Abstract
All of the common neurodegenerative disorders–Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS) and prion diseases (PrD)–are characterized by accumulation of misfolded proteins that trigger activation of microglia; brain-resident mononuclear phagocytes. This chronic form of neuroinflammation is earmarked by increased release of myriad cytokines and chemokines in patient brains and biofluids. Microglial phagocytosis is compromised early in the disease process, blocking clearance of abnormal proteins. This review identifies immune pathologies shared by the major neurodegenerative disorders. The overarching concept is that these aberrant innate immune pathways can be targeted for return to homeostasis in hopes of coaxing microglia into clearing neurotoxic misfolded proteins.
Keywords: Neurodegenerative diseases, proteinopathy, microglia, phagocytosis, neuroinflammation, innate immunity
1. Introduction
The most common neurodegenerative disorders, Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS) and prion disease (PrD) are triggered by accumulation of abnormally folded proteins that self-assemble into β-sheet structures and aggregate in the central nervous system (CNS). Interestingly, whether owed to sporadic, genetic or infectious etiologies, these abnormal proteins share “prion-like” characteristics, including their propensity for spreading (Vincent et al. 2008; Lee and Kim 2015; Hock and Polymenidou 2016; Goedert et al. 2016; Braak and Del Tredici 2016). Another key feature of these diseases is chronic neuroinflammation accompanied by neuronal injury and loss, leading to cognitive and/or motor deficits. While once regarded as epiphenomenon, there is now good evidence suggesting that inflammation and the immune response play fundamental roles in neurodegenerative disorders (López González et al. 2016). In this review, we will consider immune and inflammatory mechanisms implicated in AD, PD, ALS and PrD. Rather than exhaustively cataloguing the features specific to each disease, we broadly define pathologies of each neurodegenerative disorder in Table 1. In this review, we discuss the immunopathological similarities shared by these diseases in hopes of identifying future therapeutic target(s).
Table 1.
Summary of proteinopathies associated with the most common neurodegenerative diseases
| Pathology | Nature of abnormal protein aggregate | CNS regions most affected | Exosomal transport |
|---|---|---|---|
| Alzheimer’s Disease (AD) | Amyloid-β (Aβ) hyper phosphorylated Tau |
Hippocampus Cortex Basal forebrain Brain stem |
YES |
| Amyotrophic Lateral Sclerosis (ALS) | Superoxide dismutase 1 (SOD1) TAR DNA-binding protein 43 (TDP43) Dipeptide repeat protein (DPR) |
Motor Cortex Spinal motor neurons |
YES |
| Parkinson’s Disease (PD) | α-synuclein | Substancia Nigra Cortex Locus Coeruleus Raphe |
YES |
| Prion diseases (PrD) | Prion protein scrapie (PrPsc) | Cortex Thalamus Brain stem Cerebellum |
YES |
2. Pathogenic protein accumulation and spreading: contribution of exosomes
Exosomes are lipid bilayer endosome-derived nanovesicles mediating intercellular communication. In the CNS, exosomes encapsulate proteins, RNAs and miRNAs and are released by neurons, astrocytes, oligodendrocytes and microglia (the brain resident mononuclear phagocytes) (Bobrie et al. 2011; Hannafon and Ding 2013), where they are thought to mediate neuron-glia communication insuring neuronal development and health (Antonucci et al. 2012; Frühbeis et al. 2013a; Frühbeis et al. 2013b; Fröhlich et al. 2014). Internalization of exosomes is triggered by the interaction of various proteins and lipids with receptors on the target cell to initiate endocytosis (Morelli et al. 2004). Phagocytic cells uptake exosomes via phagocytosis or micropinocytosis (Feng et al. 2010; Fitzner et al. 2011), while non-phagocytic cells rely on clathrin-dependent and -independent endocytosis (Escrevente et al. 2011; Svensson et al. 2013).
Exosomes have been isolated from the human brain and cerebrospinal fluid (CSF) under both physiological and pathological conditions (Vella et al. 2008; Street et al. 2012). In neurodegenerative diseases, the presence of exosomes packed with abnormal or misfolded proteins suggests that the exosomal machinery is responsible for cell-to-cell “spreading” (Aguzzi and Rajendran 2009; Rajendran et al. 2014). Interestingly, exosomal spreading of misfolded proteins was first described for propagation of prions in cellular bioassays (Fevrier et al. 2004; Vella et al. 2007; Coleman et al. 2012) and in prion-infected mouse brains (Arnold et al. 1995; Alais et al. 2008). Importantly, those early discoveries showed that PrPSc containing exosomes were infectious in vitro and in vivo (Fevrier et al. 2004; Vella et al. 2007; Alais et al. 2008); spurring the study of exosomes in other neurodegenerative diseases.
In the context of AD, exosomal transmission has been described for Aβ trafficking in mice brains (Kokubo et al. 2005), and other reports suggest that exosomes may facilitate uptake and degradation of Aβ by microglia (Bulloj et al. 2010; Tamboli et al. 2010; Yuyama et al. 2012). Additionally, exosome-mediated trafficking of Aβ and phosphorylated tau has been observed in human brains and CSF samples (Rajendran et al. 2006; Sharples et al. 2008; Saman et al. 2012). Cell-to-cell tau propagation occurs in vitro (Frost et al. 2009) and in vivo (Polanco et al. 2016), and both inhibition of exosome biosynthesis and depletion of microglia halts tau spreading in a mouse model of tauopathy (Asai et al. 2015). One could conclude from these studies that microglia phagocytose exosomal tau secreted from neurons and subsequently release tau-containing exosomes, further spreading tau pathology.
Exosomes containing monomeric and oligomeric α-synuclein have been found at increased abundance in PD patient plasma and CSF (Emmanouilidou et al. 2010; Yang et al. 2015; Stuendl et al. 2016; Luo et al. 2016). Additionally, exosome-mediated secretion of α-synuclein and transmission from cell-to-cell has been demonstrated in vitro (Alvarez-Erviti et al. 2011b; Danzer et al. 2012) and in vivo (Kong et al. 2014; Tsunemi et al. 2014). These enigmatic structures also seem to accelerate α-synuclein aggregation (Grey et al. 2015; Stuendl et al. 2016), and may contribute to inclusion formation and neuronal cell death (Desplats et al. 2009).
Misfolded superoxide dismutase 1 (SOD1) and TAR DNA-binding protein 43 (TDP43) associated with ALS have also been found within exosomes (Gomes et al. 2007), and mutant SOD1 aggregates can propagate between cells via this mechanism (Münch et al. 2011; Münch and Bertolotti 2011). This suggests a role for exosomes in the intercellular spreading of abnormal proteins in ALS (Nonaka et al. 2013; Grad et al. 2014). Similarly, exosomal spreading of dipeptide repeat proteins (DPRs), characteristic of ALS and frontotemporal dementia (FTD), has been demonstrated in vitro (Ding et al. 2015; Westergard et al. 2016).
However, the source of exosomes carrying abnormal proteins is still unclear, as both neurons and reactive microglia reportedly release exosomes (Tamboli et al. 2010; Yuyama et al. 2012). Moreover, exosomes shed from peripheral mononuclear phagocytes in the circulation have the ability to cross the blood-brain barrier (BBB) and exert their effects in the CNS (Alvarez-Erviti et al. 2011b; Couch et al. 2011). This latter finding highlights the importance of understanding the influence of peripheral mechanisms on neurodegenerative diseases.
3. Exosomes as immune modulators
Aside from their role in spreading pathogenic proteins, exosomes are involved in modulating immune and inflammatory processes within the CNS. Indeed, various cell types involved in brain inflammation communicate by shedding exosomes that help to initiate and propagate inflammatory responses. In another disease paradigm–cancer, exosomes have a role in antigen cross-presentation by transferring antigens from tumor cells to dendritic cells, where they present antigen to T cells (Zitvogel et al. 1998; Wolfers et al. 2001) and exhibit other immune properties (Iero et al. 2008; Théry et al. 2009).
In AD brains, reports indicate increased levels of heat shock protein 72 (HSP72) (Hondius et al. 2016), which induces inflammation through exosomal release (Anand et al. 2010; Heppner et al. 2015). In the context of PD, astrocytes and microglia internalize cytotoxic α-synuclein, further promoting inflammation via release of exosomes containing inflammatory mediators in association with production of reactive oxygen species including nitric oxide free radical (Lee et al. 2008; Lee et al. 2010; Alvarez-Erviti et al. 2011a; Vekrellis et al. 2011). Another report indicates that α-synuclein induces production of major histocompatibility class II and tumor necrosis factor (TNF)-containing exosomes by microglia; triggering neuronal death and reinforcing the vicious cycle of neuroinflammation in PD (Chang et al. 2013).
Exosomes are highly enriched in mRNAs and small RNAs such as piwi-interacting RNA (piRNA), miRNA and tRNA transcripts (Valadi et al. 2007; Bellingham et al. 2012; Cheng et al. 2014a; Cheng et al. 2014b). Release of exosomes into biological fluids allows for small RNA transcripts to be taken up by target cells that modulate gene expression (Alvarez-Erviti et al. 2011c). Animal studies show that specific miRNAs can activate microglia associated with prion lesions (Saba et al. 2008; Montag et al. 2009; Saba et al. 2012). Furthermore, miRNA profiles are altered in exosomes isolated from CSF, blood and brains from AD (Cogswell et al. 2008; Kumar et al. 2013; Grasso et al. 2014; Gui et al. 2015), PD (Junn et al. 2009; Maciotta et al. 2013; Gui et al. 2015), and ALS patients (Gui et al. 2015). In AD patients’ CSF and brains, modulation of miRNA clusters correlates with change in genes involved in amyloid precursor protein (APP) processing (Hébert et al. 2008) and synaptic plasticity (Sarkar et al. 2016). Moreover, aberrantly expressed messenger and long non-coding RNAs discovered in CSF exosomes may be pathogenic in the PD context (Gui et al. 2015). For example, miRNAs have been shown to mediate both oxidative stress and injury to dopaminergic neurons associated with abnormal α-synuclein (Junn et al. 2009; Cho et al. 2013). It is interesting to note that in ALS, expression of several miRNAs involved in the immune response is elevated in the diseased spinal cord (Zhou et al. 2013), and miRNA-mediated Toll-like receptor (TLR) activation has been documented in both AD (Lehmann et al. 2012) and PD (He et al. 2014). This set of findings is given even more weight as TLR activation is strongly involved in immune responses during the course of neurodegenerative diseases (see section 4 below) (Letiembre et al. 2009; Zhao et al. 2010; Béraud et al. 2011; Lehmann et al. 2012; Noelker et al. 2013).
4. Receptor-mediated innate immune activation
Glial activation, including reactive microgliosis, is a common trait in neurodegenerative diseases, although the particular neuroinflammatory phenotype varies with the type of CNS pathology (Heppner et al. 2001; Sargsyan et al. 2005; Long-Smith et al. 2009; Prokop et al. 2013). Microglial activation in neurodegenerative disorders is sometimes accompanied by reactive astrocytes, lymphocytes and macrophages infiltrating from the periphery (Graves et al. 2004; Gate et al. 2010; Lewis et al. 2012). Activation of mononuclear phagocytes (microglia and hematogenous macrophages) and subsequent release of inflammatory mediators is often mediated by receptors; in the context of neurodegenerative diseases, the TLR family is particularly involved. Polymorphisms reducing the activity of TLR4 occur more frequently in AD patients than in healthy controls (Minoretti et al. 2006; Wang et al. 2011). Expression of TLR2, TLR4 and its co-receptor (CD14) are increased in AD patient brains (Fassbender et al. 2004; Liu et al. 2005; Walter et al. 2007; Letiembre et al. 2009). Remarkably, plaque-associated microglia have increased mRNA expression for TLR2 and TLR4 in a mouse model of cerebral amyloidosis (Frank et al. 2009). One study suggests that a physical interaction is required amongst CD14, TLR2 and TLR4 for stimulation of microglial responses by fibrillar Aβ (Reed-Geaghan et al. 2009). Other animal studies support a beneficial role for TLR2/4 activation in AD pathophysiology through amyloid-β (Aβ) phagocytosis (Tahara et al. 2006; Glass et al. 2010; Michaud et al. 2013), via blocking acute Aβ oligomer-mediated glial activation and memory impairment in APP/PS1 mice (Balducci et al. 2017). Stimulation of TLR4 was also reported to be beneficial by attenuating tauopathy in human tau transgenic mice (Qin et al. 2016). However, a contradictory report showed that deletion of the downstream TLR mediator, interleukin-1 receptor-associated kinase (IRAK) 4, increased Aβ clearance while reducing gliosis (Cameron et al. 2012), and a second set of findings showed that inhibition of TLR2 activation in APP/PS1 mice resulted in reduced gliosis and Aβ burden associated with learning improvement (McDonald et al. 2016). One possible explanation for this discordancy is use of different rodent models that develop different diseases with varying kinetics.
