Abstract
The adipocyte-derived hormone leptin acts via its receptor (LepRb) on central nervous system neurons to communicate the repletion of long-term energy stores, to decrease food intake, and to promote energy expenditure. We generated mice that express Cre recombinase from the calcitonin receptor (Calcr) locus (Calcrcre mice) to study Calcr-expressing LepRb (LepRbCalcr) neurons, which reside predominantly in the arcuate nucleus (ARC). Calcrcre-mediated ablation of LepRb in LepRbCalcrknockout (KO) mice caused hyperphagic obesity. Because LepRb-mediated transcriptional control plays a crucial role in leptin action, we used translating ribosome affinity purification followed by RNA sequencing to define the transcriptome of hypothalamic Calcr neurons, along with its alteration in LepRbCalcrKO mice. We found that ARC LepRbCalcr cells include neuropeptide Y (NPY)/agouti-related peptide (AgRP)/γ-aminobutyric acid (GABA) (“NAG”) cells as well as non-NAG cells that are distinct from pro-opiomelanocortin cells. Furthermore, although LepRbCalcrKO mice exhibited dysregulated expression of several genes involved in energy balance, neither the expression of Agrp and Npy nor the activity of NAG cells was altered in vivo. Thus, although direct leptin action via LepRbCalcr cells plays an important role in leptin action, our data also suggest that leptin indirectly, as well as directly, regulates these cells.
Leptin acts via calcitonin receptor‒expressing hypothalamic neurons to control feeding and energy balance.
Pharmacologic therapies to reduce appetite would be useful in treating obesity and related metabolic disorders, but our limited understanding of the neural and molecular mechanisms that regulate these processes has hindered the development of effective therapies. Of the hormones orchestrating metabolism, leptin plays a central role, as demonstrated by the hyperphagia, obesity, and diabetes produced by leptin deficiency (1–4). Adipocytes secrete leptin in proportion to lipid stores, signaling the adequacy of fat reserves to decrease appetite and permit energy expenditure.
Loss of the signaling isoform of the leptin receptor (LepRb) in Leprdb/db mice recapitulates the phenotype of leptin-deficient animals, demonstrating the essential role of LepRb signaling in leptin action (5, 6). Leptin binding to LepRb activates a noncovalently associated Jak2 tyrosine kinase, which phosphorylates three sites (Tyr985, Tyr1077, and Tyr1138) on LepRb (7, 8). Of these, only Tyr1138, which recruits and activates the latent transcription factor STAT3, plays a major role in leptin action (9–12). Thus, the leptin/STAT3‒mediated control of gene expression in LepRb cells is essential for the control of energy balance.
LepRb is expressed most highly in brain regions that control food intake, energy expenditure, and metabolism, and central nervous system (CNS) LepRb signaling is necessary and sufficient for the control of energy balance by leptin (13–15). Although the hypothalamus represents the major region by which LepRb signaling controls energy balance (16), identifying the specific sets of hypothalamic LepRb neurons important for leptin action has been challenging (17). We also incompletely understand how leptin alters gene expression and neuronal function in LepRb neurons to control energy balance.
Two distinct populations of LepRb neurons in the hypothalamic arcuate nucleus (ARC) are known to play important roles in energy balance (1, 17, 18): (1) orexigenic neurons that contain neuropeptide Y (NPY), agouti-related peptide (AgRP), and the inhibitory neurotransmitter γ-aminobutyric acid (GABA) (“NAG” neurons) and (2) anorexigenic neurons that express pro-opiomelanocortin (POMC). Leptin inhibits NAG neurons and blunts the expression of Npy and Agrp while promoting Pomc expression and activating POMC neurons. Additional groups of less well-studied populations of ARC LepRb neurons, including those that contain Ghrh, Sst, and Trh, may also contribute to leptin action (19).
The ablation of LepRb from most anatomically restricted sets of neurons examined to date (including Pomc, Sf1, Nts, Gal, Prlh, Pdyn, and Kiss1 neurons, as well as neurons in the preoptic area and ventral premammillary nucleus) modestly alters feeding and adiposity by comparison with the effects of pan-hypothalamic LepRb deletion (20–28). To identify additional molecularly defined subpopulations of LepRb neurons that participate in energy homeostasis, we used translating ribosome affinity purification (TRAP) followed by RNA sequencing (RNA-seq) (TRAP-seq)‒defined transcripts enriched in LepRb-expressing cells (25) to identify markers for potentially important populations of hypothalamic LepRb neurons. The enrichment of calcitonin receptor (Calcr)‒encoding messenger RNA (mRNA) in hypothalamic LepRb neurons (25), together with the synergy of leptin and CALCR agonists for the suppression of food intake and body weight (29, 30), led us to study Calcr-expressing LepRb cell (LepRbCalcr) neurons. Here, we identify these cells, reveal their importance for the control of energy balance by leptin, and define their transcriptional response to LepRb ablation.
Materials and Methods
Methods
Mice
Mice were bred in our colony in the Unit for Laboratory Animal Medicine at the University of Michigan; these mice and the procedures performed were approved by the University of Michigan Committee on the Use and Care of Animals and in accordance with Association for the Assessment and Approval of Laboratory Animal Care and National Institutes of Health guidelines. We purchased male and female C57BL/6 mice for experiments and breeding studies from Jackson Laboratories. Mice were bred at the University of Michigan and provided with food and water ad libitum (except as noted later) and temperature-controlled rooms on a 12-hour light-dark cycle.
We generated LepRbeGFP-L10a mice by breeding Leprcre mice (31) onto the enhanced green fluorescent protein (eGFP)–L10a background to generate double homozygous Leprcre/cre;RosaeGFP-L10a/eGFP-L10a (LepReGFP) animals, which we intercrossed to generate mice for study (25). POMC-dsRed transgenic mice (32) and NPY–green fluorescent protein (GFP) transgenic mice (33) were crossed to generate POMCdsRedNPYGFP mice.
To generate Calcrcre mice, a selection cassette containing the porcine teschoviral 2A cleavage sequence linked to Cre recombinase and a Frt-flanked neomycin resistance gene was targeted to replace the stop codon of the Calcr gene in a bacterial artificial chromosome (RP24-193M22; Children’s Hospital Oakland Research Institute). A targeting plasmid containing the Cre-containing selection cassette and ∼4-kb genomic sequence upstream and downstream of the Calcr stop codon was isolated and used for embryonic stem cell targeting by the University of Michigan Transgenic Core. Correctly targeted clones were identified by loss of native allele quantitative polymerase chain reaction (PCR) from embryonic stem cell clone DNA. Chimeric animals generated from blastocyst implantation were then bred for germline transmission of the Calcrcre allele. Flp-deleter mice were then used to remove the neomycin selection cassette. Genotyping was by allele-specific PCR.
CalcreGFP-L10a mice were produced by crossing Calcrcre mice onto the cre-inducible Rosa26eGFP-L10a background to produce Calcrcre/+;Rosa26eGFP-10a/+ mice, which were then intercrossed to generated double homozygous Calcrcre/cre;RosaeGFP-L10a/eGFP-L10a (CalcreGFP-L10a) mice that were intercrossed to generate additional CalcreGFP-L10a mice for study. Leprflox/flox mice (34) were bred to CalcreGFP-L10a mice to generate Calcrcre/+;Leprflox/+;Rosa26eGFP-L10a/+ mice, which were bred to CalcreGFP-L10a mice to produce Calcrcre/cre;Leprflox/+;Rosa26eGFP-L10a/eGFP-L10a mice. Intercrossing these mice produced littermate Calcrcre/cre;Leprflox/flox;Rosa26eGFP-L10a/eGFP-L10a [LepRbCalcrknockout (KO)] and control [Calcrcre/cre;Lepr+/+;Rosa26eGFP-L10a/eGFP-L10a (CalcreGFP-L10a)] mice for study.
