Summary
Background
Progenitor cells play critical roles in epithelial repair following ischaemic injury. Protein biomarkers have been used to identify intestinal progenitor cell subpopulations. This study aims to determine if a critical number of intestinal progenitor cells can predict tissue viability and survival to discharge of large colon volvulus (LCV) cases.
Objectives
The objectives were to (1) identify intestinal progenitor cell subpopulations using biomarkers: proliferating cell nuclear antigen (PCNA), sex determining region Y box 9 (SOX9), phospho-histone H3 (PHH3), and Ki-67, (2) define cutoff values for critical numbers of positive cells and (3) determine if survival to discharge is associated with cutoff values.
Study design
Retrospective cohort study.
Methods
Adult horses admitted to the Farm and Equine Veterinary Medical Center at NC State’s Veterinary Hospital and Peterson and Smith Equine Hospital between 2006 and 2016 that underwent an exploratory coeliotomy with a diagnosis of LCV of ≥360 degrees, had pelvic flexure biopsy, and that recovered from general anaesthesia were selected for inclusion in the study. Immunohistochemical analyses were performed and positive cells were counted. Optimal cutoff values were determined using receiver operator curves. A Fisher’s exact test was used to associate cutoff values with survival to discharge.
Results
Twenty-three cases of LCV ≥360° were included in this study. Thirteen of 23 (57%) horses survived to discharge. A cutoff of <2.1 PHH3 positive cells per crypt correctly predicted death with 100% sensitivity (95% CI; 69.15-%-100%) and 84.62% specificity (95% CI; 54.55%-99.08%). LCV cases with <2.1 PHH3 positive cells per crypt were 96.6 times more likely to die (95% CI; 4.142–2253 and p<0.001). Biomarkers PCNA, SOX9, and Ki-67 did not predict short-term survival.
Main limitations
The population size was small.
Conclusions
PHH3 immunohistochemical analysis may assist in more accurate prediction of survival to hospital discharge of LCV cases.
Keywords: horse, large colon volvulus, immunohistochemistry, phospho histone H3, intestine, stem cell
Introduction
Of the many causes of colic, intestinal strangulation is an acute and rapidly fatal subset requiring surgical intervention. Large colon volvulus (LCV) is particularly lethal, with survival to discharge ranging from 36–88% [1–3]. Studies have utilised pre-, intra and post-operative parameters to aid in prognosis determination [2, 4–6]. Nonetheless, intra-operative determination of the degree of colonic damage remains elusive to surgeons. Studies have evaluated histomorphometry of intraoperatively collected pelvic flexure biopsies to determine outcome of LCV cases [2,7,8]. However, significant parameters, such as interstitial crypt ratio, haemorrhage score, and the overall predictive capacity of histomorphometric measurements, have varied between studies [2,7,8]. An intra-operative method to determine the regenerative capacity and viability of intestinal tissue in the face of the ischaemic events associated with LCV may prove more precise and may better inform surgeons and owners to improve clinical decision making.
Resident multipotent epithelial stem cells regenerate the mucosal layer of the intestine. Within large colon mucosa, epithelial cells are arranged into crypts of Lieberkühn. Crypts are composed of mature enterocytes nearest the luminal surface, partially differentiated transit amplifying cells, and stem cells residing at the base. These critical stem cells regenerate the crypt epithelium every 5–7 days [9,10]. One study concluded that epithelial cell loss extending beyond 60% of the crypt was associated with death in LCV cases [8]. Since cell loss associated with ischaemic injury begins at the luminal surface and extends toward the crypt base with increasing severity, loss of 60% of the crypt damages the transit amplifying cells and potentially the critical resident stem cell population. Damage to these cells may result in an inability of the crypt to regenerate the epithelial lining and loss of tissue viability.
This clinical study aimed to identify if a minimum critical number of stem and progenitor cells are needed for colonic viability and determine if this information could predict short-term survival in surgical LCV cases. The specific objectives were to (1) identify stem and progenitor cells using the following protein biomarkers PHH3, SOX9, PCNA and Ki67, (2) determine cutoff values for a critical number of stem and progenitor cells for each biomarker, and (3) predict survival to discharge using determined cutoff values.
