Abstract
We determined whether deficiency of neuronal SOCS3, a potential negative regulator of leptin signaling, amplifies the chronic effects of leptin on food intake, energy expenditure, glucose, and blood pressure, and protects against adverse cardiometabolic effects of obesity. Blood pressure and heart rate were recorded by telemetry and oxygen consumption (VO2) was monitored in 22-week-old mice with nervous system SOCS3 deficiency (SOCS3–Nestin-Cre) and control mice (SOCS3flox/flox) fed normal or high fat-high fructose diet (HFFD) from 6 to 22 weeks of age. Compared to controls, SOCS3–Nestin-Cre mice had lower plasma glucose (124±7 vs 146±10mg/dl), consumed less food (3.0±0.4 vs 3.6±0.2 g/day), had similar VO2 (77±6 vs 73±3 ml/kg/min) and blood pressure (103±3 vs 107±3 mmHg) but higher heart rate (666±15 vs 602±17 bpm). In mice fed the normal diet, leptin infusion for 7 days caused similar reductions in food intake (2.3±0.1 vs 2.4±0.2 g) but greater increases in blood pressure (15±3 vs 7±2 mmHg) in SOCS3–Nestin-Cre compared to controls. Leptin reduced blood glucose concentrations in both groups. Male or female SOCS3–Nestin-Cre fed HFFD exhibited less weight gain, body fat and liver steatosis, and greater energy expenditure and heart rate compared to controls. Female SOCS3-Nestin-Cre mice fed HFFD had higher blood pressure compared to controls. Thus, neuronal SOCS3 appears to play an important role in cardiometabolic regulation since neuronal SOCS3 deficiency reduced body weight and food intake while amplifying leptin’s effects on appetite and BP and attenuating the adverse metabolic effects of HFFD.
Keywords: Food intake, blood pressure, heart rate, hypertension, obesity, liver steatosis, glucose, energy expenditure
INTRODUCTION
Suppressor of cytokine signaling 3 (SOCS3) is a protein encoded by the SOCS3 gene and is induced by various cytokines, including leptin. When activated, SOCS3 binds to the phosphorylated leptin receptor (LR) through its Src homology-2 (SH2) domain and inhibits JAK tyrosine kinase activity by its N-terminal kinase inhibitory region 1–4. This effect of SOCS3 may be an important regulator of leptin signaling and could contribute to development of resistance to leptin’s cardiometabolic effects. Peripheral leptin administration induces SOCS3 mRNA expression in hypothalamic regions 5, 6, especially in the arcuate nucleus (ARC) of the hypothalamus, which co-expresses LR and other neuropeptides that regulate food intake 5. In addition, increased SOCS3 expression is associated with attenuated leptin-induced STAT3 activation in the ARC in mice with diet-induced obesity (DIO) 7.
Increased SOCS3 expression has been suggested to play a role in body weight dysregulation in obesity since mice with neuronal deficiency of SOCS3 were reported to be resistant to DIO 8. Although these observations suggest that SOCS3 is important for regulating body weight, the role of SOCS3 in mediating the chronic blood pressure (BP) and glucose lowering effects of leptin are still unknown. There have been no previous studies, to our knowledge, that examined the role of central nervous system (CNS) SOCS3 signaling in mediating the chronic actions of leptin on appetite, thermogenesis, glucose homeostasis, and cardiovascular function. In addition, the potential role of neuronal SOCS3 deficiency in cardiovascular regulation and in modulating the chronic BP effects of leptin are unclear, especially in conditions in which SOCS3 may be activated such as in DIO.
In the present study, we inactivated SOCS3 in the entire nervous system, using a genetic approach, to determine if SOCS3 signaling is an important modulator of leptin’s chronic effects on cardiovascular and metabolic functions. We also tested the hypothesis that nervous system SOCS3 deficiency protects against the adverse metabolic effects of a chronic high fat – high fructose diet (HFFD), including weight gain, increased adiposity, impaired glucose tolerance, and increased liver lipids, while increasing BP and heart rate (HR).
Our results indicate that nervous system SOCS3 deficiency amplifies leptin’s effects to decrease plasma insulin and glucose concentrations, and to increase BP. In addition, nervous system SOCS3 deficiency attenuated body weight gain, adiposity, and liver steatosis while increasing energy expenditure and HR, but did not improve glucose tolerance in male or female mice fed HFFD. However, HFFD significantly increased BP to a greater extent in female mice with nervous system SOCS3 deficiency, suggesting a sex difference in the role of SOCS3 signaling on BP regulation in obesity.
METHODS
The experimental procedures and protocols for these studies followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
The authors declare that the raw data that support the findings of this study are available from the corresponding author upon reasonable request. Expanded Methods and additional data can be found in the online supplement.
Animals
Mice were randomly divided into different groups for acute and chronic experiments. Male and female 6- to 22-week old control SOCS3flox/flox (n=21) and SOCS3-Nestin-Cre, (n=20) mice were used in these studies. SOCS3-Nestin-Cre mice with nervous system SOCS3 deficiency were generated by crossing Nestin-Cre mice (C57Bl 6J background) that express Cre-recombinase specifically in neurons (Jackson Laboratories, B6Cg-Tg (Nestin-Cre)/1kln/J) with SOCS3flox/flox mice (generously provided by Dr. George W. Booz, University of Mississippi Medical Center). Only mice homozygous for the SOCS3flox/flox gene that also expressed Cre-recombinase were used for the group with neuronal SOCS3 deficiency (SOCS3-Nestin-Cre mice, Figure 1A). Littermate homozygous SOCS3flox/flox mice from our colony were used as controls for the experiments (See supplement for discussion of methods to validate SOCS3 deficiency).
