Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) is a heterogeneous syndrome associated with multiple pathophysiologic abnormalities, including left ventricular (LV) diastolic dysfunction, longitudinal LV systolic dysfunction, abnormal ventricular-arterial coupling, pulmonary hypertension, and right ventricular (RV) remodeling/dysfunction. However, the relative prognostic significance of each of these pathophysiologic abnormalities in HFpEF is unknown.
Methods and Results
We prospectively studied 419 patients with HFpEF using echocardiography and sphygmomanometry to assess HFpEF pathophysiologic markers. Cox proportional hazards analyses were used to determine the associations between pathophysiologic markers and outcomes. Mean age was 65±12 years; 62% were women; 39% were black; comorbidities were common; and study participants met published criteria for HFpEF. RV abnormalities were frequent: 28% had abnormal tricuspid annular plane systolic excursion, 15% had reduced RV fractional area change, and 34% had RV hypertrophy. During a median follow-up time of 18 months, 102 (24%) were hospitalized for HF and 175 (42%) experienced the composite end point of cardiovascular hospitalization or death. Decreased LV compliance, measured as reduced LV end-diastolic volume at an idealized LV end-diastolic pressure of 20 mm Hg (EDV20), and RV remodeling, as indicated by increased RV wall thickness, were the 2 pathophysiologic markers most predictive of worse outcomes: adjusted hazard ratio per 1 SD decrease in EDV20=1.39 (95% confidence interval [CI], 1.10–1.75; P=0.006), and hazard ratio per 1 SD increase in RV wall thickness=1.37 (95% CI, 1.16–1.61; P<0.001). These associations persisted after additional adjustment for markers of HF severity. By contrast, markers of LV relaxation, longitudinal LV systolic dysfunction, and ventricular-arterial coupling were not significantly associated with adverse outcomes.
Conclusions
In patients with HFpEF, reduced LV compliance and RV remodeling are the strongest pathophysiologic predictors of adverse outcomes.
Keywords: heart failure, diastolic; heart ventricles; ventricular dysfunction, right
Heart failure with preserved ejection fraction (HFpEF) represents ≈50% of prevalent cases of HF and is associated with increased mortality independent of associated cardiac dysfunction and comorbidities.1–3 No treatment has been demonstrated in clinical trials to improve outcomes in HFpEF,4–9 a fact likely attributable to the etiologic and pathophysiologic heterogeneity associated with HFpEF.10 Thus far, several pathophysiologic abnormalities have been reported in HFpEF, including (1) diastolic dysfunction, manifested both as impaired left ventricular (LV) relaxation and decreased LV compliance11,12; (2) longitudinal LV systolic dysfunction13,14; (3) abnormal ventricular-arterial (VA) coupling15; and (4) pulmonary hypertension with right ventricular (RV) remodeling and dysfunction.16–18
Impaired LV contractility19 and variably diastolic dysfunction have been independently linked to worsened outcomes in HFpEF,3,20,21 but no such association is known for other pathophysiologic abnormalities, and no previous studies have compared the relative prognostic use of pathophysiologic parameters in HFpEF. Abnormalities in RV structure and function are potent predictors of adverse outcomes in a wide variety of cardiac diseases. In patients with HF and reduced EF, RV dysfunction is a well-established and strong predictor of outcomes, including death.22–25 More recent data indicate that RV hypertrophy predicts incident HF and reduced survival even in subjects free of baseline cardiovascular disease.26 RV dysfunction is common in HFpEF, with a prevalence of ≈75% depending on the metric used; however, no previous study has linked RV dysfunction with outcomes in this population.17,27
Given the lack of proven effective treatments for HFpEF, a better understanding of the relative impact of known pathophysiologies on outcomes in at-risk HFpEF patients could help identify pathophysiologic states that would be most important to improve outcomes. We hypothesized that among the known pathophysiologic markers in HFpEF, RV abnormalities would be most predictive of adverse outcomes. Therefore, we prospectively studied the prognostic importance of a variety of pathophysiologic markers in a well-characterized HFpEF cohort.
Methods
Study Population
Between March 2008 and May 2011, consecutive patients were prospectively enrolled from the outpatient clinic of the Northwestern University HFpEF Program as part of a systematic observational study of HFpEF (ClinicalTrials.gov; NCT01030991). All patients were enrolled in the study in the outpatient setting after a hospitalization for HF. Patients were initially identified by an automated daily query of the inpatient electronic medical record at Northwestern Memorial Hospital using the following search criteria: (1) diagnosis of HF or the term heart failure in hospital notes; or (2) B-type natriuretic peptide (BNP) >100 pg/mL; or (3) administration of ≥2 doses of intravenous diuretics. Patients were offered postdischarge follow-up in a specialized HFpEF outpatient program if they met the following 3 inclusion criteria: age ≥21 years, LVEF ≥50%, and presence of HF as defined by Framingham criteria.28 Posthospitalization, HF diagnosis was confirmed in the outpatient HFpEF clinic. Consistent with previously published criteria,29 all patients were found to have ≥1 of the following 3 diagnostic hallmarks of HFpEF: grade 2 or worse LV diastolic dysfunction on echocardiography; elevated pulmonary capillary wedge pressure or LV end-diastolic pressure on invasive hemodynamic testing; or elevated BNP (>100 pg/mL). Patients were excluded if they had more than moderate valvular disease, previous cardiac transplantation, previous history of reduced LVEF <40% (ie, recovered EF), LV end-diastolic volume (EDV) >97 mL/m2, or constrictive pericarditis. All study participants gave written informed consent, and the institutional review board at Northwestern University approved the study.
Clinical Characteristics
We collected the following data in all study participants: demographics, race/ethnicity, New York Heart Association (NYHA) functional class, comorbidities, medications, vital signs, body mass index, and laboratory data, including serum sodium, blood urea nitrogen, creatinine, hemoglobin, and BNP. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease equation. Comorbidity definitions are included in the Data Supplement.
Echocardiography
All study participants underwent comprehensive 2-dimensional echocardiography with Doppler and tissue Doppler imaging using commercially available ultrasound systems with harmonic imaging (Philips iE33 or 7500; Philips Medical Systems, Andover, MA; or Vivid 7, GE Healthcare, General Electric Corp, Waukesha, WI). Blood pressure was recorded at the time of echocardiography using a digital blood pressure monitor (Omron HEM-907XL; Omron Healthcare Inc, Vernon Hills, IL). Cardiac structure and function were quantified as recommended by the American Society of Echocardiography,30–32 including the use of the biplane method of discs (as opposed to M-mode–derived formulas) for the calculation of LV volumes. All measurements were performed by an experienced research sonographer (blinded to clinical data and outcomes) using ProSolv 4.0 echocardiographic analysis software (ProSolv CardioVascular; FujiFilm, Indianapolis, IN) and verified by an experienced investigator with expertise in echocardiography.
LV Diastolic Function
Comprehensive diastolic functional assessment was performed according to published guidelines.31 LV relaxation was estimated using tissue Doppler e′ velocity. Given the contribution of RV to septal e′, we used e′ measured at the lateral mitral annulus as an estimate of LV diastolic relaxation. For the estimation of LV compliance, we calculated the end-diastolic pressure–volume relationship (EDPVR) using a single-beat method33 with the equation: LVEDP=α(LVEDV)β, where LVEDP is the LV end-diastolic pressure. EDPVR represents the nonlinear relationship between ventricular pressure and volume at end diastole and can be estimated noninvasively. The parameters α and β, which are constants that allow point measurements of nonlinear EDPVR as reviewed elsewhere,34 were calculated for each individual based on their LVEDV and LVEDP (estimated by [1.9+1.24*(lateral E/e′ ratio)]).35 These parameters were then used to calculate LVEDV at an idealized LVEDP of 20 mm Hg (EDV20) for each patient as a basis for determining whether EDPVR was related to outcomes. For the purpose of sensitivity analyses, EDV20 was recalculated using the average of septal and lateral e′ velocities to derive E/e′.
