Abstract
The insulin-like growth factor II/mannose 6-phosphate (IGF-II/M6P) receptor is a multifunctional single-transmembrane glycoprotein. Recent studies have advanced our understanding of the structure, ligand binding properties and trafficking of the IGF-II/M6P receptor. This receptor has been implicated in a variety of important cellular processes including growth and development, clearance of IGF-II, proteolytic activation of enzymes and growth factor precursors, in addition to its well-known role in the delivery of lysosomal enzymes. The IGF-II/M6P receptor, distributed widely in the central nervous system, has additional roles in mediating neurotransmitter release and memory enhancement/consolidation, possibly through activating IGF-II-related intracellular signaling pathways. Recent studies suggest that overexpression of the IGF-II/M6P receptor may have an important role in regulating the levels of transcripts and proteins involved in the development of Alzheimer’s disease (AD) – the prevalent cause of dementia affecting the elderly population in our society. It is reported that IGF-II/M6P receptor overexpression can increase the levels/processing of amyloid precursor protein leading to the generation of β-amyloid peptide, which is associated with degeneration of neurons and subsequent development of AD pathology. Given the significance of the receptor in mediating the transport and functioning of the lysosomal enzymes, it is being considered for therapeutic delivery of enzymes to the lysosomes to treat lysosomal storage disorders. Notwithstanding these results, additional studies are required to validate and fully characterize the function of the IGF-II/M6P receptor in the normal brain and its involvement in various neurodegenerative disorders including AD. It is also critical to understand the interaction between the IGF-II/M6P receptor and lysosomal enzymes in neurodegenerative processes, which may shed some light on developing approaches to detect and prevent neurodegeneration through the dysfunction of the receptor and the endosomal-lysosomal system.
Keywords: Insulin-like growth factor II receptor, Mannose 6-phosphate receptor, Endosomal–lysosomal system, Alzheimer’s disease, Neurodegenerative diseases
1. Introduction
Insulin-like growth factors I and II (IGF-I and IGF-II) are pleiotropic polypeptides that are distributed widely in various tissues/organs, including the central nervous system (CNS). They share structural homology with proinsulin and are considered to mediate a wide spectrum of physiological functions during development and in the adult. At the cellular level, both IGFs can act as autocrine and paracrine factors to regulate cellular growth, survival, differentiation and chemotaxis. As components of an endocrine system, IGFs also help to regulate general growth and metabolism [1, 2]. The biological activities of IGF-I and -II are regulated by their synthetic rates, clearance, and their binding to a family of high-affinity IGF binding proteins (IGFBP1-6) and low-affinity IGFBP-related peptides (IGFBP-rP1-4) [3]. These proteins influence the half-life of circulating and tissue IGFs by regulating their transport in the circulation, filtration and excretion by the kidneys, delivery to their target cells, and interaction with receptors [4]. Generally, binding to IGFBPs decreases the activity of IGFs while nevertheless decreasing clearance and extending their biological half-life. The evidence further suggests that IGF stimulation increases the synthetic rates of some binding proteins, thus providing a feedback regulation in controlling the activity of the growth factors at the tissue level. Adding to the complexity of IGF availability, several enzymes capable of proteolyzing IGFBPs have been identified. The cleavage of IGFBPs by IGFBP proteases plays a key role in modulating the levels of free IGFs and IGFBPs and their actions [5, 6].
The biological functions of both IGFs are mediated by specific membrane receptors referred to as the IGF-I, IGF-II and insulin receptors (Fig. 1) [1, 7]. The IGF-I receptor is a member of the tyrosine kinase receptor family with close structural homology to the insulin receptor. This receptor binds IGF-I with higher affinity than either IGF-II or insulin and is usually located at the cell surface as a heterotetramer consisting of two α (135 kDa) and two β (90 kDa) subunits held together by disulfide bonds. The α-subunits contain the extracellular ligand binding site, whereas the β-subunits have transmembrane and tyrosine kinase domains and an intracellular tyrosine autophosphorylation site [7]. Ligand binding to the extracellular α subunit induces a conformational change that triggers receptor tyrosine kinase activity and initiates autophosphorylation of tyrosine residues within the intracellular segment of the β subunit (transphosphorylation) [1, 7]. This event leads to the docking of effector and adaptor molecules and subsequent activation of various intracellular signaling cascades, including the mitogen-activated protein (MAP) kinase and phosphoinositide 3′-kinase pathways, which regulate growth, proliferation, survival, development and metabolic responses [1, 7]. Although the IGF-I receptor is the primary mediator of IGF’s physiological effects, the insulin receptor (IR), which binds both insulin and the IGFs, can also mediate certain biological actions of IGF-I and -II [7]. The IR is expressed as two isoforms, IR-A and IR-B, due to alternative splicing of exon 11 - a small exon encoding 12 amino acid residues at the carboxyl terminus of the IR α-subunit. Some studies have indicated that IGF-II binds IR-A, the isoform that excludes the exon-11 peptide, with higher affinity than IR-B in a variety of tissues and malignant cells [8, 9]. Activation of IR-A by IGF-II has been shown to stimulate mitogenic effects in IGF-I receptor-null mouse embryonic fibroblasts, possibly via the coordinated activation or deactivation of the proto-oncogenic serine kinase, Akt, glycogen synthase kinase 3-β, and extracellular-signal-regulated kinases (ERKs) [10]. The role of IR-B in mediating IGF effects remains unclear. Apart from specific IGF-I and insulin receptors, the detection of a hybrid receptor, comprising an insulin receptor αβ hemimolecule and an IGF-I receptor αβ hemimolecule, has added an additional layer of complexity to the IGF system. Although the hybrid receptors are widely distributed in certain tissues [11, 12], their signaling characteristics and/or physiological relevance in relation to the IGF-I receptor are still under investigation. Nevertheless, receptor cross-talk interactions through shared ligands, subunit combination and phosphorylation of some common substrates, are believed to be the main mechanisms by which both IGFs can mediate their functions [1, 2, 7].
Fig. 1. Structures of insulin receptor isoform A and the IGF-I and IGF-II/M6P receptors.
The IGF-I receptor and insulin receptor isoform A are members of the tyrosine kinase receptor family, which share high structural homology. Both receptors exist at the cell surface as a heterotetramer composed of two α and two β subunits joined by disulfide bonds. By contrast, the IGF-II/M6P receptor is a type I transmembrane glycoprotein dimer consisting of four structural domains, including an amino-terminal signal sequence, a large extracytoplasmic domain, a single transmembrane region and a carboxy-terminal cytoplasmic tail. Most ligands bind to the extracytoplasmic domain, and the IGF-II/M6P receptor could exist as dimers. The binding affinity of insulin, IGF-I and IGF-II to each of the three receptors differ from each other as indicated. Plg, plasminogen; uPAR, urokinase-type plasminogen activator receptor.
The IGF-II receptor, unlike the IGF-I or insulin receptors, is structurally distinct and has no intrinsic tyrosine kinase activity. It exhibits higher affinity for IGF-II than IGF-I and does not bind insulin (Fig. 1) [13, 14]. The discovery in 1987–88 [15–17] that the IGF-II receptor is identical to the cation-independent mannose 6-phosphate (M6P) receptor indicates that this receptor (i.e., the IGF-II/M6P receptor) could function in multiple biological processes. Indeed, several studies have clearly established a role for this receptor in lysosomal enzyme trafficking from the trans-Golgi network (TGN) to the endosome for their subsequent delivery to lysosomes, clearance and/or activation of a variety of growth factors and endocytosis-mediated degradation of IGF-II. A growing body of evidence further supports a role for this receptor in transmembrane signal transduction in response to IGF-II binding, but its biological relevance remains controversial. At present, unlike the IGF-I receptor, very little is known as to the physiological and/or pathological significance of the IGF-II/M6P receptor in the functioning of the CNS [13, 18]. Thus, there is a need for a better understanding of the potential implications of this unique multifunctional receptor. This review provides an overview of the current knowledge on the structure and function of the IGF-II/M6P receptor, with special emphasis on its role in CNS functions in the normal brain and in degenerative diseases. For information on earlier work on the IGF-II/M6P receptor and/or its role in the periphery, the reader is referred to several reviews [13, 18, 19].
2. Structure, ligands and trafficking of the IGF-II/M6P receptor
2.1. Primary structure of the IGF-II/M6P receptor
Structurally, the IGF-II/M6P receptor is a type 1 integral membrane glycoprotein and P-type lectin receptor with a large N-terminal extracytoplasmic domain (ectodomain), a single transmembrane domain and a short C-terminal cytoplasmic domain. The ectodomain, which protrudes into the extracellular space or the lumen of vesicles and intracellular organelles, is composed of 15 contiguous repeats of approximately 147 amino acid residues each, sharing 14–38% sequence identity. Most repeats contain eight conserved cysteines that form intramolecular disulfide bonds necessary for proper receptor folding [13, 20, 21]. Functionally, the receptor binds IGF-II and M6P-containing ligands at distinct sites: repeat 11 comprises the core IGF-II binding site, whereas repeats 3, 5, 9 and 15 bind substrates with M6P groups [20, 22–24] (Fig. 1). High-resolution crystal structure studies of repeat 11 and of a receptor fragment consisting of repeats 1–3 of the human IGF-II/M6P receptor have provided insights into the structural features of the receptor [20, 21]. These studies suggest that all 15 repeats share a similar topology consisting of a flattened β-barrel formed by nine β-strands. This overall disulfide bond-stabilized structure has been termed a mannose 6-phosphate receptor homology (MRH) domain [13]. Further analysis of the 1–3 triple-repeat crystal indicates a structure in which repeat 3 sits on the top of repeats 1 and 2, suggesting that the IGF-II/M6P receptor forms distinct structural units that stack in a back-to-front manner. In this model, the IGF-II binding site potentially resides on the opposite face of the structure relative to the principal M6P binding sites of domains 3 and 9. The ectodomain contains 19 potential glycosylation sites, of which at least two are utilized in forming the mature receptor. Post-translational modifications including phosphorylation and palmitoylation have also been reported for the receptor. The cytoplasmic domain contains motifs that are important for receptor trafficking and phosphorylation. For example, the single tyrosine-based internalization motif, YSKV, is involved in targeting plasma membrane receptors to clathrin-coated vesicles [25]. Additionally, several regions of the cytoplasmic tail can act as potential substrates for various protein kinases, including protein kinase C (PKC), cAMP-dependent protein kinase, and casein kinases I and II [26]. A truncated form of the receptor representing a proteolytic cleavage product of the receptor’s ectodomain has been identified in serum and other physiological fluids of a variety of mammalian species [27, 28]. The significance of the truncated receptor form in relation to the functioning of the IGF-II/M6P receptor under in vivo conditions remains unclear; however, it appears that proteolytic cleavage at the cell surface to release the receptor’s ectodomain may be one means to degrade and thus down-regulate levels of the IGF-II/M6P receptor [29, 30].
The human IGF-II/M6P receptor gene is located on chromosome 6 and the murine IGF-II/M6P receptor gene is located on chromosome 17, and both contain 48 exons [31]. Interestingly, the exon boundaries of the IGF-II/M6P receptor, unlike other multidomain receptors, do not correspond to its functional or structural domains: exons 1–46 encode the receptor’s ectodomain with each of its 15 domains encoded by portions of 3 to 5 separate exons. In the human, expression of the IGF-II/M6P receptor gene is biallelic, whereas in the mouse it is maternally imprinted in peripheral tissues [32] but is expressed from both parental alleles in the CNS. The IGF-II/M6P receptor is ubiquitously expressed in most cells and tissues, and a number of studies have demonstrated that levels of the receptor are developmentally regulated; expressed at higher levels during fetal development and then declining over the postnatal period [33–35].
2.2. Ligands of the IGF-II/M6P receptor
As stated above, the multifunctional IGF-II/M6P receptor binds M6P-containing ligands and IGF-II at several distinct sites (Table 1) [13, 22, 24]. Two high-affinity M6P binding sites localize to repeats 1–3 and 7–11 of the receptor’s ectodomain, with essential residues localized to domains 3 and 9 (Fig. 1). A third, lower-affinity M6P recognition site that has a preference for binding mannose 6-phosphodiesters has been demonstrated within domain 5 [23]. Recently, a fourth M6P binding site with very low ligand-binding affinity has been localized to domain 15 [24]. It has been demonstrated that one receptor can simultaneously bind two molecules of M6P and one molecule of IGF-II [13, 36]. A variety of M6P-containing ligands such as lysosomal enzymes, transforming growth factor-β (TGF-β) [37], granzyme B, glycosylated leukemia inhibitory factor (LIF) [38], proliferin [39] and thyroglobulin [40], Other ligands that may bind the receptor by a non-M6P-based interaction are retinoic acid [41], urokinase-type plasminogen activator receptor (uPAR) and plasminogen itself [42, 43]. It is noteworthy that of the receptor’s 15 ectodomain repeats, ligand-binding functions - either primary (1, 3, 5, 9, 11, 15) or secondary (1, 7, 13) - have been ascribed to every odd-numbered repeat, whereas the even-numbered domains are considered to have more passive functions involving connection, spacing, orientation or dimerization.
Table 1.
Ligands that bind the IGF-II/M6P receptor and functional consequences of their binding
| Non-M6P-containing ligands | Consequences of IGF-II/M6P receptor binding |
|---|---|
| Insulin-like growth factor-II | Endocytosis and lysosomal degradation, possible signal transduction |
| Retinoic acid | Growth inhibition and/or apoptosis |
| Urokinase-type plasminogen | Participation in TGF-h activation at the cell surface; endocytosis and activator receptor lysosomal degradation |
| Plasminogen | Conversion to plasmin and participation of TGF-β activation |
|
| |
| M6P-containing ligands | Consequences of IGF-II/M6P receptor binding |
|
| |
| Lysosomal enzymes | Endocytosis and/or trafficking to lysosomes |
| Transforming growth factor- β precursor | Cell-surface proteolytic activation |
| Leukemia inhibitory factor | Endocytosis and lysosomal degradation |
| Proliferin | Induction of endothelial cell migration and angiogenesis |
| Thyroglobulin | Endocytosis and lysosomal activation and/or degradation |
| Renin precursor | Endocytosis and proteolytic activation and/or degradation |
| Granzyme A | Targeting to lytic granules and possible role in apoptosis |
| Granzyme B | Internalization and induction of apoptosis |
| DNAse I | Possible targeting to lysosomes |
| CD26 | Internalization and T cell activation |
| Epidermal growth factor | Endocytosis and lysosomal degradation |
| Herpes simplex viral glycoprotein D | Facilitation of viral entry into cells and transmission between cells |
| Varicella-zoster viral glycoprotein I | Facilitation of viral entry into cells |
The distinct binding sites of the IGF-II/M6P receptor allow not only for simultaneous binding of IGF-II and M6P-tagged glycoproteins, but binding of one ligand has been shown to reciprocally modulate receptor affinity for other ligands [44, 45]. For example, IGF-II has been shown to prevent binding of β-galactosidase to purified IGF-II/M6P receptors, whereas several lysosomal enzymes, but not M6P, inhibit the binding of IGF-II to the receptor [46, 47]. Conversely, M6P has been shown to stimulate the binding of 125I-IGF-II to the IGF-II/M6P receptor by two-fold in a number of cell types [45, 48]. Although the physiological significance of this interaction remains to be defined, it has been suggested that reciprocal inhibition of binding of these two classes of ligand is probably caused by a steric hindrance or a conformational change in the receptor. As a consequence of this reciprocal inhibition, extracellular lysosomal enzymes may inhibit the IGF-II/M6P receptor-mediated degradation of IGF-II, whereas the presence of IGF-II would increase the concentration of extracellular lysosomal enzymes. This issue will be discussed in further detail below.
Traditionally thought to function as a monomer, the IGF-II/M6P receptor exists in the membrane as an oligomer, and simultaneous, cooperative binding by monomers is necessary to produce a high affinity for M6P-related ligands [49, 50]. The ectodomain repeat 12 is believed to be functionally important for dimerization of the receptor (Fig. 1), which apparently enhances the binding affinity of ligands that are multivalent for M6P residues and alters the kinetics of receptor internalization from the cell surface [49, 50]. As IGF-II and lysosomal enzymes act as primary ligands for the multifunctional IGF-II/M6P receptor, the following section provides a brief overview of the features that underlie the binding of these ligands to the receptor.
2.2.1. IGF-II
IGF-II, which exhibits close structural similarity with IGF-I and insulin, contains 67 amino acid residues of which 45 (62%) are identical to IGF-I [51]. The gene encoding IGF-II maps to chromosome 11p15 and comprises 9 exons and 4 promoters. Exons 7, 8 and 9 encode the prepro-IGF-II protein, whereas exons 1 to 6 are non-coding and form alternative 5′-untranslated regions [52]. The 180-residue prepro-IGF-II contains an 89-residue carboxyl-terminal (E) peptide and a 24-residue signal peptide, both of which are cleaved post-translationally to generate mature IGF-II. At the cellular level, IGF-II and its mRNA are widely distributed in many different tissues including the CNS. Although IGF-II binds to both the IGF-I and IR-A receptors, it is considered to be the best-characterized non-M6P-containing ligand of the IGF-II/M6P receptor in viviparous mammals [13]. Functionally, IGF-II binds to repeat 11 of the IGF-II/M6P ectodomain [53, 54], which contains two hydrophobic binding sites, the first being a shallow cleft located at the mouth of the β-barrel and the second one extends along an external flattened surface. The former binding site, which contains Ile1572, is crucial for the initial docking of IGF-II, whereas the latter plays a role in stabilizing IGF-II binding [55, 56]. Structural studies of the interactions between IGF-II and the receptor, confirmed by mutagenesis, demonstrate that the residues Phe19 and Leu53 of IGF-II lock into this hydrophobic pocket of the receptor [57]. Repeat 11 of the ectodomain is also sufficient to mediate internalization of IGF-II by the receptor [56]. Examination of IGF-II binding to mini-receptors containing repeats 11–12, 11–13, and 11–15 indicates that repeat 13 does not itself bind to IGF-II but contains a domain that enhances its binding to the receptor [18, 58, 59]. It is also of interest to note that IGF-II binding by the IGF-II/M6P receptor is mostly confined to viviparous mammals, as platypus [60], chicken [61] and frog [61] receptors do not demonstrate such interactions or they bind with such low affinity as to not be physiologically relevant. The sequence alignment studies of both the IGF-II/M6P receptor and IGF-II suggested that although IGF-II remains relatively unaltered during evolution, the receptor gained the ability to bind IGF-II by changing key residues located in the binding pocket. Interestingly, unlike IGF-II binding, the carbohydrate recognition function of the receptor is widely utilized by mammalian and non-mammalian species [62, 63].
