Abstract
Background
Treatment success in obesity remains low and recently, food addiction has been delineated as an underlying etiologic factor with therapeutic relevance. Specifically, current treatment focuses on reduced food intake and increase of physical activity whereas interventions for addiction encompass behavioral therapy, abstinence, and environmental interventions such as taxation, restrictions on advertising and regulation of school menus.
Content
Here, we reviewed the pertinent literature on food addiction with a specific focus on the role of high glycemic index carbohydrates in triggering addictive symptoms. Three lines of evidence support the concept of food addiction: (1) behavioral responses to certain foods are similar compared to substances of abuse; (2) food intake regulation and addiction rely on similar neurobiological circuits; (3) individuals suffering from obesity or addiction show similar neurochemical- and brain activation patterns.
High glycemic index carbohydrates elicit a rapid shift in blood glucose and insulin levels, akin to the pharmacokinetics of addictive substances. Akin to drugs of abuse, glucose and insulin signals in the mesolimbic system to modify dopamine concentration. Sugar elicits addiction-like craving and self-reported problem foods are rich in high glycemic index carbohydrates. These properties make high glycemic index carbohydrates plausible triggers for food addiction.
Summary
Food addiction is a plausible etiological factor contributing to the heterogeneous condition and phenotype of obesity. In at least a subset of vulnerable individuals, high glycemic index carbohydrates trigger addiction-like neurochemical and behavioral responses.
BACKGROUND
Obesity is among the greatest public health challenges of the 21st century. The mainstays of therapy are lifestyle changes such as diet and exercise; however, only about 5% of people with obesity are able to permanently reduce their excess body weight. A large amount of research has been dedicated to the phenomenon of obesity, but conclusive reasons for the poor long-term treatment success remain elusive.
One concept that has received increasing attention over the past 10 years is the notion of food addiction. Historically, the term addiction was reserved for drugs of abuse and encompassed the loss of control over consumption, increased motivation to consume, and persistent consumption despite negative consequences. The term is now used more broadly to also describe behavioural addictions, also known as ‘routines; or ‘behaviours’ that are habitually undertaken to attain reward - again - despite apparent negative consequences1. Individuals who develop food addiction are proposed to display symptoms analogous to those of drug addiction, including cravings for ‘problem foods’, tolerance (needing more food to satisfy cravings), limited control of food intake, unsuccessful attempts to reduce intake, as well as withdrawal symptoms2 (see Table 1). Repetitive, addiction-like behaviours resulting in over-consumption could conceptually contribute to obesity and antagonize weight-loss efforts.
Table 1.
Features of Drug- and Food Addiction
| Characteristic | Presentation in Food Addiction | Animal Data | Human Data |
|---|---|---|---|
| Tolerance | Larger amounts of food needed to achieve same affect (satiation, pleasure) | Increasing sugar intake over time when intermittent access is granted Decrease of dopamine release in response to regular chow |
Lower Nucleus accumbens activation with repeated food stimuli |
| Craving | Intense desire to consume a specific food (‘selective hunger’) | Increased lever-pushing for sugar Heightened anticipatory activation of striatum |
Specific cravings for energy-dense, or processed foods with high GI +/− fat content |
| Limited Control | Inability to regulate behavior in face of temptations and impulses | Decreased control over food-seeking despite adverse stimulus | Unsuccessful diet attempts Compulsive intake of specific foods |
| Withdrawal | Distress/dysphoria during dieting | Sucrose abstinence or opioid antagonist causes withdrawal symptoms | No convincing evidence |
| Unsuccessful attempts at behavioral control | Inability to stop or reduce intake of trigger food/larger amounts of food consumed then intended | N/A | Diet failure Compulsive food intake (e.g. bingeing) |
| Spending a lot of time to obtain/use or recover | Spending a lot of time eating/obtaining food | Increased food-seeking behaviors/locomotion | Less applicable as food is ubiquitously available |
| ot meeting other responsibilities – Social, occupational | Missing responsibilities doe to preoccupation with eating | N/A | Less applicable as food intake is socially acceptable |
| Continued despite negative consequences – health, relationship, general safety | Negative health consequences of obesity | N/A | Less applicable (except health) as food consumption is generally well accepted |
|
| |||
| Addiction transfer | Replacement of a one addictive substance for another, e.g. food for cocaine | Animal models of DA use food stimuli for conditioning/training Sucrose may replace drug, or even be preferred |
Carbohydrate craving in Post bariatric surgery, alcoholics and smokers and rehabbers crave carbs |
| Trait | Low dopamine receptor density in obese. Comorbidity of obesity and addiction | ||
Lines 1–8 are paraphrased/summarized from the 11 diagnostic DSM-V criteria for drug addiction. Addiction transfer and trait are not part of the diagnostic criteria, but are commonly cited as evidence of the overlap of obesity and addiction.