LV Systolic Function
To evaluate LV contractility, we estimated the end-systolic PVR as represented by the slope of end-systolic elastance (Ees).34 Ees is a load-independent measure of LV contractility and can be estimated using a single-beat method.36,37 The relationship between end-systolic pressure (Pes) and end-systolic volume (ESV) was defined as: Pes=Ees(ESV–V0). Using 0.9*(systolic blood pressure) at the time of echocardiography as an estimate of Pes, we estimated V0, the volume–axis intercept, for each patient. We generated the estimated ESV at an idealized Pes of 120 mm Hg (ESV120) for each patient to determine whether end-systolic PVR was associated with adverse outcomes.
Longitudinal LV systolic function was estimated using tissue Doppler s′ velocity of the lateral mitral annulus. Tissue Doppler s′ velocity of the medial (septal) mitral annulus was also recorded for comparison purposes.
VA Coupling
For the purpose of VA coupling measurements, LV quantification and estimation of Ees were performed as described above. Effective arterial elastance (Ea), which is a measure of systemic arterial stiffness, was estimated using the equation: Ea=0.9*systolic blood pressure/stroke volume.38 Stroke volume was estimated on echocardiography using the equation: stroke volume=(LV outflow tract diameter/2)2×π×LV outflow tract velocity time integral. VA coupling was defined as Ea/Ees.34
RV Assessment
Echocardiographic markers of RV structure and function, including RV end-diastolic and end-systolic area (indexed to body size),39 RV basal diameter, RV wall thickness, RV fractional area change (RVFAC), and tricuspid annular plane systolic excursion (TAPSE) were measured using 2-dimensional echocardiography and quantified as recommended by the American Society of Echocardiography.32 RV hypertrophy was defined as RV end-diastolic free wall thickness >5.0 mm (measured in the subcostal view and measurable in 362/419 [86%] of patients); abnormal TAPSE was defined as <1.6 cm, and abnormal RVFAC was defined as <35%.32 Pulmonary artery pressure (PASP) and right atrial pressure (RAP) were estimated using echocardiography as previously described.16,40
Outcomes
After enrollment, study participants were evaluated in the Northwestern HFpEF Program at least every 6 months or as clinically indicated. At each visit, intercurrent hospitalizations were documented, reviewed, and categorized as due to cardiovascular or noncardiovascular causes. For cardiovascular hospitalizations, specific causes (eg, HF, acute coronary syndrome, arrhythmia) were identified. Every 6 months, participants (or their proxy) were contacted to determine vital status with verification of deaths through query of the Social Security Death Index. Enrollment date was defined as the first visit to the outpatient HFpEF clinic. Date of last follow-up was defined as date of death or last HFpEF clinic visit. Follow-up was complete in all patients. Our primary end point was a combined outcome of cardiovascular hospitalization and death, which included hospitalization for any cardiovascular cause (including HF) and death from any cause. We also assessed the secondary outcome of HF hospitalization.
Statistical Analysis
Clinical characteristics, laboratory data, and echocardiographic parameters were summarized for the full cohort. Categorical variables are displayed as count and percentage, and continuous data with a normal distribution are displayed as mean±SD. Right-skewed data are presented as median (first to third quartiles). Intra- and interobserver variability analyses of select pathophysiologic markers (EDV20 and RV wall thickness) were conducted in a randomly selected subset of 20 HFpEF patients (Table I in the Data Supplement). A comparison of study participants with versus without measurable RV wall thickness is displayed in Table II in the Data Supplement.
For survival analyses, all pathophysiologic measurements were standardized to display hazard ratios for a 1 SD worsening of each pathophysiologic marker. We used Cox proportional hazards regression to evaluate the unadjusted relationship between the markers of HFpEF pathophysiology and outcomes (model 1). Models were then adjusted for age, sex, and clinical comorbidities, including body mass index, coronary artery disease, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease, obstructive sleep apnea, hypertension, estimated glomerular filtration rate, hemoglobin, degree of mitral regurgitation, LV mass index, and NYHA functional class (model 2). For pathophysiologic variables that were significant with P<0.05 after adjustment, we performed additional Cox regression analyses using models with further adjustment for common markers of HF severity, including estimated PASP, estimated RAP, BNP, E/e′ ratio, and left atrial volume index. Because some of these variables had missing values, we adjusted for each marker of HF severity individually to avoid sample size depletion in our multivariable models.
Multiplicative interaction terms were created to test the interaction between all covariates and the primary predictor variable (ie, RV wall thickness or EDV20) to test for interactions in the associations with outcome variables. P<0.05 for the interaction term was used as evidence of a significant interaction. Model comparison was performed using the likelihood ratio (LR) test. All analyses were performed using Stata 12 (StataCorp, College Station, TX).
Results
Baseline Characteristics
Clinical characteristics for the entire HFpEF cohort (N=419) are shown in Table 1. The average age was 65 years, 62% were women, and nearly half of the study participants were nonwhite. Comorbidities, including coronary artery disease, hypertension, hyperlipidemia, diabetes mellitus, chronic kidney disease, obesity, and smoking, were common. Advanced HF (NYHA functional class III or IV) was present in 49%, and BNP was elevated in the majority of patients. As mentioned above, all study participants were required to meet ≥1 objective criterion (elevated BNP, grade 2 or 3 diastolic dysfunction, or elevated pulmonary capillary wedge pressure) for the confirmation of HF diagnosis. Of the 419 subjects, 293 (70%) had elevated BNP and 303 (72%) had grade 2 or 3 diastolic dysfunction. Study subjects who met neither of these criteria (N=38 [9%]) were all confirmed to have elevated pulmonary capillary wedge pressure (>15 mm Hg).
Table 1.
Clinical Characteristics of the Study Cohort
| Clinical Characteristics | Total Cohort (N=419) |
|---|---|
| Age, y | 65±13 |
| Women | 260 (62) |
| Race | |
| White | 216 (52) |
| Black | 162 (39) |
| Other | 41 (10) |
| NYHA functional class | |
| I | 50 (12) |
| II | 164 (39) |
| III | 192 (46) |
| IV | 11 (3) |
| Comorbidities | |
| Coronary artery disease | 200 (48) |
| Hypertension | 323 (77) |
| Hyperlipidemia | 228 (54) |
| Diabetes mellitus | 137 (33) |
| Chronic kidney disease | 139 (33) |
| Smoker | 168 (40) |
| Atrial fibrillation | 110 (26) |
| Obesity | 222 (53) |
| Chronic obstructive pulmonary disease | 154 (37) |
| Obstructive sleep apnea | 152 (36) |
| Vital signs and laboratory data | |
| Heart rate, bpm | 71±14 |
| Systolic blood pressure, mm Hg | 125±20 |
| Diastolic blood pressure, mm Hg | 70±12 |
| Pulse pressure, mm Hg | 55±17 |
| Body mass index, kg/m2 | 32.5±9.3 |
| Serum sodium, mEq/L | 138.4±2.9 |
| Blood urea nitrogen, mg/dL | 24.5±16.2 |
| Serum creatinine, mg/dL | 1.6±1.5 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 58±27 |
| Fasting glucose, mg/dL | 119±54 |
| Hemoglobin, g/dL | 11.9±1.9 |
| B-type natriuretic peptide, pg/mL | 230 (77–520) |
| Medications | |
| ACE inhibitor or angiotensin II receptor blocker | 229 (55) |
| β-Blocker | 280 (67) |
| Calcium channel blocker | 134 (32) |
| Nitrate | 53 (13) |
| Loop diuretic | 61 (15) |
| Thiazide diuretic | 246 (59) |
| Statin | 96 (23) |
| Aspirin | 209 (50) |
Categorical variables are presented as counts and percentages; continuous variables are presented as mean±SD, and right-skewed variables are presented as median (first–third quartiles). ACE indicates angiotensin-converting enzyme; and NYHA, New York Heart Association.
