Abstract
Objective
To assess the safety and efficacy of elamipretide, an aromatic-cationic tetrapeptide that readily penetrates cell membranes and transiently localizes to the inner mitochondrial membrane where it associates with cardiolipin, in adults with primary mitochondrial myopathy (PMM).
Methods
A Study Investigating the Safety, Tolerability, and Efficacy of MTP-131 for the Treatment of Mitochondrial Myopathy (MMPOWER) was a phase I/II multicenter, randomized, double-blind, placebo-controlled trial of elamipretide in 36 participants with genetically confirmed PMM. Participants were randomized to intravenous elamipretide (0.01, 0.1, and 0.25 mg/kg/h or placebo for 2 hours in a dose-escalating sequence). The primary efficacy measure was the change in distance walked in the 6-minute walk test (6MWT) after 5 days of treatment. Other efficacy measures included changes in cardiopulmonary exercise testing parameters, in participant-reported symptoms, and in serum and urinary biomarkers. Safety, tolerability, and pharmacokinetics were also measured.
Results
Participants who received the highest dose of elamipretide walked a mean of 64.5 m farther at day 5 compared to a change of 20.4 m in the placebo group (p = 0.053). In addition, there was a dose-dependent increase in distance walked on the 6MWT with elamipretide treatment (p = 0.014). In a model that adjusted for additional covariates possibly affecting response, the adjusted change for the highest dose of elamipretide was 51.2 vs 3.0 m in the placebo group (p = 0.0297). No significant differences were observed in other efficacy and safety endpoints.
Conclusions
Elamipretide increased exercise performance after 5 days of treatment in patients with PMM without increased safety concerns. These findings, as well as additional functional and patient-reported measures, remain to be tested in larger trials with longer treatment periods to detect other potential therapeutic benefits in individuals affected by this condition.
Classification of evidence
This trial provides Class I evidence that for patients with PMM, elamipretide improved the distance walked on the 6MWT.
Primary mitochondrial disorders (PMDs) are a heterogeneous group of genetic diseases causing impaired mitochondrial respiration1–5 and are considered among the most common inherited metabolic disorders in humans, with a prevalence in adults of ≈1 in 4,300.1,6 PMDs can affect almost any organ but mostly affect those with high energy demands (nervous system, skeletal muscle, heart, kidney, and retina).1 PMDs cause several well-recognized syndromes, with muscular involvement being common in the adult patient. In a survey of 290 patients with PMD, muscle weakness, chronic fatigue, exercise intolerance, gastrointestinal problems, and balance problems were the 5 most common symptoms experienced in >75% of patients.7 Primary mitochondrial myopathy (PMM) is a PMD affecting predominantly, but not exclusively, skeletal muscle.8 PMM can be very disabling and adversely affects patients' quality of life9,10 with no available treatments to be used aside from palliative approaches.1,9,11
Elamipretide is an aromatic-cationic tetrapeptide that readily penetrates cell membranes and transiently localizes to the inner mitochondrial membrane where it associates with cardiolipin. Through this mechanism of action, elamipretide is thought to restore energy production, to reduce the production of reactive oxygen species, and ultimately to increase the energy (adenosine triphosphate [ATP]) supplied to affected cells and organs.12,13 In preclinical studies, elamipretide increased the synthesis of ATP and reduced reactive oxygen species production regardless of the specific mitochondrial abnormality causing the impaired mitochondrial respiration.14–18 There is no observed effect on normally functioning mitochondria.19
The primary objective of this trial was to evaluate the safety and efficacy of a 5-day administration of multiple ascending doses of intravenous elamipretide in patients with PMM.
Methods
Standard protocol approvals, registrations, and patient consents
A Study Investigating the Safety, Tolerability, and Efficacy of MTP-131 for the Treatment of Mitochondrial Myopathy (MMPOWER) was a phase I/II multicenter, randomized, double-blind, placebo-controlled multiple ascending-dose trial in participants with genetically confirmed PMM. The trial was conducted in 4 US sites and was approved by individual institutional review committees. Informed consent was obtained from each participant or from his or her legal representative in accordance with international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines (NCT02367014).
Trial design and participants
The trial was designed with 3 sequential ascending-dose cohorts of 12 participants, 9 of whom received elamipretide and 3 received placebo. The sample size was based on precedent set by other phase I/II studies of a similar nature and design and the number chosen to allow appropriate pharmacokinetic studies. Eligible participants were ≥16 and ≤65 years of age with PMM caused by either a nuclear DNA or mitochondrial DNA (mtDNA) mutation known to affect mitochondrial respiration (table e-1, links.lww.com/WNL/A309). The investigators reviewed both the genetic and phenotypic findings in each participant to unanimously affirm the molecular pathogenicity and genotype-phenotype correlation (table e-2). The inclusion criterion for muscle symptoms was defined as a score of ≥2 (able to walk <1,000 m on a flat surface; restricted on inclines or stairs; rest needed after 1 flight [12 steps]) on Section I, question 9 (exercise tolerance) or a score of ≥2 (mild but clear proximal weakness in hip flexion and shoulder abduction [Medical Research Council score 4 of 5]; minimal weakness in elbow flexion and knee extension [Medical Research Council score 4+ of 5, both examined with the joint at 90°]) on Section III, question 5 (myopathy) of the Newcastle Mitochondrial Disease Adult Scale (NMDAS).20 Participants also had to be able to complete a 6-minute walk test (6MWT). Medications and dietary supplements were required to be unchanged for at least a month before randomization and were to be continued during the trial. Those with either uncontrolled diabetes mellitus or significant cardiac (i.e., conduction abnormalities, cardiomyopathy, uncontrolled hypertension) or neurologic (scores ≥4 on selected neurologic questions of the NMDAS) disorders were excluded from the trial (i.e., severe ataxia, prior strokes with deficit, active seizures).
