Abstract
Exposure to psychosocial stressors increases consumption of palatable, calorically dense diets (CDD) and the risk for obesity, especially in females. While consumption of an obesogenic diet and chronic stress have both been shown to decrease dopamine 2 receptor (D2R) binding and alter functional connectivity (FC) within the prefrontal cortex (PFC) and the nucleus accumbens (NAcc), it remains uncertain how social experience and dietary environment interact to affect reward pathways critical for the regulation of motivated behavior. Using positron emission tomography (PET) and resting state functional connectivity magnetic resonance neuroimaging (rs-fMRI), in female rhesus monkeys maintained in a low calorie chow (n=18) or a dietary choice condition (chow and a CDD; n=16) for 12 months, the current study tested the overarching hypothesis that the adverse social experience resulting from subordinate social status would interact with consumption of an obesogenic diet to increase caloric intake that would be predicted by greater cortisol, lower prefrontal D2R binding potential (D2R-BP) and lower PFC-NAcc FC. Results showed that the consequences of adverse social experience imposed by chronic social subordination vary significantly depending on the dietary environment and are associated with alterations in prefrontal D2R-BP and FC in NAcc-PFC sub-regions that predict differences in caloric intake, body weight gain, and fat accumulation. Higher levels of cortisol in the chow-only condition were associated with mild inappetence, as well as increased orbitofrontal (OFC) D2R-BP and greater FC between the NAcc and the dorsolateral PFC (dlPFC) and ventromedial PFC (vmPFC). However, increased cortisol release in females in the dietary choice condition was associated with reduced prefrontal D2R-BP, and opposite FC between the NAcc and the vmPFC and dlPFC observed in the chow-only females. Importantly, the degree of these glucocorticoid-related neuroadaptations predicted significantly more total calorie intake as well as more consumption of the CDD for females having a dietary choice, but had no relation to calorie intake in the chow-only condition. Overall, the current findings suggest that dietary environment modifies the consequences of adverse social experience on reward pathways and appetite regulation and, in an obesogenic dietary environment, may reflect impaired cognitive control of food intake.
Introduction
The World Health Organization reports that the global prevalence of obesity approached 600 million adults in 2014 (WHO, 2016) and additional data suggest that 32% of men and 36% of women in the United States are obese (Flegal et al., 2010). Because the health (Hill, 2006) and economic burden (Withrow and Alter, 2011) imposed by obesity are enormous, effective programs to prevent or alleviate obesity are a high priority. Although obesity can be explained in biological terms as the consequence of prolonged positive energy imbalance, complex gene by environment interactions likely affect both sides of energy balance (Feng et al., 2010; Haslam and James, 2005; Ogden et al., 2007). Clearly, a dietary environment that includes calorically dense foods, high in fats and sugars, often leads to increased caloric intake and obesity (la Fleur et al., 2011). However, the underlying neuroadaptations resulting from exposure to an obesogenic diet that lead to sustained food intake, increasing the risk for obesity, remain unclear.
Previous studies have shown that intake of calorically dense diets (CDD) induces dopamine (DA) release and activates reward pathways similar to what has been described in psychostimulant use (Volkow et al., 2008; Wise, 2006). Decreases in DA D2 receptor (D2R) binding potential (D2R-BP) within the ventral striatum, including the nucleus accumbens (NAcc) (Haber and Knutson, 2010), involved in reward and motivational processes (Cohen et al., 2010), are predictive of an addictive phenotype (Volkow et al., 2003; Volkow and Wise, 2005) and are observed in obese humans (Wang et al., 2001). This decrease in striatal D2R-BP with drugs of abuse is attributed to increased presynaptic DA release (Volkow and Li, 2004). Prefrontal cortex (PFC) regions, which are connected with the NAcc and are involved in regulating reward and goal-directed behaviors (Haber and Knutson, 2010), are also altered in obese individuals (Volkow et al., 2008). Reduced metabolic activity in the PFC of obese individuals is associated with reductions in striatal D2R (Volkow et al., 2008). Importantly, sustained intake of CDDs reduces striatal D2R expression in obese rats, whereas experimental knockdown of D2R expression increases intake of a CDD in rats (Johnson and Kenny, 2010). Together, these data suggest that impaired DA function within the NAcc and associated changes in PFC activity may be causally linked as well as a consequence of CDD consumption (Kenny, 2011), as is the case with psychostimulant abuse (Koob and Kreek, 2007; Koob and Le Moal, 2001; Tomasi and Volkow, 2013). Additionally, obesity in humans is associated with altered PFC-striatal functional connectivity (FC), as assessed by functional MRI (fMRI) methods (Contreras-Rodriguez et al., 2017; Coveleskie et al., 2015; Stoeckel et al., 2009). In the context of food intake, this decreased corticostriatal FC may reflect lack of cognitive restraint on caloric intake (Nummenmaa et al., 2012). These neuroadaptations are characteristic of a hypodopaminergic state and may contribute to the maintenance of increased caloric intake in an obesogenic dietary environment. However, it remains unclear how these reductions in D2R relate to alterations in PFC-striatal FC and associated increased calorie intake.
It is important to note that not all individuals maintained in an obesogenic dietary environment consume excess calories. Exposure to psychosocial stressors is a risk factor for increased intake of CDDs, a phenomenon that occurs more often in women than men (Laitinen et al., 2002). Furthermore, data from animal studies show calorie consumption increases, particularly those from a CDD, when animals experience chronic social (e.g. social defeat or subordination) (Arce et al., 2010; Foster et al., 2006; Meisel et al., 1990; Michopoulos et al., 2012c; Solomon et al., 2007; Tamashiro et al., 2006; Wilson et al., 2008) or physical (e.g. restraint) stressors (Dallman et al., 2003; Hagan et al., 2003; la Fleur et al., 2005; Warne, 2009). Signals from the limbic-hypothalamic-pituitary-adrenal (LHPA) axis, such cortisol and corticotropin-releasing hormone (CRH), target DA neurons in mesolimbic regions (Harfstrand et al., 1986; Sauvage and Steckler, 2001; Swanson et al., 1983) producing a dysregulation of DA neurotransmission (Izzo et al., 2005) that increases the expression of anhedonia and the risk for developing an addictive phenotype (Anisman and Matheson, 2005; Koob and Kreek, 2007; Koob and Le Moal, 2001). Data showing that stress hormones, particularly glucocorticoids (e.g. cortisol in primates), are key signals changing food salience and increased caloric intake (Warne, 2009) are consistent with the notion that emotional feeding is sustained by activation of the LHPA axis (Michopoulos et al., 2012c; Warne, 2009). The functional consequence of chronic stress is a “reward deficiency syndrome”, characterized by reduced DA activity (Blum et al., 1996). Therefore, it is hypothesized that one biobehavioral strategy to overcome this reward deficiency may be to activate DA pathways compromised by stress exposure by consuming diets with fat and sugar to increase levels of DA in the NAcc, a finding not seen when consuming a low caloric diet or palatable food devoid of calories (Bassareo and Di Chiara, 1999; Blackburn et al., 1986; Marinelli et al., 2006; Small et al., 2003).
