Abstract
Background
Preterm birth complicates almost all triplet pregnancies and no preventive strategy has proven effective.
Objective
To determine, using individual patient data (IPD) meta-analysis, whether the outcome of triplet pregnancy is affected by prophylactic administration of 17-hydroxyprogesterone caproate (17OHPc).
Search strategy
We searched literature databases, trial registries and references in published articles.
Selection criteria
Randomised controlled trials (RCTs) of progestogens versus control that included women with triplet pregnancies.
Data collection and analysis
Investigators from identified RCTs collaborated on the protocol and contributed their IPD. The primary outcome was a composite measure of adverse perinatal outcome. The secondary outcome was the rate of birth before 32 weeks of gestation. Other pre-specified outcomes included randomisation-to-delivery interval and rates of birth at < 24, < 28 and < 34 weeks of gestation.
Main results
Three RCTs of 17OHPc versus placebo included 232 mothers with triplet pregnancies and their 696 offspring. Risk-of-bias scores and between-study heterogeneity were low. Baseline characteristics were comparable between 17OHPc and placebo groups. The rate of the composite adverse perinatal outcome was similar among those treated with 17OHPc and those treated with placebo (34% and 35%, respectively; risk ratio [RR] 0.98, 95% confidence interval [95% CI] 0.74–1.3). The rate of birth at < 32 weeks was also similar in the two groups (35% and 38%, respectively; RR 0.92, 95% CI 0.55–1.56). There were no significant between-group differences in perinatal mortality rate, randomisation-to-delivery interval, or other specified outcomes.
Conclusion
Prophylactic 17OHPc given to mothers with triplet pregnancies had no significant impact on perinatal outcome or pregnancy duration.
Tweetable abstract
17-Hydroxyprogesterone caproate had no significant impact on the outcome or duration of triplet pregnancy.
Keywords: 17-hydroxyprogesterone caproate, multiple gestation, preterm birth prevention, progestogens, triplet pregnancy
Introduction
Triplet pregnancies are at very high risk for preterm birth, neonatal morbidity and perinatal mortality. In developed countries, 90–100% of triplets deliver preterm (before 37 weeks of gestation) and 35–50% deliver very preterm (before 32 weeks).1–6 Triplets are admitted to a neonatal intensive care unit in 50–90% of cases,4,7–9 compared with about 24% of twins and 3% of singletons.10 Perinatal death occurs in 4.5–15% of triplets,4,5,7,9,11–15 compared with 2.0% of twins and 0.45% of singletons.2 Most of the excess neonatal morbidity and mortality among triplets is directly attributable to extremely preterm birth.16
Several interventions intended to prevent preterm birth have been tested in triplet pregnancy. Prophylactic bed rest17 and prophylactic cervical cerclage18,19 are ineffective. Prophylactic tocolysis20 results in preterm birth rates similar to those reported in triplet pregnancy without such therapy.17,19 Multifetal pregnancy reduction of triplets to twins, compared with continuation with triplets, may reduce the rate of extremely preterm birth21 but data are conflicting as to whether this reduces mortality among the surviving twins.21,22 Moreover, if the reduced triplet is counted as a mortality, the mortality rate with multifetal pregnancy reduction is at least one in three, much higher than in continuing triplet pregnancies.21,22 Given these shortcomings, other interventions to prevent preterm birth in triplet pregnancy are desired.
Progestogens have properties that might help to prevent preterm birth, including reduced expression of myometrial gap junctions and contraction-related proteins, reduced production of inflammatory cytokines, inhibition of cervical ripening, and reduced apoptosis in the chorion and decidua.23–25 In singleton pregnancies with high-risk conditions such as sonographic short cervix or history of previous preterm birth, vaginal progesterone or 17-hydroxyprogesterone caproate (17OHPc), respectively, reduces the rate of preterm birth.26–28 However, in twin pregnancy, neither vaginal progesterone nor 17OHPc appears effective, except perhaps in the select subgroup with short cervix.28,29 In triplet pregnancy, two randomised controlled trials (RCTs) showed no significant benefit of 17OHPc compared with placebo.30,31 One of the trials suggested that 17OHPc may actually increase the risk of perinatal loss,31 a potential ‘safety signal’ that has been raised in other investigations of 17OHPc.32 Two other RCTs tested 17OHPc33 or vaginal progesterone34 in multiple gestations, including several triplet pregnancies, but the reports did not show the results of the triplets separately.
The goal of the present investigation was to determine whether prophylactic progestogens are beneficial or harmful in triplet pregnancy by performing a meta-analysis of the individual patient data (IPD) from relevant RCTs.
Methods
Identification of studies
We identified trials by searching the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, MEDLINE and ClinicalTrials.gov for published or registered RCTs including women with triplet pregnancies randomly allocated to treatment with progestogens (including micronised progesterone and 17OHPc) versus control in the second or third trimester with the intention of preventing preterm birth. We tested various combinations of the search terms progesterone, hydroxyprogesterone, caproate, progestogen, progestin, preterm birth, perinatal morbidity, perinatal outcome, triplets and pregnancy, multiple. We reviewed the title and abstract information from retrieved citations and obtained full text citations as needed to determine relevance. We also reviewed lists of citations in related articles and review articles.
Our basic inclusion criteria were: controlled trial, triplet pregnancies included and prophylactic use of progestogen. We considered inclusion of randomised and quasi-randomised studies, studies comparing progestogen with placebo, ‘no treatment’, or ‘standard care’, and studies that were blinded (‘masked’) or unblinded (‘open-label’).
