Abstract
Objective
To develop an evidence-based guideline to help clinicians make decisions about when and how to safely taper and stop benzodiazepine receptor agonists (BZRAs); to focus on the highest level of evidence available and seek input from primary care professionals in the guideline development, review, and endorsement processes.
Methods
The overall team comprised 8 clinicians (1 family physician, 2 psychiatrists, 1 clinical psychologist, 1 clinical pharmacologist, 2 clinical pharmacists, and 1 geriatrician) and a methodologist; members disclosed conflicts of interest. For guideline development, a systematic process was used, including the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. Evidence was generated by conducting a systematic review of BZRA deprescribing trials for insomnia, as well as performing a review of reviews of the harms of continued BZRA use and narrative syntheses of patient preferences and resource implications. This evidence and GRADE quality of evidence ratings were used to generate recommendations. The team refined guideline content and recommendations through consensus and synthesized clinical considerations to address front-line clinician questions. The draft guideline was reviewed by clinicians and stakeholders.
Recommendations
We recommend that deprescribing (tapering slowly) of BZRAs be offered to elderly adults (≥ 65 years) who take BZRAs, regardless of duration of use, and suggest that deprescribing (tapering slowly) be offered to adults aged 18 to 64 who have used BZRAs for more than 4 weeks. These recommendations apply to patients who use BZRAs to treat insomnia on its own (primary insomnia) or comorbid insomnia where potential underlying comorbidities are effectively managed. This guideline does not apply to those with other sleep disorders or untreated anxiety, depression, or other physical or mental health conditions that might be causing or aggravating insomnia.
Conclusion
Benzodiazepine receptor agonists are associated with harms, and therapeutic effects might be short term. Tapering BZRAs improves cessation rates compared with usual care without serious harms. Patients might be more amenable to deprescribing conversations if they understand the rationale (potential for harm), are involved in developing the tapering plan, and are offered behavioural advice. This guideline provides recommendations for making decisions about when and how to reduce and stop BZRAs. Recommendations are meant to assist with, not dictate, decision making in conjunction with patients.
Long-term use of benzodiazepine receptor agonists (BZRAs), including benzodiazepines, zopiclone, and zolpidem, for insomnia is common in adults and the elderly in both community primary care and institutional practice.1,2 The benefits of BZRAs for insomnia can be briefly summarized by referring to the meta-analysis by Holbrook et al, which found short-term (1 day to 6 weeks) improvements in sleep onset latency of 4 minutes and an additional hour of sleep duration.3 However, chronic use of BZRAs might lead to physical and psychological dependence. In 2012, more than 30% of Canadian seniors in long-term care facilities and more than 15% living in the community used BZRAs.4 New evidence has emerged suggesting that the efficacy of BZRAs for insomnia can diminish in 4 weeks, but adverse effects might persist.5 Benzodiazepine receptor agonists were selected in a Canadian consensus process among family physicians, pharmacists, nurses, and geriatricians as the most important medication class for developing a deprescribing guideline.6 In an effort to provide evidence-based recommendations and tools to aid clinicians in reducing or stopping medications that might no longer be needed or that might be causing harm, we initiated the Deprescribing Guidelines in the Elderly project (www.open-pharmacy-research.ca/research-projects/emerging-services/deprescribing-guidelines).
Our objective was to systematically review the benefits and harms of deprescribing BZRAs, and use patient values and preferences and cost literature to develop evidence-based guidelines that assist clinicians and patients in making decisions about and taking action on reducing BZRA use.
This clinical guideline focuses on long-term use of BZRAs for insomnia. Insomnia, one of the most common complaints in primary care, is characterized by difficulty initiating or maintaining sleep accompanied by impaired daytime function.7 It is classified by the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, as insomnia disorder, which can co-occur with other conditions or occur on its own (previously known as primary insomnia in the fourth edition of the manual).7,8 In Canada, 13% of the population meets the criteria for insomnia, while the prevalence of insomnia is around 11% in the United States and Hong Kong.9,10 Benzodiazepine receptor agonists and cognitive-behavioural therapy (CBT) have emerged as 2 of the common treatments of insomnia.11 Guidelines suggest that BZRAs should only be used short term (typically up to 4 weeks) for treatment of insomnia.12,13 However, in older persons, the Canadian Geriatrics Society and the Canadian Academy of Geriatric Psychiatry Choosing Wisely recommendations and the Beers criteria recommend avoiding BZRAs altogether as first-line treatment of insomnia, and that they should only be used after failure of nonpharmacologic interventions, for as short of a duration as possible.14–16
Benzodiazepine receptor agonists attach to a site on the γ-aminobutyric acid type A receptor, but when they are used over an extended period of time, the receptor can physically change, leaving less potential for sedation but persistent amnestic effects.17 Studies detect loss of therapeutic effect in a matter of 7 to 28 days.5 Unfortunately, many patients are unaware of this effect and continue taking these agents indefinitely. This is problematic considering the potential for adverse effects (eg, falls, fractures, cognitive problems), especially in older persons.18,19 For this reason, reviews have emerged over the past number of years investigating the effectiveness of interventions to stop or reduce use of BZRAs, but none has focused specifically on patients using BZRAs for insomnia.20–23 While useful, these systematic reviews do not provide practical approaches to stopping or reducing BZRAs. Further, despite the scope of BZRA use, there are currently no evidence-based guidelines to assist clinicians in stopping or reducing use of BZRAs.
Deprescribing is the planned and supervised process of dose reduction or stopping of medication that might be causing harm or no longer providing benefit. The goal of deprescribing is to reduce medication burden and harm, while maintaining or improving quality of life. Our deprescribing guidelines, from our Deprescribing Guidelines in the Elderly project, use evidence to prioritize medications for deprescribing, to determine benefit and harms of continuing versus deprescribing a medication, and to suggest methods for approaching and implementing deprescribing plans with patients.
Our target audience includes primary care physicians, pharmacists, nurse practitioners, or other specialists who care for patients taking BZRAs for insomnia. The target population includes adults taking a BZRA to treat insomnia disorder: insomnia on its own (primary insomnia) or comorbid insomnia where underlying comorbidities are managed. This includes adults aged 18 to 64 who take BZRAs for most days of the week for more than 4 weeks12,24 and elderly adults (≥ 65 years of age) who take BZRAs regardless of duration.14–16 The guideline does not apply to those with other sleep disorders or untreated anxiety, depression, or physical and mental conditions that might be causing or aggravating insomnia. These patients should be appropriately treated for their primary conditions before considering deprescribing of BZRAs or be referred to a psychologist or psychiatrist if appropriate.12
METHODS
We used Schünemann and colleagues’ checklist for a successful guideline enterprise to construct the methods for developing deprescribing guidelines.25,26
The Guideline Development Team (GDT) comprised 8 clinicians (1 family physician and guideline chair [K.P.], 2 psychiatrists [R.S., S.D.], 1 psychologist [J.G.], 1 clinical pharmacologist [A.H.], 2 clinical pharmacists with geriatrics expertise [B.F., C.A.S.], and 1 geriatrician [C.B.]) and a GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodologist (V.W.). Searches were conducted in collaboration with a Canadian Library of Family Medicine librarian, as well as by a pharmacy resident (A.M.), a master’s student (W.T.), and a staff member. All GDT members, staff, and investigators of the deprescribing project returned conflict of interest statements at the beginning and at the end of the process. Contributors’ expertise, role descriptions, and conflicts of interest are available at CFPlus.*
We used the GRADE system for guideline development (Box 1).27 The GDT formulated the main clinical management question as follows using the PICO (population, intervention, comparison, outcome) approach: What are the effects (harms and benefits) of deprescribing BZRAs compared with continued use in adults with insomnia? We also investigated comparative effects of different deprescribing interventions. The GDT articulated definitions of deprescribing that included abrupt discontinuation, tapering, and switching or substituting therapy, among others (Box 2).