POMCdsRed;CalcreGFP-L10a mice were generated by crossing CalcreGFP-L10a mice with the POMC-dsRed transgenic mice (the generous gift of Malcolm Low, University of Michigan). Similarly, NPY-GFP;CalcrtdTomato mice were generated by crossing Calcrcre onto the cre-inducible Rosa26tdTomato (Jackson Laboratories, Bar Harbor, ME; stock no. 007909) background and then crossing with the NPY-GFP transgenic mice. We genotyped the offspring using PCR. Lepob/ob mice were produced in-house by intercrossing Lepob/+ mice from Jackson Laboratories (stock no. 000632).
Leptin treatment, salmon calcitonin treatment, and immunohistochemistry
Food was removed at the onset of the light cycle, and mice were treated 4 hours later with metreleptin [from MedImmune, Inc., Gaithersburg, MD; 5 mg/kg, intraperitoneal (i.p.)], salmon calcitonin (sCT; from Bachem, Torrance, CA; 150 μg/kg, i.p.), or vehicle and were perfused 90 minutes later. Mice were anesthetized with a lethal dose of pentobarbital and transcardially perfused with phosphate-buffered saline followed by 10% buffered formalin. Brains were removed, placed in 10% buffered formalin overnight, and dehydrated in 30% sucrose for 1 week. With use of a freezing microtome (Leica, Buffalo Grove, IL), brains were cut into 30-μm sections. Sections were treated sequentially with 1% hydrogen peroxide/0.5% sodium hydroxide, 0.3% glycine, 0.03% sodium dodecyl sulfate, and blocking solution (phosphate-buffered saline with 0.1% triton, 3% normal donkey serum). Immunostaining was performed using primary antibodies for pSTAT3 [Cell Signaling, Danvers, MA; catalog no. 9145 (RRID: AB_2491009), rabbit, 1:1000]; GFP [Aves Laboratories, Tigard, OR; catalog no. GFP1020 (RRID: AB_10000240), chicken, 1:1000]; cFos [Santa Cruz Biotechnology, Santa Cruz, CA; catalog no. sc-52 (RRID: AB_2106783), rabbit, 1:1000]; and dsRed [Takara Bio USA, San Mateo, CA; catalog no. 632496 (RRID: AB_10013483), 1:1000]. All antibodies were reacted with species-specific Alexa Fluor-488 or -568 conjugated secondary antibodies (Invitrogen, Thermo Fisher, Waltham, MA; 1:200) or processed with the avidin-biotin/diaminobenzidine (DAB) method (ABC kit; Vector Laboratories, Burlingame, CA; 1:500; DAB reagents, Sigma). Images were collected on an Olympus (Center Valley, PA) BX53F microscope. For confocal imaging, X and Y signals within neurons of the ARC were visualized with a Nikon (Melville, NY) A1 confocal system using a 40× oil objective. Green and red fluorescence signals were captured sequentially in multiple focus planes throughout the entire extent of the 30-μm tissue sections with the following scan parameters: 1024 × 1024 pixel resolution, two-line averaging, 1.8-μm optical sectioning (3.0 Airy units), and Z-step size of 0.74 μm. DAB images were pseudocolored using Photoshop software (Adobe).
In situ hybridization
Adult Calcrcre and wild-type control mice were anesthetized with isoflurane and then euthanized by decapitation. Whole brains were dissected, flash frozen in isopentane chilled on dry ice, and stored at −80°C. Next, 16-μm-thick coronal sections were cut on a cryostat (Leica), thaw-mounted to SuperFrost Plus slides, allowed to dry at −20°C for 1 hour, and then stored at −80°C. Slides were then processed for in situ hybridization using RNAScope technology per the manufacturer’s protocol (Advanced Cell Diagnostics, Newark, CA). For all slides, the multiplex fluorescent assay (320850) was used to visualize CalcR (477791) and Cre (312281-C3) probes using Amp 4 Alt-A. Images were obtained with an Olympus BX53F equipped with a QImaging Retiga 6000 monochrome camera under 40× objective. All images were processed identically in CellProfiler (35) to reduce nonspecific background. Serial images (nine per ARC) were taken and stitched together using Adobe Photoshop.
Phenotyping of LepRbCalcrKO and control mice
LepRbCalcrKO and littermate control (LepRbeGFP-L10a) mice were weaned into individual housing at 21 days and fed normal chow (Purina Laboratory Diet 5001). Weekly body weight and food intake were monitored. Blood glucose level in ad libitum‒fed mice was measured every other week from 4 to 12 weeks of age. A glucose tolerance test (2 g/kg of body weight, i.p.) and insulin tolerance test [1 unit/kg of body weight; insulin injection (Humulin; Eli Lilly), i.p.] were performed in 13- and 14-week-old mice, respectively, after a 5-hour fast that began 3 hours after the start of the light cycle. Analysis of body fat and lean mass was performed at 14 to 15 weeks of age using a Minispec LF90ll (Bruker Optics, Billerica, MA). One subset of mice (9- to 11-week-olds) were analyzed for oxygen consumption (Vo2), food intake, and locomotor activity using the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH). Leptin and insulin were assayed by commercial enzyme-linked immunosorbent assay (Crystal Chem, Elk Grove, IL).
TRAP-seq
We used anti-eGFP TRAP with hypothalamic material from CalcreGFP-L10a and LepRbCalcrKO mice (both of which express an eGFP-tagged ribosomal subunit in Calcr cells). RNA was assessed for quality using the TapeStation (Agilent, Santa Clara, CA). Samples with RNA integrity numbers of 7.5 or greater were prepared using the Illumina TruSeq mRNA Sample Prep v2 kit (catalog nos. RS-122-2001 and RS-122-2002; San Diego, CA), where 0.1 to 3 μg of total RNA was converted to mRNA using polyA purification. The mRNA was fragmented via chemical fragmentation and copied into first-strand complementary DNA (cDNA) using reverse transcription and random primers. The 3′ ends of the cDNA were adenylated, and 6-nucleotide‒barcoded adapters were ligated. The products were purified and enriched by PCR to create the final cDNA library. Final libraries were checked for quality and quantity by TapeStation (Agilent) and quantitative PCR using a Library Quantification Kit for Illumina Sequencing platforms (catalog no. KK4835; Kapa Biosystems, Wilmington, MA). They were clustered on the cBot (Illumina) and sequenced four samples per lane on a 50-cycle single-end run on a HiSeq 2000 (Illumina) using version 2 reagents according to the manufacturer’s protocols.
RNA-seq analysis
Fifty base pair single-end reads underwent quality control analysis before alignment to mouse genome build mm9 using TopHat and Bowtie alignment software (36). Differential expression was determined using Cufflinks Cuffdiff analysis, with thresholds for differential expression set to fold change >1.5 or <0.66 and a false discovery rate ≤0.05 (37). Lists of differentially expressed genes were then queried against the UniProt Database for gene ontology and protein class analysis (38).
