Abstract
Emerging evidence from human and animal studies suggest that consumption of palatable foods rich in fat and/or carbohydrates may produce deleterious influences on brain function independently of body weight or metabolic disease. Here we consider two mechanisms by which diet can impact striatal circuits to amplify food cue reactivity and impair inhibitory control. First, we review findings demonstrating that the energetic properties of foods regulate nucleus accumbens food cue reactivity, a demonstrated predictor of weight gain susceptibility, which is then sensitized by chronic consumption of an energy dense diet. Second, we consider evidence for diet-induced adaptations in dorsal striatal dopamine signaling that is associated with impaired inhibitory control and negative outcome learning.
Keywords: nucleus accumbens, dorsal striatum, dopamine, high fat diet, food reward, obesity, neural adaptation, cognition, cue reactivity, inhibitory control, ANKK1
Introduction
There has been considerable effort over the past two decades to identify and characterize behaviors and their underlying neural circuits that confer vulnerability for overeating in the modern “obesogenic” food environment. A general thesis to emerge is that enhanced reactivity to food-associated cues coupled with diminished inhibitory control produces susceptibility for overeating [1,2], particularly in an environment where salient food cues are pervasive and palatable, energy dense foods are cheap and easily obtainable. In this review we argue that the relationship between the brain and the food environment is bi-directional. In particular, there is mounting evidence that consumption of palatable foods high in fat and refined carbohydrates produces deleterious effects on neural circuits, thereby contributing to cognitive alterations permissive of overeating. Here we outline two ways in which dietary factors might negatively impact striatal circuits to produce hyper-reactivity to food cues and diminished inhibitory control.
Metabolic control of food cue reactivity in the nucleus accumbens
“Food cue reactivity”, defined as the extent to which an individual is prone to eat in the presence of food cues, has long been associated with susceptibility for weight gain [3–6]. Food cues acquire reinforcing properties via Pavlovian conditioning [7], in which a once neutral cue is associated with nutrient ingestion. Once this association is formed, food cues gain access to reward [8] and homeostatic circuits [9], thereby acquiring the ability to elicit reflexive responses such as cephalic phase responses [10], food seeking [11], and craving [6].
The nucleus accumbens (NAc) is critically involved in the formation of learned Pavlovian associations between the unconditioned rewarding properties of nutrient ingestion and conditioned cues such as the sight or flavor of the foods containing nutrients [12]. Accordingly, human neuroimaging studies have shown that NAc response to calorie-predictive food cues is associated with genetic risk for obesity [13], eating in the absence of hunger [14], poor outcomes on weight loss trials [15], unhealthy food choice [16] and weight gain susceptibility [17–19], among other factors. This raises the possibility that individual variations in NAc learning circuits mediating food cue reactivity may increase susceptibility to obesity in a food cue-laden environment.
Conditioning food cue reactivity
Work in rodents suggests that post-ingestive effects following nutrient consumption provide critical signals driving reinforcement and hence food cue reactivity. Infusing glucose directly into the gut, concomitantly to exposure to a non-caloric flavored liquid, results in lasting preferences for that flavor [20–22]. In contrast, sweetness perception in the absence of calories (or caffeine) is neither necessary nor sufficient for animals to form flavor preferences [23,24]. This flavor-nutrient conditioning occurs rapidly, even within the course of a single meal [25], demonstrating the potency with which post-oral signals transform flavors into conditioned cues.
Although it is well established that flavor-nutrient conditioning depends upon brain dopamine signaling [8], the identity of the post-oral signal supporting this learning remains a subject for debate. Direct infusion of nutrients into the gut stimulates dopamine release in the NAc and dorsal striatum (DS) [23] in a calorie-dependent manner [26]. Moreover, this effect is sensitive to glucose utilization rate, and inhibition of glucose oxidation suppresses striatal dopamine levels and reduces glucose intake [23,27]. This suggests that the post-oral “reward” signal is linked to the utilization of the nutrient as fuel.
In humans, response in the NAc to a calorie-predictive flavor is directly proportional to the magnitude of change in blood glucose that occurs when the flavor is consumed with the calorie source [28]. Since glucose availability is a requirement for its utilization this finding provides indirect support for metabolic response contributing to the reinforcing effects of nutrients in humans. Intriguingly, although pairing the flavor with calories increases the rated liking of that flavor, this change in liking does not correlate with NAc response or the change in blood glucose. This suggests that the energetic properties of foods might influence behaviors independently of perceptions of liking. Consistent with this possibility, willingness to pay for food items in an auction task correlates with NAc response and is driven by energy density independently of explicit knowledge of caloric content or rated food liking [29]. Bidding behavior was also associated with the generation of value signals in the ventromedial prefrontal cortex that were associated with actual, but not estimated energy density. Thus, NAc involvement in Pavlovian conditioning and in food choice appears to reflect the energetic characteristics of food independently of explicit awareness or liking, a notion consistent with separate substrates for explicit and implicit components of liking and incentive motivation [30–32]. If so, energy dense foods that produce large glucose excursions may well condition NAc hyper-reactivity, especially in individuals with compromised glucose metabolism. Moreover, this hyper-reactivity, which is associated with weight gain susceptibility [15,17], likely influences intake via implicit processes that may be less amenable to goal-directed behaviors such as dieting.
Effects of energy-dense diets on conditioning food cue reactivity
Emerging evidence suggests that chronic consumption of an unhealthy diet contributes to alterations in NAc-dependent learning. In humans, objectively measured energy intake was associated with greater BOLD response to anticipated food intake in the striatum independent of basal energy needs and adiposity, raising the possibility that excess caloric intake may enhance NAc food cue reactivity [33]. Accordingly, Wald and Meyers assessed flavor-glucose learning in rats that had been exposed to a high-fat, high-carbohydrate (HFHC) choice diet [34]. Learning rapidity and strength (measured by intake) was greater in HFHC-fed rats that became obese compared to chow-fed rats and HFHC-fed rats that were relatively obesity-resistant [34]. This finding points to an association between diet-induced obesity (DIO) and enhanced sensitivity to flavor-nutrient learning, though the directionality of this relationship remains unclear.
