Abstract
Rhizobial Nod factors (NFs) function as nodulation signals that trigger symbiotic responses of leguminous host plants. NFs consist of a chitin oligomer backbone carrying a fatty acid at the non-reducing end. Depending on the rhizobial strain, NFs carry additional substituents, which may determine host specificity. Transgenic suspension-cultured soybean (Glycine max [L.] Merr.) cells expressing aequorin have been used to record cytosolic [Ca2+] changes upon treatment with purified NFs and chitin fragments. Both compounds elicited an increase of cytosolic [Ca2+] at nanomolar concentrations. The shape and amplitude of cytosolic [Ca2+] changes was similar to the response elicited by un-derivatized chitin oligomers. Cells challenged first with NFs did not respond to a subsequent treatment with chitin oligomers and vice versa. Dose-response experiments showed that un-derivatized chitin oligomers were more active compared with NFs. The capacity of NFs to elicit the calcium response depended on their structure. The presence of reducing end substituents in methylfucosylated NFs from Rhizobium sp. NGR234 and the O-acetyl group at the non-reducing end in NFs from Sinorhizobium meliloti attenuated the activity to cause the calcium changes. The sulfate group in NFs from Rhizobium tropici did not affect the elicitor activity. Pentameric S. meliloti NFs were more active than tetrameric molecules, whereas trimeric or dimeric degradation products were inactive. Substituents in NFs may have the function to avoid stimulation of defense reactions mediated by the perception system for chitin oligomers.
Plants have highly sensitive chemoperception systems for signal molecules derived from pathogenic and symbiotic microorganisms (Boller, 1995). The symbiosis between legumes and rhizobial bacteria results in the formation of nitrogen-fixing root nodules. During the infection process, rhizobia secrete specific nodulation signals called Nod factors (NFs). NFs are modified lipochitooligosaccharides, i.e. chitin oligomers linked with a fatty acid replacing the N-acetyl group on their non-reducing end. Structural substitutions of NFs at the reducing or non-reducing end have been shown to influence NF activity in a host-specific manner. It is thought that these decorations influence the binding of NFs to corresponding plant receptors (Long, 1996; Cohn et al., 1998; Schultze and Kondorosi, 1998a). Moreover, NF decorations may influence the stability of NFs in the rhizosphere of the host plant and protect the molecules against hydrolysis and inactivation by plant chitinases and other glycosyl hydrolases (Staehelin et al., 1994a, 1994b, 1995, 2000; Minic et al., 1998; Schultze et al., 1998b; Ovtsyna et al., 2000).
NFs trigger a series of plant responses resulting in the formation of nodule primordia. A primary response toward NFs seems to be the opening of transmembrane channels leading to plasma membrane depolarization of root hairs (Ehrhardt et al., 1992; Felle et al., 1995). It has been proposed that the resulting increase in Ca2+ plays an important role in the signal transduction of NFs and acts as second messenger. Increases in cytosolic-free [Ca2+] in response to NFs were observed in leguminous roots hairs using different techniques, such as fluorescent dyes and Ca2+-selective microelectrodes (Ehrhardt et al., 1996; Gehring et al., 1997; De Ruijter et al., 1998; Cardenas et al., 1999; Felle et al., 1999).
Similar to other NF-inducible responses of the host plant, [Ca2+] changes in root hairs were not elicited by un-substituted chitin oligomers, i.e. the carbohydrate moiety of NFs, suggesting the presence of specific receptors for lipochitooligosaccharides (Ehrhardt et al., 1996; Gehring et al., 1997; Cardenas et al., 1999; Felle et al., 1999). Specific NF binding sites of the host plant including a lectin with apyrase activity, have been recently characterized (Etzler et al., 1999; Gressent et al., 1999). However, plants also have sensitive perception systems for un-derivatized chitin oligomers. Such perception systems have been characterized using various plant cell cultures, a convenient tool to study perception of elicitors (Boller, 1995). The receptors for chitin oligomers are assumed to play a role in detecting chitin-containing organisms, e.g. pathogenic fungi and arthropods (Boller, 1995; Stacey and Shibuya, 1997). In tomato cells, it has been shown that the sensitive perception system for chitin oligomers (Felix et al., 1993) perceives the rhizobial NFs as well (Staehelin et al., 1994a), and a high-affinity binding-site for chitin oligomers and NFs has been characterized in these cells (Baureithel et al., 1994). Similarly, a high-affinity binding protein for chitin oligomers has been identified in the plasma membrane of rice cells (Ito et al., 1997). Transgenic soybean (Glycine max (L.) Merr.) cells expressing aequorin, a protein that emits light depending on the [Ca2+] (e.g. Knight et al., 1991), have been used recently to monitor cytosolic [Ca2+] changes in response to treatment with chitin oligomers (Mithöfer et al., 1999).