Statistics
Data are reported as mean ± standard error of the mean. Statistical analyses of physiologic data were performed with Prism software (version 7). An unpaired t test was used to compare results between the two groups. Body weight gain, cumulative food intake, body length, glucose tolerance test, and insulin tolerance test were analyzed by two-way analysis of variance. P < 0.05 was considered statistically significant.
Results
LepRbCalcr neurons
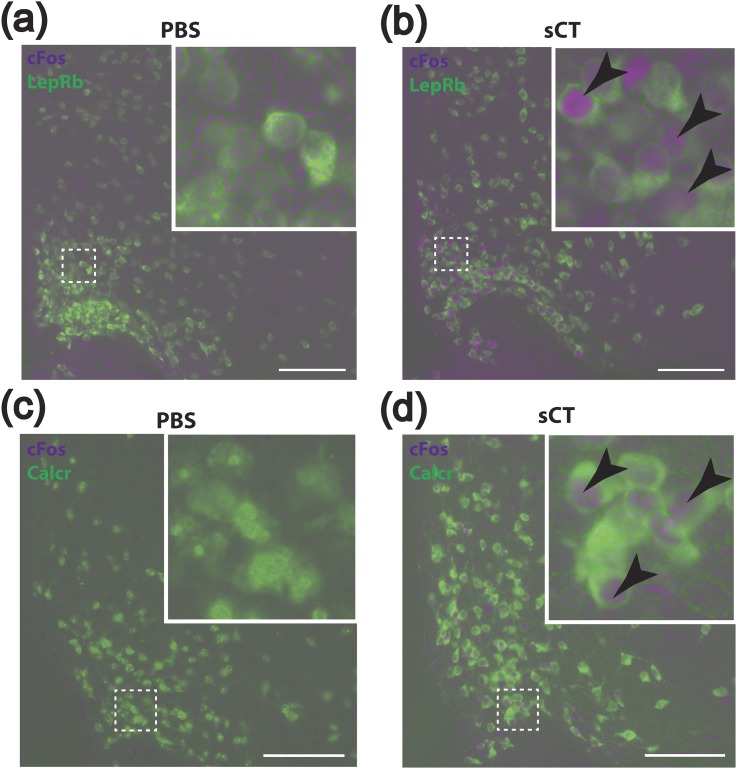
To identify the putative LepRbCalcr neurons and define their distribution and responsiveness to CALCR agonists and leptin, we treated LepRbeGFP-L10a reporter mice with sCT and examined the activation of LepRb neurons by the immunohistochemical (IHC) detection of cFos-immunoreactivity (IR) in eGFP-containing hypothalamic neurons [Fig. 1(a) and 1(b)]. This analysis revealed that sCT promoted the accumulation of cFos-IR in a large population of eGFP-expressing ARC LepRb neurons, suggesting that these cells represent LepRbCalcr cells.
Figure 1.
sCT-stimulated cFos-IR in LepRbeGFP-L10a and CalcReGFP mice. (a, b) Representative images showing colocalization of cFos-IR (purple) with GFP-IR (green) in LepRbeGFP-L10a mice treated with sCT (150 μg/kg, i.p.) or PBS vehicle. (c, d) Representative images show colocalization of cFos-IR (purple) with GFP-IR (green) in CalcreGFP-L10a mice treated with sCT (150 μg/kg, i.p.) or PBS vehicle. Insets show a digital zoom of the boxed region. Arrowheads indicate colocalized neurons. Scale bars = 100 μm. PBS, phosphate-buffered saline.
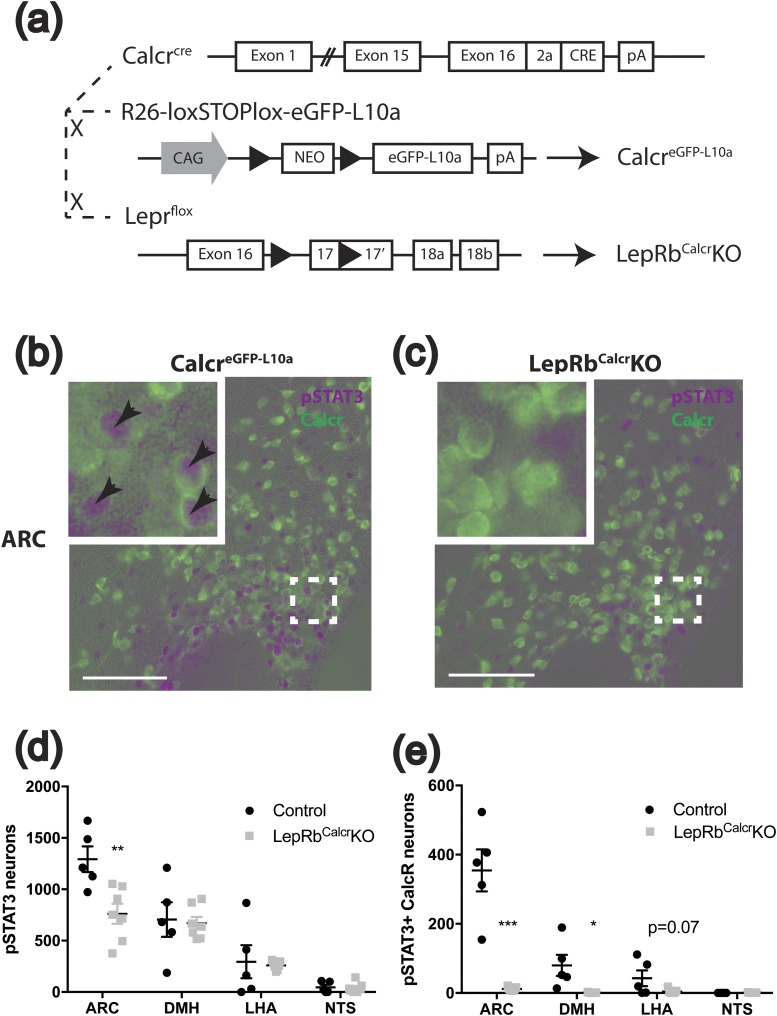
To examine the potential Calcr expression of ARC cells and to permit the manipulation of loxP-flanked (“floxed”) alleles in Calcr cells, we inserted a 2A peptide element plus the coding sequence for Cre recombinase in place of the stop codon of Calcr in mice (Calcrcre mice) [Fig. 2(a)]. In situ hybridization demonstrated the colocalization of cre and Calcr mRNA in the hypothalamus (Supplemental Fig. 1), consistent with the expected expression of cre from the endogenous Calcr locus.
Figure 2.
Generation of Calcrcre, CalcreGFP-L10a, and LepRbCalcrKO mice. (a) Schematic diagram showing the cross of Calcrcre mice with Rosa26-loxSTOPlox-eGFP-L10a (Rosa26eGFP-L10a) mice to generate CalcreGFP mice and the cross of Calcrcre with Leprflox/flox mice to generate LepRbCalcrKO mice. (b, c) Representative images showing colocalization of pSTAT3-IR (purple) with GFP-IR (green) in the ARC of 4-week-old CalcreGFP-L10a and LepRbCalcrKO mice (both on the Rosa26eGFP-L10a background) treated with leptin (5 mg/kg, i.p.) for 90 minutes. Insets show a digital zoom of the boxed region. Arrowheads indicate colocalized neurons. Scale bars = 100 μm. (d, e) pSTAT3 and GFP IR neurons and double-labeled neurons were quantified for the indicated regions and the number of cells positive for pSTAT3, and doubled-labeled pSTAT3+GFP cells are plotted by region. Graphs show mean value ± the standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001. CAG, chicken β-actin promoter; DMH, dorsomedial hypothalamic nucleus; LHA, lateral hypothalamic area; NEO, neomycin resistance cassette; NTS, nucleus of the solitary tract; pA: polyadenylation signal.