Box 1. Notes on the GRADE framework for guideline development.
This guideline was informed by a systematic review and was developed in accordance with the methods proposed by the GRADE Working Group27:
We focused our review and recommendations on outcomes important to patients, such as harms or benefits resulting from deprescribing of BZRAs, harms of continued BZRA use, patient values and preferences, and costs and resource use. Outcomes were proposed by the systematic review team and revised by the Guideline Development Team based on feasibility and the available literature
Ratings in the evidence profile tables included high, moderate, low, or very low, and depended on our confidence in the estimates of effect. Randomized controlled trials were used, and they started with a high-quality rating, but could be rated down by limitations in any of 4 domains: risk of bias, inconsistency, indirectness, and imprecision. Publication bias could not be rated owing to the paucity of studies
The GRADE Working Group outlines appropriate wording for recommendations depending on the rating of strength and confidence in the evidence. A strong recommendation with implications for patients (phrased as “we recommend ...”) implies that all patients in the given situation would want the recommended course of action, and only a small proportion would not. A weak recommendation (phrased as “we suggest ...”) implies that most patients would wish to follow the recommendation, but some patients would not. Clinicians must help patients make management decisions consistent with patients’ values and preferences. Implications for clinicians are similar such that a strong recommendation implies all or most patients should receive the intervention. A weak recommendation should prompt a clinician to recognize that different choices will be appropriate for individual patients
BZRA—benzodiazepine receptor agonist, GRADE—Grading of Recommendations Assessment, Development and Evaluation.
Box 2. Definitions of BZRA deprescribing.
Deprescribing BZRAs can include the following:
Abruptly stopping the BZRA (ie, abrupt discontinuation)
Tapering the BZRA dose (ie, gradually reducing the dose until complete cessation of the BZRA)
Recommending CBT (ie, a CBT program for insomnia with the aim of stopping or reducing BZRA use in the process)
Combining tapering and CBT
- Reducing BZRA use with the following approaches:
- -Using a lower dose of BZRA compared with baseline
- -Using BZRAs only as needed
Providing substitutive therapy (ie, discontinuing the BZRA and replacing it with an alternative agent [eg, melatonin] either abruptly or by cross-tapering)
BZRA—benzodiazepine receptor agonist, CBT—cognitive-behavioural therapy.
Before running our search we conducted a scoping review to assess the body of available evidence. We then conducted a systematic review to assess the effects of different approaches to deprescribing BZRAs. The methods and results of the systematic review can be found at CFPlus.*
The systematic review focused on important outcomes relevant to patients, caregivers, and health care providers. Primary outcomes included sleep quality, effect on cognition (improvement or worsening), adverse drug withdrawal events, cessation rate (proportion of patients who completely stop BZRAs), and harms (daytime sedation, balance, motor vehicle accidents, falls, mortality, dependence). Secondary outcomes included BZRA pill burden (average BZRA dose) and patient satisfaction. The GRADE evidence tables that provide a summary of estimates on patient-important and critical outcomes for decision making can be found at CFPlus.*
We formulated clinical recommendations from the evidence tables using confidence in estimated effects and taking into account benefits and harms of BZRA deprescribing, patient preferences and values, harms associated with continued BZRA use, and resource implications. The GDT members met via teleconference to review clinical recommendations and voting was conducted by e-mail. All votes were sent to the project coordinator; unanimous agreement was sought; 80% agreement among the 9 GDT members was considered the cutoff for consensus. All GDT members agreed with the final recommendations.
RECOMMENDATIONS
The recommendations (Box 3) apply to adults aged 18 and older including elderly adults living in the community or in long-term care facilities who take BZRAs for the purpose of treating primary insomnia or comorbid insomnia where all potential underlying comorbidities are effectively managed. These recommendations do not apply to those with other sleep disorders or untreated anxiety, depression, or physical and mental conditions that might be causing or aggravating insomnia.
Box 3. Recommendations.
For elderly adults (≥ 65 y) who use BZRAs, we recommend the following:
Taper the BZRA dose slowly (strong recommendation, low-quality evidence)
For adults (18 to 64 y) who have used BZRAs most days of the week for > 4 wk, we suggest the following:
Taper the BZRA dose slowly (weak recommendation, low-quality evidence)
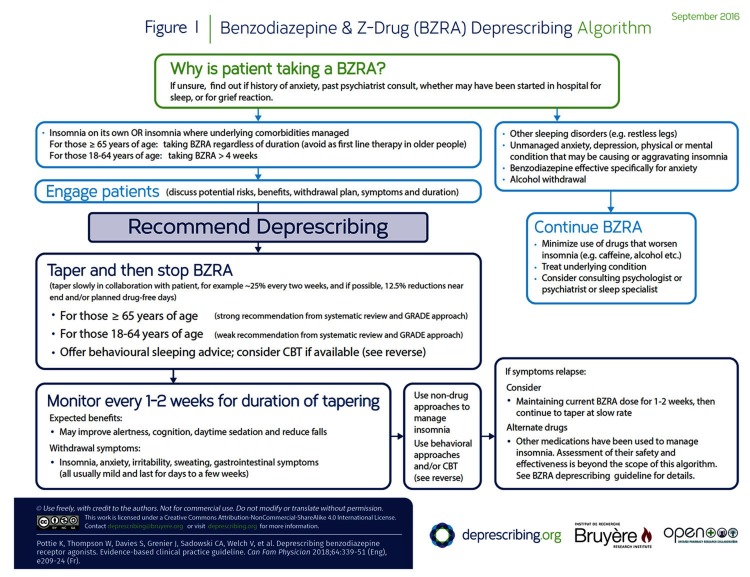
The rationale for the recommendations is outlined in Table 1.28,29 The algorithm developed for this guideline is provided in Figure 1.
Table 1.
Evidence to recommendations table: What are the effects of deprescribing BZRAs compared with continuous use in insomnia for adults ≥ 18 y who use BZRAs for insomnia on its own or for comorbid insomnia with underlying comorbidities managed (specifically, adults 18–64 y using BZRAs for most nights of the week for > 4 wk, or adults ≥ 65 y taking BZRAs for any duration as first-line therapy)?
