Significance
Polyphosphate kinases (PPKs) are involved in many metabolic processes in bacteria, including pathogenic species. As these enzymes are not present in animals, they are a prime target for the development of novel antibiotics. The detailed knowledge of the mechanism of action and structure–function relationships of these enzymes is of utmost importance for the identification and design of new pharmaceutically active compounds and the rational improvement of lead structures. In addition, PPKs use inexpensive and stable polyphosphate as a phosphate donor and phosphorylate nucleoside 5′-mono- as well as 5′-diphosphates. This makes them of special interest for application in ATP regeneration systems, which can be efficiently coupled to ATP-consuming enzymes in environmentally friendly and sustainable biotechnological processes.
Keywords: kinase, polyphosphate, enzyme structure, kinetics
Abstract
Inorganic polyphosphate is a ubiquitous, linear biopolymer built of up to thousands of phosphate residues that are linked by energy-rich phosphoanhydride bonds. Polyphosphate kinases of the family 2 (PPK2) use polyphosphate to catalyze the reversible phosphorylation of nucleotide phosphates and are highly relevant as targets for new pharmaceutical compounds and as biocatalysts for cofactor regeneration. PPK2s can be classified based on their preference for nucleoside mono- or diphosphates or both. The detailed mechanism of PPK2s and the molecular basis for their substrate preference is unclear, which is mainly due to the lack of high-resolution structures with substrates or substrate analogs. Here, we report the structural analysis and comparison of a class I PPK2 (ADP-phosphorylating) and a class III PPK2 (AMP- and ADP-phosphorylating), both complexed with polyphosphate and/or nucleotide substrates. Together with complementary biochemical analyses, these define the molecular basis of nucleotide specificity and are consistent with a Mg2+ catalyzed in-line phosphoryl transfer mechanism. This mechanistic insight will guide the development of PPK2 inhibitors as potential antibacterials or genetically modified PPK2s that phosphorylate alternative substrates.
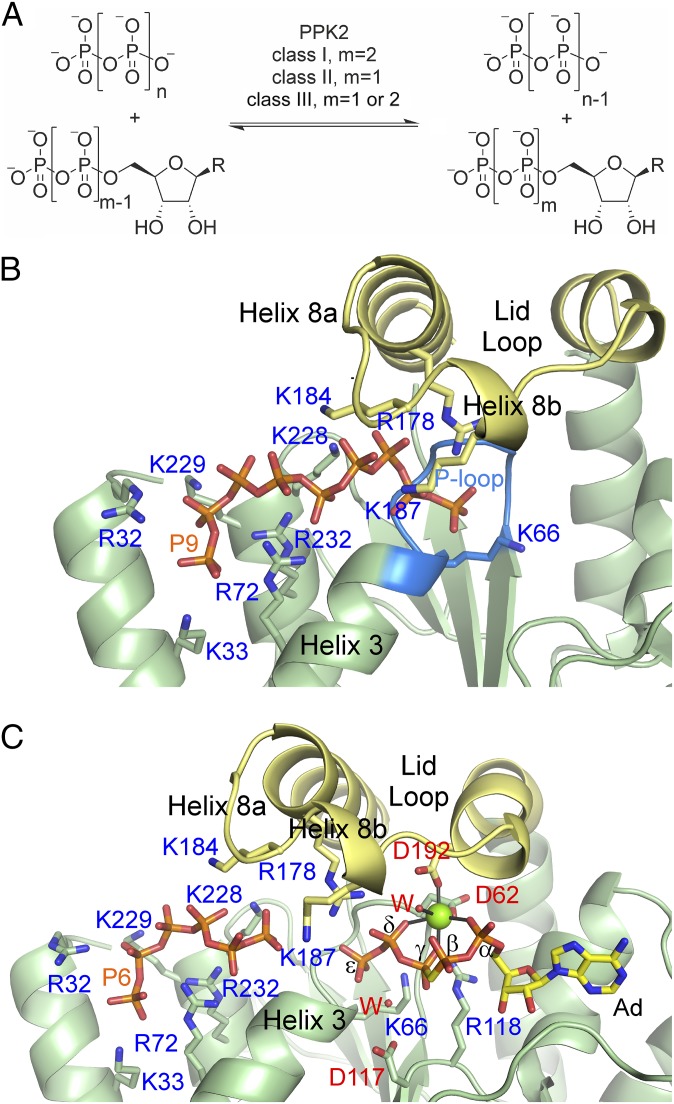
Polyphosphate (polyP) is an inorganic linear polymer of tens to thousands of phosphoryl monomers and is found in all branches of life (1). The biological functions of polyP are numerous, including energy storage, metal chelation, buffering, DNA uptake, gene regulation, and the bacterial stringent response to nutrient deficiency (1, 2). Its importance in processes crucial for bacterial pathogens such as biofilm formation, persistence, motility, quorum sensing, and synthesis of virulence factors has made the enzymes involved in its biosynthesis and utilization promising targets for the development of new antibiotics (2–7). In nature, polyP is synthesized and degraded by polyphosphate kinases (PPKs) that catalyze the reversible transfer of the terminal phosphoryl residue from nucleoside 5′-triphosphates to polyP. Two large, structurally unrelated families of bacterial PPKs have been characterized so far: PPK1 enzymes that favor polyP synthesis (3, 8) and the PPK2 family that favors nucleotide phosphorylation. PPK2s can be phylogenetically subdivided into three classes (9): class I preferentially phosphorylates nucleoside diphosphates (10–12), class II converts nucleoside monophosphates into diphosphates (11, 13–15), and class III can phosphorylate either nucleoside mono- or diphosphates (9) (Fig. 1A). A small subgroup of PPKs that favors the phosphorylation of pyrimidine nucleobases instead of purine nucleobases, which has been designated as PPK3, clusters phylogenetically with the PPK2 family (class I) (16).