Here, we present results obtained with these soybean cells challenged with a set of differently modified NFs. The data indicate that chitin oligomers and NFs induce cytosolic [Ca2+] responses of the same type and that structurally different NFs differ in their activity to elicit this response.
RESULTS
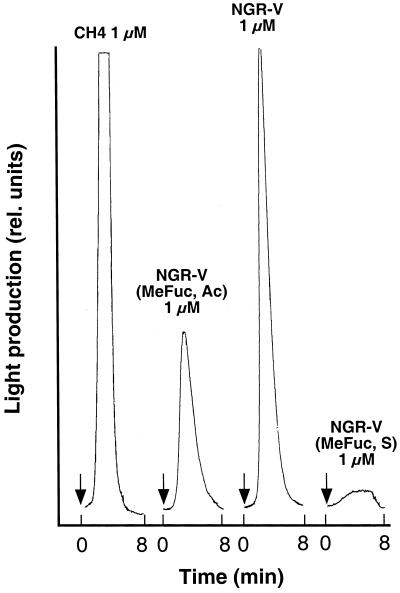
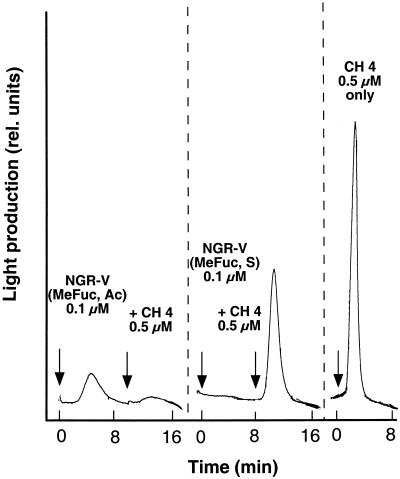
Transgenic plant cells expressing aequorin were used to visualize cytosolic [Ca2+] changes. The calcium-sensing protein aequorin emits light in the presence of cytosolic [Ca2+] upon addition of elicitors to suspension-cultured soybean cell (see Mithöfer et al., 1999). When cells were challenged with Nod New Guinea Rhizobium (NGR)-V(Me-Fuc, Ac), a pentameric NF from Rhizobium NGR234 (Fig. 1), a luminescence corresponding to an increase of cytosolic [Ca2+] was observed. The shape and amplitude of this response was similar to the transient light emission elicited by un-derivatized chitin oligomers, i.e. the non-substituted carbohydrate backbone of NFs (Fig. 2; see also Mithöfer et al., 1999), whereas β-glucan elicitors induced a different response with a second increase in cytosolic [Ca2+] (Mithöfer et al., 1999). Thus, both chitin oligomers and the structurally related rhizobial NFs had the capacity to elicit cytosolic [Ca2+] changes in suspension-cultured soybean cells. The transient increase in light emission of the aequorin expressing soybean cells was different upon treatment with differently substituted NGR factors (Fig. 1). Compared with the acetylated NodNGR-V(Me-Fuc, Ac), the NFs from strain NGRΔNodZ1 lacking a reducing-end substitution showed significantly higher elicitor activity. In contrast, the cells responded only very weakly when they were treated with the sulfated NodNGR-V(Me-Fuc, S) (Fig. 2).
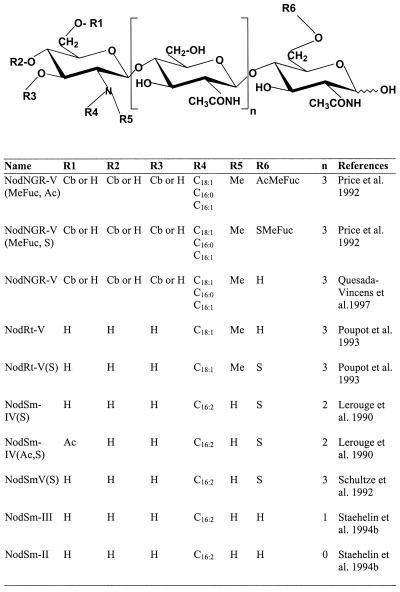
Figure 1.
General scheme of the Nod factors used in this study. Nod factors were purified from Rhizobium sp. NGR234 and NGRΔNodZ1 (NodNGR factors), R. tropici CFN 299 (NodRt factors), or S. meliloti 1,021 (pEK327) (NodSm factors). NodSm-III and NodSm-II are hydrolytic degradation products of NodSm factors. Cb, Carbamoyl; Fuc, fucosyl; Me, methyl; S, sulfate; Ac, acetyl.
Figure 2.
Kinetics of Nod factor-induced enhancement of the cytosolic Ca2+ concentration in soybean cells expressing aequorin. Ca2+- mediated luminescence was determined after addition of three differently substituted Nod factors and chitotetraose (CH4). Arrows indicate when the effectors were added.