2.2.2. Lysosomal enzymes
To date, approximately 60 lysosomal hydrolases have been identified [64], and the majority of them are transported to lysosomes via the IGF-II/M6P receptor [62]. Equilibrium dialysis experiments have shown that the receptor can bind 2 moles of M6P or 1 mole of β-galactosidase or equivalent lysosomal enzyme bearing multiple M6P moieties [13]. Site-directed mutagenesis studies combined with pentamannosyl phosphate-agarose chromatography and binding affinity analyses have identified 5 amino acids in both repeat 3 (Q392, S431, R435, E460 and Y465) and repeat 9 (Q1292, H1329, R1334, E1354 and Y1360) that are essential for carbohydrate recognition by the bovine IGF-II/M6P receptor [13, 65]. Structure-based sequence alignment analysis of repeat 5 has also revealed 4 key residues (Gln, Arg, Glu and Tyr) that are necessary for carbohydrate binding, but the affinity of this site for M6P is approximately 300-fold lower than those of repeats 3 and 9 [23]; this site has now been identified as having a preference for binding mannose 6-phosphodiesters [66]. The C-terminal M6P binding site located on domain 9 exhibits optimal binding at pH 6.4–6.5, whereas the N-terminal M6P binding site of domain 3 shows a higher optimal binding pH of 6.9–7.0. Furthermore, the C-terminal site is highly specific for M6P and M6P phosphomonoester, whereas the N-terminal site binds M6P phosphodiester and M6P-sulfate with lower affinity than M6P [13, 67]. A recent study revealed that domain 15 has 3 of the five canonical ligand-binding residues and does bind M6P ligands, but with very low affinity [24, 66]; the physiological relevance of this property is unclear at this time. Thus, it is apparent that carbohydrate-binding sites of the IGF-II/M6P receptor recognize a great diversity of ligands over a relatively broad pH range [68].
2.3. Trafficking of the IGF-II/M6P receptor
Under normal conditions, the IGF-II/M6P receptors are localized mostly in the TGN and endosomal compartments and to a lesser extent (~10%) on the plasma membrane, but the receptors continuously shuttle between the surface and intracellular pools [18, 59]. Several agents, including growth factors, enzymes and chemical compounds, have been shown to modulate cellular recycling and routing of the IGF-II/M6P receptor. For example, a rapid and transient redistribution of IGF-II/M6P receptors from internal pools to the cell surface is induced in human fibroblasts by IGF-I, IGF-II and epidermal growth factor (EGF) [69]. The most striking effects on the distribution of the IGF-II/M6P receptor have been observed in rat adipocytes and H-35 hepatoma cells, wherein insulin causes a major subcellular relocalization of receptors from internal membranes to the cell surface [70, 71]. Glucose also increases IGF-II binding to the IGF-II/M6P receptor as a result of increased receptor cell-surface localization in two insulin-secreting cell lines (RINm5F and HIT) and human erythroleukemia K562 cell line [72]. Furthermore, the lysosomal enzyme β-glucuronidase has been shown to increase the rate of receptor internalization from the cell surface by stimulating or stabilizing receptor dimerization [73], whereas some major histocompatibility complex class I-derived peptides have been shown to inhibit receptor internalization in insulin-stimulated rat adipose cells [74]. Although the underlying mechanism(s) remains to be established, several kinases and phosphatases have been suggested to regulate the redistribution of cellular IGF-II/M6P receptors [19]. There is evidence that PKC-mediated serine phosphorylation or okadaic acid inhibition of serine phosphatases increases the proportion of receptors on the plasma membrane [72, 75, 76]. The cytoplasmic domain of the bovine IGF-II/M6P receptor contains three serine residues (i.e., Ser19, Ser85 and Ser156) whose phosphorylation correlates with the localization of the receptor in the TGN and clathrin-coated vesicles [77–79]. However, disruption of these three phosphorylation sites by mutagenesis had no detectable effect on the sorting of lysosomal enzymes by the bovine or murine IGF-II/M6P receptor [25].
3. Functions of the IGF-II/M6P receptor
The IGF-II/M6P receptor is actively involved in the regulation of cellular homeostasis either by transporting a diverse group of extracellular ligands into the cell via clathrin-coated vesicles for their subsequent activation/degradation or by targeting molecules such as lysosomal enzymes to endosomes/lysosomes for their subsequent functions. Binding of IGF-II to the receptor located on the plasma membrane may also exert some biological effects under certain circumstances by activating specific signal transduction pathways. Although the precise function of the IGF-II/M6P receptor may vary depending on the ligands and/or cell type under consideration, here we provide a brief overview of the general functions of IGF-II/M6P receptors that have been studied rather extensively over the years [26].
3.1. IGF-II/M6P receptor in sorting of lysosomal enzymes
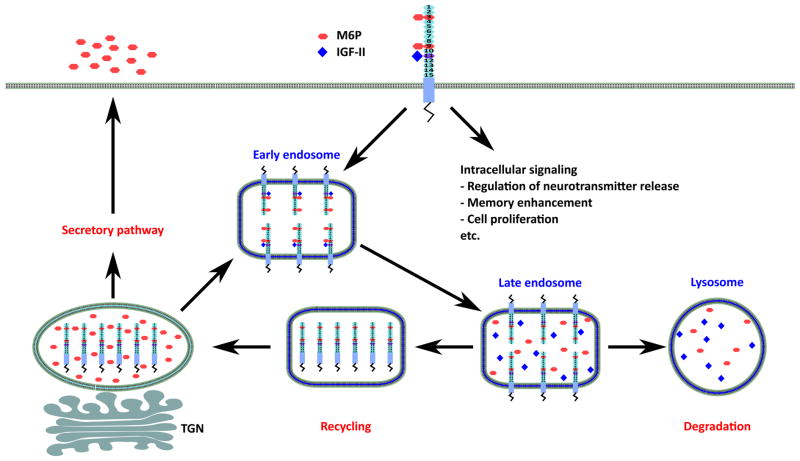
Although the segregation and transport of lysosomal enzymes are believed to be mediated by a number of selective receptors, several lines of evidence demonstrate a critical role for IGF-II/M6P receptor in intracellular sorting of newly synthesized lysosomal enzymes. The lysosomal enzymes, after being synthesized in the rough endoplasmic reticulum (ER), enter the lumen of the ER by means of an amino-terminal signal peptide. Co-translational glycosylation occurs on selected asparagine residues by transfer of pre-formed oligosaccharides rich in mannose residues. The lysosomal enzymes are then moved by vesicular transport to the Golgi stack, where phosphorylation of selected mannose residues on the 6-position is performed in a two-step process. First, a phosphotransferase transfers N-acetylglucosamine-1-phosphate from UDP-GlcNAc to a mannose residue on the lysosomal enzyme, resulting in a phosphodiester intermediate. Subsequently, N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase removes N-acetylglucosamine, yielding a mannose 6-phosphomonoester that can bind to the receptor. As the lysosomal enzymes acquire their complement of M6P residues in the cis-Golgi, they become capable of binding to the IGF-II/M6P receptors that are also being directed through the Golgi stacks. Sorting of lysosomal enzymes away from other transmembrane and secreted protein cargo usually occurs in the TGN, where clathrin-coated vesicles are found to arise from the tubular structures. The vesicles containing lysosomal enzymes are then trafficked to late endosomes, where the acidic milieu triggers the dissociation of lysosomal enzymes from the IGF-II/M6P receptor. While the lysosomal enzymes are subsequently delivered to lysosomes by vesicular trafficking, the unoccupied IGF-II/M6P receptors are either targeted to the cell surface or recycled to the TGN to engage in another round of transport of ligands (Fig. 2) [13, 26]. A minor population of lysosomal enzymes escapes the intracellular sorting and gets secreted through the constitutive secretory trafficking pathway; these molecules bind to cell-surface IGF-II/M6P receptors and are transported to the lysosomes via clathrin-coated endocytic vesicles. The endocytic pathway that leads to delivery of the enzymes to lysosomes utilizes the same late-endosome/pre-lysosomal intermediates that are utilized in the intracellular biosynthetic pathway [80]. Recent evidence suggests that IGF-II/M6P receptor can also mediate transcytosis of lysosomal enzymes across the blood-brain barrier (BBB) in neonatal mice [81], thus providing an underlying basis for the treatment of lysosomal storage disorders characterized by neurological impairment arising from deficits in specific lysosomal enzymes [82].
Fig. 2. Cellular roles of IGF-II/M6P receptor.
Newly synthesized lysosomal enzymes are targeted within the trans-Golgi network for sorting to lysosomes by the posttranslational addition of M6P residues. IGF-II/M6P receptors, possibly interacting with GGA/AP-1, mediate the recruitment of lysosomal hydrolases to clathrin-coated vesicles, following which enzyme-receptor complexes are delivered to endosomal compartments. Lysosomal enzymes dissociate from M6P receptors within the low-pH environment of late endosomes and are subsequently delivered to lysosomes. Recycling of IGF-II/M6P receptors from late endosomes to the Golgi from early endosomes is thought to be mediated by retromer. Cell-surface IGF-II/M6P receptors also function in the capture and activation/degradation of extracellular M6P-bearing ligands, as well as in the clearance and degradation of the non-glycosylated IGF-II polypeptide hormone through clathrin-dependent endocytosis. IGF-II/M6P receptor may mediate intracellular signal transduction following IGF-II binding to regulate neurotransmitter release memory enhancement and cell proliferation.
Site-directed mutagenesis experiments have shown that binding of clathrin-associated proteins to an acidic-cluster-dileucine amino acid (AC-LL) motif within the cytoplasmic tail of the M6P receptors is required for efficient clathrin-mediated transport of lysosomal enzymes to endosomal compartments [18, 83]. Previously, interactions between the clathrin adaptor protein AP1 and the dileucine-based sorting signals of M6P receptors, in conjunction with ADP-ribosylation factor, were thought to mediate clathrin-coat assembly on vesicles budding from the TGN [13, 84, 85]. Although a role for AP1 in the transport of M6P receptors from TGN-to-endosome has not been ruled out, several studies have provided strong evidence that members of the clathrin-associated Golgi-localized, γ-ear-containing, ADP-ribosylation factor-binding (GGA) protein family mediate M6P receptor sorting into vesicles budding from the TGN [18, 86, 87]. The GGAs, which comprise three members in mammals (GGA1, GGA2 and GGA3) and two members in yeast (Gga1p and Gga2p), are monomeric, multi-domain, cytoplasmic adaptor proteins consisting of four domains: an amino-terminal VHS (for Vps27, Hrs, STAM homology) domain, a GAT (for GGA and TOM homology) domain, a connecting hinge segment, and a carboxyl-terminal GAE (for γ-adaptin ear homology domain) domain [18, 85, 86, 88, 89]. The GAT domain binds ADP-ribosylation factor-GTP complexes and mediates recruitment of GGAs from the cytosol onto the TGN. The VHS domain interacts specifically with the AC-LL motif in the cytoplasmic tails of the M6P receptors. The GAE domain binds a subset of the accessory factors that interacts with the ear domain of AP-1, whereas the recruitment of clathrin triskeletons to budding vesicles is most likely mediated through clathrin-binding motifs of the hinge and GAE domain [18, 86, 87, 90]. Taken together, these findings suggest that GGAs are sorting proteins that recruit IGF-II/M6P receptors into clathrin-coated vesicles at the TGN for their transport to endosomes [18, 91]. On the other hand, receptor recycling back to the TGN from endosomes appears to involve an interaction between the cytoplasmic tail of the receptor and a complex of adaptor proteins termed as retromers. The retromers are composed of two subcomplexes: one consisting of Vps35p, Vps29p and Vps26p protein involve in cargo selection and the other comprising SNX1/SNX2/-SNX5-SNX6 dimers that regulate the formation of membrane tubules [92]. Several other proteins have also been implicated in the retrieval of the IGF-II/M6P receptor, including AP1, tail-interacting protein of 47 kDa (TIP47) and phosphofurin acidic cluster-sorting protein 1 (PACS-1) [18, 85, 93]. There is evidence that PACS-1 acts as a connector between the IGF-II/M6P receptor and AP1 to facilitate recycling of the receptor from early endosomes to the TGN [26], whereas TIP47 recycles receptors from late endosomes by binding Rab9, a late endosome GTPase [13, 18].
3.2. IGF-II/M6P receptor in the clearance/activation of extracellular ligands
Earlier studies using a variety of cultured cells, including rat adipocytes [94], L6 rat myoblasts [95], rat C6 glial cells [46] and mouse L cells [96] have demonstrated that IGF-II/M6P receptor mediates internalization and subsequent degradation of IGF-II. This is further substantiated by genetic experiments [97–99], which showed that deletion of the IGF-II/M6P receptor gene in mice led to prenatal death, but the fetuses were ~30% larger than normal [99] with increased levels of serum IGF-II but no alteration in IGF-II mRNA expression [97]. Interestingly, this phenotype can be rescued by simultaneous disruption of the genes for either IGF-II itself or the IGF-I receptor [98], thus indicating that increased levels of local IGF-II in the absence of IGF-II/M6P receptors produce a proliferative and hypertrophic response mediated via the IGF-I receptor.
In addition to IGF-II, IGF-II/M6P receptors regulate the internalization followed by degradation or activation of a variety of other ligands, including proliferin (a prolactin-related murine protein) [39], glycosylated human LIF [38], pre-prorenin [100] and epidermal growth factor receptor [101]. The cell-surface IGF-II/M6P receptor is believed to facilitate activation of latent TGF-β [102, 103], the precursor of the potent growth effector that regulates differentiation and growth of many cell types, by complex interactions involving IGF-II/M6P receptor, plasminogen and urokinase-type plasminogen activator receptor (uPAR). The ability of plasminogen and uPAR to bind the IGF-II/M6P receptor at distinct sites (localized to domain 1) from the latent TGF-β provides a plausible model in which urokinase binding to uPAR first facilitates the conversion of plasminogen to plasmin, which in turn proteolytically activates IGF-II/M6P receptor-bound TGF-β precursor [18, 42].
3.3. IGF-II/M6P receptor in mediating the biological effects of IGF-II
Unlike its function as a clearance receptor (in the clearance of IGF-II), the role of the IGF-II/M6P receptor in mediating certain biological effects of IGF-II by triggering an intracellular signaling cascade remains controversial. Because the IGF-II/M6P receptor lacks intrinsic catalytic activity, most of the biological effects of IGF-II have been attributed either to the IGF-I receptor [13] or IR-A [8]. Nonetheless, a number of studies over the years have suggested that certain biological effects of IGF-II are being triggered directly via the IGF-II/M6P receptor, including calcium influx in mouse embryo fibroblasts [104] and rat calvarial osteoblasts [105], increased protein phosphorylation [106] and alkalinization in proximal renal tubule cells [107], stimulation of Na+/H+ exchange and inositol trisphosphate production in canine kidney cells [108], increased amino acid uptake in muscle cells [109], increased glycogen synthesis in hepatoma cells [110], proteoglycan synthesis in human chondrosarcoma cells [111], calcium mobilization in rabbit articular chondrocytes [112], cell motility in rhabdomyosarcoma cells [113, 114], aromatase activity in placenta cytotrophoblasts [115], migration of human extravillous trophoblasts [116], insulin exocytosis by pancreatic β cells [117], and regulation of endogenous acetylcholine and GABA release from the adult rat brain [118, 119].
Since the cytoplasmic tail of IGF-II/M6P receptor lacks an intrinsic kinase domain, the intracellular mechanisms by which the receptor can mediate the range of biological effects listed above remain unclear. However, a number of studies using cell-free experimental systems or live cells have provided evidence for an interaction of the IGF-II/M6P receptor with heterotrimeric G proteins [72, 118, 120]. This is supported by the evidence that i) a cytoplasmic 14-residue region (Arg2410-Lys2423) of the human IGF-II/M6P receptor, which exhibits some sequence similarity with mastoparan - a small peptide in wasp venom that can directly activate Gi/Go proteins, is able to activate Giα protein [121, 122], and ii) the existence of sequence homology between the C-terminal Ser2424-Ile2451 region of the IGF-II/M6P receptor and part of the pleckstrin homology domain of several proteins that bind Gβγ and inhibit its stimulatory action on adenylyl cyclase activity [120]. At the functional level, there is evidence to suggest that IGF-II, acting via a G protein, can stimulate Ca2+ influx in 3T3 fibroblasts and CHO cells [123, 124], increase exocytosis of insulin from pancreatic β cells [117], promote migration of extravillous trophoblast cells [116], and enhance PKC-induced phosphorylation of intracellular proteins [72]. Notwithstanding these results, the IGF-II/M6P receptor, under certain conditions, failed to interact with G protein or to couple with a Giα in some studies [125, 126], thus raising doubt about the physiological relevance of the interaction between the IGF-II/M6P receptor and G proteins.