Fig. 1.
PPK2 catalysis and substrate binding. (A) Phosphotransfer reactions catalyzed by PPK2 classes. R, nucleobase. (B) PolyP binding to FtPPK2. The lid loop is shown in yellow and the Walker A motif (P-loop) is shown in blue. P9, nonaphosphate. (C) Active-site region of the FtPPK2:AMPPCPPP:polyP complex. The lid loop is shown in yellow. Ad, adenine moiety; P6, hexaphosphate; W, water.
The structure and mechanism of the PPK1 enzyme from Escherichia coli are well characterized (3, 17). Although not a genuine membrane enzyme, PPK1 seems to be associated with the membrane (18), which makes the purification process difficult. The mechanism is proposed to proceed via a phosphorylated enzyme intermediate (8), which is supported by a crystal structure with β,γ-imidoadenosine 5′-triphosphate (AMPPNP) bound in the active site [Protein Data Bank (PDB) ID code 1XDP] (3). In a few cases, PPK1 has been used as a catalyst for ATP regeneration, e.g., as so-called “energy beads” in the form of active inclusion bodies (19). Due to their preference for nucleotide phosphorylation, PPK2 enzymes are more often used for ATP, or—in the case of PPK2 classes II and III—ADP regeneration (20–24); in addition, they are generally easier to purify, as they are soluble cytoplasmic enzymes (25).
Several bacterial PPK2s have been structurally characterized, including the class I enzymes from Sinorhizobium meliloti (PDB ID code 3CZQ) (11) and Francisella tularensis (PDB ID code 4YEG) (26), the class II enzyme from Pseudomonas aeruginosa (PDB ID code 3CZP) (11), and the class III enzyme (9) from Arthrobacter aurescens (PDB ID code 3RHF). However, the absence of substrate-bound structures has made rationalization of the PPK2 mechanism and substrate specificity difficult (9). Here we compare different enzyme-ligand complexes of a class I PPK2 and a class III PPK2. Combining thermodynamic and kinetic analyses with these structures, we propose a model for substrate binding and discrimination as well as a mechanism for PPK2s.
Results
Three-Dimensional Structures.
We report here the crystal structure of Meiothermus ruber (Mr) PPK2 as the archetypal class III PPK2 (9) to a resolution of 1.90 Å. The overall structure is consistent with the general PPK2 fold as described for other class I and II PPK2s (9, 11), which belong to the family of the P-loop kinases and typically contain two conserved sequence motifs (Walker A and Walker B, phosphate-binding loops) and a flexible lid loop built of two α-helices (SI Appendix, Fig. S1). MrPPK2 forms a crystallographic tetramer; each protomer consists of a five-stranded parallel β-sheet surrounded by 10 α-helices. There is a high degree of similarity between the overall structure of MrPPK2 and the previously reported PPK2 structures (26) (SI Appendix, Table S2), the closest being that of the class III A. aurescens enzyme (PDB ID code 3RHF) with an rmsd of 1.3 Å over 268 residues, compared with the class I F. tularensis (Ft) (PDB ID code 5LLB) structure with an rmsd of 1.5 Å over 239 aligned residues.
To gain insight into the substrate-binding mode of PPK2 enzymes, FtPPK2 was cocrystallized with polyP, revealing nine phosphoryl residues bound in a channel between helix 3 of the protein core and helix 8a of the lid loop (Fig. 1B). The polyP is precisely positioned by a constellation of positively charged residues, many of which are conserved across all PPK2s (SI Appendix, Fig. S1), and two phosphoryl residues are juxtaposed to the catalytically important P-loop (Walker A motif, Fig. 1B). This polyP channel is conserved in all of the available PPK2 structures including MrPPK2, and a similar structural feature has been described for PPK1 enzymes (3).
In the absence of reported nucleotide-bound PPK2 structures, the question remained how this polyP channel connects to the active site and how each class of PPK2 recognizes its particular nucleotide(s). To address this, we determined additional structures with nucleotide ligands. Cocrystallization of FtPPK2 with polyP and the nonhydrolyzable ATP analog β,γ-methylene adenosine 5ʹ-triphosphate (AMPPCP) gave a structure featuring a Mg2+ bound by the active site residues Asp62FtPPK2 (Asp66MrPPK2, Walker A motif) and Asp192FtPPK2 (Asp196MrPPK2, lid loop, Fig. 1C and SI Appendix, Fig. S2C). The electron density map (SI Appendix, Fig. S3A) clearly indicates that, during the crystallization, two additional phosphoryl units have been transferred onto the terminus of AMPPCP, yielding β,γ-methylene adenosine 5ʹ-pentaphosphate (AMPPCPPP). Oxygens of the α-, γ-, and δ-phosphoryl groups occupy three ligand sites around the magnesium ion (Fig. 1C and SI Appendix, Fig. S2C). The remaining Mg2+ ligand is poorly resolved and modeled as a water. The complete conservation of the aspartyl residues throughout all PPK2 classes (SI Appendix, Fig. S1) and the octahedral coordination pattern led us to hypothesize that this is the native position of the Mg2+ during catalysis. Beyond the ε-phosphate position of the nucleotide ligand in the FtPPK2-AMPPCPPP structure, there is a short break (∼7.0 Å) in the electron density and then further density traverses the polyP binding channel, consistent with the binding of six more phosphoryl residues (Fig. 1C, P6). Interactions of the lid loop region with the two substrates and the active-site Mg2+ closes the lid loop by an ∼4.5-Å movement of helix 8b (in comparison with the unliganded structure). The PPK1 catalyzed transfer of pyrophosphate to GDP to form G4P has been reported (27), but efforts to detect FtPPK2 catalyzed formation of AMPPCPPP in free solution (as opposed to in crystals) were unsuccessful.
