Abstract
Background
Sleep-related movement disorders (SRMD) have been shown to increase the risk of cardiovascular diseases. However, the relationship between SRMD and stroke remains unclear.
Aim
To explore the relationship between SRMD and stroke in the general population.
Design
Two cohorts of patients with SRMD and without SRMD were followed up for the occurrence of hemorrhagic and ischemic stroke.
Methods
The study cohort enrolled 604 patients who were initially diagnosed as SRMD between 2000 and 2005. 2,416 age- and sex-matched patients without prior stroke were selected as the comparison cohort. A Cox-proportional hazard regression analysis was performed for multivariate adjustment.
Results
Patients with SRMD had a higher risk for developing all-cause stroke [adjusted hazard ratio (HR) = 2.29, 95% confidence interval (CI) = 1.42–3.80]. Patients of below 45 years old had the greatest stroke risk (HR = 4.03, 95% CI = 3.11–5.62), followed by patients aged ≥65 years (HR = 2.64, 95% CI = 1.12–3.44) and 45–64 years (HR = 1.07, 95% CI = 1.02–1.71). The age-stratified analysis suggested that the increased risk of hemorrhagic stroke was more significant than ischemic stroke among all age groups. Furthermore, males with SRMD were at greater risk to develop all-cause stroke (HR = 2.98, 95% CI = 1.74–4.50) than that of females (HR = 1.94, 95% CI = 1.01–3.77).
Conclusions
Patients with SRMD were found to have an increased risk of all-cause stroke along with a higher possibility of hemorrhagic stroke over ischemic stroke.
Introduction
Accumulating evidence suggests that sleep disorders may be associated with the occurrence of stroke.1 For example, obstructive sleep apnea (OSA) increases the risk of atherosclerosis and plaque formation by increasing inflammatory mediators which is linked to a higher risk of stroke.2,3 In this regard, continuous positive airway pressure (CPAP) which is an effective therapeutic management of OSA, has been recommended for stroke prevention and the use of CPAP has been demonstrated to reduce the increased incidence of refractory hypertension.4,5 In contrast, the relationship between sleep-related movement disorders (SRMD) and stroke remains to be elucidated and hence, a large-scale longitudinal study is needed to provide the epidemiological evidence.
Among sleep disorders, there are two major types of limb movements. One is restless legs syndrome (RLS) which leads to an urge to move legs that worsens at night and at rest, is partially relieved with movement.6 The other is periodic limb movement disorder (PLMD) which is defined as leg movements seen during polysomnography and last 0.5–10 s and occur every 5–90 s and at least four movements occur consecutively with at least an 8 μV increase in amplitude from baseline as seen in the limb movement leads.7 These movements may be associated with an arousal and can cause excessive daytime sleepiness in the presence of sleep disruption at night. It has been reported that about 80% of RLS patients were found to have PLMD during sleep.8 The prevalence of RLS and PLMD was reported to be 5.5 and 3.9% respectively in cross-sectional studies of general population in a large telephone survey in Europe and heart diseases were reported to be associated with both types of SRMD.9 Although a positive correlation between SRMD and cardiovascular diseases has been demonstrated,10 only a few studies have been focused on cerebrovascular complications of SRMD in specific patient groups at a small scale.9,11–13 Moreover, the SRMD were regarded as the post-stroke complications in most of the studies,14,15 and the potential predictive value of the development of SRMD in stroke prevention has not been explored.16
On the other hand, stroke is considered to be one of the most common causes of death and disability in Taiwan and worldwide. Though the major risk factors in stroke have been established, up to 40% of stroke patients may have health conditions other than that of the established stroke risk factors, which predispose a selected population to stroke.17,18 A detailed study on the relationship between SRMD and stroke is absolutely necessary before an effective management for SRMD is strongly recommended for stroke prevention. With this background, the present study was aimed to ascertain whether patients with SRMD had an elevated risk of developing stroke during more than 5 years of follow-up, using data from the Taiwan National Health Insurance Research Database.