Randomization and masking
Assignment to treatment groups within each cohort was determined by a computer-generated random sequence using an Interactive Web-Response System to assign identical glass vials containing either the elamipretide or a placebo, which consisted of the same formulation without elamipretide. The pharmacists, trial staff, sponsor, and participants were blinded to the treatment given.
Procedures and outcomes
Participants were randomized within 40 days of the screening visit. Baseline evaluation of functional, participant-reported, and safety assessments was completed within 24 hours of treatment. Three escalating doses (0.01, 0.10, and 0.25 mg/kg/h) were infused intravenously over 2 hours for 5 consecutive days in each of the 3 consecutive escalating-dose cohorts, after which the same measures obtained at baseline were completed for the analyses of efficacy. Follow-up testing was also done 2 days after treatment cessation.
The primary efficacy measure was the change in distance walked in the 6MWT. The 6MWT was chosen as the primary outcome because physical fatigue and exercise intolerance are among the most common symptoms of patients with PMM, regardless of the underlying genetic defect.21,22 In addition, skeletal muscle requires high levels of ATP synthesis for minimal activity, and those needs increase as much as 30-fold during exercise.15 Additional efficacy measures included change in physiologic parameters measured during cardiopulmonary exercise testing (CPET) and changes in the values of exploratory biomarkers (serum glutathione levels, fibroblast growth factor-21 [FGF-21], urine 8-isoprostane, and 8-hydroxy-2-deoxyguanosine).
CPET is a complete assessment of aerobic functional performance providing an integrated assessment of cardiovascular, pulmonary, and skeletal muscle physiologic reserve capacity. It was performed on a stationary upright bicycle with ECG and hemodynamic monitoring. Blood samples to measure serum lactate were collected before and within 10 minutes of completion of the exercise. Because of the physical demands of the test, it was performed at the option of the participant.
Participant-reported changes in symptoms were obtained with 2 tools. The modified NMDAS (a rating scale to allow evaluation of the progression of mitochondrial disease symptoms and quality of life in adults) consisted of Sections I and III, with the exception of Section III, question 10 of the NMDAS. The Daily Symptom Questionnaire was also completed, evaluating 6 symptoms on a 0 to 10 scale (0 = none/no symptoms and 10 = very severe symptoms): abdominal pain, limitation of activities, muscle pain, muscle weakness, physical fatigue, mental fatigue.
Apart from the Daily Symptom Questionnaire, all other efficacy assessments were performed at baseline (day 1), at the end of treatment (day 5), and at the end of the trial (day 7).
Safety and tolerability endpoints included adverse events and changes from baseline in vital signs, ECGs, and clinical laboratory evaluations. Dose escalation for the drug was approved by an independent scientific medical board after review of each dose cohort safety data.
Statistical analysis
All analyses were performed with SAS version 9.3 (SAS Institute Inc, Cary, NC). Continuous variables were summarized with descriptive statistics, and categorical variables were summarized with frequency counts and percentages. The study design and nonbinary endpoints do not allow number needed to treat calculation or absolute risk reduction assessment.
In this phase I/II trial, no adjustments were made to the stated significance level to account for the multiple efficacy measures or multiple dose groups. In general, placebo was pooled across dosing cohorts. The primary analysis for efficacy used an analysis of covariance (ANCOVA) model that included treatment as a factor and baseline as a covariate. In addition, for the change in the 6MWT distance, a mixed model for repeated-measures analysis that included treatment, visit, distance walked at screening, distance walked at baseline, and a treatment-by-visit interaction was conducted. This model was also used to test a linear dose effect. A post hoc analysis was also completed on the primary efficacy endpoint of change in 6MWT distance to determine whether other covariates beyond baseline distance contributed to the change in distance walked. Covariates considered included treatment, baseline 6MWT distance, a baseline 6MWT distance-by-treatment interaction, screening 6MWT distance, sex, height, weight, and randomization cohort. A backward elimination approach was used to include only those factors with a significance level equal to p ≤ 0.1 in the final model. The final ANCOVA model derived with the backward elimination approach included distance walked in baseline 6MWT, treatment, baseline 6MWT-by-treatment interaction, and sex. Confidence intervals are provided in the tables when appropriate.
Primary research question of this trial
The primary research question is whether the short-term intravenous use of elamipretide is safe and well tolerated and if it improves exercise performance in adult patients with PMM. This trial provides Class I evidence that a trial of intravenous elamipretide in adults with PMM is well tolerated and improves exercise performance in a dose-dependent manner compared to placebo.