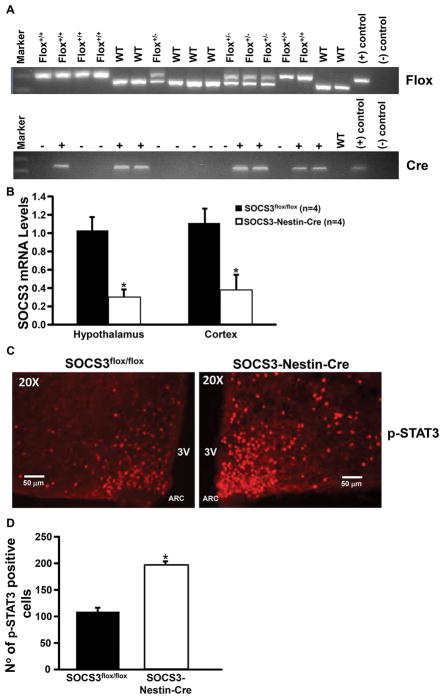
Figure 1.
Genotype confirmation using polymerase chain reaction (PCR) from tail snip samples with analysis indicating presence or absence of Cre-recombinase and SOCS3flox/flox and immunofluorescence analysis. (A) Top: +/+ indicates a homozygous, +/− heterozygous results and WT, wild-type based on the 3 control bands for WT, +, and – primers (far right). Bottom: + expresses Cre-recombinase and – does not express Cre-recombinase based on + and − control bands (far right). Positive (+) and negative (−) DNA samples. (B) SOCS3 mRNA levels quantified by qRT-PCR in hypothalamic and cortex fractions of SOCS3-Nestin-Cre and SOCS3flox/flox control mice (n=4/per group). (C) 20x view of representative immunohistochemical staining of p-STAT3 expression. (D) Quantification of pSTAT3 positive cells in arcuate nucleus of homozygous SOCS3-Nestin-Cre and SOCS3flox/flox control mice (n=3 per group). *p<0.05 compared to SOCS3flox/flox mice.
Food and water were offered ad libitum throughout the experiment and room temperature was maintained at 23 to 24oC. A normal sodium intake of ~460 μmol/day was maintained during the leptin infusion experiments via the constant saline infusion combined with sodium-deficient rodent chow as previously described 9. All mice were allowed to recover for 8 to 10 days after the surgery before baseline measurements were taken.
High-Fat High-Fructose diet (HFFD)
Separate groups of control male (n=6) and female (n=6) SOCS3flox/flox mice and male (n=6) and female (n=7) SOCS3-Nestin-Cre mice were individually housed and fed a HFFD (Harlan Teklad TD-0881, 4.7 Kcal/g, 45% fat) and water was replaced by degassed 7UP (Pepper/Seven Up, Inc, Plano, TX; 0.416 kcal/mL) starting at 6 weeks of age until 26 weeks of age. Consumption of a HFFD diet mimics the obesogenic Western diet, including high fructose intake from beverages, and leads to many characteristics of the metabolic syndrome including increased body weight, increased adiposity, hyperglycemia, hyperinsulinemia and hyperleptinemia in rodents 10–13.
Experimental Design and Methods
Acute Leptin Injection
To determine whether nervous system SOCS3 inactivation amplifies the acute effects of leptin on appetite, food intake was measured 2, 4, 15 and 24 hours after an intraperitoneal leptin (5 mg/kg) or vehicle injection (saline, 0.3 ml) between 5 and 6 pm in separate groups of non-instrumented and non-fasted male and female 22-week-old SOCS3flox/flox (n=7) and SOCS3-Nestin-Cre (n=6) mice fed control diet.
Chronic Leptin Infusion
After an 8–10 day post-surgery recovery period plus 5 days of stable baseline control measurements, leptin was added to the saline vehicle and infused at 2 μg/kg/min in male and female SOCS3flox/flox (n=6) and SOCS3-Nestin-Cre (n=6) mice for 7 days followed by a 5-day post-treatment period during which only saline vehicle was infused. BP, HR were measured by telemetry, 24 hrs/day (see supplement), and food and water intake were recorded daily. Blood samples (100 μL) were collected via a tail snip after 6 hours of fasting (8:00 am to 2:00 pm) during the control period (day 5), on the last day of leptin infusion (day 7 of treatment), and at the end of the post-treatment period for measurements of plasma glucose, leptin and insulin concentrations.
Oxygen Consumption and Motor Activity
In separate experiments, male and female 22-week-old SOCS3flox/flox (n=6–12) and SOCS3-Nestin-Cre (n=6–13) mice fed the control or the HFFD were placed individually in metabolic cages (AccuScan Instruments Inc, Columbus, OH) equipped with oxygen sensors to measure oxygen consumption (VO2) and infrared beams to determine motor activity. After 4–6 days of acclimatization, VO2 and animal activity were recorded for 3 consecutive control days and then during 7 days of leptin infusion (4 μg/kg/min, i.p.) and a 5-day post-treatment period (see supplement).
Oral Glucose Tolerance Test (GTT)
D-glucose (3 mg/kg of lean body mass plus 1 mg/kg of fat mass) was administered by gavage after a 5-h fast in male and female 22-week-old SOCS3flox/flox (n=6–8) and SOCS3-Nestin-Cre (n=7–9) mice fed a control or HFFD. Blood samples were collected by tail snip, and blood glucose was measured using glucose strips (ReliOn) at baseline, 15, 30, 60, 90, and 120 minutes after glucose administration.
Body Weight, Body Composition, and Liver Composition
Body weight was measured twice a week from 6 until 20 weeks of age, and weekly changes in body composition were analyzed using magnetic resonance imaging (4-in-1 EchoMRI-900TM, Echo Medical System, Houston, TX). Whole livers from male and female SOCS3flox/flox (n=5) and SOCS3-Nestin-Cre (n=5) mice fed a HFFD were harvested to determine liver fat and lean mass composition using EchoMRI and Oil Red-O staining (see supplement).
