnr.23927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oberlander JG, Woolley CS. 17beta-Estradiol Acutely Potentiates Glutamatergic Synaptic Transmission in the Hippocampus through Distinct Mechanisms in Males and Females. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36(9):2677–90. doi: 10.1523/JNEUROSCI.4437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato SM, Woolley CS. Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model. eLife. 2016;5:e19109. doi: 10.7554/eLife.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabatadze N, Smejkalova T, Woolley CS. Distribution and Posttranslational Modification of Synaptic ERalpha in the Adult Female Rat Hippocampus. Endocrinology. 2013;154:819–30. doi: 10.1210/en.2012-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alaluf S, Mulvihill ER, McIlhinney RA. The metabotropic glutamate receptor mGluR4, but not mGluR1 alpha, is palmitoylated when expressed in BHK cells. J Neurochem. 1995;64(4):1548–55. doi: 10.1046/j.1471-4159.1995.64041548.x. [DOI] [PubMed] [Google Scholar]
- 48.Pickering DS, Taverna FA, Salter MW, Hampson DR. Palmitoylation of the GluR6 kainate receptor. Proc Natl Acad Sci U S A. 1995;92(26):12090–4. doi: 10.1073/pnas.92.26.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meitzen J, Perry AN, Westenbroek C, Hedges VL, Becker JB, Mermelstein PG. Enhanced striatal β1-adrenergic receptor expression following hormone loss in adulthood is programmed by both early sexual differentiation and puberty: a study of humans and rats. Endocrinology. 2013;154:1820–31. doi: 10.1210/en.2012-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meitzen J, Luoma J, Stern C, Mermelsten PG. β1-adrenergic receptors activate two distinct signaling pathways in striatal neurons. Journal of neurochemistry. 2011;116:984–95. doi: 10.1111/j.1471-4159.2010.07137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arambula SE, Belcher SM, Planchart A, Turner SD, Patisaul HB. Impact of Low Dose Oral Exposure to Bisphenol A (BPA) on the Neonatal Rat Hypothalamic and Hippocampal Transcriptome: A CLARITY-BPA Consortium Study. Endocrinology. 2016;157(10):3856–3872. doi: 10.1210/en.2016-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Keefe JA, Handa RJ. Transient elevation of estrogen receptors in the neonatal rat hippocampus. Brain research Developmental brain research. 1990;57(1):119–27. doi: 10.1016/0165-3806(90)90191-z. [DOI] [PubMed] [Google Scholar]
- 53.O’Keefe JA, Li Y, Burgess LH, Handa RJ. Estrogen receptor mRNA alterations in the developing rat hippocampus. Brain research Molecular brain research. 1995;30(1):115–24. doi: 10.1016/0169-328x(94)00284-l. [DOI] [PubMed] [Google Scholar]
- 54.Solum DT, Handa RJ. Localization of estrogen receptor alpha (ER alpha) in pyramidal neurons of the developing rat hippocampus. Brain research Developmental brain research. 2001;128(2):165–75. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- 55.Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. The Journal of comparative neurology. 1997;388(4):603–12. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 56.Loy R, Gerlach JL, McEwen BS. Autoradiographic localization of estradiol-binding neurons in the rat hippocampal formation and entorhinal cortex. Brain research. 1988;467(2):245–51. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- 57.Alexander A, Irving AJ, Harvey J. Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology. 2017;113(Pt B):652–660. doi: 10.1016/j.neuropharm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Koss WA, Frick KM. Sex differences in hippocampal function. Journal of neuroscience research. 2017;95(1–2):539–562. doi: 10.1002/jnr.23864. [DOI] [PubMed] [Google Scholar]
