Abstract
The photorespiratory pathway is comprised of enzymes localized within three distinct cellular compartments: chloroplasts, peroxisomes, and mitochondria. Photorespiratory enzymes are encoded by nuclear genes, translated in the cytosol, and targeted into these distinct subcellular compartments. One likely means by which to regulate the expression of the genes encoding photorespiratory enzymes is coordinated temporal control. We have previously shown in Arabidopsis that a circadian clock regulates the expression of the nuclear genes encoding both chloroplastic (Rubisco small subunit and Rubisco activase) and peroxisomal (catalase) components of the photorespiratory pathway. To determine whether a circadian clock also regulates the expression of genes encoding mitochondrial components of the photorespiratory pathway, we characterized a family of Arabidopsis serine hydroxymethyltransferase (SHM) genes. We examined mRNA accumulation for two of these family members, including one probable photorespiratory gene (SHM1) and a second gene expressed maximally in roots (SHM4), and show that both exhibit circadian oscillations in mRNA abundance that are in phase with those described for other photorespiratory genes. In addition, we show that SHM1 mRNA accumulates in light-grown seedlings, although this response is probably an indirect consequence of the induction of photosynthesis and photorespiration by illumination.
Ser hydroxymethyltransferase (SHMT; EC 2.1.2.1) is a tetramer of pyridoxal 5′-phosphate-containing 53-kD subunits that catalyzes the reversible conversion of Ser and tetrahydrofolate (THF) to Gly and 5,10-methylene THF (Schirch, 1982). Methylene THF can be converted to methyl-, methenyl- and formyl-THF, which serve as the primary sources of one-carbon (C1) units of differing oxidation states for biosynthetic reactions in amino acid, purine, pyrimidine, and lipid synthesis (Cossins, 1987). Eukaryotes have both cytosolic and mitochondrial SHMT isoforms (Cossins, 1987), and evidence is accumulating for the intracellular partitioning of Ser and Gly metabolism (Narkewicz et al., 1996; Kastanos et al., 1997).
The plant photorespiratory pathway provides a novel role for SHMT. The primary controlling step in photosynthesis is the fixation of CO2, which is catalyzed by Rubisco (Bowes, 1991). In addition to catalyzing the carboxylation of its substrate, ribulose-1,5-bisphosphate (RuBP), to initiate the Calvin cycle, Rubisco also catalyzes the oxygenation of RuBP to initiate the photorespiratory pathway (Leegood et al., 1995; Douce and Neuberger, 1999). The oxygenation of RuBP yields one molecule each of 2-phosphoglycolate and 3-phosphoglycerate. The photorespiratory phosphoglycolate is recycled through a complex series of reactions occurring in chloroplasts, peroxisomes, and mitochondria. In the mitochondria of a photorespiring plant leaf, SHMT is associated with the Gly decarboxylase complex (GDC; Douce and Neuberger, 1999). GDC catalyzes the decarboxylation of photorespiratory Gly, derived from phosphoglycolate, to yield NH3, CO2, and a C1 unit that is transferred to THF to form methylene THF (Oliver and McIntosh, 1995). The CO2 from the Gly decarboxylation is evolved as photorespiratory CO2. The C1 unit from the methylene THF is transferred by SHMT to a second molecule of Gly to form Ser, which is recycled into the chloroplastic Calvin cycle after conversion to phosphoglycerate.
Consistent with roles of SHMT and GDC in photorespiration, mutants deficient in mitochondrial SHMT or GDC exhibit symptoms of chlorosis under normal atmospheric conditions (Leegood et al., 1995). Under elevated CO2 concentrations, which suppress photorespiration, Arabidopsis or barley mutants deficient in SHMT (Somerville and Ogren, 1981) or in GDC (Somerville and Ogren, 1982; Blackwell et al., 1990) grow normally. This demonstrates that mitochondrial SHMT and GDC are only essential in the metabolic reactions of photorespiration. In non-photorespiratory conditions, C1-THF mediated biosynthesis is not impaired, implying activity of other SHMT isoforms (Somerville and Ogren, 1981). Pea has two mitochondrial SHMT isoforms, and a third plastid isozyme was postulated to account for SHMT activity in non-photosynthetic tissue (e.g. roots) and in the dark (Turner et al., 1992a). This is consistent with the retention of about 15% of non-mitochondrial SHMT activity in the Arabidopsis stm mutant (Somerville and Ogren, 1981).
The coordination of the photorespiratory pathway poses an interesting regulatory problem. One key aspect of this coordinated regulation is the temporal control of the nuclear genes encoding photorespiratory proteins that are targeted into these three distinct subcellular compartments: chloroplasts, peroxisomes, and mitochondria. We previously showed in Arabidopsis that a circadian clock regulates the expression of the nuclear genes encoding both chloroplastic and peroxisomal components of the photorespiratory pathway. The genes encoding the chloroplastic small subunit of Rubisco (RBCS) and Rubisco activase (RCA) exhibit oscillations in mRNA abundance that peak around dawn (Pilgrim and McClung, 1993). Similarly, the mRNA abundance of the peroxisomal photorespiratory catalase (CAT2) exhibits a maximum at the same circadian phase as RBCS and RCA (Zhong et al., 1994; Zhong and McClung, 1996).
To determine whether a circadian clock also regulates the expression of genes encoding mitochondrial components of the photorespiratory pathway, we set out to characterize Arabidopsis genes encoding Ser hydroxymethyltransferase (SHM). In prokaryotes, single genes encode SHMT functions. In animals and fungi, two SHMT isoforms (cytosolic and mitochondrial) are encoded by two distinct nuclear genes (Martini et al., 1987, 1989; Garrow et al., 1993; McNeil et al., 1994). To our surprise, the Arabidopsis genome includes at least five SHM genes. Our prediction of clock regulation of SHM expression was accurate: Both the Arabidopsis genes examined, including one probable photorespiratory gene (SHM1) and a second gene expressed maximally in roots (SHM4), show circadian oscillations in mRNA abundance.
RESULTS
Ser Hydroxymethyltransferase Is Encoded by a Gene Family in Arabidopsis
Initial isolation of an Arabidopsis SHM gene was carried out using a pea mitochondrial SHM gene probe (Turner et al., 1992a) to screen an Arabidopsis cDNA library. Although the original designation of a mutant lacking mitochondrial SHMT activity as stm (Somerville and Ogren, 1981) suggested that we name these genes STM, that name had also been used for a gene identified by a developmental mutant, shoot meristemless (Barton and Poethig, 1993). To avoid further confusion with this STM gene, which encodes a member of the KNOTTED class of homeodomain proteins (Long et al., 1996), we refer to the Arabidopsis genes encoding SHMT as SHM. Preliminary sequence analysis indicated that the two cDNA clones identified by hybridization with the pea SHMT gene corresponded to a single gene, designated SHM1.