We have earlier shown that activation of the IGF-II/M6P receptor by Leu27IGF-II, an IGF-II analog that preferentially binds to the IGF-II/M6P receptor as opposed to the IGF-I or insulin receptors, can induce depolarization of the basal forebrain cholinergic neurons and potentiate acetylcholine release from the adult rat hippocampus by a G protein-sensitive, PKCα-dependent pathway [118, 127]. More recently we reported that brain IGF-II/M6P receptors, as observed for several G-protein coupled receptors, are associated with β-arrestin 2. Following stimulation with Leu27IGF-II, the receptors are translocated from detergent-resistant to detergent-soluble membrane fractions along with a portion of β-arrestin 2 [128]. Activation of the IGF-II/M6P receptor by IGF-II was also found to cause cardiac cell pathological hypertrophy via Gαq-mediated increased phosphorylation of PKCα and calcium/calmodulin-dependent protein kinase II (CaMKII) [129]. Furthermore, IGF-II in cultured HEK293 cells can promote rapid membrane recruitment and activation of sphingosine kinase, leading to the production of extracellular sphingosine 1-phosphate (S1P), the ligand for G protein-coupled S1P receptors [130, 131]. This triple-membrane-spanning model of sphingosine kinase-dependent SIP receptor transactivation provides a general mechanism for the activation of G protein-dependent signaling pathways by non-classical G protein-coupled receptors [59, 131] but its physiological significance in relation to IGF-II/M6P receptor remains to be established.
3.4. IGF-II/M6P receptor in regulation of cell proliferation/death
It has long been suggested that overexpression of the IGF-II/M6P receptor acts as a growth inhibitor [132], whereas loss of the receptor function is associated with cell proliferation and progression of tumorigenesis [133–135]. This is supported by the evidence that IGF-II/M6P receptor plays a critical role in regulating the pericellular levels of IGF-II, a mitogen that is overexpressed in a number of human cancers [135, 136]. As stated above, the IGF-II/M6P receptor facilitates activation of the growth inhibitor TGF-β [42, 102, 137], and modulates the uptake/targeting of lysosomal enzymes, and glycosylated LIF [38], all of which require tight control to avoid the development and growth of tumors. Several studies have demonstrated that cytotoxic T cells kill target cells by granzyme B, which is taken up by perforin- and IGF-II/M6P receptor-based internalization mechanisms [138, 139]. Nevertheless, this conclusion has also been called into question [140]. Heterozygosity at the IGF-II/M6P receptor locus due to the loss of one allele and somatic mutations in the remaining allele have also been associated with a variety of cancers [141, 142]. Additionally, microsatellite instability within the IGF-II/M6P receptor gene has been shown to occur in a large number of gastrointestinal cancers [143], and single-nucleotide polymorphisms within the receptor gene have been suggested to increase the risk of developing cancers [144].
4. IGF-II/M6P receptor in neurodegenerative diseases
4.1. Distribution of IGF-II/M6P receptor in CNS
IGF-II/M6P receptor is widely but selectively distributed throughout the CNS (Table 2). Earlier studies using in vitro receptor autoradiography and membrane binding assays have shown the presence of specific 125I-IGF-II binding sites in various neuroanatomic regions of the brain, with particular enrichment in the choroid plexus, as well as in cortical areas, hippocampus, hypothalamus, cerebellum and certain brainstem nuclei of the adult rat brain [145–147]. A high to moderate density of specific 125I-IGF-II labeling, as revealed by in vitro receptor autoradiography, was apparent in various regions of the spinal cord [148]. Subsequent studies using Western blotting and immunohistochemistry have demonstrated that very high levels of the IGF-II/M6P receptor are expressed in the striatum, deeper layers (layers IV and V) of the cortex, pyramidal and granule cell layers of the hippocampus, selected thalamic nuclei, Purkinje cells of the cerebellum, pontine nucleus, and motor neurons of the brainstem as well as spinal cord [149–152]. Moderate neuronal labeling is noted primarily in the olfactory bulb, basal forebrain areas, hypothalamus, superior colliculus, midbrain areas and granule cells of the cerebellum, whereas relatively low intensity of labeling is apparent in the outer layer of the cortex, stratum lacunosum moleculare of Ammon’s horn, the molecular layer of the dentate gyrus and cerebellum [149–151]. Although most of the staining appears to be associated with neurons and their processes, non-neuronal ependymal cells also seem to express moderate levels of the receptor [149, 150]. Occasionally, IGF-II/M6P receptor immunoreactivity is evident in normal astrocytes, but its presence in resident microglia remains to be established [151, 152]. The distributional profile of the IGF-II/M6P receptor in the brain exhibits striking similarity with the distribution of the 46-kDa cation-dependent M6P receptor, but the relative intensity of the IGF-II/M6P receptor immunoreactivity was found to be greater, particularly in the basal forebrain and cerebral cortex, than that of the cation-dependent M6P receptor [153]. In keeping with protein profiles, high levels of IGF-II/M6P receptor mRNA have been demonstrated in various regions of the adult rat brain by Northern blot and RNAse protection assays [35, 154]. It is also of interest that IGF-II/M6P receptor expression in the brain is developmentally regulated, with high prenatal levels preceding a sharp postnatal decline, which is less acute in humans as compared to rat [155, 156]. The widespread distribution of the IGF-II/M6P receptor in the CNS suggests that one of its functions could relate to a “housekeeping” role in transporting intracellular or recapturing secreted lysosomal enzymes. The receptor may also participate in regulating the levels or functions of LIF, TGF-β and retinoic acid, which are known to modulate the activities of the nervous system [26, 102, 157, 158]. Additionally, the receptor may have a role in regulating the release of neurotransmitter/modulators [118, 119]. Several lines of experimental evidence suggest that IGF-II, which exhibits coordinated expression with the IGF-II/M6P receptor during development, has been shown to promote the growth, proliferation, and/or differentiation of a variety of neuronal phenotypes as well as glial cells under in vitro conditions [159–162]. Given the evidence that most, but not all, biological effects of IGF-II are mediated via IGF-I or insulin receptor, it is likely that mitogenic/growth-promoting effects of IGF-II during development are regulated by IGF-I receptor or insulin receptor, whereas the IGF-II/M6P receptor may act to stabilize or regulate local levels of IGF-II [26]. IGF-II/M6P receptor levels are also differentially altered following various surgical or pharmacological manipulations, thus indicating a potential role for the receptor in lesion-induced degenerative or regenerative processes [163–166]. As this review focuses primarily on recent developments on the potential role of the receptor in neurodegenerative diseases, especially in relation to Alzheimer’s disease (AD), the reader is referred to several earlier reviews that describe the role of the receptor in regulating the functions of the brain [26, 127, 167, 168].
Table 2.
Summary of the distribution of IGF-II/M6PR immunoreactivity in the adult brain
| Brain region | IGF-II/M6P receptor | ||
|---|---|---|---|
| Lateral septal nucleus | + | ||
| Medial septal nucleus | +++ | ||
| Nucleus of diagonal band of Broca | +++ | ||
| Caudate-putamen | − to + | ||
| Globus pallidus | + | ||
| Cerebral cortex, layers | I | II–III | IV–VI |
| Neocortex | + | + | +++ |
| Piriform/entorhinal | − to + | +++ | − to + |
| Amygdala | +++ | ||
| Stratum pyramidale | ++ | ||
| Strata oriens/radiatum | − to + | ||
| Molecular layer | + | ||
| Granule cell layer | + | ||
| Dorsal nuclei | + | ||
| Posterior nuclei | + | ||
| Ventral nuclei | ++ | ||
Relative intensity levels of the immunoreactivity for IGF-II/M6P receptor were estimated by visual inspection of stained sections under a light microscope. High (+++), intermediate (++), low (+), and undetectable (−) levels of immunoreactive intensity were discerned (Adapted from Konishi et al., 2005).
Unlike the normal brain, neurodegenerative diseases associated with aging constitute a set of pathological conditions characterized by progressive loss of neurons and synapses in selected areas of the nervous system. Brain cell death in degenerative disorders varies in type, location and rate of loss depending upon the disorders [169]. Even though it is unclear why certain brain regions are vulnerable in different degenerative disorders, it is also a fact that some neurons in targeted regions survive despite the loss of adjacent neurons [170]. The major neurodegenerative diseases that are associated with aging are AD, Parkinson disease (PD) and Huntington disease (HD). Other age-associated neurodegenerative diseases include amyotrophic lateral sclerosis (ALS), frontotemporal lobar degeneration, lysosomal storage diseases (LSDs), etc. The etiology of these diseases is either genetic and/or sporadic and the factors that trigger degeneration of neurons are considered to be multifactorial i.e., genetic, environmental, or endogenous factors related to aging. Nevertheless, neuronal loss in some of these diseases occurs via a shared mechanism triggered by an accumulation of misfolded toxic protein(s) - a possible consequence of abnormalities in synthesis, intracellular trafficking and/or clearance mechanisms [64, 171, 172]. This is partly supported by altered activity/functioning of the endosomal/lysosomal (EL) system which plays a critical role in the generation, and metabolism of proteins involved in various neurodegenerative diseases, including AD, PD and HD. Since IGF-II/M6P receptor is involved in the trafficking and sorting of lysosomal enzymes and some of their substrates to the EL system - the potential implication of this receptor in regulating the function of the EL system has been studied to a greater extent in relation to AD pathology, which shares several themes (such as protein misfolding) with other neurodegenerative diseases.
4.2. IGF-II/M6P receptor and AD pathology
AD is a progressive neurodegenerative disorder characterized by severe memory loss followed by deterioration of higher cognitive functions such as language, praxis, and judgment. Although in most cases AD develops sporadically after 65 years of age, a small proportion of cases corresponds to the early-onset (<65 years), autosomal-dominant form of the disease. Mutations in three genes, the amyloid precursor protein (APP) gene on chromosome 21, the presenilin 1 (PSEN1) gene on chromosome 14 and the presenilin 2 (PSEN2) gene on chromosome 1, have been identified as the cause of a large proportion of early-onset familial AD cases [173, 174]. Additionally, inheritance of the ε4 allele of the apolipoprotein E (APOE) gene on chromosome 19 increases the risk of late-onset/sporadic AD [175]. The neuropathological features associated with AD include the presence of extracellular β-amyloid (Aβ) peptide-containing neuritic plaques, intracellular tau-positive neurofibrillary tangles, and the loss of synapses and neurons in defined brain regions [176–178]. Structurally, neuritic plaques contain a compact deposit of proteinaceous amyloid filaments surrounded by dystrophic neurites, activated microglia, and fibrillary astrocytes. The principal component of neuritic/amyloid fibrils is the β-amyloid (Aβ) peptide, which is generated from APP [176, 177]. Pathological changes that characterize AD, together with the constitutive production of Aβ in the normal brain [179–181], indicate that an overproduction and/or a lack of degradation may increase Aβ levels which, in turn, contribute to neuronal loss and development of AD. Indeed, enhanced production of Aβ peptide has been associated with familial AD cases, whereas decreased clearance of the peptide has been linked to sporadic AD [182, 183].
The brain regions that are affected in AD include the basal forebrain, hippocampus, entorhinal cortex, neocortex and certain brainstem nuclei. Of all these regions, the basal forebrain, which provides the major cholinergic input to the hippocampus and the neocortex, is known to be most severely affected in AD pathology [184, 185]. Additionally, the loss of these neurons has been suggested to contribute to the progressive memory impairment associated with AD [185–187]. Given the evidence that the IGF-II/M6P receptor can potentiate acetylcholine release [118], enhance short- and long-term memory [188, 189], and also regulate the function of the EL system, which is involved in Aβ metabolism [190, 191], we and others have evaluated levels/distribution of IGF-II/M6P receptor in brains of individuals with AD. In general, IGF-II/M6P receptor levels are not significantly altered in AD brains compared to age-matched controls but are found to be decreased in the hippocampus of patients with two copies of the APOE e4 alleles [192] or increased in the cortex of patients with PSEN1 mutations [193]. Transgenic (Tg) mice expressing familial AD-linked mutant APP, on the other hand, showed increased levels/expression of IGF-II/M6P receptor in the affected regions of the brain at early but not late stages compared to age-matched controls [152] (unpublished data). Notwithstanding these results, IGF-II/M6P receptor is present in a subset of Aβ-containing neuritic plaques and activated astrocytes in both AD brains and mutant APP transgenic mice [152, 192], thus suggesting a potential role for the receptor in Aβ metabolism. In contrast to the receptor, IGF-II mRNA/peptide levels are decreased in AD brains [194, 195] and APP transgenic mice [195]. Additionally, enhancing IGF-II levels in the brain has been shown to ameliorate Aβ-containing neuritic plaques, synaptic deficits and cognitive impairments in two different lines of mutant APP transgenic mice [195, 196]. Since IGF-II can enhance working memory via the IGF-II/M6P receptor [188], it is likely that the receptor may have a role in regulating both cognitive functions and Aβ metabolism associated with AD pathology.
4.2.1. IGF-II/M6P receptor and EL system in AD
The EL system is part of the cell’s central vacuolar system where secretory and membrane proteins/lipids are synthesized, modified, trafficked to appropriate cellular compartments and then eventually degraded. It is comprised of the endocytic pathway and lysosomal system including five major compartments: early endosomes, late endosomes, autophagic vacuoles, lysosomes and residual bodies [197]. Along the endocytic pathway, endosomes sort cargos through tubular protrusions for recycling or through intraluminal vesicles for degradation. Sorting of cargos involves fission of endosomal transport intermediate vesicles and fusion with other endosomes or with lysosomes [198]. Endosomes are able to regulate numerous pathways in the cell by sorting, processing, recycling, activating, silencing and degrading a variety of substances and receptors [199]. Lysosomes are intracellular organelles that contain about 60 enzymes crucial for degradation and recycling of macromolecules delivered by endocytosis, phagocytosis or autophagy [200]. Therefore, the EL system is not only important for protein trafficking and processing, but also the key component of the autophagy system involved in cellular homeostasis and protection [64, 201, 202].
A variety of experimental approaches over the last decade have indicated that the EL system, which acts as one of the hubs for APP metabolism, is drastically altered in “at risk” neurons of AD brains. The changes associated with early-endosomes, which precede the clinical symptoms and substantial deposition of Aβ peptides, include increased volumes and increased expression of proteins involved in endocytosis/ recycling (such as Rab5 and rababtin) as well as certain lysosomal enzymes. These alterations likely reflect increased rates of endocytosis and endosomal recycling of proteins involved in AD [190, 191]. Coincidentally, changes in the lysosomal system, which possibly occur after endosomal abnormalities, are reflected by a robust proliferation of lysosomes as well as expression of all classes of lysosomal hydrolases, including cathepsins B and D [203, 204]. Transgenic mice overproducing Aβ peptides also exhibit an up-regulation of endosomal markers as well as lysosomal enzymes in selected brain regions [152]. Since cathepsins can directly influence APP processing and Aβ metabolism [205, 206], it is possible that enhanced levels of these enzymes may lead to intracellular accumulation of the peptides in vulnerable cells. This is partly supported by the evidence that pharmacological inhibition of cathepsin B or deletion of the cathepsin B gene has been shown to reduce Aβ burden in mutant APP transgenic mice [207, 208]. Additionally, as the leakage of lysosomal enzymes into the cytoplasm often leads to cell death [209, 210], it has also been suggested that chronic activation of lysosomes may have a role in the degeneration of neurons in AD brains. This notion is partly supported by two distinct lines of evidence i) increased levels of Aβ peptide trigger the release of lysosomal enzymes cathepsin D and β-hexosaminidase into the cytosol prior to degeneration of neurons [211, 212] and ii) preventing lysosomal breakdown protects neurons against toxicity [213]. At present, the mechanism underlying the activation of the EL system has not been clearly established, but it is likely to be regulated by receptors involved in the trafficking of molecules/enzymes to the lysosomes.
Previous studies have reported that IGF-II/M6P receptor levels, in general, are not markedly altered in most AD brain regions compared to controls [192, 193]. Conversely, the levels of the receptor are increased at early but not later stages of mutant APP-Tg mice [152] (unpublished data). Considering the multifunctional role of the IGF-II/M6P receptor, it is possible that unaltered receptor levels may reflect a rapid receptor turnover or a compromise in its other functions at the expense lysosomal enzyme delivery. Alternatively, a subset of the lysosomal enzymes could possibly be transported by other sorting receptors such as the cation-dependent M6P receptor or sortilin A receptor. In this regard, the cation-dependent M6P receptor, whose levels are elevated in vulnerable neurons of the AD brain, has been shown to redirect certain lysosomal hydrolases to early endosomes and increase the secretion of Aβ peptides in cultured fibroblasts [214, 215]. Thus further studies are required to define the relative significance of IGF-II/M6P receptor vs. cation-dependent M6P receptor in regulating the transport of lysosomal enzymes in AD pathology.