Materials and Methods
Study population and inclusion criteria
Medical records from 2006–2016 were collected from North Carolina State University Equine Hospital and Peterson and Smith Equine Hospital for recovered surgical equine LCV cases with volvulus of ≥360°. Intraoperative pelvic flexure biopsies were used for the study, and case survival was defined as horses that survived to discharge from the hospital.
Specimen Preparation
Intraoperative, full thickness pelvic flexure biopsies were immediately fixed in 10% neutral buffered formalin at room temperature (approximately 21°C). Fixation times were no less than 24 hours long and duration was unknown for most samples. Samples were then embedded in paraffin wax and sectioned at approximately 5 to 8 µm thickness and mounted on positively-charged glass slides. Sectioned samples were stained with haematoxylin and eosin to verify and evaluate crypt positioning. For immunohistochemical analysis, sections were deparaffinised, and rehydrated. Heat induced epitope retrieval was performed by placing slides into citrate Target Retrieval Solutiona for 30 s at 119°C followed by 90°C for 10 s in a Pascal pressure chambera. Slides were cooled to room temperature and moved to a DakoAutostainerPlusa after which they were incubated in a peptide-blocking agent, Background Busterb, for 30 minutes. Primary antibodies were selected that have been shown to positively identify stem and progenitor cells in normal equine tissue [9]. Primary antibodies were applied to tissue sections diluted in Common Antibody Diluentc. mouse α-PCNAd was diluted to 1:3500, rabbit α-SOX9e was diluted 1:200, rabbit α-phospho-histone H3e was diluted to 1:500, and mouse α-Ki67f was diluted to 1:500. Primary antibody incubation was performed for 30 minutes at room temperature. Slides were then incubated for 30 minutes in a 1:1 dilution of ImmPRESS™ (Peroxidase) Polymer anti-rabbit IgG reagentg followed by 30 minutes in ImmPact DAB peroxidase (HPR) substrate solutiong.
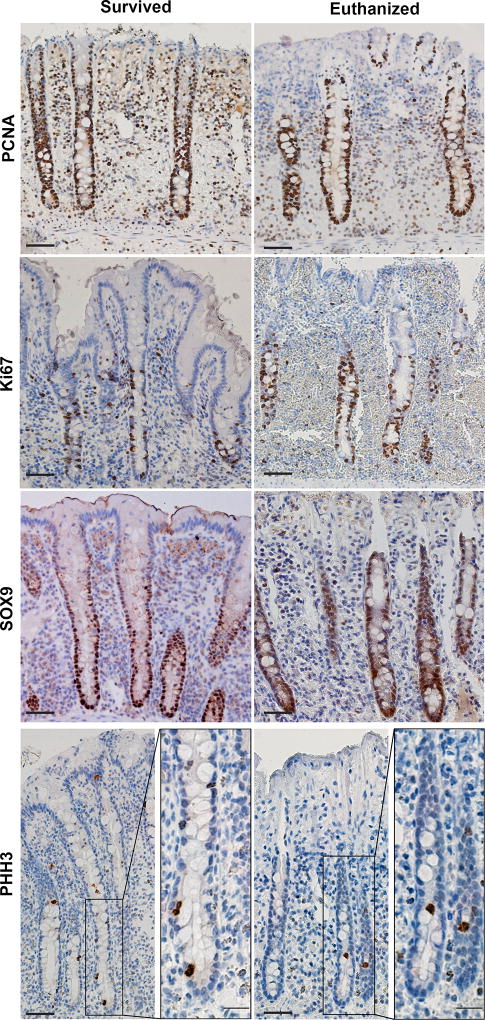
Investigators analysing the histologic slides were blinded to case outcome. For each slide evaluated, only well-oriented crypts were used to obtain cell counts. A well-oriented crypt was identified as one with a crypt base closely apposed to the muscularis mucosa layer and that extended and opened fully into the gut lumen (Fig 1). For each case of LCV, investigators imaged 6–10 well-oriented crypts for each protein biomarker and averaged the number of positive cells counted per crypt [11]. All images were captured using a light microscope (Olympus IX81)h fitted with a digital camera (Hamamatsu)i. The objective lensesh used were X10 and X20 with numerical apertures of 0.3 and 0.5, respectively. The cell counter feature in ImageJ (a public domain, Java-based image processing program developed at the National Institutes of Health and available as freeware from http://rsbweb.nih.gov/ij)j was used to count the cells.