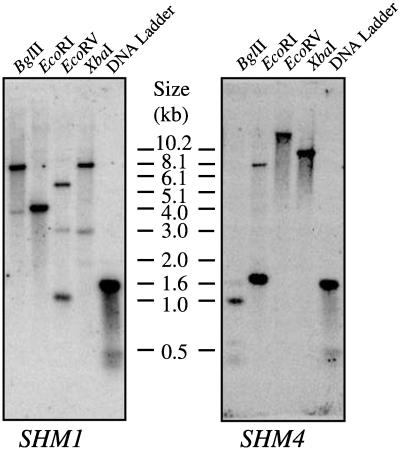
Our initial BLAST searches (Altschul et al., 1990) with these cDNA sequences identified two classes of expressed sequence tags (ESTs), one of which corresponded to SHM1 and the other to a related gene designated SHM4. That these two were distinct SHM genes was confirmed by complete sequence determination of the longest EST clone of each class. cDNA inserts were used as probes for Southern analysis on Arabidopsis ecotype Columbia genomic DNA to confirm the presence of two distinct Arabidopsis SHM genes (Fig. 1). Each of the four enzymes cut within the genomic sequence of SHM1 and the multiple bands are consistent with the known SHM1 genomic sequence, in which the faint bands hybridize with only a very short region of the probe. Similarly, BglII and EcoRI cut within the genomic sequence of SHM4 and are predicted to yield the fragments observed. EcoRV and XbaI do not cut within the SHM4 sequence, which is consistent with the detection of a single large fragment. Neither the SHM1 nor the SHM4 hybridization probes employed cross-hybridize with other SHM sequences under the stringency conditions employed.
Figure 1.
Southern analysis of Arabidopsis SHM1 and SHM4. Ecotype Columbia genomic DNA was digested with BglII, EcoRI, EcoRV, or XbaI and hybridized at high stringency with gene-specific probes to SHM1, and, after the membrane was stripped, to SHM4. The molecular mass marker was a 1-kb DNA ladder (Gibco-BRL, Gaithersburg, MD) and the corresponding molecular masses are indicated.
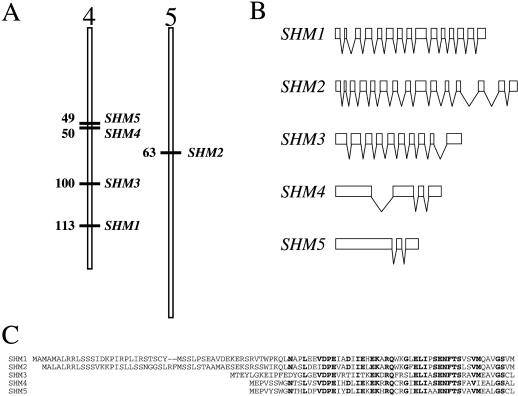
Subsequent BLAST searches with our two SHM cDNA sequences identified genomic clones for these two SHM genes, as well as genomic and EST clones for three more Arabidopsis SHM genes. As of December 20, 1999, we had identified five distinct SHM gene sequences (Fig. 2; Table I), which we designated SHM1 through SHM5. These five genes are highly related, both at the nucleotide and the deduced amino acid levels (Table II). Four of the SHM genes (SHM1, SHM3, SHM4, and SHM5) lie on chromosome IV and the fifth, SHM2, lies on chromosome V (Fig. 2A). As determined using PSORT (http://psort.nibb.ac.jp:8800/helpwww.html; Nakai and Kanehisa, 1992), two of these genes, SHM1 and SHM2, encode amino-terminal sequence extensions predicted to target the proteins into the mitochondrial matrix (Fig. 2C). The remaining three SHM genes encode putative proteins that lack recognizable targeting sequences and thus are predicted to be cytosolic. The coding sequences of both SHM1 and SHM4, as predicted from the complete cDNA sequences, confirm the predictions of the annotated genomic sequences (GenBank accession nos. AL035538 and Z97335, respectively). The longest ESTs corresponding to SHM1 (GenBank accession no. T44375) and SHM4 (GenBank accession no. Z24502) begin 41 and 58 nt, respectively, upstream of the predicted ATG initiation codons. These SHM1 and SHM4 cDNA sequences predict 3′-untranslated regions extending 116 and 234 nt, respectively, beyond the termination codons.
Figure 2.
Characterization of the five Arabidopsis SHM genes and SHMT proteins. A, Map positions of the five Arabidopsis SHM genes, based on the current (January 3, 2000) Arabidopsis Genome Initiative maps (http://www.Arabidopsis.org/chromosomes/). The numbers beside the chromosomes indicate map positions in centiMorgans (cM). B, Intron/exon structures of the five Arabidopsis SHM genes. White boxes denote coding regions and bent lines indicate introns (5′- and 3′-untranslated regions are not indicated). C, Amino acid alignment of the amino termini of the five Arabidopsis SHMTs, indicating the amino-terminal extensions of SHMT1 and SHMT2 that encode putative mitochondrial targeting sequences. Residues indicated in bold are conserved among at least four of the five sequences.
Table I.
SHM sequences used in this study
| Species | Subcellular Localizationa | GenBank Accession Nos.
|
|
|---|---|---|---|
| cDNA | Genomic | ||
| Arabidopsis | |||
| SHM1 | m | nd | AL035538 |
| SHM2 | m | nd | AF007270 |
| SHM3 | c | nd | AL034567 |
| SHM4 | c | nd | Z97335 |
| SHM5 | c | nd | AL035528 |
| Caenorhabditis briggsae | c | nd | ftp |
| Caenorhabditis elegans | c | nd | U00048 |
| Candida albicans | c | AF009966 | nd |
| m | AF009965 | nd | |
| Encephalitozoon cuniculi | c | nd | AJ005644 |
| Escherichia coli | J01620 | nd | |
| Flaveria pringlei | |||
| SHM1 | m | Z25859 | nd |
| SHM2 | m | Z25860 | nd |
| Homo sapiens | c | L23928 | nd |
| m | L11932 | nd | |
| Mus musculus | |||
| SHM1 | c | X94478 | nd |
| SHM2 | c | X94479 | nd |
| Neurospora crassa | c | nd | M81918 |
| Oryctolagus cuniculus | c | Z11846 | nd |
| m | X91902 | nd | |
| Ovis aries | c | X80024 | nd |
| Pisum sativum | m | M87649 | nd |
| Saccharomyces cerevisiae | c | nd | L22529 |
| m | nd | L22528 | |
| Schizosaccharomyces pombe | c | nd | Z98601 |
| Solanum tuberosum | m | Z25863 | nd |
c, Cytoplasmic; m, mitochondrial; nd, not deposited in GenBank; ftp, ftp://genome.wustl.edu/pub/gsc1/sequence/st.louis/briggsae/finish/mel_32/G18D16.seq.
Table II.
Similarity and identity values (%) among the Arabidopsis SHMs
| SHM1 | SHM2 | SHM3 | SHM4 | SHM5 | |
|---|---|---|---|---|---|
| SHM1 | – | 76.7 | 61.7 | 61.1 | 60.6 |
| SHM2 | 85.3 (91.7) | – | 61.1 | 58.0 | 56.5 |
| SHM3 | 61.3 (75.1) | 60.8 (74.5) | – | 58.8 | 58.5 |
| SHM4 | 57.4 (73.0) | 56.8 (73.4) | 59.8 (75.7) | – | 84.1 |
| SHM5 | 55.9 (71.2) | 55.0 (70.4) | 56.5 (73.1) | 81.1 (89.4) | – |
Nucleotide identities are indicated to the upper right of the diagonal. Amino acid identities (similarities in parentheses) are indicated to the lower left of the diagonal.