Interestingly, TLR4 is elevated in brains of patients suffering from α-synucleopathies (Letiembre et al. 2009) and in PD mouse models, where its ablation impairs phagocytic uptake of α-synuclein and enhances neurodegeneration (Cookson 2009; Stefanova et al. 2011; Fellner et al. 2013). In addition, the TLR4 pathway has been shown to regulate PARK2 transcription in mononuclear phagocytes (Tran et al. 2011) and a polymorphism in CD14 is a risk factor for late-onset PD (Wahner et al. 2007; Deleidi and Gasser 2013). These data begin to suggest a beneficial role for TLR4 in PD by mediating microglial clearance of α-synuclein. TLR2 expression is increased in neurons and in microglia residing in disease-relevant PD brain areas and in transgenic Thy1.2-α-synuclein mouse brains (Doorn et al. 2014; Drouin-Ouellet et al. 2014; Dzamko et al. 2016). In addition, misfolded α-synuclein has been shown to increase expression of TLR2 and other TLRs and to activate microglia in culture (Béraud et al. 2011) and in mouse brains expressing human α-synuclein (Béraud et al. 2011; Kim et al. 2013). Misfolded α-synuclein seems to dually activate TLR1/2 signaling (Daniele et al. 2015), and recent data support the notion that neuron-secreted α-synuclein induces activation of TLR2 and subsequent inflammatory responses in microglia, causing neurodegeneration (Kim et al. 2016; Dzamko et al. 2016).
In similar fashion, TLR2 and TLR4 expression is increased in reactive microglia in the ALS spinal cord (Casula et al. 2011), and mutant SOD1 activates microglia via TLR2/4 and the CD14 co-receptor, potentiating neurotoxicty (Liu et al. 2009; Zhao et al. 2010). Strikingly, TLR4 antagonists rescued ALS mouse-derived neurons in culture (De Paola et al. 2016), while TLR4 deletion increased survival in ALS transgenic mice (Lee et al. 2015), suggesting that aberrantly elevated microglial TLR4 signaling contributes to ALS pathology.
With regard to prionopathies, dominant-negative TLR4 mice present with accelerated infection after PrPsc inoculation (Spinner et al. 2008), while TLR2 deficiency switches prion-induced microglial activation from neurotoxic to neuroprotective (Wang et al. 2015a). On the other hand, TLR1/2 expression is responsive to recombinant prion fibrils in mixed neuronal/glial cultures, and glial activation inhibits prion replication (Kang et al. 2016). Altogether, these studies highlight the role of TLR-dependent innate immune activation and its interference with PrP infection.
Immune receptors other than TLRs have been found to incur risk for neurodegenerative disorders (Doty et al. 2014). Triggering receptor expressed on myeloid cells 2 (TREM2) took the field by storm after genome-wide association studies (GWAS) identified rare variants as risk factors for AD (Guerreiro et al. 2013; Jonsson et al. 2013; Lill et al. 2015), PD (Rayaprolu et al. 2013; Lill et al. 2015), and ALS (Cady et al. 2014; Lill et al. 2015). The TREM2-neurodegenerative diseases risk relationship has been confirmed by other groups in the cases of PD (Feng et al. 2014; Mengel et al. 2016) and ALS (Lattante et al. 2013; Chen et al. 2015). TREM2 expression is also increased in spinal cord samples from ALS patients and SOD1G93A mice (Cady et al. 2014). It is interesting to note that prion infection elevates TREM2 in microglia; however, TREM2 deficiency does not modulate microglia phenotype (Zhu et al. 2015), and no association between TREM2 variants and prion-CJD has been found (Slattery et al. 2014). Convergent reports have shown that TREM2 is elevated by plaque-associated mononuclear phagocytes in brains of AD model mice (Frank et al. 2008; Melchior et al. 2010; Jay et al. 2015). However, the impact of TREM2 on cerebral amyloidosis is still under debate, with some studies reporting reduced Aβ pathology in 4 month-old TREM2 deficient APP/PS1 mice (Jay et al. 2015), while TREM2 deficiency in 5XFAD mice results in increased Aβ accumulation at 8 months of age but no effect at an earlier age (Wang et al. 2015b; Rivest 2015; Wang et al. 2016b). Just recently, a study reconciled these reports, showing that TREM2 deficiency ameliorates Aβ deposition early but exacerbates it at a later age through reduction of inflammation-related gene expression and decrease of Aβ uptake by mononuclear phagocytes in APP/PS1-21 mice (Jay et al. 2016).
In addition to TLR and TREM2, GWAS studies have identified CD33 as another immune receptor conferring risk for AD (Hollingworth et al. 2011; Naj et al. 2011). CD33 expression is elevated in AD patients’ brains (Malik et al. 2013; Walker et al. 2015; Li et al. 2015b), where it is thought to modulate microglial activation and inhibit Aβ clearance (Griciuc et al. 2013; Bradshaw et al. 2013). An interesting study recently showed that the CD33 risk allele (rs3865444C), which is associated with increased CD33 expression, is linked to increased TREM2 surface expression on mononuclear phagocytes. This is intriguing, given that TREM2 mRNA expression is associated with increased Aβ load (Chan et al. 2015). Strong evidence exists for an association between PD risk and increased CD33 expression, and increased CD33 expression on peripheral monocytes is associated with greater disease burden (Chan et al. 2016). Taken together, it seems that innate immunity–once thought to be completely dispensable for evolution of neurodegenerative disorders–is actually at the epicenter of these syndromes.
Microglia also express various receptors for neurotransmitters, including glutamate, GABA, norepinephrine, cannabinoid, and acetylcholine receptors that mediate neuroprotective or neurotoxic effects depending on the particular receptor system (for review see Liu et al. 2016). Certain types of cannabinoid receptors are increased in postmortem tissue from ALS patients and in rat models of PD (Concannon et al. 2015), and overexpression of cannabinoid receptors has a neuroprotective effect in the 6-hydroxydopamine model of PD (Ternianov et al. 2012). Others have shown that cannabinoid agonists protect against nigrostriatal cell loss in the MPTP mouse model of PD (Price et al. 2009) and inhibit Aβ-induced microglial activation in vitro and Aβ toxicity in vivo (Ramirez et al. 2005). Interestingly, depletion of adrenergic signaling decreases microglial Aβ phagocytic capacity (Heneka et al. 2010), and indirect activation of microglial glutamate receptors by Aβ has been described (Taylor et al. 2002; Taylor et al. 2003). These studies underline the potential role of neurotransmitters as microglial modulators in neurodegenerative diseases.
5. Gliosis, glial dysfunction and the complement pathway
Direct activation of microglia by pathogenic proteins has been reported for fibrillary PrP (Peyrin et al. 1999; Veerhuis et al. 2002; Sisková et al. 2009; Zhu et al. 2015), Aβ (Yu and Ye 2015; Tu et al. 2015), tau (Chen et al. 2016), α-synuclein (Zhang et al. 2005; Béraud et al. 2011; Fellner et al. 2013) and SOD1 (Roberts et al. 2013; Kinsella et al. 2016). In a terminal mouse model of PrPsc infection, activation of astrocytes and microglia is observed in brain areas showing vacuolization and pathological accumulation of PrP. Activated microglia also present with elevated endocytic and lysosomal activity, accompanied by recruitment of limited numbers of peripheral macrophages (Williams et al. 1994a; Williams et al. 1994b) and CD4+ T cells (Betmouni et al. 1996; Togo et al. 2002; Brochard et al. 2009). Initial microglial activation coincides with changes in neuronal morphology early during the disease process (Williams et al. 1997; Jeffrey et al. 2000)–before neuronal loss and clinical signs (Williams et al. 1997; Giese et al. 1998; Betmouni and Perry 1999). This suggests that injured neurons further exacerbate local glial activation.
With regards to other neurodegenerative diseases, molecular imaging studies have revealed widespread microglial activation in the AD brain (Edison et al. 2008), and clinico-pathological studies support a strong correlation between microglial abundance and disease severity (McGeer et al. 1987; Bornemann et al. 2001; Bolmont et al. 2008; Okello et al. 2009; Arends et al. 2000; Vehmas et al. 2003; Cagnin et al. 2006), suggesting that reactive microglia play a salient role in AD pathogenesis. In terms of PD, patients present with chronic neuroinflammation characterized by focal activation of microglia and astrocytes, and infiltration of lymphocytes (McGeer et al. 1988; Mirza et al. 2000; Imamura et al. 2003; Orr et al. 2005; Brochard et al. 2009; Hirsch and Hunot 2009). It is noteworthy that microglia predominantly segregate near degenerated dopaminergic neurons in PD patient brains (Banati et al. 1998). In ALS, deletion of mutant SOD1 in astrocytes and microglia slows progression of the disease in an ALS mouse model without affecting disease onset (Boillée et al. 2006; Yamanaka et al. 2008), suggesting that reactive glia modify neuronal toxicity induced by mutant SOD1 (Nagai et al. 2007).
A new type of glial cell, namely the aberrant astrocyte-like cell (AbA), has been reported in rodent models of ALS (Trias et al. 2017). AbAs seem to originate from phagocytic microglia and express both astrocytic markers (i.e., GFAP, S100β and Cx43) and microglial markers (such as Iba1 and CD163), but do not express glutamate transporter. Interestingly, their emergence in the degenerating spinal cord is coincident with ALS disease onset in rodent models of the disease (Trias et al. 2017). A few studies have reported peculiar astrocytes populations in ALS patients suggesting that AbAs or a related type of reactive glial cell may also arise in human disease, but this remains to be proven (Trias et al. 2017). There is undoubtedly interest in exploring the role(s) of AbAs and other aberrant glial cell phenotypes in the context of neurodegenerative disease.
Mounting evidence now indicates that microglia become dysfunctional in the ageing brain and during exposure to build-up of misfolded proteins. Recent studies have identified AD risk polymorphisms in a variety of innate immune genes, not only including TREM2 (Guerreiro et al. 2013; Jonsson et al. 2013; Benitez et al. 2014) and CD33 (Griciuc et al. 2013; Bradshaw et al. 2013), but also CLU (which encodes clusterin), PICALM (encoding phosphatidylinositol binding clathrin assembly protein) (Harold et al. 2009) and HLA-DRB5–DRB1 (human leukocyte antigen – antigen D related) (Lambert et al. 2013). A common phenotype linking TREM2 and other innate immune gene risk alleles (e.g., CR1, CD33, MS4A4, MS4A6A, CD2AP, EPHA1) is modulation of microglial phagocytosis. Interestingly, variants of these genes are also risk factors for other degenerative diseases–i.e. TREM2 for ALS (Cady et al. 2014), and HLA-DRB5–DRB1 for PD (International Parkinson Disease Genomics Consortium et al. 2011).
In the context of neurodegenerative diseases, microglia lose their ability to phagocytose Aβ (Hickman et al. 2008; Mawuenyega et al. 2010), α-synuclein (Bliederhaeuser et al. 2016) and infectious prion proteins (Sisková et al. 2009). Interestingly, hyperphosphorylated tau has recently been shown to promote microglial degeneration (Sanchez-Mejias et al. 2016), and also to activate microglia towards increased phagocytosis of insoluble Aβ (Chen et al. 2016). In the mutant SOD1 mouse model of ALS, changes have been reported in microglial molecular signature(s) including loss of P2ry12, Tmem119, Olfml3, transcription factors Egr1, Atf3, Jun, Fos, and Mafb, and the upstream regulators Csf1r, Tgfb1, and Tgfbr1 (Butovsky et al. 2015). This altered microglial phenotype seems to be accompanied by compromised phagocytosis (Butovsky et al. 2015).
Recent reports have shed light on the role of the complement pathway as a regulator of glial phagocytosis in neurodegenerative diseases. The complement cascade is strongly activated in brains of AD patients and rodent models of the disease (Bradt et al., 1998; Tacnet-Delorme et al., 2001; Fan and Tenner, 2004; Sim et al. 2007; Loeffler et al., 2008), and complement receptor 1 has been identified as a genetic risk factor for AD (Lambert et al., 2009; Crehan et al., 2012). Complement cascade components C1q, C3 or C3 receptor regulate microglial synaptic pruning in AD (Hong et al. 2016). Others have shown that astrocyte-derived C3 interacts with microglial C3R to mediate Aβ pathology and neuroinflammation in APP/PS1 model mice (Lian et al. 2016). Additionally, clearance of Aβ from the peripheral circulation is complement-mediated (Brubaker et al. 2017; Crane et al. 2017). Complement pathway activation has also been reported in PD (Yamada et al. 1992; McGeer et al. 2004; Finehout et al. 2005; Wang et al. 2011), and C1q has been implicated in neuromelanin clearance in the PD context (Depboylu et al. 2011); yet, it deserves noting that the contribution of C1q to cognitive impairment is still under debate (Carbutt et al. 2016).
