Abstract
Grade 3 endometrioid and uterine serous carcinomas (USC) account for the vast majority of endometrial cancer deaths. The purpose of this study was to determine folic acid receptor alpha (FRα) expression in these biologically aggressive (Type II) endometrial cancers, and evaluate FRα as a targetable receptor for IMGN853 (Mirvetuximab soravtansine). The expression of FRα was evaluated by immunohistochemistry (IHC) and flow cytometry in 90 endometrioid and USC samples. The in vitro cytotoxic activity and bystander effect were studied in primary uterine cancer cell lines expressing differential levels of FRα. In vivo antitumor efficacy of IMGN853 was evaluated in xenograft/patient derived xenograft (PDX) models. Semi-quantitative IHC analysis indicated that 41% of the USC patients overexpress FRα. Further, overexpression of FRα (ie, 2+) was detected via flow cytometry in 22% (2/9) of primary endometrioid and in 27% (3/11) of primary USC cell lines. Increased cytotoxicity was seen with IMGN853 treatment compared to control in 2+ expressing uterine tumor cell lines. In contrast, tumor cell lines with low FRα showed no difference when exposed to IMGN853 versus control. IMGN853 induced bystander killing of FRα = 0 tumor cells. In an endometrioid xenograft model (END(K)265), harboring 2+ FRα, IMGN853 treatment showed complete resolution of tumors (p< 0.001). Treatment with IMGN853 in USC PDX model (BIO(K)1), expressing 2+ FRα, induced 2-fold increase in median survival (p< 0.001). IMGN853 shows impressive anti-tumor activity in biologically aggressive FRα 2+ uterine cancers. This preclinical data suggests that patients with chemotherapy resistant/recurrent endometrial cancer overexpressing FRα may benefit from this treatment.
Keywords: mirvetuximab soravtansine, IMGN853, Folic Acid Receptor, Antibody drug conjugate (ADC), uterine cancer
INTRODUCTION
Uterine cancer is the most common gynecologic cancer in the United States (1,2) with 61,380 cases leading to an estimated 10,920 deaths in 2017 (1, 2). Mortality rates for uterine cancer have increased by more than 100% in the last 2 decades (3), emphasizing the need to identify novel treatments for advanced/recurrent disease. Therapy selection for these tumors depends upon the type of uterine cancer, eg. type I versus type II. Type I tumors are well, or moderately differentiated tumors with endometrioid histology and are typically associated with obesity and excess estrogen. At early FIGO stages the striking majority of these tumors are successfully treated with surgery alone (4–6). Type II/biologically aggressive tumors, conversely, harbor multiple histopathologic subtypes (ie., poorly differentiated endometrioid tumors, serous, clear cell and carcinosarcomas) and are considered, even at early stages, to be highly aggressive neoplasms with high risk of relapse and poor overall survival. The poor clinical features of these tumors typically necessitate treatment with surgery, radiation and chemotherapy (4,7–12). Collectively, biologically aggressive subtypes represent only about 25% of all uterine cancers, but account for more than 50% of all uterine cancer deaths (13,14). This poor prognosis emphasizes the need to identify novel treatment options for patients with recurrent type II disease resistant to conventional chemotherapy.
Antibody drug conjugates (ADC) are a class of targeted therapy combining an antibody targeting a surface receptor with a toxic payload, thereby allowing selective delivery of a chemotherapeutic agent to tumor cells. One example of a successful ADC is TDM1 (Kadcyla, Genentech/Roche) which has shown excellent preclinical results in uterine serous and ovarian cancer cells overexpressing HER2/neu receptors (15,16) and is currently Food and Drugs Administration (FDA) and European Medical Agency (EMA) approved for the treatment of HER2-positive metastatic breast cancer patients who previously were treated with trastuzumab and a taxane chemotherapy. A more recently developed ADC, IMGN853 (Immunogen, Waltham, MA), is composed of a humanized antibody (M9346A) with high affinity to folic acid receptor alpha (FRα) attached via a cleavable disulfide-containing hydrophilic linker (sulfo-SPDB), to the maytansinoid DM4, a potent microtubule toxin (17,18) IMGN853 binds with selectivity to tumor cells expressing FRα and is internalized by receptor mediated endocytosis. Importantly, once intracellular, IMGN853 is degraded by acidic lysosomes allowing DM4 to inhibit microtubules, resulting in cell-cycle arrest and apoptosis. IMGN853 can also induce bystander cytotoxic activity. This action is considered to be particularly important for the activity of the ADC against tumors with heterogeneous expression of FRα (ie, negative or low FRα expressing malignant cells intermixed with FRα highly expressing tumor cells) (18).
FRα is a glycosylphosphatidylinositol-anchored high-affinity folate receptor that localizes to the apical surface of epithelia and shows a restricted distribution pattern in normal tissues, with expression limited to a variety of polarized epithelia, such as those found in the choroid plexus, kidney, uterus, ovary, lung, and placenta (19–21). Unlike normal tissues, FRα may localize to the basolateral side in many tumors, and accordingly, epithelial ovarian cancer (EOC) and endometrial cancer have recently been identified as a target for anti-folic acid receptor agents. Several reports have indicated an increased expression of FRα in a large number of patients with EOC (19–21), and uterine cancer (22–24) and consistent with this view, the activity of IMGN853 is currently being tested in Phase II/III clinical trials with promising activity reported in platinum-resistant ovarian cancer patients (25–27).
The objective of this study was to evaluate the expression of FRα in biologically aggressive endometrial cancers and to examine the preclinical anti-tumor activity of IMGN853 against primary endometrioid and USC cell lines with differential FRα expression. We demonstrate for the first time that IMGN853 is highly active, both in vitro as well as in vivo, against poorly differentiated, biologically aggressive endometrial tumors which cause the overwhelming majority of endometrial cancer deaths. Clinical studies with IMGN853 in patients harboring FRα overexpressing endometrial tumors resistant to chemotherapy are warranted.
