Angiotensin II (AngII) is the most important endocrine ligand in the renin angiotensin system (RAS), contributing to the development of several cardiovascular diseases including hypertension 1. AngII mediates its signal transduction and functions via the AngII receptors 2. Historically, the presence of two subtypes of AngII receptors were pharmacologically recognized based on the sensitivity to the first orally-active non-peptide AngII receptor antagonist, losartan. The losartan-sensitive receptor was termed AT1 receptor. It was assumed to be a heterotrimeric G protein-coupled receptor (GPCR) as it generates inositol triphosphate and diacylglycerol leading to intracellular Ca2+ elevation and protein kinase C activation, respectively. Most known physiological and pathophysiological functions of AngII including stimulation of vasoconstriction and salt and water reabsorption are mediated through the AT1 receptor. The losartan-insensitive receptor was termed AT2 receptor, whereas its G protein-coupling remains unclear 1, 3, 4. In 1991, two research groups in the United States independently isolated cDNA (termed AGTR1) encoding the mammalian AT1 receptor 5, 6. Subsequently, rat AT2 receptor cDNA (AGTR2) was cloned in 19937, 8. These pioneer works revealed complete amino acid sequences of the AngII receptor subtypes belonging to the seven-transmembrane GPCR superfamily. In the early nineties, several studies reported that AT1 receptor elicits tyrosine phosphorylation of multiple proteins as well as activation of mitogen-activated protein kinase (p42/p44 MAPK)/extracellular signal regulated kinase (ERK1/2) in various cell types including vascular smooth muscle cells (VSMC). The early nineties also saw the establishment of the concept that AngII via the AT1 receptor has a direct action on cardiac myocytes, fibroblasts and VSMCs causing hypertrophic and fibrotic cardiovascular remodeling 9, 10. The cardiovascular remodeling caused by AngII appeared to be at least partially independent from the hypertensive action of AngII 11. These findings lead to identification of common signaling mechanisms shared by AT1 receptor and a growth factor receptor which has an intrinsic tyrosine kinase activity 12–15. Interestingly, AT1 receptor can be activated by mechanical stretch contributing to cardiac hypertrophy 16, 17. The mechano-sensor concept of the AT1 receptor has been expanded to mediate myogenic vasoconstriction 18–20. Another key discovery from the early nineties is NAD(P)H oxidase-dependent reactive oxygen species (ROS) generation through the AT1 receptor activation in VSMC 21. This finding lead to a major (yet controversial) concept that ROS mediate cardiovascular pathophysiology including those involving the RAS. The finding was also significant as it is an important foundation for the well acknowledged concept established in the late nineties that AngII acts as a pro-inflammatory cytokine via the AT1 receptor 22. The basic understanding remains solid and unchanged that the AT1 receptor signaling contributes to hypertension and various cardiovascular complications via activation of protein kinases, generation of ROS, and subsequent induction of remodeling and inflammation 2, 23. However, there has been astonishing progress elucidating various novel components and pathways in the AngII/AT1 receptor signal transduction for the past two decades. AT1 receptor interacts and signals with G proteins and β-arrestin. In addition, AT1 receptor communicates with growing numbers of AT1 receptor-interacting proteins including other GPCRs (heterodimer formation). AT1 receptor appears to activate several new signaling cascades including the Wnt/β-catenin pathway, Notch pathway and Hippo pathways. Moreover, AT1 receptor mediates additional posttranslational protein modification including acetylation/deacetylation, S-nitrosylation, O-GlcNAcylation and SUMOylation (reviewed recently 2). Crystal structures of the AT1 and AT2 receptors have also been recently demonstrated 24, 25. However, further research is desired regarding the physiological and pathophysiological roles of these new components and signaling pathways. Here, based on the 2017 Lewis K. Dahl Memorial Lecture, we will describe noteworthy recent concepts of the AT1 receptor signal transduction in mediating vascular pathophysiology. We will also discuss controversies, limitations and future directions of the AT1 receptor research.
Transactivation of Growth Factor Receptor via a Disintegrin Metalloprotease 17 (ADAM17)
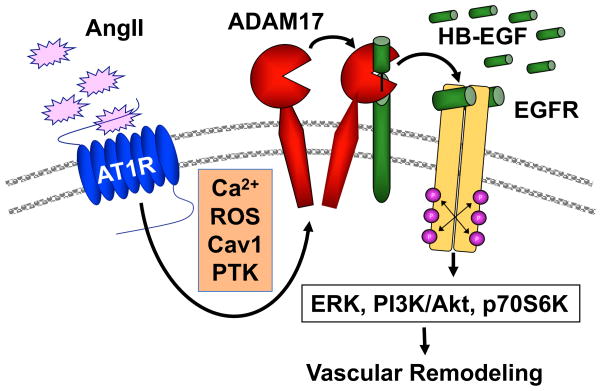
It has been demonstrated that AngII activates ERK1/2 via AT1 receptor-mediated transactivation of epidermal growth factor receptor (EGFR) in VSMC in vitro 26. The EGFR transactivation also mediates activation of other downstream kinases including Akt, p70 S6 kinase and p38 MAPK, and subsequent hypertrophic responses in VSMC 27–30 (Figure 1). Note that there are many other classical as well as novel pathways shown to potentially mediate vascular remodeling in vivo (reviewed in detail in the reference 31). Moreover, while the EGFR transactivation cascade is well acknowledged in VSMCs, whether it has any significance in vascular pathophysiology linked to AngII had not been studied. Recently our group was able to demonstrate the critical roles the cascade play in AngII-induced hypertensive cardiovascular remodeling.
Figure 1.
