Abstract
Aim:
The aim of this study is to find out the anti-Alzheimer’s activity of isolated karanjin and embelin.
Materials and Methods:
Karanjin isolated from Pongamia pinnata (L.) pierre and embelin from Embelia ribes Burm.f. and their purity was confirmed by ultraviolet spectrophotometric and Thin layer chromatography based study. Anti-Alzheimer’s activity of isolated compounds were evaluated through elevated plus maze and Morris water maze model on Swiss albino mice. Diazepam (1 mg/kg body weight, intraperitoneally) was used for the induction of Alzheimer’s like effects (amnesia) on Swiss albino mice and piracetam (200 mg/kg body weight, oral) used as a standard treatment.
Results:
In EPM, embelin and karanjin decrease the transfer latency time in dose dependent manner and escape latency time in MWM method. A significant (P < 0.01) reduction in amnesia with an anti-Alzheimer’s effect found when results of isolated compounds were compared with standard and vehicle control. Diazepam (1 mg/kg) treated group showed significant increase in escape latency and transfer latency when compared with vehicle control; which indicates impairment in learning and memory.
Conclusion:
Both isolated compounds and standard significantly reversed the amnesia induced by diazepam and improved learning and memory of mice in dose and time dependent manner. This study supports the ethnobotanical use of these two plants in India for the management of nerve or brain related problems.
Keywords: Anti-Alzheimer’s, Diazepam, Embelia ribes, Embelin, Karanjin, Pongamia pinnata
Introduction
Alzheimer’s, the most common form of dementia and also a progressive neurodegenerative disorder mostly affects the older people.[1] Several mechanisms contribute to the development of Alzheimer’s they include oxidative stress, senile plaque deposition, cholinergic deficit, neurofibrillary tangle formation,[2,3] cerebral oxidative stress, neuroinflammation, cholinergic dysfunction; accompanied by psychological and pathophysiological complications such as anxiety, depression, concentration problems and motor disturbances.[4]
According to the National Institute on Aging “in USA Alzheimer’s is the third cause of death in older people”[5] and according to the Alzheimer’s Association of India, there are 44 million people in the World living with dementia and more than 4 million people have some form of dementia in India.[6] At present, the Food and Drug Administration approved two classes of drugs to treat the cognitive symptoms of Alzheimer’s Cholinesterase inhibitors (generally used to treat mild-to-moderate Alzheimer’s disease symptoms) and N-methyl-D-aspartate receptor antagonists (used to treat moderate-to-severe Alzheimer’s condition).[7] Many other types of allopathic drugs (Donepezil, rivastigmine, galantamine, tacrine and memantine) are also used to treat symptoms of Alzheimer’s. Despite the availability of a wide range of drugs in the allopathic medicine system, most of the drugs are employed for symptomatic relief and are short lived and more over these drugs produce severe side effects[8,9] such as gastrointestinal pain, liver problems, nausea, diarrhea and severe vomiting etc., In addition, memantine use has been associated with side effects, such as mental confusion, constipation, dizziness and headache.[10]
In this study, we have selected two Indian traditional medicinal plants (Embelia ribes Burm.f. and Pongamia pinnata (L.) pierre), which are used traditionally to treat mental problems and also used as brain tonic in different areas of India.[11,12,13] Embelin (benzoquinone) from E. ribes and karanjin (flavonoid) from P. pinnata were isolated for their medicinal use validation in the management of neurodegenerative diseases. Previous studies on these plants showed there antioxidant, antistress, neuroprotective and antianxiety activity with low/negligible toxicity.[14,15,16,17,18,19]
Materials and Methods
Plant material and extraction
Authenticated seeds of P. pinnata and fruits of E. ribes were procured from “Jankalyan herbs,” E7, Industrial area, Haridwar, Uttrakhand, India. Plant material was grinded and then extracted from powdered form of plant material by Soxhlet extraction method.[20,21]
Isolation of karanjin from Pongamia pinnata extract
Coarsely powdered seeds of P. pinnata (300 g) were boiled in 1 lt. of methanol for 3 h. An oily residue obtained after 3 h. This oily residue was washed twice with petroleum ether and after washing a yellow thick residue was obtained, which was dissolved in methanol and kept for 48 h. After 48 h, white precipitate was obtained, which was again dissolved in minimum quantity of methanol; after filtration, it was dried and was recrystallize again with methanol.[20]
Isolation of embelin from Embelia ribes
Coarsely powdered fruits (500 g) of E. ribes were extracted for 12 h in Soxhlet apparatus with n-hexane. After 12 h of extraction, solvent was evaporated using steam bath and petroleum ether was added in the residue and was filtered. After filtration, remaining residue was mixed with cold petroleum ether in separating funnel and it was again filtered. After that, filtrate residue was dried and crystallized with chloroform to obtain pure embelin.[21,22]
Identification of karanjin and embelin