An initial search in February 2014 located four potentially eligible trials. Three of these were placebo-controlled trials of 17OHPc.30,31,33 The fourth was a trial of vaginal progesterone in 81 women with twin pregnancy and three with triplet pregnancy.34 Because there were only three participants with triplet pregnancies in that trial and because vaginal progesterone is fundamentally different from 17OHPc,35 we decided to focus on trials using 17OHPc.
The investigators of the three trials identified in the initial search collaborated on the development of the protocol and agreed to share their patient-level data. After the protocol was finalised, a second search for trials was undertaken in November 2014.
The protocol called for exclusion of a trial for any of these reasons: investigator(s) decline to provide data; more than 10% attrition or exclusion of women after randomisation; incomplete reporting of reasons for withdrawals and protocol violations; imbalance in drop-outs across groups; incomplete reporting of all the study’s pre-specified outcomes; outcomes of interest not made available for analysis.
Registration, protocol and approval
Before any analysis was undertaken, the protocol was registered on the International Prospective Register of Systematic Reviews (Prospero). The full protocol is available on the Prospero website, www.crd.york.ac.uk/PROSPERO, #CRD42014010330. The included studies had approval from their local Institutional Review Boards before the studies began. De-identified individual case data were used for the present analysis. The study is reported according to guidelines of the PRISMA-IPD statement.36
Assessment of bias
Risks of bias for each study were assessed using the risk-of-bias tool of the Cochrane collaboration,37 including these components: sequence generation (i.e. computer-generated random number, use of random number table or other truly random process); allocation concealment (i.e. web-based or telephone central randomisation or consecutively numbered sealed opaque envelopes); blinding of participants, study personnel and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. Each item of potential bias was scored as low, high or unclear based on criteria specified by the Cochrane Handbook.
For each included study, all the elements of potential bias were scored independently by four of the authors (CAC, SC, AL, ES), with majority opinion prevailing. A fifth author (BWJM) was designated to break any ties.
Outcome measures
Outcome measures were specified in advance of any analysis.
The primary outcome was a composite measure of adverse perinatal outcome, defined as any one or more of the following: miscarriage (fetal death and/or spontaneous expulsion at < 20 weeks of gestation); fetal death at ≥ 20 weeks of gestation; neonatal death (death at ≤ 28 days of life in an infant born alive at ≥ 20 weeks of gestation); respiratory distress syndrome requiring ventilation for ≥ 24 hours; bronchopulmonary dysplasia; intraventricular haemorrhage grade 3 or 4; necrotising enterocolitis stage 2 or 3; culture-proven sepsis.
The secondary outcome was the rate of very preterm birth (< 32 weeks of gestation). Other outcomes specified in advance were: each of the individual component outcomes of the primary outcome; rates of extremely preterm birth (< 28 weeks of gestation) and early preterm birth (< 34 weeks of gestation); rates of pregnancy loss (birth, miscarriage, or fetal death) at < 24, < 28, < 32 and < 34 weeks of gestation; and time from randomisation to delivery.
Data collection and synthesis
The investigators of all eligible RCTs provided individual participant data as either a spreadsheet or as a data file from a standard statistical package, along with a document specifying the definitions used. The data collected included relevant baseline characteristics and outcomes of interest. Data quality was assessed by comparison of the numbers published and the data shared by the investigators. In case of any questions or discrepancies, the principal investigator was contacted for clarification and corrections were made if necessary. After all questions were resolved, the data were combined into a single file for analysis.
Analysis
Our approach to data analysis followed that used in a similar IPD meta-analysis by our group concerning the effects of progestogens in twin pregnancy.29 In the present study, the overall effects of 17OHPc treatment were estimated in the pooled IPD. Descriptive comparisons between studies were conducted to assess between-study differences. Missing data were not an issue, so a complete-case analysis was performed.
The effectiveness of progestogen treatment was estimated using mixed models for binomial outcomes with a log link, thus resulting in risk ratios (RR) with 95% confidence interval (95% CI). A random intercept (to account for differences in prevalence between studies) and a random slope (to account for differences in treatment effect between studies) were included in these models. In the analysis on newborn level, we incorporated a compound symmetric residual error variance to account for the non-independence of neonates from one mother.39 When convergence problems occurred due to low numbers in any of the studies, a fixed slope and/or intercept was used instead of a random slope and/or intercept.
Time-to-delivery analysis was performed with Kaplan–Meier and Cox proportional hazards regression analysis. This analysis was stratified by study to account for dependency between data originating from the same study.40
Heterogeneity across trials was assessed using the I2 measure, with values interpreted as follows: 0% indicates no observed heterogeneity; 25%, 50% and 75% indicate low, moderate and high heterogeneity, respectively.41 A Number-Needed-to-Treat calculation was planned for associations found to be statistically significant.
Pre-specified subgroup analyses of the effect of 17OHPc on the primary outcome were conducted in the following subgroups: women with a cervical length < 30th centile versus ≥ 30th centile (in studies where transvaginal cervical length measurement was specified in the protocol); women with versus without a previous spontaneous preterm birth at < 37 weeks; women with body mass index < 25 kg/m2 versus ≥ 25 kg/m2); and women of various race/ethnicity groups. Additional subgroup analyses, not pre-specified by the protocol, compared subgroups based on chorionicity (monochorionic, dichorionic, trichorionic) and based on usage of assisted reproductive techniques. Subgroup effects were investigated by adding an interaction term between the subgrouping variable and treatment group in the regression model.
Statistical analyses were performed using R software, version 3.0.1 (The R Foundation for Statistical Computing, 2012).