To dissociate diet-induced from pre-existing differences associated with obesity, Robinson and colleagues examined cue reactivity in rats before and after chronic exposure to a palatable “junk-food” diet [35]. Rats subsequently identified as susceptible to diet-induced obesity (DIO-prone) displayed enhanced conditioned approach to sucrose-predicting food cues prior to diet exposure and independent of initial body weight. Following diet exposure, both DIO-prone and DIO-resistant rats displayed cross-sensitization to amphetamine and down-regulation of striatal dopamine D2 receptors (D2Rs). Thus, while enhanced food cue reactivity precedes obesity and may confer vulnerability to overeating and diet-induced weight gain, other adaptations in dopamine function may occur as a direct consequence of palatable diet consumption, regardless of weight gain [36].
Further support comes from a recent study showing that NAc insulin modulates flavor-nutrient learning and enhances dopamine release, an effect that is abolished by HFHC diet [37]. Given that insulin also acts in the hypothalamus to promote satiety [38], this suggests a potential mechanism by which diet may influence circuits important for reward learning and homeostasis. Indeed, a number of nutritionally-regulated hormones involved in hypothalamic control of feeding and glucose homeostasis—including glucagon-like peptide 1 [39], amylin [40], leptin [41], and ghrelin [42]—also modulate mesocorticolimbic dopamine signaling. Accordingly, recent data suggest that hypothalamic feeding circuits are regulated by sensory input [9], and may integrate information from reward circuits about nutritional and hedonic properties of food to direct metabolic learning and memory [43]. This metabolic learning regulates food choice and is susceptible to genetic and environmental factors, such as overnutrition [43].
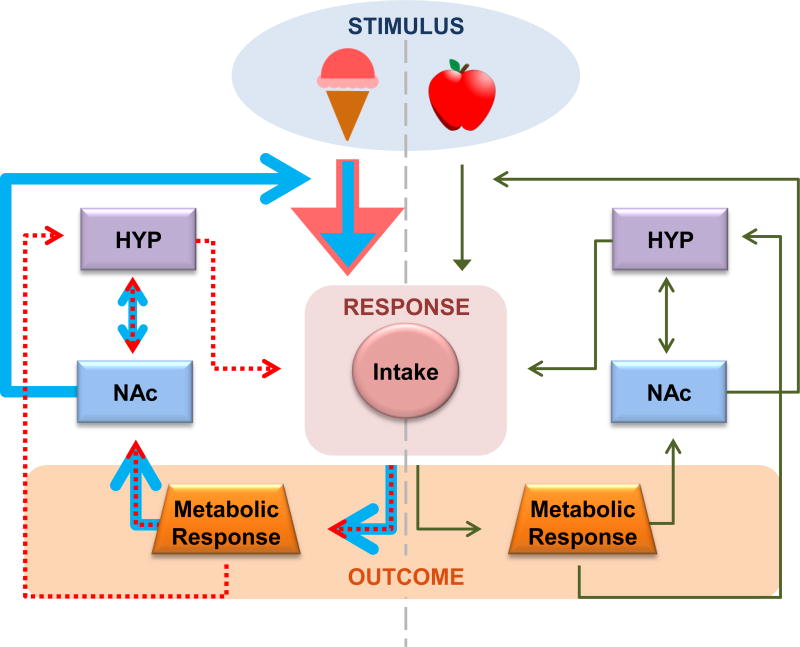
In the modern environment, humans are presented with a daily barrage of food-associated cues in the form of advertisements, most of which promote energy-dense foods high in fat and sugar [44]. Emerging evidence supports the possibility that energy-dense foods and beverages, which produce larger glucose and insulin excursions, will be more effective at driving NAc food cue reactivity and cue-potentiated feeding. Chronic consumption of foods high in fat and carbohydrates may in turn alter this learning by disrupting glucose metabolism and insulin sensitivity, or by directly altering dopamine signaling. Thus, an environment in which palatable foods are readily available and food cues are pervasive may propel a vicious cycle whereby energy-dense foods, via their effect on the NAc, turn neutral cues into powerful conditioned stimuli that drive excessive intake. Excessive intake may in turn alter NAc-mediated learning and behavior, culminating in even greater susceptibility to overeating (FIGURE 1).
Figure 1. Diet-induced alterations in NAc-mediated learning drive a vicious cycle of overeating.
In healthy individuals (green arrows), feeding is regulated by homeostatic and hedonic processes. The NAc facilitates associative learning and promotes adaptive behavioral responses to procure nutrient-rich foods during energy deficit. Energy-dense foods (blue arrows) promote supranormal metabolic responses and DA release in the NAc, thereby strengthening the incentive salience of these foods. Chronic consumption (red arrows) leads to metabolic disturbances (e.g. insulin insensitivity) and neural adaptations (e.g. down-regulated D2Rs), which reduce sensitivity to homeostatic signals and impair NAc-mediated associative learning. Thus, consumption of energy-dense foods promotes the formation of strong cue associations which drive reflexive food seeking irrespective of metabolic outcomes and homeostatic need.