4.2.2. IGF-II/M6P receptor in APP processing and Aβ metabolism
Aβ peptides, the principal components of neuritic plaques, are a group of hydrophobic peptides containing 39–43 amino acid residues. These peptides are generated from proteolysis of APP, which is processed under normal conditions either by non-amyloidogenic α-secretase or amyloidogenic β-secretase pathways [190, 191]. The α-secretase pathway is mediated by a set of proteases that cleaves APP within the Aβ domain, yielding soluble APPα and a 10-kDa C-terminal fragment (CTF-α), which can be processed further by γ-secretase to generate Aβ17-40/Aβ17-42 [191]. The candidates that act as α-secretase are tumor necrosis factor-α converting enzyme (TACE or ADAM-17), ADAM-9, ADAM-10 or MDC-9. The amyloidogenic β-secretase pathway, on the other hand, cleaves APP to generate soluble APPβ and an Aβ-containing C-terminal fragment (CTF-β), which is further processed via γ-secretase to release full-length Aβ1-40/Aβ1-42 peptides [191]. While the β-secretase is a transmembrane aspartyl protease called BACE1, γ-secretase is a multi-transmembrane aspartyl protease complex made of presenilins (PS1 or PS2), nicastrin, PEN-2 and APH-1 [216–218]. Evidence suggests that non-amyloidogenic α-secretase processing occurs mostly in the secretory pathway and at the cell surface, whereas amyloidogenic BACE1 processing of APP mainly occurs in the endocytic pathway [190, 191]. The relative efficiencies of the two alternative modes of APP processing in the brain can be influenced by a variety of factors including classical neurotransmitters, neuropeptides and growth factors.
Recently, using well-characterized mouse L-cells deficient in expression of the murine IGF-II/M6P receptor (MS cells) and corresponding MS9II cells that overexpress the human IGF-II/M6P receptor, we reported that overexpression of the IGF-II/M6P receptor increases the steady-state levels of App, Bace1, Psen1, Ncstn and Aph1a transcripts [219]. In addition, the levels of APP holo-protein and its cleaved products APP-CTFs (APP-CTFα and APP-CTFβ) were increased in MS9II cells, suggesting a potential role for the receptor in regulating the levels of APP and its processing. As for secretase, while BACE1 proteins were increased, the steady-state levels of PS1 and APH-1 were not altered in MS9II cells when compared to MS cells [219]. The activities of both β- and γ-secretases, as well as secretory Aβ1-40/Aβ1-42 levels, were significantly higher in MS9II cells than MS cells [220]. Conversely, levels of the Aβ degrading enzyme IDE, but not neprilysin, were lower in MS9II cells indicating that overexpression of the IGF-II/M6P receptor may also influence clearance of Aβ-related peptides [220]. At the cellular level, subsets of APP, BACE1 and PS1 in the perinuclear region were found to be co-localized with IGF-II/M6P receptor in MS9II cells [220]. Additionally, receptor overexpression promoted APP localization in certain tubular structures, whose exact nature remains unclear. The significance of the IGF-II/M6P receptor overexpression on APP metabolism is highlighted by two lines of evidence i) siRNA-targeted decrease of the receptor level led to a concomitant reduction of APP, APP-CTFs and Aβ levels in MS9II cells and ii) activation of the receptor by its agonist Leu27IGF-II did not significantly affect the levels or processing of the APP in MS9II cells [220]. Notwithstanding these results, the functional implications of normal levels of the IGF-II/M6P receptor on neuronal Aβ metabolism remain to be evaluated.
At present, the mechanism(s) by which IGF-II/M6P receptor overexpression regulates APP levels or processing remains unclear. A number of earlier studies have indicated that lipid-raft microdomains and non-raft regions of lipid bilayers play critical roles in the efficiency of amyloidogenic and non-amyloidogenic processing of APP, respectively [221–224]. Since the relative amount of full-length APP is found to be markedly higher in raft vs non-raft fractions in MS9II cells compared to MS cells, it is likely that IGF-II/M6P receptor overexpression may partly enhance Aβ production in MS9II cells by promoting raft microdomain association of APP [220]. Alternatively, given the evidence that IGF-II/M6P receptors are involved in the intracellular trafficking of lysosomal enzymes such as cathepsins B and D, which are known to regulate Aβ metabolism [59, 203, 225], it is possible that receptor overexpression can influence amyloidogenic processing of APP by altering the levels and/or redistribution of the enzymes within the EL compartments, as in the case of cation-dependent M6P receptor overexpression [214].
Evidence suggests that trafficking of both APP and BACE1 within the cells is intimately associated with Aβ production. Newly synthesized APP and BACE1 are first transported to the cell surface via the secretory pathway and then internalized into endosomes where BACE cleaves APP in an acidic environment leading to Aβ production. APP can also be retrieved from endosomes back to TGN by binding to sortilin A receptor via PACS1, thus preventing its processing into Aβ peptides [191, 226]. Interestingly, retrograde transport of BACE1 from endosomes to TGN, as observed for IGF-II/M6P receptor, is mediated by binding to GGA proteins and the retromer complex [227, 228]. Dysfunction of the retromer complex not only causes endosomal accumulation of BACE1 but also increases production of APP-CTFβ and soluble APPβ [227, 229]. Since retrieval of the IGF-II/M6P receptor from endosomes to TGN overlaps with that of APP and BACE1, it is possible that overexpression of the receptor alters the trafficking dynamics in a manner that is conducive to amyloidogenic processing of APP within endosomal compartments leading to increased production of Aβ peptides.
4.2.3. IGF-II/M6P receptor and the cholinergic system
The IGF-II/M6P receptor is known to be widely but selectively distributed in various neuronal populations in the brain including the basal forebrain cholinergic neurons that are preferentially vulnerable in AD pathology [150]. Given the significance of the basal forebrain and acetylcholine in learning and memory processing, it has long been suggested that loss of these neurons and their innervations to the hippocampus and cortex contribute to the progressive memory impairment associated with AD patients [185]. Indeed, decreased levels of choline acetyltransferase activity, choline uptake and acetylcholine levels in the hippocampus and cortical regions of AD brains correlate positively with the clinical severity of dementia [185]. At present, however, the underlying cause of neurodegeneration of the basal forebrain cholinergic neurons remains unclear. Some earlier studies have shown that IGF-II/M6P receptor can enhance neuronal survival and increase the activity of acetylcholine transferase, the enzyme responsible for the synthesis of acetylcholine, in mouse primary septal cultured neurons [160]. N-nitrosodiethylamine-induced loss of neurons, on the other hand, has been shown to reduce both acetylcholine transferase and IGF-II/M6P receptor mRNAs [230]. We have reported that expression of the IGF-II/M6P receptor is increased in surviving cholinergic and non-cholinergic neurons in the basal forebrain region following in vivo administration of the immunotoxin 192 IgG-saporin [231]. Apart from influencing the viability of cholinergic neurons, there is evidence that activation of the IGF-II/M6P receptor can potentiate endogenous acetylcholine release from the hippocampus and cortex by a G protein-sensitive, PKCα-dependent pathway [118, 232]. Prenatal choline supplementation has also been shown to increase the expression of IGF-II and IGF-II/M6P receptor and enhance IGF-II-induced acetylcholine release in adult rat hippocampus and frontal cortex [233]. Thus, it would be of interest to determine whether the altered levels and/or signaling of the IGF-II/M6P receptor is the cause or consequence of the loss of basal forebrain cholinergic neuronal loss observed in AD brains.
4.2.4. IGF-II/M6P receptor and memory enhancement in AD
Apart from potentiating acetylcholine release, the IGF axis and the IGF-II/M6P receptor are involved in the retention and consolidation of memory processing [188, 189]. Using multiple approaches, it has recently been demonstrated that inhibitory avoidance learning in rat [188] and extinction learning in mouse [234] require increased synthesis of IGF-II. Administration of IGF-II directly into the hippocampus also enhanced memory retention and prevent forgetting [188]. This effect, which requires new protein synthesis, the function of the activity-regulated cytoskeletal-associated protein, and glycogen-synthase kinase-3, is mediated via the IGF-II/M6P receptor [167, 188]. Intriguingly, systemic administration of IGF-II has also been shown to enhance the retention and persistence of working, short-term as well as long-term memories via activation of the IGF-II/M6P receptors [189]. In a parallel study, it has been shown that IGF-II acts as a downstream regulator of IκB kinase/nuclear factor of κB (IKK/NF-κB)-dependent synapse development and remodeling process. This effect, which appears to be mediated by the IGF-II/M6P receptor via the MEK/ERK signaling pathway, provides a morphological correlate of memory enhancement coupled to IGF-II [235]. The involvement of IGF-II in cognitive function is further reinforced by three lines of evidence: i) IGF-II mRNA/protein levels are decreased in the brains of AD patients as well as transgenic mice overexpressing mutant APP [194, 236], ii) direct administration or adenovirus-mediated delivery of IGF-II into the brain has been shown to ameliorate cognitive deficits as well as other AD-related pathology [195, 196], and iii) an IGF-II polymorphism has been associated with human cognitive function [237]. Collectively, these findings not only suggest a novel role for IGF-II in cognitive functions but also indicate the possibility that activation of the receptor by IGF-II or its mimetic may have therapeutic relevance in the treatment of AD pathology.
4.2.5. IGF-II/M6P receptor and neuroprotection in AD
Although IGF-II has been shown to protect neurons against a variety of toxic agents [238–241], the significance of the IGF-II/M6P receptor in mediating the effects of IGF-II remains unclear. A recent study, however, showed that ameliorating effects of IGF-II against glucocorticoid-induced toxicity can partly be mediated via IGF-II/M6P receptor [241]. A protective role for the receptor has also been suggested by the evidence that cultured PC12 cells that are resistant to Aβ-mediated toxicity [242] or brain neurons that survive 192 IgG-saporin-induced toxicity express high levels of the IGF-II/M6P receptors [231]. Additionally, TGFβ1, which is known to be activated by IGF-II/M6P receptor, has been shown to protect cultured neurons against Aβ-mediated toxicity both under in vitro and in vivo conditions [243–245]. Notwithstanding these results, overexpression of the IGF-II/M6P receptor was found to render MS9II cells more vulnerable to staurosporine-induced toxicity than IGF-II/M6P receptor lacking MS cells [220]. Thus, more work is needed to define the significance of the receptor in relation to degeneration of neurons observed in AD brains.
5. IGF-II/M6P receptor in lysosomal storage disorders (LSDs)
LSDs represent two groups of inherited metabolic neurodegenerative diseases triggered by a deficiency of lysosomal enzymes or components integral to lysosomal function, the mucolipidoses and the mucopolysaccharidoses [246]. Nearly two-thirds of the more than 50 LSDs involve the CNS, where neuronal dysfunction or loss results in mental retardation, progressive motor degeneration, and premature death [247, 248]. The majority of LSDs are caused by loss of function of lysosomal hydrolases [247], and most of these enzymes are transported by the IGF-II/M6P receptor. Dysfunction in any of these components may cause lysosomal deficits, leading to accumulation of degradative products and ultimately lead to LSD. I-cell disease (mucolipidosis type II) is one of the LSDs caused by deficiency of N-acetylglucosamine phosphotransferase, which catalyzes the first step in M6P addition to N-linked oligosaccharides on the lysosomal enzymes, leading to defective lysosomal delivery of enzymes, secretion of those enzymes into the extracellular space, and accumulation of products destined to be degraded in dense inclusion bodies within cells [249]. Deficiencies of M6P-tagged lysosomal enzymes in some of the LSDs have been rectified by enzyme replacement therapy (ERT) where exogenously administered enzymes are delivered to the EL system via the cell-surface IGF-II/M6P receptor [250]. Recently discovered M6P analogs may improve both the affinity of the specific recombinant enzyme for the IGF-II/M6P receptor and the stability of the M6P moiety in the blood circulation and consequently enhance ERT efficacy and reduce enzyme dosage required for an efficient treatment [251, 252]. In addition, a newly-designed chimeric protein of IGF-II fused to β-glucuronidase to deliver enzyme via the IGF-II binding site on the IGF-II/M6P receptor can further increase the efficiency and improve delivery to resistant sites [253].
ERT has become well established as a useful therapeutic approach for several of the mucopolysaccharidoses (MPSs), a group of LSDs characterized by inherited deficiencies in a single lysosomal enzyme [250]. The effectiveness of this therapy depends on the expression of functional IGF-II/M6P receptors on the surface of the affected cells [250, 253]. The first disorder to be treated by this means was Gaucher disease, an inherited deficiency of the lysosomal enzyme glucocerebrosidase. Although there are now ERT approaches available for a number of the MPSs, including Fabry disease, Pompe disease, and MPS I, II and IV, the clinical effectiveness of these therapies has not matched the success attained with Gaucher disease. Even more problematic has been the nearly complete failure of ERT strategies to help correct brain dysfunction caused by CNS storage conditions in these diseases because the M6P-tagged replacement enzyme delivered intravenously does not transit the blood-brain barrier (BBB) [250, 253, 254]. The principal reason for this appears to be that the IGF-II/M6P receptors are not expressed on the BBB beyond the early perinatal period. Some hope for the application of brain-targeted ERT for the LSDs has been provided recently through the use of a chimeric form of sulphamidase coupled to the BBB-binding domain of apolipoprotein B [255]. The chimeric enzyme restored sulphamidase activity in the brains of sulphamidase-deficient mice with MPS IIIA. This highly engineered protein was taken up by binding of the apolipoprotein B portion of the chimera to the low-density lipoprotein receptor on the BBB and moved across into brain cells via transcytosis. Thus, in this case, the solution did not involve the IGF-II/M6P receptors.
Niemann-Pick disease covers a heterogenous group of three distinct lysosomal lipid storage diseases with autosomal recessive inheritance, i.e., Niemann-Pick type A, Niemann-Pick type B and Niemann-Pick type C (NPC). Niemann-Pick disease types A and B, caused by mutations in the gene coding for the lysosomal enzyme acid sphingomyelinase, result in the progressive accumulation of sphingomyelin and other lipids in the lysosomes of various tissues [256, 257]. NPC disease, which accounts for the majority of the cases of Niemann-Pick disease, is caused by mutation of either NPC1 gene located on chromosome 18 or NPC2 gene located on chromosome 14. This disease is characterized by a defect in intracellular cholesterol trafficking, which leads to accumulation of unesterified cholesterol in lysosomes. The buildup of cholesterol triggers hepatomegaly with foamy macrophage infiltration and chronic neurologic deterioration, leading to seizures, supranuclear ophthalmoplegia and progressive loss of motor and intellectual function. Neuropathologically, impaired lipid trafficking results in the degeneration of neurons, activation of glial cells and the presence of intracellular neurofibrillary tangles [256, 258, 259]. Interestingly, the brains of patients with NPC contain increased levels of Aβ-related peptides and often display extracellular deposition of the peptide – thus exhibiting some striking similarity with AD brain pathology [260, 261].
Earlier studies have shown that altered levels of cholesterol can influence distribution/trafficking of IGF-II/M6P receptors within cells [262–264]. In fact, it has been reported that cholesterol accumulation in cultured cells induced by treatment with U18666A (an amphiphilic drug that induces an NPC-like phenotype at the cellular level) or siRNA-mediated NPC1 depletion can lead to redistribution of the IGF-II/M6P receptors to endosomes and impair its retrograde transport from late endosomes to the trans-Golgi network [262, 265, 266]. Studies from Npc1−/− mice, which recapitulate most of the pathological features of NPC disease, revealed that IGF-II/M6P receptor levels are not altered in the brain even though cathepsins B and D levels/activity are dramatically increased in the same regions [258, 267, 268]. At the cellular level, IGF-II/M6P receptor immunoreactivity is found to be somewhat decreased in neurons of the Npc1−/− mouse brains. Additionally, the majority of activated astrocytes, but not microglia, express higher levels of IGF-II/M6P receptor indicating that decreased neuronal levels may partially be compensated by glial expression of the receptor [268]. Since the subcellular distribution of the IGF-II/M6P receptor in Npc1−/− mouse brains has not yet been established, it remains unclear whether the increased levels/activity of the lysosomal enzymes may be associated with the altered levels/distribution of the receptor [258, 268].
6. IGF-II/M6P receptor in other neurodegenerative diseases
Since IGF-II/M6P receptor is widely distributed throughout the brain, it is expected that altered levels and/or functioning of the receptor, may have a role in various neurodegenerative disorders in addition to AD. PD, the second most common neurodegenerative disorder, is characterized by akinesia, rigidity, tremor and postural abnormalities. The sensorimotor symptoms are often accompanied by autonomic dysfunction, dementia, and cognitive deficits. Etiologically, PD is heterogeneous; only a minority (8–10%) segregates with genetic abnormalities, whereas the majority of cases are believed to be sporadic. The neuropathological features associated with PD include: i) loss of dopaminergic neurons of the substantia nigra pars compacta projecting to the striatum; and ii) the presence of intracellular inclusions known as Lewy bodies, composed of insoluble aggregates of a protein called α-synuclein, which plays a critical role in the degeneration of neurons [269]. It is reported that lysosomes and lysosomal enzyme cathepsin D are fundamental regulators of α-synuclein degradation through the chaperone-mediated autophagy pathway [270, 271]. Some recent studies have shown that several point mutations of Vps35, a subunit of retromer complex that plays an important role in the trafficking of the IGF-II/M6P receptor, can lead to the manifestation of late-onset PD in many ethnic groups [272–274]. Using a transgenic Drosophila model, it has been demonstrated that interference with the retromer function can lead to aberrant maturation of cathepsin D and subsequent accumulation of α-synuclein in the late-endosome/lysosome compartments [275]. Additionally, the missense Vps35 mutation (i.e., D620N) did not alter steady-state levels of IGF-II/M6P receptor in the fibroblasts of PD patients, but triggered a deficit in retromer-dependent trafficking of the receptor and its ligand cathepsin D, which may underlie impaired degradation of α-synuclein resulting in the loss of neurons and development of PD pathology [276]. Interestingly, IGF-II/M6P receptor mRNA levels were not altered either in the frontal cortex or basal ganglia but decreased in the amygdala region of PD brains compared to control brains [277]. Although IGF-II/M6P receptor protein expression has not yet been evaluated in patients with PD, it appears that altered trafficking/function rather than levels of the receptor may have a role in the development of disease pathology. Impaired trafficking of the IGF-II/M6P receptor, as observed in PD-linked Vps35 mutation, may also contribute to the development of juvenile Batten disease - an autosomal recessive disorder with onset at 5–8 years of age. Hallmarks of this disease include lysosomal accumulation of undigested materials, degeneration of neurons in the brain and eye, blindness, seizures and development of cognitive and motor deficits, leading to death. This disease is caused by mutation of the gene encoding CLN3, a multipass transmembrane glycoprotein with unknown function. Using genetic manipulation, it has been shown that loss of CLN3 function triggers accumulation of IGF-II/M6P receptor in the trans-Golgi network, which leads to defective maturation/transport and activity of lysosomal cathepsins. Thus, dysfunction of lysosomal clearance mechanisms may underlie the development of devastating Batten disease [278].
