Abstract
The prevalence of obesity has increased globally over the last two decades. Although the body mass index (BMI) has been a convenient and simple index of obesity at the population level, studies have shown that obesity defined by BMI alone is a remarkably heterogeneous condition with varying cardiovascular (CV) and metabolic manifestations across individuals. Adipose tissue is an exquisitely active metabolic organ engaged in cross-talk between various systems; perturbation of which results in a pathologic response to positive caloric balance in susceptible individuals that directly and indirectly contributes to CV and metabolic disease. Inadequate subcutaneous adipose tissue expansion in the face of dietary triglycerides leads to visceral and ectopic fat deposition, inflammatory/adipokine dysregulation, and insulin resistance. Conversely, preferential fat storage in the lower body depot may act as a metabolic buffer and protect other tissues from lipotoxicity caused by lipid overflow and ectopic fat. Translational, epidemiologic, and clinical studies over the past 30 years have clearly demonstrated a strong link between visceral and ectopic fat and the development of a clinical syndrome characterized by atherogenic dyslipidemia, hyperinsulinemia/glucose intolerance, hypertension, atherosclerosis, and adverse cardiac remodeling/heart failure. This relationship is even more nuanced when clinical entities such as metabolically healthy obesity phenotype and the obesity paradox are considered. Although it is clear that the accumulation of visceral/ectopic fat is a major contributor to CV and metabolic risk above and beyond the BMI, implementation of fat distribution assessment into clinical practice remains a challenge. Anthropometric indices of obesity are easily implemented but newer imaging-based methods offer improved sensitivity and specificity for measuring specific depots. Lifestyle, pharmacologic, and surgical interventions allow a multidisciplinary approach to overweight/obesity that may improve outcomes and align with a public health message to combat the growing epidemic of obesity worldwide and build healthier lives, free of cardiovascular diseases.
Keywords: obesity, waist circumference, adiposity, cardiovascular disease, imaging techniques
Journal Subject Codes: obesity, epidemiology, remodeling, risk factors, magnetic resonance imaging
Obesity: Classification and the Scope of the Problem
The prevalence of obesity has doubled in over 70 countries since 1980 and continues to increase in most areas globally. Recent age-standardized estimates of global obesity prevalence report that at least 30% of men and 35% of women are obese in many countries worldwide including in North America, the Middle East, Asia, and Australia.1, 2 In the United States, obesity is now prevalent in greater than 35% of individuals in four states (Mississippi, Arkansas, Louisiana, and West Virginia) and present in at least 20% of the population in all other states (http://stateofobesity.org/adult-obesity).
Obesity is defined by body mass index (BMI), calculated as the weight in kilograms divided by the height in meters, squared, and stratified into categories according to the World Health Organization (Table 1).3 The purpose of this classification is to help clinicians and researchers standardize terminology and clinical severity based on a dose dependent relationship between BMI and health outcomes such as mortality. However, this relationship is in actuality a “J-shaped” curve, with increasing mortality also seen among individuals classified as underweight. In one systematic review incorporating data from 19 prospective studies of ~1.5 million adults, the lowest mortality was observed at a BMI of ~23 kg/m2 with higher rates of mortality at either end of the BMI spectrum and with clear inflection points after a BMI of 30 kg/m2 (the cutoff for “obesity”) and below 18.5 kg/m2 (the cutoff for “underweight”).4
Table 1.
World Health Organization Classification of Obesity
| Classification | Body Mass Index (kg/m2) |
|---|---|
| Underweight | <18.5 |
| Normal weight | 18.5–24.9 |
| Overweight | 25–29.9 |
| Obese | |
| Class I | 30–34.9 |
| Class II | 35–39.9 |
| Class III | ≥40 |
Modified from reference 3
Although the BMI has been a convenient and simple index to monitor the growth in obesity prevalence at the population level, many metabolic and clinical studies have revealed that obesity, when defined on the basis of the BMI alone, is a remarkably heterogeneous condition.5 For instance, patients with similar body weight or BMI values have been shown to display markedly different co-morbidities and levels of health risk.6 Thus, if one were to evaluate critically the performance of BMI as a biomarker, it would fall short in several areas. First, the BMI has never emerged as a component of the Framingham or Pooled Cohort Equation7 cardiovascular disease (CVD) risk scores, because it has not demonstrated sufficient improvement in discriminatory capacity over traditional risk factors.
Second, although a proportion of individuals with obesity will develop type 2 diabetes and/or CVD, a significant minority will remain free of cardiometabolic disease during their lifetime. In one study from the National Health and Human Nutrition Examination Surveys (NHANES), 51.3% of overweight and 31.7% of obese adults were determined to be metabolically healthy.8 This phenotype, termed metabolically healthy obesity (MHO) has been associated with younger age, non-Hispanic black race/ethnicity, higher physical activity and cardiorespiratory fitness levels, better overall nutritional quality and, with low levels of abdominal visceral adipose tissue and ectopic fat.9
Third, the relationship of BMI with health outcomes is further complicated by the concept of an “obesity paradox” in which overweight and obese people with established disease have a better prognosis compared with normal weight people.10 At least four obesity-related paradoxes in relation to mortality risk have been identified, including the classic obesity paradox (obesity is protective in chronic disease states), the pre-obesity paradox (overweight is protective in normal populations), the fat-but-fit concept (obesity is not a risk factor for mortality in fit individuals) and MHO.11–13 An obesity paradox has been observed in both cardiovascular diseases (hypertension, coronary heart disease14, heart failure, peripheral vascular disease, and stroke15) and non-cardiovascular diseases (e.g. end-stage renal disease, cancer, and chronic obstructive pulmonary disease), and in particular among the elderly.16 The obesity paradox concept is controversial, however, because its very existence has been questioned due to concerns about selection, survival, treatment, and confounding biases causing the paradoxical observations.12, 17–20 Several hypotheses have been proposed to explain the obesity paradox, including inability of BMI to differentiate between central and peripheral fat deposits, un-measured or unable to be-measured confounding factors, the effect of cardiorespiratory fitness (CRF)21, the benefit of surplus energy reserves to help combat the catabolic state of disease, and a deficiency of lean mass (sarcopenia) contributing to adverse health outcomes. These important limitations create an opportunity for new adiposity-related biomarkers to emerge that will impact clinical cardiovascular care while improving on the inherent shortcomings of BMI assessment.
In this review, we aim to describe the incredible cardiovascular and metabolic heterogeneity of obesity, including our evolving understanding of its biologic and mechanistic underpinnings, epidemiology, clinical challenges, and implications for management.
The Biology of Adipose Tissue Dysfunction: Adiposopathy
The concept of “adiposopathy”, or “sick fat” - a pathologic response by the adipose tissue organ to a stimulus - is a relatively recent concept in medicine.22 In the past, the adipose organ was thought to be relatively inert and act primarily as a storage depot for excess energy in the form of triglycerides with the sole function of building up or breaking down excess lipid into free fatty acids and glycerol based on the body’s metabolic needs and anabolic/catabolic balance. In more recent years, the adipose organ has been recognized to be quite metabolically active and engaged in cross-talk between various organ systems. Perturbation of this highly regulated system results in a pathologic response by adipose tissue to positive caloric balance in susceptible individuals that directly and indirectly contributes to cardiovascular and metabolic disease. The three central tenets of adiposopathy are: 1) deposition of ectopic fat (fat stores in body locations where fat is not physiologically stored, such as the liver, pancreas, heart, and skeletal muscle) and a shift to visceral adipose tissue distribution (fat storage in the intraperitoneal and retroperitoneal spaces); 2) inflammatory and adipokine dysregulation; and 3) insulin resistance. The presence or absence of adiposopathy, therefore, may help explain the heterogeneity of obesity and its manifestations since the pathogenic potential of excess body fat is conditioned on adipose tissue dysfunction/ectopic fat deposition rather than simply on increased fat mass alone.
Body Fat Distribution: A Key Factor in Obesity Heterogeneity
The ability of the abdominal subcutaneous depot to expand is the physiologic response to a positive energy balance leading to an increased demand for triglyceride storage in adipose tissue. It is important to note, however, that the body’s response to excess energy accumulation is not uniform – there is significant individual variation in how much and where fat is deposited or stored. Studies in rodents where activation of mitochondrial pathways results in massive expansion in the subcutaneous fat pad via hyperplasia resulted in decreased lipid oxidation, increased storage, and a compensatory healthy adipose tissue expansion with preserved glucose tolerance.23 Similarly, transplantation of adipose tissue from the subcutaneous flank depot in mice into the visceral cavity resulted in decreased body weight, total fat mass, and glucose and insulin levels; whereas these effects were observed to a lesser extent when subcutaneous fat was transplanted to the subcutaneous area and no changes seen when visceral fat was transplanted into the visceral area.24
Humans exhibit remarkable variability in body fat distribution for a given BMI. Susceptibility to store fat either subcutaneously or viscerally is in part determined by genetics first demonstrated in overfeeding studies of monozygotic twins by Bouchard and colleagues over two decades ago.25 For example, Figure 1 shows T1-weighted MRI coronal abdominal images in two persons, one individual with a BMI of 25 kg/m2 (normal weight) and one individual with a BMI of 30 kg/m2 (obese). Knowledge of the BMI in isolation fails to demonstrate the extreme variation in intraabdominal (or visceral) fat distribution between the two subjects. Furthermore, the individual with the lower BMI has a significantly greater burden of visceral adipose tissue (VAT, red shaded) whereas the subject with the higher BMI has more abdominal subcutaneous adipose tissue (SAT, blue shaded). Variation in visceral fat that is not always readily apparent from anthropometric data alone is a key element to risk assessment, due to the fact that VAT and SAT differ greatly in their functional significance and response to weight gain. Imaging studies using computed tomography conducted more than 25 years ago had shown that among equally obese individuals, men and women with high levels of VAT were characterized by an atherogenic dyslipidemia, hyperinsulinemia, and glucose intolerance whereas obese individuals with low levels of VAT did not show these metabolic abnormalities compared to non-obese controls.5 In one study, individuals matched for abdominal SAT with low or high VAT had different levels of glucose tolerance, whereas those matched for VAT had similar glucose tolerance testing between high and low SAT.26 Further evidence for a contributory role of visceral adiposity to type 2 diabetes comes from data in the Dallas Heart Study where visceral fat mass was shown to be independently associated with the development of both type 2 diabetes and prediabetes in a cohort of otherwise healthy obese individuals.27 Neither BMI nor waist circumference were associated with incident diabetes in this study. VAT in obese persons has been associated with dyslipidemia, atherosclerosis, and adipocytokine dysfunction whereas SAT seems to be relatively neutral in this population.28 Furthermore, differences in gene expression, inflammatory milieu, and development of traditional risk factors may also differ between depots.29
Figure 1. Extreme Variation in Abdominal Fat Distribution.