MATERIALS AND METHODS
Establishment of endometrioid and serous uterine cancer cell lines
Approval for this study was obtained through the Institutional Review Board (IRB). All patients signed consent before tissue collection per institutional guidelines. Nine primary endometrial endometrioid and 11 primary uterine serous cell lines were established from fresh tumor biopsy samples as described previously (28–31). In brief, tumors were processed by mechanical disruption in an enzymatic solution of 0.14% collagenase type I (Sigma) and 0.01% DNase (Sigma) in RPMI 1640. The resulting solution was incubated for 45 minutes at room temperature while stirring. The samples were then washed with RPMI 1640/10% FBS and plated in Petri dishes using RPMI 1640, 10% FBS, 1% penicillin with streptomycin and 1% amphotericin. The cell lines were kept in an incubator at 37 degrees Celsius with 5% CO2. The cell cultures were continually monitored for growth. Primary cell lines with limited passages (<50) were utilized in the experiments. Tumors were initially staged per the International Federation of Gynecology and Obstetrics (FIGO) staging system.
Tissue Microarray
A retrospective, stage I–IV uterine (USC) cohort represented in tissue microarray (TMA) format, was used in this study (USC N = 70). Cases were collected between 1981 and 2014. Briefly, representative areas from primary tumors were selected in hematoxylin/eosin–stained preparations by a pathologist and 0.6 mm cores were obtained using a needle and arrayed in a recipient block. To increase representation and capture possible marker heterogeneity, 4 cores obtained from different areas of each tumor were included in the TMAs. Sections of the resultant TMA were cut and transferred to glass slides for histology processing and staining. Tissues were collected with specific consent or waived consent under an approved Yale Human Investigation committee protocol.
Immunostaining of Tissue Microarrays, and Cell Blocks of Primary Endometrial Endometrioid and Uterine Serous Cancer Cell Lines
Formalin-fixed paraffin-embedded cell lines, patient derived xenograft (PDX) tumors and human xenograft tumors were evaluated by immunohistochemistry (IHC) for FRα using anti-FOLR1 alpha antibody (FOLR1-2.1(353.2.1, Immunogen). Five μm cell pellet and tissue sections were prepared and along with the TMAs, stained with FOLR1-2.1 using Ventana Medical System’s Discovery Ultra instrument. H-score was calculated by determining the level of FRα expression (ie, intensity level of membrane staining on each cell (0–3, 0 = negative, 1 = weak, 2 = moderate and 3 = strong), and the percentage of cells in a representative field at each staining intensity (0–100%) as follows: [1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+)], with a final score, ranging from 0 to 300. Appropriate positive (KB cell line) and negative controls (namalwa cell line) were used with each case. All staining was evaluated and scored by a board-certified pathologist.
Determination of folic acid receptor expression in endometrioid and uterine serous cancer cell lines
Primary endometrial endometrioid and USC cell lines were analyzed by flow cytometry for FRα expression after being cultured in vitro for up to 50 passages. Briefly, the endometrial endometrioid, mixed endometrioid/clear cells and serous uterine cancer cell lines were incubated with 2.5 μg/mL of M9346A for 120 min at 7 degrees Celsius. For staining, a fluorescein isothiocyanate-conjugated goat anti-human F(ab1)2 immunoglobulin (FITC) was used as a secondary reagent (BioSource International, Camarillo, CA). Comparisons were made between the isotype control Mab and the cell line stained with M9346A while the corresponding cell blocks were analyzed for FRα by IHC. Analysis was conducted with a FACScalibur, using Cell Quest software (BD Biosciences, San Diego, CA). Cell viability was determined by identifying all viable and all non-viable cells, identified by incubating with propidium iodide (PI) staining (2μl of 500 μg/mL stock solution in PBS). Data analysis was performed using Cell Quest (BD Biosciences) and Prism 7.01. Cell lines with a Mean fluorescence index (MFI) greater than 50 were determined to have 2+ expression of FRα, while cell lines with an MFI of 20 to 50 were noted to have 1+ and 20 or less was 0.
Cell Viability Assays in endometrial endometrioid and USC cell lines
Endometrial endometrioid and uterine serous cancer cell lines were plated at log phase of growth in 6-well tissue culture plates at a density of 20,000–40,000 cells per well in RPMI 1640 media (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Mediatech, Manassas, VA), and 1 % amphotericin (Life Technologies, Carlsbad, CA) as previously described (28). Cells were incubated at 37ºC, 5% CO2 for 24 hours. After this brief incubation, cells were treated with either IMGN853 (M9346A-sulfo-SPDB-DM4), non-targeting ADC isotype control (chKTI-sulfo-SPDB-DM4) or the naked Mab M9346A (Immunogen, Waltham, MA). IMGN853, ADC isotype control and M9346A were used at an equivalent antibody concentrations to determine the IC50. After 72 hours of incubation, cells were harvested, centrifuged and stained with propidium iodide (2μl of 500 μg/mL stock solution in PBS). Analysis was performed using a flow cytometry based assay to quantify percent viable cells as a mean ± SEM relative to untreated cells as 100% viable controls. A minimum of three independent experiments per cell line was performed.
Bystander Effect
Briefly, a 1:1 ratio of 2+ FRα expressing uterine endometrioid cells (i.e., END(K)265) and FRα negative uterine serous cells (i.e., ARK4) stably transfected with a Green Fluorescence Protein (GFP) plasmid (pCDH-CMV-MCSEF1-copGFP, a gift from Dr. Simona Colla, MDACC), were mixed (40,000 cells/well of each cell type) and plated in 6-well plates (2 mL/well). After an overnight incubation, IMGN853 or isotype control ADC at a concentration of 0.1μg/ml or vehicle were added. After a 72-hour incubation, cells were harvested, centrifuged and stained with propidium iodide (2μl of 500 μg/mL stock solution in PBS) to identify percentage of cell killing. Analysis was performed using flow cytometry based assay to quantify cell killing as a mean ± SEM relative to untreated cells. After determining the percentage of cell killing the formula, percentage of cell killing in negative FRα cells/percentage of cell killing in positive FRα cells, was utilized to calculate cell death in the low FRα tumor cells (ARK4) relative to tumor cells with high FRα expression (END(K)265).