Our previous studies show that socially subordinate adult female rhesus monkeys, maintained in long-established stable social groups, are mildly inappetent compared with more dominant group mates when fed laboratory chow, but consume nearly twice as many calories as do dominant females when given a choice between chow and a CDD (Arce et al., 2010; Michopoulos et al., 2012c). More recent analyses show that the dietary environment affects DA neurochemistry and appetite in adult females immediately following new social group formation and the acquisition of new social ranks, with lower D2R-BP in the orbital PFC (OFC) was associated with increased caloric intake (Michopoulos et al., 2016). The present study extends these initial observations of these same female rhesus monkeys to determine how social experience and dietary environment interact to affect prefrontal D2R-BP and corticostriatal FC, using positron emission tomography (PET) neuroimaging and resting state (rs)-fMRI, respectively, after one year in a chow or a dietary choice condition. The current study tested the overarching hypothesis that the adverse social experience resulting from subordinate status would interact with consuming an obesogenic diet to increase caloric intake, body weight and body fat. We also hypothesized that greater calorie intake in an obesogenic dietary environment would be predicted by lower prefrontal D2R-BP and weakened PFC-NAcc FC. Specifically, the dorsolateral PFC (dlPFC), ventromedial PFC (vmPFC), OFC, and anterior cingulate cortex (ACC) were assessed due to their connections with the NAcc and their role in incentive-based behavioral responses, goal-directed behavior, reward coding, impulse control and salience/value of food, and anticipation of the benefit/risk ratio of rewards (Haber and Knutson, 2010; Knutson et al., 2005; Yacubian et al., 2006).
Methods
Subjects and group formation
Adult female rhesus monkeys (n=34) living in one of six breeding groups located at the Yerkes National Primate Research Center (YNPRC) Field Station in Lawrenceville, Georgia were selected as subjects based on age and familiarity with other females. As described previously (Michopoulos et al., 2016), females were removed from their natal groups to form new social groups of four to six females each. Briefly, a sequential group formation process was employed to introduce females in indoor-outdoor pens measuring approximately 144 ft2 (12×12 ft) with a randomized order of introduction, a process that results in immediate formation of a dominance hierarchy (Jarrell et al., 2008; Snyder-Mackler et al., 2016). The Emory University Institutional Animal Care and Use Committee approved all procedures in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for Care and Use of Laboratory Animals.”
Experimental Design
Females were randomly assigned to be a member of a new social group (4 to 6 monkeys per group) and these groups were then randomly assigned to have either access to the standard, low calorie monkey chow (4 groups, n = 18 monkeys) or access to a choice dietary environment wherein both the chow and a CDD were available (3 groups, n = 16 monkeys). The caloric composition of the chow diet (3.45 kcal/g; Lab Diets, St. Louis MO, #5038) was 12% fat, 18% protein, and 4.14% sugar carbohydrate and 65.9% fiber carbohydrate (including 42.4% starch). The calories of the CDD (4.47 kcal/g; Research Diets, New Brunswick NJ, #D07091204S) were distributed as 36% fat, 18% protein, 16.4% sugar carbohydrate and 29.6% fiber-starch carbohydrate. While the chow diet contains a higher concentration of starch compared with the CDD, data show that starch intake is associated with reduced appetite and less body weight gain compared with intake of sugars in CDD (Aller et al., 2011). The rationale for providing access to both the chow and CDD is based on well-established data showing that dietary choice sustains intake of high caloric diets (la Fleur et al., 2010) and more closely models the human dietary environment.
All females had access to experimental diets ad libitum via previously validated automated feeders that allow for quantification of caloric intake in socially housed, free feeding monkeys (Arce et al., 2010; Wilson et al., 2008). Briefly, activation of a radio-frequency antenna by an identification chip within each animal’s wrist signaled a computer to dispense a single pellet of food via a pellet dispenser. Each social group had access to two automated feeders and a computer recorded each feeding event in a log. Validation of the feeding system showed that high-ranking females in these small social groups rarely took a pellet from more subordinate animals and pellets were never discarded (Wilson et al., 2008). The observation that subordinate females consumed more calories in a dietary choice condition underscores that higher ranking animals did not restrict access to these automated feeding systems (Michopoulos et al., 2012c). Calorie intake was monitored continually for 12 months following group formation to determine how status-related differences in social behavior and dietary environment predicts caloric intake.
Physiology
All animals were trained and habituated to being removed from their group for conscious venipuncture using previously described procedures (Walker et al., 1982). Blood samples were obtained within 10 minutes from entering the animal area to provide baseline cortisol measures, although the training and habituation minimizes arousal (Blank et al., 1983). Diurnal plasma cortisol and sensitivity to glucocorticoid negative feedback were determined as part of a dexamethasone sensitivity test (Michopoulos et al., 2012b). Samples were obtained 1 and 4 hr after sunrise and again 1 hr before sunset, with the evening samples followed by administration of dexamethasone (0.125 mg/kg, IM). Samples were obtained again the following morning 1 and 4 hr after sunrise. Plasma levels of cortisol were measured by LC-ESI-tandem mass spectrometry using a Discovery 5cm × 2.1mm C18 column (Supelco, PA) eluted at flow rate of 0.5 ml/min at the YNPRC Biomarkers Core. The intra- and inter-assay coefficient of variation (CV%) was 1.21% and 5.78%, respectively. Body weight was measured every two weeks and estimates of body fat, obtained using dual x-ray absorptiometry (DEXA, Norland), were assessed at baseline prior to the diet intervention and again at 12 months, coincident with the neuroimaging scans. Total activity levels were assessed using Actical accelerometers (Phillips Respironics, Bend Oregon) at baseline and again at 12 months. Accelerometers were attached to a primate collar (Primate Products), which the females wore for 10 to 12 days.
Behavioral data
Throughout the study period, social and solitary behaviors were obtained from weekly 30-minute group focal observations using an established ethogram (Jarrell et al., 2008). The outcome of dyadic agonistic interactions determined the relative rank of females within each social group (Bernstein and Gordon, 1974). In addition to prosocial behaviors (frequency and duration of proximity and grooming initialed and received), rates of agonistic behavior (aggression and submission initiated and received) were recorded. Cumulative rates and durations of social behaviors from study start to 12 months were summarized by performing a Principal Components Analysis (PCA) with Direct Oblimin rotation. In addition, a female’s previous social rank in her natal group, as well as total activity levels derived from the Actical accelerometers, were included. Inclusion of activity measures was based on assumption that females moving about their enclosure could not engage in social behavior. Raw values were z-transformed prior to inclusion in the PCA. Two components emerged from this analysis (Table 1) that were labeled social engagement and adverse experience. Social engagement was comprised of positive loading of social behaviors (time spent in proximity; proximity initiated to others; time spent grooming others; and time others groomed the female) and a negative loading of total activity level at 12 months. Adverse social experience was comprised of positive loading of natal group rank, rates of aggression received, submissive behaviors, and total activity levels and negative loadings of proximity initiated to others and time others groomed the female.
Table 1.
Loading scores for social behaviors, previous social rank, and activity levels for the PCA.
| Behavior | Social Engagement | Adverse Experience |
|---|---|---|
| Natal group social rank | 0.694 | |
| Total time in proximity to others | 0.959 | |
| Frequency of initiation to others | 0.758 | −0.441 |
| Duration of grooming others | 0.770 | |
| Duration of other grooming female | 0.740 | −.449 |
| Frequency of aggression towards others | −.496 | |
| Frequency of aggression received from others | 0.886 | |
| Frequency of submission towards others | 0.956 | |
| Total activity levels | −0.593 | 0.460 |
Acquisition and analyses of D2R-BP
A subset of females comprised of the six highest and six lowest ranking females from each dietary condition (n=12 for both groups; 24 total) received a PET scan 12 to 14 months after new group formation and imposition of the dietary conditions to assess D2R-BP. There were no differences in age at scan, natal group rank, or social engagement PCA scores between subjects in the LCD-only and Choice conditions (see Supplemental Table 1). [18F]-fallypride was used as the D2R radioligand because it provides robust in vivo measures of both striatal and extra-striatal D2R binding, due to its high D2R affinity (Riccardi et al., 2008; Vandehey et al., 2010). [18F]-fallypride-PET has been validated previously in monkeys and in humans (Mukherjee et al., 2002). Quantitative PET images were acquired using a MicroPET Focus 220 scanner system (CTI Concorde Microsystems LLC, Knoxville, TN) and cyclotron located in the YNPRC Imaging Core. PET imaging occurred at the same time of day to control for any diurnal effects (Krajnak et al., 2003). Animal anesthesia (isoflurane 1 to 2% to effect) and monitoring followed standard veterinary practices (Michopoulos et al., 2014). A transmission scan was obtained with a germanium-68 source for attenuation correction of the emission data. [18F]-fallypride was infused over 1 min. Emission data were collected continuously over 120 min after injection and then binned into appropriate time frames. Structural MR images were obtained within three weeks of the PET scan using a 3T magnet (Siemens Trio) for co-registration of PET and calculation of D2R-BP in prefrontal region(s) of interest (ROIs).