Soaking MrPPK2 crystals with the substrates AMP, ADP, ATP, and the transition state mimic adenosine 5′-pentaphosphate (A5P) resulted in a series of nucleotide-bound structures (Fig. 2 and SI Appendix, Table S1). Soaking with A5P resulted in a structure of MrPPK2 with ADP and pyrophosphate (PPi) bound at the active site. Some of these structures revealed Mg2+, coordinated either by the active-site Asp residues (Asp66MrPPK2, Walker A motif, and Asp196MrPPK2, lid loop) and/or at alternative positions (e.g., Fig. 2B), predominantly bound to nucleoside phosphates. For MrPPK2, a structure containing an intact polyP chain could not be solved, but the corresponding polyP-binding channel is delineated by a series of individual phosphate ions observed in the structure of the MrPPK2 Asn121Asp variant soaked with ATP (SI Appendix, Fig. S2B).
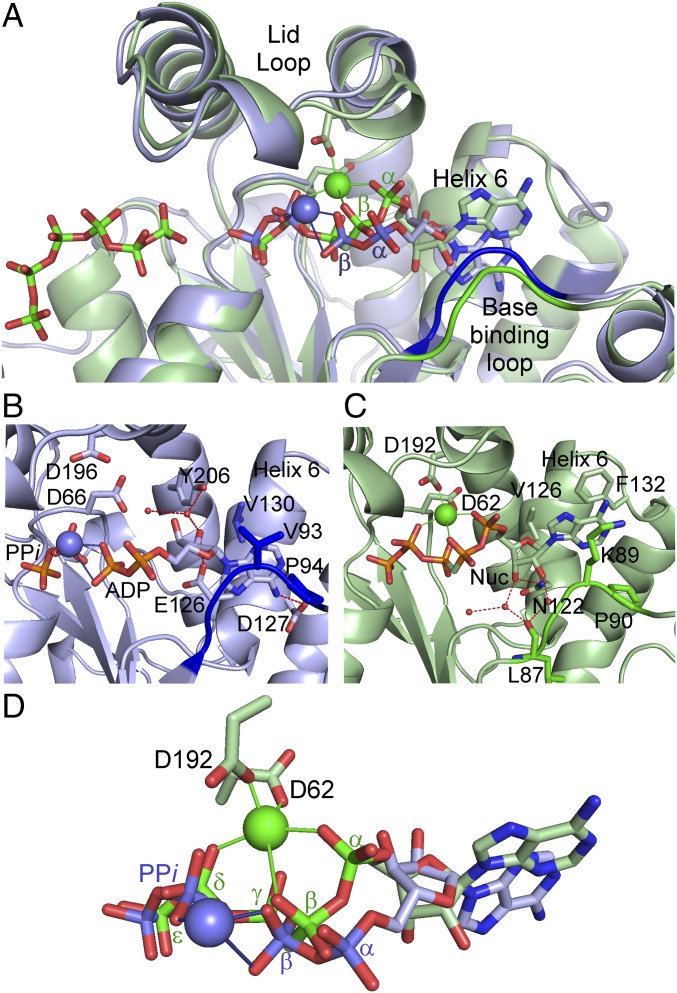
Fig. 2.
PPK2 nucleotide-binding. (A) Overlay of MrPPK2:ADP:PPi complex (blue) with the FtPPK2:AMPPCPPP:polyP complex (green). (B) Detail of nucleotide interactions for MrPPK2. (C) Detail of nucleotide interactions for FtPPK2. Magnesium ions shown as larger spheres, water molecules as smaller red spheres. Nuc, AMPPCPPP nucleotide. (D) Overlay of bound nucleotide conformations derived from the MrPPK2:ADP:PPi complex (blue) and the FtPPK2:AMPPCPPP:polyP complex (green).