The neuro-biologic basis for food addiction in animals appears robust; however, findings derived from human studies are more heterogeneous.3–5 Controversial topics include: (1) the applicability of all DSM-5 criteria for addiction to food (Table 1); (2) the validity of food addiction as a model for overeating (e.g. food is required to sustain life, craving and withdrawal are physiologic reactions and should not be interpreted as pathological “addiction”; and a threshold between normal adaptation and pathologic deviation is not defined); (3) the association of food addiction with obesity (addictive like symptoms and behavioural patterns are inconsistently observed); and (4) lack of research identifying the addictive agent in food (most studies in humans are based on mixed foods, typically fast-foods, or food cues).
Uncovering the role of food addiction for obesity, and identifying possible triggers bears major importance for identifying effective therapeutic strategies: Treatment approaches for obesity and addiction are fundamentally different, the latter including behavioral therapy, abstinence and environmental control including taxation, restrictions on advertising and regulation of school menus. In other words, while food intake is essential for sustaining life, the number of specific chemical or nutrient-based triggers of food addition might be limited and accordingly could be restricted or even avoided altogether.
Here, we performed a targeted review of the literature (1) to outline the neurobiological and behavioral basis of food addiction, (2) to explore its possible connection with obesity, and (3) to highlight the possible role of high glycemic index (GI) carbohydrates in triggering addictive symptoms.
A NEUROBIOLOGICAL BASIS FOR FOOD ADDICTION
1. The Mesolimbic Reward System
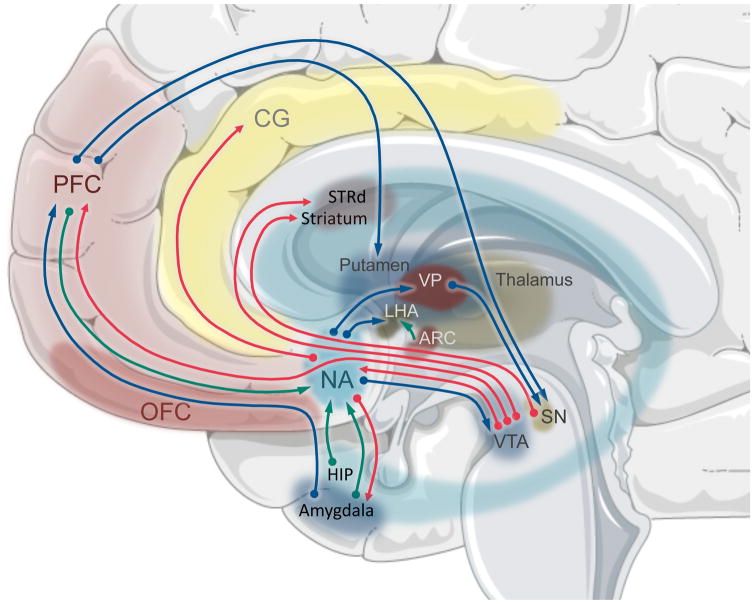
When considering the neurobiology of addiction, it is noteworthy that drugs of abuse take effect by “hijacking” brain pathways for natural reward and aversion reactions. Specifically, the mesolimbic reward system (Figure 1) is ontogenetically evolved to steer organisms toward seeking favorable, potentially life- or kindred-sustaining stimuli; for example, high caloric foods in times of sparse food supply, sweet foods (representing non-toxic energy supplies), and other natural rewards like water and sex. Dopaminergic projections extend from the ventral tegmental area (VTA) and substantia nigra (SN) to a network of interconnected brain areas with specific functions in reward processing. The nucleus accumbens (NAcc) plays a central role and processes reward and salience. The amygdala and hippocampus are involved in forming memories of stimulus-reward relationships. The orbitofrontal cortex regulates decision making and reward/punishment anticipation. The prefrontal cortex and anterior cingulate gyrus provide inhibitory control and emotional regulation. As a whole, the mesolimbic reward system plays a pivotal role in food intake regulation (Figure 1).
Figure 1. Brain areas and transmitters of the mesolimbic reward system.