Results
Demographic and other baseline characteristics
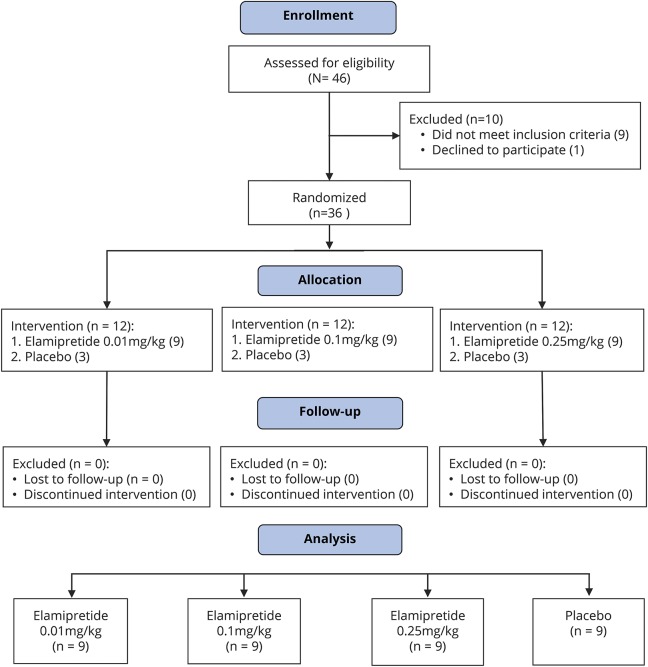
Forty-six participants were screened and 36 participants were randomized between February 2015 and April 2016. Ten screened participants were excluded because of significant cardiac disease, inability to complete the 6MWT, morbid obesity, not being on a stable medical regimen, or unwillingness to participate in the trial. None of the randomized participants dropped out or were lost to follow-up (figure 1).
Figure 1. CONSORT 2010 flowchart for the MMPOWER trial.
Forty-six participants were screened for the trial. Ten were excluded because of major health concerns, including cardiac and neurologic disorders (arrhythmias, severe ataxia), inability to perform the 6MWT, morbid obesity, recent changes in medical management, deemed unstable, or unwilling to participate in the trial procedures. Of the 36 participants enrolled, none dropped out or were lost to follow-up. All participants performed the required trial procedures and were included, and their information was used in the final analysis. CONSORT = Consolidated Standards of Reporting Trials; MMPOWER = A Study Investigating the Safety, Tolerability, and Efficacy of MTP-131 for the Treatment of Mitochondrial Myopathy; 6MWT = 6-minute walk test.
Overall, the majority of participants in this trial were white (97.2%) and female (83.3%) with a mean age of 42.5 years. Demographics of each treatment group were generally similar to those of the overall population, with some variability observed in overall age and age ranges, sex distribution, smoking status, weight, and body mass index (table e-1, links.lww.com/WNL/A309). The most common genetic abnormalities of PMM were mtDNA single deletions, mtDNA transfer RNA mutations, and nuclear DNA POLG mutations (table e-2). Similar nutritional supplements were being used in all but 1 elamipretide-treated participant. The average daily dose administered was 1.4 mg for the 0.01–mg/kg/h dose cohort, 12.7 mg for the 0.10–mg/kg/h dose cohort, and 29.6 mg for the 0.25–mg/kg/h dose cohort.
Efficacy findings
Participants who received the highest dose of elamipretide walked 64.5 m farther at day 5 compared to 20.4 m farther in the placebo group (p = 0.053) (table 1); however, there was no difference between the highest-dose and placebo groups 2 days after stopping treatment (61.7 vs 38.5 m, respectively; p = 0.387). On the basis of the mixed model for repeated measures, at day 5, there was a significant dose-related increase in the change in distance walked in the 6MWT (p = 0.014). The final post hoc ANCOVA model derived with the backward elimination approach, which included distance walked in baseline 6MWT, treatment, baseline 6MWT-by-treatment interaction, and sex as factors, resulted in an adjusted 51.2-m increase in the distance walked in highest-dose group at day 5 compared to a 3.0-m increase for placebo-treated participants (p = 0.0297) (figure 2), with the greatest apparent benefit for participants with a relatively shorter distance walked at baseline in a dose-dependent manner (figure e-1, links.lww.com/WNL/A308).
Table 1.