Signal transduction mechanism of EGFR transactivation by AngII in vascular smooth muscle cells leading to vascular remodeling. PTK; protein tyrosine kinase, PI3K; Phosphoinositide 3-kinase, p70S6K; p70 S6 kinase. Please note that in addition to this cascade both classical and novel pathways have been shown to contribute to AngII-mediated vascular remodeling (reviewed in detail recently in the reference 31).
Upon 2 week AngII infusion in mice, activation of EGFR is mainly observed in coronary arteries in the cardiac section. Erlotinib is a clinically utilized selective EGF receptor kinase inhibitor. Treatment with erlotinib markedly attenuated vascular EGFR activation, vascular medial hypertrophy and perivascular fibrosis induced by AngII infusion, whereas AngII-induced hypertension was unaltered. Interestingly, AngII-induced cardiac hypertrophy was also prevented by the EGFR inhibitor 32. These data suggest that vascular EGFR transactivation mediate cardiovascular remodeling induced by AngII independently from hypertension. In addition, erlotinib prevented development of abdominal aortic aneurysm (AAA) induced by co-treatment of AngII and a lysyl oxidase inhibitor, β-aminopropianitrile 33. Others also demonstrated that in EGFR inactivated mutant mice, AngII-induced cerebral arteriolar hypertrophy but not hypertension was attenuated 34. In smooth muscle-targeted and inducible EGFR silencing mice, vascular hypertrophy and fibrosis induced by AngII infusion were also attenuated and development of hypertension was partially inhibited. However, AngII-induced cardiac hypertrophy was not prevented 35. Taken together, these data suggest that EGFR transactivation is critical for AngII-mediated cardiovascular complications and that distinct cell types including VSMC and cardiac myocytes may be involved in the EGFR-dependent pathophysiology.
In vitro studies have demonstrated that a metalloprotease, ADAM17, mediates AngII-induced EGFR transactivation via generation of mature form of heparin-binding EGF-like growth factor 36, 37. AT1 receptor activates ADAM17 via Tyr702 phosphorylation through unidentified kinase 38. Src family kinase is the potential candidate as it phosphorylates and activates ADAM17 in response to mechanical stretch in rat myoblasts 39. In addition, several Ser/Thr kinases are implicated in ADAM17 activation in other cell systems 40. We have utilized Sm22α-mediated conditional ADAM17 knockout mice to ask what role VSMC ADAM17 plays in hypertension and associated cardiovascular remodeling induced by AngII. Compared with wild type littermate control mice, vascular hypertrophy, perivascular fibrosis and cardiac hypertrophy but not hypertension induced by AngII infusion were blunted in the ADAM17 silenced mice. The phenotype is associated with inhibition of vascular EGFR activation. Systemic ADAM17 inhibition by neutralizing antibody also attenuated AngII-induced cardiovascular remodeling but not hypertension in wild type mice 41. In addition, development of AAA induced by AngII plus β-aminopropianitrile was also blunted in VSMC ADAM17 silenced mice or wild type mice treated with ADAM17 antibody 42. While Sm22α-mediated ADAM17 knockdown could partially reduce cardiac myocyte ADAM17 expression 41, others have reported that AngII-induced cardiac hypertrophy was not altered in cardiomyocyte-targeted ADAM17 silenced mice 43. These data further support the concept that the VSMC ADAM17/EGFR transactivation mainly mediates cardiovascular pathology including cardiac hypertrophy induced by AngII.
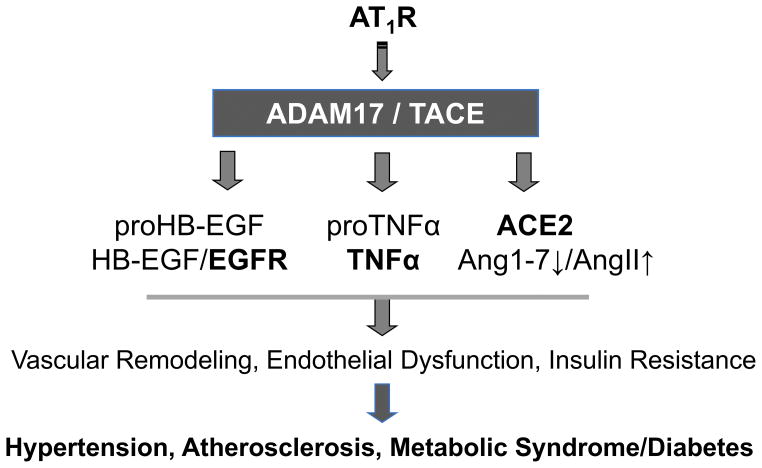
It should be noted that ADAM17 has many other substrates beside EGFR ligands including tissue necrosis factor α (TNFα) 44. In TNFα knockout mice, AngII-induced hypertension and cardiac hypertrophy were blunted 45. Transplant experiment with TNFα knockout mice suggest a partial involvement of TNFα produced in kidney in AngII-induced hypertension 46. Smooth muscle-derived TNFα has been shown to positively contribute to blood pressure responses 47. Another important substrate for ADAM17 is angiotensin converting enzyme 2 (ACE2). ACE2 cleavage by ADAM17 inactivates ACE2 leading to reduced Ang(1–7) generation and enhanced AngII retention. This concept has been shown to be involved in DOCA-salt induced neurogenic hypertension 48. Subsequent study demonstrated neuronal AT1 receptor mediating the ADAM17-dependent ACE2 inactivation 49. Therefore, in addition to EGFR transactivation, it is important to further investigate the potential participation of TNFα generation and ACE2 inactivation as consequences of ADAM17 activation, leading to hypertension, cardiovascular remodeling as well as other types of pathophysiology associated with enhancement of the RAS (Figure 2).