Complement activation has also been found in plaques in PrD patients’ brains (Ishii et al. 1984; Nawrocka-Kunecka et al. 2005; Kovacs et al. 2004) and in brain tissue from scrapie-infected rodents (Lv et al. 2015). Additionally, several reports indicate that soluble prion oligomers activate the complement cascade (Dumestre-Perard et al. 2007; Sim et al. 2007), and complement activation occurs early on in the course of prion pathogenesis in rodent models (Klein et al. 2001; Zabel et al. 2007; Michel et al. 2012; Michel et al. 2013). Similarly, high levels of various complement proteins were found in ALS patient CSF and motor endplates (Annunziata et al. 1985; Tsuboi et al. 1994; Bahia El Idrissi et al. 2016) and in rodent models of the disease (Lobsiger et al. 2007, Heurich et al. 2011). While debatable (Lobsiger at al. 2013), it has been proposed that aberrant complement activation in ALS impacts recruitment of macrophages and leads to motor neuron injury (Woodruff et al. 2008, Wang et al. 2017, Lee et al. 2017).
6. On the role of anti-inflammatory mediators
In concert with microglia, activated astrocytes can produce various inflammatory and neurotoxic factors including cytokines and free radicals; triggering a vicious cycle of inflammation that likely exacerbates neurodegeneration (Johnston et al. 2011). Myriad cytokines and chemokines have been detected in brains and CSF of patients suffering from neurodegenerative disorders–leading to the general acceptance that chronic neuroinflammation is a driving force for disease progression (Heneka et al. 2015, Andreasson et al. 2016). Pro-inflammatory mediators have been the center of attention, and their role(s) in neurodegenerative diseases have been extensively reviewed elsewhere (Heneka et al. 2015; Hooten et al. 2015; Alam et al. 2016; López González et al. 2016). However, anti-inflammatory cytokines and other immune suppressive molecules can also be detrimental in neurodegenerative diseases (Weitz and Town 2012; Colangelo et al. 2014; Doty et al. 2014). In this section, we will focus on anti-inflammatory responses that have long been overlooked as culprits in neurodegenerative disorders and are only just recently gaining attention from the scientific community. Two primary examples are the cardinal anti-inflammatory immunosuppressive cytokines, interleukin-10 (IL-10) (Lobo-Silva et al. 2016) and transforming growth factor β (TGF-β) (Zheng et al. 2016), which are elevated in a number of neurodegenerative disorders (see Table 2).
Table 2.
Roles of anti-inflammatory mediators in neurodegenerative disorders
| IL-10 pathway | Experimental Effects | |||
|---|---|---|---|---|
| Human | Rodent | Overexpression | Blockade/deletion | |
| AD |
|
|
|
|
| ALS |
|
|
|
|
| PD |
|
Not Reported |
|
Not Reported |
| PrD |
|
|
Not Reported | Not Reported |
| TGF-β pathway | Experimental Effects | |||
| Human | Rodent | Overexpression | Blockade/deletion | |
| AD |
|
|
|
|
| ALS |
|
|
|
|
| PD |
|
|
|
|
| PrD | Not Reported |
|
|
Not Reported |
Interestingly, a functional polymorphism within the human IL10 gene has been associated with risk for late-onset AD in some (but not all) populations (Lio et al. 2003; Depboylu et al. 2003; Scassellati et al. 2004; Arosio et al. 2004; Ma et al. 2005; Ramos et al. 2006; Vural et al. 2009). Strikingly, all elements of the IL-10 signaling pathway are abnormally elevated in AD patients’ sera and brains and in rodent models of the disease (Angelopoulos et al. 2008; Loewenbrueck et al. 2010; Gezen-Ak et al. 2013; Asselineau et al. 2015; Guillot-Sestier et al. 2015a; Guillot-Sestier et al. 2015b), and recent GWAS/integrative genomics studies validate these findings (Zhang et al. 2013; Li et al. 2015a). Aged microglia have elevated expression of IL-10/STAT3 pathway genes (Sierra et al. 2007; Henry et al. 2009; Kingery et al. 2013), and IL-10 abundance is increased in plasma and in reactive glia surrounding amyloid-β plaques in aged mouse models of AD (Apelt and Schliebs 2001; Guillot-Sestier et al. 2015a). We and others studied the effects of the IL-10 pathway on cerebral amyloidosis using animal models. On one hand, Il10 genetic ablation in APP/PS1 mice activates mononuclear phagocytes to clear cerebral amyloid, without coming at the cost of bystander neuronal injury (Guillot-Sestier et al. 2015a). Indeed, synaptic integrity and cognitive deficits are rescued in APP/PS1/IL-10−/− mice compared to APP/PS1/IL-10+/+ controls (Guillot-Sestier et al. 2015a). In complementary fashion, cerebral Il10 overexpression using adeno-associated virus in TgCRND8 and Tg2576 mice exacerbates Aβ plaque number and size and decreases synaptic protein abundance; worsening cognitive impairment (Chakrabarty et al. 2015).
Several studies have explored the risk relationship between neurodegenerative diseases and the IL-10 pathway. Similar to the case of human AD, polymorphisms within IL10 that affect levels of cytokine production correlates with PD in some but not all populations (Bialecka et al. 2007; Infante et al. 2008; Bialecka et al. 2008; Pascale et al. 2011; Iyer and Cheng 2012; Li et al. 2012; Nie et al. 2013; Jin et al. 2014). Interestingly, the G1082A polymorphism in the IL10 promoter that correlates with high IL-10 production is related to the age of PD onset (Håkansson et al. 2005), and IL-10 is elevated in PD patient sera (Brodacki et al. 2008; Rentzos et al. 2009). Remarkably, infusion of IL-10 into the substancia nigra of rats protects against LPS-induced cell death of dopaminergic neurons (Arimoto et al. 2007). In a similar manner, administration of cDNA coding for human IL10 into the striatum of PD animal models seems to play a protective role on the nigrostriatal dopaminergic system in (6-OHDA) rats (Johnston et al. 2008) and MPTP-treated mice (Joniec-Maciejak et al. 2014). In ALS patients’ spinal cords, IL-10RA expression is elevated, and IL-10 immunoreactivity is associated with TDP-43 inclusions in motor neurons (Berjaoui et al. 2015). Further, in a mouse model of ALS, glial cells overexpress IL-10 in presymptomatic disease (Gravel et al. 2016). In this model, blocking Il10 increases inflammation and exacerbates, while overexpression of Il10 delays disease onset (Gravel et al. 2016). However, it is not known if the protective role of IL-10 in PD/ALS animal models is durable, and whether it may repress beneficial microglial responses at later disease stages. Interestingly, CNS and CSF IL-10 expression is also increased in another neurodegenerative disorder: Creutzfeldt-Jakob disease (Stoeck et al. 2005).
Second only to IL-10, TGF-β is the other major anti-inflammatory, immunosuppressive cytokine in the body (Li et al. 2008). Release of TGF-β by microglia, astrocytes and oligodendroglia is elevated in response to CNS injury (Constam et al. 1992; Finch et al. 1993), and TGF-β1 levels are increased in AD patients’ CSF and serum (Chao et al. 1994b; Chao et al. 1994a). TGF-βR1 immunoreactivity is associated with amyloid-β plaques (van der Wal et al. 1993), and is elevated in AD patient reactive microglia vs. controls (Lippa et al. 1998). In TgCRND8 transgenic mouse model of AD, brain TGF-β1 is elevated, where it amplifies Aβ-induced neurodegeneration (Salins et al. 2008). This suggests that the AD brain may try to over-compensate for Aβ insult by inappropriately producing high levels of TGF-β1 (Wyss-Coray et al. 1997; Wyss-Coray and Mucke 2002), thereby inducing non-productive low-level neuroinflammation that impairs amyloid-β clearance. Moreover, this “cloud” of brain TGF-β1 acts as an inhibitory signal to keep peripheral innate cells with Aβ clearance aptitude out of the CNS. In support of this, our group has previously demonstrated that genetic blockade of peripheral TGF-β-Smad 2/3 signaling in the Tg2576 transgenic mouse model of cerebral amyloidosis promotes mononuclear phagocyte homing to the CNS and resolution of Aβ deposits (Town et al. 2008; Town 2009; Rezai-Zadeh et al. 2009; Town 2010; Gate et al. 2010). Interestingly, when overexpressing TGF-β1 under the GFAP promoter in hAPP transgenic mice, others reported promotion of microgliosis and repartitioning of Aβ deposits from brain parenchyma to cerebral vessels in transgenic mice overexpressing hAPPV717F (Wyss-Coray et al. 1997; Wyss-Coray et al. 2000; Wyss-Coray et al. 2001). Interestingly, TGF-β1 stimulated cultured microglial cells to clear synthetic Aβ, suggesting that the in vivo effect of astrocyte TGF-β1 overexpression might be mediated by activated microglia (Wyss-Coray et al. 2001). In another study, reduced neuronal TGF-β signaling in hAPP mice increased amyloid-β load and neurodegeneration in vivo (Tesseur et al. 2006), likely due to neuronal cell-autonomous effects of TGF-β removal. These apparent discordancies highlight the exquisite cell-dependent contextual effects of TGF-β, and that TGF-β-driven responses often follow a U-curve pattern.
In the context of PD, TGF-β1 levels are elevated in patient striatum and CSF samples (Mogi et al. 1995; Vawter et al. 1996; Nagatsu et al. 2000), and mice bilaterally infused into the Substantia Nigra with α-synuclein have increased TGF-β striatal mRNA levels (Sznejder-Pacholek et al. 2016). Importantly, TGF-β1 is known to be neuroprotective and neurotrophic for dopaminergic neurons (Krieglstein et al. 1995; Unsicker et al. 1996; Knöferle et al. 2010). In Parkinsonian rats, TGF-β1-releasing extra-adrenal chromaffin cell grafts have shown functional benefit in terms of regeneration (Espejo et al. 2001; Fernandez-Espejo et al. 2005; Galan-Rodriguez et al. 2008), and in the same rodent model, TGF-β1 potentiates GDNF-mediated dopamine trophic effects (Gonzalez-Aparicio et al. 2010). Strikingly, inhibition of TGF-β signaling worsened Parkinsonism in mice given MPTP, while increasing TGF-β signaling reduced Parkinson disease-related pathologies and motor deficits (Tesseur et al. 2017). Similarly, TGF-β1 is increased in plasma and CSF of ALS patients and positively correlates with disease stage and duration (Houi et al. 2002; Iłzecka et al. 2002; Iwasaki et al. 2003). In addition, phosphorylation of the proximal TGF-β downstream effector, Smad2/3, is increased in neuronal and glial nuclei of sporadic and familial ALS patients and in mutant human SOD1 transgenics (Nakamura et al. 2008). In vitro activation of TGF-β/Smad 2/3 signaling reduces aggregate formation of cytoplasmic mis-localized TDP-43 (Nakamura et al. 2013), and bioinformatics has identified modulation of TGF-β signaling pathway as a contributor to motor neuron degeneration in ALS (Phatnani et al. 2013). Increased expression of miR-155 in mSOD1 mice–mimicking increased miR-155 found in the spinal cords of both familial and sporadic ALS–is accompanied by loss of TGF-β1 and TGF-βR1 expression amongst changes in other innate immune molecules. At least in this context then, phagocytic capacity is suppressed. Conversely, genetic ablation of miR-155 in mutant human SOD1 transgenic mice restores abnormal microglial phenotype and endorses beneficial functions (Butovsky et al. 2015). Other reports show that astrocyte-derived TGF-β1 disrupts the neuroprotective function of microglia and accelerates ALS progression in SOD1 mice (Endo et al. 2015). Furthermore, pharmacological inhibition of TGF-β signaling extends survival of SOD1 transgenics (Endo et al. 2015), indicating that overabundance of astrocytic TGF-β is detrimental in the ALS context (Endo and Yamanaka 2015). Interestingly, TGF-β1 mRNA and protein levels were enhanced in the ME7 murine model of prion disease (Betmouni et al. 1999), suggesting TGF-β1 pathway activation in the context of prionopathy (Cunningham et al. 2002). More specifically, this early report suggests that TGF-β1 controls microglial phenotype in the context of prion diseases. Indeed, a more recent ME7 mouse gene screening strategy placed TGF-β as a key factor promoting the microglial pro-neurogenic response during chronic neurodegeneration (De Lucia et al. 2016).
Targeting production of cytokines themselves could also be of therapeutic value. Endogenous over-production of anti-inflammatory cytokines (e.g., IL-10 and TGF-β) is likely reflective of an attempt to protect neurons from inflammatory bystander damage. But timing is everything. During early stages of neurodegenerative disease, this response is likely beneficial. However, as the disease becomes chronic, immunosuppression proves to be maladaptive. Mounting evidence shows that chronic dysregulation of immune homeostasis is deleterious. For example, we and others showed that inhibiting IL-10/STAT3 signaling dramatically mitigates Alzheimer-like pathology (Guillot-Sestier et al. 2015a), while brain overexpression of Il10 produces complementary effects (Chakrabarty et al. 2015; Michaud and Rivest 2015). Blockade of TGF-β-Smad 2/3 signaling in peripheral macrophages causes brain infiltration of these cells and mitigation of cerebral β-amyloidosis (Town et al. 2008). Likewise, elevation of TGF-β is deleterious in ALS (Endo et al. 2015; Endo and Yamanaka 2015), and others have shown that microglia can be rebalanced to ameliorate pathology in a transgenic ALS mouse model (Gravel et al. 2016; Wang et al. 2016a). Interestingly, reduced inflammation in aged APP/PS1-TREM2 deficient mice is associated with reduced amyloid-β clearance (Jay et al. 2016).