| DECISION DOMAIN | SUMMARY OF REASON FOR DECISION | SUBDOMAINS INFLUENCING DECISION |
|---|---|---|
| QoE: Is there high- or moderate-quality evidence? Yes □ No ☑ | The QoE for the benefits of deprescribing is low to moderate The QoE for the harms of deprescribing is low to moderate |
Key reason for downgrading is risk of bias The QoE from RCTs for benefits of deprescribing is low The QoE from RCTs for harms of deprescribing is moderate (sleep quality) |
| Balance of benefits and harms: Is there certainty that the benefits outweigh the harms? Yes ☑ No □ |
Effects of interventions on cessation rate
Sleep quality outcomes
Anxiety
Other harms of deprescribing (eg, adverse drug withdrawal effects)
Effect of deprescribing on cognition
Adverse events for the elderly
|
Is the baseline risk of benefit of deprescribing similar across subgroups? Yes ☑ No □
Should there be separate recommendations for subgroups based on risk levels? Yes □ No ☑
Yes ☑ No □
Should there be separate recommendations for subgroups based on harms of continued use? Yes ☑ No □
|
| Values and preferences: Is there confidence in the estimate of relative importance of outcomes and patient preferences? Yes □ No ☑ |
Patients tend to rate the benefits of BZDs higher than physicians do and rate the risks lower. Those patients interested in stopping BZDs see potential improvements in thinking and memory as benefits, as well as obtaining more natural sleep and feeling proud of themselves for having stopped. Factors associated with increased likelihood of stopping BZRA use include higher education level, lower intake or potency of BZDs, and lower anxiety sensitivity scores. Of those who fail BZD discontinuation, many describe having experienced such failure as difficulty in sleeping within a few days of stopping |
Perspective taken: Evidence suggests there are patients who wish to discontinue BZRAs to avoid the harms of long-term use. There are others who might be hesitant and might fail owing to difficulty sleeping after stopping Source of values and preferences: Scoping review on subjects including the elderly Source of variability, if any: Education levels, potency of BZRA, and anxiety sensitivity scores Method for determining values satisfactory for this recommendation? Yes ☑ No □ All critical outcomes measured? Yes ☑ No □ |
| Resource implications: Are the resources worth the expected net benefit? Yes ☑ No □ |
Cost implications
|
Feasibility: Is tapering BZRA intervention generally available? Yes ☑ No □
Opportunity cost: Is this intervention and its effects worth withdrawing or not allocating resources from other interventions? Yes ☑ No □ Economic and preventive benefits for harms: Is there a lot of variability in resource requirements across settings? Yes □ No ☑
|
| Overall strength of recommendation in older persons (≥ 65 y): strong Overall strength of recommendation in adults < 65 y: weak | There is low-quality evidence that deprescribing interventions improve cessation rates of BZRAs at 3 mo. Compared with continuation of BZRAs, tapering these drugs does not result in any difference in withdrawal symptom scores (low-quality evidence). Those who taper BZRAs might have more problems sleeping than those who continue; however, there is no difference at 12 mo (very low-quality evidence). Our systematic review found that deprescribing of BZRAs did not worsen anxiety. Despite low-quality evidence surrounding deprescribing, our recommendation was rated as strong in older persons owing to evidence surrounding harms of continued BZRA use specifically in older persons (associated increased risk of falls, cognitive impairment, motor vehicle accidents) and resultant resource implications. We also considered literature on patient preferences suggesting patients might value regaining control over sleep and potentially avoiding adverse effects of BZRAs |
|
BWSQ—benzodiazepine withdrawal symptom questionnaire, BZD—benzodiazepine, BZRA—benzodiazepine receptor agonist, CBT—cognitive-behavioural therapy, COPD—chronic obstructive pulmonary disease, NNT—number needed to treat, QoE—quality of evidence, RCT—randomized controlled trial.
Figure 1.
Benzodiazepine & Z-Drug (BZRA) Deprescribing Algorithm
In this section of the guideline, we summarize the evidence reviews (ie, systematic review of deprescribing studies, review of reviews of BZRA harms, patient values and preferences, and cost and resource implications literature) that support the GRADE-based recommendations. Additional details of the evidence reviews and references can be found at CFPlus.*
Our systematic review results suggest that slow tapering of BZRAs improves cessation rates at 3 and 12 months (compared with continuation or usual care) and does not result in differences in withdrawal symptom scores. Table 228,30–38 provides examples of tapering. Adding CBT to the tapering intervention improves cessation rates versus tapering alone, but this improvement is not maintained in the long term. Combining CBT and tapering does not appear to ameliorate withdrawal symptoms or affect sleep outcomes more so than tapering alone. Tapering of BZRAs might result in more problems sleeping compared with continuation, but there was no difference between these groups at 12 months. Using melatonin does not appear to improve cessation rates; studies employing zopiclone as a means of tapering from benzodiazepines did not produce usable data to inform recommendations.
Table 2.
Deprescribing methods from eligible trials in systematic review
| STUDY | TAPERING STRATEGY | NOTES |
|---|---|---|
| Baillargeon et al, 200330 | Reduce dose by 25% every 1–2 wk until stopped | Patients followed up weekly; could remain at same dose if they experienced withdrawal symptoms |
| Belleville et al, 200731 | Reduce dose by 25% every 2 wk until lowest available dosage is reached; then introduce drug-free nights progressively | Drug-free nights planned in advance Rate of taper could vary depending on presence of withdrawal symptoms |
| Curran et al, 200328 | Reduce dose by 25%–50% every 2 wk until stopped | Rate specific to patient’s original dose and BZRA |
| Garfinkel et al, 199932 | Reduce dose by 50% for 2 wk; then reduce by 25% for 2 wk, and then discontinue | NA |
| Habraken et al, 199733 | Reduce dose by 25% weekly for 3 wk; then reduce by 12.5% for 2 wk | All participants took lorazepam |
| Morin et al, 200434 | Reduce dose by 25% every 2 wk until lowest available dosage is reached; then introduce drug-free nights | Drug-free nights planned in advance Specific instructions could vary based on withdrawal symptoms |
| Pat-Horenczyk et al, 199835 | Reduce dose by 50% for 1 wk; then discontinue | All subjects took zopiclone or flurazepam |
| Shapiro et al, 199536 | Switch to zopiclone for 4 wk and then recommend abrupt cessation of zopiclone | All patients switched from BZDs through 1 of the following methods: stopping BZD for 3 d and then starting zopiclone; directly switching to zopiclone; or overlapping existing BZD with zopiclone for 3-8 d, and then stopping BZD and continuing zopiclone |
| Vissers et al, 200737 | Reduce dose by 25% every 2 wk for 6 wk; then reduce by 12.5% for 2 wk and then stop | First converted to diazepam and stabilized for 2 wk |
| Voshaar et al, 200338 | Reduce dose by 25% weekly for 4 wk; participants could choose to split last step into 12.5% reduction for 4 d | First converted to diazepam and stabilized for 2 wk |
BZD—benzodiazepine, BZRA—benzodiazepine receptor agonist, NA—not applicable.
Observational studies have shown that BZRAs are associated with various harms. These deserve special note because patients rely on health care professionals to provide them with this information. Associated harms include physical dependence, drowsiness, balance issues, falls, fractures, cognitive impairment, memory disorders (including anterograde amnesia), functional impairment, and motor vehicle accidents. Evidence demonstrating harms of BZRAs comes largely from older persons, suggesting they might be at higher risk of adverse effects compared with younger adults (however, adverse effects such as dependence and somnolence have also been demonstrated in younger adults). More information on ranges of frequency ratios for harms and the evidence reviews can be found at CFPlus.*
Older persons are often reluctant to consult with a physician about poor sleep owing to fear of receiving medication that they associate with drowsiness or losing control over what they consider to be a natural process. Some patients would like to stop using BZRAs but worry about insomnia, while others state strongly that they would like to continue using BZRAs. Patients tend to rate the benefits of BZRAs higher than physicians do and the risks lower; they commonly state they are reassured that BZRAs are safe because otherwise their physicians would not prescribe them. Those interested in stopping BZRA use see potential improvements in thinking and memory as benefits, as well as obtaining more natural sleep (evidence reviews are available at CFPlus).* Spending on benzodiazepines is high; $330 million was spent on BZRAs in Canada in 2012 to 2013.29 Using CBT to manage insomnia instead of BZRAs would result in considerable savings owing to decreased risk of falls and related consequences (evidence reviews are available at CFPlus).*
Based on the lack of evidence of substantial harm of deprescribing, the evidence of potential harm associated with continuing a BZRA (particularly in the elderly), along with resultant resource implications and the feasibility of tapering interventions, we rated the recommendation to deprescribe BZRAs in older patients as strong. The recommendation to deprescribe a BZRA in the younger population was rated as weak owing to lower risk of adverse effects associated with continuing BZRA use.
Clinical considerations
Community and institutional long-term care clinicians at our implementation sites clearly expressed 2 key concerns when applying this deprescribing guideline. First, approaching the patient and getting “buy-in” about reducing BZRA use is challenging. Second, clinicians wanted to know what other approaches could be used instead for insomnia. This section discusses clinically relevant findings on engaging patients, attitudes toward medications, other comorbid conditions, and tapering schedules.
What patient attitudes should a clinician expect?