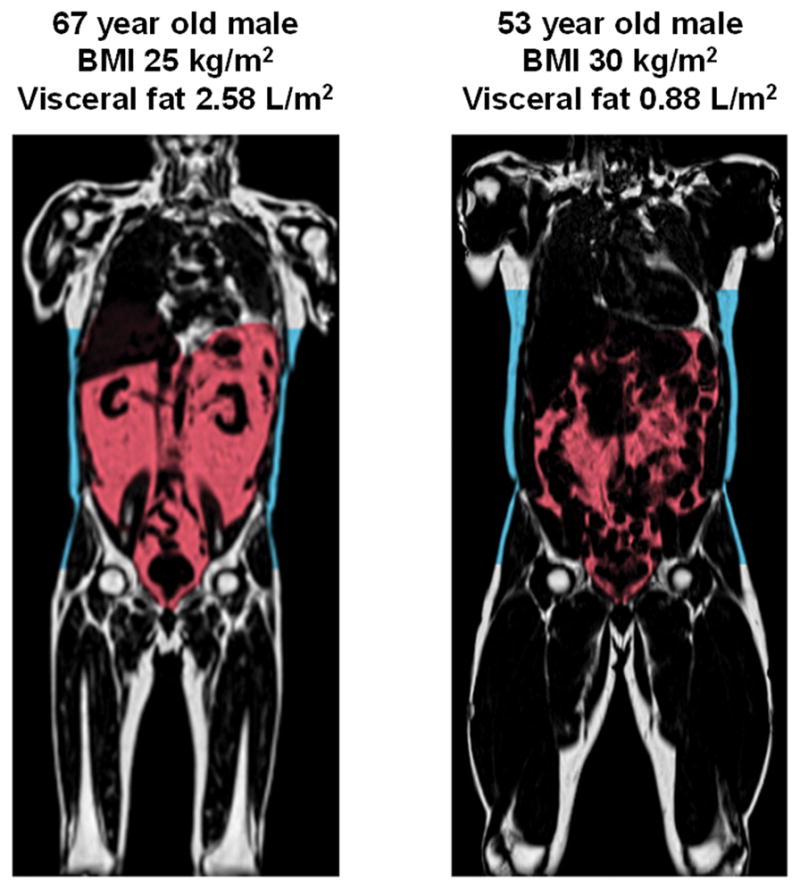
T1-weighted coronal neck-to-knee magnetic resonance images demonstrating variation in visceral fat (shaded red) and abdominal subcutaneous fat (shaded blue) between two individuals with normal weight (left) and obesity (right). Images courtesy of Advanced MR Analytics (Linköping, Sweden)
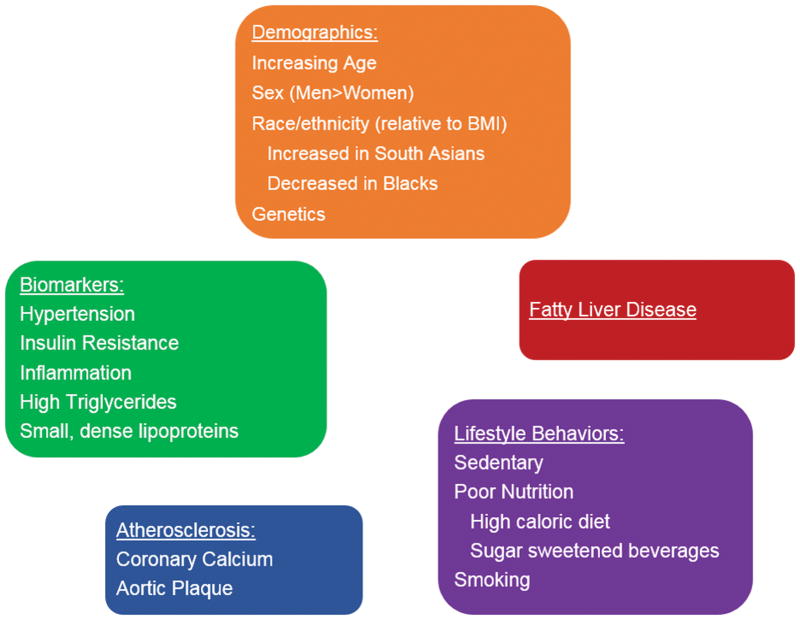
The sine qua non of adiposopathy is the accumulation of fat in the abdominal cavity in the form of visceral adipose tissue. There are several factors, both modifiable and non-modifiable, associated with excess VAT (Figure 2). Some factors may predispose individuals to higher levels of VAT for a given BMI whereas others are consequences of dysfunctional adiposity related to VAT. Sex-based differences in VAT may relate to higher levels of estrogens and lower levels of testosterone in women compared with men; expansion of visceral adiposity in women after menopause have been postulated as one cause for increased cardiovascular risk seen during this stage of a women’s lifespan. Race-based differences in VAT are well known and have important clinical implications for classification and risk assessment of obesity and metabolic syndrome. For example, it has been observed that Asian Americans, in particular South Asians, manifest type 2 diabetes at lower BMI levels compared with whites.30, 31 This may be explained, in part, by racial/ethnic differences in visceral adiposity even when adjusted for differences in body composition.32 These observations have led to the International Diabetes Federation to recommend race/ethnic-specific cutoffs for waist circumference in the diagnosis of metabolic syndrome (Table 2).33 Black individuals are less likely to be viscerally obese and have increased lipolytic activity and more efficient clearance of dietary triglycerides compared with whites.34 Nevertheless, the relationships between visceral adiposity and adverse cardiometabolic traits persist in blacks.
Figure 2. Factors Associated with Increased Visceral Adiposity.
Both modifiable and non-modifiable factors both contribute to and result from excess visceral adiposity and ectopic fat deposition
Table 2.
Ethnicity-Specific Cut-off Values for Waist Circumference
| Ethnic Group | Waist Circumference (marker of central obesity) |
|---|---|
| European origin | |
| Men | ≥94 cm |
| Women | ≥80 cm |
| South Asians | |
| Men | ≥90 cm |
| Women | ≥80 cm |
| Chinese | |
| Men | ≥90 cm |
| Women | ≥80 cm |
| Japanese | |
| Men | ≥85 cm |
| Women | ≥90 cm |
| Ethnic South and Central Americans | Use South Asian recommendations until more specific data are available |
| Sub-Saharan Africans | Use European data until more specific data are available |
| Eastern Mediterranean and Middle East (Arab) populations | Use European data until more specific data are available |
Modified from reference 33
From an epidemiological standpoint, associations have been shown between visceral adiposity and incident hypertension,35 atherosclerotic CVD,36 and cardiac dysfunction/failure, characterized by concentric remodeling, lower cardiac output, and higher systemic vascular resistance.37 Retroperitoneal fat, in particular, has been of considerable interest due to its proximity to the kidneys and potential local paracrine effects on renal sodium handling and downstream impact on blood pressure. VAT, and in particular retroperitoneal fat, had a graded association with incident hypertension in the Dallas Heart Study even after adjusting for traditional hypertension risk factors.35 Further research shows that both intraperitoneal and retroperitoneal fat may also impact blood pressure variability over the short- and long-term such that greater amounts of visceral fat are linked to persistently elevated, less variable systolic blood pressure with potential contribution to cardiac hypertrophy and failure.38 Excess visceral adiposity, in particular concomitant with diabetes, has also been linked to decrements in systolic and diastolic strain rate (a measure of myocardial deformation), excess intra-myocardial triglyceride, and prolonged myocardial T1 by MRI (a measure of relaxation time).39 Other localized fat depots around the heart, including pericardial and epicardial fat, have also been shown to associate with coronary events in the general population independent of traditional risk factors. However, the precise role of these localized fat depots in development of the atherosclerotic process is controversial. The presence of excessive pericardial or epicardial fat incidentally seen on a CT scan performed to evaluate coronary calcium or for obstructive coronary heart disease may complement the information gained from these tests for use in CVD risk assessment and prognostication.