The main mesolimbic input is derived from the ventral tegmental area (VTA) and substantia nigra (SN) to the nucleus accumbens (NAcc). The NAcc plays a central role in processessing reward and salience. The amygdala, hippocampus (Hip) are involved in forming memories of stimulus-reward relationships. The orbitofrontal cortex (OFC) regulates decision-making and reward/punishment anticipation. The prefrontal cortex (PFC) and anterior cingulate gyrus (CG) provide inhibitory control and emotional regulation. Projections exist between the different areas and are depicted by arrows: dopamine – red, GABA – blue, glutamate – green. In addition, direct connections to the hypothalamic nuclei regulate homeostatic food intake: lateral hypothalamic area (LHA) arcuate nucleus (ARC); and the ventral pallidum (VP).
Transgenic mice that lack dopamine signaling demonstrate a complete loss of food-seeking behavior and die of starvation. Restoring dopamine production in the dorsal striatum reinstates feeding on regular chow, whereas restoration of dopamine production in the NAcc reinstates motivational behavior. Replacement of dopamine to either region restores preference for sucrose or a palatable diet. 6 While dopamine is a critical neurotransmitter in the mesolimbic system, numerous other neurotransmitter families are involved and modify dopamine concentration. For example, local infusion of opioid agonists increase food motivation and ad libitum food intake, 7 and hormones like insulin, leptin, ghrelin and GLP-1 modify natural and drug reward. 8 In addition to hedonic input through VTA and SN, the mesolimbic system receives direct projections from hypothalamic nuclei that regulate energy homeostasis.
2. Neurobiology of Substance Addiction
Hijacking the above-outlined mesolimbic systems, drugs of abuse signal through a variety of different pathways that ultimately converge to increase dopamine concentration in the NAcc. The supra-physiologic dopamine concentrations initially increase salience and therefore motivation towards drug-related cues to reinforce drug-taking. However, repeated drug use results in blunted dopamine release in the NAcc over time. 9 Instead, drug-related cues (e.g. images, situations) produce an anticipatory dopamine release in the dorsal striatum (caudate/putamen) and baso-lateral amygdala 10. The resulting shift is critical as cue-based activation increases, drug seeking and craving upsurge due to the heightened anticipatory reward. At the same time, the blunted activation in response to actual consumption is associated with the need for increased intake to achieve the same level of reward. As this behaviour becomes progressively elicited by drug-related cues, it is ultimately consolidated as a habit. 9 Over time, habitual drug consumption leads to functional impairments in the prefrontal, dorsolateral and inferior cortices, leading to increased compulsivity and reduced executive control of drug intake. 11 Morphologically, drug addiction has been associated with low density of dopamine receptors in animal and human studies. The low density of dopamine receptors can be the result of a combination of pre-existing low dopamine receptor availability in vulnerable individuals (e.g. genetic polymorphisms), 12 and down regulation of dopamine receptors 13 in drug tolerance, where drug consumption no longer elicits a positive effect and rather mitigates a negative state to avoid dysphoria and withdrawal. 14
3. Neurobiology of Food Addiction
The food addiction model asserts that excessive consumption of problem foods may have similar phenotypic characteristics and the implicit notion of the same neurobiological framework links food and drug addiction. Conceptually, the neurobiology of consuming a problem food would increase dopamine concentration in the mesolimbic system and consequently increase salience and food motivation. Over time, dopamine signalling would shift from the NAcc to the dorsal striatum and perpetuate craving and food seeking. Consumption would become habitual and compulsive as prefrontal control is altered. As dopamine receptors are down-regulated, food intake would become driven by the need to avoid withdrawal symptoms rather than by pleasure and homeostatic needs. In line with research of chronic drug use, dopamine receptor levels may represent a vulnerability marker and/or central dopamine and receptor concentrations are modified by excessive intake over time.
Indeed, NAcc dopamine neurons are activated by novel food rewards and with repeated exposure the associated activation decreases over time, and predictive cues of the food begin to induce more pronounced striatal activation. 15 The resulting cue-based signaling along with a decreased consummatory response has been proposed to drive craving and habitual food intake. 16 Furthermore, Gearhardt et al. have shown that humans with high self-reported symptoms of food addiction had elevated activation in the mesolimbic reward system in response to food cues, and reduced activation in inhibitory regions in response to food intake. 17 Importantly, these responses are similar to those observed in drug-dependent individuals when viewing drug cues. 17
Simply put, from a neurobiological perspective the intended function of the mesolimbic systems is to ensure food intake towards favourable energy sources. These life-sustaining responses can be exaggerated to the point of addictive-like patterns. Assuming a continuous biological spectrum of activation patterns and associated behaviours to triggering food sources, the controversy regarding food addiction becomes a matter of defining a threshold of normal adaptation vs. pathological addiction.