Results
Study selection is summarised in the flow diagram (see Supplementary material, Figure S1). The updated search for trials in November 2014 yielded 620 records (452 unique records), of which four met our inclusion criteria, with no new trials identified in the updated search. The Supplementary material (Table S1) gives a brief synopsis of the four studies. Three trials tested 17OHPc versus placebo30,31,33 in a total of 232 women and one tested vaginal progesterone versus placebo in three women with triplet pregnancies.34 As no additional trials testing vaginal progesterone in triplets were found in the updated search, we affirmed our preliminary decision to exclude the latter trial from the meta-analysis.
Data sets were obtained from the three remaining studies. Regarding the planned subgroup analysis with stratification by cervical length, one of the trials measured cervical length per protocol at the time of randomisation or the next visit,33 another measured cervical length per protocol at 24–26 weeks of gestation,31 and the third trial did not provide cervical length data.30 Otherwise, the data available from all three trials were comparable.
Risks of bias were scored as Low for all three studies, as summarised in the Supplementary material (Figure S2).
Baseline characteristics of the subjects at the time of enrolment were comparable between studies (Table 1). There were no significant differences between those allocated to 17OHPc versus those allocated to placebo regarding maternal demographics, gestational age, or other baseline characteristics.
Table 1.
Baseline characteristics of the mothers
| Trial | Treatment | Group, pooled* | |||
|---|---|---|---|---|---|
| NICHD-MFMU30 (n = 134) |
Obstetrix31 (n = 81) |
AMPHIA33 (n = 17) |
17-hydroxy-progesterone caproate (n=136) |
Placebo (n=96) |
|
| Maternal age, years | 31.0 ± 5.1 | 33.4 ± 5.1 | 32.8 ± 5.3 | 32.0 ± 5.4 | 32.0 ± 5.0 |
| Body mass index, kg/m2 | 24.6 (22.1–29.4) | 24.2 (21.1–27.4) | 23.7 (21.2–25.5) | 23.9 (21.4–28.2) | 25.1 (22.1–28.9) |
| Nulliparous | 71 (53) | 56 (69) | 9 (53) | 76 (56) | 49 (51) |
| Gestational age (weeks) | 19.0 ± 1.3 | 19.9 ± 2.3 | 16.5 ± 1.5 | 19.1 ± 2.0 | 19.1 ± 1.9 |
| Race | |||||
| White | 110 (82) | 56 (69) | 14 (82) | 102 (75) | 78 (81) |
| Black | 11 (8) | 3 (4) | 1 (6) | 9 (7) | 6 (6) |
| Asian | 2 (1) | 5 (6) | 1 (6) | 7 (5) | 1 (1) |
| Other | 11 (8) | 17 (21) | 1 (6) | 18 (13) | 11 (11) |
| Smoking | 6 (4) | 0 | 2 (12) | 2 (1) | 6 (6) |
| Alcohol | 6 (4) | 30 (37)** | 0 | 20 (15) | 16 (17) |
| ART conception | 95 (71) | 71 (91) | 10 (59) | 107 (79) | 69 (73) |
| Prior preterm birth | 7 (5) | 14 (17) | 1 (6) | 10 (7) | 12 (12) |
| Chorionicity | |||||
| Trichorionic | 96 (81) | 81 (100) | 11 (65) | 112 (85) | 76 (89) |
| Dichorionic | 16 (14) | 0 (0) | 5 (29) | 12 (9) | 9 (11) |
| Monochorionic | 6 (5) | 0 (0) | 1 (6) | 7 (5) | 0 (0) |
Data are mean ± SD, median (interquartile range), or n (%).
No significant differences between 17-hydroxyprogesterone caproate and placebo (P > 0.05 for all comparisons). Imbalance in numbers is due to 2 : 1 randomisation ratio in one of the trials.
All cases of alcohol use were reported as ‘rare’ in this trial.
Abbreviations: ART, assisted reproduction techniques; RCT, randomised clinical trial; NICHD-MFMU,, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Materanl-Fetal Medicine Units Network; AMPHIA, 17-α hydroxyprogesterone caproate in Multiple pregnancies to Prevent Handicapped InfAnts
The primary outcome (composite adverse perinatal outcome) occurred with similar frequency in those treated with 17OHPc and those treated with placebo (RR 0.98, 95% CI 0.74–1.3, P = 0.77), as summarised in Table 2. There were no significant differences in any of the individual components of the primary outcome or in other serious neonatal complications.
Table 2.
Perinatal outcomes
| 17-hydroxy-progesterone caproate n = 408 |
Placebo n=288 |
i2 | Risk ratio (95% CI) |
P value | |
|---|---|---|---|---|---|
| Composite adverse perinatal outcome* | 140/408 (34) | 101/288 (35) | 0% | 0.98 (0.79–1.2) | 0.79 |
| Perinatal death** | 25/408 (6.1) | 14/288 (4.9) | 72% | 1.3 (0.37–4.2)*** | 0.71 |
| Miscarriage < 20 weeks | 3 (0.7) | 4 (1.3) | |||
| Fetal death ≥ 20 weeks | 3 (0.7) | 6 (2.1) | |||
| Neonatal death ≥ 20 weeks | 19 (4.7) | 4 (1.3) | |||
| Respiratory distress syndrome | 115/395 (29) | 83/278 (30) | 44% | 0.99 (0.65–1.5) | 0.93 |
| Bronchopulmonary dysplasia | 26/392 (6.6) | 28/278 (10) | 0 | 0.68 (0.38–1.2) | 0.11 |
| Intraventricular haemorrhage (grade 3 or 4) | 6/391 (1.5) | 7/278 (2.5) | 0 | 0.37 (0.089–1.5)*** | 0.17 |
| Necrotising enterocolitis (stage 2 or 3) | 10/394 (2.5) | 8/278 (2.9) | 23% | 0.94 (0.31–2.8)*** | 0.91 |
| Neonatal sepsis, culture-proven | 12/394 (3.1) | 8/278 (2.9) | 21% | 0.99 (0.24–4.0) | 0.97 |
| Outcomes not included in the composite: | |||||
| Periventricular leukomalacia | 0/376 (0) | 1/264 (0.4) | NC | NC | NC |
| Retinopathy of prematurity | 5/247 (2.0) | 7/193 (3.6) | 0 | 0.56 (0.18–1.7) *** | 0.32 |
Composite adverse perinatal outcome defined as perinatal death, respiratory distress syndrome, bronchopulmonary dysplasia and/or intraventricular haemorrhage grade 3 or 4, necrotising enterocolitis, or culture-proven neonatal sepsis.