The dlPFC was selected for its involvement in executive function and working memory for evaluation, comparison and selection of incentive-based behavioral responses (Haber and Knutson, 2010), goal-directed behavior, reward coding, impulse control and salience/value of food (Grabenhorst et al., 2008; Kuhnen and Knutson, 2005; Rolls and McCabe, 2007). The vmPFC was chosen because it is involved in emotional and motivational salience assessment, prosocial behavior, stress, and emotional regulation (Ma et al., 2010; Rilling et al., 2002; Rilling et al., 2004). The OFC was analyzed because it has been implicated in goal-directed behavior, reward coding, impulse control and salience/value of food (Grabenhorst et al., 2008; Kuhnen and Knutson, 2005; Rolls and McCabe, 2007) and prosocial behavior (Rilling et al., 2002; Rilling et al., 2004). Finally, the ACC ROI was investigated due to its involvement in emotional appraisal/regulation, immediacy, and anticipation of the benefit/risk ratio of rewards (Haber and Knutson, 2010; Knutson et al., 2005; Yacubian et al., 2006) as well as integration of value across different stimuli (Blair et al., 2006).
Prefrontal ROIs were based on neuroanatomical definitions previously published in rhesus by our group (Embree et al., 2013; Parr et al., 2012). ROIs were manually traced on both hemispheres [left(l) and right(r)] for each monkey after realignment of sagittal, coronal and axial orthogonal planes into stereological space (see Supplemental Figure 1). Rhesus macaque brain atlases (Paxinos et al., 2000; Saleem and Logothetis, 2007) were used to guide ROI tracing within structural MRI images in coronal and sagittal views (Embree et al., 2013). Sub-regions of the PFC were drawn, including the dlPFC (-Brodmanns area- BA 46), vmPFC (BA 32), OFC (BA 11, 13) and the medial PFC (mPFC)/ACC (BA 24). The NAcc was traced using an adaptation of the technique applied to macaques (Li et al., 2013b) and humans (Mawlawi et al., 2001; Narendran et al., 2006). The non-vermis cerebellar gray matter served as reference region due to the relative absence of D2R binding sites. Regional measures D2R-BP were determined using the simplified reference tissue method (SRTM; CER served as reference tissue), a composite parameter that serves as an index for D2 receptor availability (Lammertsma and Hume, 1996; Mintun et al., 1984).
Resting State fMRI Neuroimaging
A subset of high and low ranking females who received D2R PET scans comprised from each dietary condition (n=8 for each diet condition, 16 total) also received a rs-fMRI scan within one month of D2R PET scan at 12 months. There were no differences in age at scan, natal group rank, or social engagement PCA scores between subjects in the LCD-only and Choice conditions (see Supplemental Table 1). Neuroimaging scans were collected using a 3T Siemens Magnetom Trio Tim scanner (Siemens Med. Sol., Malvern, PA, USA), and an 8-channel phase array coil. Subjects were transported from their social groups at the YNPRC Field Station in Lawrenceville, GA to the YNPRC Imaging Center in Atlanta, GA, either the morning of the scan or the day prior to the scan. Two 15 minute rs-fMRI (T2*-weighted) scans were acquired to measure temporal changes in regional blood-oxygen-level dependent (BOLD) signal during a single session that also included T1- and T2-weighted structural MRI scans for registration purposes (T1-weighted MRI scans were collected using a 3D magnetization prepared rapid gradient echo (3D-MPRAGE) parallel image sequence: TR/TE = 2600/3.38msec, FoV: 128mmx128mm, voxel size: 0.5mm3 isotropic, 1 average, GRAPPA acceleration factor of R=2; T2-weighted MRI scans were collected using a 3D fast spin-echo sequence: TR/TE = 3200/373msec, FoV: 128mmx128mm, voxel size: 0.5mm3 isotropic, 1 average, GRAPPA acceleration factor of R=2). Subjects were scanned under isoflurane anesthesia (1% to effect, inhalation) following induction with telazol (2.16–3.82 mg/kg, I.M.) and intubation to ensure lack of motion artifacts. This dose (and higher doses) of isoflurane has previously been used to report coherent patterns of BOLD fluctuations in rhesus macaques, including sensory, motor, visual and cognitive-task related systems (Birn et al., 2014; Hutchison et al., 2013; Li et al., 2013a; Tang and Ramani, 2016; Vincent et al., 2007). Importantly, these patterns are similar to those observed in awake, behaving monkeys (Vincent et al., 2007). To ensure no potential confounding effects of anesthetic induction dose on FC, bivariate correlations were generated between FC values and mg/kg dosage of telazol. None of these correlations were statistically significant at p<0.05. For physiological monitoring, animals were fitted with an oximeter, electrocardiograph, rectal thermometer and blood pressure monitor. Additionally, an I.V. catheter was placed to administer dextrose/NaCl (0.45%) in order to maintain hydration. All subjects were scanned in the same supine placement and orientation on an MRI-compatible heating pad, using a custom-made head holder with ear bars and a mouth piece. A vitamin E capsule was taped to the right temple to mark the right side of the brain. Subjects were returned to their social groups upon completion of the scan and full recovery from anesthesia. BOLD-weighted functional images were collected using a single-shot gradient-echo-planar imaging (EPI) sequence (400 volumes, FoV: 105 mm × 105 mm, slice thickness: 1.5 mm, matrix size: 70 × 70, TR/TE: 2290/25msec, 2×15min, voxel size: 1.5mm3 isotropic) to analyze FC between brain regions. The rs-fMRI scans were collected following the T1-MRI scan (which lasted about 30 min) to standardize time from initial anesthesia to 45 minutes for all subjects. An additional short reverse-phase encoding scan was also acquired for unwarping susceptibility-induced distortions in the EPI images, using previously published methods (Andersson et al., 2003). The first 3 volumes were removed from each scan to allow for scanner equilibrium, resulting in a total of 794 concatenated volumes.