Recent work to clarify the genetics of obesity/central obesity lend support to the potential causality of excess adiposity for adverse cardiovascular outcomes. A technique called Mendelian Randomization that seeks to use natural genetic variation to assess the causality of modifiable risk factors has been used to demonstrate a “causal” relationship between genetic risk for higher BMI and hypertension, coronary heart disease, type 2 diabetes,40 and atrial fibrillation,41 and separately, genetic risk for central obesity (as a surrogate for visceral adiposity) and coronary heart disease and type 2 diabetes.42 Advancements in methods to detect small amounts of metabolites in large population based screening studies (“metabolomics”) have also contributed to a better understanding of the metabolic milieu surrounding obesity. Studies evaluating the relationships between metabolomic pathways and imaging-based assessments of VAT independent of BMI or glucose tolerance are ongoing.
Lower body subcutaneous adipose tissue (LBAT) is defined as fat contained in body areas below the trunk and abdomen and includes the gluteal, femoral, and peripheral leg regions. It can be measured on a dual x-ray absorptiometry (DXA) scan that may be performed to evaluate bone density for osteoporosis or to assess composition of total body fat and fat-free mass. Although it is less frequently discussed in the clinical literature, this adipose tissue depot is very important with regard to cardiovascular and metabolic disease risk. Epidemiological studies have demonstrated that greater LBAT is associated with a lower cardiac risk factor burden43, lower risk of incident CVD36 and cancer44, and that individuals with more LBAT have less left ventricular concentric remodeling, greater cardiac output, and lower systemic vascular resistance37 when adjusted for their body size. LBAT can therefore be considered as the diametric opposite of VAT. LBAT may impart these beneficial/protective effects by acting as a metabolic buffer of the influx of dietary lipids and protecting other tissues from lipotoxicity caused by lipid overflow and ectopic fat deposition. Indeed, it was reported >25 years ago that women with a high femoral adipose tissue lipoprotein lipase activity were characterized by higher levels of HDL-cholesterol, suggesting a link between the triglyceride clearance capacity of lower body fat and a healthier lipid profile.45 Accordingly, investigations of femoral adipose tissue biopsies in lactating women have suggested that lipid mobilization is turned on during that period, suggesting that gluteal femoral fat may represent an energy reserve used to meet the demands of lactation.46 Longitudinal studies of changes in LBAT with a weight loss intervention have shown that greater LBAT loss is associated with increases in diastolic blood pressure despite an overall decrease in body weight. However, other studies have not confirmed that selective reduction of LBAT worsens risk factors for diabetes and cardiovascular disease.47 Overall, these and other data suggest that depot-specific fat loss with preservation or shifting of fat stores to the lower body subcutaneous depot may have beneficial health effects on CVD risk factors and outcomes despite being BMI- and overall weight-neutral. Although there are data that indicate a favorable shift in fat distribution from visceral to subcutaneous adipose depots is associated with improvements in hepatic and peripheral tissue insulin sensitivity with certain medications (e.g. thiazolidinediones), there are no large, randomized controlled trials evaluating use of these medications as a strategy to alter body fat distribution-related CVD risk.
Brown adipose tissue (BAT) is a subtype of adipocyte characterized by large number of mitochondria and increased expression of uncoupling protein-1 (UCP-1), a key mediator of thermogenesis. It is responsible for heat generation in response to cold exposure, primarily in non-human species such as rodents.48 In humans, BAT is thought to play a minor role in adipose physiology given its relatively small quantity and remote location (e.g. supraclavicular, paravertebral) observable with 18F-fluorodeoxyglucose positron-emission tomographic/computed tomographic imaging49 in newborns or cold-exposed adults.50 The amount of human brown fat is inversely proportional to body weight and overweight/obese individuals tend to have less BAT mass and consequently less cold-induced thermogenesis. Cold stimulation in humans, however, leads to enhanced energy expenditure and weight loss, with increased glucose and fatty acid uptake in BAT, but not in other metabolically-active tissues like skeletal muscle or white fat.51 Recent advances in understanding the molecular heterogeneity of brown versus white (classical) adipose tissue suggest that one potential way to improve adipose tissue function may be through “browning” or “beiging” of white fat, which seeks to effect a transition in white adipose tissue to attain the gene expression patterns and immunohistochemical characteristics of brown fat with improved metabolic consequences. Beiging of white adipose tissue can be affected by cold, certain pharmaceuticals, such as thiazolidinediones and beta adrenergic agonists, and exercise.52 Rodent models have shown that beiging of white adipose tissue can resist diet induced obesity and improve metabolism.52 In humans, increasing BAT mass and/or activity and beiging of white adipocytes has been suggested to have the potential to increase energy expenditure, improve glucose and lipid metabolism, and improve body weight. However, translating the modulation of BAT physiology from animal model to human as a target for obesity and cardiometabolic disease has proven complex, since improved glycemic metabolism in BAT appears to be limited to conditions of prolonged cold exposure53 and muscle glucose utilization during shivering seems to be a much more robust contributor to systemic glucose utilization than BAT.54 Further investigation into the physiological differences between brown, beige, and white adipose depots and the potential contribution of brown/beige fat activation to treating metabolic disorders in humans will continue to enhance our understanding of obesity heterogeneity in the future.
Mechanisms of Adipose Effects on the Cardiovascular and Metabolic Systems
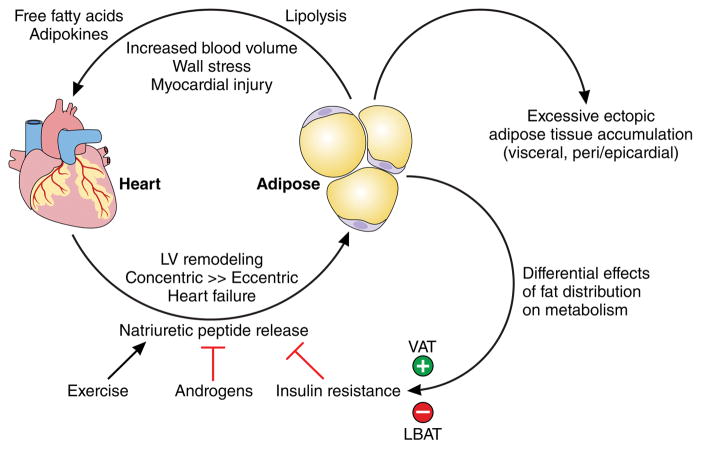
The mechanisms underpinning the pathogenic effects of adipocyte hypertrophy (as a response to excess caloric intake) on the cardiovascular and metabolic systems are well documented.55 A multitude of pathways including, impaired adipogenesis, adipokine dysregulation, inflammation (including macrophage polarization), increased circulating free fatty acids, oxidative stress, adipose tissue hypoxia, lipotoxicity (both local and systemic), and altered energy storage/ectopic fat deposition can directly promote atherosclerosis and endothelial cell dysfunction and indirectly lead to cardiometabolic disease through modulation of risk factors such as hypertension, diabetes, dyslipidemia, and the metabolic syndrome.22 Appreciating the full pathogenic potential of adipose tissue requires an integrated perspective, recognizing the importance of ‘cross-talk’ and interactions between adipose tissue and other body systems.55 Visceral obesity, for example, has a number of local effects on the myocardium including inducing cardiomyocyte hypertrophy, myocardial fibrosis, and activation of inflammatory pathways relating to macrophage infiltration and cytokine gene expression.56 One current model for adiposity-related cardiac dysfunction integrates these data into a potential etiologic pathway leading from obesity to heart failure (Figure 3). Excessive fat accumulation in adipose tissue and ectopic sites, such as the viscera, pericardium/epicardium, and liver, result in increasing circulating blood volume and local and systemic pro-atherogenic inflammatory factors which act to increased stroke volume, cardiac wall stress, and myocardial injury leading to concentric left ventricular hypertrophy, left ventricular remodeling, and ultimately diastolic and systolic cardiac failure.
Figure 3. Mechanistic Model for Effects of Obesity on Cardiac Dysfunction and Heart Failure.
Model for adiposity-related cardiac dysfunction integrates our understanding of body fat distribution into a potential etiologic pathway leading from obesity to heart failure. Excessive fat accumulation in visceral and ectopic sites result in increasing circulating blood volume and local and systemic pro-atherogenic inflammatory factors which act to increased stroke volume, cardiac wall stress, and myocardial injury leading to concentric left ventricular hypertrophy, left ventricular remodeling, and ultimately diastolic and systolic cardiac failure. Natriuretic peptides released by cardiomyocytes may exert differential effects on fat metabolism in a positive feedback loop modified by factors such as exercise, sex hormones, and insulin resistance. LBAT=lower body subcutaneous adipose tissue; VAT=visceral adipose tissue
Further data regarding a cardiac-adipose axis come from the observations that obesity, and in particular greater VAT, is associated with lower natriuretic peptide levels in asymptomatic individuals (so-called “natriuretic peptide deficiency”) whereas more LBAT is associated with higher natriuretic peptide levels.57 Moreover, natriuretic peptides have been shown to increase after gastric bypass surgery induced weight loss by up to 50%.58 From a mechanistic standpoint, atrial natriuretic and B-type natriuretic peptides (BNP) bind to NPR-A receptors on adipose tissue and stimulate lipolysis59, promote browning of adipocytes60, regulate body fat distribution by activation of peroxisome proliferator-activated receptor gamma gene expression61, enhance adiponectin secretion from adipocytes62, and improve human skeletal muscle oxidation fat and glucose utilization.63 Mice that overexpress or are treated with exogenous infusions of natriuretic peptide exhibit reduced fat mass, improved glucose tolerance, and enhanced energy expenditure 60, suggesting that the “lipomobilizing” effects of natriuretic peptides may have salutary consequences on body fat metabolism and distribution. These experimental data provide evidence that natriuretic peptides released by cardiomyocytes may indeed exert beneficial effects on fat metabolism in a positive feedback loop resulting in differential effects of fat distribution on metabolism partly mediated through the heart. The question of whether the cardiac benefits of increasing circulating levels of endogenous natriuretic peptides, as seen in a recent heart failure trial64, are in part mediated by metabolic and/or adipose modulation remains unanswered.