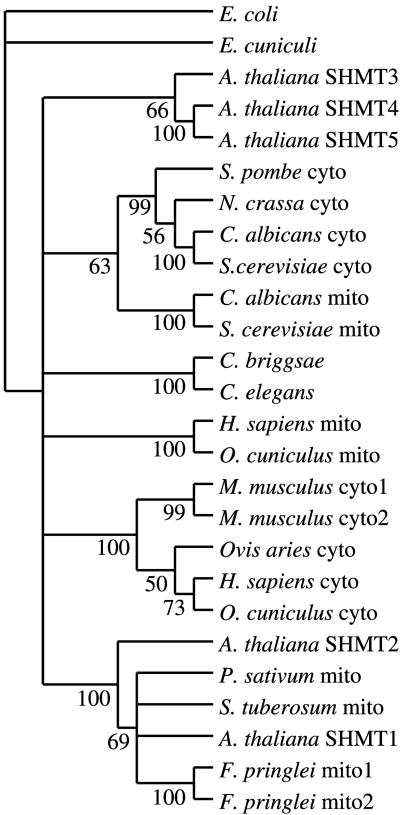
We compared the putative amino acid sequences of Arabidopsis SHMTs with other eukaryotic SHMTs in GenBank (Table I), aligned the predicted amino acid sequences of the deduced proteins using the PileUp and Pretty programs (data not shown; Program Manual for the Wisconsin Package, Version 10, Genetics Computer Group, Madison, WI), and used these alignments to hypothesize the phylogenetic relationships among these sequences (Fig. 3) using parsimony supported by bootstrap (100 replicates) analysis (Felsenstein, 1985). The two putative mitochondrial proteins, SHMT1 and SHMT2, are more closely related to each other than to the other three Arabidopsis SHMTs and cluster robustly with other known plant mitochondrial SHMTs, which is consistent with the predictions of PSORT. SHMT3, SHMT4, and SHMT5 form a clade that is robustly supported by bootstrap analysis. Within that clade, SHMT4 and SHMT5 are more closely related to each other than to SHMT3. The remainder of the tree is congruent with known phylogenetic relationships with fungal, nematode, and mammalian clades. As observed in the plant sequences, mitochondrial isoforms from fungi and mammals cluster distinctly from cytosolic isoforms. The single SHMT from a protozoan, E. cuniculi, is not predicted to be more closely related to other eukaryotic SHMTs than to the single prokaryotic SHMT, that encoded by the E. coli glyA gene, which was included as the outgroup in the analysis.
Figure 3.
Hypothesized phylogenetic relationships among eukaryotic SHMs. GenBank accession numbers of sequences are given in Table I. The sequences were aligned using the PileUp program, and the tree was constructed using the heuristic search algorithm of PAUP* version 4.02b. The numbers next to the nodes are bootstrap values (Felsenstein, 1985) for 100 replicates, and represent the percentage of times that the branch appears in a tree. Cyto, Cytosolic isozyme; mito, mitochondrial isozyme.
Analysis of intron-exon structures of the five Arabidopsis SHM genes (Fig. 2B; Table III) was consistent with the distribution into two distinct subgroups (SHM1 and SHM2 versus SHM4 and SHM5), and further suggested that SHM3 is distinct from the SHM4-SHM5 clade. SHM1 and SHM2 each contain 14 introns, and the intron positions are absolutely conserved between these two genes. SHM3 has nine introns, seven of which are at unique positions. Two SHM3 introns, the fourth and eighth, are at positions occupied by the eighth and twelfth introns of SHM1 and SHM2. SHM4 and SHM5 share two conserved introns: one is at a position distinct from those of the SHM1 and SHM2 introns and the final one is at a position conserved with that of the thirteenth introns of SHM1 and SHM2. SHM4 has an additional intron not shared by SHM5 or any other Arabidopsis SHM gene. Intron lengths are not conserved among these genes, although most are small (approximately 100 nt): the largest intron, unique to SHM4, is 409 nt.
Table III.
Positions and lengths of introns in Arabidopsis SHM genes
| Intron no. | Intron Length (nt)
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
| Intron | Ma | Ta | Ka | Rc | Ec | Vb | Ma | Qa | Gb | Qa | Ka | Eb | Ka | Ea | Qa | Qa | Qa | Qa | Ka | Gb | Gb | Gb | Gb |
| Positiond
|
29
|
46
|
75
|
110
|
116
|
142
|
168
|
183
|
208
|
214
|
245
|
269
|
286
|
311
|
329
|
346
|
358
|
368
|
394
|
398
|
419
|
432
|
469
|
| SHM1 | 106e | 171 | 95 | –f | 104 | 79 | – | 84 | – | 87 | 78 | – | – | 87 | – | 81 | – | 82 | 80 | – | – | 81 | 83 |
| SHM2 | 79 | 88 | 91 | – | 80 | 93 | – | 116 | – | 172 | 81 | – | – | 90 | – | 164 | – | 130 | 328 | – | – | 287 | 84 |
| SHM3 | – | – | – | 88 | – | – | 84 | – | 79 | – | 112 | – | 97 | – | 86 | – | 93 | – | 107 | – | 244 | – | – |
| SHM4 | – | – | – | – | – | – | – | – | – | – | – | 409 | – | – | – | – | – | – | – | 91 | – | 93 | – |
| SHM5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 85 | – | 75 | – |
Intron follows the codon encoding the indicated amino acid residue.
Intron inserted between the first and second nucleotides of the codon encoding the indicated amino acid residue.
Intron inserted between the second and third nucleotides of the codon encoding the indicated amino acid residue.
Based on Arabidopsis SHM1, to which all the others were aligned. The amino acid residue listed is that of Arabidopsis SHMT1.
Intron length in nt.
Intron not present.
Organ-Specific Expression of the SHM Genes
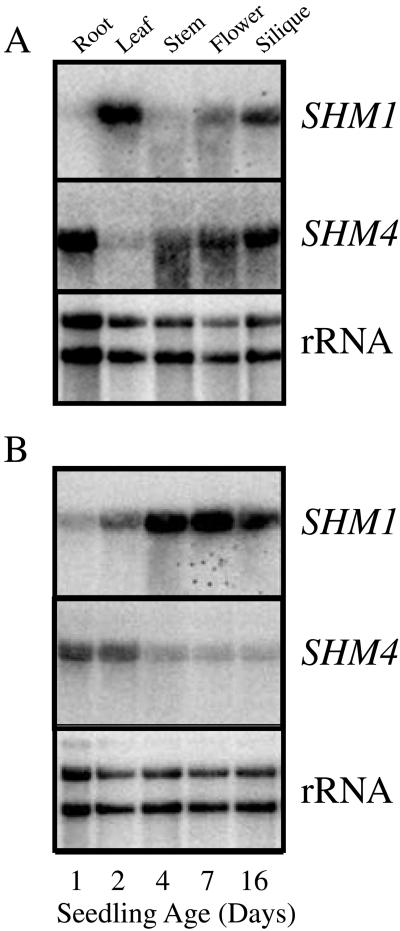
In an initial examination of the expression of individual SHM genes, SHM1 and SHM4 were selected as representatives of mitochondrial and cytosolic SHMTs, respectively. Northern analysis of expression was performed on RNAs extracted from different organs (Fig. 4A). Both genes produced transcripts of the sizes predicted by the cDNAs (excluding polyA tails, the mRNA lengths were predicted to be approximately 1.7 and approximately 1.8 kb for SHM1 and SHM4, respectively). SHM1 mRNA was most abundant in leaves, less abundant in flowers and siliques (about 64% and 56%, respectively, the level detected in leaves, when normalized to rRNA abundance), and barely detectable in roots and inflorescence stems. When normalized to rRNA abundance, SHM4 mRNA was most abundant in flowers; less abundant in roots, inflorescence stems, and siliques (50%, 40%, and 60% the levels in flowers, respectively); and barely detectable in leaves. These expression patterns are consistent with a photorespiratory role for SHM1 and a non-photorespiratory role for SHM4 in C1 metabolism. Presumably, one of the other SHMs provides cytosolic non-photorespiratory functions in leaves.