Echocardiographic Characteristics
Table 2 summarizes the echocardiographic characteristics of the study cohort. The majority of study participants had concentric LV remodeling, as demonstrated by increased LV mass/volume ratio and increased relative wall thickness. LV hypertrophy, as defined by elevated LV mass index, was present in 59% of the cohort. Hemodynamic estimates by echocardiography demonstrated increased PASP, RAP, and E/e′ ratio—evidence of pulmonary hypertension, fluid overload, and elevated LV filling pressures, respectively. LV diastolic function was abnormal based on reduced tissue Doppler e′ velocity and EDV20, increased left atrial volume index, and a high frequency of grade 2 or worse diastolic function in the study participants. Although LVEF was preserved (mean 61%), longitudinal LV systolic function as measured by tissue Doppler s′ velocity was decreased.
Table 2.
Echocardiographic Characteristics and Pathophysiologic Markers of the Study Cohort
| Parameters | Total Cohort (N=419) | Normal Range |
|---|---|---|
| Left heart parameters | ||
| LV end-systolic volume index, mL/m2 | 16.3±6.5 | <31 |
| LV end-diastolic volume index, mL/m2 | 40.9±11.5 | <76 |
| Relative wall thickness | 0.51±0.15 | ≤0.42 |
| LV mass/volume ratio | 2.6±1.1 | … |
| LV mass, g | 210±79 | <163 in women, <225 in men |
| LV mass index, g/m2 | 104±38 | <96 in women, <116 in men |
| LV hypertrophy | 246 (59) | … |
| Left atrial volume index, mL/m2 | 34.2±14.3 | <28 |
| Mitral regurgitation | … | |
| None | 246 (59) | |
| Mild | 114 (27) | |
| Moderate | 59 (14) | |
| LV ejection fraction, % | 61±7 | >55 |
| Stroke volume, mL | 50±15 | >60 |
| Cardiac index, L/min per m2 | 2.9±1.1 | >2.0 |
| Hemodynamics | ||
| Pulmonary artery systolic pressure, mm Hg | 43.8±15.3 | <30 |
| Right atrial pressure, mm Hg | 7.7±4.1 | <5 |
| E/e′ lateral annulus | 13.3±7.9 | <10 |
| LV diastolic function parameters | ||
| e′ lateral annulus, cm/s | 9.3±3.9 | Varies by age |
| End-diastolic volume20, mL | 85.8±28.5 | … |
| LV systolic function parameters | ||
| s′ lateral annulus, cm/s | 8.5±2.7 | Varies by age |
| End-systolic elastance, mm Hg/mL | 2.3±0.7 | … |
| End-systolic volume120, mL | 37.7±17.2 | … |
| Ventricular-arterial coupling | ||
| Ea/Ees | 1.6±0.2 | … |
| RV structural and functional parameters | ||
| RV basal diameter, cm | 3.9±0.7 | ≤4.2 |
| RV length, cm | 8.0±1.1 | <8.0 in women, <8.8 in men |
| RV outflow tract diameter, PLAX, cm | 3.4±0.6 | ≤3.3 |
| RV end-systolic area index, cm2/m2 | 8.0±2.8 | <6.5 in women, <6.9 in men |
| RV end-diastolic area index, cm2/m2 | 13.9±3.8 | <12.6 |
| RV wall thickness, mm | 5.1±0.9 | ≤5.0 |
| RV/LV maximum diameter ratio | 0.98±0.20 | <0.67 |
| RV fractional area change, % | 43±7 | ≥35 |
| Tricuspid annular plane systolic excursion, cm | 2.0±0.6 | ≥1.6 |
Continuous variables are presented as mean±SD. LV indicates left ventricular; PLAX, parasternal long axis; and RV, right ventricular.
Increased RV wall thickness, indicative of RV hypertrophy, was present in 34% of the study participants. RV chamber size was increased in approximately one third of the cohort (eg, RV basal diameter >4.2 cm in 30% of the study participants). Although the majority of study patients had TAPSE and RVFAC values within the normal range, 28% had reduced TAPSE (<1.6 cm) and 14% had reduced RVFAC (<35%).
Clinical Outcomes
The median follow-up time was 17.7 months (25th–75th percentile, 9.5–30.0 months). During the follow-up period, 138 patients (33%) were hospitalized for a cardiovascular reason, 102 (24%) were hospitalized for HF, 59 (14%) died, and 175 (42%) experienced the composite end point of cardiovascular hospitalization or death.
In unadjusted Cox proportional hazards models (model 1), conventional hemodynamic markers of HF severity (estimated PASP, estimated RAP, log BNP, and E/e′) were significantly associated with an increased risk for the combined outcome of cardiovascular hospitalization and death as well as HF hospitalization alone (Table 3). Furthermore, in unadjusted analyses, multiple parameters of RV structure/function and LV parameters (EDV20 and s′ velocity) were associated with the composite outcome. Of the conventional markers of HF severity, estimated RAP and BNP remained predictive of outcomes after adjustment for clinical covariates (model 2).
Table 3.
Association of Pathophysiologic Markers with Outcomes in Heart Failure with Preserved Ejection Fraction
| Predictor Variables | Combined Outcome
|
Heart Failure Hospitalization
|
||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
|
| ||||||||
| Unadjusted (Model 1) | Adjusted (Model 2)* | Unadjusted (Model 1) | Adjusted (Model 2)* | |||||
| Hemodynamic markers | ||||||||
| Estimated PASP, mm Hg (N=299) | 1.31 (1.10–1.55) | 0.002 | 1.04 (0.85–1.26) | 0.71 | 1.34 (1.07–1.67) | 0.01 | 1.04 (0.81–1.32) | 0.78 |
| Estimated RAP, mm Hg (N=373) | 1.35 (1.16–1.58) | <0.001 | 1.22 (1.02–1.45) | 0.03 | 1.48 (1.22–1.79) | <0.001 | 1.25 (1.00–1.56) | 0.05 |
| Log BNP, pg/mL | 1.71 (1.45–2.02) | <0.001 | 1.45 (1.13–1.85) | 0.003 | 1.79 (1.45–2.21) | <0.001 | 1.56 (1.14–2.13) | 0.006 |
| E/e′ ratio | 1.29 (1.14–1.46) | <0.001 | 1.10 (0.95–1.28) | 0.21 | 1.36 (1.18–1.57) | <0.001 | 1.17 (0.98–1.39) | 0.08 |
| LA volume index, mL/m2 | 1.16 (1.02–1.34) | 0.025 | 1.02 (0.85–1.21) | 0.86 | 1.20 (1.02–1.42) | 0.029 | 1.06 (0.85–1.31) | 0.62 |
| LV diastolic function | ||||||||
| Lateral e′ velocity, cm/s† | 1.14 (0.96–1.36) | 0.14 | 0.94 (0.76–1.15) | 0.54 | 1.12 (0.9–1.39) | 0.31 | 0.93 (0.71–1.22) | 0.60 |
| EDV20, mL† | 1.29 (1.08–1.54) | 0.005 | 1.39 (1.10–1.75) | 0.006 | 1.29 (1.03–1.62) | 0.03 | 1.67 (1.22–2.30) | 0.001 |
| LV systolic function | ||||||||
| Lateral s′ velocity, cm/s† | 1.26 (1.06–1.51) | 0.01 | 1.07 (0.88–1.31) | 0.49 | 1.17 (0.94–1.45) | 0.16 | 0.96 (0.75–1.22) | 0.72 |
| Ees, mm Hg/mL | 1.08 (0.93–1.26) | 0.33 | 1.07 (0.91–1.27) | 0.40 | 1.10 (0.91–1.34) | 0.33 | 1.20 (0.96–1.49) | 0.11 |