We also performed Oil Red-O staining in frozen liver sections of SOCS3flox/flox (n=5) and SOCS3-Nestin-Cre (n=5) mice fed a HFFD to assess liver lipid deposits. Sections (10 μm thick) were fixed in 10% buffered formalin for 5 minutes and stained for 10 minutes with 0.5% Oil Red-O in 60% isopropyl alcohol. The slides were washed several times in water and counterstained in Mayer’s hematoxylin for 30 seconds and mounted in aqueous mounting media.
Plasma Hormones and Glucose Measurements
Plasma leptin and insulin concentrations were measured with ELISA kits (R&D Systems and Crystal Chem Inc., respectively), and plasma glucose concentrations were determined using the glucose oxidation method (Beckman glucose analyzer 2).
Statistical Analyses
Results are expressed as means ± SEM. Data were analyzed by paired t test or 1-way ANOVA with repeated measures followed by Dunnett’s post hoc test for comparisons between control and experimental values within each group when appropriate. Comparisons between different groups were made by unpaired t test or 1-way ANOVA followed by Dunnett’s post hoc test when appropriate. Statistical significance was accepted at a level of P<0.05.
RESULTS
Expression of SOCS3 in the Hypothalamus and Cortex of SOCS3flox/flox and SOCS3-Nestin-Cre Mice
The qRT-PCR analysis showed that expression of SOCS3 mRNA in the hypothalamus and cortex of SOCS3-Nestin-Cre was approximately 70 and 62 % lower, respectively, compared to control mice (Figure 1B).
p-STAT3 Immunofluorescence in the Hypothalamus of SOCS3flox/flox and SOCS3-Nestin-Cre Mice After Acute Leptin Injection
After acute i.p. leptin injection, p-STAT3 staining was markedly enhanced in SOCS3-Nestin-Cre compared to control mice (Figures 1C and D). Since STAT3 is the major signaling pathway for the effects of leptin to reduce food intake, increased p-STAT3 in the ARC provides additional confirmation of the functional effects of neuronal SOCS3 deficiency in SOCS3-Nestin-Cre compared to control SOCS3flox/flox mice.
Effect of CNS SOCS3 Deletion on Body Length, Body Weight, and Visceral Fat
Mice with neuronal SOCS3 deletion showed ~22% reduction in food intake (2.4±0.1 vs. 3.2±0.5 g) and a tendency for lower body weight (20.4±1.4 vs. 25.3±2.5 g), lean (14.2±0.6 vs. 17.1±2.7 g) and fat mass (3.1±0.6 vs. 4.5±0.5 g) compared to control mice as early as 10 weeks of age, and these differences persisted until 22 weeks of age when experiments were initiated (Figure S1). The small reductions in body weight, lean and fat mass were not statistically significant. Body length (nasal to anal distance) at 22 weeks of age was shorter in male and female SOCS3-Nestin-Cre mice compared to SOCS3flox/flox control mice (8.3±0.1 vs. 9.2±0.9 cm).
Acute and Chronic Effects of Leptin on Food Intake and Body Weight
Compared to vehicle injection, acute i.p. leptin injection reduced 24-hour food intake in SOCS3flox/flox mice but caused a greater reduction in SOCS3-Nestin-Cre mice (Figure 2A). The acute anorexic effect of leptin was observed as early as 4 hours post-injection in SOCS3-Nestin-Cre mice but only after 16 hours in SOCS3flox/flox mice. These results indicate that SOCS3 deficiency amplifies the acute effects of leptin to reduce appetite. No changes in body weight were observed after acute leptin or saline vehicle injections (data not shown).
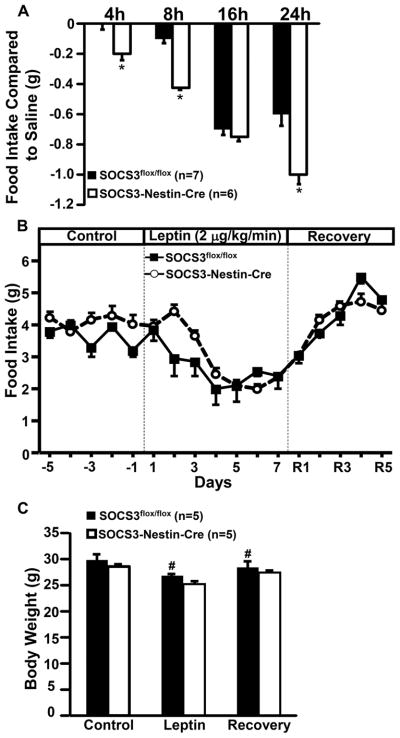
Figure 2.
Food intake and body weight responses to acute and chronic leptin infusion in male and female SOCS3flox/flox (n=10) SOCS3-Nestin-Cre (n=10) mice. (A) Twenty-four hour food intake response to acute ip leptin injection, (B) Effect of leptin infusion (2 μg/kg/min, iv) for 7 days on food intake, and (C) body weight response to leptin infusion (2 μg/kg/min, iv) for 7 days. *p<0.05 compared to SOCS3flox/flox mice. #p<0.05 compared to control period.
Chronic i.v. leptin infusion for 7 consecutive days markedly reduced food intake in SOCS3flox/flox and SOCS3-Nestin-Cre mice (Figure 2B). The reduction in food intake was associated with approximately 10% weight loss in both groups (Figure 2C). After stopping leptin infusion, food intake and body weight returned to baseline values in both groups (Figures 2B and C).
In order to determine whether the presence of Cre-recombinase per se may induce a phenotypic response to chronic leptin infusion we previously investigated the anorexigenic effect of leptin in Nestin-Cre mice. Chronic leptin infusion in these mice reduced food intake and body weight to the same extent observed in control SOCS3flox/flox mice, thus indicating that the presence of Cre-recombinase by itself does not alter leptin’s ability to suppress appetite and to promote weight loss14. There were no significant sex differences in the anorexic effect of acute or chronic leptin infusion (Figure S2).