Comparing MrPPK2 and FtPPK2 nucleotide-bound structures (Fig. 2 and SI Appendix, Fig. S4), the nucleoside moiety is sandwiched in a pocket between a base-binding loop (residues 87–90FtPPK2/91–94MrPPK2) and helix 6. In MrPPK2 (Fig. 2B), this pocket includes hydrophobic interactions from Val93MrPPK2 and Pro94MrPPK2 from the base-binding loop and Val130MrPPK2 from helix 6. In FtPPK2, interactions come from pi-stacking the purine against Phe132FtPPK2 and hydrophobic interactions from Lys89FtPPK2 and Pro90FtPPK2 from the base-binding loop (Fig. 2C). Other interactions contribute to the orientation of the nucleoside moieties: in MrPPK2, the adenine 6-amino group forms a hydrogen bond with Asp127MrPPK2 and the ribose 2′-hydroxyl hydrogen bonds to Glu126MrPPK2 and via a water to Tyr206MrPPK2; in FtPPK2, the ribose interacts with Asn122FtPPK2 via a hydrogen-bonding network (9). Some nucleotide-binding residues are conserved across PPK2 subclasses (SI Appendix, Figs. S1 and S4), but interpretation of sequence conservation over multiple PPK2 subclasses is complicated by the different phosphorylation states of the preferred nucleotide substrates that enforce alternative interactions on the conserved residues. Between the MrPPK2 and FtPPK2 structures, this results in different binding modes for the nucleoside moieties that adopt “flipped” orientations relative to each other (Fig. 2 A and D). Compared with FtPPK2, the flipped conformation of the adenosine moiety shifts the MrPPK2 base-binding pocket ∼4.5 Å deeper into the protein. A structural overlay of MrPPK2 and FtPPK2 (Fig. 2A) shows that the different nucleotide conformations (Fig. 2D) result in the α-phosphate of AMP in MrPPK2 and the β-phosphate of the nucleotide substrate in FtPPK2 occupying similar positions (∼1.6 Å apart) relative to the presumed catalytic Mg2+-binding site in FtPPK2. We hypothesize that the functional importance of fine-tuning the nucleotide-binding site is to position the nucleophilic phosphate on an ideal trajectory for nucleophilic attack of the polyP terminal residue.
The structures of MrPPK2 complexed with nucleotides (AMP, ADP, ATP, and ADP plus PPi) show them bound in very similar positions and conformations. In the wild-type MrPPK2:ATP complex, but not in the corresponding structure of the Asn121Asp variant, a second equivalent of ATP is bound near the active site (SI Appendix, Fig. S3C). The two triphosphate chains are bound to Mg2+, and while the first ATP is bound to the active site in a similar manner to the AMP and ADP structures, the adenosine moiety of the second ATP protrudes from the active site (SI Appendix, Fig. S3 C and D), making contacts with the neighboring protomer. The residues contacting the second ATP are not well conserved in FtPPK2 or other PPK2s and the physiological relevance of the second equivalent of ATP is unclear, but the hypothesis of ATP-mediated regulation is under investigation.
Nevertheless, as there is no polyP chain bound in these structures, there remains a degree of uncertainty as to how MrPPK2 precisely positions the nucleotide to achieve two phosphorylation steps: in addition to catalyzing the phosphorylation of AMP, MrPPK2 also catalyzes the conversion of ADP to ATP, but at a reduced rate (Table 1 and SI Appendix, Figs. S5 and S6). For MrPPK2 catalysis in the direction of nucleotide phosphorylation, a relatively small conformational adjustment of the 5′-substituent (either phosphate or diphosphate) positions the terminal phosphate on a favored trajectory for nucleophilic attack on the polyP, possibly with the assistance of an active-site bound Mg2+.
Table 1.
Biochemical characterization of wild-type and variant PPK2s
| Protein | Nucleotide turnover* | PolyP binding | ||||||
| Source | Variant | kcat (×10−1⋅s−1) | KM (mM) | R2 | kcat/KM (μM⋅s−1) | Kd (μM) | ∆G (kcal⋅mol−1)† | Binding stoichiometry |
| Ft | WT | 31.7 ± 2.20‡ | 0.546 ± 0.079‡ | 0.99‡ | 5,788‡ | 0.62 ± 0.19 | −8.52 ± 0.32 | 0.67 ± 0.05 |
| Mr | WT | 70.7 ± 3.86§ | 0.033 ± 0.009§ | 0.93 | 214,242 | ND | ND | ND |
| 2.46 ± 0.08¶ | 0.246 ± 0.027¶ | 0.99 | 100,000 | ND | ND | ND | ||
| Ft | D62A | 0.159 ± 0.018 | 2.47 ± 0.746 | 0.91 | 6.43 | ND | ND | ND |
| Ft | D117N | 0.724 ± 0.047 | 4.03 ± 0.532 | 0.99 | 18.0 | 0.47 ± 0.03 | −8.74 ± 0.04 | 0.40 ± 0.04 |
| Mr | N121D | 24.9 ± 0.82§ | 0.075 ± 0.010§ | 0.98 | 33,200 | ND | ND | ND |
| 0.147 ± 0.008¶ | 0.358 ± 0.065¶ | 0.97 | 4,106 | ND | ND | ND | ||
| Ft | D192A | 0.015 ± 0.002 | 3.21 ± 0.870 | 0.94 | 0.47 | ND | ND | ND |
| Ft | K66A | 0.053 ± 0.008 | 12.3 ± 4.12 | 0.96 | 0.44 | 3.58 ± 0.80 | −7.46 ± 0.12 | 0.43 ± 0.01 |
| Ft | R118A | 0.022 ± 0.07 | 6.18 ± 3.54 | 0.83 | 0.37 | 0.98 ± 0.10 | −8.20 ± 0.06 | 0.60 ± 0.01 |
| Ft | R178A | 0.031 ± 0.004 | 3.25 ± 1.05 | 0.92 | 0.95 | 7.50 ± 0.20 | −7.00 ± 0.20 | 0.69 ± 0.07 |
ND, not determined; WT, wild type.