Figure 4.
Spatial and developmental regulation of SHM1 and SHM4 expression. A, Organ-specific SHM1 and SHM4 mRNA accumulation. RNA extracted from isolated organs of mature Arabidopsis plants grown under constant illumination (80 μE m−2 s−1 white light) was glyoxalated, resolved by agarose gel electrophoresis, blotted onto nylon membranes, and probed sequentially (see Fig. 1 legend) with Arabidopsis SHM1, SHM4, or rDNA, as indicated. B, Developmental regulation of SHM1 and SHM4 mRNA accumulation. Total RNA was isolated from Arabidopsis seedlings grown under constant illumination for 1, 2, 4, 7, or 16 d after release from stratification. Each panel was probed sequentially (see Fig. 1 legend) with Arabidopsis SHM1, SHM4, or rDNA, as indicated.
Developmental Accumulation of SHM1 and SHM4 mRNAs
Both SHM1 and SHM4 mRNAs were detected in whole seedlings 1 to 16 d after release from stratification, although the patterns of mRNA accumulation for the two genes were different (Fig. 4B). SHM1 mRNA accumulated to progressively higher levels throughout the 16-d time frame. In contrast, SHM4 mRNA was most abundant in the youngest seedlings and declined in abundance progressively over the 16-d period. However, this may simply reflect the relatively greater growth of shoots than roots in seedlings on plates, as SHM4 is restricted to the roots in these seedlings (data not shown). In 3-week-old mature plants, SHM1 mRNA was present at approximately equal abundance in light-grown cotyledons and in the first four leaves, and at slightly reduced abundance in the fifth and sixth leaves (Fig. 5A). Very little SHM1 mRNA was present in the roots. In contrast, SHM4 mRNA was not detectable in the cotyledons and leaves, but could be detected at low abundance in the roots (data not shown).
Figure 5.
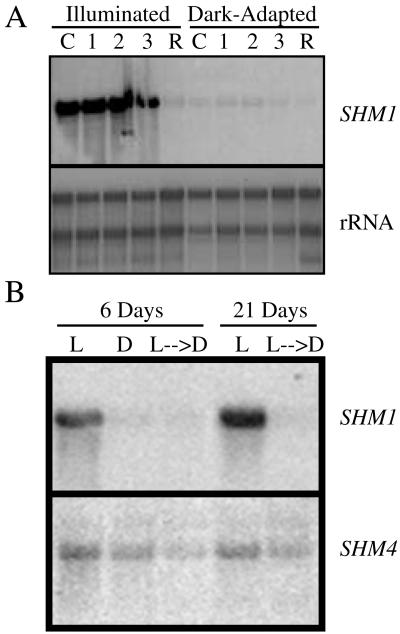
Developmental and light regulation of SHM1 expression and mRNA accumulation. A, Developmental regulation of SHM1 mRNA accumulation in illuminated (80 μE m−2 s−1 white light) and dark-adapted seedlings. RNA from leaves of different ages was isolated from mature Arabidopsis plants grown under constant illumination or dark-adapted for 24 h. Sources of RNA are as follows: C, Cotyledons; l, the first two leaves; 2, the third and fourth leaves; 3, the fifth and sixth leaves; R, roots. The panel was probed sequentially (see Fig. 1 legend) with SHM1, SHM4, or rDNA, as indicated. B, Light regulation of SHM1 mRNA accumulation. Total RNA was isolated from 6-d-old seedlings grown under the following light conditions: L, Constant illumination (80 μE m−2 s−1 white light) for 6 d; D, constant darkness for 6 d; L → D, constant light for 5 d followed by constant darkness for 1 d; L, constant light for 21 d; L → D, constant light for 20 d followed by constant darkness for 1 d.
Light Regulation of SHM1 mRNA Accumulation
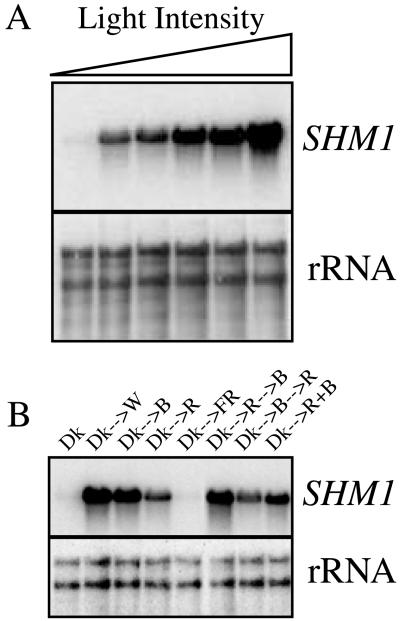
In contrast to the high levels of SHM1 mRNA detected in the aerial tissues of illuminated plants (Fig. 5A), SHM1 mRNA was greatly reduced following dark adaptation of plants for 24 h (Fig. 5A). Similarly, SHM1 mRNA was abundant in whole 6-d-old seedlings or 21-d-old plants, but was greatly reduced following dark adaptation for 24 h (Fig. 5B). The accumulation of SHM1 mRNA in illuminated seedlings was dependent upon irradiance, increasing 24-fold over the etiolated level at 2 μE m−2 s−1 and a further 10-fold (244-fold relative to the etiolated value) as the fluence rate was increased to 80 μE m−2 s−1, and was not apparently saturated at the highest rate tested (Fig. 6A). SHM1 mRNA accumulated in response to continuous illumination for 24 h with either red (34 μE m−2 s−1) or blue light (18 μE m−2 s−1), but not with far-red (34 μE m−2 s−1) light (Fig. 6B). Simultaneous illumination with red and blue light was more effective than illumination with red alone. When we first illuminated with red and then with blue, or first illuminated with blue and then with red, the ultimate mRNA abundance was consistent with that of the response to the single treatment with the light presented last. Pretreatment with blue light did not potentiate the effectiveness of red light in the induction of SHM1 mRNA.
Figure 6.
Effects of light fluence and spectral quality on SHM1 expression. A, Light fluence response of SHM1 mRNA accumulation. Total RNA was isolated from seedlings grown in constant darkness for 4 d and subjected to constant illumination for 24 h with white light at varying light intensities. Light intensities were, from left to right: 0, 2, 8, 15, 30, and 80 μE m−2 s−1. The panel was probed sequentially (see Fig. 1 legend) with SHM1 or rDNA as indicated. The induction relative to the SHM1 mRNA abundance in etiolated seedlings, arbitrarily defined as 1, was 24-, 32-, 85-, 109-, and 244-fold, respectively. B, Spectral quality response of SHM1 mRNA accumulation. RNA was isolated from seedlings grown in constant darkness (Dk) and subsequently transferred to 80 μE m−2 s−1 white light. Dk-->W, to 18 μE m−2 s−1 blue light; Dk-- >B, to 34 μE m−2 s−1 red light; Dk-->R, to 34 μE m−2 s−1 far-red light; Dk-->FR; Dk-->R+B, 34 μE m−2 s−1 red plus 18 μE m−2 s−1 blue light for 24 h. Additional etiolated seedlings were transferred to red light for 24 h followed by blue light for 24 h (Dk-->R-->B) or to blue light for 24 h followed by red light for 24 h (Dk-->B-->R). The panel was probed sequentially (see Fig. 1 legend) with SHM1 or rDNA.