In vivo treatment of xenograft model of endometrial endometrioid cancer
The in vivo antitumor activity of IMGN853, ADC isotype control and M9346A was compared in xenograft models with a grade 3 endometrioid/clear cell tumor harboring 2+ FRα expression (END(K)265). Six to eight week old CB-17/SCID mice were given a single subcutaneous injection of 15 × 106 END(K)265 cells in approximately 300 μl of a 1:1 solution of sterile PBS containing cells and Matrigel (BD Biosciences). Once the average group size tumor volume was approximately 0.159 cm3, the mice were randomized into treatment groups (ie, 6 to 7 per group); each group was treated with either IMGN853 (5 mg/kg), isotype control (5 mg/kg), M9346A (5 mg/kg) or PBS. Drug dosages were chosen according to previous studies conducted on xenograft models (18,32). All treatment drugs were given as retro-orbital intravenous (IV) weekly injections for two doses based on prior literature (18,32). Tumor measurements were recorded twice weekly initially, then once weekly. Tumor volume was determined using the formula (A2 * B)/2, where B represented the largest tumor diameter size and A was the smaller perpendicular tumor diameter. Mice were sacrificed when the group’s average tumor volume reached 1.0 cm3. Animal care and euthanasia were carried out according to the rules and regulations as set forth by the Institutional Animal Care and Use Committee (IACUC).
In vivo model of uterine serous cancer
Efficacy of IMGN853 in vivo was evaluated on a patient-derived xenograft (PDX) model of uterine serous cancer, BIO(K)1, as described in Methods. Briefly, patient tissue collection was completed per institutional guidelines. BIO(K)1 was obtained from a core biopsy sample of metastatic serous uterine cancer prior to therapy. Tissue from the biopsy was directly engrafted subcutaneously on the flank region of CB-17/SCID mice using 3–5 mm3 fragments, 150 μl of Matrigel (BD Biosciences) was placed into the same subcutaneous region. These tumor xenografts were serially passaged and expanded in the CB-17/SCID mice until adequate tumor volumes were obtained to initiate a study with IMGN853, ADC isotype control and M9346A; utilizing 5–6 mice per group. Tumor measurements and treatments were completed as previously outlined. Mice were sacrificed when tumor volume reached 1.5 cm3.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 7.01 (GraphPad Software, Inc. San Diego, CA). The differences in the inhibition of proliferation in the endometrial endometrioid and uterine serous cell lines after exposure to treatments were evaluated by two-tailed unpaired student t-test. In all in vivo experiments aimed at analyzing survival and tumor growth 6 animals per group were used. This group size was calculated as the minimal group size to observe a difference in survival of 50% with 80% chance at significance of 0.05. Overall survival data were analyzed and plotted using the Kaplan-Meier method. Survival curves were compared using the log-rank test. Differences in all comparisons were considered statistically significant with a two-sided p-value of less than 0.05.
RESULTS
Folic acid receptor alpha expression in uterine serous cancer patient samples by Tissue Microarray (TMA)
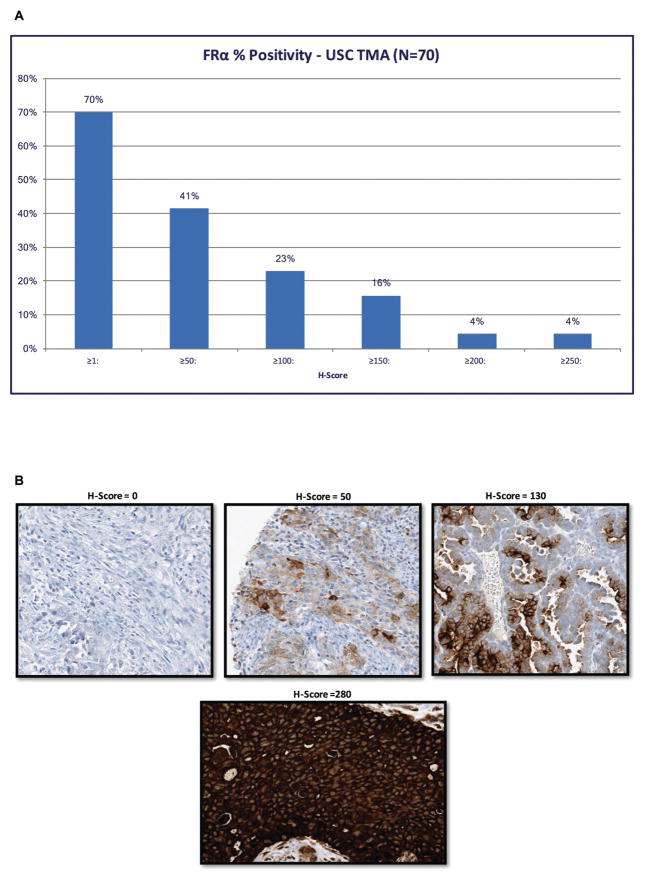
A tissue microarray containing 70 uterine serous cancer samples was used to semi-quantitatively analyze FRα expression by IHC. 41% of these biologically aggressive tumors were found to express FRα with an H-score ≥ 50 (Figure 1A). A representative IHC image of the H-Score range is presented in Figure 1B.
FIGURE 1.
Results of folic acid receptor IHC on TMAs of uterine serous cancer (USC). (A) FRα percent positivity in USC cases, 70 patient samples stained for FRα, H-Score was used to quantify the FRα immunohistochemistry (IHC) staining. Forty-one percent of the patients had an H-Score of 50 or higher. TMA, Tissue Microarray. (B) Representative immunohistochemistry of the FRα expression in uterine serous carcinoma with the corresponding semi-quantitative H-Score from ImmunoGen using the Ventana method.