LINKING FOOD ADDICTION TO OBESITY
1. Epidemiologic Overlap of Food Addiction
As a behavioural phenomenon, symptom capture requires self-reported psychometric tools and The Yale Food Addiction Scale (YFAS) has been established as a reliable tool to identify those individuals who exhibit addictive symptoms with the consumption of foods 18. Individuals with obesity have higher rates of food addiction when compared to non-obese control populations, as assessed by YFAS. Specifically, in a meta-analysis, Pursey at al. demonstrate that the prevalence of food addiction increased with BMI from 10% in normal-weight to about 25% in people with obesity (higher with increasing BMI). 19 Furthermore, people with obesity who have higher YFAS scores show decreased weight loss responses to treatment. 20 Nonetheless, obesity is a heterogeneous phenotype and the overlap with food addiction is incomplete: according to Pursey et al., a majority of obese individuals do not show a distinct addiction phenotype, and conversely a minority of lean individuals report addictive symptoms. In addition to heterogeneity in objectively studying behavior in obese patients (‘obesity ethology’), the dissociation may be increased by methodological issues relating to sensitivity and specificity of the YFAS, and a lack of reliable definitions of food addiction.
2. Neurobiological Overlap of Addiction and Obesity
In a meta-analysis of 87 functional neuroimaging studies, Garcia et al. reported similar brain activation patterns in response to reward in participants with obesity, substance addiction and non-substance addiction. 21 Wang et al. 22 and other groups demonstrated a negative correlation of striatal dopamine transporters with body-mass index (BMI). Assuming obesity as a proxy of habitual overeating, this may parallel dopamine receptor paucity (trait) or down-regulation in response to habitual intake (tolerance) described in drug addiction. The resulting dopamine signal deficiency has been postulated to promote compensatory pathological eating to activate reward circuits. 23 Thus, functional neuroimaging studies demonstrate a shared neurobiological framework of obesity and addiction.
3. Shared Vulnerability for of Addiction and Obesity
A shared vulnerability for addiction and obesity is suggested by genetic polymorphisms and observations of addiction transfer. For example, Carpenter found an association of higher BMI with the TaqI A1 allele of the dopamine D2 receptor (DRD2), a polymporphism associated with cocaine, alcohol, and opioid use. 24 Another circumstantial piece of evidence is addiction transfer from drugs to high GI carbohydrates and vice versa. For example, people with alcoholism display higher sweet preference and cravings, which is further increased by abstinence. 25 Subsequent to bariatric surgery, when the imposed anatomical and physiological barriers restrict food intake, patients often manifest new substance addictions. 26 Several studies report associations of these new substance disorders with pre-operative food addiction symptoms, and an addiction-transfer from food addiction has been proposed. 27 Fowler et al. 28 found that self-reported problems specifically with intake of high glycaemic index/high carbohydrate, low fat foods was associated with an increased risk for developing substance addictions postoperatively, suggesting an addiction-transfer.
Collectively these findings indicate a significant clinical and neurobiological overlap between addiction and obesity.
WHAT TRIGGERS FOOD ADDICTION?
1. Behavioral Addiction
Considerable debate remains around the triggering mechanism of food addiction. Hebebrand et al. and others have argued that food addiction may be a behavioral addiction, analogous to gambling disorder that was recently included among addiction disorders in the DSM-5 catalog. Behavioral addictions are thought to be mediated by Pavlovian conditioning and habit formation, 29 ultimately also converging on the mesolimbic reward system through the VTA. Akin to chemical addiction, behavioral addictions modulate function and plasticity of the mesolimbic reward system and manifest in symptoms including craving, impaired control over the behavior, tolerance, withdrawal, and high rates of relapse 30. So strictly speaking no chemical trigger is necessary to elicit addictive symptoms. Indeed, the majority of human food addiction literature has relied on cue-based paradigms such as food pictures, or mixed meals, and allows no conclusions toward possible chemical triggers.
However, food contains a variety of compounds that may serve as chemical or metabolic triggers. It is noteworthy that all commonly suspected problem foods share nutritive properties, suggesting a chemical or metabolic link rather than a mere behavioral phenomenon.
2. Commonly Suspected Trigger Foods
When Theron Randolph first proposed the concept of food addiction in the 1950s, 31 he reported addictive consumption of common foods with high energy density, such as corn, milk, and potatoes. Randolph postulated that the rapid shifts in metabolic fuels that follow consumption of these foods are akin to the pharmacokinetic properties of drugs of abuse, and may trigger addictive behaviours. The modern food addiction literature has focused on processed, energy-dense foods with high GI and fat content (i.e. fast-foods and sweets). Schulte et al.2 asked healthy participants how likely they were to experience food addiction-type problems with a list of 35 foods. Highly processed foods containing either mixed macronutrients or pure high GI carbohydrates ranked highest. Further, the group found that glycemic load (the product of carbohydrate amount and glycemic index), 32 fat and salt content of food items predicted problem rating. While these foods at first glance seem rather distinct from what Randolph proposed in the 1950s, they share an important physiologic property. Processed carbohydrates, corn and potatoes all have a high GI and cause rapid shifts in blood glucose, insulin and other metabolic fuels and hormones. These rapid shifts are pharmacokinetically akin to the rapid shifts in neurotransmitters seen after consumption of substances of abuse.