Neural adaptations in the dorsal striatum
The nigrostriatal pathway is critically involved in reward-seeking behaviors, including feeding [45,46]. Dopamine release is observed in the DS during food consumption in rodents and humans [47–49], and intact DS dopamine signaling is required for the expression of normal ingestive behavior [50]. Evidence from preclinical studies in rodents consistently report alterations in DS dopamine function in diet-induced obese rodents, including diminished dopamine D2 receptor expression [51,52] and dopamine release [53]. Interestingly, administration of the gastrointestinal messenger oleoylethanolamide restores nutrient-stimulated dopamine release in high-fat fed mice, while simultaneously eliminating motivational deficits during flavorless intragastric feeding and increasing oral intake of low-fat emulsions [53]. These findings suggest that DS dopamine acts as a critical sensor of the nutritional value of ingested calories, and provide support for the notion that excess intake of high-calorie foods may represent a compensatory response to diminished nutrient sensitivity.
The relationship between human obesity and DS circuitry is less clear. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies assessing baseline binding potential (BP) for dopamine ligands report decreased receptor availability [54,55] and evoked dopamine release [56] in morbid obesity, and increased receptor availability [57–59] and evoked dopamine release [60] in overweight and mild obesity. One interpretation of these findings is that there is a non-linear relationship between obesity and dopaminergic tone: decreased dopamine tone (increased receptor availability) is associated with enhanced phasic responses in overweight/mild obesity, while in severe obesity, increased tone (decreased receptor availability) is associated with blunted phasic responses [61]. This is consistent with evidence of a non-linear relationship between dopamine-dependent functions, such as reward sensitivity, and BMI [62]. However, this interpretation is complicated by conflicting reports of increased [60] and decreased [63] striatal dopamine release in mildly obese subjects with similar BMI range, which suggests that other factors likely contribute to inter-study discrepancies, such as differences in radiotracer characteristics, heterogeneity between studies with respect to nutritional status, genetic variation, metabolic disturbances, and diet.
FMRI studies have also demonstrated alterations in DS function in association with obesity. Cross-sectional studies consistently report enhanced blood oxygen level-dependent (BOLD) response in the DS in response to calorie-predictive cues in overweight/mild obesity [64–67]. In contrast, DS BOLD response to the receipt of a predicted food is decreased in association with overweight/obesity [57,67–71], and is predictive of future weight gain [72,73].
Although the BOLD signal is not a direct measure of neurotransmitter release, there is evidence that these differences in DS responsivity are related to alterations in dopaminergic function. First, the relationship between BMI and BOLD DS response is stronger in individuals who are at genetic risk for reduced D2R signaling capacity by virtue of possessing the A1 allele of the Taq1A polymorphism [72–74]. Furthermore, while studies consistently report a negative association between BMI/weight gain DS response to the receipt of a predicted palatable food [57,67,68,71], when receipt is unpredicted, this association is positive [75]. According to animal literature, dopamine neurons produce bursts of action potentials in response to unexpected food rewards [76]. After repeated pairings with a neutral cue, dopamine neurons begin to fire in response to the reward-predictive cue and cease responding to the receipt of food [77]. Thus, DS BOLD responses to expected and unexpected reward receipt may reflect distinct temporal aspects of dopamine dynamics.
Consistent with this possibility, a recent study by Burger and Stice [78] found that during exposure to repeated pairings of palatable food receipt and cues that predict palatable food receipt, striatal BOLD responses to cues increased, while responses to food receipt decreased. Additionally, the slopes of increases and decreases in striatal response to cues and food receipt observed across learning trials predicted future weight gain, and heightened responsivity to initial receipt accounted for a substantial proportion of variance in future weight gain.
Collectively then, extant data from neuroimaging studies is consistent with the model that overweight and mild obesity is characterized by DS hyper-reactivity to food cues and hypo-reactivity to food receipt, which may reflect greater propensity for cue-reward learning and food reward habituation, respectively.
Considerable evidence suggests that dietary factors contribute to dopamine dysregulation by producing neuroadaptations in DS, which may potentiate overeating by impairing dopamine-dependent learning and cognition; a proposal that very much parallels models of vicious cycles observed in drug addiction [79]. First, decreased DS response to food receipt appears to be consequential rather than causal as it is associated with weight gain over time [71], but not risk for obesity by virtue of parental obesity [80]. Second, the effect is driven primarily by individuals who are at genetic risk for decreased D2R density [72,73]. The single-nucleotide polymorphism that gives rise to this risk (SNP; rs1800497; Accession Number: NP_848605.1) is located 9.5 kb downstream from D2R in exon 9 of the ANKK1 gene (ankyrin repeats and kinase domain containing 1 gene) and causes an amino acid substitution within the C-terminal [81]. The encoded protein, ANKK1, belongs to a family of receptor-interacting protein (RIP) serine/threonine kinases. RIP kinases are of interest because they have emerged as essential sensors of cellular stress, initiating responses to various environmental factors, including nutrient ingestion, by activating transcription factors such as NF-κB and AP-1 [82]. NF-κB response elements exist in the D2R promoter region and NF-κB is a necessary and sufficient signal to induce DRD2 expression [83,84]. Though much remains to be understood about the relationship between the Taq1A polymorphism, ANKK1, and dopaminergic function, these findings suggest a potential mechanism whereby diet-induced interactions between ANKK1 and NF-κB could enhance risk for adaptations in the dopamine system, particularly in individuals with the A1 allele.