| ESV120, mL | 1.21 (0.97–1.50) | 0.09 | 0.84 (0.70–1.02) | 0.08 | 0.83 (0.67–1.03) | 0.09 | 0.79 (0.62–1.02) | 0.07 |
| Ventricular-arterial coupling | ||||||||
| Ea/Ees | 1.04 (0.89–1.22) | 0.58 | 1.02 (0.86–1.21) | 0.78 | 1.07 (0.88–1.31) | 0.48 | 1.03 (0.83–1.27) | 0.82 |
| RV structure and function | ||||||||
| RV wall thickness, mm (N=362) | 1.50 (1.32–1.71) | <0.001 | 1.37 (1.16–1.61) | <0.001 | 1.55 (1.32–1.81) | <0.001 | 1.43 (1.17–1.75) | 0.001 |
| RV basal diameter, cm | 1.27 (1.10–1.47) | 0.001 | 1.26 (1.04–1.52) | 0.017 | 1.33 (1.11–1.59) | 0.002 | 1.21 (0.95–1.55) | 0.12 |
| RV/LV diameter ratio | 1.32 (1.12–1.55) | 0.001 | 1.18 (0.98–1.41) | 0.08 | 1.34 (1.09–1.65) | 0.005 | 1.27 (1.00–1.61) | 0.047 |
| RVEDAI, cm2/m2 | 1.26 (1.10–1.44) | 0.001 | 1.28 (1.05–1.56) | 0.02 | 1.30 (1.10–1.53) | 0.002 | 1.41 (1.09–1.82) | 0.009 |
| RVESAI, cm2/m2 | 1.25 (1.10–1.42) | 0.001 | 1.23 (1.01–1.49) | 0.04 | 1.30 (1.11–1.53) | 0.001 | 1.33 (1.04–1.69) | 0.02 |
| RVFAC, %† | 1.18 (1.02–1.37) | 0.02 | 1.05 (0.88–1.25) | 0.60 | 1.27 (1.06–1.53) | 0.01 | 1.08 (0.86–1.35) | 0.52 |
| TAPSE, cm† | 1.19 (1.02–1.39) | 0.03 | 1.09 (0.91–1.3) | 0.33 | 1.37 (1.11–1.68) | 0.003 | 1.30 (1.02–1.67) | 0.04 |
BNP indicates B-type natriuretic peptide; CI, confidence interval; Ea, effective arterial elastance; Ees, end-systolic elastance; EDV, end-diastolic volume; ESV, end-systolic volume; HR, hazards ratio; LA, left atrial; LV, left ventricular; PASP, pulmonary artery systolic pressure; RAP, right atrial pressure; RV, right ventricular; RVEDAI, RV end-diastolic area index; RVESAI, RV end-systolic area index; RVFAC, RV fractional area change; and TAPSE, tricuspid annular plane systolic excursion.
Adjusted for age, sex, and clinical comorbidities, which include body mass index, coronary artery disease, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disorder, obstructive sleep apnea, hypertension, glomerular filtration rate, hemoglobin concentration, degree of mitral regurgitation, LV mass index, and New York Heart Association functional class.
HRs are reported as per SD increase in predictor variable except when noted by † in which case HRs are reported as per SD decrease in predictor variable. Outcome tallies for variables with missing data for the combined outcome and heart failure hospitalization are as follows: PASP (N=123, N=73); RAP (N=148, N=90); RV wall thickness (N=141, N=87).
Association of Reduced LV Compliance With Worse Outcomes
Lower EDV20 (reflecting a stiffer LV) was associated with both the combined outcome of cardiovascular hospitalization and death, as well as HF hospitalization, after adjustment for clinical covariates (Table 3; Figure I in the Data Supplement). Additional models adjusting for markers of HF severity (estimated PASP, estimated RAP, log BNP, and E/e′) did not attenuate the prognostic utility of EDV20 (Table 4). Recalculation of EDV20 using average e′ or septal e′ velocity (for the calculation of E/e′) did not change any of the results. Testing for interactions (Table III in the Data Supplement) demonstrated that there was a significant interaction between EDV20 and hemoglobin. EDV20 was associated with the composite outcome in patients who were not anemic (hazard ratio [HR], 1.74; 95% confidence interval [CI], 1.13–2.40; P=0.001), but there was no association in those who were anemic (HR, 1.17; 95% CI, 0.95–1.46; P=0.13). The interaction between EDPVR and hemoglobin level is demonstrated in Figure 1.
Table 4.
Association of Pathophysiologic Markers with Outcomes After Adjustment for Hemodynamic Markers of Heart Failure Severity
| Predictor Variables | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Model 2* Plus PASP | Model 2 Plus RAP | Model 2 Plus Log BNP | Model 2 Plus E/e′ | Model 2 Plus LAVI | ||||||
| Combined outcome | ||||||||||
| EDV20, mL† | 1.64 (1.21–2.22) |
0.002 | 1.35 (1.05–1.71) |
0.018 | 1.36 (1.08–1.71) |
0.008 | 1.36 (1.07–1.74) |
0.012 | 1.39 (1.10–1.75) |
0.006 |
| RV wall thickness, mm | 1.56 (1.26–1.92) |
<0.001 | 1.34 (1.12–1.61) |
0.001 | 1.37 (1.17–1.61) |
<0.001 | 1.40 (1.14–1.72) |
0.001 | 1.37 (1.17–1.61) |
<0.001 |
| RV basal diameter, cm | 1.21 (0.91–1.53) |
0.12 | 1.25 (1.01–1.56) |
0.04 | 1.15 (0.94–1.40) |
0.18 | 1.25 (1.03–1.51) |
0.02 | 1.24 (1.03–1.50) |
0.02 |
| RVEDAI, cm2/m2 | 1.27 (1.00–1.61) |
0.05 | 1.25 (1.00–1.57) |
0.05 | 1.18 (0.96–1.44) |
0.12 | 1.37 (1.06–1.78) |
0.02 | 1.24 (1.03–1.50) |
0.03 |
| RVESAI, cm2/m2 | 1.28 (1.01–1.61) |
0.04 | 1.18 (0.95–1.48) |
0.14 | 1.13 (0.92–1.38) |
0.24 | 1.21 (0.99–1.48) |
0.06 | 1.19 (0.99–1.43) |
0.06 |
| Heart failure hospitalization | ||||||||||
| EDV20, mL† | 1.67 (1.13–2.49) |
0.011 | 1.62 (1.16–2.27) |
0.005 | 1.70 (1.24–2.33) |
0.001 | 1.62 (1.17–2.26) |
0.004 | 1.69 (1.24–2.33) |
0.001 |
| RV wall thickness, mm | 1.56 (1.20–2.04) |
0.001 | 1.40 (1.12–1.74) |
0.003 | 1.38 (1.13–1.69) |
0.002 | 1.40 (1.14–1.72) |
0.001 | 1.42 (1.16–1.74) |
0.001 |
| RV/LV diameter ratio | 1.28 (0.97–1.69) |
0.08 | 1.36 (1.07–1.73) |
0.01 | 1.29 (1.05–1.60) |
0.02 | 1.36 (1.10–1.69) |
0.005 | 1.37 (1.11–1.70) |
0.003 |
| RVEDAI, cm2/m2 | 1.39 (1.02–1.90) |
0.04 | 1.34 (1.00–1.79) |
0.05 | 1.28 (0.98–1.66) |
0.07 | 1.37 (1.06–1.78) |
0.02 | 1.38 (1.07–1.79) |
0.01 |
| RVESAI, cm2/m2 | 1.34 (0.99–1.81) |
0.06 | 1.27 (0.96–1.69) |
0.10 | 1.22 (0.95–1.56) |
0.13 | 1.30 (1.01–1.67) |
0.04 | 1.31 (1.02–1.17) |
0.03 |
| TAPSE, cm† | 1.47 (1.09–2.00) |
0.01 | 1.33 (1.02–1.74) |
0.03 | 1.26 (0.98–1.62) |
0.07 | 1.30 (1.02–1.66) |
0.04 | 1.30 (1.02–1.67) |
0.04 |
BNP indicates B-type natriuretic peptide; CI, confidence interval; EDV, end-diastolic volume; ESV, end-systolic volume; HR, hazards ratio; LV, left ventricular; PASP, pulmonary artery systolic pressure; RAP, right atrial pressure; RV, right ventricular; RVEDAI, RV end-diastolic area index; RVESAI, RV end-systolic area index; and TAPSE, tricuspid annular plane systolic excursion.