While fat intake per se does not cause rapid metabolic shifts, dietary fat content has been linked to food addiction in several studies and it seems that fat-intake does contribute to brain activation and addictive behaviors. In an elegant set of experiments, Hoch et al. 33 demonstrated increased food seeking and mesolimbic brain activation in rats in response to a mixed meal dependent on the ratio of carbohydrate to fat: Maximum behaviours were triggered by diets containing ~ 35% fat and ~ 45% carbohydrate, while sugar alone or fat alone triggered minimal responses. Hoch further assessed food seeking and brain activation in response to potato chips (with similar macronutrient composition) and found the largest response, suggesting a role of other ingredients or palatability in triggering behaviours and brain activation. Literature on the role of fat as an isolated macronutrient in food addiction is sparse. Animal literature was recently reviewed by Avena et al., 34 and dietary fat has been associated with binge eating and increased body weight in rats, 35,36 likely via effects on the opioid system and/or by enhancing palatability. 35,37 However, bingeing on fat-rich foods does not induce opiate-like withdrawal symptoms after the food is removed, as seen in sugar binging. 38 To our knowledge, no isolated fat, e.g. butter or oil, has been proposed in association food addiction in humans, and no macro-nutrient selective studies using fat only have been performed and high GI carbohydrates have received considerably more attention.
SUGAR, ARTIFICIAL SWEETENERS AND HIGH GLYCEMIC INDEX CARBOHYDRATES
Sugar elicits addiction-like craving, compulsive food seeking, and withdrawal in rats and has therefore been used in substance abuse models for some time. Several reviews have summarized the addictive properties of sugar 39–41 and glycemic index (GI). 42 In addition, non-nutritive sweeteners have been proposed as a possible trigger for food addiction, because their intake is associated with increased preference and cravings for sweet foods, and weight gain. 43
1. Sugar and Food Addiction
Extensive evidence in animal models suggests that sugar may be an addictive agent in highly palatable foods. Rats given intermittent access to sugar show behavioral signs of addiction, such as binge consumption, tolerance, and cross-sensitization to other drugs of abuse. 44 Bingeing on sucrose produces a repeated increase of dopamine akin to drugs of abuse, rather than the gradual decline over time that is typical for natural rewards. 45 Mu-opioid receptor binding 46 is increased in a similar manner to drugs of abuse. When the sugar is removed from the diet or when an opiate antagonist is administered, rats experience signs of opiate-like withdrawal44, such as anxiety, teeth chattering, and aggression. Two properties of sugar participate in mediating these manifestations: hedonic sweetness, and homeostatic rapid metabolic shifts following its ingestion. Studies relying on intra-gastric administration, the use of artificial sweeteners, and high GI carbohydrates without sweet taste can help untangle these factors.
2. Non-nutritive Sweeteners and Food Addiction
Non-nutritive sweeteners elicit an intense sweet taste, but do not evoke a rise in blood glucose. In other words, the sweet perception is dissociated from nutritive satisfaction. In rats, intense sweetness from both nutritive and non-nutritive sweeteners surpasses cocaine and nicotine reward and elicits strong food-seeking behaviors. 3 These data suggest that sweet taste alone can mediate reward and craving. In addition, dissociating sweet taste from nutritive satisfaction may elicit compensatory sweet cravings to restore the anticipated effect, and ultimately condition alterations in homeostatic control. Indeed, rats exposed to non-nutritive sweeteners display increased compensatory intake of sugar sweetened foods (not chow) and excess weight gain if allowed access to such foods. 43 To distinguish the effects of palatable vs. nutritive signalling, Tellez et al. 49 used a paradigm of licking sucralose during intra-gastric glucose or sucralose administration in rats. Sucralose taste increased dopamine concentration in the ventral striatum (NAcc) regardless of the intra-gastric infusion whereas dorsal striatum dopamine release occurred only with the nutritive infusion of glucose.