DS adaptations have important functional implications. The DS plays a key role in instrumental learning [12,85,86], as well a number of other cognitive functions including habit formation [45,87–89], working memory [90,91], inhibitory control [92,93] and negative outcome learning [94,95], raising the possibility that diet-induced alterations in dopamine function lead to cognitive and behavioral deficits. Accordingly, DS response to milkshake receipt is inversely associated with self-reported impulsivity in overweight/obese but not healthy-weight individuals [68]. In contrast, patients with anorexia nervosa, who exert excessive inhibitory control over feeding, engage the DS more than healthy controls when making food choices, and fronto-striatal connectivity in these patients correlates with food intake [96]. Obese individuals also show impaired working memory [97], as well as negative, but not positive outcome learning [97,98]. This latter finding is of particular interest because negative outcome learning has been specifically associated with striatal D2 signaling [99]. Accordingly, rats with extended access to a palatable high-fat diet display down-regulated DS D2Rs accompanied by reward deficits and compulsive-like food-seeking characterized by insensitivity to negative outcomes [52]. Moreover, lentivirus-mediated knockdown of DS D2Rs rapidly accelerated the development of these behavioral deficits, suggesting a direct link between diet, DS dopamine adaptations, and impulsive, inflexible behavioral patterns.
Revealingly, many of these dopamine-dependent cognitive functions have been identified as risk factors for overeating [100–102], and even targets for behavioral interventions for obesity [103,104]. As such, pre-existing and/or diet-induced alterations in dopamine signaling may produce impairments in executive function, which may increase susceptibility for overeating and weight gain. Consistently, A1 carriers display greater behavioral inflexibility, working memory deficits, and impaired negative outcome learning [98,105–107], and are at greater risk for developing a variety of psychiatric disorders related to impaired striatal dopamine function including smoking [108], alcoholism [109], and obesity [110,111].
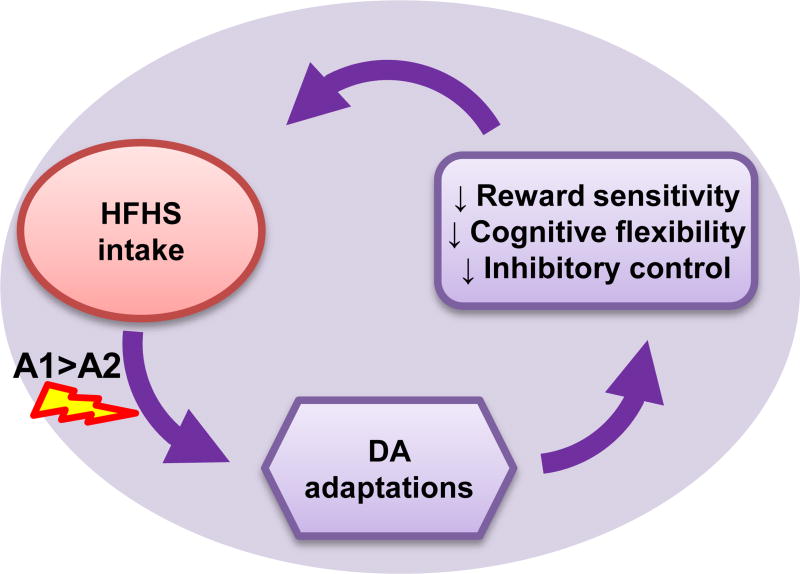
Collectively then, accumulating data suggests that A1 carriers, who constitute 30–40% of the population, possess an increased genetic vulnerability to diet-induced dopamine adaptions, and these adaptations are associated with cognitive impairments that may increase predisposition to obesity (FIGURE 2). Unfortunately, verification of this hypothesis is complicated by the fact that all rodents are A1 homozygotes. Therefore, development of a transgenic “non-carrier” mouse would be of interest to rigorously test this hypothesis and determine the mechanisms by which ANKK1 variants influence D2R expression.
Figure 2. Genetic and dietary factors influence DS function to increase risk for overeating and obesity.
Chronic intake of a diet high in fat and sugar leads to neuroadaptations that disrupt striatal DA signaling. These adaptations are associated with cognitive impairments that perpetuate impulsive and inflexible feeding habits (purple). Genetic variants affecting DA signaling capacity (e.g. TaqIA polymorphism) enhance risk for diet-induced DA adaptations associated with overeating and the development of dopamine-dependent cognitive impairments.
Conclusions
The power of energy dense foods, via their effects on mesoaccumbens and nigrostriatal circuits, to condition cue-reward associations and motivate appetitive behavior is adaptive when food is scarce or its availability unpredictable. In modern societies where energy dense foods and food cues are abundant, these mechanisms can become a liability, promoting energy intake that far exceeds metabolic needs. Indeed, evidence from preclinical and clinical studies suggest that obesity is associated with distinct alterations in ventral (NAc) and DS dopamine signaling, and in behaviors and cognitive functions governed by these circuits. In particular, heightened food cue reactivity and cue-induced dopamine signaling in the NAc have been reported in relation to obesity. In addition, obesity is associated with diminished DS dopamine signaling in response to food receipt and greater DS response to anticipated food reward, in parallel with reward hyposensitivity and impulsive, inflexible behavior. Revealingly, while NAc reactivity to food cues was found to predict subsequent snacking, NAc response was associated with increased BMI only in individuals reporting low self-control [14]. Taken together, the findings presented here are consistent with a model of obesity that is characterized by hypersensitivity to conditioned food cues in combination with hyposensitivity to reward receipt and weakened inhibitory control over appetitive behaviors. Moreover, there is now clear evidence that at least some of the differential brain effects observed in obesity occur as a consequence of high-fat/high-carbohydrate diet consumption, and that some of these adaptations exacerbate behaviors that confer initial vulnerability for overeating and weight gain. It is therefore critical to determine the precise mechanisms underlying these diet-induced adaptations and to evaluate methods for their reversal.
Highlights.