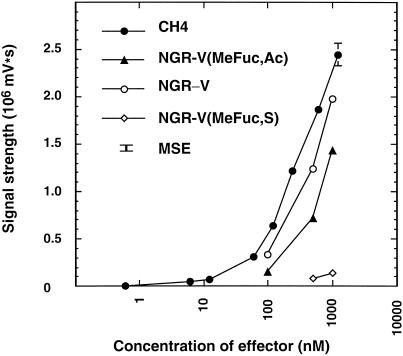
To quantify the elicitor activity of the differently modified NFs, dose-response curves were established (Fig. 3). Like CH4 (N,N′,N",N‴-tetraacetylchitotetraose), the NFs stimulated light emission in a concentration-dependent manner. As observed previously (Mithöfer et al., 1999), the cells responded in a log-linear way even at the highest concentrations of elicitors tested, namely in the micromolar range. The non-fucosylated NodNGR-V was almost equally active, followed by NGR-V(Me-Fuc, Ac), the NF carrying an acetyl group on the Fuc residue. Very weak responses were observed with NGR-V(Me-Fuc, S), the NF carrying a sulfate group on the Fuc residue (Fig. 3).
Figure 3.
Dose-response relationships for the enhancement of the cytosolic Ca2+ concentration in soybean cells expressing aequorin. Ca2+-mediated luminescence was determined in cells treated with increasing concentrations of chitotetraose (CH 4) and three differently substituted Nod factors. Mean values and se are given for three independent measurements. Where not indicated, se are smaller than the symbols. MSE, mean se
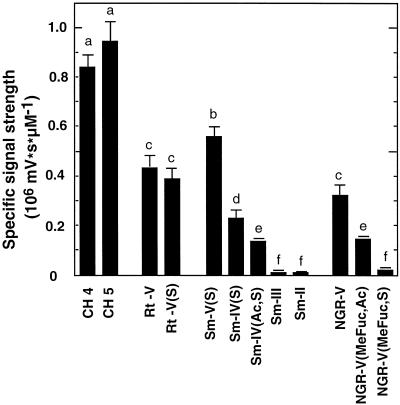
To compare the specific elicitor activity of differently substituted NFs, soybean cells expressing aequorin were incubated with chitooligomers or NFs in a concentration range between 10 and 100 nm. Under these conditions, the light-emission was linear to the concentration of the elicitor. Compared with un-derivatized CH4 or CH5 (N,N′,N",N‴,N‴′-pentaacetylchitopentaose), the NFs had generally a weaker activity in inducing luminescence. The NFs eliciting the strongest responses were Rt-V, Rt-V(S), Sm-V(S), and NGR-V. The Sm-IV NFs and NGR-V(Me-Fuc, Ac) elicited significantly weaker responses. Responses elicited by NGR-V(Me-Fuc, S) and by hydrolysis products from Sm-NFs were at background levels (Fig. 4).
Figure 4.
Standardized response factors of enhancement of the cytosolic Ca2+ concentration in soybean cells expressing aequorin. Ca2+-mediated luminescence was determined upon treatment with chitooligomers (chitotetraose, CH4, and chitopentaose, CH5) and various Nod factors (10–100 nm; see Fig. 1). Mean values and se are given for three independent measurements. Treatments superscribed by the same letters are not significantly different (P < 0.05; ANOVA followed by Student-Newman-Keuls test).
It has been found that plant cells lose their capacity to respond a second time to the same type of elicitor (“refractory behavior”), whereas they remain sensitive to another type of elicitor, perceived by another receptor (e.g. Felix et al., 1993; Staehelin et al., 1994a; Felix et al., 1998). To investigate whether calcium responses induced by NFs and chitooligomers have this refractory behavior, cells were treated with NFs and subsequently with CH4. Different concentrations of NFs and CH4 were chosen to remain in a range where a linear relationship between stimulus and response was observed. Cells challenged first with NodNGR-V(Me-Fuc, Ac) were refractory to a subsequent treatment with CH4 (Fig. 5). Similarly, after stimulation with CH4, the cells were unresponsive to NodNGR-V(Me-Fuc, Ac) (not shown). The same was observed with respect to NodRt-V and NodRt-V(S) (data not shown). Conversely, NodNGR-V(Me-Fuc, S) eliciting only very weak responses did not suppress the response of subsequently added CH4 (Fig. 5).
Figure 5.
Kinetics of Nod factor-induced enhancement of the cytosolic Ca2+-concentration in soybean cells expressing aequorin. Ca2+-mediated luminescence was determined after treatment with two differently substituted Nod factors and the subsequent addition of chitotetraose (CH 4). Arrows indicate when the effectors were added.