Materials and methods
Data source
This study was conducted with an aim to investigate the association between the SRMD and the subsequent stroke using the data deduced from Longitudinal Health Insurance Database (LHID) which is a sub-database of National Health Insurance (NHI) program, Taiwan that covers over 99% of Taiwanese citizens (23 million). The LHID contains the records of inpatients, outpatients and ambulatory care services, covering the period from 1996 to 2010. The diagnostic coding system accepted by the NHI was the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The data in the LHID have been de-identified before releasing for research. This study protocol was reviewed by Institutional Review Board of Tri-Service General Hospital (TSGHIRB No.: 2-104-05-045).
Study sample
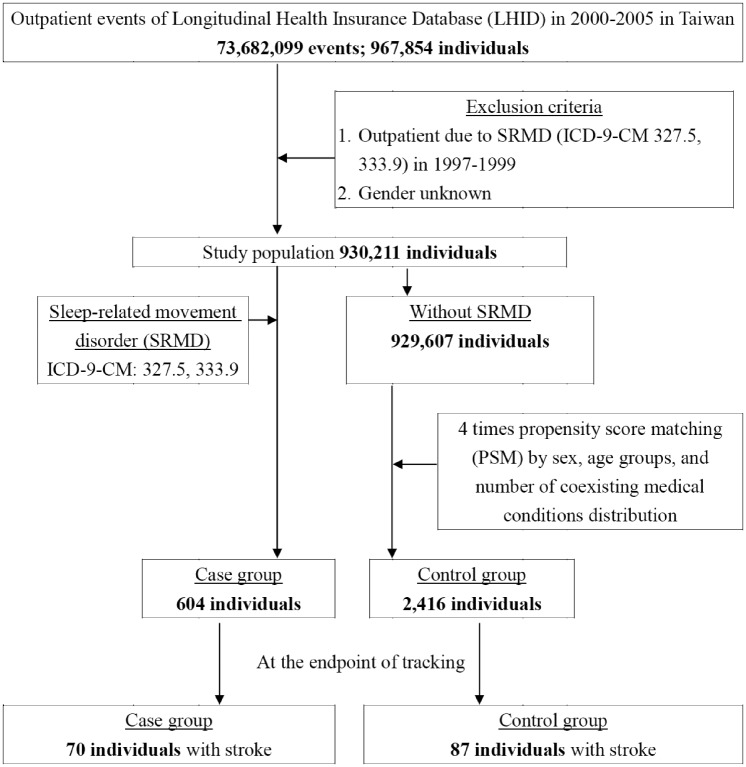
The study group and a comparison cohort were selected from the LHID (as shown in Figure 1). The study group comprised all patients who had been diagnosed for SRMD based on ICD-9-CM codes 327.5 or 333.9 for the first time from 2000 to 2005 (N = 604). Among all the SRMD patients, 492 (81.5%) and 112 (18.5%) had PLMD (ICD-9-CM 327.5) and RLS (ICD-9-CM 333.9), respectively. The index date of SRMD cohort was the first date of SRMD diagnosis. We randomly selected 2416 subjects (a sample size 4-fold that of the SRMD group) and the index date of these age- and sex-matched comparison cohort was randomly assigned a month and a day with the same year of the matched cases. Each patient was then followed up from the index date until the occurrence of stroke. For those who did not have stroke, the last day of follow-up was defined as the date of insurance withdrawal or the last day of the study period (December 31, 2010).
Figure 1.
The flowchart of study sample selection from National Health Insurance Research Database in Taiwan.
Definitions of stroke subtypes by ICD classification
The stroke subtypes were classified into hemorrhagic stroke (ICD-9-CM 430–432) and ischemic stroke (ICD-9-CM 433–437). The computed tomography (CT) or magnetic resonance imaging (MRI) was used to further confirm the diagnosis of stroke and to confirm that the patient was a new stroke case rather than someone with a history of stroke. The stroke-associated comorbidity was defined that the individual with a history of the comorbidity before the index date and it included hypertension (ICD-9-CM 401-405), diabetes mellitus (DM, ICD-9-CM 250), ischemic heart disease (IHD, ICD-9-CM 410-414), hyperlipidemia (ICD-9-CM 272), atrial fibrillation (AF, ICD-9-CM 427.3) and obstructive sleep apnea (OSA, ICD-9-CM 780.5).