Striatal circuits involved in associative learning are altered in obesity
Differences in neural reactivity to food cues and reward confer risk for overeating
Excess intake of dietary fat and sugar alters striatal dopamine function
Diet-induced adaptations lead to cognitive impairments that may potentiate risk
Acknowledgments
The authors would like to thank Susanne La Fleur, Serge Luquet and Ivan de Araujo for discussions and comments during manuscript development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest
References
- 1.Sweet LH, Hassenstab JJ, McCaffery JM, Raynor HA, Bond DS, Demos KE, Haley AP, Cohen RA, Del Parigi A, Wing RR. Brain response to food stimulation in obese, normal weight, and successful weight loss maintainers. Obesity (Silver Spring) 2012;20:2220–5. doi: 10.1038/oby.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat. Neurosci. 2005;8:555–60. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 3.Schachter S. Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science. 1968;161:751–6. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- 4.Rodin J, Slochower J. Externality in the nonobese: effects of environmental responsiveness on weight. J. Pers. Soc. Psychol. 1976;33:338–44. doi: 10.1037//0022-3514.33.3.338. [DOI] [PubMed] [Google Scholar]
- 5.Meyer MD, Risbrough VB, Liang J, Boutelle KN. Pavlovian conditioning to hedonic food cues in overweight and lean individuals. Appetite. 2015;87:56–61. doi: 10.1016/j.appet.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Nederkoorn C, Jansen A. Cue reactivity and regulation of food intake. Eat. Behav. 2002;3:61–72. doi: 10.1016/s1471-0153(01)00045-9. [DOI] [PubMed] [Google Scholar]
- 7.Darvas M, Wunsch AM, Gibbs JT, Palmiter RD. Dopamine dependency for acquisition and performance of Pavlovian conditioned response. Proc. Natl. Acad. Sci. U. S. A. 2014;111:2764–9. doi: 10.1073/pnas.1400332111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touzani K, Bodnar RJ, Sclafani A. Glucose-conditioned flavor preference learning requires co-activation of NMDA and dopamine D1-like receptors within the amygdala. Neurobiol. Learn. Mem. 2013;106:95–101. doi: 10.1016/j.nlm.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Chen Y, Lin Y-C, Kuo T-W, Knight ZA. Sensory Detection of Food Rapidly Modulates Arcuate Feeding Circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. Using calcium imaging to record activity in hypothalamic AgRP and POMC neurons in awake behaving mice, the authors found that the sensory detection of food was sufficient to rapidly reverse the activation state of these neurons induced by energy deficit. Food palatability influenced the magnitude of the response, suggesting that the response contains information about the hedonic properties or energy content of food acquired through learned associations. This study provides evidence that hypothalamic neurons are not merely sensors of circulating homeostatic signals, but are strongly regulated by sensory input and may integrate information related to the nutritional content and hedonic properties of food. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nederkoorn C, Smulders FT, Jansen A. Cephalic phase responses, craving and food intake in normal subjects. Appetite. 2000;35:45–55. doi: 10.1006/appe.2000.0328. [DOI] [PubMed] [Google Scholar]
- 11.Weingarten H. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science (80-.) 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 12.O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–4. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 13.van der Klaauw AA, von dem Hagen EAH, Keogh JM, Henning E, O’Rahilly S, Lawrence AD, Calder AJ, Farooqi IS. Obesity-associated melanocortin-4 receptor mutations are associated with changes in the brain response to food cues. J. Clin. Endocrinol. Metab. 2014;99:E2101–6. doi: 10.1210/jc.2014-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63:415–22. doi: 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 15.Murdaugh DL, Cox JE, Cook EW, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–21. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, Schur EA. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am. J. Clin. Nutr. 2012;96:989–99. doi: 10.3945/ajcn.112.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012;32:5549–52. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geha PY, Aschenbrenner K, Felsted J, O’Malley SS, Small DM. Altered hypothalamic response to food in smokers. Am. J. Clin. Nutr. 2013;97:15–22. doi: 10.3945/ajcn.112.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring) 2014;22:2544–51. doi: 10.1002/oby.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zukerman S, Ackroff K, Sclafani A. Post-oral glucose stimulation of intake and conditioned flavor preference in C57BL/6J mice: a concentration-response study. Physiol. Behav. 2013;109:33–41. doi: 10.1016/j.physbeh.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackroff K, Sclafani A. Flavor change and food deprivation are not critical for post-oral glucose appetition in mice. Physiol. Behav. 2015;140:23–31. doi: 10.1016/j.physbeh.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holman EW. Immediate and delayed reinforcers for flavor preferences in rats. Learn. Motiv. 1975;6:91–100. [Google Scholar]
- 23.Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. J. Neurosci. 2010;30:8012–23. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–41. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning in rats by glucose but not a non-metabolizable glucose analog. Physiol. Behav. 2014;133:92–8. doi: 10.1016/j.physbeh.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira JG, Tellez LA, Ren X, Yeckel CW, de Araujo IE. Regulation of fat intake in the absence of flavour signalling. J. Physiol. 2012;590:953–72. doi: 10.1113/jphysiol.2011.218289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tellez La, Ren X, Han W, Medina S, Ferreira J, Yeckel C, de Araujo IE. Glucose Utilization Rates Regulate Intake Levels of Artificial Sweeteners. J. Physiol. 2013;00:1–18. doi: 10.1113/jphysiol.2013.263103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Araujo IE, Lin T, Veldhuizen MG, Small DM. Metabolic regulation of brain response to food cues. Curr. Biol. 2013;23:878–83. doi: 10.1016/j.cub.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Tang DW, Fellows LK, Dagher A. Behavioral and neural valuation of foods is driven by implicit knowledge of caloric content. Psychol. Sci. 2014;25:2168–76. doi: 10.1177/0956797614552081. fMRI was used to measure brain response while participants bid for a chance to purchase and eat food items displayed on a screen. Willingness to pay was determined by true caloric density rather than individual estimates of caloric content and was reflected in NAc response. [DOI] [PubMed] [Google Scholar]