Rs-fMRI data preprocessing
Rs-fMRI is a useful tool to investigate FC between brain regions, based on evidence that BOLD-related activity in neural circuits during resting state recapitulates task-evoked activations in humans (Cordes et al., 2000), even in anesthetized macaques (Margulies et al., 2009; Vincent et al., 2007). All raw data were preprocessed using the FMRIB Software Library [FSL, Oxford, UK, RRID: SCR_002823; (Smith et al., 2004; Woolrich et al., 2009)], in addition 4dfp tools (ftp://ftp.imaging.wustl.edu/pub/raichlab/4dfp_tools/) and an in-house built Nipype pipeline [based on (Gorgolewski et al., 2011)] with modifications of previously published methods (Fair et al., 2009; Fair et al., 2007; Fair et al., 2012; Iyer et al., 2013). Some methods were adapted specifically for the rhesus monkey brain (Miranda-Dominguez et al., 2014). After file conversion, in order to reduce noise and artifact, functional imaging series were unwarped using a reverse phase-encoding distortion correction method (Andersson et al., 2003), slice-time corrected (for the even vs. odd slice intensity differences due to interleaved acquisition), motion-corrected (rigid body motion correction within-run, linear registration from EPI to T1, and nonlinear registration from T1 to template applied all in one resampling step), and signal normalized to a whole brain mode value gradient of 1000. Next, the EPI functional time series were concatenated and rigid-body co-registered to the subject’s averaged T1-weighted structural image. This was then transformed to conform to the 112RM-SL (RM: Rhesus Macaque; SL: Saleem-Logothetis coordinate space) atlas (McLaren et al., 2010), using rigid-body registration followed by linear (FLIRT) and non-linear registration (FNIRT) methods in FSL. This 112RM-SL atlas is an average of 112 male and female adult monkeys (McLaren et al., 2010; McLaren et al., 2009), in F99 space, and the EPI images were transformed into F99 space following previously published protocols (Miranda-Dominguez et al., 2014) in one interpolation step for the region of interest (ROI) analysis described below. Additional preprocessing steps included functional signal detrending, temporal low-pass filtering (f < 0.1Hz) via a second order Butterworth filter, and nuisance regression of rigid body head motion parameters in 6 directions, the global whole-brain signal, the ventricular and white matter functional signal (averaged from a ventricle- and a white matter mask, respectively), and their first-order derivatives (Fair et al., 2009; Fair et al., 2007; Fair et al., 2012; Miranda-Dominguez et al., 2014). Analyses were conducted with the removal of frames having framewise displacement (FD) value greater than 0.2 mm (Power et al., 2012; Power et al., 2014). Additionally, all imaging data were visually inspected upon preprocessing completion, to determine if any series had unsatisfactory co-registration or significant BOLD signal dropout.
Analysis of FC between NAcc and subdivisions of the PFC
The NAcc ROI was selected due to its involvement in reward processing (Cohen, Asarnow et al. 2010). Sub-regions of the PFC were assessed, including the dlPFC (BAs 9, 46), vmPFC (BAs 14, 25, 32), OFC (BAs 11, 13), and ACC (BA 24). The PFC ROIs were defined based on previously published anatomical parcellations (Lewis and Van Essen, 2000; Markov et al., 2012) mapped onto the cortical surface of the 112RM-SL atlas (registered to F99 space; see Supplemental Figure 2). Each ROI was manually edited in both the 112RM-SL atlas and each of the subjects to exclude overlapping ROIs, non-brain tissue or signal dropout). The resting state BOLD time series were then correlated ROI by ROI for each subject. For this, the time course of the BOLD signal was averaged across the voxels within each ROI, and then correlated with the time course of all other ROIs. Correlation coefficients (r-values) between ROIs were extracted from these correlation matrices using Matlab (MathWorks Inc., Natick, MA, RRID: SCR_001622).
Statistical analyses
Data were summarized as mean ± standard error. Non-normal distributions were log-transformed before analysis. PCA scores (described above) were used to reflect a composite of social experience during the 12 month interval rather than to the average rates of behavior for a specific month. We chose to use these PCA scores, rather than categorical ranks (e.g., high vs. low), to better assess the “dosing” effect of social experience provided by including these continuous variables in our statistical models. To test the hypothesis that a choice dietary condition would result in increased caloric intake, body weight and body fat accumulation, ANOVAs were used to assess differences in food intake, body weight and body fat mass, and behavioral phenotypes due to dietary condition (choice vs. chow-only). To test the hypothesis that greater adverse social experience would be associated with greater plasma cortisol concentrations, linear regressions were used to assess relations between social experience and plasma cortisol concentrations.
Because caloric intake varied significantly by diet condition (see results below) and consumption of a CDD is known to impact neurochemistry of reward pathways (Guo et al., 2014; Johnson and Kenny, 2010), predictors of caloric intake were examined separately for each dietary condition. Using the bivariate correlations, we tested the a priori hypothesis that greater caloric intake in subjects in the choice dietary condition would be predicted by greater plasma cortisol concentrations, decreased D2R-BP, and decreased FC between PFC subregions (dlPFC, vmPFC, OFC, and ACC) and NAcc. In order to generate explanatory models of the diet- and experience-induced neuroadaptations that emerged, Fisher-z transformation of Pearson correlation values were used to generate composite r-values for parameters of glucocorticoid signaling, D2R-BP, and NAcc-PFC FC (Silver and Dunlap, 1987).
Results
Effects of dietary environment on behavioral and endocrine phenotypes
Social engagement (F1,33=0.085, p=0.77) and adverse social experience component scores (F1,33=0.052, p=0.82) did not vary significantly between the two dietary conditions. Increased social engagement was associated with decreased adverse social experience (r32=−0.38; p=0.026) and greater sensitivity to glucocorticoid negative feedback inhibition on cortisol secretion as assessed by dexamethasone sensitivity test (r32=0.41; p=0.015), evidenced by lower morning cortisol following dexamethasone administration. However, this enhanced glucocorticoid sensitivity for socially engaged females did not vary significantly by dietary condition (r32=−0.07, p>0.05).
Effects of dietary environment on calorie intake and body weight
Females in the dietary choice condition consumed more total calories (CDD plus chow) than females in the chow-only condition (F1,33=4.59, p=0.040; Figure 1A). Females in the choice diet condition ate more of the CDD (57% of total caloric intake) than the chow (p=0.011; Figure 1A). This increased caloric intake in females maintained in the choice dietary environment was associated with greater absolute body weight (F1,33=5.30, p=0.028), greater increases in body weight (F1,33=8.62, p=0.006; Figure 1B), trunk fat (F1,33=5.14, p=0.031; Figure 1C), and total body fat (F1,33=5.38, p=0.028; Figure 1D) over the 12 month period.
Figure 1.
Females in the dietary choice condition consumed more total calories during the 12 month study (A), gained more body weight (B), had an increase in trunk fat (C), and body fat (D) compared to females maintained in the chow-only (LCD) dietary environment. Asterisks denote significant differences between dietary conditions (p<0.05) and # denotes greater consumption of the CDD compared to the LCD in the Choice subjects (p<0.05). Error bars indicate standard error of the mean (SEM).
Predictors of caloric intake in chow-only dietary condition
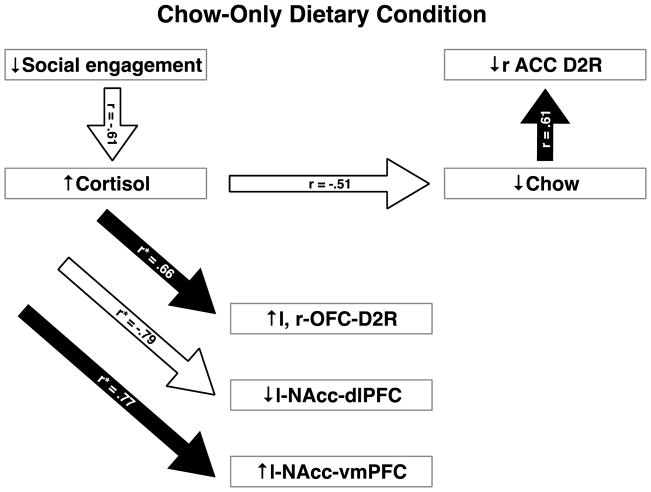
The impaired glucocorticoid regulation shown by less socially engaged females (r16=−0.61, p=0.008) predicted mild inappetence, as higher cortisol concentrations the morning following dexamethasone administration were negatively related to chow intake (r16=−0.51, p 0.031; see Figure 2). Higher pre-dexamethasone morning cortisol levels also were associated with increased D2R BP in the right (r10=0.66, p=0.021) and left OFC (r10=0.67, p=0.016; composite r10=0.66, p=0.018). No other significant relationships were found between D2R-BP with cortisol and other PFC regions or the NAcc (all p>0.05).