Adjusted for age, sex, and clinical comorbidities, which include body mass index, coronary artery disease, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disorder, obstructive sleep apnea, hypertension, glomerular filtration rate, hemoglobin concentration, degree of mitral regurgitation, LV mass index, and New York Heart Association functional class.
HRs are reported as per SD increase in predictor variable except when noted by † in which case HRs are reported as per SD decrease in predictor variable.
Figure 1.
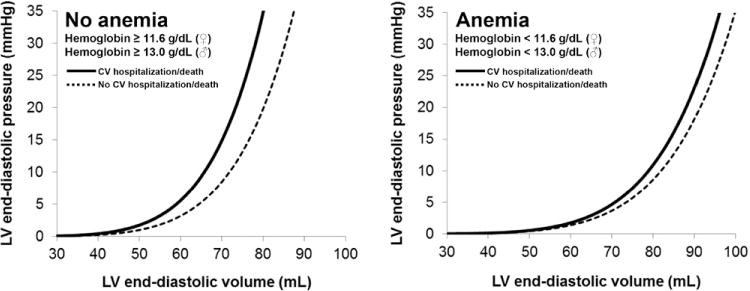
LV end-diastolic pressure–volume relationships stratified by the combined outcome of cardiovascular hospitalization or death. In the group of heart failure with preserved ejection fraction (HFpEF) patients who were not anemic (N=224, 53% of the cohort), lower EDV20 was significantly associated with adverse events, as shown by the end-diastolic pressure–volume relationship (EDPVR) curves. The EDPVR curve was shifted up and to the left, reflecting a stiffer LV, in nonanemic patients who had an adverse event during follow-up. The association between LV stiffness (EDV20) and outcomes was not present in HFpEF patients who were anemic at baseline (N=195, 47% of the cohort). CV indicates cardiovascular; and EDV20, left ventricular (LV) end-diastolic volume at an idealized LV end-diastolic pressure of 20 mm Hg.
Using an alternative equation proposed for estimating LVEDP in subjects with atrial fibrillation,41 we recalculated EDV20 in 14% of the cohort who had atrial fibrillation at the time of echocardiography. Replacing the revised estimates of EDV20 for patients in atrial fibrillation did not affect the relationship between EDV20 and outcomes (adjusted HR for the combined outcome of cardiovascular hospitalization or death=1.37, 95% CI, 1.08–1.74; P=0.008).
To exclude the possibility that patients with HFpEF due to amyloidosis were driving the association between EDV20 and worse outcomes, we repeated our analyses after removing patients with cardiac amyloidosis (N=15) from the cohort. Baseline characteristics and echocardiographic parameters were not significantly different compared with the entire cohort. Cox proportional hazards modeling (Tables IV and V in the Data Supplement) showed that the removal of patients with cardiac amyloidosis did not eliminate the association between EDV20 and the primary outcome (HR per 1 SD decrease in EDV20=1.32; 95% CI, 1.04–1.67; P=0.02), which was not significantly different than the entire cohort (HR, 1.39; 95% CI, 1.10–1.75; P=0.006). The adjustment for markers of HF severity did not blunt this association.
Association of RV Abnormalities With Worse Outcomes
Among measurements of RV structure and function, RV wall thickness was the strongest predictor of outcomes (Figure 2; Figure I in the Data Supplement). Additionally, RV end-diastolic area index, RV end-systolic area index, and RV basal diameter, all measures of RV dilatation, were associated with worse outcomes on unadjusted analyses and remained associated with worse outcomes after adjustment for clinical covariates (Table 3). Of the RV function parameters, TAPSE was predictive of HF hospitalization but not the primary (composite) outcome, whereas RVFAC did not remain significantly associated with outcomes after multivariable adjustment (Table 3). Excluding patients with chronic obstructive pulmonary disease did not eliminate the aforementioned associations between RV parameters and outcomes (data not shown). Excluding patients with cardiac amyloidosis (N=15) also did not change the association between increased RV wall thickness and worse outcomes (Tables III and IV in the Data Supplement).
Figure 2.
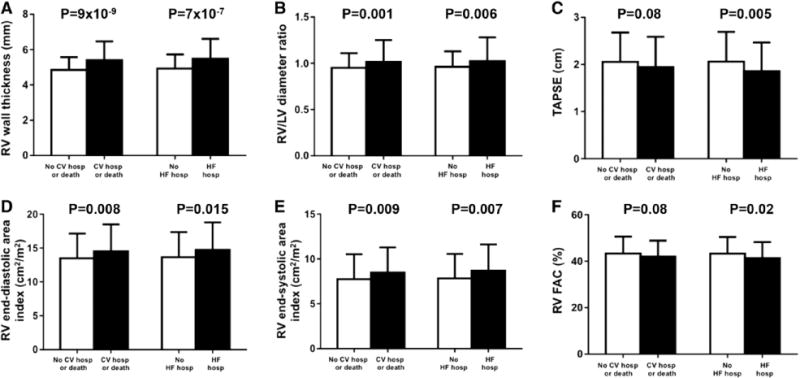
Bar graphs comparing RV structural and functional parameters by the presence or absence of the primary (CV hospitalization or death) and secondary outcomes (HF hospitalization). RV wall thickness (A), RV/LV maximal diameter ratio (B), TAPSE (C), RV end-diastolic area index (D), RV end-systolic area index (E), and RVFAC (F). CV indicates cardiovascular; FAC, fractional area change; HF, heart failure; hosp, hospitalization; LV, left ventricular; RV, right ventricular; and TAPSE, tricuspid annular plane systolic excursion.
The prognostic utility of RV wall thickness for all outcomes, and TAPSE for HF hospitalization, were not significantly attenuated by adjustment for HF severity (Table 4). The associations between RV dilation and outcomes were somewhat attenuated after adjustment for markers of HF severity (Table 4). There was an interaction between RV wall thickness and LV mass index in predicting the composite outcome (Table III in the Data Supplement). When adjusted for all the covariates, including LV mass index, in model 2, RV wall thickness was more predictive of the composite outcome in patients without LV hypertrophy (adjusted HR, 1.56; 95% CI, 1.19–2.04; P=0.001) compared with those with LV hypertrophy (adjusted HR, 1.31; 95% CI, 1.06–1.62; P=0.014).
Additive Prognostic Importance of Reduced EDV20 and RV Abnormalities
Figure 3 shows the Kaplan–Meier curves for the composite end point, stratified by median EDV20 and median RV wall thickness. Because EDV20 and RV wall thickness were the 2 strongest predictors of outcomes, we evaluated whether these 2 parameters were correlated, but found no significant relationship between them (r=−0.02; P=0.67).
Figure 3.