Effects of Chronic Leptin Infusion on VO2 and Motor activity
At baseline, SOCS3-Nestin-Cre mice exhibited higher VO2 compared to control mice (Figure 3A). Chronic leptin infusion increased VO2 by approximately 15% and 20% in SOCS3flox/flox and SOCS3-Nestin-Cre mice, respectively (Figure 3B). At baseline, no differences in motor activity were observed between groups (SOCS3-Nestin-Cre: 87±18 vs. SOCS3flox/flox: 78±6 m/day). Chronic leptin infusion significantly reduced motor activity in both groups, however this effect was more pronounced in SOCS3-Nestin-Cre mice (SOCS3-Nestin-Cre: 47±8 vs. SOCS3flox/flox: 60±8 m/day).
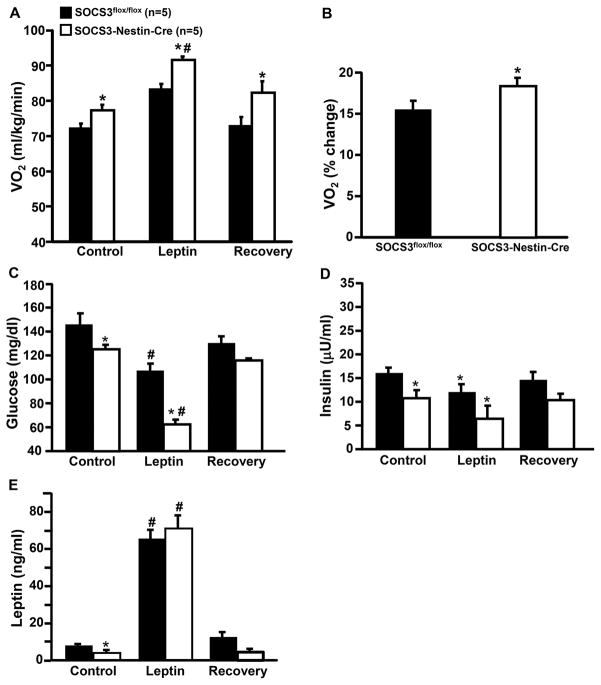
Figure 3.
Oxygen consumption and blood hormones in response to leptin infusion (2 μg/kg/min, iv) for 7 days). (A) Oxygen consumption (VO2), (B) changes in VO2 in response to leptin compared to baseline, (C) blood glucose, (D) insulin, and (E) leptin levels in male and female SOCS3flox/flox and SOCS3-Nestin-Cre mice. *p<0.05 compared to SOCS3flox/flox mice. #p<0.05 compared to baseline.
Effects of Chronic Leptin Infusion on Plasma Glucose, Insulin and Leptin Concentrations
At baseline, SOCS3-Nestin-Cre mice showed significantly lower plasma glucose, insulin and leptin concentrations (Figure 3C–E). Chronic intravenous leptin infusion increased plasma leptin concentrations similarly in SOCS3flox/flox and SOCS3-Nestin-Cre mice (Figure 3E) to concentrations that were similar to those observed in severe obesity.
Leptin infusion in SOCS3flox/flox and SOCS3-Nestin-Cre mice reduced plasma glucose and insulin concentrations. However, leptin’s effects on glucose concentration was significantly greater in SOCS3-Nestin-Cre mice (Figures 3C and D). These results indicate that neuronal system SOCS3 deficiency may amplify the impact of chronic hyperleptinemia to reduce plasma insulin and glucose concentrations.
MAP and HR Responses to Chronic Leptin Infusion
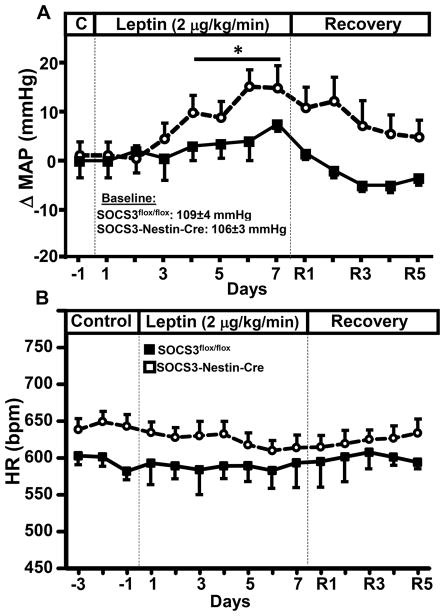
Mean arterial pressure (MAP) and HR were measured 24 hrs/day by telemetry (see supplement). SOCS3-Nestin-Cre and SOCS3flox/flox mice showed similar MAP at baseline (Figure 4A). Chronic leptin infusion caused a gradual and modest elevation in MAP in both groups, despite decreased food intake and weight loss which would normally be associated with reduction in MAP. The elevation in BP during leptin infusion, however, was potentiated in SOCS3-Nestin-Cre mice; on day 7 of treatment, leptin increased MAP by ~6 mmHg in SOCS3flox/flox mice and ~15 mmHg in SOCS3-Nestin-Cre mice. These data indicate that suppression of neuronal SOCS3 exacerbates the chronic effects of leptin to increase arterial pressure. Our previous observations showed that expression of Cre-recombinase in neurons did not alter the BP responses to leptin 9, 15. SOCS3-Nestin-Cre mice also exhibited increased HR compared to SOCS3flox/flox mice (Figure 4A) but there were no significant changes in HR during chronic leptin infusion (Figure 4B). Since there were no significant sex differences for the metabolic and cardiovascular responses to leptin, Figures 4A and 4B represent combined data from male and female mice.
Figure 4.
Mean arterial pressure (MAP) and heart rate (HR) response to leptin infusion (2 μg/kg/min, iv) for 7 days). (A) Delta MAP and (B) HR in response to leptin in male and female SOCS3flox/flox and SOCS3-Nestin-Cre mice. *p<0.05 compared to SOCS3flox/flox mice.