Perinatal death defined as miscarriage at < 20 weeks, fetal death ≥ 20 weeks, or neonatal death ≥ 20 weeks. Numbers appear not to reconcile with primary literature because Combs et al.31 reported nine neonates born at 200/7 to 236/7 weeks as ‘midtrimester pregnancy loss’ whereas the definitions used here classify eight of those as neonatal deaths and one as a fetal death.
Random slope and/or intercept component removed from the analytical model due to convergence issues.
NC, not calculated due to 0 outcomes in some studies.
The secondary outcome (birth at < 32 weeks of gestation) also occurred with similar frequency in the two groups (35% and 38%, respectively; RR 0.92, 95% CI 0.55–1.56), as shown in Table 3. There were no significant differences in the rates of preterm birth or pregnancy loss (birth, miscarriage, or fetal death) before various gestational age milestones (24, 28, 32, 34 weeks).
Table 3.
Rates of preterm birth or pregnancy loss at various gestational ages
| 17-hydroxy-progesterone caproate n=136 |
Placebo n=96 |
i2 | Risk ratio (95% CI) |
P value | |
|---|---|---|---|---|---|
| Birth < 34 weeks | 86 (63) | 64 (67) | 4% | 0.95 (0.78–1.2) | 0.59 |
| Birth < 32 weeks | 48 (35) | 36 (38) | 63% | 0.92 (0.55–1.56) | 0.77 |
| Birth < 28 weeks | 15 (11) | 12 (12) | 0% | 0.88 (0.43–1.8) | 0.73 |
| Birth < 24 weeks | 4 (3) | 2 (2) | 3% | 1.4 (0.26–7.6)** | 0.69 |
| Pregnancy loss < 34 weeks* | 86 (63) | 65 (68) | 36% | 0.93 (0.68–1.3) | 0.68 |
| Pregnancy loss < 32 weeks* | 49 (36) | 37 (39) | 65% | 0.91 (0.53–1.6) | 0.74 |
| Pregnancy loss < 28 weeks* | 16 (12) | 13 (14) | 24% | 0.87 (0.44–1.7) | 0.69 |
| Pregnancy loss < 24 weeks* | 5 (4) | 3 (3) | 34% | 1.1 (0.29-4.8)*** | 0.82 |
Data expressed as n (%)
Pregnancy loss defined as miscarriage, fetal death, or early preterm birth before the given gestational age
Due to low numbers, analysis is a log-binomial model combining all data rather than random effects model accounting for the origin of data from individual studies.
Kaplan–Meier survival curves of the two groups are shown in Figure 1. The median time from randomisation to delivery was similar in those receiving 17OHPc (median 97 days, interquartile range 79–109 days) and those receiving placebo (median 95 days, interquartile range 78–106 days, hazard ratio 0.96, 95% CI 0.72–1.3, P = 0.78).
Figure 1.
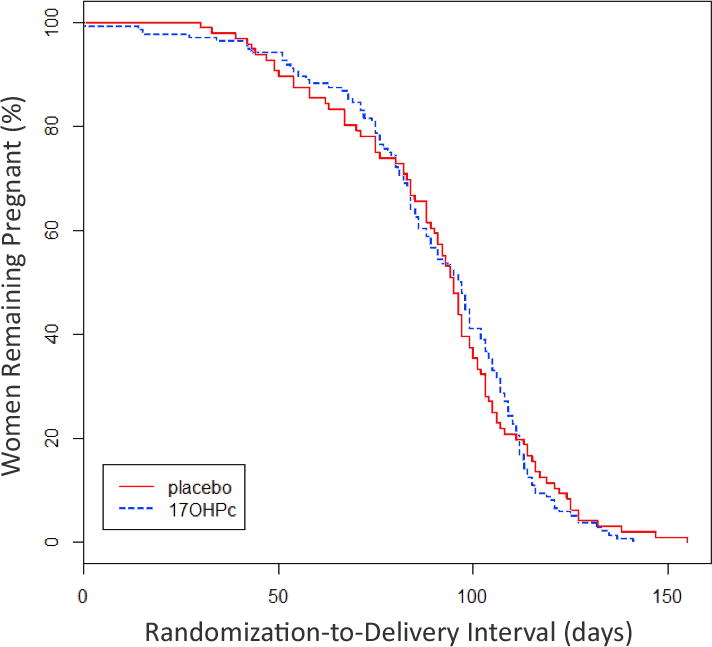
Kaplan–Meier curves for time from randomisation to delivery. P = 0.88, log-rank test; 17OHPc, 17-hydroxyprogesterone caproate.
Subgroup analyses on the rate of the primary outcome are summarised in the Supplementary material (Table S2). There was no significant interaction between treatment allocation and any of the subgroups.
Discussion
Principal finding
The principal finding of this study is that prophylactic 17OHPc given to mothers with triplet pregnancies had no significant impact on perinatal outcome or pregnancy duration. To our knowledge, this is the first meta-analysis concerning the effect of progestogens in triplet pregnancies.