Kinetic parameters were determined by HPLC analysis of initial rates of product formation. All rates for FtPPK2 were measured for phosphorylation of ADP.
Calculated at 20 °C.
Data taken from Batten et al. (26).
For phosphorylation of AMP.
For phosphorylation of ADP.
Oligomerization of PPK2s in Solution.
In the crystalline state, both Ft and MrPPK2s are tetrameric. Oligomerization of PPK2s in the functionally important solution state was determined using a combination of size exclusion chromatography and right-angle light scattering (RALS) (SI Appendix, Figs. S7–S9). This showed that FtPPK2 behaves as a monomer in the absence of substrate, but addition of polyP promoted dimerization. In the absence of substrates, MrPPK2 is trimeric in solution, but forms tetramers in the presence of polyP, which in turn form higher multimers of the tetrameric form (i.e., containing 8, 12, and 16 protomers). We hypothesize that the polyP (25 residues average length) is sufficiently long to bridge between the protomer active sites, inducing the formation of the observed higher oligomers (SI Appendix, Fig. S10).
Activity and PolyP Binding of Sequence Variants.
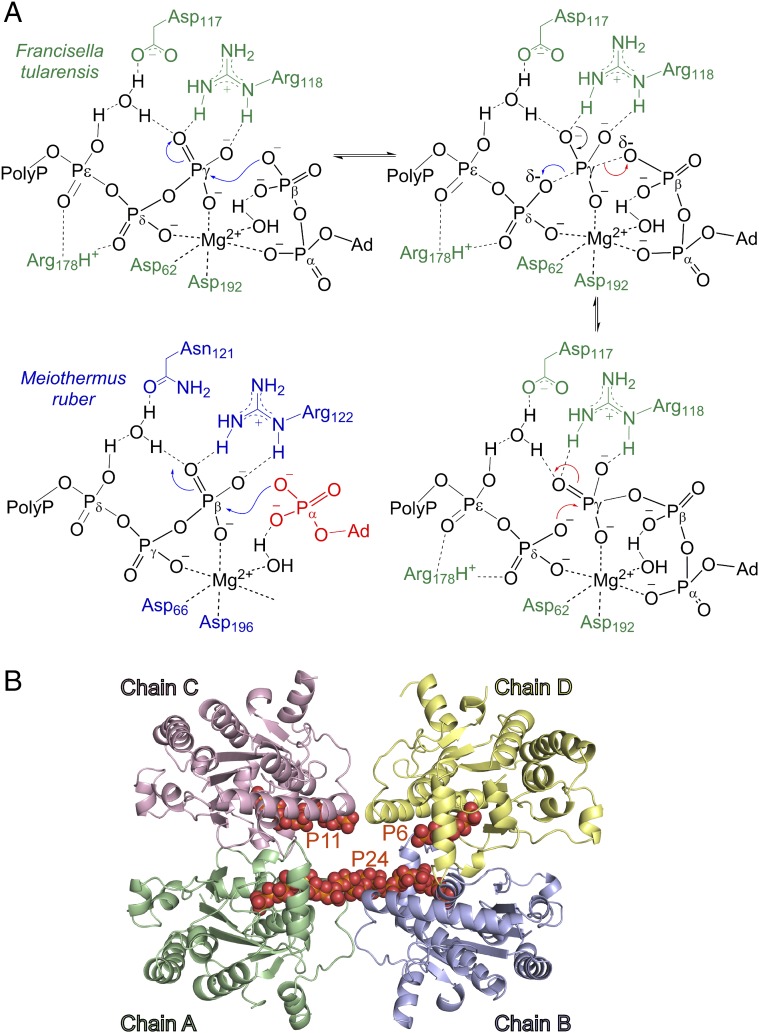
To complement the structural studies, the PPK2 wild types and several sequence variants were analyzed for activity (SI Appendix, Figs. S5, S6, and S11), and the polyP dissociation constant was determined using isothermal calorimetry (ITC) (Table 1). As expected, Lys66AlaFtPPK2 (Walker A motif) and Arg178AlaFtPPK2 (lid loop) showed weaker polyP binding and slower turnover relative to the wild-type enzyme. FtPPK2 Asp117 and MrPPK2 Asn121 occupy equivalent positions in the structures and to probe the functional significance of this difference, the variant Asp117AsnFtPPK2 was prepared. Asp117AsnFtPPK2 shows a decreased kcat, but as expected, polyP binding is unaffected; the reciprocal MrPPK2 variant (Asn121AspMrPPK2) also shows decreased activity. Both variants were crystallized to analyze the effect of the mutation on the active site. The overall structures as well as the active sites are very similar with rmsds for Cα atoms of 0.394 Å and 0.557 Å for FtPPK2 and MrPPK2, respectively. As described above, soaking Asn121AspMrPPK2 with ATP resulted in a structure with the nucleotide bound in the same manner as the wild-type ATP complex. The structure of the Asp117AsnFtPPK2 variant cocrystallized with polyP (average length 25) revealed a 24-mer polyP stretching between protomers A and B (Fig. 3B). This structure may explain the polyP/FtPPK2-binding stoichiometry of ∼0.5 observed by ITC (Table 1) and provides a mechanistic rationale for the formation of higher multimers observed in the RALS experiments (SI Appendix, Figs. S7–S10). Consistent with its proposed role, Arg118AlaFtPPK2 (Walker B motif) showed little or no effect on the Kd for polyP, and in the structures this arginine makes contacts only with the nucleotide phosphates.