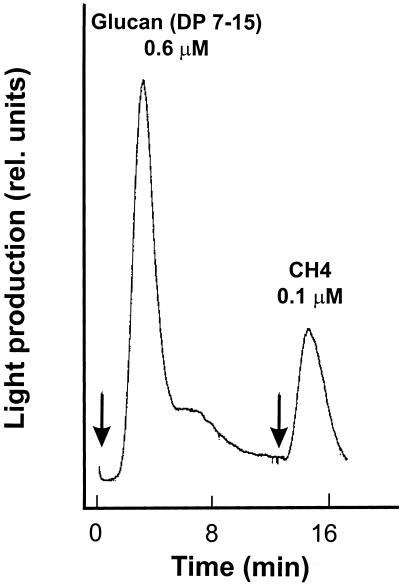
As a positive control, cells pretreated with β-glucan elicitors (degree of polymerization, 7–15) were subsequently treated with CH4. The cells showed both responses and thus were not refractory (Mithöfer et al., 1999; Fig. 6).
Figure 6.
Kinetics of Nod factor-induced enhancement of the cytosolic Ca2+-concentration in soybean cells expressing aequorin. Ca2+-mediated luminescence was determined after treatment with 0.6 μm glucans (degree of polymerization, 7–15) and the subsequent addition of 0.1 μm chitotetraose (CH 4). Arrows indicate when the effectors were added.
DISCUSSION
Using soybean cells transgenic for the calcium-sensing protein aequorin, we show in this study that NFs elicit Ca2+-dependent luminescence, namely a sharp increase of cytosolic [Ca2+]. When cells are challenged with NFs, they lose their responsiveness to a subsequent treatment with un-derivatized chitin oligomers. This is reminiscent of earlier results obtained with suspension-cultured tomato cells. In this system, representing a non-host of rhizobia, NFs elicited a similar response as chitin oligomers with respect to transient alkalinization of the culture medium (Staehelin et al., 1994a) and correspondingly, the high-affinity binding site of these cells for chitin oligomers exhibited binding of NFs as well (Baureithel et al., 1994). Our data indicate that cell cultures of soybean, a host of rhizobia, perceive chitin oligomer elicitors and the structurally related NFs at submicromolar concentrations in a very similar way. Responsiveness to both CH4 and NFs has also been found in other leguminous model systems. Both CH4 and NFs stimulate in soybean roots the expression of the early nodulin gene enod40 (Minami et al., 1996) and induce the activity of a specific chitinase isoenzyme (Xie et al., 1999). Moreover, Medicago cell cultures respond to CH4 and NFs with expression of genes that are assumed to play a role in plant defense reactions against pathogens (Savouré et al., 1997). In contrast, there are examples of specific responsiveness to NFs, but not to CH4. Legume root hairs specifically perceive NFs, whereas un-derivatized chitin oligomers are inactive in inducing early root hair responses, such as cytosolic free [Ca2+] changes (Ehrhardt et al., 1996; Gehring et al., 1997; Felle et al., 1999). Furthermore, they induce a different pattern of rearrangements of the cytoskeleton (Cardenas et al., 1999).
Our data show that activity of NFs in inducing cytosolic [Ca2+] changes vary in function of the length and the substituents of the chitooligomer backbones. The responses elicited by sulfated or non-sulfated Rt factors are one-half as strong as those elicited by un-derivatized chitin oligomers. Thus, the sulfate group at the reducing end has no effect on the activity of Rt factors. The activity of O-acetylated NodSm-IV(Ac, S) is significantly reduced compared with NodSm-IV(S), indicating that the presence of an O-acetyl group at the non-reducing end reduces the calcium response.
Substituents at the reducing end influence the elicitor activity of NGR factors. When a methyl-Fuc residue with an acetyl group is present in NGR factors, the calcium response is attenuated compared with NGR factors lacking this modification. The presence of a sulfate decoration in NodNGR-V(Me-Fuc, S) reduces its elicitor activity to background levels.
Decorations of NFs have been shown to influence host specificity in certain interactions of legumes with rhizobia. The effect of the fucosyltransferase NodZ, which is required for fucosylation of NFs, has been investigated on various host plants (Stacey et al., 1994; Stokkermans et al., 1995; Lopez-Lara et al., 1996; Quesada-Vincens et al., 1997). O-acetylation at the reducing end of NFs plays an important role in nodule formation of certain pea lines harboring sym2A (Firmin et al., 1993; Geurts et al., 1997; Ovtsyna et al., 1998), whereas O-acetylation at the non-reducing end promotes nodulation of Medicago falcata (Ardourel et al., 1994).
How can these effects of NF substituents be explained? First, the substituents could influence the solubility of the NFs. Un-substituted chitooligomers are more water soluble than lipochitooligomers, and these molecules form micellar structures in water (Goedhart et al., 1999; Gonzalez et al., 1999). Polar substituents like sulfate groups may disrupt these micelles thus changing solubility of NFs. Second, chitooligomers and NFs may interact with receptors of the same type. Our observations concerning the refractory behavior of NFs and CH4 point into this direction. From this perspective, differences in NF responses could be explained by different affinities of NFs and chitooligomers. Third, chitooligomers and NFs may bind to different receptors having structural relationships. According to this model, NFs would elicit responses of different intensities according to their substituents whereas blocking receptors of chitooligomers and vice versa.