Earlier studies indicated that 125I-IGF-II receptor binding was markedly increased in the spinal cords of patients with ALS - a fatal neurodegenerative disease caused by selective motor neuron death [279]. Using transgenic rat exhibiting loss of motor neurons, it has been demonstrated that IGF-II/M6P receptor expression is upregulated in reactive astrocytes towards the end-stages of disease pathology but not in pre-symptomatic animals suggesting a potential protective response against the loss of IGF-related trophic support [280]. In contrast to ALS, 125I-IGF-II receptor binding sites were not found to be altered either in the hippocampus or cerebellum following voluntary consumption of alcohol [146], but the receptor mRNA and binding sites are decreased in the cerebellar vermis and/or anterior cingulate gyrus of human alcoholics – often associated with loss of neurons as well as cognitive and motor deficits [281]. Multiple sclerosis (MS), an autoimmune inflammatory disease of the CNS, is characterized by demyelination, axonal loss, and neuronal death in selected brain regions. It is widely accepted that infiltration of inflammatory cells plays major roles in MS pathogenesis possibly via serine protease granzyme B secreted from granules of cytotoxic T cells [282, 283]. Interestingly, M6P treatment was found to attenuate granzyme B-mediated cell death by blocking the IGF-II/M6P receptor-dependent endocytosis, which suggests potential new targets for the treatment of MS [284]. However, no alteration was evident in 125I-IGF-II receptor binding sites in the cerebral cortex of MS patients [147], thus the significance of the receptor in the development of disease pathology, if any, remains to be determined. More recently, IGF-II/M6P receptor has been shown to be expressed highly in microglial nodules in human brains with human immunodeficiency virus encephalitis. Under an in vitro paradigm in which IGF-II/M6P receptor expression can be enhanced in microglia by interferon-γ, it has been shown to be a positive modulator of human immunodeficiency virus infection thus suggesting a contribution of the receptor in the persistence and spreading of viral-related pathology in the brain [285].
7. Conclusion
Recent studies have advanced our understanding of the structure, ligand binding properties and trafficking of the IGF-II/M6P receptor. This receptor plays a critical role in many cellular processes including growth and development, clearance of IGF-II, proteolytic activation of enzymes and growth factor precursors, apart from regulating trafficking of the lysosomal enzymes. This receptor, which is widely distributed throughout the brain, is also involved in IGF-II-mediated memory enhancement/ consolidation process as well as regulation of neurotransmitter release via G protein-dependent signaling mechanisms. Overexpression of the receptor has been shown to influence not only the functioning of the EL system but also the levels and metabolism of APP/Aβ-related peptides – thus suggesting its possible implication in AD pathology. Considering the significance of the receptor in regulating the trafficking of the lysosomal enzymes and other molecules, it is likely that the receptor may have a role not only in LSD but also in other neurodegenerative disorders that are associated with abnormal functioning of the EL system. However, further studies are needed to validate and fully characterize the significance of the IGF-II/M6P receptor in the normal brain and various neurodegenerative diseases to understand its full implications in regulating neuronal functioning.
Acknowledgments
This work was supported by grants from Natural Sciences and Engineering Research Council of Canada (#203518) and Canadian Institutes of Health Research (MOP-84480) to SK and R01AG019070 grant from National Institutes on Aging to GT and National Cancer Institute grant CA91885 to RM. YW is a recipient of Doctoral Awards from Alzheimer Society of Canada and a Graduate Studentship Award from Alberta Innovates - Health Solutions.
References
- 1.Adams TE, et al. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci. 2000;57(7):1050–93. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rother KI, Accili D. Role of insulin receptors and IGF receptors in growth and development. Pediatr Nephrol. 2000;14(7):558–61. doi: 10.1007/s004670000351. [DOI] [PubMed] [Google Scholar]
- 3.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20(6):761–87. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 4.Duan C, Xu Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol. 2005;142(1–2):44–52. doi: 10.1016/j.ygcen.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Bunn RC, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab. 2003;14(4):176–81. doi: 10.1016/s1043-2760(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 6.Lelbach A, Muzes G, Feher J. The insulin-like growth factor system: IGFs, IGF-binding proteins and IGFBP-proteases. Acta Physiol Hung. 2005;92(2):97–107. doi: 10.1556/APhysiol.92.2005.2.1. [DOI] [PubMed] [Google Scholar]
- 7.Dupont J, LeRoith D. Insulin and insulin-like growth factor I receptors: similarities and differences in signal transduction. Horm Res. 2001;55(Suppl 2):22–6. doi: 10.1159/000063469. [DOI] [PubMed] [Google Scholar]
- 8.Frasca F, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19(5):3278–88. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sciacca L, et al. In IGF-I receptor-deficient leiomyosarcoma cells autocrine IGF-II induces cell invasion and protection from apoptosis via the insulin receptor isoform A. Oncogene. 2002;21(54):8240–50. doi: 10.1038/sj.onc.1206058. [DOI] [PubMed] [Google Scholar]
- 10.Scalia P, et al. Regulation of the Akt/Glycogen synthase kinase-3 axis by insulin-like growth factor-II via activation of the human insulin receptor isoform-A. J Cell Biochem. 2001;82(4):610–8. doi: 10.1002/jcb.1196. [DOI] [PubMed] [Google Scholar]
- 11.Bailyes EM, et al. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem J. 1997;327(Pt 1)(1):209–15. doi: 10.1042/bj3270209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federici M, et al. Distribution of insulin/insulin-like growth factor-I hybrid receptors in human tissues. Mol Cell Endocrinol. 1997;129(2):121–6. doi: 10.1016/s0303-7207(97)04050-1. [DOI] [PubMed] [Google Scholar]
- 13.Dahms NM, Hancock MK. P-type lectins. Biochim Biophys Acta. 2002;1572(2–3):317–40. doi: 10.1016/s0304-4165(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 14.Massague J, Czech MP. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982;257(9):5038–45. [PubMed] [Google Scholar]
- 15.Morgan DO, et al. Insulin-like growth factor II receptor as a multifunctional binding protein. Nature. 1987;329(6137):301–7. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- 16.Oshima A, et al. The human cation-independent mannose 6-phosphate receptor. Cloning and sequence of the full-length cDNA and expression of functional receptor in COS cells. J Biol Chem. 1988;263(5):2553–62. [PubMed] [Google Scholar]
- 17.MacDonald RG, et al. A single receptor binds both insulin-like growth factor II and mannose-6-phosphate. Science. 1988;239(4844):1134–7. doi: 10.1126/science.2964083. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4(3):202–12. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 19.Kiess W, et al. Insulin-like growth factor II (IGF-II) and the IGF-II/mannose-6-phosphate receptor: the myth continues. Horm Res. 1994;41(Suppl 2)(SUPPL 2):66–73. doi: 10.1159/000183963. [DOI] [PubMed] [Google Scholar]
- 20.Brown J, et al. Structure of a functional IGF2R fragment determined from the anomalous scattering of sulfur. EMBO J. 2002;21(5):1054–62. doi: 10.1093/emboj/21.5.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson LJ, Dahms NM, Kim JJ. The N-terminal carbohydrate recognition site of the cation-independent mannose 6-phosphate receptor. J Biol Chem. 2004;279(32):34000–9. doi: 10.1074/jbc.M404588200. [DOI] [PubMed] [Google Scholar]
- 22.Braulke T. Type-2 IGF receptor: a multi-ligand binding protein. Horm Metab Res. 1999;31(2–3):242–6. doi: 10.1055/s-2007-978725. [DOI] [PubMed] [Google Scholar]
- 23.Reddy ST, et al. Identification of a low affinity mannose 6-phosphate-binding site in domain 5 of the cation-independent mannose 6-phosphate receptor. J Biol Chem. 2004;279(37):38658–67. doi: 10.1074/jbc.M407474200. [DOI] [PubMed] [Google Scholar]
- 24.Olson LJ, et al. Identification of a fourth mannose 6-phosphate binding site in the cation-independent mannose 6-phosphate receptor. Glycobiology. 2015;25(6):591–606. doi: 10.1093/glycob/cwv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen HJ, Yuan J, Lobel P. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J Biol Chem. 1997;272(11):7003–12. doi: 10.1074/jbc.272.11.7003. [DOI] [PubMed] [Google Scholar]
- 26.Hawkes C, Kar S. The insulin-like growth factor-II/mannose-6-phosphate receptor: structure, distribution and function in the central nervous system. Brain Res Brain Res Rev. 2004;44(2–3):117–40. doi: 10.1016/j.brainresrev.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Kiess W, et al. Type II insulin-like growth factor receptor is present in rat serum. Proc Natl Acad Sci U S A. 1987;84(21):7720–4. doi: 10.1073/pnas.84.21.7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonald RG, et al. Serum form of the rat insulin-like growth factor II/mannose 6-phosphate receptor is truncated in the carboxyl-terminal domain. J Biol Chem. 1989;264(6):3256–61. [PubMed] [Google Scholar]
- 29.Clairmont KB, Czech MP. Extracellular release as the major degradative pathway of the insulin-like growth factor II/mannose 6-phosphate receptor. J Biol Chem. 1991;266(19):12131–4. [PubMed] [Google Scholar]
- 30.Leksa V, et al. Soluble M6P/IGF2R released by TACE controls angiogenesis via blocking plasminogen activation. Circ Res. 2011;108(6):676–85. doi: 10.1161/CIRCRESAHA.110.234732. [DOI] [PubMed] [Google Scholar]
- 31.Killian JK, Jirtle RL. Genomic structure of the human M6P/IGF2 receptor. Mamm Genome. 1999;10(1):74–7. doi: 10.1007/s003359900947. [DOI] [PubMed] [Google Scholar]
- 32.Kalscheuer VM, et al. The insulin-like growth factor type-2 receptor gene is imprinted in the mouse but not in humans. Nat Genet. 1993;5(1):74–8. doi: 10.1038/ng0993-74. [DOI] [PubMed] [Google Scholar]
- 33.Funk B, et al. Expression of the insulin-like growth factor-II/mannose-6-phosphate receptor in multiple human tissues during fetal life and early infancy. J Clin Endocrinol Metab. 1992;75(2):424–31. doi: 10.1210/jcem.75.2.1379254. [DOI] [PubMed] [Google Scholar]
- 34.Matzner U, von Figura K, Pohlmann R. Expression of the two mannose 6-phosphate receptors is spatially and temporally different during mouse embryogenesis. Development. 1992;114(4):965–72. doi: 10.1242/dev.114.4.965. [DOI] [PubMed] [Google Scholar]
- 35.Sklar MM, et al. Developmental expression of rat insulin-like growth factor-II/mannose 6-phosphate receptor messenger ribonucleic acid. Endocrinology. 1992;130(6):3484–91. doi: 10.1210/endo.130.6.1317785. [DOI] [PubMed] [Google Scholar]
- 36.Westlund B, Dahms NM, Kornfeld S. The bovine mannose 6-phosphate/insulin-like growth factor II receptor. Localization of mannose 6-phosphate binding sites to domains 1–3 and 7–11 of the extracytoplasmic region. J Biol Chem. 1991;266(34):23233–9. [PubMed] [Google Scholar]
- 37.Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991;88(2):580–4. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanchard F, et al. Mannose 6-Phosphate/Insulin-like growth factor II receptor mediates internalization and degradation of leukemia inhibitory factor but not signal transduction. J Biol Chem. 1999;274(35):24685–93. doi: 10.1074/jbc.274.35.24685. [DOI] [PubMed] [Google Scholar]
- 39.Lee SJ, Nathans D. Proliferin secreted by cultured cells binds to mannose 6-phosphate receptors. J Biol Chem. 1988;263(7):3521–7. [PubMed] [Google Scholar]
- 40.Scheel G, Herzog V. Mannose 6-phosphate receptor in porcine thyroid follicle cells. Localization and possible implications for the intracellular transport of thyroglobulin. Eur J Cell Biol. 1989;49(1):140–8. [PubMed] [Google Scholar]
- 41.Kang S, et al. The retinoid X receptor agonist 9-cis-retinoic acid and the 24-hydroxylase inhibitor ketoconazole increase activity of 1,25-dihydroxyvitamin D3 in human skin in vivo. J Invest Dermatol. 1997;108(4):513–8. doi: 10.1111/1523-1747.ep12289736. [DOI] [PubMed] [Google Scholar]
- 42.Godar S, et al. M6P/IGFII-receptor complexes urokinase receptor and plasminogen for activation of transforming growth factor-beta1. Eur J Immunol. 1999;29(3):1004–13. doi: 10.1002/(SICI)1521-4141(199903)29:03<1004::AID-IMMU1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 43.Olson LJ, et al. Structure of uPAR, plasminogen, and sugar-binding sites of the 300 kDa mannose 6-phosphate receptor. EMBO J. 2004;23(10):2019–28. doi: 10.1038/sj.emboj.7600215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waheed A, et al. Mannose 6-phosphate/insulin like growth factor II receptor: the two types of ligands bind simultaneously to one receptor at different sites. Biochem Biophys Res Commun. 1988;152(3):1248–54. doi: 10.1016/s0006-291x(88)80419-4. [DOI] [PubMed] [Google Scholar]
- 45.Nissley P, Kiess W. Reciprocal modulation of binding of lysosomal enzymes and insulin-like growth factor-II (IGF-II) to the mannose 6-phosphate/IGF-II receptor. Adv Exp Med Biol. 1991;293:311–24. doi: 10.1007/978-1-4684-5949-4_28. [DOI] [PubMed] [Google Scholar]
- 46.Kiess W, et al. Insulin-like growth factor-II (IGF-II) inhibits both the cellular uptake of beta-galactosidase and the binding of beta-galactosidase to purified IGF-II/mannose 6-phosphate receptor. J Biol Chem. 1989;264(8):4710–4. [PubMed] [Google Scholar]
- 47.Hille-Rehfeld A. Mannose 6-phosphate receptors in sorting and transport of lysosomal enzymes. Biochim Biophys Acta. 1995;1241(2):177–94. doi: 10.1016/0304-4157(95)00004-b. [DOI] [PubMed] [Google Scholar]
- 48.Macdonald RG. Mannose-6-Phosphate Enhances Cross-Linking Efficiency between Insulin-Like Growth Factor-Ii (Igf-Ii) and Igf-Ii/Mannose-6-Phosphate Receptors in Membranes. Endocrinology. 1991;128(1):413–421. doi: 10.1210/endo-128-1-413. [DOI] [PubMed] [Google Scholar]
- 49.Byrd JC, MacDonald RG. Mechanisms for high affinity mannose 6-phosphate ligand binding to the insulin-like growth factor II/mannose 6-phosphate receptor. J Biol Chem. 2000;275(25):18638–46. doi: 10.1074/jbc.M000010200. [DOI] [PubMed] [Google Scholar]
- 50.Byrd JC, et al. Dimerization of the insulin-like growth factor II/mannose 6-phosphate receptor. J Biol Chem. 2000;275(25):18647–56. doi: 10.1074/jbc.M001273200. [DOI] [PubMed] [Google Scholar]
- 51.Brissenden JE, Ullrich A, Francke U. Human chromosomal mapping of genes for insulin-like growth factors I and II and epidermal growth factor. Nature. 1984;310(5980):781–4. doi: 10.1038/310781a0. [DOI] [PubMed] [Google Scholar]
- 52.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 53.Dahms NM, Wick DA, Brzycki-Wessell MA. The bovine mannose 6-phosphate/insulin-like growth factor II receptor. Localization of the insulin-like growth factor II binding site to domains 5–11. J Biol Chem. 1994;269(5):3802–9. [PubMed] [Google Scholar]
- 54.Garmroudi F, MacDonald RG. Localization of the insulin-like growth factor II (IGF-II) binding/cross-linking site of the IGF-II/mannose 6-phosphate receptor to extracellular repeats 10–11. J Biol Chem. 1994;269(43):26944–52. [PubMed] [Google Scholar]
- 55.Zaccheo OJ, et al. Kinetics of insulin-like growth factor II (IGF-II) interaction with domain 11 of the human IGF-II/mannose 6-phosphate receptor: function of CD and AB loop solvent-exposed residues. J Mol Biol. 2006;359(2):403–21. doi: 10.1016/j.jmb.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 56.Garmroudi F, et al. Truncated forms of the insulin-like growth factor II (IGF-II)/mannose 6-phosphate receptor encompassing the IGF-II binding site: characterization of a point mutation that abolishes IGF-II binding. Mol Endocrinol. 1996;10(6):642–51. doi: 10.1210/mend.10.6.8776724. [DOI] [PubMed] [Google Scholar]
- 57.Brown J, et al. Structure and functional analysis of the IGF-II/IGF2R interaction. EMBO J. 2008;27(1):265–76. doi: 10.1038/sj.emboj.7601938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hassan AB. Keys to the hidden treasures of the mannose 6-phosphate/insulin-like growth factor 2 receptor. Am J Pathol. 2003;162(1):3–6. doi: 10.1016/S0002-9440(10)63791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El-Shewy HM, Luttrell LM. Insulin-like growth factor-2/mannose-6 phosphate receptors. Vitam Horm. 2009;80:667–97. doi: 10.1016/S0083-6729(08)00624-9. [DOI] [PubMed] [Google Scholar]
- 60.Killian JK, et al. M6P/IGF2R imprinting evolution in mammals. Mol Cell. 2000;5(4):707–16. doi: 10.1016/s1097-2765(00)80249-x. [DOI] [PubMed] [Google Scholar]