After isolation, karanjin and embelin were identified and confirmed using ultraviolet (UV)-spectrometer (Shimadzu model no – 1700) followed by thin-layer chromatography (TLC). Dried isolated compounds were dissolved in ethanol and was then scanned at 200–400 nm wavelengths in UV-spectrometer. In TLC, silica gel was used as a stationary phase, N-propanol: butanol: ammonia (11:3:6) was used as a mobile phase for embelin and two mobile phases toulene: Ethyl acetate (16:4) and ethyl acetate: methanol: distilled water (10:3:7) was used for Karanjin. Then, both the isolated compounds were compared with standard compounds for their confirmation.[22,23,24]
Drugs and dose selection
For this study, doses of isolated compounds and drugs were selected on the basis of previous studies.[18,25,26,27] For induction of amnesia, 1 mg/kg intraperitoneally diazepam (10 mg anxol injection, Svizera Healthcare) and piracetam (200 mg/kg body weight, intraperitoneally) was used as standard. Karanjin (25 and 50 mg/kg body weight) and embelin (100 and 200 mg/kg body weight) were given orally.
Animals
Male Swiss albino mice (20–25 g) were used for this study. All experimental procedure were reviewed and approved by the Institutional ethical committee (1145/Po/a/07/CPCSEA) for ethical use of animals. All animals were housed in groups of six mice per cages and maintained under standard environmental condition 25°C ± 2°C temperature, 12:12 h light and dark cycle, and 45%–55% relative humidity, with free access of food and water (ad libitum). Food and water was withdrawn during the experiment. All the experiments were carried out during the light period (0800:1600 h).
Treatment schedule
Animals were divided in 2 group (Group 1: Karanjin and Group 2: Embelin), each group consist of 5 subgroups; in each subgroup 6 animals were used. Subgroup 01 (vehicle control: Acacia suspension 1 ml/kg oral), subgroup 02 (diazepam control: 1 mg/kg intraperitoneal), subgroup 3 (karanjin 25 mg/kg or embelin 100 mg/kg, oral), subgroup 4 (50 mg/kg karanjin or 200 mg/kg embelin oral) and subgroup 5 standard (piracetam 200 mg/kg intraperitoneal).
Anti-Alzheimer’s activity
Anti-Alzheimer’s activity of karanjin and embelin were studied by in vivo diazepam-induced amnesia on mice and the effect of isolated compounds elevated by plus maze model and Morris water maze (MWM) model.
Elevated plus maze test
Elevated plus maze (EPM) consist of four arms elevated 30 cm above the floor, with each arm positioned at 90° relative to the adjacent arms. Two of the arms were enclosed with high walls (30 cm × 7 cm × 20 cm), and the other arms were connected through a central area (7 cm × 7 cm) to form a plus sign. The maze floor and the walls of enclosed arms were painted black. Animals were treated 60 min before the test with vehicle, isolated test compound, piracetam and diazepam. Each mouse was individually placed on the central platform facing toward an open arm. The frequency and duration of entries into the open and closed arms were observed for 5 min. An entry was counted when all four paws of the mouse entered an open or closed arm. Subsequently, the percentage of time spent (duration) in the open arms and percentage of the number of open arm entries were calculated for each animal. The apparatus was thoroughly cleaned after each trial. Diazepam and isolated compound (karanjin and embelin) dose was continued for up to 8 days and daily readings are taken on EPMT. On the 9th day, the readings were taken without dosing, for evaluating the transfer latency keeping the period of 60 s as a cutoff criterion.[28]
Morris water maze test
MWM is a swimming-based model where the animal learns to escape on to a hidden platform. It consist of a round pool, about 6 feet in diameter and about 3 feet deep. It was filled with tap water, which should be close to 26°C. The tank was divided into four equal quadrants with the help of two threads fixed at right angles to each other on the rim of the pool. Escape platform was placed in the center of the pool. During training, it must be exposed, one inch above the water, that teaches the mouse that there was a platform and that was the way to get out of the water. Later, after the animal was trained and ready for testing, the escape platform would be just below the surface of the water and not be visible because the water should be made opaque with milk. The mouse was gently placed in the water between the quadrants, facing the wall of the pool, with the drop location changing for each trial and allowed 120 sec. to locate the submerged platform. Then, it was allowed to stay on the platform for 20 sec. If it failed to find the platform within 120 s, it was gently guided onto the platform and allowed to remain there for 20 sec. On the day 4, escape latency time to locate the hidden platform in the water maze was noted as index of acquisition or learning. Dose of diazepam and herbal isolates (karanjin and embelin) were continued till 8 days. On the 9th day, the readings were taken without dosing to evaluate the retention memory of animals.[29,30]
Statistical analysis
All the results were expressed as mean ± standard error of the mean. The data was analyzed using ANOVA and Student’s (unpaired) t-test. P < 0.01 was considered as statistically significant.