Figure 2.
Potential roles of ADAM17 activation in cardiovascular pathophysiology. In addition to EGFR transactivation, ADAM17 may contribute to endothelial dysfunction and insulin resistance by producing TNFα and inhibiting ACE2.
Involvement of Caveolin 1 in AngII-induced Vascular Remodeling
Caveolae are a specific type of small lipid raft at the plasma membrane and serve as important signal transduction platforms 50. The roles of caveolin 1 (Cav1), a major component protein in caveolae in AT1 receptor signal transduction has been extensively studied 51. However, limited information has been available regarding the role of Cav1-mediated AngII signaling in vascular pathophysiology. It has been shown that in Cav1+/− mice, AngII-induced hypertension and decline in nitric oxide were partially blunted 52. We have recently examined the involvement of Cav1 in AngII-induced vascular remodeling with Cav1 knockout (Cav1−/−) mice. In Cav1−/− mice, AngII infusion causes hypertension and cardiac hypertrophy similar to the control Cav1+/+ mice. However, AngII-induced vascular hypertrophy and perivascular fibrosis are attenuated in Cav1−/− mice. Protection of vascular remodeling seen in Cav1−/− mice may involve two mechanisms according to our in vitro analyses. Cav1 silencing in VSMC attenuated ADAM17 activation, EGFR transactivation, protein synthesis and collagen synthesis induced by AngII. In addition, Cav1 silencing in endothelial cells prevented induction of vascular endothelial cell adhesion molecule and leukocyte adhesion induced by TNFα 53. We also reported that Cav1 knockout mice were protected from AAA formation induced by AngII, which were associated with reduced inflammatory cytokines and oxidative stress 54. However, several problematic baseline phenotypes are also associated with Cav1−/− mice including cardiac hypertrophy and pulmonary hypertension 50. Further experiments such as those with cell type specific knockout mice are needed before considering any intervention toward Cav1 function.
ER Stress and Cardiovascular Remodeling
ER stress is caused by adaptive responses to an excess of misfolded proteins leading to unfolded protein response (UPR). UPR mediates specific signaling pathways which lead to induction of protein chaperones and attenuation of protein synthesis to reduce misfolded proteins. Sustained ER stress also activates c-Jun N-terminal kinase and nuclear factor-kB causing inflammatory responses. Several disease conditions including those occurring in the cardiovascular system are associated with enhancement of ER stress 55. It has been demonstrated that AngII stimulation causes ER stress/UPR in the target organs including vasculature, heart and brain 56–58. CCAAT-enhancer-binding protein homologous protein (CHOP) is a critical transcriptional factor induced by UPR. CHOP−/− mice are protected from AngII-induced hypertension and cardiovascular pathology 59. Our investigation has demonstrated that AngII mediated ER stress responses are attenuated if the Cav1/ADAM17/EGFR pathway is inhibited pharmacologically and or genetically 32, 33, 41, 42. One potential interpretation is that ER stress causes ADAM17 gene induction and enhances EGFR transactivation as a positive feed-back mechanism, where inhibition of either ER stress or the transactivation cascade results in suppression of vascular remodeling induced by AngII 32. Alternatively, suppression of protein synthesis and hypertrophic/fibrotic remodeling reduce the rate of protein misfolding 41. In addition, whether the UPR in response to AngII stimulation is sufficient to attenuate misfolding to maintain protein homeostasis (proteostasis) remains unknown due to a lack of study to directly evaluate protein misfolding. It has been well documented that imbalance among protein folding, UPR and clearance of misfolded proteins by proteasome pathway or autophagy lead to aggregation of specific sets of proteins causing neurodegenerative diseases. Enhancement of protein aggregates were shown in mice hearts infused with AngII as well as aged mouse hearts. Nearly a hundred proteins are identified as commonly enriched aggregated proteins 60. It is interesting to speculate that these proteins cause specific proteotoxicity and “protein aggregate responses” thus enhancing cardiovascular pathophysiology induced by AngII.
Mitochondrial Signaling of AngII
Due to its significant contribution to mitochondrial ROS production, AngII-induced mitochondrial dysfunction has been strongly implicated in cardiovascular diseases, metabolic diseases and aging 61, 62. Indeed, inhibition of mitochondrial ROS can attenuate vascular dysfunction and hypertension induced by AngII 63, 64. Moreover, AngII-infused mice showed cardiac hypertrophy and diastolic dysfunction associated with reduced cardiac ATP production and glucose oxidation, suggesting a role for AngII signal transduction in mitochondrial dysfunction 65. However, mitochondrial targeted treatment such as antioxidant peptide or mitochondrial catalase transgene have no effect on AngII-induced hypertension, whereas these interventions can inhibit cardiac hypertrophy 66, 67. Regarding the molecular mechanism by which AngII increases mitochondrial ROS, the contribution of Nox2-derived cytosolic ROS has been demonstrated 64. In addition, AngII has been shown to inhibit mitochondrial Sirt3 and SOD2 via S-glutathionylation and acetylation, respectively, thus enhancing mitochondrial ROS generation 68. There are a few reports available regarding the relationship between AngII pathophysiology and mitophagy. An E3 ubiquitin ligase autophagy protein 5 (Atg5) mediates formation of autophagosomes and autophagy. AngII increases cardiac Atg5 expression, autophagy and mitophagy in infiltrated macrophages. In Atg5+/− mice, reduction in macrophage mitophagy is associated with enhancement of cardiac hypertrophy and oxidative stress 69. However, in swine model of renovascular hypertension, AT1 receptor blocker attenuated myocardial mitophagy and increased mitochondrial biogenesis 70.