Folic acid receptor expression in primary uterine cancer cell lines by flow cytometry
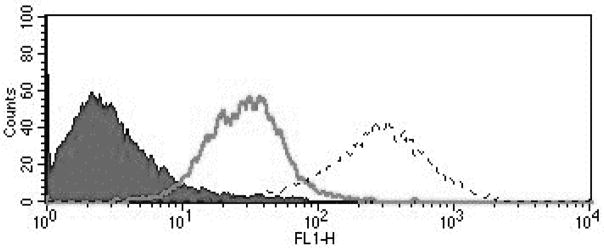
Nine primary endometrial endometrioid and 11 primary uterine serous cell lines were established from fresh tumor biopsy samples as described in Methods. Stage, grade, histology and primary site of the tumors is displayed in Supplementary Table S1 and Supplementary Table S2. Cell lines with a Mean fluorescence index (MFI) greater than 50 were determined to have 2+ expression of FRα, while cell lines with an MFI of 20 to 50 were noted to have 1+ and 20 or less was 0. Twenty-two percent of the endometrioid cell lines were determined to have 2+ FRα expression while 27% of the uterine serous cancer cell lines were determined to have 2+ FRα expression by flow cytometry. Figure 2 depicts a representative flow cytometry histogram of primary uterine cancer cell lines showing 0, 1+, 2+ folic acid alpha receptor (FRα) expression (Figure 2). Similar flow cytometry histograms were generated for the USC cell lines. Supplementary Fig. S1 and Supplementary Fig. S2 show representative IHC of endometrioid and USC cell blocks stained with FRα. The corresponding H-scores and flow cytometry scores are given for each image.
FIGURE 2.
Flow cytometry histograms of primary uterine cancer cell lines showing 0 (END(K)153, black), 1+ (END(K)34, solid gray line), 2+ (END(K)265, dashed line) folic acid alpha receptor (FRα) expression. FRα 1+ and 2+ show significantly higher mean fluorescence intensity versus the 0 FRα expression cell lines.
In vitro cytotoxicity in endometrial endometrioid and CC/Endometrioid cancer cell lines
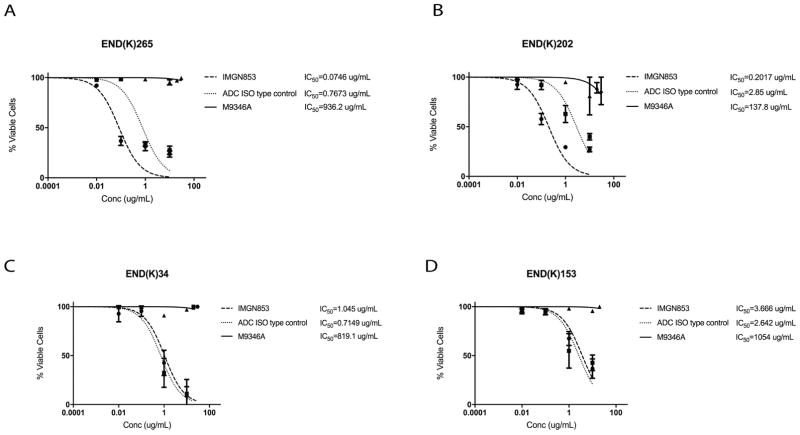
Cell cytotoxicity was tested with IMGN853, ADC isotype control and M9346A in pure and mixed endometrial endometrioid cell lines harboring high FRα expression (2+ cell lines by flow cytometry). When treating the high FRα expressing cell lines, END(K)265 (G3, endometrioid/clear cell) and END(K)202 (G1, endometrioid), with IMGN853 there was a 10-fold (p< 0.001) and 14-fold (p< 0.01) increase in cell cytotoxicity, respectively, compared to the ADC isotype control, Figure 3a and 3b. When the low expressing FRα cell lines, END(K)34 (FRα=1+) and END(K)153 (FRα=0), were treated with IMGN853, there was no increase in cell cytotoxicity when compared to the ADC isotype control group (p=0.9309 and p=0.7010) Figure 3c and 3d. The IC50 of M9346A (ie, naked MAb) was extrapolated to be 900 to 10,000 fold higher than IMGN853 IC50 for all cell lines as there is no activity of the naked MAb in the in vitro studies.
FIGURE 3.
Determination by IC50 of IMGN853 cytotoxicity compared to controls, ADC isotype and M9346A, in endometrial endometrioid cancer. (A) High FRα expressing (2+) endometrial endometrioid cell lines, END(K)265 (endometrioid/clear cell, FIGO grade 3) and (B) END(K)202 (endometrioid, FIGO grade 1). IMGN853 shows significantly lower IC50s when compared to ADC isotype control (p<0.001, and 0.01) in both cell lines. (C) Endometrial endometrioid cell lines with lower FRα expression, END(K)34 (FRα=1+) and (D) END(K)153 (FRα=0), show no difference in the IC50s of IMGN853 and ADC isotype (p=0.9309 and 0.7010). M9346A antibody was inactive against these cell lines.