Results
Percentage yield of isolated compounds
Needle-shaped off-white crystals of karanjin and glistening orange color embelin were isolated. 0.9% karanjin and 2.6% embelin were isolated from P. pinnata and E. ribes, respectively.
Determination of karanjin and embelin by TLC
Rf values of isolated compounds were compared with standard compounds revealed that, both isolated test compounds showed similar Rf value as standard compounds. From Figure 1a and b, karanjin shows Rf value 0.69 (Ethanol: Ethyl acetate – 16:4 v/v), 0.71 (Ethyl acetate: Methanol: Distilled water – 10:3:7) and embelin showed Rf value 0.36 (N-propanol: Butanol: Ammonia – 11: 3:6).
Figure 1.
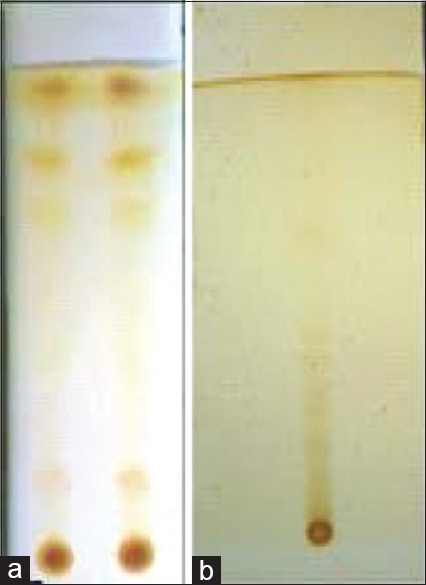
(a) Thin-layer chromatography fingerprint (Rf = 0.69 and 0.71) of karanjin in Ethanol: ethyl acetate (16:4 v/v) and Ethyl acetate: methanol: distilled water (10:3:7) solvent system, (b) Thin-layer chromatography fingerprint of Embelin (Rf = 0.36) in N-propanol: butanol: ammonia (11: 3:6) solvent system
UV spectral analysis of karanjin and embelin
Isolated karanjin and embelin showed λmax at 259 nm and 291 nm, which was comparable to that of standard karanjin and embelin. Isolated concentration of karanjin was 0–20 μg/ml and embelin was 0–7 μg/ml found in extract, when compared with standard.
Anti-Alzheimer’s activity
Embelin and karanjin show dose and time dependent memory and learning behavior changes on amnesia-induced mice when compared to piracetam-treated group. Both compounds revert the effects of diazepam-induced amnesia on EPM test and MWM test.
Effect of karanjin and embelin on elevated plus maze test
From Table 1, effect of vehicle, diazepam, piracetam (200 mg/kg), karanjin (25 and 50 mg/kg) and embelin (100 and 200 mg/kg) on transfer latency were evaluated on day 1, day 5 and at the end of 8th day. Transfer latency on the 9th day without drug treatments reflected the retention memory of animals. Diazepam (1 mg/kg i. p.)-treated group showed significant increase in transfer latency on the day 1, 5 and 8 when compared with vehicle control mice indicating impairment in learning and memory. Karanjin (25 and 50 mg/kg p. o.) and embelin (100 and 200 mg/kg) decreased the transfer latency on all the observation days. Both isolated compound decrease transfer latency in dose- and time-dependent manner [Figure 2] when compared with piracetam (200 mg/kg i. p.), karanjin at 50 mg/kg (5.66 ± 0.33) showed decrease in transfer latency, which is equal to piracetam-treated group on day 8 (5.66 ± 0.31); embelin (200 mg/kg body weight) also showed a good decrease in transfer latency (6.16 ± 0.60) on day 8th. Piracetam (200 mg/kg ip) and both isolated compound significantly (P < 0.01) reversed diazepam-induced amnesia when compared with standard and control. This indicates improved learning and memory in treated mice.