Statistical analysis
The continuous variables were presented as mean and standard deviation (SD) and the categorical variables as number and percentages. The differences between the study group and the comparison cohort in the distribution of demographic characteristics (age and sex) and comorbidities were examined by t-test (age) and chi-square test (sex and comorbidities). The incidence density of stroke was measured for SRMD and comparison cohorts that the total stroke event divided by the total sum of follow-up years (per 1000 person-years). Cox proportional hazard regression analysis was performed to calculate the adjusted hazard ratios (HR) with 95% of confidence intervals (CI) for stroke risk between SRMD and comparison cohorts. To investigate the interaction of covariates in relation to the association between SRMD and stroke, we have calculated the adjusted HR stratified by age (<45, 45–64, ≥65 years), sex and follow-up time. We have also measured and compared the cumulative incidence curves between SRMD and comparison cohorts by Kaplan–Meier method and tested the difference in curves by log-rank test. All statistical analyses were performed with SPSS software version 22.0. A two-tailed P values of <0.05 was considered as statistically significant.
Results
A total of 604 patients diagnosed with SRMD and 2416 age- and sex-matched controls for comparison were included in this cohort study. Demographic characteristics of both groups were presented in Table 1. There were no significant differences in distribution of age, sex and comorbidities between the study group with SRMD and the comparison cohort. The mean age was about 57 years and the majority of participants in two cohorts were male (56.29%).
Table 1.
Baseline demographic status and comorbidity compared between SRMD and comparison groups
| Variable | SRMD cohort | Comparison cohort | P-value |
|---|---|---|---|
| N = 604 (%) | N = 2,416 (%) | ||
| Age, years (SD)a | 57.11 (16.51) | 56.64 (15.87) | 0.779 |
| <45 | 216 (35.76) | 864 (35.76) | |
| 45-64 | 277 (45.86) | 1,108 (45.86) | |
| ≥65 | 111 (18.38) | 444 (18.38) | |
| Sex | 0.999 | ||
| Female | 264 (43.71) | 1,056 (43.71) | |
| Male | 340 (56.29) | 1,360 (56.29) | |
| Comorbidity | |||
| Hypertension | 155 (25.66) | 628 (25.99) | 0.868 |
| DM | 148 (24.50) | 600 (24.83) | 0.866 |
| IHD | 100 (16.56) | 395 (16.35) | 0.902 |
| Hyperlipidemia | 132 (21.85) | 509 (21.07) | 0.672 |
| AF | 12 (1.99) | 50 (2.07) | 0.898 |
| OSA | 68 (11.26) | 279 (11.55) | 0.842 |
at-test.
Our data showed that over 5-year follow-up, 11.6% of SRMD patients (n = 70) developed all-cause stroke with an overall rate of 31.31 cases per 1,000 person-years, of which 14 were hemorrhagic and 56 were ischemic stroke. In the comparison cohort, 87 non-SRMD individuals (3.6%) had stroke and 6 and 81 were hemorrhagic and ischemic respectively. The results revealed that the patients with SRMD had a 2.29 times (95% CI = 1.42–3.80, P < 0.001) higher risk to develop all-cause stroke compared with individuals without SRMD (Table 2). In order to explore whether the SRMD is an age-dependent risk factor for all-cause stroke, the patients were divided into 3 groups according to their age, namely <45, 45–64 and ≥65 years. The results showed that in comparison with the age/sex matched controls, the SRMD patients aged <45 years exhibited the highest risk of developing all-cause stroke (HR = 4.03, 95% CI = 3.11–5.62, P < 0.001), followed by the patients aged ≥65 years (HR = 2.64, 95% CI = 1.12–3.44, P < 0.001) and 45–64 years (HR = 1.07, 95% CI = 1.02–1.71, P < 0.001). As the majority (81.5%) of the SRMD cohort has PLMD, supposedly the increased risk of stroke is mainly ascribed to the PLMD component of the disease, rather than the RLS component.
Table 2.