- 59.Duarte-Guterman P, Yagi S, Chow C, Galea LA. Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Hormones and behavior. 2015;74:37–52. doi: 10.1016/j.yhbeh.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 60.Luine V. Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents. The Journal of steroid biochemistry and molecular biology. 2016;160:189–95. doi: 10.1016/j.jsbmb.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin B, Pearson M, Brenneman R, Golden E, Keselman A, Iyun T, Carlson OD, Egan JM, Becker KG, Wood W, 3rd, Prabhu V, de Cabo R, Maudsley S, Mattson MP. Conserved and differential effects of dietary energy intake on the hippocampal transcriptomes of females and males. PloS one. 2008;3(6):e2398. doi: 10.1371/journal.pone.0002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider JS, Anderson DW, Sonnenahalli H, Vadigepalli R. Sex-based differences in gene expression in hippocampus following postnatal lead exposure. Toxicology and applied pharmacology. 2011;256(2):179–90. doi: 10.1016/j.taap.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winkler JM, Fox HS. Transcriptome meta-analysis reveals a central role for sex steroids in the degeneration of hippocampal neurons in Alzheimer’s disease. BMC systems biology. 2013;7:51. doi: 10.1186/1752-0509-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trabzuni D, Ramasamy A, Imran S, Walker R, Smith C, Weale ME, Hardy J, Ryten M. Widespread sex differences in gene expression and splicing in the adult human brain. Nature communications. 2013;4:2771. doi: 10.1038/ncomms3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, Rubbi L, Arnold AP, de Vries GJ, Forger NG, Pellegrini M, Vilain E. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biology of sex differences. 2014;5:8. doi: 10.1186/2042-6410-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braun JE, Madison DV. A novel SNAP25-caveolin complex correlates with the onset of persistent synaptic potentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20(16):5997–6006. doi: 10.1523/JNEUROSCI.20-16-05997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takayasu Y, Takeuchi K, Kumari R, Bennett MV, Zukin RS, Francesconi A. Caveolin-1 knockout mice exhibit impaired induction of mGluR-dependent long-term depression at CA3-CA1 synapses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21778–83. doi: 10.1073/pnas.1015553107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaudreault SB, Blain JF, Gratton JP, Poirier J. A role for caveolin-1 in post-injury reactive neuronal plasticity. Journal of neurochemistry. 2005;92(4):831–9. doi: 10.1111/j.1471-4159.2004.02917.x. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, Liang Z, Liu J, Zou W, Li X, Wang Y, An L. Downregulation of caveolin-1 contributes to the synaptic plasticity deficit in the hippocampus of aged rats. Neural regeneration research. 2013;8(29):2725–33. doi: 10.3969/j.issn.1673-5374.2013.29.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lievens PM, Kuznetsova T, Kochlamazashvili G, Cesca F, Gorinski N, Galil DA, Cherkas V, Ronkina N, Lafera J, Gaestel M, Ponimaskin E, Dityatev A. ZDHHC3 Tyrosine Phosphorylation Regulates Neural Cell Adhesion Molecule Palmitoylation. Molecular and cellular biology. 2016;36(17):2208–25. doi: 10.1128/MCB.00144-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ponimaskin E, Dityateva G, Ruonala MO, Fukata M, Fukata Y, Kobe F, Wouters FS, Delling M, Bredt DS, Schachner M, Dityatev A. Fibroblast growth factor-regulated palmitoylation of the neural cell adhesion molecule determines neuronal morphogenesis. J Neurosci. 2008;28(36):8897–907. doi: 10.1523/JNEUROSCI.2171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(49):12758–68. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kilpatrick CL, Murakami S, Feng M, Wu X, Lal R, Chen G, Du K, Luscher B. Dissociation of Golgi-associated DHHC-type Zinc Finger Protein (GODZ)- and Sertoli Cell Gene with a Zinc Finger Domain-beta (SERZ-beta)-mediated Palmitoylation by Loss of Function Analyses in Knock-out Mice. The Journal of biological chemistry. 2016;291(53):27371–27386. doi: 10.1074/jbc.M116.732768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsutsumi R, Fukata Y, Noritake J, Iwanaga T, Perez F, Fukata M. Identification of G protein alpha subunit-palmitoylating enzyme. Molecular and cellular biology. 2009;29(2):435–47. doi: 10.1128/MCB.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du K, Murakami S, Sun Y, Kilpatrick C, Luscher B. DHHC7 Palmitoylates Glut4 and Regulates Glut4 Membrane Translocation. The Journal of biological chemistry. 