Our review of the literature suggests that there are a range of attitudes toward BZRA discontinuation. While some patients show reluctance, other patients appreciate the opportunity to taper and stop BZRA use in order to regain control over sleep and minimize potential for adverse effects. Indeed, our systematic review demonstrates that many patients can successfully discontinue BZRAs, with approximately 60% to 80% of patients able to stop BZRA use as a result of a deprescribing intervention. This is consistent with other systematic review evidence demonstrating a mean success rate of 25% to 80% for BZRA tapering (compared with 10% to 20% cessation rates with usual care when deprescribing is not initiated).39
In general, the decision to continue, reduce, or discontinue a medication is based on a balance of knowledge about its indication and effectiveness, as well as risks of use (actual or potential side effects), drug interactions, pill burden, and cost. Patient or family values and preferences play an important role in shared decision making with regard to continuing, tapering, or stopping medications. To facilitate these initial discussions we developed a patient pamphlet, which is available at CFPlus.*
What is causing insomnia?
When insomnia is caused by a comorbid condition, treatment should first be aimed at optimizing treatment of that condition.12,24 Insomnia might occur on its own, might be related to a psychiatric illness (eg, an anxiety disorder or major depressive disorder), or might be related to another sleep-wake disorder (eg, sleep apnea or restless legs syndrome).40–44 Other comorbid medical conditions such as respiratory diseases or pain disorders45 might also initiate or exacerbate insomnia (such as nocturia, nocturnal pruritus).46–50 Insomnia might be related to substance use (eg, caffeine, alcohol) or pharmacologic treatment of other conditions (eg, antidepressants for major depressive disorder).42 Contributory comorbid mental health conditions need to be considered as part of a comprehensive diagnostic evaluation before initiating treatment or considering deprescribing.50 Once the reason for insomnia has been clearly determined, appropriate treatment can be instituted and the potential for deprescribing BZRAs considered more clearly.
How do I engage patients in deprescribing BZRAs?
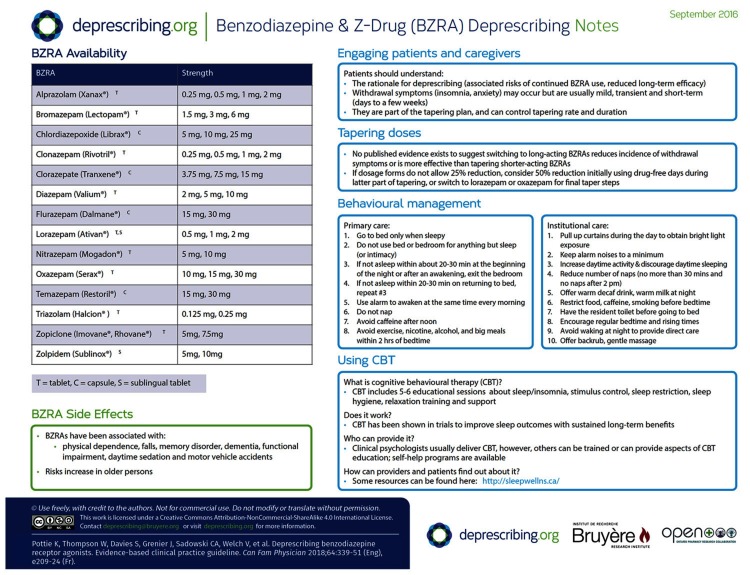
The GDT members believed that engaging patients in a discussion about BZRAs and their goals and preferences regarding BZRA use was an important first step. The need to engage patients and establish goals and preferences related to BZRA use and deprescribing was also echoed by clinicians at our implementation sites. Ensuring patient values and preferences are incorporated into shared decision making surrounding deprescribing has been highlighted as being critical given the preference-sensitive nature of these decisions.51 Patients have indicated they would be more amenable to deprescribing if there were a clear plan for tapering and they knew what to expect.52 The rationale for deprescribing should be clearly explained, a tapering plan should be negotiated, and the following evidence should be discussed:
risks of ongoing BZRA use (eg, falls, memory impairment, motor vehicle accidents) and potential benefits of discontinuation (eg, reduced fall risk, less daytime sedation, improvement in thinking and memory);
therapeutic effect of BZRAs might be lost within 4 weeks owing to receptor changes (but amnestic effects persist)5; and
mild, short-term (a few days to weeks) adverse drug withdrawal effects can be expected during tapering.39
Our systematic review found that tapering strategies were often accompanied by such brief education interventions informing patients about the withdrawal process, sleep hygiene, and withdrawal symptoms.28,31 Such engagement strategies (aimed at educating patients regarding BZRA use and involving them in tapering plans) have been employed as part of tapering interventions with success. Randomized controlled trials involving educational and motivational tools about the risks of BZRA use, the benefits of deprescribing, and the tapering process have been shown to be effective in deprescribing BZRAs.53,54
How should tapering be approached?
A tapering deprescribing strategy should be offered to all eligible patients. However, our systematic review did not identify trials that compared different tapering strategies. Very gradual dose reductions to lowest available doses (eg, 25% reduction every 2 weeks and a slower taper of 12.5% every 2 weeks near the end of stopping), followed by periodic drug-free days were used successfully in clinical trials (Table 2).28,30–38 Switching to long-acting BZRAs (eg, diazepam) has not been shown to reduce incidence of withdrawal symptoms or improve cessation rates more than tapering shorter-acting BZRAs does.55,56 Patients using lower doses at baseline and using BZRAs for a shorter duration tend to have greater cessation rates and lower risk of restarting use of their BZRAs.57–59 Psychological distress and worse general health at baseline appears to increase risk of needing to restart BZRA use.60,61 When deciding on tapering doses and rates, consider using a slower rate with those more likely to have a higher risk of relapse (eg, long-term use or history of psychological distress). Some clinicians would recommend tapering over several months. Such patients might require closer monitoring and support. It has been the GDT members’ experience that other patients might be able to simply stop taking their BZRAs with no or minimal ill effect, and some medical situations might require this, but care should still be taken to monitor for adverse drug withdrawal effects (see monitoring section below for more detail). Some evidence suggests that zolpidem might be less likely than a standard BZRA to cause tolerance, and therefore fewer withdrawal effects upon discontinuation, but there is likely to be a mixture of beneficial effects and adverse effects depending on the patient and duration of treatment.62,63
What withdrawal symptoms can be expected and how should they be dealt with?
Concern over potential for withdrawal symptoms is a key reason why prescribers often do not approach patients about deprescribing BZRAs. Our systematic review found that there was no difference in overall BZRA withdrawal symptom scores for tapering compared with usual care or continuation of BZRAs. The tapering group reported more trouble sleeping at 3 months compared with continuation of BZRAs (mean difference of 16.1 higher on a 100-point scale of “trouble sleeping,” 95% CI 15.0 to 17.2), but any difference in reports of trouble sleeping was no longer present at 12 months. In many cases, when withdrawal symptoms occur they are mild and short term (lasting a few days and up to approximately 4 weeks).39 In studies detailing benzodiazepine withdrawal symptoms, such symptoms tend to appear and peak more quickly (1 to 2 days) and be more severe with abruptly stopping short-acting benzodiazepines compared with after tapering long-acting benzodiazepines (4 to 10 days).64,65 Gradual taper of short-acting agents does not eliminate withdrawal symptoms but ameliorates their severity, with symptoms beginning to appear once doses are reduced to about 25% of baseline.55 While common, resulting insomnia is typically mild, and patients should be assured that there is no difference in insomnia compared with usual care or continuation of BZRAs at 12 months. Behavioural management education for insomnia (Figure 1 and patient pamphlet at CFPlus)* should be offered to patients to address insomnia during tapering. Other common withdrawal symptoms reported in the literature include irritability, sweating, gastrointestinal symptoms, and anxiety.39 Patients should be reassured that if these symptoms occur they are typically mild and short term (lasting days to weeks), and that discomfort is usually temporary.39 Severe withdrawal symptoms (eg, seizures) do not appear to occur with tapering but have been reported rarely in patients stopping very high doses without tapering39 or who have underlying seizure disorders. Patients should be aware of the potential for withdrawal effects and these can be monitored throughout the tapering process (see monitoring section below for more detail).