The persistent turnover of mesenteric triglycerides in spite of hyperinsulinemia as seen with obesity delivers glycerol and fatty acids directly into the portal circulation, providing both a gluconeogenic substrate and energy for gluconeogenesis in the liver.65 Glycerol contributes about 10% to total glucose production after an overnight fast in healthy non-obese participants, but recent data has demonstrated that visceral adiposity may significantly alter glucose production from glycerol. A study of obese adults without diabetes showed that individuals with high visceral fat had less incorporation of orally-ingested, biologically-labeled glycerol via hepatic gluconeogenesis compared with similarly BMI-matched obese persons with low visceral fat.66 These results suggested a dilution effect from excess unlabeled endogenous glycerol substrates from the VAT contributing to gluconeogenesis. Furthermore, high visceral fat individuals who were non-fasting had even less exogenous glycerol incorporation into glucose. Overall, these findings provide intriguing evidence that excess VAT may act as a “constitutively fed state” and result in increased risk for hyperglycemia and type 2 diabetes through overstimulation of hepatic gluconeogenesis by chronic delivery of glycerol arising from mesenteric triglyceride turnover directly into the portal circulation and to the liver. A more thorough discussion of the multiple and complex mechanisms to explain how obesity can cause cardiovascular and metabolic disease is reviewed elsewhere.67
Cardiovascular Risk of the Metabolically Healthy Obese Patient
The concept of “metabolically healthy obesity” has been heavily debated as the prevalence of presumably MHO subjects is largely determined by clinical cut-offs used to define abnormal levels of risk factors.68 For example, having a blood pressure <130/85 mmHg does not mean that blood pressure is optimally controlled for maximum risk reduction. For instance, studies that have shown that MHO individuals nevertheless had an increased CVD event rate, also found that these individuals were characterized by subtle differences in their risk factor profile compared to healthy non-obese individuals.
MHO individuals have been estimated to account for as much as 30–40% of the obese adult population.69 A meta-analysis of 8 studies (N = 61,386 patients with 3,988 events) designed to critically evaluate all-cause mortality or cardiovascular events (or both) in relation to clinical characteristics of 6 patient groups defined by BMI category (normal weight/overweight/obese) and metabolic status (healthy/unhealthy) found that MHO was nevertheless associated with an increased risk for events compared with metabolically healthy normal-weight (RR, 1.24; 95% CI, 1.02 to 1.55) when studies with ≥10 years of follow-up were considered.70 All metabolically unhealthy groups had a similarly elevated risk: normal weight (RR, 3.14; CI, 2.36 to 3.93), overweight (RR, 2.70; CI, 2.08 to 3.30), and obese (RR, 2.65; CI, 2.18 to 3.12). The findings from this meta-analysis suggest that there is no healthy pattern of increased weight since, compared with metabolically healthy normal-weight individuals, obese persons are at increased risk for adverse long term outcomes even in the absence of metabolic abnormalities. However, the relationship between metabolic health and obesity may be more nuanced, since a population-based prospective cohort study from Norway with 12 years of follow-up found that in relation to acute myocardial infarction, obesity without metabolic abnormalities did not confer substantial excess risk, not even for severe or long-lasting obesity; whereas for heart failure, even MHO was associated with increased risk, particularly for long-lasting or severe obesity.71 More recently, investigators used a sample of 7,579 fasting adults from the 1999–2006 NHANES survey to compare all-cause mortality in six mutually exclusive groups: 1) metabolically healthy and normal weight (referent group), 2) metabolically healthy and overweight, 3) MHO, 4) metabolically abnormal and normal weight, 5) metabolically abnormal and overweight, and 6) metabolically abnormal and obese.72 The unweighted median follow-up was 103 months with 770,568 person-months of follow-up and an incidence rate of 1.18 deaths per 1000 person-months. When compared with those who were metabolically healthy and with a normal BMI, all other metabolic and weight groups showed an increased mortality risk. There was no evidence of effect modification by physical activity or demographic characteristics. A more recent meta-analysis assessing the risks of cardiovascular events and all-cause mortality in MHO individuals was recently published with data derived from 22 prospective studies, involving 584,799 participants using metabolically healthy normal weight as the reference group. This study showed that the MHO phenotype was associated with a higher risk of CVD events (HR 1.60, 95% CI 1.38 to 1.84), but not all-cause mortality (HR 1.07, 95% CI 0.92 to 1.25).73
There are, however, inherent limitations associated with these meta-analyses which have an obvious influence on the conclusions reached. As mentioned before, the lack of a standard definition for MHO makes study comparisons difficult and is an important factor contributing to the substantial heterogeneity observed between studies. Many studies have defined the MHO phenotype based on various combinations of four conventional metabolic criteria: 1) blood pressure, 2) HDL cholesterol, 3) triglycerides and 4) fasting plasma glucose. Other components less commonly used to define MHO have been insulin resistance (HOMA-IR) and C-reactive protein. Furthermore, most studies were performed in predominantly Caucasian populations. In addition, the term “healthy” may not be suitable to describe secular trends in populations because of the possibility of underestimating the long-term effects of obesity, as well as minimizing the public health importance of maintaining a normal weight. Weight gain, independent of one’s particular baseline risk factor profile, is an important risk factor for the development of metabolic abnormalities. However, no studies to date have reported data regarding weight changes during the follow-up. Another limitation of these meta-analyses was inadequate adjustment for many potentially important confounding factors such as age, sex, physical activity, regional body fat distribution and duration of disease.
Ultimately, the notion of a MHO phenotype may depend on the context of the disease/outcome for which it impacts. For example, whereas an obese patient may be metabolically normal for the endocrinologist, obesity-related health outcomes may be very different in the view of the orthopedic surgeon. Indeed, from a clinical perspective, anthropometric-based classification schemes for excess adiposity do not incorporate assessments of obesity-related comorbidity and impairment in a patient’s functional status. One alternative paradigm that had been developed is a clinical staging system that ranks individuals with excess adiposity on a 5-point ordinal scale, while incorporating obesity-related comorbidities and functional status into the global clinical patient assessment (Table 3).74 Investigators examined the ability of this paradigm, called the Edmonton obesity staging system, in predicting mortality using data from the NHANES III (1988–1994) and the NHANES IV (1999–2004) surveys, with mortality follow-up through 2006 in overweight or obese adults. The obesity staging system was independently associated with increased mortality even after adjustment for contemporary methods of classifying adiposity. It may offer improved clinical utility as an adjunctive tool to current anthropometric classification systems in assessing obesity-related risk.75 Limitations of this staging system include lack of visceral adiposity assessment, one of the major cardiovascular risk factors of obesity76, and the fact that comorbidities within the Edmonton obesity staging system, such as diabetes and osteoarthritis, were arbitrarily assigned to be equivalent in terms of their burden of illness. It is not yet clear whether certain comorbidities should be given more emphasis when assessing risk for clinical health outcomes. Importantly, this staging system includes an assessment of functional status, which is an under-recognized predictor of adverse health outcomes independent of adiposity. Low exercise capacity has been associated with worse outcomes independent of traditional risk factors77 and a higher level of cardiorespiratory fitness substantially attenuated the adverse effects of obesity in a number of studies.78–80 Studies have also demonstrated that cardiorespiratory fitness could play a central role in the prognosis of MHO individuals.81, 82 On that basis, it has been argued that a greater emphasis should be placed on improving cardiorespiratory fitness rather than weight loss per se in the primary and secondary prevention of CVD in the presumably MHO patient. Finally, although such overweight/obesity phenotypes do exist, we postulate that the prevalence of overweight/obese patients with optimal level of CVD risk factors have been overestimated in most studies.
Table 3.
The 5-Point Edmonton Obesity Staging System
| Stage | Obesity-Related Health Status |
|---|---|
| 0 | No apparent risk factors (blood pressure, serum lipid and fasting glucose levels within normal range), physical symptoms, psychopathology, functional limitations and/or impairment of well-being related to obesity |
| 1 | Presence of obesity-related subclinical risk factors (borderline hypertension, impaired fasting glucose levels, elevated levels of liver enzymes), mild physical symptoms (dyspnea on moderate exertion, occasional aches and pains, fatigue), mild psychopathology, mild functional limitations and/or mild impairment of well-being |
| 2 | Presence of established obesity-related chronic disease (hypertension, type 2 diabetes, sleep apnea, osteoarthritis), moderate limitations in activities of daily living and/or well-being |
| 3 | Established end-organ damage such as myocardial infarction, heart failure, stroke, significant psychopathology, significant functional limitations and/or impairment of well-being |
| 4 | Severe (potentially end stage) disabilities from obesity-related chronic diseases, severe disabling psychopathology, severe functional limitations and/or severe impairment of well-being |
Modified from reference 74
Furthermore, since chronic, non-communicable diseases have always been seen as a continuum rather than a categorical phenomenon, MHO should be considered as a temporary/transition state for most obese individuals rather than a permanent state. For example, in a study with a 20 year follow-up, approximately half of the MHO adults became unhealthy by the end of the study.83 The increased risk for CVD events among MHO participants was particularly high over the long-term and similar risk estimates were observed when MHO was defined by other approaches. A recent study considering the full range of possible definitions for MHO suggested that the risk associated with the MHO phenotype increased with longer follow-up times.84 This systematic review and meta-analysis of prospective studies suggests that obese participants classified on the basis of different metabolic parameters as “metabolically healthy” phenotype are indeed characterized by subtle abnormalities and that they are on the path of developing an altered risk factor profile compared with metabolically healthy normal-weight participants. Nevertheless, their risk was lower than that of metabolically unhealthy obese participants, suggesting an intermediate risk state. In summary, the data available suggest that the MHO phenotype as defined in most studies is not a benign condition over the long-term.