Summary of change from baseline in distance walked (meters) in the 6MWT after 5 days of treatment and 2 days after treatment
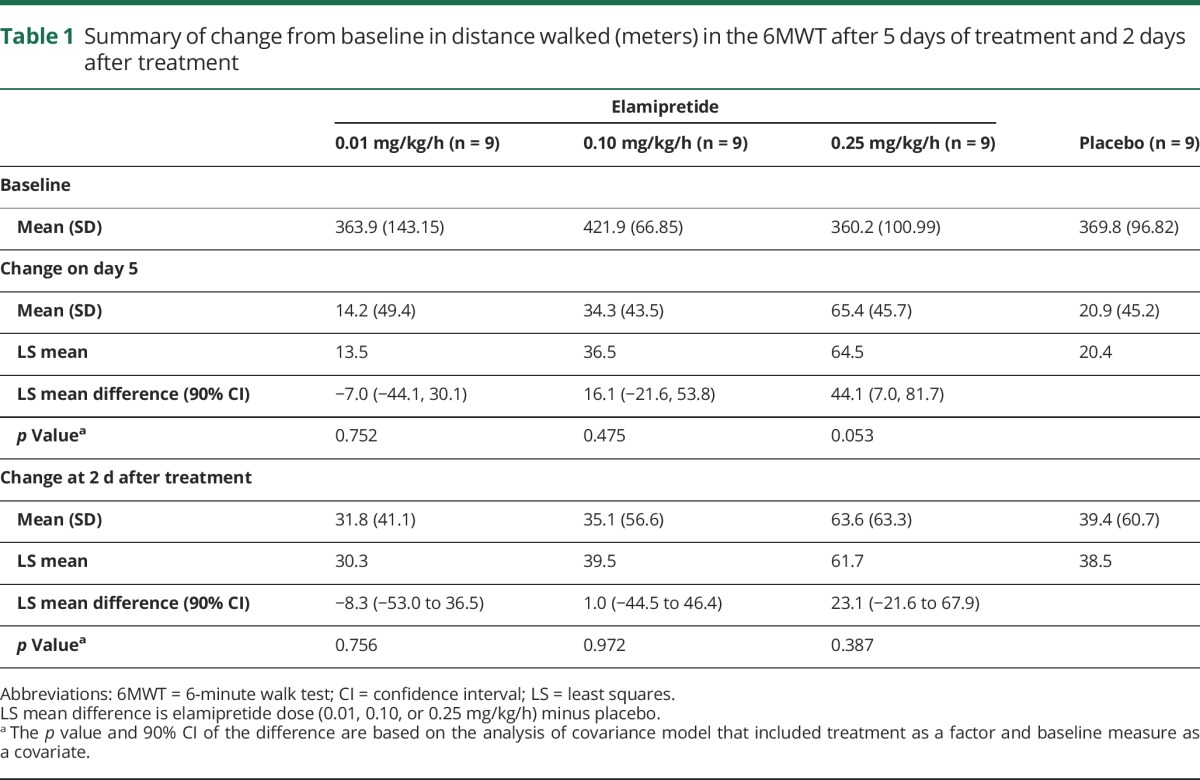
Figure 2. Change in distance walked after 5 days of treatment with elamipretide.
The p value is the comparison of the high-dose arms with placebo for the heterogeneous slope model. Change in distance walked after 5 days of treatment with elamipretide is represented. There is a clear upward dose response between the distance walked and the dose escalation in the treatment arms. In a backward elimination model to adjust for additional possible confounders (sex and the baseline distance walked–by–treatment interaction), the difference in distance walked (at the average value of baseline distance walked in the study of ≈380 m) was a 3.0-m increase for the placebo-treated participants compared to 51.2 m for the highest-dose group (p = 0.0297).
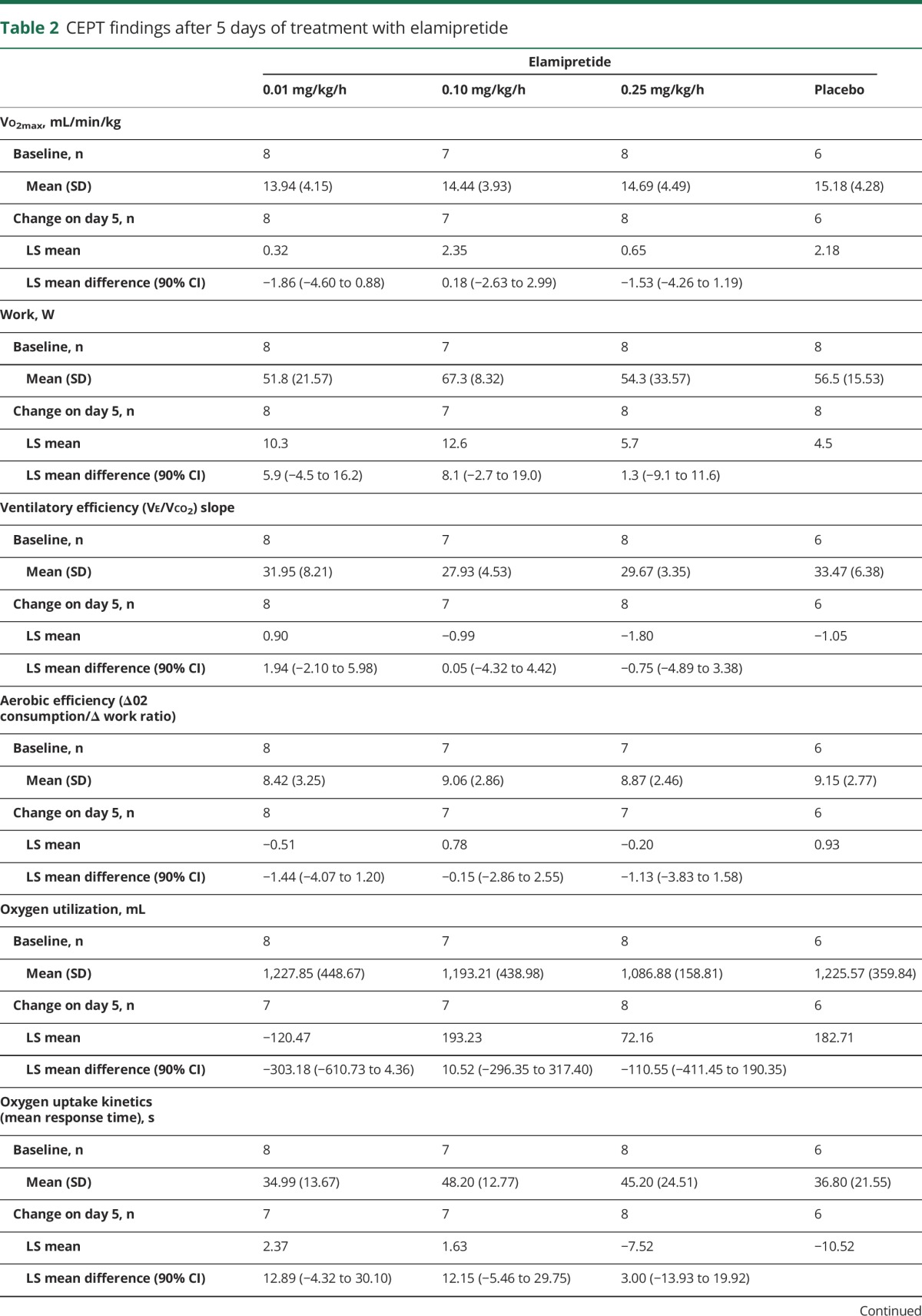
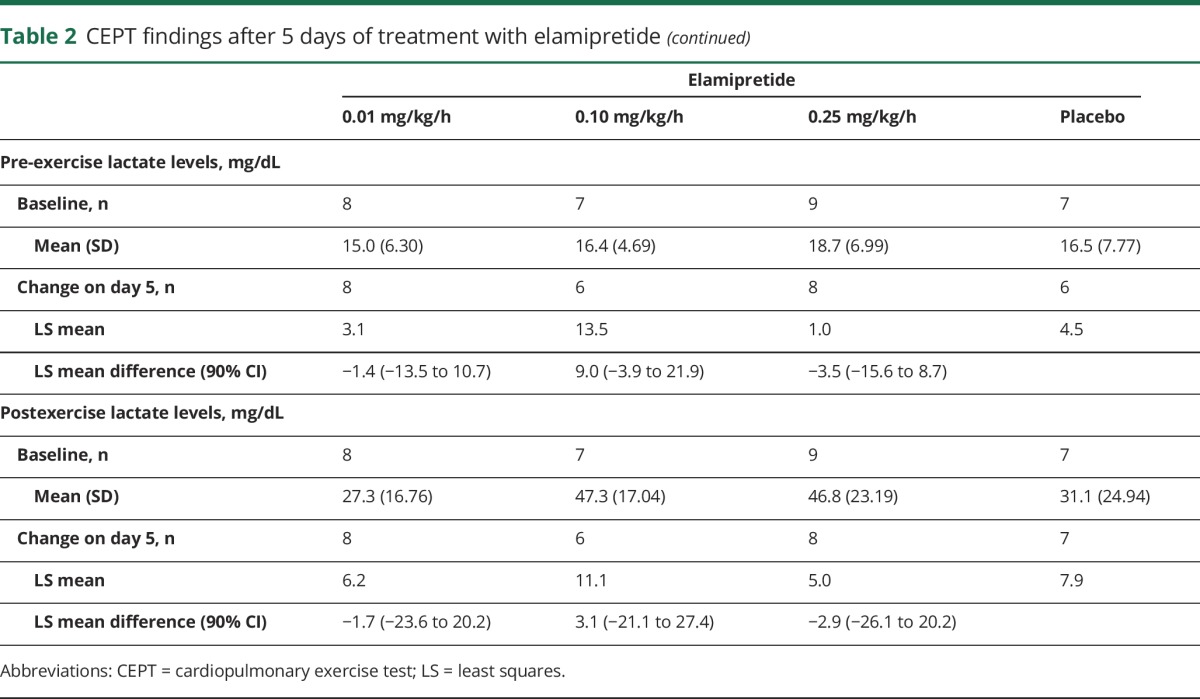
For most parameters, baseline CPET values were similar between treatment groups (table 2). Adjusted mean Vo2 max increased over time for all treatment groups; however, those changes were not significantly different from those seen with placebo. A significant positive correlation was observed with increasing distance walked during the 6MWT (change from baseline) and increasing Vo2 max measurements (Spearman correlation coefficient 0.4022, p = 0.0249) (table 2). Changes in other CPET parameters varied during the trial and between treatment groups, with no significant differences observed compared to placebo.
Table 2.
CEPT findings after 5 days of treatment with elamipretide
The modified NMDAS symptom scores were not significantly different between any elamipretide dose group and placebo. Small decreases in the mean total score, for most individual items, and for Current Function (Section I) and Current Clinical Assessment (Section III), progressing toward improvement, were observed on day 5 across treatment groups and placebo (table e-3, links.lww.com/WNL/A309). Total and individual item scores of the Daily Symptom Questionnaire also showed no differences between the treated and placebo groups; however, changes toward improvements were observed at most visits for all treatment groups. In addition, a significant negative correlation was observed between the change from baseline in both distance walked during the 6MWT and Daily Symptom Questionnaire limitations on activities on day 5 (Spearman correlation coefficient = −0.3962, p = 0.0167). There were no significant differences in levels of biomarkers (FGF-21, glutathione, 8-isoprostane, and 8-hydroxy-2-deoxyguanosine) between treatment groups (table e-3).