- 61.Clairmont KB, Czech MP. Chicken and Xenopus mannose 6-phosphate receptors fail to bind insulin-like growth factor II. J Biol Chem. 1989;264(28):16390–2. [PubMed] [Google Scholar]
- 62.Nadimpalli SK, Amancha PK. Evolution of mannose 6-phosphate receptors (MPR300 and 46): lysosomal enzyme sorting proteins. Curr Protein Pept Sci. 2010;11(1):68–90. doi: 10.2174/138920310790274644. [DOI] [PubMed] [Google Scholar]
- 63.Nolan CM, et al. Mannose 6-phosphate receptors in an ancient vertebrate, zebrafish. Dev Genes Evol. 2006;216(3):144–51. doi: 10.1007/s00427-005-0046-3. [DOI] [PubMed] [Google Scholar]
- 64.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19(8):983–97. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 65.Hancock MK, et al. Identification of residues essential for carbohydrate recognition by the insulin-like growth factor II/mannose 6-phosphate receptor. J Biol Chem. 2002;277(13):11255–64. doi: 10.1074/jbc.M109855200. [DOI] [PubMed] [Google Scholar]
- 66.Chavez CA, et al. Domain 5 of the cation-independent mannose 6-phosphate receptor preferentially binds phosphodiesters (mannose 6-phosphate N-acetylglucosamine ester) Biochemistry. 2007;46(44):12604–17. doi: 10.1021/bi7011806. [DOI] [PubMed] [Google Scholar]
- 67.Marron-Terada PG, et al. Recognition of Dictyostelium discoideum lysosomal enzymes is conferred by the amino-terminal carbohydrate binding site of the insulin-like growth factor II/mannose 6-phosphate receptor. Biochemistry. 2000;39(9):2243–53. doi: 10.1021/bi992226o. [DOI] [PubMed] [Google Scholar]
- 68.Bohnsack RN, et al. Cation-independent mannose 6-phosphate receptor: a composite of distinct phosphomannosyl binding sites. J Biol Chem. 2009;284(50):35215–26. doi: 10.1074/jbc.M109.056184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Braulke T, et al. Regulation of the mannose 6-phosphate/IGF II receptor expression at the cell surface by mannose 6-phosphate, insulin like growth factors and epidermal growth factor. EMBO J. 1989;8(3):681–6. doi: 10.1002/j.1460-2075.1989.tb03426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oka Y, et al. Insulin activates the appearance of insulin-like growth factor II receptors on the adipocyte cell surface. Proc Natl Acad Sci U S A. 1984;81(13):4028–32. doi: 10.1073/pnas.81.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Appell KC, I, Simpson A, Cushman SW. Characterization of the stimulatory action of insulin on insulin-like growth factor II binding to rat adipose cells. Differences in the mechanism of insulin action on insulin-like growth factor II receptors and glucose transporters. J Biol Chem. 1988;263(22):10824–9. [PubMed] [Google Scholar]
- 72.Zhang Q, Berggren PO, Tally M. Glucose increases both the plasma membrane number and phosphorylation of insulin-like growth factor II/mannose 6-phosphate receptors. J Biol Chem. 1997;272(38):23703–6. doi: 10.1074/jbc.272.38.23703. [DOI] [PubMed] [Google Scholar]
- 73.York SJ, et al. The rate of internalization of the mannose 6-phosphate/insulin-like growth factor II receptor is enhanced by multivalent ligand binding. J Biol Chem. 1999;274(2):1164–71. doi: 10.1074/jbc.274.2.1164. [DOI] [PubMed] [Google Scholar]
- 74.Stagsted J, et al. Inhibition of internalization of glucose transporters and IGF-II receptors. Mechanism of action of MHC class I-derived peptides which augment the insulin response in rat adipose cells. J Biol Chem. 1993;268(30):22809–13. [PubMed] [Google Scholar]
- 75.Braulke T, Mieskes G. Role of protein phosphatases in insulin-like growth factor II (IGF II)-stimulated mannose 6-phosphate/IGF II receptor redistribution. J Biol Chem. 1992;267(24):17347–53. [PubMed] [Google Scholar]
- 76.Hu KQ, et al. Modulation of the insulin growth factor II/mannose 6-phosphate receptor in microvascular endothelial cells by phorbol ester via protein kinase C. J Biol Chem. 1990;265(23):13864–70. [PubMed] [Google Scholar]
- 77.Corvera S, et al. Insulin action inhibits insulin-like growth factor-II (IGF-II) receptor phosphorylation in H-35 hepatoma cells. IGF-II receptors isolated from insulin-treated cells exhibit enhanced in vitro phosphorylation by casein kinase II. J Biol Chem. 1988;263(7):3116–22. [PubMed] [Google Scholar]
- 78.Meresse S, et al. Phosphorylation of the cytoplasmic domain of the bovine cation-independent mannose 6-phosphate receptor. Serines 2421 and 2492 are the targets of a casein kinase II associated to the Golgi-derived HAI adaptor complex. J Biol Chem. 1990;265(31):18833–42. [PubMed] [Google Scholar]
- 79.Meresse S, Hoflack B. Phosphorylation of the cation-independent mannose 6-phosphate receptor is closely associated with its exit from the trans-Golgi network. J Cell Biol. 1993;120(1):67–75. doi: 10.1083/jcb.120.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neufeld EF. From serendipity to therapy. Annu Rev Biochem. 2011;80:1–15. doi: 10.1146/annurev.biochem.031209.093756. [DOI] [PubMed] [Google Scholar]
- 81.Urayama A, et al. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci U S A. 2004;101(34):12658–63. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Urayama A, et al. Mannose 6-phosphate receptor-mediated transport of sulfamidase across the blood-brain barrier in the newborn mouse. Mol Ther. 2008;16(7):1261–6. doi: 10.1038/mt.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boker C, von Figura K, Hille-Rehfeld A. The carboxy-terminal peptides of 46 kDa and 300 kDa mannose 6-phosphate receptors share partial sequence homology and contain information for sorting in the early endosomal pathway. J Cell Sci. 1997;110( Pt 8):1023–32. doi: 10.1242/jcs.110.8.1023. [DOI] [PubMed] [Google Scholar]
- 84.Dell’Angelica EC, Payne GS. Intracellular cycling of lysosomal enzyme receptors: cytoplasmic tails’ tales. Cell. 2001;106(4):395–8. doi: 10.1016/s0092-8674(01)00470-6. [DOI] [PubMed] [Google Scholar]
- 85.Mullins C, Bonifacino JS. The molecular machinery for lysosome biogenesis. BioEssays. 2001;23(4):333–43. doi: 10.1002/bies.1048. [DOI] [PubMed] [Google Scholar]
- 86.Hirst J, Lindsay MR, Robinson MS. GGAs: roles of the different domains and comparison with AP-1 and clathrin. Mol Biol Cell. 2001;12(11):3573–88. doi: 10.1091/mbc.12.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Puertollano R, et al. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292(5522):1712–6. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- 88.Zhu Y, et al. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science. 2001;292(5522):1716–8. doi: 10.1126/science.1060896. [DOI] [PubMed] [Google Scholar]
- 89.Hirsch DS, et al. Arf regulates interaction of GGA with mannose-6-phosphate receptor. Traffic. 2003;4(1):26–35. doi: 10.1034/j.1600-0854.2003.40105.x. [DOI] [PubMed] [Google Scholar]
- 90.Misra S, et al. Structural basis for acidic-cluster-dileucine sorting-signal recognition by VHS domains. Nature. 2002;415(6874):933–7. doi: 10.1038/415933a. [DOI] [PubMed] [Google Scholar]
- 91.Collins BM, Watson PJ, Owen DJ. The structure of the GGA1-GAT domain reveals the molecular basis for ARF binding and membrane association of GGAs. Dev Cell. 2003;4(3):321–32. doi: 10.1016/s1534-5807(03)00037-6. [DOI] [PubMed] [Google Scholar]
- 92.Seaman MN. The retromer complex - endosomal protein recycling and beyond. J Cell Sci. 2012;125(Pt 20):4693–702. doi: 10.1242/jcs.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Orsel JG, et al. Recognition of the 300-kDa mannose 6-phosphate receptor cytoplasmic domain by 47-kDa tail-interacting protein. Proc Natl Acad Sci U S A. 2000;97(16):9047–51. doi: 10.1073/pnas.160251397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oka Y, Rozek LM, Czech MP. Direct demonstration of rapid insulin-like growth factor II Receptor internalization and recycling in rat adipocytes. Insulin stimulates 125I-insulin-like growth factor II degradation by modulating the IGF-II receptor recycling process. J Biol Chem. 1985;260(16):9435–42. [PubMed] [Google Scholar]
- 95.Kiess W, et al. An antibody that blocks insulin-like growth factor (IGF) binding to the type II IGF receptor is neither an agonist nor an inhibitor of IGF-stimulated biologic responses in L6 myoblasts. J Biol Chem. 1987;262(26):12745–51. [PubMed] [Google Scholar]
- 96.Nolan CM, et al. Binding of insulin-like growth factor II (IGF-II) by human cation-independent mannose 6-phosphate receptor/IGF-II receptor expressed in receptor-deficient mouse L cells. Cell Regul. 1990;1(2):197–213. doi: 10.1091/mbc.1.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lau MM, et al. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 1994;8(24):2953–63. doi: 10.1101/gad.8.24.2953. [DOI] [PubMed] [Google Scholar]
- 98.Ludwig T, et al. Mouse mutants lacking the type 2 IGF receptor (IGF2R) are rescued from perinatal lethality in Igf2 and Igf1r null backgrounds. Dev Biol. 1996;177(2):517–35. doi: 10.1006/dbio.1996.0182. [DOI] [PubMed] [Google Scholar]
- 99.Wang ZQ, et al. Regulation of embryonic growth and lysosomal targeting by the imprinted Igf2/Mpr gene. Nature. 1994;372(6505):464–7. doi: 10.1038/372464a0. [DOI] [PubMed] [Google Scholar]
- 100.Saris JJ, et al. High-affinity prorenin binding to cardiac man-6-P/IGF-II receptors precedes proteolytic activation to renin. Am J Physiol Heart Circ Physiol. 2001;280(4):H1706–15. doi: 10.1152/ajpheart.2001.280.4.H1706. [DOI] [PubMed] [Google Scholar]
- 101.Todderud G, Carpenter G. Presence of mannose phosphate on the epidermal growth factor receptor in A-431 cells. J Biol Chem. 1988;263(34):17893–6. [PubMed] [Google Scholar]
- 102.Ghahary A, et al. Cellular response to latent TGF-beta1 is facilitated by insulin-like growth factor-II/mannose-6-phosphate receptors on MS-9 cells. Exp Cell Res. 1999;251(1):111–20. doi: 10.1006/excr.1999.4561. [DOI] [PubMed] [Google Scholar]
- 103.Villevalois-Cam L, et al. The hepatocyte is a direct target for transforming-growth factor beta activation via the insulin-like growth factor II/mannose 6-phosphate receptor. J Hepatol. 2003;38(2):156–63. doi: 10.1016/s0168-8278(02)00378-1. [DOI] [PubMed] [Google Scholar]
- 104.Nishimoto I, et al. Insulin-like growth factor II stimulates calcium influx in competent BALB/c 3T3 cells primed with epidermal growth factor. Characteristics of calcium influx and involvement of GTP-binding protein. J Biol Chem. 1987;262(25):12120–6. [PubMed] [Google Scholar]
- 105.Martinez DA, et al. Identification of functional insulin-like growth factor-II/mannose-6-phosphate receptors in isolated bone cells. J Cell Biochem. 1995;59(2):246–57. doi: 10.1002/jcb.240590213. [DOI] [PubMed] [Google Scholar]
- 106.Hammerman MR, Gavin JR., 3rd Binding of insulin-like growth Factor ii and multiplication-stimulating activity-stimulated phosphorylation in basolateral membranes from dog kidney. J Biol Chem. 1984;259(21):13511–7. [PubMed] [Google Scholar]
- 107.Mellas J, Gavin JR, 3rd, Hammerman MR. Multiplication-stimulating activity-induced alkalinization of canine renal proximal tubular cells. J Biol Chem. 1986;261(31):14437–42. [PubMed] [Google Scholar]
- 108.Rogers SA, Hammerman MR. Insulin-like growth factor II stimulates production of inositol trisphosphate in proximal tubular basolateral membranes from canine kidney. Proc Natl Acad Sci U S A. 1988;85(11):4037–41. doi: 10.1073/pnas.85.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shimizu M, et al. Insulin and insulinlike growth factor receptors and responses in cultured human muscle cells. Am J Physiol. 1986;251(5 Pt 1):E611–5. doi: 10.1152/ajpendo.1986.251.5.E611. [DOI] [PubMed] [Google Scholar]
- 110.Hari J, et al. The receptor for insulin-like growth factor II mediates an insulin-like response. EMBO J. 1987;6(11):3367–71. doi: 10.1002/j.1460-2075.1987.tb02658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takigawa M, et al. Insulin-like growth factors I and II are autocrine factors in stimulating proteoglycan synthesis, a marker of differentiated chondrocytes, acting through their respective receptors on a clonal human chondrosarcoma-derived chondrocyte cell line, HCS-2/8. Endocrinology. 1997;138(10):4390–400. doi: 10.1210/endo.138.10.5265. [DOI] [PubMed] [Google Scholar]
- 112.Poiraudeau S, et al. Different mechanisms are involved in intracellular calcium increase by insulin-like growth factors 1 and 2 in articular chondrocytes: voltage-gated calcium channels, and/or phospholipase C coupled to a pertussis-sensitive G-protein. J Cell Biochem. 1997;64(3):414–22. [PubMed] [Google Scholar]
- 113.El-Badry OM, et al. Insulin-like growth factor II acts as an autocrine growth and motility factor in human rhabdomyosarcoma tumors. Cell Growth Differ. 1990;1(7):325–31. [PubMed] [Google Scholar]
- 114.Minniti CP, et al. The insulin-like growth factor II (IGF-II)/mannose 6-phosphate receptor mediates IGF-II-induced motility in human rhabdomyosarcoma cells. J Biol Chem. 1992;267(13):9000–4. [PubMed] [Google Scholar]
- 115.Nestler JE. Insulin-like growth factor II is a potent inhibitor of the aromatase activity of human placental cytotrophoblasts. Endocrinology. 1990;127(5):2064–70. doi: 10.1210/endo-127-5-2064. [DOI] [PubMed] [Google Scholar]
- 116.McKinnon T, et al. Stimulation of human extravillous trophoblast migration by IGF-II is mediated by IGF type 2 receptor involving inhibitory G protein(s) and phosphorylation of MAPK. J Clin Endocrinol Metab. 2001;86(8):3665–74. doi: 10.1210/jcem.86.8.7711. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Q, et al. Insulin-like growth factor II signaling through the insulin-like growth factor II/mannose-6-phosphate receptor promotes exocytosis in insulin-secreting cells. Proc Natl Acad Sci U S A. 1997;94(12):6232–7. doi: 10.1073/pnas.94.12.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hawkes C, et al. Single transmembrane domain insulin-like growth factor-II/mannose-6-phosphate receptor regulates central cholinergic function by activating a G-protein-sensitive, protein kinase C-dependent pathway. J Neurosci. 2006;26(2):585–96. doi: 10.1523/JNEUROSCI.2730-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amritraj A, et al. Leu27 insulin-like growth factor-II, an insulin-like growth factor-II analog, attenuates depolarization-evoked GABA release from adult rat hippocampal and cortical slices. Neuroscience. 2010;170(3):722–30. doi: 10.1016/j.neuroscience.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 120.Ikezu T, et al. In vivo coupling of insulin-like growth factor II/mannose 6-phosphate receptor to heteromeric G proteins. Distinct roles of cytoplasmic domains and signal sequestration by the receptor. J Biol Chem. 1995;270(49):29224–8. doi: 10.1074/jbc.270.49.29224. [DOI] [PubMed] [Google Scholar]
- 121.Higashijima T, Burnier J, Ross EM. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J Biol Chem. 1990;265(24):14176–86. [PubMed] [Google Scholar]
- 122.Okamoto T, Nishimoto I. Analysis of stimulation-G protein subunit coupling by using active insulin-like growth factor II receptor peptide. Proc Natl Acad Sci U S A. 1991;88(18):8020–3. doi: 10.1073/pnas.88.18.8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matsunaga H, et al. Activation of a calcium-permeable cation channel by insulin-like growth factor II in BALB/c 3T3 cells. American Journal of Physiology. 1988;255(4 Pt 1):C442–6. doi: 10.1152/ajpcell.1988.255.4.C442. [DOI] [PubMed] [Google Scholar]
- 124.Pfeifer A, et al. Cyclic GMP-dependent protein kinase blocks pertussis toxin-sensitive hormone receptor signaling pathways in Chinese hamster ovary cells. J Biol Chem. 1995;270(16):9052–9. doi: 10.1074/jbc.270.16.9052. [DOI] [PubMed] [Google Scholar]
- 125.Sakano KI, et al. The Design, Expression, and Characterization of Human Insulin-Like Growth Factor-Ii (Igf-Ii) Mutants Specific for Either the Igf-Ii Cation-Independent Mannose 6-Phosphate Receptor or Igf-I Receptor. Journal of Biological Chemistry. 1991;266(31):20626–20635. [PubMed] [Google Scholar]
- 126.Korner C, et al. Mannose 6-phosphate/insulin-like growth factor II receptor fails to interact with G-proteins. Analysis of mutant cytoplasmic receptor domains. J Biol Chem. 1995;270(1):287–95. doi: 10.1074/jbc.270.1.287. [DOI] [PubMed] [Google Scholar]
- 127.Hawkes C, et al. Heterotrimeric G proteins and the single-transmembrane domain IGF-II/M6P receptor: functional interaction and relevance to cell signaling. Mol Neurobiol. 2007;35(3):329–45. doi: 10.1007/s12035-007-0021-2. [DOI] [PubMed] [Google Scholar]
- 128.Amritraj A, et al. Single-Transmembrane Domain IGF-II/M6P Receptor: Potential Interaction with G Protein and Its Association with Cholesterol-Rich Membrane Domains. Endocrinology. 2012;153(10):4784–98. doi: 10.1210/en.2012-1139. [DOI] [PubMed] [Google Scholar]
- 129.Chu CH, et al. Activation of insulin-like growth factor II receptor induces mitochondrial-dependent apoptosis through G(alpha)q and downstream calcineurin signaling in myocardial cells. Endocrinology. 2009;150(6):2723–31. doi: 10.1210/en.2008-0975. [DOI] [PubMed] [Google Scholar]