Clinical Implications of Obesity Heterogeneity
Measuring Adiposity
There are many anthropometric methods available clinically to assess a patient’s adiposity that have been used for decades and have a large body of literature detailing their relationships with markers of cardiometabolic heath, outcomes, and interventions to reduce body weight (Table 4).85 Several prospective studies including a recent analysis of the EPIC cohort have shown that within each BMI category an increased waist circumference was associated with an increased mortality risk.86, 87 It is important to point out that, when used in isolation, waist circumference is also an index of total body fat which only shows slightly stronger associations with obesity-related clinical outcomes compared to BMI. It is really in the context of the patient BMI that waist circumference provides incremental information to the clinician. For instance, a waist circumference of 102 cm in a middle aged man with a BMI of 25 kg/m2 clearly indicates the presence of abdominal obesity whereas the same waist value in a man with a BMI of 32 kg/m2 rather suggests “overall obesity” which is associated with a different health risk. In a large international cardiometabolic CT imaging study (INSPIRE ME IAA), it was found that within every single BMI unit category, an increased waist circumference was predictive of an increased level of visceral adipose tissue.88 As visceral obesity is often accompanied by more liver fat content driving an increased production of triglyceride-rich very low density lipoproteins, it has been proposed that the combined presence of an elevated waistline and of increased triglyceride levels was associated with a high probability (~80%) for the patient to be characterized by high levels of visceral adipose tissue.89 Several studies have since confirmed the notion that the so-called “hypertriglyceridemic waist” phenotype is a simple and quick clinical tool to screen for the presence of excess visceral adipose tissue/ectopic fat.90 Therefore, within every BMI category, the presence of an increased waist circumference accompanied by elevated hypertriglyceridemia (above 2 mmol/l in men and 1.5 mmol/l in women) should flag the need for further investigation as it may identify patients with increased levels of VAT and liver fat.
Table 4.
Potential Clinical Utility of Methods to Assess Adiposity
| Method | Clinical Use | Surrogate for Visceral Adiposity |
|---|---|---|
| Body mass index | +++ | + |
| Waist circumference | +++ | ++ |
| Waist-height ratio | ++ | ++ |
| Waist-hip ratio | ++ | ++ |
| Hypertriglyceridemic waist | +++ | ++ |
| Computed tomography (CT) | ??? | +++ |
| Magnetic resonance imaging (MRI) | ??? | +++ |
| Dual x-ray absorptiometry (DXA) | ??? | +++ |
Modified from reference 85
Although anthropometric indices of central obesity (such as waist circumference and waist-height or –hip ratios) are easy to implement clinically, their correlation with direct imaging-based assessments of visceral adiposity are modest; furthermore, these indices incorporate both the abdominal subcutaneous and visceral depots which, as discussed above, are anatomically and functionally distinct. Newer imaging-based methods offer more sensitivity and specificity for measuring specific adipose depots including visceral and ectopic fat. However, the two most widely utilized methods in research (CT and MRI) have significant drawbacks limiting their use in clinical practice. CT imaging can be done rapidly and interpreted using commercial software that segments the adipose depots and measures their area or volume. CT segmentation is based on the difference in Hounsfield units (a CT measurement of radiodensity) between adipose tissue and other soft tissues. However, CT exposes the subject to radiation so it is not ideal for serial assessments over time or to evaluate change after an intervention. MRI imaging is more time consuming and expensive, but does not involve radiation, and may therefore be used for serial assessments over time. Growing interest in this area has seen the emergence of research-based companies specializing in advanced body fat imaging, such as Advanced MR Analytics (Linköping, Sweden, www.amra.se), that offer cloud-based, 3-D volumetric assessments of body fat distribution using a rapid 6 minute MRI scan. However, both CT and MRI imaging modalities are cost-intensive and require a specially trained technologist to administer the exams, making them less attractive for office based assessment. Dual x-ray absorptiometry (DXA), historically used to measure bone mineral density and body composition, is now being investigated as a lower cost, lower radiation alternative to MRI and CT. It has been recently shown that DXA assessment of visceral adiposity using a commercial software program was highly accurate compared with the gold-standard MRI in >2000 multiethnic men and women in the Dallas Heart Study.91 DXA can be an office based procedure and given its low radiation profile is ideal for serial assessments. However, currently, measurement of VAT using DXA is not reimbursed by most commercial insurances or Medicare.
Therapeutic Implications
Recent guidelines from the American Heart Association, American College of Cardiology, and The Obesity Society on the management of overweight and obesity in adults92 recommend measurement of waist circumference and height and weight to calculate BMI at least annually, but are silent on assessment of body fat distribution or visceral adiposity. Other professional societies such as the International Atherosclerosis Society which recently held a Visceral Adiposity Working Group session, are becoming increasingly focused on integrating more detailed assessments of body fat distribution into risk assessment and treatment paradigms. For example, at a Think Tank93 on metabolic syndrome sponsored by the American College of Cardiology and American Association of Clinical Endocrinologists in 2015, visceral adiposity/ectopic fat was included in a novel staging system for the metabolic syndrome and incorporated as a specific risk factor for metabolic syndrome in the absence of any other diagnostic criteria. Furthermore, the staging system recommended specific therapies to address visceral adiposity/ectopic fat including increasing physical activity, improved nutritional choices, and obesity prevention.
A growing body of evidence on the lifestyle, pharmacologic, and surgical interventions for visceral adiposity suggests that a multidisciplinary approach to risk stratification in obese individuals and targeted treatment strategies may improve outcomes while limiting therapies to persons who are most likely to benefit. First, it is important to emphasize that even in the absence of weight loss, targeting the lifestyle of viscerally obese patients will be associated with clinical benefits. For instance, lifestyle intervention studies using physical activity/exercise have reported improvements in indices of plasma glucose-insulin homeostasis which were more closely related to the loss of visceral adipose tissue than to body weight or fat loss.94, 95 Furthermore, in the landmark PREDIMED trial involving 7,447 patients with 50% of them having type 2 diabetes, it was reported that an approach favoring the adherence to a Mediterranean diet (by providing olive oil and nuts to patients) was associated with a significant reduction in CVD outcomes over the 4.8 year intervention period.96 It is important to point out that such remarkable clinical benefits were observed in the absence of weight loss. Although no similar physical activity/exercise randomized trial has been conducted in abdominally obese patients, studies that have compared the CVD event rates in physically active vs. physically inactive men and women with/without abdominal obesity and with features of the metabolic syndrome have shown a remarkable impact of physical activity on reducing CVD, even among those who were abdominally obese.97 These results are critical to emphasize the importance of targeting lifestyle per se in abdominally obese patients, even in the absence of weight loss. Thus, improving nutritional quality, increasing physical activity level, and improving cardiorespiratory fitness (as an index of regular participation in vigorous physical activity/exercise) are important and clinically relevant targets in abdominally obese patients. Simple tools to assess “lifestyle vital signs” in clinical practice have been proposed and this topic is currently a fertile area of investigation.
In addition to the clinical benefits of lifestyle, weight loss in viscerally obese patients has also been reported to predict improvements in several markers of cardiometabolic risk. For instance, clinical studies in overweight and obese patients show that visceral, and not abdominal subcutaneous, adiposity reduction drives the benefits of a 1-year lifestyle modification program.94 Multiple systematic reviews and meta-analyses have been published detailing the effects of diet and exercise on visceral fat reduction in the short term (usually <12 weeks). Both very-low calorie diet98 and exercise99 produce preferential loss of VAT, with aerobic (rather than resistance) exercise having the greatest benefits.100 Unpublished meta-analysis data including 12 studies between 2000 and 2014 evaluated the impact of a sustained intervention (≥6 months) on VAT and demonstrated significant reductions in VAT area with both lifestyle and pharmacologic interventions. Exercise showed a greater, more sustained impact on changes in visceral adiposity compared with pharmacological therapies. In addition, because exercise training can potentially increase lean body mass and since VAT represents a relatively minor component of total body fat, it has also be reported that a reduction of VAT with a lifestyle modification program including regular exercise could even be observed in the absence of body weight loss. In those situations (visceral loss and lean body mass gain), the measurement of waist circumference may allow the clinician to perceive that diabetogenic/atherogenic visceral adipose tissue was lost. On that basis, it has been suggested that waist loss may be a more preferable therapeutic target than weight loss, particularly in high risk prediabetic or diabetic obese patients who have more visceral adipose tissue at any BMI than nondiabetic individuals. Loss of visceral adipose tissue in high risk viscerally obese patients is a marker that the size of ectopic fat depots has been reduced. Indeed, lifestyle intervention studies have shown a reduction in liver, cardiac and skeletal muscle fat and these changes also contributed to the improvements in the cardiometabolic risk profile, although the specific contribution of each of these ectopic fat depots is currently under investigation.101
Multiple medications are now FDA-approved for sustained treatment of obesity as adjuncts to lifestyle modification to induce weight loss. Medications such as sustained-release phentermine/topiramate, liraglutide, lorcaserin, naltrexone-bupropion, and orlistat all help reduce body weight to varying degrees by ~2 to 10 kg over 6 months to 1 year (Table 5).102 Few data are available describing the effects of these medications on visceral and ectopic fat depots. Preliminary data in small dedicated sub-studies within larger randomized trials of some medications such as liraglutide103 and naltrexone/bupropion have showed promising results with greater reductions in visceral fat compared with subcutaneous fat. Whether these reductions in visceral adiposity translate to improvement in surrogate outcomes (e.g. lipid levels, inflammatory markers, markers of cardiac injury or hemodynamic stress) in high risk individuals remains an open question. A randomized, placebo-controlled clinical trial has been recently launched to address this question using liraglutide in order to better assess its impact on body fat distribution and markers of cardiometabolic risk in a population of overweight and obese individuals at high risk for CVD (Clinicaltrials.gov number, NCT03038620).