Incidence of stroke and stroke subtype and multivariate Cox proportional hazards regression analysis measured hazard ratio for study cohort
| SRMD cohort |
Comparison cohort |
Crude HR (95% CI) | Adjusted HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Event | PYs | Rate | Event | PYs | Rate | ||
| All stroke | 70 | 2,236 | 31.31 | 87 | 10,332 | 8.42 | 5.02 (3.98–6.38)*** | 2.29 (1.42–3.80)*** |
| Hemorrhagic stroke | 14 | 2,236 | 6.26 | 6 | 10,332 | 0.58 | 6.76 (4.53–8.34)* | 4.25 (1.96–6.61)* |
| Ischemic stroke | 56 | 2,236 | 25.04 | 81 | 10,332 | 7.84 | 5.00 (4.31–5.61) ** | 2.04 (1.95–2.87)** |
| <45 years | ||||||||
| All stroke | 21 | 930 | 22.58 | 9 | 3,679 | 2.45 | 6.60 (6.12–7.80)*** | 4.03 (3.11–5.62)*** |
| Hemorrhagic stroke | 7 | 930 | 7.53 | 3 | 3,679 | 0.82 | 8.45 (7.14–8.69)** | 6.77 (4.88–7.64)* |
| Ischemic stroke | 14 | 930 | 15.05 | 6 | 3,679 | 1.63 | 6.57 (5.43–7.33)** | 3.92 (3.24–5.59)** |
| 45–64 years | ||||||||
| All stroke | 7 | 1,025 | 6.83 | 21 | 4,758 | 4.41 | 3.39 (3.70–4.46)*** | 1.07 (1.02–1.71)*** |
| Hemorrhagic stroke | 4 | 1,025 | 3.90 | 2 | 4,758 | 0.42 | 5.93 (3.27–7.54)** | 3.25 (1.11–4.87)* |
| Ischemic stroke | 3 | 1,025 | 2.93 | 19 | 4,758 | 3.99 | 3.07 (2.87–4.77)** | 1.04 (1.00–1.62)* |
| ≧65 years | ||||||||
| All stroke | 42 | 281 | 149.47 | 57 | 1,895 | 30.08 | 5.51 (3.30–6.21)*** | 2.64 (1.12–3.44)*** |
| Hemorrhagic stroke | 3 | 281 | 10.68 | 1 | 1,895 | 0.53 | 6.84 (4.37–9.01)*** | 4.27 (2.56–6.36)** |
| Ischemic stroke | 39 | 281 | 138.79 | 56 | 1,895 | 29.55 | 5.32 (4.75–6.00)*** | 2.11 (1.70–3.05)** |
| Male | ||||||||
| All stroke | 35 | 1,261 | 27.76 | 36 | 5,670 | 6.35 | 5.61 (4.21–6.86)*** | 2.98 (1.74–4.50)*** |
| Hemorrhagic stroke | 7 | 1,261 | 5.55 | 3 | 5,670 | 0.53 | 7.68 (4.07–8.49)** | 5.55 (2.10–6.82)* |
| Ischemic stroke | 28 | 1,261 | 22.20 | 33 | 5,670 | 5.82 | 5.51 (4.81–5.77)** | 2.74 (2.15–3.28)* |
| Female | ||||||||
| All stroke | 35 | 975 | 35.90 | 51 | 4,662 | 10.94 | 4.53 (2.46–19.42)*** | 1.94 (1.01–3.77)** |
| Hemorrhagic stroke | 7 | 975 | 7.18 | 3 | 4,662 | 0.64 | 6.38 (4.25–8.21)** | 3.69 (1.98–6.00)* |
| Ischemic stroke | 28 | 975 | 28.72 | 48 | 4,662 | 10.30 | 4.49 (3.15–5.06)*** | 1.82 (1.33–2.41)** |
Model adjusted for age, sex, hypertension, DM, IHD, hyperlipidemia, AF and OSA.
P < 0.05; **P < 0.01; ***P < 0.001.