We bred Calcrcre to the cre-inducible Rosa26eGFP-L10a background (25) (CalcreGFP-L10a mice) to express an eGFP-tagged L10a ribosomal subunit specifically in Calcr cells, permitting their IHC detection and analysis. The ARC of these mice not only contains a large population of GFP-containing cells, but treatment of CalcreGFP-L10a mice with sCT also promoted the accumulation of cFos-IR only in GFP-IR cells in the ARC of these mice [Fig. 1(c) and 1(d)], consistent with the direct regulation of these cells by CALCR agonists and with the overlap of Calcr expression with LepRb in the ARC.
We thus bred CalcreGFP-L10a onto the Leprflox background to generate littermate Calcrcre/cre;Leprflox/flox;Rosa26eGFP-L10a/eGFP-L10a (LepRbCalcrKO) mice and control CalcreGFP-L10a mice to define the distribution of LepRbCalcr cells and their roles in leptin action [Fig. 2(a)]. We treated these mice with leptin and perfused them for the IHC analysis of pSTAT3 and GFP [Fig. 2(b)–2(e); Supplemental Fig. 2]; because pSTAT3 reveals cell-autonomous LepRb signaling (39) [unlike cFos, which reflects cellular activity that can be altered directly or trans-synaptically (40)], neurons that contain both GFP and pSTAT3 represent LepRbCalcr cells. We found that most LepRbCalcr neurons lie in the ARC (consistent with the sCT stimulation of cFos in LepRb neurons in this area) and that LepRbCalcr neurons represent approximately 30% of ARC LepRb neurons. Smaller numbers of LepRbCalcr cells were also observed in the lateral hypothalamic area [LHA; as previously reported (41)] and the dorsomedial hypothalamic nucleus (DMH). Although the nucleus of the solitary tract contains both LepRb and Calcr cells, we detected no overlap between these populations in that region. As expected, LepRbCalcrKO mice exhibited no pSTAT3-IR in GFP-expressing Calcr neurons, consistent with the predicted ablation of LepRb from LepRbCalcr neurons in these animals.
Leptin action via LepRbCalcr neurons controls feeding and energy balance
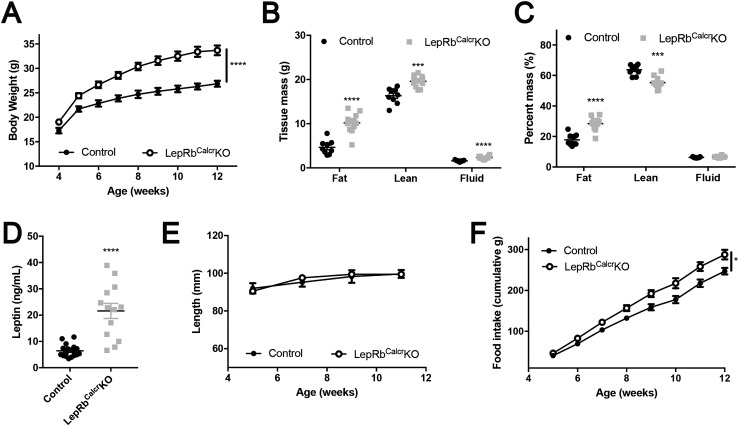
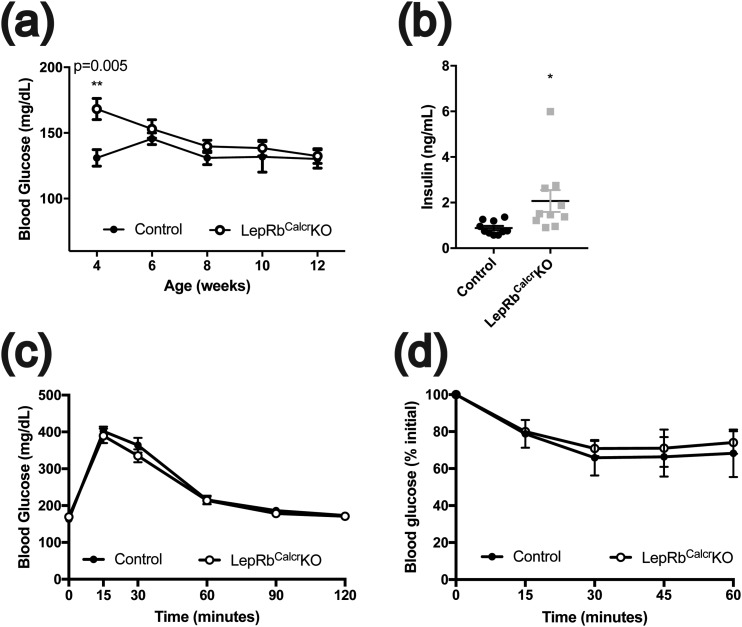
To understand the role for LepRbCalcr neurons in leptin action, we examined parameters of weight and metabolic control in LepRbCalcrKO mice (Fig. 3; Supplemental Fig. 3). We found increased body weight in both male and female LepRbCalcrKO mice compared with controls (Fig. 3A; Supplemental Fig. 3A). The excess weight in LepRbCalcrKO mice reflected a more than doubling of their adipose mass (Fig. 3B–3C; Supplemental Fig. 3B–3C); circulating leptin concentrations were similarly increased (Fig. 3D; Supplemental Fig. 3D). Although LepRbCalcrKO mice displayed increased lean mass on the basis of absolute weight, body length was not altered compared with that of controls (Fig. 3E; Supplemental Fig. 3E), suggesting that the disruption of leptin action in these animals did not impair the function of the hypothalamic melanocortin system [disruption of which increases linear growth (42, 43)]. LepRbCalcrKO mice of both sexes consumed more food than controls (Fig. 3F; Supplemental Fig. 3F). Thus, disruption of direct leptin action on LepRbCalcr neurons caused hyperphagic obesity.
Figure 3.
Leptin action via LepRbCalcr neurons controls energy balance. Shown are (A) body weight at ages 4 to 12 weeks, (B, C) body composition at 14 to 15 weeks of age, (D) serum leptin concentration at 10 weeks of age, (E) body length from 5 to 11 weeks of age, and (F) cumulative food intake from 5 to 12 weeks of age for male CalcreGFP-L10a (Control) and LepRbCalcrKO mice. Shown is mean value ± the standard error of the means. *P < 0.05; ***P < 0.001; ****P < 0.0001 by (A, F) analysis of variance or (all other panels) t test.