Table 5.
Pharmacotherapy for Long-Term Obesity Management in the United States in 2017
| Drug (generic) | Dosage | Mean Weight Loss above Lifestyle | Common Side Effects | Contraindications | Special Populations |
|---|---|---|---|---|---|
| Phentermine/Topiramate | 7.5/46 mg daily | 6.6 kg, 1 year | Insomnia, dry mouth, nausea | Pregnancy, multiple drug interactions | Young, no CVD risk factors |
| Liraglutide | 3.0 mg daily | 5.8 kg, 1 year | Nausea, vomiting, pancreatitis | Medullary thyroid cancer, MEN-2 | Type 2 diabetes |
| Lorcaserin | 10 mg bid | 3.6 kg, 1 year | Headache, nausea | Pregnancy, multiple drug interactions | CVD risk factors |
| Naltrexone/Bupropion | 32/360 mg qid | 4.8 kg, 1 year | Nausea, constipation | Seizure, eating disorder, drug or ETOH withdrawal | Addiction disorders |
| Orlistat (Rx and OTC) | 60/120 mg tid | 2.9–3.4 kg, 1 year | Steatorrhea, flatulence, fecal discharge | Cyclosporine, malabsorption, pregnancy, certain meds | Available over-the-counter |
Modified from reference 102
Surgical therapies for weight loss such as gastric bypass, gastric sleeve, and laparoscopic gastric banding are being increasingly used to treat severe obesity and type 2 diabetes.104 Both gastric bypass and gastric sleeve significantly reduce visceral fat by ~40–50% with more modest reduction in abdominal subcutaneous fat (~10%).105 Peripheral glucose utilization in both skeletal muscle and in adipose tissue is also improved after bariatric surgery. In a recent meta-analysis of 6 studies, diabetes remission rates after surgery ranged from 66.7% with gastric bypass to 28.6% with gastric banding. Improvements in glycated hemoglobin, fasting blood glucose, and reduction in the need for glucose lowering therapies were also seen with bariatric surgery.106 Multiple observational case series also suggest long term benefits for heart failure with both preserved and reduced ejection fraction after bariatric surgery.107 Evidence that sustained weight loss through surgery can modulate visceral and epicardial fat108 also supports a potential role for bariatric surgery in the treatment of obesity-related cardiovascular risk.
Unanswered Questions, Future Directions, and Public Health Messaging
Although it is clear that the accumulation of visceral and ectopic body fat is a major contributor to cardiovascular and metabolic risk above and beyond the BMI, implementation of fat distribution assessment into clinical practice remains a challenge. Future endeavors should focus on better understanding the factors that influence an individual’s body fat distribution profile in order to answer why a person preferentially accumulates fat in one depot over another. Although evidence of genetic predisposition is available, molecular mechanisms responsible for such genetic susceptibility are largely speculative at this time. In addition, how type and makeup of diet, or other lifestyle factors play a role will be important areas of investigations for the future. Emerging technologies allow assessment of visceral and lower body adiposity that is faster, cheaper, and at less risk than ever before. A focus on implementation of visceral and lower body fat measurements into clinical practice should be a priority over the next 5–10 years and clinical assessment of visceral and lower body adiposity should be incorporated into risk paradigms in order to refine risk evaluation and develop improved and effective preventive and therapeutic strategies for high risk obesity. Drug and device companies have a unique opportunity to develop targeted interventions that reduce visceral fat and induce fat re-distribution that could result in body fat remodeling to a more acceptable, healthier body fat profile without necessarily requiring overall or substantial weight loss.
The study of the causes and consequences of excess visceral adiposity/ectopic fat have major clinical implications considering the worldwide epidemic of type 2 diabetes. The current confusion around the health risk of overweight/obesity clearly shows the need to better define its health risk and the importance of measuring/targeting visceral adiposity/ectopic fat and its environmental and genetic determinants. We need to go beyond body weight, BMI, dietary caloric restriction, and generalized weight loss to help patients in clinical practice and in our public health messages. As an example, a national educational campaign on the “waist of the nation” would put this simple index of abdominal obesity on the radar screen of its citizens, the way similar campaigns were conducted in the 1970’s to emphasize the risk of hypertension. However, improving behaviors at the national level is a daunting objective and will require that the healthy behaviors become the easy, default decisions. To help achieve this ambitious goal, on the basis of the scientific evidence available, we propose that it is time to at least align our public health and clinical messages and approaches in our combat against obesity. The impact of obesity on cardiovascular and metabolic diseases is more than just skin deep and we, as a scientific community, have only scratched the surface. So much more can be learned to help combat the growing epidemic of obesity worldwide and build healthier lives, free of cardiovascular diseases for the future.
Acknowledgments
Sources of Funding
Dr. Neeland is supported by grant K23 DK106520 from the NIH/National Institute of Diabetes and Digestive and Kidney Diseases and by the Dedman Family Scholarship in Clinical Care at UT Southwestern Medical Center. Dr. Després’ work has been supported by the Canadian Institutes of Health Research and by the “Fondation de l’Institut Universitaire de Cardiologie et de Pneumologie de Québec - Université Laval”.
Abbreviations
- BMI
body mass index
- CRF
cardiorespiratory fitness
- CT
computed tomography
- CVD
cardiovascular disease
- DXA
dual x-ray absorptiometry
- LBAT
lower body subcutaneous adipose tissue
- MHO
metabolically healthy obesity
- MRI
magnetic resonance imaging
- SAT
subcutaneous adipose tissue
- VAT
visceral adipose tissue
Footnotes
Disclosures
Dr. Neeland has received honoraria, consulting/speaking fees, and other research support from Boehringer-Ingelheim (significant), a research grant from Novo Nordisk (significant), and is a member of the scientific advisory board of Advanced MR Analytics (modest). Dr. Poirier has received honoraria and consulting/speaking fees from Abbott Vascular, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck, Novo Nordisk, Pfizer, Roche, Sanofi-Aventis, Servier, and Valeant (significant). Dr. Després is the scientific director of the International Chair on Cardiometabolic Risk which is supported by the “Fondation de l’Université Laval” (significant).