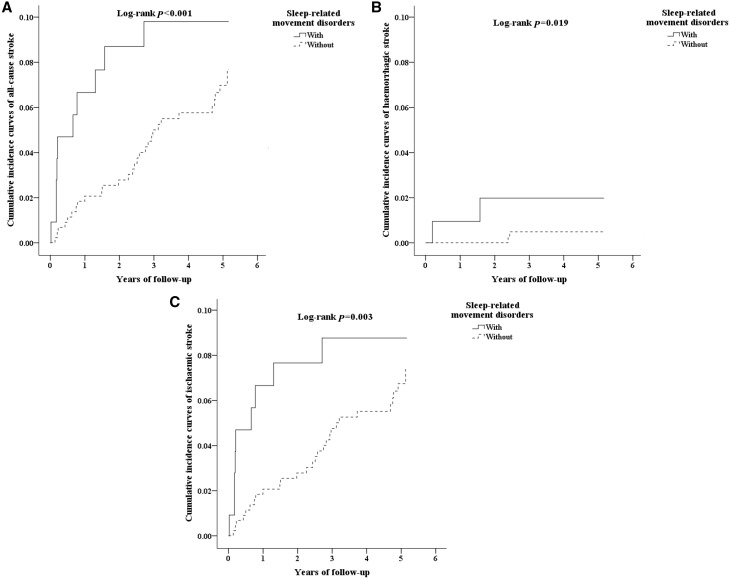
We have further examined if SRMD is a sex-dependent risk factor for developing stroke. The Cox regression analysis revealed that the increased risk of all-cause stroke in SRMD males (HR = 2.98, 95% CI = 1.74–4.50, P < 0.001) was greater than that of females (HR = 1.94, 95% CI = 1.01–3.77, P < 0.001). Then, we have analyzed the incidence of stroke and the stroke subtypes by using the multivariate Cox proportional hazards regression analysis based on time intervals. Table 3 shows the impact of time on the risk of developing stroke. Patients with SRMD were most likely to develop all-cause stroke within one year (stroke of any type, HR = 3.01, 95% CI = 1.49–4.56, P < 0.001; hemorrhagic stroke, HR = 4.38, 95% CI = 2.80–5.07, P < 0.05; ischemic stroke, HR = 2.98, 95% CI = 2.01–4.79, P < 0.01) after diagnosis with time-dependent decline characteristic (Table 3). However, the increased risk of hemorrhagic stroke in the second follow-up year (HR = 4.44, 95% CI = 2.76–5.33, P < 0.05) was even higher than the first one. Further, the Kaplan–Meier analysis showed that, compared to the matched controls, the patients with SRMD had significantly higher incidence of all-cause stroke (log-rank test P < 0.001, Figure 2A), hemorrhagic stroke (log-rank test P = 0.019, Figure 2B) and ischemic stroke (log-rank test P = 0.003, Figure 2C).
Table 3.
Incidence of stroke and stroke subtype and multivariate Cox proportional hazards regression analysis measured hazard ratio for study cohort by various time intervals
| SRMD cohort |
Comparison cohort |
Crude HR (95% CI) | Adjusted HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Event | PYs | Rate | Event | PYs | Rate | ||
| Follow <1 year | ||||||||
| All stroke | 45 | 123 | 365.85 | 27 | 229 | 117.90 | 4.77 (3.81–5.48)*** | 3.01 (1.49-4.56)*** |
| Hemorrhagic stroke | 6 | 123 | 48.78 | 2 | 229 | 8.73 | 5.73 (4.63–6.99)** | 4.38 (2.80–5.07)* |
| Ischemic stroke | 39 | 123 | 317.07 | 25 | 229 | 109.17 | 4.41 (3.94–5.01)** | 2.98 (2.01–4.79)** |
| Follow ≧1, <2 years | ||||||||
| All stroke | 14 | 175 | 80.00 | 17 | 297 | 57.24 | 4.12 (3.37–4.68)*** | 2.25 (1.31–2.97)*** |
| Hemorrhagic stroke | 5 | 175 | 28.57 | 2 | 297 | 6.73 | 5.71 (4.06–6.81)** | 4.44 (2.76–5.33)* |
| Ischemic stroke | 9 | 175 | 51.43 | 15 | 297 | 50.51 | 3.98 (2.76–4.46)* | 2.04 (1.48–3.07)* |
| Follow ≧2 years | ||||||||
| All stroke | 11 | 1,938 | 5.68 | 43 | 9,806 | 4.39 | 3.79 (2.84–4.23)*** | 1.87 (1.11–2.69)*** |
| Hemorrhagic stroke | 3 | 1,938 | 1.55 | 2 | 9,806 | 0.20 | 5.30 (3.80–6.04)* | 3.77 (1.85–5.32)* |
| Ischemic stroke | 8 | 1,938 | 4.13 | 41 | 9,806 | 4.18 | 3.50 (2.51–4.02)* | 1.59 (1.08-2.81)* |
Model adjusted for age, sex, hypertension, DM, IHD, hyperlipidemia, AF and OSA.
P < 0.05; **P < 0.01; ***P < 0.001.
Figure 2.