- 130.El-Shewy HM, et al. Insulin-like growth factors mediate heterotrimeric G protein-dependent ERK1/2 activation by transactivating sphingosine 1-phosphate receptors. J Biol Chem. 2006;281(42):31399–407. doi: 10.1074/jbc.M605339200. [DOI] [PubMed] [Google Scholar]
- 131.El-Shewy HM, et al. The insulin-like growth factor type 1 and insulin-like growth factor type 2/mannose-6-phosphate receptors independently regulate ERK1/2 activity in HEK293 cells. J Biol Chem. 2007;282(36):26150–7. doi: 10.1074/jbc.M703276200. [DOI] [PubMed] [Google Scholar]
- 132.O’Gorman DB, et al. Insulin-like growth factor-II/mannose 6-phosphate receptor overexpression reduces growth of choriocarcinoma cells in vitro and in vivo. Endocrinology. 2002;143(11):4287–94. doi: 10.1210/en.2002-220548. [DOI] [PubMed] [Google Scholar]
- 133.DaCosta SA, Schumaker LM, Ellis MJ. Mannose 6-phosphate/insulin-like growth factor 2 receptor, a bona fide tumor suppressor gene or just a promising candidate? J Mammary Gland Biol Neoplasia. 2000;5(1):85–94. doi: 10.1023/a:1009571417429. [DOI] [PubMed] [Google Scholar]
- 134.Oates AJ, et al. The mannose 6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2R), a putative breast tumor suppressor gene. Breast Cancer Res Treat. 1998;47(3):269–81. doi: 10.1023/a:1005959218524. [DOI] [PubMed] [Google Scholar]
- 135.Osipo C, Dorman S, Frankfater A. Loss of insulin-like growth factor II receptor expression promotes growth in cancer by increasing intracellular signaling from both IGF-I and insulin receptors. Exp Cell Res. 2001;264(2):388–96. doi: 10.1006/excr.2000.5121. [DOI] [PubMed] [Google Scholar]
- 136.Toretsky JA, Helman LJ. Involvement of IGF-II in human cancer. J Endocrinol. 1996;149(3):367–72. doi: 10.1677/joe.0.1490367. [DOI] [PubMed] [Google Scholar]
- 137.Yang L, Tredget EE, Ghahary A. Activation of latent transforming growth factor-beta1 is induced by mannose 6-phosphate/insulin-like growth factor-II receptor. Wound Repair Regen. 2000;8(6):538–46. doi: 10.1046/j.1524-475x.2000.00538.x. [DOI] [PubMed] [Google Scholar]
- 138.Motyka B, et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell. 2000;103(3):491–500. doi: 10.1016/s0092-8674(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 139.Veugelers K, et al. Granule-mediated killing by granzyme B and perforin requires a mannose 6-phosphate receptor and is augmented by cell surface heparan sulfate. Mol Biol Cell. 2006;17(2):623–33. doi: 10.1091/mbc.E05-07-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dressel R, et al. Granzyme-mediated cytotoxicity does not involve the mannose 6-phosphate receptors on target cells. J Biol Chem. 2004;279(19):20200–10. doi: 10.1074/jbc.M313108200. [DOI] [PubMed] [Google Scholar]
- 141.Hu CK, et al. Loss of heterozygosity of M6P/IGF2R gene is an early event in the development of prostate cancer. Prostate Cancer Prostatic Dis. 2006;9(1):62–7. doi: 10.1038/sj.pcan.4500842. [DOI] [PubMed] [Google Scholar]
- 142.Jang HS, et al. Clinical significance of loss of heterozygosity for M6P/IGF2R in patients with primary hepatocellular carcinoma. World J Gastroenterol. 2008;14(9):1394–8. doi: 10.3748/wjg.14.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Souza RF, et al. Microsatellite instability in the insulin-like growth factor II receptor gene in gastrointestinal tumours. Nat Genet. 1996;14(3):255–7. doi: 10.1038/ng1196-255. [DOI] [PubMed] [Google Scholar]
- 144.Savage SA, et al. Analysis of genes critical for growth regulation identifies Insulin-like Growth Factor 2 Receptor variations with possible functional significance as risk factors for osteosarcoma. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1667–74. doi: 10.1158/1055-9965.EPI-07-0214. [DOI] [PubMed] [Google Scholar]
- 145.Kar S, Chabot JG, Quirion R. Quantitative autoradiographic localization of [125I]insulin-like growth factor I, [125I]insulin-like growth factor II, and [125I]insulin receptor binding sites in developing and adult rat brain. J Comp Neurol. 1993;333(3):375–97. doi: 10.1002/cne.903330306. [DOI] [PubMed] [Google Scholar]
- 146.Marinelli PW, Gianoulakis C, Kar S. Effects of voluntary ethanol drinking on [125I]insulin-like growth factor-I, [125I]insulin-like growth factor-II and [125I]insulin receptor binding in the mouse hippocampus and cerebellum. Neuroscience. 2000;98(4):687–95. doi: 10.1016/s0306-4522(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 147.Wilczak N, et al. Insulin-like growth factor II receptors in human brain and their absence in astrogliotic plaques in multiple sclerosis. Brain Res. 2000;863(1–2):282–8. doi: 10.1016/s0006-8993(00)02153-3. [DOI] [PubMed] [Google Scholar]
- 148.Hawkes C, Kar S. Insulin-like growth factor-II/Mannose-6-phosphate receptor in the spinal cord and dorsal root ganglia of the adult rat. Eur J Neurosci. 2002;15(1):33–9. doi: 10.1046/j.0953-816x.2001.01864.x. [DOI] [PubMed] [Google Scholar]
- 149.Couce ME, Weatherington AJ, McGinty JF. Expression of insulin-like growth factor-II (IGF-II) and IGF-II/mannose-6-phosphate receptor in the rat hippocampus: an in situ hybridization and immunocytochemical study. Endocrinology. 1992;131(4):1636–42. doi: 10.1210/endo.131.4.1396308. [DOI] [PubMed] [Google Scholar]
- 150.Hawkes C, Kar S. Insulin-like growth factor-II/mannose-6-phosphate receptor: widespread distribution in neurons of the central nervous system including those expressing cholinergic phenotype. J Comp Neurol. 2003;458(2):113–27. doi: 10.1002/cne.10578. [DOI] [PubMed] [Google Scholar]
- 151.Fushimi S, Shirabe T. Expression of insulin-like growth factors in remyelination following ethidium bromide-induced demyelination in the mouse spinal cord. Neuropathology. 2004;24(3):208–18. doi: 10.1111/j.1440-1789.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 152.Amritraj A, et al. Altered levels and distribution of IGF-II/M6P receptor and lysosomal enzymes in mutant APP and APP + PS1 transgenic mouse brains. Neurobiol Aging. 2009;30(1):54–70. doi: 10.1016/j.neurobiolaging.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 153.Konishi Y, Fushimi S, Shirabe T. Immunohistochemical distribution of cation-dependent mannose 6-phosphate receptors in the mouse central nervous system: comparison with that of cation-independent mannose 6-phophate receptors. Neurosci Lett. 2005;378(1):7–12. doi: 10.1016/j.neulet.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 154.Nissley P, Kiess W, Sklar M. Developmental expression of the IGF-II/mannose 6-phosphate receptor. Mol Reprod Dev. 1993;35(4):408–13. doi: 10.1002/mrd.1080350415. [DOI] [PubMed] [Google Scholar]
- 155.Sara VR, Carlsson-Skwirut C. The role of the insulin-like growth factors in the regulation of brain development. Prog Brain Res. 1988;73:87–99. doi: 10.1016/S0079-6123(08)60499-9. [DOI] [PubMed] [Google Scholar]
- 156.Valentino KL, Ocrant I, Rosenfeld RG. Developmental expression of insulin-like growth factor-II receptor immunoreactivity in the rat central nervous system. Endocrinology. 1990;126(2):914–20. doi: 10.1210/endo-126-2-914. [DOI] [PubMed] [Google Scholar]
- 157.Murphy M, et al. Cytokines which signal through the LIF receptor and their actions in the nervous system. Prog Neurobiol. 1997;52(5):355–78. doi: 10.1016/s0301-0082(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 158.Thompson Haskell G, et al. Retinoic acid signaling at sites of plasticity in the mature central nervous system. J Comp Neurol. 2002;452(3):228–41. doi: 10.1002/cne.10369. [DOI] [PubMed] [Google Scholar]
- 159.Knusel B, et al. Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. J Neurosci. 1990;10(2):558–70. doi: 10.1523/JNEUROSCI.10-02-00558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Konishi Y, et al. Insulin-like growth factor II promotes in vitro cholinergic development of mouse septal neurons: comparison with the effects of insulin-like growth factor I. Brain Res. 1994;649(1–2):53–61. doi: 10.1016/0006-8993(94)91048-0. [DOI] [PubMed] [Google Scholar]
- 161.Liu JP, Lauder JM. S-100 beta and insulin-like growth factor-II differentially regulate growth of developing serotonin and dopamine neurons in vitro. J Neurosci Res. 1992;33(2):248–56. doi: 10.1002/jnr.490330208. [DOI] [PubMed] [Google Scholar]
- 162.Neff NT, et al. Insulin-like growth factors: putative muscle-derived trophic agents that promote motoneuron survival. J Neurobiol. 1993;24(12):1578–88. doi: 10.1002/neu.480241203. [DOI] [PubMed] [Google Scholar]
- 163.Walter HJ, et al. Distinct sites of insulin-like growth factor (IGF)-II expression and localization in lesioned rat brain: possible roles of IGF binding proteins (IGFBPs) in the mediation of IGF-II activity. Endocrinology. 1999;140(1):520–32. doi: 10.1210/endo.140.1.6463. [DOI] [PubMed] [Google Scholar]
- 164.Dore S, et al. Impact of neonatal kainate treatment on hippocampal insulin-like growth factor receptors. Neuroscience. 1999;91(3):1035–43. doi: 10.1016/s0306-4522(98)00646-0. [DOI] [PubMed] [Google Scholar]
- 165.Beilharz EJ, et al. Insulin-like growth factor II is induced during wound repair following hypoxic-ischemic injury in the developing rat brain. Brain Res Mol Brain Res. 1995;29(1):81–91. doi: 10.1016/0169-328x(94)00232-4. [DOI] [PubMed] [Google Scholar]
- 166.Stephenson DT, Rash K, Clemens JA. Increase in insulin-like growth factor II receptor within ischemic neurons following focal cerebral infarction. J Cereb Blood Flow Metab. 1995;15(6):1022–31. doi: 10.1038/jcbfm.1995.128. [DOI] [PubMed] [Google Scholar]
- 167.Alberini CM, Chen DY. Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci. 2012;35(5):274–83. doi: 10.1016/j.tins.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O’Kusky J, Ye P. Neurodevelopmental effects of insulin-like growth factor signaling. Front Neuroendocrinol. 2012;33(3):230–51. doi: 10.1016/j.yfrne.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jellinger KA. Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med. 2010;14(3):457–87. doi: 10.1111/j.1582-4934.2010.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Double KL, et al. Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Prog Neurobiol. 2010;92(3):316–29. doi: 10.1016/j.pneurobio.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 171.Wang X, et al. Dysregulation of protein trafficking in neurodegeneration. Mol Neurodegener. 2014;9:31. doi: 10.1186/1750-1326-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lim J, Yue Z. Neuronal aggregates: formation, clearance, and spreading. Dev Cell. 2015;32(4):491–501. doi: 10.1016/j.devcel.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bertram L, Tanzi RE. The genetics of Alzheimer’s disease. Prog Mol Biol Transl Sci. 2012;107:79–100. doi: 10.1016/B978-0-12-385883-2.00008-4. [DOI] [PubMed] [Google Scholar]
- 174.Karch CM, Cruchaga C, Goate AM. Alzheimer’s Disease Genetics: From the Bench to the Clinic. Neuron. 2014;83(1):11–26. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Poirier J, et al. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993;342(8873):697–9. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 176.Selkoe DJ. Biochemistry and molecular biology of amyloid beta-protein and the mechanism of Alzheimer’s disease. Handb Clin Neurol. 2008;89:245–60. doi: 10.1016/S0072-9752(07)01223-7. [DOI] [PubMed] [Google Scholar]
- 177.Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110(4):1129–34. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 178.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68(1):1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Selkoe DJ. Alzheimer’s disease. Cold Spring Harb Perspect Biol. 2011;3(7) doi: 10.1101/cshperspect.a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Haass C, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359(6393):322–5. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 181.Seubert P, et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992;359(6393):325–7. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 182.Saido TC, Iwata N. Metabolism of amyloid beta peptide and pathogenesis of Alzheimer’s disease. Towards presymptomatic diagnosis, prevention and therapy. Neurosci Res. 2006;54(4):235–53. doi: 10.1016/j.neures.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 183.Mawuenyega KG, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Auld DS, et al. Alzheimer’s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68(3):209–45. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 185.Francis PT, et al. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66(2):137–47. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kar S, et al. Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J Psychiatry Neurosci. 2004;29(6):427–41. [PMC free article] [PubMed] [Google Scholar]
- 187.Ladner CJ, Lee JM. Pharmacological drug treatment of Alzheimer disease: the cholinergic hypothesis revisited. J Neuropathol Exp Neurol. 1998;57(8):719–31. doi: 10.1097/00005072-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 188.Chen DY, et al. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469(7331):491–7. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stern SA, et al. Enhancement of memories by systemic administration of insulin-like growth factor II. Neuropsychopharmacology. 2014;39(9):2179–90. doi: 10.1038/npp.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283(44):29615–9. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Haass C, et al. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med. 2012;2(5):a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kar S, et al. Cellular distribution of insulin-like growth factor-II/mannose-6-phosphate receptor in normal human brain and its alteration in Alzheimer’s disease pathology. Neurobiol Aging. 2006;27(2):199–210. doi: 10.1016/j.neurobiolaging.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 193.Cataldo AM, et al. Presenilin mutations in familial Alzheimer disease and transgenic mouse models accelerate neuronal lysosomal pathology. J Neuropathol Exp Neurol. 2004;63(8):821–30. doi: 10.1093/jnen/63.8.821. [DOI] [PubMed] [Google Scholar]
- 194.Rivera EJ, et al. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8(3):247–68. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 195.Pascual-Lucas M, et al. Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice. EMBO Mol Med. 2014;6(10):1246–62. doi: 10.15252/emmm.201404228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mellott TJ, et al. IGF2 ameliorates amyloidosis, increases cholinergic marker expression and raises BMP9 and neurotrophin levels in the hippocampus of the APPswePS1dE9 Alzheimer’s disease model mice. PLoS One. 2014;9(4):e94287. doi: 10.1371/journal.pone.0094287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nixon RA, Mathews PM, Cataldo AM. The neuronal endosomal-lysosomal system in Alzheimer’s disease. J Alzheimers Dis. 2001;3(1):97–107. doi: 10.3233/jad-2001-3114. [DOI] [PubMed] [Google Scholar]
- 198.Gautreau A, Oguievetskaia K, Ungermann C. Function and regulation of the endosomal fusion and fission machineries. Cold Spring Harb Perspect Biol. 2014;6(3) doi: 10.1101/cshperspect.a016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Appelqvist H, et al. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5(4):214–26. doi: 10.1093/jmcb/mjt022. [DOI] [PubMed] [Google Scholar]
- 201.Nixon RA. Endosome function and dysfunction in Alzheimer’s disease and other neurodegenerative diseases. Neurobiol Aging. 2005;26(3):373–82. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 202.Nixon RA, Yang DS. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb Perspect Biol. 2012;4(10) doi: 10.1101/cshperspect.a008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nixon RA, Cataldo AM. Lysosomal system pathways: genes to neurodegeneration in Alzheimer’s disease. J Alzheimers Dis. 2006;9(3 Suppl):277–89. doi: 10.3233/jad-2006-9s331. [DOI] [PubMed] [Google Scholar]
- 204.Cataldo AM, et al. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000;157(1):277–86. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chevallier N, et al. Cathepsin D displays in vitro beta-secretase-like specificity. Brain Res. 1997;750(1–2):11–9. doi: 10.1016/s0006-8993(96)01330-3. [DOI] [PubMed] [Google Scholar]
- 206.Schechter I, Ziv E. Cathepsins S, B and L with aminopeptidases display beta-secretase activity associated with the pathogenesis of Alzheimer’s disease. Biol Chem. 2011;392(6):555–69. doi: 10.1515/BC.2011.054. [DOI] [PubMed] [Google Scholar]