Chitinases and other glycosyl hydrolases are able to cleave NFs thereby inactivating their biological activity to stimulate host plant responses, such as root hair deformation (Heidstra et al., 1994; Staehelin et al., 1994b). Hydrolytic degradation also inactivates NFs in inducing responses that are mediated by the receptor for un-derivatized chitin oligomers, i.e. the alkalinization response of tomato cells (Staehelin et al., 1994a). Here, we have shown that NodSm-III and NodSm-II, degradation products from Sm factors, have a strongly reduced activity in inducing cytosolic [Ca2+] changes in soybean cells. Thus, shortening of the chitin oligomer backbone of NFs seems to affect binding to the postulated binding sites of soybean cells. Cleavage of NFs by host plant hydrolases could have the function to inactivate excess amounts of NFs (Staehelin et al., 1995), thereby avoiding the stimulation of defense reactions mediated by the perception system for chitin oligomers. “Poorly” decorated NFs could act in the same way as “chitin elicitors,” thereby activating defense responses inhibiting rhizobial infection. Nod factor cleaving hydrolases of the host plant could have the function to inactivate this elicitor activity (Staehelin et al., 1995; Ovtsyna et al., 2000).
Taken together, we suggest that the receptor for chitin oligomers, which plants have evolved to perceive chitin-containing organisms, can interfere with NF signaling in the interaction of legumes with rhizobia. Certain decorations of NFs can be seen as a symbiotic adaptation to avoid over-stimulation of the perception system for chitin oligomers, adding a new dimension of complexity to chemical communication between rhizobia and their host plants.
MATERIALS AND METHODS
Plant Material
Photo-autotrophic cell suspension cultures of soybean (Glycine max [L.] Merr.; SB-P; Horn et al., 1983) carrying the stably integrated plasmid pGNAAequ/neo2 expressing a transgene for apoaequorin (line 6.6.12) were cultivated as described (Mithöfer et al., 1999).
NFs and Chitin Oligomers
To test whether structural modifications in NFs influence their activity to elicit the observed [Ca2+] changes, the soybean cells were treated with differently substituted NFs purified from Rhizobium NGR234 (NGR factors), its mutant strain NGRΔNodZ1, Rhizobium tropici (Rt factors), and Sinorhizobium meliloti (Sm factors; formerly named Rhizobium meliloti [Rm] factors). These NFs differed in various chemical modifications (Fig. 1). The pentameric NGR factors are modified at the reducing end with a 2-O-methyl-Fuc carrying either a 4-O-acetyl substitution, i.e. NodNGR-V(Me-Fuc, Ac) or a 3-O-sulfate group, i.e. NodNGR-V(Me-Fuc, S). NFs from Rhizobium sp. NGR234 and its mutant strain NGRΔNodZ1 were purified as described (Price et al., 1992). The strain NGRΔNodZ 1 lacking the fucosyltransferase NodZ produces NGR factors without a terminal reducing end modification, i.e. NodNGR-V (Quesada-Vincens et al., 1997). Using similar HPLC running conditions, sulfated and non-sulfated NFs from R. tropici strain CFN 299 (Poupot et al., 1993) were purified. These NFs are either non-modified at the reducing end, i.e. NodRt-V, or decorated with a sulfate group, i.e. NodRt-V(S).
Three sulfated NFs carrying a C16:2 fatty acid were purified from S. meliloti, the pentameric NodSm-V(S), the tetrameric NodSm-IV(S), and the NodSm-IV(Ac, S), having an additional O-acetyl substitution at the non-reducing end. Moreover, two acylated degradation products derived from Sm factors without O-acetyl group were prepared, the lipotrisaccharide NodSm-III and the lipodisaccharide NodSm-II (Fig. 1). The NFs from S. meliloti (Lerouge et al., 1990; Schultze et al., 1992) and their hydrolytic degradation products (Staehelin et al., 1994b) were purified from strain 1021(pEK327) by reverse-phase HPLC, using isocratic conditions with 35% (v/v) acetonitrile/water, 40 mm ammonium acetate as the mobile phase (Staehelin et al., 1994b). De-acetylated NodSm-IV(S) was obtained from purified NodSm-IV(Ac, S) after incubation at 50 mm Tris-HCl (pH 10.5) at 37°C for 16 h. The purified Sm factors were desalted on a C18 column (Machery Nagel, Düren, Germany, Polygosil C18, 60–4063, particle size 40–63 μm) equilibrated with H2O, using 100% (v/v) methanol for elution. NFs were quantified either by determining the dry weight of the purified material or by measuring their absorption (peak area) at a given wavelength, followed by comparison with known standards.
The chitin oligomers N,N′,N",N‴-tetraacetylchitotetraose (CH4) and N,N′,N",N‴,N‴′-pentaacetylchitopentaose (CH5) were obtained from Seikagaku Corporation (Tokyo).