(A) The cumulative incidence curves of all-cause stroke for the individual with and without sleep-related movement disorders. (B) The cumulative incidence curves of hemorrhagic stroke for the individual with and without sleep-related movement disorders. (C) The cumulative incidence curves of ischemic stroke for the individual with and without sleep-related movement disorders.
The demographic status and comorbidity among the younger age group (<45 years) with PLMD and RLS were shown in Supplementary Tables S1 and S2. A lower prevalence of all the comorbidities including hypertension, DM, IHD, AF and OSA among the 216 SRMD patients aged < 45 (Supplementary Tables S1) compared to the whole SRMD cohort (Table 1) may suggest that most cases in this age group of SRMD are primary. Interestingly, the prevalence of all the comorbidities did not show significant difference between PLMD and RLS groups of patients aged < 45, except for OSA which was not found in all RLS patients (Supplementary Table S2). Additionally, SRMD patients aged < 45 seemed to have the highest risk of either type of stroke two years after being diagnosed, but the event numbers were small (Supplementary Table S3). This is not consistent with the whole SRMD cohort in which patients were most likely to develop all-cause stroke within the first year after diagnosis (Table 3).
Discussion
In this population-based longitudinal study, we have found that the patients with SRMD had a significantly higher risk for developing all-cause stroke. In particular, the SRMD patients of below 45 years old were at the highest risk for developing either hemorrhagic or ischemic stroke than that of all other age groups. Among the all age groups, the age-stratified analysis suggests that the increased risk of hemorrhagic stroke is more significant than the ischemic stroke.
Since many evidences suggest that sleep disorders are associated with higher risk of cardiovascular events, including stroke, a recent prospective study with a population of relatively small scale revealed that the RLS can be a predictor of subcortical stroke.13 Those patients with first-ever stroke were asked to complete a sleep questionnaire and not all the diagnosis of RLS could be confirmed by previous medical records. The memory bias could therefore exist in those patients with brain insults. In contrast, the diagnosis of SRMD in the present study was made according to standard criteria in our nation-wide health care system,6,7 and the time interval between diagnosis making of SRMD and the subsequent stroke could be collected correctly. This can partially explain why the prevalence of SRMD in our population is lower than others. It suggests that the disease state of SRMD in our case group may be more severe than those who were evaluated by primary screening instruments or questionnaires in other studies. Moreover, the racial differences in the prevalence of PLMD between Caucasian and African Americans has been reported.19 The present study is the first one to present the prevalence of SRMD in a nation-wide population (in Taiwan) where Han Chinese accounts for about 98%.
Sleep quality has been linked to an increased risk of cardiovascular diseases.20–22 RLS and PLMD were shown to be associated with increased risk for cardiovascular disease in specified populations.23–25 The systemic manifestations of SRMD may also affect the cerebrovascular circulation. In this context, a recent prospective study illustrated that the duration of RLS was an independent predictor of the burden of silent cerebral small vessel disease, a risk factor for clinical stroke.26 The sympathetic hyperactivity associated with RLS may lead to non-dipping nocturnal blood pressure and atherosclerotic plaque rupture, leading to heart disease and stroke.10 In the present study, the age groups of <45 years and ≥ 65 years patients with SRMD have a higher risk of stroke than the group of 45–64 years, which suggests that the people of different ages are differentially influenced by SRMD. Young patients with cerebrovascular malformation, e.g. may be predisposed to a much higher risk of stroke if they have SRMD.27 Aged cerebral arteries may be affected more significantly by hemodynamic disturbance in SRMD patients older than 65 years.
Previous research showed that the prevalence of RLS was 25.3% amongst Taiwanese end-stage renal disease (ESRD) patients,28,29 significantly higher than the general population. RLS was demonstrated to be associated with cardio/cerebrovascular events and mortality in ESRD, regarding severity of RLS.30 It may therefore suggest that ESRD patients have a higher risk of stroke when they developed SRMD. Moreover, the patients with iron-deficiency anemia (IDA) are associated with an increased risk of RLS and the response rate to intravenous iron supplement reached 76%.31 A population-based study showed a positive correlation between ischemic stroke and IDA.32 A future study is required to demonstrate if IDA patients have a further higher risk of stroke when they develop SRMD.