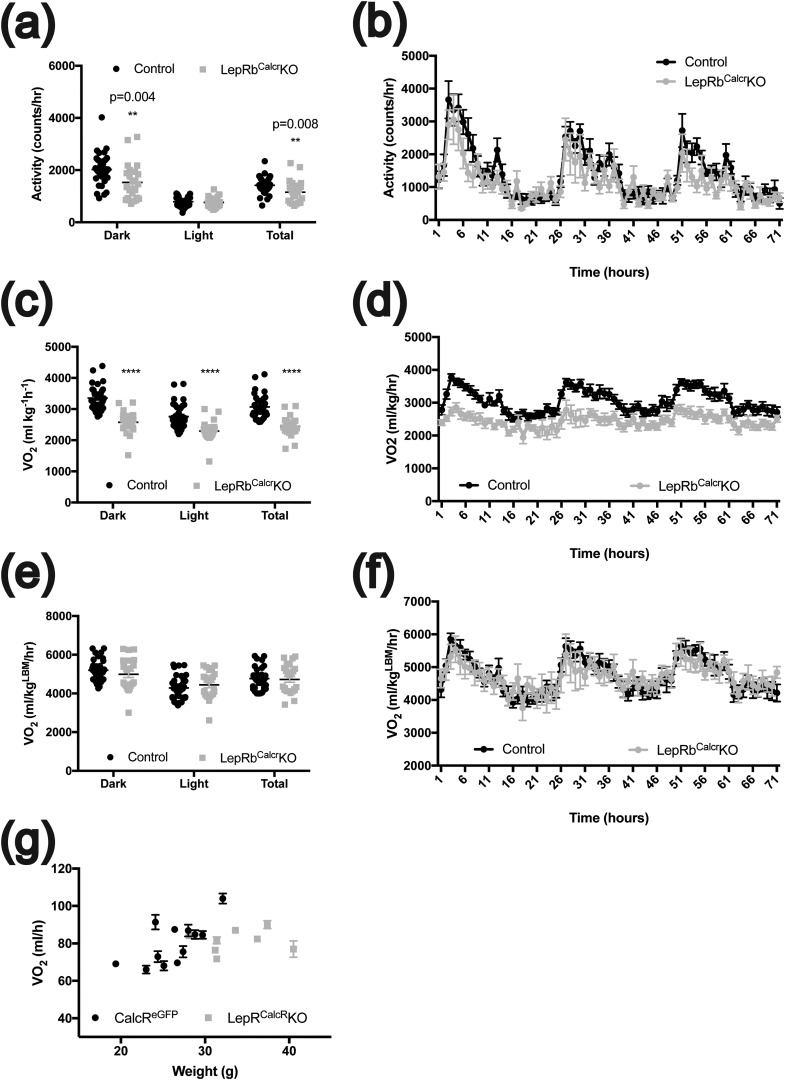
To determine whether the impairment of energy expenditure might also contribute to the obesity of LepRbCalcrKO mice, we used metabolic cages to quantify locomotor activity and Vo2 in male LepRbCalcrKO mice (Fig. 4). Twenty-four‒hour locomotor activity was decreased in LepRbCalcrKO mice because of decreased activity during the dark cycle. Although Vo2 by LepRbCalcrKO mice was decreased when normalized to total body mass, this difference disappeared when normalized to lean body mass, suggesting that energy expenditure was only minimally affected by the ablation of LepRb in LepRbCalcr neurons, consistent with the relatively modest contribution of normal ambulatory activity to total energy expenditure in small rodents. Indeed, the relationship between Vo2 and body weight was similar to that between LepRbCalcrKO mice and their controls [Fig. 4(g)].
Figure 4.
Control of activity and energy expenditure by leptin action on LepRbCalcr neurons. The 9- to 11-week-old male CalcreGFP-L10a mice (Controls; n = 12) and LepRbCalcrKO mice (n = 8) were subjected to Comprehensive Laboratory Animal Monitoring System (Columbus Instruments) analysis to determine (a, b) locomotor activity; (c, d) Vo2 normalized to total body mass; and (e, f) Vo2 adjusted to lean body mass. Data are shown for (a, c, e) dark cycle (Dark), light cycle (Light), and 24 hours (Total) and (b, d, f) the entire hour-by-hour 72-hour period. (g) Graph shows 24-hour Vo2 consumption rate plotted against body weight for each animal. (b, d, f, g) Mean ± standard error of the mean is shown. **P < 0.01; ****P < 0.0001.
We also examined parameters of glucose homeostasis in male and female LepRbCalcrKO mice (Fig. 5; Supplemental Fig. 4). Although ad libitum‒fed glucose concentrations were not different between LepRbCalcrKO mice and controls at most ages, serum insulin concentrations were increased in both male and female LepRbCalcrKO mice, suggesting that the hyperinsulinemia of these animals normalized their blood glucose by compensating for some degree of insulin resistance. Although we detected no alteration in glycemic excursions in response to an i.p. glucose challenge or insulin challenge in male mice, female mice showed subtle alterations in these measures. These findings are consistent with the more modest impairments in insulin tolerance and glucose homeostasis that accompany obesity relative to the florid insulin resistance and glucose intolerance that accompany complete leptin or LepRb deficiency in mice (44), suggesting that increased food intake and resultant obesity (rather than direct interference with glycemic control by leptin) represent the main physiologic alterations in LepRbCalcrKO mice.
Figure 5.
Relatively preserved glycemic control in LepRbCalcrKO mice. Shown are (a) blood glucose concentrations from 4 to 12 weeks of age, (b) serum insulin concentrations at 10 weeks of age, (c) glycemic excursions during glucose tolerance test (2 g/kg glucose, i.p.) at 12 weeks of age, and (d) insulin tolerance test (1 U/kg insulin, i.p.) at 13 weeks of age for male CalcreGFP-L10a (Control) and LepRbCalcrKO mice. Mean ± standard error of the mean is shown. *P < 0.05; **P < 0.01 by t test.
The control of gene expression by leptin in hypothalamic Calcr neurons
Because LepRbCalcrKO and control mice contain Calcrcre and Rosa26eGFP-L10a, their Calcr neurons express an eGFP-tagged L10a ribosomal subunit, permitting the retrieval of Calcr neuron‒derived ribosomes and their associated mRNA by TRAP. Thus, to understand the potential overlap of LepRbCalcr neurons with previously defined populations of LepRb neurons and to understand the roles of leptin action on LepRbCalcr neurons for the control of gene expression in LepRbCalcr neurons, we used TRAP to purify mRNA from the hypothalamic Calcr neurons of LepRbCalcrKO and control mice and subjected it to RNA-seq (TRAP-seq). This analysis defined 739 genes that were significantly enriched in hypothalamic Calcr neurons relative to nonCalcr hypothalamic cells (Table 1; Supplemental Table 1).
Table 1.