Aequorin Luminescence
Measurement of aequorin luminescence was performed according to Mithöfer et al. (1999). Briefly, 24 h prior to each experiment, cells were treated with coelenterazine (10 μm) in order to reconstitute active, Ca2+-sensitive aequorin in the cytosol. One batch of suspension-cultured cells was separated into aliquots, and each of the aliquots was treated with a different NF or CH4 preparation. For each assay, 0.1 mL of these “reconstituted cells” was carefully pipetted into a transparent polypropylene tube at room temperature, effectors were added in suitable amounts, and light emission at 470 nm was monitored over time using a luminometer (LKB 1250 Wallac Pharmacia Biotech, Uppsala, Sweden). Visualization and integration of peaks was performed using a chromatopac C-R4A (Shimadzu, Kyoto). In order to verify that the concentration of reconstituted aequorin was not limiting under any of the experimental conditions, cells were occasionally challenged with ice or dimethyl sulfoxide inducing maximal calcium responses (Mithöfer et al., 1999). These tests showed that the maximal consumption of aequorin never exceeded more than 10% of the total amount.
Statistics
Analyses of variance and Student-Newman-Keuls tests were performed using the software SigmaStat (Jandel Scientific, San Rafael, CA).
ACKNOWLEDGMENTS
We thank Dr. Eva Kondorosi (Institut des Sciences Végétales, Centre National de la Recherche Scientifique, Gif-sur-Yvette, France) for Sinorhizobium meliloti strain 1021 (pEK327). NFs from this strain were purified at the ISV. We thank Dr. Espéranza Martinez-Romero (Universidad Nacional Autonoma de Mexico, Cuernavaca, Morelos, Mexico) for providing Rhizobium tropici strain CFN 299. Our special thanks go to Prof. Dr. W.J. Broughton (University of Geneva) for providing purified Nod factors from Rhizobium sp. NGR234. We are indebted to Chantal Ebel (Friedrich Miesch Institute, Basel) for technical help with the aequorin cells.
Footnotes
This work was supported by the Swiss National Foundation and by a Roche Foundation fellowship (to J.M.).
LITERATURE CITED
- Ardourel M, Demont N, Debellé F, Maillet F, De Billy F, Promé J-C, Dénarié J, Truchet G. Rhizobium meliloti lipooligosaccharide nodulation factors: different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell. 1994;6:1357–1374. doi: 10.1105/tpc.6.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baureithel K, Felix G, Boller T. Specific, high affinity binding of chitin fragments to tomato cells and membranes: competitive inhibition of binding by derivatives of chitooligosaccharides and a Nod factor of Rhizobium. J Biol Chem. 1994;269:17931–17938. [PubMed] [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:198–214. [Google Scholar]
- Cardenas L, Feijo J, Kunkel JG, Sanchez F, Holdaway-Clarke T, Hepler P, Quinto C. Rhizobium Nod factors induce increases in intracellular free calcium and extracellular calcium influxes in bean root hairs. Plant J. 1999;19:347–352. doi: 10.1046/j.1365-313x.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- Cohn J, Day RB, Stacey G. Legume nodule organogenesis. Trends Plant Sci. 1998;3:105–110. [Google Scholar]
- De Ruijter NCA, Rook MB, Bisseling T, Emons AMC. Lipochito-oligosaccharides re-initiate root hair tip growth in Vicia sativa with high calcium and spectrin-like antigen at the tip. Plant J. 1998;13:341–350. [Google Scholar]
- Ehrhardt DW, Atkinson EM, Long SR. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science. 1992;256:998–1000. doi: 10.1126/science.10744524. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Etzler ME, Kalsi G, Ewing NN, Roberts NJ, Day RB, Murph JB. A Nod factor binding lectin with apyrase activity. Proc Natl Acad Sci USA. 1999;96:5856–5861. doi: 10.1073/pnas.96.10.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Baureithel K, Boller T. Desensitization of the perception system for chitin fragments in tomato cells. Plant Physiol. 1998;117:643–650. doi: 10.1104/pp.117.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Regenass M, Boller T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 1993;4:307–316. [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. Nod signal-induced plasma membrane potential changes in alfalfa root hairs are differentially sensitive to structural modifications of the lipochitooligosaccharide. Plant J. 1995;7:939–947. [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. Nod factors modulate the concentration of cytosolic free calcium differently in growing and non-growing root hairs of Medicago sativa L. Planta. 1999;209:207–212. doi: 10.1007/s004250050624. [DOI] [PubMed] [Google Scholar]
