Summary
The autonomic nervous system (ANS) conveys neuronal input from the brain to the stomach. We investigated mechanisms through which urocortin 1 (UCN1) injected intracerebroventricularly (ICV, 300 pmol/rat) inhibits circulating ghrelin in rats. This was achieved by assessing (1) the induction of c-fos gene expression as a marker of neuronal activation in specific hypothalamic and caudal brainstem regulating ANS; (2) the influence of vagotomy and pharmacological blockade of central and peripheral α- and β-adrenergic receptor (AR) on ICV UCN1 -induced reduction of plasma ghrelin levels (determined by ELISA); and (3) the relevance of this pathway in the feeding response to a fast in rats. UCN1 increased c-fos mRNA expression in key brain sites influencing sympathetic activity namely the hypothalamic paraventricular and ventromedial nuclei, locus coeruleus, nucleus of the solitary tract, and rostral ventrolateral medulla, by 16-, 29-, 6-, 37-, and 13-fold, respectively. In contrast, the dorsal motor nucleus of the vagus had little c-fos mRNA expression and ICV UCN1 induced a similar reduction in acylated ghrelin in the sham-operated (31%) and vagotomized (41%) rats. An intraperitoneal (IP) injection of either a non-selective α- or selective α2-AR antagonist reduced, while a selective α2-AR agonist enhanced ICV UCN1-induced suppression of plasma acylated ghrelin levels. In addition, IP injection of a non-selective β- or selective β1-AR agonist blocked, and selective β1-AR antagonist augmented, the ghrelin response to ICV UCN1. The IP injections of a selective α1- or non-selective β or β2-AR antagonists, or any of the pretreatments given ICV had no effect. ICV UCN1 reduced the 2-h food intake in response to a fast by 80%, and this effect was partially prevented by a selective α2-AR antagonist. These data suggest that ICV UCN1 reduces plasma ghrelin mainly through the brain sympathetic component of the ANS and peripheral AR specifically α2-AR activation and inactivation of β1-AR. The α2-AR pathway contributes to the associated reduction in food intake.
Keywords: Acylated ghrelin, Adrenergic receptors, Food intake, Urocortin 1, Stress, Rikkunshito
1. Introduction
Stress is well established to influence feeding behaviors and metabolism in both humans and rodents (Patterson and Abizaid, 2013). Corticotropin-releasing factor (CRF) plays a key role in coordinating the hormonal, autonomic, behavioral and visceral components of the acute stress response (Stengel et al., 2010a). In rodents, CRF administered into the brain induces stress-like behaviors, including increased anxiety-like manifestations and food intake suppression (Stengel and Taché, 2014; Zorrilla et al., 2003). Urocortin 1 (UCN1) is a member of the mammalian CRF-related peptide that is mainly localized in the Edinger–Westphal nucleus, and to a lesser extent, in the olfactory bulb, supraoptic nucleus, ventromedial hypothalamus (VMH) and lateral hypothalamic area (Kozicz et al., 1998; Shah et al., 2013). Evidence suggests that experimental stressors activate UCN1 neurons in the Edinger-Westphal nucleus (Gaszner et al., 2004). Both CRF and UCN1 mediate their actions through the activation of CRF receptor subtypes 1 and 2 (CRF1 and CRF2, respectively); however, CRF and UCN1 exhibit differential binding affinities. UCN1 displays high affinity to both CRF receptor subtypes, while CRF is a preferential CRF1 agonist and has a low affinity to CRF2 (Vaughan et al., 1995).
It is well documented that UCN1 injected intracerebroventricularly (ICV) is more potent than CRF to suppress fasting- or dark phase-induced food intake without inducing conditioned taste aversion or visceral illness in rodents (Benoit et al., 2000; Smagin et al., 1998; Spina et al., 1996). ICV UCN1-induced food intake inhibition action is mediated mainly through the activation of brain CRF2 in rats (Smagin et al., 1998; Yakabi et al., 2011). Several brain sites expressing high density of CRF2 (Bittencourt et al., 1999) and regulate the autonomic nervous system (ANS) (Saper, 2002) have been identified to be responsive to UCN1, which results in a CRF2-mediated anorexigenic response in rats, namely the lateral septum (Bakshi et al., 2007), paraventricular nucleus of the hypothalamus (PVN) (Currie et al., 2001), VMH (Chen et al., 2012; Ohata et al., 2000), and dorsal raphe (Weitemier and Ryabinin, 2006). In addition, based on the observation that UCN1 injected into the fourth brain ventricle is still able to reduce food intake in chronically decerebrated rats (Daniels et al., 2004), hindbrain structures are believed to be involved in this response. This is consistent with the earlier report that the nucleus tractus solitarius (NTS) is a brainstem site that is responsive to UCN1 (Grill et al., 2000).
Several potential mechanisms could participate in the anorexic effects of ICV UCN1. The ICV injection of UCN1 induces a CRF2-mediated inhibition of gastric emptying (Martinez et al., 2004; Yakabi et al., 2011) and hyperglycemia (Grill et al., 2000) in rodents. Both effects are known to reduce feeding (Cha et al., 2008; Phillips and Powley, 1996). In addition, we have recently reported that UCN1 ICV acts through the CRF2 receptor to decrease circulating acylated ghrelin (Yakabi et al., 2011), which is the only known orexigenic hormone that is produced peripherally by gastric endocrine X/A cells but acts centrally (Hosoda et al., 2002; Muller and Tschop, 2013). Of functional relevance, we have shown that the exogenous injection of ghrelin or the ghrelin enhancer rikkunshito (Takeda et al., 2012) restored food intake in ICV UCN1-injected rats (Yakabi et al., 2011). However, the mechanism(s) through which the circulating ghrelin is suppressed by the central administration of UCN1 is yet to be elucidated. Changes in the ANS activity influence gastric ghrelin secretion (Hosoda and Kangawa, 2008), and various local classical neurotransmitters and neuropeptides are reportedly to influence ghrelin release (de la Cour et al., 2007; Stengel et al., 2011). Previous reports indicate that centrally injected UCN1 effects on gastric function involve the ANS (Czimmer et al., 2006; De Fanti and Martinez, 2002).
In the present study, we first delineated the ANS pathway(s) that contributes to the decreased circulating ghrelin induced by ICV injection of UCN1 in rats. This was achieved by surgical approach (vagotomy) and mapping the induction of c-fos gene expression as a marker of neuronal activation (Krukoff, 1993), in specific hypothalamic (PVN, VMH) and brainstem [LC (locus coeruleus), NTS, DMN (dorsal motor nucleus), RVLM (rostral ventrolateral medulla)] nuclei that regulate the ANS (Saper, 2002; Toth et al., 1999; Travagli et al., 2006). Then, we assessed the related peripheral adrenergic mechanisms using the pharmacological blockade of central and peripheral α- and β-adrenergic receptors (ARs). Lastly, we examined the functional implications of ghrelin suppression in the inhibition of food intake induced by ICV UCN1 using blockade of identified AR pathways alone or in combination with a ghrelin receptor antagonist or the ghrelin enhancer, rikkunshito (Takeda et al., 2012).