7. Conclusions
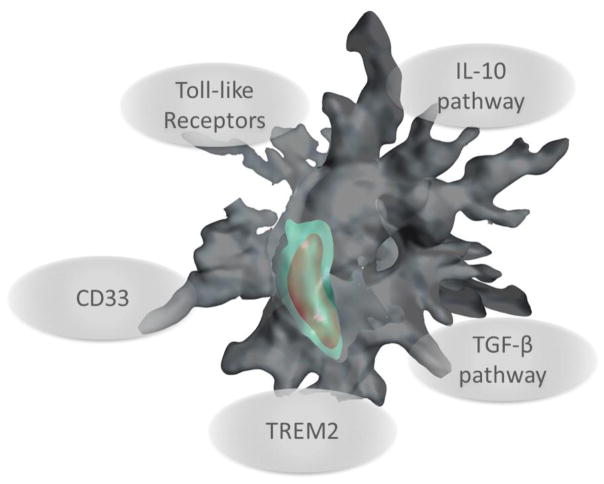
It is now becoming clear that broad-based anti-inflammatory therapeutics (i.e., non-steroidal anti-inflammatory drugs) will ultimately not be the best way forward as an AD treatment (Szekely and Zandi 2010; Miguel-Álvarez et al. 2015). These negative findings have raised awareness to the ironic notion that blocking immunosuppressive pathways may actually be beneficial as a therapeutic approach for neurodegenerative diseases that share common denominators, such as neurotoxic misfolded proteins. Rewiring the cerebral innate immune response by inhibiting actions of key anti-inflammatory cytokines could allow the brain to return to a physiological state, and may therefore represent a therapeutic approach (Figure 1).
Figure 1.
Targeting innate immune receptors and anti-inflammatory pathways to promote innate immunity against neurodegenerative diseases. The cartoon depicts a mononuclear phagocyte (gray) engulfing abnormal proteins (red) into a phagolysosome (green).
The literature detailed in this review demonstrates that neuroinflammation is not always damaging; in principle, innate immunity could actually be harnessed to treat neurodegenerative diseases. Specifically targeting the beneficial responses and dampening detrimental neuroinflammation has become the critical question in neurodegenerative disease research. Another important question is when to therapeutically intervene–at early or late clinical stages. With respect to modeling microglial phenotypes in the dish, it is particularly important to keep in mind that microglia obtained from rodent neonates have a macrophage-like profile that may not represent human adult or senescent microglial physiology. Finally, specifically targeting recruitment of blood-borne monocytes to promote phagocytosis of misfolded proteins is an area that is garnering intense interest (Wisniewski et al. 1991; Gate et al. 2010; Depboylu et al. 2012; Weitz and Town 2016; Prinz and Priller 2017). Embedded within this last question is the need to better understand the relative contribution of CNS vs peripheral mononuclear phagocytes to neurodegenerative diseases.
With an eye toward future research, an emerging area is the contribution of the gut microbiome to neurodegenerative diseases. While it’s early days yet, a broader understanding of how gut commensals contribute to evolution of AD and PD is becoming increasingly important (for review, see Ghaisas et al 2016; Sherwin et al. 2017). Indeed, through the so-called gut-brain axis, the gastrointestinal tract communicates with the CNS to support or destabilize neuronal health. Defining the role(s) of the innate immune system in gut-brain communication may lead to new strategies to alter gut microbiota populations in order to manage neurodegenerative diseases.
Acknowledgments
We thank Drs. Tara M. Weitz and Kevin R. Doty for helpful discussion. M-V.G-S. was supported by a BrightFocus Foundation Alzheimer’s Disease Research Fellowship Award (A2015309F) and an Alzheimer’s Association, California Southland Chapter Young Investigator Award. This work was supported by the National Institute on Neurologic Disorders and Stroke (1R01NS076794-01, to T.T.), an Alzheimer’s Association Zenith Fellows Award (ZEN-10-174633, to T.T.), and an American Federation of Aging Research/Ellison Medical Foundation Julie Martin Mid-Career Award in Aging Research (M11472, to T.T.). We are grateful for startup funds from the Zilkha Neurogenetic Institute, which helped to make this work possible.
References
- Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Alais S, Simoes S, Baas D, et al. Mouse neuroblastoma cells release prion infectivity associated with exosomal vesicles. Biol Cell. 2008;100:603–615. doi: 10.1042/BC20080025. [DOI] [PubMed] [Google Scholar]
- Alam Q, Alam MZ, Mushtaq G, et al. Inflammatory Process in Alzheimer“s and Parkinson”s Diseases: Central Role of Cytokines. Current pharmaceutical design. 2016;22:541–548. doi: 10.2174/1381612822666151125000300. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Couch Y, Richardson J, et al. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011a;69:337–342. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiology of disease. 2011b;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011c;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Anand PK, Anand E, Bleck CKE, et al. Exosomal Hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PLoS ONE. 2010;5:e10136. doi: 10.1371/journal.pone.0010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson KI, Bachstetter AD, Colonna M, et al. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J Neurochem. 2016 Sep;138(5):653–93. doi: 10.1111/jnc.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelopoulos P, Agouridaki H, Vaiopoulos H, et al. Cytokines in Alzheimer’s disease and vascular dementia. Int J Neurosci. 2008;118:1659–1672. doi: 10.1080/00207450701392068. [DOI] [PubMed] [Google Scholar]
- Annunziata P, Volpi N. High levels of C3c in the cerebrospinal fluid from amyotrophic lateral sclerosis patients. Acta Neurol Scand. 1985;72(1):61–4. doi: 10.1111/j.1600-0404.1985.tb01548.x. [DOI] [PubMed] [Google Scholar]
- Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31:1231–1240. doi: 10.1038/emboj.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelt J, Schliebs R. Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain research. 2001;894:21–30. doi: 10.1016/S0006-8993(00)03176-0. [DOI] [PubMed] [Google Scholar]
- Arends YM, Duyckaerts C, Rozemuller JM, et al. Microglia, amyloid and dementia in alzheimer disease. A correlative study. Neurobiology. 2000;21:39–47. doi: 10.1016/S0197-4580(00)00094-4. [DOI] [PubMed] [Google Scholar]
- Arimoto T, Choi D-Y, Lu X, et al. Interleukin-10 protects against inflammation-mediated degeneration of dopaminergic neurons in substantia nigra. Neurobiology. 2007;28:894–906. doi: 10.1016/j.neurobiolaging.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Arnold JE, Tipler C, Laszlo L, et al. The abnormal isoform of the prion protein accumulates in late-endosome-like organelles in scrapie-infected mouse brain. J Pathol. 1995;176:403–411. doi: 10.1002/path.1711760412. [DOI] [PubMed] [Google Scholar]
- Arosio B, Trabattoni D, Galimberti L, et al. Interleukin-10 and interleukin-6 gene polymorphisms as risk factors for Alzheimer’s disease. Neurobiology. 2004;25:1009–1015. doi: 10.1016/j.neurobiolaging.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Asai H, Ikezu S, Tsunoda S, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nature neuroscience. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselineau D, Benlhassan K, Arosio B, et al. Interleukin-10 Production in Response to Amyloid-β Differs between Slow and Fast Decliners in Patients with Alzheimer’s Disease. Journal of Alzheimer’s disease: JAD. 2015;46:837–842. doi: 10.3233/JAD-142832. [DOI] [PubMed] [Google Scholar]
- Bahia El Idrissi N, Bosch S, Ramaglia V, et al. Complement activation at the motor end-plates in amyotrophic lateral sclerosis. J Neuroinflammation. 2016;13(1):72. doi: 10.1186/s12974-016-0538-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci C, Frasca A, Zotti M, et al. Toll-like receptor 4-dependent glial cell activation mediates the impairment in memory establishment induced by β-amyloid oligomers in an acute mouse model of Alzheimer’s disease. Brain Behav Immun. 2017;60:188–197. doi: 10.1016/j.bbi.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson’s disease. Mov Disord. 1998;13:221–227. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012;40:10937–10949. doi: 10.1093/nar/gks832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez BA, Jin SC, Guerreiro R, et al. Missense variant in TREML2 protects against Alzheimer’s disease. Neurobiology. 2014;35:1510e19–26. doi: 10.1016/j.neurobiolaging.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berjaoui S, Povedano M, Garcia-Esparcia P, et al. Complex Inflammation mRNA-Related Response in ALS Is Region Dependent. Neural Plast. 2015;2015:573784. doi: 10.1155/2015/573784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betmouni S, Perry VH. The acute inflammatory response in CNS following injection of prion brain homogenate or normal brain homogenate. Neuropathology and applied neurobiology. 1999;25:20–28. doi: 10.1046/j.1365-2990.1999.00153.x. [DOI] [PubMed] [Google Scholar]
- Betmouni S, Perry VH, Gordon JL. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience. 1996;74:1–5. doi: 10.1016/0306-4522(96)00212-6. [DOI] [PubMed] [Google Scholar]
- Béraud D, Maguire-Zeiss KA. Misfolded α-synuclein and Toll-like receptors: therapeutic targets for Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S17–20. doi: 10.1016/S1353-8020(11)70008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béraud D, Twomey M, Bloom B, et al. α-Synuclein Alters Toll-Like Receptor Expression. Front Neurosci. 2011;5:80. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecka M, Klodowska-Duda G, Kurzawski M, et al. Interleukin-10 (IL10) and tumor necrosis factor alpha (TNF) gene polymorphisms in Parkinson’s disease patients. Parkinsonism Relat Disord. 2008;14:636–640. doi: 10.1016/j.parkreldis.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Bialecka M, Klodowska-Duda G, Kurzawski M, et al. Interleukin-10 gene polymorphism in Parkinson’s disease patients. Arch Med Res. 2007;38:858–863. doi: 10.1016/j.arcmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Bliederhaeuser C, Grozdanov V, Speidel A, et al. Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta neuropathologica. 2016;131:379–391. doi: 10.1007/s00401-015-1504-2. [DOI] [PubMed] [Google Scholar]
- Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- Boillée S, Yamanaka K, Lobsiger CS, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, et al. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornemann KD, Wiederhold KH, Pauli C, et al. Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice. The American journal of pathology. 2001;158:63–73. doi: 10.1016/S0002-9440(10)63945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Potential Pathways of Abnormal Tau and α-Synuclein Dissemination in Sporadic Alzheimer“s and Parkinson”s Diseases. Cold Spring Harbor perspectives in biology. 2016 doi: 10.1101/cshperspect.a023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw EM, Chibnik LB, Keenan BT, et al. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nature neuroscience. 2013;16:848–850. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer’s disease beta-peptide. J Exp Med. 1998;188:431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadière B, Prigent A, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. The Journal of clinical investigation. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodacki B, Staszewski J, Toczyłowska B, et al. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neuroscience letters. 2008;441:158–162. doi: 10.1016/j.neulet.2008.06.040. [DOI] [PubMed] [Google Scholar]
- Brubaker WD, Crane A, Johansson JU, et al. Peripheral complement interactions with amyloid β peptide: Erythrocyte clearance mechanisms. Alzheimers Dement. 2017 doi: 10.1016/j.jalz.2017.03.010. S1552–5260(17)30149–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloj A, Leal MC, Xu H, et al. Insulin-degrading enzyme sorting in exosomes: a secretory pathway for a key brain amyloid-beta degrading protease. Journal of Alzheimer’s disease: JAD. 2010;19:79–95. doi: 10.3233/JAD-2010-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Cialic R, et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol. 2015;77:75–99. doi: 10.1002/ana.24304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady J, Koval ED, Benitez BA, et al. TREM2 variant p.R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol. 2014;71:449–453. doi: 10.1001/jamaneurol.2013.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A, Kassiou M, Meikle SR, Banati RB. In vivo evidence for microglial activation in neurodegenerative dementia. Acta neurologica Scandinavica Supplementum. 2006;185:107–114. doi: 10.1111/j.1600-0404.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- Cameron B, Tse W, Lamb R, et al. Loss of interleukin receptor-associated kinase 4 signaling suppresses amyloid pathology and alters microglial phenotype in a mouse model of Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:15112–15123. doi: 10.1523/JNEUROSCI.1729-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbutt S, Duff J, Yarnall A, et al. Variation in complement protein C1q is not a major contributor to cognitive impairment in Parkinson’s disease. Neurosci Lett. 2015;594:66–9. doi: 10.1016/j.neulet.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier AE, Ubhi K, Spencer B, et al. Differential effects of UCHL1 modulation on alpha-synuclein in PD-like models of alpha-synucleinopathy. PLoS ONE. 2012;7:e34713. doi: 10.1371/journal.pone.0034713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula M, Iyer AM, Spliet WGM, et al. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience. 2011;179:233–243. doi: 10.1016/j.neuroscience.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Chakrabarty P, Li A, Ceballos-Diaz C, et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron. 2015;85:519–533. doi: 10.1016/j.neuron.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, White CC, Winn PA, et al. CD33 modulates TREM2: convergence of Alzheimer loci. Nature neuroscience. 2015;18:1556–1558. doi: 10.1038/nn.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, White CC, Winn PA, et al. Trans-pQTL study identifies immune crosstalk between Parkinson and Alzheimer loci. Neurol Genet. 2016;2:e90. doi: 10.1212/NXG.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Lang H, Geng N, et al. Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neuroscience letters. 2013;548:190–195. doi: 10.1016/j.neulet.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Chao CC, Ala TA, Hu S, et al. Serum cytokine levels in patients with Alzheimer’s disease. Clin Diagn Lab Immunol. 1994a;1:433–436. doi: 10.1128/cdli.1.4.433-436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Frey WH, et al. Transforming growth factor beta in Alzheimer’s disease. Clin Diagn Lab Immunol. 1994b;1:109–110. doi: 10.1128/cdli.1.1.109-110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang X, Song L, Le W. Autophagy dysregulation in amyotrophic lateral sclerosis. Brain Pathol. 2012;22:110–116. doi: 10.1111/j.1750-3639.2011.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Abud EA, Yeung ST, et al. Increased tauopathy drives microglia-mediated clearance of beta-amyloid. Acta Neuropathol Commun. 2016;4:63. doi: 10.1186/s40478-016-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen Y, Wei Q, et al. Assessment of TREM2 rs75932628 association with amyotrophic lateral sclerosis in a Chinese population. Journal of the neurological sciences. 2015;355:193–195. doi: 10.1016/j.jns.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014a doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Sun X, Scicluna BJ, et al. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int. 2014b;86:433–444. doi: 10.1038/ki.2013.502. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Phatnani H, Kuligowski M, et al. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20960–20965. doi: 10.1073/pnas.0911405106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Liu G, Jin SM, et al. MicroRNA-205 regulates the expression of Parkinson’s disease-related leucine-rich repeat kinase 2 protein. Human molecular genetics. 2013;22:608–620. doi: 10.1093/hmg/dds470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell JP, Ward J, Taylor IA, et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. Journal of Alzheimer’s disease: JAD. 2008;14:27–41. doi: 10.3233/JAD-2008-14103. [DOI] [PubMed] [Google Scholar]