What nondrug approaches can be used to help with insomnia?
A variety of behaviour management strategies and interventions such as CBT have been used to help with insomnia and can be considered as nondrug alternatives should insomnia recur during or after deprescribing of BZRAs.66 Cognitive-behavioural therapy for treatment of insomnia has been widely studied and demonstrates long-term improvements in sleep outcomes.67–70 Our systematic review found that, when used as part of a deprescribing intervention, CBT combined with tapering improved post-intervention BZRA cessation rates compared with tapering alone. This is consistent with the current evidence base.21 However, the improved BZRA cessation rates following deprescribing were not sustained at 3 months and CBT did not improve clinical outcomes. Deprescribing studies typically employed 4 to 6 CBT sessions delivered by clinical psychologists every 1 to 2 weeks.
Behavioural interventions and CBT for insomnia are reviewed in more detail elsewhere.67,68 While CBT might be difficult to access for many patients owing to cost and availability, online and self-help options are available.69,71 Brief interventions and Internet-based CBT have also been shown to be effective in improving sleep outcomes.67–69,72 The website sleepwellns.ca is an example of such an online resource; a link to this site is highlighted in our decision-support algorithm (Figure 1) and patient information pamphlet (CFPlus),* in which we offer behaviour management advice. With training, various health care professionals can also provide CBT or its components.
What monitoring needs to be done, how often, and by whom?
Tapering will reduce, but might not eliminate, withdrawal symptoms.28,34 A monitoring plan should be developed in conjunction with the patient. At each step in the taper (approximately every 1 to 2 weeks for the duration) monitor for severity and frequency of adverse drug withdrawal symptoms (anxiety, irritability, sweating, gastrointestinal symptoms, insomnia), potential benefits (eg, less daytime sedation, improved cognition, fewer falls), and mood, sleep quality, and changes in sleep. This can be done at a scheduled appointment or through a telephone call (by physician, psychologist, pharmacist, or nurse). If desired, withdrawal symptoms can be monitored using the types of scales used in clinical trials (eg, benzodiazepine withdrawal symptom questionnaire or Clinical Institute Withdrawal Assessment for Benzodiazepines scale)73 or via clinical assessment. If withdrawal symptoms occur at a severity and frequency that is bothersome for a patient, consider maintaining the current BZRA dose for 1 to 2 weeks before attempting the next dose reduction; then continue to taper at a slower rate.
What if insomnia returns or persists?
If insomnia persists, consideration should be given to using behavioural management techniques or CBT.66,67 There are no medications for primary or chronic insomnia in the elderly that are proven to be safe and effective. A 2015 US evidence synthesis by the Agency for Healthcare Research and Quality on management of insomnia disorder reported little to no long-term efficacy evidence for pharmacologic treatments.67 A 2016 evidence-based guideline from the American College of Physicians strongly recommends CBT for chronic insomnia and includes a weak recommendation for shared decision making concerning other nonpharmacologic and pharmacologic options.66
Comorbidities
When offering BZRA deprescribing it is essential to be vigilant for pre-existing or incident depression or anxiety disorders. Psychiatric comorbidities are common in insomnia.74,75 In a 6-year longitudinal study using national data,76 individuals who both had insomnia and were taking a benzodiazepine had a 5-fold increased risk of developing depression and a 3-fold risk of developing an anxiety disorder compared with individuals who did not have insomnia and did not use benzodiazepines. This increased comorbidity might be due to pre-existing risk factors that initially triggered insomnia but might also relate to a biologic or psychological reaction to the consequences of insomnia or to pharmacologic changes associated with long-term BZRA use.77
Depression and anxiety disorders are prevalent in people with insomnia using benzodiazepines. Stopping or reducing a drug with anxiolytic properties (ie, most benzodiazepines) might unmask a previously undiagnosed anxiety disorder. In addition, a change in the behavioural routine of taking a hypnotic medication at bedtime might also provoke a psychological response with anxiety. Cognitive-behavioural therapy might be useful in this instance.66
Where depression or anxiety is present, it might be necessary to initiate pharmacologic- or psychotherapy-based treatment. In the context of insomnia, treatment guidelines13,78 recommend antidepressants that can provide sedation directly.
Where patients exhibit features of an anxiety disorder, a first step would be clinical inquiry for anxiety disorders. In the case of generalized anxiety disorder, a condition dominated by worry in which sleep disturbance is an accessory diagnostic feature, psychological therapy (eg, CBT) or pharmacologic treatment are effective options. First-line medications include serotonin or serotonin-norepinephrine reuptake inhibitors and pregabalin.79–81 There is still a need for high-quality direct evidence for adjunctive medications in benzodiazepine deprescribing for insomnia.
Medicolegal considerations
The Canadian Medical Protective Association reported that sedatives were among the most common drugs in the 215 medicolegal cases surrounding medication use between 2005 and 2010 (proportion not reported).82 Inadequate assessment of adverse effects and not properly evaluating potential effects of drug use in older persons were commonly implicated. Further, 16 of 49 medicolegal cases of opioid-related adverse events involved benzodiazepines.83 Given the potential for adverse effects with BZRA use, appropriateness and opportunities for deprescribing should be routinely addressed by prescribers on an ongoing basis.82,84
Clinical and stakeholder review
External clinical review of the guideline was conducted by a practising geriatrician and a pharmacist using the AGREE II (Appraisal of Guidelines for Research and Evaluation) Global Rating Scale tool.85 Relevant stakeholder organizations were invited to similarly review and endorse the guideline. Modifications were made to address reviewer comments. This guideline has been endorsed by the College of Family Physicians of Canada and the Canadian Pharmacists Association.
How this deprescribing guideline relates to other clinical practice guidelines for insomnia and BZRAs
Guidelines recommending BZRA use for insomnia suggest that attempts to discontinue therapy should be made after 4 weeks.12,24 Several organizations recommend avoiding BZRAs altogether in older persons as first-line therapy for insomnia.14–16 The 2016 American College of Physicians evidence-based guideline for treatment of insomnia strongly recommends the use of CBT as first-line treatment of insomnia.66
The joint clinical practice guideline from the American Geriatrics Society and the British Geriatrics Society on falls prevention suggests that reduction or withdrawal of sedative hypnotics (such as BZRAs) should be pursued as part of multifactorial falls reduction interventions, along with other components such as exercise and adaptation of the home environment.86 Systematic reviews and meta-analyses have shown that multifactorial falls prevention strategies (involving medication review or targeted medication [eg, BZRA] withdrawal) reduce fall rates.87,88
A BZRA deprescribing guideline works in conjunction with current treatment guidelines because it offers clinicians evidence-based recommendations and clinical considerations to help them deprescribe BZRAs.
Gaps in knowledge
Insomnia is a common and often complex condition. Practitioners need tools to assist them in both diagnosis and treatment of insomnia, recognizing anxiety and other psychiatric conditions, as well as specific forms of insomnia. Our research provides an estimated strategy for tapering BZRAs. We did not identify studies that compared various tapering regimens head-to-head. There is also limited evidence for optimal shared decision-making approaches related to deprescribing BZRAs. Finally, most studies did not evaluate patient-important outcomes such as quality of life or function. Thus, studies comparing patient engagement approaches and tapering strategies (eg, duration of tapering), and focusing on patient-important outcomes (eg, quality of life and function) are needed to help guide effective deprescribing approaches. There is also a need to study BZRA deprescribing in relation to the potential dependence period at around 4 weeks of BZRA use, as well as the cost-effectiveness of BZRA deprescribing interventions. More implementation work is needed to improve the process of tapering, as well as consideration of linking it to multifactorial interventions for improving health in elderly adults.
Next steps
The GDT will provide routine guideline updates as new evidence emerges that might change the recommendations. Prospective evaluation of the effects of this and other deprescribing guidelines will be part of a research strategy in the future.