2. Materials and methods
2.1. Animals
Eight-week-old male Sprague-Dawley rats (weight, 240–280g) were purchased from Japan SLC, Inc. (Shizuoka, Japan). All animals were housed in polycarbonate cages in room with controlled conditions of ambient temperature (23 ± 3°C), humidity (50 ± 20%), and lighting (12-h light:dark cycle starting at 7:00 PM). Animals were maintained with water and standard laboratory food ad libitum. Access to the standard laboratory food was removed 16 h before experiments, which were conducted between 1 and 5 h after the beginning of the light cycle to avoid the influences of diurnal rhythms. All experimental procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals and approved by the Laboratory Animal Committee of Tsumura & Co. (Tokyo, Japan).
2.2. Surgery
2.2.1. Intracerebroventricular cannula
Rats under sodium pentobarbital (50 mg/kg, intraperitoneal, IP) anesthesia were placed on a stereotaxic apparatus (Japan SLC Inc., Shizuoka, Japan). A stainless steel guide cannula (AG-8; Eicom, Kyoto, Japan) was implanted into the right lateral ventricle using the following coordinates derived from the rat brain atlas (Paxinos and Watson, 1998): 0.8 mm posterior and 1.4 mm lateral from the bregma and 3.4 mm ventral from the skull surface. Rats were singly housed after the surgery and had at least a recovery period of 5 days before the start of the treatment.
2.2.2. Subdiaphragmatic vagotomy
The surgery was performed at least 10 days after ICV cannulation as previously described (Hosoda and Kangawa, 2008; Takeda et al., 2008) in pentobarbital-anesthetized rats (40 mg/kg, IP). Following a laparotomy, both vagal trunks located at the lower esophagus were resected, and a pyloroplasty was performed to widen the pylorus and prevent pyloric stenosis. Sham operations consisted of the opening of the abdomen and exposure of the internal organs. The abdomen was closed by sutures. Animals had at least 6 days of recovery time and maintained under standard ad libitum food.
2.3. Drugs and treatments
Rat UCN1 was purchased from Peptide Institute, Inc. (Osaka, Japan). Phentolamine hydrochloride (non-selective α-AR antagonist), prazosin hydrochloride (selective α1-AR antagonist), yohimbine hydrochloride (selective α2-AR antagonist), propranolol (non-selective β-AR antagonist), atenolol (selective β1-AR antagonist), ICI-118,551 (selective β2-AR antagonist), synephrine (non-selective α-AR agonist), phenylephrine hydrochloride (selective α1-AR agonist), clonidine hydrochloride (selective α2-AR agonist), isoproterenol hydrochloride (non-selective β-AR agonist), denopamine (selective β1AR agonist), and salbutamol (selective β2-AR agonist) were all purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The growth hormone secretagogue receptor type 1a (GHS-R1a) antagonist, [D-Lys3]-GHRP-6, was purchased from Bachem, Inc. (Torrance, CA, USA). These compounds were dissolved in saline when injected IP and in phosphate-buffered saline (PBS) when injected ICV. Rikkunshito, which is a Japanese kampo medicine, was supplied from Tsumura & Co. (Tokyo, Japan) in the form of a powdered extract obtained by spray-drying a hot water extract mixture of the following eight crude drugs: Atractylodis lanceae rhizoma (4.0 g), Ginseng radix (4.0 g), Pinelliae tuber (4.0 g), Poria (4.0 g), Zizyphi fructus (2.0 g), Aurantii nobilis pericarpium (2.0 g), Glycyrrhizae radix (1.0 g), and Zingiberis rhizoma (0.5 g). Rikkunshito was dissolved in distilled water for oral administration. Components of rikkunshito for binding assay were dissolved in dimethyl sulfoxide (DMSO, final dilution: 1%). Other analytical reagents were highest-purity commercially available products.
Treatments were performed on lightly hand-restrained rats in the following volumes: 10 µL/rat for ICV injection, 1 mL/kg for IP or intravenous (IV) injection through tail vein, and 10 mL/kg for orogastric administration. Rats were handled daily more than 5 days prior to treatment to minimize stress during the procedure.
2.4. Ghrelin determination
Rats were euthanized by decapitation, and trunk blood (approximately 4 mL) was collected in cold polypropylene tubes containing 8.0 mg ethylenediaminetetraacetic acid and 0.8 mg aprotinin. Samples were centrifuged at 10,000 × g at 4°C for 3 min. The supernatant was acidified with 1M HCl (1/10 volume) and stored at −80°C until the ghrelin assays were performed. Plasma ghrelin levels were determined using active ghrelin and des-acyl ghrelin Enzyme-Linked Immunoassay Kits (Mitsubishi Chemical Corp., Tokyo, Japan). The detection limits for the acylated and des-acyl ghrelin were 2.7 fmol/mL and 12.3 fmol/mL, respectively. The intraassay coefficients of variation for the acylated and des-acyl ghrelin were 0.8–4.8% and 2.2–5.5%, respectively, and the interassay coefficients of variation for the acylated ghrelin and des-acyl ghrelin were 2.8–6.4% and 1.9–9.0%, respectively.
2.5. Binding affinity of rikkunshito for ARs
For the α2A-AR binding assay, insect Sf9 cells expressing human recombinant α2A-AR were homogenized in modified Tris–HCl buffer and aliquots were incubated with 1 nM [3H] MK-912 for 60 min at 25 °C. Nonspecific binding was estimated in the presence of 10 µM WB-4101 (Uhlen et al., 1994). For α2B-AR, CHO-K1 cells stably transfected with a plasmid encoding human α2B-AR were homogenized in modified Tris–HCl buffer using standard techniques and aliquots were incubated with 2.5 nM [3H] rauwolscine for 60 min at 25°C. Nonspecific binding was estimated in the presence of 10 µM prazosin (Uhlen et al., 1998). For α2C-AR, insect Sf9 cells expressing human recombinant α2C-AR were homogenized in modified Tris–HCl buffer and aliquot was incubated with 1 nM [3H] MK-912 for 60 min at 25 °C. Nonspecific binding was estimated in the presence of 10 µM WB-4101 (Uhlen et al., 1994). Radio binding assays for α1 and β-AR are detailed in Supplementary Material 1. The half-maximal inhibitory concentration (IC50) values were determined by nonlinear, least-squares regression analysis using the MathIQ™ statistical software (ID Business Solutions Ltd., Surrey, UK).
Supplementary Material 1 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.09.003.
2.6. Experimental protocols
All experiments were performed in 16 h-fasted rats with no access to food post ICV injection except otherwise mentioned.
2.6.1. c-Fos gene expression in rat brain induced by ICV UCN
Rats with chronic ICV cannula were injected ICV either with PBS or UCN1 (300 pmol/rat) and were anesthetized 1 h later with sodium pentobarbital (50 mg/kg, IP). The brain was collected and processed for c-fos mRNA detected by in situ hybridization as detailed previously (Yakabi et al., 2011) (Supplementary Material 2). c-Fos mRNA-positive cells in the PVN, VMH, LC, NTS, DMN and RVLM were unilaterally counted.
Supplementary Material 2 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.09.003.