- Firmin JL, Wilson KE, Carlson RW, Davies AE, Downie JA. Resistance to nodulation of cv Afghanistan peas is overcome by nodX, which mediates an O-acetylation of the Rhizobium leguminosarum lipo-oligosaccharide nodulation factor. Mol Microbiol. 1993;10:351–360. doi: 10.1111/j.1365-2958.1993.tb01961.x. [DOI] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Kabbara AA, Parish RW, Boukli NM, Broughton WJ. Rapid, plateau-like increases in intracellular free calcium are associated with Nod-factor-induced root-hair deformation. Mol Plant Microbe Interact. 1997;10:791–802. [Google Scholar]
- Geurts R, Heidstra R, Hadri AE, Downie JA, Franssen H, van Kammen A, Bisseling T. Sym2 of pea is involved in a nodulation factor-perception mechanism that controls the infection process in the epidermis. Plant Physiol. 1997;115:351–359. doi: 10.1104/pp.115.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedhart J, Rohrig H, Hink M, van Hoek A, Visser A, Bisseling T, Gadella T. Nod factors integrate spontaneously in biomembranes and transfer rapidly between membranes and to root hairs, but transbilayer flip-flop does not occur. Biochem. 1999;38:10898–10907. doi: 10.1021/bi990714q. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Bernabe M, Espinoza J, Tejero-Mateo P, Gil-Serrano A, Mantegazza N, Imberty A, Driguez H, Jimenez-Barbero J. Solvent dependent conformational behavior of lipooligosaccharides related to Nod factors. Carbohydr Res. 1999;318:10–19. doi: 10.1016/s0008-6215(99)00082-8. [DOI] [PubMed] [Google Scholar]
- Gressent F, Drouillard S, Mantegazza N, Samain E, Geremia R, Canut H, Niebel A, Driguez H, Ranjeva R, Cullimore J, Bono J-J. Ligand specificity of a high-affinity binding site for lipo-chitooligosaccharidic Nod factors in Medicago cell suspension cultures. Proc Natl Acad Sci USA. 1999;96:4707–4709. doi: 10.1073/pnas.96.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidstra R, Geurts R, Franssen H, Spaink HP, van Kammen A, Bisseling T. Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol. 1994;105:787–797. doi: 10.1104/pp.105.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn ME, Sherrard JH, Widham JM. Photoautotrophic growth of soybean cells in suspension culture. Plant Physiol. 1983;72:426–429. doi: 10.1104/pp.72.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Kaku H, Shibuya N. Identification of a high-affinity binding protein for N-acetylchitooligosaccharide elicitor in the plasma membrane of suspension-cultured rice cells by affinity labeling. Plant J. 1997;12:347–356. doi: 10.1046/j.1365-313x.1997.12020347.x. [DOI] [PubMed] [Google Scholar]
- Knight M, Campbell A, Smith S, Trewavas A. Transgenic plant aequorin reports the effect of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Promé J-C, Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990;344:781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Long SR. Rhizobium symbiosis: Nod factors in perspective. Plant Cell. 1996;8:1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lara IM, Blok-Tip L, Quinto C, Garcia ML, Stacey G, Bloemberg GV, Lamers GEM, Lugtenberg BJJ, Thomas-Oates JE, Spaink HP. NodZ of Bradyrhizobium extends the nodulation host range of Rhizobium by adding a fucosyl residue to nodulation factors. Mol Microbiol. 1996;21:397–408. doi: 10.1046/j.1365-2958.1996.00644.x. [DOI] [PubMed] [Google Scholar]
- Minami E, Kouchi H, Cohn JR, Ogawa T, Stacey G. Expression of the early nodulin, ENOD40, in soybean roots in response to various lipo-chitin signal molecules. Plant J. 1996;10:23–32. doi: 10.1046/j.1365-313x.1996.10010023.x. [DOI] [PubMed] [Google Scholar]
- Minic Z, Brown S, De Kouchkovsky Y, Schultze M, Staehelin C. Purification and characterization of a novel chitinase-lysozyme, of another chitinase, both hydrolyzing Rhizobium meliloti Nod factors, and of a pathogenesis-related protein from Medicago sativa roots. Biochem J. 1998;332:329–335. doi: 10.1042/bj3320329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithöfer A, Ebel J, Bhagwat AA, Boller T, Neuhaus-Url G. Transgenic aequorin monitors cytosolic calcium transients in soybean cells challenged with β-glucan or chitin elicitors. Planta. 1999;207:566–574. [Google Scholar]
- Ovtsyna AO, Geurts R, Bisseling T, Lugtenberg BJJ, Tikhonovich IA, Spaink HP. Restriction of host range by the sym2 allele of Afghan pea is non-specific for the type of modification at the reducing terminus of nodulation signals. Mol Plant Microbe Interact. 1998;11:418–422. [Google Scholar]