- Colangelo AM, Alberghina L, Papa M. Astrogliosis as a therapeutic target for neurodegenerative diseases. Neuroscience letters. 2014;565:59–64. doi: 10.1016/j.neulet.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Coleman BM, Hanssen E, Lawson VA, Hill AF. Prion-infected cells regulate the release of exosomes with distinct ultrastructural features. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:4160–4173. doi: 10.1096/fj.11-202077. [DOI] [PubMed] [Google Scholar]
- Concannon RM, Okine BN, Finn DP, et al. Differential upregulation of the cannabinoid CB(2) receptor in neurotoxic and inflammation-driven rat models of Parkinson’s disease. Exp Neurol. 2015;269:133–41. doi: 10.1016/j.expneurol.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Constam DB, Philipp J, Malipiero UV, et al. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992;148:1404–1410. [PubMed] [Google Scholar]
- Cookson MR. alpha-Synuclein and neuronal cell death. Mol Neurodegener. 2009;4:9. doi: 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch Y, Alvarez-Erviti L, Sibson NR, et al. The acute inflammatory response to intranigral α-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. Journal of neuroinflammation. 2011;8:166. doi: 10.1186/1742-2094-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane A, Brubaker WD, Johansson JU, et al. Peripheral complement interactions with amyloid β peptide in Alzheimer’s disease: 2. Relationship to Aβ immunotherapy. Alzheimers Dement. 2017 doi: 10.1016/j.jalz.2017.04.015. S1552–5260(17)32898–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Boche D, Perry VH. Transforming growth factor beta1, the dominant cytokine in murine prion disease: influence on inflammatory cytokine synthesis and alteration of vascular extracellular matrix. Neuropathol Appl Neurobiol. 2002;28(2):107–19. doi: 10.1046/j.1365-2990.2002.00383.x. [DOI] [PubMed] [Google Scholar]
- Daniele SG, Béraud D, Davenport C, et al. Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders. Sci Signal. 2015;8:ra45. doi: 10.1126/scisignal.2005965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer KM, Kranich LR, Ruf WP, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia C, Rinchon A, Olmos-Alonso A, et al. Microglia regulate hippocampal neurogenesis during chronic neurodegeneration. Brain Behav Immun. 2016;55:179–90. doi: 10.1016/j.bbi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola M, Sestito SE, Mariani A, et al. Synthetic and natural small molecule TLR4 antagonists inhibit motoneuron death in cultures from ALS mouse model. Pharmacol Res. 2016;103:180–187. doi: 10.1016/j.phrs.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Deleidi M, Gasser T. The role of inflammation in sporadic and familial Parkinson’s disease. Cell Mol Life Sci. 2013;70:4259–4273. doi: 10.1007/s00018-013-1352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depboylu C, Du Y, Muller U, et al. Lack of association of interleukin-10 promoter region polymorphisms with Alzheimer’s disease. Neuroscience letters. 2003;342:132–134. doi: 10.1016/S0304-3940(03)00231-3. [DOI] [PubMed] [Google Scholar]
- Depboylu C, Schäfer MK, Arias-Carrión O, et al. Possible involvement of complement factor C1q in the clearance of extracellular neuromelanin from the substantia nigra in Parkinson disease. J Neuropathol Exp Neurol. 2011;70(2):125–32. doi: 10.1097/NEN.0b013e31820805b9. [DOI] [PubMed] [Google Scholar]
- Depboylu C, Stricker S, Ghobril J-P, et al. Brain-resident microglia predominate over infiltrating myeloid cells in activation, phagocytosis and interaction with T-lymphocytes in the MPTP mouse model of Parkinson disease. Experimental neurology. 2012;238:183–191. doi: 10.1016/j.expneurol.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee H-J, Bae E-J, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Ma M, Teng J, et al. Exposure to ALS-FTD-CSF generates TDP-43 aggregates in glioblastoma cells through exosomes and TNTs-like structure. Oncotarget. 2015;6:24178–24191. doi: 10.18632/oncotarget.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorn KJ, Moors T, Drukarch B, et al. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol Commun. 2014;2:90. doi: 10.1186/s40478-014-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty KR, Guillot-Sestier M-V, Town T. The role of the immune system in neurodegenerative disorders: Adaptive or maladaptive? Brain research. 2014 doi: 10.1016/j.brainres.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin-Ouellet J, St-Amour I, Saint-Pierre M, et al. Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson’s disease. Int J Neuropsychopharmacol. 2014 doi: 10.1093/ijnp/pyu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N, Gysbers A, Perera G, et al. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta neuropathologica. 2016 doi: 10.1007/s00401-016-1648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison P, Archer HA, Gerhard A, et al. Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiology of disease. 2008;32:412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo F, Komine O, Fujimori-Tonou N, et al. Astrocyte-derived TGF-β1 accelerates disease progression in ALS mice by interfering with the neuroprotective functions of microglia and T cells. Cell Rep. 2015;11:592–604. doi: 10.1016/j.celrep.2015.03.053. [DOI] [PubMed] [Google Scholar]
- Endo F, Yamanaka K. Astrocytic TGF-β1: detrimental factor in ALS. Oncotarget. 2015;6:15728–15729. doi: 10.18632/oncotarget.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo EF, Gonzalez-Albo MC, Moraes JP, et al. Functional regeneration in a rat Parkinson“s model after intrastriatal grafts of glial cell line-derived neurotrophic factor and transforming growth factor beta1-expressing extra-adrenal chromaffin cells of the Zuckerkandl”s organ. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:9888–9895. doi: 10.1523/JNEUROSCI.21-24-09888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, Tenner AJ. Complement C1q expression induced by Abeta in rat hippocampal organotypic slice cultures. Exp Neurol. 2004;185:241–253. doi: 10.1016/j.expneurol.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Walter S, Kuhl S, et al. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, et al. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Zhao W-L, Ye Y-Y, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- Feng S-J, Nie K, Gan R, et al. Triggering receptor expressed on myeloid cells 2 variants are rare in Parkinson’s disease in a Han Chinese cohort. Neurobiology. 2014;35:1780e11–2. doi: 10.1016/j.neurobiolaging.2014.01.142. [DOI] [PubMed] [Google Scholar]
- Fernandez-Espejo E, Armengol JA, Flores JA, et al. Cells of the sympathoadrenal lineage: biological properties as donor tissue for cell-replacement therapies for Parkinson’s disease. Brain research Brain research reviews. 2005;49:343–354. doi: 10.1016/j.brainresrev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Fevrier B, Vilette D, Archer F, et al. Cells release prions in association with exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Laping NJ, Morgan TE, et al. TGF-beta 1 is an organizer of responses to neurodegeneration. J Cell Biochem. 1993;53:314–322. doi: 10.1002/jcb.240530408. [DOI] [PubMed] [Google Scholar]
- Finehout EJ, Franck Z, Lee KH. Complement protein isoforms in CSF as possible biomarkers for neurodegenerative disease. Dis Markers. 2005;21:93–101. doi: 10.1155/2005/806573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzner D, Schnaars M, van Rossum D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- Frank S, Burbach GJ, Bonin M, et al. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia. 2008;56:1438–1447. doi: 10.1002/glia.20710. [DOI] [PubMed] [Google Scholar]
- Frank S, Copanaki E, Burbach GJ, et al. Differential regulation of toll-like receptor mRNAs in amyloid plaque-associated brain tissue of aged APP23 transgenic mice. Neuroscience letters. 2009;453:41–44. doi: 10.1016/j.neulet.2009.01.075. [DOI] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich D, Kuo WP, Frühbeis C, et al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond, B, Biol Sci. 2014 doi: 10.1098/rstb.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeis C, Fröhlich D, Kuo WP, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013a;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeis C, Fröhlich D, Kuo WP, Krämer-Albers E-M. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013b;7:182. doi: 10.3389/fncel.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Rodriguez B, del-Marco A, Flores JA, et al. Grafts of extra-adrenal chromaffin cells as aggregates show better survival rate and regenerative effects on parkinsonian rats than dispersed cell grafts. Neurobiology of disease. 2008;29:529–542. doi: 10.1016/j.nbd.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Gambuzza ME, Sofo V, Salmeri FM, et al. Toll-like receptors in Alzheimer’s disease: a therapeutic perspective. CNS Neurol Disord Drug Targets. 2014;13:1542–1558. doi: 10.2174/1871527313666140806124850. [DOI] [PubMed] [Google Scholar]
- Gate D, Rezai-Zadeh K, Jodry D, et al. Macrophages in Alzheimer’s disease: the blood-borne identity. J Neural Transm. 2010;117:961–970. doi: 10.1007/s00702-010-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gezen-Ak D, Dursun E, Hanağası H, et al. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. Journal of Alzheimer’s disease: JAD. 2013;37:185–195. doi: 10.3233/JAD-130497. [DOI] [PubMed] [Google Scholar]
- Ghaisas S, Maher J, Kanthasamy A. Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther. 2016 Feb;158:52–62. doi: 10.1016/j.pharmthera.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese A, Brown DR, Groschup MH, et al. Role of microglia in neuronal cell death in prion disease. Brain Pathol. 1998;8:449–457. doi: 10.1111/j.1750-3639.1998.tb00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Masuda-Suzukake M, Falcon B. Like prions: the propagation of aggregated tau and α-synuclein in neurodegeneration. Brain: a journal of neurology. 2016 doi: 10.1093/brain/aww230. [DOI] [PubMed] [Google Scholar]
- Gomes C, Keller S, Altevogt P, Costa J. Evidence for secretion of Cu, Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neuroscience letters. 2007;428:43–46. doi: 10.1016/j.neulet.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aparicio R, Flores JA, Fernandez-Espejo E. Antiparkinsonian trophic action of glial cell line-derived neurotrophic factor and transforming growth factor β1 is enhanced after co-infusion in rats. Experimental neurology. 2010;226:136–147. doi: 10.1016/j.expneurol.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Grad LI, Pokrishevsky E, Silverman JM, Cashman NR. Exosome-dependent and independent mechanisms are involved in prion-like transmission of propagated Cu/Zn superoxide dismutase misfolding. Prion. 2014;8:331–335. doi: 10.4161/19336896.2014.983398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso M, Piscopo P, Confaloni A, Denti MA. Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules. 2014;19:6891–6910. doi: 10.3390/molecules19056891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel M, Béland L-C, Soucy G, et al. IL-10 Controls Early Microglial Phenotypes and Disease Onset in ALS Caused by Misfolded Superoxide Dismutase 1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36:1031–1048. doi: 10.1523/JNEUROSCI.0854-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves MC, Fiala M, Dinglasan LAV, et al. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:213–219. doi: 10.1080/14660820410020286. [DOI] [PubMed] [Google Scholar]