Recent studies also demonstrated that AngII regulates mitochondrial morphology. Mitochondrial fission and fusion are key regulatory mechanisms required for mitochondrial homeostasis as well as quality control under stress. Accumulating evidence suggest the causal relationship between mitochondrial fragmentation/fission and cardiovascular/metabolic diseases. Mitochondrial fission and fusion are regulated by multiple distinct proteins distributed in cytosol, ER and mitochondrial outer and inner membranes, of which GTPases, dynamin-related protein 1 (Drp1) and mitofusion 1/2 are central mediators of fission and fusion, respectively 71. In cultured VSMC and neuronal cell line SH-SY5Y, AngII stimulation caused mitochondrial fission which was associated with Drp1 Ser616 phosphorylation 72, 73. Moreover, pharmacological inhibition of Drp1 by mdivi1 attenuated AngII-induced mitochondrial ROS production and VSMC proliferation 73. However, it should be noted that mdivi1 is known to inhibit mitochondrial respiration at complex I and modulate ROS production 74.
During the lecture, our unpublished data utilizing both pharmacological and genetic manipulations including those obtained with conditional knockout mice were presented. These data support two novel signal transduction concepts regarding the mitochondrial dynamics dictating vascular pathophysiology induced by AngII or TNFα. 1) In VSMCs in vitro and in vivo, AngII activation of AT1 receptor causes mitochondrial fragmentation via the EGFR transactivation. Mitochondrial fission appears to be an essential step for cardiovascular remodeling (but not hypertension) induced by AngII. 2) In endothelial cells in vitro and in vivo, TNFα induces mitochondrial fragmentation via a mechanism distinct from EGFR transactivation. Endothelial mitochondrial fragmentation significantly influences TNFα signal transduction. Moreover, inhibition of mitochondrial fragmentation prevents inflammatory responses induced by TNFα infusion in mice including leukocyte adhesion. Further research is warranted to answer several fundamental questions. Why do vascular pathogens cause mitochondrial fission and what is the consequence to mitochondrial homeostasis and cellular phenotype in cardiovascular diseases? What is the essential “forward grade” signaling mechanism utilized by the receptors that cause vascular mitochondrial fragmentation? Finally, we need to explore the other essential “retro grade” signaling mechanism by which mitochondrial fragmentation mediate vascular remodeling and inflammation.
Cell Type Specific AT1 Receptor Signal Transduction
Although the literature presented here strongly suggests that VSMC (and perhaps partially via endothelial) AT1 receptor signaling mechanisms mediate AngII pathophysiology in the vasculature including hypertension and vascular remodeling, there are noteworthy findings challenging these concepts. We are aware of the accumulating findings suggesting the importance of several distinct immune cell populations in mediating hypertension and endothelial dysfunction in response to AngII 75. However, caution is required when interpreting the findings in this field 76. Many of the strategies utilized manipulate a specific subset of immune cells by removing their presence in mice. As such it is difficult to specify if the outcomes are due to initiation of AngII signal transduction in the immune cell, if the immune cell’s function lay downstream of AT1 receptor signal transduction originally elicited in other cell types, or removing the specific immune cell type is affecting the phenotype independently from the RAS. Deletion of AT1 receptor on bone marrow-derived cells augmented hypertension, renal inflammation and injury in mice 77. Bone marrow AT1 receptor appears dispensable for AngII-induced enhancement of atherosclerosis in apoE−/− mice 78. A few studies are available utilizing immune cell targeted conditional AT1 receptor knockout mice. In T cell AT1 knockout mouse, no alteration was detected in hypertension induced by AngII. Moreover, AngII-induced renal injury was enhanced in the knockout mice 79. Macrophage AT1 receptor deletion also indicate the role of macrophage AT1 receptor in renal protection 80. These data thus challenge the concept that inactivation of the AT1 receptor on inflammatory T cell or macrophage is protective against hypertension and end organ damage. The findings also indicate that while T cells and macrophages enhance AngII causing hypertension and end-organ damage, these actions are independent from immune cell RAS and likely regulated through the peripheral AT1 receptor. However, additional investigation is needed to explore the protective AT1 receptor signal transduction in the immune cells.
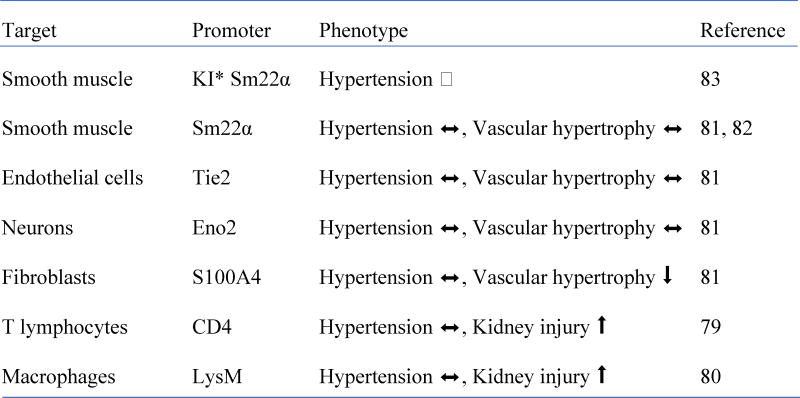
Conditional AT1 receptor knockout mice have also been utilized to study the requirement of AT1 receptor in VSMC, endothelial cell and fibroblast to mediate hypertension and vascular remodeling (Table 1). Sm22α-Cre deletion of VSMC AT1, Tie2-Cre deletion of endothelial (and hematopoietic) AT1, or Eno2-Cre deletion of neuronal AT1 did not alter hypertension or vascular medial hypertrophy induced by AngII infusion. In contrast, S100A4 Cre deletion of fibroblast AT1 attenuated vascular hypertrophy but not hypertension induced by AngII 81. However, there is a concern in the interpretation of these data. While these findings confirm no alteration of hypertension by “transgenic” Sm22α-Cre deletion of VSMC AT1 in AngII-induced hypertension 82, more effective silencing of AT1 receptor using Cre that is regulated by endogenous Sm22α (“knock-in”) shows significant reduction in hypertension induced by AngII infusion 83. However, whether AngII-induced vascular remodeling is attenuated in the mice remains to be studied. Expression of S100A4 in VSMC has been demonstrated 84. Our mass spectrometry analysis of cultured rat VSMC lysates detected protein fragments derived from S100A4 (unpublished observation), thus Cre under control of S100A4 promoter may delete smooth muscle AT1 receptors in addition to those on fibroblasts. In relation to these issues (insufficiency and non-specific targeting), a critical limitation common in these studies are lack of confirmation of AT1 receptor “protein” silencing in the target cells/tissues. This is because reliable AT1 receptor antibody has not yet been available 85, 86. Therefore, further effort is desired to specify AT1 receptor-expressing cell types involved in AngII-induced cardiovascular pathophysiology.