Fig 1.
Representative immunohistochemical images from cases of large colon volvulus. Protein biomarker expression of PCNA, Ki67, SOX9 and PHH3 from LCV cases that survived and that were euthanised. Scale bar 50 micrometers, scale bar insets 20 micrometers.
Data Analysis
All statistical analyses were performed using Prismk. D’Agostino-Pearson omnibus normality test was performed on raw data obtained from counting cells positive for each biomarker. The mean positive cell counts per case for each biomarker were analysed using receiver operator curves (ROC) to determine a cutoff value of stem and progenitor cells for use in further analysis to predict survival. The optimal cutoff value for each biomarker was determined by using the equation d = (1 − sensitivity)2 + (1 − specificity)2, where the cutoff that corresponds to the smallest value of d was chosen. A Fisher’s exact test using the Woolf logit method to determine the confidence interval was then used to determine the association of biomarker cutoff values with survival to discharge. A p-value ≤0.05 was considered statistically significant.
Results
Twenty-three horses met the criteria for inclusion. The ages of the horses ranged from 2 years to 23 years with a median of 10 years. Seventeen of the horses were mares, and 6 were geldings. Seven breeds were represented: 7 Thoroughbred, 6 Quarter horse, 4 Warmblood, 2 Paso Fino, 2 Arabians, one Paint, and one Tennessee Walking horse. Of the 23 horses, 13 survived until discharge (57%). Through the course of this study, a total of 53 LCV samples were admitted for potential inclusion, however 30 of these samples were omitted due to over-fixation of the samples leading to poor biomarker stain uptake and inability to properly evaluate the presence of the biomarkers. The over-fixation of samples was attributed to prolonged storage of the samples in formalin. Of the 23 included samples, 8 horses had colonic resection and anastomosis, and of these cases, 4 (50%) were euthanised or died within days of recovery for various reasons including laminitis (n = 2), endotoxaemia (n = 1), or reported post-operative ileus (n = 1). Of the 15 cases that were not resected, 6 (40%) were euthanised due to multiple organ failure (n = 1), laminitis (n = 1), endotoxaemia (n = 1), and general worsening signs of prognosis such as prolonged increased heart rate, respiratory rate, and diarrhoea (n = 3).
Positive cell count values of PCNA, SOX9 and Ki67 were all normally distributed. Mean ± s.d. values for PCNA, SOX9 and Ki67 were 61.89 ± 9.61, 28.20 ± 14.78 and 15.39 ± 9.08 positive cells per crypt, respectively. Values of PHH3 cell counts were not normally distributed. The median value for PHH3 was 2.56 (interquartile range, 0.875–3.8).
Using receiver operator characteristic (ROC) curve analysis, the only biomarker found to have a statistically significant cutoff value was PHH3. Cases with <2.10 average PHH3 positive cells per crypt predicted death in LCV cases with 100% sensitivity (95% confidence interval (CI); 69.15%-100%) and 84.62% specificity (95% CI; 54.55%-98.08%) with an area under the curve (AUC) of 0.93 (standard error (s.e.) 0.053, 95% CI; 0.83–1.04) and a significant p value equal to 0.0005 (Table 1, Supplementary Item 1.
Table 1.