2.6.2. Effects of ICV UCN1 on acylated and des-acyl ghrelin plasma levels in sham or vagotomized rats
Chronically ICV cannulated rats with sham or subdiaphragmatic vagotomy performed 6 days earlier were divided into two groups (n = 8–10/group) and injected ICV with either PBS or UCN1 (300 pmol/rat). Rats were euthanized by decapitation 2 h after ICV injection. The trunk blood was collected for the determination of acylated and des-acyl ghrelin levels in plasma. The ICV dose of UCN1 was selected, based on our previous dose-response studies showing the maximal suppression of 24-h food intake in rats and reduced fasting ghrelin levels at 1-h and 2-h post-ICV injection compared to ICV PBS (Yakabi et al., 2011).
2.6.3. Effects of AR antagonists, agonists or rikkunshito on ICV UCN 1-induced reduction of plasma acylated ghrelin levels
Chronically ICV-cannulated rats were divided into five groups (n = 8–17/group) and injected ICV with PBS or UCN1 (300 pmol/rat) after the following treatments given either IP 15 min before or ICV simultaneously: saline, non-selective α-AR antagonist (phentolamine, 5 mg/kg, IP or 0.05 mg/rat, ICV), selective α1-AR antagonist (prazosin, 5 mg/kg, IP or 0.50 mg/rat, ICV), selective α2-AR antagonist (yohimbine, 5 mg/kg, IP or 0.04 mg/rat, ICV). The regimen of AR blockade was based on previous reports (Becker et al., 1999; Hosoda and Kangawa, 2008). In other sets of experiments, the IP pretreatment were given either 15 min before or simultaneously ICV UCN1 with the following β-AR antagonists: non-selective (propranolol, 3 mg/kg), β1-selective (atenolol, 10 mg/kg), or β2-selective (ICI-118,551, 0.1 mg/kg) (Hosoda and Kangawa, 2008; Zhao et al., 2010); the following β-AR agonists: non-selective (isoproterenol, 0.1 mg/kg), β1-selective (denopamine, 0.1 mg/kg) or β2-selective (salbutamol, 0.1 mg/kg); or the following α-AR agonists: non-selective (synephrine, 5 mg/kg), α1-selective (phenylephrine, 5 mg/kg), or α2-selective (clonidine, 5 mg/kg) (Hosoda and Kangawa, 2008). In the last set of study, the following pretreatments were given 1 h before ICV UCN1: orogastric gavage with distilled water (10 mL/kg) or rikkunshito (0.5 g/kg or 1.0 g/kg). This regimen of administration was based on our previous studies (Takeda et al., 2008; Yakabi et al., 2011). In all experiments, the trunk blood was collected 2 h after the ICV injection to determine the acylated ghrelin levels.
2.6.4. Effects of a selective α2-AR antagonist on ICV UCN 1-induced reduction of food intake
Chronically ICV-cannulated rats were pretreated IP with a selective α2-AR antagonist (yohimbine, 5 mg/kg) or vehicle and 15min later injected ICV with UCN1 (300 pmol/rat). To assess the effects of the ghrelin antagonist on the selective α2-AR antagonist activities, rats were first injected IP with saline or the selective α2-AR antagonist, and 15 min later, rats received an IV injection of either saline or [D-Lys3]-GHRP-6 (3.7 mg/kg) followed 1 min later by ICV PBS or UCN1 (300 pmol/rat). Preweighed chow was placed in each cage, and the 2-h cumulative food intake was monitored immediately after ICV injection.
2.7. Statistical analysis
All values are presented as the mean ± standard error of the mean. Statistical analyses of the mean values of two groups were performed using Student’s t-test or the Aspin-Welch t-test. The mean values of multiple groups were determined by two-way factorial analysis of variance (ANOVA) followed by the Bonferroni post hoc test or one-way analysis of variance (ANOVA) followed by Dunnett’s test or Steel’s test. For all tests, probability (P) values of <0.05 were considered statistically significant.
3. Results
3.1. ICV UCN1 injection induces c-fos mRNA expression in specific brain nuclei
ICV UCN1 (300 pmol/rat) did not influence c-fos mRNA expression in the DMN while increasing the number of labeled cells in the PVN, VMH, LC, NTS and RVLM by 16-, 29-, 6-, 37- and 13-fold, respectively, compared with ICV vehicle-injected rats as monitored 1 h after the peptide injection by in situ hybridization (Supplementary Fig. S1 and Table S1).
Supplementary Table S1 and Fig. S1 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.09.003.
3.2. Vagotomy did not influence ICV UCN1-induced reduction of plasma acylated and des-acyl ghrelin levels
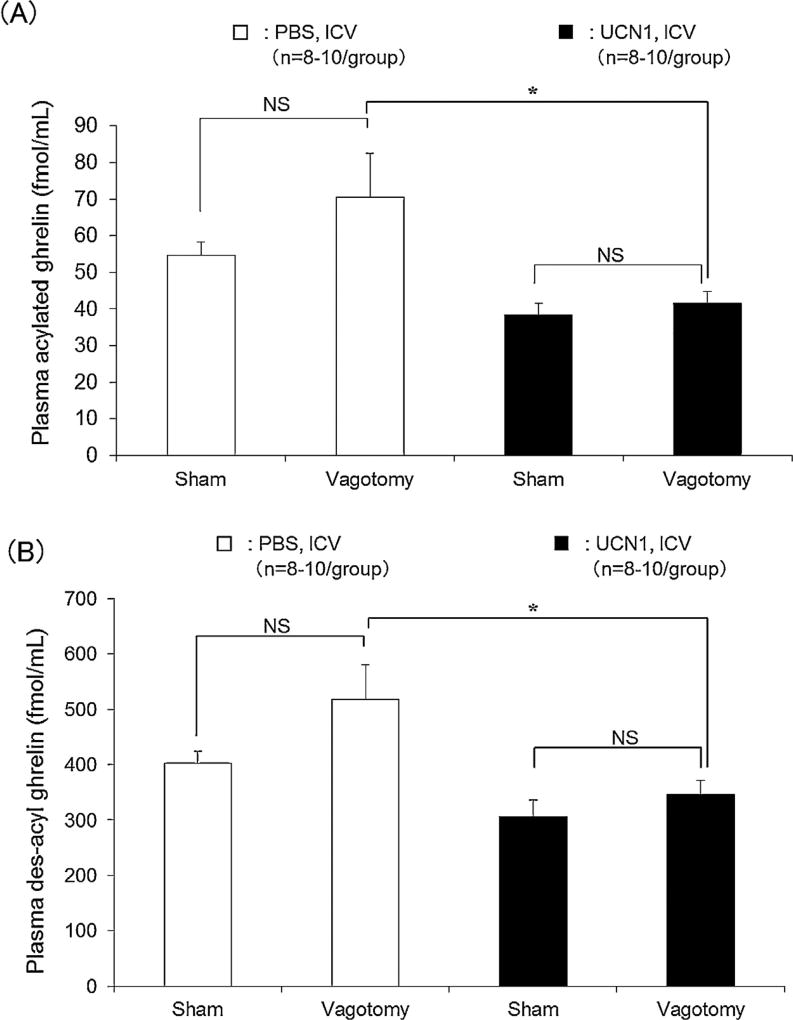
In the sham-operated, fasted rats, UCN1 (300 pmol/rat, ICV) reduced the plasma levels of acylated and des-acyl ghrelin by 31% and 25%, respectively, compared to ICV vehicle injection as monitored at 2 h after the injection (Fig. 1A and B). Similarly, in the rats with subdiaphragmatic vagotomy, ICV UCN1 significantly decreased plasma acylated and des-acyl ghrelin levels by 41% (P < 0.05) and 33% (P < 0.05), respectively (Fig. 1A and B, n = 8–10). The magnitude of the reduction did not significantly differ between the sham and vagotomized groups. It is to note that the vagotomized rats injected ICV with vehicle showed a trend to have an increase in acylated ghrelin (21%) and des-acyl ghrelin (22%), which did not reach significance by using the two-way factorial analysis of variance (ANOVA) followed by the Bonferroni post hoc test (Fig. 1A and 1B). As acylated ghrelin is the active form of the peptide that activates GHS-R1a to stimulate food intake (Stengel et al., 2010b), in all subsequent experiments, we limited the plasma determination to the acylated form of ghrelin.