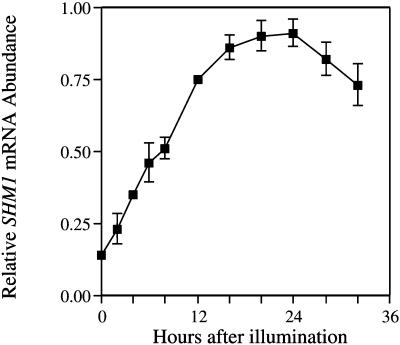
To determine whether the accumulation of SHM1 mRNA in illuminated seedlings represents a direct response to light, we transferred 8-d-old etiolated seedlings into continuous white light (125 μE m−2 s−1) and measured SHM1 mRNA abundance over the next 32 h. As can be seen in Figure 7, SHM1 mRNA accumulated gradually to a peak about 24 h following the onset of illumination. Light-regulated genes such as LHCB and CAT2 exhibit an acute response to light within about 4 h of the onset of illumination (Zhong et al., 1994, 1998; Millar and Kay, 1996). In contrast, SHM1 did not exhibit an acute induction, suggesting that the accumulation of SHM1 mRNA in the light is an indirect response to illumination, and may represent a consequence of the stimulation of photosynthesis and photorespiration in the light.
Figure 7.
Accumulation of SHM1 mRNA in etiolated seedlings transferred into continuous white light. Seeds were allowed to imbibe, stratified at 4°C in the dark for 3 d, grown at 22°C for 8 d in the dark, and then released into continuous white light (125 μE m−2 s−1). SHM1 mRNA abundance was quantified by slot-blot analysis. Data are presented as means ± se of the mean derived from eight replicates. The most abundant signal was defined as 1.0 (arbitrary units), and other abundances are expressed relative to that value.
In contrast to the light responsiveness of SHM1 mRNA, SHM4 mRNA abundance was unaffected by light. It was detected at low abundance in roots of both illuminated and dark-adapted plants (Fig. 5B).
Circadian Regulation of SHM1 and SHM4 mRNA Abundance
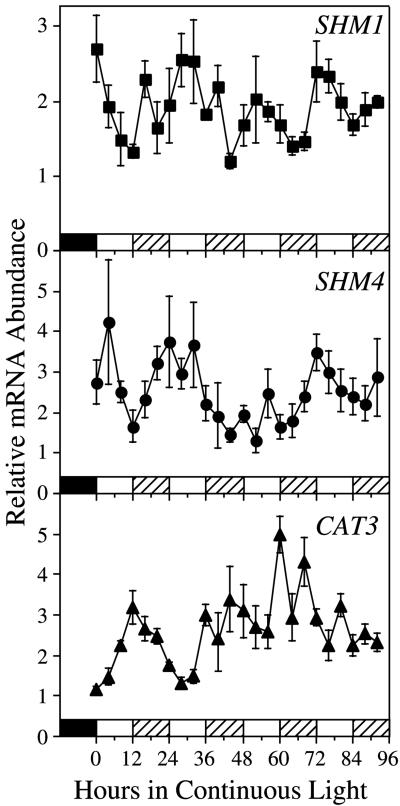
When etiolated seedlings were transferred into continuous light, SHM1 mRNA accumulated to a peak about 24 h after the onset of illumination and then declined slightly, which is reminiscent of the pattern observed with transcripts such as LHCB and CAT2, which are regulated by the circadian clock (Tavladoraki et al., 1989; Zhong et al., 1994, 1998; Millar and Kay, 1996). To directly assess whether a circadian clock controls SHM1 and SHM4 mRNA abundance, we measured mRNA abundance in whole seedlings germinated and grown for 7 d in 12-h light:12-h dark cycles and then released into continuous light. Oscillations with periods in the circadian range were observed for both SHM1 and SHM4 mRNA abundance measured in preparations from seedlings harvested over four circadian cycles in continuous light (Fig. 8).
Figure 8.
Circadian regulation of SHM1 and SHM4 mRNA abundance. Circadian oscillations are detected in both SHM1 and SHM4 mRNA abundance in seedlings entrained on 12-h light:12-h dark cycles and released into continuous light (130 μE m−2 s−1 white light). Slot blots loaded with total RNA (1 μg) were hybridized with SHM gene-specific probes. Following autoradiography, the membrane was stripped and rehybridized to a CAT3-specific probe. The blots were again stripped and rehybridized to a ribosomal DNA probe. SHM and CAT3 mRNA levels are expressed relative to rRNA, with the lowest value expressed as 1.0 (arbitrary units). The error bars indicate se of the mean, based on three independent RNA preparations for each time point. rRNA showed minimal fluctuations with no circadian periodicity. The experiment was repeated with similar results. The bars at the base of the graphs indicate the light-dark regimen. The filled bar indicates the last night of the entraining light-dark cycle before seedlings were released into continuous light. White bars indicate subjective day and hatched bars indicate subjective night during the continuous light treatment.
mRNA abundance for both SHM1 and SHM4 was maximal in the morning, which is consistent with the phase observed for mRNA abundance of other photosynthetic and photorespiratory genes (McClung and Kay, 1994; Kreps and Kay, 1997). Statistical analysis of the time series data by fast Fourier transform-nonlinear least squares (FFT-NLLS) (see “Materials and Methods”; see also Plautz et al., 1997; Zhong et al., 1997) detected significant circadian oscillations in both SHM1 and SHM4 mRNA abundance. The period lengths (mean ± sd) of the oscillations were 26.0 ± 2.1 h for SHM1 and 23.8 ± 1.2 h for SHM4. This experiment was repeated with similar results. For comparison, the blots were stripped and rehybridized to a CATALASE 3 (CAT3)-specific probe. As previously established (Zhong and McClung, 1996), CAT3 oscillates with a dusk-specific phase approximately 12 h out-of-phase with the peaks in SHM mRNA abundance. In this experiment, the CAT3 period is 24.9 ± 2.7 h. Because SHM and CAT3 exhibit out-of-phase oscillations in abundance in the same RNA samples assayed on the same blots, it is clear that the oscillations in SHM mRNA abundance cannot result from unequal loading or from other experimental artifacts.
In mature plants, SHM4 mRNA is found in roots but not in rosette leaves (Figs. 4A and 5A), suggesting that this might represent a circadian rhythm in a root-expressed gene. However, the data shown in Figure 8 represent RNA populations from entire seedlings. Accordingly, we harvested 10-d-old seedlings similar to those analyzed in the circadian protocol of Figure 8 at the circadian phase of maximal SHM mRNA abundance, separated the seedlings into roots and shoots, and extracted RNA. Northern analysis showed that SHM4 mRNA accumulation in these young seedlings is restricted to the roots (data not shown). Therefore, the circadian oscillation detected in SHM4 mRNA abundance reflects a circadian rhythm in root-specific SHM4 mRNA accumulation.
DISCUSSION
It has become increasingly apparent that circadian clocks are used to coordinate the temporal expression of many plant processes (McClung and Kay, 1994; Kreps and Kay, 1997; Lumsden and Millar, 1998). Photorespiration is a complex metabolic pathway in which the plant must coordinate the expression of nuclear genes whose products are targeted to three distinct subcellular compartments: the chloroplast, the peroxisome, and the mitochondrion. In Arabidopsis, a circadian clock gates expression of at least two chloroplastic components of the photorespiratory pathway. Rubisco is the bifunctional enzyme that initiates both the photosynthetic carbon assimilation pathway and the competing photorespiratory pathway. The RBCS genes, which encode the small subunit of Rubisco, exhibit robust circadian oscillations in mRNA abundance (Pilgrim and McClung, 1993). Transcription of the RCA gene, which encodes Rubisco activase, a key regulator of Rubisco, is regulated by a circadian clock (Pilgrim and McClung, 1993; Liu et al., 1996). A circadian clock also regulates the mRNA abundance of the photorespiratory catalase gene CAT2 (Zhong et al., 1994), a peroxisomal component of the photorespiratory pathway. Moreover, a circadian clock gates the response of CAT2 to environmental stimuli such as light (Zhong et al., 1998). To determine whether a circadian clock coordinates the temporal expression of mitochondrial components of the photorespiratory pathway with these chloroplastic and peroxisomal components, we set out to characterize the SHM genes encoding Ser hydroxymethyltransferase, an essential mitochondrial matrix component of the photorespiratory pathway.
