Abstract
The S-locus-encoded S receptor kinase (SRK) is an intrinsic plasma membrane protein that is viewed as the primary stigma determinant of specificity in the self-incompatibility response of Brassica spp. We analyzed two self-compatible mutant strains that express low levels of the S-locus glycoprotein (SLG), a cell wall-localized protein also encoded at the S locus that is coordinately expressed with SRK. We found that mutant stigmas synthesized wild-type levels of SRK transcripts but failed to produce SRK protein at any of the developmental stages analyzed. Furthermore, SRK was shown to form aberrant high-molecular mass aggregates when expressed alone in transgenic tobacco (Nicotiana tabacum) plants. This aggregation was prevented in tobacco plants that co-expressed SRK and SLG, but not in tobacco plants that co-expressed SRK and SLR1, an SLG-related secreted protein not encoded at the S locus. In analyses of protein extracts under reducing and non-reducing conditions, evidence of intermolecular association was obtained only for SLG, a fraction of which formed disulfide-linked oligomers and was membrane associated. The data indicate that, at least in plants carrying the S haplotypes we analyzed, SRK is an inherently unstable protein and that SLG facilitates its accumulation to physiologically relevant levels in Brassica stigmas.
Plants possess a large number of genes encoding transmembrane receptor-like protein kinases (Stone and Walker, 1995; Becraft, 1998; Hardie, 1999). These genes can be classified into distinct families on the basis of the sequence of their predicted extracellular domain (Becraft, 1998; Hardie, 1999) and are thought to play important roles in a variety of biological processes in view of the diverse expression patterns exhibited by their transcripts. However, a biological function is actually known for only a few of these genes, and attempts to identify the receptor protein and investigate its biochemical properties have been made for an even smaller subset of the genes. Among these are the Brassica S-locus receptor kinase (SRK) (Stein et al., 1991), which functions in the self-incompatibility response, and the Arabidopsis CLAVATA1 (CLV1) protein, which is required for normal development of the shoot meristem (Clark et al., 1997). SRK, a member of the S gene family, which is characterized by an “S” domain containing a conserved array of Cys residues, has been shown to be an integral membrane protein in Brassica stigmas (Delorme et al., 1995; Stein et al., 1996), to be targeted to the plasma membrane when expressed in transgenic tobacco (Nicotiana tabacum) plants (Stein et al., 1996), and as predicted from its sequence, to be oriented in the plasma membrane with its “S” domain to the outside of the cell (Letham et al., 1999). CLV1, the predicted extracellular domain of which contains Leu-rich repeats, has been shown to occur in complexes with other proteins in vivo (Trotochaud et al., 1999) and to require CLV2 for its stability (Jeong et al., 1999). CLV2 is predicted to be a transmembrane protein with an extracellular domain containing Leu-rich repeats and a very short cytoplasmic domain lacking a kinase domain (Jeong et al., 1999).
The Brassica self-incompatibility response prevents the development of genetically related pollen on the epidermal (papillar) cells of the stigma (for review, see Nasrallah and Nasrallah, 1993; Nasrallah et al., 1994a). This response is controlled genetically by haplotypes of the S locus, and a self-incompatibility (SI) response is instigated if the pollen and pistil are derived from plants sharing an identical S haplotype. Recent work has demonstrated that specificity in the SI response is determined by two highly polymorphic proteins encoded by the S locus: the S receptor kinase discussed above determines SI specificity in the stigma (Takasaki et al., 2000), and the SCR (S-locus Cys-rich) protein, a small highly polymorphic Cys-rich protein expressed specifically in anthers and proposed to be a ligand for SRK, is necessary and sufficient for SI specificity in pollen (Schopfer et al., 1999).
In addition to SRK and SCR, the S locus encodes a third protein, the S-locus glycoprotein (SLG). SLG shares a high degree of sequence similarity with the SRK ectodomain (Nasrallah et al., 1987; Stein et al., 1991; Kusaba et al., 1997), is expressed specifically and coordinately with SRK in stigmatic papillar cells (Stein et al., 1996), and accumulates in the papillar cell walls to high levels (Kandasamy et al., 1989), often reaching a 100-fold excess over SRK. However, the role of SLG is not understood. Its requirement for SI has been questioned on the basis that self-incompatible plants homozygous for some S haplotypes express low levels of SLG (Tantikanjana et al., 1993, 1996; Gaude et al., 1995), that an S haplotype seems to lack an SLG gene (Okazaki et al., 1999), and that sequence analysis of some SLG/SRK gene pairs reveals a more robust correlation between sequence divergence and SI specificity for SRK than for SLG (Kusaba and Nishio, 1999; Kusaba et al., 2000). Nevertheless, it remains possible that SLG performs another function in SI. Such a role is suggested from the fact that the majority of Brassica S haplotypes analyzed contains a highly expressed SLG gene and that this gene also occurs in self-incompatible strains of Raphanus (Sakamoto et al., 1998) and thus has persisted through events of speciation. Furthermore, transgenic plants that express both SLG and SRK exhibit an enhanced SI response relative to transgenic plants that express SRK alone (Takasaki et al., 2000).
We have been analyzing the expression of SRK/SLG transcripts and proteins in Brassica mutant strains that exhibit a stigma-specific breakdown of SI to elucidate properties of the SRK receptor and define parameters required for its proper maturation and function. In this paper, we report on our analysis of two mutant strains bearing defects in the structure or expression of the SLG gene. We show that SRK does not accumulate in stigma cells when SLG expression is dramatically reduced, providing a biochemical basis for the requirement of SLG in SI. Together with results of expression studies in transgenic tobacco plants, our data reveal that the SRK isoforms we analyzed require accessory molecules for their accumulation and proper maturation. Thus, these isoforms may be inherently unstable, as has been demonstrated for CLV1 (Jeong et al., 1999) as well as for many of the receptors and other intrinsic membrane proteins analyzed in animal systems (Yoshimura et al., 1990; Ward and Kopito, 1994; Centrella et al., 1996). The requirement of molecules related to the receptor extracellular domain may represent a common mechanism for the proper maturation and accumulation of plant receptor protein kinases.
RESULTS
Analysis of Self-Compatible Mutant Brassica Strains
Two self-compatible (SC) mutant Brassica strains that exhibit defects in the structure or expression of SLG were used in this study: (a) Brassica campestris (syn. B. rapa) strain homozygous for scf1, a recessive mutation at a trans-acting locus unlinked to the S locus that leads to a dramatic reduction in the levels of SLG transcripts and transcripts encoded by two other stigma-specific members of the S gene family, but does not affect the levels of SRK transcripts (Nasrallah et al., 1992) and (b) a B. oleracea mutant designated ΔS-1668 that carries a deletion encompassing the SLG gene. This mutant was identified in a screen of F1 plants generated by using γ-irradiated pollen from a self-incompatible S13S13 plant to pollinate stigmas from plants homozygous for the Sf1 haplotype and selecting for mutant self-compatible plants in the otherwise self-incompatible F1 generation (Nasrallah et al., 2000). The Sf1 haplotype is a naturally occurring non-functional (self-fertile) haplotype that carries a null SRK allele and as such does not encode SRK protein, but it does contain a functional SLG gene and thus encodes SLG protein (Nasrallah et al., 1994b). Previous DNA gel-blot analysis of the ΔS-1668 strain had shown that it carries a mutant S13 haplotype in which all but 500 bp at the 5′ end of the SLG13 gene was deleted but which retained an intact SRK13 gene (Boyes et al., 1997). Thus, this strain is expected to express transcripts and proteins derived from SRK13 but to lack the 1.6-kb transcripts derived from SLG13. ΔS-1668 also produces SLGf1 protein but not SRKf1 protein due to the presence of the Sf1 haplotype.
We had previously reported that the scf1 mutation causes the stigma to be receptive to self-pollen but does not affect the pollination phenotype of the male gametophyte (Nasrallah et al., 1992). Pollination analysis revealed that ΔS-1668 is a highly self-fertile strain, routinely producing >300 pollen tubes/stigma upon self-pollination. Furthermore, ΔS-1668 stigmas were fully compatible with pollen derived from plants bearing the S13 haplotype (>300 pollen tubes produced/stigma), in contrast to wild-type S13Sf1 and S13S13 stigmas, which inhibited the development of S13-derived pollen. However, ΔS-1668 pollen failed to germinate on stigmas carrying the S13 haplotype, an incompatible reaction identical to that exhibited by pollen from wild-type S13S13 and S13Sf1 plants. Thus, DNA encompassed by the deletion in ΔS-1668 is required for SI in the stigma but not in pollen.