In human imaging studies, decreases in stress-related cortisol levels and hippocampus activation have been observed in response to sucrose, but not saccharose, 50 and habitual intake of artificially sweetened beverages decreases amygdala activation. 51 Both can be interpreted as correlates of stimulus-reward disconnect. Epidemiologic studies show an association between artificial sweetener intake and increased BMI, but the possibility of confounding and reverse causation cannot be excluded 52. Raben et al. did not find increased caloric or sugar consumption after intake of artificial sweeteners in a 10-week interventional study in 20 overweight participants. 53
In summary, artificial sweeteners have been shown to alter food reward and food cravings in some but not all studies. Behavioral data on binge consumption, tolerance, cross-sensitization and withdrawal is not available for artificial sweeteners. Thus, artifical sweeteners cannot be excluded as etiologic factors of food addiction.
3. High Glycemic Index Carbohydrates and Food Addiction
High GI carbohydrates elicit the most pronounced metabolic response of all macronutrients. In analogy to the pharmacology of addictive drugs: blood glucose and insulin levels rise and fall quickly – with associated shifts in other metabolic fuels and hormones. The blood glucose excursion is tightly associated with changes in insulin levels. 54 Glucose and insulin both signal directly and indirectly to the mesolimbic system. Insulin increases dopamine reuptake in the presynaptic membrane, and suppresses food-motivated behavior 55. In addition, insulin receptors are found on neurons projecting from the hypothalamus to the VTA. 56 Glucose modulates SN dopamine neuronal activity by the actions of an ATP-sensitive potassium channels. 57 In addition, the mesolimbic system receives direct projections from other glucose-sensing brain areas: Domingos et al. 58 showed that melanin-concentrating hormone (MCH)-expressing neurons in the lateral hypothalamus respond to extracellular glucose levels and project to dopaminergic neurons in the striatum and midbrain regions. While mice show a preference for sucrose over the non-nutritive sweetener, sucralose, transgenic mice lacking MCH neurons do not show this preference.
There are at least 5 studies indicating unique central activation patterns in response to high GI carbohydrates: (1) Spring and colleagues showed a preference for carbohydrate beverage over a taste-matched mixed carbohydrate and protein beverage in 61 overweight women with “carbohydrate-craving”. 59 Insulin and glucose levels have been associated with altered brain activity in regions associated with reward processing. (2) Page at al. found that mild hypoglycemia preferentially activated limbic-striatal brain regions in response to food cues, and produced a greater desire for high-calorie foods. 60 In another study, (3) Page demonstrated increased connectivity between the hypothalamus and striatum in response to glucose, but not fructose ingestion. These alterations were associated with higher excursions in blood glucose and insulin levels 61 (4) Anthony et al. found than insulin infusion increased metabolism in ventral striatum and prefrontal cortex, and decreased metabolism in right amygdala/hippocampus and cerebellar vermis. 62 Insulin’s effect was attenuated in ventral striatum and prefrontal cortex in the insulin-resistant subjects. The authors concluded that brain insulin resistance exists in regions mediating appetite and reward, diminishing the link between intake control and energy balance. (5) Lennerz et al., showed NAcc activation in response to nutrient-matched milk shakes with high- versus low- glycemic index.63
Together, these data indicate a role of nutrient signaling in addiction that is independent of hedonic taste signals. Non-nutritive sweeteners mimic some of the properties of nutritive carbohydrate and seem to increase the propensity for developing addictive behaviors toward carbohydrate in some studies. This notion bears similarity to the gateway drug theory, where use of a less deleterious drug can increase the risk for using more potent substances. 64 However, the data on non-nutritive sweeteners is heterogeneous and more studies are needed.
DIAGNOSTIC AND MANAGEMENT IMPLICATIONS
As outlined above, there is a need to translate our partial (mechanistic) and neurobiological understanding of how nutrients contribute to food addiction and obesity. Even if not all DSM-5 criteria are applicable, this does not discredit the phenomenon. It merely underlines the importance of more targeted diagnostic criteria and the development of thresholds for healthy adaptation vs. pathological addiction. The concept of food addiction may open new avenues for obesity prevention, treatment, and public health policy. 65 Current obesity therapy focuses on moderation of food intake and increase of physical activity whereas therapeutic approaches for addiction encompass behavioral therapy and abstinence. One cannot abstain from food; however, at least in a subset of vulnerable individuals, high GI carbohydrates can be considered a specific trigger that can be reduced or avoided. Other successful strategies to fight addiction are environmental interventions, and restrictions on advertising and/or taxation have all been proven successful in reducing, for example smoking prevalence.66 It is therefore no surprise that taxation of sugar-sweetened beverages has been proposed 67; clarification of the specific role of food addiction will be paramount to make informed public health decisions.