Fig. 3.
PPK2 mechanism and interaction with polyP. (A) Proposed mechanism of FtPPK2 and the modified nucleotide-binding mode for MrPPK2 that permits phosphorylation of the α-phosphate (highlighted in red). Blue curly arrows indicate the forward reaction (ATP formation), red curly arrows the reverse reaction, and black curly arrows are common to both. (B) Structure of FtPPK2 Asp117Asn variant cocrystallized with polyP. Labels indicate the polyP lengths: P6, P11, and P24.
Discussion
Different mechanisms leading to the AMPPCPPP complex with FtPPK2 can be envisaged; in one scenario, diphosphate transfer arises from the nucleophilic attack of AMPPCP on the terminal residue of polyP forming a cross-linked intermediate, followed by hydrolysis of the Pε-O-Pζ link under crystallization conditions (SI Appendix, Fig. S12). Soaking A5P into MrPPK2 crystals also resulted in a chemical reaction: the electron density is consistent with bound ADP and PPi ligands, likely derived from A5P. The overlay of FtPPK2:AMPPCPPP:PolyP6 with MrPPK2:ADP:PPi (Fig. 2A) or with MrPPK2-N121D:ATP:Pi (SI Appendix, Fig. S2B) clearly identifies the course of the polyP-binding channel, which passes under the lid loop to the nucleotide-binding site.
Comparison of the ligand-bound Mr and FtPPK2 structures with the unliganded PPK2 structures present in the Protein Data Bank using DALI (28) showed (SI Appendix, Table S2) the highest degree of similarity between the class I enzymes (Ft and S. meliloti, rmsd 0.9 Å over 249 residues) and the class III enzymes (Mr and A. aurescens, rmsd 1.3 Å over 268 residues). The class II P. aeruginosa PPK2 catalytic domain shows less similarity to either ligand-bound structure (rmsd 1.7 Å to Mr over 249 residues and 1.7 Å to Ft over 241 residues).
The nucleotide-binding site of exclusively AMP-phosphorylating class II PPK2s may be inferred from residues functionally conserved in both classes I and II that participate in enzyme–nucleotide interactions. Characteristic for class I PPK2s is the pi-stacking interaction of the adenine base with Phe132FtPPK2; this residue is conserved in PPK2 classes I and II, but not in class III, and suggests that other class I PPK2s and class II PPK2s bind the nucleotide purine and ribose in a similar manner to FtPPK2 (SI Appendix, Fig. S1). In contrast, MrPPK2 recognizes the adenine amino group through interactions with Asp127MrPPK2, which is conserved solely in class III PPK2s; it is replaced in class I and II PPK2s (SI Appendix, Fig. S1) with an arginine residue, the side chain of which is directed away from the active site. Class III PPK2s have been proposed to be phylogenetically closest to a PPK2 ancestor (9), but the radically different conformations of the ribose moiety observed in nucleotide-bound complexes of Ft and MrPPK2s (Fig. 2D) emphasize the evolutionary divergence required to accommodate nucleotide substrates in different phosphorylation states (i.e., either AMP or ADP).
Understanding the mechanism of phosphoryl transfer reactions is a long-standing and important biochemical problem (29, 30). Evidence from a range of techniques (31), including linear free-energy relationships and kinetic isotope effects (32), have led to the tentative conclusion that, during substitution at a singly substituted phosphate (such as the γ-phosphate of ATP), the reaction likely proceeds via a “loose” transition state with a small degree of bond formation from the incoming nucleophile and a large degree of bond cleavage to the leaving group (29). Our proposed mechanism for PPK2s (Fig. 3A) features the in-line nucleophilic attack of the nucleotide on polyP that is activated by binding to the active-site Lewis acidic Mg2+ ion. Arg178FtPPK2/Arg182MrPPK2 from the lid loop orients the polyP chain in the active site. In FtPPK2, ADP coordinated to the Mg2+ via the α-phosphate may allow nucleophilic attack by the β-phosphate, which is not directly coordinated with Mg2+, on the terminal phosphorus of polyP (Fig. 3A). Analogously, during MrPPK2-catalyzed formation of ADP from AMP, the alternate flipped conformation of the adenosine positions the α-phosphate in the nucleophilic site. Formation of a trigonal bipyramidal transition state may be stabilized by hydrogen-bonding to a water molecule coordinated by Asp117FtPPK2/Asn121MrPPK2 (Figs. 1C and 3A); alternatively, this Asx-water pair may protonate and thus stabilize this structure. Collapse of the transition state yields polyPn-1 and ATP or ADP for FtPPK2 or MrPPK2, respectively. The proximal polyP residue (Fig. 1B) lies between the P-loop (Walker A motif) and Lys66FtPPK2/Lys70MrPPK2, which likely stabilizes the transition state by hydrogen-bonding to (or possibly by protonating) leaving groups (33, 34). Arg118FtPPK2/Arg122MrPPK2 hydrogen-bonds to the γ-phosphate position in FtPPK2, which may polarize the P-O bonds, increasing the electrophilicity of this phosphate in the direction of triphosphate synthesis. This mechanism resembles that developed for thymidylate kinase (34, 35), a close structural relative of the bacterial PPK2s (SI Appendix, Fig. S13) (36).