In vitro cytotoxicity in uterine serous cancer
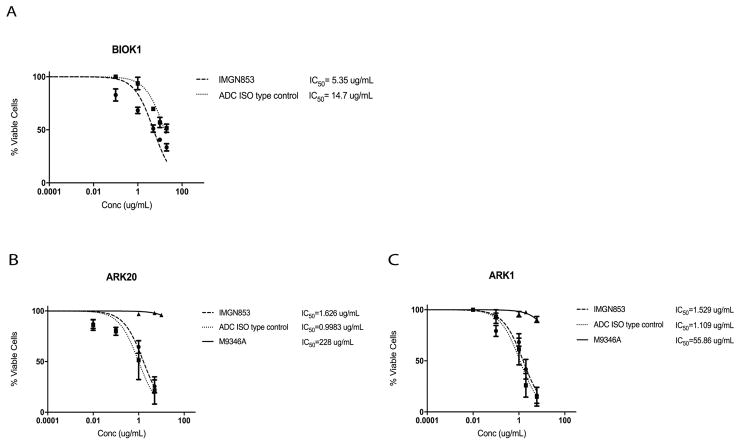
IMGN853 cytotoxicity was tested in uterine serous cancer cell lines expressing high FRα (FRα=2+ by flow cytometry). BIO(K)1 tumor cells, a uterine serous tumor harvested from a patient derived xenograft model with FRα 2+ expressivity, showed a 2.7-fold increase in cell cytotoxicity when compared to the ADC isotype control (p<0.05) (Figure 4a). The low expressing FRα cell lines, ARK20 (FRα=1+) and ARK1 (FRα=0), showed no difference in cell viability when treated with IMGN853 or ADC isotype control, the naked MAb does not show activity (Figure 4b and 4c). Two additional primary USC cell lines with FRα=2+ by flow cytometry (ie, END(K)149 and ARK19) showed a 4.6-fold and a 3.6-fold increase in cell cytotoxicity, respectively, when compared to the ADC isotype control (p<0.05) (Supplementary Fig. S3).
FIGURE 4.
Determination by IC50 of IMGN853 cytotoxicity compared to controls, ADC isotype and M9346A, in uterine serous carcinoma (USC). (A) High FRα expressing (2+) uterine serous cancer cell line harvested from a patient derived xenograft model. IMGN853 shows significantly lower IC50 when compared to ADC isotype control (2.7 fold decrease, p<0.05). (B) Uterine serous cancer cell lines with lower FRα, ARK20 (FRα=1+) and (C) ARK1 (FRα=0) show no difference in the IC50s of IMGN853 and ADC isotype control. M9346A antibody was inactive against these cell lines.
Bystander Effect in vitro
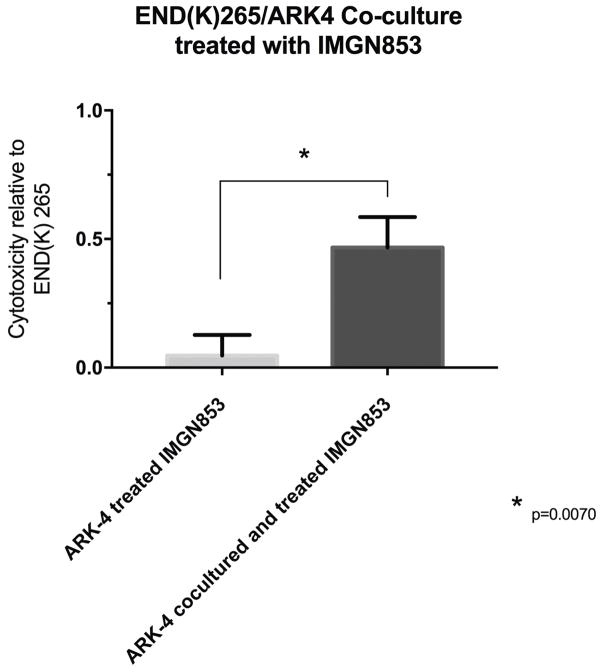
The ability of IMG853 to induce a bystander killing effect against endometrial tumors with heterogeneous FRα expression was tested by admixing END(K)265 (ie, high FRα expression) in vitro with low/negligible FRα expressing cells (i.e., GFP-ARK4 cells) for 72 hours. As shown in Figure 5, a 10-fold increase in cytotoxicity of ARK4 cells was seen when ARK4 and END(K)265 were treated as co-cultures with 0.1 μg/mL of IMGN853 when compared to IMGN853-treated ARK4 monocultures (p<0.01).
FIGURE 5.
Bystander effect assay. Gray bar: Low FRα expressing cells, ARK4 (GFP-ARK4 cells) treated with IMGN853 at 0.1 μg/mL. Black bar: Low FRα expressing cells (ARK4 with GFP) co-cultured in 1:1 fashion with high FRα expressing cells (END(K)265), and treated with IMGN853 at 0.1 μg/mL. Ten-fold increase in ARK4 cell cytotoxicity seen relative to END(K)265 (p=0.01).