Analysis of SRK Transcripts and Proteins in SLG-Deficient Mutants
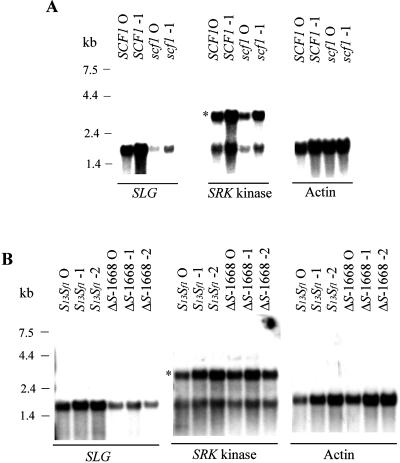
We performed RNA gel-blot analysis of wild-type and mutant stigmas at different stages of development to determine if the levels and developmental regulation of SRK transcripts were similar in wild-type and mutant plants. As illustrated in Figure 1A, scf1 stigmas from open flowers and from buds at 1 d prior to anthesis (−1 stage) exhibited a depletion of the 1.6-kb SLG transcripts relative to wild-type SCF1 controls (Fig. 1A, left panel). In contrast, the levels of the 3.0-kb SRK transcripts were comparable between the mutant and wild-type stigmas (Fig. 1A, center panel). Similarly, ΔS-1668 stigmas, which are null for SLG13 (and only produced a low level of SLGf1 transcripts) accumulated SRK13 transcripts to levels indistinguishable from control S13Sf1 stigmas (Fig. 1B, center panel). The genotype and S transcript species produced by both wild-type and mutant strains used in this study are shown in Table I.
Figure 1.
RNA gel-blot analysis of Brassica strains. Poly(A+) RNA was prepared from stigmas isolated from wild-type SCF1 and mutant scf1 homozygotes (A) and wild-type S13Sf1 and mutant ΔS-1668 plants (B) (approximately 3 μg of RNA per lane for both experiments). The blots were hybridized sequentially with a DNA probe specific for SLG (derived from the 3′-untranslated region of SLG) and a probe corresponding to the kinase domain of SRK, followed by a Brassica actin probe to confirm equal loading of RNA. The developmental stage of isolated stigmas is indicated above the lanes as 0 (open flowers at anthesis) or as negative numbers corresponding to days before anthesis. The asterisk in the center panel indicates the position of the 3.0-kb SRK transcripts. The 1.5-kb band detected with the SRK probe probably corresponds to a related kinase gene. Molecular length markers in kb are indicated to the left.
Table I.
Wild-type and mutant Brassica strains analyzed in this paper
| Strain (Phenotype) | S Haplotype | Transcript Species |
|---|---|---|
| SCF1SCF1 (SI) | S8S8 | Wild-type levels of SRK8, SLG8, SLR1, and SLR2 transcripts |
| scf1scf1 (SC) | S8S8 | Wild-type levels of SRK8 transcripts; low levels of SLG8, SLR1, and SLR2 transcripts |
| S13Sf1 (SI) | S13Sf1 | Wild-type levels of SRK13, SLG13, SLGf1, SLR1, and SLR2 transcripts; no SRKf1 transcripts |
| ΔS-1668 (SC) | S13ΔSf1 | Wild-type levels of SRK13, SLGf1, SLR1, and SLR2 transcripts; no SRKf1 and SLG13 transcripts |
| ΔS-55 (SC) | S13ΔSf1 | Wild-type levels of SLGf1, SLR1, and SLR2 transcripts; no SRKf1, SRK13, and SLG13 transcripts |
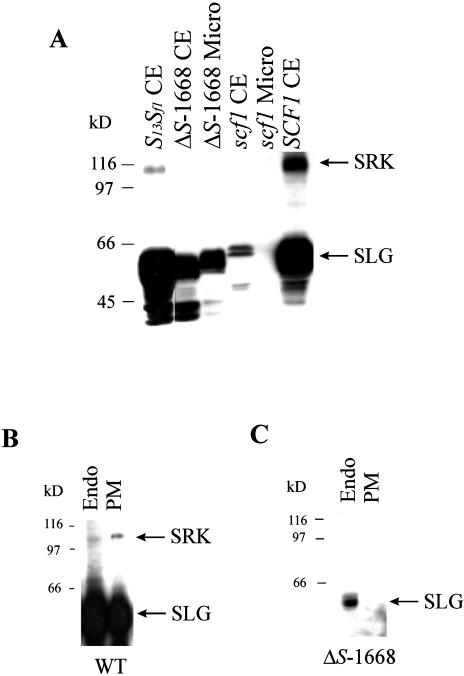
To determine if attenuation of SLG transcripts (and thus SLG protein) in the mutant strains affected the levels of SRK protein, we performed protein immunoblot analysis of open flower stigmas from wild-type and mutant plants using monoclonal antibody MAb/H8. We have previously demonstrated that MAb/H8 detects SRK as a discrete band of approximately 108 kD and SLG as a cluster of glycoforms in the size range of approximately 40 to 65 kD (Stein et al., 1996). Figure 2A shows that the approximately 108-kD SRK protein, which is clearly visible in whole cell extracts of S13Sf1 and SCF1 control stigmas, was undetectable in stigmas of the ΔS-1668 and scf1 mutants, either in whole cell extracts or in microsome fractions. Furthermore, whereas SRK is enriched in plasma membrane fractions obtained from wild-type self-incompatible stigmas (Fig. 2B), it remains undetectable in plasma membrane fractions purified from mutant stigmas (as shown for ΔS-1668 in Fig. 2C). The low level of SRK visible in the endosome fraction in Figure 2B is probably reflective of its presence in the secretory pathway in transit to the plasma membrane.
Figure 2.
Immunoblot analysis of SLG and SRK in Brassica stigmas. A, Whole cell extracts (CE) or microsome fractions (Micro) prepared from S13Sf1, ΔS-1668, scf1scf1, and SCF1SCF1 stigmas were subjected to immunoblot analysis using MAb/H8. Each lane contains 50 μg of protein. B and C, Microsome fractions isolated from wild-type (WT) stigmas (B) and from ΔS-1668 stigmas (C) were partitioned into endomembrane-enriched (Endo) and plasma membrane-enriched (PM) fractions and the blot probed with MAb/H8. Each lane contains 10 μg of protein. SRK is detected in wild-type but not in mutant stigma extracts. SLG observed in ΔS-1668 stigmas is the product of the SLGf1 gene. The lower level of SRK in S13Sf1 plants is due to the presence of only one functional copy of the SRK gene. Molecular mass markers in kD are indicated to the left of each panel.
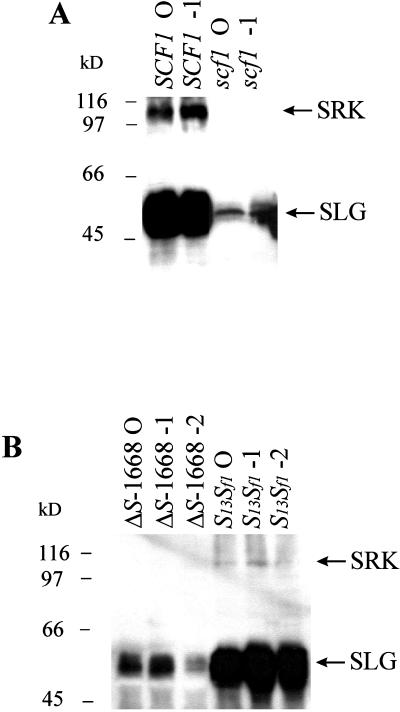
Instability of membrane proteins has been shown to be developmentally regulated (Kearse et al., 1994). To test if SRK is initially expressed in mutant stigmas at early stages of flower bud development and subsequently degraded as the stigmas mature, we performed immunoblot analysis of stigmas at various maturation phases. SRK was undetectable in scf1 stigmas (Fig. 3A) and ΔS-1668 stigmas (Fig. 3B) at all developmental stages tested, which is in contrast to wild-type stigmas in which SRK was detected throughout development. The consistent correlation we observed between the diminished levels of SLG protein and the absence of detectable SRK protein despite the presence of wild-type levels of SRK transcripts indicates that post-transcriptional processes regulate SRK accumulation in Brassica stigmas.
Figure 3.
Developmental analysis of SLG and SRK in Brassica stigmas. Microsome fractions (40 μg of protein in each lane) isolated from stigmas of wild-type SCF1 and mutant scf1 homozygotes (A) and wild-type S13Sf1 and mutant ΔS-1668 plants (B) at various developmental stages were subjected to immunoblot analysis using MAb/H8. Developmental stage designation is as in Figure 1.