Strengths of the study
One strength of the study is the moderately large number of triplets (696 total) included in the primary efficacy analysis of adverse perinatal outcome. The meta-analysis has greater statistical power than any of the individual studies to detect between-group differences, allowing us to conclude that 17OHPc did not improve the perinatal outcome of triplet pregnancy. Similarly, for the analysis of interval from randomisation-to-delivery, the hazard ratio of 0.96 with a narrow 95% CI (0.72–1.3) allows us to conclude that 17OHPc did not prolong triplet pregnancy.
Meta-analysis of individual patient-level data offers several advantages over meta-analysis of aggregated data from published reports. First, IPD meta-analysis allows the specification of unified outcomes and subgroups even if they were defined differently or not presented at all in the included trials. Second, it allows for standardisation of inclusion/exclusion criteria and analysis across studies, independent of bias that may arise through selective reporting.42 Third, IPD meta-analysis allows for exploration of differential treatment effects in relevant subgroups (i.e. treatment covariate interactions);43 as it includes more detail than aggregated data, it has greater statistical power to carry out informative subgroup analyses.44 Fourth, it allows time-to-event analysis whereas aggregated data only allows a pooled estimate of treatment effects at specified cut-points, e.g. delivery before 28, 32, or 34 weeks of gestation; this may be important because small differences in time-to-delivery attributable to progestogens may have been missed in the individual trials owing to relatively small sample size.
Limitations of the study
The analysis of the effect of 17OHPc on perinatal mortality has low statistical power owing to the small number of deaths. Though we no found significant effect (RR 1.3, 95% CI 0.37–4.2), we estimate post hoc that 696 subjects yields a power of only 10% to find a significant difference at P < 0.05 in the observed mortality rates (6.1% versus 4.9%). Hence, we cannot draw definitive conclusions about the safety of 17OHPc in triplet pregnancy.
Similarly, power to detect differences between the various subgroups was limited, owing to the small numbers of subjects in some of the subgroups. This was especially true for the subgroups based on cervical length because the protocol of the largest trial did not include cervical length measurement and the other trial protocols differed in the timing of the measurement.
Another limitation is that all three of the included studies tested only a single dose of 17OHPc, namely 250 mg weekly. But in the absence of any evidence of efficacy we do not recommend using higher doses.
Another limitation is that we did not have data regarding use of progestogens in early pregnancy. The majority of the pregnancies were conceived by assisted reproductive techniques, which often involve progestogen supplementation in the first trimester. The two largest trials excluded women with a plan to use non-study progestogens after 16 weeks30 or any pre-enrolment use of progestogens after 15 weeks of gestation.31 But we cannot exclude the possibility that residual progestogen from first-trimester usage may have influenced the results. However, this seems unlikely in light of the lack of a significant interaction between assisted reproductive techniques and treatment-group (see Supplementary material, Table S2).
Interpretation (in light of other evidence)
In 2003, two studies were published showing that progestogens reduced the rate of preterm birth in women with singleton pregnancies at high risk. One study used 17OHPc in women with a history of previous preterm birth27 and the other used vaginal progesterone in women with various risk factors, including previous preterm birth in 93% of them.26 Since that time, there have been eight RCTs testing vaginal progesterone34,45–51 and eight RCTs testing 17OHPc30,31,33,52–56 in women with multiple gestations. None of the 16 individual trials showed benefit of progestogens in reducing early preterm birth. Our recent IPD meta-analysis of trials in twin pregnancies found that neither vaginal progesterone nor 17OHPc reduced the rate of preterm birth in unselected women.29 Subgroup analysis suggested a possible benefit of vaginal progesterone, but not 17OHPc, in reducing perinatal morbidity and early preterm birth in the subgroup of twin pregnancies with a sonographically short cervix.28,29 This result is similar to findings with singleton pregnancies with a short cervix, where vaginal progesterone, but not 17OHPc, appears to reduce the risk of preterm birth.28,57
We do not know why prophylactic 17OHPc is effective in singleton pregnancies with previous preterm birth27 but not in multiple gestations. The pharmacokinetics of the drug differ between singleton58 and multiple59 gestations, the half-life averaging 16 ± 6 days versus 10 ± 4 days, respectively. A shorter half-life might be expected to yield lower serum levels in multiple gestation. In singleton pregnancy, the risk of recurrent preterm birth is higher with lower serum levels of 17OHPc,60 but paradoxically, in twin pregnancy, the risk of preterm birth is lower with lower serum levels.61 Hence, pharmacokinetics do not entirely explain the failure of 17OHPc to prevent preterm birth in multiple gestations. It seems likely that the factors that trigger parturition in multiple gestations (such as uterine distension or fetal signalling) are simply different from those triggering recurrent preterm birth in singletons (such as inflammation or maternal factors) and such factors may not be responsive to prophylactic progestogen treatment.
Regardless of the reason for the failure, the results of all the studies to date point to the consistent conclusion that prophylactic progestogens are ineffective in unselected women with multiple gestation.
Conclusions
We found no evidence that prophylactic 17OHPc given to mothers with triplet pregnancy had a significant impact on perinatal outcome or pregnancy duration. Given potential safety concerns32,62 and the lack of any evidence of efficacy, 17OHPc should not be used in triplet pregnancy except in controlled clinical trials. Vaginal progesterone has not been adequately studied in triplet pregnancy. For planning possible future trials, it appears unlikely that 17OHPc would be effective. Extrapolating from experience with twin pregnancy,28,29 a trial of vaginal progesterone for triplet pregnancy complicated by a sonographic short cervix may be warranted.