Receiver operator characteristic curve analysis to determine biomarker cutoff values. Data represents information for 23 horses diagnosed with LCV. A value of area under the curve (AUC) equal to 1 denotes 100% sensitivity and specificity. Phosphohistone H3 (PHH3) was the only biomarker found to have a statistically significant cutoff value. Cases with <2.10 average PHH3 positive cells per crypt predicted death in LCV cases.
| Biomarker | PCNA | SOX9 | Ki67 | PHH3 |
|---|---|---|---|---|
| Cutoff Value | 62.50 | 32.35 | 19.50 | 2.10 |
| AUC | 0.64 | 0.5 | 0.4 | 0.93 |
| Standard Error | 0.12 | 0.13 | 0.13 | 0.053 |
| 95% CI | 0.41–0.88 | 0.21–0.72 | 0.12–0.58 | 0.83–1.04 |
| P value | 0.25 | NA | NA | 0.0005 |
| Sensitivity % (95% CI) | 60 (26.24–87.84) | 60 (26.24–87.84) | 60 (26.24–87.84) | 100 (69.15–100) |
| Specificity % (95% CI) | 61.54 (31.58–86.14) | 38.46 (13.86–68.42) | 38.46 (13.86–68.42) | 84.62 (54.55–98.08) |
A Fisher’s Exact test determined that cases with <2.10 average PHH3 positive cells per case were 96.60 times more likely to die before discharge (95% CI; 4.14–2253 p value ≤0.05). Biomarkers PCNA, SOX9, and Ki-67 were unable to significantly predict death (Table 2, Fig 2).
Table 2.
Fisher’s exact test analysis of biomarker cutoff values associated with death in cases of LCV. Data represents information for 23 horses diagnosed with LCV. The odds ratio for each biomarker represents the odds a horse with LCV will not survive to discharge.
| Biomarker | Cutoff Value | No. (%) Horses | OR | 95% CI | P Value |
|---|---|---|---|---|---|
| PCNA | <62.5 | 11/23(47.8) | 2.4 | 0.44–13.0 | 0.44 |
| ≥62.5 | 12/23(52.2) | ||||
| PHH3* | <2.1 | 12/23(52.2) | 96.6 | 4.14–225 | <0.0001 |
| ≥2.1 | 11/23(47.8) |
Asterisk indicates a variable found to be statistically significant (p<0.05).
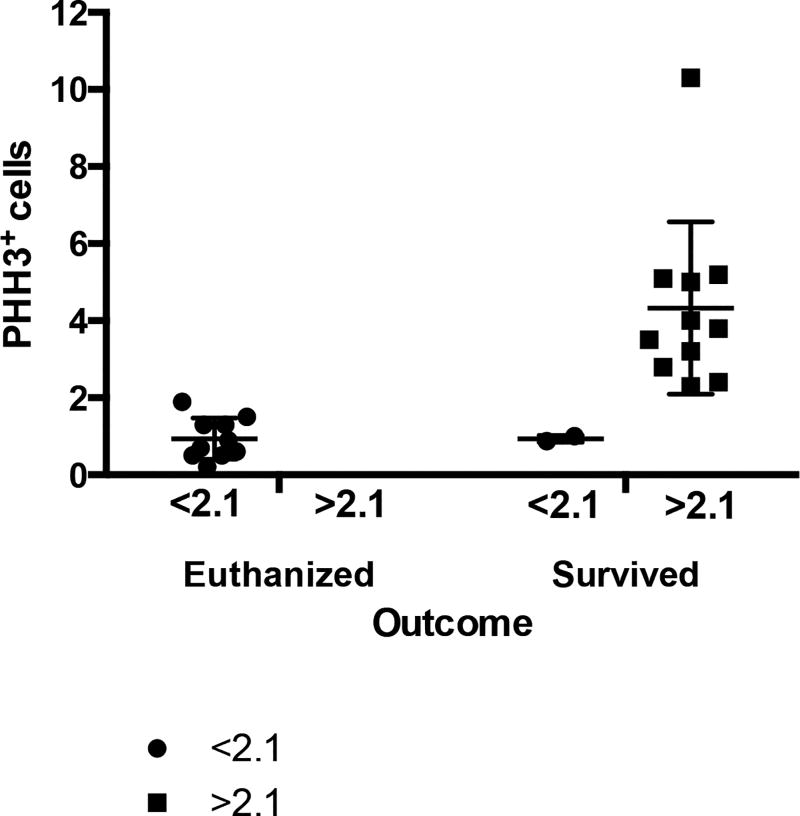
Fig 2.