Conclusion
The use of BZRAs for insomnia disorder in aging adults has been associated with falls, dementia, motor vehicle accidents, and physical addiction. This guideline outlines these harms and, through a systematic review, demonstrates the efficacy of deprescribing using tapering regimens in patients who were willing to enter deprescribing trials. Many patients are willing to stop taking BZRAs when they can expect improvements in cognition and reductions in other side effects. The tools provided with this guideline—a decision-support algorithm and a corresponding patient information pamphlet—are intended to support clinicians in engaging with patients about this important topic and implementing deprescribing plans with them. A credible guideline developed with a rigorous evidence-based approach arms the clinician with a clear case for discussions about BZRA deprescribing with patients.
Acknowledgments
We thank members of the Cochrane research team, Elli Polemiti, Sonia Hussain, and Olanrewaju Medu, who conducted the systematic review upon which these recommendations were based. We also thank the staff at the Bruyère Research Institute in Ottawa, Ont, librarian Lynn Dunikowski, and the stakeholders and peer reviewers (Dr Cara Tannenbaum and Lawrence Jackson), whose thoughtful comments helped to improve the quality of this manuscript. Funding for this guideline was provided by the Government of Ontario.
Editor’s key points
▸ Benzodiazepine receptor agonist (BZRA) prescribing for insomnia is common in both community and long-term care.
▸ The efficacy of BZRAs for insomnia can be diminished in as little as 4 weeks.
▸ Use of BZRAs is associated with increased risk of falls, motor vehicle accidents, memory problems, and daytime sedation—risks that might be increased in the elderly. Choosing Wisely Canada recommends that BZRAs should be avoided as first-line treatment of insomnia in older persons.
▸ A systematic review of BZRA deprescribing in patients demonstrated successful outcomes. The most common withdrawal symptoms were mild, short-term (days to weeks) insomnia, anxiety, and restlessness. There was no evidence of severe withdrawal (eg, seizures) symptoms.
▸ This guideline recommends that deprescribing of BZRAs (by tapering) be offered to all adults who take BZRAs, especially those aged 65 and older. Discuss with your patients and their caregivers the harms of continued use, decreased efficacy over time, tapering options, recommendations for monitoring, and potential withdrawal symptoms.
Footnotes
Descriptions of contributors’ expertise, roles, and conflicts of interest; methods and results of the systematic review and related references; GRADE evidence tables; ranges of frequency ratios for harms; evidence reviews and related references; a patient information pamphlet on deprescribing of benzodiazepine receptor agonists; and an easy-to-print version of the algorithm are available at www.cfp.ca. Go to the full text of the article online and click on the CFPlus tab.
Contributors
All authors made substantial contributions to the conception and design of the guideline; the acquisition, analysis, and interpretation of data; and drafting the article, revising it critically for important intellectual content, and approving the final version.
Competing interests
Dr Farrell received research funding to develop this guideline; received financial payments from the Institute for Healthcare Improvement and The Commonwealth Fund for a deprescribing guidelines summary; and from the Ontario Long Term Care Physicians Association, the Ontario Pharmacists Association, and the Canadian Society of Hospital Pharmacists for speaking engagements. Dr Boyd received funding from Patient-Centered Outcomes Research Institute for a project related to improving patient-centred care for people with multiple chronic conditions and funding from the National Institutes of Health for a project related to medication regimen complexity in home health care. Dr Sadowski is the primary investigator on an unrestricted grant from Pfizer Canada related to finding novel strategy to address the underdiagnosis and undertreatment of overactive bladder and urinary tract symptoms and is a member of the Alberta Expert Committee on Drug Evaluation and Therapeutics. None of the other authors has any competing interests to declare.
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to www.cfp.ca and click on the Mainpro+ link.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de mai 2018 à la page e209.
This article has been peer reviewed.
References
- 1.Anthierens S, Tansens A, Petrovic M, Christiaens T. Qualitative insights into general practitioners views on polypharmacy. BMC Fam Pract. 2010;11:65. doi: 10.1186/1471-2296-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander GC, Sayla MA, Holmes HM, Sachs GA. Prioritizing and stopping prescription medicines. CMAJ. 2006;174(8):1083–4. doi: 10.1503/cmaj.050837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162(2):225–33. [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Institute for Health Information. Drug use among seniors on public drug programs in Canada, 2012. Ottawa, ON: Canadian Institute for Health Information; 2014. Available from: https://secure.cihi.ca/free_products/Drug_Use_in_Seniors_on_Public_Drug_Programs_2012_EN_web.pdf. Accessed 2018 Mar 20. [Google Scholar]
- 5.Vinkers CH, Olivier B. Mechanisms underlying tolerance after long-term benzodiazepine use: a future for subtype-selective GABA receptor modulators? Adv Pharmacol Sci. 2012;2012:416864. doi: 10.1155/2012/416864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell B, Tsang C, Raman-Wilms L, Irving H, Conklin J, Pottie K. What are priorities for deprescribing for elderly patients? Capturing the voice of practitioners: a modified Delphi process. PLoS One. 2015;10(4):e0122246. doi: 10.1371/journal.pone.0122246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 8.American Academy of Sleep Medicine . International classification of sleep disorders. Diagnostic and coding manual. Darien, IL: American Academy of Sleep Medicine; 2001. [Google Scholar]
- 9.Chung KF, Yeung WF, Ho FY, Yung KP, Yu YM, Kwok CW. Cross-cultural and comparative epidemiology of insomnia: the diagnostic and statistical manual (DSM), international classification of diseases (ICD) and international classification of sleep disorders (ICSD) Sleep Med. 2015;16(4):477–82. doi: 10.1016/j.sleep.2014.10.018. Epub 2015 Jan 21. [DOI] [PubMed] [Google Scholar]
- 10.Morin CM, LeBlanc M, Bélanger L, Ivers H, Mérette C, Savard J. Prevalence of insomnia and its treatment in Canada. Can J Psychiatry. 2011;56(9):540–8. doi: 10.1177/070674371105600905. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health. National Institutes of Health State-of-the-Science Conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep. 2005;28(9):1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 12.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24(11):1577–601. doi: 10.1177/0269881110379307. Epub 2010 Sep 2. [DOI] [PubMed] [Google Scholar]
- 14.Canadian Academy of Child and Adolescent Psychiatry, Canadian Academy of Geriatric Psychiatry, Canadian Psychiatric Association. Thirteen things physicians and patients should question. Toronto, ON: Choosing Wisely Canada; 2017. Available from: www.choosingwiselycanada.org/recommendations/psychiatry/. Accessed 2018 Mar 21. [Google Scholar]
- 15.Canadian Geriatrics Society. Geriatrics: five things physicians and patients should question. Toronto, ON: Choosing Wisely Canada; 2017. Available from: https://choosingwiselycanada.org/geriatrics/. Accessed 2018 Mar 21. [Google Scholar]