Table 1.
Effect of karanjin and embelin on plus maze test
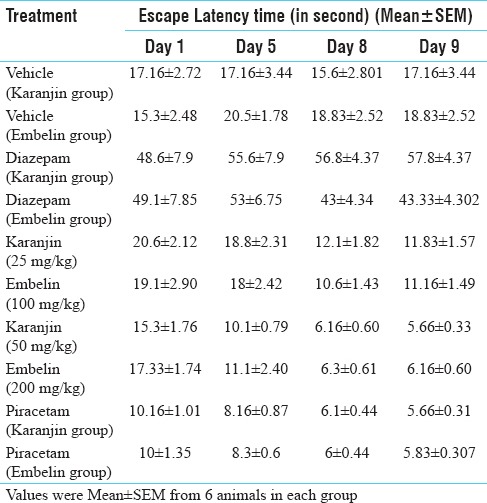
Figure 2.
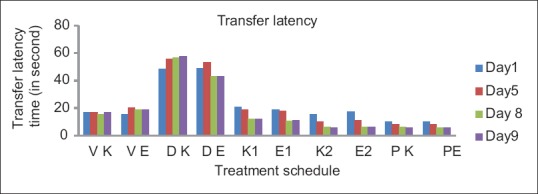
Effect of karanjin and embelin on transfer latency. Data are expressed as means ± standard error of the mean (n = 6), P < 0.01, when compared with the control and standard group
Effect of karanjin and embelin on Morris water maze test
Effect of vehicle, diazepam, piracetam (200 mg/kg i. p.), karanjin (25 and 50 mg/kg p. o) and embelin (100 and 200 mg/kg) on retention of memory were evaluated on day 1st, 5th and day 8th [Table 2]. Escape latency on day 9th, without drug treatment reflected the retention memory of the animals. Diazepam (1 mg/kg ip)-treated group showed significant increase in escape latency on day 1st, 5th and day 8th when compared with vehicle control mice indicating impairment in learning and memory. It was evident [from the Table 2 and Figure 3] that karanjin, embelin and piracetam significantly (P < 0.01) decreased the escape latency in a dose dependent manner, when compared with control and standard. Embelin (200 mg/kg) and karanjin (50 mg/kg) showed approximately similar activity as that of standard drug piracetam (200 mg/kg) on day 9th. Both the isolated compound and standard significantly reversed the amnesia induced by diazepam indicating improved learning and memory in mice.
Table 2.
Effect of karanjin and embelin on Morris water maze test
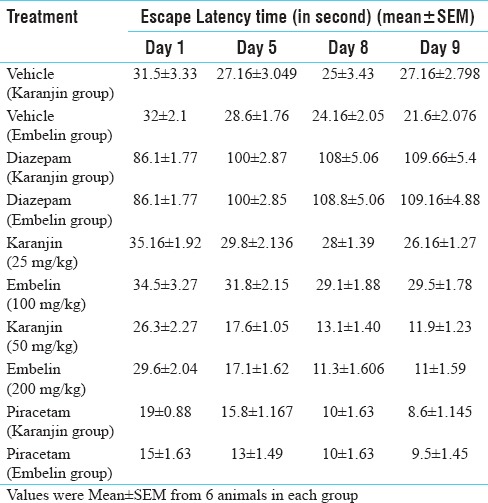
Figure 3.