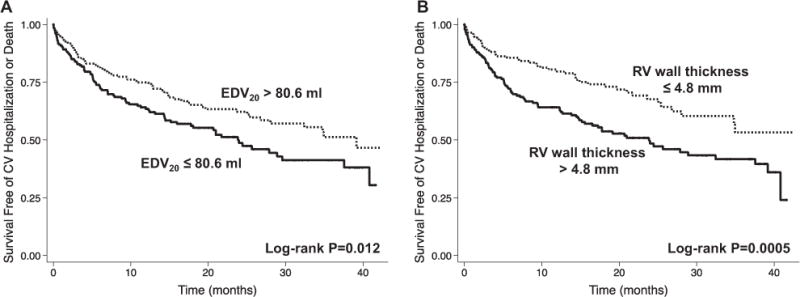
Kaplan–Meier survival curves for cardiovascular hospitalization or death, stratified by the median values of EDV20 and RV wall thickness. A, Patients with reduced LV compliance (ie, stiffer LV). B, Patients with increased RV wall thickness were more likely to have an adverse outcome (cardiovascular hospitalization or death) during follow-up. EDV20 indicates left ventricular (LV) end-diastolic volume at an idealized LV end-diastolic pressure of 20 mm Hg; and RV, right ventricular.
The addition of these predictors to the multivariable model (model 2) showed that RV wall thickness and EDV20 have predictive ability beyond the baseline model (LR test P=0.0003 and 0.005 for RV wall thickness and EDV20, respectively). RV wall thickness was superior to EDV20 when both were included in the multivariable model (LR test P=0.002 when RV wall thickness added to model 2 plus EDV20; LR test P=0.09 when EDV20 added to model 2 plus RV wall thickness). We also tested the additive value of RV wall thickness and EDV20 on top of conventional echocardiographic predictors of worse outcomes (LVEF, E/e′ ratio, LA volume index, TAPSE) and comorbidities. Both RV wall thickness (LR test P=0.002) and EDV20 (LR test P=0.039) demonstrated incremental prognostic value over conventional echocardiographic characteristics.
HFpEF patients with EDV20 below the median (<80.6 mL) and RV wall thickness above the median (>4.8 mm) had worse outcomes compared with either abnormality alone (Figure 4). Similarly, higher RV wall thickness and lower TAPSE were also additive for the outcome of HF hospitalization (Figure 5).
Figure 4.
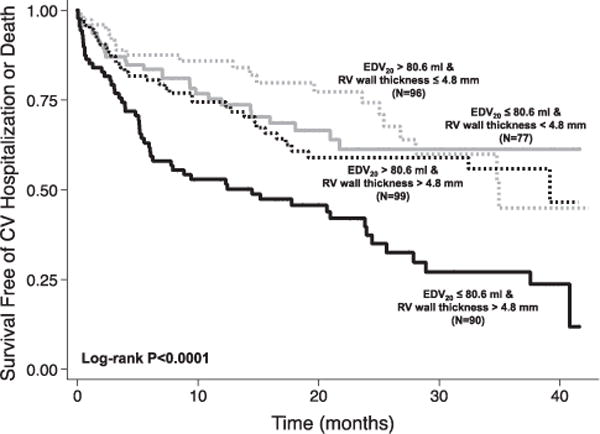
Kaplan–Meier survival curves for cardiovascular hospitalization or death according to the presence or absence of reduced LV compliance and increased RV wall thickness. Patients with both reduced LV compliance and increased LV wall thickness had worse outcomes, indicating an additive effect of both abnormalities on prognosis. EDV20 indicates left ventricular (LV) end-diastolic volume at an idealized LV end-diastolic pressure of 20 mm Hg; and RV, right ventricular.
Figure 5.
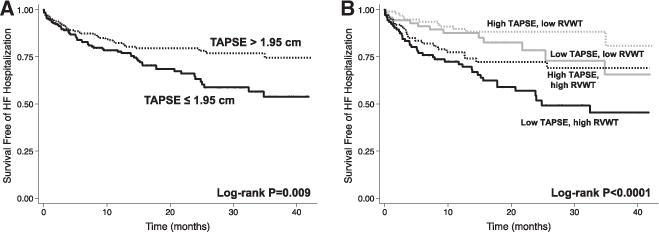
Kaplan–Meier survival curves for heart failure hospitalization according to the presence or absence of RV dysfunction and increased RVWT. A, Heart failure hospitalization stratified by median TAPSE showing that patients with heart failure with preserved ejection fraction with lower TAPSE were more likely to be hospitalized for heart failure. B, Patients with low TAPSE and increased RVWT were most likely to be hospitalized for heart failure, showing the additive effect of both RV hypertrophy and dysfunction. RVWT indicates right ventricular wall thickness; and TAPSE, tricuspid annular plane systolic excursion. High/low TAPSE defined by median value (1.95 cm); and high/low RVWT defined by median value (4.8 mm).
Discussion
In a contemporary HFpEF population, we prospectively evaluated the relationship between markers of different pathophysiologies associated with HFpEF and adverse outcomes. We found that lower LV compliance (determined by reduced EDV20) and increased RV remodeling (indicated by increased RV wall thickness) were both independently associated with the primary composite outcome of cardiovascular hospitalization or death, as well as HF hospitalization. Importantly, these parameters predicted outcomes after adjustment for age, sex, a wide range of comorbidities, and markers of HF severity. In addition, reduced EDV20 and increased RV wall thickness were uncorrelated and had an additive association with outcomes, implying that these pathophysiologic changes are independent indicators of adverse prognosis in HFpEF. Conversely, impaired LV relaxation, longitudinal LV systolic function, and VA coupling did not predict outcomes, indicating that although these pathophysiologies may play a role in HFpEF development or exercise intolerance, they do not necessarily herald future morbidity and mortality. Collectively, these results offer insight into the most important pathophysiologies in HFpEF. Given that previously hospitalized HFpEF patients have poor prognosis, patients with increased RV wall thickness or reduced EDV20 represent those in the greatest need of intervention.
The role of the RV in HFpEF has been incompletely described. RV hypertrophy, defined electrocardiographically, has been linked to worse outcomes in pulmonary hypertension due to HFpEF.42 Given the exquisite afterload dependence of RV, it is expected that RV dysfunction would occur in the setting of elevated pulmonary pressures,43 and in pulmonary arterial hypertension, RV dysfunction has been associated with a worse prognosis, even in those patients with improvement in pulmonary vascular resistance on therapy.44 RV systolic and diastolic dysfunction have both been shown to be prevalent in HFpEF,17,27 but this has not been clearly associated with poor outcomes. In an echocardiographic study, reduced TAPSE, as a marker of RV systolic dysfunction, was linked to mortality in those with HF with reduced EF, but not in those with HFpEF.45 In the present study, we showed an association between TAPSE and HF hospitalization, suggesting that RV dysfunction did drive some outcomes in HFpEF. However, RV wall thickness was a much stronger predictor of outcomes even after controlling for elevated pulmonary pressures, suggesting an independent association between RV wall thickness and outcomes in patients with HFpEF.
RV hypertrophy is thought to be an adaptive response to chronically elevated left-sided filling pressures and, consequently, would be expected to precede RV dysfunction. This in turn leads to RV decompensation, overt RV failure, and, predictably, worse outcomes. As such, metrics of frank RV dysfunction would be expected to be a stronger predictor of outcomes in HFpEF, contrary to our findings. Two possibilities exist for this phenomenon. First, longer follow-up (allowing for the progression of RV pathology) of this cohort could reveal a stronger association with echocardiographic measures of RV dysfunction and outcomes. Alternatively, better metrics of RV function, such as RV strain imaging,46 or RV EF measured using 3-dimensional echocardiography or cardiac MRI, may more accurately reflect the degree of RV dysfunction in these individuals and consequently show a stronger association with outcomes. Regardless, as in patients with other cardiopulmonary disorders, we demonstrated for the first time that abnormalities of RV structure and function are associated with worse outcomes in patients with HFpEF.