- Ovtsyna AO, Schultze M, Tikhonovich I, Spaink H, Kondorosi E, Kondorosi A, Staehelin C. Nod factors of Rhizobium leguminosarum bv viciae and their fucosylated derivatives stimulate a Nod factor cleaving activity in pea roots and are hydrolyzed in vitro by plant chitinases at different rates. Mol Plant Microbe Interact. 2000;13:799–807. doi: 10.1094/MPMI.2000.13.8.799. [DOI] [PubMed] [Google Scholar]
- Poupot R, Martinez-Romero E, Promé J-C. Nodulation factors from Rhizobium tropici are sulfated or non-sulfated chitopentasaccharides containing an N-methyl-N-acylglucosamine terminus. Biochemistry. 1993;32:10430–10435. doi: 10.1021/bi00090a019. [DOI] [PubMed] [Google Scholar]
- Price NPJ, Relic B, Talmont F, Lewin A, Promé D, Pueppke SG, Maillet F, Dénarié J, Promé J-C, Broughton WJ. Broad host-range Rhizobium species strain NGR234 secretes a family of carbamoylated, and fucosylated, nodulation signals that are O-acetylated or sulphated. Mol Microbiol. 1992;6:3575–3584. doi: 10.1111/j.1365-2958.1992.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Quesada-Vincens D, Fellay R, Nasim T, Viprey V, Burger U, Promé J-C, Broughton WJ, Jabbouri S. Rhizobium sp. strain NGR234 NodZ protein is a fucosyltransferase. J Bacteriol. 1997;179:5087–5093. doi: 10.1128/jb.179.16.5087-5093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savouré A, Sallaud C, El-Turk J, Zuanazzi J, Ratet P, Schultze M, Kondorosi A, Esnault R, Kondorosi E. Distinct response of Medicago suspension cultures and roots to Nod factors and chitin oligomers in the elicitation of defense-related responses. Plant J. 1997;11:277–287. [Google Scholar]
- Schultze M, Kondorosi A. Regulation of symbiotic root nodule development. Annu Rev Genet. 1998a;32:33–57. doi: 10.1146/annurev.genet.32.1.33. [DOI] [PubMed] [Google Scholar]
- Schultze M, Quiclet-Sire B, Kondorosi E, Virelizier H, Glushka JN, Endre G, Géro SD, Kondorosi A. Rhizobium meliloti produces a family of sulfated lipooligosaccharides exhibiting different degrees of plant host specificity. Proc Natl Acad Sci USA. 1992;89:192–196. doi: 10.1073/pnas.89.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze M, Staehelin C, Brunner F, Genetet I, Legrand M, Fritig B, Kondorosi E, Kondorosi A. Plant chitinase/lysozyme isoforms show distinct substrate specificity and cleavage site preference towards lipochitooligosaccharide Nod signals. Plant J. 1998b;16:571–580. [Google Scholar]
- Stacey G, Luka S, Sanjuan J, Banfalvi Z, Niewkoop AJ, Chun JZ, Forsberg LS, Carlson R. nodZ, a unique host-specific nodulation gene, is involved in the fucosylation of the lipooligosaccharide nodulation signal of Bradyrhizobium japonicum. J Bacteriol. 1994;176:620–633. doi: 10.1128/jb.176.3.620-633.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G, Shibuya N. Chitin recognition in rice and legumes. Plant Soil. 1997;194:161–169. [Google Scholar]
- Staehelin C, Granado J, Müller J, Wiemken A, Mellor RB, Felix G, Regenass M, Broughton WJ, Boller T. Perception of Rhizobium nodulation factors by tomato cells and inactivation by root chitinases. Proc Natl Acad Sci USA. 1994a;91:2196–2200. doi: 10.1073/pnas.91.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin C, Schultze M, Kondorosi E, Kondorosi A. Lipo-chitooligosaccharide nodulation signals from Rhizobium meliloti induce their rapid degradation by the host plant alfalfa. Plant Physiol. 1995;108:1607–1614. doi: 10.1104/pp.108.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin C, Schultze M, Kondorosi E, Mellor RB, Boller T, Kondorosi A. Structural modifications in Rhizobium meliloti Nod factors influence their stability against hydrolysis by root chitinases. Plant J. 1994b;5:319–330. [Google Scholar]
- Staehelin C, Schultze M, Tokuyasu K, Poinsot V, Promé J-C, Kondorosi E, Kondorosi A. N-deacetylation of Sinorhizobium meliloti Nod factors increases their stability in the Medicago sativa rhizosphere and decreases their biological activity. Mol Plant Microbe Interact. 2000;13:72–79. doi: 10.1094/MPMI.2000.13.1.72. [DOI] [PubMed] [Google Scholar]
- Stokkermans TJW, Ikeshita S, Cohn J, Carlson RW, Stacey G, Ogawa T, Peters NK. Structural requirements of synthetic and natural product lipo-chitin oligosaccharides for induction of nodule primordia on Glycine soja. Plant Physiol. 1995;108:1587–1595. doi: 10.1104/pp.108.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie ZP, Staehelin C, Wiemken A, Broughton WJ, Müller J, Boller T. Symbiosis-stimulated chitinase isoenzymes of soybean (Glycine max (L.) Merr.) J Exp Bot. 1999;50:327–333. [Google Scholar]