Supplementary Material
Figure S1. Flow diagram of study identification and selection. 17OHPc, 17-hydroxyprogesterone caproate.
Figure S2. Quality summary: risks of bias for each included study.
Table S1. Overview of clinical trials testing prophylactic progestogens in triplet pregnancy.
Table S2. Rates of composite adverse perinatal outcome in various subgroups.
Acknowledgments
The authors express their gratitude to the hundreds of women with multiple gestations who have consented to participate in randomised trials of progestogens and to the dozens of co-investigators, collaborators, and research personnel who conducted the original trials.
Funding
No separate funding was obtained for this study. Each of the authors has salary support through their individual institutional affiliations.
Footnotes
Protocol registration: International Prospective Register of Systematic Reviews (Prospero) # CRD42014010330; www.crd.york.ac.uk/PROSPERO
Disclosure of interests
CAC, TJG and KM disclosed that the Obstetrix Collaborative Research Network received a donation of hydroxyprogesterone caproate (Makena®) from KV Pharmaceuticals (now Lumara Health) through an unrestricted grant for a clinical trial not related to the present study. The granting company had no involvement in the planning, conduct, or writing of the present study, or the decision to submit the paper for publication. The investigators reported no other disclosures. The ICMJE disclosure forms are available as online supporting information.
Contribution to authorship
CAC, ES and BWJM were involved in the concept and initial design of the study. CAC, SNC, ACL, TJG, KM, DR, ET and AT were involved in the design, performance and reporting of one of the trials included in the meta-analysis and in making their individual patient data available for meta-analysis. CAC wrote the initial drafts of the protocol and the manuscript, with significant contributions from ES. ES performed the statistical analyses in collaboration with CAC and BWJM. CAC, ES, SNC, ACL, TJG, KM, DR, ET, AT and BWJM participated in face-to-face meetings, phone calls, and/or e-mail exchanges at all phases of the study, were involved in making critical revisions to the protocol and to the manuscript, and approved the final version.
Details of ethics approval
Each study included in the meta-analysis had institutional review board approval and informed consent from all subjects.
Supporting Information
The following supplementary materials are available for this article.
Additional Supporting Information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author.
References
- 1.Geipel A, Berg C, Katalinic A, Plath H, Hansmann M, Germer U, Gembruch U. Prenatal diagnosis and obstetric outcomes in triplet pregnancies in relation to chorionicity. BJOG. 2005;112:554–8. doi: 10.1111/j.1471-0528.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 2.Luke B, Brown MB. The effect of plurality and gestation on the prevention or postponement of infant mortality: 1989-1991 versus 1999-2001. Twin Res Hum Genet. 2007;10:514–20. doi: 10.1375/twin.10.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuppa AA, Scorrano A, Cota F, D’Andrea V, Fracchiolla A, Romagnoli C. Neonatal outcomes in triplet pregnancies: assisted reproduction versus spontaneous conception. J Perinat Med. 2007;35:339–43. doi: 10.1515/JPM.2007.066. [DOI] [PubMed] [Google Scholar]
- 4.Battin M, Wise M, DeZoete A, Stone P. Infant and perinatal outcomes of triplet pregnancy in Auckland: better than expected? J NZ Med Assoc. 2009;122:39–47. [PubMed] [Google Scholar]
- 5.Tandberg A, Bjorge T, Nygard O, Bordahl PE, Skjaerven R. Trends in incidence and mortality for triplets in Norway 1967–2006: the influence of assisted reproductive technologies. BJOG. 2010;117:667–75. doi: 10.1111/j.1471-0528.2010.02530.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Mathews TJ. Births: final data for 2011. Nat Vital Stat Rep. 2013;62:1–69. [PubMed] [Google Scholar]
- 7.Al-Suleiman SA, Al-Jama FE, Rahman J, Rahman MS. Obstetric complications and perinatal outcome in triplet pregnancies. J Obstet Gynaecol. 2006;26:200–4. doi: 10.1080/01443610500508295. [DOI] [PubMed] [Google Scholar]
- 8.Spencer JV, Ingardia CJ, Nold CJ, Borgida AF, Herson VC, Egan JFX. Perinatal and neonatal outcomes of triplet gestations based on placental chorionicity. Am J Perinatol. 2009;26:587–90. doi: 10.1055/s-0029-1220776. [DOI] [PubMed] [Google Scholar]
- 9.Chibber R, Fouda M, Shishtawy W, Shishtawy W, Al-Dossry M, Al-Hijji J, et al. Maternal and neonatal outcome in triplet, quadruplet and quintuplet gestations following ART: a 11-year study. Arch Gynecol Obstet. 2013;288:759–67. doi: 10.1007/s00404-013-2796-x. [DOI] [PubMed] [Google Scholar]
- 10.Lemos EV, Zhang D, van Voorhis J, Hu XH. Healthcare expenses associated with multiple vs singleton pregnancies in the United States. Am J Obstet Gynecol. 2013;209:586.e1–11. doi: 10.1016/j.ajog.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Adegbite AL, Ward SB, Bajoria R. Perinatal outcome of spontaneously conceived triplet pregnancies in relation to chorionicity. Am J Obstet Gynecol. 2005;193:1463–71. doi: 10.1016/j.ajog.2005.02.098. [DOI] [PubMed] [Google Scholar]