- 16.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46. doi: 10.1111/jgs.13702. Epub 2015 Oct 8. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg HC, Chiu TH. Time course for development of benzodiazepine tolerance and physical dependence. Neurosci Biobehav Rev. 1985;9(1):123–31. doi: 10.1016/0149-7634(85)90038-7. [DOI] [PubMed] [Google Scholar]
- 18.Sithamparanathan K, Sadera A, Leung L. Adverse effects of benzodiazepine use in elderly people: a meta-analysis. Asian J Gerontol Geriatr. 2012;7(2):107–11. [Google Scholar]
- 19.Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. doi: 10.1136/bmj.38623.768588.47. Epub 2005 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parr JM, Kavanagh DJ, Cahill L, Mitchell G, Young RM. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction. 2009;104(1):13–24. doi: 10.1111/j.1360-0443.2008.02364.x. Epub 2008 Oct 31. [DOI] [PubMed] [Google Scholar]
- 21.Gould RL, Coulson MC, Patel N, Highton-Williamson E, Howard RJ. Interventions for reducing benzodiazepine use in older people: meta-analysis of randomised controlled trials. Br J Psychiatry. 2014;204(2):98–107. doi: 10.1192/bjp.bp.113.126003. [DOI] [PubMed] [Google Scholar]
- 22.Voshaar RC, Couvée JE, van Balkom AJ, Mulder PG, Zitman FG. Strategies for discontinuing long-term benzodiazepine use: meta-analysis. Br J Psychiatry. 2006;189:213–20. doi: 10.1192/bjp.189.3.213. [DOI] [PubMed] [Google Scholar]
- 23.Pollmann AS, Murphy AL, Bergman JC, Gardner DM. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol. 2015;16(1):19. doi: 10.1186/s40360-015-0019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishnan K, Scheid DC. Treatment options for insomnia. Am Fam Physician. 2007;76(4):517–26. [PubMed] [Google Scholar]
- 25.Schünemann HJ, Wierioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014;186(3):E123–42. doi: 10.1503/cmaj.131237. Epub 2013 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrell B, Pottie K, Rojas-Fernandez CH, Bjerre LM, Thompson W, Welch V. Methodology for developing deprescribing guidelines: using evidence and GRADE to guide recommendations for deprescribing. PLoS One. 2016;11(8):e0161248. doi: 10.1371/journal.pone.0161248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curran HV, Collins R, Fletcher S, Kee SE, Woods B, Iliffe S. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33(7):1223–37. doi: 10.1017/s0033291703008213. [DOI] [PubMed] [Google Scholar]
- 29.Morgan S, Smolina K, Mooney D, Raymond C, Bowen M, Gorczynski C, et al. The Canadian Rx atlas. 3rd ed. Vancouver, BC: University of British Columbia, Centre for Health Services and Policy Research; 2013. [Google Scholar]
- 30.Baillargeon L, Landreville P, Verreault R, Beauchemin J, Grégoire JP, Morin CM. Discontinuation of benzodiazepines among older insomniac adults treated with cognitive-behavioural therapy combined with gradual tapering: a randomized trial. CMAJ. 2003;169(10):1015–20. [PMC free article] [PubMed] [Google Scholar]
- 31.Belleville G, Guay C, Guay B, Morin CM. Hypnotic taper with or without self-help treatment of insomnia: a randomized clinical trial. J Consult Clin Psychol. 2007;75(2):325–35. doi: 10.1037/0022-006X.75.2.325. [DOI] [PubMed] [Google Scholar]
- 32.Garfinkel D, Zisapel N, Wainstein J, Laudon M. Facilitation of benzodiazepine discontinuation by melatonin: a new clinical approach. Arch Intern Med. 1999;159(20):2456–60. doi: 10.1001/archinte.159.20.2456. [DOI] [PubMed] [Google Scholar]
- 33.Habraken H, Soenen K, Blondeel L, Van Elsen J, Bourda J, Coppens E, et al. Gradual withdrawal from benzodiazepines in residents of homes for the elderly: experience and suggestions for future research. Eur J Clin Pharmacol. 1997;51(5):355–8. doi: 10.1007/s002280050213. [DOI] [PubMed] [Google Scholar]
- 34.Morin CM, Bastien C, Guay B, Radouco-Thomas M, Leblanc J, Vallières A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161(2):332–42. doi: 10.1176/appi.ajp.161.2.332. [DOI] [PubMed] [Google Scholar]
- 35.Pat-Horenczyk R, Hacohen D, Herer P, Lavie P. The effects of substituting zopiclone in withdrawal from chronic use of benzodiazepine hypnotics. Psychopharmacology (Berl) 1998;140(4):450–7. doi: 10.1007/s002130050789. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro CM, Sherman D, Peck DF. Withdrawal from benzodiazepines by initially switching to zopiclone. Eur Psychiatry. 1995;10(Suppl 3):145s–51s. doi: 10.1016/0924-9338(96)80096-4. [DOI] [PubMed] [Google Scholar]
- 37.Vissers FHJA, Knipschild PG, Crebolder HFJM. Is melatonin helpful in stopping the long-term use of hypnotics? A discontinuation trial. Pharm World Sci. 2007;29(6):641–6. doi: 10.1007/s11096-007-9118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voshaar RCO, Gorgels WJ, Mol AJ, van Balkom AJ, van de Lisdonk EH, Breteler MH, et al. Tapering off long-term benzodiazepine use with or without group cognitive−behavioural therapy: three-condition, randomised controlled trial. Br J Psychiatry. 2003;182:498–504. doi: 10.1192/bjp.182.6.498. [DOI] [PubMed] [Google Scholar]
- 39.Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919–34. doi: 10.1517/14740338.2014.925444. Epub 2014 Jun 6. [DOI] [PubMed] [Google Scholar]
- 40.Okuji Y, Matsuura M, Kawasaki N, Kometani S, Shimoyama T, Sato M, et al. Prevalence of insomnia in various psychiatric diagnostic categories. Psychiatry Clin Neurosci. 2002;56(3):239–40. doi: 10.1046/j.1440-1819.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- 41.Neylan T, Reynolds C, Kupfer D. Sleep disorders. In: Hales RE, Yudofsky SC, editors. Textbook of clinical psychiatry. Washington, DC: American Psychiatric Publishing; 2003. pp. 975–1000. [Google Scholar]
- 42.Wooten V, Buysse D. Sleep in psychiatric disorders. In: Chokroverty S, editor. Sleep disorders medicine: basic sciences, technical considerations, and clinical aspects. 3rd ed. New York, NY: Saunders; 2009. [Google Scholar]
- 43.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146(2):105–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 44.Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry. 2000;41(6):469–78. doi: 10.1053/comp.2000.16568. [DOI] [PubMed] [Google Scholar]
- 45.Pigeon W. Insomnia as a risk factor for disease. In: Sateia MJ, Buysse DJ, editors. Insomnia: diagnosis and treatment. London, UK: Informa Healthcare; 2010. pp. 31–41. [Google Scholar]
- 46.Chokroverty S. Clinical companion to sleep disorders medicine. 2nd ed. Boston MA: Butterworth-Heinemann; 2000. [Google Scholar]
- 47.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. doi: 10.1378/chest.09-0791. Epub 2009 Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merlino G, Gigli GL, Valente M. Sleep disturbances in dialysis patients. J Nephrol. 2008;21(Suppl 13):S66–70. [PubMed] [Google Scholar]
- 49.De Santo RM, Bartiromo M, Cesare CM, Cirillo M. Sleep disorders occur very early in chronic kidney disease. J Nephrol. 2008;21(Suppl 13):S59–65. [PubMed] [Google Scholar]
- 50.Grenier J. A decision tree approach to the differential diagnosis of insomnia. In: Billard M, editor. Sleep: physiology, investigations and medicine. New York, NY: Springer; 2003. pp. 191–9. [Google Scholar]
- 51.Jansen J, Naganathan V, Carter SM, McLachlan AJ, Nickel B, Irwig L, et al. Too much medicine in older people? Deprescribing through shared decision making. BMJ. 2016;353:i2893. doi: 10.1136/bmj.i2893. [DOI] [PubMed] [Google Scholar]
- 52.Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30(10):793–807. doi: 10.1007/s40266-013-0106-8. [DOI] [PubMed] [Google Scholar]
- 53.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890–8. doi: 10.1001/jamainternmed.2014.949. [DOI] [PubMed] [Google Scholar]