- 30.Castro DC, Berridge KC. Advances in the neurobiological bases for food “liking” versus “wanting”. Physiol. Behav. 2014;136:22–30. doi: 10.1016/j.physbeh.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J. Neurosci. 2014;34:4239–50. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 33.Burger KS, Stice E. Elevated energy intake is correlated with hyperresponsivity in attentional, gustatory, and reward brain regions while anticipating palatable food receipt. Am. J. Clin. Nutr. 2013;97:1188–94. doi: 10.3945/ajcn.112.055285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wald HS, Myers KP. Enhanced flavor-nutrient conditioning in obese rats on a high-fat, high-carbohydrate choice diet. Physiol. Behav. 2015;151:102–10. doi: 10.1016/j.physbeh.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 35**.Robinson MJ, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, Berridge KC, Ferrario CR. Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity. Neuropsychopharmacology. 2015;40:2113–2123. doi: 10.1038/npp.2015.71. This study showed heightened food cue reactivity in rats prone to diet-induced obesity prior to obesity onset. This suggests that decreases in NAc D2R mRNA can be a consequence of eating a junk-food diet, regardless of weight gain or individual predisposition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van de Giessen E, la Fleur SE, Eggels L, de Bruin K, van den Brink W, Booij J. High fat/carbohydrate ratio but not total energy intake induces lower striatal dopamine D2/3 receptor availability in diet-induced obesity. Int. J. Obes. (Lond) 2013;37:754–7. doi: 10.1038/ijo.2012.128. [DOI] [PubMed] [Google Scholar]
- 37*.Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, de Vaca SC, Reith MEA, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat. Commun. 2015;6:8543. doi: 10.1038/ncomms9543. The authors demonstrated that insulin amplifies striatal dopamine release by modulating cholinergic interneuron excitability via insulin receptors. The effect of insulin on dopamine release was oppositely modulated by caloric restriction and obesogenic diet, and behavioral studies demonstrated a critical role for insulin signaling in the acquisition of flavor-glucose preferences. This paper is the first to implicate insulin as a reward signal in addition to a satiety signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulingkamp R, Pagano T, Hung D, Raffa R. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci. Biobehav. Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 39.Wang X-F, Liu J-J, Xia J, Liu J, Mirabella V, Pang ZP. Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons. Cell Rep. 2015;12:726–33. doi: 10.1016/j.celrep.2015.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mietlicki-Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ, Roitman MF, Hayes MR. Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology. 2015;40:372–85. doi: 10.1038/npp.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Plasse G, van Zessen R, Luijendijk MCM, Erkan H, Stuber GD, Ramakers GMJ, Adan RAH. Modulation of cue-induced firing of ventral tegmental area dopamine neurons by leptin and ghrelin. Int. J. Obes. (Lond) 2015;39:1742–9. doi: 10.1038/ijo.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cone JJ, Roitman JD, Roitman MF. Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J. Neurochem. 2015;133:844–56. doi: 10.1111/jnc.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Zhang Y, Liu G, Yan J, Zhang Y, Li B, Cai D. Metabolic learning and memory formation by the brain influence systemic metabolic homeostasis. Nat. Commun. 2015;6:6704. doi: 10.1038/ncomms7704. The authors found that Drosophila developed metabolic memories to balance food choice with caloric intake by which they were guided towards a preference for normal rather than high-caloric environments. This learning was impaired by NF-κB-dependent hypothalamic inflammation resulting from chronic overnutrition. Moreover, mice with a genetic predisposition to obesity were particularly vulnerable to the effects of overnutrition. These findings provide evidence that metabolic learning plays a role in controlling metabolic homeostasis and is susceptible to genetic and environmental influences such as overnutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mink M, Evans A, Moore CG, Calderon KS, Deger S. Nutritional imbalance endorsed by televised food advertisements. J. Am. Diet. Assoc. 2010;110:904–10. doi: 10.1016/j.jada.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37:2456–66. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang G-J, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn. Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rada P, Avena NM, Barson JR, Hoebel BG, Leibowitz SF. A high-fat meal, or intraperitoneal administration of a fat emulsion, increases extracellular dopamine in the nucleus accumbens. Brain Sci. 2012;2:242–53. doi: 10.3390/brainsci2020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira JG, Tellez LA, Ren X, Yeckel CW, de Araujo IE. Regulation of fat intake in the absence of flavour signalling. J. Physiol. 2012;590:953–72. doi: 10.1113/jphysiol.2011.218289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 50.Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, Palmiter RD. Dopamine Production in the Caudate Putamen Restores Feeding in Dopamine-Deficient Mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 51.Narayanaswami V, Thompson AC, Cassis LA, Bardo MT, Dwoskin LP. Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int. J. Obes. (Lond) 2013;37:1095–103. doi: 10.1038/ijo.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson P, Kenny P. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tellez L, Medina S, Han W, Ferreira J, Licona-Limón P, Ren X, Lam T, Schwartz G, Araujo I. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science. 2013;341:800–802. doi: 10.1126/science.1239275. [DOI] [PubMed] [Google Scholar]
- 54.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 55.de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, van de Laar A, Fliers E, Serlie MJ, Booij J. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2011;1:37. doi: 10.1186/2191-219X-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van de Giessen E, Celik F, Schweitzer DH, van den Brink W, Booij J. Dopamine D2/3 receptor availability and amphetamine-induced dopamine release in obesity. J. Psychopharmacol. 2014;28:866–73. doi: 10.1177/0269881114531664. [DOI] [PubMed] [Google Scholar]