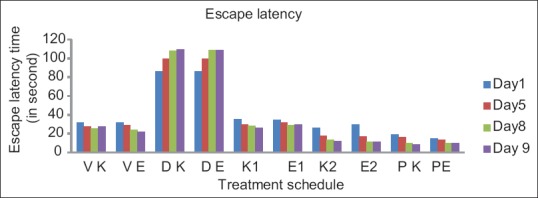
Effect of karanjin and embelin on escape latency. Data are expressed as means ± standard error of the mean (n = 6), P < 0.01 when compared with the control and standard group. where VK – Vehicle for karanjin group, VE – Vehicle for embelin group, DK – Diazepam for karanjin group, DE – Diazepam for embelin group, K1 – Karanjin (25 mg/kg), K2 – Karanjin (50 mg/kg), E1 – Embelin (100 mg/kg), E2 – Embelin (200 mg/kg), PK – Piracetam for karanjin group, PE - Piracetam for embelin group
Discussion
Neurodegenerative disorders are becoming major issues in aged peoples or in working peoples in industrialized countries. Oxidative stress of today’s lifestyle is a common causative factor for neurodegenerative disorders;[3] illness is another factor for oxidative stress. Many scientific studies indicate that in neurodegenerative disorder, oxidative stress induces overproduction of reactive oxygen species and reactive nitrogen species; this type of molecules damage protein, lipid and nucleic acids.[31,32,33] Brain tissues are extremely sensitive to oxidative stress due to its high oxygen consumption, iron content, polyunsaturated fatty acids and low antioxidant capacity.[34,35] Moreover, the hippocampus and amygdala are more sensitive to oxidative injury[36] and excessive oxidative stress on these soft tissues can lead to memory deficits by impairing hippocampal synaptic plasticity.[37] Now a days natural drugs or natural drug sources are seeking attention as complementary and alternative medicine for neurodegenerative disorders. At present, galantamine (from bulbs of Galanthus caucasicus), a plant derived alkaloid is used to treat vascular dementia and Alzheimer’s.[38]
In some parts of India, P. pinnata and E. ribes are used by the traditional healers to treat nerve and memory related disorders, but till now, there is no valid scientific proof behind their effective use in memory related disorders. Some earlier studies on E. ribes shows its antianxiety, antidepressant, antioxidant, neuroprotective[9,11,12] and anticonvulsant activity.[11] Embelin from E. ribes shows neuroprotective,[13] antioxidant[14,39,40] and anticonvulsant effect.[15] Karanjin from seeds of P. pinnata inhibits oxidative stress[17] and showed antioxidant activity and tissue protectant activity.[41,42]
Alzheimer’s is a type of dementia that causes problems with memory, thinking, and behavior.[6] This disease is mainly related with memory and behavior disturbance, so in present study, it has focused on exploring the potentials of karanjin and embelin in reversing the memory deficits. Anti-Alzheimer’s effects of embelin and karanjin were studied on a diazepam induced amnesia mouse model. MWM and EPM model was used to evaluate the effects of embelin and karanjin on memory and learning behavior of diazepam treated mouse model. Diazepam, a long acting classical benzodiazepine, exhibits anxiolytic, sedative, muscle relaxant and anticonvulsant effect. The administration of diazepam produces transient memory deficit and causes amnesia. Amnesia induced by diazepam is widely used as a primary screening test for anti-Alzheimer’s agents and amnesic effects may be associated with inappropriate behavior.[43] Diazepam induces amnesia at higher dose (1 mg/kg) by reduction of hippocampal corticosterone concentration and at lower dose (0.5 and 0.25 mg/kg) it revert the symptoms of amnesia or shows memory enhancing effect on mice.[44] Diazepam acts on the brain hippocampus as GABA ergic agent[45,46] or work as benzodiazepine receptor agonist.[49,50] In some studies, diazepam showed recurrent inhibition of pyramidal neuron firing in a dose dependent manner in rat hippocampal slices[51] during amnesic effect[45,46] and reduced rat hippocampus paired-pulse inhibition.[46] Diazepam reduces SOD activity of rat brain[48] and antioxidant defense (TBARS, isozymes of superoxide dismutase and glutathione reductase) in the brain cerebellum and brain stem.[47]
Brain hippocampus is the main part of the brain, which is responsible for memory and limbic function of animal. Neurological and memory disorders are linked with hippocampal damage by accident or by free-radical generation in the brain during diseased condition. Diazepam induces amnesia or amnesia-like effects by acting on brain hippocampus or by disturbing brain antioxidant homeostasis. Antioxidant activity of neuroprotective drugs plays a significant role during reversion of neurodegenerative effects. Isolated compounds of this study: Embelin (benzoquinone) a phenolic compound and karanjin (flavonoid) both have free radical neutrilizing activity or antioxidant activity.[14,39,40,41,42]
Results of this study showed significant (P < 0.01) anti-Alzheimer’s activity of isolated compounds karanjin and embelin. TLC and UV-spectral study shows that both isolated compounds are homogenous and pure, when compared with standard compounds. Isolated karanjin showed λmax at 259 nm and embelin showed λmax at 291 nm. Some earlier studies also find λmax at same wavelength.[27,28,29] Diazepam (1 mg/kg body weight) induces dementia or loss of memory followed by increase in transfer latency and escape latency in diazepam-treated animals. Piracetam (200 mg/kg), karanjin (25, 50 mg/kg) and embelin (100, 200 mg/kg p. o.) demonstrated improvement in learning and memory and inhibited the symptoms of diazepam induced memory loss in experimental animals. Karanjin (25, 50 mg/kg) and embelin (100, 200 mg/kg) pretreatment for 8 days decreased the transfer latency on the 8th and 9th day. Decrease in transfer latency on day 8 indicates the positive effect of isolated compounds on the learning process while decrease in transfer latency on the 9th day indicates retention of memory. Both these parameters were increased in a dose dependent manner in EPM model. In MWM, both isolated compounds (karanjin and embelin) decreased the escaped latency in dose dependent manner on day 8 and day 9; this indicates learning behavior and retention of memory, respectively.