- Grey M, Dunning CJ, Gaspar R, et al. Acceleration of α-synuclein aggregation by exosomes. J Biol Chem. 2015;290:2969–2982. doi: 10.1074/jbc.M114.585703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A, Serrano-Pozo A, Parrado AR, et al. Alzheimer’s Disease Risk Gene CD33 Inhibits Microglial Uptake of Amyloid Beta. Neuron. 2013:1–13. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y, Liu H, Zhang L, et al. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6:37043–37053. doi: 10.18632/oncotarget.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot-Sestier M-V, Doty KR, Gate D, et al. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015a;85:534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot-Sestier M-V, Doty KR, Town T. Innate Immunity Fights Alzheimer’s Disease. Trends Neurosci. 2015b;38:674–681. doi: 10.1016/j.tins.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannafon BN, Ding W-Q. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. 2013;14:14240–14269. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nature genetics. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson A, Westberg L, Nilsson S, et al. Investigation of genes coding for inflammatory components in Parkinson’s disease. Mov Disord. 2005;20:569–573. doi: 10.1002/mds.20378. [DOI] [PubMed] [Google Scholar]
- He X, Jing Z, Cheng G. MicroRNAs: new regulators of Toll-like receptor signalling pathways. Biomed Res Int. 2014;2014:945169. doi: 10.1155/2014/945169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert SS, Horré K, Nicolaï L, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Nadrigny F, Regen T, et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci USA. 2010;107:6058–63. doi: 10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Prinz M, Aguzzi A. Pathogenesis of prion diseases: possible implications of microglial cells. Prog Brain Res. 2001;132:737–750. doi: 10.1016/S0079-6123(01)32114-3. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nature reviews Neuroscience. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Heurich B, El Idrissi NB, Donev RM, et al. Complement upregulation and activation on motor neurons and neuromuscular junction in the SOD1 G93A mouse model of familial amyotrophic lateral sclerosis. J Neuroimmunol. 2011;235(1–2):104–9. doi: 10.1016/j.jneuroim.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, Khoury El J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Hock E-M, Polymenidou M. Prion-like propagation as a pathogenic principle in frontotemporal dementia. J Neurochem. 2016;138(Suppl 1):163–183. doi: 10.1111/jnc.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nature genetics. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondius DC, van Nierop P, Li KW, et al. Profiling the human hippocampal proteome at all pathologic stages of Alzheimer’s disease. Alzheimers Dement. 2016;12:654–668. doi: 10.1016/j.jalz.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten KG, Beers DR, Zhao W, Appel SH. Protective and Toxic Neuroinflammation in Amyotrophic Lateral Sclerosis. Neurotherapeutics. 2015;12:364–375. doi: 10.1007/s13311-014-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houi K, Kobayashi T, Kato S, et al. Increased plasma TGF-beta1 in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2002;106:299–301. doi: 10.1034/j.1600-0404.2002.01301.x. [DOI] [PubMed] [Google Scholar]
- Iero M, Valenti R, Huber V, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, et al. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta neuropathologica. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Infante J, Garcia-Gorostiaga I, Sanchez-Juan P, et al. Inflammation-related genes and the risk of Parkinson’s disease: a multilocus approach. Eur J Neurol. 2008;15:431–433. doi: 10.1111/j.1468-1331.2008.02092.x. [DOI] [PubMed] [Google Scholar]
- International Parkinson Disease Genomics Consortium. Nalls MA, Plagnol V, et al. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Haga S, Yagishita S, Tateishi J. The presence of complements in amyloid plaques of Creutzfeldt-Jakob disease and Gerstmann-Straussler-Scheinker disease. Appl Pathol. 1984;2(6):370–379. [PubMed] [Google Scholar]
- Iwasaki Y, Ichikawa Y, Igarashi O, et al. Plasma TGFbeta1 in ALS patients. Acta Neurol Scand. 2003;108:221. doi: 10.1034/j.1600-0404.2003.00164.x. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iłzecka J, Stelmasiak Z, Dobosz B. Transforming growth factor-Beta 1 (tgf-Beta 1) in patients with amyotrophic lateral sclerosis. Cytokine. 2002;20:239–243. doi: 10.1006/cyto.2002.2005. [DOI] [PubMed] [Google Scholar]
- Jay TR, Hirsch AM, Broihier ML, et al. Disease progression-dependent effects of TREM2 deficiency in a mouse model of Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016 doi: 10.1523/JNEUROSCI.2110-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TR, Miller CM, Cheng PJ, et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. The Journal of experimental medicine. 2015;212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey M, Halliday WG, Bell J, et al. Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie-infected murine hippocampus. Neuropathology and applied neurobiology. 2000;26:41–54. doi: 10.1046/j.1365-2990.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- Jin J, Wu P, Li W, et al. Interleukin-10-1082A/G and -592C/A polymorphisms with risk of Parkinson’s disease: a meta-analysis. Int J Neurosci. 2014;124:852–858. doi: 10.3109/00207454.2014.880910. [DOI] [PubMed] [Google Scholar]
- Johnston H, Boutin H, Allan SM. Assessing the contribution of inflammation in models of Alzheimer’s disease. Biochemical Society transactions. 2011;39:886–890. doi: 10.1042/BST0390886. [DOI] [PubMed] [Google Scholar]
- Johnston LC, Su X, Maguire-Zeiss K, et al. Human interleukin-10 gene transfer is protective in a rat model of Parkinson’s disease. Mol Ther. 2008;16:1392–1399. doi: 10.1038/mt.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joniec-Maciejak I, Ciesielska A, Wawer A, et al. The influence of AAV2-mediated gene transfer of human IL-10 on neurodegeneration and immune response in a murine model of Parkinson’s disease. Pharmacol Rep. 2014;66:660–669. doi: 10.1016/j.pharep.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junn E, Lee K-W, Jeong BS, et al. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S-G, Kim C, Cortez LM, et al. Toll-like receptor-mediated immune response inhibits prion propagation. Glia. 2016;64:937–951. doi: 10.1002/glia.22973. [DOI] [PubMed] [Google Scholar]
- Kim C, Ho D-H, Suk J-E, et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Lee H-J, Masliah E, Lee S-J. Non-cell-autonomous Neurotoxicity of α-synuclein Through Microglial Toll-like Receptor 2. Exp Neurobiol. 2016;25:113–119. doi: 10.5607/en.2016.25.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingery ND, Ohsumi TK, Borowsky ML, et al. The microglial sensome revealed by direct RNA sequencing. Nature neuroscience. 2013:1–12. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella S, König H-G, Prehn JHM. Bid Promotes K63-Linked Polyubiquitination of Tumor Necrosis Factor Receptor Associated Factor 6 (TRAF6) and Sensitizes to Mutant SOD1-Induced Proinflammatory Signaling in Microglia. eNeuro. 2016 doi: 10.1523/ENEURO.0099-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MA, Kaeser PS, Schwarz P, et al. Complement facilitates early prion pathogenesis. Nat Med. 2001;7(4):488–492. doi: 10.1038/86567. [DOI] [PubMed] [Google Scholar]
- Knöferle J, Ramljak S, Koch JC, et al. TGF-beta 1 enhances neurite outgrowth via regulation of proteasome function and EFABP. Neurobiology of disease. 2010;38:395–404. doi: 10.1016/j.nbd.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Kokubo H, Saido TC, Iwata N, et al. Part of membrane-bound Abeta exists in rafts within senile plaques in Tg2576 mouse brain. Neurobiology. 2005;26:409–418. doi: 10.1016/j.neurobiolaging.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Kong SMY, Chan BKK, Park J-S, et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Human molecular genetics. 2014;23:2816–2833. doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Gasque P, Ströbel T, et al. Complement activation in human prion disease. Neurobiol Dis. 2004;15(1):21–8. doi: 10.1016/j.nbd.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Suter-Crazzolara C, Fischer WH, Unsicker K. TGF-beta superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J. 1995;14:736–742. doi: 10.1002/j.1460-2075.1995.tb07052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Dezso Z, MacKenzie C, et al. Circulating miRNA biomarkers for Alzheimer’s disease. PLoS ONE. 2013;8:e69807. doi: 10.1371/journal.pone.0069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature genetics. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattante S, Le Ber I, Camuzat A, et al. TREM2 mutations are rare in a French cohort of patients with frontotemporal dementia. Neurobiology. 2013;34:2443e1–2. doi: 10.1016/j.neurobiolaging.2013.04.030. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Suk J-E, Bae E-J, et al. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Suk J-E, Patrick C, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Lee JD, Phipps S, et al. Absence of toll-like receptor 4 (TLR4) extends survival in the hSOD1 G93A mouse model of amyotrophic lateral sclerosis. Journal of neuroinflammation. 2015;12:90. doi: 10.1186/s12974-015-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim H-J. Prion-like Mechanism in Amyotrophic Lateral Sclerosis: are Protein Aggregates the Key? Exp Neurobiol. 2015;24:1–7. doi: 10.5607/en.2015.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Kumar V, Fung JN, et al. Pharmacological inhibition of complement C5a-C5a1 receptor signalling ameliorates disease pathology in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. Br J Pharmacol. 2017;174(8):689–699. doi: 10.1111/bph.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann SM, Krüger C, Park B, et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nature neuroscience. 2012;15:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Liu Y, Walter S, et al. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiology. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Lewis C-A, Manning J, Rossi F, Krieger C. The Neuroinflammatory Response in ALS: The Roles of Microglia and T Cells. Neurol Res Int. 2012;2012:803701. doi: 10.1155/2012/803701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, He Q, Li R, et al. Interleukin-10 promoter polymorphisms in Chinese patients with Parkinson’s disease. Neuroscience letters. 2012;513:183–186. doi: 10.1016/j.neulet.2012.02.033. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008 Apr;28(4):468–76. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Li X, Long J, He T, et al. Integrated genomic approaches identify major pathways and upstream regulators in late onset Alzheimer’s disease. Sci Rep. 2015a;5:12393. doi: 10.1038/srep12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Shen N, Zhang S, et al. CD33 rs3865444 Polymorphism Contributes to Alzheimer’s Disease Susceptibility in Chinese, European, and North American Populations. Mol Neurobiol. 2015b;52:414–421. doi: 10.1007/s12035-014-8880-9. [DOI] [PubMed] [Google Scholar]
- Lian H, Litvinchuck A, Chiang ACA, et al. Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. J Neurosci. 2016;36(2):577–589. doi: 10.1523/JNEUROSCI.2117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill CM, Rengmark A, Pihlstrøm L, et al. The role of TREM2 R47H as a risk factor for Alzheimer“s disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and Parkinson”s disease. Alzheimers Dement. 2015;11:1407–1416. doi: 10.1016/j.jalz.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio D, Licastro F, Scola L, et al. Interleukin-10 promoter polymorphism in sporadic Alzheimer’s disease. Genes and immunity. 2003;4:234–238. doi: 10.1038/sj.gene.6363964. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Fujiwara H, Mann DM, et al. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. The American journal of pathology. 1998;153:1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Leak RK, Hu X. Neurotransmitter receptors on microglia. Stroke and Vascular Neurology. 2016;1(2):52–58. doi: 10.1136/svn-2016-000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hao W, Dawson A, et al. Expression of amyotrophic lateral sclerosis-linked SOD1 mutant increases the neurotoxic potential of microglia via TLR2. J Biol Chem. 2009;284:3691–3699. doi: 10.1074/jbc.M804446200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Walter S, Stagi M, et al. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain: a journal of neurology. 2005;128:1778–1789. doi: 10.1093/brain/awh531. [DOI] [PubMed] [Google Scholar]
- Lobo-Silva D, Carriche GM, Castro AG, et al. Balancing the immune response in the brain: IL-10 and its regulation. Journal of neuroinflammation. 2016;13:297. doi: 10.1186/s12974-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobsiger CS, Boillée S, Cleveland DW. Toxicity from different SOD1 mutants dysregulates the complement system and the neuronal regenerative response in ALS motor neurons. Proc Natl Acad Sci U S A. 2007;104(18):7319–26. doi: 10.1073/pnas.0702230104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobsiger CS, Boillée S, Pozniak C, et al. C1q induction and global complement pathway activation do not contribute to ALS toxicity in mutant SOD1 mice. Proc Natl Acad Sci U S A. 2013 Nov 12;110(46):E4385–92. doi: 10.1073/pnas.1318309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler DA, Camp DM, Bennett DA. Plaque complement activation and cognitive loss in Alzheimer’s disease. J Neuroinflammation. 2008;5:9. doi: 10.1186/1742-2094-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenbrueck KF, Tigno-Aranjuez JT, Boehm BO, et al. Th1 responses to beta-amyloid in young humans convert to regulatory IL-10 responses in Down syndrome and Alzheimer’s disease. Neurobiology. 2010;31:1732–1742. doi: 10.1016/j.neurobiolaging.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson’s disease. Prog Neurobiol. 2009;89:277–287. doi: 10.1016/j.pneurobio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- López González I, Garcia-Esparcia P, Llorens F, Ferrer I. Genetic and Transcriptomic Profiles of Inflammation in Neurodegenerative Diseases: Alzheimer, Parkinson, Creutzfeldt-Jakob and Tauopathies. Int J Mol Sci. 2016;17:206. doi: 10.3390/ijms17020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H-T, Zhang J-P, Miao F. Effects of pramipexole treatment on the α-synuclein content in serum exosomes of Parkinson’s disease patients. Exp Ther Med. 2016;12:1373–1376. doi: 10.3892/etm.2016.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y, Chen C, Zhang BY, et al. Remarkable Activation of the Complement System and Aberrant Neuronal Localization of the Membrane Attack Complex in the Brain Tissues of Scrapie-Infected Rodents. Mol Neurobiol. 2015;52(3):1165–1179. doi: 10.1007/s12035-014-8915-2. [DOI] [PubMed] [Google Scholar]