Figure 2.
Bivariate correlations between social behavior, measures of cortisol release, D2R-BP, and NAcc-PFC FC for females maintained in the chow-only condition for 12 months. The designation of r* reflects a composite correlation coefficient. All correlations are significant (p < 0.05). See text for details.
In addition, high post-dexamethasone cortisol values also predicted reduced rs-MRI FC between the left NAcc and the dlPFC (r6=−0.74, p=0.037), as did pre-dexamethasone morning cortisol (r6=−0.85, p=0.007; composite r6=−0.79, p=0.018; Figure 2). In contrast, higher pre-dexamethasone morning (r6=0.75, p=0.033) and evening cortisol (r6=0.80, p=0.017) significantly predicted increased left NAcc-vmPFC FC (composite r6=0.77, p=0.025). However, none of these glucocorticoid-dependent associations of D2R-BP or NAcc-PFC FC were directly related to chow intake (p>0.05). Chow intake was positively associated with increased D2R-BP in the right ACC (r10=0.61, p=0.035). However, binding potential in the right ACC was unrelated to any parameter of cortisol release (all p>0.05).
Predictors of caloric intake in dietary choice condition
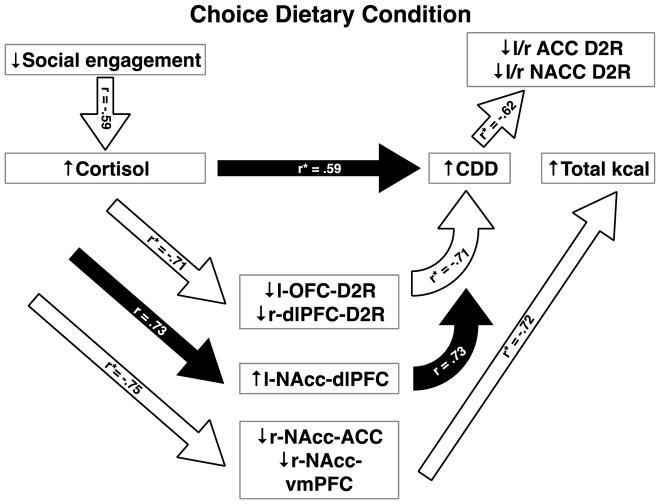
A very different pattern emerges in females maintained for 12 months in an obesogenic, dietary choice environment (Figure 3). Under these dietary conditions, the reduced sensitivity to glucocorticoid negative feedback observed in less socially engaged females (r14=−0.59, p=0.017) reflecting impaired glucocorticoid negative feedback regulation, is associated with significantly increased calorie intake, quite unlike the chow-only condition. Specifically, pre-dexamethasone morning (r14=0.631, p<0.01) and evening cortisol (r14=0.55, p=0.027) predict greater intake of the CDD. Post-dexamethasone plasma morning (r14=0.65, p=0.006) and mid-day cortisol (r14=0.52, p=0.039) predict more total caloric intake (CDD plus chow). These associations yield a composite significant positive relation between cortisol concentrations and caloric intake for the dietary choice females (r14=0.59, p=0.015).
Figure 3.
Bivariate correlations between social behavior, measures of cortisol release, D2R-BP, and NAcc-PFC FC for females maintained in dietary choice condition for 12 months. The designation of r* reflects a composite correlation coefficient. All correlations are significant (p < 0.05). See text for details.
Higher concentrations of cortisol for females in the dietary choice condition also predicts reduced D2R BP in several PFC ROIs. Pre-dexamethasone morning (r10=−0.62, p=0.030) and evening cortisol (r10=−0.80, p=0.002) predicts significantly lower D2R-BP in the left OFC (composite r10=−0.73, p=0.007), an effect opposite to that observed in chow-only females. Furthermore, pre-dexamethasone evening cortisol also predicts significantly lower D2R-BP in the right dlPFC (r10=−0.67, p=0.017). Together these association between parameters of cortisol release and D2R-BP in the OFC and dlPFC yielded a significant composite association (r10=−0.71, p=0.010, Figure 3).
Increased parameters of cortisol release also predicted differences in NAcc-PFC FC. Notably, pre-dexamethasone morning (r6=−0.75, p=0.033) and evening cortisol (r6=−0.78, p=0.023) predicted reduced right NAcc-mPFC FC. In addition, pre-dexamethasone morning cortisol also predicted reduced left NAcc-OFC FC (r6=−0.73, p=0.040) and reduced right NAcc-vmPFC FC (r6=−0.74, p=0.036), with the latter opposite to what was observed in chow-only females. Together these associations between parameters of cortisol release and reduced NAcc-PFC FC yielded a significant composite association (r6=−0.75, p=0.033, Figure 3). In contrast, pre-dexamethasone evening cortisol predicted increased left NAcc-dlPFC FC (r6=0.73, p=0.040), a pattern again opposite to that observed in chow-only females.
Unlike the consequences of the greater cortisol for females in the chow-only condition, differences in D2R-BP and NAcc-PFC FC associated with increased cortisol secretion predicted increased caloric intake. Reduced D2R BP in the left OFC (r10=−0.65, p=0.022) and both the left (r10=−0.59, p=0.043) and right dlPFC (r10=−0.72, p=0.008) predicted significantly more CDD intake (composite r10=−0.71, p=0.010). Furthermore, increased left NAcc-dlPFC FC also predicted greater CDD intake (r6=0.73, p=0.042). Increased total calorie intake was significantly related to reduced FC between the right NAcc-mPFC (r6=−0.73, p=0.042) and right NAcc-vmPFC (r6=−0.71, p=0.047; composite r6=−0.72, p=0.046). Unlike these cortisol-related changes in brain function, the FC between the left NAcc and OFC was unrelated to any parameter of calorie intake (all p>0.05).
Greater calorie intake of the CDD intake predicted decreased D2R-BP in the right (r10=−0.63, p=0.030) and left ACC (r10=−0.62, p=0.031) as well as the right (r10=−0.63, p=0.028) and left NAcc (r10=−0.59, p=0.040). While these lower levels of D2R-BP (composite r10=−0.62, p=0.031, Figure 3) were associated with more CDD consumption, D2R-BP in other corticostriatal regions was unrelated to any parameter of food intake (p>0.05). It is important to note the effect of calorie intake on D2R-BP in the right ACC was opposite to that observed in chow-only females for whom reduced binding potential was associated with reduced chow intake.
Discussion
Our overarching hypothesis for this study was that the adverse social experience resulting from subordinate status would interact with consuming an obesogenic diet to increase caloric intake, body weight and body fat and that the greater intake, particularly from a CDD, would be predicted by lower prefrontal D2R-BP and reduced PFC-NAcc FC. In general, the data from our exploratory analysis support this broad hypothesis by showing that the consequences of adverse social experience, imposed by chronic social subordination, vary significantly by the dietary environment and are associated with differences in caloric intake that is predicted by lower D2R-BP and FC in NAcc-PFC regions in a diet-dependent manner. Less social engagement, a characteristic of social subordination in small groups of female rhesus monkeys (Snyder-Mackler et al., 2016), is associated with more cortisol release, but the consequences of greater cortisol concentrations differed between females maintained in a chow-only condition compared to those maintained in a dietary choice condition, with both chow and a CDD available. This hypercortisolemia in the chow-only condition was associated with mild inappetence, as well as increased OFC D2R-BP and greater FC between the NAcc and the dlPFC and vmPFC. However, greater cortisol release in females in the obesogenic dietary condition was associated with more extensive and, in some cases, opposite relationships to that observed in chow-only condition. Notably, D2R-BP was reduced in the OFC and dlPFC, and NAcc FC was reduced with the vmPFC, but increased with the dlPFC, opposite to the outcomes for the chow-only females. Importantly, these glucocorticoid-associated neuroadaptations predicted significantly more calorie intake of the CDD or CDD plus chow combined for the choice females, but had no relation to calorie intake in the chow-only condition. Together these data show how the dietary environment interacts with variation in social experience, particularly being socially engaged, to shape how the brain responds to higher cortisol secretion and food intake.