In vivo antitumor activity
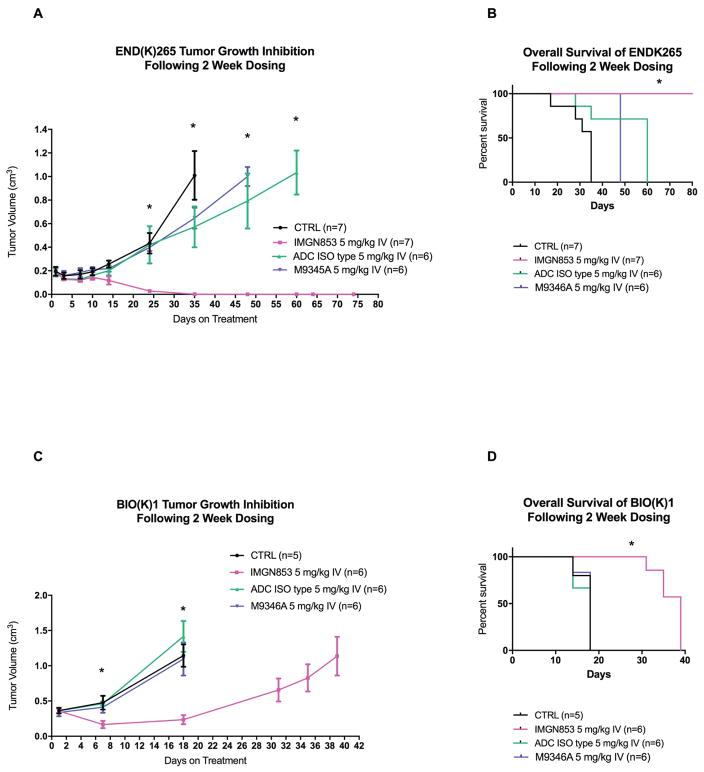
In vivo experiments comparing the antitumor activity of IMGN853 versus ADC isotype control, PBS and M9346A were conducted using both high grade endometrioid/clear cell xenografts (ie, END(K)265) as well as a uterine serous tumor PDX model (ie, BIO(K)1). As described in the Methods section, all treatments were given twice, one week apart by retro-orbital injection at a concentration of 5 mg/kg. The treatment, IMGN853 5 mg/kg IV (intravenous), showed inhibition of tumor growth in the high grade endometrioid/clear cell xenograft model (END(K)265) beginning on day 14. A statistical significance between IMGN853 and the controls: ADC isotype control, M9346A and PBS, was detected on day 24 and beyond (p<0.01, 0.001, 0.001), Figure 6a. IMGN853 had a significantly longer overall survival (p<0.001) when compared to the ADC ISO type control, M9346A and PBS control in the high grade endometrial endometrioid model (END(K)265); no tumor recurrence or death was identified at the time of final analysis. Median survival for IMGN853 was greater than 78 days versus 60 days, 48 days and 35 days for the ADC isotype control, M9346A and PBS control, respectively (p<0.001, 0.001, and 0.001), Figure 6b. In vivo antitumor activity of IMGN853 was also seen in the uterine serous cancer PDX model. IMGN853 5 mg/kg IV treatment showed inhibition of tumor growth in the uterine serous tumor BIO(K)1 patient derived xenograft model beginning on day 7. A statistical significance between IMGN853 and the controls, ADC isotype control, M9346A and PBS was detected at day 7 (p<0.001, 0.05, 0.05), Figure 6c. The group treated with IMGN853 had a significantly longer overall survival (p<0.001) when compared to the ADC isotype control, M9346A and PBS control. Median survival for IMGN853 was 39 days versus the median survival of 18 days for ADC isotype control, M9346A and PBS control, Figure 6d.
FIGURE 6.
In vivo efficacy of IMGN853. (A) Antitumor activity of IMGN853 was compared to controls, PBS CTRL, ADC isotype, and M9345A, in endometrial endometrioid xenograft tumor models with END(K)265 (FRα=2+). Mice were treated intravenously with two doses administered once a week as described in Methods. A significant difference in tumor growth inhibition was detected on day 24 (p<0.01) in the IMGN853-treated group when compared to the other control groups. (B) Overall survival of IMGN853 was compared to controls, PBS CTRL, ADC isotype, and M9345A in the endometrial endometrioid xenograft tumor models with END(K)265 (FRα=2+). A significant overall survival (0.0005) advantage was seen with IMGN853 when compared to the controls. At the time of analysis (80 days) no tumor recurrence or death had occurred in the IMGN853 treated group. (C) Antitumor activity of IMGN853 was compared to controls, PBS CTRL, ADC isotype, and M9345A in uterine serous cancer xenograft tumor models of BIO(K)1 (FRα=2+). Mice were treated intravenously with two doses administered once a week as described in Methods. A significant difference in tumor growth inhibition was detected on day 7 (p<0.001) in IMGN853-treated group when compared to the other control groups. (D): Overall survival of IMGN853 treated xenograft model of the uterine serous cancer, BIO(K)1. A significant overall survival (p<0.001) advantage was seen with IMGN853 compared to controls. Median survival for IMGN853 was 39 days compared to 18 days for ADC isotype, M9346A and PBS CTRL.
DISCUSSION
Uterine tumor types with poor prognosis (eg, uterine serous, clear cell, carcinosarcomas and grade 3 endometrioid) (13,14) have led to increased mortality rates in the last two decades (33). Even with extensive cytoreductive surgery followed by radiation and chemotherapy, these uterine cancer patients have a dismal 30–50% 5 year survival (8,9,13,14). There is a dire need for novel targeted therapies in Type II endometrial cancer patients given their increased risk of recurrence and poor overall survival despite conventional therapy.
Folic acid receptor is a potentially targetable surface protein in the treatment of aggressive gynecologic cancers (17–24). Consistent with this view, recent studies have shown promising preclinical data with FRα antibody linked to toxic payloads in platinum-resistant ovarian cancers (25–27). Accordingly, IMGN853 has been employed in a clinical phase I study by Moore et al. who enrolled and treated 46 recurrent EOC patients (PS2 ≥25%) with IMGN853 administered intravenously once every 3 weeks at 6.0 mg/kg AIBW (26,27). Moore et al. found that patients receiving IMGN853 with 3 or less prior lines of therapy achieved a 39% objective response rate and progression free-survival of 6.7 months. Impressively, IMGN853 was well tolerated with minimal low grade (1 to 2) toxicity (eg, diarrhea, blurred vision and fatigue) and limited adverse treatment-related events in 22% of the patients (26,27). As a result of these findings, IMGN853 is now undergoing evaluation as a single-agent in a randomized phase III trial (FORWARD I trial) comparing the safety and efficacy of IMGN853 to a single-agent chemotherapy in women with platinum-resistant FRα positive advanced EOC.
Given the encouraging clinical activity in platinum resistant EOC, and in an effort to optimize the use of IMGN853 against other gynecologic tumors, we initially investigated the frequency of FRα expression in biologically aggressive (ie, Type II) endometrial cancers by IHC. Using a TMA containing 70 USC samples, we found that up to 41% of patients with uterine serous cancer have high expression of FRα. Moreover, 22% (2/9) of the primary endometrioid mixed clear cell, along with 27% (3/11) of the primary USC cell lines available to this study overexpressed FRα by flow cytometry and IHC. These samples, via IHC, showed ≥ 25% staining at an intensity level of 2+. Given this finding these tumors would be eligible for targeting by the IMGN853 ADC in the clinical setting based on Moore et al.’s inclusion criteria used in the IMGN853 phase I study for EOC (26,27). These levels of FRα expression are consistent with prior literature in Type I and Type II endometrial cancer (23,24) and further support FRα as a targetable surface protein in the treatment of biologically aggressive endometrial tumors.