- 57.Cosgrove KP, Veldhuizen MG, Sandiego CM, Morris ED, Small DM. Opposing relationships of BMI with BOLD and dopamine D2/3 receptor binding potential in the dorsal striatum. Synapse. 2015;69:195–202. doi: 10.1002/syn.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD. Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol. Psychiatry. 2014;19:1078–84. doi: 10.1038/mp.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunn JP, Kessler RM, Feurer ID, Volkow ND, Patterson BW, Ansari MS, Li R, Marks-Shulman P, Abumrad NN. Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care. 2012;35:1105–11. doi: 10.2337/dc11-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Kessler RM, Zald DH, Ansari MS, Li R, Cowan RL. Changes in dopamine release and dopamine D2/3 receptor levels with the development of mild obesity. Synapse. 2014;68:317–20. doi: 10.1002/syn.21738. The authors used [18F]fallypride PET imaging to measure dopamine receptor availability at baseline and following an amphetamine challenge in human subjects ranging from lean to mildly obese. Mild obesity was associated with increased amphetamine-induced striatal dopamine release, but not with changes in D2 receptor levels. This study is the first to directly assess changes in phasic dopamine release associated with the development of obesity. [DOI] [PubMed] [Google Scholar]
- 61*.Horstmann A, Fenske WK, Hankir MK. Argument for a non-linear relationship between severity of human obesity and dopaminergic tone. Obes. Rev. 2015;16:821–30. doi: 10.1111/obr.12303. An outstanding review of the literature examining dopamine signaling in human ingestive behavior and obesity. The authors propose overweight/mild obesity and severe obesity are associated with different states of dopaminergic tone and phasic dopamine responses in the striatum. Alterations in this dopaminergic signaling is hypothesized to give rise to deficits in feedback-dependent behavior, such as reward and punishment, as well as different domains of cognition. [DOI] [PubMed] [Google Scholar]
- 62.Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42:131–8. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Wang G-J, Tomasi D, Convit A, Logan J, Wong CT, Shumay E, Fowler JS, Volkow ND. BMI modulates calorie-dependent dopamine changes in accumbens from glucose intake. PLoS One. 2014;9:e101585. doi: 10.1371/journal.pone.0101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 65.Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–21. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, Nuutila P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One. 2012;7:e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J. Abnorm. Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Babbs RK, Sun X, Felsted J, Chouinard-Decorte F, Veldhuizen MG, Small DM. Decreased caudate response to milkshake is associated with higher body mass index and greater impulsivity. Physiol. Behav. 2013;121:103–11. doi: 10.1016/j.physbeh.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frank GKW, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, O’Reilly RC. Anorexia Nervosa and Obesity are Associated with Opposite Brain Reward Response. Neuropsychopharmacology. 2012;37:2031–2046. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green E, Jacobson A, Haase L, Murphy C. Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Res. 2011;1386:109–17. doi: 10.1016/j.brainres.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J. Neurosci. 2010;30:13105–9. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–52. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73*.Stice E, Burger KS, Yokum S. Reward Region Responsivity Predicts Future Weight Gain and Moderating Effects of the TaqIA Allele. J. Neurosci. 2015;35:10316–24. doi: 10.1523/JNEUROSCI.3607-14.2015. This prospective fMRI study found that elevated orbitofrontal cortex response to cues signaling the impending receipt of milkshake predicted future weight gain in adolescents. Additionally, elevated caudate response to milkshake receipt predicted weight gain in carriers of the Taq1A A2/A2 allele, while the inverse relationship was observed in A1 carriers. Moreover, brain response to receipt and anticipated receipt of monetary reward did not predict weight gain. This study demonstrates that alterations in reward region responsivity to food cues and receipt confer weight gain vulnerability and suggests distinct pathways to obesity depending on genetic predisposition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50:1618–25. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75*.Sun X, Kroemer NB, Veldhuizen MG, Babbs AE, de Araujo IE, Gitelman DR, Sherwin RS, Sinha R, Small DM. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J. Neurosci. 2015;35:7964–76. doi: 10.1523/JNEUROSCI.3884-14.2015. Using fMRI, the authors demonstrated a positive association between caudate response to the unexpected milkshake receipt and future weight gain in individuals with diminished dopamine D2 signaling by virtue of possessing the A1 allele of the Taq1A polymorphism. In contrast, amygdale response to milkshake receipt when sated predicted future weight gain only A1 non-carriers who possess greater dopamine D2 signaling capacity. Thus study provides compelling evidence that distinct brain mechanisms confer weight gain susceptibility depending on individual differences in dopamine signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J. Neurophysiol. 1994;72:1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- 77.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–5. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 78.Burger KS, Stice E. Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. Neuroimage. 2014;99:122–8. doi: 10.1016/j.neuroimage.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 80.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J. Neurosci. 2011;31:4360–6. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum. Mutat. 2004;23:540–5. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 82.Meylan E, Tschopp J. The RIP kinases: crucial integrators of cellular stress. Trends Biochem. Sci. 2005;30:151–159. doi: 10.1016/j.tibs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Bontempi S, Fiorentini C, Busi C, Guerra N, Spano P, Missale C. Identification and characterization of two nuclear factor-kappaB sites in the regulatory region of the dopamine D2 receptor. Endocrinology. 2007;148:2563–70. doi: 10.1210/en.2006-1618. [DOI] [PubMed] [Google Scholar]