Enrichment for Genes Expressed in Hypothalamic Calcr and LepRb Neurons and Alterations in Calcr Neuron Gene Expression Between Control and LepRbCalcrKO Mice
| Gene |
Enrichment (Fold)
|
Fold Change (Calcr)
|
|
|---|---|---|---|
| Calcr | LepRb | LepRbCalcrKO/Control | |
| Fkbp6 | 1.54 | 1.44 | 4.31 |
| Slc25a31 | 0.76 | 1.46 | 3.61 |
| BC002163 | 1.71 | 1.52 | 2.21 |
| Abcb1a | 0.55 | 4.27 | 2.02 |
| Trpa1 | 0.93 | 1.32 | 1.70 |
| Krt27 | 2.32 | 3.29 | 1.54 |
| Rspo4 | 1.19 | 2.62 | 1.51 |
| Dnajc22 | 1.11 | 0.60 | 1.50 |
| Npy | 10.62 | 21.54 | 1.44 |
| Agrp | 11.59 | 41.89 | 0.99 |
| Calcr | 8.62 | 4.00 | 1.12 |
| Sst | 5.49 | 1.70 | 0.97 |
| Ghrh | 3.73 | 8.96 | 1.08 |
| Klf14 | 3.13 | 4.55 | 0.66 |
| Rab3b | 2.04 | 1.58 | 0.66 |
| Gm5177 | 1.94 | 0.53 | 0.65 |
| Cartpt | 1.52 | 9.38 | 0.64 |
| Serpina3n | 1.79 | 7.35 | 0.60 |
| Skor2 | 8.86 | 1.21 | 0.60 |
| Gstm3 | 3.85 | 1.92 | 0.58 |
| Ndufs5 | 1.60 | 1.44 | 0.54 |
| Procr | 3.21 | 4.99 | 0.51 |
| Serpina3i | 4.13 | 10.59 | 0.45 |
TRAP was performed on CalcreGFP-L10a and LepRbCalcrKO mice; the resultant TRAP and supernatant mRNA was subjected to RNA-seq. Shown are enrichment values for Calcr neurons and for LepRb neurons [from (25)] in Control (untreated or vehicle-treated) mice, along with changes in gene expression in Calcr neurons between Control and LepRbCalcrKO mice for genes found to be differentially expressed, along with other genes of interest. Note that not all genes shown were enriched in Calcr neurons in Control mice; genes shown that were not enriched in Control Calcr neurons were enriched in LepRbCalcrKO Calcr neurons.
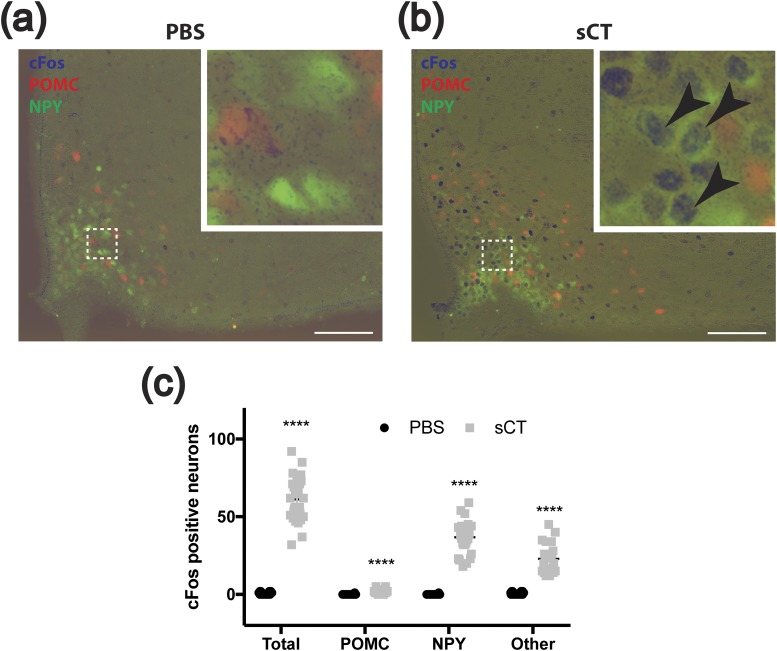
Agrp and Npy represented the mRNA species most highly enriched in hypothalamic Calcr neurons (whereas Pomc was poorly recovered), suggesting the potential overlap of LepRbCalcr neurons with ARC NAG (but not POMC) cells. Sst, Trh, and Ghrh were also enriched in hypothalamic Calcr neurons, suggesting that other groups of ARC LepRb cells might contribute to the overall population of LepRbCalcr neurons. To test this possibility directly, we treated mice containing NPY-GFP and POMC-dsRed reporter transgenes with sCT to examine the induction of cFos in NAG and POMC cells as an indicator of their potential CALCR expression (Fig. 6). This analysis revealed the sCT-mediated induction of cFos-IR in essentially all ARC NPY-GFP neurons and a population of cells that does not colocalize with either POMC or NPY, but few POMC-dsRed cells. Furthermore, Calcrcre-dependent reporters failed to colocalize with POMC-dsRed cells but marked all NPY-GFP neurons (as well as some additional ARC Calcr cells) (Supplemental Fig. 5). Thus, these data are consistent with the notion that ARC Calcr neurons consist of NAG cells along with an additional population of non-POMC neurons.
Figure 6.
sCT-induced cFos in ARC NPY neurons. (a, b) Shown are representative images of cFos-IR (blue), dsRed-IR (red), and GFP-IR (green) in the ARC of POMCdsRedNPYGFP mice after treatment with (a) vehicle (PBS) or (b) sCT (150 μg/kg, i.p.). Insets show a digital zoom of the boxed area. Arrowheads indicate colocalized neurons. Scale bars = 100 μm. (c) Graph shows counts of ARC cFos-IR (Total), those dual-labeled with dsRed-IR (POMC), those dual-labeled with GFP-IR (NPY), and those not colocalized with either (Other). Shown are mean value ± the standard error of the means. ****P < 0.0001 by t test. PBS, phosphate-buffered saline.
We used TRAP-seq to compare the expression of genes enriched in hypothalamic Calcr neurons from LepRbCalcrKO and control mice (Table 1), revealing genes that were dysregulated in these mice. Regulated transcripts include several genes that we have shown to be enriched in hypothalamic LepRb neurons, including members of the Serpina3 gene family, which are also regulated by leptin in LepRb neurons (25, 45). Thus, these may represent important transcriptional targets for leptin in the control of energy balance. Interestingly, however, although the lack of leptin or LepRb throughout the body increased Agrp and Npy expression many-fold (11), the expression of these genes was not dysregulated in LepRbCalcrKO mice. This suggests that the control of Agrp and Npy gene expression by leptin was mediated indirectly by leptin action on other neurons (rather than by the direct action of leptin on these cells). Although leptin suppressed the activity of NAG neurons, rendering the examination of leptin-stimulated cFos in these cells moot, leptin-deficient Lepob/ob mice (or LepRb-deficient Leprdb/db mice) (46) demonstrated increased cFos in the medial basal ARC (previously shown to colocalize with NAG cells) (Supplemental Fig. 6). In contrast, ARC cFos was normal in LepRbCalcrKO mice, consistent with the control of NAG neuron electrical activity by LepRb neurons that lie afferent to NAG cells (47).
Discussion
We identified a population of LepRbCalcr neurons that includes NAG neurons and additional undefined non-POMC cells, demonstrated their importance for the control of feeding and energy balance by leptin, and defined the LepRbCalcr neuron transcriptome and its regulation by leptin. In addition to revealing the overlap between genes dysregulated in Calcr neurons in LepRbCalcrKO mice and those controlled by leptin in LepRb neurons (45), this analysis also revealed that direct leptin action on LepRbCalcr neurons is dispensable for the control of gene expression and cFos in these cells. Thus, although direct leptin action on these cells plays an important role in the control of energy balance by leptin, leptin also indirectly controls the function of LepRbCalcr cells.
Although the ablation of LepRb from many previously examined hypothalamic cell types modestly, if at all, altered energy balance (20, 48–50), LepRbCalcrKO mice demonstrated hyperphagia and increased body weight and adiposity. Although LepRbCalcrKO mice displayed slightly decreased ambulatory activity, their energy expenditure (normalized to lean mass) was similar to that of control animals and their glucose homeostasis remained relatively normal (especially compared with that of completely leptin- or LepRb-deficient animals) (44), suggesting that the control of food intake (rather than metabolic rate and glycemic control) represents the main effect of direct leptin action on LepRbCalcr neurons.