- 54.Vicens C, Bejarano F, Sempere E, Mateu C, Fiol F, Socias I, et al. Comparative efficacy of two interventions to discontinue long-term benzodiazepine use: cluster randomised controlled trial in primary care. Br J Psychiatry. 2014;204(6):471–9. doi: 10.1192/bjp.bp.113.134650. Epub 2014 Feb 13. [DOI] [PubMed] [Google Scholar]
- 55.Schweizer E, Rickels K, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines. II. Effects of gradual taper. Arch Gen Psychiatry. 1990;47(10):908–15. doi: 10.1001/archpsyc.1990.01810220024003. [DOI] [PubMed] [Google Scholar]
- 56.Denis C, Fatseas M, Lavie E, Auriacombe M. Pharmacological interventions for benzodiazepine mono-dependence management in outpatient settings. Cochrane Database Syst Rev. 2013;(6):CD005194. doi: 10.1002/14651858.CD005194.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gorgels WJ, Oude Voshaar RC, Mol AJ, van de Lisdonk EH, van Balkom AJ, Breteler MH, et al. Predictors of discontinuation of benzodiazepine prescription after sending a letter to long-term benzodiazepine users in family practice. Fam Pract. 2006;23(1):65–72. doi: 10.1093/fampra/cmi065. Epub 2005 Aug 17. [DOI] [PubMed] [Google Scholar]
- 58.Cormack MA, Owens RG, Dewey ME. The effect of minimal interventions by general practitioners on long-term benzodiazepine use. J R Coll Gen Pract. 1989;39(327):408–11. [PMC free article] [PubMed] [Google Scholar]
- 59.Morin CM, Bélanger L, Bastien C, Vallières A. Long-term outcome after discontinuation of benzodiazepines for insomnia: a survival analysis of relapse. Behav Res Ther. 2005;43(1):1–14. doi: 10.1016/j.brat.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 60.O’Connor K, Marchand A, Brousseau L, Aardema F, Mainguy N, Landry P, et al. Cognitive-behavioural, pharmacological and psychosocial predictors of outcome during tapered discontinuation of benzodiazepine. Clin Psychol Psychother. 2008;15(1):1–14. doi: 10.1002/cpp.556. [DOI] [PubMed] [Google Scholar]
- 61.Voshaar RC, Gorgels WJ, Mol AJ, van Balkom AJ, Mulder J, van de Lisdonk EH, et al. Predictors of long-term benzodiazepine abstinence in participants of a randomized controlled benzodiazepine withdrawal program. Can J Psychiatry. 2006;51(7):445–52. doi: 10.1177/070674370605100706. [DOI] [PubMed] [Google Scholar]
- 62.Soldatos CR, Dikeos DG, Whitehead A. Tolerance and rebound insomnia with rapidly eliminated hypnotics: a meta-analysis of sleep laboratory studies. Int Clin Psychopharmacol. 1999;14(5):287–303. [PubMed] [Google Scholar]
- 63.Van Rijnsoever C, Täuber M, Choulli MK, Keist R, Rudolph U, Mohler H, et al. Requirement of α5-GABA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci. 2004;24(30):6785–90. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Busto U, Sellers EM, Naranjo CA, Cappell H, Sanchez-Craig M, Sykora K. Withdrawal reaction after long-term therapeutic use of benzodiazepines. N Engl J Med. 1986;315(14):854–9. doi: 10.1056/NEJM198610023151403. [DOI] [PubMed] [Google Scholar]
- 65.Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47(10):899–907. doi: 10.1001/archpsyc.1990.01810220015002. [DOI] [PubMed] [Google Scholar]
- 66.Qaseem A, Kansagara D, Forciea M, Cooke M, Denberg TD, Clinical Guidelines Committee of the American College of Physicians Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–33. doi: 10.7326/M15-2175. Epub 2016 May 3. [DOI] [PubMed] [Google Scholar]
- 67.Agency for Healthcare Research and Quality . Effective Health Care Program: management of insomnia disorder. Rockville, MD: Agency for Healthcare Research and Quality; 2015. Available from: https://effectivehealthcare.ahrq.gov/sites/default/files/related_files/insomnia_executive.pdf. Accessed 2018 Mar 22. [Google Scholar]
- 68.Buysse DJ. Insomnia. JAMA. 2013;309(7):706–16. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seyffert M, Lagisetty P, Landgraf J, Chopra V, Pfeiffer PN, Conte ML, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139. doi: 10.1371/journal.pone.0149139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 71.Get your sleep back. Sleepwell Nova Scotia [website]; 2018. Available from: http://sleepwellns.ca/get-your-sleep-back. Accessed 2018 Mar 22.
- 72.Ritterband LM, Thorndike FP, Gonder-Frederick LA, Magee JC, Bailey ET, Saylor DK, et al. Efficacy of an Internet-based behavioral intervention for adults with insomnia. Arch Gen Psychiatry. 2009;66(7):692–8. doi: 10.1001/archgenpsychiatry.2009.66. Erratum in: Arch Gen Psychiatry 2010;67(3):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tyrer P, Murphy S, Riley P. The benzodiazepine withdrawal symptom questionnaire. J Affect Disord. 1990;19(1):53–61. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- 74.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–9. doi: 10.1016/j.jad.2011.01.011. Epub 2011 Feb 5. [DOI] [PubMed] [Google Scholar]
- 75.Roth T, Jaeger S, Jin R, Kalsekar A, Stang PE, Kessler RC. Sleep problems, comorbid mental disorders, and role functioning in the National Comorbidity Survey Replication. Biol Psychiatry. 2006;60(12):1364–71. doi: 10.1016/j.biopsych.2006.05.039. Epub 2006 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chung KH, Li CY, Kuo SY, Sithole T, Liu WW, Chung MH. Risk of psychiatric disorders in patients with chronic insomnia and sedative-hypnotic prescription: a nationwide population-based follow-up study. J Clin Sleep Med. 2015;11(5):543–51. doi: 10.5664/jcsm.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bateson AN. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des. 2002;8(1):5–21. doi: 10.2174/1381612023396681. [DOI] [PubMed] [Google Scholar]
- 78.Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord. 2009;117(Suppl 1):S26–43. doi: 10.1016/j.jad.2009.06.041. Epub 2009 Aug 11. [DOI] [PubMed] [Google Scholar]
- 79.Bandelow B, Lichte T, Rudolf S, Wiltink J, Beutel ME. The German guidelines for the treatment of anxiety disorders. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):363–73. doi: 10.1007/s00406-014-0563-z. [DOI] [PubMed] [Google Scholar]
- 80.Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1. doi: 10.1186/1471-244X-14-S1-S1. Epub 2014 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403–39. doi: 10.1177/0269881114525674. Epub 2014 Apr 8. [DOI] [PubMed] [Google Scholar]
- 82.Canadian Medical Protective Association [website]. Risk management in elderly patients: medication issues. Ottawa, ON: Canadian Medical Protective Association; 2011. Available from: www.cmpa-acpm.ca/en/advice-publications/browse-articles/2011/risk-management-in-elderly-patients-medication-issues. Accessed 2018 Mar 22. [Google Scholar]
- 83.Canadian Medical Protective Association [website]. Adverse events—physician-prescribed opioids. Ottawa, ON: Canadian Medical Protective Association; 2009. Available from: www.cmpa-acpm.ca/en/advice-publications/browse-articles/2009/adverse-events-physician-prescribed-opioids. Accessed 2018 Mar 27. [Google Scholar]
- 84.Greenfield D, Brown J. Current medicolegal status of prescribing benzodiazepines: a special case. Natl Crim Justice Ref Serv. 1995:41–52. [Google Scholar]
- 85.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. The Global Rating Scale complements the AGREE II in advancing the quality of practice guidelines. J Clin Epidemiol. 2012;65(5):526–34. doi: 10.1016/j.jclinepi.2011.10.008. Epub 2011 Dec 19. [DOI] [PubMed] [Google Scholar]
- 86.Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148–57. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- 87.Gillespie LD, Robertson M, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coussement J, De Paepe L, Schwendimann R, Denhaerynck K, Dejaeger E, Milisen K. Interventions for preventing falls in acute- and chronic-care hospitals: a systematic review and meta-analysis. J Am Geriatr Soc. 2008;56(1):29–36. doi: 10.1111/j.1532-5415.2007.01508.x. Epub 2007 Nov 20. [DOI] [PubMed] [Google Scholar]