Next, we confirmed the preclinical activity of IMGN853 in endometrial cancer patients using multiple primary Type II endometrial cancer cell lines with differential FRα expression. We found that tumor cells overexpressing FRα (2+) were highly sensitive to IMGN853 with a 2.7-fold to 14-fold increase in cytotoxicity when compared to ADC isotype control. The activity was detected against all FRα 2+ expressing Type II tumor cell lines available for testing including endometrioid, mixed endometrioid/clear cells and uterine serous tumors. In contrast, endometrioid and USC cell lines with low FRα by flow cytometry, (ie, FRα = 1+ or FRα=0) showed no difference in the IC50s when exposed to IMGN853 vs ADC isotype control. These results suggest that the in vitro cytotoxic effect of IMGN853 is largely FRα mediated, and a specific threshold of FRα receptor expression on tumor cells must be present for the induction of IMGN853 cytotoxic activity against endometrial cancers.
One of the major challenges in utilizing highly targeted agents such as ADCs is represented by the potentially high heterogeneity of FRα receptor expression in tumors, both at the primary or metastatic sites. Importantly, lysosomal processing of the ADC IMGN853 results in production of the highly potent catabolites lysine-sulpho-SPDB-DM4, DM4 and DM4-Me. The latter two are cell membrane permeable and can be effluxed to the tumor microenvironment, inducing a potent bystander effect (18,32). Consistent with this view, we found IMGN853 to induce bystander killing of low/negative FRα expressing tumor cells admixed with FRα overexpressing tumor cells. These results strongly suggest that IMGN853 may be active in the treatment of recurrent endometrial cancer patients harboring tumors with heterogeneous FRα expression.
Importantly, in vivo experiments using xenograft and PDX models of 2+ FRα Type II endometrial cancer established in our laboratory demonstrated that two injections of IMGN853 ADC (5mg/kg) one week apart were highly effective in inducing complete resolution of tumor in the xenograft endometrioid/CC model. The two dose treatment also doubled the survival of mice harboring BIO(K)1, a PDX USC model, when compared to animals treated with control ADC. Taken together these results demonstrate, for the first time, in vivo activity of IMGN853 against multiple biologically aggressive and difficult to treat histological types of endometrial cancer.
In conclusion, we have demonstrated that IMGN853 is a novel ADC with impressive activity against Type II endometrial cancer with high (2+) FRα expression. Due to its cleavable linker, IMGN853 may be active against heterogeneous FRα expressing endometrial cancer. Clinical studies with IMGN853 in biologically aggressive endometrial cancer patients harboring disease resistant to standard salvage chemotherapy are warranted.
Supplementary Material
Acknowledgments
Financial support: This work was supported in part by R01 CA154460-01 and U01 CA176067-01A1 grants from NIH, and grants from the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation and the Guido Berlucchi Foundation to A.D. Santin. This investigation was also supported by NIH Research Grant CA-16359 from the NCI to A.D. Santin.
Footnotes
Conflict of interest: The authors declare that they have no competing interests.
Disclosure of Potential Conflicts of Interest. Ashley Morneault and Jose F. Ponte are employees of Immunogen, Waltham, MA. The remaining authors declare no conflict of interest or previous publications. All of the authors fulfill the conditions required for authorship.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Duong LM, Wilson RJ, Ajani UA, Singh SD, Eheman CR. Trends in Endometrial Cancer Incidence Rates in the United States, 1999–2006. J Womens Health. 2011;20:1157–1163. doi: 10.1089/jwh.2010.2529. [DOI] [PubMed] [Google Scholar]
- 4.Rose PG, Ali S, Moslemi-Kebria M, Simpkins F. Paclitaxel, Carboplatin, and Bevacizumab in Advanced and Recurrent Endometrial Carcinoma. Int J Gynecol Cancer. 2017;27:452–458. doi: 10.1097/IGC.0000000000000891. [DOI] [PubMed] [Google Scholar]
- 5.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 6.Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards R, et al. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control. 2010;21:1851–1856. doi: 10.1007/s10552-010-9612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creasman WT, Ali S, Mutch DG, Zaino RJ, Powell MA, Mannel RS, et al. Surgical-pathological findings in type 1 and 2 endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study on GOG-210 protocol. Gynecol Oncol. 2017;145:519–525. doi: 10.1016/j.ygyno.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voss MA, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, et al. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol. 2012;124:15–20. doi: 10.1016/j.ygyno.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Greven KM, Randall M, Fanning J, Bahktar M, Duray P, Peters A, et al. Patterns of failure in patients with stage I, grade 3 carcinoma of the endometrium. Int J Radiat Oncol Biol Phys. 1990;19:529–34. doi: 10.1016/0360-3016(90)90477-2. [DOI] [PubMed] [Google Scholar]
- 10.Straughn JM, Huh WK, Orr JW, Kelly FJ, Roland PY, Gold MA, et al. Stage IC adenocarcinoma of the endometrium: survival comparisons of surgically staged patients with and without adjuvant radiation therapy☆☆Presented at the 33rd Annual Meeting of Gynecologic Oncologists, Miami, FL, March 2002. Gynecol Oncol. 2003;89:295–300. doi: 10.1016/s0090-8258(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 11.Creutzberg CL, van Putten WLJ, Wárlám-Rodenhuis CC, van den Bergh ACM, De Winter KAJ, Koper PCM, et al. Outcome of High-Risk Stage IC, Grade 3, Compared With Stage I Endometrial Carcinoma Patients: The Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol. 2004;22:1234–1241. doi: 10.1200/JCO.2004.08.159. [DOI] [PubMed] [Google Scholar]