Formation of Disulfide-Linked Oligomers of S-Locus Glycoprotein in the Stigma
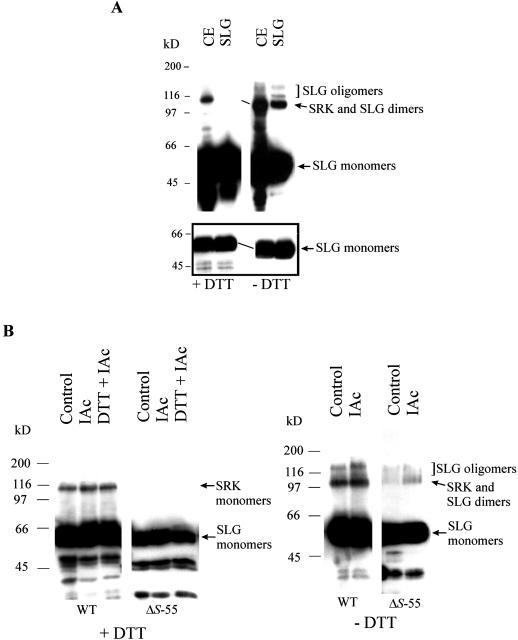
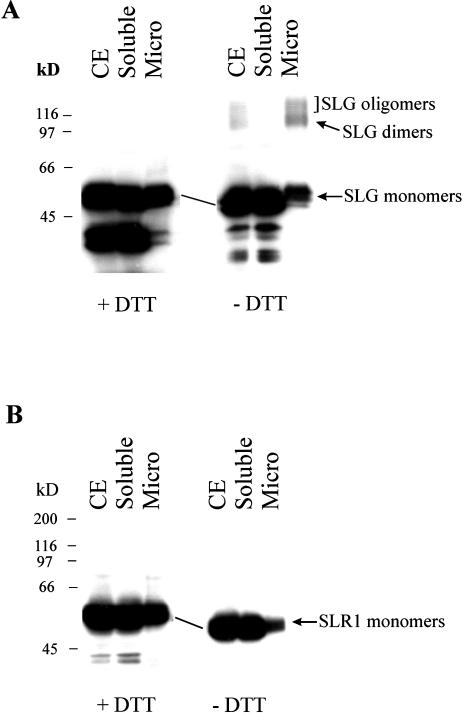
The observation that SRK does not accumulate in the absence of SLG is suggestive of an interaction between the two proteins. To test the possibility that such an interaction might occur through the formation of inter-molecular disulfide bonds, stigma cell extracts were subjected to SDS-PAGE analysis under reducing (+dithiothreitol [DTT]) and non-reducing (−DTT) conditions, followed by immunoblot analysis. As shown in Figure 4A, the apparent molecular mass of both SLG and SRK observed under non-reducing conditions was decreased by approximately 5 to 10 kD relative to that observed under reducing conditions. In addition, we observed a significant difference in electrophoretic mobility between reduced and alkylated SLG relative to unreduced and alkylated SLG using acid-urea gel electrophoresis (data not shown). Both electrophoretic properties are indicative of intra-molecular disulfide bonding (Hollecker, 1997). This occurrence of intra-molecular disulfide bonds appears to be a general feature of proteins within the S-gene family. Similar electrophoretic shifts were also noted for SLG and SRK from the S8, S13, and S22 haplotypes (data not shown) as well as for the S-locus related SLR1 glycoprotein (see below), a molecule that is expressed specifically in papillar cells and accumulates to high levels in the cell wall like SLG but is encoded by a gene unlinked to the S locus (Umbach et al., 1990).
Figure 4.
Analysis of stigma proteins under reducing and non-reducing conditions. Stigma proteins were separated by SDS-PAGE under reducing (+DTT) or non-reducing (−DTT) conditions and subjected to immunoblot analysis using MAb/H8. A, Wild-type stigma whole cell extract (25 μg, CE) and isoelectric focusing-purified SLG (2 μg, SLG). The box shows the SLG signal after a short exposure of the immunoblot to x-ray film. The oblique lines indicate the observed differences in mobility of SRK and SLG under reducing and non-reducing conditions. B, Whole cell extracts obtained from wild-type (WT; 20 μg of protein) or ΔS-55 (50 μg of protein) stigmas treated with buffer alone (Control), buffer with 100 mm IAc, or buffer with 50 mm DTT and 100 mm IAc (DTT + IAc).
It is interesting that there was a significant enhancement of the SRK-containing 108-kD band in wild-type stigma extracts run in the absence of DTT (Fig. 4A). This enhancement could result from the formation of SLG oligomers, possibly homodimers, which would be expected to migrate at approximately the same position as SRK. Indeed, SLG fractions isolated by preparative isoelectric focusing and shown to be free of contaminating proteins by silver staining (see “Materials and Methods”) were also found to contain an approximately 108-kD species upon electrophoresis under non-reducing conditions (Fig. 4A). These results strongly suggest that the apparent enhancement in SRK signal under non-reducing conditions is due to the presence of SLG homodimers. However, neither this study nor another study that also suggested the occurrence of SLG dimers (Doughty et al., 1998) can categorically rule out the possibility that the approximately 108-kD SLG fraction represents heterodimers (or oligomers) between SLG and one or more unidentified stigma protein(s) with the same pI point and molecular mass as SLG.
In addition to the approximately 108-kD band, stigma whole cell extracts running under non-reducing conditions also contained minor bands that migrated with an apparent molecular mass of approximately 120 kD and approximately 140 kD (Fig. 4A). Because bands with the same mass were also observed in purified SLG fractions under non-reducing conditions (Fig. 4A), these bands likely represent higher-order SLG oligomers. It should be noted that we did not detect immunoreactive bands >200 kD in size that might represent SRK homodimers or SRK complexed with other as-yet-unidentified molecules, contrary to a recent study that reported the detection of >200-kD SRK species upon cross-linking of un-pollinated stigma extracts (Giranton et al., 2000). If such SRK complexes prove to be of general occurrence in Brassica strains, our inability to detect these complexes would suggest that their formation is not mediated by disulfide bridges.
To ascertain that disulfide bonds did not artificially arise during preparation of stigma extracts, Brassica stigmas were pretreated with a high concentration of iodoacetate (IAc) in the presence or absence of DTT. IAc quenches free sulfhydryl side chains on proteins (Hollecker, 1997) and will hence prevent their participation in disulfide bond formation during preparation of stigma extract. The analysis was carried out using wild-type stigmas as well as stigmas obtained from a deletion mutant designated ΔS-55. ΔS-55 is a self-compatible strain identified in the same screen as ΔS-1668. The ΔS-55 strain is deleted for both SLG13 and SRK13 (Nasrallah et al., 2000) and possesses the Sf1 haplotype with its non-functional SRKf1 gene and its functional SLGf1 gene (see Table I). This plant hence produces only SLGf1 protein and allows unambiguous characterization of SLG properties in native tissue.
Stigma proteins were either reduced and alkylated by immersion in buffer containing both DTT and IAc or alkylated in the absence of DTT by immersion in buffer containing IAc alone. Control stigmas were immersed in buffer containing no DTT or IAc for an identical time period. The results of this experiment are shown in Figure 4B. Stigma extracts in which proteins were reduced and alkylated by treatment of stigmas with DTT and IAc prior to extraction exhibited the expected electrophoretic patterns under reducing conditions: i.e. wild-type stigma extracts exhibited the approximately 108-kD SRK band and the cluster of SLG forms (Fig. 4B, +DTT lanes), and ΔS-55 stigma extracts exhibited SLG monomers (Fig. 4B, +DTT lanes). Pretreatment of wild-type or ΔS-55 mutant stigmas with high concentration of IAc did not prevent the approximately 5- to 10-kD shift in mobility of SLG and SRK observed under reducing versus non-reducing conditions (Fig. 4B, compare +DTT and −DTT lanes) and also did not prevent the formation of SLG oligomeric forms under non-reducing conditions (Fig. 4B, −DTT lanes). Hence, the observed disulfide-bonded forms of SLG do not result from oxidative processes induced during cell extraction.
Identification of a Membrane-Associated Fraction of SLG
Figure 4, A and B demonstrate that only a fraction of the SLG population is represented as oligomers. Such a result might arise if the disulfide bond-induced oligomerization is dynamic in nature or it may be indicative of a heterogeneous SLG population, only a subset of which is competent for oligomerization. Experiments testing the electrophoretic behavior of SLG in soluble or microsome fractions obtained from ΔS-55 stigmas under non-reducing conditions revealed that it is only the microsome fraction-associated SLG that is capable of forming disulfide bond-mediated oligomers (Fig. 5A). The result is all the more striking since the bulk of SLG is retained in the soluble fraction. Hence, SLG occurs as a heterogeneous population in Brassica stigmas: Only a subset of SLG glycoforms can associate with the microsome fraction and it is this subset that forms disulfide-linked oligomers. This property of SLG membrane retention was also observed for SLR1 (Fig. 5B) and as such may be a general feature of the “soluble” S-family proteins. However, the capacity to form inter-molecular disulfide bonds is perhaps distinctive of SLG since we have failed to detect oligomeric forms of SLR1 under non-reducing conditions (Fig. 5B).
Figure 5.
Oligomerization of membrane-associated SLG. Whole cell extract (CE), soluble, and microsome (Micro) fractions obtained from ΔS-55 stigmas were subjected to electrophoresis under reducing (+DTT) or non-reducing (−DTT) conditions followed by immunodetection using MAb/H8 (A) or anti-SLR1 serum (B). Each lane contains 100 μg of protein. The oblique lines indicate the observed differences in mobility of SLG and SLR1 under reducing and non-reducing conditions.