Safety evaluation
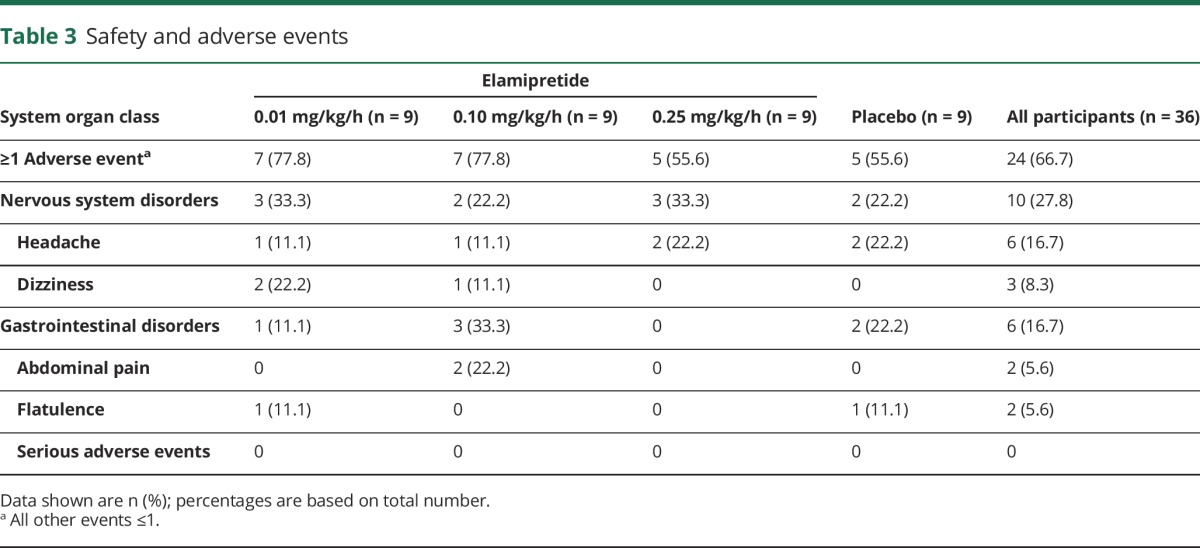
The most common adverse event was headache (6 [16.7%] participants), followed by dizziness (3 [8.3%] participants). For participants treated with the highest elamipretide dose or placebo, the most common adverse event was headache (2 [22.2%] participants in each group). There were no differences in adverse events between the treated and placebo groups (table 3). No deaths, serious adverse events (e.g., deaths, hospitalizations), or adverse events leading to participant discontinuation were reported in this trial. There were also no clinically significant differences in vital signs, blood chemistries, and ECG findings between the elamipretide- and placebo-treated participants.
Table 3.
Safety and adverse events
Discussion
In this randomized, placebo-controlled clinical trial of elamipretide in patients with PMM, a dose-dependent improvement in exercise performance was observed, as measured by the 6MWT. These results support preclinical animal studies in which elamipretide improved exercise performance in aged mice.15 In these preclinical animal studies, improved exercise performance was observed only in animals with decreased performance, not in normally functioning animals. This finding was also observed in this trial. The final post hoc ANCOVA model derived with the backward elimination approach included a baseline 6MWT-by-treatment interaction as one of the factors. The inclusion of this interaction as a factor is supportive of participants with a greater degree of impairment having a greater improvement in distance walked in the 6MWT after treatment with elamipretide. This was also suggested by a similar prespecified subgroup analysis in which participants treated with elamipretide who walked <350 m at screening showed greater improvement (i.e., a greater increase in distance walked) on day 5 (range 8.40–32.57 m) compared to those who walked ≥350 m at screening (range 2.19–9.91 m).
The 6MWT was selected as the primary efficacy assessment because of its relevance to the day-to-day functioning of patients with PMM. Muscular weakness, cardiovascular limitations, and gait disorders can influence 6MWT performance, and any or all can be present in PMM. Limitations of the 6MWT are its variability and the ability of the technician coaching during the test to influence the participants. The trial minimized these concerns by excluding participants with severe cardiovascular or neurologic impairments and using a standardized protocol for performing the test. Some initial training effect has been observed for the 6MWT in neuromuscular and chronic conditions.23 A screening 6MWT was done for each participant before the baseline test as a way to reduce the likelihood of observing a training effect in the evaluation of the effect of elamipretide after the 5-day treatment period.
The trial also evaluated the potential effect of elamipretide on numerous CPET parameters. Interpretation of the CPET results is complicated by participants dropping out (missing data from 7 participants), by several other participants stopping the test before reaching their maximal predicted heart rate because of muscle symptoms and fatigue, and by technical problems at the sites. There was a correlation between the change in distance walked in the 6MWT and peak oxygen consumption in all the participants, similar to what has been observed in other advanced chronic conditions such as heart disease.23
Two participant-reported tools were used in this trial, the NMDAS and the Daily Symptom Questionnaire. The NMDAS was created to measure the chronic progression of mitochondrial disease. The tool was not created or validated for the PMM patient population or for the purpose of measuring a short-term treatment effects. Likewise, the Daily Symptom Questionnaire is a symptom assessment without any prior validation. The further evaluation of elamipretide with a fit-for-purpose, specific symptom assessment tool might be more sensitive to detect clinically meaningful changes in clinical trials.