SUMMARY
In summary, food addiction is –at least in some individuals– a plausible causal factor contributing to obesity. The concept of food addiction may reveal new avenues for intervention on an individual and public health level, especially if specific triggers can be identified and mechanisms clarified. High GI carbohydrates are a possible trigger mediating neurochemical responses similar to addiction. As a neuro-psycho-biological entity, food addiction requires an evidence-based, multi-disciplinary classification system to ultimately improve assessment and management.
Acknowledgments
We thank Dr. David Ludwig for stimulating discussions and critical revision of the manuscript.
LITERATURE
- 1.Marks I. Behavioural (non-chemical) addictions. Br J Addict. 1990;85:1389–1394. doi: 10.1111/j.1360-0443.1990.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 2.Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vella SL, Pai N. What is in a name? Is food addiction a misnomer? Asian J Psychiatr. 2017;25:123–126. doi: 10.1016/j.ajp.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Ziauddeen H, Fletcher PC. Is food addiction a valid and useful concept? Obes Rev. 2013;14:19–28. doi: 10.1111/j.1467-789X.2012.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corsica JA, Pelchat ML. Food addiction: true or false? Curr Opin Gastroenterol. 2010;26:165–169. doi: 10.1097/MOG.0b013e328336528d. [DOI] [PubMed] [Google Scholar]
- 6.Szczypka MS, et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 7.Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: Map based on microinjection Fos plumes. Brain Research. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- 8.Engel JA, Jerlhag E. Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs. 2014;28:875–886. doi: 10.1007/s40263-014-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vereczkei A, et al. Multivariate Analysis of Dopaminergic Gene Variants as Risk Factors of Heroin Dependence. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–25. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 14.Nader MA, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: Initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- 15.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry. 2013;73:804–810. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Gearhardt AN, et al. Neural correlates of food addiction. Arch Gen Psychiatry. 2011;68:808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52:430–436. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Pursey KM, Stanwell P, Gearhardt AN, Collins CE, Burrows TL. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients. 2014;6:4552–4590. doi: 10.3390/nu6104552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burmeister JM, Hinman N, Koball A, Hoffmann DA, Carels RA. Food addiction in adults seeking weight loss treatment. Implications for psychosocial health and weight loss. Appetite. 2013;60:103–110. doi: 10.1016/j.appet.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Garcia I, et al. Reward processing in obesity, substance addiction and non-substance addiction. Obes Rev. 2014;15:853–869. doi: 10.1111/obr.12221. [DOI] [PubMed] [Google Scholar]
- 22.Volkow ND, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 23.Curtis C, Davis C. A qualitative study of binge eating and obesity from an addiction perspective. Eat Disord. 2014;22:19–32. doi: 10.1080/10640266.2014.857515. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter CL, Wong AM, Li Z, Noble EP, Heber D. Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. Obes (Silver Spring) 2013;21:13. doi: 10.1002/oby.20202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Junghanns K, Veltrup C, Wetterling T. Craving shift in chronic alcoholics. Eur Addict Res. 2000;6:64–70. doi: 10.1159/000019012. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Wu LT. Substance use after bariatric surgery: A review. J Psychiatr Res. 2016;76:16–29. doi: 10.1016/j.jpsychires.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Wu LT. Substance use after bariatric surgery: A review. J Psychiatr Res. 2016;76:16–29. doi: 10.1016/j.jpsychires.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler L, Ivezaj V, Saules KK. Problematic intake of high-sugar/low-fat and high glycemic index foods by bariatric patients is associated with development of post-surgical new onset substance use disorders. Eat Behav. 2014;15:505–508. doi: 10.1016/j.eatbeh.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Hebebrand J, et al. ‘Eating addiction’, rather than ‘food addiction’, better captures addictive-like eating behavior. Neurosci Biobehav Rev. 2014;47:295–306. doi: 10.1016/j.neubiorev.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011;61:1109–1122. doi: 10.1016/j.neuropharm.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Randolph TG. The descriptive features of food addiction; addictive eating and drinking. Q J Stud Alcohol. 1956;17:198–224. [PubMed] [Google Scholar]
- 32.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–23. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 33.Hoch T, Kreitz S, Gaffling S, Pischetsrieder M, Hess A. Fat/carbohydrate ratio but not energy density determines snack food intake and activates brain reward areas. Sci Rep. 2015;5 doi: 10.1038/srep10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avena NM, Bocarsly ME, Hoebel BG. Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Methods Mol Biol. 2012;829:351–365. doi: 10.1007/978-1-61779-458-2_23. [DOI] [PubMed] [Google Scholar]