- 207.Hook VY, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J Biol Chem. 2008;283(12):7745–53. doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- 208.Kindy MS, et al. Deletion of the cathepsin B gene improves memory deficits in a transgenic ALZHeimer’s disease mouse model expressing AbetaPP containing the wild-type beta-secretase site sequence. J Alzheimers Dis. 2012;29(4):827–40. doi: 10.3233/JAD-2012-111604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27(50):6434–51. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- 210.Johansson AC, et al. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis. 2010;15(5):527–40. doi: 10.1007/s10495-009-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang AJ, et al. Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Abeta1–42 pathogenesis. J Neurosci Res. 1998;52(6):691–8. doi: 10.1002/(SICI)1097-4547(19980615)52:6<691::AID-JNR8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 212.Ditaranto K, Tekirian TL, Yang AJ. Lysosomal membrane damage in soluble Abeta-mediated cell death in Alzheimer’s disease. Neurobiol Dis. 2001;8(1):19–31. doi: 10.1006/nbdi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 213.Yamashima T. Reconsider Alzheimer’s disease by the ‘calpain-cathepsin hypothesis’--a perspective review. Prog Neurobiol. 2013;105:1–23. doi: 10.1016/j.pneurobio.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 214.Mathews PM, et al. Alzheimer’s disease-related overexpression of the cation-dependent mannose 6-phosphate receptor increases Abeta secretion: role for altered lysosomal hydrolase distribution in beta-amyloidogenesis. J Biol Chem. 2002;277(7):5299–307. doi: 10.1074/jbc.M108161200. [DOI] [PubMed] [Google Scholar]
- 215.Cataldo AM, et al. Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathologic evidence for a mechanism of increased beta-amyloidogenesis. J Neurosci. 1997;17(16):6142–51. doi: 10.1523/JNEUROSCI.17-16-06142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wolfe MS. Gamma-secretase: structure, function, and modulation for Alzheimer’s disease. Curr Top Med Chem. 2008;8(1):2–8. doi: 10.2174/156802608783334024. [DOI] [PubMed] [Google Scholar]
- 217.Nathalie P, Jean-Noel O. Processing of amyloid precursor protein and amyloid peptide neurotoxicity. Curr Alzheimer Res. 2008;5(2):92–9. doi: 10.2174/156720508783954721. [DOI] [PubMed] [Google Scholar]
- 218.Vassar R, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 219.Wang Y, Thinakaran G, Kar S. Overexpression of the IGF-II/M6P receptor in mouse fibroblast cell lines differentially alters expression profiles of genes involved in Alzheimer’s disease-related pathology. PLoS One. 2014;9(5):e98057. doi: 10.1371/journal.pone.0098057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang Y, et al. Overexpression of the Insulin-Like Growth Factor II Receptor Increases beta-Amyloid Production and Affects Cell Viability. Mol Cell Biol. 2015;35(14):2368–84. doi: 10.1128/MCB.01338-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ehehalt R, et al. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160(1):113–23. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kojro E, et al. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98(10):5815–20. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vetrivel KS, et al. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279(43):44945–54. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vetrivel KS, et al. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J Biol Chem. 2005;280(27):25892–900. doi: 10.1074/jbc.M503570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Haque A, Banik NL, Ray SK. New insights into the roles of endolysosomal cathepsins in the pathogenesis of Alzheimer’s disease: cathepsin inhibitors as potential therapeutics. CNS Neurol Disord Drug Targets. 2008;7(3):270–7. doi: 10.2174/187152708784936653. [DOI] [PubMed] [Google Scholar]
- 226.Buggia-Prevot V, Thinakaran G. Sorting the role of SORLA in Alzheimer’s disease. Sci Transl Med. 2014;6(223):223fs8. doi: 10.1126/scitranslmed.3008562. [DOI] [PubMed] [Google Scholar]
- 227.He X, et al. GGA proteins mediate the recycling pathway of memapsin 2 (BACE) J Biol Chem. 2005;280(12):11696–703. doi: 10.1074/jbc.M411296200. [DOI] [PubMed] [Google Scholar]
- 228.Cuartero Y, et al. Retromer regulates postendocytic sorting of beta-secretase in polarized Madin-Darby canine kidney cells. Traffic. 2012;13(10):1393–410. doi: 10.1111/j.1600-0854.2012.01392.x. [DOI] [PubMed] [Google Scholar]
- 229.Okada H, et al. Proteomic identification of sorting nexin 6 as a negative regulator of BACE1-mediated APP processing. FASEB J. 2010;24(8):2783–94. doi: 10.1096/fj.09-146357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.de la Monte SM, Tong M. Mechanisms of nitrosamine-mediated neurodegeneration: potential relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2009;17(4):817–25. doi: 10.3233/JAD-2009-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hawkes C, et al. Up-regulation of cation-independent mannose 6-phosphate receptor and endosomal-lysosomal markers in surviving neurons after 192-IgG-saporin administrations into the adult rat brain. Am J Pathol. 2006;169(4):1140–54. doi: 10.2353/ajpath.2006.051208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kar S, et al. Insulin-like growth factors-I and -II differentially regulate endogenous acetylcholine release from the rat hippocampal formation. Proc Natl Acad Sci U S A. 1997;94(25):14054–9. doi: 10.1073/pnas.94.25.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Napoli I, Blusztajn JK, Mellott TJ. Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippocampus and frontal cortex. Brain Res. 2008;1237:124–35. doi: 10.1016/j.brainres.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 234.Agis-Balboa RC, et al. A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J. 2011;30(19):4071–83. doi: 10.1038/emboj.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Schmeisser MJ, et al. IkappaB kinase/nuclear factor kappaB-dependent insulin-like growth factor 2 (Igf2) expression regulates synapse formation and spine maturation via Igf2 receptor signaling. J Neurosci. 2012;32(16):5688–703. doi: 10.1523/JNEUROSCI.0111-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Amtul Z, et al. Oleic acid ameliorates amyloidosis in cellular and mouse models of Alzheimer’s disease. Brain Pathol. 2011;21(3):321–9. doi: 10.1111/j.1750-3639.2010.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Alfimova MV, et al. Association of the insulin-like growth factor II (IGF2) gene with human cognitive functions. Genetika. 2012;48(8):993–8. [PubMed] [Google Scholar]
- 238.Pu SF, et al. Insulin-like growth factor-II increases and IGF is required for postnatal rat spinal motoneuron survival following sciatic nerve axotomy. J Neurosci Res. 1999;55(1):9–16. doi: 10.1002/(SICI)1097-4547(19990101)55:1<9::AID-JNR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 239.Silva A, et al. Growth factor effects on survival and development of calbindin immunopositive cultured septal neurons. Brain Res Bull. 2000;51(1):35–42. doi: 10.1016/s0361-9230(99)00188-4. [DOI] [PubMed] [Google Scholar]
- 240.Jarvis K, et al. Beta-amyloid toxicity and reversal in embryonic rat septal neurons. Neurosci Lett. 2007;423(3):184–8. doi: 10.1016/j.neulet.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Martin-Montanez E, et al. Involvement of IGF-II receptors in the antioxidant and neuroprotective effects of IGF-II on adult cortical neuronal cultures. Biochim Biophys Acta. 2014;1842(7):1041–51. doi: 10.1016/j.bbadis.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 242.Li Y, Xu C, Schubert D. The up-regulation of endosomal-lysosomal components in amyloid beta-resistant cells. J Neurochem. 1999;73(4):1477–82. doi: 10.1046/j.1471-4159.1999.0731477.x. [DOI] [PubMed] [Google Scholar]
- 243.Prehn JH, et al. Protective effect of transforming growth factor-beta 1 on beta-amyloid neurotoxicity in rat hippocampal neurons. Mol Pharmacol. 1996;49(2):319–28. [PubMed] [Google Scholar]
- 244.Ren RF, Flanders KC. Transforming growth factors-beta protect primary rat hippocampal neuronal cultures from degeneration induced by beta-amyloid peptide. Brain Res. 1996;732(1–2):16–24. doi: 10.1016/0006-8993(96)00458-1. [DOI] [PubMed] [Google Scholar]
- 245.Chen JH, et al. Protection of TGF-beta1 against neuroinflammation and neurodegeneration in Abeta1-42-induced Alzheimer’s disease model rats. PLoS One. 2015;10(2):e0116549. doi: 10.1371/journal.pone.0116549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bellettato CM, Scarpa M. Pathophysiology of neuropathic lysosomal storage disorders. J Inherit Metab Dis. 2010;33(4):347–62. doi: 10.1007/s10545-010-9075-9. [DOI] [PubMed] [Google Scholar]
- 247.Prada CE, Grabowski GA. Neuronopathic lysosomal storage diseases: clinical and pathologic findings. Dev Disabil Res Rev. 2013;17(3):226–46. doi: 10.1002/ddrr.1116. [DOI] [PubMed] [Google Scholar]
- 248.Meikle PJ, et al. Prevalence of lysosomal storage disorders. JAMA. 1999;281(3):249–54. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 249.Dittmer F, et al. I-cell disease-like phenotype in mice deficient in mannose 6-phosphate receptors. Transgenic Res. 1998;7(6):473–83. doi: 10.1023/a:1008823315416. [DOI] [PubMed] [Google Scholar]
- 250.Lachmann RH. Enzyme replacement therapy for lysosomal storage diseases. Curr Opin Pediatr. 2011;23(6):588–93. doi: 10.1097/MOP.0b013e32834c20d9. [DOI] [PubMed] [Google Scholar]
- 251.Zhu Y, et al. Conjugation of mannose 6-phosphate-containing oligosaccharides to acid alpha-glucosidase improves the clearance of glycogen in pompe mice. J Biol Chem. 2004;279(48):50336–41. doi: 10.1074/jbc.M409676200. [DOI] [PubMed] [Google Scholar]
- 252.Gary-Bobo M, et al. Mannose 6-phosphate receptor targeting and its applications in human diseases. Curr Med Chem. 2007;14(28):2945–53. doi: 10.2174/092986707782794005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Grubb JH, Vogler C, Sly WS. New strategies for enzyme replacement therapy for lysosomal storage diseases. Rejuvenation Res. 2010;13(2–3):229–36. doi: 10.1089/rej.2009.0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sly WS, Vogler C. The final frontier -- crossing the blood-brain barrier. EMBO Mol Med. 2013;5(5):655–7. doi: 10.1002/emmm.201302668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sorrentino NC, et al. A highly secreted sulphamidase engineered to cross the blood-brain barrier corrects brain lesions of mice with mucopolysaccharidoses type IIIA. EMBO Mol Med. 2013;5(5):675–90. doi: 10.1002/emmm.201202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tang Y, Li H, Liu JP. Niemann-Pick Disease Type C: from molecule to clinic. Clin Exp Pharmacol Physiol. 2010;37(1):132–40. doi: 10.1111/j.1440-1681.2009.05235.x. [DOI] [PubMed] [Google Scholar]
- 257.Schuchman EH, Wasserstein MP. Types A and B Niemann-Pick disease. Best Pract Res Clin Endocrinol Metab. 2015;29(2):237–47. doi: 10.1016/j.beem.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 258.Rosenbaum AI, Maxfield FR. Niemann-Pick type C disease: molecular mechanisms and potential therapeutic approaches. J Neurochem. 2011;116(5):789–95. doi: 10.1111/j.1471-4159.2010.06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Maulik M, et al. Role of Cholesterol in APP Metabolism and Its Significance in Alzheimer’s Disease Pathogenesis. Mol Neurobiol. 2012 doi: 10.1007/s12035-012-8337-y. [DOI] [PubMed] [Google Scholar]
- 260.Malnar M, et al. Bidirectional links between Alzheimer’s disease and Niemann-Pick type C disease. Neurobiol Dis. 2014;72(Pt A):37–47. doi: 10.1016/j.nbd.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 261.Mattsson N, et al. Amyloid-beta metabolism in Niemann-Pick C disease models and patients. Metab Brain Dis. 2012;27(4):573–85. doi: 10.1007/s11011-012-9332-8. [DOI] [PubMed] [Google Scholar]
- 262.Kobayashi T, et al. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1(2):113–8. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 263.Ohashi M, et al. Arrested maturing multivesicular endosomes observed in a Chinese hamster ovary cell mutant, LEX2, isolated by repeated flow-cytometric cell sorting. J Cell Sci. 2000;113( Pt 12):2187–205. doi: 10.1242/jcs.113.12.2187. [DOI] [PubMed] [Google Scholar]
- 264.Miwako I, et al. Cholesterol requirement for cation-independent mannose 6-phosphate receptor exit from multivesicular late endosomes to the Golgi. J Cell Sci. 2001;114(Pt 9):1765–76. doi: 10.1242/jcs.114.9.1765. [DOI] [PubMed] [Google Scholar]
- 265.Ganley IG, Pfeffer SR. Cholesterol accumulation sequesters Rab9 and disrupts late endosome function in NPC1-deficient cells. J Biol Chem. 2006;281(26):17890–9. doi: 10.1074/jbc.M601679200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ikeda K, et al. Drug-induced phospholipidosis is caused by blockade of mannose 6-phosphate receptor-mediated targeting of lysosomal enzymes. Biochem Biophys Res Commun. 2008;377(1):268–74. doi: 10.1016/j.bbrc.2008.09.121. [DOI] [PubMed] [Google Scholar]
- 267.Liao G, et al. Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in Npc1 −/− mouse brain. Am J Pathol. 2007;171(3):962–75. doi: 10.2353/ajpath.2007.070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Amritraj A, et al. Increased Activity and Altered Subcellular Distribution of Lysosomal Enzymes Determine Neuronal Vulnerability in Niemann-Pick Type C1-Deficient Mice. Am J Pathol. 2009 doi: 10.2353/ajpath.2009.081096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kalia LV, Lang AE. Lancet. 2015. Parkinson’s disease. [DOI] [PubMed] [Google Scholar]
- 270.Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47(36):9678–87. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Qiao L, et al. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol Brain. 2008;1:17. doi: 10.1186/1756-6606-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zimprich A, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89(1):168–75. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sharma M, et al. A multi-centre clinico-genetic analysis of the VPS35 gene in Parkinson disease indicates reduced penetrance for disease-associated variants. J Med Genet. 2012;49(11):721–6. doi: 10.1136/jmedgenet-2012-101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tsika E, et al. Parkinson’s disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Hum Mol Genet. 2014;23(17):4621–38. doi: 10.1093/hmg/ddu178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Miura E, et al. VPS35 dysfunction impairs lysosomal degradation of alpha-synuclein and exacerbates neurotoxicity in a Drosophila model of Parkinson’s disease. Neurobiol Dis. 2014;71:1–13. doi: 10.1016/j.nbd.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 276.Follett J, et al. The Vps35 D620N mutation linked to Parkinson’s disease disrupts the cargo sorting function of retromer. Traffic. 2014;15(2):230–44. doi: 10.1111/tra.12136. [DOI] [PubMed] [Google Scholar]
- 277.Tong M, Dong M, de la Monte SM. Brain insulin-like growth factor and neurotrophin resistance in Parkinson’s disease and dementia with Lewy bodies: potential role of manganese neurotoxicity. J Alzheimers Dis. 2009;16(3):585–99. doi: 10.3233/JAD-2009-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Metcalf DJ, et al. Loss of the Batten disease gene CLN3 prevents exit from the TGN of the mannose 6-phosphate receptor. Traffic. 2008;9(11):1905–14. doi: 10.1111/j.1600-0854.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 279.Dore S, et al. Distribution and levels of insulin-like growth factor (IGF-I and IGF-II) and insulin receptor binding sites in the spinal cords of amyotrophic lateral sclerosis (ALS) patients. Brain Res Mol Brain Res. 1996;41(1–2):128–33. doi: 10.1016/0169-328x(96)00081-2. [DOI] [PubMed] [Google Scholar]
- 280.Dagvajantsan B, et al. Up-regulation of insulin-like growth factor-II receptor in reactive astrocytes in the spinal cord of amyotrophic lateral sclerosis transgenic rats. Tohoku J Exp Med. 2008;214(4):303–10. doi: 10.1620/tjem.214.303. [DOI] [PubMed] [Google Scholar]
- 281.de la Monte SM, et al. Insulin and insulin-like growth factor resistance in alcoholic neurodegeneration. Alcohol Clin Exp Res. 2008;32(9):1630–44. doi: 10.1111/j.1530-0277.2008.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2(6):401–9. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 283.Neumann H, et al. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25(6):313–9. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- 284.Haile Y, et al. Granule-derived granzyme B mediates the vulnerability of human neurons to T cell-induced neurotoxicity. J Immunol. 2011;187(9):4861–72. doi: 10.4049/jimmunol.1100943. [DOI] [PubMed] [Google Scholar]
- 285.Suh HS, et al. Insulin-like growth factor 2 receptor is an IFNgamma-inducible microglial protein that facilitates intracellular HIV replication: implications for HIV-induced neurocognitive disorders. Am J Pathol. 2010;177(5):2446–58. doi: 10.2353/ajpath.2010.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]