Table 1.
Phenotype of conditional AT1 receptor knockout mice infused with AngII
KI (Knock-in)
Perspectives
Here, we summarized the noteworthy novel concepts and progresses in AT1 receptor signal transduction in mediating cardiovascular pathophysiology. The AT1 receptor signal transduction appears to remain a central component in cardiovascular pathophysiology. To conquer cardiovascular complications and improve the prognoses of hypertensive patients, we have to further clarify the complexity of the AT1 signal transduction. Better molecular tools should be developed, and additional effort is required in order to answer cell/tissue type specific roles that AT1 receptor plays in cardiovascular and metabolic diseases. This seems particularly important in cardiac myocytes, fibroblasts, adipocytes and immune cell subsets. Organelle signal communication such as those involving ER, mitochondria and exosomes 87 as well as balance among protein synthesis, misfolding, aggregation and the “proteo”-toxicity are important questions to ask for their relevance in AngII pathophysiology. We also expect that unbiased system biology and bioinformatics approaches will further shed light on previously unrecognized AT1 receptor signal transduction for the next decade. Finally, we strongly hope that this article helps the researcher to further explore novel molecular mechanisms that RAS plays in cardiovascular diseases and that these studies will lead to a remarkable translation into effective therapies.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health grants HL128324 (S.E. and V.R.), HL133248 (S.E.), DK111042 (R.S. and S.E.), and American Heart Association grants 16GRNT30410007 (S.E.), 16GRNT30130013 (V.R.), 16POST3051004 (T.K.).
Footnotes
Disclosures
None
References
- 1.Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, Thomas WG International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli [corrected] Pharmacol Rev. 2015;67:754–819. doi: 10.1124/pr.114.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawai T, Forrester SJ, O’Brien S, Baggett A, Rizzo V, Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol Res. 2017;125:4–13. doi: 10.1016/j.phrs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bumpus FM, Catt KJ, Chiu AT, DeGasparo M, Goodfriend T, Husain A, Peach MJ, Taylor DG, Jr, Timmermans PB. Nomenclature for angiotensin receptors. A report of the Nomenclature Committee of the Council for High Blood Pressure Research. Hypertension. 1991;17:720–1. doi: 10.1161/01.hyp.17.5.720. [DOI] [PubMed] [Google Scholar]
- 4.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–72. [PubMed] [Google Scholar]
- 5.Sasaki K, Yamano Y, Bardhan S, Iwai N, Murray JJ, Hasegawa M, Matsuda Y, Inagami T. Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature. 1991;351:230–3. doi: 10.1038/351230a0. [DOI] [PubMed] [Google Scholar]
- 6.Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351:233–6. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- 7.Mukoyama M, Nakajima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem. 1993;268:24539–42. [PubMed] [Google Scholar]
- 8.Kambayashi Y, Bardhan S, Takahashi K, Tsuzuki S, Inui H, Hamakubo T, Inagami T. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem. 1993;268:24543–6. [PubMed] [Google Scholar]
- 9.Sadoshima J, Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–23. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest. 1992;90:456–61. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morishita R, Gibbons GH, Ellison KE, Lee W, Zhang L, Yu H, Kaneda Y, Ogihara T, Dzau VJ. Evidence for direct local effect of angiotensin in vascular hypertrophy. In vivo gene transfer of angiotensin converting enzyme. J Clin Invest. 1994;94:978–84. doi: 10.1172/JCI117464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schieffer B, Paxton WG, Marrero MB, Bernstein KE. Importance of tyrosine phosphorylation in angiotensin II type 1 receptor signaling. Hypertension. 1996;27:476–80. doi: 10.1161/01.hyp.27.3.476. [DOI] [PubMed] [Google Scholar]
- 13.Griendling KK, Ushio-Fukai M, Lassegue B, Alexander RW. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension. 1997;29:366–73. doi: 10.1161/01.hyp.29.1.366. [DOI] [PubMed] [Google Scholar]
- 14.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–72. [PubMed] [Google Scholar]
- 15.Eguchi S, Frank GD, Mifune M, Inagami T. Metalloprotease-dependent ErbB ligand shedding in mediating EGFR transactivation and vascular remodelling. Biochem Soc Trans. 2003;31:1198–202. doi: 10.1042/bst0311198. [DOI] [PubMed] [Google Scholar]
- 16.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–84. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 17.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 18.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schleifenbaum J, Kassmann M, Szijarto IA, Hercule HC, Tano JY, Weinert S, Heidenreich M, Pathan AR, Anistan YM, Alenina N, Rusch NJ, Bader M, Jentsch TJ, Gollasch M. Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries. Circ Res. 2014;115:263–72. doi: 10.1161/CIRCRESAHA.115.302882. [DOI] [PubMed] [Google Scholar]