Body Weight, Body Composition and Liver Fat of SOCS3flox/flox and SOCS3-Nestin-Cre Mice Fed a HFFD
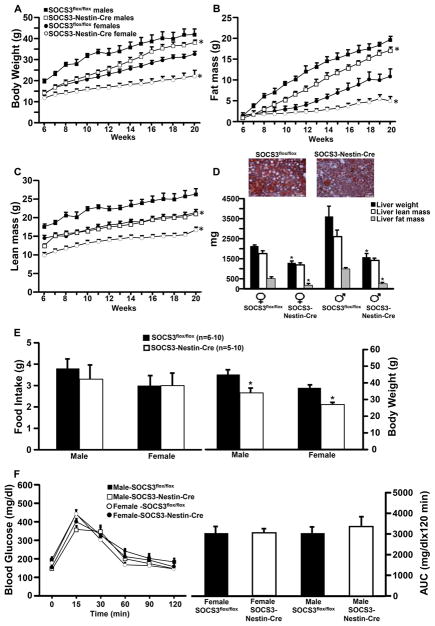
To examine the potential role of SOCS3 in obesity, control mice and SOCS3-Nestin-Cre mice were placed on HFFD for 14 weeks. Male and female SOCS3-Nestin-Cre mice exhibited significantly lower weight gain on HFFD from 6 to 20 weeks compared to control SOCS3flox/flox mice (Figure 5A). This reduced weight gain was associated with reduced fat mass (Figure 5B).
Figure 5.
Body weight, fat and lean mass, and liver lipid analysis in male (n=6) and female (n=6) SOCS3flox/flox mice and male (n=6) and female (n=6) SOCS3-Nestin-Cre mice fed a HFFD. Liver fat and lean mass were assessed using EchoMRI. (A) Body weight, (B) body fat, (C) body lean mass, (D) SOCS3flox/flox and SOCS3-Nestin-Cre mice liver sections stained for Oil Red O and visualized at 20X magnification and liver weight and lean and fat mass, (E) food intake and body weight and (F) blood glucose concentration measured over 120 minutes post gavage during an oral glucose tolerance test (GTT), and blood glucose area under the GTT curve (AUC) for male and female SOCS3flox/flox and SOCS3-Nestin-Cre mice fed a HFFD.*p<0.05 compared to SOCS3flox/flox mice.
Female SOCS3-Nestin-Cre and SOCS3flox/flox mice exhibited significant lower lean mass than their male counterparts (Figure 5C). Male and female SOCS3-Nestin-Cre mice also had significantly lower lean mass compared to their respective controls (Figure 5C). Although male SOCS3-Nestin-Cre mice were approaching the body weight and fat mass of male SOCS3flox/flox mice at 20 weeks of age, female SOCS3-Nestin-Cre mice had progressively lower body weight than female control mice.
Mice were sacrificed at 22 weeks of age and livers harvested from male and female SOCS3-Nestin-Cre mice weighed significantly less than livers from control mice. Compared to controls, male and female SOCS3-Nestin-Cre mice had significantly less liver fat accumulation as measured by EchoMRI (Figure 5D). These results indicate that inactivation of neuronal SOCS3 attenuates the effects of HFFD on body fat and liver fat accumulation.
Food Intake, Body Weight and Glucose Tolerance of SOCS3flox/flox and SOCS3-Nestin-Cre in Mice Fed a HFFD
Daily averages of food intake in SOCS3-Nestin-Cre and SOCS3flox/flox mice were not significantly different at 22 weeks of age in male (3.3±0.7 vs. 3.8±0.5 g) or female mice (3.1±0.6 vs. 3.3±0.5 g, Figure 5E). However, body weight was significantly lower in SOCS3-Nestin-Cre mice compared to SOCS3flox/flox mice (Male: 34±3 vs. 45±3 g and female: 27±1 vs. 37±2 g, Figure 5E). There were no significant differences in glucose tolerance test (GTT) at 20 weeks of age in male or female SOCS3-Nestin-Cre mice compared to control SOCS3flox/flox mice (Figure 5F).
Cardiometabolic Responses to HFFD in SOCS3flox/flox and SOCS3-Nestin-Cre Mice
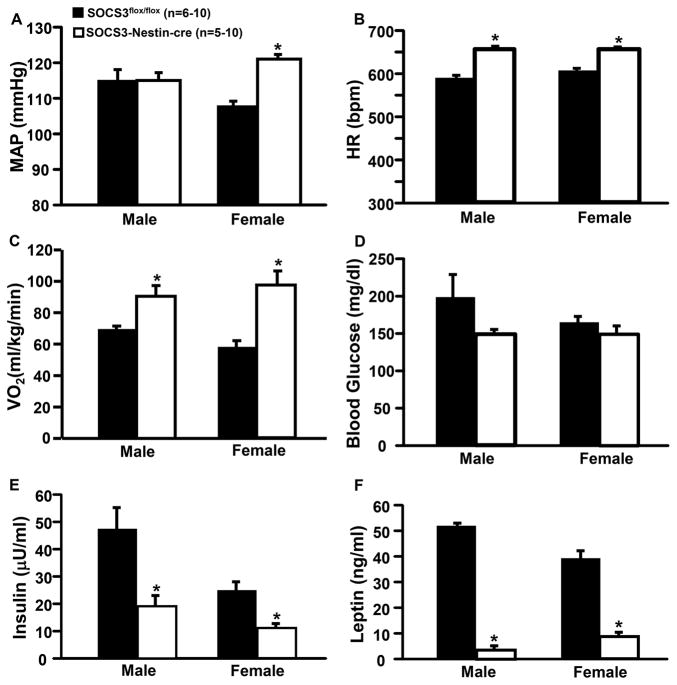
Male SOCS3-Nestin-Cre mice had similar MAP (115±2 vs. 116±1 mmHg) but higher HR (657±3 vs. 592±3 bpm) compared to control SOCS3flox/flox mice fed HFFD (Figures 6A and B). In females, however, SOCS3-Nestin-Cre mice had higher MAP (121±1 vs. 108±1 mmHg) and HR (655±2 vs. 606±5 bpm) compared to female controls (Figures 6A and B).