Scatter dot plot of horses that were subjected to euthanasia or survived based on PHH3 biomarker expression. Phosphohistone H3 biomarker expression was measured from biopsies obtained from cases of large colon volvulus, indicating that most horses with a PHH3 cell count of <2.1 were likely to die. Interquartile range and median are represented by horizontal lines. Error bars represent standard deviation, and dots represent individual values.
Of the 12 horses predicted to die as determined by the cutoff value of <2.10 PHH3 positive cells per crypt, death prior to discharge was correctly predicted in 10 cases (83.33%), and 11 horses were accurately predicted to survive to discharge (100%). No horse was falsely predicted to survive short-term that ultimately died. Therefore, PHH3 accurately predicted short-term survival in 21 of 23 horses, with an accuracy of 91.30%.
Discussion
This study successfully used immunohistochemical techniques in pelvic flexure biopsy samples derived from clinical cases of LCV to determine short-term outcome. Of the currently known and previously optimised antibodies used to identify protein biomarkers of progenitor cells in equine intestine, PHH3 was found to accurately predict short-term survival in 91.30% of the cases. With future development of other, more rapid, antibody-based technologies, the identification of biomarkers to determine tissue viability holds promise for clinical use, both in determination of the need for intestinal resection as well as an improved prognostic indicator.
Accurate determination of both short-term and long-term prognosis in cases of LCV has continued to elude clinicians and has spurred the need for improved methods to determine outcome. Pre-operative and post-operative parameters have been used as indicators of survival [2, 4–6]. Pre-operative values of increased packed cell volume, heart rate, and blood lactate concentration have been shown to predict decreased LCV survival time [2, 4, 5, 6]. Similarly, operative and post-operative parameters of resection, relaparotomy, post-operative colic, as well as packed cell volume and heart rate at 24 and 48 hours post-operative are associated with decreased LCV survival time [2, 4–6].
In contrast to the pre-operative measured parameters, most intraoperative methods have focused on utilising gross changes in tissue appearance or subjective measures to determine tissue viability [1, 2, 7, 8]. Other studies have utilised histologic evaluations of intestinal biopsies in an attempt to better evaluate tissue damage [2, 7, 8]. Specifically, increases to interstitial-to-crypt ratio as well as degree of haemorrhage and loss of glandular epithelium have each been associated with poor case outcome [7, 8]. As in this study, these previous studies utilised pelvic flexure biopsies from equine LCV surgical cases as they have also been shown to represent changes that occur throughout the ascending colon [12]. However, at least one study questioned the utility of pelvic flexure histologic measurements to determine outcome [2]. The discrepancy between these studies may be the result of limitations of histologic evaluation. Histomorphometry evaluates tissue at a specific time point, and does not directly indicate cellular processes and the ability of a cell to overcome injury in order to contribute to repair following correction of a strangulating lesion.
Few studies have evaluated cellular activity and specific measures of tissue viability following intestinal ischaemic injury in horses. Rowe et al., used the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) technique to count the number of apoptotic cells as a means to determine tissue injury [13]. Although the study identified increases in apoptotic cells in both horses with simple obstruction or strangulating obstruction compared to normal horses, no conclusions were made as to whether apoptosis affects intestinal function or case outcome.