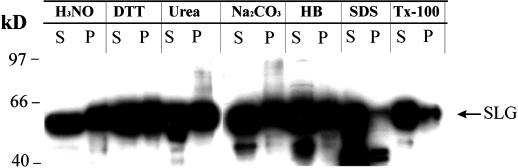
To determine the nature of the forces resulting in SLG-membrane association, we attempted to disrupt this association by treating stigma microsome fractions with various chemicals. The microsome fractions for this experiment were prepared using a relatively hypotonic buffer (lacking glycerol) to prevent the formation of intact membrane vesicles, which might trap proteins inside. As shown in Figure 6, all of the treatments resulted in some release of the membrane-associated SLG, but in most cases only to an extent equivalent to that achieved simply by re-extracting microsomes with the homogenization buffer (HB) (Fig. 6). However, significantly greater dissociation of SLG from the microsomes was achieved by treatment with detergents: SDS treatment resulted in near complete release of SLG from the membranes (Fig. 6). It should be noted that SLG membrane association is also insensitive to the inclusion of 50 mm DTT in the extraction buffer prior to stigma homogenization (data not shown) and hence the association of SLG with the membrane fraction is non-covalent in nature and is probably mediated by hydrophobic forces. Similar membrane associative properties have been described for animal Cys string proteins, which are predicted to be soluble proteins but nonetheless associate with cellular membranes via the Cys string domain (Mastrogiacomo et al., 1998).
Figure 6.
Chemical treatment of stigma microsome fractions. Equal amounts of microsome fraction obtained from Brassica stigmas were treated with 1 m H3NO, 50 mm DTT, 8 m urea, 0.1 m Na2CO3, 0.2% (w/v) SDS, 1% (v/v) Triton X-100 (Tx-100), or HB (see “Materials and Methods”) and centrifuged at 100,000g for 1 h to obtain supernatant (S) and pellet (P) fractions. The fractions were subjected to electrophoresis on a 10% (w/v) polyacrylamide gel and the immunoblot was probed with MAb/H8.
Co-Expression of SRK with SLG or SLR1 in Transgenic Tobacco Plants
To investigate further the effect of SLG on SRK accumulation, we used a heterologous tobacco expression system. We had previously shown that transformation of tobacco with chimeric genes consisting of the cauliflower mosaic virus (CaMV) 35S promoter fused to either SRK cDNA (Stein et al., 1996) or SLG cDNA (Perl-Treves et al., 1993) resulted in the production of SLG and SRK proteins that were indistinguishable from stigma-expressed proteins on reducing SDS-PAGE gels. Furthermore, heterologous expression studies are now recognized as an essential and convenient tool for the biochemical analysis of plant proteins (for review, see Frommer and Ninnemann, 1995) and have been performed using tobacco plants (Kaye et al., 1998; Veena Reddy and Sopory, 1999), as well as yeast (Chen and Halkier, 1999; Montamat et al., 1999), Xenopus (Cao et al., 1992; Maurel et al., 1993), and mammalian COS cells (Kammerloher et al., 1994). Most pertinent to this study, expression of storage proteins of the maize kernel in transgenic tobacco plants was used to demonstrate that β-zein expression has a stabilizing effect on δ-zein (Bagga et al., 1997).
Therefore we retransformed SRK6-expressing transgenic tobacco plants previously generated in our laboratory (Stein et al., 1996) with a chimeric gene consisting of the double CaMV 35S promoter fused to the coding region of SLG6, and generated 12 independent transformants (designated [SRK+SLG]) that expressed both SLG and SRK. Two classes of control transgenic plants were also generated: One class consisted of nine independent transgenic plants (designated [SRK]) expressing only SRK that were produced by retransforming the SRK-expressing plants with vector lacking the SLG transgene, and a second class comprised of nine independent transformants (designated [SLG]) expressing only SLG that were obtained by introducing the SLG transgene into tobacco plants containing vector lacking SRK6. In addition, the SRK6-expressing tobacco plants were retransformed with another chimeric gene construct consisting of the SLR1 cDNA inserted downstream of a double CaMV 35S promoter as a control for the specificity of any effect SLG might have on SRK properties. Four independent transformants that expressed SRK and SLR1 (designated [SRK+SLR1]) were obtained and used for the analyses.
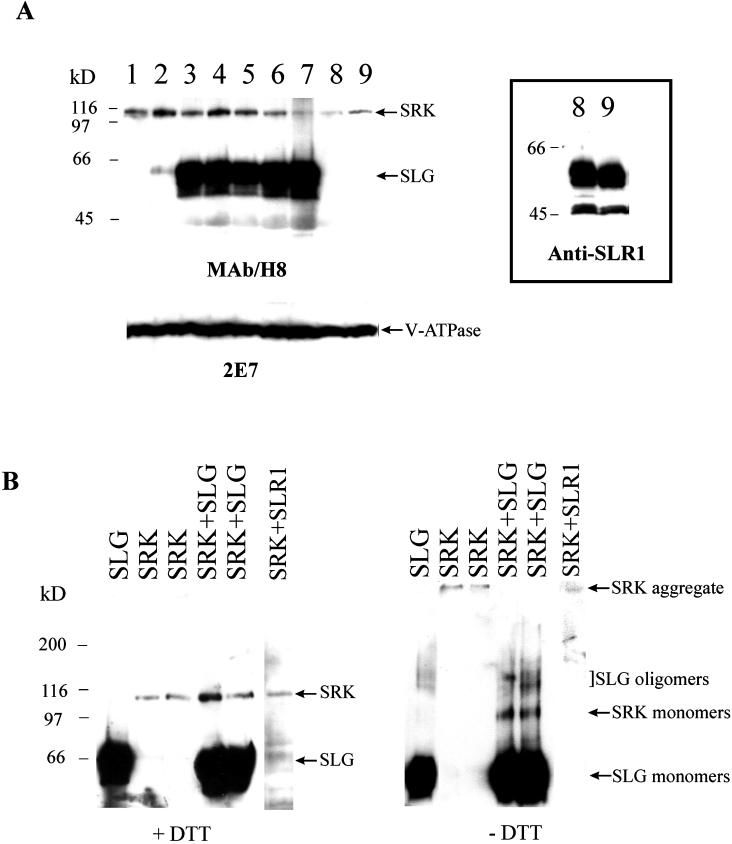
Microsome fractions obtained from all independent [SRK+SLG], [SRK], and [SRK+SLR1] transformants were subjected to immunoblot analysis with MAb/H8. As shown in Figure 7A (lanes 1–7), the [SRK+SLG] lines produced SRK at levels similar to those produced in the [SRK] transformants. They also produced high amounts of SLG, a significant fraction of which was associated with the microsome fraction as observed in Brassica stigmas (Fig. 5A). Similarly, the [SRK+SLR1] plants expressed SRK (Fig. 7A, lanes 8 and 9) as well as high amounts of SLR1, a fraction of which was also membrane associated (Fig. 7A, boxed panel).
Figure 7.
Immunoblot analysis of transgenic tobacco plants. A, Microsome fractions (150 μg of protein in each lane) were isolated from transgenic tobacco seedlings that express either SRK alone (lanes 1 and 2), both SRK and SLG (lanes 3–7), or both SRK and SLR1 (lanes 8 and 9). Each lane represents an independent transformant. The blot was sequentially probed with MAb/H8 to identify SLG and SRK (top panel) and with the anti-vacuolar H+-ATPase 2E7 antibody as a loading control (bottom panel). The boxed panel to the right shows samples from plants represented in lanes 8 and 9 probed with anti-SLR1 serum to demonstrate the accumulation of SLR1 protein. B, Microsome fractions (150 μg of protein in each lane) isolated from transgenic tobacco seedlings expressing SLG alone, SRK alone, SRK and SLG, or SRK and SLR1 were subjected to electrophoresis under reducing (+DTT) and non-reducing (−DTT) conditions followed by immunodetection with MAb/H8. The results shown are representative of each class of plants and each lane represents an independent transformant.
To determine if the SLG and SRK proteins produced in transgenic tobacco displayed electrophoretic properties similar to those observed in Brassica stigmas, microsome fractions isolated from the [SRK+SLG], [SRK], [SRK+SLR1], and [SLG] tobacco plants were tested by immunoblot analysis following SDS-PAGE under reducing and non-reducing conditions. As shown in Figure 7B (left panel), tobacco-expressed SLG and SRK migrated to their expected positions under reducing conditions. In addition, under non-reducing conditions, the SLG protein expressed in either the [SLG] or [SLG+SRK] plants exhibited the same approximately 5- to 10-kD difference in electrophoretic mobility relative to reduced SLG as observed in Brassica stigmas. Furthermore, a fraction of unreduced SLG migrated as bands of approximately 120 kD, which likely represent SLG oligomers similar to those observed in Brassica stigmas, because bands of similar size appear in extracts from tobacco plants expressing SLG alone (Fig. 7B, compare the lanes “SLG” and “SRK+SLG” in the right panel).