Figure 6.
Cardiometabolic responses to HFFD in male and female in SOCS3flox/flox (n=6–10) and SOCS3-Nestin-Cre (n=5–10) mice. Blood pressure and heart rate (HR) in SOCS3flox/flox and SOCS3flox/flox/Nestin-Cre mice were measured for 12/12 hrs day/night for 5 consecutive days. (A) Mean arterial pressure (MAP), (B) HR, (C) oxygen consumption (VO2), (D) blood glucose, (E) plasma insulin, and (F) plasma leptin concentrations. *p<0.05 compared to SOCS3flox/flox mice.
Compared to control mice, SOCS3-Nestin-Cre mice had higher oxygen consumption (VO2) in male (94±12 vs. 69±6 ml/kg/min) and female mice fed HFFD (97±15 vs. 58±8 ml/kg/min) (Figure 6C). There were no significant differences in blood glucose concentrations in SOCS3-Nestin-Cre compared to control mice (male: 148±8 vs. 198±31 mg/dl and female 149±12 vs. 164±12 mg/dl) (Figure 6D). However, plasma insulin (Figure 6E) and leptin concentrations (Figure 6F) were significantly lower in male and female SOCS3-Nestin-Cre compared to SOCS3flox/flox control mice at 22 weeks of age. These results indicate that nervous system SOCS3 deficiency reduces weight gain in mice fed HFFD, attenuates increased fat mass, and elevates energy expenditure, but does not improve glucose tolerance during an acute glucose load in male or female mice.
DISCUSSION
We demonstrated that nervous system SOCS3 deficiency is associated with amplified responses to leptin’s effects to raise BP. In addition, neuronal SOCS3 deletion attenuated body weight gain while increasing energy expenditure and HR without altering glucose tolerance in male or female mice fed a HFFD for 14 weeks. Although nervous system SOCS3 deficiency did not improve glucose tolerance after administration of an oral glucose load in mice fed a HFFD, liver lipid accumulation as well as fasting plasma insulin and glucose concentrations were reduced suggesting that liver regulation of glucose metabolism was improved. We also found that on a HFFD, female SOCS3-Nestin-Cre mice exhibited significantly elevated BP compared to control mice on a HFFD, suggesting sex differences for the role of neuronal SOCS3 signaling in BP regulation in obesity.
Our results therefore indicate that neuronal SOCS3 signaling may play an important role in modulating the chronic actions of leptin on metabolic and cardiovascular function. Data from the present study also support our previous findings that enhanced leptin signaling may be a key link between obesity and development of hypertension. One important finding of the present study is that there were sex differences in the effect of neuronal SOCS3 deficiency on BP regulation in DIO. While male mice with neuronal SOCS3 deficiency had similar MAP compared to male controls on a HFFD, female SOCS3-Nestin-Cre mice placed on a HFFD had elevated MAP compared to female control mice. Thus, neuronal deficiency of SOCS3 made female mice more susceptible to the hypertensive effects of a HFFD compared to males. The mechanisms for these sex differences in BP effects of neuronal SOCS3 deficiency are unclear and await further investigation.
Role of SOCS3 in Regulating Energy Balance and Adiposity
SOCS3-Nestin-Cre mice were lighter after weaning compared to control mice of similar age and exhibited greater increases in energy expenditure in response to chronic leptin administration, suggesting that nervous system SOCS3 contributes to normal regulation of body weight.
Mori et al. previously showed that mice lacking SOCS3 in neurons had enhanced leptin signaling 8. The authors observed greater reduction in food intake and weight loss after an acute leptin injection, and lesser weight gain when mice were placed on a high fat diet. In the present study, we observed that when SOCS3-Nestin-Cre mice were fed a HFFD for 14 weeks they exhibited attenuated weight gain and less adiposity compared to control mice. However, the reduced weight gain and adiposity of SOCS3-Nestin-Cre mice fed HFFD observed in our study may not be due entirely to reduced appetite since food intake at 22 weeks of age was similar to that found in control mice fed HFFD. On normal chow or on a HFFD, SOCS3-Nestin-Cre mice had significantly higher VO2 compared to SOCS3flox/flox mice on the same diets, suggesting that nervous system deficiency of SOCS3 may also increase metabolic rate. This enhanced VO2 in mice with nervous system SOCS3 deficiency may be related to enhanced leptin signaling; previous observations indicate that leptin increases VO2 and we found in the present study that leptin injection increased the number and intensity of neurons positively stained for p-STAT3 in the ARC substantially more in SOCS3-Nestin-Cre than in control mice.
Nervous system SOCS3 deficiency was also associated with reduced liver lipids in male and female mice as assessed by oil red-O staining and Echo-MRI. This large reduction in liver lipids may protect these mice against HFFD-induced hepatic insulin resistance since fasting insulin levels were markedly lower while plasma glucose concentration was similar, or slightly lower, when compared to control mice. Thus, reduction in neuronal SOCS3 appears to have an important protective effect against liver steatosis in DIO, suggesting that SOCS3 signaling may exacerbate development of fatty liver and hepatic insulin resistance in obesity.
Role of SOCS3 in Glucose Homeostasis
Another important finding of the present study is that nervous system SOCS3 deficiency reduced fasting insulin and glucose levels while amplifying leptin’s effects to reduce fasting plasma glucose concentration in male and female mice fed a normal diet. We previously showed that leptin administration markedly enhances peripheral tissue glucose utilization via insulin dependent and independent mechanisms 16, 17. Some of leptin’s acute effects on tissue glucose utilization may involve local actions in peripheral tissues, but CNS actions also play an important role in leptin’s chronic effects to stimulate peripheral tissue glucose utilization by mechanisms that are still unclear 18–20. Pedroso et al.21 observed improved GTT in mice placed on a high fat diet with SOCS3 deficiency in leptin receptor expressing cells; this is consistent with our observation of enhanced effects of leptin to reduce fasting insulin and glucose levels in SOCS3-Nestin-Cre mice.