References
- 1.Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Arnlov J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Furst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 4.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: An update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA. Obesity. Nat Rev Dis Primers. 2017;3:17034. doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 7.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 8.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 9.Despres JP. Body fat distribution and risk of cardiovascular disease: An update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 10.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 11.Lainscak M, von Haehling S, Doehner W, Anker SD. The obesity paradox in chronic disease: Facts and numbers. J Cachexia Sarcopenia Muscle. 2012;3:1–4. doi: 10.1007/s13539-012-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hainer V, Aldhoon-Hainerova I. Obesity paradox does exist. Diabetes Care. 2013;36(Suppl 2):S276–281. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Standl E, Erbach M, Schnell O. Defending the con side: Obesity paradox does not exist. Diabetes Care. 2013;36(Suppl 2):S282–286. doi: 10.2337/dcS13-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ZJ, Zhou YJ, Galper BZ, Gao F, Yeh RW, Mauri L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: A systematic review and meta-analysis. Heart. 2015;101:1631–1638. doi: 10.1136/heartjnl-2014-307119. [DOI] [PubMed] [Google Scholar]
- 15.Bagheri M, Speakman JR, Shabbidar S, Kazemi F, Djafarian K. A dose-response meta-analysis of the impact of body mass index on stroke and all-cause mortality in stroke patients: A paradox within a paradox. Obes Rev. 2015;16:416–423. doi: 10.1111/obr.12272. [DOI] [PubMed] [Google Scholar]
- 16.Kamel HK, Iqbal MA. Body mass index and mortality among hospitalized elderly patients. Arch Intern Med. 2001;161:1459–1460. doi: 10.1001/archinte.161.11.1459. [DOI] [PubMed] [Google Scholar]
- 17.Banack HR, Kaufman JS. Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Ann Epidemiol. 2015;25:342–349. doi: 10.1016/j.annepidem.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Lajous M, Banack HR, Kaufman JS, Hernan MA. Should patients with chronic disease be told to gain weight? The obesity paradox and selection bias. Am J Med. 2015;128:334–336. doi: 10.1016/j.amjmed.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loprinzi P, Smit E, Lee H, Crespo C, Andersen R, Blair SN. The “fit but fat” paradigm addressed using accelerometer-determined physical activity data. N Am J Med Sci. 2014;6:295–301. doi: 10.4103/1947-2714.136901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loprinzi PD. Application of the “fat-but-fit” paradigm in predicting 10-yr risk for an atherosclerotic cardiovascular disease (ASCVD) event using the pooled cohort risk equations among US adults. Int J Cardiol. 2016;202:297–299. doi: 10.1016/j.ijcard.2015.09.057. [DOI] [PubMed] [Google Scholar]
- 21.McAuley PA, Beavers KM. Contribution of cardiorespiratory fitness to the obesity paradox. Prog Cardiovasc Dis. 2014;56:434–440. doi: 10.1016/j.pcad.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 23.Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, Scherer PE. Mitoneet-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18:1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 26.Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab. 2002;282:E657–663. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- 27.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM, de Lemos JA. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 2013;21:E439–447. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Divoux A, Karastergiou K, Xie H, Guo W, Perera RJ, Fried SK, Smith SR. Identification of a novel lncrna in gluteal adipose tissue and evidence for its positive effect on preadipocyte differentiation. Obesity (Silver Spring) 2014;22:1781–1785. doi: 10.1002/oby.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Araneta MR, Kanaya AM, Hsu WC, Chang HK, Grandinetti A, Boyko EJ, Hayashi T, Kahn SE, Leonetti DL, McNeely MJ, Onishi Y, Sato KK, Fujimoto WY. Optimum BMI cut points to screen Asian Americans for type 2 diabetes. Diabetes Care. 2015;38:814–820. doi: 10.2337/dc14-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, Massien C, Almeras N, Despres JP. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: The International Study of Prediction of Intra-abdominal Adiposity and its Relationship with Cardiometabolic Risk/Intra-abdominal Adiposity. Am J Clin Nutr. 2012;96:714–726. doi: 10.3945/ajcn.112.035758. [DOI] [PubMed] [Google Scholar]
- 32.Shah AD, Kandula NR, Lin F, Allison MA, Carr J, Herrington D, Liu K, Kanaya AM. Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: Results from the MASALA and MESA studies. Int J Obes (Lond) 2016;40:639–645. doi: 10.1038/ijo.2015.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 34.Despres JP, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: The Health, Risk factors, Exercise training, and Genetics (HERITAGE) Family Study. Arterioscler Thromb Vasc Biol. 2000;20:1932–1938. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 35.Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, Khera A, McGuire DK, de Lemos JA, Turer AT. The relationship of body mass and fat distribution with incident hypertension: Observations from the Dallas Heart Study. J Am Coll Cardiol. 2014;64:997–1002. doi: 10.1016/j.jacc.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 36.Neeland IJ, Turer AT, Ayers CR, Berry JD, Rohatgi A, Das SR, Khera A, Vega GL, McGuire DK, Grundy SM, de Lemos JA. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015;65:2150–2151. doi: 10.1016/j.jacc.2015.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, Berry JD, Khera A, McGuire DK, Vega GL, Grundy SM, de Lemos JA, Drazner MH. Relation of regional fat distribution to left ventricular structure and function. Circulation. Cardiovascular Imaging. 2013;6:800–807. doi: 10.1161/CIRCIMAGING.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yano Y, Vongpatanasin W, Ayers C, Turer A, Chandra A, Carnethon MR, Greenland P, de Lemos JA, Neeland IJ. Regional fat distribution and blood pressure level and variability: The Dallas Heart Study. Hypertension. 2016;68:576–583. doi: 10.1161/HYPERTENSIONAHA.116.07876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, Ariga R, Thomas S, Francis J, Rodgers C, Clarke W, Sabharwal N, Antoniades C, Schneider J, Robson M, Clarke K, Karamitsos T, Rider O, Neubauer S. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol. 2016;68:53–63. doi: 10.1016/j.jacc.2016.03.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyall DM, Celis-Morales C, Ward J, Iliodromiti S, Anderson JJ, Gill JMR, Smith DJ, Ntuk UE, Mackay DF, Holmes MV, Sattar N, Pell JP. Association of body mass index with cardiometabolic disease in the UK Biobank: A mendelian randomization study. JAMA Cardiol. 2017;2:882–889. doi: 10.1001/jamacardio.2016.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatterjee NA, Giulianini F, Geelhoed B, Lunetta KL, Misialek JR, Niemeijer MN, Rienstra M, Rose LM, Smith AV, Arking DE, Ellinor PT, Heeringa J, Lin H, Lubitz SA, Soliman EZ, Verweij N, Alonso A, Benjamin EJ, Gudnason V, Stricker BH, van der Harst P, Chasman DI, Albert CM. Genetic obesity and the risk of atrial fibrillation-causal estimates from mendelian randomization. Circulation. 2017;135:741–754. doi: 10.1161/CIRCULATIONAHA.116.024921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emdin CA, Khera AV, Natarajan P, Klarin D, Zekavat SM, Hsiao AJ, Kathiresan S. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA. 2017;317:626–634. doi: 10.1001/jama.2016.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 44.Gupta A, Pandey A, Ayers C, Beg MS, Lakoski SG, Vega GL, Grundy SM, Johnson DH, Neeland IJ. An analysis of individual body fat depots and risk of developing cancer: Insights from the Dallas Heart Study. Mayo Clin Proc. 2017;92:536–543. doi: 10.1016/j.mayocp.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pouliot MC, Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional variation in adipose tissue lipoprotein lipase activity: Association with plasma high density lipoprotein levels. Eur J Clin Invest. 1991;21:398–405. doi: 10.1111/j.1365-2362.1991.tb01387.x. [DOI] [PubMed] [Google Scholar]
- 46.Rebuffe-Scrive M, Enk L, Crona N, Lonnroth P, Abrahamsson L, Smith U, Bjorntorp P. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J Clin Invest. 1985;75:1973–1976. doi: 10.1172/JCI111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janiszewski PM, Kuk JL, Ross R. Is the reduction of lower-body subcutaneous adipose tissue associated with elevations in risk factors for diabetes and cardiovascular disease? Diabetologia. 2008;51:1475–1482. doi: 10.1007/s00125-008-1058-0. [DOI] [PubMed] [Google Scholar]
- 48.Loh RKC, Kingwell BA, Carey AL. Human brown adipose tissue as a target for obesity management; beyond cold-induced thermogenesis. Obes Rev. 2017;18:1227–1242. doi: 10.1111/obr.12584. [DOI] [PubMed] [Google Scholar]
- 49.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SH, Plutzky J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab J. 2016;40:12–21. doi: 10.4093/dmj.2016.40.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blondin DP, Labbe SM, Phoenix S, Guerin B, Turcotte EE, Richard D, Carpentier AC, Haman F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593:701–714. doi: 10.1113/jphysiol.2014.283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- 56.Murase T, Hattori T, Ohtake M, Abe M, Amakusa Y, Takatsu M, Murohara T, Nagata K. Cardiac remodeling and diastolic dysfunction in dahls.Z-lepr(fa)/lepr(fa) rats: A new animal model of metabolic syndrome. Hypertens Res. 2012;35:186–193. doi: 10.1038/hr.2011.157. [DOI] [PubMed] [Google Scholar]
- 57.Neeland IJ, Winders BR, Ayers CR, Das SR, Chang AY, Berry JD, Khera A, McGuire DK, Vega GL, de Lemos JA, Turer AT. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J Am Coll Cardiol. 2013;62:752–760. doi: 10.1016/j.jacc.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arora P, Reingold J, Baggish A, Guanaga DP, Wu C, Ghorbani A, Song Y, Chen-Tournaux A, Khan AM, Tainsh LT, Buys ES, Williams JS, Heublein DM, Burnett JC, Semigran MJ, Bloch KD, Scherrer-Crosbie M, Newton-Cheh C, Kaplan LM, Wang TJ. Weight loss, saline loading, and the natriuretic peptide system. J Am Heart Assoc. 2015;4:e001265. doi: 10.1161/JAHA.114.001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: A new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 60.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 mapk to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, Yamahara K, Taura D, Inuzuka M, Sonoyama T, Nakao K. Natriuretic peptides/cgmp/cgmp-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, Okazaki H, Asai M, Nagamachi Y, Maeda N, Shintani Y, Minamino T, Asakura M, Kishimoto I, Funahashi T, Tomoike H, Kitakaze M. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 63.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I, de Glisezinski I, Lieske S, Reinke J, Beckmann B, Langin D, Jordan J, Moro C. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122:4675–4679. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR Investigators P-H, Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 65.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neeland IJ, Hughes C, Ayers CR, Malloy CR, Jin ES. Effects of visceral adiposity on glycerol pathways in gluconeogenesis. Metabolism. 2017;67:80–89. doi: 10.1016/j.metabol.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diaz-Melean CM, Somers VK, Rodriguez-Escudero JP, Singh P, Sochor O, Llano EM, Lopez-Jimenez F. Mechanisms of adverse cardiometabolic consequences of obesity. Curr Atheroscler Rep. 2013;15:364. doi: 10.1007/s11883-013-0364-2. [DOI] [PubMed] [Google Scholar]