- 84.Fiorentini C, Guerra N, Facchetti M, Finardi A, Tiberio L, Schiaffonati L, Spano P, Missale C. Nerve growth factor regulates dopamine D(2) receptor expression in prolactinoma cell lines via p75(NGFR)-mediated activation of nuclear factor-kappaB. Mol. Endocrinol. 2002;16:353–66. doi: 10.1210/mend.16.2.0773. [DOI] [PubMed] [Google Scholar]
- 85.Hart G, Leung BK, Balleine BW. Dorsal and ventral streams: the distinct role of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiol. Learn. Mem. 2014;108:104–18. doi: 10.1016/j.nlm.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corbit LH, Janak PH. Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning. Eur. J. Neurosci. 2010;31:1312–21. doi: 10.1111/j.1460-9568.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McNamee D, Liljeholm M, Zika O, O’Doherty JP. Characterizing the associative content of brain structures involved in habitual and goal-directed actions in humans: a multivariate FMRI study. J. Neurosci. 2015;35:3764–71. doi: 10.1523/JNEUROSCI.4677-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liljeholm M, Tricomi E, O’Doherty JP, Balleine BW. Neural correlates of instrumental contingency learning: differential effects of action-reward conjunction and disjunction. J. Neurosci. 2011;31:2474–80. doi: 10.1523/JNEUROSCI.3354-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gourley SL, Olevska A, Gordon J, Taylor JR. Cytoskeletal determinants of stimulus-response habits. J. Neurosci. 2013;33:11811–6. doi: 10.1523/JNEUROSCI.1034-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chatham CH, Frank MJ, Badre D. Corticostriatal output gating during selection from working memory. Neuron. 2014;81:930–42. doi: 10.1016/j.neuron.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Backman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, Neely AS, Virta J, Laine M, Rinne JO. Effects of Working-Memory Training on Striatal Dopamine Release. Science (80-. ) 2011;333:718–718. doi: 10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- 92.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 93.Tschernegg M, Pletzer B, Schwartenbeck P, Ludersdorfer P, Hoffmann U, Kronbichler M. Impulsivity relates to striatal gray matter volumes in humans: evidence from a delay discounting paradigm. Front. Hum. Neurosci. 2015;9:384. doi: 10.3389/fnhum.2015.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frank MJ, Seeberger LC, O’reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–3. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 95.Frank MJ, Hutchison K. Genetic contributions to avoidance-based decisions: striatal D2 receptor polymorphisms. NeuroScience. 2009;164:131–140. doi: 10.1016/j.neuroscience.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Foerde K, Steinglass JE, Shohamy D, Walsh BT. Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nat. Neurosci. 2015;18:1571–1573. doi: 10.1038/nn.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coppin G, Nolan-Poupart S, Jones-Gotman M, Small DM. Working memory and reward association learning impairments in obesity. Neuropsychologia. 2014;65:146–55. doi: 10.1016/j.neuropsychologia.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sevgi M, Rigoux L, Kühn AB, Mauer J, Schilbach L, Hess ME, Gruendler TOJ, Ullsperger M, Stephan KE, Brüning JC, et al. An Obesity-Predisposing Variant of the FTO Gene Regulates D2R–Dependent Reward Learning. J. Neurosci. 2015;35:12584–92. doi: 10.1523/JNEUROSCI.1589-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cox SML, Frank MJ, Larcher K, Fellows LK, Clark CA, Leyton M, Dagher A. Striatal D1 and D2 signaling differentially predict learning from positive and negative outcomes. Neuroimage. 2015;109:95–101. doi: 10.1016/j.neuroimage.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 100.Houben K, Nederkoorn C, Jansen A. Eating on impulse: the relation between overweight and food-specific inhibitory control. Obesity (Silver Spring) 2014;22:E6–8. doi: 10.1002/oby.20670. [DOI] [PubMed] [Google Scholar]
- 101.van den Akker K, Jansen A, Frentz F, Havermans RC. Impulsivity makes more susceptible to overeating after contextual appetitive conditioning. Appetite. 2013;70:73–80. doi: 10.1016/j.appet.2013.06.092. [DOI] [PubMed] [Google Scholar]
- 102.Epstein LH, Jankowiak N, Fletcher KD, Carr KA, Nederkoorn C, Raynor HA, Finkelstein E. Women who are motivated to eat and discount the future are more obese. Obesity (Silver Spring) 2014;22:1394–9. doi: 10.1002/oby.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lawrence NS, O’Sullivan J, Parslow D, Javaid M, Adams RC, Chambers CD, Kos K, Verbruggen F. Training response inhibition to food is associated with weight loss and reduced energy intake. Appetite. 2015;95:17–28. doi: 10.1016/j.appet.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guerrieri R, Nederkoorn C, Jansen A. Disinhibition is easier learned than inhibition. The effects of (dis)inhibition training on food intake. Appetite. 2012;59:96–9. doi: 10.1016/j.appet.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 105.Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318:1642–5. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- 106.Fagundo AB, Fernández-Aranda F, de la Torre R, Verdejo-García A, Granero R, Penelo E, Gené M, Barrot C, Sánchez C, Alvarez-Moya E, et al. Dopamine DRD2/ANKK1 Taq1A and DAT1 VNTR polymorphisms are associated with a cognitive flexibility profile in pathological gamblers. J. Psychopharmacol. 2014;28:1170–7. doi: 10.1177/0269881114551079. [DOI] [PubMed] [Google Scholar]
- 107.Berryhill ME, Wiener M, Stephens JA, Lohoff FW, Coslett HB. COMT and ANKK1-Taq-Ia genetic polymorphisms influence visual working memory. PLoS One. 2013;8:e55862. doi: 10.1371/journal.pone.0055862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N, Li MD. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology. 2009;34:319–30. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- 109.Connor JP, Young RM, Saunders JB, Lawford BR, Ho R, Ritchie TL, Noble EP. The A1 allele of the D2 dopamine receptor gene region, alcohol expectancies and drinking refusal self-efficacy are associated with alcohol dependence severity. Psychiatry Res. 2008;160:94–105. doi: 10.1016/j.psychres.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 110.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J. R. Soc. Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoactive Drugs. 2000;32(Suppl:i–iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]