A surprising finding of our current study was nervous system SOCS3 deficiency did not improve glucose tolerance in male or female mice fed a HFFD despite attenuating liver fat accumulation. Our results differ from those of Pedroso et al. who found improved GTT in SOCS3-Nestin-Cre mice fed high fat diet 21. One potential explanation for this apparent discrepancy is that we used high fat plus high fructose diet whereas Pedroso et al. used only a high fat diet in their DIO protocol.
Although our results suggest that SOCS3 may not play a major role in the development of HFFD-induced glucose intolerance in male or female mice, neuronal SOCS3 is important in DIO-induced liver fat accumulation and development of liver insulin resistance. Further studies are needed, however, to determine the specific neurons and efferent mechanisms that link leptin with CNS SOCS3 and peripheral glucose metabolism.
Role of SOCS3 in Modulating the Chronic Blood Pressure and Heart Hate Effects of Leptin
Chronic leptin infusion in rodents raises MAP and HR mainly via activation of the sympathetic nervous system (SNS) 22, 23. The stimulatory effect of leptin on SNS activity appears to be mediated in large part by activation of the proopiomelanocortin-melanocortin 4 receptor (POMC-MC4R) axis. In a previous study we showed that leptin receptor deletion specifically in POMC neurons abolished the chronic effects of leptin on MAP and HR, and acute and chronic MC4R antagonism prevent leptin-induced increases in renal SNS activity, arterial pressure and HR 9. In the present study we found that nervous system SOCS3 deficiency exacerbated the BP response to chronic leptin infusion.
In addition, we also found that female mice with nervous system SOCS3 deficiency fed a HFFD had increased BP compared to female control mice, whereas no differences were observed in male mice with nervous system SOCS3 deficiency compared to control male mice fed a HFFD. This finding suggests a sex difference for the effects of nervous system SOCS3 signaling on BP regulation in obesity caused by feeding a HFFD. Our results are consistent with prior findings of Manrique et al. 24 who reported sex differences in the cardiovascular responses to a HFFD diet. However, the mechanisms by which female mice with nervous system SOCS3 deficiency are more susceptible to HFFD induced elevations in BP are still unknown.
Previous studies by Gao et al. 25 and Dhillon et al. 26 showed that estrogen (E2) triggers excitatory inputs to POMC neurons and suppresses neuropeptide Y (NPY) via activation of ERα. The effects of E2 on food intake, energy expenditure and body weight have also been suggested to be mediated by melanocortin 4 receptor (MC4R) activation27. Thus, one potential explanation for the finding that HFFD increased BP to a greater extent in female than male SOCS3-Nestin-cre mice fed HFFD is that E2 may contribute to activation of the POMC-MC4R axis and suppression of NPY neurons activity. However, further experiments are needed to test these potential mechanisms for sex differences in BP responses to HFFD in mice with neuronal SOCS3 deficiency.
A previous study by Metlakunta et al. suggested that SOCS3 deficiency may increase sensitivity of the PI3K and STAT3 pathways for acute leptin signaling in the hypothalamus 28. The importance of PIK3 in mediating the BP effects of leptin is supported by the observation that pharmacological blockade of PI3K almost completely prevented the acute effects of leptin to increase RSNA 29. We also showed that mice with CNS IRS2 deficiency, which reduces PI3K signaling, are unresponsive to the chronic BP effects of leptin 14. Thus, blockade of the inhibitory effects of SOCS3 signaling on leptin-induced activation of the IRS2/PI3K pathway could be an important mechanism by which CNS SOCS3 deficiency amplifies leptin’s effects on BP.
In summary, we demonstrated that chronic leptin infusion caused greater increases in BP in mice with nervous system SOCS3 deficiency, compared to control mice. Neuronal SOCS3 deficiency also reduced fasting insulin and glucose in mice fed a normal diet and this was associated with reduced liver fat. Compared to controls, male and female mice with nervous system SOCS3 deficiency also had attenuated weight gain, increased energy expenditure and decreased liver fat accumulation when fed a normal or HFFD. When female SOCS3-Nestin-Cre mice were fed a HFFD, BP increased significantly more than in control mice fed a HFFD. Thus, nervous system SOCS3 deficiency reduces body weight and food intake, and protects against liver fat accumulation and liver insulin resistance while amplifying leptin’s effects on BP, and exacerbating the effects of a HFFD to raise BP in females.
PERSPECTIVES
These observations provide insights into potential pathways by which leptin may differentially regulate BP, glucose homeostasis, VO2 and appetite. Our findings also suggest that nervous system SOCS3 deficiency may exacerbate the adverse cardiovascular effects of obesity induced by a chronic HFFD in female but not in male mice; however the mechanisms for these sex differences remain unknown. Further studies are needed to assess the mechanisms responsible for sex differences in the role of neuronal SOCS3 signaling in metabolic and cardiovascular regulation and their role in obesity-induced cardiometabolic dysfunction.
Supplementary Material
Novelty and Significance.
What is new?
Nervous system SOCS3 deficiency amplifies the chronic effects of leptin to raise BP and to lower plasma glucose;
SOCS3 deficiency in the entire nervous system attenuates weight gain, liver fat infiltration and fasting insulin levels in response to HFFD;
There are important sex differences in the role of SOCS3 signaling on BP regulation during dietary-induced obesity
What is relevant?
Unraveling the signaling pathways by which leptin differentially regulates body weight, liver steatosis, glucose, and BP may provide an important information that could lead to new therapeutic approaches for the treatment of obesity and hypertension.
Summary
SOCS3 signaling in the entire nervous system is important in modulating the long-term cardiometabolic actions of leptin and may contribute to some of the adverse metabolic effects of a high fat-high fructose diet.