The findings of this study suggests the possible neuroprotective role of these compounds and their source plants in neurodegenerative problems. Therefore, these two plants or both isolated compounds of this study may prove useful in the treatment of the patients suffering with Alzheimer disease and memory related disorders. However, further investigation needs to be warranted to explore the possible involvement of these plants on the neurotransmitter release. Furthermore, the plants may be further investigated for establishing the detailed mechanism of action.
Conclusion
In this study, karanjin and embelin showed learning and memory improvement in a dose-dependent manner. This finding suggests that both isolated compounds (and their source plant) can be used in the treatment of Alzheimer’s and other neurodegenerative disease. This study also supports traditional use of P. pinnata and E. ribes in neurodegenerative or memory related disorders. Hence, these two medicinal plants can be used as a valuable source of therapeutic agents for neurodegenerative disease management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Beuscher L, Beck C. A literature review of spirituality in coping with early-stage Alzheimer's disease. J Clin Nurs. 2008;17:88–97. doi: 10.1111/j.1365-2702.2007.02126.x. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Parikh V, Singh M. Pharmacological basis of drug therapy of Alzheimer's disease. Indian J Exp Biol. 1997;35:1146–55. [PubMed] [Google Scholar]
- 3.Ismail MS, Connie B, Kimberly M. Benefits of early Pharmacological treatment in Alzheimer Disease Psychiatric Times; 1 April. 2007. [Last accessed on 2016 Mar 04]. Available from: http://www.psychiatrictimes.com/articles/benefits-early-pharmacological-treatment-alzheimer-disease .
- 4.Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–80. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- 5.NIH. USA: National Institute on Aging; 2016. [Last accessed on 2016 Mar 05]. Available from: https://www.nia.nih.gov/alzheimers/publication/alzheimers-disease-fact-sheet . [Google Scholar]
- 6.Chicago: Alzheimer's Association; 2016. [Last accessed on 2016 Mar 05]. c2016. Available from: http://www.alz.org/in/dementia-alzheimers-en.asp . [Google Scholar]
- 7.Osborn GG, Saunders AV. Current treatments for patients with Alzheimer disease. J Am Osteopath Assoc. 2010;110:S16–26. [PubMed] [Google Scholar]
- 8.Schneider LS, Insel PS, Weiner MW Alzheimer's Disease Neuroimaging Initiative. Treatment with cholinesterase inhibitors and memantine of patients in the Alzheimer's disease neuroimaging initiative. Arch Neurol. 2011;68:58–66. doi: 10.1001/archneurol.2010.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase inhibitors in mild cognitive impairment: A systematic review of randomised trials. PLoS Med. 2007;4:e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowotny P, Kwon JM, Goate AM. Chichester: John Wiley & Sons Ltd; 2001. Alzheimer Disease eLS. [Google Scholar]
- 11.Thippeswamy BS, Nagakannan P, Shivasharan BD, Mahendran S, Veerapur VP, Badami S, et al. Protective effect of embelin from Embelia ribes burm. Against transient global ischemia-induced brain damage in rats. Neurotox Res. 2011;20:379–86. doi: 10.1007/s12640-011-9258-7. [DOI] [PubMed] [Google Scholar]
- 12.Badole SL, Patil KY. Pongamia pinnata (Linn.) Pierre and inflammation. In: Watson RR, Preedy VR, Zibadi S, editors. Ployphenols in Human Health and Diseases. Vol. 1. USA: Academic Press; 2014. pp. 463–5. [Google Scholar]
- 13.Asadulla S, Ramandang, Rajasekharan Pharmacognosy of Embelia ribes burm. IJRPC. 2011;1:1236–51. [Google Scholar]
- 14.Tiwari KS, Pandey KY. Ayurvedic drugs in prevention and management of age related cognitive decline: A review. Int J Pharma Sci Drug Res. 2012;4:183–90. [Google Scholar]