Diastolic dysfunction is known to be an important pathophysiologic contributor to HFpEF,11 and various markers of diastolic dysfunction have been linked to poor prognosis in HFpEF. The results from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity Echocardiographic Substudy (CHARMES) indicated that diastolic dysfunction as measured by mitral inflow parameters using Doppler echocardiography predicted worse outcomes in HFpEF.20 Other proxies for diastolic dysfunction, such as BNP, PASP, and E/e′ ratio, have also been linked to worse prognosis, but these are all load-dependent measures.21 Alternatively, Ohtani et al21 proposed diastolic wall strain as a load-independent, noninvasive measurement of diastolic stiffness and showed that this value predicted worse outcomes in HFpEF. However, it has been suggested that diastolic wall strain is likely to be a marker of systolic function because it is also an index of the degree of LV wall thickening.10
To our knowledge, ours is the first study to demonstrate echocardiographic estimation of LV diastolic chamber compliance (ie, EDV20) as independently prognostic in HFpEF. These data illustrate the fact that although many pathophysiologic factors contribute to HFpEF, a stiff LV is important in dictating the natural history of HFpEF. Patients with cardiac amyloidosis are a subset of patients that can have particularly severe diastolic dysfunction (due to stiff ventricles) that drives outcomes.47 However, we showed that even after excluding those with cardiac amyloidosis (N=15), EDV20 remained significantly associated with both composite outcome and HF hospitalization.
The reasons underlying the interaction between anemia and EDV20 in the prediction of outcomes in HFpEF (Figure 1) are unknown. Whereas reduced LV compliance was predictive of outcomes in nonanemic HFpEF patients, the association was eliminated in anemic HFpEF patients. As shown in Figure 1, LV volumes were larger in HFpEF patients with anemia, likely owing to increased cardiac output and increased circulating blood volume in anemic HFpEF patients. Although previous studies have shown an association between anemia and diastolic dysfunction,48,49 these prior studies were based on mitral inflow, pulmonary vein flow, and E/e′ characteristics, all of which can be altered by volume overload alone and not necessarily intrinsic diastolic dysfunction of the LV myocardium. Thus, in anemic HFpEF patients, LV compliance (as measured by EDV20) may not be as important in predicting outcomes compared with other factors such as extracardiac causes of volume overload or anemia-related comorbidities.
The fact that tissue Doppler s′ velocity and Ees, markers of longitudinal LV systolic function and chamber contractility, respectively, failed to emerge as predictors of adverse outcomes in our study further emphasizes the prognostic importance of diastolic pathophysiology, but it should be noted that, based on our findings, reduced LV diastolic compliance, and not impaired LV relaxation, seems to drive outcomes in HFpEF. Systolic function and VA coupling parameters have previously failed to predict outcomes. In a community-based cohort of HFpEF patients, Ees, Ea, and EF all failed to predict mortality, though stress-corrected midwall fractional shortening was predictive of adverse events.19 Nonetheless, LV systolic dysfunction likely contributes to the development of HFpEF syndrome. Indeed, systolic dysfunction and diastolic dysfunction frequently coincide, implying shared risk factors and pathophysiology. However, our results suggest that although risk factors may be generally shared, underlying disease and pathways of disease progression are not necessarily uniform across all HFpEF patients. Those with increased RV wall thickness and significantly reduced LV compliance as indicated by a lower EDV20 are at greatest risk for poor outcomes and are in greatest need of targeted interventions.
Strengths and Limitations
The strengths of our study include the prospective and standardized recruitment of high-risk HFpEF patients and the use of detailed, quantitative echocardiographic phenotyping to evaluate HFpEF pathophysiologies. Our study also included detailed adjudication of adverse events, and follow-up was complete on all patients. Thus, for the first time to our knowledge, we were able to determine the relative prognostic importance of several HFpEF pathophysiologies. Finally, the recruitment of patients posthospitalization provided a unique opportunity to study the pathophysiologic predictors of rehospitalization in a prospective fashion. Postdischarge, HF patients are at highest risk for adverse outcomes and have the most urgent need for effective therapies.2
Our study has some potential limitations. First and foremost, our study mainly provides pathophysiologic insights—the parameters we studied are not necessarily clinically useful markers for risk prediction. Although RV wall thickness and EDV20 provided incremental prognostic information, the increase in log LRs was modest; thus, there may be little clinical use in measuring markers such as EDV20. Second, because we do not have data on the duration of HF, RV remodeling and dysfunction may represent a late pathophysiologic change that could explain its association with poor outcomes. However, the adjustment of HF severity did not nullify the association between RV remodeling and dysfunction and adverse outcomes. Third, although we performed detailed echocardiographic quantification, we did not measure the indices of RV diastolic function, which could have provided additional insight into the pathophysiology of RV dysfunction in HFpEF. RV wall thickness was also not measurable in 13.6% of the study participants because of lack of adequate subcostal views; however, the clinical characteristics among those with immeasurable RV wall thickness were similar to those with measurable RV wall thickness (Table II in the Data Supplement).
The use of EDV20 as a measure of LV compliance is also a limitation, because it is based on a single-beat, noninvasive methodology that requires the estimation of both LVEDV by the biplane method of discs and LVEDP by the E/e′ ratio, which may be unreliable at values of 8 to 15. In addition, 14% of the cohort was in atrial fibrillation at the time of echocardiography; in these patients, E/e′ ratio was still used to calculate EDV20, although the calculation of EDV20 has not been validated in the setting of atrial fibrillation. However, we showed good reproducibility of EDV20 measurement, and any error in the single-beat estimation of EDPVR (EDV20) would have most likely reduced our ability to show an association between reduced LV compliance and worse outcomes.
Finally, all the study participants were recruited from a single academic medical center. However, Northwestern Memorial Hospital serves a large, diverse urban environment. Although the participants in our cohort were younger than those described in epidemiological and registry HFpEF studies, the frequencies of comorbidities were similar, and our cohort was more racially diverse. Therefore, our cohort may represent the broader population of HFpEF patients.
Conclusions
Among the pathophysiologic abnormalities that contribute to the heterogeneous syndrome of HFpEF, reduced LV compliance and RV abnormalities are the strongest predictors of adverse outcomes. These specific pathophysiologies may represent targets for novel management strategies and therapies aimed at reducing morbidity and mortality in HFpEF.
Supplementary Material
CLINICAL PERSPECTIVE.
Heart failure with preserved ejection fraction (HFpEF) represents ≈50% of prevalent cases of HF, but no treatment has improved outcomes in clinical trials. This fact is likely because of the pathophysiologic heterogeneity of HFpEF. Abnormalities in left ventricular (LV) diastolic function, longitudinal LV systolic function, ventricular-arterial (VA) coupling, and right ventricular (RV) structure and function have all been reported in HFpEF and likely contribute to the pathogenesis of the HFpEF syndrome. However, the relative contribution of these pathophysiologic abnormalities to adverse outcomes is not well understood. We aimed to study the prognostic importance prospectively of each of these pathophysiologies in a well-characterized HFpEF cohort. We determined that lower LV compliance (as estimated by a reduced LV end-diastolic volume at an idealized pressure of 20 mm Hg [EDV20]) and increased RV remodeling (as indicated by increased RV wall thickness) are associated with both HF hospitalization and the composite outcome of cardiovascular hospitalization or death. Moreover, these associations were independent of age, sex, comorbidities, and markers of HF severity. Furthermore, these 2 pathophysiologic markers were uncorrelated and were additive in their association with adverse outcomes. Conversely, impaired LV relaxation, longitudinal LV systolic dysfunction, and abnormal VA coupling were not independently associated with adverse outcomes. These results suggest that both reduced LV compliance and worse RV remodeling are the most important pathophysiologies driving outcomes in HFpEF. Thus, targeting these 2 pathophysiologic domains may be the key to improving the track record of HFpEF clinical trials.