- 20.Blodow S, Schneider H, Storch U, Wizemann R, Forst AL, Gudermann T, Mederos y Schnitzler M. Novel role of mechanosensitive AT1B receptors in myogenic vasoconstriction. Pflugers Arch. 2014;466:1343–53. doi: 10.1007/s00424-013-1372-3. [DOI] [PubMed] [Google Scholar]
- 21.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 22.Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257–66. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- 23.Vukelic S, Griendling KK. Angiotensin II, from vasoconstrictor to growth factor: a paradigm shift. Circ Res. 2014;114:754–7. doi: 10.1161/CIRCRESAHA.114.303045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Unal H, Gati C, Han GW, Liu W, Zatsepin NA, James D, Wang D, Nelson G, Weierstall U, Sawaya MR, Xu Q, Messerschmidt M, Williams GJ, Boutet S, Yefanov OM, White TA, Wang C, Ishchenko A, Tirupula KC, Desnoyer R, Coe J, Conrad CE, Fromme P, Stevens RC, Katritch V, Karnik SS, Cherezov V. Structure of the Angiotensin receptor revealed by serial femtosecond crystallography. Cell. 2015;161:833–44. doi: 10.1016/j.cell.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Han GW, Batyuk A, Ishchenko A, White KL, Patel N, Sadybekov A, Zamlynny B, Rudd MT, Hollenstein K, Tolstikova A, White TA, Hunter MS, Weierstall U, Liu W, Babaoglu K, Moore EL, Katz RD, Shipman JM, Garcia-Calvo M, Sharma S, Sheth P, Soisson SM, Stevens RC, Katritch V, Cherezov V. Structural basis for selectivity and diversity in angiotensin II receptors. Nature. 2017;544:327–332. doi: 10.1038/nature22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273:8890–6. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 27.Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, Marumo F, Hirata Y, Inagami T. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J Biol Chem. 1999;274:36843–51. doi: 10.1074/jbc.274.52.36843. [DOI] [PubMed] [Google Scholar]
- 28.Eguchi S, Dempsey PJ, Frank GD, Motley ED, Inagami T. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J Biol Chem. 2001;276:7957–62. doi: 10.1074/jbc.M008570200. [DOI] [PubMed] [Google Scholar]
- 29.Eguchi S, Iwasaki H, Hirata Y, Frank GD, Motley ED, Yamakawa T, Numaguchi K, Inagami T. Epidermal growth factor receptor is indispensable for c-Fos expression and protein synthesis by angiotensin II. Eur J Pharmacol. 1999;376:203–6. doi: 10.1016/s0014-2999(99)00357-x. [DOI] [PubMed] [Google Scholar]
- 30.Forrester SJ, Kawai T, O’Brien S, Thomas W, Harris RC, Eguchi S. Epidermal Growth Factor Receptor Transactivation: Mechanisms, Pathophysiology, and Potential Therapies in the Cardiovascular System. Annu Rev Pharmacol Toxicol. 2016;56:627–53. doi: 10.1146/annurev-pharmtox-070115-095427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiological Review. 2018 doi: 10.1152/physrev.00038.2017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takayanagi T, Kawai T, Forrester SJ, Obama T, Tsuji T, Fukuda Y, Elliott KJ, Tilley DG, Davisson RL, Park JY, Eguchi S. Role of epidermal growth factor receptor and endoplasmic reticulum stress in vascular remodeling induced by angiotensin II. Hypertension. 2015;65:1349–55. doi: 10.1161/HYPERTENSIONAHA.115.05344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obama T, Tsuji T, Kobayashi T, Fukuda Y, Takayanagi T, Taro Y, Kawai T, Forrester SJ, Elliott KJ, Choi E, Daugherty A, Rizzo V, Eguchi S. Epidermal growth factor receptor inhibitor protects against abdominal aortic aneurysm in a mouse model. Clin Sci (Lond) 2015;128:559–65. doi: 10.1042/CS20140696. [DOI] [PubMed] [Google Scholar]
- 34.Chan SL, Umesalma S, Baumbach GL. Epidermal growth factor receptor is critical for angiotensin II-mediated hypertrophy in cerebral arterioles. Hypertension. 2015;65:806–12. doi: 10.1161/HYPERTENSIONAHA.114.04794. [DOI] [PubMed] [Google Scholar]
- 35.Schreier B, Hunerberg M, Mildenberger S, Rabe S, Bethmann D, Wickenhauser C, Gekle M. Deletion of the EGF receptor in vascular smooth muscle cells prevents chronic angiotensin II-induced arterial wall stiffening and media thickening. Acta Physiol (Oxf) 2018 doi: 10.1111/apha.12996. [DOI] [PubMed] [Google Scholar]
- 36.Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, Frank GD, Inagami T, Higashiyama S, Thomas WG, Eckhart AD, Dempsey PJ, Eguchi S. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J Biol Chem. 2005;280:26592–9. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- 37.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133–7. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 38.Elliott KJ, Bourne AM, Takayanagi T, Takaguri A, Kobayashi T, Eguchi K, Eguchi S. ADAM17 silencing by adenovirus encoding miRNA-embedded siRNA revealed essential signal transduction by angiotensin II in vascular smooth muscle cells. J Mol Cell Cardiol. 2013;62:1–7. doi: 10.1016/j.yjmcc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niu A, Wen Y, Liu H, Zhan M, Jin B, Li YP. Src mediates the mechanical activation of myogenesis by activating TNFalpha-converting enzyme. J Cell Sci. 2013;126:4349–57. doi: 10.1242/jcs.125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45:146–69. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayanagi T, Forrester SJ, Kawai T, Obama T, Tsuji T, Elliott KJ, Nuti E, Rossello A, Kwok HF, Scalia R, Rizzo V, Eguchi S. Vascular ADAM17 as a Novel Therapeutic Target in Mediating Cardiovascular Hypertrophy and Perivascular Fibrosis Induced by Angiotensin II. Hypertension. 