Figure 1.
Effects of vagotomy on ICV UCN1-induced decrease in plasma levels of (A) acylated and (B) des-acyl ghrelin in rats. Sub-diaphragmatic vagotomy with pyloroplasty or sham operation was performed 6 days before the experiments. Rats were euthanized 2 h after ICV vehicle (PBS) or UCN1 (300 pmol/rat) administration, and blood samples were collected. All values are presented as the mean ± standard error of the mean (SEM) (n = 8–10/group). Significance was identified using the Bonferroni post hoc test following two-way analysis of variance (ANOVA). *P < 0.05 vs. vagotomy + PBS-treated group.
3.3. Effects of IP injection or ICV injection of AR antagonists, agonists or rikkunshito on ICV UCN1-induced decreases in plasma acylated ghrelin
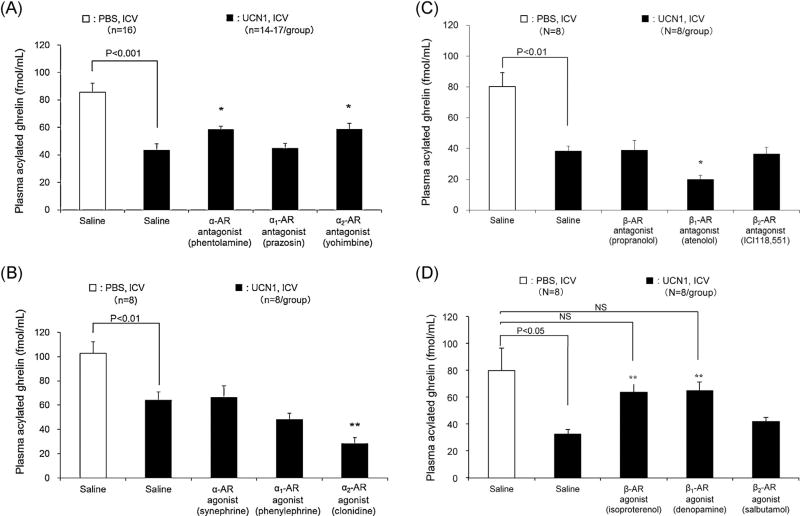
In IP vehicle-pretreated, fasted rats, ICV UCN1 (300 pmol/rat, n = 38) induced a significant, 50–59% reduction of fasted plasma levels of acylated ghrelin compared with ICV vehicle (Fig. 2A–D, P < 0.001, P < 0.01, P < 0.01 and P < 0.05, respectively [n = 8–14]). IP pretreatment with the non-selective α-AR antagonist (phentolamine) or selective α2-AR antagonist (yohimbine) before ICV UCN1 increased significantly and similarly plasma levels of acylated ghrelin compared with those of the vehicle pretreated-plus-ICV UCN1 group (58.5 ± 2.5 fmol/mL, 58.6 ± 4.5 fmol/mL vs. 43.5 ± 4.5 fmol/mL respectively, P < 0.05), while the selective α1-AR antagonist (prazosin) had no effect (44.9 ± 3.6 fmol/mL; Fig. 2A). However, in the phentolamine- or yohimbine-pretreated rats, the values remained significantly lower than those of the control group (IP saline + ICV PBS: 85.5 ± 6.7 fmol/mL, Dunnett’s test: P < 0.05; Fig. 2A, n = 14–17/group). Conversely, the IP injection of the selective α2-AR agonist (clonidine, P < 0.01), unlike a non-selective α-AR agonist (synephrine) or a selective α1-AR agonist (phenylephrine), further decreased the plasma acylated ghrelin levels induced by ICV UCN1 (Fig. 2B, n = 8/group and Supplementary Fig. S2, n = 8/group). In a preliminarily study, the selective α2-AR antagonist did not influence the acylated ghrelin levels in fasted rats. Furthermore, a previous report has shown no significant changes in the fasting levels of ghrelin induced by the non-selective α-AR antagonist phentolamine (Hosoda and Kangawa, 2008).
Figure 2.
Effects of IP pretreatment with (A) α-AR antagonists (n = 14–17/group), (B) α-AR agonists (n = 8/group), (C) β-AR antagonists (n = 8/group), and (D) β-AR agonists (n = 8/group) on plasma acylated ghrelin levels inhibited by ICV UCN1 in rats. IP injection was performed 15 min before ICV UCN1 (300 pmol/rat) or vehicle, and trunk blood samples were collected 2 h later. All values are presented as the mean ± SEM. Significance was determined using Student’s t-test or one-way ANOVA followed by post hoc Dunnett’s test: *P < 0.05 and **P < 0.01 vs. UCN1/saline-treated group.
Supplementary Fig. S2 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.09.003.
With regard to the influences of β-ARs, we found that the selective β1-AR antagonist (atenolol, P < 0.05), but not the non-selective β-AR antagonist (propranolol) or the selective β2-AR antagonist (ICI-118,551), further decreased the plasma acylated ghrelin levels induced by ICV UCN1 (Fig. 2C, n = 8/group). Conversely, the non-selective β-AR agonist (isoproterenol, P < 0.01) or the selective β1-AR agonist (denopamine, P < 0.01), but not the selective β2-AR agonist (salbutamol), prevented the decreased plasma acylated ghrelin levels elicited by ICV UCN1, leading to acylated ghrelin values not significantly different from those of ICV saline-treated rats (Fig. 2D, n = 8/group).