- 12.Bajoria R, Ward SB, Adegbite AL. Comparative study of perinatal outcome of dichorionic and trichorionic iatrogenic triplets. Am J Obstet Gynecol. 2006;194:415–24. doi: 10.1016/j.ajog.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Getahun D, Amre DK, Ananth CV, Demissie K, Rhoads GG. Temporal changes in rates of stillbirth, neonatal and infant mortality among triplet gestations in the United States. Am J Obstet Gynecol. 2006;195:1506–11. doi: 10.1016/j.ajog.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Alran S, Sibony O, Luton D, Touitou S, Fourchotte V, Feraud O, et al. Maternal and neonatal outcome of 93 consecutive triplet pregnancies with 71% vaginal delivery. Acta Obstet Gynecol Scand. 2004;83:554–9. doi: 10.1111/j.0001-6349.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 15.Weissman A, Ulanovsky I, Burke Y, Makhoul IR, Blazer S, Drugan A. Triplet pregnancies—a three-decade perspective: do we fare better? Eur J Obstet Gynecol Reprod Biol. 2013;170:82–4. doi: 10.1016/j.ejogrb.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Garite TJ, Clark RH, Elliott JP, Thorp JA. Twins and triplets: the effect of plurality and growth on neonatal outcome compared with singleton infants. Am J Obstet Gynecol. 2004;191:700–7. doi: 10.1016/j.ajog.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 17.Crowther CA, Han S. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database Syst Rev. 2010:CD000110. doi: 10.1002/14651858.CD000110.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss A, Heer IM, Janssen U, Dannecker C, Hillemanns P, Muller-Egloff S. Routine cervical cerclage in higher order multiple gestation—does it prolong the pregnancy? Twin Res. 2002;5:67–70. doi: 10.1375/1369052022910. [DOI] [PubMed] [Google Scholar]
- 19.Rebarber A, Roman AS, Istwan N, Rhea D, Stanziano G. Prophylactic cerclage in the management of triplet pregnancies. Am J Obstet Gynecol. 2005;193:1193–6. doi: 10.1016/j.ajog.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 20.Elliott JP, Flynn MJ, Kaemmerer EL, Radin TG. Terbutaline pump tocolysis in high-order multiple gestation. J Reprod Med. 1997;42:687–94. [PubMed] [Google Scholar]
- 21.Wimalasundera R, Trew G, Fisk NM. Reducing the incidence of twins and triplets. Best Pract Res Clin Obstet Gynaecol. 2003;17:309–29. doi: 10.1016/s1521-6934(02)00135-9. [DOI] [PubMed] [Google Scholar]
- 22.van de Mheen L, Everwijn SM, Knapen MFCM, Oepkes D, Engels M, Manten GTR, et al. The effectiveness of multifetal pregnancy reduction in trichorionic triplet gestation. Am J Obstet Gynecol. 2014;211:536.e1–6. doi: 10.1016/j.ajog.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Murtha AP, Feng L, Yonish B, Leppert PC, Shomberg DW. Progesterone protects fetal chorion and maternal decidua cells from calcium-induced death. Am J Obstet Gynecol. 2007;196:257.e1–5. doi: 10.1016/j.ajog.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Hirota Y, Cha J, Dey SK. Prematurity and the puzzle of progesterone resistance. Nature Med. 2010;5:529–31. doi: 10.1038/nm0510-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–86. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- 26.da Fonseca EB, Bittar RE, Carvalho MHB, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–24. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 27.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 α-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Nicolaides K, Conde-Agudelo A, Tabor A, O’Brien JM, Cetingoz E, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124.e1–19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuit E, Stock S, Rode L, Rouse E, Lim AC, Norman JE, et al. Effectiveness of progestogens to improve perinatal outcome in twin pregnancies: an individual participant data meta-analysis. BJOG. 2015;122:27–37. doi: 10.1111/1471-0528.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caritis SN, Rouse DJ, Peaceman AM, Sciscione A, Momirova V, Spong CY, et al. Prevention of preterm birth in triplets using 17α-hydroxyprogesterone caproate, a randomized controlled trial. Obstet Gynecol. 2009;113:285–92. doi: 10.1097/AOG.0b013e318193c677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Combs CA, Garite T, Maurel K, Das A, Porto M, Obstetrix Collaborative Research Network Failure of 17-hydroxyprogesterone to reduce neonatal morbidity or prolong triplet pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2010;203:248.e1–9. doi: 10.1016/j.ajog.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien JM. Medication safety is still an issue in obstetrics 50 years after the Kefauver-Harris amendments: the case of progestogens. Ultrasound Obstet Gynecol. 2013;42:247–53. doi: 10.1002/uog.12456. [DOI] [PubMed] [Google Scholar]
- 33.Lim AC, Schuit E, Bloemenkamp K, Bernardus RE, Duvekot JJ, Erwich JJHM, et al. 17 α-hydroxyprogesterone caproate for the prevention of adverse neonatal outcome in multiple pregnancies. Obstet Gynecol. 2011;118:513–20. doi: 10.1097/AOG.0b013e31822ad6aa. [DOI] [PubMed] [Google Scholar]
- 34.Wood S, Ross S, Tang S, Miller L, Sauve R, Brant R. Vaginal progesterone to prevent preterm birth in multiple pregnancy: a randomized controlled trial. J Perinat Med. 2012;40:593–9. doi: 10.1515/jpm-2012-0057. [DOI] [PubMed] [Google Scholar]
- 35.Romero R, Stanczyk FZ. Progesterone is not the same as 17-hydroxprogesterone caproate: implications for obstetrical practice. Am J Obstet Gynecol. 2013;208:421–6. doi: 10.1016/j.ajog.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for a ststematic review and meta-analysis of individual participant data. The PRISMA-IPD statement. JAMA. 2014;313:1657–65. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration; 2011. [Google Scholar]
- 38.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–91. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed? BJOG. 2004;111:213–19. doi: 10.1111/j.1471-0528.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 40.Smith CT, Williamson PR, Marson AG. Investigating heterogeneity in an individual patient data meta-analysis of time to event outcomes. Stat Med. 2005;24:1307–19. doi: 10.1002/sim.2050. [DOI] [PubMed] [Google Scholar]