Populations of LepRb- and Calcr-expressing neurons intersect in the LHA [as previously reported (41)], in the DMH, and most significantly in the ARC, where LepRbCalcr neurons consist mainly of a large population of non-POMC ARC neurons, including NAG and other ARC neurons. Although Calcr is widely expressed outside the CNS (including in the kidney, bone, and gastrointestinal tract), most LepRb resides within the CNS, and CNS LepRb mediates the effects of leptin on energy balance (14, 51, 52); thus, it is unlikely that LepRbCalcr cells exist outside the CNS or that they contribute to energy balance.
Although the distributed nature of LepRbCalcr neurons renders it difficult to ascertain the specific subpopulation(s) of the cells that contribute most strongly to the phenotype of LepRbCalcrKO mice, the deletion of LepRb from NAG cells must contribute because deletion of LepRb from NAG cells using Agrpcre (LepRbAgrpKO mice) causes mild obesity (20, 48–50). Because the reported increase in body weight and adiposity is more modest in LepRbAgrpKO mice than in LepRbCalcrKO mice, however, other LepRbCalcr neurons must contribute to the control of energy balance by leptin. Additional analysis of non-NAG, non-POMC ARC LepRbCalcr neurons is required to determine how these or the small groups of LepRbCalcr cells in the DMH and/or LHA contribute to energy homeostasis.
Amylin, which acts via CALCR in combination with a receptor activity‒modifying protein, stimulates a leptin-activated population of LHA LepRb neurons and could contribute to synergy between leptin and amylin. In contrast, leptin inhibits (46, 53) whereas sCT activates orexigenic NAG neurons, suggesting that leptin and CALCR agonists antagonize each other’s actions on NAG cells and would not be predicted to contribute to the synergy. Thus, the leptin/amylin synergy is mediated by either the relatively small population of non-NAG LepRbCalcr cells or by indirect but convergent effects of leptin and amylin on different cell populations. If sCT action on NAG neurons is orexigenic, this suggests that sCT action on other cells must dominate its effects on the NAG cells. CALCR agonists directly activate neurons in the brainstem satiety system and in other areas of the brain (e.g., the PVH) (54, 55); either of these or non-NAG ARC Calcr neurons could mediate such effects. However, determining whether CALCR-mediated activation of NAG neurons blunts the overall anorectic action of sCT and other CALCR agonists will require deleting Calcr from these cells in future studies. Furthermore, because the expression of receptor activity‒modifying proteins specifically in NAG and other LepRbCalcr neurons is not known, the regulation of these cell types by amylin agonists relative to CALCR agonists cannot be predicted with any certainty.
Direct leptin action on LepRbCalcr neurons contributes to their control of gene expression because deletion of LepRb in these cells increases the expression of eight genes and decreases the expression of 10 genes. Because STAT3 mediates important LepRb-dependent transcriptional control and STAT3 primarily increases gene expression, genes exhibiting decreased expression in Calcr neurons of LepRbCalcrKO mice more likely contribute to the phenotype of these mice and to the control of energy balance. Of the genes exhibiting decreased expression in LepRbCalcrKO mice, several are not enriched in LepRb neurons, suggesting they are primarily expressed in non-LepRb Calcr neurons that may be affected secondarily. Similarly, although both hypothalamic Calcr and LepRb neurons contain Cartpt, most of the LepRb-expressed Cartpt derives from POMC cells, which do not contain Calcr, suggesting that Cartpt expression in non-LepRb Calcr neurons might also be controlled indirectly by leptin action on LepRbCalcr cells.
Of the transcripts enriched in both hypothalamic Calcr and LepRb neurons and altered in expression in LepRbCalcrKO mice, at least two (Serpina3i and Serpina3n) are controlled by leptin in LepRb neurons (45). Indeed, these two transcripts are some of the most highly and coordinately regulated by leptin in LepRb neurons (45), suggesting their regulation by direct leptin action in LepRbCalcr neurons and also their potential importance in leptin action.
In contrast, although LepRb expression in LepRbCalcr neurons (which include NAG neurons) is important for the control of feeding and energy balance, it is not required for the control of Agrp and Npy expression. Furthermore, although the total absence of leptin action increased medial basal ARC cFos, consistent with increased activation of NAG cells, ARC cFos was normal in LepRbCalcrKO mice. Thus, although direct leptin action on LepRbCalcr neurons is important for energy balance, the control of Agrp and Npy expression and the activity of medial basal ARC cFos was mediated indirectly by leptin action on other LepRb neurons, potentially including the presumptive DMH LepRbvGat cells that control inhibitory inputs to NAG cells (47).
Overall, our findings defined a population of LepRbCalcr neurons that plays a crucial role in the control of feeding and energy balance by leptin and suggest the importance of direct and indirect control of these cells. In the future, it will be important to define the identity and roles of subpopulations of these cells and to determine the mechanisms by which indirect leptin action on these cells controls gene expression (and potential other functions) in these cells.
Supplementary Material
Acknowledgments
We thank members of the M.G.M. and D.P.O. laboratories for helpful discussions.
Financial Support: Research support was provided by the Michigan Diabetes Research Center [National Institutes of Health (NIH) Grant P30 DK020572, including the Molecular Genetics, Animal Phenotyping, and Clinical Cores]; the Michigan Mouse Metabolic Phenotyping Center, the American Diabetes Association (to M.G.M. and J.N.F.); the Marilyn H. Vincent Foundation (to M.G.M.); and NIH Grants DK056731 (to M.G.M.), DK097861 (to M.B.A.), and GM007315 (to W.P.). MedImmune, Inc. provided funding for the TRAP-seq experiments.
Author Contributions: J.M.A., C.P., J.N.F., J.C.J., and G.S. researched and analyzed data and edited the manuscript. W.P., M.B.A., and D.P.O. designed the experiments, researched and analyzed the data, and wrote and edited the manuscript. J.T., C.J.R., and M.G.M. designed the experiments and wrote and edited the manuscript. M.G.M. is the guarantor of the manuscript.
Disclosure Summary: J.T. and C.J.R. are employees of MedImmune, Inc. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- AgRP
agouti-related peptide
- ARC
arcuate nucleus
- Calcr
calcitonin receptor
- cDNA
complementary DNA
- CNS
central nervous system
- DAB
avidin-biotin/diaminobenzidine
- DMH
dorsomedial hypothalamic nucleus
- eGFP
enhanced green fluorescent protein
- GABA
γ-aminobutyric acid
- GFP
green fluorescent protein
- IHC
immunohistochemical
- i.p.