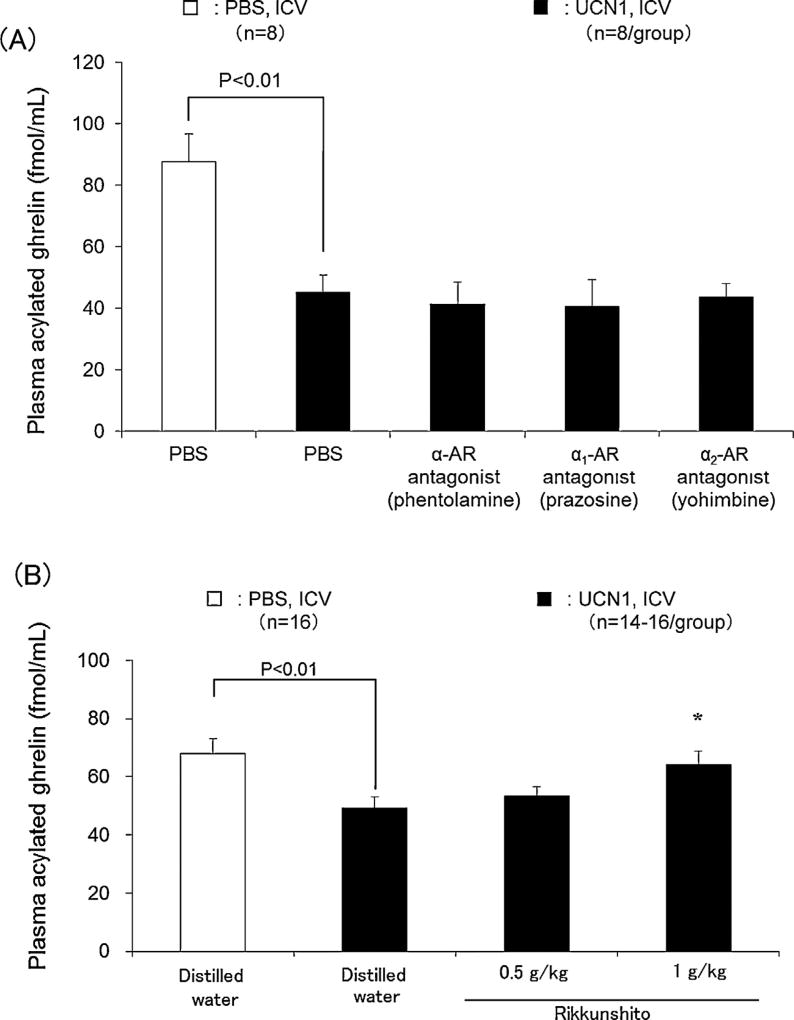
When injected ICV, the non-selective α-AR antagonist (phentolamine), the selective α1-AR antagonist (prazosin) and the selective α2-AR antagonist (yohimbine) did not influence the reduction of plasma acylated ghrelin levels induced by ICV UCN1 (Fig. 3A, n = 8/group). Lastly, the oral administration of rikkunshito significantly inhibited (P < 0.05) the decreased plasma acylated ghrelin levels when given at 1 g/kg, while at a dose of 0.5 g/kg, it had no effect (Fig. 3B, n = 14–16/group).
Figure 3.
Effects of ICV with (A) selective α-AR antagonists (n = 8/group) and (B) orogastric administration of rikkunshito (n = 14–16/group) on acylated ghrelin levels inhibited by ICV UCN1 in rats. ICV was performed simultaneously with UCN1 (300 pmol/rat) or vehicle. Distilled water or rikkunshito was orogastrically administered (10 mL/kg) 1 h before ICV injection of PBS or UCN1 (300 pmol/rat), the rats were euthanized 2 h later, and blood samples were collected. All values are presented as the mean ± SEM. Significance was determined using Student’s t-test or ANOVA followed by post hoc Dunnett’s tests: *P < 0.05 vs. UCN1 + Distilled water-treated group.
3.4. Effects of selective α2-antagonist (yohimbine) in combination with [D-Lys3]-GHRP-6 on decreased food intake induced by ICV UCN1
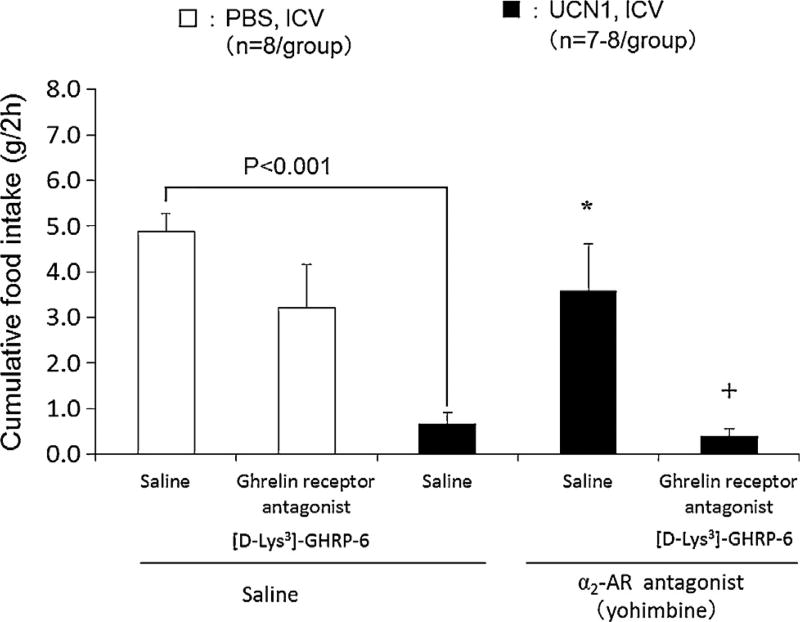
In the control-pretreated rats, ICV UCN1 induced a significant 87% reduction of the cumulative 2-h food intake response to overnight fasting (control: IP saline, IV saline, ICV PBS: 4.9 ± 0.4 g/2 h; IP saline, IV saline, ICV UCN1: 0.7 ± 0.3 g/2 h, P < 0.001; Fig. 4, n = 7–8/group). IP pre-treatment with the selective α2-AR antagonist (yohimbine) prevented ICV UCN1 inhibitory effects (IP yohimbine, IV saline, ICV UCN1: 3.6 ± 1.0 g/2h, P < 0.05) while not influencing significantly the feeding response to the fast (IP yohimbine, IV saline, ICV PBS: 6.8 ± 1.0 g/2h vs. IP saline, IV saline, ICV PBS: 6.4 ± 0.7 g/2h). [D-Lys3]-GHRP-6 injected IV did not influence the 2-h cumulative food intake in vehicle-treated control rats while completely blocking the normalization of feeding induced by IP yohimbine in ICV UCN1-treated rats (Fig. 4, n = 7–8/group).
Figure 4.
Effects of selective α2-AR antagonist (yohimbine) alone or in combination with a ghrelin receptor antagonist on food intake in ICV UCN1-treated rats. Saline or yohimbine (5 mg/kg) was administrated intraperitoneally 15 min before ICV injection of PBS or UCN1 (300 pmol/rat). Saline or [D-Lys3]-GHRP-6 (3.7 mg/kg) was administrated intravenously into the tail vein 1 min after ICV administration of PBS or UCN1 (300 pmol/rat). Each bar represents the mean ± SEM (n = 7–8/group). Significance was determined using Steel’s post hoc test following one-way ANOVA analysis *P < 0.05 compared with the UCN1 /saline group. Significance was determined using Aspin-welch t-test. +P < 0.05 vs. the UCN1/selective α2-AR antagonist (yohimbine) group. Significance between PBS and UCN1/saline was determined using Student’s t-test.
3.5. In vitro binding assays of rikkunshito components on ARs
The AR binding-inhibitory activities (IC50) of the crude drug components contained in rikkunshito were tested in transfected cells with human AR subtypes. As shown in Table 1, glycycoumarin 6-, 8-shogaol and 10-gingerol, and eudesmol display inhibitory activity to α2A-AR and differential activities on α1-AR or β1,3-AR (Supplementary Table S2).
Table 1.