It is interesting that the electrophoretic behavior of tobacco-expressed SRK under non-reducing conditions is specifically modified when SRK is co-expressed with SLG. When [SRK] and [SRK+SLR1] extracts were analyzed under non-reducing conditions, SRK protein did not migrate to the expected position. Instead, under optimal protein-blotting conditions, SRK was detected as a very high molecular mass band at the top of the separating gel (Fig. 7B, the “SRK” and “SRK+SLR1” lanes in the right panel). This SRK band exceeds in mass that expected for SRK dimers and likely consists of multimeric aggregates of SRK. It is significant that no such SRK aggregates were detected in extracts of the [SRK+SLG] plants (Fig. 7B, the “SRK+SLG” lanes in the right panel); rather, in these extracts, the mobility of SRK was restored to that exhibited by stigma SRK run under non-reducing conditions. It should be noted that we have never detected SRK aggregates in non-reduced extracts of wild-type Brassica stigmas that express substantial levels of SLG.
DISCUSSION
The analysis of Brassica self-compatible mutants described in this paper suggests that the SRK receptor protein kinase isoforms we analyzed are regulated post-transcriptionally and may be inherently unstable molecules. In two independent mutant strains, there was a correlation between the depletion of SLG protein and the failure of stigmas to accumulate detectable levels of SRK protein despite the synthesis of normal amounts of SRK transcripts. The breakdown of SI in the scf1 and ΔS-1668 mutant strains may thus be a direct consequence of the absence of the SRK receptor in mutant stigmas. These molecular defects are associated with a breakdown of SI in the stigmas but not the pollen of the mutant strains. Therefore, our results also show that the SLG and SRK genes function in the stigma but not in pollen, in support of biochemical (Stein et al., 1996), genetic (Nasrallah et al., 1992, 1994b; Goring et al., 1993), and transgenic (Toriyama et al., 1991; Conner et al., 1997; Stahl et al., 1998; Cui et al., 2000; Takasaki et al., 2000) studies.
Taken together with the observation that co-expression of SLG (but not SLR1) with SRK in tobacco cells prevents the aggregation of SRK, our results suggest that in the strains we used and with the SRK/SLG alleles we analyzed, SLG plays a major role in the stabilization of SRK molecules, possibly by facilitating their proper maturation. Such a role would provide a molecular basis for the breakdown of SI in plants that express little or no SLG, and for the observed but as-yet-unexplained enhancement in the intensity of the acquired SI response in transgenic B. campestris plants that express both SRK and SLG relative to transgenic plants that express SRK alone (Takasaki et al., 2000).
Post-transcriptional regulation of proteins is a well-known phenomenon. In particular, proteins that are part of heterodimers or higher-order complexes are often degraded when another protein in the complex is absent (Halban and Irminger, 1994; Wickner et al., 1999). Thus, our results provide circumstantial evidence that, in the strains we analyzed and in the tobacco expression system, SRK interacts with SLG either directly or indirectly. Such an interaction would presumably occur through the extracellular domain (ectodomain) of SRK, which occupies the same topological space as SLG (Letham et al., 1999). It would not, however, be mediated by disulfide bridges between the conserved Cys residues contained in both the SRK ectodomain and SLG, because we found no evidence for the occurrence of SLG-SRK disulfide-linked dimers in stigma extracts and in transgenic tobacco plants expressing SRK and SLG. Further, because SRK and SLG are coordinately regulated in papillar cells, the interaction might occur between the immature proteins either co-translationally or as they migrate through the secretory pathway, or between the mature proteins at the papillar cell surface to which both are targeted.
Several transmembrane proteins have been shown to be inherently unstable, with a substantial fraction of the newly synthesized protein targeted for degradation (Yoshimura et al., 1990; Ward and Kopito, 1994; Centrella et al., 1996). By analogy to processes described in the maturation of receptors in animal systems, SLG may assist in SRK folding by transient binding as described for receptor-associated protein in the folding and trafficking of the low density lipoprotein and very low density lipoprotein receptors (Savonen et al., 1999). An oligomerization-assisted folding mechanism alternatively may operate as described for the T-cell receptor (TCR) complex (Bonifacino and Klausner, 1994), procollagen (Bulleid et al., 1997), and the secreted immunoglobulin, IgM (Reddy and Corley, 1998). It is interesting that both the receptor-associated protein and ε- and ζ-subunits of TCR are very stable molecules compared with the corresponding unstable low density lipoprotein/very low density lipoprotein receptors or α-, β-, and δ-TCR subunits (Bonifacino and Klausner, 1994; Savonen et al., 1999). With this perspective, it is interesting to note that SLG is a highly stable protein and its accumulation is insensitive to the absence of SRK both in Brassica stigmas (Nasrallah et al., 1994b) and transgenic tobacco plants (Perl-Treves et al., 1993; this study). The accumulation of the SRK isoforms we analyzed may hence be viewed as correlated with the co-expression of highly stable SLG protein.
Based on our results, it is possible to infer some features required for the stabilization of SRK in Brassica stigmas expressing the S haplotypes investigated in this study. First, the amount of SLG protein appears to be critical, because SRK does not accumulate in scf1 stigmas that do produce low levels of SLG. Second, qualitative properties of SLG may be important since, in ΔS-1668 stigmas, molecules contributed by the Sf1 haplotype, SLGf1 in particular, failed to complement the mutation in SLG13 and to allow the accumulation of SRK13. Thus, only some allelic forms of SLG might contribute to the stabilization of a particular SRK protein. It is also possible that only a subset of SLG functions in SRK stabilization, namely the SLG fraction that is membrane associated and capable of dimerizing. Membrane association of SLG would limit its diffusion to the two-dimensional space of the membrane and hence is likely to influence the frequency and character of SLG interaction with the transmembrane SRK protein. In this regard it is of interest to note that membrane-bound and soluble forms of various growth factors have been shown to display different potencies in activating the corresponding transmembrane receptors and can hence have distinct functional roles (Miyoshi et al., 1997; Takemura et al., 1997; Mueller et al., 1999).
It is interesting that SLG-related molecules cannot effect stabilization of SRK in the strains we analyzed. Several secreted SLG-related proteins are expressed in Brassica papillar cells. These include soluble glycoproteins encoded by the SLR1 (Umbach et al., 1990) and SLR2 (Boyes et al., 1991; Tantikanjana et al., 1996) genes that share approximately 70% sequence identity with SLG8 and SLG13, and also possibly SLG-like soluble forms of SRK, designated sSRK, which were predicted based on the occurrence of truncated SRK transcripts (Stein et al., 1991) and were indeed detected in at least one Brassica strain (Giranton et al., 1995). However, in the mutant stigmas we analyzed, such SLG-related molecules could not substitute for SLG in allowing normal accumulation of SRK. In addition, the formation of aberrant SRK aggregates in transgenic tobacco was prevented specifically by co-expression of SLG, but not by co-expression of SLR1, which is consistent with a specific role for SLG in the stabilization of SRK.
Whether the various allelic forms of SRK will all prove to require accessory molecules for their accumulation to physiologically relevant levels remains to be determined. S haplotypes are extremely diverse, and SRK and SLG genes exhibit extraordinarily high levels of sequence polymorphisms (Nasrallah et al., 1987; Chen and Nasrallah, 1990; Stein et al., 1991; Kusaba et al., 1997). Remarkably, they can also vary in their organization and in the classes of transcripts and proteins they produce (Tantikanjana et al., 1993, 1996; Gaude et al., 1995; Cabrillac et al., 1999). It is thus possible that some allelic forms of SRK are inherently more stable than the SRK8 and SRK13 analyzed in our study, or that some SRK genes produce relatively high levels of sSRK that might contribute to stabilization of the full-length receptor. It is also possible that in some strains, other classes of S proteins that share a high degree of sequence identity with SLG may contribute to the stabilization of SRK. For example, the stigmas of B. oleracea S2S2 homozygotes produce low levels of secreted SLG (Tantikanjana et al., 1993, 1996; Gaude et al., 1995). However, these stigmas express SLR2, a protein that shares >90% sequence identity with SLG2 (Boyes et al., 1991) and hence could potentially substitute for SLG2. S2S2 stigmas also express a membrane-anchored form of SLG2 consisting of the SLG2 S domain fused to a transmembrane domain and a short cytoplasmic tail (Tantikanjana et al., 1993). This protein, which is structurally similar to the CLV2 protein (Jeong et al., 1999), would have the same diffusional constraints as SRK and therefore might be effective in stabilizing SRK even when present at relatively low levels.