Acknowledgments
Sources of Funding
Supported by the American Heart Association Scientist Development Grant (#0835488N) and National Institutes of Health grant (R01 HL107557), both to S.J.S.
Footnotes
The Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.113.000854/-/DC1.
Disclosures
None.
References
- 1.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Campbell RT, Jhund PS, Castagno D, Hawkins NM, Petrie MC, McMurray JJ. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-preserved, and I-PRESERVE? J Am Coll Cardiol. 2012;60:2349–2356. doi: 10.1016/j.jacc.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 4.Setaro JF, Zaret BL, Schulman DS, Black HR, Soufer R. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981–986. doi: 10.1016/0002-9149(90)90937-v. [DOI] [PubMed] [Google Scholar]
- 5.Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction > or = 40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol. 1997;80:207–209. doi: 10.1016/s0002-9149(97)00320-2. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, PEP-CHF Investigators The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 8.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, I-PRESERVE Investigators Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 9.Shah RV, Desai AS, Givertz MM. The effect of renin-angiotensin system inhibitors on mortality and heart failure hospitalization in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis. J Card Fail. 2010;16:260–267. doi: 10.1016/j.cardfail.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1716–1717. doi: 10.1093/eurheartj/ehs124. [DOI] [PubMed] [Google Scholar]
- 11.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 12.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE, I-PRESERVE Investigators Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 13.Liu YW, Tsai WC, Su CT, Lin CC, Chen JH. Evidence of left ventricular systolic dysfunction detected by automated function imaging in patients with heart failure and preserved left ventricular ejection fraction. J Card Fail. 2009;15:782–789. doi: 10.1016/j.cardfail.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Shah SJ. Evolving approaches to the management of heart failure with preserved ejection fraction in patients with coronary artery disease. Curr Treat Options Cardiovasc Med. 2010;12:58–75. doi: 10.1007/s11936-009-0060-2. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 16.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puwanant S, Priester TC, Mookadam F, Bruce CJ, Redfield MM, Chandrasekaran K. Right ventricular function in patients with preserved and reduced ejection fraction heart failure. Eur J Echocardiogr. 2009;10:733–737. doi: 10.1093/ejechocard/jep052. [DOI] [PubMed] [Google Scholar]
- 18.Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P, Rich S. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–265. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 19.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, Ostergren J, McKelvie RS, Investigators of the CHARM Echocardiographic Substudy-CHARMES Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence: results from the CHARM Echocardiographic Substudy-CHARMES. J Am Coll Cardiol. 2007;49:687–694. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 21.Ohtani T, Mohammed SF, Yamamoto K, Dunlay SM, Weston SA, Sakata Y, Rodeheffer RJ, Roger VL, Redfield MM. Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1742–1749. doi: 10.1093/eurheartj/ehs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavazzi A, Berzuini C, Campana C, Inserra C, Ponzetta M, Sebastiani R, Ghio S, Recusani F. Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure. J Heart Lung Transplant. 1997;16:774–785. [PubMed] [Google Scholar]
- 23.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 24.Meluzin J, Spinarová L, Hude P, Krejcí J, Kincl V, Panovský R, Dusek L. Prognostic importance of various echocardiographic right ventricular functional parameters in patients with symptomatic heart failure. J Am Soc Echocardiogr. 2005;18:435–444. doi: 10.1016/j.echo.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, White M, Aban IB, Mujib M, Dell’Italia LJ, Ahmed A. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–258. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawut SM, Barr RG, Lima JA, Praestgaard A, Johnson WC, Chahal H, Ogunyankin KO, Bristow MR, Kizer JR, Tandri H, Bluemke DA. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA)–right ventricle study. Circulation. 2012;126:1681–1688. doi: 10.1161/CIRCULATIONAHA.112.095216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris DA, Gailani M, Vaz Pérez A, Blaschke F, Dietz R, Haverkamp W, Özcelik C. Right ventricular myocardial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24:886–897. doi: 10.1016/j.echo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 28.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 29.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 30.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MSJ, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Klotz S, Hay I, Dickstein ML, Yi GH, Wang J, Maurer MS, Kass DA, Burkhoff D. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006;291:H403–H412. doi: 10.1152/ajpheart.01240.2005. [DOI] [PubMed] [Google Scholar]
- 34.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–H512. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 35.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 36.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 37.Senzaki H, Chen CH, Kass DA. Single-beat estimation of end-systolic pressure-volume relation in humans. A new method with the potential for noninvasive application. Circulation. 1996;94:2497–2506. doi: 10.1161/01.cir.94.10.2497. [DOI] [PubMed] [Google Scholar]
- 38.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willis J, Augustine D, Shah R, Stevens C, Easaw J. Right ventricular normal measurements: time to index? J Am Soc Echocardiogr. 2012;25:1259–1267. doi: 10.1016/j.echo.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Ommen SR, Nishimura RA, Hurrell DG, Klarich KW. Assessment of right atrial pressure with 2-dimensional and Doppler echocardiography: a simultaneous catheterization and echocardiographic study. Mayo Clin Proc. 2000;75:24–29. doi: 10.4065/75.1.24. [DOI] [PubMed] [Google Scholar]
- 41.Temporelli PL, Scapellato F, Corrà U, Eleuteri E, Imparato A, Giannuzzi P. Estimation of pulmonary wedge pressure by transmitral Doppler in patients with chronic heart failure and atrial fibrillation. Am J Cardiol. 1999;83:724–727. doi: 10.1016/s0002-9149(98)00978-3. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal R, Shah SJ, Foreman AJ, Glassner C, Bartolome SD, Safdar Z, Coslet SL, Anderson AS, Gomberg-Maitland M. Risk assessment in pulmonary hypertension associated with heart failure and preserved ejection fraction. J Heart Lung Transplant. 2012;31:467–477. doi: 10.1016/j.healun.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–990. doi: 10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 44.van de Veerdonk MC, Kind T, Marcus JT, Mauritz G-J, Heymans MW, Bogaard H-J, Boonstra A, Marques KMJ, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 45.Damy T, Kallvikbacka-Bennett A, Goode K, Khaleva O, Lewinter C, Hobkirk J, Nikitin NP, Dubois-Randé JL, Hittinger L, Clark AL, Cleland JG. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. J Card Fail. 2012;18:216–225. doi: 10.1016/j.cardfail.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 46.La Gerche A, Jurcut R, Voigt JU. Right ventricular function by strain echocardiography. Curr Opin Cardiol. 2010;25:430–436. doi: 10.1097/HCO.0b013e32833b5f94. [DOI] [PubMed] [Google Scholar]
- 47.Klein AL, Hatle LK, Taliercio CP, Oh JK, Kyle RA, Gertz MA, Bailey KR, Seward JB, Tajik AJ. Prognostic significance of Doppler measures of diastolic function in cardiac amyloidosis. A Doppler echocardiography study. Circulation. 1991;83:808–816. doi: 10.1161/01.cir.83.3.808. [DOI] [PubMed] [Google Scholar]
- 48.Brucks S, Little WC, Chao T, Rideman RL, Upadhya B, Wesley-Farrington D, Sane DC. Relation of anemia to diastolic heart failure and the effect on outcome. Am J Cardiol. 2004;93:1055–1057. doi: 10.1016/j.amjcard.2003.12.062. [DOI] [PubMed] [Google Scholar]
- 49.Nair D, Shlipak MG, Angeja B, Liu HH, Schiller NB, Whooley MA. Association of anemia with diastolic dysfunction among patients with coronary artery disease in the Heart and Soul Study. Am J Cardiol. 2005;95:332–336. doi: 10.1016/j.amjcard.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