2017 doi: 10.1074/jbc.M116.747139. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Charych EI, Jiang LX, Lo F, Sullivan K, Brandon NJ. Interplay of palmitoylation and phosphorylation in the trafficking and localization of phosphodiesterase 10A: implications for the treatment of schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(27):9027–37. doi: 10.1523/JNEUROSCI.1635-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci. 2009;29(11):3590–602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–91. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luoma JI, Boulware MI, Mermelstein PG. Caveolin proteins and estrogen signaling in the brain. Mol Cell Endocrinol. 2008;290(1–2):8–13. doi: 10.1016/j.mce.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christensen A, Micevych P. CAV1 siRNA reduces membrane estrogen receptor-alpha levels and attenuates sexual receptivity. Endocrinology. 2012;153(8):3872–7. doi: 10.1210/en.2012-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions, Molecular endocrinology (Baltimore. Md) 2002;16(1):100–15. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 82.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278(4):2701–12. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 83.Egawa J, Pearn ML, Lemkuil BP, Patel PM, Head BP. Membrane lipid rafts and neurobiology: age-related changes in membrane lipids and loss of neuronal function. The Journal of physiology. 2016;594(16):4565–79. doi: 10.1113/JP270590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Head BP, Patel HH, Tsutsumi YM, Hu Y, Mejia T, Mora RC, Insel PA, Roth DM, Drummond JC, Patel PM. Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22(3):828–40. doi: 10.1096/fj.07-9299com. [DOI] [PubMed] [Google Scholar]
- 85.Kong MM, Hasbi A, Mattocks M, Fan T, O’Dowd BF, George SR. Regulation of D1 dopamine receptor trafficking and signaling by caveolin-1. Molecular pharmacology. 2007;72(5):1157–70. doi: 10.1124/mol.107.034769. [DOI] [PubMed] [Google Scholar]
- 86.Shmuel M, Nodel-Berner E, Hyman T, Rouvinski A, Altschuler Y. Caveolin 2 regulates endocytosis and trafficking of the M1 muscarinic receptor in MDCK epithelial cells. Molecular biology of the cell. 2007;18(5):1570–85. doi: 10.1091/mbc.E06-07-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stern CM, Mermelstein PG. Caveolin regulation of neuronal intracellular signaling. Cell. 2010;67(22):3785–95. doi: 10.1007/s00018-010-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 2010;11(3):161–75. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- 89.Ohno Y, Kashio A, Ogata R, Ishitomi A, Yamazaki Y, Kihara A. Analysis of substrate specificity of human DHHC protein acyltransferases using a yeast expression system. Mol Biol Cell. 2012;23:4543–51. doi: 10.1091/mbc.E12-05-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hou H, John Peter AT, Meiringer C, Subramanian K, Ungermann C. Analysis of DHHC acyltransferases implies overlapping substrate specificity and a two-step reaction mechanism. Traffic. 2009;10(8):1061–73. doi: 10.1111/j.1600-0854.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- 91.Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44(6):987–96. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Fukata M, Sekiya A, Murakami T, Yokoi N, Fukata Y. Postsynaptic nanodomains generated by local palmitoylation cycles. Biochemical Society transactions. 2015;43(2):199–204. doi: 10.1042/BST20140238. [DOI] [PubMed] [Google Scholar]
- 93.Fukata Y, Dimitrov A, Boncompain G, Vielemeyer O, Perez F, Fukata M. Local palmitoylation cycles define activity-regulated postsynaptic subdomains. The Journal of cell biology. 2013;202(1):145–61. doi: 10.1083/jcb.201302071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yokoi N, Fukata Y, Sekiya A, Murakami T, Kobayashi K, Fukata M. Identification of PSD-95 Depalmitoylating Enzymes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36(24):6431–44. doi: 10.1523/JNEUROSCI.0419-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]