It is significant that the correlation we observed between SRK and SLG is similar to that observed between the Arabidopsis CLV1 receptor kinase and the CLV2 protein (Jeong et al., 1999), even though the SRK and CLV1 receptors belong to very different families of receptor protein kinases. Analysis of clv2 mutant strains revealed a dramatic (> 90%) decrease in the levels of CLV1 protein, although CLV1 transcript levels were unaffected (Jeong et al., 1999). It is intriguing that the residual CLV1 protein was detected as a novel high-Mr complex that was absent in wild-type plants (Jeong et al., 1999). The strong parallels between these results and the ones described in this paper indicate that plant transmembrane receptor kinases are characterized by the same inherent instability described for receptors in animal systems. Further, the requirement of molecules related to the receptor extracellular domain, either in the form of a soluble protein (as in the case of SRK) or of a membrane-anchored protein (as in the case of CLV1), may represent a common mechanism for the sustained accumulation of plant receptor protein kinases.
MATERIALS AND METHODS
Plant Material and Pollination Assays
Brassica oleracea plants bearing the S6, S13, and Sf1 haplotypes and the Brassica campestris (syn. B. rapa) scf1 mutant strain have been described previously (Nasrallah et al., 1988, 1992, 1994b). Pollination phenotypes of reciprocal crosses involving ΔS-1668, S13Sf1, and S13S13 plants were determined by monitoring pollen tube behavior by UV-fluorescence microscopy (Kho and Baer, 1968).
Isolation of RNA and Protein from Brassica Stigmas
Isolation of poly(A+) RNA from Brassica stigmas and subsequent gel-blot analysis were performed as described (Stein et al., 1991). The gel blots were hybridized with a probe derived from SLG (probes derived from several SLG genes produce equivalent hybridization signals; an SLG13 3′-untranslated region probe was used in this study), and a probe corresponding to the kinase domain of SRK (again kinase probes derived from several SRK alleles are equivalent; an SRK6 kinase domain probe was used in this study). An actin probe was used as a loading control.
Stigma protein extracts were prepared by homogenizing stigmas in buffer containing 30 mm Tris (tris[hydroxy-methyl]aminomethane)-HCl, pH 7.5, 75 mm NaCl, 10 mm EDTA, and 10% (v/v) glycerol. The buffer was supplemented with 5 mm ascorbate, 2.5 mm potassium metabisulfite, 1 mm phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 μg/mL pepstatin A just before use. Whole cell extracts and microsome samples were isolated using conditions described previously (Stein et al., 1996). Plasma membrane-enriched fractions were prepared by two-phase partitioning of stigma microsome pellets using a scaled-down version of the protocol previously described for tobacco (Nicotiana tabacum) tissue (Stein et al., 1996).
Purification of SLG6 from S6S6 stigmas by isoelectric focusing was performed on a pH 3.5 to 9.5 gradient in flat beds of Sephadex G50 (Pharmacia Biotech, Piscataway, NJ) as described by Nasrallah et al. (1985). The purity of the SLG fraction was determined by silver staining of various amounts of purified SLG following electrophoresis under reducing conditions.
Alkylation of Stigma Proteins Using IAc
Brassica stigmas obtained from open flowers were incubated with 100 mm IAc in the presence or absence of 50 mm DTT in the above-mentioned extraction buffer (10 stigmas in 30 μL of extraction buffer) containing 0.05% (v/v) Tween 20 as a surfactant. Alkylation of the stigma proteins was carried out for 20 min at room temperature in the dark. Stigmas immersed in extraction buffer lacking DTT and indole-3-acetic acid were used as a control. After the incubation period, the stigmas were homogenized in the respective buffers to obtain whole cell extracts as described above that were subjected to electrophoresis under reducing or non-reducing conditions.
Chemical Treatment of Stigma Microsome Fractions
Microsome fractions were isolated from B. oleracea S6S6 stigmas as described above using an HB consisting of 50 mm Tris-HCl, pH 7.5, 100 mm NaCl, and 10 mm EDTA along with the anti-oxidative and protease inhibitor supplements. Equal quantities of microsome pellets were resuspended in the following solutions: (a) 1 m hydroxylamine (H3NO, prepared in HB and pH adjusted to 7.1 using NaOH), a deacylating agent, to test for the presence of acyl moieties on SLG that may result in its membrane attachment; (b) 50 mm DTT in HB to test for the covalent attachment of SLG to integral membrane proteins via disulfide bonds; (c) 8 m urea in HB, to test for hydrogen bond-mediated SLG-membrane association; (d) 0.1 m Na2CO3, pH 11.5, to test for ion-sensitive peripheral attachment of SLG to membranes; (e) 0.2% (w/v) SDS in HB to test the sensitivity of SLG-membrane attachment to treatment with ionic detergent; (f) 1% (v/v) Triton X-100 in HB to test the sensitivity of SLG-membrane attachment to treatment with non-ionic detergent; and (g) HB with no additives as a control for the extent of SLG released during resuspension of the microsome pellet. Equal volumes of all solutions were used to resuspend the microsome pellets. All treatments were incubated for 16 to 18 h at 4°C except for the SDS treatment, which was carried out at room temperature. The samples were subsequently centrifuged at 100,000g for 1 h and the supernatant and pellet fractions were analyzed by SDS-PAGE to determine the extent of SLG solubilization.
Co-Expression of SLG or SLR1 with SRK in Transgenic Tobacco
SLG6 or SLR1 cDNA was inserted between the duplicated CaMV 35S promoter (Kay et al., 1987) and nos terminator of a pBIN19 (Bevan, 1984) derived plant transformation vector bearing a hygromycin-resistance cassette. Cells of Agrobacterium tumefaciens strain GV3101 (Katavic et al., 1994) containing these constructs were used as described (Horsch et al., 1988) to transform tobacco (N. tabacum cv Petit Havana) plants expressing SRK6 (Stein et al., 1996) or tobacco plants previously transformed with vector lacking the SRK6 transgene. As an additional control, we used the vector backbone to transform the SRK6-expressing tobacco plants.
Kanamycin- and hygromycin-resistant transformants were analyzed by DNA gel-blot and immunoblot analysis to confirm the presence and expression of SLG6 or SLR1 transgene. Seeds obtained from the primary transformants were surface sterilized and germinated on Murashige and Skoog medium (Murashige and Skoog, 1962), containing 50 mg/L kanamycin and 10 mg/L hygromycin. Tobacco shoot tissue from seedlings grown for 30 to 45 d on selection medium was used to obtain microsome fractions as described previously (Stein et al., 1996).
Protein-Gel Electrophoresis and Immunoblot Analysis
Samples containing equal amounts of protein were resolved by SDS-PAGE on 7.5% (w/v) or 10% (w/v) gels and electroblotted onto PVDF membranes using a semi-dry transfer technique. Protein quantification was carried out according to the Bradford technique (Bradford, 1976) using the Bio-Rad (Hercules, CA) dye reagent. Bovine serum albumin was used as a standard for protein quantification. Electrophoresis was performed either under reducing conditions by inclusion of DTT (100 mm) or under non-reducing conditions by omission of DTT from the protein-loading buffer. All experiments entailing the comparison of protein mobility under reducing versus non-reducing conditions were performed on the same gel with empty lanes separating the reduced versus non-reduced samples (to limit diffusion of DTT). The monoclonal antibody MAb/H8, which recognizes SLG and SRK (Kandasamy et al., 1989; Stein et al., 1996), was used at a concentration of 1:50 and the polyclonal anti-SLR1 serum (Umbach et al., 1990) was used at a concentration of 1:1,000. The 2E7 serum, which recognizes vacuolar H+-ATPase (Ward et al., 1992), served to verify equal loading between lanes for the transgenic tobacco microsome samples and was used at a concentration of 1:500. Immunoblots were developed using the Boehringer Mannheim (Indianapolis) chemiluminescence western-blotting kit according to the manufacturer's instructions.
ACKNOWLEDGMENT
We thank Dr. Heven Sze for the antibodies against vacuolar H+-ATPase.
Footnotes
This work was supported by the National Institutes of Health (grant no. GM57527) and National Science Foundation (grant no. IBN–9631921).