Several lines of evidence suggest that SHM1 encodes a photorespiratory SHMT. First, the predicted SHMT1 protein includes an N-terminal extension with the characteristics of a mitochondrial targeting signal. Second, SHM1 mRNA is abundant in leaves. Third, SHM1 mRNA accumulation is positively regulated by light. Similar light inducibility has been established for the genes that encode the P, H, and T subunits of the GDC complex (Kim and Oliver, 1990; Macherel et al., 1990; Kim et al., 1991; Turner et al., 1992b, 1993; Srinivasan and Oliver, 1995) and pea SHMT (Turner et al., 1993).
To test for circadian regulation we examined the pattern of accumulation of SHM1 mRNA in seedlings entrained to a light-dark cycle and then released into continuous light. Circadian oscillations in SHM1 mRNA abundance persisted over four cycles in continuous conditions, establishing circadian clock control over the temporal pattern of SHM1 mRNA abundance. Thus, a circadian clock(s) controls the expression of nuclear genes that encode photorespiratory pathway components that are targeted into each of the three distinct subcellular compartments housing photorespiratory reactions. The peak in mRNA abundance for SHM1, which encodes a putative photorespiratory SHMT, is gated to the early subjective morning, which is the same phase as observed for other mRNAs encoding photorespiratory or photosynthetic products (e.g. LHCB, RBCS, RCA, and CAT2; McClung and Kay, 1994). Evidently, circadian clock regulation is employed by the plant to coordinate metabolic capacity in the three compartments involved in the complex photorespiratory pathway. However, we emphasize that we have only established an oscillation in SHM mRNA abundance and have not shown that there are corresponding oscillations in SHMT protein abundance and SHMT activity.
The interplay between the circadian clock and light in the regulation of SHM1 is complex. SHM1 fails to exhibit an acute (4-h) induction in response to illumination with continuous white light, which suggests that SHM1 is not directly responding to light. In contrast, both phytochromes A and B contribute to the acute induction of Arabidopsis LHCB (CAB2) mRNA by light (Karlin-Neumann et al., 1988; Anderson et al., 1997). The transfer of etiolated seedlings into continuous white light induces circadian oscillations in LHCB (Tavladoraki et al., 1989; Millar and Kay, 1996) and CATALASE (Zhong et al., 1994, 1998) mRNAs. It is likely that the accumulation of SHM1 mRNA observed after 24 h of illumination with white, red, or blue light represents sampling during the peak of the circadian oscillation in SHM1 mRNA, because the phase of the oscillation of SHM1 mRNA is similar to the phases of the oscillations in LHCB and CATALASE 2 mRNAs, both of which peak about 24 h after the onset of illumination.
We attribute the failure of continuous illumination with far-red light to result in accumulation of SHM1 mRNA 24 h after the onset of illumination to a failure of far-red light to induce circadian oscillations of this transcript. The induction of circadian oscillations of bean LHCB mRNA by a pulse of red light is greatly attenuated by a subsequent pulse of far-red light (Tavladoraki et al., 1989), and a pulse of far-red light prevents the accumulation of wheat LHCB mRNA in the next circadian cycle (Nagy et al., 1988). It is possible that SHM1 mRNA abundance is responding directly to temporal signals from the circadian clock. However, it is also possible that SHM1 mRNA abundance is responding indirectly to the circadian clock via the induction of circadian oscillations in photosynthetic and photorespiratory carbon metabolism. For example, both stomatal aperture (Somers et al., 1998) and CO2 assimilation (E.V. Kearns, A.G. Fett-Neto, and C.R. McClung, unpublished observations) oscillate in a circadian fashion in Arabidopsis and other plants (Lumsden and Millar, 1998).
What is the relationship of the SHM genes to the stm mutation, which yields a defect in mitochondrial SHMT activity (Somerville and Ogren, 1981)? It seems unlikely that stm represents a loss-of-function mutation in an SHM gene, as the presence of two genes encoding mitochondrial SHMTs suggests that loss of function of either alone would be insufficient to eliminate mitochondrial SHMT activity. Of course, organ-specific expression of SHM1 or SHM2 could indicate that only one of these genes plays a photorespiratory role. The mRNA abundance and size for an Arabidopsis SHM cDNA that corresponds to SHM1 (R. Hell, personal communication) was unaltered in the stm mutant (Beckmann et al., 1997). This raises the intriguing possibility that the stm mutation affects a locus required for SHMT activity but distinct from the SHM loci encoding the SHMT proteins.
Prokaryotes generally have a single gene encoding SHMT, whereas eukaryotes commonly have two SHM genes, one encoding cytosolic SHMT and the second encoding mitochondrial SHMT. However, we found at least five SHM genes in Arabidopsis. SHM1 is most similar to SHM2 on the basis of both amino acid sequence and intron-exon structure. Both SHM1 and SHM2 protein products are predicted to be targeted into the mitochondrial matrix. The remaining three SHM genes are predicted to encode cytosolic SHMTs. On the basis of both amino acid sequence and intron-exon structure, SHM4 and SHM5 are more related to each other than to SHM3. SHM4 mRNA accumulates in roots and not in leaves. In addition, SHM4 mRNA is not light responsive. SHM4 is unlikely to play a role in photorespiration, more likely being involved in C1 metabolism in the roots. Nonetheless, a circadian clock also regulates the accumulation of SHM4 mRNA in the roots of seedlings. At this time we cannot explain the biological significance of this clock regulation of SHM4. The first step necessary to elaborate such a hypothesis will entail the establishment of a metabolic role for SHM4, potentially through the analysis of loss-of-function mutations of SHM4. It will also be interesting to explore the regulatory mechanisms by which the clock exerts control of SHM4 mRNA abundance in the roots, as most studies of clock-regulated gene expression have addressed expression only in aboveground tissues (McClung and Kay, 1994; Kreps and Kay, 1997; Lumsden and Millar, 1998).
MATERIALS AND METHODS
DNA Extraction and Analysis
For genomic DNA or RNA extraction, Arabidopsis ecotype Columbia plants were grown as described by Pilgrim and McClung (1993). We initially used a cDNA clone of a pea mitochondrial SHMT gene (Turner et al., 1992a) as a hybridization probe to identify Arabidopsis cDNAs from a λgt10 library using standard techniques (Ausubel et al., 1999). Preliminary sequence analysis indicated that these cDNA clones corresponded to a single gene, designated SHM1, although neither clone was full-length. Subsequently, ESTs (124O13T7, GenBank accession no. T44375; and 160C23T7, GenBank accession no. T88275) corresponding to full-length cDNAs of SHM1 and a second, related gene designated SHM4, respectively, became available from the Arabidopsis Biological Resource Center (Ohio State University, Columbus) and were used in further experiments.