2016;68:949–955. doi: 10.1161/HYPERTENSIONAHA.116.07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawai T, Takayanagi T, Forrester SJ, Preston KJ, Obama T, Tsuji T, Kobayashi T, Boyer MJ, Cooper HA, Kwok HF, Hashimoto T, Scalia R, Rizzo V, Eguchi S. Vascular ADAM17 (a Disintegrin and Metalloproteinase Domain 17) Is Required for Angiotensin II/beta-Aminopropionitrile-Induced Abdominal Aortic Aneurysm. Hypertension. 2017;70:959–963. doi: 10.1161/HYPERTENSIONAHA.117.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan D, Takawale A, Shen M, Samokhvalov V, Basu R, Patel V, Wang X, Fernandez-Patron C, Seubert JM, Oudit GY, Kassiri Z. A Disintegrin and Metalloprotease-17 Regulates Pressure Overload-Induced Myocardial Hypertrophy and Dysfunction Through Proteolytic Processing of Integrin beta1. Hypertension. 2016;68:937–48. doi: 10.1161/HYPERTENSIONAHA.116.07566. [DOI] [PubMed] [Google Scholar]
- 44.Reiss K, Saftig P. The “a disintegrin and metalloprotease” (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol. 2009;20:126–37. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–51. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD. Tumor necrosis factor-alpha produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension. 2014;64:1275–81. doi: 10.1161/HYPERTENSIONAHA.114.03863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroetsch JT, Levy AS, Zhang H, Aschar-Sobbi R, Lidington D, Offermanns S, Nedospasov SA, Backx PH, Heximer SP, Bolz SS. Constitutive smooth muscle tumour necrosis factor regulates microvascular myogenic responsiveness and systemic blood pressure. Nat Commun. 2017;8:14805. doi: 10.1038/ncomms14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia H, Sriramula S, Chhabra KH, Lazartigues E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res. 2013;113:1087–1096. doi: 10.1161/CIRCRESAHA.113.301811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O, Poglitsch M, Lazartigues E. Clinical Relevance and Role of Neuronal AT1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ Res. 2017;121:43–55. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chidlow JH, Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86:219–25. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ushio-Fukai M, Alexander RW. Caveolin-dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension. 2006;48:797–803. doi: 10.1161/01.HYP.0000242907.70697.5d. [DOI] [PubMed] [Google Scholar]
- 52.Lobysheva I, Rath G, Sekkali B, Bouzin C, Feron O, Gallez B, Dessy C, Balligand JL. Moderate caveolin-1 downregulation prevents NADPH oxidase-dependent endothelial nitric oxide synthase uncoupling by angiotensin II in endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:2098–105. doi: 10.1161/ATVBAHA.111.230623. [DOI] [PubMed] [Google Scholar]
- 53.Forrester SJ, Elliott KJ, Kawai T, Obama T, Boyer MJ, Preston KJ, Yan Z, Eguchi S, Rizzo V. Caveolin-1 Deletion Prevents Hypertensive Vascular Remodeling Induced by Angiotensin II. Hypertension. 2017;69:79–86. doi: 10.1161/HYPERTENSIONAHA.116.08278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takayanagi T, Crawford KJ, Kobayashi T, Obama T, Tsuji T, Elliott KJ, Hashimoto T, Rizzo V, Eguchi S. Caveolin 1 is critical for abdominal aortic aneurysm formation induced by angiotensin II and inhibition of lysyl oxidase. Clin Sci (Lond) 2014;126:785–94. doi: 10.1042/CS20130660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–28. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest. 2012;122:3960–4. doi: 10.1172/JCI64583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kassan M, Galan M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol. 2012;32:1652–61. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spitler KM, Webb RC. Endoplasmic reticulum stress contributes to aortic stiffening via proapoptotic and fibrotic signaling mechanisms. Hypertension. 2014;63:e40–5. doi: 10.1161/HYPERTENSIONAHA.113.02558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kassan M, Ait-Aissa K, Radwan E, Mali V, Haddox S, Gabani M, Zhang W, Belmadani S, Irani K, Trebak M, Matrougui K. Essential Role of Smooth Muscle STIM1 in Hypertension and Cardiovascular Dysfunction. Arterioscler Thromb Vasc Biol. 2016;36:1900–9. doi: 10.1161/ATVBAHA.116.307869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayyadevara S, Mercanti F, Wang X, Mackintosh SG, Tackett AJ, Prayaga SV, Romeo F, Shmookler Reis RJ, Mehta JL. Age- and Hypertension-Associated Protein Aggregates in Mouse Heart Have Similar Proteomic Profiles. Hypertension. 2016;67:1006–13. doi: 10.1161/HYPERTENSIONAHA.115.06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res. 2011;89:31–40. doi: 10.1093/cvr/cvq285. [DOI] [PubMed] [Google Scholar]
- 62.Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal. 2013;19:1085–94. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of Thioredoxin 2. Hypertension. 2009;54:338–44. doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal. 2014;20:281–94. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mori J, Basu R, McLean BA, Das SK, Zhang L, Patel VB, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Fail. 2012;5:493–503. doi: 10.1161/CIRCHEARTFAILURE.112.966705. [DOI] [PubMed] [Google Scholar]