Binding assays for α2-adrenergic receptors by rikkunshito components (IC50 values).
| Rikkunshito components | α2-adrenergic receptor subtypes
|
||||
|---|---|---|---|---|---|
| A | B | C | NS | ||
| Glysyrrhizae Radix | Glycycoumarin | 5.2 ± 0.7 | 39.4 ± 3.3 | 14.5 ± 0.3 | — |
| Zingiberis | 6-Shogaol | 24.7 ± 2.4 | — | — | — |
| Rhizoma | 8-Shogaol | 5.7 ± 0.8 | — | 6.1 ± 0.5 | — |
| 10-Gingerol | 5.4 ± 0.2 | 30.9 ± 1.9 | 6.6 ± 0.8 | — | |
| Aurantii Nobilis | Heptamethoxyflavone | — | — | — | — |
| pericarpium | Synephrine | — | — | — | 11.2 ± 1.2 |
| Atractylodis Lanceae Rhizoma | Eudesmol | 37.2 ± 4.4 | — | — | — |
(−): Indicates more than 100 µmol/Las IC50 values.
NS: Non selective.
Each value indicates the mean ± SEM of three samples as µmol/L
Supplementary Table S2 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.09.003.
4. Discussion
We found that ICV UCN1 -induced reduction of plasma ghrelin levels was not altered by vagotomy and attenuated by the peripheral blockade of α2-AR and restored by the activation of β1-AR in rats. Conversely, α2-AR agonist and β1AR antagonist enhanced the inhibitory effect of ICV UCN1. This α2-AR-ghrelin inhibitory pathway has functional relevance since yohimbine prevented the anorexic effect of ICV UCN1 and the α2-AR action was abolished by the ghrelin receptor antagonist.
UCN1 injected ICV at 300 pmol/rat reproducibly decreased by 50–59% plasma levels of acylated ghrelin in fasted rats consistent with our previous report (Saegusa et al., 2011; Yakabi et al., 2011). UCN1 action is brain-mediated and does not reflect peptide leakage into the periphery since systemic injection of UCN1 increases the total ghrelin plasma levels in fed rats or had no effect in fasted state (Wang et al., 2006, 2013). It has been well established that the majority of circulating ghrelin is produced by X/A-like cells located in the gastric mucosa, as indicated by the pronounced reduction of circulating ghrelin after gastrectomy (Ariyasu et al., 2001; Mizutani et al., 2009). The stomach receive prominent vagal inner-vation through efferent projections from DMN neurons (Berthoud et al., 1991). Central vagal activation (Stengel et al., 2010a) and peripheral acetylcholine administration (Shrestha et al., 2009) reportedly increase ghrelin release, while muscarinic receptor antagonists suppress ghrelin secretion in fasted rats (Hosoda and Kangawa, 2008). However, ICV UCN1 action is unlikely to be mediated by alterations of vagal pathway as reported for the inhibitory influence of intracisternally injected UCN1 on gastric emptying in rats (Czimmer et al., 2006). This is supported by the observation that subdiaphragmatic vagotomy did not alter the magnitude of ghrelin decrease induced by ICV UCN1. Moreover c-fos mRNA, which is used as a marker of autonomic neuronal activation (Krukoff, 1993), was not induced in DMN neurons by ICV UCN1 (Supplementary Fig. S1 and Table S1) as previously observed (Yakabi et al., 2011).
In contrast, there are compelling reports documenting that ICV injection of UCN1 activates the core group of autonomic brain structures regulating sympathetic outflow, namely the PVN, LC, NTS and RVLM, as monitored by the robust induction of Fos immunoreactivity (Bittencourt et al., 1999; Daniels et al., 2004) or c-fos gene expression including under similar conditions associated with decreased ghrelin plasma levels (Yakabi et al., 2011) and as well as in the present study (Supplementary Fig. S1 and Table S1). We have previously reported that ICV UCN1-induced decreased circulating levels of acylated ghrelin are mediated by brain CRF2 (Yakabi et al., 2011). Other function data support that activation of CRF2 in the PVN stimulates sympathetic nerve activity in rat the viscera (Li et al., 2010). These data provide neuroanatomical and functional support for a supra spinal sites of action of ICV UCN1 to activate sympathetic outflow. However, it cannot be ruled out that an additional thoracic site regulating sympathetic nerve (Quinson et al., 2001) can also be involved in ICV UCN1 action.
Activation of sympathetic pathways release nore-pinephrine from nerve endings that bind to ARs, which are classified into α- and β-ARs based on their responses to various catecholamines. Previous studies in rats have indicated that fasting plasma levels of ghrelin are stimulated by α-AR antagonists and β-AR agonists and inhibited by α-AR agonists (Hosoda and Kangawa, 2008). In the present study, a pharmacologic approach using peripheral administered selective α- and β-AR subtype antagonists and agonists revealed that α2-AR antagonist and β1AR agonist raise, while α2-AR agonist and β1-AR antagonist further suppress, the lowered ghrelin plasma levels under conditions of ICV UCN1 injection in fasted rats. In support of this assertion, the peripheral injection of the α-AR antagonist, phentolamine, and the selective α2-AR antagonist, yohimbine, partially prevented ICV UCN1-induced reduction of plasma acylated ghrelin. Conversely, the activation of α2-AR with IP injection of clonidine exerted inhibitory effects, as shown by the dose-related further decreased of ghrelin plasma levels in ICV UCN1-treated rats (Supplementary Fig. S2). Although peripherally administered the selective α2-AR antagonist yohimbine crosses the blood-brain barrier (Szemeredi et al., 1991), however it is likely that yohimbine exerts its action peripherally, because, when administered ICV, this α2-AR antagonist did not influence the decreased ghrelin levels in response to ICV UCN1.
Additional pharmacological studies established the specificity toward peripheral α2-ARs since under the same conditions, the α1-AR antagonist (prazosin) or β-AR antagonists (propanolol or the β2-AR antagonist, ICI-118,551) had either no effect or further (the β1 -AR antagonist, atenolol) enhanced the inhibitory effects of ICV UCN1. Likewise, the activation of other α- or β-AR subtypes by agonists could not mimic the inhibitory effects of UCN1, as shown by the lack of effect of IP injection of α-AR agonist, synephrine, the α1-AR agonist, phenylephrine, or the β2-AR agonist, salbutamol. In addition, we showed that the IP injection of non-selective β-AR agonist, isoproterenol or the selective β1-AR agonist, denopamine, restored the basal plasma levels of acylated ghrelin in ICV UCN1-injected rats. This may represent a direct action on X/A like cells. This finding is supported by a recent study using a culture ghrelinoma cells line showing that ghrelin release is also stimulated selectively by the activation of β1-AR agonist and inhibited by β1-AR antagonist, atenonol (Zhao et al., 2010). Other in vivo studies have shown that noradrenalin raises the ghrelin levels measured in effluent of microdialysis probe implanted into the rat gastric submucosa (de la Cour et al., 2007). Additionally, electrically stimulated postganglionic sympathetic axons projecting from the celiac ganglions of rats increases ghrelin release (Mundinger et al., 2006). Taken together, it may be speculated that the α2-AR antagonist, yohimbine-induced partial reversal of decreased levels of ghrelin under conditions of sympathetic activation by ICV UCN1 may reflect the balance between the inhibitory and stimulatory action of peripheral α2-AR and β1-AR activation, respectively. However, the cellular mechanisms by which the peripheral α2-ARs contribute to reduce the decline of ghrelin plasma levels in ICV UCN1 -treated rats will require further investigation. The α2-AR genes were expressed in the rat stomach under our conditions (Supplementary Fig. S3) were consistent with a previous study showing α2A-AR, α2B-AR and α2c-AR mRNA levels in the gastric mucosa of rats with a more abundant expression of the message for α2A-AR (Gyires et al., 2007). This suggests that α2-ARs may directly or indirectly regulate gastric ghrelin secretion.