We have previously reported that adult female monkeys maintained on a chow diet exhibit distinct, rank-related metabolic phenotypes including mild inappetence, reduced body weight and fat, and increased activity levels (Michopoulos et al., 2012a; Michopoulos et al., 2012c). These observations were replicated and extended in the current project, as the impaired glucocorticoid regulation shown by less socially engaged females significantly predicted less chow intake, consistent with a large body of data showing stressor exposure attenuates chow intake in laboratory animals (Harris et al., 1998; Heinrichs and Richard, 1999; Krahn et al., 1990). These higher cortisol levels also were associated with increased D2R BP in the OFC and reduced FC between the NAcc and the dlPFC, possibly reflecting impaired top down inhibition of striatal-dependent behaviors that would otherwise lead to an addictive-like phenotype under the right circumstances. In contrast, higher cortisol was associated with increased NAcc-vmPFC FC, which may reflect ability to correctly assess behaviorally risky situations – an adaptive response for chronically stressed animals. However, none of these glucocorticoid-related associations were directly related to chow intake.
A very different series of outcomes were observed for females in the dietary choice condition, as hypercortisolemia was associated with greater overall food intake in this obesogenic dietary condition. These current data corroborate previous findings implicating glucocorticoids as key signals for altering food salience and increasing caloric intake in a complex dietary environment (Warne, 2009), and are consistent with the notion that increased caloric intake of CDD in the face of stressor exposure is sustained by activation of the LHPA axis (Michopoulos et al., 2012c; Warne, 2009). Additionally, higher concentrations of cortisol were associated with reduced D2R-BP in the left OFC and dlPFC as well as reduced FC between the NAcc and the right ACC, left vmPFC, and left OFC. In contrast, FC between the right and left NAcc and dlPFC was significantly higher in relation to increased cortisol concentrations. Signals from the stress axis, including cortisol and CRH, act on DA neurons in corticostriatal and mesolimbic regions (Harfstrand et al., 1986; Sauvage and Steckler, 2001; Swanson et al., 1983) to alter DA neurotransmission (Izzo et al., 2005). Importantly, this disruption in DA function increases the expression of anhedonia and the risk for developing an addictive phenotype (Anisman and Matheson, 2005; Koob and Kreek, 2007; Koob and Le Moal, 2001), suggesting that glucocorticoids may act to increase caloric intake in the current study by dysregulating DA neurotransmission. While we have previously shown that antagonism of CRH receptor 1 reduces caloric intake in a choice dietary environment in subordinate female rhesus monkeys (Moore et al., 2015), it remains to be determined how direct manipulation of the stress signals impacts reward neuroadaptations described in the current study.
Reduced D2R BP within the OFC, dlPFC, and NAcc was significantly associated with increased caloric intake, particularly of the CDD, in females exposed to the dietary choice condition. These data highlight the importance of dopaminergic function in the PFC and NAcc for behavioral responses to food (Petrovich, 2011). Lower levels of prefrontal D2R in females exposed to the dietary choice condition may facilitate increased caloric intake by increasing compulsivity and disrupting the ability to inhibit motivated behavior, as the dlPFC is critical for incentive-based behavioral responses (Haber and Knutson, 2010) and the OFC for goal-directed behavior, reward coding, impulse control and salience/value of food (Grabenhorst et al., 2008; Kuhnen and Knutson, 2005; Rolls and McCabe, 2007). Lower metabolic activity in the OFC, dlPFC, and NAcc is associated with increased food craving (Wang et al., 2009) and body mass index in humans, and is predictive of reduced executive function (Volkow et al., 2009). Importantly, lower prefrontal D2R levels have been linked to reduced sensitivity to reward in obese individuals, which may facilitate overeating (Davis et al., 2009), and decreased dopaminergic function in rodents has been linked to increased appetite (Cordeira et al., 2010; Geiger et al., 2008).
Differences in FC between corticostriatal regions were also significantly associated with increased total caloric intake and CDD consumption in females exposed to the dietary choice condition. Specifically, greater NAcc-dlPFC FC predicted greater CDD intake and reduced FC between the NAcc-ACC and NAcc-vmPFC predicted greater overall calorie intake. These results are divergent from previous findings in women indicating that FC between the NAcc and vmPFC and mPFC is increased in women who have high body mass index (Contreras-Rodriguez et al., 2015; Coveleskie et al., 2015). It is important to note, however, that existing rs-fMRI studies in the context of eating have focused on obesity as a surrogate measure of food intake as opposed to actual measures of calorie consumption assessed in the current study. To our knowledge, no studies have specifically investigated dlPFC connectivity with NAcc in the context of differences in food intake in an obesogenic environment, but studies in both drug addiction and obesity have shown alterations in NAcc FC with other prefrontal regions. For example, some studies show increased FC between NAcc and PFC subregions in both obesity (Contreras-Rodriguez et al., 2015; Coveleskie et al., 2015) and drug addiction (Ma et al., 2010; Wilcox et al., 2011), whereas other studies in drug addiction show decreased corticostriatal FC (Upadhyay et al., 2010; Wang et al., 2010). Although dlPFC-NAcc FC has not been explicitly investigated or was not significant in these previous studies, our finding that greater NAcc-dlPFC FC predicted greater CDD intake may reflect decreased functioning of reward circuitry and loss of PFC top-down inhibitory control, which in drug addiction studies is thought to precipitate the transition from using drugs into the development of addiction (Koob and Le Moal, 2008). Future studies are clearly necessary to characterize changes that occur in the initial periods of CDD access and worsen over time to facilitate greater caloric intake in dietary environments wherein CDD are available. Such studies will be critical for disentangling neuroadaptations specific to eating behavior as opposed to obesity, a consequence of increased caloric intake.
Overall, the patterns of FC and reduced D2R BP described in the current study suggest that these neuroanatomical alterations may together reflect lack of cognitive restraint of feeding as each of these regions is involved in executive function and impulse control. Critically, because the glucocorticoid-related changes in D2R and FC were not observed in the chow-only condition – and, indeed, OFC D2R BP as well as NAcc-dlPFC and NAcc-vmPFC FC were opposite in the choice compared with the chow-only condition – the current data underscore the hypothesis that an obesogenic diet interacts with experience-dependent increases in cortisol to promote feeding and increase the risk for obesity (Warne, 2009). The interactions, and underlying mechanisms, between how a complex dietary environment and social experience affect the brain and behavior highly warrant future elucidation and underscore the necessity for considering dietary environment in any translational animal model of adverse health outcomes in humans.