- Ma SL, Tang NL, Lam LC, Chiu HF. The association between promoter polymorphism of the interleukin-10 gene and Alzheimer’s disease. Neurobiology. 2005;26:1005–1010. doi: 10.1016/j.neurobiolaging.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Maciotta S, Meregalli M, Torrente Y. The involvement of microRNAs in neurodegenerative diseases. Front Cell Neurosci. 2013;7:265. doi: 10.3389/fncel.2013.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Simpson JF, Parikh I, et al. CD33 Alzheimer’s risk-altering polymorphism, CD33 expression, and exon 2 splicing. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:13320–13325. doi: 10.1523/JNEUROSCI.1224-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani S, Gordon R, Macmaw JK, et al. J Neuroimmunol. 2014;276(1–2):213–8. doi: 10.1016/j.jneuroim.2014.09.005. Elevation of the terminal complement activation products C5a and C5b-9 in ALS patient blood. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CL, Hennessy E, Rubio-Araiz A, et al. Inhibiting TLR2 activation attenuates amyloid accumulation and glial activation in a mouse model of Alzheimer’s disease. Brain Behav Immun. 2016;58:191–200. doi: 10.1016/j.bbi.2016.07.143. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson“s and Alzheimer”s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neuroscience letters. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Melchior B, Garcia AE, Hsiung BK, et al. Dual induction of TREM2 and tolerance-related transcript, Tmem176b, in amyloid transgenic mice: implications for vaccine-based therapies for Alzheimer’s disease. ASN neuro. 2010;2:e00037. doi: 10.1042/AN20100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel D, Thelen M, Balzer-Geldsetzer M, et al. TREM2 rare variant p.R47H is not associated with Parkinson’s disease. Parkinsonism Relat Disord. 2016;23:109–111. doi: 10.1016/j.parkreldis.2015.11.026. [DOI] [PubMed] [Google Scholar]
- Michaud J-P, Hallé M, Lampron A, et al. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1941–1946. doi: 10.1073/pnas.1215165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud J-P, Rivest S. Anti-inflammatory signaling in microglia exacerbates Alzheimer’s disease-related pathology. Neuron. 2015;85:450–452. doi: 10.1016/j.neuron.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Michel B, Ferguson A, Johnson T, et al. Genetic depletion of complement receptors CD21/35 prevents terminal prion disease in a mouse model of chronic wasting disease. J Immunol. 2012;189(9):4520–4527. doi: 10.4049/jimmunol.1201579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B, Ferguson A, Johnson T, et al. Complement protein C3 exacerbates prion disease in a mouse model of chronic wasting disease. Int Immunol. 2013;25(12):697–702. doi: 10.1093/intimm/dxt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Álvarez M, Santos-Lozano A, Sanchis-Gomar F, et al. Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer’s disease: a systematic review and meta-analysis of treatment effect. Drugs Aging. 2015;32:139–147. doi: 10.1007/s40266-015-0239-z. [DOI] [PubMed] [Google Scholar]
- Minoretti P, Gazzaruso C, Vito CD, et al. Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer’s disease. Neuroscience letters. 2006;391:147–149. doi: 10.1016/j.neulet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Mirza B, Hadberg H, Thomsen P, Moos T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience. 2000;95:425–432. doi: 10.1016/S0306-4522(99)00455-8. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, et al. Transforming growth factor-beta 1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson’s disease. Neuroscience letters. 1995;193:129–132. doi: 10.1016/0304-3940(95)11686-q. [DOI] [PubMed] [Google Scholar]
- Montag J, Hitt R, Opitz L, et al. Upregulation of miRNA hsa-miR-342-3p in experimental and idiopathic prion disease. Mol Neurodegener. 2009;4:36. doi: 10.1186/1750-1326-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli AE, Larregina AT, Shufesky WJ, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- Muth C, Schröck K, Madore C, et al. Activation of microglia by retroviral infection correlates with transient clearance of prions from the brain but does not change incubation time. Brain Pathol. 2016 doi: 10.1111/bpa.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch C, Bertolotti A. Self-propagation and transmission of misfolded mutant SOD1: prion or prion-like phenomenon? Cell Cycle. 2011;10:1711. doi: 10.4161/cc.10.11.15560. [DOI] [PubMed] [Google Scholar]
- Münch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nature neuroscience. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J Neural Transm. 2000;(Suppl):143–151. [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nature genetics. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Ito H, Wate R, et al. Phosphorylated Smad2/3 immunoreactivity in sporadic and familial amyotrophic lateral sclerosis and its mouse model. Acta neuropathologica. 2008;115:327–334. doi: 10.1007/s00401-007-0337-z. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kaneko S, Ito H, et al. Activation of transforming growth factor-β/Smad signaling reduces aggregate formation of mislocalized TAR DNA-binding protein-43. Neurodegener Dis. 2013;11:182–193. doi: 10.1159/000338151. [DOI] [PubMed] [Google Scholar]
- Nawrocka-Kunecka A, Papierz W, Liberski PP. Complement factors C1q and C3b in brains with Creutzfeldt-Jakob disease. Pol J Pathol. 2005;56(3):127–9. [PubMed] [Google Scholar]
- Nie K, Zhang Y, Gan R, et al. Polymorphisms in immune/inflammatory cytokine genes are related to Parkinson’s disease with cognitive impairment in the Han Chinese population. Neuroscience letters. 2013;541:111–115. doi: 10.1016/j.neulet.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Noelker C, Morel L, Lescot T, et al. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep. 2013;3:1393. doi: 10.1038/srep01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka T, Masuda-Suzukake M, Arai T, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Norris KL, Hao R, Chen L-F, et al. Convergence of Parkin, PINK1, and α-Synuclein on Stress-induced Mitochondrial Morphological Remodeling. J Biol Chem. 2015;290:13862–13874. doi: 10.1074/jbc.M114.634063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello A, Edison P, Archer HA, et al. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology. 2009;72:56–62. doi: 10.1212/01.wnl.0000338622.27876.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Mizuno Y, et al. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain: a journal of neurology. 2005;128:2665–2674. doi: 10.1093/brain/awh625. [DOI] [PubMed] [Google Scholar]
- Pascale E, Passarelli E, Purcaro C, et al. Lack of association between IL-1β, TNF-α, and IL-10 gene polymorphisms and sporadic Parkinson’s disease in an Italian cohort. Acta Neurol Scand. 2011;124:176–181. doi: 10.1111/j.1600-0404.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Peyrin JM, Lasmézas CI, Haïk S, et al. Microglial cells respond to amyloidogenic PrP peptide by the production of inflammatory cytokines. Neuroreport. 1999;10:723–729. doi: 10.1097/00001756-199903170-00012. [DOI] [PubMed] [Google Scholar]
- Phatnani HP, Guarnieri P, Friedman BA, et al. Intricate interplay between astrocytes and motor neurons in ALS. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E756–65. doi: 10.1073/pnas.1222361110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco JC, Scicluna BJ, Hill AF, Götz J. Extracellular Vesicles Isolated from the Brains of rTg4510 Mice Seed Tau Protein Aggregation in a Threshold-dependent Manner. J Biol Chem. 2016;291:12445–12466. doi: 10.1074/jbc.M115.709485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DA, Martinez AA, Seillier A, et al. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur J Neurosci. 2009;29:2177–86. doi: 10.1111/j.1460-9568.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nature neuroscience. 2017 doi: 10.1038/nn.4475. [DOI] [PubMed] [Google Scholar]
- Prokop S, Miller KR, Heppner FL. Microglia actions in Alzheimer’s disease. Acta neuropathologica. 2013;126:461–477. doi: 10.1007/s00401-013-1182-x. [DOI] [PubMed] [Google Scholar]
- Qin Y, Liu Y, Hao W, et al. Stimulation of TLR4 Attenuates Alzheimer’s Disease-Related Symptoms and Pathology in Tau-Transgenic Mice. J Immunol. 2016;197:3281–3292. doi: 10.4049/jimmunol.1600873. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Bali J, Barr MM, et al. Emerging roles of extracellular vesicles in the nervous system. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:15482–15489. doi: 10.1523/JNEUROSCI.3258-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez BG, Blazquez C, Gomez del Pulgar T, et al. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–13. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos EM, Lin MT, Larson EB, et al. Tumor necrosis factor alpha and interleukin 10 promoter region polymorphisms and risk of late-onset Alzheimer disease. Archives of neurology. 2006;63:1165–1169. doi: 10.1001/archneur.63.8.1165. [DOI] [PubMed] [Google Scholar]
- Rayaprolu S, Mullen B, Baker M, et al. TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener. 2013;8:19. doi: 10.1186/1750-1326-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzos M, Nikolaou C, Andreadou E, et al. Circulating interleukin-10 and interleukin-12 in Parkinson’s disease. Acta Neurol Scand. 2009;119:332–337. doi: 10.1111/j.1600-0404.2008.01103.x. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2009;4:462–475. doi: 10.1007/s11481-009-9166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. TREM2 enables amyloid β clearance by microglia. Cell Res. 2015;25:535–536. doi: 10.1038/cr.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K, Zeineddine R, Corcoran L, et al. Extracellular aggregated Cu/Zn superoxide dismutase activates microglia to give a cytotoxic phenotype. Glia. 2013;61:409–419. doi: 10.1002/glia.22444. [DOI] [PubMed] [Google Scholar]
- Saba R, Goodman CD, Huzarewich RLCH, et al. A miRNA signature of prion induced neurodegeneration. PLoS ONE. 2008;3:e3652. doi: 10.1371/journal.pone.0003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba R, Gushue S, Huzarewich RLCH, et al. MicroRNA 146a (miR-146a) is over-expressed during prion disease and modulates the innate immune response and the microglial activation state. PLoS ONE. 2012;7:e30832. doi: 10.1371/journal.pone.0030832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salins P, He Y, Olson K, et al. TGF-beta1 is increased in a transgenic mouse model of familial Alzheimer’s disease and causes neuronal apoptosis. Neuroscience letters. 2008;430:81–86. doi: 10.1016/j.neulet.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Saman S, Kim W, Raya M, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanagi T, Yuasa S, Nakamura Y, et al. Appearance of phagocytic microglia adjacent to motoneurons in spinal cord tissue from a presymptomatic transgenic rat model of amyotrophic lateral sclerosis. Journal of neuroscience research. 2010;88:2736–2746. doi: 10.1002/jnr.22424. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mejias E, Navarro V, Jimenez S, et al. Soluble phospho-tau from Alzheimer’s disease hippocampus drives microglial degeneration. Acta neuropathologica. 2016;132:897–916. doi: 10.1007/s00401-016-1630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia. 2005;51:241–253. doi: 10.1002/glia.20210. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Jun S, Rellick S, et al. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain research. 2016;1646:139–151. doi: 10.1016/j.brainres.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scassellati C, Zanardini R, Squitti R, et al. Promoter haplotypes of interleukin-10 gene and sporadic Alzheimer’s disease. Neuroscience letters. 2004;356:119–122. doi: 10.1016/j.neulet.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Sharples RA, Vella LJ, Nisbet RM, et al. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:1469–1478. doi: 10.1096/fj.07-9357. [DOI] [PubMed] [Google Scholar]
- Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2017 doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Sim RB, Kishore U, Villiers CL, et al. C1q binding and complement activation by prions and amyloids. Immunobiology. 2007;212(4–5):355–62. doi: 10.1016/j.imbio.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Sisková Z, Page A, O’Connor V, Perry VH. Degenerating synaptic boutons in prion disease: microglia activation without synaptic stripping. The American journal of pathology. 2009;175:1610–1621. doi: 10.2353/ajpath.2009.090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery CF, Beck JA, Harper L, et al. R47H TREM2 variant increases risk of typical early-onset Alzheimer’s disease but not of prion or frontotemporal dementia. Alzheimers Dement. 2014;10:602–608e4. doi: 10.1016/j.jalz.2014.05.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner DS, Cho IS, Park SY, et al. Accelerated prion disease pathogenesis in Toll-like receptor 4 signaling-mutant mice. J Virol. 2008;82:10701–10708. doi: 10.1128/JVI.00522-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Fellner L, Reindl M, et al. Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons. The American journal of pathology. 2011;179:954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeck K, Bodemer M, Ciesielczyk B, et al. Interleukin 4 and interleukin 10 levels are elevated in the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Archives of neurology. 2005;62:1591–1594. doi: 10.1001/archneur.62.10.1591. [DOI] [PubMed] [Google Scholar]