The complete sequences of EST clones 124O13 (SHM1) and 160C23 (SHM4) were determined by cycle sequencing reactions with the DyeDeoxy Terminator sequencing reaction mixture (Applied Biosystems, Foster City, CA). All samples were amplified in a programmable thermal controller (MJ Research, Watertown, MA) by the following cycle-sequencing program: 96°C for 1 min, followed by 24 cycles of 96°C for 30 s, 50°C for 15 s, and 60°C for 4 min. Samples were held at 4°C when cycles were completed. Products were purified using Sephadex columns (Quik-Spin G-50, Boehringer Mannheim, Indianapolis) or Centri-spin 20 columns (Princeton Separations, Adelphia, NJ) according to the manufacturers' instructions. DNA eluates were dried in a vacuum centrifuge and sequenced using sequenators (373A, Applied Biosystems). The GCG package (Genetics Computer Group) was used for computer analysis of nucleic acid and deduced amino acid sequences. PAUP* was used for phylogenetic analysis (Phylogenetic Analysis Using Parsimony, version 4.02b, Sinauer Associates, Sunderland, MA).
Genomic DNA prepared from frozen tissue by the method of Dellaporta et al. (1983) was digested in the presence of 10 mm spermidine (Dellaporta et al., 1983). Southern analysis was by standard methods (Ausubel et al., 1999). Inserts of EST plasmid clones derived from the vector λPRL2 (Newman et al., 1994) were amplified by PCR with T7 and M13 primers. Approximate quantities were determined by agarose gel electrophoresis, and PCR-amplified products to be used as hybridization probes specific for each gene were isolated by agarose gel electrophoresis and radiolabeled with [α-32P]dCTP by the random primer method using the Klenow fragment of DNA polymerase I (Feinberg and Vogelstein, 1984). For comparisons of the genomic hybridization patterns of the SHM genes, each membrane was hybridized sequentially with probes specific for each gene. After each hybridization, the membranes were stripped (Pilgrim and McClung, 1993). The membranes were exposed to x-ray film or to phosphor imager screens (Molecular Dynamics, Sunnyvale, CA) and then examined with a phosphor imager employing imaging software (Imagequant, Molecular Dynamics).
RNA Preparation and Analysis
Total RNA was prepared from frozen plant tissue via phenolic RNA extraction as described previously (Zhong et al., 1994). Samples were quantified by spectrophotometry. For northern analysis, samples (8 μg of total RNA per lane) were resolved on formaldehyde-agarose gels (Zhong et al., 1994). Alternatively, glyoxalated RNA samples (10 μg per lane) were analyzed as described previously (Learned, 1996). Slot-blot analyses were as described previously (Zhong et al., 1994). rDNA (Richards and Ausubel, 1988) was used as a control probe for normalization of RNA loading. The Arabidopsis LHCB1*3 (CAB1) probe was as described previously (Pilgrim and McClung, 1993). Membranes were exposed to a phosphor imager screen (Molecular Dynamics) for 24 h to 2 weeks. Relative mRNA abundance of slot blots was quantified using the phosphor imager with imagine software.
For evaluation of circadian rhythmicity, time series data were quantitatively evaluated by an iterative, coupled FFT-NLLS multicomponent cosine estimation algorithm as previously described (Plautz et al., 1997; Zhong et al., 1997). FFT-NLLS provides estimates of periods, phases, and amplitudes of rhythmic components, along with associated estimates of joint parameter confidence limits and of the statistical significance of rhythmic amplitudes.
ACKNOWLEDGMENTS
We thank Todd Michael, Lorenzo Sempere, Mary Lou Guerinot, Tom Jack, and Mark McPeek for helpful discussions and for critical reading of the manuscript. Erin Connolly provided invaluable assistance with RNA analyses, and Jessicah Phillips provided excellent technical assistance. We are grateful to the Arabidopsis Biological Resource Center at Ohio State University for seed stocks and for EST clones.
Footnotes
This work was supported by grants from the National Science Foundation (to C.R.M.) and by an institutional grant from the American Cancer Society, administered through the Norris Cotton Cancer Center at Dartmouth. M.H. and J.E.P. were supported by Howard Hughes Undergraduate Research Internships, S.D.K. was supported through the National Science Foundation Research Experience for Undergraduates Program, and S.D.K. and J.M.G. were supported through the Richter Foundation at Dartmouth.
LITERATURE CITED
- Altschul SF, Gish W, Miller W, Meyers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anderson SL, Somers DE, Millar AJ, Hanson K, Chory J, Kay SA. Attenuation of phytochrome A and B signaling pathways by the Arabidopsis circadian clock. Plant Cell. 1997;9:1727–1743. doi: 10.1105/tpc.9.10.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Greene Publishing Associates/Wiley Interscience; 1999. [Google Scholar]
- Barton MK, Poethig RS. Formation of the shoot apical meristem in Arabidopsis thaliana: an analysis of development in the wild type and in the shoot meristemless mutant. Development. 1993;119:823–831. [Google Scholar]
- Beckmann K, Dzuibany C, Biehler K, Fock H, Hell R, Migge A, Becker TW. Photosynthesis and fluorescence quenching, and the mRNA levels of plastidic glutamine synthetase or of mitochondrial serine hydroxymethyltransferase (SHMT) in the leaves of the wild-type and of the SHMT-deficient stm mutant of Arabidopsis thaliana in relation to the rate of photorespiration. Planta. 1997;202:379–386. doi: 10.1007/s004250050140. [DOI] [PubMed] [Google Scholar]
- Blackwell RD, Murray AJS, Lea PJ. Photorespiratory mutants of the mitochondrial conversion of glycine to serine. Plant Physiol. 1990;94:1316–1322. doi: 10.1104/pp.94.3.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G. Growth at elevated CO2: photosynthetic responses mediated through Rubisco. Plant Cell Environ. 1991;14:795–806. [Google Scholar]
- Cossins EA. Folate biochemistry and the metabolism of one-carbon units. In: Davies DD, editor. The Biochemistry of Plants. New York: Academic Press; 1987. pp. 317–353. [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- Douce R, Neuberger M. Biochemical dissection of photorespiration. Curr Opin Plant Biol. 1999;2:214–222. doi: 10.1016/S1369-5266(99)80038-7. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence intervals on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Garrow TA, Brennert AA, Whitehead VM, Chen X-N, Duncan RG, Korenberg JR, Shane B. Cloning of human cDNAs encoding mitochondrial and cytosolic serine hydroxymethyltransferases and chromosomal location. J Biol Chem. 1993;268:11910–11916. [PubMed] [Google Scholar]
- Karlin-Neumann GA, Sun L, Tobin EM. Expression of light-harvesting chlorophyll a/b-protein genes is phytochrome-regulated in etiolated Arabidopsis thaliana seedlings. Plant Physiol. 1988;88:1323–1331. doi: 10.1104/pp.88.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastanos EK, Woldman YY, Appling DR. Role of mitochondrial and cytoplasmic serine hydroxymethyltransferase isozymes in de novo purine synthesis in Saccharomyces cerevisiae. Biochemistry. 1997;36:14956–14964. doi: 10.1021/bi971610n. [DOI] [PubMed] [Google Scholar]
- Kim Y, Oliver DJ. Molecular cloning, transcriptional characterization, and sequencing of cDNA encoding the H-protein of the mitochondrial glycine decarboxylase complex in peas. J Biol Chem. 1990;265:848–853. [PubMed] [Google Scholar]