Acknowledgments
The authors were supported by grants from the National Heart, Lung, and Blood Institute (P01 HL51971) and the National Institute of General Medical Sciences (P20 GM104357 and U54 GM115428) of the National Institutes of Health.
The work performed through the UMMC Molecular and Genomics Facility is supported, in part, by funds from the COBRE (P20 GM104357). The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
References
- 1.Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, Stahl N. Enhancing leptin response by preventing sh2-containing phosphatase 2 interaction with ob receptor. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6061–6066. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr Socs3 mediates feedback inhibition of the leptin receptor via tyr985. The Journal of biological chemistry. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 3.Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J, Tavernier J. Identification of the y985 and y1077 motifs as socs3 recruitment sites in the murine leptin receptor. FEBS Lett. 2000;486:33–37. doi: 10.1016/s0014-5793(00)02205-5. [DOI] [PubMed] [Google Scholar]
- 4.Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 5.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of socs-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 6.Olofsson LE, Unger EK, Cheung CC, Xu AW. Modulation of agrp-neuronal function by socs3 as an initiating event in diet-induced hypothalamic leptin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E697–706. doi: 10.1073/pnas.1218284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG, Jr, Xu AW. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes. 2010;59:894–906. doi: 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nature medicine. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 9.do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Softic S, Gupta MK, Wang GX, Fujisaka S, O’Neill BT, Rao TN, Willoughby J, Harbison C, Fitzgerald K, Ilkayeva O, Newgard CB, Cohen DE, Kahn CR. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. The Journal of clinical investigation. 2017;127:4059–4074. doi: 10.1172/JCI94585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Y, Burrington CM, Graff EC, Zhang J, Judd RL, Suksaranjit P, Kaewpoowat Q, Davenport SK, O’Neill AM, Greene MW. Metabolic phenotype and adipose and liver features in a high-fat western diet-induced mouse model of obesity-linked nafld. American journal of physiology. Endocrinology and metabolism. 2016;310:E418–439. doi: 10.1152/ajpendo.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, Sela BA. Fructose-induced fatty liver disease: Hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005;45:1012–1018. doi: 10.1161/01.HYP.0000164570.20420.67. [DOI] [PubMed] [Google Scholar]
- 13.do Carmo JM, da Silva AA, Morgan J, Jim Wang YX, Munusamy S, Hall JE. Inhibition of soluble epoxide hydrolase reduces food intake and increases metabolic rate in obese mice. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2012;22:598–604. doi: 10.1016/j.numecd.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.do Carmo JM, da Silva AA, Wang Z, Freeman NJ, Alsheik AJ, Adi A, Hall JE. Regulation of blood pressure, appetite, and glucose by leptin after inactivation of insulin receptor substrate 2 signaling in the entire brain or in proopiomelanocortin neurons. Hypertension. 2016;67:378–386. doi: 10.1161/HYPERTENSIONAHA.115.06153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.do Carmo JM, da Silva AA, Wang Z, Freeman NJ, Alsheik AJ, Adi A, Hall JE. Regulation of blood pressure, appetite, and glucose by leptin after inactivation of insulin receptor substrate 2 signaling in the entire brain or in proopiomelanocortin neurons. Hypertension. 2015 doi: 10.1161/HYPERTENSIONAHA.115.06153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva AA, Tallam LS, Liu J, Hall JE. Chronic antidiabetic and cardiovascular actions of leptin: Role of cns and increased adrenergic activity. American journal of physiology. Regulatory, integrative and comparative physiology. 2006;291:R1275–1282. doi: 10.1152/ajpregu.00187.2006. [DOI] [PubMed] [Google Scholar]
- 17.do Carmo JM, Hall JE, da Silva AA. Chronic central leptin infusion restores cardiac sympathetic-vagal balance and baroreflex sensitivity in diabetic rats. American journal of physiology. Heart and circulatory physiology. 2008;295:H1974–1981. doi: 10.1152/ajpheart.00265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent progress in hormone research. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- 19.Coppari R, Bjorbaek C. Leptin revisited: Its mechanism of action and potential for treating diabetes. Nature reviews. Drug discovery. 2012;11:692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denroche HC, Huynh FK, Kieffer TJ. The role of leptin in glucose homeostasis. J Diabetes Investig. 2012;3:115–129. doi: 10.1111/j.2040-1124.2012.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedroso JA, Buonfiglio DC, Cardinali LI, Furigo IC, Ramos-Lobo AM, Tirapegui J, Elias CF, Donato J., Jr Inactivation of socs3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Molecular metabolism. 2014;3:608–618. doi: 10.1016/j.molmet.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: Role of adrenergic activity. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 23.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 24.Manrique C, DeMarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, Hayden MR, Sowers JR. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a western diet. Endocrinology. 2013;154:3632–3642. doi: 10.1210/en.2013-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and stat3 signaling in obese animals. Nature medicine. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 26.Dhillon SS, Belsham DD. Estrogen inhibits npy secretion through membrane-associated estrogen receptor (er)-alpha in clonal, immortalized hypothalamic neurons. International journal of obesity. 2011;35:198–207. doi: 10.1038/ijo.2010.124. [DOI] [PubMed] [Google Scholar]
- 27.Polidori C, Geary N. Estradiol treatment fails to affect the feeding responses to melanocortin-3/4 receptor agonism or antagonism in ovariectomized rats. Peptides. 2002;23:1697–1700. doi: 10.1016/s0196-9781(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 28.Metlakunta AS, Sahu M, Yasukawa H, Dhillon SS, Belsham DD, Yoshimura A, Sahu A. Neuronal suppressor of cytokine signaling-3 deficiency enhances hypothalamic leptin-dependent phosphatidylinositol 3-kinase signaling. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;300:R1185–1193. doi: 10.1152/ajpregu.00794.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic pi3k and mapk differentially mediate regional sympathetic activation to insulin. The Journal of clinical investigation. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.