In addition, several biomarkers, including FGF-21, glutathione, and urinary isoprostanes, were measured. These biomarkers are limited by their lack of specificity and unproven ability to discriminate treatment effects.24,25
Other factors may have clouded the interpretation of the trial results. Numerous supplements and antioxidants were taken long term by the enrolled participants. Only some of these compounds have been tested in clinical trials, with no clear proven efficacy or positive effect (e.g., dichloroacetate, creatine, dimethylglycine, CoQ10, and idebenone).11,26–28 In addition, while participants were instructed to maintain their normal diet, daily caffeine, and fiber intake, as well as their normal activity/exercise level throughout the trial period, baseline differences in these participant attributes could have an impact on functional performance and treatment benefit results. There were too few participants in this trial to determine any differential effect resulting from the use of these supplements.
Finally, the small number of participants in this trial created a challenge in determining whether differences in efficacy responses were affected by the different genetic abnormalities or the degree of heteroplasmy present in those with mtDNA encoding abnormalities. Heteroplasmy was not measured in all participants, and of those participants with heteroplasmy levels available, it was determined in various tissues, making meaningful comparisons challenging.
There was no significant improvement in distance walked in the 6MWT when the participants were retested 2 days after the cessation of treatment. This could be the result of the short half-life of the drug. Larger studies are required to determine whether longer periods of treatment will further enhance exercise performance and to determine the duration of the effect of elamipretide. In addition, long-term daily intravenous administration is not practical in patients with PMM. A subcutaneous formulation of elamipretide for long-term use is being studied.
The improvements in exercise performance and the well-tolerated safety profile of elamipretide in this trial are encouraging. Despite the inherent limitations of a small phase I/II trial, this trial supports the proposed mechanism of action of elamipretide, improving ATP synthesis regardless of the underlying genetic defect impairing mitochondrial respiration. The results justify a larger prospective trial to determine the effect of long-term administration of the drug. This trial should include other measures of physical functioning and assessment of the disabling symptoms of fatigue and exercise intolerance in this patient population.
Acknowledgment
Amanda Remse and Anthony Aiudi, Stealth BioTherapeutics, edited the manuscript and tables for nonintellectual content. Statistical analysis was conducted by Dean Rutty, Everest Clinical Research Corp, Markham, Ontario, Canada. The authors thank Jeffrey S. Finman, PhD, Jupiter Point Pharma Consulting, LLC for statistical consultation; the patients and families for their participation; and the Mito Action and United Mitochondrial Disease Foundation for helping with recruitment. They also acknowledge Harvard Catalyst (https://catalyst.harvard.edu).
Glossary
- ANCOVA
analysis of covariance
- ATP
adenosine triphosphate
- CPET
cardiopulmonary exercise testing
- FGF-21
fibroblast growth factor-21
- MMPOWER
A Study Investigating the Safety, Tolerability, and Efficacy of MTP-131 for the Treatment of Mitochondrial Myopathy
- mtDNA
mitochondrial DNA
- NMDAS
Newcastle Mitochondrial Disease Adult Scale
- PMD
primary mitochondrial disorder
- PMM
primary mitochondrial myopathy
- 6MWT
6-minute walk test
Footnotes
Author contributions
Amel Karaa: trial concept and design, trial supervision, acquisition of data, interpretation of data, drafted and finalized the manuscript, tables and figures, has access to all the data, and takes responsibility for the data accuracy and analysis and the conduct of the research. Richard Haas: trial design, trial supervision, acquisition of data, interpretation of data, edited the manuscript. Amy Goldstein: trial design, trial supervision, acquisition of data, analysis and interpretation of data, edited the manuscript. Jerry Vockley: trial concept, trial supervision, and data interpretation, edited the manuscript. W. Douglas Weaver: trial design, edited the manuscript, tables and figures for intellectual content, edited the statistical analysis plan. Bruce Cohen: trial concept, trial design, trial supervision, acquisition of data, data interpretation, edited the manuscript.
Study funding
Funded by Stealth BioTherapeutics, Newton, MA. All payments from Stealth BT were directly pertaining to travel for investigator meetings and the conduct of this clinical trial. As part of the trial, the investigators and coordinators have also received a mini iPad to conduct trial procedures.