More important than the amount of cell death that occurs within injured tissues is, arguably, the capacity of tissue to regenerate. Immediately following severe ischaemic injury, when epithelial cells are sloughed into the lumen of the intestine, the mucosa responds by contracting in order to decrease the surface area. Remaining epithelial cells elongate in an attempt to cover exposed basement membrane and bridge defects between adjoining cells, a process called restitution. Stem cell differentiation and self-renewal is then critical to first re-populate lost glandular epithelial cells within the crypt and ultimately, the luminal epithelial cells. This process is essential to intestinal repair and recovery of normal intestinal function. A previous study indicated that damage to the colon crypt impairs the ability of the colon to survive. In that study, greater than 60% glandular epithelial loss was associated with increased mortality [8]. We surmise that this association with glandular epithelial cell loss was due to a reduction of the intestinal stem and progenitor cells that reside deep within the crypt. For this reason, we selected protein biomarkers known to identify progenitor type cells in active stages of cellular proliferation for this study.
Normally, intestinal stem cells differentiate into new mature enterocytes through an active cycle of cellular division. The cell cycle consists of a resting state, Gap 0 (G0) phase, three phases collectively termed interphase, and the mitotic (M) phase. When a cell is in interphase it increases in size during gap 1 (G1), then moves into synthesis phase (S) for DNA replication, and continues into gap 2 (G2) for further cell growth. Following interphase, the stem cell transitions into M phase for cell division into two asymmetrical daughter cells, one to replace the parent stem cell and one to become a transit-amplifying cell. Most transit-amplifying cells continue to differentiate into either an absorptive or secretory cell lineage as they migrate toward the luminal surface. During these different stages of the cell cycle, protein biomarkers are used to identify cells within specific stages [9]. Proliferating cell nuclear antigen is a general marker of cell proliferation and therefore identifies cells in all active stages of the cell cycle, G1, S, G2 and M. Similarly, Ki67 is strictly associated with proliferation and is expressed in all stages except G0. However, Ki67 expression is generally accepted to be more specific than that of PCNA, a finding consistent with the data obtained in this study with the mean number of PCNA expressing cells measured to be 61.89 and that of Ki67 only 15.39 per crypt. The more discrete expression of Ki67 within a crypt may be attributed to the suspected functional role of Ki67 to regulate the progression of cells through each stage of cellular division [14, 15]. However, the broad expression of PCNA and Ki67 throughout the cell cycle may explain the lack of significance found in this study for use as prognostic indicators.
Compared to PCNA and Ki67, SOX9 is considered to be a selective biomarker, identifying discrete populations of cells near the crypt base [15]. SOX9 has been shown to play a critical role as a transcription factor within intestinal stem cells [17]. However, although SOX9 is a marker of stem cells within the crypt base, it is also expressed in a subpopulation of transit amplifying cells. This may have contributed to the lack of significance of SOX9 positive cell number with survival. Therefore, because the protein biomarkers PCNA, SOX9 and Ki-67 were unable to predict death, examination of a more specific biomarker of active proliferation, PHH3, was evaluated.
PHH3 expression is limited to a small number of proliferating cells specifically within the G2 to M phase of the cell cycle, unlike the other biomarkers evaluated [18]. This unique expression pattern may have contributed to the significance found in association with PHH3 expression and case outcome. Horses were 96.6 times more likely to die when the average PHH3 count was less than 2.1 cells per crypt. This biomarker of proliferation was the single significant biomarker to associate cellular expression with survival to discharge following surgical correction of a LCV.
Interestingly, PCNA, Ki-67 and PHH3 have all been used to determine prognosis in human cases of cancer [14, 15, 18]. Contrary to our study utilising these biomarkers to indicate regenerative potential, these studies associated increased signs of proliferation with poor prognosis, as would be expected for cancer. A recent study directly compared the histologic protein expression Ki-67 to PHH3 and concluded that PHH3 was superior to Ki-67 as a prognostic indicator in human breast cancer patients [18]. The finding of PHH3 as a more robust indicator of prognosis is likely due to the discrete expression pattern of PHH3 during mitosis [18]. In fact, cells undergoing apoptosis cannot express PHH3, unlike the biomarker Ki-67 that may label cells that ultimately die [20]. These additional studies of cell proliferation markers further underscore the significance of PHH3 as a more specific biomarker of the capacity of tissue to regenerate.