- Kim Y, Shah K, Oliver DJ. Cloning and light-dependent expression of the gene coding for the P-protein of the glycine decarboxylase complex from peas. Physiol Plant. 1991;81:501–506. [Google Scholar]
- Kreps JA, Kay SA. Coordination of plant metabolism and development by the circadian clock. Plant Cell. 1997;9:1235–1244. doi: 10.1105/tpc.9.7.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learned RM. Light suppresses 3-hydroxy-3-methylglutaryl-coenzyme A reductase gene expression in Arabidopsis thaliana. Plant Physiol. 1996;110:645–655. doi: 10.1104/pp.110.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood RC, Lea PJ, Adcock MD, Häusler RE. The regulation and control of photorespiration. J Exp Bot. 1995;46:1397–1414. [Google Scholar]
- Liu Z, Taub CC, McClung CR. Identification of an Arabidopsis thaliana ribulose-1,5-bisphosphate carboxylase/oxygenase activase (RCA) minimal promoter regulated by phytochrome and the circadian clock. Plant Physiol. 1996;112:43–51. doi: 10.1104/pp.112.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature. 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- Lumsden PJ, Millar AJ. Biological Rhythms and Photoperiodism in Plants. Oxford: BIOS Scientific Publishers; 1998. [Google Scholar]
- Macherel D, Lebrun M, Gagnon J, Neuburger M, Douce R. cDNA Cloning, primary structure and gene expression for H-protein, a component of the glycine-cleavage system (glycine decarboxylase) of pea (Pisum sativum) leaf mitochondria. Biochem J. 1990;268:783–789. doi: 10.1042/bj2680783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini F, Angelaccio S, Pascarella S, Barra D, Bossa F, Schirch V. The primary structure of rabbit liver cytosolic serine hydroxymethyltransferase. J Biol Chem. 1987;262:5499–5509. [PubMed] [Google Scholar]
- Martini F, Maras B, Tanci P, Angelaccio S, Pascarella S, Barra D, Bossa F, Schirch V. The primary structure of rabbit liver mitochondrial serine hydroxymethyltransferase. J Biol Chem. 1989;264:8509–8519. [PubMed] [Google Scholar]
- McClung CR, Kay SA. Circadian rhythms in Arabidopsis thaliana. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 615–637. [Google Scholar]
- McNeil JB, McIntosh EM, Taylor BV, Zhang FR, Tang S, Bognar AL. Cloning and molecular characterization of three genes, including two genes encoding serine hydroxymethyltransferases, whose inactivation is required to render yeast auxotrophic for glycine. J Biol Chem. 1994;269:9155–9165. [PubMed] [Google Scholar]
- Millar AJ, Kay SA. Integration of circadian and phototransduction pathways in the network controlling CAB gene transcription in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:15491–15496. doi: 10.1073/pnas.93.26.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F, Kay SA, Chua N-H. A circadian clock regulates transcription of the wheat Cab-1 gene. Genes Dev. 1988;2:376–382. [Google Scholar]
- Nakai K, Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkewicz MR, Sauls SD, Tjoa SS, Teng C, Fennessey PV. Evidence for intracellular partitioning of serine and glycine metabolism in Chinese hamster ovary cells. Biochem J. 1996;313:991–996. doi: 10.1042/bj3130991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T, de Bruijn FJ, Green P, Keegstra K, Kende H, McIntosh L, Ohlrogge J, Raihkel N, Somerville S, Thomashow M, Retzel E, Somerville C. Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 1994;106:1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver DJ, McIntosh CA. The biochemistry of the mitochondrial matrix. In: Levings CS, Vasil IK, editors. The Molecular Biology of Plant Mitochondria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 237–280. [Google Scholar]
- Pilgrim ML, McClung CR. Differential involvement of the circadian clock in the expression of genes required for ribulose-1,5-bisphosphate carboxylase/oxygenase synthesis, assembly, and activation in Arabidopsis. Plant Physiol. 1993;103:553–564. doi: 10.1104/pp.103.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plautz JD, Straume M, Stanewsky R, Jamison CF, Brandes C, Dowse HB, Hall JC, Kay SA. Quantitative analysis of Drosophila period gene transcription in living animals. J Biol Rhythms. 1997;12:204–217. doi: 10.1177/074873049701200302. [DOI] [PubMed] [Google Scholar]
- Richards EJ, Ausubel FM. Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell. 1988;53:127–136. doi: 10.1016/0092-8674(88)90494-1. [DOI] [PubMed] [Google Scholar]
- Schirch L. Serine hydroxymethyltransferase. Adv Enzymol. 1982;53:83–112. doi: 10.1002/9780470122983.ch3. [DOI] [PubMed] [Google Scholar]
- Somers DE, Webb AAR, Pearson M, Kay SA. The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development. 1998;125:485–494. doi: 10.1242/dev.125.3.485. [DOI] [PubMed] [Google Scholar]
- Somerville CR, Ogren WL. Photorespiration-deficient mutants of Arabidopsis thaliana lacking mitochondrial serine transhydroxymethylase activity. Plant Physiol. 1981;67:666–671. doi: 10.1104/pp.67.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville CR, Ogren WL. Mutants of the cruciferous plant Arabidopsis thaliana lacking glycine decarboxylase activity. Biochem J. 1982;202:373–380. doi: 10.1042/bj2020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Oliver DJ. Light-dependent and tissue-specific expression of the H-protein of the glycine decarboxylase complex. Plant Physiol. 1995;109:161–168. doi: 10.1104/pp.109.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavladoraki P, Kloppstech K, Argyroudi-Akoyunoglou J. Circadian rhythm in the expression of the mRNA coding for the apoprotein of the light-harvesting complex of photosystem II. Plant Physiol. 1989;90:665–672. doi: 10.1104/pp.90.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SR, Hellens R, Ireland R, Ellis N, Rawsthorne S. The organisation and expression of the genes encoding the mitochondrial glycine decarboxylase complex and serine hydroxymethyltransferase in pea (Pisum sativum) Mol Gen Genet. 1993;236:402–408. doi: 10.1007/BF00277140. [DOI] [PubMed] [Google Scholar]
- Turner SR, Ireland R, Morgan C, Rawsthorne S. Identification and localization of multiple forms of serine hydroxymethyltransferase in pea (Pisum sativum) and characterization of a cDNA encoding a mitochondrial isoform. J Biol Chem. 1992a;267:13528–13534. [PubMed] [Google Scholar]
- Turner SR, Ireland R, Rawsthorne S. Cloning and characterization of the P subunit of glycine decarboxylase from pea (Pisum sativum) J Biol Chem. 1992b;267:5355–5360. [PubMed] [Google Scholar]
- Zhong HH, McClung CR. The circadian clock gates expression of two Arabidopsis catalase genes to distinct and opposite circadian phases. Mol Gen Genet. 1996;251:196–203. doi: 10.1007/BF02172918. [DOI] [PubMed] [Google Scholar]
- Zhong HH, Painter JE, Salomé PA, Straume M, McClung CR. Imbibition, but not release from stratification, sets the circadian clock in Arabidopsis seedlings. Plant Cell. 1998;10:2005–2017. doi: 10.1105/tpc.10.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong HH, Resnick AS, Straume M, McClung CR. Effects of synergistic signaling by phytochrome A and cryptochrome 1 on circadian clock-regulated catalase expression. Plant Cell. 1997;9:947–955. doi: 10.1105/tpc.9.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong HH, Young JC, Pease EA, Hangarter RP, McClung CR. Interactions between light and the circadian clock in the regulation of CAT2 expression in Arabidopsis. Plant Physiol. 1994;104:889–898. doi: 10.1104/pp.104.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]