Despite the current data showing the importance of the dietary environment on shaping neural responses to differences in social experience, the study has several limitations. While the magnitude of the significant correlations was indicated by relatively high effect sizes, the fact that subsets of females from each diet condition contributed to PET and MRI imaging data limitations of the current study. Moreover, the low sample size used for these analyses is also a study limitation; however, the fact that these significant relationships were observed between cortisol, FC, and D2R-BP with these reduced samples sizes for females in the choice dietary conditions warrants further investigation of the synergistic effects of diet and adverse social experience on appetite regulation. Because were testing a priori hypotheses regarding associations between social experience, plasma cortisol, appetite and neuroimaging endpoints in this largely exploratory analysis, we did not correct for multiple comparisons. Thus, a concern that a significant correlation may be spurious is noted. Future studies are necessary with a larger sample size in order to replicate and extend these findings. Another limitation is that PFC ROIs assessed are not completely congruent between PET and rs-fMRI data analyses due to differences in image resolution, data processing and analytical pipelines. Furthermore, the present exploratory analysis is largely one of associations, despite the dietary intervention. To infer causality, the social experience and the dietary environment must be manipulated in the same females. For example, our group has previously manipulated social ranks in female monkeys and has shown that social experience associated with specific ranks has causal effects on immune gene regulation (Snyder-Mackler et al., 2016). Thus, a similar manipulation that would include a diet switch could be applied to the paradigm used in the present study to determine how diet and social experience causally shape brain function to regulate appetite. Additionally, the experiment was conducted exclusively in female monkeys, limiting whether the result of females, that are more at risk to overeat in the face of psychosocial stressors in an obesogenic dietary environment, is generalizable to males (Laitinen et al., 2002). Finally, despite the low levels of anesthesia used in this study, there may be potential isoflurane effects on BOLD signal compared to studies investigating awake states. However, the dose of isoflurane used in this study (and higher doses) has previously been used to report coherent patterns of BOLD fluctuations in rhesus macaques, including sensory, motor, visual and cognitive-task related systems (Birn et al., 2014; Hutchison et al., 2013; Li et al., 2013a; Tang and Ramani, 2016; Vincent et al., 2007). Future studies are necessary to understand the impact of psychosocial stressor exposure and the dietary environment on reward pathways and appetite regulation in males.
Supplementary Material
Research Highlights.
Chronic social subordination interacts with obesogenic dietary environment to facilitate increased food intake
Corticostriatal dopamine 2 receptor (D2R) binding and functional connectivity (FC) predict differences in caloric intake that are dependent on the dietary environment
Greater cortisol in the dietary choice condition was associated with lower prefrontal D2R-BP and NAcc-vmPFC FC.
This effect was not present in females in the chow-only diet condition
These consequences on reward pathways and appetite regulation may reflect impaired cognitive control of food intake.
Acknowledgments
The current study would not have been possible without the expert technical assistance of Jennifer Whitley, Jessica Johnson, Angela Tripp, Brandon Hughes, Juliet Brown, Paul Chen, Jordan Kohn, Patrick Ulam, Rebecca Herman, Venkatagiri Krishnamurthy, and Jonathan Lowe, as well as the dedication of the animal husbandry and veterinary staff at the YNPRC. Our study was supported by NIH grants DK096983 (MW), HD085850 (VM), ORIP/OD P51OD011132 (YNPRC), S10OD010757-01 (YNPRC Biomarkers Core). The YNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Footnotes
Disclosure
All authors have nothing to disclose.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aller EE, Abete I, Astrup A, Martinez JA, van Baak MA. Starches, sugars and obesity. Nutrients. 2011;3:341–369. doi: 10.3390/nu3030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003;20:870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neuroscience and biobehavioral reviews. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiology & behavior. 2010;101:446–455. doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP. The function of aggression in primate societies. Am Sci. 1974;62:304–311. [PubMed] [Google Scholar]
- Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM, Shelton SE, Alexander AL, Pine DS, Slattery MJ, Davidson RJ, Fox AS, Kalin NH. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Molecular psychiatry. 2014;19:915–922. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Increased dopamine metabolism in the nucleus accumbens and striatum following consumption of a nutritive meal but not a palatable non-nutritive saccharin solution. Pharmacology, biochemistry, and behavior. 1986;25:1095–1100. doi: 10.1016/0091-3057(86)90091-2. [DOI] [PubMed] [Google Scholar]
- Blair K, Marsh AA, Morton J, Vythilingam M, Jones M, Mondillo K, Pine DC, Drevets WC, Blair JR. Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. J Neurosci. 2006;26:11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MS, Gordon TP, Wilson ME. Effects of capture and venipuncture on serum levels of prolactin, growth hormone and cortisol in outdoor compound-housed female rhesus monkeys (Macaca mulatta) Acta endocrinologica. 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nature neuroscience. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Rodriguez O, Martin-Perez C, Vilar-Lopez R, Verdejo-Garcia A. Ventral and Dorsal Striatum Networks in Obesity: Link to Food Craving and Weight Gain. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Contreras-Rodriguez O, Martin-Perez C, Vilar-Lopez R, Verdejo-Garcia A. Ventral and Dorsal Striatum Networks in Obesity: Link to Food Craving and Weight Gain. Biological psychiatry. 2017;81:789–796. doi: 10.1016/j.biopsych.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30:2533–2541. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Coveleskie K, Gupta A, Kilpatrick LA, Mayer ED, Ashe-McNalley C, Stains J, Labus JS, Mayer EA. Altered functional connectivity within the central reward network in overweight and obese women. Nutr Diabetes. 2015;5:e148. doi: 10.1038/nutd.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LM, Michaelides M, Cheskin LJ, Moran TH, Aja S, Watkins PA, Pei Z, Contoreggi C, McCullough K, Hope B, Wang GJ, Volkow ND, Thanos PK. Bromocriptine administration reduces hyperphagia and adiposity and differentially affects dopamine D2 receptor and transporter binding in leptin-receptor-deficient Zucker rats and rats with diet-induced obesity. Neuroendocrinology. 2009;89:152–162. doi: 10.1159/000170586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree M, Michopoulos V, Votaw JR, Voll RJ, Mun J, Stehouwer JS, Goodman MM, Wilson ME, Sanchez MM. The relation of developmental changes in brain serotonin transporter (5HTT) and 5HT1A receptor binding to emotional behavior in female rhesus monkeys: effects of social status and 5HTT genotype. Neuroscience. 2013;228:83–100. doi: 10.1016/j.neuroscience.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang YF, Mostofsky S, Castellanos FX, Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Glass TA, Curriero FC, Stewart WF, Schwartz BS. The built environment and obesity: a systematic review of the epidemiologic evidence. Health Place. 2010;16:175–190. doi: 10.1016/j.healthplace.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. American journal of physiology. 2006;290:R1284–1293. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, Pothos EN. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. 2011;5:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18:1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD. Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Molecular psychiatry. 2014;19:1078–1084. doi: 10.1038/mp.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. The American journal of physiology. 1998;275:R1928–1938. doi: 10.1152/ajpregu.1998.275.6.R1928. [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Richard D. The role of corticotropin-releasing factor and urocortin in the modulation of ingestive behavior. Neuropeptides. 1999;33:350–359. doi: 10.1054/npep.1999.0047. [DOI] [PubMed] [Google Scholar]
- Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocrine reviews. 2006;27:750–761. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp. 2013;34:2154–2177. doi: 10.1002/hbm.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SP, Shafran I, Grayson D, Gates K, Nigg JT, Fair DA. Inferring functional connectivity in MRI using Bayesian network structure learning with a modified PC algorithm. NeuroImage. 2013;75:165–175. doi: 10.1016/j.neuroimage.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo E, Sanna PP, Koob GF. Impairment of dopaminergic system function after chronic treatment with corticotropin-releasing factor. Pharmacology, biochemistry, and behavior. 2005;81:701–708. doi: 10.1016/j.pbb.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiology & behavior. 2008;93:807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature neuroscience. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nature reviews. 2011;12:638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. The American journal of psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biological psychiatry. 1990;27:1094–1102. doi: 10.1016/0006-3223(90)90046-5. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Rosewell KL, Duncan MJ, Wise PM. Aging, estradiol and time of day differentially affect serotonin transporter binding in the central nervous system of female rats. Brain research. 2003;990:87–94. doi: 10.1016/s0006-8993(03)03441-3. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Luijendijk MC, van Rozen AJ, Kalsbeek A, Adan RA. A free-choice high-fat high-sugar diet induces glucose intolerance and insulin unresponsiveness to a glucose load not explained by obesity. Int J Obes (Lond) 2011;35:595–604. doi: 10.1038/ijo.2010.164. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, van Rozen AJ, Luijendijk MC, Groeneweg F, Adan RA. A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int J Obes (Lond) 2010;34:537–546. doi: 10.1038/ijo.2009.257. [DOI] [PubMed] [Google Scholar]
- Laitinen J, Ek E, Sovio U. Stress-related eating and drinking behavior and body mass index and predictors of this behavior. Prev Med. 2002;34:29–39. doi: 10.1006/pmed.2001.0948. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. The Journal of comparative neurology. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Li CX, Patel S, Auerbach EJ, Zhang X. Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neuroscience letters. 2013a;541:58–62. doi: 10.1016/j.neulet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Gao L, Wang XL, Chen L, Fang W, Ge SN, Gao GD. Deep brain stimulation of the bilateral nucleus accumbens in normal rhesus monkey. Neuroreport. 2013b;24:30–35. doi: 10.1097/WNR.0b013e32835c16e7. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. NeuroImage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- Markov NT, Ercsey-Ravasz M, Ribeiro Gomes A, Lamy C, Magrou L, Vezoli J, Misery P, Falchier A, Quilodran R, Gariel M. A weighted and directed interareal connectivity matrix for macaque cerebral cortex. Cerebral cortex. 2012;24:17–36. doi: 10.1093/cercor/bhs270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Kastman EK, Bendlin BB, Johnson SC. Rhesus macaque brain morphometry: a methodological comparison of voxel-wise approaches. Methods. 2010;50:157–165. doi: 10.1016/j.ymeth.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Oakes TR, Kroenke CD, Kohama SG, Matochik JA, Ingram DK, Johnson SC. A population-average MRI-based atlas collection of the rhesus macaque. NeuroImage. 2009;45:52–59. doi: 10.1016/j.neuroimage.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Hays TC, Del Paine SN, Luttrell VR. Induction of obesity by group housing in female Syrian hamsters. Physiology & behavior. 1990;47:815–817. doi: 10.1016/0031-9384(90)90002-l. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Diaz MP, Wilson ME. Social change and access to a palatable diet produces differences in reward neurochemistry and appetite in female monkeys. Physiology & behavior. 2016 doi: 10.1016/j.physbeh.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012a;37:1071–1085. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Perez Diaz M, Embree M, Reding K, Votaw JR, Mun J, Voll RJ, Goodman MM, Wilson M, Sanchez M, Toufexis D. Oestradiol alters central 5-HT1A receptor binding potential differences related to psychosocial stress but not differences related to 5-HTTLPR genotype in female rhesus monkeys. Journal of neuroendocrinology. 2014;26:80–88. doi: 10.1111/jne.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Hormones and behavior. 2012b;62:389–399. doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012c;37:1479–1490. doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- Miranda-Dominguez O, Mills BD, Grayson D, Woodall A, Grant KA, Kroenke CD, Fair DA. Bridging the gap between the human and macaque connectome: a quantitative comparison of global interspecies structure-function relationships and network topology. J Neurosci. 2014;34:5552–5563. doi: 10.1523/JNEUROSCI.4229-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CJ, Johnson ZP, Higgins M, Toufexis D, Wilson ME. Antagonism of corticotrophin-releasing factor type 1 receptors attenuates caloric intake of free feeding subordinate female rhesus monkeys in a rich dietary environment. Journal of neuroendocrinology. 2015;27:33–43. doi: 10.1111/jne.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse (New York, NY) 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse (New York, NY) 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, Nuutila P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PloS one. 2012;7:e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Parr LA, Boudreau M, Hecht E, Winslow JT, Nemeroff CB, Sanchez MM. Early life stress affects cerebral glucose metabolism in adult rhesus monkeys (Macaca mulatta) Dev Cogn Neurosci. 2012;2:181–193. doi: 10.1016/j.dcn.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. The rhesus monkey brain in sterotaxic coordinates. Academic Press; San Diego: 2000. [Google Scholar]
- Petrovich GD. Forebrain circuits and control of feeding by learned cues. Neurobiology of learning and memory. 2011;95:152–158. doi: 10.1016/j.nlm.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi P, Baldwin R, Salomon R, Anderson S, Ansari MS, Li R, Dawant B, Bauernfeind A, Schmidt D, Kessler R. Estimation of baseline dopamine D2 receptor occupancy in striatum and extrastriatal regions in humans with positron emission tomography with [18F] fallypride. Biological psychiatry. 2008;63:241–244. doi: 10.1016/j.biopsych.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. Opposing BOLD responses to reciprocated and unreciprocated altruism in putative reward pathways. Neuroreport. 2004;15:2539–2543. doi: 10.1097/00001756-200411150-00022. [DOI] [PubMed] [Google Scholar]
- Rolls ET, McCabe C. Enhanced affective brain representations of chocolate in cravers vs. non-cravers. The European journal of neuroscience. 2007;26:1067–1076. doi: 10.1111/j.1460-9568.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis N. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Academic Press; London; Burlington, MA: 2007. [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Silver NC, Dunlap WP. Averaging correlation coefficients: should Fisher’s z transformation be used. Journal of Applied Psychology. 1987;72:146–148. [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snyder-Mackler N, Sanz J, Kohn JN, Brinkworth JF, Morrow S, Shaver AO, Grenier JC, Pique-Regi R, Johnson ZP, Wilson ME, Barreiro LB, Tung J. Social status alters immune regulation and response to infection in macaques. Science (New York, NY) 2016;354:1041–1045. doi: 10.1126/science.aah3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. American journal of physiology. 2007;292:R283–290. doi: 10.1152/ajpregu.00330.2006. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, 3rd, Horwitz B. Effective connectivity of a reward network in obese women. Brain research bulletin. 2009;79:388–395. doi: 10.1016/j.brainresbull.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiology & behavior. 2006;89:536–542. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Tang CY, Ramani R. fMRI and Anesthesia. Int Anesthesiol Clin. 2016;54:129–142. doi: 10.1097/AIA.0000000000000081. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Critical reviews in biochemistry and molecular biology. 2013;48:1–19. doi: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandehey NT, Moirano JM, Converse AK, Holden JE, Mukherjee J, Murali D, Nickles RJ, Davidson RJ, Schneider ML, Christian BT. High-affinity dopamine D2/D3 PET radioligands 18F-fallypride and 11C-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab. 2010;30:994–1007. doi: 10.1038/jcbfm.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. The Journal of clinical investigation. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nature reviews. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical transactions of the Royal Society of London. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, Logan J, Wong C, Thanos PK, Ma Y. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity. 2009;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature neuroscience. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770–12776. doi: 10.1523/JNEUROSCI.3192-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong CT, Thanos PK, Geliebter A, Biegon A, Fowler JS. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne JP. Shaping the stress response: interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Molecular and cellular endocrinology. 2009;300:137–146. doi: 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- WHO, W.H.O. Obesity and Overweight Fact Sheet 2016. 2016. [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug and alcohol dependence. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiology & behavior. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philosophical transactions of the Royal Society of London. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. 2011;12:131–141. doi: 10.1111/j.1467-789X.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Buchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.