- 41.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riley RD, Lambert PC, bo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 43.Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials. 2005;2:209–217. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
- 44.Thompson SG, Higgins JP. Treating individuals 4: can meta-analysis help target interventions at individuals most likely to benefit? Lancet. 2005;365:341–6. doi: 10.1016/S0140-6736(05)17790-3. [DOI] [PubMed] [Google Scholar]
- 45.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–9. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 46.Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, et al. Progesterone for the prevention of preterm birth in tin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373:2034–40. doi: 10.1016/S0140-6736(09)60947-8. [DOI] [PubMed] [Google Scholar]
- 47.Cetingoz E, Cam C, Sakalli M, Karateke Ates, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet. 2011;283:423–9. doi: 10.1007/s00404-009-1351-2. [DOI] [PubMed] [Google Scholar]
- 48.Rode L, Klein K, Nicolaides KH, Krampl-Bettelheim E, Tabor A. Prevention of preterm delivery in twin gestations (PREDICT): a multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol. 2011;38:272–80. doi: 10.1002/uog.9093. [DOI] [PubMed] [Google Scholar]
- 49.Aboulghar MM, Aboulghar MA, Amin YM, Al-Inany HG, Mansour RT, Serour GI. The use of vaginal natural progesterone for prevention of preterm birth in IVF/ICSI pregnancies. Reprod Biomed Online. 2012;25:133–8. doi: 10.1016/j.rbmo.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Serra V, Perales A, Meseguer J, Parilla JJ, Lara C, Bellver J, et al. Increased doses of vaginal progesterone for the prevention of preterm birth in twin pregnancies: a randomised controlled double-blind multicentre trial. BJOG. 2013;120:50–7. doi: 10.1111/j.1471-0528.2012.03448.x. [DOI] [PubMed] [Google Scholar]
- 51.Brizot ML, Hernandez W, Liao AW, Bittar RE, Francisco RPV, Krebs VLJ, Mugaib M. Vaginal progesterone for the prevention of preterm birth in twin gestations: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 52.Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, Thom EA, Spong CY, et al. A trial of 17 α-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–61. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 53.Briery CM, Veillon EW, Klauser CK, Martin RW, Chauhan SP, Magann EF, Morrison JC. Progesterone does not prevent preterm births in women with twins. South Med J. 2009;102:900–4. doi: 10.1097/SMJ.0b013e3181afee12. [DOI] [PubMed] [Google Scholar]
- 54.Combs CA, Garite T, Maurel K, Das A, Porto M, Obstetrix Collaborative Research Network 17-hydroxyprogesterone caproate for twin pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2011;204:221–8. doi: 10.1016/j.ajog.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 55.Senat M, Porcher R, Winer N, Vayssiere C, Deruelle P, Capelle M, et al. Prevention of preterm delivery by 17α-hydroxyprogesterone caproate in asymptomatic twin pregnancies with a short cervix: a randomized controlled trial. Am J Obstet Gynecol. 2013;208:194.e1–8. doi: 10.1016/j.ajog.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 56.Awwad J, Usta IM, Ghazeeri G, Yacoub N, Succar J, Hayek S, et al. A randomized controlled double-blind clinical trial of 17-hydroxyprogesterone caproate for the prevention of preterm birth in twin gestation (PROGESTWIN): evidence for reduced neonatal morbidity. BJOG. 2015;122:71–9. doi: 10.1111/1471-0528.13031. [DOI] [PubMed] [Google Scholar]
- 57.Grobman WA, Thom EA, Spong CY, Iams JD, Saade GR, Mercer BM, et al. 17 α-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;207:390.e1–8. doi: 10.1016/j.ajog.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caritis SN, Sharma S, Venkataramanan R, Hankins GD, Miodovnik M, Hebert MF, et al. Pharmacology and placental transport of 17-hydroxyprogesterone caproate in singleton gestation. Am J Obstet Gynecol. 2012;207:398.e1–8. doi: 10.1016/j.ajog.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caritis SN, Sharma S, Venkataramanan R, Rouse DJ, Peaceman AM, Sciscione A, et al. Pharmacokinetics of 17-hydroxyprogesterone in multifetal gestation. Am J Obstet Gynecol. 2011;205:40.e1–8. doi: 10.1016/j.ajog.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caritis SN, Venkataramanan R, Thom E, Harper M, Klebanoff MA, Sorokin Y, et al. Relationship between 17α hydroxyprogesterone caproate concentration and spontaneous preterm birth. Am J Obstet Gynecol. 2014;201:126.e1–6. doi: 10.1016/j.ajog.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caritis SN, Simhan H, Zhao Y, Rouse DJ, Peaceman AM, Sciscione A, et al. Relationship between 17-hydroxyprogesterone caproate concentrations and gestational age at delivery in twin gestation. Am J Obstet Gynecol. 2012;207:396.e1–8. doi: 10.1016/j.ajog.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Brien JM. Progestogen safety in multiple gestations: application of the Bradford Hill criteria. BJOG. 2015;122:610–14. doi: 10.1111/1471-0528.13277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow diagram of study identification and selection. 17OHPc, 17-hydroxyprogesterone caproate.
Figure S2. Quality summary: risks of bias for each included study.
Table S1. Overview of clinical trials testing prophylactic progestogens in triplet pregnancy.
Table S2. Rates of composite adverse perinatal outcome in various subgroups.