intraperitoneal
- IR
immunoreactivity
- KO
knockout
- LepRb
leptin receptor
- LepRbCalcr
Calcr-expressing leptin receptor
- LHA
lateral hypothalamic area
- mRNA
messenger RNA
- NAG
neuropeptide Y/agouti-related peptide/γ-aminobutyric acid
- NPY
neuropeptide Y
- PCR
polymerase chain reaction
- POMC
pro-opiomelanocortin
- RNA-seq
RNA sequencing
- sCT
salmon calcitonin
- TRAP
translating ribosome affinity purification
- TRAP-seq
translating ribosome affinity purification followed by RNA sequencing
- Vo2
oxygen consumption
References
- 1. Wisse BE, Kim F, Schwartz MW. Physiology: an integrative view of obesity. Science. 2007;318(5852):928–929. [DOI] [PubMed] [Google Scholar]
- 2. Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514(5):518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedman JM. A tale of two hormones. Nat Med. 2010;16(10):1100–1106. [DOI] [PubMed] [Google Scholar]
- 4. Allison MB, Myers MG Jr. 20 years of leptin: connecting leptin signaling to biological function. J Endocrinol. 2014;223(1):T25–T35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chua SC Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271(5251):994–996. [DOI] [PubMed] [Google Scholar]
- 6. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. [DOI] [PubMed] [Google Scholar]
- 7. Banks AS, Davis SM, Bates SH, Myers MG Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275(19):14563–14572. [DOI] [PubMed] [Google Scholar]
- 8. Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Münzberg H, Myers MG Jr. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282(42):31019–31027. [DOI] [PubMed] [Google Scholar]
- 9. Björnholm M, Münzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjørbaek C, Myers MG Jr. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117(5):1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patterson CM, Villanueva E, Greenwald-Yarnell M, Rajala MW, Gonzales IE, Saini N, Jones JC, Myers MG. Leptin action via LepR-b Tyr1077 contributes to the control of energy balance and female reproduction. Mol Metab. 2012;1(1-2):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG Jr. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421(6925):856–859. [DOI] [PubMed] [Google Scholar]
- 12. Piper ML, Unger EK, Myers MG Jr, Xu AW. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol. 2008;22(3):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kowalski TJ, Liu SM, Leibel RL, Chua SC Jr. Transgenic complementation of leptin-receptor deficiency: I. rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 2001;50(2):425–435. [DOI] [PubMed] [Google Scholar]
- 14. Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108(8):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, Elmquist JK. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423(2):261–281. [PubMed] [Google Scholar]
- 16. Ring LE, Zeltser LM. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest. 2010;120(8):2931–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flak JN, Myers MG Jr. Minireview: CNS mechanisms of leptin action. Mol Endocrinol. 2016;30(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15(10):1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA, Rosen ED, Lowell BB, Tsai LT. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983–991. [DOI] [PubMed] [Google Scholar]
- 21. Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. [DOI] [PubMed] [Google Scholar]
- 22. Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG Jr. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14(3):313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laque A, Yu S, Qualls-Creekmore E, Gettys S, Schwartzenburg C, Bui K, Rhodes C, Berthoud HR, Morrison CD, Richards BK, Münzberg H. Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol Metab. 2015;4(10):706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dodd GT, Worth AA, Nunn N, Korpal AK, Bechtold DA, Allison MB, Myers MG Jr, Statnick MA, Luckman SM. The thermogenic effect of leptin is dependent on a distinct population of prolactin-releasing peptide neurons in the dorsomedial hypothalamus. Cell Metab. 2014;20(4):639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allison MB, Patterson CM, Krashes MJ, Lowell BB, Myers MG Jr, Olson DP. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Mol Metab. 2015;4(4):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donato J Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121(1):355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu S, Francois M, Huesing C, Munzberg H. The hypothalamic preoptic area and body weight control. Neuroendocrinology. 2018;106(2):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu S, Qualls-Creekmore E, Rezai-Zadeh K, Jiang Y, Berthoud HR, Morrison CD, Derbenev AV, Zsombok A, Münzberg H. Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J Neurosci. 2016;36(18):5034–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA. 2008;105(20):7257–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trevaskis JL, Coffey T, Cole R, Lei C, Wittmer C, Walsh B, Weyer C, Koda J, Baron AD, Parkes DG, Roth JD. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology. 2008;149(11):5679–5687. [DOI] [PubMed] [Google Scholar]
- 31. Patterson CM, Leshan RL, Jones JC, Myers MG Jr. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 2011;1378:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29(43):13684–13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–115. [DOI] [PubMed] [Google Scholar]
- 34. McMinn JE, Liu SM, Dragatsis I, Dietrich P, Ludwig T, Eiden S, Chua SC Jr. An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm Genome. 2004;15(9):677–685. [DOI] [PubMed] [Google Scholar]
- 35. Lamprecht MR, Sabatini DM, Carpenter AE. CellProfiler: free, versatile software for automated biological image analysis. Biotechniques. 2007;42(1):71–75. [DOI] [PubMed] [Google Scholar]
- 36. Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Magrane M, Consortium U.. UniProt Knowledgebase: a hub of integrated protein data. Database (Oxford). 2011;2011:bar009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145(11):4880–4889. [DOI] [PubMed] [Google Scholar]
- 40. Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14(3):173–213. [DOI] [PubMed] [Google Scholar]
- 41. Li Z, Kelly L, Heiman M, Greengard P, Friedman JM. Hypothalamic amylin acts in concert with leptin to regulate food intake. Cell Metab. 2016;23(5):945. [DOI] [PubMed] [Google Scholar]
- 42. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165–168. [DOI] [PubMed] [Google Scholar]
- 43. Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. [DOI] [PubMed] [Google Scholar]
- 44. Rossetti L, Massillon D, Barzilai N, Vuguin P, Chen W, Hawkins M, Wu J, Wang J. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J Biol Chem. 1997;272(44):27758–27763. [DOI] [PubMed] [Google Scholar]
- 45. Allison MB, Pan W, MacKenzie A, Patterson C, Shah K, Barnes T, Cheng W, Rupp A, Olson DP, Myers MG Jr. Defining the transcriptional targets of leptin reveals a role for Atf3 in leptin action [published online ahead of print March 13, 2018]. Diabetes. doi: 10.2337/db17-1395. [DOI] [PMC free article] [PubMed]
- 46. Münzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Björnholm M, Elmquist J, Sleeman M, Cowley MA, Myers MG Jr. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, Madara JC, Campbell JN, Kroeger D, Scammell TE, Tannous BA, Myers MG Jr, Andermann ML, Krashes MJ, Lowell BB. Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci. 2016;19(12):1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG Jr, Schwartz GJ, Chua SC Jr. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luo N, Marcelin G, Liu SM, Schwartz G, Chua S Jr. Neuropeptide Y and agouti-related peptide mediate complementary functions of hyperphagia and reduced energy expenditure in leptin receptor deficiency. Endocrinology. 2011;152(3):883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Egan OK, Inglis MA, Anderson GM. Leptin signaling in AgRP neurons modulates puberty onset and adult fertility in mice. J Neurosci. 2017;37(14):3875–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–1271. [DOI] [PubMed] [Google Scholar]
- 52. Chua SC Jr, Liu SM, Li Q, Sun A, DeNino WF, Heymsfield SB, Guo XE. Transgenic complementation of leptin receptor deficiency: II. increased leptin receptor transgene dose effects on obesity/diabetes and fertility/lactation in lepr-db/db mice. Am J Physiol Endocrinol Metab. 2004;286(3):E384–E392. [DOI] [PubMed] [Google Scholar]
- 53. Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. [DOI] [PubMed] [Google Scholar]
- 54. Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord. 2001;25(7):1005–1011. [DOI] [PubMed] [Google Scholar]
- 55. Chait A, Suaudeau C, De Beaurepaire R. Extensive brain mapping of calcitonin-induced anorexia. Brain Res Bull. 1995;36(5):467–472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.