Supplementary Fig. S3 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.09.003.
Irrespective of the mechanisms involved, we showed that the α2-AR signaling pathway has functional relevance to ghlerin secretion. ICV UCN1 decreased the feeding response to an overnight fast consistent with previous reports (Benoit et al., 2000; Smagin et al., 1998; Spina et al., 1996; Yakabi et al., 2011). This was prevented by the α2-AR antagonist, yohimbine. In addition, the simultaneous administration of a ghrelin receptor antagonist and α2-AR antagonist completely abolished the effects achieved by α2-AR antagonist administration alone. Likewise, rikkunshito established as an enhancer of endogenous ghrelin secretion (Takeda et al., 2012) administered orogastrically also increased plasma acylated ghrelin inhibited by ICV UCN1. This was associated with the suppression of the reductions in food intake (Supplementary Fig. S4) was consistent with our previous data (Yakabi et al., 2011). We reported recently that the decreased food intake following ICV UCN1 injection was improved by the administration of exogenous acylated ghrelin (Yakabi et al., 2011). Collectively, these findings point to a role of ghrelin receptor activation to restore feeding behavior under conditions of decreased circulating ghrelin induced by ICV UCN1.
Supplementary Fig. S4 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.09.003.
Lastly, in order to validate further our hypothesis that the UCN1-induced reduction in food intake involved the activation of peripheral α2-ARs, we assessed whether rikkunshito functions as a α2-AR antagonist. Rikkunshito is a mixture of herbal ingredients; therefore, in this study, we examined its 34 components using an in vitro AR binding assay. We found that several components, namely glycycoumarin, 6- and 8-shogaol, 10-gingerol and eudesmol, functioned as α2-AR antagonists. These results suggest that the effects of rikkunshito on decreased food intake in response to ICV UCN1 administration may be induced through the α2-AR antagonist property of some of its specific components.
In conclusion, the present findings indicate that ICV UCN1 -induced reduction of fasting plasma levels of acylated ghrelin is independent from the vagus nerve, while it is associated with the activation of specific brain nuclei influencing sympathetic pathways. The peripheral AR effectors mediating ghrelin decline after ICV UCN1 involve the activation of α2-ARs and the dampening of β1-AR stimulatory effect on ghrelin release. The α2-ARs activation and related ghrelin decline contribute to the inhibition of feeding response to a fast induced by ICV UCN1. It suggests that α2-AR antagonists may improve alterations of food intake linked with suppression of ghrelin under conditions of brain CRF receptor activation.
Supplementary Material
Acknowledgments
This work was supported in part by Saitama Medical Center, Saitama Medical University (Japan), a grant from Tsumura & Co. (Japan) and the Senior Research Career Scientist Award (YT).
Role of the funding source
There was no impact from funding source(s) on any aspect of the work with the current manuscript (design, data collection, analysis, interpretation, writing, or submission).
Footnotes
Conflict of interest
Prof. Koji Yakabi and Prof. Yvette Taché received research grant support from Tsumura & Co. Dr. Yumi Harada, Dr. Seiichi lizuka, and Dr. Tomohisa Hattori are employed by Tsumura & Co. Prof. Kiyoshige Takayama, Dr. Shoki Ro, Ms. Mitsuko Ochiai, and Dr. Lixin Wang have no conflicts of interests to declare.
References
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Newman SM, Smith-Roe S, Jochman KA, Kalin NH. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J. Neurosci. 2007;27:10568–10577. doi: 10.1523/JNEUROSCI.3044-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Hamon M, Benoliel JJ. Prevention by 5-HT1A receptor agonists of restraint stress- and yohimbine-induced release of cholecystokinin in the frontal cortex of the freely moving rat. Neuropharmacology. 1999;38:525–532. doi: 10.1016/s0028-3908(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Thiele TE, Heinrichs SC, Rushing PA, Blake KA, Steeley RJ. Comparison of central administration of corticotropin-releasing hormone and urocortin on food intake, conditioned taste aversion, and c-Fos expression. Peptides. 2000;21:345–351. doi: 10.1016/s0196-9781(00)00153-4. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol. 1991;260:R200–R207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J. Comp. Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- Cha SH, Wolfgang M, Tokutake Y, Chohnan S, Lane MD. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16871–16875. doi: 10.1073/pnas.0809255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Hover CV, Lindberg D, Li C. Central urocortin 3 and type 2 corticotropin-releasing factor receptor in the regulation of energy homeostasis: critical involvement of the ventromedial hypothalamus. Front Endocrinol. (Lausanne) 2012;3:180. doi: 10.3389/fendo.2012.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie PJ, Coscina DV, Bishop C, Coiro CD, Koob GF, Rivier J, Vale W. Hypothalamic paraventricular nucleus injections of urocortin alter food intake and respiratory quotient. Brain Res. 2001;916:222–228. doi: 10.1016/s0006-8993(01)02851-7. [DOI] [PubMed] [Google Scholar]
- Czimmer J, Million M, Tache Y. Urocortin 2 acts centrally to delay gastric emptying through sympathetic pathways while CRF and urocortin 1 inhibitory actions are vagal dependent in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G511–G518. doi: 10.1152/ajpgi.00289.2005. [DOI] [PubMed] [Google Scholar]
- Daniels D, Markison S, Grill HJ, Kaplan JM. Central structures necessary and sufficient for ingestive and glycemic responses to Urocortin I administration. J. Neurosci. 2004;24:11457–11462. doi: 10.1523/JNEUROSCI.2702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fanti BA, Martinez JA. Central urocortin activation of sympathetic-regulated energy metabolism in Wistar rats. Brain Res. 2002;930:37–41. doi: 10.1016/s0006-8993(01)03401-1. [DOI] [PubMed] [Google Scholar]
- de la Cour CD, Norlen P, Hakanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdial-ysis study. Regul. Pept. 2007;143:118–126. doi: 10.1016/j.regpep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Gaszner B, Csernus V, Kozicz T. Urocortinergic neurons respond in a differentiated manner to various acute stressors in the Edinger-Westphal nucleus in the rat. J. Comp. Neurol. 2004;480:170–179. doi: 10.1002/cne.20343. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Markison S, Ginsberg A, Kaplan JM. Long-term effects on feeding and body weight after stimulation of forebrain or hindbrain CRH receptors with urocortin. Brain Res. 2000;867:19–28. doi: 10.1016/s0006-8993(00)02193-4. [DOI] [PubMed] [Google Scholar]