- 15.Nazam Ansari M, Bhandari U, Islam F, Tripathi CD. Evaluation of antioxidant and neuroprotective effect of ethanolic extract of Embelia ribes burm in focal cerebral ischemia/reperfusion-induced oxidative stress in rats. Fundam Clin Pharmacol. 2008;22:305–14. doi: 10.1111/j.1472-8206.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 16.Mahendran S, Thippeswamy BS, Veerapur VP, Badami S. Anticonvulsant activity of embelin isolated from Embelia ribes. Phytomedicine. 2011;18:186–8. doi: 10.1016/j.phymed.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Sangwan S, Rao DV, Sharma RA. A review on Pongamia Pinnata (L.) Pierre: A great versatile, leguminous plant. Nature Sci. 2010;8:130–39. [Google Scholar]
- 18.Sikarwar MS, Patil MB. Antidiabetic activity of pongamia pinnata leaf extracts in Alloxan-induced diabetic rats. Int J Ayurveda Res. 2010;1:199–204. doi: 10.4103/0974-7788.76780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manigauha A, Patel S, Monga J, Ali H. Evaluation of anticonvulsant activity of Pongamia pinnata Linn in experimental animals. Int J Pharm Tech Res. 2009;4:1119–21. [Google Scholar]
- 20.Katekhaye SD, Kale MS, Laddha KS. A simple and improved method for isolation of karanjin from Pongamia pinnata Linn. seed oil. Indian J Nat Prod Res. 2012;3:131–4. [Google Scholar]
- 21.Suthar M, Patel R, Hapani K, Patel A. Screening of Embelia ribes for antifungal activity. Int J Pharma Sci Drug Res. 2009;1:203–6. [Google Scholar]
- 22.The Merck Index. 14th ed. USA: Merch Research Laboratories, Merck and Co Inc; 2006. An Encyclopedia of Chemicals, Drugs and Biologicals; p. 3559. [Google Scholar]
- 23.Lanjhiyana S, Patra KC, Ahirwar D, Rana AC, Garabadu D, Lanjhiyana SK. Development and validation of a HPTLC method for determination of Karanjin in Pongamia pinnata: A novel India medicinal plant. Der Pharma Sinica. 2012;3:144–7. [Google Scholar]
- 24.Patel R, Kalindi H, Patel A. Screening of Embelia ribes for anti fungal activity. Int J Pharma Sci Drug Res. 2009;1:203–6. [Google Scholar]
- 25.Vismaya, Belagihally SM, Rajashekhar S, Jayaram VB, Dharmesh SM, Thirumakudalu SK, et al. Gastroprotective properties of karanjin from karanja (Pongamia pinnata) seeds; role as antioxidant and H, K-ATPase inhibitor. Evid Based Complement Alternat Med. 2011;2011:747246. doi: 10.1093/ecam/neq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamrakar AK, Yadav PP, Tiwari P, Maurya R, Srivastava AK. Identification of pongamol and karanjin as lead compounds with antihyperglycemic activity from Pongamia pinnata fruits. J Ethnopharmacol. 2008;118:435–9. doi: 10.1016/j.jep.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Souravi K, Rajasekharan PE. A review on the pharmacology of Embelia ribes Burm F-A threatened medicinal plant. IJPBS. 2014;5:443. [Google Scholar]
- 28.Thippeswamy BS, Mishra B, Veerapur VP, Gupta G. Anxiolytic activity of Nymphaea alba linn. In mice as experimental models of anxiety. Indian J Pharmacol. 2011;43:50–5. doi: 10.4103/0253-7613.75670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiran G, Rao GS, Krishan NS, Bhatla N, Jahnavi SV, Ayesha Begum S, et al. Memory enhancing activity of Ageratum conyzoides L. on corticosterone induced dementia in mice. Int J Pharmacol Screen Meth. 2012;2:77–81. [Google Scholar]