- 35.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–8. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corwin RL, et al. Limited Access to a Dietary Fat Option Affects Ingestive Behavior But Not Body Composition in Male Rats. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 37.Avena NM. The study of food addiction using animal models of binge eating. Appetite. 2010;55:734–737. doi: 10.1016/j.appet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bocarsly ME, Berner LA, Hoebel BG, Avena NM. Rats that binge eat fat-rich food do not show somatic signs or anxiety associated with opiate-like withdrawal: implications for nutrient-specific food addiction behaviors. Physiol Behav. 2011;104:865–872. doi: 10.1016/j.physbeh.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westwater ML, Fletcher PC, Ziauddeen H. Sugar addiction: the state of the science. Eur J Nutr. 2016;55:55–69. doi: 10.1007/s00394-016-1229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed SH, Guillem K, Vandaele Y. Sugar addiction: pushing the drug-sugar analogy to the limit. Curr Opin Clin Nutr Metab Care. 2013;16:434–439. doi: 10.1097/MCO.0b013e328361c8b8. [DOI] [PubMed] [Google Scholar]
- 41.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience and Biobehavioral Reviews. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thornley S, McRobbie H, Eyles H, Walker N, Simmons G. The obesity epidemic: is glycemic index the key to unlocking a hidden addiction? Med Hypotheses. 2008;71:709–714. doi: 10.1016/j.mehy.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiol Behav. 2010;100:55–62. doi: 10.1016/j.physbeh.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008;94:309–315. doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 46.Colantuoni C, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 47.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lesage MG. Toward a nonhuman model of contingency management: Effects of reinforcing abstinence from nicotine self-administration in rats with an alternative nondrug reinforcer. Psychopharmacology (Berl) 2009;203:13–22. doi: 10.1007/s00213-008-1362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tellez LA, et al. Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci. 2016;19:465–70. doi: 10.1038/nn.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tryon MS, et al. Excessive sugar consumption may be a difficult habit to break: A view from the brain and body. J Clin Endocrinol Metab. 2015;100:2239–2247. doi: 10.1210/jc.2014-4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudenga KJ, Small DM. Amygdala response to sucrose consumption is inversely related to artificial sweetener use. Appetite. 2012;58:504–507. doi: 10.1016/j.appet.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swithers SE. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends in Endocrinology and Metabolism. 2013;24:431–441. doi: 10.1016/j.tem.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raben A, Vasilaras TH, Christina Møller A, Astrup A. Sucrose compared with artificial sweeteners: Different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins DJ, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–6. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 55.Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R388–94. doi: 10.1152/ajpregu.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Frontiers in Neuroendocrinology. 2010;31:104–112. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Röper J, Ashcroft FM. Metabolic inhibition and low internal ATP activate K-ATP channels in rat dopaminergic substantia nigra neurones. Pflugers Arch Eur J Physiol. 1995;430:44–54. doi: 10.1007/BF00373838. [DOI] [PubMed] [Google Scholar]
- 58.Domingos AI, et al. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spring B, et al. Abuse potential of carbohydrates for overweight carbohydrate cravers. Psychopharmacology (Berl) 2008;197:637–647. doi: 10.1007/s00213-008-1085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Page KA, et al. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 2011;121:4161–4169. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Page KA, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. Jama. 2013;309:63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anthony K, et al. Attenuation of Insulin-Evoked Responses in Brain Networks Controlling Appetite and Reward in Insulin Resistance: The Cerebral Basis for Impaired Control of Food Intake in Metabolic Syndrome? Diabetes. 2006;55:2986–2992. doi: 10.2337/db06-0376. [DOI] [PubMed] [Google Scholar]
- 63.Lennerz BS, et al. Effects of dietary glycemic index on brain regions related to reward and craving in men. Am J Clin Nutr. 2013;98 doi: 10.3945/ajcn.113.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kandel ER, Kandel DB. A Molecular Basis for Nicotine as a Gateway Drug. N Engl J Med. 2014;371:932–943. doi: 10.1056/NEJMsa1405092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gearhardt A, Roberts M, Ashe M. If sugar is addictive…what does it mean for the law? J Law Med Ethics. 2013;1:46–49. doi: 10.1111/jlme.12038. [DOI] [PubMed] [Google Scholar]
- 66.Levy DT, Ellis Ja, Mays D, Huang A-T. Smoking-related deaths averted due to three years of policy progress. Bulletin of the World Health Organization. 2013 doi: 10.2471/BLT.12.113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brownell KD, et al. The public health and economic benefits of taxing sugar-sweetened beverages. N Engl J Med. 2009;361:1599–605. doi: 10.1056/NEJMhpr0905723. [DOI] [PMC free article] [PubMed] [Google Scholar]