LITERATURE CITED
- Bagga S, Adams HP, Rodriguez FD, Kemp JD, Sengupta-Gopalan C. Coexpression of the maize δ-zein and β-zein genes results in stable accumulation of δ-zein in endoplasmic reticulum-derived protein bodies formed by β-zein. Plant Cell. 1997;9:1683–1696. doi: 10.1105/tpc.9.9.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft PW. Receptor kinases in plant development. Trends Plant Sci. 1998;3:384–388. [Google Scholar]
- Bevan MW. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Klausner RD. Degradation of proteins retained in the ER. In: Ciechanover AJ, Schwartz AL, editors. Modern Cell Biology: Cellular Proteolytic Systems. New York: John Wiley & Sons; 1994. pp. 137–160. [Google Scholar]
- Boyes DC, Chen CH, Tantikanjana T, Esch JJ, Nasrallah JB. Isolation of a second S-locus related cDNA from Brassica oleracea: genetic relationships between the S-locus and two related loci. Genetics. 1991;127:221–228. doi: 10.1093/genetics/127.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Nasrallah ME, Vrebalov J, Nasrallah JB. The self-incompatibility (S) haplotypes of Brassica contain highly divergent and rearranged sequences of ancient origin. Plant Cell. 1997;9:237–247. doi: 10.1105/tpc.9.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bulleid NJ, Dalley JA, Lees JF. The C-propeptide domain of procollagen can be replaced with a transmembrane domain without affecting trimer formation or collagen triple helix folding during biosynthesis. EMBO J. 1997;16:6694–6701. doi: 10.1093/emboj/16.22.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrillac D, Delorme V, Garin J, Ruffio-Chable V, Giranton J-L, Dumas C, Gaude T, Cock MJ. The S15 self-incompatibility haplotype in Brassica oleracea includes three S gene family members expressed in stigmas. Plant Cell. 1999;11:971–986. doi: 10.1105/tpc.11.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YW, Anderova M, Crawford NM, Schroeder JI. Expression of an outward-rectifying potassium channel from maize mRNA and complementary RNA in Xenopus oocytes. Plant Cell. 1992;4:961–969. doi: 10.1105/tpc.4.8.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M, Ji C, Casinghino S, McCarthy TL. Rapid flux in transforming growth factor-β receptors on bone cells. J Biol Chem. 1996;271:18616–18622. doi: 10.1074/jbc.271.31.18616. [DOI] [PubMed] [Google Scholar]
- Chen C-H, Nasrallah JB. A new class of S sequences defined by a pollen recessive self-incompatibility allele of Brassica oleracea. Mol Gen Genet. 1990;222:241–248. doi: 10.1007/BF00633824. [DOI] [PubMed] [Google Scholar]
- Chen SX, Halkier BA. Functional expression and characterization of the myrosinase MYR1 from Brassica napus in Saccharomyces cerevisiae. Protein Expr Purif. 1999;17:414–420. doi: 10.1006/prep.1999.1158. [DOI] [PubMed] [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997;89:575–585. doi: 10.1016/s0092-8674(00)80239-1. [DOI] [PubMed] [Google Scholar]
- Conner JA, Tantikanjana T, Stein JC, Kandasamy MK, Nasrallah JB, Nasrallah ME. Transgene-induced silencing of S-locus genes and related genes in Brassica. Plant J. 1997;11:809–823. [Google Scholar]
- Cui Y, Bi Y-M, Brugiere N, Arnoldo MA, Rothstein SJ. The S locus glycoprotein and the S receptor kinase are sufficient for self-pollen rejection in Brassica. Proc Natl Acad Sci USA. 2000;97:3713–3717. doi: 10.1073/pnas.050480297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme V, Giranton J-L, Hatzfeld Y, Friry A, Heizmann P, Ariza MJ, Dumas C, Gaude T, Cock JM. Characterization of the S locus genes, SLG and SRK, of the Brassica S3 haplotype: identification of a membrane-localized protein encoded by the S locus receptor kinase gene. Plant J. 1995;7:429–440. doi: 10.1046/j.1365-313x.1995.7030429.x. [DOI] [PubMed] [Google Scholar]
- Doughty J, Dixon S, Hiscock SJ, Willis AC, Parkin IAP, Dickinson HG. PCP-A1, a defensin-like Brassica pollen coat protein that binds the S locus glycoprotein, is the product of gametophytic gene expression. Plant Cell. 1998;10:1333–1347. doi: 10.1105/tpc.10.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer WB, Ninnemann O. Heterologous expression of genes in bacterial, fungal, animal, and plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:419–444. [Google Scholar]
- Gaude T, Rougier M, Heizmann P, Ockendeon DJ, Dumas C. Expression level of the SLG gene is not correlated with the self-incompatibility phenotype in the class II S haplotypes of Brassica oleracea. Plant Mol Biol. 1995;27:1003–1014. doi: 10.1007/BF00037027. [DOI] [PubMed] [Google Scholar]
- Giranton J-L, Ariza MJ, Dumas C, Cock JM, Gaude T. The S locus receptor kinase gene encodes a soluble glycoprotein corresponding to the SRK extracellular domain in Brassica oleracea. Plant J. 1995;8:827–834. doi: 10.1046/j.1365-313x.1995.8060827.x. [DOI] [PubMed] [Google Scholar]
- Giranton JL, Dumas C, Cock JM, Gaude T. The integral membrane S-locus receptor kinase of Brassica has serine/threonine kinase activity in a membranous environment and spontaneously forms oligomers in planta. Proc Natl Acad Sci USA. 2000;97:3759–3764. doi: 10.1073/pnas.070025097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goring DR, Glavin TL, Schafer U, Rothstein SJ. An S receptor kinase gene in self-compatible Brassica napus has a 1-bp deletion. Plant Cell. 1993;5:531–539. doi: 10.1105/tpc.5.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban PA, Irminger J-C. Sorting and processing of secretory proteins. Biochem J. 1994;299:1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. Plant protein serine threonine kinases: classification and functions. Annu Rev Plant Phys Plant Mol Biol. 1999;50:97–131. doi: 10.1146/annurev.arplant.50.1.97. [DOI] [PubMed] [Google Scholar]
- Hollecker M. Counting integral numbers of residues by chemical modification. In: Creighton TE, editor. Protein Structure: A Practical Approach. New York: IRL Press; 1997. pp. 151–164. [Google Scholar]
- Horsch RB, Fry J, Hoffmann N, Neidermeyer J, Rogers SG, Fraley RT. Leaf disc transformation. In: Gelvin SB, Schilperoort RA, editors. Plant Molecular Biology Manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 1–9. [Google Scholar]
- Jeong S, Trotochaud AE, Clark SE. The Arabidopsis CLAVATA2 gene encodes a receptor-like required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell. 1999;11:1925–1933. doi: 10.1105/tpc.11.10.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerloher W, Fischer U, Piechottka GP, Schäffner AR. Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 1994;6:187–199. doi: 10.1046/j.1365-313x.1994.6020187.x. [DOI] [PubMed] [Google Scholar]
- Kandasamy MK, Paolillo DJ, Faraday CD, Nasrallah JB, Nasrallah ME. The S-locus specific glycoproteins of Brassica accumulate in the cell wall of developing stigma papillae. Dev Biol. 1989;134:462–472. doi: 10.1016/0012-1606(89)90119-x. [DOI] [PubMed] [Google Scholar]
- Katavic V, Haughn GW, Reed D, Martin M, Kunst L. In planta transformation of Arabidopsis thaliana. Mol Gen Genet. 1994;245:363–370. doi: 10.1007/BF00290117. [DOI] [PubMed] [Google Scholar]
- Kay R, Chan A, Daly M, McPherson J. Duplication of the CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science. 1987;236:1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- Kaye C, Neven L, Hofig A, Li QB, Haskell D, Guy C. Characterization of a gene for spinach CAP160 and expression of two spinach cold-acclimation proteins in tobacco. Plant Physiol. 1998;116:1367–1377. doi: 10.1104/pp.116.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse KP, Roberts JL, Munitz TI, Wiest DL, Nakayama T, Singer A. Developmental regulation of alpha-beta T cell antigen receptor expression results from differential stability of nascent TCR-alpha proteins within the endoplasmic reticulum of immature and mature T cells. EMBO J. 1994;13:4504–4514. doi: 10.1002/j.1460-2075.1994.tb06772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho YO, Baer J. Observing pollen tubes by means of fluorescence. Euphytica. 1968;17:298–302. [Google Scholar]
- Kusaba M, Matsushita M, Okazaki K, Satta Y, Nishio T. Sequence and structural diversity of the S locus genes from different lines with the same self-recognition specificities in Brassica oleracea. Genetics. 2000;154:413–420. doi: 10.1093/genetics/154.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba M, Nishio T. Comparative analysis of S haplotypes with very similar SLG alleles in Brassica rapa and Brassica oleracea. Plant J. 1999;17:83–91. doi: 10.1046/j.1365-313x.1999.00355.x. [DOI] [PubMed] [Google Scholar]