In conclusion, the importance of polyP metabolism for the virulence of bacterial pathogens (1, 5) has led to its proposal as a potential target for antibacterial discovery (37). The phosphorylation of a variety of (nucleotide) substrates driven by inexpensive and plentiful polyP leading to nucleoside di- and triphosphates has numerous prospective biotechnological applications (22–24, 38), and not only as biocatalysts for ATP regeneration systems. The mechanisms of catalysis and substrate selectivity described here provide a useful foundation for progress in both these areas.
Materials and Methods
Detailed synthetic procedures for the preparation of nucleotides and analogs, the expression and purification of wild-type and variant PPK2 proteins, and biochemical studies are described in SI Appendix, SI Materials and Methods. Purified PPK2 proteins were crystallized using the vapor diffusion method. Substrates and analogs were introduced either by cocrystallization or soaking, and structures were determined by X-ray diffraction experiments, model building, and refinement as detailed in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Diamond Light Source for access to beamlines i24, i04, and i04-1 under proposal mx8889 and the staff of the Swiss Light Source for their support. This work was supported by funds from the US Defense Threat Reduction Agency Grant HDTRA1-11-1-0007; Defence Science and Technology Laboratory Grant DSTLX1000097311; Engineering and Physical Sciences Research Council Grant EP/M507623/1; Human Frontiers of Science Program Grant RGP0025/2016; and Deutsche Forschungsgemeinschaft Grant RTG1976.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.A.S. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID codes 5LC9, 5LCD, 5LDB, 5LD1, 5MAQ, 5LL0, 5LLB, 5LLF, 5O6K, and 5O6M).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710741115/-/DCSupplemental.
References
- 1.Brown MR, Kornberg A. Inorganic polyphosphate in the origin and survival of species. Proc Natl Acad Sci USA. 2004;101:16085–16087. doi: 10.1073/pnas.0406909101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao NN, Gómez-García MR, Kornberg A. Inorganic polyphosphate: Essential for growth and survival. Annu Rev Biochem. 2009;78:605–647. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Huang W, Lee SS, Xu W. Crystal structure of a polyphosphate kinase and its implications for polyphosphate synthesis. EMBO Rep. 2005;6:681–687. doi: 10.1038/sj.embor.7400448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achbergerová L, Nahálka J. Polyphosphate: An ancient energy source and active metabolic regulator. Microb Cell Fact. 2011;10:63. doi: 10.1186/1475-2859-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerdes K, Maisonneuve E. Bacterial persistence and toxin-antitoxin loci. Annu Rev Microbiol. 2012;66:103–123. doi: 10.1146/annurev-micro-092611-150159. [DOI] [PubMed] [Google Scholar]
- 6.Shum KT, et al. Aptamer-mediated inhibition of Mycobacterium tuberculosis polyphosphate kinase 2. Biochemistry. 2011;50:3261–3271. doi: 10.1021/bi2001455. [DOI] [PubMed] [Google Scholar]
- 7.Chuang YM, Belchis DA, Karakousis PC. The polyphosphate kinase gene ppk2 is required for Mycobacterium tuberculosis inorganic polyphosphate regulation and virulence. MBio. 2013;4:e00039-13. doi: 10.1128/mBio.00039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn K, Kornberg A. Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J Biol Chem. 1990;265:11734–11739. [PubMed] [Google Scholar]
- 9.Motomura K, et al. A new subfamily of polyphosphate kinase 2 (class III PPK2) catalyzes both nucleoside monophosphate phosphorylation and nucleoside diphosphate phosphorylation. Appl Environ Microbiol. 2014;80:2602–2608. doi: 10.1128/AEM.03971-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishige K, Zhang H, Kornberg A. Polyphosphate kinase (PPK2), a potent, polyphosphate-driven generator of GTP. Proc Natl Acad Sci USA. 2002;99:16684–16688. doi: 10.1073/pnas.262655299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nocek B, et al. Polyphosphate-dependent synthesis of ATP and ADP by the family-2 polyphosphate kinases in bacteria. Proc Natl Acad Sci USA. 2008;105:17730–17735. doi: 10.1073/pnas.0807563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindner SN, Vidaurre D, Willbold S, Schoberth SM, Wendisch VF. NCgl2620 encodes a class II polyphosphate kinase in Corynebacterium glutamicum. Appl Environ Microbiol. 2007;73:5026–5033. doi: 10.1128/AEM.00600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi X, Rao NN, Kornberg A. Inorganic polyphosphate in Bacillus cereus: Motility, biofilm formation, and sporulation. Proc Natl Acad Sci USA. 2004;101:17061–17065. doi: 10.1073/pnas.0407787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Rao NN, Shiba T, Kornberg A. Inorganic polyphosphate in the social life of Myxococcus xanthus: Motility, development, and predation. Proc Natl Acad Sci USA. 2005;102:13416–13420. doi: 10.1073/pnas.0506520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonting CF, Kortstee GJ, Zehnder AJ. Properties of polyphosphate: AMP phosphotransferase of Acinetobacter strain 210A. J Bacteriol. 1991;173:6484–6488. doi: 10.1128/jb.173.20.6484-6488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahálka J, Pätoprstý V. Enzymatic synthesis of sialylation substrates powered by a novel polyphosphate kinase (PPK3) Org Biomol Chem. 2009;7:1778–1780. doi: 10.1039/b822549b. [DOI] [PubMed] [Google Scholar]