Limitations
There are few limitations in this study. First of all, neither the different severities of SRMD nor the health information such as intensity of tobacco use, alcohol consumption, exercise habits nor diet that may influence the risk of SRMD and stroke were provided by the database. Secondly, the data may also include unidentified recurrent patients who may have had SRMD and stroke before 1996 when NHI was started.33 Nevertheless, we only included the patients with new incidences of SRMD and stroke in order to increase the accuracy of the diagnosis of SRMD and stroke. Although our study depended on ICD codes, which may be different from other studies, the NHI administration in Taiwan has a cross-checking system to evaluate the precision of records from different hospitals.34 The last, in some categories, the number of stroke outcomes, particularly for hemorrhagic strokes was small.
Conclusions
The SRMD were linked to an increased risk of all-cause stroke. The increased risk of hemorrhagic stroke is more significant than ischemic stroke in all the age groups. The SRMD patients below the age of 45 years old were at the highest risk of developing either hemorrhagic or ischemic stroke. Future intervention studies are required to investigate whether stroke risks can be reduced by providing effective treatments for SRMD patients, especially for young people.
Conflict of interest: None declared.
Supplementary Material
Acknowledgements
This work was supported by grants from the Tri-Service General Hospital (TSGH-C104-084; TSGH-C105-084; TSGH-C100-101; TSGH-C101-080; TSGH-C103-085; TSGH-C104-083; TSGH-C105-085; TSGH-C106-007-S05), Ministry of Science and Technology (MOST104-2314-B-016-017-MY3), Teh-Tzer Study Group for Human Medical Research Foundation (A1031031). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Supplementary material
Supplementary material is available at QJMED online.
References
- 1. Mims KN, Kirsch D.. Sleep and stroke. Sleep Med Clinic 2016; 11:39–51. [DOI] [PubMed] [Google Scholar]
- 2. Kwon Y, Duprez DA, Jacobs DR, Nagayoshi M, McClelland RL, Shahar E, et al. Obstructive sleep apnea and progression of coronary artery calcium: the multi-ethnic study of atherosclerosis study. J Am Heart Assoc 2014; 3:e001241.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stanke-Labesque F, Pepin JL, Gautier-Veyret E, Levy P, Back M.. Leukotrienes as a molecular link between obstructive sleep apnoea and atherosclerosis. Cardiovasc Res 2014; 101:187–93. [DOI] [PubMed] [Google Scholar]
- 4. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008; 118:1080–111. [DOI] [PubMed] [Google Scholar]
- 5. Williams SK, Ravenell J, Jean-Louis G, Zizi F, Underberg JA, McFarlane SI, et al. Resistant hypertension and sleep apnea: pathophysiologic insights and strategic management. Curr Diabetes Reports 2011; 11:64–9. [DOI] [PubMed] [Google Scholar]
- 6. Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med 2014; 15:860–73. [DOI] [PubMed] [Google Scholar]
- 7. Littner MR, Kushida C, Anderson WM, Bailey D, Berry RB, Hirshkowitz M, et al. Practice parameters for the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep 2004; 27:557–9. [DOI] [PubMed] [Google Scholar]
- 8. Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P.. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord 1997; 12:61–5. [DOI] [PubMed] [Google Scholar]
- 9. Ohayon MM, Roth T.. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res 2002; 53:547–54. [DOI] [PubMed] [Google Scholar]
- 10. Walters AS, Rye DB.. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep 2009; 32:589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson KN, Bhatia KP, Losseff NA.. A case of restless legs syndrome in association with stroke. Sleep 2005; 28:147–8. [PubMed] [Google Scholar]
- 12. Walters AS, Moussouttas M, Siddiqui F, Silveira DC, Fuentes K, Wang L, et al. Prevalence of stroke in restless legs syndrome: initial results point to the need for more sophisticated studies. Open Neurol J 2010; 4:73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta A, Shukla G, Mohammed A, Goyal V, Behari M.. Restless legs syndrome, a predictor of subcortical stroke: a prospective study in 346 stroke patients. Sleep Med 2015. [DOI] [PubMed] [Google Scholar]