- Kusaba M, Nishio T, Satta Y, Hinata K, Ockendon D. Stricking sequence similarity in inter- and intra-specific comparisons of class I SLG alleles from Brassica oleracea and Brassica campestris: implications for the evolution and recognition mechanism. Proc Natl Acad Sci USA. 1997;94:7673–7678. doi: 10.1073/pnas.94.14.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letham DLD, Blissard GW, Nasrallah JB. Production and characterization of the Brassica oleracea self-incompatibility locus glycoprotein and receptor kinase in a baculovirus infected insect cell culture system. Sex Plant Reprod. 1999;12:179–187. [Google Scholar]
- Mastrogiacomo A, Kohan SA, Whitelegge JP, Gundersen CB. Intrinsic membrane association of Drosophila cysteine string proteins. FEBS Lett. 1998;436:85–91. doi: 10.1016/s0014-5793(98)01092-8. [DOI] [PubMed] [Google Scholar]
- Maurel C, Reizer J, Schroeder JI, Chrispeels MJ. The vacuolar membrane protein γ-TIP creates water specific channels in Xenopus oocytes. EMBO J. 1993;12:2241–2247. doi: 10.1002/j.1460-2075.1993.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi E, Higashiyama S, Nakagawa T, Hayashi N, Taniguchi N. Membrane-anchored heparin-binding epidermal growth factor-like growth factors acts as a tumor survival factor in a hepatoma cell line. J Biol Chem. 1997;272:14349–14355. doi: 10.1074/jbc.272.22.14349. [DOI] [PubMed] [Google Scholar]
- Montamat F, Maurousset L, Tegeder M, Frommer W, Delrot S. Cloning and expression of amino acid transporters from broad bean. Plant Mol Biol. 1999;41:259–268. doi: 10.1023/a:1006321221368. [DOI] [PubMed] [Google Scholar]
- Mueller C, Corazza N, Trachsel-Loseth S, Eugster H-P, Buhler-Jungo M, Brunner T, Imboden MA. Noncleavable transmembrane mouse tumor necrosis factor-α (TNFα) mediates effects distinct from those of wild-type TNFα in vitro and in vivo. J Biol Chem. 1999;274:38112–38118. doi: 10.1074/jbc.274.53.38112. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays for tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nasrallah JB, Doney RC, Nasrallah ME. Biosynthesis of glyproteins involved in the pollen-stigma interaction of incompatibility in developing flowers of Brassica oleracea L. Planta. 1985;165:100–107. doi: 10.1007/BF00392217. [DOI] [PubMed] [Google Scholar]
- Nasrallah JB, Kao T-H, Chen C-H, Goldberg ML, Nasrallah ME. Amino-acid sequence of glycoproteins encoded by three alleles of the S locus of Brassica oleracea. Nature. 1987;326:617–619. [Google Scholar]
- Nasrallah JB, Nasrallah ME. Pollen-stigma signaling in the sporophytic self-incompatibility response. Plant Cell. 1993;5:1325–1335. doi: 10.1105/tpc.5.10.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah JB, Rundle SJ, Nasrallah ME. Genetic evidence for the requirement of the Brassica S-locus receptor kinase gene in the self-incompatibility response. Plant J. 1994b;5:373–384. [Google Scholar]
- Nasrallah JB, Stein JC, Kandasamy MK, Nasrallah ME. Signaling the arrest of pollen tube development in self-incompatible plants. Science. 1994a;266:1505–1508. doi: 10.1126/science.266.5190.1505. [DOI] [PubMed] [Google Scholar]
- Nasrallah JB, Yu S-M, Nasrallah ME. Self-incompatibility genes of Brassica oleracea: expression, isolation and structure. Proc Natl Acad Sci USA. 1988;85:5551–5555. doi: 10.1073/pnas.85.15.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah ME, Kandasamy MK, Chang M-C, Stadler Z, Lim S, Nasrallah JB. Identifying genes for pollen-stigma recognition in Crucifers. Ann Bot Suppl A. 2000;85:125–132. [Google Scholar]
- Nasrallah ME, Kandasamy MK, Nasrallah JB. A genetically defined trans-acting locus regulates S-locus function in Brassica. Plant J. 1992;2:497–506. [Google Scholar]
- Okazaki K, Kusaba M, Ockendon DJ, Nishio T. Characterization of S tester lines in Brassica oleracea: polymorphisms of restriction fragment length of SLG homologues and isoelectric points of S-locus glycoproteins. Theor Appl Genet. 1999;98:1329–1334. [Google Scholar]
- Perl-Treves R, Howlett B, Nasrallah ME. Self-incompatibility related glycoproteins of Brassica are produced and secreted by transgenic tobacco cell cultures. Plant Sci. 1993;92:99–110. [Google Scholar]
- Reddy PS, Corley RB. Assembly, sorting, and exit of oligomeric proteins from the endoplasmic reticulum. Bioessays. 1998;20:546–554. doi: 10.1002/(SICI)1521-1878(199807)20:7<546::AID-BIES5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Kusaba M, Nishio T. Polymorphism of the S-locus glycoprotein gene (SLG) and the S-locus related gene (SLR1) in Raphanus sativus L. and self-incompatible ornamental plants in the Brassicaceae. Mol Gen Genet. 1998;258:397–403. doi: 10.1007/s004380050747. [DOI] [PubMed] [Google Scholar]
- Savonen R, Obermoeller LM, Trausch-Azar JS, Schwartz AL, Bu GJ. The carboxyl-terminal domain of receptor-associated protein facilitates proper folding and trafficking of the very low density lipoprotein receptor by interaction with the three amino-terminal ligand-binding repeats of the receptor. J Biol Chem. 1999;274:25877–25882. doi: 10.1074/jbc.274.36.25877. [DOI] [PubMed] [Google Scholar]
- Schopfer CR, Nasrallah ME, Nasrallah JB. The male determinant of self-incompatibility in Brassica. Science. 1999;286:1697–1700. doi: 10.1126/science.286.5445.1697. [DOI] [PubMed] [Google Scholar]
- Stahl RJ, Arnoldo MA, Glavin TL, Goring DR, Rothstein SJ. The self-incompatibility phenotype in Brassica is altered by the transformation of a mutant S locus receptor kinase. Plant Cell. 1998;10:209–218. doi: 10.1105/tpc.10.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JC, Dixit R, Nasrallah ME, Nasrallah JB. SRK, the stigma-specific S locus receptor kinase of Brassica, is targeted to the plasma membrane in transgenic tobacco. Plant Cell. 1996;8:429–445. doi: 10.1105/tpc.8.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci USA. 1991;88:8816–8820. doi: 10.1073/pnas.88.19.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Walker JC. Plant protein kinase families and signal transduction. Plant Physiol. 1995;108:451–457. doi: 10.1104/pp.108.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki T, Hatakeyama K, Suzuki G, Watanabe M, Isogai A, Hinata K. The S receptor kinase determines self-incompatibility in Brassica stigma. Nature. 2000;403:913–916. doi: 10.1038/35002628. [DOI] [PubMed] [Google Scholar]
- Takemura T, Kondo S, Homma T, Sakai M, Harris RC. The membrane-bound form of heparin-binding epidermal growth factors-like growth factor promotes survival of cultured renal epithelial cells. J Biol Chem. 1997;272:31036–31042. doi: 10.1074/jbc.272.49.31036. [DOI] [PubMed] [Google Scholar]
- Tantikanjana T, Nasrallah ME, Nasrallah JB. The Brassica S gene family: molecular characterization of the SLR2 gene. Sex Plant Reprod. 1996;9:107–116. [Google Scholar]
- Tantikanjana T, Nasrallah ME, Stein JC, Chen C-H, Nasrallah JB. An alternative transcript of the S locus glycoprotein gene in a class II pollen-recessive self-incompatibility haplotype of Brassica oleracea encodes a membrane-anchored protein. Plant Cell. 1993;5:657–666. doi: 10.1105/tpc.5.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriyama K, Stein JC, Nasrallah ME, Nasrallah JB. Transformation of Brassica oleracea with an S-locus gene from B. campestris changes the self-incompatibility phenotype. Theor Appl Genet. 1991;81:769–776. doi: 10.1007/BF00224988. [DOI] [PubMed] [Google Scholar]
- Trotochaud AE, Hao T, Guang W, Yang Z, Clark SE. The CLAVATA1 receptor-like kinase requires CLAVATA3 for its assembly into a signaling complex that includes KAPP and a Rho-related protein. Plant Cell. 1999;11:393–405. doi: 10.1105/tpc.11.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Lalonde BA, Kandasamy MK, Nasrallah JB, Nasrallah ME. Immunodetection of protein glycoforms encoded by two independent genes of the self-incompatibility multigene family of Brassica. Plant Physiol. 1990;93:739–747. doi: 10.1104/pp.93.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veena Reddy VS, Sopory SK. Glyoxalase I from Brassica juncea: molecular cloning, regulation, and its overexpression confer tolerance in transgenic tobacco under stress. Plant J. 1999;17:385–395. doi: 10.1046/j.1365-313x.1999.00390.x. [DOI] [PubMed] [Google Scholar]
- Ward CL, Kopito RR. Intracellular turnover of cystic fibrosis transmembrane conductance regulator: inefficient processing and rapid degradation of wild-type and mutant proteins. J Biol Chem. 1994;269:25710–25718. [PubMed] [Google Scholar]
- Ward JM, Reinders A, Hsu H-T, Sze H. Dissociation and reassembly of the vacuolar H+-ATPase complex from oat roots. Plant Physiol. 1992;99:161–169. doi: 10.1104/pp.99.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S, Maurizi MR, Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science. 1999;286:1888–1893. doi: 10.1126/science.286.5446.1888. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, D'Andrea AD, Lodish HF. Friend spleen-focus forming virus glycoprotein GD55 interacts with the erythropoietin receptor in the endoplasmic-reticulum and affects receptor metabolism. Proc Natl Acad Sci USA. 1990;87:4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]