The major limitation of this study was the population size. This was partly due to the exclusion of cases that were not permitted to recover from general anaesthesia. An owner’s decision to euthanise intra-operatively may be influenced by multiple factors and many times is based on financial concerns or perceived poor prognosis of survival. A further cause of case exclusion was the result of over fixation due to an extended storage of samples in formalin. These samples were collected prior to the development of the immunohistochemical techniques used in this study [9]. Ideal tissue fixation for immunohistochemical assays varies depending on the thickness of the tissue but is approximately 24 hours in formalin. The tissue should then be transferred and stored in 70% ethanol for further processing at a later date. Future LCV case sampling will utilise these improved fixation techniques, thereby increasing the available data set. Additional case numbers are critical to improve our knowledge of the accuracy of the technique to predict survival to discharge. In addition, the ability of the technique to predict long-term survival should be assessed in the future.
Although immunohistochemical techniques are limited by the same delay from tissue acquisition to biopsy evaluation as histomorphometric analyses, the long-term goal is to confidently identify a protein biomarker or multiple markers used in a fast acting immunoassay diagnostic test such as an ELISA, the technology used in SNAP tests. Additionally, although expression of PCNA, Ki-67 and SOX9 in histologic sections were not associated with case outcome in this study, these findings may be more associated with the sensitivity of the testing method since genes associated with these proteins have been shown to play a critical role in cellular regeneration and stem cell function.
This study has taken a novel approach to determine the regenerative capacity of intestinal tissue in the hope to make an association with case outcome. A more precise prediction of case outcome will improve medical decision making for both owners and surgeons regarding outcome of surgery, the welfare of the horse, and the decisions regarding financial investments made to recover the horse.
Supplementary Material
Acknowledgments
The authors would like to thank the histopathology laboratory at NCSU College of Veterinary Medicine for their processing of all biopsy specimens.
Sources of funding
This work was funded by a generous grant from NC State CVM Equine Health Program (CRK), George H. Hitchings New Investigator Award in Health Research (CRK) and the NIH Special Emphasis Research Career Award (SERCA, K01OD0199, LMG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Authors’ declaration of interests
No competing interests have been declared.
Ethical animal research
Procedures were approved by the North Carolina State University Institutional Care and Use Committee. Mucosal biopsies were obtained with the consent of the owners.
Authorship
L. Gonzalez and C. Kucera contributed with study design, study execution, data analysis and interpretation, and preparation of the manuscript. L. Stranahan and F. Hughes contributed with study execution. A. Blikslager contributed with study design. All authors gave their final approval of the manuscript.
Manufacturers’ addresses
DakoCytomation, Glostrup, Denmark.
Innovex Biosciences, Richmond, California, USA.
Biogenex, Fremont, California, USA.
Abcam, Cambridge, Massachusetts, USA.
EMD Millipore, Temecula, California, USA.
Dako, Carpinteria, California, USA.
Vector Laboratories, Burlingame, California, USA.
Olympus, Tokyo, Japan.
Hamamatsu, Japan.
National Institutes of Health, Bethesda, Maryland, USA.
GraphPad Software, La Jolla, California, USA.