- 12.Young MR, Higgins SA, Ratner E, Yu JB, Mani S, Silasi DA, et al. Adjuvant carboplatin, paclitaxel, and vaginal cuff brachytherapy for stage III endometrial cancer: analysis of outcomes and patterns of recurrence based on pathologic characteristics. Int J Gynecol Cancer. 2015;25:431–439. doi: 10.1097/IGC.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fader AN, Seamon LG, Escobar PF, Frasure HE, Havrilesky LA, Zanotti KM, et al. Minimally invasive surgery versus laparotomy in women with high grade endometrial cancer: A multi-site study performed at high volume cancer centers. Gynecol Oncol. 2012;126:180–185. doi: 10.1016/j.ygyno.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Clement PB, Young RH. Non-endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2004;11:117–142. doi: 10.1097/00125480-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 15.English DP, Bellone S, Schwab CL, Bortolomai I, Bonazzoli E, Cocco E, et al. T-DM1, a novel antibody-drug conjugate, is highly effective against primary HER2 overexpressing uterine serous carcinoma in vitro and in vivo. Cancer Med. 2014;3:1256–65. doi: 10.1002/cam4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicoletti R, Lopez S, Bellone S, Cocco E, Schwab CL, Black JD, et al. T-DM1, a novel antibody-drug conjugate, is highly effective against uterine and ovarian carcinosarcomas overexpressing HER2. Clin Exp Metastasis. 2015;32:29–38. doi: 10.1007/s10585-014-9688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann Oncol. 2015;26:2034–2043. doi: 10.1093/annonc/mdv250. [DOI] [PubMed] [Google Scholar]
- 18.Ab O, Whiteman KR, Bartle LM, Sun X, Singh R, Tavares D, et al. IMGN853, a Folate Receptor-α (FRα)-Targeting Antibody-Drug Conjugate, Exhibits Potent Targeted Antitumor Activity against FRα-Expressing Tumors. Mol Cancer Ther. 2015;14:1605–1613. doi: 10.1158/1535-7163.MCT-14-1095. [DOI] [PubMed] [Google Scholar]
- 19.Wu M, Gunning W, Ratnam M. Expression of folate receptor a in relation to cell type, malignancy, and differentiation in ovary, uterus and cervix. Cancer Epidemiol Biomarkers Prev. 1999;8:775–82. [PubMed] [Google Scholar]
- 20.Miotti S, Canevari S, Ménard S, Mezzanzanica D, Porro G, Pupa SM, et al. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer. 1987;39:297–303. doi: 10.1002/ijc.2910390306. [DOI] [PubMed] [Google Scholar]
- 21.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 22.O’Shannessy DJ, Somers EB, Smale R, Fu YS. Expression of folate receptor-α (FRA) in gynecologic malignancies and its relationship to the tumor type. Int J Gynecol Pathol. 2013;32:258–268. doi: 10.1097/PGP.0b013e3182774562. [DOI] [PubMed] [Google Scholar]
- 23.Dainty LA, Risinger JI, Morrison C, Chandramouli GV, Bidus MA, Zahn C, et al. Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecol Oncol. 2007;105:563–70. doi: 10.1016/j.ygyno.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 24.Senol S, Ceyran AB, Aydin A, Zemheri E, Ozkanli S, Kösemetin D, et al. Folate receptor α expression and significance in endometrioid endometrium carcinoma and endometrial hyperplasia. Int J Clin Exp Pathol. 2015;8:5633–5641. [PMC free article] [PubMed] [Google Scholar]
- 25.Serpe L, Gallicchio M, Canaparo R, Dosio F. Targeted treatment of folate receptor-positive platinum-resistant ovarian cancer and companion diagnostics, with specific focus on vintafolide and etarfolatide. Pharmgenomics Pers Med. 2014;7:31–42. doi: 10.2147/PGPM.S58374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore KN, Martin LP, O’Malley DM, Matulonis UA, Konner JA, et al. Safety and Activity of Mirvetuximab Soravtansine (IMGN853), a Folate Receptor Alpha-Targeting Antibody-Drug Conjugate, in Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer: A Phase I Expansion Study. J Clin Oncol. 2016;35:1112–1118. doi: 10.1200/JCO.2016.69.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore KN, Borghaei H, O’Malley DM, Jeong W, Seward SM, Bauer TM, et al. Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor α-targeting antibody-drug conjugate, in patients with solid tumors. Cancer. 2017;123:3080–3087. doi: 10.1002/cncr.30736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roque DM, Bellone S, Buza N, Romani C, Cocco E, Bignotti E, et al. Class III β-tubulin overexpression in ovarian clear cell and serous carcinoma as a maker for poor overall survival after platinum/taxane chemotherapy and sensitivity to patupilone. Am J Obstet Gynecol. 2013;209:62.e1–62.e9. doi: 10.1016/j.ajog.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A. 2013;110:2916–2921. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Sahwi K, Bellone S, Cocco E, Cargnelutti M, Casagrande F, Bellone M, et al. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. Br J Cancer. 2010;102:134–143. doi: 10.1038/sj.bjc.6605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santin AD, Rose GS, Hiserodt JC, Fruehauf J, Eck LM, Garcia RI, et al. Effects of cytokines combined with high-dose gamma irradiation on the expression of major histocompatibility complex molecules and intercellular adhesion molecule-1 in human ovarian cancers. Int J Cancer. 1996;65:688–694. doi: 10.1002/(SICI)1097-0215(19960301)65:5<688::AID-IJC21>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Ponte JF, Ab O, Lanieri L, Lee J, Coccia J, Bartle LM, et al. Mirvetuximab Soravtansine (IMGN853), a Folate Receptor Alpha-Targeting Antibody-Drug Conjugate, Potentiates the Activity of Standard of Care Therapeutics in Ovarian Cancer Models. Neoplasia. 2016;18:775–784. doi: 10.1016/j.neo.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120:383–397. doi: 10.1097/AOG.0b013e3182605bf1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.