- 17.Kumble KD, Ahn K, Kornberg A. Phosphohistidyl active sites in polyphosphate kinase of Escherichia coli. Proc Natl Acad Sci USA. 1996;93:14391–14395. doi: 10.1073/pnas.93.25.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akiyama M, Crooke E, Kornberg A. The polyphosphate kinase gene of Escherichia coli. Isolation and sequence of the ppk gene and membrane location of the protein. J Biol Chem. 1992;267:22556–22561. [PubMed] [Google Scholar]
- 19.Nahálka J, Gemeiner P, Bucko M, Wang PG. Bioenergy beads: A tool for regeneration of ATP/NTP in biocatalytic synthesis. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:515–521. doi: 10.1080/10731190600862886. [DOI] [PubMed] [Google Scholar]
- 20.Resnick SM, Zehnder AJ. In vitro ATP regeneration from polyphosphate and AMP by polyphosphate:AMP phosphotransferase and adenylate kinase from Acinetobacter johnsonii 210A. Appl Environ Microbiol. 2000;66:2045–2051. doi: 10.1128/aem.66.5.2045-2051.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kameda A, et al. A novel ATP regeneration system using polyphosphate-AMP phosphotransferase and polyphosphate kinase. J Biosci Bioeng. 2001;91:557–563. doi: 10.1263/jbb.91.557. [DOI] [PubMed] [Google Scholar]
- 22.Kulmer ST, Gutmann A, Lemmerer M, Nidetzky B. Biocatalytic cascade of polyphosphate kinase and sucrose synthase for synthesis of nucleotide-activated derivatives of glucose. Adv Synth Catal. 2017;359:292–301. [Google Scholar]
- 23.Schwander T, Schada von Borzyskowski L, Burgener S, Cortina NS, Erb TJ. A synthetic pathway for the fixation of carbon dioxide in vitro. Science. 2016;354:900–904. doi: 10.1126/science.aah5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mordhorst S, Siegrist J, Müller M, Richter M, Andexer JN. Catalytic alkylation using a cyclic S-adenosylmethionine regeneration system. Angew Chem Int Ed. 2017;56:4037–4041. doi: 10.1002/anie.201611038. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Ishige K, Kornberg A. A polyphosphate kinase (PPK2) widely conserved in bacteria. Proc Natl Acad Sci USA. 2002;99:16678–16683. doi: 10.1073/pnas.262655199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batten LE, et al. Biochemical and structural characterization of polyphosphate kinase 2 from the intracellular pathogen Francisella tularensis. Biosci Rep. 2015;36:e00294. doi: 10.1042/BSR20150203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroda A, Kornberg A. Polyphosphate kinase as a nucleoside diphosphate kinase in Escherichia coli and Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1997;94:439–442. doi: 10.1073/pnas.94.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holm L, Rosenström P. Dali server: Conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545–W549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lassila JK, Zalatan JG, Herschlag D. Biological phosphoryl-transfer reactions: Understanding mechanism and catalysis. Annu Rev Biochem. 2011;80:669–702. doi: 10.1146/annurev-biochem-060409-092741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirby AJ, Nome F. Fundamentals of phosphate transfer. Acc Chem Res. 2015;48:1806–1814. doi: 10.1021/acs.accounts.5b00072. [DOI] [PubMed] [Google Scholar]
- 31.Stockbridge RB, Wolfenden R. The intrinsic reactivity of ATP and the catalytic proficiencies of kinases acting on glucose, N-acetylgalactosamine, and homoserine: A thermodynamic analysis. J Biol Chem. 2009;284:22747–22757. doi: 10.1074/jbc.M109.017806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cleland WW, Hengge AC. Enzymatic mechanisms of phosphate and sulfate transfer. Chem Rev. 2006;106:3252–3278. doi: 10.1021/cr050287o. [DOI] [PubMed] [Google Scholar]
- 33.Reinstein J, Schlichting I, Wittinghofer A. Structurally and catalytically important residues in the phosphate binding loop of adenylate kinase of Escherichia coli. Biochemistry. 1990;29:7451–7459. doi: 10.1021/bi00484a014. [DOI] [PubMed] [Google Scholar]
- 34.Ostermann N, et al. Insights into the phosphoryltransfer mechanism of human thymidylate kinase gained from crystal structures of enzyme complexes along the reaction coordinate. Structure. 2000;8:629–642. doi: 10.1016/s0969-2126(00)00149-0. [DOI] [PubMed] [Google Scholar]
- 35.Gardberg A, Shuvalova L, Monnerjahn C, Konrad M, Lavie A. Structural basis for the dual thymidine and thymidylate kinase activity of herpes thymidine kinases. Structure. 2003;11:1265–1277. doi: 10.1016/j.str.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Leipe DD, Koonin EV, Aravind L. Evolution and classification of P-loop kinases and related proteins. J Mol Biol. 2003;333:781–815. doi: 10.1016/j.jmb.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 37.Singh M, et al. Establishing virulence associated polyphosphate kinase 2 as a drug target for Mycobacterium tuberculosis. Sci Rep. 2016;6:26900. doi: 10.1038/srep26900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andexer JN, Richter M. Emerging enzymes for ATP regeneration in biocatalytic processes. ChemBioChem. 2015;16:380–386. doi: 10.1002/cbic.201402550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.