- Gyires K, Zadori ZS, Shujaa N, Minorics R, Falkay G, Matyus P. Analysis of the role of central and peripheral alpha2-adrenoceptor subtypes in gastric mucosal defense in the rat. Neurochem. Int. 2007;51:289–296. doi: 10.1016/j.neuint.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kangawa K. The autonomic nervous system regulates gastric ghrelin secretion in rats. Regul. Pept. 2008;146:12–18. doi: 10.1016/j.regpep.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Kangawa K. Ghrelin and the regulation of food intake and energy balance. Mol. Interv. 2002;2:494–503. doi: 10.1124/mi.2.8.494. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J. Comp. Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Krukoff TL. Expression of c-fos in studies of central autonomic and sensory systems. Mol. Neurobiol. 1993;7:247–263. doi: 10.1007/BF02769178. [DOI] [PubMed] [Google Scholar]
- Li X, Fan M, Shen L, Cao Y, Zhu D, Hong Z. Excitatory responses of cardiovascular activities to urocortin3 administration into the PVN of the rat. Auton. Neurosci. 2010;154:108–111. doi: 10.1016/j.autneu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J. Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, Kato I, Fujimiya M. Localization of acyl ghrelin-and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G974–G980. doi: 10.1152/ajpgi.00147.2009. [DOI] [PubMed] [Google Scholar]
- Muller TD, Tschop MH. Ghrelin - a key pleiotropic hormone-regulating systemic energy metabolism. Endocr. Dev. 2013;25:91–100. doi: 10.1159/000346590. [DOI] [PubMed] [Google Scholar]
- Mundinger TO, Cummings DE, Taborsky GJ., Jr Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology. 2006;147:2893–2901. doi: 10.1210/en.2005-1182. [DOI] [PubMed] [Google Scholar]
- Ohata H, Suzuki K, Oki Y, Shibasaki T. Urocortin in the ventromedial hypothalamic nucleus acts as an inhibitor of feeding behavior in rats. Brain Res. 2000;861:1–7. doi: 10.1016/s0006-8993(99)02378-1. [DOI] [PubMed] [Google Scholar]
- Patterson ZR, Abizaid A. Stress induced obesity: lessons from rodent models of stress. Front. Neurosci. 2013;7:130. doi: 10.3389/fnins.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am. J. Physiol. 1996;271:R766–R769. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- Quinson N, Robbins HL, Clark MJ, Furness JB. Locations and innervation of cell bodies of sympathetic neurons projecting to the gastrointestinal tract in the rat. Arch. Histol. Cytol. 2001;64:281–294. doi: 10.1679/aohc.64.281. [DOI] [PubMed] [Google Scholar]
- Saegusa Y, Takeda H, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Nahata M, Hattori T, Asaka M. Decreased plasma ghrelin contributes to anorexia following novelty stress. Am. J. Physiol. Endocrinol. Metab. 2011;301:E685–E696. doi: 10.1152/ajpendo.00121.2011. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Shah NS, Pugh PC, Nam H, Rosenthal DT, van Wijk D, Gaszner B, Kozicz T, Kerman IA. A subset of presympathetic-premotor neurons within the centrally projecting Edinger-Westphal nucleus expresses urocortin-1. J. Chem. Neuroanat. 2013;52:25–35. doi: 10.1016/j.jchemneu.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha YB, Wickwire K, Giraudo SQ. Direct effects of nutrients, acetylcholine, CCK, and insulin on ghrelin release from the isolated stomachs of rats. Peptides. 2009;30:1187–1191. doi: 10.1016/j.peptides.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagin GN, Howell LA, Ryan DH, De Souza EB, Harris RB. The role of CRF2 receptors in corticotropin-releasing factor- and urocortin-induced anorexia. Neuroreport. 1998;9:1601–1606. doi: 10.1097/00001756-199805110-00063. [DOI] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel-Stengel M, Wang L, Shaikh A, Lambrecht NW, Rivier J, Tache Y. Abdominal surgery inhibits circulating acyl ghrelin and ghrelin-O-acyltransferase levels in rats: role of the somatostatin receptor subtype 2. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G239–G248. doi: 10.1152/ajpgi.00018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Luckey A, Yuan PQ, Wang L, Tache Y. Cold ambient temperature reverses abdominal surgery-induced delayed gastric emptying and decreased plasma ghrelin levels in rats. Peptides. 2010a;31:2229–2235. doi: 10.1016/j.peptides.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Tache Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides. 2010b;31:357–369. doi: 10.1016/j.peptides.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Taché YF. CRF and urocortin peptides as modulators of energy balance and feeding behavior during stress. Front. Neurosci. 2014;8 doi: 10.3389/fnins.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szemeredi K, Komoly S, Kopin IJ, Bagdy G, Keiser HR, Goldstein DS. Simultaneous measurement of plasma and brain extracellular fluid concentrations of catechols after yohimbine administration in rats. Brain Res. 1991;542:8–14. doi: 10.1016/0006-8993(91)90990-d. [DOI] [PubMed] [Google Scholar]
- Takeda H, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Sae-gusa Y, Nahata M, Hattori T, Asaka M. Rikkunshito as a ghrelin enhancer. Methods Enzymol. 2012;514:333–351. doi: 10.1016/B978-0-12-381272-8.00021-0. [DOI] [PubMed] [Google Scholar]
- Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, Asaka M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134:2004–2013. doi: 10.1053/j.gastro.2008.02.078. [DOI] [PubMed] [Google Scholar]
- Toth ZE, Gallatz K, Fodor M, Palkovits M. Decussations of the descending paraventricular pathways to the brainstem and spinal cord autonomic centers. J. Comp. Neurol. 1999;414:255–266. [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen S, Dambrova M, Nasman J, Schioth HB, Gu Y, Wikberg-Matsson A, Wikberg JE. [3H]RS79948-197 binding to human, rat, guinea pig and pig alpha2A-, alpha2B- and alpha2C-adrenoceptors. Comparison with MK912, RX821002, rauwolscine and yohimbine. Eur. J. Pharmacol. 1998;343:93–101. doi: 10.1016/s0014-2999(97)01521-5. [DOI] [PubMed] [Google Scholar]
- Uhlen S, Porter AC, Neubig RR. The novel alpha-2 adrenergic radioligand [3H]-MK912 is alpha-2C selective among human alpha-2A, alpha-2B and alpha-2C adrenoceptors. J. Pharmacol. Exp. Ther. 1994;271:1558–1565. [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St-Pierre DH, Tache Y. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G611–G620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- Wang L, Stengel A, Goebel-Stengel M, Shaikh A, Yuan PQ, Tache Y. Intravenous injection of urocortin 1 induces a CRF2 mediated increase in circulating ghrelin and glucose levels through distinct mechanisms in rats. Peptides. 2013;39:164–170. doi: 10.1016/j.peptides.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Urocortin 1 in the dorsal raphe regulates food and fluid consumption, but not ethanol preference in C57BL/6J mice. Neuroscience. 2006;137:1439–1445. doi: 10.1016/j.neuroscience.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Yakabi K, Noguchi M, Ohno S, Ro S, Onouchi T, Ochiai M, Takabayashi H, Takayama K, Harada Y, Sadakane C, Hattori T. Urocortin 1 reduces food intake and ghrelin secretion via CRF(2) receptors. Am. J. Physiol. Endocrinol. Metab. 2011;301:E72–E82. doi: 10.1152/ajpendo.00695.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, Goldstein JL, Zigman JM. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15868–15873. doi: 10.1073/pnas.1011116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Tache Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol. Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.