- 30.Nunez J. Morris water maze experiment. J Vis Exp. 2008 doi: 10.3791/897. pii: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 32.Lee B, Cao R, Choi YS, Cho HY, Rhee AD, Hah CK, et al. The CREB/CRE transcriptional pathway: Protection against oxidative stress-mediated neuronal cell death. J Neurochem. 2009;108:1251–65. doi: 10.1111/j.1471-4159.2008.05864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer's disease. Biochim Biophys Acta. 2000;1502:139–44. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 34.Choi BH. Oxygen, antioxidants and brain dysfunction. Yonsei Med J. 1993;34:1–0. doi: 10.3349/ymj.1993.34.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–23. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 36.Candelario-Jalil E, Al-Dalain SM, Castillo R, Martínez G, Fernández OS. Selective vulnerability to kainate-induced oxidative damage in different rat brain regions. J Appl Toxicol. 2001;21:403–7. doi: 10.1002/jat.768. [DOI] [PubMed] [Google Scholar]
- 37.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–43. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Mathew M, Subramanian S. In vitro evaluation of anti-Alzheimer effects of dry ginger (Zingiber officinale roscoe) extract. Indian J Exp Biol. 2014;52:606–12. [PubMed] [Google Scholar]
- 39.Joshi R, Kamat JP, Mukherjee T. 2007. radical scavenging reactions and antioxidant activity of embelin; pp. 125–34. [DOI] [PubMed] [Google Scholar]
- 40.Gupta R, Sharma AK, Sharma MC, Gupta RS. Antioxidant activity and protection of pancreatic β-cells by embelin in streptozotocin-induced diabetes. J Diabetes. 2012;4:248–56. doi: 10.1111/j.1753-0407.2012.00187.x. [DOI] [PubMed] [Google Scholar]
- 41.Arshad N, Rashid N, Absar S, Abbasi MS, Saleem S, Bushra Mirza B. UV-absorption studies of interaction of karanjin and karanjachromene with ds.DNA: Evaluation of binding and antioxidant activity. Cent Eur J Chem. 2013;11:2040–7. [Google Scholar]
- 42.Ghosh A, Mandal S, Banerji A, Kar M, Banerji J. A new chalcone from Pongamia pinnata and its antioxidant properties. Nat Prod Commun. 2009;4:209–10. [PubMed] [Google Scholar]
- 43.Kiran G, Rao GS. Memory enhancing activity of Ageratum conyzoides on corticosterone induced dementia in mice. IJPSM. 2012;2:133–6. [Google Scholar]
- 44.Béracochéa D, Tronche C, Coutan M, Dorey R, Chauveau F, Piérard C, et al. Interaction between diazepam and hippocampal corticosterone after acute stress: Impact on memory in middle-aged mice. Front Behav Neurosci. 2011;5:14. doi: 10.3389/fnbeh.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adamec RE, McNaughton B, Racine R, Livingston KE. Effects of diazepam on hippocampal excitability in the rat: Action in the dentate area. Epilepsia. 1981;22:205–15. doi: 10.1111/j.1528-1157.1981.tb04103.x. [DOI] [PubMed] [Google Scholar]
- 46.Rock DM, Taylor CP. Effects of diazepam, pentobarbital, phenytoin and pentylenetetrazol on hippocampal paired-pulse inhibition in vivo . Neurosci Lett. 1986;65:265–70. doi: 10.1016/0304-3940(86)90272-7. [DOI] [PubMed] [Google Scholar]
- 47.Musavi S, Kakkar P. Diazepam induced early oxidative changes at the subcellular level in rat brain. Mol Cell Biochem. 1998;178:41–6. doi: 10.1023/a:1006834706117. [DOI] [PubMed] [Google Scholar]
- 48.Prabhakar S, Saraf MK, Banik A, Anand A. Bacopa monniera selectively attenuates suppressed superoxide dismutase activity in diazepam induced amnesic mice. Ann Neurosci. 2011;18:8–13. doi: 10.5214/ans.0972.7531.1118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saraf MK, Kishore K, Thomas KM, Sharma A, Singh M. Role of platelet activating factor in triazolobenzodiazepines-induced retrograde amnesia. Behav Brain Res. 2003;142:31–40. doi: 10.1016/s0166-4328(02)00365-0. [DOI] [PubMed] [Google Scholar]
- 50.Tomaz C, Dickinson-Anson H, McGaugh JL. Basolateral amygdala lesions block diazepam-induced anterograde amnesia in an inhibitory avoidance task. Proc Natl Acad Sci U S A. 1992;89:3615–9. doi: 10.1073/pnas.89.8.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HK, Dunwiddie TV, Hoffer BJ. Interaction of diazepam with synaptic transmission in the in vitro rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1979;309:131–6. doi: 10.1007/BF00501220. [DOI] [PubMed] [Google Scholar]