Disclosure
A. Karaa: research grant, reimbursement for travel, and consulting payments from Stealth BioTherapeutics, Sanofi Genzyme, and Shire; research grant and reimbursement for travel from Protalix and REATA; consulting payments from MitoBridge; on the medical advisory board of MitoAction and scientific and medical advisory board of the United Mitochondrial Disease Foundation; board member of Rare New England and the Mitochondrial Medicine Society; an investigator in the North American Mitochondrial Disease Consortium. R. Haas: research grant, reimbursement for travel, and consulting payments from Stealth BioTherapeutics; scientific and medical advisory board of the United Mitochondrial Disease Foundation; advisory board for MitoBridge; clinical trial funding from Edison Pharmaceuticals, Stealth BioTherapeutics, Horizon Pharma (previously Raptor), and Sarepta; grant funding from Food and Drug Administration Orphan Products grant 1RO1FD004147 and NIH U54 NS078059. A. Goldstein: research grant, reimbursement for travel, consulting payments from Stealth BioTherapeutics; United Mitochondrial Disease Foundation Scientific and Medical Advisory Board; Data Safety Monitoring Board, University of Pittsburgh; Editorial Board for the Journal of Child Neurology and Pediatric Neurology; consultant for Biomarin; an investigator in the North American Mitochondrial Disease Consortium; president of the Mitochondrial Medicine Society. J. Vockley: research grant, reimbursement for travel, consulting payments from Stealth BioTherapeutics. W. Weaver: was a paid consultant during the trial and now is employed by Stealth BioTherapeutics and holds stock in the company. B. Cohen: research grant, reimbursement for travel, and consulting payments from Stealth BioTherapeutics; research grants from Reata Pharmaceuticals, BioElectron Technology (Edison Pharma), and Horizon Pharma (Raptor Pharma); received travel support from Reata; consultant with MitoBridge and Wellstat Pharma; United Mitochondrial Disease Foundation Board of Trustees; an investigator for the North American Mitochondrial Disease Consortium. Go to Neurology.org/N for full disclosures.
References
- 1.Parikh S, Goldstein A, Koening MK, et al. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med 2015;17:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naviaux R. Developing a systematic approach to the diagnosis and classification of mitochondrial disease. Mitochondrion 2004;55:351–361. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi G, Cortopassi G. Oxidative stress in inherited mitochondrial diseases. Free Radic Biol Med 2015;88:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat AH, Dar KB, Anees S, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases: a mechanistic insight. Biomed Pharmacother 2015;74:101–110. [DOI] [PubMed] [Google Scholar]
- 5.Arun S, Liu L, Donmez G. Mitochondrial biology and neurological diseases. Curr Neuropharmacol 2016;14:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorman G, Schaefer A, Ng Y, et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol 2015;77:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zolkipli-Cunningham Z, Stoddart A, McCormick E, Xio R, Falk M. Mitochondrial disease patients' motivations and barriers to participate in clinical trials. Presented at the UMDF Mitochondrial Medicine 2015 International Symposium; June 20, 2015; Herndon, VA.
- 8.Hirano M, Mancuso M. Mitochondrial myopathy. In: National Organization of Rare Disorder, Physician Guide [online]. Available at: https://rarediseases.org/physician-guide/mitochondrial-myopathy/. Accessed October 1, 2017. [Google Scholar]
- 9.Karaa A, Kriger J, Grier J, et al. Mitochondrial disease patients' perception of dietary supplements' use. Mol Gen Metab 2016;119:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorman G, Elson J, Newman J, et al. Perceived fatigue is highly prevalent and debilitating in patients with mitochondrial disease. Neuromuscul Disord 2015;25:563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeffer G, Majamaa K, Turnbull D, et al. Treatment for mitochondrial disorders. Cochrane Database Syst Rev 2012;4:CD004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szeto H, Birk A. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther 2014;96:672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szeto H. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 2014;171:2029–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szeto HH, Liu S, Soong Y, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 2011;22:1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel M, Kruse S, Percival J, et al. Mitochondrial-targeted peptide rapidly improves mitochondrial energetic and skeletal muscle performance in aged mice. Aging Cell 2013;12:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birk A, Liu S, Soong Y, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 2013;24:1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown DA, Hale SL, Baines CP, et al. Reduction of early reperfusion injury with the mitochondria-targeting peptide Bendavia. J Cardiovasc Pharmacol 2014;19:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stauffer B, Sparagna G, Chau S, et al. MTP131, a cardiolipin targeting peptide, improves mitochondrial activity in the failing human heart. Eur J Heart Fail Abstr Suppl 2016;18:289. [Google Scholar]
- 19.Eirin A, Ebrahimi B, Zhang X, et al. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res 2014;103:461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elson JL, Cadogan M, Apabhai S, et al. Initial development and validation of a mitochondrial disease quality of life scale. Neuromuscul Disord 2013;23:324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarnopolsky M. Exercise testing in metabolic myopathies. Phys Med Rehabil Clin N Am 2012;23:173–186. [DOI] [PubMed] [Google Scholar]
- 22.Mancuso M, Angelini C, Bertini E, et al. Fatigue and exercise intolerance in mitochondrial diseases: literature revision and experience of the Italian Network of Mitochondrial Diseases. Neuromuscul Disord 2012;22:S226–S229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opasich C, Pinna GD, Mazza A, et al. Six-minute walking performance in patients with moderate-to-severe heart failure: is it a useful indicator in clinical practice? Eur Heart J 2001;22:488–496. [DOI] [PubMed] [Google Scholar]
- 24.Steele H, Horvath R, Taylor R. The swinging pendulum of biomarkers in mitochondrial disease: the role of FGF-21. Neurology 2016;87: 2286–2287. [DOI] [PubMed] [Google Scholar]
- 25.Suomalainen A, Elo J, Pietiläinen K, et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol 2011;10:806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerr DS. Review of clinical trials for mitochondrial disorders: 1997–2012. Neurotherapeutics 2013;10:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiMauro S, Hirano M, Schon E. Approaches to the treatment of mitochondrial disease. Muscle Nerve 2006;34:265–283. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve 2007;35:235–242. [DOI] [PubMed] [Google Scholar]