- 14. Sechi G, Agnetti V, Galistu P, Murgia B, Marrosu F, Puligheddu M, et al. Restless legs syndrome and periodic limb movements after ischemic stroke in the right lenticulostriate region. Parkinsonism Related Disord 2008; 14:157–60. [DOI] [PubMed] [Google Scholar]
- 15. Kim JS, Lee SB, Park SK, Han SR, Kim YI, Lee KS.. Periodic limb movement during sleep developed after pontine lesion. Mov Disord 2003; 18:1403–5. [DOI] [PubMed] [Google Scholar]
- 16. Unrath A, Kassubek J.. Symptomatic restless leg syndrome after lacunar stroke: a lesion study. Mov Disord 2006; 21:2027–8. [DOI] [PubMed] [Google Scholar]
- 17. Whisnant JP. Modeling of risk factors for ischemic stroke. The Willis Lecture. Stroke 1997; 28:1840–4. [DOI] [PubMed] [Google Scholar]
- 18. Donnan GA, Fisher M, Macleod M, Davis SM.. Stroke. Lancet 2008; 371:1612–23. [DOI] [PubMed] [Google Scholar]
- 19. Scofield H, Roth T, Drake C.. Periodic limb movements during sleep: population prevalence, clinical correlates, and racial differences. Sleep 2008; 31:1221–7. [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson CL, Redline S, Emmons KM.. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health 2015; 36:417–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Osonoi Y, Mita T, Osonoi T, Saito M, Tamasawa A, Nakayama S, et al. Poor sleep quality is associated with increased arterial stiffness in Japanese patients with type 2 diabetes mellitus. BMC Endocr Disord 2015; 15:29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Del Brutto OH, Mera RM, Zambrano M, Del Brutto VJ, Castillo PR.. Association between sleep quality and cardiovascular health: a door-to-door survey in rural Ecuador. Environ Health Prev Med 2014; 19:234–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cuellar NG. The effects of periodic limb movements in sleep (PLMS) on cardiovascular disease. Heart & Lung 2013; 42:353–60. [DOI] [PubMed] [Google Scholar]
- 24. Koo BB, Blackwell T, Ancoli-Israel S, Stone KL, Stefanick ML, Redline S, et al. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation 2011; 124:1223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trenkwalder C, Allen R, Hogl B, Paulus W, Winkelmann J.. Restless legs syndrome associated with major diseases: A systematic review and new concept. Neurology 2016; 86:1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferri R, Cosentino FI, Moussouttas M, Lanuzza B, Arico D, Bagai K, et al. et al. Silent cerebral small vessel disease in restless legs syndrome. Sleep 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehanna R, Jankovic J.. Movement disorders in cerebrovascular disease. Lancet Neurol 2013; 12:597–608. [DOI] [PubMed] [Google Scholar]
- 28. Lin CH, Chen ML, Wu VC, Li WY, Sy HN, Wu SL, et al. Association of candidate genetic variants with restless legs syndrome in end stage renal disease: a multicenter case-control study in Taiwan. Eur J Neurol 2014; 21:492–8. [DOI] [PubMed] [Google Scholar]
- 29. Lin CH, Wu VC, Li WY, Sy HN, Wu SL, Chang CC, et al. Restless legs syndrome in end-stage renal disease: a multicenter study in Taiwan. Eur J Neurol 2013; 20:1025–31. [DOI] [PubMed] [Google Scholar]
- 30. Lin CH, Sy HN, Chang HW, Liou HH, Lin CY, Wu VC, et al. Restless legs syndrome is associated with cardio/cerebrovascular events and mortality in end-stage renal disease. Eur J Neurol 2015; 22:142–9. [DOI] [PubMed] [Google Scholar]
- 31. Mehmood T, Auerbach M, Earley CJ, Allen RP.. Response to intravenous iron in patients with iron deficiency anemia (IDA) and restless leg syndrome (Willis-Ekbom disease). Sleep Med 2014; 15:1473–6. [DOI] [PubMed] [Google Scholar]
- 32. Chang YL, Hung SH, Ling W, Lin HC, Li HC, Chung SD.. Association between ischemic stroke and iron-deficiency anemia: a population-based study. PloS One 2013; 8:e82952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng TM. Reflections on the 20th anniversary of Taiwan's single-payer National Health Insurance System. Health Affairs 2015; 34:502–10. [DOI] [PubMed] [Google Scholar]
- 34. Huang SK, Tsai SL, Hsu MT.. Ensuring the sustainability of the Taiwan National Health Insurance. J Formosan Med Assoc 2014; 113:1–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.