- 68.Despres JP. What is “metabolically healthy obesity”?: From epidemiology to pathophysiological insights. J Clin Endocrinol Metab. 2012;97:2283–2285. doi: 10.1210/jc.2012-2081. [DOI] [PubMed] [Google Scholar]
- 69.Phillips CM. Metabolically healthy obesity: Definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14:219–227. doi: 10.1007/s11154-013-9252-x. [DOI] [PubMed] [Google Scholar]
- 70.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159:758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 71.Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trondelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–1078. doi: 10.1016/j.jacc.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 72.Loprinzi PD, Frith E. Cardiometabolic healthy obesity paradigm and all-cause mortality risk. Eur J Intern Med. 2017;43:42–45. doi: 10.1016/j.ejim.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 73.Zheng R, Zhou D, Zhu Y. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: A systematic review and meta-analysis. J Epidemiol Community Health. 2016;70:1024–1031. doi: 10.1136/jech-2015-206948. [DOI] [PubMed] [Google Scholar]
- 74.Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes (Lond) 2009;33:289–295. doi: 10.1038/ijo.2009.2. [DOI] [PubMed] [Google Scholar]
- 75.Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton Obesity Staging System to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ. 2011;183:E1059–1066. doi: 10.1503/cmaj.110387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56:369–381. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 77.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 78.Huot M, Arsenault BJ, Gaudreault V, Poirier P, Perusse L, Tremblay A, Bouchard C, Despres JP, Rheaume C. Insulin resistance, low cardiorespiratory fitness, and increased exercise blood pressure: Contribution of abdominal obesity. Hypertension. 2011;58:1036–1042. doi: 10.1161/HYPERTENSIONAHA.111.180349. [DOI] [PubMed] [Google Scholar]
- 79.Rheaume C, Arsenault BJ, Belanger S, Perusse L, Tremblay A, Bouchard C, Poirier P, Despres JP. Low cardiorespiratory fitness levels and elevated blood pressure: What is the contribution of visceral adiposity? Hypertension. 2009;54:91–97. doi: 10.1161/HYPERTENSIONAHA.109.131656. [DOI] [PubMed] [Google Scholar]
- 80.Rheaume C, Arsenault BJ, Dumas MP, Perusse L, Tremblay A, Bouchard C, Poirier P, Despres JP. Contributions of cardiorespiratory fitness and visceral adiposity to six-year changes in cardiometabolic risk markers in apparently healthy men and women. J Clin Endocrinol Metab. 2011;96:1462–1468. doi: 10.1210/jc.2010-2432. [DOI] [PubMed] [Google Scholar]
- 81.Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: Impact of cardiorespiratory fitness. Diabetes Care. 2005;28:391–397. doi: 10.2337/diacare.28.2.391. [DOI] [PubMed] [Google Scholar]
- 82.Ortega FB, Cadenas-Sanchez C, Sui X, Blair SN, Lavie CJ. Role of fitness in the metabolically healthy but obese phenotype: A review and update. Prog Cardiovasc Dis. 2015;58:76–86. doi: 10.1016/j.pcad.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Bell JA, Hamer M, Sabia S, Singh-Manoux A, Batty GD, Kivimaki M. The natural course of healthy obesity over 20 years. J Am Coll Cardiol. 2015;65:101–102. doi: 10.1016/j.jacc.2014.09.077. [DOI] [PubMed] [Google Scholar]
- 84.Eckel N, Meidtner K, Kalle-Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:956–966. doi: 10.1177/2047487315623884. [DOI] [PubMed] [Google Scholar]
- 85.Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, Poirier P. Assessing adiposity: A scientific statement from the American Heart Association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 86.Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami HO, Ebbert JO, English DR, Gapstur SM, Giles GG, Horn-Ross PL, Park Y, Patel AV, Robien K, Weiderpass E, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hartge P, Bernstein L, Berrington de Gonzalez A. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89:335–345. doi: 10.1016/j.mayocp.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lassale CTI, Moons KGM, Sweeting M, Boer J, Johnson L, Huerta JM, Agnoli C, Freisling H, Weiderpass E, Wennberg P, van der ADL, Arriola L, Benetou V, Boeing H, Bonnet F, Colorado-Yohar SM, Engström G, Eriksen AK, Ferrari P, Grioni S, Johansson M, Kaaks R, Katsoulis M, Katzke V, Key TJ, Matullo G, Melander O, Molina-Portillo E, Moreno-Iribas C, Norberg M, Overvad K, Panico S, Quirós JR, Saieva C, Skeie G, Steffen A, Stepien M, Tjønneland A, Trichopoulou A, Tumino R, van der Schouw YT, Verschuren WMM, Langenberg C, Di Angelantonio E, Riboli E, Wareham NJ, Danesh J, Butterworth AS. Separate and combined associations of obesity and metabolic health with coronary heart disease: A pan-european case-cohort analysis. Eur Heart J. 2017 Aug 14; doi: 10.1093/eurheartj/ehx448. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nazare JA, Smith J, Borel AL, Aschner P, Barter P, Van Gaal L, Tan CE, Wittchen HU, Matsuzawa Y, Kadowaki T, Ross R, Brulle-Wohlhueter C, Almeras N, Haffner SM, Balkau B, Despres JP Investigators IMI. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study) Am J Cardiol. 2015;115:307–315. doi: 10.1016/j.amjcard.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 89.Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Almeras N, Bergeron J, Gaudet D, Tremblay G, Prud’homme D, Nadeau A, Despres JP. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179–184. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 90.Lemieux I, Poirier P, Bergeron J, Almeras N, Lamarche B, Cantin B, Dagenais GR, Despres JP. Hypertriglyceridemic waist: A useful screening phenotype in preventive cardiology? Can J Cardiol. 2007;23(Suppl B):23B–31B. doi: 10.1016/s0828-282x(07)71007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neeland IJ, Grundy SM, Li X, Adams-Huet B, Vega GL. Comparison of visceral fat mass measurement by dual-x-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: The Dallas Heart Study. Nutr Diabetes. 2016;6:e221. doi: 10.1038/nutd.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines and The Obesity Society. Circulation. 2014;129:S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sperling LS, Mechanick JI, Neeland IJ, Herrick CJ, Despres JP, Ndumele CE, Vijayaraghavan K, Handelsman Y, Puckrein GA, Araneta MR, Blum QK, Collins KK, Cook S, Dhurandhar NV, Dixon DL, Egan BM, Ferdinand DP, Herman LM, Hessen SE, Jacobson TA, Pate RR, Ratner RE, Brinton EA, Forker AD, Ritzenthaler LL, Grundy SM. The Cardiometabolic Health Alliance: Working toward a new care model for the metabolic syndrome. J Am Coll Cardiol. 2015;66:1050–1067. doi: 10.1016/j.jacc.2015.06.1328. [DOI] [PubMed] [Google Scholar]
- 94.Borel AL, Nazare JA, Smith J, Almeras N, Tremblay A, Bergeron J, Poirier P, Despres JP. Visceral and not subcutaneous abdominal adiposity reduction drives the benefits of a 1-year lifestyle modification program. Obesity (Silver Spring) 2012;20:1223–1233. doi: 10.1038/oby.2011.396. [DOI] [PubMed] [Google Scholar]
- 95.Borel AL, Nazare JA, Baillot A, Almeras N, Tremblay A, Bergeron J, Poirier P, Despres JP. Cardiometabolic risk improvement in response to a 3-yr lifestyle modification program in men: Contribution of improved cardiorespiratory fitness vs. weight loss. Am J Physiol Endocrinol Metab. 2017;312:E273–E281. doi: 10.1152/ajpendo.00278.2016. [DOI] [PubMed] [Google Scholar]
- 96.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 97.Broekhuizen LN, Boekholdt SM, Arsenault BJ, Despres JP, Stroes ES, Kastelein JJ, Khaw KT, Wareham NJ. Physical activity, metabolic syndrome, and coronary risk: The EPIC-NORFOLK prospective population study. Eur J Cardiovasc Prev Rehabil. 2011;18:209–217. doi: 10.1177/1741826710389397. [DOI] [PubMed] [Google Scholar]
- 98.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: Findings from a systematic review. Int J Obes (Lond) 2008;32:619–628. doi: 10.1038/sj.ijo.0803761. [DOI] [PubMed] [Google Scholar]
- 99.Verheggen RJ, Maessen MF, Green DJ, Hermus AR, Hopman MT, Thijssen DH. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: Distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17:664–690. doi: 10.1111/obr.12406. [DOI] [PubMed] [Google Scholar]
- 100.Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: A systematic review and meta-analysis. PLoS ONE. 2013;8:e56415. doi: 10.1371/journal.pone.0056415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Despres JP. Obesity and cardiovascular disease: Weight loss is not the only target. Can J Cardiol. 2015;31:216–222. doi: 10.1016/j.cjca.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 102.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD, Endocrine S. Pharmacological management of obesity: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 103.Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rossner S, Savolainen MJ, Van Gaal L. Safety, tolerability and sustained weight loss over 2 years with the once-daily human glp-1 analog, liraglutide. Int J Obes (Lond) 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Piche ME, Auclair A, Harvey J, Marceau S, Poirier P. How to choose and use bariatric surgery in 2015. Can J Cardiol. 2015;31:153–166. doi: 10.1016/j.cjca.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 105.Dadson P, Landini L, Helmio M, Hannukainen JC, Immonen H, Honka MJ, Bucci M, Savisto N, Soinio M, Salminen P, Parkkola R, Pihlajamaki J, Iozzo P, Ferrannini E, Nuutila P. Effect of bariatric surgery on adipose tissue glucose metabolism in different depots in patients with or without type 2 diabetes. Diabetes Care. 2016;39:292–299. doi: 10.2337/dc15-1447. [DOI] [PubMed] [Google Scholar]
- 106.Puzziferri N, Roshek TB, 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: A systematic review. JAMA. 2014;312:934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodriguez Flores M, Aguilar Salinas C, Piche ME, Auclair A, Poirier P. Effect of bariatric surgery on heart failure. Expert Rev Cardiovasc Ther. 2017;15:567–579. doi: 10.1080/14779072.2017.1352471. [DOI] [PubMed] [Google Scholar]
- 108.Gaborit B, Jacquier A, Kober F, Abdesselam I, Cuisset T, Boullu-Ciocca S, Emungania O, Alessi MC, Clement K, Bernard M, Dutour A. Effects of bariatric surgery on cardiac ectopic fat: Lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol. 2012;60:1381–1389. doi: 10.1016/j.jacc.2012.06.016. [DOI] [PubMed] [Google Scholar]