- Street JM, Barran PE, Mackay CL, et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012;10:5. doi: 10.1186/1479-5876-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuendl A, Kunadt M, Kruse N, et al. Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain: a journal of neurology. 2016;139:481–494. doi: 10.1093/brain/awv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson KJ, Christianson HC, Wittrup A, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288:17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely CA, Zandi PP. Non-steroidal anti-inflammatory drugs and Alzheimer’s disease: the epidemiological evidence. CNS Neurol Disord Drug Targets. 2010;9:132–139. doi: 10.2174/187152710791012026. [DOI] [PubMed] [Google Scholar]
- Sznejder-Pachołek A, Joniec-Maciejak I, Wawer A, et al. The effect of α-synuclein on gliosis and IL-1α, TNFα, IFNγ, TGFβ expression in murine brain. Pharmacol Rep. 2017;69(2):242–251. doi: 10.1016/j.pharep.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Tacnet-Delorme P, Chevallier S, Arlaud GJ. Beta-amyloid fibrils activate the C1 complex of complement under physiological conditions: evidence for a binding site for A beta on the C1q globular regions. J Immunol. 2001;167:6374–6381. doi: 10.4049/jimmunol.167.11.6374. [DOI] [PubMed] [Google Scholar]
- Tahara K, Kim HD, Jin JJ, et al. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain: a journal of neurology. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamboli IY, Barth E, Christian L, et al. Statins promote the degradation of extracellular amyloid {beta}-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J Biol Chem. 2010;285:37405–37414. doi: 10.1074/jbc.M110.149468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DL, Diemel LT, Cuzner ML, et al. Activation of group II metabotropic glutamate receptors underlies microglial reactivity and neurotoxicity following stimulation with chromogranin A, a peptide up-regulated in Alzheimer’s disease. J Neurochem. 2002;82:1179–91. doi: 10.1046/j.1471-4159.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Diemel LT, Pocock JM. Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci. 2003;23:2150–60. doi: 10.1523/JNEUROSCI.23-06-02150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternianov A, Perez-Ortiz JM, Solesio ME, et al. Overexpression of CB2 cannabinoid receptors results in neuroprotection against behavioral and neurochemical alterations induced by intracaudate administration of 6-hydroxydopamine. Neurobiol Aging. 2012;33:421e1–16. doi: 10.1016/j.neurobiolaging.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Tesseur I, Zou K, Esposito L, et al. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer’s pathology. The Journal of clinical investigation. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews Immunology. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol. 2002;124(1–2):83–92. doi: 10.1016/S0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- Town T. Alternative Abeta immunotherapy approaches for Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2009;8:114–127. doi: 10.2174/187152709787847306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T. Alzheimer’s Disease Beyond Abeta. Expert review of neurotherapeutics. 2010;10:671–675. doi: 10.1586/ern.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Laouar Y, Pittenger C, et al. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nature medicine. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TA, Nguyen AD, Chang J, et al. Lipopolysaccharide and tumor necrosis factor regulate Parkin expression via nuclear factor-kappa B. PLoS ONE. 2011;6:e23660. doi: 10.1371/journal.pone.0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias E, Ibarburu S, Barreto-Núñez R, Barbeito L. Significance of aberrant glial cell phenotypes in pathophysiology of amyotrophic lateral sclerosis. Neuroscience letters. 2017;636:27–31. doi: 10.1016/j.neulet.2016.07.052. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Yamada T. Increased concentration of C4d complement protein in CSF in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1994;57(7):859–61. doi: 10.1136/jnnp.57.7.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi T, Hamada K, Krainc D. ATP13A2/PARK9 regulates secretion of exosomes and α-synuclein. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:15281–15287. doi: 10.1523/JNEUROSCI.1629-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J, Chen B, Yang L, et al. Amyloid-β Activates Microglia and Regulates Protein Expression in a Manner Similar to Prions. J Mol Neurosci. 2015;56:509–518. doi: 10.1007/s12031-015-0553-2. [DOI] [PubMed] [Google Scholar]
- Unsicker K, Suter-Crazzalora C, Krieglstein K. Growth factor function in the development and maintenance of midbrain dopaminergic neurons: concepts, facts and prospects for TGF-beta. Ciba Found Symp. 1996;196:70–80. doi: 10.1002/9780470514863.ch6. discussion 80–4. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- van der Wal EA, Gómez-Pinilla F, Cotman CW. Transforming growth factor-beta 1 is in plaques in Alzheimer and Down pathologies. Neuroreport. 1993;4:69–72. doi: 10.1097/00001756-199301000-00018. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Dillon-Carter O, Tourtellotte WW, et al. TGFbeta1 and TGFbeta2 concentrations are elevated in Parkinson’s disease in ventricular cerebrospinal fluid. Experimental neurology. 1996;142:313–322. doi: 10.1006/exnr.1996.0200. [DOI] [PubMed] [Google Scholar]
- Veerhuis R, Hoozemans JJM, Janssen I, et al. Adult human microglia secrete cytokines when exposed to neurotoxic prion protein peptide: no intermediary role for prostaglandin E2. Brain research. 2002;925:195–203. doi: 10.1016/s0006-8993(01)03273-5. [DOI] [PubMed] [Google Scholar]
- Vehmas AK, Kawas CH, Stewart WF, Troncoso JC. Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiology. 2003;24:321–331. doi: 10.1016/S0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- Vekrellis K, Xilouri M, Emmanouilidou E, et al. Pathological roles of α-synuclein in neurological disorders. Lancet Neurol. 2011;10:1015–1025. doi: 10.1016/S1474-4422(11)70213-7. [DOI] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Lawson VA, et al. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol. 2007;211:582–590. doi: 10.1002/path.2145. [DOI] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Nisbet RM, et al. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J. 2008;37:323–332. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- Vincent B, Cisse MA, Sunyach C, et al. Regulation of betaAPP and PrPc cleavage by alpha-secretase: mechanistic and therapeutic perspectives. Curr Alzheimer Res. 2008;5:202–211. doi: 10.2174/156720508783954749. [DOI] [PubMed] [Google Scholar]
- Vural P, Değirmencioğlu S, Parildar-Karpuzoğlu H, et al. The combinations of TNFalpha-308 and IL-6 -174 or IL-10 -1082 genes polymorphisms suggest an association with susceptibility to sporadic late-onset Alzheimer’s disease. Acta Neurol Scand. 2009;120:396–401. doi: 10.1111/j.1600-0404.2009.01230.x. [DOI] [PubMed] [Google Scholar]
- Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Archives of neurology. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- Walker DG, Whetzel AM, Serrano G, et al. Association of CD33 polymorphism rs3865444 with Alzheimer’s disease pathology and CD33 expression in human cerebral cortex. Neurobiology. 2015;36:571–582. doi: 10.1016/j.neurobiolaging.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao D, Pan B, et al. Toll-like receptor 2 deficiency shifts PrP106-126-induced microglial activation from a neurotoxic to a neuroprotective phenotype. J Mol Neurosci. 2015a;55:880–890. doi: 10.1007/s12031-014-0442-0. [DOI] [PubMed] [Google Scholar]
- Wang L-Z, Yu J-T, Miao D, et al. Genetic association of TLR4/11367 polymorphism with late-onset Alzheimer’s disease in a Han Chinese population. Brain research. 2011;1381:202–207. doi: 10.1016/j.brainres.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hancock AM, Bradner J, et al. Complement 3 and factor h in human cerebrospinal fluid in Parkinson’s disease, Alzheimer’s disease, and multiple-system atrophy. Am J Pathol. 2011;178:1509–1516. doi: 10.1016/j.ajpath.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015b;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Duan W, Wang W, et al. scAAV9-VEGF prolongs the survival of transgenic ALS mice by promoting activation of M2 microglia and the PI3K/Akt pathway. Brain research. 2016a;1648:1–10. doi: 10.1016/j.brainres.2016.06.043. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ulland TK, Ulrich JD, et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. The Journal of experimental medicine. 2016b;213:667–675. doi: 10.1084/jem.20151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HA, Lee JD, Lee KM, et al. Complement C5a-C5aR1 signalling drives skeletal muscle macrophage recruitment in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. Skelet Muscle. 2017;7(1):10. doi: 10.1186/s13395-017-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz TM, Town T. Microglia in Alzheimer“s Disease: It”s All About Context. International journal of Alzheimer’s disease. 2012;2012:314185. doi: 10.1155/2012/314185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz TM, Town T. Amyloid Cascade into Clarity. Immunity. 2016;45:717–718. doi: 10.1016/j.immuni.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Westergard T, Jensen BK, Wen X, et al. Cell-to-Cell Transmission of Dipeptide Repeat Proteins Linked to C9orf72-ALS/FTD. Cell Rep. 2016;17:645–652. doi: 10.1016/j.celrep.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Lucassen PJ, Ritchie D, Bruce M. PrP deposition, microglial activation, and neuronal apoptosis in murine scrapie. Experimental neurology. 1997;144:433–438. doi: 10.1006/exnr.1997.6424. [DOI] [PubMed] [Google Scholar]
- Williams AE, Lawson LJ, Perry VH, Fraser H. Characterization of the microglial response in murine scrapie. Neuropathology and applied neurobiology. 1994a;20:47–55. doi: 10.1111/j.1365-2990.1994.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Williams AE, van Dam AM, Man-A-Hing WK, et al. Cytokines, prostaglandins and lipocortin-1 are present in the brains of scrapie-infected mice. Brain research. 1994b;654:200–206. doi: 10.1016/0006-8993(94)90480-4. [DOI] [PubMed] [Google Scholar]
- Wisniewski HM, Barcikowska M, Kida E. Phagocytosis of beta/A4 amyloid fibrils of the neuritic neocortical plaques. Acta neuropathologica. 1991;81:588–590. doi: 10.1007/BF00310142. [DOI] [PubMed] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature medicine. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- Woodruff TM, Costantini KJ, Crane JW, et al. The complement factor C5a contributes to pathology in a rat model of amyotrophic lateral sclerosis. J Immunol. 181(12):8727–34. doi: 10.4049/jimmunol.181.12.8727. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Sanan DA, et al. Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. The American journal of pathology. 2000;156:139–150. doi: 10.1016/S0002-9440(10)64713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Yan F, et al. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nature medicine. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Masliah E, Mallory M, et al. Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer’s disease. Nature. 1997;389:603–606. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/S0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Yamada T, McGeer PL, McGeer EG. Lewy bodies in Parkinson’s-disease are recognized by antibodies to complement proteins. Acta Neuropathologica. 1992;84:100–104. doi: 10.1007/BF00427222. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillée S, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nature neuroscience. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Keene CD, Peskind ER, et al. Cerebrospinal Fluid Particles in Alzheimer Disease and Parkinson Disease. Journal of neuropathology and experimental neurology. 2015;74:672–687. doi: 10.1097/NEN.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Ye RD. Microglial Aβ receptors in Alzheimer’s disease. Cellular and molecular neurobiology. 2015;35:71–83. doi: 10.1007/s10571-014-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated Exosome Secretion Promotes Clearance of Amyloid-beta by Microglia. J Biol Chem. 2012;287:10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel MD, Heikenwalder M, Prinz M, et al. Stromal complement receptor CD21/35 facilitates lymphoid prion colonization and pathogenesis. J Immunol. 2007;179(9):6144–6152. doi: 10.4049/jimmunol.179.9.6144. [DOI] [PubMed] [Google Scholar]
- Zhang B, Gaiteri C, Bodea L-G, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153:707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Henkel JS, et al. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia. 2010;58:231–243. doi: 10.1002/glia.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Zhou X-W, Wang J-Z. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl Neurodegener. 2016;5:7. doi: 10.1186/s40035-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Guan Y, Chen Y, et al. miRNA-9 expression is upregulated in the spinal cord of G93A-SOD1 transgenic mice. Int J Clin Exp Pathol. 2013;6:1826–1838. [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Herrmann US, Falsig J, et al. A neuroprotective role for microglia in prion diseases. The Journal of experimental medicine. 2016;213:1047–1059. doi: 10.1084/jem.20151000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Herrmann US, Li B, et al. Triggering receptor expressed on myeloid cells-2 is involved in prion-induced microglial activation but does not contribute to prion pathogenesis in mouse brains. Neurobiology. 2015;36:1994–2003. doi: 10.1016/j.neurobiolaging.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature medicine. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]