- 66.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–46. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dikalova AE, Itani HA, Nazarewicz RR, McMaster WG, Flynn CR, Uzhachenko R, Fessel JP, Gamboa JL, Harrison DG, Dikalov SI. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ Res. 2017;121:564–574. doi: 10.1161/CIRCRESAHA.117.310933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao W, Li Y, Jia L, Pan L, Li H, Du J. Atg5 deficiency-mediated mitophagy aggravates cardiac inflammation and injury in response to angiotensin II. Free Radic Biol Med. 2014;69:108–15. doi: 10.1016/j.freeradbiomed.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Zhang X, Li ZL, Crane JA, Jordan KL, Pawar AS, Textor SC, Lerman A, Lerman LO. Valsartan regulates myocardial autophagy and mitochondrial turnover in experimental hypertension. Hypertension. 2014;64:87–93. doi: 10.1161/HYPERTENSIONAHA.113.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee H, Yoon Y. Mitochondrial fission: regulation and ER connection. Mol Cells. 2014;37:89–94. doi: 10.14348/molcells.2014.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by protein kinase C{delta} under oxidative stress conditions in vivo. Mol Biol Cell. 2011;22:256–65. doi: 10.1091/mbc.E10-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim S, Lee SY, Seo HH, Ham O, Lee C, Park JH, Lee J, Seung M, Yun I, Han SM, Lee S, Choi E, Hwang KC. Regulation of mitochondrial morphology by positive feedback interaction between PKCdelta and Drp1 in vascular smooth muscle cell. J Cell Biochem. 2015;116:648–60. doi: 10.1002/jcb.25016. [DOI] [PubMed] [Google Scholar]
- 74.Bordt EA, Clerc P, Roelofs BA, Saladino AJ, Tretter L, Adam-Vizi V, Cherok E, Khalil A, Yadava N, Ge SX, Francis TC, Kennedy NW, Picton LK, Kumar T, Uppuluri S, Miller AM, Itoh K, Karbowski M, Sesaki H, Hill RB, Polster BM. The Putative Drp1 Inhibitor mdivi-1 Is a Reversible Mitochondrial Complex I Inhibitor that Modulates Reactive Oxygen Species. Dev Cell. 2017;40:583–594. e6. doi: 10.1016/j.devcel.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–33. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rudemiller NP, Crowley SD. Interactions Between the Immune and the Renin-Angiotensin Systems in Hypertension. Hypertension. 2016;68:289–96. doi: 10.1161/HYPERTENSIONAHA.116.06591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension. 2010;55:99–108. doi: 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koga J, Egashira K, Matoba T, Kubo M, Ihara Y, Iwai M, Horiuchi M, Sunagawa K. Essential role of angiotensin II type 1a receptors in the host vascular wall, but not the bone marrow, in the pathogenesis of angiotensin II-induced atherosclerosis. Hypertens Res. 2008;31:1791–800. doi: 10.1291/hypres.31.1791. [DOI] [PubMed] [Google Scholar]
- 79.Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, Sparks MA, Yan M, Howell DN, Gomez JA, Spurney RF, Coffman TM, Crowley SD. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res. 2012;110:1604–17. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang JD, Patel MB, Griffiths R, Dolber PC, Ruiz P, Sparks MA, Stegbauer J, Jin H, Gomez JA, Buckley AF, Lefler WS, Chen D, Crowley SD. Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Invest. 2014;124:2198–203. doi: 10.1172/JCI61368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poduri A, Rateri DL, Howatt DA, Balakrishnan A, Moorleghen JJ, Cassis LA, Daugherty A. Fibroblast Angiotensin II Type 1a Receptors Contribute to Angiotensin II-Induced Medial Hyperplasia in the Ascending Aorta. Arterioscler Thromb Vasc Biol. 2015;35:1995–2002. doi: 10.1161/ATVBAHA.115.305995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sparks MA, Parsons KK, Stegbauer J, Gurley SB, Vivekanandan-Giri A, Fortner CN, Snouwaert J, Raasch EW, Griffiths RC, Haystead TA, Le TH, Pennathur S, Koller B, Coffman TM. Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension. 2011;57:577–85. doi: 10.1161/HYPERTENSIONAHA.110.165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sparks MA, Stegbauer J, Chen D, Gomez JA, Griffiths RC, Azad HA, Herrera M, Gurley SB, Coffman TM. Vascular Type 1A Angiotensin II Receptors Control BP by Regulating Renal Blood Flow and Urinary Sodium Excretion. J Am Soc Nephrol. 2015;26:2953–62. doi: 10.1681/ASN.2014080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choe N, Kwon DH, Shin S, Kim YS, Kim YK, Kim J, Ahn Y, Eom GH, Kook H. The microRNA miR-124 inhibits vascular smooth muscle cell proliferation by targeting S100 calcium-binding protein A4 (S100A4) FEBS Lett. 2017;591:1041–1052. doi: 10.1002/1873-3468.12606. [DOI] [PubMed] [Google Scholar]
- 85.Elliott KJ, Kimura K, Eguchi S. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type-1 receptor protein. Hypertension. 2013;61:e31. doi: 10.1161/HYPERTENSIONAHA.111.00943. [DOI] [PubMed] [Google Scholar]
- 86.Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension. 2013;61:253–8. doi: 10.1161/HYPERTENSIONAHA.112.203679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eguchi S, Rizzo V. Organelles in health and diseases. Clin Sci (Lond) 2017;131:1–2. doi: 10.1042/CS20160610. [DOI] [PubMed] [Google Scholar]