References
- 1.Snyder JR, Pascoe JR, Olander HJ, Spier SJ, Meagher DM, Bleifer DR. Strangulation volvulus of the ascending colon in horses. J. Am. Vet. Med. Assoc. 1989;195:757–764. [PubMed] [Google Scholar]
- 2.Levi O, Affolter VK, Benak J, Kass PH, Le Jeune SS. Use of pelvic flexure biopsy scores to predict short-term survival after large colon volvulus. Vet. Surg. 2012;41:582–588. doi: 10.1111/j.1532-950X.2012.00994.x. [DOI] [PubMed] [Google Scholar]
- 3.Ducharme NG, Hackett RP, Ducharme GR, Long S. Surgical treatment of colic results in 181 horses. Vet. Surg. 1983;12:206–209. [Google Scholar]
- 4.Suthers JM, Pinchbeck GL, Proudman CJ, Archer DC. Survival of horses following strangulating large colon volvulus. Equine Vet. J. 2013;45:219–223. doi: 10.1111/j.2042-3306.2012.00620.x. [DOI] [PubMed] [Google Scholar]
- 5.Proudman CJ, Edwards GB, Barnes J, French NP. Modelling long-term survival of horses following surgery for large intestinal disease. Equine Vet. J. 2005;37:366–370. doi: 10.2746/0425164054529328. [DOI] [PubMed] [Google Scholar]
- 6.Johnston K, Holcombe SJ, Hauptman JG. Plasma lactate as a predictor of colonic viability and survival after 360° volvulus of the ascending colon in horses. Vet. Surg. 2007;36:563–567. doi: 10.1111/j.1532-950X.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez LM, Fogel CA, Baker WT, Hughes FE, Law JM, Motsinger-Reif AA, Blikslager AT. Operative factors associated with short-term outcome in horses with large colon volvulus: 47 cases from 2006 to 2013. Equine Vet. J. 2015;47:279–284. doi: 10.1111/evj.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Hoogmoed L, Snyder JR, Pascoe JR, Olander HJ. Use of pelvic flexure biopsies to predict survival after large colon torsion in horses. Vet. Surg. 2000;29:572–577. doi: 10.1053/jvet.2000.17836. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez LM, Kinnin LA, Blikslager AT. Characterization of discrete equine intestinal epithelial cell lineages. Am. J. Vet. Res. 2015;76:358–366. doi: 10.2460/ajvr.76.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker N, van der Wetering M, Clevers H. The intestinal stem cell. Gene Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am. J. Physiol.-Gastr. L. 2007;293:1013–1022. doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- 12.van Hoogmoed L, Snyder JR, Pascoe JR, Olander HJ. Evaluation of uniformity of morphological injury of the large colon following severe colonic torsion. Equine Vet. J. 2000;32(Suppl. 32):98–100. doi: 10.1111/j.2042-3306.2000.tb05343.x. [DOI] [PubMed] [Google Scholar]
- 13.Rowe EL, White NA, Buechner-Maxwell V, Robertson JL, Ward DL. Detection of apoptotic cells in intestines from horses with and without gastrointestinal tract disease. Am. J. Vet. Res. 2003;64:982–988. doi: 10.2460/ajvr.2003.64.982. [DOI] [PubMed] [Google Scholar]
- 14.Malkas LH, Herbert BS, Waleed A, Dobrolecki LE, Liu Y, Agarwal B, Hoelz D, Badve S, Schnaper L, Arnold RJ, Mechref Y, Novoty MV, Loehrer P, Goulet RJ, Hickey RJ. A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. PNAS. 2006;103:19472–19477. doi: 10.1073/pnas.0604614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholzen T, Gerdes J. The ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez LM, Williamson I, Piedrahita JA, Blikslager AT, Magness ST. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLOS One. 2013;8:e66465. doi: 10.1371/journal.pone.0066465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roche KC, Gracz AD, Liu XF, Newton V, Akiyama H, Magness ST. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology. 2015;149:1553–1563. doi: 10.1053/j.gastro.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerring Z, Pearson JE, Morrin HR, Robinson BA, Harris GC, Walker LC. Phosphohistone H3 outperforms Ki67 as a marker of outcome for breast cancer patients. Histopathology. 2015;67:538–547. doi: 10.1111/his.12678. [DOI] [PubMed] [Google Scholar]
- 19.Hans F, Demitrov F. Histone H3 phosphorylation and cell division. Oncogene. 2001;20:3021–3027. doi: 10.1038/sj.onc.1204326. [DOI] [PubMed] [Google Scholar]
- 20.Alkhasawneh A, Reith JD, Toro TZ, Ayed AO, Lu X, George TJ, Duckworth LV. Interobserver variability of mitotic index and utility of PHH3 for risk stratification in gastrointestinal stromal tumors. Am. J. Clin. Pathol. 2015;143:385–392. doi: 10.1309/AJCPAPH28VHZEKNQ. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.